Note: Descriptions are shown in the official language in which they were submitted.
~ WO 96/Ojl38 2 1 9 7 ~ ~ 5 p
METHOD AND APPARATUS FOR PRODUCING
hY~KO~ PEROXIDB FROM nY~kO~ AND OXYGEN
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a method and apparatus
for producing hydrogen peroxide by injecting minute
bubbles of hydLvg~., and oxygen into a liquid stream of an
aqueous solution of water and an inorganic acid and a
Group VIII metal catalyst in which the liquid stream
flows at high velocity.
2. Descrimtion of the Related Art
It is known that a mixture of gaseous oxygen
and gaseous hydrogen forms an explosive material.
Virtually all currently produced hydL~g~,- peroxide is
produced by indirectly combining hydrogen and oxygen.
Thus, the primary conventional industrial method for
production of hydL Ugell peroxide uses a chemical agent
first for the reduction or hyd~g~l~ation. Typically,
alkylanthraquinone, such as ethyl or tertiary butyl
anthraquinone, is used as the chemical agent. This
working solution contains organic solvents such as di-
isobutylcarbinol and methyl naphthalene. Oxidation of
the int ~ te product of the reduction reaction
produces hYdL ~gen peroxide and the original
alkylanthraquinone. The anthraquinone is recycled back
through the process. This method has the shortcoming
that there is a considerable loss of the anthr~l;n~nP
and the organic solvents from oxidation and thermal
degradation of these organic materials. The presence of
these organics with oxygen and hydrogen peroxide presents
safety hazards from their potential reactions. This
destruction of organics involves high operating losses
for the currently practiced commercial process.
Various methods have been attempted to directly
combine oxygen and hydrogen to OV~L the above-
described problems. The direct combination of hydrogen
and oxygen to form hydrogen peroxide has the advantage of
2~765~ ~
Wo~/0513X PCT~S95/103~
not requiring the use of any organic or combustible
materials. The direct process, however, does require
careful control of the gaseous mixture of oxygen and
hydrogen so that they are always outside the explosive
range.
There have been numerous patents issued for the
direct combination of hydrogen and oxygen to produce
hydrogen peroxide. U.S. Patents 4,347,232 and g,336,240
employ an two phase system through the use of organic
ou-~uullds to form a second phase. However, the organics
can react with oxygen or hydrogen peroxide to render
these processes llneconr - ic~l.
Other patents employ a fixed bed catalyst
within a reaction vessel which has low conversion per
pass or low reactor productivity (see for example, U.S.
Patents 4,336,239 and 5,082,647).
U.S. Patent 5,169,618 ~'618 patent) to
Marischino desaribes establishing a pulse-flow regime ln
a catalyst bed. The '618 patent has the limitation of~0 low conversion of hydrogen peroxide per pass and high
t costs.
u.s. Patent 4,996,039 describes first absorbing
hydLuy~ll into the aqueous reaction mixture with a
catalyst; dropping the pressure to remove all the
hydrogen in the gas phase; and then introducing the
oxygen in order to produce hy~Lugell peroxide. This
technique minimizes the presence of hYdLUg~n in the
gaseous phase. This process is expensive to repressure
the reactor with oxygen and doesn't lend itself to
continuous processing.
Continuous modes of operation for the direct
combination process have also been proposed, as disclosed
in U.S. Patents 4,009,252; 4,279,883; 4,681,751; and
4,772,458. These patents employ a catalyst as a slurry
in an agitated reactor. These patents have the drawback
of having either low conversion per pass or low
volumetric efficiency either of which is nnecn~- ~c~l.
~ W096l0sl38 ~I~ 7 ~ 5 5 pcT~s95'10304
U.S. Patent 4,661,337 ('337 patent) describes a
process for direct combination for producing hydlogen
peroxide of increased concentration. The volume of the
reaction mixture occupies a small portion of the
available volume of the reactor. It is taught in the
'337 patent that the layer of reaction mixture has a
thickness of no more that 2 millimeters. This patent has
the disadvantage that the majority of the reactor is in
the gas phase in which no hydrogen peroxide if formed.
