Note: Descriptions are shown in the official language in which they were submitted.
2195663
WO 96/03399 PCT/EP95/02841
Dihydrobenzofurans
Field of application of the invention
The invention relates to novel compounds which are used
in the pharmaceutical industry for the production of
medicaments.
Known technical background
International Patent Application W092/12961 describes
benzamides having PDE-inhibiting properties. -
International Patent Application W093/25517 discloses
trisubstituted phenyl derivatives as selective PDE-IV
inhibitors. - International Patent Application WO
94/02465 describes inhibitors of c-AMP
phosphodiesterase and of TNF.
Description of the invention
It has been found that the benzamides described in
greater detail below, which differ from the previously
published compounds by completely different
substitution in positions 2 and 3 on the benzamide,
have surprising and particularly advantageous
properties.
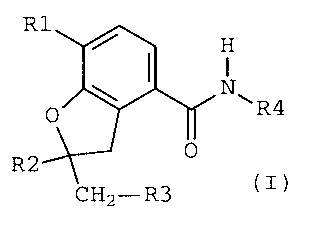
The invention thus relates to compounds of the formula
I(see attached formula sheet I) in which
R1 is 1-6C-alkoxy, 3-7C-cycloalkoxy, 3-7C-
cycloalkylmethoxy, benzyloxy or completely or
partially fluorine-substituted 1-4C-alkoxy,
R2 is 1-4C-alkyl and
R3 is hydrogen or 1-4C-alkyl,
or
R2 and R3, together and including the two carbon atoms
to which they are bonded, are a 5-, 6- or 7-
2195663
- 2 -
membered hydrocarbon ring, if desired interrupted
by an oxygen atom,
R4 is phenyl, pyridyl, R41-, R42- and R43-substituted
phenyl or R44-, R45-, R46- and R47-substituted
pyridyl, where
R41 is hydroxyl, halogen, cyano, carboxyl,
trifluoromethyl, 1-4C-alkyl, 1-4C-alkoxy,
1-4C-alkoxycarbonyl, 1-4C-alkylcarbonyl, 1-
4C-alkylcarbonyloxy, amino, mono- or di-1-4C-
alkylamino or 1-4C-alkylcarbonylamino,
R42 is hydrogen, hydroxyl, halogen, amino,
trifluoromethyl, 1-4C-alkyl or 1-4C-alkoxy,
R43 is hydrogen, halogen, 1-4C-alkyl or 1-4C-
alkoxy,
R44 is hydroxyl, halogen, cyano, carboxyl, 1-4C-
alkyl, 1-4C-alkoxy, 1-4C-alkoxycarbonyl or
amino,
R45 is hydrogen, halogen, amino or 1-4C-alkyl,
R46 is hydrogen or halogen and
R47 is hydrogen or halogen,
the salts of these compounds, and the N-oxides of the
pyridines and their salts.
1-6C-alkoxy is a radical which, beside the oxygen atom,
contains a straight-chain or branched alkyl radical
having 1 to 6 carbon atoms. Alkyl radicals having 1 to
6 carbon atoms which may be mentioned here are, for
example, the hexyl, isohexyl (2-methylpentyl), neohexyl
(2,2-dimethylbutyl), pentyl, isopentyl (3-methylbutyl),
neopentyl (2,2-dimethylpropyl), butyl, isobutyl, sec-
butyl, tert-butyl, propyl, isopropyl, ethyl and methyl
radicals.
3-7C-cycloalkoxy is, for example, cyclopropyloxy,
cyclobutyloxy, cyclopentyloxy, cyclohexyloxy and
cycloheptyloxy, of which cyclopropyloxy, cyclobutyloxy
and cyclopentyloxy are preferred.
2195663
- 3 -
3-7C-cycloalkylmethoxy is, for example, cyclopropyl-
methoxy, cyclobutylmethoxy, cyclopentylmethoxy, cyclo-
hexylmethoxy and cycloheptylmethoxy, of which
cyclopropylmethoxy, cyclobutylmethoxy and cyclopentyl-
methoxy are preferred.
Completely or partially fluorine-substituted 1-4C-
alkoxy radicals which may be mentioned are, for
example, the 1,2,2-trifluoroethoxy, the 2,2,3,3,3-
pentafluoropropoxy, the perfluoroethoxy and in
particular the 1,1,2,2-tetrafluoroethoxy, the
trifluoromethoxy, the 2,2,2-trifluoroethoxy and the
difluoromethoxy radicals.
5-, 6- or 7-membered hydrocarbon rings, if desired
interrupted by an oxygen atom, which may be mentioned
are the cyclopentane, the cyclohexane, the
cycloheptane, the tetrahydrofuran and the
tetrahydropyran rings. If R2 and R3, together and
including the two carbon atoms to which they are
bonded, form a 5-, 6- or 7-membered ring, a spiro
compound is present.
1-4C-alkyl is straight-chain or branched alkyl radicals
having 1 to 4 carbon atoms. Examples which may be
mentioned are the butyl, isobutyl, sec-butyl, tert-
butyl, propyl, isopropyl, ethyl and methyl radicals.
Halogen within the meaning of the present invention is
bromine, chlorine or fluorine.
1-4C-alkoxy is a radical which, beside the oxygen atom,
contains one of the abovementioned 1-4C-alkyl radicals.
Examples which may be mentioned are the methoxy and the
ethoxy radicals.
1-4C-alkoxycarbonyl is a carbonyl group to which one of
the abovementioned 1-4C-alkoxy radicals is bonded.
2195663
- 4 -
Examples which may be mentioned are the methoxycarbonyl
(CH3O-CO-) and the ethoxycarbonyl radicals (CH3CH2O-CO).
1-4C-alkylcarbonyl is a carbonyl group to which one of
the abovementioned 1-4C-alkyl radicals is bonded. An
example which may be mentioned is the acetyl radical
(CH3CO-).
1-4C-alkylcarbonyloxy radicals contain, beside the
oxygen atom, one of the abovementioned 1-4C-
alkylcarbonyl radicals. An example which may be
mentioned is the acetoxy radical (CH3CO-O-).
Mono- or di-1-4C-alkylamino radicals which may be
mentioned are, for example, the methylamino, the
dimethylamino and the diethylamino radicals.
A 1-4C-alkylcarbonylamino radical which may be
mentioned is, for example, the acetylamido radical
(NH-CO-CH3) .
Exemplary, R41-, R42- and R43-substituted phenyl
radicals which may be mentioned are the radicals
2-acetylphenyl, 2-aminophenyl, 2-bromophenyl, 2-chloro-
phenyl, 2,3-dichlorophenyl, 2,4-dichlorophenyl, 4-di-
ethylamino-2-methylphenyl, 4-bromo-2-trifluoromethyl-
phenyl, 2-carboxy-5-chlorophenyl, 3,5-dichloro-2-hy-
droxyphenyl, 2-bromo-4-carboxy-5-hydroxyphenyl, 2,6-di-
chlorophenyl, 2,5-dichlorophenyl, 2,4,6-trichloro-
phenyl, 2,4,6-trifluorophenyl, 2,6-dibromophenyl,
2-cyanophenyl, 4-cyano-2-fluorophenyl, 2-fluorophenyl,
2,4-difluorophenyl, 2,6-difluorophenyl, 2-chloro-
6 fluorophenyl, 2-hydroxyphenyl, 2-hydroxy-4-methoxy-
phenyl, 2,4-dihydroxyphenyl, 2-methoxyphenyl, 2,3-di-
methoxyphenyl, 2,4-dimethoxyphenyl, 2,6-dimethoxy-
phenyl, 2-dimethylaminophenyl, 2-methylphenyl,
2-chloro-6-methylphenyl, 2,4-dimethyiphenyl, 2,6-di-
methylphenyl, 2,3-dimethylphenyl, 2-methoxycarbon-
ylphenyl, 2-trifluoromethylphenyl, 2,6-dichioro-
2195663
- 5 -
4-methoxyphenyl, 2,6-dichloro-4-cyanophenyl, 2,6-di-
chloro-4-aminophenyl, 2,6-dichloro-4-methoxycarbonyl-
phenyl, 4-acetylamino-2,6-dichlorophenyl and 2,6-di-
chloro-4-ethoxycarbonylphenyl.
Exemplary, R44-, R45-, R46- and R47-substituted pyridyl
radicals which may be mentioned are the radicals
3,5-dichloropyrid-4-yl, 2,6-diaminopyrid-3-yl, 4-amino-
pyrid-3-yl, 3-methylpyrid-2-yl, 4-methylpyrid-2-yl,
5-hydroxypyrid-2-yl, 4-chloropyrid-3-yl, 3-chloropyrid-
2-yl, 3-chloropyrid-4-yl, 2-chloropyrid-3-yl,
2,3,5,6-tetrafluoropyrid-4-yl, 3,5-dichloro-2,6-difluo-
ropyrid-4-yl, 3,5-dibromopyrid-2-yl, 3,5-dibromopyrid-
4-yl, 3,5-dichloropyrid-4-yl, 2,6-dichloropyrid-3-yl,
3,5-dimethylpyrid-4-yl, 3-chloro-2,5,6-trifluoropyrid-
4-yl and 2,3,5-trifluoropyrid-4-yl.
