Note: Descriptions are shown in the official language in which they were submitted.
WO 96/07601 02199263 PCTIIB95/00728
CHILDPRODF BLISTER PACKAGE WITH DESSICANT
Technical Field
The present invention regards a blister pack-
age,, particularly a blister package providing preparation
cavities and drying agent cavities for packaging moisture
sensitive solid or semi-solid pharmaceutical prepara-
tions.
The expression blister package is used herein
in its usual meaning, i.e. for any type of sheet package,
preferably made of plastics and aluminum, which have
cavities for the uptake of the solid to be packaged in
the pan sheet (usually in the plastic sheet) and which
are closed by sealing with a second sheet, a so called
cover sheet, preferably a coated aluminum foil, whereby
the product at its offtake can be pushed'through the
cover sheet by pressure on the pan sheet.
Background Art
Today, blister packages are a preferred pack-
aging form for solid or semi-solid pharmaceutical prepa-
rations, respectively. They have the advantage that the
preparations.remain cleanly packed until they are used
and furthermore - because of the usual labeling on the
back side and/or on the top side - the risk of a mix-up
is much reduced. Further advantages of the blister pack-
age are a reduced abrasion of the product, the possibil-
ity to.get single units under retention of_the primary
package, water vapor resistance etc.
Today used blister packages have a certain
=~ viater vapor permeability due to the plastic sheet making
them unsuitable for the packaging of moisture sensitive
= products. The minimizing of the water vapor permeability
by thickening the foil is only limitedly possible because
of the'technical practicability and the handling (pushing
through)". '
WO 96/07601 W'521 9926 3 PCT/IB95/00728
2
Therefore, the moisture permeability with the
today generally usual packages can only be improved by
the material selection, i.e. as much as possible moisture
impermeable laminate sheets. In this respect particularly
multilayer sheets have turned out to be suitable.
These known packages, however, are not suit-
able
for very moisture sensitive preparations or for mod-
erately moisture sensitive preparations destined for
countries with humid-hot climate.
Several attempts have already been made to
provide packages for the dry storage of pharmaceutical
preparations.
A known procedure exclusively uses aluminum
composite films as pan sheet and cover sheet. An addi-
tional drying effect can be achieved by providing two
cavities connected with each other for air exchange, from
which one comprises a drying agent and the other one the
pharmaceutical preparation. Such a package has the disad-
vantage that it is relatively expensive and that, par-
ticularly because of the lack of transparency, the risk
for the erroneous intake of the drying agent instead of
the effective substance comprising preparation exists.
Besides of the possibly occurring side effects of some
drying agents the non-co.nsummation of the effective sub-
stance comprising preparation (pharmaceutical prepara-
tion) can very badly influence the state of health of the
patient.
There have also already attempts been made to
reduce the moisture intake of blister packages without
renouncing the transparency of the foil of at least one
side of the package.
EP-A-O 466 068 describes a blister package ~=
in which each cavity for a pharmeceutical preparation is
connected with .a cavity for a drying agent. Additionally
a perforation can be provided enabling the'separation of
each combination of a pharmaceutical preparation and a
drying agent.
WO 96107601 02 a 992 7 3 PCTIlB95/00725
3
The disadvantage of this blister package is
that by accident the drying agent can easily be consumed,
particularly with a separated unit.
FR-A-2 593 152 also describes a blister pack-
age with drying agent whereby several cavities for a
pharmaceutical preparation are connected with one cavity
for drying agents.
While for this package the risk of the acci-
dental intake of the drying agent is reduced because of
lo its exposed location and/or its largeness, this package
bears the disadvantage that the drying effect is badly
influenced if the pharmeceutical preparations are con-
sumed in the wrong sequence.
FR-A-2 660 634 discloses a package comprising
a drying agent whereby between the drying agent and the
cover sheet a membrane is located to keep the drying
agent at the desires place. The offtake of the charge is
not performed by pushing the charge through the cover
sheet but by peeling the cover sheet off. A peeling of
the cover sheet from the drying agent is made more diffi-
cult by additional sealings.
Also known are children-resistant packages.
DE-OS-24 04 232 discloses a children-
resistant package, however without any means to keep the
pharmaceutical preparation dry. Besides of a removable
tear resistant sheet on the cover sheet preventing push-
ing of the charge through the cover sheet, the cavities
are thus formed, that a child cannot open them by biting
on the blister.
US-5 172 812 discloses a children-resistant
package with a rupturable cover sheet and with a paper-
board material peelably laminated to the sheet of rup-
turable material. Furthermore a die cut is provided
"misregistered" with the-opening so that only a part of
the paperboard material is peeled off from the opening so
that the tablet can only be removed if pressure is ap-
r''s
. - iti=I'. it'' . .- = r .."! .