In all the above described patents, there is a
separate continuous gaseous phase in which it is
n~cessAry to inject an inert gas such as nitrogen, argon
or helium in order to remain outside the explosive range
of hydrogen and oxygen.
Other attempts for the direct formulation of
hydrogen peroxide use liquid filled reactors without a
continuous gas phase. U.S. Patent 5,104,635 describes a
liquid filled reactor with two internal membranes which
each are permeable only for hydrogen and oxygen,
respectively. This reaction system requires con~ rable
capital for the use of the membranes.
U.s. Patent No. 4,279,8&3 describes a process
for preparing hydLug~n peroxide in an aqueous medium.
The aqueous medium contains dissolved llydLug~l and a
platinum-group catalyst having absorbed thereto hydrogen.
Inert nitrogen and argon are blown into the aqueous
medium so that no dissolved oxygen is present in the
aqueous medium during the llydtoye~l absorbing treatment.
Oxygen gas is injected into the medium after the
absorption of the hYdLUge~ on the catalyst and the
gaseous zone and liquid zones are stirred. This patent
has the shortcoming of requiring an injection of an inert
gas into the reactor during the hydrogen absorption phase
to prevent an explosion between the hydrogen and oxygen
gases.
U.S. Patent No. 5,194,242 ('242 patent)
describes a process for preparing hydLog~ peroxide in
2 1 9~G55
WO96/0~138 PCT~JSg~/10304
which an acidic agueous solution fills an elongated
reaction zone in a tubular reactor. A catalyst is
provided to the reaction zone. Oxygen together with
recycled gas and then hydL~g~., are dLsp2rsed into the
solution in proportions that are above the lower
flammability limit for hydrogen and oxygen and are
maintained at a temperature and PLe~L~ until the
reaction mixture has decreased to below the lower
flammability limit for the hydL~g~ll and oxygen mixture.
The parti21 pressure of hydrogen and oxygen is super-
ai ~ ric in the range of about 20 to about 400 psi.
The aqueous solution flows through the reactor at liguid
velocity at rate from about 4 to about 8 ft~sec.
In the '242 patent, the ratio of the flow of
the aqueous medium to the aggreg2te flow of the hydrogen
and oxygen is such that a g2s phase regime of large
elongated bubbles may be produced, which, if reacted
violently would not be surrounded by sufficient liquid
volume to cool the gas mixture, resulting in elevated
20 t~ LULe and pressure which can result in an explosion
of the gas mixture. Patentees provide no teaching of the
importance of operating in a regime in which small
discrete individual bubbles exist which can be uuell~hed
by the surrounding medium. It is desirable to provide a
25 safe direct combination process for producing hYdLO~en
peroxide which has low manu~acturing costs.
O~JECTS OF TF~ INV.~NTION
It i5 thus a primary object of the invention to
provide a process for the reaction of oxy~en and hydL~n
which is efficient and safe.
It is a further related object of the invention
to provide a process which operates in the fl hl~
range of oxygen~hydrogen mixtures so as to benefit from
increased reaction rate, without sacrificing the safety
aspects of the process.
It is still a further related object of the
invention to substantially completely react hY~L~n
~ wos6/osl38 2 ~ 9 7 6 5 5 PCT~S9sll03n4
during the process in order to maximize the efficiency of
utilization of that expensive reactant.
It is still a further related object of the
invention to min;m;ze or avoid entirely the necessity of
recycling spent gases from the reaction to the reaction
zone.
It is still a further object of the invention
to carry out the process with minute bubbles of hydL ~g~
and oxygen supplied to the reaction zone at a rate and in
such a way as to obviate the risk of explosion.