Suitable salts of compounds of the formula I -
depending on substitution - are all acid addition
salts, but in particular all salts with bases.
Particular mention may be made of the pharmacologically
tolerable salts of the inorganic and organic acids and
bases customarily used in pharmacy. Pharmacologically
nontolerable salts which, for example, are initially
formed as process products during the preparation of
the compounds according to the invention on the
industrial scale, are converted into pharmacologically
tolerable salts by processes known to the person
skilled in the art. Those which are suitable are, on
the one hand, water-soluble and water-insoluble acid
addition salts with acids such as, for example,
hydrochloric acid, hydrobromic acid, phosphoric acid,
nitric acid, sulfuric acid, acetic acid, citric acid,
D-gluconic acid, benzoic acid, 2-(4-hydroxybenzoyl)-
benzoic acid, butyric acid, sulfosalicylic acid, maleic
acid, lauric acid, malic acid, fumaric acid, succinic
acid, oxalic acid, tartaric acid, embonic acid, stearic
acid, toluenesulfonic acid, methanesulfonic acid or 3-
hydroxy-2-naphthoic acid, it being possible for the
2195663
- 6 -
acids to be employed in salt preparation - depending on
whether it is a mono- or polybasic acid and depending
on what salt is required - in an equimolar quantitative
ratio or one differing therefrom.
On the other hand, salts with bases are also especially
suitable. Examples of basic salts which may be
mentioned are lithium, sodium, potassium, calcium,
aluminum, magnesium, titanium, ammonium, meglumine or
guanidinium salts, here too the bases being employed
during salt preparation in an equimolar quantitative
ratio or one differing therefrom.
Compounds of the formula I to be emphasized are those
in which
R1 is 1-4C-alkoxy, 3-5C-cycloalkoxy, 3-5C-
cycloalkylmethoxy, or completely or partially
fluorine-substituted 1-4C-alkoxy,
R2 is 1-4C-alkyl and
R3 is hydrogen or 1-4C-alkyl,
or
R2 and R3, together and including the two carbon atoms
to which they are bonded, are a cyclopentane,
cyclohexane, tetrahydrofuran or tetrahydropyran
ring,
R4 is phenyl, pyridyl, R41-, R42- and R43-substituted
phenyl or R44-, R45-, R46- and R47-substituted
pyridyl, where
R41 is halogen, cyano, carboxyl, 1-4C-alkyl,
1-4C-alkoxy, or 1-4C-alkoxycarbonyl,
R42 is hydrogen, halogen, 1-4C-alkyl or 1-4C-
alkoxy,
R43 is hydrogen, halogen, 1-4C-alkyl or 1-4C-
alkoxy,
R44 is halogen or 1-4C-alkyl,
R45 is hydrogen or halogen,
R46 is hydrogen or halogen and
R47 is hydrogen or halogen,
2195663
_ 7 -
the salts of these compounds, and the N-oxides of the
pyridines and their salts.
Compounds of the formula I particularly to be
emphasized are those in which
R1 is 1-4C-alkoxy, 3-5C-cycloalkoxy, 3-5C-
cycloalkylmethoxy, or completely or partially
fluorine-substituted 1-4C-alkoxy,
R2 is 1-4C-alkyl and
R3 is hydrogen or 1-4C-alkyl,
or
R2 and R3, together and including the two carbon atoms
to which they are bonded, are a cyclopentane,
cyclohexane, tetrahydrofuran or tetrahydropyran
ring,
R4 is 2-bromophenyl, 2,6-dichloro-4-ethoxycarbonyl-
phenyl, 2,6-dimethoxyphenyl, 4-cyano-2-fluoro-
phenyl, 2,4,6-trifluorophenyl, 2-chloro-6-methyl-
phenyl, 2,6-dimethylphenyl, 2-chloro-6-fluoro-
phenyl, 2,6-difluorophenyl, 2,6-dichlorophenyl,
3,5-dichloropyrid-4-yl, 3-methylpyrid-2-yl,
2-chloropyrid-3-yl, 3,5-dibromopyrid-2-yl, 3,5-di-
fluoropyrid-4-yl, 2-chlorophenyl, 2,3,5,6-tetra-
fluoropyrid-4-yl, 3-chloro-2,5,6-trifluoropyrid-
4-yl, 3,5-dichloro-2,6-difluoropyrid-4-yl or
2,6-dichloropyrid-3-yl,
the salts of these compounds, and the N-oxides of the
pyridines and their salts.
Preferred compounds of the formula I are those in which
R1 is methoxy, ethoxy, cyclopropylmethoxy,
difluoromethoxy, trifluoromethoxy or 2,2,2-
trifluoroethoxy,
R2 is methyl or ethyl and
R3 is hydrogen or methyl,
or
R2 and R3, together and including the two carbon atoms
to which they are bonded, are a cyclopentane,
2195663
- 8 -
cyclohexane, tetrahydrofuran or tetrahydropyran
ring,
R4 is 2-bromophenyl, 2,6-dichloro-4-ethoxycarbonyl-
phenyl, 2,6-dimethoxyphenyl, 4-cyano-2-fluoro-
phenyl, 2,4,6-trifluorophenyl, 2-chloro-6-methyl-
phenyl, 2,6-dimethylphenyl, 2-chloro-6-fluoro-
phenyl, 2,6-difluorophenyl, 2,6-dichlorophenyl,
3,5-dichloropyrid-4-yl, 3-methylpyrid-2-yl,
2-chloropyrid-3-yl, 3,5-dibromopyrid-2-yl, 3,5-di-
fluoropyrid-4-yl, 2-chlorophenyl, 2,3,5,6-tetra-
fluoropyrid-4-yl, 3-chloro-2,5,6-trifluoropyrid-
4-yl, 3,5-dichloro-2,6-difluoropyrid-4-yl or
2,6-dichloropyrid-3-yl,
the salts of these compounds, and the N-oxides of the
pyridines and their salts.
Particularly preferred compounds of the formula I are
those in which
R1 is methoxy, ethoxy, cyclopropylmethoxy,
difluoromethoxy, trifluoromethoxy or 2,2,2-
trifluoroethoxy,
R2 is methyl or ethyl and
R3 is hydrogen or methyl,
or
R2 and R3, together and including the two carbon atoms
to which they are bonded, are a cyclopentane,
cyclohexane, tetrahydrofuran or tetrahydropyran
ring,
R4 is 3,5-dichloropyrid-4-yl, 2,6-dichlorophenyl or
2,6-difluorophenyl,
the salts of these compounds, and the N-oxides of the
pyridines and their salts.
