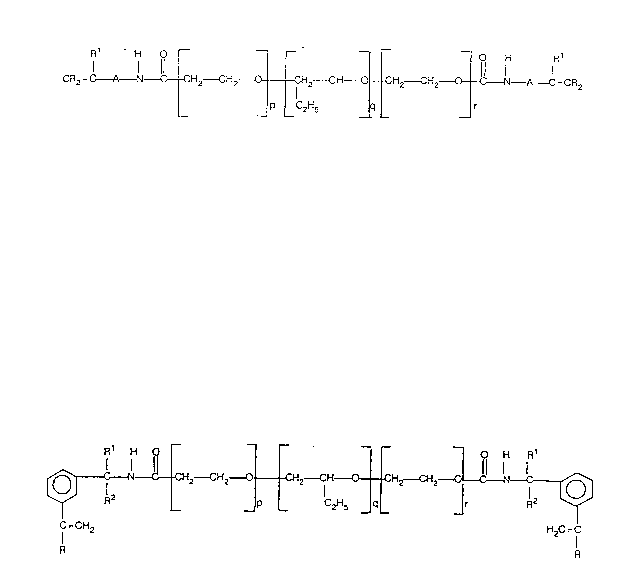Note: Descriptions are shown in the official language in which they were submitted.
21~6908
NOVEL ASSOCIATIVE MONOMERS AND POLYMERS
This invention relates to novel non-ionic urethane monomers and emulsion
and solution polymers and copolymers comprising the non-ionic urethane monomers.
The polymers comprising these novel monomers thicken, modify rheology, stabilize,
suspend particles, control viscosity and otherwise enhance the physical
properties of latex systems, coating compositions, sizings and printing pastes,
paints, paper coatings, industrial coatings, petroleum drilling fluids, cleaners,
detergents, personal care products and other aqueous solution or emulsion
products. -
Various polymeric materials based on mono and dicarboxylic acids are known.The properties of these polymers, in particular, the thickening efficiency of
these polymers, has been improved by the inclusion of various surfactant monomers
to produce associative copolymer thickening properties. These ~associative
thickeners (polymers comprising associative monomers) have found use in
materials such as lattices used as carpet and textile backing adhesives and in
similar applications. Such copolymers are described in U.S. Patent No.
-A-5,013,789, issued May 7, 1991, to Barron, et al. Surfactant monomers suitable
for making these associative copolymer thickeners are disclosed in U.S. Patent
No. -A-5,011,978, issued April 30, 1991, to Barron, et al.
Other surfactant -nc ~rS and copolymers prepared from such monomers which
are characterized by associative thickening properties are disclosed in U.S.
Patent No. -A-4,743,698, issued May 10, 1988, to Ruffner, et al., and U.S. Patent
No. -A-4,600,761, issued July 15, 1986, to Ruffner, et al. The Ruffner monomers
are copolymers prepared from an urethane reaction product of a non-ionic
monohydric surfactant or a sorbitan fatty ester. The Barron monomer and
copolymers are derived from the reaction product of a non-ionic amine-containing
surfactant with an unsaturated isocyanate. When preparing copolymers of these
monomers with acrylic acid, the urea (or urethane) monomer is incorporated into
the backbone of the polymer along with the comonomers.
An associative thickener copolymer disclosed in U.S Patent No.
-A-4,514,552, issued April 30, 1985, to Shay, et al., and reissued as U.s- Patent
No. -~-Re33,156, January 30, 1990, to Shay, et al., comprises an acrylic
- 219690~
backbone, together with urethane side chains modified with poly(alkoxy)
(surfactant) moieties. The surfactant moieties contain C6-C22 alkyl or C~-C22
aryl terminal groups to provide hydrophobicity to the side chains. A
propoxylated alkali-soluble thickener disclosed in U.S. Patent No. -A-5,191,051,
issued March 2, 1993 to Shay, et al., is an aqueous emulsion copolymer comprising
about 0.S to 40 weight percent of a monoethylenically unsaturated, hydrophobic
nonionic monomer which is the reaction product of a monohydric or monoamine-
terminated polypropoxylated or polybutoxylated hydrophobe with a
monoethylenically unsaturated monomer having a single group which is reactive
under the conditions employed, and provided said monoethylenically unsaturated,
hydrophobic nonionic monomer is not the reaction product of a monohy~dric or
monoamine-terminated polyethoxylated hydrophobe with a monoethylenically
unsaturated monomer having a single group which is reactive under the conditions
employed.
Other polymers having associative thickening properties are disclosed in
U.S. Patent Nos. -A-4,792,343, issued December 20, 1988, to Hawe, et al.;
-A-4,384,096, issued May 17, 1983, to Sonnabend; -A-3,657,17S, issued April 18,
1972, to Zimmerman; and -A-S,102,936, issued April 7, 1992, to Huth, et al.