SUMMARY OF THE INVENTION
Briefly described, the invention comprises a
method and apparatus for producing hydrogen peroxide in
which hydLug~ll and oxygen are separately injected into a
liquid filled reactor to form a plurality of discrete
individual bubbles in a continuous rapidly flowing liquid
stream. Each bubble is ~uLL~u-,ded by a continuous liquid
phase such that if the hydrogen and oxygen gas reacted,
there is sufficient liquid available to quench/cool down
the reaction in order to prevent an explosion ~r u~ayatillg
throughout the reactor. It has been found that it is
critical to maintain the ratio of the volume of flow of
aqueous medium to the a~yL~yate volume of flow of
hYdL~gen and oxygen, at a high value so as to avoid
uncontrolled reaction of 1-ydLogen and oxygen bubbles to
form water. By controlling the ratio of the volume of
flow of aqueous medium to the volume of flow of hydLogen
and oxygen, both ;n~pppn~pntly~ and, in the aggregate,
there is sufficient liquid volume present to quench any
runaway reaction that might take place. It is also
important to maintain the flow velocity of aqueous medium
at at least ten feet per second to obtain a dispersed
bubbly flow regime. The prevention of a reaction from
p~n~; ng allows the concentration of unreacted hydrogen
and oxygen within the reactor to be within the explosive
range.
W096/0~13~ 2 ~ q 7 6 5 ~ PCT~S9~10~4
Preferably, a pipeline reactor i5 used having a
plurality of passes within the reactor. The pipeline
reactor can be formed of a plurality of tubes arranged
vertically or horizontally and connected with curved
(elbow) tubes. The liquid stream can be formed of water,
a dilute acid and a Group VIII metal catalyst. The Group
VIII metal catalyst can be platinum or palladium or a
mixture of the two on an inert support such as alumina,
silica or carhon. The liquid stream fills the reactor.
Fine dispersed hydrogen gas bubbles can be dissolved into
the flowing liquid stream. ~fter the hydrogen is
dissolved, finely dispersed oxygen gas bubbles are
injected into the liguid stream for reacting with
hydrogen to form hydrogen peroxide. After this first
reaction is complete, multiple injections of first
hydrogen and then oxygen can be used to raise the
concentration of h~dLO~ peroxide y~uduued to a
predetermined level. The number of in~ections of
hydrogen and oxygen bubbles can be varied for producing
ZO the desired cuncellL~tion of the ~ydLo~ peroxide.
The present invention has the advantage of
avoiding a continuous gas phase between the hydrogen and
oxygen and having full utilization of the entire volume
of the reactor. The production of l1YdL UY~I peroxide
occurs in the liquid phase between the dissolved hydLug~l
gas and oxygen in the presence of a catalyst. The method
prevents an explosive reaction from PYp~n~ing, thereby
allowing the concentration of unreacted hydrogen and
oxygen to be within the explosive range.
The invention will be more fully described by
reference to the following drawing.
RRTEF DESCRIPTION OF T~ DRAWINGS
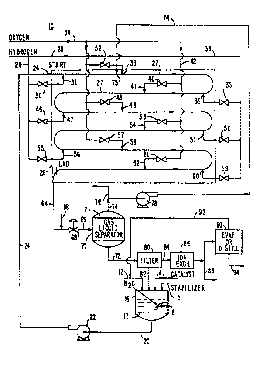
Fig. l is a schematic diagram of an apparatus
for con~in~ lc~y producing hydrogen peroxide from
hydrogen and oxygen according to an environment of the
invention.
~ WO96/05138 2 1 ~ 7 6 5 ~ PCT~Sg511030~
Fig. 2 is a schematic diagram of an apparatus
for producing hydrogen peroxide in a semicontinuous batch
process according to another Pmho~;r~nt of the invention.
DETATmTn DESCRIPTION OF T~E INVENTION
Fig. 1 illustrates a schematic view of the
apparatus 10 for producing hydrogen peroxide from
hydroy~n and oxygen. Water 12 and catalyst 14 are added
to tank 16. Catalyst 14 is preferably a supported Group
VIII metal catalyst. Preferably, catalyst 14 is
palladium or platinum or mixtures thereof. Examples of a
support useful for supporting the catalyst in a dispersed
fashion are carbon, silica and alumina. It will be
appreciated that other catalysts known in the art of
hydrogen peroxide production can be used in the present
invention.
Preferably, an amount of hydrogen peroxide
stabilizer 15 is added to tank 16. Stabilizer 15 can be
an acidic solution having a Ph in the range of about 2 to
7. Examples of acids useful for the present invention
are hydrochloric, phosph~ric~ and other commercially
available inorganic acids. Typically, the amount of
stabilizer 15 added to water is less than about 1% of the
reaction medium. Solution 17 within tank 16 can be
stirred with an automatic or manual agitation means 18.