Exemplary compounds according to the invention are
listed in the following tables:
2195663
- 9 -
Table 1
Compounds of the formula I (see attached formula sheet
I) with R4=3,5-dichloropyrid-4-yl and the following
further substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5 CH3 H
OCHZC3H5 CH3 H
OCF2H CH3 H
OCF3 CH3, H
OCH2CF3 CH3 H
OCH3 C2H5 CH
0CZH5 C2H5 CH3
OCH2C3H5 CZH5 CH3
OCF2H CZH5 CH3
OCF3 C2H5 CH3
OCHZCF3 C2H5 CH3
OCH3 CH2CH2CH2
0C2H5 CH2CH2CH2
0CH2C3H5 CH2CH2CH2
OCF2H CH2CH2CH2
OCF3 CH2CH2CH2
OCH2CF3 CH2CHZCH2
OCH3- CH2CH2CH2CH2
0C2H5 CH2CH2CH2CH2
OCH2C3H5 CH2CH2CH2CH2
OCF2H CH2CH2CHZCH2
OCF3 CHZCH2CHZCHZ
OCH2CF3 CH2CH2CH2CH2
OCH3 CH2-0-CH2
0C2H5 CH2-0-CH2
OCH2C3H5 CH2-0-CH2
OCF2H CHZ-0-CH2
OCF3 CH2-0-CH2
OCHZCF3 CHZ-0-CH2
OCH3 CH2CH2-0
0C2H5 CH2CH2-0
OCH2C3H5 CH2CH2-0
OCFZH CH2CH2-0
2195663
- 10 -
Continuation of Table 1
RI R2 R3
OCF3 CH2CH2-0
OCHZCF3 C-H 2CH2-0
OCH3 CH2CH2-0-CH2
0CZH5 CH2CH2-0-CH2
OCH2C3H5 CH2CH2-0-CH2
OCF2H CHZCH2-0-CH2
OCF3 CH2CH2-0-CH2
OCHZCF3 CHZCH2-0-CH2
Table 2
Compounds of the formula I (see attached formula sheet
I) with R4=2,6-dichlorophenyl and the following further
substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5_ CH3 H
OCH2C3H5 CH3 H
OCF2H CH3 H
OCF3 CH3 H
OCH2CF3 CH3 H
OCH3 C2H5 CH3
0C2H5 C2H5 CH3
OCH2C3H5 C2H5 CH3
OCF2H C2H5 CH3
OCF3 CZH5 CH3
OCH2CF3 C2H5 CH3
OCH3 CH2CH2CH2
0CZH5 CHZCH2CH2
OCH2C3H5 CHZCH2CH2
OCF2H CHZCHZCH2
OCF3 CH2CH2CH2
2195663
-~~-
Continuation of Table 2
R1 R2 R3
OCHZCF3 Cti2C112CH2
OCH3 CH2CH2CH2CH2
0C2H5 CHZCH2CH2CH2
OCH2C3H5 CH2CH2CH2CH2
OCF2H CH2CH2CH2CH2
OCF3 CH2CH2CHZCH2
OCHZCF3 CH2CH2CH2CH2
OCH3 CH2-0-CH2
0C2H5 CH2-0-CH2
OCH2C3H5 CH2-0-CH2
OCF2H CH2-0-CH2
OCF3 CH2-0-CH2
OCH2CF3 CH2-0-CH2
OCH3 CH2CH2-0
OC2H5 CH2CH2-0
OCH2C3H5 CH2CH2-0
OCF2H CH2CH2-0
OCF3 CH2CH2-0
OCH2CF3 CH2CH2-0
OCH3 CH2CH2-0-CH2
0CZH5_ CHZCH2-0-CH2
OCH2C3H5 CH2CH2-0-CH2
OCF2H CH2CH2-0-CH2
OCF3 CH2CH2-0-CH2
OCH2CF3 CH2CH2-0-CH2
2195663
- 12 -
Table 3
Compounds of the formula I (see attached formula sheet
I) with R4-2,6-difluorophenyl and the following further
substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5 CH3 H
OCH2C3H5 CH3 H
OCF2H CH3 H
OCF3 CH3 H
OCHZCF3 CH3 H
OCH3 CZH5 CH3
0C2H5 CZH5 CH3
OCH2C3H5 C2H5 CH3
OCF2H CZH5 CH3
OCF3 C2H5 CH3
OCH2CF3 C2H5 CH3
OCH3 CH2CH2CH2
0CZH5 CH2CH2CH2
OCH2C3H5 CH2CH2CH2
OCF2H CH2CH2CH2
OCF3 CH2CH2CH2
OCH2CF3 CH2CH2CH2
OCH3 - CH2CHZCHZCH2
0CZH5 CH2CH2CH2CH2
OCH2C3H5 CH2CH2CH2CH2
OCFZH CH2CH2CHZCH2
OCF3 CHZCH2CHZCH2
OCH2CF3 CH2CH2CH2CH2
OCH3 CH2-0-CH2
0C2H5 CH2-0-CH2
OCH2C3H5 CH2-0-CH2
OCF 2H CH2-0-CH2
OCF3 CH2-0-CHZ
OCH2CF3 CH2-0-CH2
OCH3 CH2CH2-0
0C2H5 CH2CH2-0
OCH2C3H5 CH2CH2-0
OCF2H CH2CH2-0
13 2195663
- -
Continuation of Table 3
RI R2 R3
OCF3 CH2CH2-0
OCH2CF3 CH2CH2-0
OCH3 CH2 CH2-0-CH2
0C2H$ CH2CH2-0-CH2
OCHZC3H5 CH2CH2-0-CH2
OCF2H CH2CH2-0-CHZ
OCF3 CH2CH2-0-CH2
OCH2CF3 CH2CH2-0-CH2
Table 4
Compounds of the formula I (see attached formula sheet
I) with R4=2-chloropyrid-3-yl and the following further
substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5 CH3 H
OCHZC3H5 CH3 H
OCFZH CH3 H
OCF3 CH3 H
OCH2CF3 CH3 H
OCH3 C2H5 CH3
0C2H5- C2H5 CH3
OCH2C3H5 C2H5 CH3
OCF2H C2H5 CH3
OCF3 C2H5 CH3
OCH2CF3 C2H5 CH3
OCH3 CH2CH2CH2
0C2H5 CH2CH2CH2
OCH2C3H5 CH2CH2CH2
OCF2H CH2CH2CH2
OCF3 CH2 CH2CH2
OCHZCF3 CH2CH2CH2
OCH3 CH2CH2CH2 CH2
0C2H5 CH2CHZCHZCH2
OCH2C3H5 CH2CH2CH2CH2
OCF2H CH2CH2CH2 CH2
OCF3 CH2CH2CHZCH2
2195663
- 14 -
Continuation of Table 4
R1 R2 R3
OCH2CF3 CH2CH2CH2CH2
OCH3 CH2-0-CH2
0C2H5 CH2-0-CH2
OCHZC3H5 CH2-0-CH2
OCF2H CH2-0-CH2
OCF3 CH2-0-CH2
OCH2CF3 CH2-0-CH2
OCH3 CH2CH2-0
0C2H5 CH2CH2-0
OCH2C3H5 CH2CH2-0
OCF2H CH2CH2-0
OCF3 CH2CH2-0
OCH2CF3 CH2CH2-0
OCH3 CH2CH2-0-CH2
0C2H5 CH2CH2-0-CH2
OCH2C3H5 CH2CH2-0-CH2
OCF2H CH2CH2-0-CH2
OCF3 CH2CH2-0-CH2
OCH2CF3 CH2CH2-0-CH2
Table 5
Compounds of the formula I (see attached formula sheet
I) with R4=2-chloro-6-methylphenyl and the following
further substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5 CH3 H
OCH2C3H5 CH3 H
OCF2H CH3 H
OCF3 CH3 H
OCH2CF3 CH3 H
OCH3 C2H5 CH3
0C2H5 C2H5 CH3
OCHZC3H5 C2H5 CH3
OCF2H C2H5 CH3
OCF3 C2H5 CH3
OCH2CF3 C2H5 CH3
2195663
- 15 -
Continuation of Table 5
RI R2 R3
OCH3 CH2CH2CH2
0C2H$ CH2CH2CH2
OCH2C3H5 CH2CH2CH2
OCF2H CH2CH2CH2
OCF3 CHZCH2CH2
OCH2CF3 CH2CHZCH2
OCH3 CH2CH2CH2CH2
0C2H5 CH2CH2CH2CH2
OCH2C3H5 CH2CH2CH2CH2
OCFZH CHZCH2CH2CH2
OCF3 CH2CH2CHZCH2
OCH2 CF3 CH2CH2CH2CH2
OCH3 CH2-0-CH2
0C2H5 CH2-0-CH2
OCH2C3H5 CH2-0-CH2
OCF2H CH2-0-CH2
OCF3 CH2-0-CH2
OCH2CF3 CH2-0-CHZ
OCH3 CH2CH2-0
0CZH5 CH2CH2-0
OCH2C3H5 CHZCH2-0
OCFZH CH2CH2-0
OCF3 CH2CH2-0
OCH2CF3 CHZCH2-0
0CH3 CH2CH2-0-CH2
0C2H5 CHZCH2-0-CH2
OCH2C3H5 CH2CHZ-0-CHZ
OCF2H CH2CH2-0-CH2
0CF3 CH2CH2-0-CH2
OCH2CF3 CH2CH2-0-CH2
2195663
- 16 -
Table 6
Compounds of the formula I (see attached formula sheet
I) with R4=2-chloro-6-fluorophenyl and the following
further substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5 CH3 H
OCH2C3H5 CH3 H
OCr"2H CH3 H
OCF3 CH3 H
OCH2CF3 CH3 H
OCH3 C2H5 CH3
0C2H5 C2H5 CH3
OCH2C3H5 C2H5 CH3
OCFZH C2H5 CH3
OCF3 CZH5 CH3
OCH2CF3 C2H5 CH3
OCH3 CH2CH2CH2
0CZH5 CH2CH2CH2
OCH2C3H5 CH2CH2CH2
OCF2H CH2CH2CH2
OCF3 CH2CH2CH2
OCH2CF3 CH2CH2CH2
OCH3 - CH2CH2CH2CH2
0C2H5 CHZCH2CHZCH2
OCH2C3H5 CH2CH2CH2CH2
OCF2H CH2CH2CH2CH2
OCF3 CHZCH2CH2CH2
OCH2CF3 CH2CH2CH2CH2
OCH3 CH2-0-CH2
0C2H5 CH2-0-CH2
OCH2C3H5 CH2-0-CH2
OCF2H CH2-0-CH2
OCF3 CH2-0-CH2
OCH2CF3 CH2-0-CH2
OCH3 CH2CH2-0
0C2H5 CH2CH2-0
OCH2C3H$ CH2CH2-0
OCF2H CH2CH2-0
2195663
-17-
Continuation of Table 6
RI R2 R3
OCF3 CH2CH2-0
OCHZCF3 CH2CH2-0
OCH3 CH2CH2-0-CH2
0C2H5 CH2CH2-0-CH2
OCH2C3H5 CH2CH2-0-CH2
OCF2H CH2CH2-0-CH2
OCF3 CH2CH2-0-CH2
OCHZCF3 CHZCH2-0-CH2
Table 7
Compounds of the formula I (see attached formula sheet
I) with R4=3,5-difluoropyrid-4-yl and the following
further substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5 CH3 H
OCHZC3H5 CH3 H
OCF2H CH3 H
OCF3 CH3 H
OCH2CF3 CH3 H
OCH3 C2H5 CH3
0CZH5 C2H5 CH3
OCH2C3H5 C2H5 CH3
OCF2H CZH5 CH3
OCF3 C2H5 CH3
OCH2CF3 C2H5 CH3
OCH3 CHZCH2CH2
0C2H5 CH2CH2CH2
OCH2C3H5 CH2CH2CH2
OCF2H CHZCH2CH2