It has now been discovered that a variety of polymeric materials having
superior associative thickening efficiency may be prepared from the novel non-
ionic urethane monomers of this invention. As a further benefit, these polymers
have lower volatile organic compound residues than polymers known in the art for
thickening compositions such as carpet backing adhesives. In one aspect, the
monomer is the reaction product of approximately equimolar amounts of selected
non-ionic, monohydric surfactants and a monoethylenically unsaturated isocyanate
which reaction product may subsequently be polymerized to yield a polymer'
backbone comprising urethane moieties. In another aspect of this invention,
selected non-ionic, multihydric surfactants are reacted with approximately two
moles of a monoethylenically unsaturated isocyanate to provide a multifunctional
monomer having associative thickening potential, as well as potential for
crosslinking polymers so as to further enhance thickening efficiency.
As used herein the term "thickening efficiency" re_ers to the relative
increase in viscosity or thickening effect produced by the addition of a minimum
amount of a thickening agent of this invention to an aqueous system, e.g., an
2196908
aqueous dispersion of a polymeric material or other insolu~le materials, with
that produced by a same amount of another thickenin~ agent
Such monomers may be advantageously produced from surfactants comprising
poly(ethylene oxide)(butylene oxide) ~lock C1-C4 alkoxy-terminated chains in lieu
of the C6-C30 hydrocarbon terminal groups that have been traditionally used as
the hydrophobe in associative monomers. Thus, the monomers of this invention
surprisingly provide associative thickening properties to their acrylic
copolymers in the absence of the hydrophobic moieties used in the prior art.
In addition to unexpected thickening efficiencies, the polymers prepared
from the monomers herein provide a variety of benefit in applications such as
carpet backing adhesives, including a reduction in the volatile organic chemical
content of the finished carpet and improve adhesive tensile strength and froth
stability, relative to conventional thickeners.
This invention provides novel monofunctional and multifunctional ~r~- -rs
comprising either Structure I (monofunctional~:
R1 H 0
I ll
R2=C ~ C----CL---CH2--~oH2----~ CH--~CH~ O ~2
' X C2~1s y
Structure I
wherein R and Rl are H, -CH3 or -CH2CH3; R2 is a C1-C4 linear ~r branched alkyl
group; y is an integer from l to 50; x is an integer from l to S0; the sum of x
y is less than or equal to lO0; and A is-a C2-C20 alkyl, aryl, ~1 or
alkylene group; or Structure II ~multifunctional):
C A N~}o CH2 CH2 0 Cll2--CH~CHZ--CH2~ A C=C~2
--- P C2~5 _ q _ _ r
Structure 11
wherein R and R1 are H, -CH3 or -CHzCH3; q is an integer from l to 50; p and r
are, independently, integers from l to S0; the sum of p + q + r is less tnan or
equal to 150; and A is a Cz-C20 alkyl, aryl, alkaryl or alkylene group.
2196~08
Also p~ovided in the present tnvention are carpet coâtir.g CompoSitiGns cornprising 10 to 70%
by weight of a latex binder, 30 to 90% by weight of at least c)ne filler, and 0.5 to 15% by weight of at
least one thickener, wherein the thickener is a polymer of a non-ionic urethane monomer comprising at
least two repeating units of the structure:
R R 1 H (:?
l ~-c
--'C--'C A N H2---CH2----C~--c~--cH2-~}-R3
R R n x C2Hs y
wherein ~, Rl and R2 are, independently, H, -CH3 or -CH2CH3; R3 is a Cl-C4 linear
or branched alkyl group; y is an integer from 1 to 50; x is an integer from 1 to
50; the sum of x ~ y is less than or equal to 100; A is a C2-C20 alkyl, aryl or
alkaryl alkylene group; and n is at least 1 ..
In another embodiment of ~e present invention, there is provided carpet coating compositions
co,.,~ g 10 to 70% by weight of a latex binder, 30 to 90% by weight of at least one filler, and 0.5
to 15% by weight of at least one thickener, wherein the thickener is a copolymer comprising:
~ a) 30 to 99 mole percent monoethylenically unsaturated C3-C~-
monocarboxylic acid, monoethylenically unsaturated C4-C8-dicarboxylic acid,
esters of monoethylenically unsaturated C4-C~-dicarboxylic acids, esters of
monoethylenically unsaturated C3-C8-monocarboxylic acid, vinyl esters of
saturated Cl-C8-monocarboxylic acid or a mixture thereof; and
(b) ~.1 to 70 mole percent of a non-ionic urethane ~r -r
comprising the structure:
Rt H O
1 ~1
C~2=C A N---C_--C---CH2--~CH2----CI--CH--~CH2-~}-R2
C2Hs y
wherein R and Rl are, independently, H, -CH3 or -CH2CH3; R2 is a Cl-C4 linear or
branched alkyl group; y is an integer from 1 to SO; x is an integer from 1 to 50;
the sum of x + y is less than or equal to lOOi and A is a C2-C20 alkyl, aryl or
alkaryl alkylene group.