Solution stream 20 is circulated with
recirculating pump 22 as input stream 24. Liquid stream
24 is received at pipeline reactor 26 and fills pipes 27
of pipeline reactor 26. Liquid stream 24 has a flow
velocity of greater than 10 feet per second for avoiding
the presence of a continuous gas phase or elongated
bubbles within reactor 26. It is known that dispersed
~ bubble regime occurs when water has a velocity of greater
that 10 feet per second. "The Flow of Complex Mixtures
~ of Pipes." G.W. Govier, Robert Kreiger pllhli~h;~g
Company, Malaber, Florida, page 523. Preferably, the
flow velocity of liquid stream 24 is in the range of more
than 10 feet per second to about 50 feet per second.
_ .. . , . _ _, ,, _, ,,,,,,,,,, , . _ _ , . _ _ _ _
W0~0~l38 2 1 ~ 7 6 5 ~ pCT/US9~1030~
Most preferably, the flow velocity of liquid stream 24 is
in the range of about 11 to about 20 feet per second.
Pipeline reactor 26 is preferably formed of a
plurality of pipes 27 joined with a 180~ bend. Joined
pipes 27 can be arranged either vertically or
hori~ontally. The length and diameter 27 of pipes are
predet~rm;n~d for providing the desired flow velocity.
Preferably, pipes 27 are formed of a commercially
available heavy wall pipe such as a U.S. schedule 160
which has a wall thickness form 0.44 to 1.31 inches over
the size range of 3 to 12 inches. Pipes 27 useful for
practice of the present invention can have diameter in
the range of 2 inches to at least 12 inches. The
preferred length of pipeline reactor 26 can vary widely
dependiny on the number of joined pipes 27 used in the
reactor. Typically, joined pipes 27 have a total length
in the range of about 50 to about 60,000 feet.
Preferably, joined pip2S 27 have a length of about 2,000
to about 45,00c feet.
It will be appreciated that the number of pipes
27 used in pipeline reactor 26 can be varied to change
the number of injection points or passes in the pipeline
reactor 26. Preferably, the number of passes of pipeline
reactor 26 is between about six and about 48 passes.
The pipeline reactor 26 operates at a ~ uLa
in the range of about between 30 to abou* 300 atm. The
reaction t~ ~uLe normally is in the range of ooc to
60~C. The lower the temperature of the aqueous medium
the higher the solubility of hydrogen is in solution.
Similarly, the higher the pressure, the greater the
solubility of hydrogen is in solution. The reaction
temperature can be maintained by providing jacketing on
each pipe 27 or by installing the entire pipeline reactor
26 within a vessel in which a refrigerant is being
evaporated or cold liquid solution is circulated.
It has now been found that it is important to
maintain the ratio of the volumetric flows of the aqueous
~ WO9~0~138 2 1 97~ PCT~S9~/10304
reaction medium and the aggregate flows of the gaseous
hydrogen and oxygen at of broadly from 300 to 30,
preferably from 200 to 40 and most desirably from 150 to
50. If the flow ratios are maintained within these
ranges and the velocity of the flowing liquid is
maintained at above 10 feet per second, as described
above, a regime of tiny bubbles surrounded by adequate
aqueous medium is created and the risk of runaway
explosion by reaction of bubbles is substantially
obviated. Statistically, the local increase of the
temperature due to the reaction of hydrogen and oxygen
bubbles to form water is desirably less than 3~C. This
is achieved with the volumetric flow rates and velocities
of the invention.
Broadly, the volumetric ratio of oxygen to
11YdL ug~n may be in the range of 1:1 to 50:1. The art has
recognized that higher ratios of oxygen to hydLuyen may
be desirable to force the reaction to completion. It is
recognized that oxygen is by far the cheaper of the
reactants and accordingly, excess oxygen can be vented
from the system without excessive economic penalty.
Desirably, the reaction ta~es place in the flammable
range, the volume ratio of oxygen to hydrogen being from
l:1 to 20:1.