OCF3 CH2CHZCH2
OCH2CF3 CHZCH2CH2
OCH3 CH2CHZCH2CH2
0CZH5 CH2CHZCHZCHZ
2195663
-18-
Continuation of Table 7
RI R2 R3
OCH2C3H5 CHZCH2CHZCH2
OCF2H CH2CH2CH2CH2
OCF3 CH2CH2CH2CH2
OCH2CF3 CH2CH2CH2CH2
OCH3 CH2-0-CH2
0C2H5 CHZ-0-CH2
OCHZC3H5 CHZ-0-CH2
OCF2H CH2-0-CH2
OCF3 CHZ-0-CH2
OCH2CF3 CH2-0-CH2
OCH3 CH2CH2-0
0C2H5 CH2CH2-0
OCHZC3H5 CH2CH2-D
OCF2H CH2CH2-0
OCF3 CH2CH2-0
OCH2CF3 CH2CH2-0
OCH3 CH2CH2-0-CH2
0C2H5 CH2CH2-0-CH2
OCH2C3H5 CH2CH2-0-CH2
OCFZH CH2CH2-0-CH2
OCF3 CHZCH2-0-CHZ
OCH2CF3 CHZCH2-0-CHZ
Table 8
Compounds of the formula I (see attached formula sheet
I) with R4=2-chlorophenyl and the following further
substituents meanings:
RI R2 R3
OCH3 CH3 H
0C2H5 CH3 H
OCH2C3H5 CH3 H
OCF2H CH3 H
OCF3 CH3 H
OCH2CF3 CH3 H
OCH3 C2H5 CH3
2195663
- 19 -
Continuation of Table 8
RI R2 R3
0C2H5 C2H5 CH3
OCH2C3H5 C2H5 CH3
OCF2H C2H5 CH3
OCF3 C2H5 CH3
OCH2CF3 C2H5 CH3
OCH3 CH2CH2CH2
CCF3 CH2CHZCH2
OCH2CF3 CH2CHZCH2
OCH3 CHZCH2CH2CH2
0C2H5 CHZCH2CH2CH2
0CH2C3H5 CHZCHZCH2CH2
OCF2H CH2CH2CH2CH2
0C2H5. CHZCHZCH2
OCHZC3H5 CHZCHZCH2
OCF2H CH2CH2CH2
OCF3 CH2CHZCH2CH2
OCHZCF3 CH2CHZCH2CH2
OCH3 CH2-0-CH2
0CZH5 CH2-0-CH2
OCHZC3H5 CH2-0-CH2
OCFZH CH2-0-CHZ
OCF3 - CH2-0-CN2
OCH2CF3 CH2-0-CHZ
OCH3 CHZCH2-0
0C2H5 CHZCHZ-0
OCH2C3H5 CH2CH2-0
OCF2H CH2CH2-0
OCF3 CHZCH2-0
OCHZCF3 CH2CH2-0
OCH3 CH2CH2-0-CH2
OC2H5 CH2CH2-0-CH2
OCH2C3H5 CHZCH2-0-CH2
OCFZH CH2CH2-0-CH2
OCF3 CHZCH2-0-CH2
OCH2CF3 CHZCH2-0-CH2
2195663
- 20 -
and the salts of the compounds mentioned in the tables.
The compounds of the formula I are chiral compounds if
the substituents -R2 and -CH2R3 are not identical. The
invention therefore comprises both the pure enantiomers
and their mixtures in any mixing ratio, including the
racemates.
The invention further relates to a process for the
preparation of the compounds of the formula I and their
salts, and also the N-oxides of the pyridines and their
salts. The process comprises reacting compounds of the
formula II (see attached formula sheet I), in which R1,
R2 and R3 have the meanings indicated above and X is a
suitable leaving group, with amines R4-NH2, and, if
desired, then converting compounds of the formula I
obtained into their salts and/or converting pyridines
obtained into the N-oxides and, if desired, then into
the salts, or, if desired, then converting salts of the
compounds of the formula I obtained into the free
compounds.
The person skilled in the art is familiar on the basis
of his expert knowledge with suitable leaving groups X.
For example, starting materials are suitable acid
halides of the formula II (X=Cl or Br). Otherwise, the
reaction is carried out, for example, as described in
the following examples, or in a manner familiar per se
to the person skilled in the art (e.g. as described in
the International Patent Application WO 92/12961).
The N-oxidation is carried out in a manner which is
also familiar to the person skilled in the art, e.g.
with the aid of m-chloroperoxybenzoic acid in
dichloromethane at room temperature. The person skilled
in the art is familiar on the basis of his expert
knowledge with the reaction conditions which are
specifically necessary for carrying out the process.
2195663
- 21 -
The isolation and purification of the substances
according to the invention is carried out in a manner
known per se, for example by distilling off the solvent
in vacuo and recrystallizing the residue obtained from
a suitable solvent or subjecting it to one of the
customary purification methods, such as, for example,
column chromatography on a suitable support material.
Salts are obtained by dissolving the free compound in a
suitable solvent, e.g. in a chlorinated hydrocarbon,
such as methylene chloride or chloroform, or a low
molecular weight aliphatic alcohol (ethanol,
isopropanol), which contains the desired acid or base,
or to which the desired acid or base is then added. The
salts are obtained by filtering, reprecipitating,
precipitating with a nonsolvent for the addition salt
or by evaporating the solvent. Salts obtained can be
converted by basification or by acidification into the
free compounds which, in turn, can be converted into
salts. In this manner, pharmacologically nontolerable
salts can be converted into pharmacologically tolerable
salts.
The compounds of the formula II can be prepared
according to the general reaction scheme on the
attached formula sheet II. By way of example, the
preparation of compounds of the formula II is described
in the following examples under "starting compounds".
The preparation of further compounds of the formula II
can be carried out in an analogous manner.
The amines R4-NH2 are known, or they can be prepared in
a known manner.
The following examples illustrate the invention in
greater detail, without restricting it.
2195663
- 22 -
The abbreviation RT stands for room temperature, h
stands for hour(s), min for minute(s) and m.p. for
melting point.
Examnes
Final products
1. 2 3-Dihydro-2 2-dimethyl-7-methoxvbenzofuran-
4-carbggylic acid-N-3.5-dichloro-4-pyridyla?nide
0.22 g of sodium hydride (80 % in paraffin) is
suspended in 20 ml of anhydrous THF and a solution of
0.5 g of 4-amino-3,5-dichloropyridine in 5 ml of abs.
THF is then added dropwise with stirring. The mixture
is stirred for 30 min and a solution of 2,3-dihydro-
2,2-dimethyl-7-methoxybenzofuran-4-carbonyl chloride
(prepared from 0.8 g of 2,3-dihydro-2,2-dimethyl-7-
methoxybenzofuran-4-carboxylic acid, see example Al) in
10 ml of abs. THF is then added dropwise. After 10 min,
it is poured onto water, adjusted to pH 4 with 2N HC1,
extracted 3 times with ethyl acetate, and the combined
extracts are dried over sodium sulfate and filtered.
The oily residue which remains after concentrating in a
rotary evaporator is chromatographed on a silica gel
column using dichloromethane/methanol (98:2). The
chromatographically pure fractions are combined,
concentrated and crystallized using diethyl ether.
0.7 g of the title compound of m.p. 140-142 C is
obtained.