3a
-- 21969~8
In yet another embodiment of the present invention, a method is provided for reducing the
volatile organic chemical contents of carpet, comprising the steps:
a. fabricating carpet from a plurality of yarn loops or tufts inserted into a backing; and
b. coating the backing with a coating composition comprising carpet coating compositions
comprising 10 to 70% by weight of a latex binder, 30 to 90~/c by weight of at least
one filler, and 0.5 to 15% by weight of at least one thickener, wherein the thickener is
a polymer of a non-ionic urethane monomer comprising at least two repeating units of
the structure:
R Rl H O
1 ~
---~C-----C ~ N H2---CH2-------C~----C~--CH2-~}-R3
~ R2 n x CzHs y
wherein R, ~1 and R2 are, independently, ~, -CH3 or -CH2CH3; R3 is a Cl-C4 linear
or branched alkyl group; y is an integer from l to 50; x is an integer from l to
SO; the sum of x ~ y is less than or equal to lOO; A is a C2-C20 alkyl, aryl or
alkaryl alkylene group; and n is at least l.
and wherein said polymer is used to replace a th~ t7ner having a volatile organic
chemical content of at least 1.0 mg/m2.
In another preferred embodiment of the present invention, there is provided a method for
- reducing the volatile organic chemical contents of carpet, CO.~-p~ 1g the steps:
a. fabricating carpet from a plurality of yarn loops or tufts inserted into a backing; and
b. coating the backing with a coating composition comprising carpet coating compositions
complising 10 to 70% by weight of a latex binder, 30 to 90% by weight of at least
one filler, and 0.5 to 15% by weight of at least one thickener, wherein the thickener is
a copolymer comprising:
3b
219690~
~ a) 30 to 99 mole percent monoethylenically unsaturated C3-C8-
monocarboxylic acid, monoethylenically unsaturated C4-C8-dicarboxylic acid,
esters of monoethylenically unsaturated C4-C8-dicarboxylic acids, esters of
monoethylenically unsaturated C3-C8-monocarboxylic acid, vinyl esters of
saturated Cl-C8-monocarboxylic acid or a mixture thereof; and
(b) 0.1 to 70 mole percent of a non-ionic urethane ~on- er
comprising the structure:
Rl H O
CR2= ~ Z~2 0 CH~2 ~R2
x C2Hs y
wherein R and Rl are, independently, H, -CH3 or -C~2CH3; R2 is a C1-C4 linear or
branched alkyl group; y is an inteqer from 1 to 50; x is an integer from 1 to 50;
the sum of x ~ y is less than or equal to 100; and A is a C2-C20 alkyl, aryl or
alkaryl alkylene group
and wherein the copolymer is used to replace a thickener having a volatile organic
chemical content of at least 1.0 mg/m2.
3c
21 96908
The monomers are preferably the urethane reaction products of
monoethylenically unsaturated aliphatic isocyanates, or of the reaction product
of organic diisocyanates and a hydroxy ester of a C2-C4 epoxide and acrylic or
methacrylic acid, with non-ionic surfactants. The monofunctional monomer may be
prepared from a monohydric surfactant and the multifunctional monomer may be
prepared from a di- or multi-hydric surfactant. The isocyanate may be selected
from isocyanatoethylmethylacrylate and alpha-, alpha-dimethyl-m-isopropenyl-
benzylisocyanate. The non-ionic surfactant is a polyglycol, preferably
comprising either a monohydric butylene oxide/ethylene oxide, C1-C4 alkoxy-
terminated block polymer, or a dihydric ethylene oxide/butylene oxide/ethylene
oxide triblock polymer.
This invention also provides solution and emulsion polymers and copolymers
comprising the novel monomers of this invention.
The novel monomers used in preparing the polymers of this invention are
urethane reaction products of a monoethylenically unsaturated isocyanate and non-
ionic surfactants comprising C1-C4 alkoxy-terminated, block copolymers of 1,2-
butylene oxide and 1,2-ethylene oxide.
Preferred isocyanates for use in these monomers have the formulations shown
in Structures III and IV below:
R1
CR2=C J~ N=C=O
Structure 111
wherein R and R1 are H,-CH3 or -CH2CH3; and A is a C2-C20 alkyl, aryl or alkaryl
alkylene group; and
Z~O--A--N~H2
Structure IV
2196908
wherein A is a c2-c20 alkyl, aryl, or alkaryl alkylene group, and z is a c2-c5
ethylenically unsaturated alkylene group selected from the group consisting of
H o
Il 1~
CH2=CH-,-CH2=CH (CH3)-, CH2=C(Cl)-, CH3CH=CH-, and C~2=C--C-O-CH2-CH2-.
Suitable isocyanates for preparing the monomers of this invention include
alpha, alpha-dimethyl-m-isopropenylbenzylisocyanate ( TMI") and isocyanatoethyl
methacrylate (~IEM ), and the reaction product of organic diisocyanates with a
hydroxy ester of a C2-C4 monoepoxide. Other vinyl-substituted monoisocyanates
are suitable for use herein, provided such compounds are reactive with the mono-
or multi-hydric non-ionic surfactants described herein. Examples of such
isocyanates are provided in U.S. Pat. Nos. 4,514,522 and 4,~43,698 and U.S. Re
33,156.