The foregoing ratios of the flows of aqueous
medium to the aggregate volumetric flows of hydLuge-l and
oxygen, in turn set the ratios of the volume of aqueous
medium to each of the volumes of hydLugen and oxygen,
respectively, at the several hydrogen and oxygen gas
inlets. Broadly, the ratio of the volumetric flow of
aqueous medium to the volumetric flow of hydLuy~n at each
hydrogen gas inlet, is broadly from 15,000 to 60. The
ratio of the volumetric flow of aqueous medium to the
volumetric flow of oxygen is from about 15,000 to 60.
A gaseous stream of hydrogen 28 is injected by
valve 30 into a flowing liquid stream 24 at point 31.
Liquid stream 24 flows bctween points 31 and 33 of pipe
... ... . , , . , .. . . . _ _ _ _ _ _ _ _ _ _ _ . _ _ _
wo g6,05l38 2 ! 9 i7 f~i 5 5 p~ruS9ra/103~
27. Hydrogen is dissolved in a liquld stream 24. A
gaseous stream of oxygen 3g is injected by valve 32 into
liquid stream 24 at point 33. Within pipeline reactor
26, the dissolved hydrogen 28 reacts with the gaseous
oxygen 34 to form hydrogen peroxide in solution.
Preferably, gaseous hydLuyel~ 28 and gaseous
oxygen 34 are sparged into liquid stream 24 by a small
diameter nozzle for producing a plurality of minute
bubbles. Preferably, the nozzle has a diameter in the
range of about 0.001 inches to about 0.25 inches to
produce fine minute bubbles which are ~uLLuullded by
rapidly flowing liquid stream 24. The minute bubbles of
hydrogen and o~ygen are of a size which is small enough
to be surrounded by flowing liquid stream 24. ~he volume
of liquid stream is sufficiently large and continuous so
that in the event of any explosion of a single bubble the
~uLLuunding liguid can expeditiously quench the ~Yplosi~n
within the bubble to prevent the propagation of the
explosion thL uuyhuu~ the entire regime of the reactor.
The flow rate of liquid stream 24 and the injection of
minute bubbles provide a dispersed bubbly regime in
liquid stream 24.
Addltional gaseous hydL~y~n 28 can be injected
at a plurality of passes through pipeline reactor 26 with
25 respective valves 35, 46, 50, 55 and 59 at points 36, 47,
51, 56 and 60 for dissolution into liquid stream 24.
Additional gaseous oxygen 34 can be injected downstream
of hydrogen in~ection points 36, 47, 51, 56 and 60 with
respcctive valves 40, 48, 53, 57 and 61 at respective
points 41, 49, 54, 58 and 62 for reacting with the
dissolved hydrogen.
Desirably, the point of oxygen introduction is
sufficiently distanced from that of l~ydLùyull injection to
permit the hyd~ug~ll to have become distributed throughout
the aqueous medium as tiny dispersed bubbles and to
dissolve in the aqueous medium. Desirably, the second
volume of hydrogen and subsequent volumes of IIYdLUYU~I
~ WO96/05l38 2 1 9 7 6 5 5 PcTn59s/l0304
introduced along the elongated reaction zone are
introduced after about 50% of the previously introduced
hydrogen has been reacted with oxygen and preferably
after at least 75% of the previously introduced hydrogen
has been reacted.
After the multi-pass reaction, stream 64 flows
from pipeline reactor 26. In the event the off-gas from
the reactor is in the flammable range, a diluent gas 66
can be added to stream 64. An example of a diluent gas
useful for practice of the invention is nitrogen.
A pressure letdown valve 68 can be used before
gas-liquid separator 70 for reducing the pressure of the
inlet mlxture 69 to gas-liquid separator 70. Gas liquid
separator 70 separates liquid 72 from gas 74. Gas 74
containing unreacted oxygen, nitrogen and some unreacted
hydlu~ell from separator 70 can be recycled with recycled
gas compressor 78 and can be injected at point 75 into
liquid stream 24. Alternatively, separated gas 74 can be
purged with valve 76. It will be appreciated that a gas
liquid separator useful for practice of the present
invention is known in the art. Stream 64 can be received
at additional pipeline reactors 26 for cnnnectin~ the
reactors in series before gas liquid separator 70.