2. 2 3-Dihydro-7-methoxybenzofuran-2-sairo-1'-cyc o-
aeõ*a^-4-carboxvlic acid-N-3.5-dichloro-4-8Yridvl-
amide
Analogously to Example 1, 0.55 g of sodium hydride
(80 % in paraffin) in 50 ml of abs. THF, 1.5 g of 4-
amino-3,5-dichloropyridine in 20 ml of abs. THF and
2.5 g of 2,3-dihydro-7-methoxybenzofuran-2-spiro-
2195663
- 23 -
1'-cyclopentane-4-carbonyl chloride give 1.4 g of the
title compound of m.p. 168-170 C (from diethyl ether).
3. 7-Difluoromethoxv-2 3-dihxdrobenzofuran-2-sviro-
1'-cyclopentane-4-carboxvlic acid-N-3.5-dichioro-
4-p,vridylamide
Analogously to Example 1, 0.15 g of sodium hydride
(80 % in paraffin) in 20 ml of abs. THF, 0.41 g of 4-
amino-3,5-dichloropyridine in 10 ml of abs. THF and a
solution of 7-difluoromethoxy-2,3-dihydrobenzofuran-2-
spiro-1'-cyclopentane-4-carbonyl chloride (prepared
from 0.7 g of 7-difluoromethoxy-2,3-dihydrobenzofuran-
2-spiro-1'-cyclopentane-4-carboxylic acid, see Example
Cl) in 10 ml of abs. THF give, after chromatography
(silica gel, eluent: ethyl acetate/petroleum ether
4:6), 0.15 g of the title compound of m.p. 152-153 C
(from diisopropyl ether).
4. 2 3-dihydro-7-methoxvbenzofuran -2-sE7iro-1'-cyc o-
hexane-4-carboxvlic acid-N-3 5-dichloro-4-pyridv -
amide
Analogously to Example 1, 0.46 g of sodium hydride
(80 %_in paraffin) in 20 ml of abs. THF, 1.24 g of 4-
amino- 3, 5 -dichloropyridine in 20 ml of abs. THF and a
solution of 2,3-dihydro-7-methoxybenzofuran-2-spiro-1'-
cyclohexane-4-carbonyl chloride (prepared from 2 g of
2,3-dihydro-7-methoxybenzofuran-2-spiro-1'-cyclohexane-
4-carboxylic acid, see Example D1) in 20 ml of abs. THF
give 2.9 g of the title compound of m.p.: 169-170 C.
5. 2 3-Dihydro-7-methoxvbenzofuran-2-sFiro-1'-
(4'-oxacyclohexane)-4-carboxylic acid-N-3,5-di-
3 5 chloro - 4 -pyridylamide
Analogously to Example 1, 0.22 g of sodium hydride in
ml of abs. THF, 0.62 g of 4-amino-3,5-di-
chloropyridine in 20 ml of abs. THF and a solution of
2195663
- 24 -
2,3-dihydro-7-methoxybenzofuran-2-spiro-l'-(4'-oxa-
cyclohexane)-4-carbonyl chloride (prepared from 1 g of
2,3-dihydro-7-methoxybenzofuran-2-spiro-1'-(4'-oxa-
cyclohexane)-4-carboxylic acid, see Example El) in
10 ml of abs. THF give 0.3 g of the title compound of
m.p.: 208-210 C.
6. 2,2-Diethyl-2.3-dihydro-7-methoxvbenzofuran-4-car-
boxvlic acid-N-3.5-dichloro-4-gyri lamide
Analogously to Example 1, 0.3 g of sodium hydride (80 ~
in paraffin) in 20 ml of abs. THF, 0.8 g of 4-amino-
3,5-dichloropyridine in 10 ml of abs. THF and a
solution of 2,2-diethyl-2,3-dihydro-7-methoxy-
benzofurancarbonyl chloride (prepared from 1.2 g of
2,2-diethyl-2,3-dihydro-7-methoxybenzofuran-4-car-
boxylic acid, see Example Fl) in 20 ml of THF give,
after chromatography (silica gel, dichloromethane,
methanol 98:2), 0.9 g of the title compound of m.p.:
171-172 C.
7. 2.3-Dihydro-7-methoxybenzofuran-2-sviro-
1'-cyclopentane-4-carboxylic acid-2,6-dichloro-
anilide
A solution of 0.65 g of 2,6-dichloroaniline and 0.7 ml
of triethylamine in 20 ml of dioxane is warmed to
40-50 C and a solution of 2,3-dihydro-7-
methoxybenzofuran-2-spiro-1'-cyclopentane-4-carbonyl
chloride (prepared from 1 g of 2,3-dihydro-7-
methoxybenzofuran-2-spiro-1'-cyclopentane-4-carboxylic
acid, see Example Al) in 10 ml of dioxane is then added
dropwise. The mixture is stirred at 50 C for 1 h, then
it is poured onto water and extracted with ethyl
acetate. The organic extract is dried over sodium
sulfate and concentrated, and the residue is
chromatographed on a silica gel column using ethyl
acetate/petroleum ether 4:6. The chromatographically
pure fractions are combined, concentrated and
2195663
- 25 -
crystallized using diisopropyl ether. 0.2 g of the
title compound of m.p.: 172-174 C is obtained.
8. 2,3-Dihydro-7-methoxvbenzofuran-2-spiro-1'-cy;lo-
pentane-4-carboxylic acid-2,6-difluoroanilide
Analogously to Example 7, 0.65 ml of
2,6-difluoroaniline, 0.9 ml of triethylamine and
2,3-dihydro-7-methoxybenzofuran-2-spiro-1'-cyclopen-
tane-4-carbonyl chloride (prepared from 1.5 g of
carboxylic acid, see Example Al) give 1.2 g of the
title compound of m.p.: 142-145 C (from diisopropyl
ether).
Starting compounds
Al: 2,3-Dihvdro-2,2-dimethvl-7-methoxybenzofuran-
4-carbonyl chloride
0.8 g of 2,3-dihydro-2,2-dimethyl-7-methoxybenzofuran-
4-carboxylic acid is heated to boiling under reflux for
1 h in a mixture of 50 ml of abs. toluene and 3 ml of
thionyl chloride. The solvent is distilled off in a
rotary evaporator and toluene (about 30 ml) is then
added a further 2 times and the mixture is concentrated
again. The residue is dried in a high vacuum and
employed in Example 1 without further purification.
A2: 2.3-Dihydro-2.2-dimethyl-7-methoxvbenzofuran-4-
carboxylic acid
5.5 g of methyl 2,3-dihydro-2,2-dimethyl-7-methoxy-
benzofuran-4-carboxylate are stirred at 60 C for 2 h in
a mixture of 150 ml of ethanol and 50 ml of 2N NaOH.
The ethanol is distilled off, the residue is taken up
in the water and the solution is adjusted to a pH of 4
with 2N HC1. The product which is precipitated hereby
is filtered off with suction, washed with water and
2195663
- 26 -
dried. 4.7 g of the title compound of m.p. 147-149 C
are obtained.
A3: Methy 1 2 3-dihvdro-2 2-dimethyl-7-methoxvbenzofu-
ran-4-carboxv ate
15.6 g of methyl 3-hydroxy-4-methoxy-2-(2-methyl-2-
propenyl)benzoate are dissolved in 250 ml of abs.
dichloromethane and the solution is treated with 3 ml
of concentrated sulfuric acid. The mixture is stirred
at RT for 12 h, then it is treated with water and a pH
of 5 is set in the aqueous phase by addition of 2N
NaOH. After separating off the organic phase, the
aqueous phase is extracted a further 2 times with ethyl
acetate. The combined organic phases are concentrated
and the oily residue is chromatographed on a silica gel
column using ethyl acetate/petroleum ether (4:6). The
chromatographically pure fractions of the product with
Rf = 0.6 are combined and concentrated. 6.6 g of the
title compound of m.p. 65-67 C are obtained.
A4: Mp* yl-3-hvdroxy-4-methoxv-2-(2-methvl-2-vro-
penyl) -benzoate
20 g of methyl 4-methoxy-3-(2-methyl-2-propenyl-
oxy)benzoate are dissolved in 60 ml of quinoline and
the mixture is heated at 180-190 C for 2 h. After
cooling, it is treated with ethyl acetate and the
quinoline is extracted using 2N HC1. The organic phase
is washed a further 2 times with water and
concentrated. The oily residue is chromatographed on a
silica gel column using ethyl acetate/petroleum ether
(4:6). The chromatographically pure fractions are
combined, concentrated and dried in a high vacuum.
15.6 g of the title compound are obtained as a pale
yellow oil.
A5: Methyl 4-methogy-3-(2-methyl-2-FroBenyloxv)ben-
zoate
2195663
- 27 -
22 g of methyl 3-hydroxy-4-methoxybenzoate are
dissolved in 200 ml of anhydrous DMF and 41 g of ground
potassium carbonate and 14.7 ml of 3-chloro-2-methyl-
propene are then added. The mixture is stirred at 60 C
for 5 h. After cooling, the precipitate is filtered off
with suction, water is added to the filtrate and it is
then extracted 3 times with ethyl acetate. The residue
which remains after concentrating the extracts is
crystallized using petroleum ether. 21 g of the title
compound of m.p. 62-63 C are obtained.