CA 02199263 2005-08-03
4
plied in a specific manner. This document does not disclose
a drying agent comprising package.
US-3 780 856 and US-3 835 995 both disclose
blister packages with a cover sheet that is to be peeled
off. In both packages a tear-off strip is provided where the
cover sheet is not sealed to the carrier layer.
Short description of the invention
The problem of the present invention thus was to
provide a blister package for the dry storage of
pharmaceutical preparations, for which the accidental intake
of the drying agent is prevented.
This problem is solved by a blister package with a
pan sheet with preparation cavities and drying agent
cavities as well as a cover sheet sealed to said pan sheet,
characterized in that the blister package in the region of
the drying agent cavities is reinforced thus, that the
withdrawing of the charge, i.e. a drying agent carried by
said drying agent cavity, with usual finger pressure is
prevented, and that the reinforcement over the drying agent
cavities cannot be removed by applying usual finger means.
In the inventive blister package each cavity comprising a
pharmaceutical preparation is connected to at least one
cavity comprising a drying agent, and at least one sheet,
particularly the cover sheet is reinforced in the region of
the drying agent thus that the reinforcement over the drying
agent cavities cannot be removed by usual application of
usual finger means as for example pressing, pushing or
peeling off with the fingers or the finger nails.
CA 02199263 2005-08-03
4a
In a preferred embodiment, the invention provides
a blister package with a pan sheet with preparation cavities
and drying agent cavities as well as a cover sheet sealed to
said pan sheet, characterized in that the blister package in
the region of the drying agent cavities is provided with at
least one protection sheet on the cover sheet and is
reinforced thus, that the withdrawing of the charge, i.e. a
drying agent carried by said drying agent cavity, with usual
finger pressure is prevented, and that the reinforcement
over the drying agent cavities cannot be removed by applying
usual finger means.
By this package it is possible to secure the
sufficiently dry storage of the tablets during the whole
desired consummation period of the pharmaceutical
preparation under consideration of the climatic situation
and to simultaneously hinder the often even dangerous
accidental intake of the drying agent. By not consuming the
essential effective agent as well as by
side effects of the drying agent the organism of a possi-
bly already weakened patient may be further weakened.
Examples for an inventive blister package
are shown in the Figures 1 to 6.
- -
Short description of the drawings
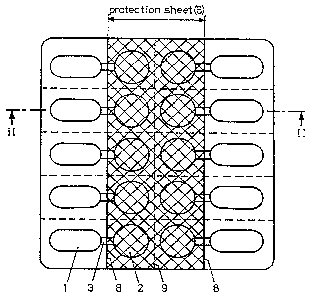
Figure 1 is a top view on a blister package
in which each pharmaceutical preparation cavity is con-
nected to a drying agent cavity by a connecting passage,
1.o and with an additional protection sheet being placed on
the coversheet in the region of the drying agent,
Figure 2 is a section along the line II-II of
the blister package of Figure 1,
Figure 3 shows the structure of a specific
protection sheet against withdrawing simultaneously serv-
ing as child-resistant,
Figure 4 is a top view on a blister package
with a specific withdrawing-protection sheet according to
Figure 3,
Figure 5 is a section along line V-V of the
blister package of Figure 4 during the peeling of.f of the
protection sheet from the preparation side, and
Figure 6 shows a blister package in which two
preparation cavities are connected to one drying agent
cavi ty .
Detailed description of the invention
The inventive blister package has a pan sheet
4 with cavities 1 for the uptake of effective substance
comprising preparations and cavities 2 for the uptake of
drying agents as well as a cover sheet 5_sealed to said
pan sheet 4, characterized in that the blister package in
the region of-the drying agent cavities 2 is reinforced
thus, that the withdrawing of a drying agent carried by
said drying agent cavity 2 with usual finger pressure is
prevented, and that the reinforcement over the drying
agent cavities 2 cannot be removed by applying usual
Dr. RRldp AMENDED SHEET
19.08.1996
CA 02199263 2005-08-03
5a
finger means. Usually each cavity 1 is connected with at least
one cavity 2 by a connecting means 3, thus that an air
exchange is of course possible, but not a direct contact of
the effect-
WO 96107601 02199263 PCTYM95/00728
6
tive substance comprising preparation with the drying
agent. Preferably said connecting means 3 is a connecting
passage-like cavity in the formed pan sheet 4 of the
blister package, which is particularly a plastic sheet.
The lifetime of the package can be controlled
by the amount of drying agent used, dependent on the en-
vironmental conditions.
The amount of drying agent results from its
water absorbing capacity, the blister design, the used
foil quality,.the provided storage conditions and the de-
sired shelf life of the product.