Separated liquid 72 containing the l-ydLugell
peroxide product in the aqueous solution of catalyst and
acid is passed to filter 80 for recovering the catalyst
as a filter cake 82. Filter cake 82 can be-added to tank
16 for recycling the catalyst. Filtrate 84 includes the
hydrogen peroxide product and the aqueous acid water
solution. Filtrate 84 is received at ion exchange
apparatus 86 for removing the acid from the filtrate.
Hydrogen peroxide product 88 from ion exchange
apparatus 8~ can be directly used as a hydrogen peroxide
~ product. Alternatively, hydrogen peroxide product 88 can
be received at column 90 for conc~l,LL~Ling the hYdLU~
peroxide product 88 in order to produce a cuncenLL~ted
hydrogen peroxide product 94. Column 90 can be an
WO~6~5138 2 1 9 7 6 5 5
12
evaporation or distillation column. Water 92 removed
from column 9C can be recycled into water stream 12 as
make-up water.
The concentration of hydrogen peroxide product
82 produced by pipeline reactor 26 depends on the number
of injections of hydrogen and oxygen in the passes of
pipeline reactor 26. Preferably, hydrogea peroxide
product 82 has a concentration in the range of about 2%
to about 30~ of hydrogen peroxide in solution.
Preferably, hydrogen peroxide product 94 has up to 70
concentration.
The present invention has the advantage of
providing an economical and safe process for producing
hydrogen peroxide. The process does not specifically
inject an inert gas or chemical agent within the reactor,
thereby reducing costs. The entire regime of the
pipeline reactor comprises a dispersed bubbly regime in a
rapidly flowing liquid stream for preventing the
formation of an explosive gas phase with the reactor.
The entire pipeline is utilized for the production of the
hydrogen peroxide. In addition, the high surface to
volume relationship of the reactor provides l~Yr~nqive
removal of heat from the reactor.
F~Y~MPT.~
Continuous Proces~
A circulating aqueous stream of a suqp~n~d
group VIII metal catalyst deposited on an inert carrier
with an acid stabilizer is delivered at a pressure of 200
di -sphFres (3000 psi) to the first of two tubular
reactors operated in series at a flow rate of 195,000
pounds per hour per reactor in the reactor shown in Fig.
1. The reactor consists of 4" c~h~d~ 160 pipes 100
feet long, each connected together by 180~ U bends. The
liquid flow rate has a liquid velocity of 13 feet/second.
The liquid stream is introduced into the reactor at 15~C.
At the reactor inlet 27.2 pounds per hour of
h~uge,- gas is injected through a nozzle to form fine
~ WO96~5138 2 ~ 9 7 6 5 5 PCT~S95/10304
13
individual bubbles in the liquid stream flowing at 13
feet/second. This produces a bubbly flow regime with a
continuous liquid phase and small evenly dispersed
individual bubbles. Recycled gas from the gas-liquid
separator can be injected into the process fluid. This
is followed by the injection of 432 lbs/hr. of oxygen as
finely dispersed bubbles which reacts with the hYdL Ug~ll
to form hydL~g~l~ peroxide. This is followed by repeated
injections for each reactor of firct hydrogen and the
oxygen to form hydrogen peroxide of increasing
concentration. The heat of reaction is removed by the
circulation of cooled water (or refrigerant) outside the
reactor pipes.
After passing through the second reactor, the
effluent flows through a pressure letdown valve before a
gas-liquid separator. Nitrogen or other diluent gas is
added to the reactor effluent as needed to assure the
exit gas from the separator is outside the
explosive/flammable limits of hydrogen and oxygen. This
gas can be either recycled to the first reactor or vented
to ai ,~-re.
The liquid phase is filtered to remove the
5llcppn~pd catalyst slurry so that it can be r~cllcpPn~Pd
in the aqueous medium. This is done in a mix tank where
the concel,LL~tion of each ingredient is checked and
adjusted as needed. This includes the acid used as a
stabilizer for hydrogen peroxide. The filtrate from the
filter that contains the desired hydrogen peroxide
product passes over an ion exchange or equal agent to
remove residual acid values from the hydrogen peroxide
product. This product can then be used directly or can
be concentrated in an evaporator or distillation column
to concentrated in an evaporator or distillation column
to conc~nLL~tions up to 70% following conventional
practice. A total production of 100,000,000 pounds per
year of hydrogen peroxide can be produced from these two
reactors.