B1: 2.3-DihSsdro-7-methoxybenzofuran-2-spiro-
1'-cyclopentane-4-carbonyl chloride
The title compound is prepared analogously to starting
compound Al from 2,3-dihydro-7-methoxybenzofuran-2-
spiro-1'-cyclopentane-4-carboxylic acid in 50 ml of
abs. toluene and 3 ml of thionyl chloride and reacted
further without further purification.
B2: 2.3-dihydro-7-methoxvbenzofuran-2-spiro-1'-cyclo-
aentane-4-carboxvlic acid
2.6 g of methyl 2,3-dihydro-7-methoxybenzofuran-2-
spiro-i'-cyclopentane-4-carboxylate are hydrolyzed
analogously to Example A2 in 50 ml of ethanol and 10 ml
of 2N NaOH. 2.3 g of the title compound of m.p. 166-
168 C are obtained.
B3: Methyl 2,3-dihydro-7-methoxvbenzofuran-2-spiro-1'-
c,vcloaentane-4-carboxylat@
10.2 g of methyl 2-cyclopenten-1-ylmethyl-3-hydroxy-4-
methoxybenzoate are dissolved in 500 ml of anhydrous n-
hexane and treated with about 5 g of Amberlyst 15. The
mixture is stirred at RT for 3 days, filtered and
concentrated. The residue is chromatographed on a
silica gel column using ethyl acetate/petroleum ether
2195663
- 28 -
(4:6), the chromatographically pure fractions are
combined and concentrated and the residue is dried in a
high vacuum. 7.2 g of the title compound are obtained
as a yellow oil.
B4: Methyi 2-cvclopenten-l-ylmethyl-3-hydroxv-4-meth-
o2Wbenzoate
12.7 g of methyl 3-(2-methylenecyclopentyloxy)-4-
methoxybenzoate are treated with 50 ml of quinoline and
the mixture is stirred at 190 C for 1 h. After cooling,
it is treated with water, adjusted to pH 3 using 2N HC1
and extracted with ethyl acetate. The residue which
remains after concentrating the solvent is
chromatographed on a silica gel column using ethyl
acetate/petroleum ether (4:6). The chromatographically
pure fractions are concentrated and dried in a high
vacuum. 10.2 g of the title compound are obtained as a
yellow oil.
B5: methyl 3- (2-methSrlenecyclopentyloxy) -4-methoxy-
benzoate
28.5 g of inethyltriphenylphosphonium bromide are
suspended under nitrogen in 300 ml of anhydrous THF and
the mixture is cooled to -40 C. 50 ml of n-butyllithium
(1.6M) in n-hexane are then added dropwise with
stirring. After stirring at -20 to -10 for 30 min, a
solution of 20 g of methyl 4-methoxy-3-(2-oxo-
cyclopentyloxy)benzoate in 100 ml of abs. THF is added
dropwise. The mixture is then allowed to warm to RT and
is stirred for a further 1 h. It is poured onto water
and extracted with ethyl acetate. The oil which remains
after concentrating the organic phase is
chromatographed on a silica gel column using ethyl
acetate/petroleum ether (4:6). The chromatographically
pure fractions are combined, concentrated and dried in
a high vacuum. 12.7 g of the title compound are
obtained as a colorless oil.
2195663
- 29 -
B6: Methyl 4-methoxv-3-(2-oxocyclopentyloxy)benzoate
23.8 g of inethyl 3-hydroxy-4-methoxybenzoate are
dissolved in 200 ml of anhydrous DMF and the solution
is treated with 35 g of potassium carbonate (ground)
and 13 ml of 2-chlorocyclopentanone. The mixture is
stirred at 60 C for 3 h, then the solid is filtered off
with suction and the filtrate is concentrated in vacuo.
The residue is chromatographed on a silica gel column
using ethyl acetate/petroleum ether (4:6). The
chromatographically pure fractions are combined,
concentrated and dried in a high vacuum. 24.3 g of the
title compound are obtained as a pale yellow oil.
Cl: 7-Difluoromethoxy-2 3-dihvdrobenzofuran 2-spiro-
1'-cyclopentane-4-carbonyl chloride
Analogously to Example Al, 0.7 g of 7-difluoromethoxy-
2,3-dihydrobenzofuran-2-spiro-1'-cyclopentane-4-car-
boxylic acid are reacted in a mixture of 20 ml of abs.
toluene and 2 ml of thionyl chloride and reacted
without further purification.
C2: 7-Difluoromethoxy-2 3-dihydrobenzofuran-l-spiro-
1'-cyclopentane-4-carboxvlic acid
Analogously to Example A2, 2.4 g of ethyl 7-di-
fluoromethoxy-2,3-dihydrobenzofuran-2-spiro-1'-
cyclopentane-4-carboxylate gives 2 g of the title
compound of m.p.: 143-145 C.
C3: Ethyl 7-difluoromethoxy-2 3-dihydrobenzofuran-2-
spi*-o-i'-cyclopentane-4-carboxvlate
2.7 g of ethyl 2,3-dihydro-7-hydroxybenzofuran-2-spiro-
1'-cyclopentane-4-carboxylate are dissolved in 70 ml of
dioxane, 3 ml of 50 % NaOH solution and 0.1 g of
2195663
- 30 -
benzyltrimethylammonium chloride are added and then
difluorochloromethane is passed into the mixture with
stirring at 70-75 C until the reaction has ended (about
1 h). After cooling, the mixture is poured onto water
and extracted 3 times with 100 ml of ethyl acetate.
After drying over sodium sulfate, the organic phase is
concentrated and the residue is chromatographed on a
silica gel column using ethyl acetate/petroleum ether
4:6. The chromatographically pure fractions are
combined, concentrated and dried in a high vacuum. 2.4
g of the title compound are obtained as a pale yellow
oil.
C4: Ethyl 2.3-dihydro-7-hydroxvbenzofuran-2-sairo-1'-
cvlo8entane-4-carboxylate
4.1 g of ethyl 7-benzyloxy-2,3-dihydrobenzofuran-2-
spiro-1'-cyclopentane-4-carboxylate, 0.5 g of Pd/C
(10 %) and 20 ml of cyclohexane are heated to boiling
under reflux for 4 h in 100 ml of toluene. After
cooling, the mixture is filtered and the filtrate is
concentrated to dryness. 2.7 g of the title compound
are obtained as a pale brown oil.
C5: Ethyl 7-benzyloxy-2.3-dihydrobenzofuran-2-spiro-
1'-r~.rclo8entane-4-carboxylate
10 g of inethyltriphenylphosphonium bromide-sodium amide
mixture (FLUKA 69500) are suspended in 100 ml of abs.
THF under an N2 atmosphere and stirred at RT for 0.5 h.
A solution of 7 g of ethyl 4-benzyloxy-3-(2-oxo-
cyclopentyloxy)benzoate in 20 ml of abs. THF is then
added dropwise in the course of 30 min. The mixture is
stirred at RT for 2 h, then it is poured onto water and
extracted 3 times with 100 ml of ethyl acetate. After
drying over sodium sulfate, the organic phase is
CA 02195663 2005-08-08
- 31 -
concentrated to dryness. For rearrangement, the oily
residue is stirred at 190 C for 1.5 h. After coolif,
it is mixed with 100 ml of toluene, 10 g of Amberlyst
15 (anhydrous) are added and the mixture is stirred at
80 C for 3 h. It is then filtered and washed with
methanol, and the filtrate is concentrated to dryness.
The residue is chromatographed on a silica gel column
using ethyl acetate/petroleum ether 4:6. The fractions
containing the main product (Rt - 0.8) are combined,
concentrated and dried in a high vacuum. 4.1 g of the
title compound are obtained as a pale yellow oil.
C6: Et]2yl 4-benzvloxy-3-(2-oxocyclopentyloxv)benzoate
Analogously to Example B6, 34 g of ethyl 4-benzyloxy-3-
hydroxybenzoate, 35 g of potassium carbonate and 15 ml
of 2-chlorocyclopentanone give 36 g of the title
compound as a colorless oil.
Dl: 2.3-Dihvdro-7-methoxybenzofuran-2-spiro-1'-cyclo-
hexane-4-carbonvl chloride
Analogously to Example Al, 2 g of 2,3-dihydro-7-
methoxybenzofuran-2-spiro-1'-cyclohexane-4-carboxylic
acid are reacted with 5 ml of thionyl chloride in 50 ml
of toluene.
D2; 2.3-Dihxdro-7-methogybenzofuran-2-s,piro-1'-cyclo-
hexane-4-carboxvlic acid
Analogously to Example Al, 10.3 g of methyl 2,3-di-
hydro-7-methoxybenzofuran-2-spiro-1'-cy,clohexane-
4-carboxylate give 9 g of the title compound of m.p.:
171-173 C.