For space reasons it can be advantageous if
more than one cavity 1 for a pharmaceutical preparation
is connected with one cavity 2 for a drying agent (e.g.
as represented in Figure 6). Since the drying agent ad-
vantageously is also applied in the form of tablets
whereby the dosage as well as the introduction into the
cavities 2 is facilitated and additionally a clogging up
of the passages by loose particles is diminished or
2o avoided, respectively, for such an embodiment possibly
larger drying agent tablets are used.
Suitable as drying agent are all solid, pref-
erably pharmaceutically harmless substances such as mo-
lecular sieves and silica gel. Considered as essential
thereby is that the package comprises additional security
elements preventing the accidental intake of the drying
agent instead of the effective substance comprising
preparation. Such a security measure can for example be
the sealing of an additional plastic sheet in the drying
agent region, particularly onto the cover sheet S. This
additional sheet prevents a pushing through of the drying
agent through the cover sheet 5. Preferably one addi-
tional sheet simultaneously covers several drying agent
comprising cavi=ties 2, resulting in an improved adhesion
thereof. This foil can be clear or colored. Measures on
the side of the pan sheet 4 such as local coloring, local
reinforcement of the sheet or covering hood, respec-
WO 96/07601 9- 2199263 PCT/IB95/00728
7
tively, etc. are possible but technically much more ex-
pansive.
Particularly if an additional protection
sheet 6 shall simultaneously cover several drying agent
cavities 2, it is preferred that the drying agent cavi-
ties 2 are situated adjacently, particularly in one or
several adjacent rows.
The preferred inventive blister packages with
enhanced security against withdrawing of the drying
lo agents provide on the cover sheet side at least in the
region of the cavities 2 for the uptake of drying agent a
protection sheet 6.
The protection sheet 6 can be of any shape,
provided that the withdrawing of the drying agent is ef-
fectively prevented. Suitable are e.g. plastic foils
which, due to their toughness prohibit a pushing through.
Because of their toughness polyethylene (PE) sheets or
polyethylene composite films, respectively, for example a
laminate of polyethylene (PE) and polyethyleneterephtha-
late (PET) sheet (PET/PE sheet) are preferred.
Also suitable are cover sheets 5 of a multi-
layer laminate with the protection sheet 6 being already
comprised (cf. Fig. 3). For example such sheets, which
are commercially available, inside (contact to the pan
sheet 4) have a hot-melt layer, e.g. a thermoplastic lac-
quer. As further layers an aluminum foil, an adhesive, a
polyester sheet, e.g. polyethyleneterephthalate (PET), an
adhesion enhancer, e.g. another solid adhesive layer, a
paper layer and possibly an imprint follow. A sheet with
e.g. 20 pm thickness of the Al-layer and 12 pm thickness
of the polyester sheet and an adhesive with restricted
adhesion in the laminate is e.g. available from Neher,
Kreuzlingen, Switzerland, under the name Peel-Push foil.
The-adhesive can either be thus construed
that it only provides restricted laminate adhesion so
that a separation between the cover sheet 5 and the pro-
tection sheet 6 is possible by hand, or it is thus that
WO 96/07601 021992 63 PCT/IB95/00728
8
the adhesion of the laminate is so strong that the multi-
layer laminate is peeled off as such, i.e. cover sheet 5
and protection sheet 6 together.
When such a multilayer laminate with re-
stricted laminate adhesion is used, the whole cover sheet
region is covered, that is also the preparation region.
Such a cover is considered as child-resistant. A usual
pressing through of the tablet through the laminate is
not possible.
For the inventive blister package it must be
secured that the protection sheet part of the multilayer
laminate can only be peeled off from the effective sub-
stance comprising preparations but not f-rom the drying
agent.
=15 This is for example secured in that (as shown
in Figures 4 and 5) in the effective substance comprising
preparation part opening aids are provided, e.g. seal
free zones 7, as well as a perforation or a cross section
8 to the drying agent region. By the perforation or the
crosssection 8, respectively, it is secured that, start-
ing from the seal free zone 7, the protection sheet part
of the multilayer laminate is only peeled off in the re-
gion of the effective substance comprising preparations.
The seal free zones 7 are preferably located directly at
the perforation or the cross section 8, or at the inter-
section of the perforation or the cross section 8 and the
perforation or the cross section 9, so that the peel off
direction necessarily leads away from the drying agent.
The seal free zone 7 is e.g. made in that the sealing
tool, e.g. a stamp, provides respective "cold" parts,
and/or by deep drawing of the package form in the respec-
tive region, so that no contact can be made and at the
same time the seizing of the loose sheet part is facili-
tated, or by punching out the respective zone from the =
pan sheet prior to sealing it with the cover sheet. Ad-
vantageously also between two pharmaceutical preparation
cavities and/or between two drying agent cavities a per-
WO 96/07601 2 PCT/IB95/00728
9
foration or cross section 9, respectively, is provided.