WO9~05138 2 ~ q 7 6 5 '~ PCT~Sg~lo~ --
~MPLE II
8atch Semicontinuous Process
Fig. 2 illustrates an alternate method of
operating the process of the invention in a batch,
semicontinuous fashion. A fresh batch of reaction medium
consisting of a group VIII metal catalyst on an inert
support in an acidic aqueous solution, is charged through
valve 1 to separator 2. The solution is charged to the
reactor via valve 3 and recirculating pump 4. Once the
system is filled, flow of fresh solution is stopped by
closing valve l. The ~les~u ~ in the system is increased
by closing valve 5.
The velocity of the medium is maintained at 10
feet per second or more. Hydrogen is injected at
injection system 6. The amount of hydrogen introduced i5
at or less than the solubility limit in the flowing
medium. Oxygen is introduced at point 8, at a sufficient
distance downstream (pipe length 7) to ensure the
absorption of hydrogen. At full capacity, hydrogen flow
is about 15 pounds per hour and oxygen flow is
approximately 250 pounds per hour. Sufficient pipe
length 9 is provided downstream of the oxygen injection
to permit the maximum conversion to hYdL~g~ll peroxide.
The pipeline is cooled by a coolant on the outside of the
pipe to maintain an operating temperature between 5 and
30~C.
The reactor effluent passes to separator 2 to
disengage the gas 10 from the liquid. The exit gas is
outside the flammable range so it can be recycled back to
the reactor by recycle ~_ u55u~ 11. As needed, fresh
oxygen 12 is added to the recycle stream before it is
injected at point 8. If the exit gas is in flammable
region, nitrogen 13 is injected into the effluent stream.
Effluent gas 14 is then vented from the system.
Pump 4 recirculates the liquid medium until thc
hydrogen peroxide reaches its desired concentration,
desirably between 4-15% by weight, preferably 5-8%. Gas
~ W096/0s138 6 5 ~ PCT~S~5/10304
injection will continue for from one to three hours. The
reactor system, including the separator, pump and piping
is then drained. The system is then refilled with a
fresh reactor charge following the procedure outlined
above. This batch semicontinuous procedure produces from
l,000,000-l,500,000 pounds per year of hydrogen peroxide
product. With smaller or larger diameter pipe of the
same length, lower or higher quantities, respectively, of
hydrogen peroxide are produced.
EXAMP1E III
Nodified Batch Semicontinuous Process
The batch semicontinuous process described in
Example II can be carried out in a modified way to reduce
both the capital costs and operating costs of the
reaction system. The basic flow diagram of Fig. 2 is
used with the exception that a second reaction medium
charge pump is provided in parallel with recirculating
pump 4 in order to fill the system and the pressure let
down valve 5, rather than being at the effluent of the
reactor pipeline upstream of the separator, is downstream
of the separator in the gas effluent line.
In operation, the operating ~L~s~uLe in the
separator remains high (1,000-4,000 psi) throughout the
course of the reaction. Pressure is maintained by "head"
gas above the separator liquid. The gas to be recycled
enters recycle ~ essor upstream of the pressure
letdown valve. The exit gas in line lO pas~es through a
PLeS~ULe letdown valve. By configuring the process -in
this way, any residual dissolved gas in the reactor
effluent stays in solution while passing through the
separator because the latter remains at pressure. Thus,
~ it is not n~c~qs~ry to redissolve gases in the
recirculating reactor effluent and both the separated
liquid and unreacted gas need not be repressurized as in
Example II. Configuring the process thusly should reduce
the capital and operating costs of the recirculating
W~09C/05138 2 1 9 ~ 6 5 5 Pc~/usgs/~03o~ -
16
pump, the recycle compressor, the separator and other
parts of the process.
While the invention has been described with
reference to the preferred ~mhcd;r-~t, this description
is not intended to be limiting. It will be appreciated
by those of ordinary skill in the art that modificaticns
may be made without departing from the spirit and scope
of the invention.