D3: Methyl 2.3-dihydro-7-methoxybenzofuran-2-sairo-1'-
cyclohexane-4-carboxvlate
*'h'gdeulglk
2195663
- 32 -
Analogously to Example B3, 17 g of methyl 2-cyclo-
hexen-1-ylmethyl-3-hydroxy-4-methoxybenzoate in 500 ml
of n-hexane and 15 g of Amberlyst 15 (4 h at 60 C) give
10.3 g of the title compound as a yellow oil.
D4: Methyl 2-cvclohexen-l-vlmethyl-3-hydroxv-4-meth-
oxy-benzoate
Analogously to Example B4, 21 g of methyl 3-(2-
methylenecyclohexyloxy) -4-methoxybenzoate (reaction 2 h
at 190 C) give 17 g of the title compound as a yellow
oil.
D5: Methyl 3-(2-methylenecvclhexyloxy)-4-methoxv-
benzoate
43.8 g of inethyltriphenylphosphonivm bromide in 300 ml
of abs. dimethoxyethane are treated in portions under
nitrogen with 3.6 g of sodium hydride (80 % in
paraffin) . The mixture is stirred at RT for 1 h and a
solution of 30 g of methyl 4-methoxy-3-(2-oxo-
cyclohexyloxy)benzoate is then slowly added dropwise.
The mixture is stirred at RT overnight and then worked
up analogously to Example B5. 21 g of the title
compound are obtained as a colorless oil.
D6: Methyl 4-methoxy-3-(2-oxocyclohexyloxy)benzoate
Analogously to Example B6, 25 g of methyl 3-hydroxy-4-
methoxybenzoate, 41 g of potassium carbonate and
17.5 ml of 2-chlorocyclohexanone in 200 ml of DMF give
32.9 g of the title compound as a pale yellow oil.
El: 2.3-Dihydro-7-methoxl.rbenzofuran-2-spiro-1'-
(4'-oxacyclohexane)-4-carbonvl chloride
Analogously to Example Al, 1 g of 2,3-dihydro-7-
methoxybenzofuran-2-spiro-1'-(4'-oxacyclohexane)-4-car-
boxylic acid are reacted in a mixture of 50 ml of
2195663
- 33 -
toluene and 5 ml of thionyl chloride and further
processed without further purification.
E2: 2 -Dihydro-7-methoxvbenzofuran-2-sairo-1'-
(4'-oxacyclohexane)-4-carboxvlic acid
Analogously to Example A2, 1.3 g of methyl 2,3-dihydro-
7-methoxybenzofuran-2-spiro-1'-(4'-oxacyclohexane)-
4-carboxylate are hydrolyzed in a mixture of 50 ml of
methanol and 10 ml of 1N sodium hydroxide solution. 1 g
of the title compound of m.p.: 194-196 C is obtained.
E3: Methyl 2 3-dihydro-7-methoxvbenzofuran-2-spiro-1'-
(4'-oxacyclohexane)-4-carboxvlate
3.6 g of methyl 4-methoxy-3-(4-methylenetetrahydro-
pyran-3-yloxy)benzoate are dissolved in 50 ml of
quinoline and the solution is stirred at 190-200 C for
1 h. After cooling, it is poured onto water, and the
mixture is adjusted to pH 3 using 2 N hydrochloric acid
and extracted 3 times with ethyl acetate. After drying
over sodium sulfate, the organic phase is concentrated
to dryness in vacuo and the residue (2.9 g) is
dissolved in 150 ml of n-hexane. The solution is
treated with 2.9 g of Amberlyst 15 and vigorously
stirred at 60 C for 4 h. It is then filtered, the
filtrate is concentrated in vacuo and the oily residue
is chromatographed on a silica gel column using ethyl
acetate/petroleum ether 4:6. The chromatographically
pure fractions are combined, concentrated and dried in
a high vacuum. 1.3 g of the title compound are obtained
as a pale yellow oil.
E4: Methyl 4-methoxy-3-(4-methylenetetrahydropyran-3-
yloxy)benzoate
18.2 g of inethyltriphenylphosphonium bromide are
suspended in 200 ml of dimethoxyethane under a nitrogen
atmosphere and 1.5 g of sodium hydride (80 $ in
2195663
- 34 -
paraffin) are then added in portions. The mixture is
stirred at RT for 3 h and a solution of 13 g of methyl
4-methoxy-3-(4-oxotetrahydropyran-3-yloxy)benzoate are
then added dropwise in the course of 30 min. The
mixture is stirred overnight, then poured onto water
and extracted 3 times with ethyl acetate. After drying
over sodium sulfate, the organic phase is concentrated
in vacuo and the residue is chromatographed on a silica
gel column using ethyl acetate/petroleum ether 4:6. The
chromatographically pure fractions are combined,
concentrated and dried in a high vacuum. 3.6 g of the
title compound are obtained as a pale yellow oil.
E5: Methyl 4-methoxy-3-(4-oxotetrajydrovvran-3-yloxy)-
benzoate
Analogously to Example B6, 36.4 g of methyl 3-hydroxy-
4-methoxybenzoate, 50 g of potassium carbonate and 27 g
of 3-chlorotetrahydropyran-4-one in 200 ml of DMF give
11.5 g of the title compound as a pale yellow oil.
Fl: 2,2-Diethvl-2.3-dihXdro-7-methoxybenzofuran-4-
caronvl chloride
Analogously to Example Al, 1.2 g of 2,2-diethyl-2,3-
dihydro-7-methoxybenzofuran-4-carboxylic acid are
reacted in a mixture of 10 ml of toluene and 2 ml of
thionyl chloride.
F2: 2.2-Diethyl-2.3-dihvdro-7-methoxvbenzofuran-4-
carboxvlic acid
1.5 g of methyl 2,2-diethyl-2,3-dihydro-7-methoxy-
benzofuran-7-carboxylate are hydrolyzed in a mixture of
20 ml of ethanol and 5 ml of 2N sodium hydroxide
solution analogously to Example A2 and worked up. 1.2 g
of the title compound of m.p.: 152-154 C are obtained.
2195663
- 35 -
F3: Methyl 2 2-diethyl-2.3-dihvdro-7-methoxvbenzo-
furan-4-carboxvlate
of methyltriphenylphosphonium bromide-sodium amide
5 mixture (FLUKA 69500) are added to 100 ml of abs. THF
at about 10 C under protective gas (nitrogen), and the
mixture is warmed to RT and stirred for about 30 min. A
solution of 5.3 g of methyl 4-methoxy-3-(1-methyl-2-
oxobutoxy)benzoate is then added dropwise. The mixture
10 is stirred at RT for 1 h, then poured onto water and
extracted 3 times with about 50 ml of ethyl acetate.
The combined extracts are dried over sodium sulfate and
concentrated, and the oily residue is dried in a high
vacuum. The oil obtained (3.8 g) is stirred at 190-
200 C for 1 h, cooled and dissolved in 100 ml of
toluene. The solution is treated with 5 g of Amberlyst
15 and vigorously stirred at 80 C overnight. The
Amberlyst is then filtered off, the solution is
concentrated and the residue is chromatographed on a
silica gel column using ethyl acetate/petroleum ether
4:6. The chromatographically pure fractions are
combined and concentrated, and the residue is dried in
a high vacuum. 1.5 g of the title compound are obtained
as a pale yellow oil.
-
F4: Methyl 4-methoxv-3-(1-methyl-2-oxobutoxy)benzoate
Analogously to Example B6, 48.5 g of methyl 3-hydroxy-
4-methoxybenzoate, 83 g of potassium carbonate and
43.9 g of 2-bromopentan-3-one in 200 ml of DMF give
68 g of the title compound of m.p.: 63-65 C (stirring
with petroleum ether).
Commercial utility
The compounds according to the invention have useful
pharmacological properties which make them commercially
utilizable. As cyclic nucleotide phosphodiesterase
(PDE) inhibitors (to be precise of type IV), they are
2195663
- 36 -
suitable on the one hand as bronchial therapeutics (for
the treatment of airway obstructions on account of
their dilating but also on account of their respiratory
rate- and respiratory drive-increasing action), but on
the other hand especially for the treatment of
disorders, in particular of inflammatory nature, e.g.
of the airways (asthma prophylaxis), of the skin, of
the intestine, of the eyes and of the joints, which are
mediated by mediators such as histamine, PAF (platelet-
activating factor), arachidonic acid derivatives such
as leukotrienes and prostaglandins, cytokines,
interleukins IL-1 to IL-12, alpha-, beta- and gaamna-
interferons, tumor necrosis factor (TNF) or oxygen free
radicals and proteases. The compounds according to the
invention are distinguished here by a low toxicity, a
good enteral absorption (high bioavailability), a wide
therapeutic range and the absence of significant side
effects.