The perforation or the cross section 8, respectively, as
well as the perforation or cross section 9 preferably
concern at least the protection foil part of the multi-
layer laminate. Particularly a perforation 9 through the
whole blister package can be provided so that single
units can be separated. Since the drying agent region be-
cause of the protection foil is secured against withdraw-
ing also for a 1:1 combination (one effective substance
comprising preparation per drying agent tablet) no risk
of withdrawing exists.
Important, however, is that the paper + PET
complex can only be peeled off from the region of the
preparation. This is achieved in that the opening aids 7
are exclusively present in the preparation field. If a
sheet with an adhesive providing strong laminate adhesion
between cover sheet 5 and protection sheet 6 is used, a
perforation.or cross section 8 as well as a perforation
or cross section 9 must be provided to ensure that the
multilayer laminate is only peeled off from one effective
substance preparation, which then is laid open.
As a further protection measure, particularly
with the multilayer laminate, the paper layer, particu-
larly in the region over the drying agent, can be colored
or provided with a warning imprint. Advantageous is also
a label in the product region, e.g. specification of the
weekdays etc., on which the respective tablet shall be
consumed. Such a label additionally diminishes the prob-
ability that the sequence of the intake is thus that -
e.g. for packages with several units for which one unit
comprises more than one preparation cavity 1 per drying
agent cavity 2 - all but one tablet of one unit are con-
sumed at the beginning of the consumption, the one re-
maining tablet,.=however, is consumed as the last one of
all units, so that the drying agent - because of the en-
hanced humidity access through the ruptured area of the
WO 96/07601 02199263 PCT/IB95/00728
lo
cover sheet 6 over the tablet cavities 1 - is extremely
strained.
Furthermore, with the package a monitoring
device can be provided indicating the complete consump-
tion of the drying agent or the enhancement of the humid-
ity, respectively. Known in this respect is e.g. the sil-
ica gel with indicator, a silica gel colored with CoC12
to show the water absorption. Because of the side effects
that may occur with the intake of CoC12, silica gel with
1o indicator preferably should only be used in inventive
blister packages providing the additional protection to
prevent the intake of the drying agent.
The pan sheet itself should - as already men-
tioned above - be as little moisture permeable as possi-
ble. Furthermore it should be sealable as well as also
having a good compatibility with the product. A today
usual sheet for such applications is a combination sheet
of PVC (polyvinylchloride),PE (polyethylene) and PVDC
(polyvinylidenechloride). Such a sheet is e.g. sold under
the name Alfoil , Tristar , Aclar , etc. and extensively
used for pharmaceuticals.
Also as cover sheet a usual sheet can be
used, particularly one comprising an aluminium layer or
an aluminium foil such as a usually used polymer coated
aluminium foil or the above mentioned multilayer lami-
nate, respectively.
The pan sheet is formed according to the
blister design with usual methods and after the introduc-
tion of the drying agent and the product conneted with
the cover foil in a manner usual for blister packages ,
particularly sealed.
Example 1:.Protection against withdrawing for
blister packages
Onto the cover sheet, a usual aluminum sheet,
in the region of the part to be protected against with-
drawing (drying agent region) a protection sheet of
WO 96/07601 02199263 PCT/I[B95100728
11
PET/PE (thickness about 60 pm) is sealed at about 120-
130 C. Due to the toughness of the PET/PE sheet a pushing
through of the drying agent is not possible. The thus se-
cured blister package then can be perforated as desired,
preferably between each unit.
Example 2: Protection against withdrawing by
a multilayer laminate
The filled pan sheet is sealed at about 200 C
with a multilayer laminate (Peel-Push-Folie from Neher,
Kreuzlingen, Switzerland) according to Figure 3 as cover
sheet and protection sheet instead of an aluminum sheet
thus, that, in the product region at the perforation line
to the drying agent region and at the perforation line
between the units, for each unit at least one seal free
zone is formed. If the seal is made by a stamp, this is
achieved by "cold" zones on the stamp itself.
Then a perforation is made between the prepa-
2o ration cavities and the drying agent cavities.
The withdrawing of.the product is made in
several steps:
- optionally separation of the blister yard
along the perforation lines
- peeling off of the outside cover sheet lay-
ers, here paper + PET-complex, at the seal free corners
- pressing through of the product through the
aluminum-foil fixed at the lower part by heat-sealing.
In that the seal free zones are situated at
the border line between the drying agent region and the
product region, so that the peeling takes place away from
. the drying agent region, the protection sheet part of the
multilayer laminate can exclusively be peeled off in the
product region..-This is not possible in the region of the
drying agents since any opening aid such as a seal free
zone is lacking.