The surfactants useful in preparing the monomers herein are butylene
oxide/ethylene oxide polymers terminated with one or more hydroxy groups which
are available for reaction with the isocyanate compounds. The monohydric
butylene oxide/ethylene oxide polymers are terminated with one hydroxy group and
one alkoxy group, having one to four carbon atoms. The multihydric butylene
oxide/ethylene oxide polymers are terminated with more than one hydroxy group.
In a preferred embodiment, an ethylene oxide/butylene oxide/ethylene oxide
block copolymer is the surfactant used to prepare the multifunctional ~on~ ~-s.
In another preferred embodiment, a butylene oxide/ethylene oxide block copolymer
i5 used to prepare the monofunctional monomer.
The butylene oxide polymers and copolymers have a molecular weight range
between about S00 and 6,100, preferably between 1,000 and 3,000, most preferably
between 1,000 and 1,500.
Suitable butylene oxide/ethylene oxide polymers may be obtained from the
Dow Chemical Company under the tradename Polyglycol ~-50-6600 (dihydric
surfactant) and Polyglycol BL50-1500 (monohydric surfactant)~
Butylene oxide/ethylene oxide copolymers suitable for use herein have a
molar ratio of 3 to l, preferably 1 to 1, of butylene oxide to ethylene oxide.
Any other non-ionic surfactants comprising butylene oxide units and having
the formula shown in Structures V or VI, below, may be employed herein:
' TM
2196908
,
R~H2 ::H2~{~H--~2
C2Hs y --x
Structure V
wherein R is a C1 to C4 linear or branched alkoxy group; y is an integer from 1
to 50; x is an integer from l to 50; and the sum of x + y is less than or equal
to 100; and
HO ~H2~C~12~c~2~ 2~2--O
p , C2Hs q r
Structure \/1
wherein q is an integer from 1 to 50; p and r are, independently, integers from
1 to 50 and the sum of p + q + r is less than or equal to 150.
The novel monomers may be prepared using standard procedures known in the
art for reaction of an alcohol with an isocyanate group (e.g., addition
reaction). Suitable reactions conditions include, but are not limited to, the
conditions described in the Examples herein. Using such conditions, essentially
quantitative yields are obtained and no by-products are formed. Therefore,
purification of the monomer is an optional step which may be accomplished by any
means known in the art.
Homopolymers and copolymers comprising the novel monomers of this invention
are characterized by enhanced thickening efficiency in aqueous systems and are
particularly usefu~ in commercial aqueous systems such as carpet backing
coatings, adhesives, suspension and dispersion systems for piqments and paints,
coatings, industrial muds, clays and ores and other slurries.
The polymers of this invention are characterized by an extremely low
volatile organic chemical content. This provides a tremendous improvement over
conventional polymers used in the carpet industry which has experienced safety
and environmental pressures to reduce the amount of volatile organic chemicals
released from carpets into homes and offices. The polymers herein may be used
as the sole rheology modifier or may be used in conjunction with other polymeric
thickeners, whether associative in nature or traditional polymeric thickeners,
and other rheology modifiers, such as surfactants.
Alone or in combination with other polymeric thickeners, dispersants and
gelling agents, the polymers of this invention are useful in coatings, (e.g.,
2I9690~
paper coatings), textile sizings, textile printing pastes, paints and any
industrial coatings where a water soluble resin can be utilized. They are also
useful as thickening agents in latex based adhesives, where clays, other fillers,
pigments and the like are present. In addition, alkali soluble emulsion polymers
and copolymers find application in cleaners, laundry detergents, lotions,
toothpastes, and other cosmetic and personal care products. In petroleum
exploration, acrylic copolymers are used as drilling fluid additives for
viscosity control and as bentonite extenders for enhancing performance of the
drilling fluid, e.g., water soluble alkali metal polyacrylates copolymers are
useful additives in drilling fluids based on fresh water.
The polymers of this invention may be prepared using conventional free-
radical polymerization processes, preferably an aqueous solution polymerization
process or an aqueous emulsion polymerization process, including but not limited
to, the processes described in the Examples herein.
Following emulsion polymerization, the copolymer may be hydrolyzed by
treatment with alkali to form a neutralized salt of the copolymer in solution for
certain adhesive, coating and particle suspension applications requiring an
alkaline thic~ening polymer.
Upon addition of an alkaline material such as an alkali metal hydroxide,
sodium carbonate, or other bases such as ammonium hydroxide, methylamine or
diethylamine, at least some of the free carboxy groups in the polymer are
neutralized, rendering the copolymer soluble in water. At a low p~, e.g. a pH
below about 5.5, the emulsion polymer exists in the form of discrete, water
insoluble particles, having an average particle size of about 0.1 to 5 microns.
Average molecular weights of the copolymers are estimated to be between about
100,000 and 5,000,000. Preferred copolymers have an average molecular weight of
from about 500,000 to 1,000,000.