On account of their PDE-inhibiting properties, the
compounds according to the invention can be employed in
human and veterinary medicine as therapeutics, it being
possible to use them, for example, for the treatment
and prophylaxis of the following diseases: acute and
chronic (in particular inflammatory and allergen-
induced) airway disorders of varying genesis
(bronchitis, allergic bronchitis, bronchial asthma);
dermatoses (especially of proliferative, inflammatory
and allergic nature) such as, for example, psoriasis
(vulgaris), toxic and allergic contact eczema, atopic
eczema, seborrheic eczema, lichen simplex, sunburn,
pruritus in the anogenital area, alopecia areata,
hypertrophic scars, discoid lupus erythematosus,
follicular and widespread pyodermias, endogenous and
exogenous acne, acne rosacea and other proliferative,
inflammatory and allergic skin disorders; disorders
which are based on an excessive release of TNF and
leukotrienes, for example disorders of the arthritic
type (rheumatoid arthritis, rheumatoid spondylitis,
2195663
- 37 -
osteoarthritis and other arthritic conditions),
disorders of the immune system (AIDS), types of shock
[septic shock, endotoxin shock, Gram-negative sepsis,
toxic shock syndrome and ARDS (adult respiratory
distress syndrome)] and also generalized inflammations
in the gastrointestinal region (Crohn's disease and
ulcerative colitis); disorders which are based on
allergic and/or chronic, faulty immunological reactions
in the region of the upper airways (pharynx, nose) and
the adjacent regions (paranasal sinuses, eyes), such as
allergic rhinitis/sinusitis, chronic rhinitis
sinusitis, allergic conjunctivitis and also nasal
polyps; but also disorders of the heart which can be
treated by PDE inhibitors, such as, for example,
cardiac insufficiency, or disorders which can be
treated on account of the tissue-relaxing action of the
PDE inhibitors, such as colics of the kidneys and of
the ureters in connection with kidney stones; or
alternatively disorders of the CNS, such as, for
example, depression or arteriosclerotic dementia.
The invention further relates to a method for the
treatment of mammals, including humans, which are
suffering from one of the abovementioned diseases. The
method comprises administering to the sick mammal a
therapeutically active and pharmacologically tolerable
amount of one or more of the compounds according to the
invention.
The invention further relates to the compounds
according to the invention for use in the treatment
and/or propylaxis of the diseases mentioned.
The invention also relates to the use of the compounds
according to the invention for the production of
medicaments which are employed for the treatment and/or
prophylaxis of the diseases mentioned.
2195663
- 38 -
The invention furthermore relates to medicaments for
the treatment and/or prophylaxis of the diseases
mentioned, which contain one or more of the compounds
according to the invention.
The medicaments are prepared by processes known per se
which are familiar to the person skilled in the art. As
medicaments, the compounds according to the invention
(= active compounds) are either employed as such, or
preferably in combination with suitable pharmaceutical
auxiliaries, e.g. in the form of tablets, coated
tablets, capsules, suppositories, patches, emulsions,
suspensions, gels or solutions, the active compound
content advantageously being between 0.1 and 95 ~.
The person skilled in the art is familiar on account of
his expert knowledge with the auxiliaries which are
suitable for the desired pharmaceutical formulations.
Beside solvents, gel-forming agents, ointment bases and
other active compound excipients, for example,
antioxidants, dispersants, emulsifiers, preservatives,
solubilizers or permeation promoters can be used.
For the treatment of disorders of the respiratory
tract, the compounds according to the invention are
preferably also administered by inhalation. To this
end, these are either administered directly as powders
(preferably in micronized form) or by atomizing
solutions or suspensions which contain them. With
respect to the preparations and administration forms,
reference is made, for example, to the details in
European Patent 163 965.
For the treatment of dermatoses, the administration of
the compounds according to the invention takes place,
in particular, in the form of those medicaments which
are suitable for topical application. For the
production of the medicaments, the compounds according
to the invention (= active compounds) are preferably
2195663
- 39 -
mixed with suitable pharmaceutical auxiliaries and
processed further to give suitable pharmaceutical
formulations. Suitable pharmaceutical formulations
which may be mentioned are, for example, powders,
emulsions, suspensions, sprays, oils, ointments, fatty
ointments, creams, pastes, gels or solutions.
The medicaments according to the invention are prepared
by methods known per se. The dosage of the active
compounds is carried out in the order of magnitude
customary for PDE inhibitors. Thus topical application
forms (such as, for example, ointments) for the
treatment of dermatoses contain the active compounds in
a concentration of, for example, 0.1-99 %. The dose for
administration by inhalation is customarily between
0.01 and 0.5 mg/kg. The customary dose in the case of
systemic therapy is between 0.05 and 2 mg/kg per day.
Biological investigations
In the investigation of PDE IV inhibition at the
cellular level, the activation of inflammatory cells is
ascribed particular importance. An example which may be
mentioned is the FMLP (N-formyl-methionyl-leucyl-
phenylalanine)-induced superoxide production of
neutrophilic granulocytes, which can be measured as
luminol-potentiated chemoluminescence. (Mc Phail LC,
Strum SL, Leone PA and Sozzani S, The neutrophil
respiratory burst mechanism. In "Immunology Series" 57:
47-76, 1992; ed. Coffey RG (Marcel Decker, Inc., New
York-Basle-Hong Kong)).
Substances which inhibit the chemoluminescence and the
cytokine secretion and the secretion of proinflammatory
mediators of inflammatory cells, in particular
neutrophilic and eosinophilic granulocytes, are those
which inhibit PDE IV. This isoenzyme of the
phosphodiesterase families is particularly represented
in granulocytes. Its inhibition leads to the raising of
2195663
- 40 -
the intracellular cyclic AMP concentration and thus to
the inhibition of cellular activation. PDE IV
inhibition by the substances according to the invention
is thus a central indicator of the suppression of
inflammatory processes (Giembycz MA, Could isoenzyme-
selective phosphodiesterase inhibitors render
bronchodilatory therapy redundant in the treatment of
bronchial asthma?. Biochem Pharmacol 43: 2041-2051,
1992; Torphy TJ et al., Phosphodiesterase inhibitors;
new opportunities for treatment of asthma. Thorax 46:
512-523, 1991; Schudt C et al., Zardaverine: a cyclic
AMP PDE III/IV inhibitor. In "New Drugs for Asthma
Therapy", 379-402, Birkhauser Verlag Basel 1991; Schudt
C et al., Influence of selective phosphodiesterase
inhibitors on human neutrophil functions and levels of
cAMP and Cai. Naunyn-Schmiedebergs Arch Pharmacol 344:
682-690, 1991; Nielson CP et al., Effects of selective
phosphodiesterase inhibitors on polynnorphonuclear
leukocyte respiratory burst. J Allergy Clin Immunol 86:
801-808, 1990; Schade et al., The specific type III and
IV phosphodiesterase inhibitor zardaverine suppresses
formation of tumor necrosis factor by macrophages.
European Journal of Pharmacology 230: 9-14, 1993).
1. Inhibition of PDE IV activity
Methodolocv
The activity test was carried out according to the
method of Bauer and Schwabe, which was adapted to
microtiter plates (Naunyn-Schmiedeberg's Arch.
Pharmacol. 311, 193-198, 1980). Here the PDE reaction
takes place in the first step. In a second step, the
5'-nucleotide formed is cleaved to the uncharged
nucleoside by a 5'-nucleotidase of the snake venom of
Ophiophagus hannah (king cobra). In the third step, the
nucleoside is separated from the remaining charged
substrate on ion-exchange columns. The columns are
eluted with 2 ml of 30 mM aamnonium formate (pH 6.0)
2195663
- 41 -
directly into minivials to which is additionally added
2 ml of scintillator fluid for counting.
The inhibitory values determined for the compounds
according to the invention can be seen from the
following Table A, from which the numbers of the
compounds correspond to the numbers of the Examples.
Table A
Inhibition of PDE IV activity
Comvound -1Qg ICU
1 8.47
2 9.42
3 9.87
4 9.09
5 8.57
6 8.63
7 8.57
8 8.42
2195663
- 42 -
FORMULA SHEET I
R1
H
R4 ( I )
R2
CH2-R3
R1
x
(II)
R2
CH2-R3
2195663
- 43 -
FORMTJLA SHEET II
CO2CH3
P.2 0 R2
x
0 R3
R3 Ri
K2CO3
DMF
C02CH3
H2=PPh3
OH R2
R1 )NrcI
R3
CO2CH3
R2
K2C03 ~
DMF
O R3
R1
C 02C H3 ZAhinolin
R2
OH R3
R1
HE)
CO2CH3 CO-X
~ R2 R2
1. NaOH
O R3 2. SOC12 O R3
R1 R1 (II)