The homopolymers comprise at least two moles of the monofunctional or
multi-functional associative monomers of this invention The homopolymers are
preferably polymerized neat, or at low solids (e.g., less than about 15%) in an
aqueous polymerization process.
The copolymers comprise:
(a) 30 to 99 mole percent monoethylenically unsaturated C3-C8-
monocarboxylic acid, monoethylenically unsaturated C4-C8-dicarboxylic acid,
2I96908
esters of monoethylenically unsaturated c4-CB-dicar~oxylic acids, esters of
monoethylenically unsaturated C3-C8-monocarboxylic acid, vinyl esters of
saturated C1-C8-monocarboxylic acid or a mixture thereof; and
(b) O.l to 70 mole percent of the non-ionic urethane monomers of this
invention.
In a preferred embodiment, an emulsion copolymer comprises O.l to 20 mole
percent of monomer (b) and 80 to 99 mole percent of monomer (a), wherein monomer
(a) comprises less than 80% mono- or di-carboxylic acid. In a preferred
embodiment the emulsion copolymer is prepared from 30 to 99 mole percent alkyl
esters of carboxylic acid monomers, preferably methyl acrylate or ethyl acrylate.
In general, hydrophobic monomers are selected for preparation of emulsion
copolymers.
The (a) monomer may be selected from alpha, beta-ethylenically unsaturated
carboxylic acid monomers having the general formula
R1
RCH=C---COOH
where R is H and R1 is H, or an alkyl group containing from l to 4 carbon atoms,
or -CH2COOX; R is -COOX and R1 is H, and X is H or an alkyl group containing from
l to 4 carbon atoms.
Examples of these acid monomers include such monobasic acids as acrylic,
methacrylic, crotonic, and acyloxypropionic acid. Dibasic acid monomers include
maleic, fumaric, and itaconic acid. Monomers which are monoesters of dibasic
acids, such as the monobutyl ester of maleic acid can also be used to advantage.
Acrylic and methacrylic acid are preferred acid monomers.
The (a) monomer also may be selected from non-ionic alpha,beta-
ethylenically unsaturated monomers, of the formula:
CH2 = CYZ
where Y is H and Z is CN, Cl, - COOR, --C6H4R,
O O
31 1~
~CR2, ~NH2
-- 2196908
or --CH=cH2; Y and Z are cl; and Y is cH3 and z is CN,
o
~NH2
- CH=CH2, --C6H4R, or --COORl; and R is H, Cl, Br or an alkyl group containing
from 1 to 4 carbon atoms; R1 is an alkyl group containing from 1 to 12 carbon
atoms, or hydroxyalkyl group containing from 2 to 8 carbon atoms, and R2 is an
alkyl group containing from 1 to 8 carbon atoms.
Examples of these nonionic monomers are the C1-C18 alkyl and C2-C18
hydroxyalkyl esters of acrylic and methacrylic acids, such as methyl acrylate,
ethyl acrylate, butyl acrylate, 2-ethyl hexyl acrylate, methyl methacrylate,
ethyl methacrylate, butyl methacrylate, 2-hydroxybutyl acrylate, 2-hydroxyethyl
methacrylate, 2-hydroxypropyl methacrylate, lauryl methacrylate, styrene,
butadiene, isoprene, vinyl chloride, vinylidene chloride, acrylonitrile,
methacrylonitrile, vinyl acetate, vinyl butyrate, vinyl caprolate, p-chloro
styrene, isopropyl styrene, vinyl toluene and the like. The preferred monomers
are acrylate and methacrylate esters alone or mixtures thereof with styrene,
acrylonitrile, or vinyl acetate.
In a preferred embodiment, a solution copolymer is prepared from a mixture
of monomers comprising 0.1 to 20 mole percent of monomer (b) and 70 to 99.9 mole
percent of monomer (a), wherein monomer (a) comprises at least 90 mole percent
carboxylic acid monomer, most preferably acrylic acid.
The copolymers may further comprise about 1 to 30 mole percent of at least
one optional comonomer in addition to the (a) and (b) monomers described above.
Examples of suitable, preferred comonomers are described above and also include
any monomer that is susceptible to free radical polymerization with the (a) and
(b) monomers.
Preferred optional comonomers are water soluble and may be polymerized by
a solution polymerization process. Water soluble is defined herein the mean
comonomers which have a minimum solubility of 5% by weight in water at 25~C.
Such comonomers include acrylic, methacrylic, and ethacrylic acid, alpha-chloro-
acrylic acid, alpha-cyano-acrylic acid, beta-methyl-acrylic acid (crotonic acid),
alpha-phenyl-acrylic acid, beta-acryloxy propionic acid, sorbic acid, alpha-
chloro sorbic acid, angelic acid, cinnamic acid, p-chloro cinnamic acid, beta-
- 2196908
styryl-acrylic acid (1-carboxy-4-phenylbutadiene-1,3), itaconic acid, citraconic
acid, mesaconic acid, glutaconic acid, aconitic acid, fumaric acid, and
tricarboxy ethylene.
Also useful herein are the corresponding anhydride monomers formed by the
elimination of one molecule of water from two carboxylic acids located on the
same polycarboxylic acid molecule. The preferred carboxylic acid monomers for
use in this invention are the monoethylenically unsaturated acrylic acids having
a substituent selected from hydrogen, halogen and hydroxyl groups, monovalent
alkylene radicals, monovalent aryl radicals, monovalent aralkyl radicals,
monovalent alkaryl radicals and monovalent cycloaliphatic radicals.
Other comonomers useful herein include acrylamide, methacrylamide,
acrylonitrile, N,N-dialkylaminoalkyl acrylates and methacrylates, ethylenically
unsaturated quaternary ammonium salts, such as N,N,N-trimethyl inC Lhyl
methacrylate methylsulfate or halide, 2-hydroxy-3-methacryloxypropyltrimethyl-
ammonium methyl sulfate or halide, vinylbenzyltrialkylammonium methylsulfate or
halide, sodium or ammonium styrene sulfonate, vinyl pyrrolidone, hydroxy alkyl
acrylates and methacrylates, sodium 2-acrylamide-2-methylpropane sulfonate, and
the like. Other comonomers that are particularly useful herein include
diallylamine, hydroxypropyl acrylate, hydroxyethylacrylate and vinyl acetate.
It will be recognized by the practitioner that other comonomers may be
useful herein, including substantially water insoluble comonomers when the
copolymer is prepared in a solvent or by an emulsion polymerization process.
Such comonomers include, for example, C2-C30 olefins.
The following examples, in which all parts are by weight unless otherwise
indicated, are presented as a means of further describing the preparation and use
of the novel copolymers of this invention, and should not be considered as -
limiting the scope of the invention.
Example 1
This example illustrates the preparation of a monofunctional non-ionic
urethane monomer from a nonionic, monohydric surfactant and a monoethylenically
unsaturated aliphatic isocyanate
A total of 103.5 g (0.1050 moles) of polyglycol BL50-1500 (1,500 mol. wt.
monohydric, poly(butylene oxide) poly(ethylene oxide) surfactant;, o~tained from
the Dow Chemical Company, Freeport, Texas, was slowly heated in a 1-liter reactor
2196908
fitted with a thermometer, stirrer, refl~x condenser, ar.d heating mantle. When
the temperature of the surfactant reached 55~c, 0.206 g of a monomethyl ether of
hydroquinone and 0 200 g of stannous octoate were added. The solution was
maintained at 55~C and 21.14 g (0.1035 mole) of alpha,alpha-dimethyl-m-
isopropenyl benzyl isocyanate ("TMI") (obtained from American Cyanamid
Corporation, Wayne, New Jersey) were added dropwise from an addition funnel over
about a 45 minute period while the reaction mixture was maintained at 55~C to
60~C. The reaction mixture was then cooked 2 hours at 57~c. The final product
after cooling was a viscous solution.
Example 2
Using the procedure of Example 1, additional monomers were prepared for
comparative purposes from TMI and the group of non-ionic surfactants listed in
Table I, and described below. Each of the monomers was copolymerized by the
method of Example 6, below, with acrylic acid at a weight ratio of 5:95
monomer:acrylic acid and the copolymers were tested for solids, pH, viscosity and
thickening efficiency. Thickening efficiency was measured in a 82% solids, 600
load carpet precoat compound. Thic~ening efficiency was measured as the grams
of polymer (on a dry weight basis) added to a carpet precoat compound to yield
a precoat compound viscosity of 13,000 to 14,000 cps using a Brookfield RVT
viscometer with a $6 spindle at 20 rpm. Results are shown in Table I.
Copolymer A
A urethane monomer was prepared from TMI and monohydric methoxypoly
(ethylene) glycol (mol. wt. about 750) (Sample TB90017, obtained from Dow
Chemical). The monomer was polymerized with acrylic acid (5:95 monomer:acrylic-
acid). The thickening efficiency was only about one-half of the efficiency of
a control polymer prepared from acrylic acid and the monomer of Example l in a
S:95 weight ratio.
Copolymer B
A urethane monomer was prepared from TMI and a monohydric, poly~butylene)
oxide (mol. wt. about 750) ~Polyglycol XU-13428 ~ sample ~2067 obtained from
219690~
Dow Chemical). The thickening efficiency of the urethane/acrylic acid copolymer
(s) was only about one-quarter of the control polymer efficiency.
Copolymer C
A urethane monomer was prepared from T~I and l-butanol (obtained from
Aldrich Chemical company)~ The thickening efficiency of the urethane/acrylic
acid copolymer (C) was only 19.15, compared to 0.98 for the control polymer
efficiency.
Copolymer D
A urethane monomer was prepared from TMI and 1-hexanol (obtained from
Aldrich Chemical Company). The thickening efficiency of the urethane/acrylic
acid copolymer ~D) was only about one-half of the control polymer efficiency.
Thus, the exceptional thickening efficiency obtained with the control
polymer requires the presence of an associative monomer residue characterized by:
(A) a terminal C1-C4 alkyl group, (B) ethylene oxide spacers within the butylene
oxide groups to move the terminal Cl-C4 alkyl group and the butylene oxide groups
away from the hydrophilic polymer backbone, (C) more than one butylene oxide
group, and (D) a polyglycol sidechain component which isolates the termin~l Cl-C4
alkoxy group from the polymer backbone.
Example 3
This example illustrates the preparation of a multifunctional non-ionic
urethane monomer from one mole of a non-ionic multihydric surfactant and two
moles of a monoethylenically unsaturated aliphatic isocyanate.
A total of 210 g (0.1050 moles) of Polyglycol B50-6600 (6,600 mol. wt.
dihydric, poly(ethylene oxide)poly(butylene oxide)poly~ethylene oxide) triblock
surfactant, obtained from the Dow Chemical Company) was used in place of the
surfactant in the process of Example 1. The urethane monomer prepared from TMI
and the Polyglycol B50-6600 surfactant was an off-white wax having a solids
content of 100 percent, and a pH of 7.1. The preparation of acrylic acid
copolymer (5:9s weight ratio) was carried out by the process described in Example
6.
2196~0~
Example 4
This example illustrates the preparation of homopolymers of the urethane
monomer of Examples 1 and 3.
-
Part A
A sample of 45 g of the monomer of Example 1 and 255 g of water werecharged to a l-liter reactor fitted with a thermometer, stirrer, reflux condenser
and heating mantle. The monomer was heated to 70~C and an azo initiator
azo-bis-(2-methylpropane nitrile) was added. The reaction was permitted to
continue for 2 hours during which the viscosity of the reaction mixture
increased. The reaction mixture was cooled to room temperature and the product
recovered.
The product had a melting ~oint in excess of 240~C which contrasts with the
melting point of the monomer of Example 1 (i.e., about 42~C) and indicates the
monomer had been successfully polymerized.
The product was tested in solution at 13% solids for thickening efficiency
in the precoat composition of Example 2. When used in the carpet precoating
compound at a level of 69.04 g, the product solution exhibited a viscosity of
3,910 cps This viscosity contrasts with that of 69.04 g of a 13~ solids
solution of the monomer of Example 1, which was measured to be 1,830 cps, and
indicates the formation of a homopolymer.
Part B
The monomer of Example 3 is polymerized by the method described in Part A.
The product is viscous, has a higher melting point and exhibits greater
thickening efficiency than the monomer of Example 3.
Example 5
This example illustrates the preparation of copolymers from the urethane
monomers of Examples 1 and 2 by an emulsion polymerization processes.
A mixture of 198.S g water and 6 1 g of sodium lauryl sulfate was charged
to a l-liter reactor fitted with a thermometer, stirrer, condenser, and heating
mantle.
2196908
~.
A pre-emulsion of monomers was prepared in a beaker by mixing 262.7 g
water, 22.8 g of urethane monomer prepared according to Example 1, 91.4 g (1.06
moles) methacrylic acid, 114.1 g (1.14 moles) ethyl acrylate, 262.1 g water and
6.1 g sodium lauryl sulfate.
The reactor charge was heated to 80~c and 0.44 g potassium persulfate in
48.46 g water was added. The monomer pre-emulsion was added from an addition
funnel at constant rates over 90 minutes while maintaining the reaction mixture
at 80~C. The latex formed was cooked at 88~C for 1 hour, 100 g water were added,
and the mixture was cooled to 30~C.
A solution of the methacrylate/ethylacrylate/urethane copolymer emulsion
was prepared by adding 145 g of a 25 percent solution of sodium hydroxide (0.906
eq.) to the latex with mixing over 10 minutes. The mixture was heated to 90 to
95~C and cooked at that temperature for 16 hours to yield a sodium
polyacrylate/urethane copolymer solution.
The solutions containing the emulsion polymerization copolymers prepared
from the monomers of Examples 1, 2 and 3 were tested for thickening efficiency
and other characteristics. Results are shown in Table II, below.
2196908
D ~ O ~J D- g ,,~
X I=r G~ m,D O C~ w ~ ~ ~ -
D _ 2
Q~ o~
~ ~ ~ ~ ~o ~ o o
v~ ~ v~ ~
m m m O O O m
~~ ~ ~ ~ , I <DI ,~I O ~DI ~~.
Q ~- ~n ~~
Ia ~ ~0
~ ~ ~
o ~ --o ~~
., z
o~
V. 8 o 8 ~o o 8 ~o
.:~, ~ o
~ -- 2
3 o
o-- ~ O _ -- ~, -- o 3
2196908
~? ~,? C~ ~? ~?, ~ ?
D C) ~ ~ <') ~-? O O O O O o
. . - ~ ~ ~ -- m m
c ~ cr 0~ ~ o o c" ~
O O
2 - o O ~~ O O m
_ Q C'>
Q ,D ~ D D D D ¦~J
a~ c
- ~o
'~ o
~ _ _ ~ _ ID -- I
..
x 8 g 8 o o o
o
- n ~
o ~ o 3
2196~08
Example ~
A solution polymerization process was used to polymerize the urethane
monomers of this invention.
A mixture of 568 5 9 water and 4 . 5 g of the ~Irethane monomer of Example 1
was charged to a l liter reactor fitted with a thermometer, stirrer, condenser
and heating mantle.
The charge was heated to 80~C and a mixture of 0. 45 g divinyl benzene and
105.45 g acrylic acid (95 mls) was added dropwise over about 90 minutes along
with 0,23 g sodium persulfate in solution (28 mls). The temperature was
maintained at 80~C and the reaction was permitted to continue for one hour with
a post-addition of sodium persulfate during the first 15 minutes. Following
poly~tterization, 91 g of water and a solution containing 40 g of ammonium
hydroxide were added to the copolymer solution,
Similar comparative copolymers were prepared from the urethane monomers of
Example 2. Results are shown in Table I.
Example 7
The polymer of Examples 1 and 6 ~Table 1, "Control" solution copolymer) is
used as an efficient thickener in the carpet coatinq compositions shown in Table
III, below,
Table nl
CARPET COATING COMPOSmON
Parts by Weightt
1~.9.~" .t Control C D E f
Styrene But-' ~ne atexa 188.5 188.5 188.5 188.5 188.5
OURA-BOND~ H Adhesive ~ 8.0
Calcium Ca-bonate FillerC200.0199.0 199.0 199.0 199.0
Jute Colord -- -- -- .1 --
Froth Aid CF 1885-A * 2.s 2 s -- 2.5 2.5
Table I Thickenere -- 12.0 12.0 12.0 4.0
Paragum P-178 * 12.0 -- -- -- --
a. Dow 8300 latex is obtained from Oow Chemical Company, Midland, Michigan.
b DURA-80NO H adhesive is obta~ned from National Starch and Chemical Company,
[)ridge. , New Jersey
c. Grade D-90 filler is obtained from Georgia Marble, Dallon, Georgia.
d. Jute color is obtained trom Standard Adhesive, Dalton, Georgia. and Froth Aid from Cham-
Tex, Charlotte. North Carolina.
e. See Examples 1 and 6, and -control~ copolymer of Table 1.
t. The thickener of the pr;or art. Paragum P-178 thickener, is obtained from Patachem Inc., Si.. lpso.. il'e, South
Carolina.
9. The amount of thickener(s) used in each sample is adjusted to provide a coatmg viscosity
of 13,00010 14,000 cps by the method of Example 2.
* TM
17
2196908
The coating is applied to the carpet at 20 oz /sq. yd. The coating is
aerated with 30% air prior to coating the carpet.
The copolymer of this invention is an efficient thickener in a carpet back
coating composition, having performance equivalent to a commercial thickener used
in carpet coating compositions.
Example 8
The polymers of Examples l and 6 (Table I, "control" solution copolymer)
were tested in an environmental test chamber for total volatile organic chemical
emissions according to the "Standard Test Method for Determining TVOC Emission
Factors from Carpet under Defined Test Conditions Using Small Environmental
Chambers:Modification l. Adhesive Products," as prepared by the Carpet Policy
Dialogue, EPA, and the Adhesive and Sealant Council, finalized October, 1992.
A commercial carpet backing thickener (see Example 7) was also evaluated
for comparative purposes. Results are shown in Table IV, below.
Table IV
C~ J~ I Chambe- Testa
Sample Elapsed Exposure HourCo ",ald~h~de Emission TVOC Emiss;on Factor
Factor ~g/m' - hr IL9/m' - hr
98 45 4122 62
Paragum P-178 Polyme~- 4 N/A 29143
Col ~ N al Thickene-24 N/A 11 9û
48 N/A 9 52
Total TVOC C ~;ssions 5028 mg/m'
nd 54 05
Examples 1 & 6 Solution 4 N/A 1057
Copolymer 24 N/A 4 05
48 N/A 5 23
Total TVOC C ";is;ons 0 735 mg/m'
a) nd = non-detectable (less than 120 ~-g/m2-hour); N/A - not analyzed; TVOC = total
volatile organic chem;cals
b) The copolymer was the 'control' sample Table 1
The polymers remained packaged until immediately prior to chamber
evaltlation. At ~hat time, the polymer was applied at a load of 0.42 m2/m2 to astainless steel plate with a l/4" short nap roller held at a 45~ angle relative
18
2196gO8
to the surface of the plate. The plate was then placed in a stainless steel tray
and loaded into the S~3 environmental chamber. Testing was completed at 50.0
+ 2.0% relative humidity and 23.0 + l.O~C.
The results demonstrate the significantly lower volatile organic chemical
content of the polymers of this invention relative to a polymer used commercially
as a thickener in carpet coating compositions.
Although preferred embodiments of the invention have been described herein,
it will be understood by those skilled in the art that variations, modifications, and
equivalents may be made thereto without departing from the spirit of the invention
or the scope of the appended claims.