Note: Descriptions are shown in the official language in which they were submitted.
W096/09503 2 2 0 ~ 5 ~ 6 PCT~S95/059OS
-
Description
ACID-BASE F~ELS FOR SELF HEATING FOOD CONTATN~-C
Technical Field
The present invention relates to exothermic compositions
for warming a sealed food container and, more particularly, to
S a dry acid-base composition that has a long shelf life and
generates heat on contact with water.
Backqround of the Invention
There is a demand for meal packages that include a
heating medium. These meals can be used by hikers, climbers,
forest fire fighting crews, etc. The largest demand is for
tasty, convenient meals for military personnel to carry while
in action on maneuvers. The self-contained heat source
eliminates the necessity to carry stoves and fuel, and the
meal can be heated without a hot fire or flame which can be
easily detected by infra-red detectors by the enemy.
The most widely used heating medium is based on the reac-
tion of quicklime and water. However, in order to provide an
optimum package, weight and cost are principal considerations.
Also important is the ability to heat the food without causing
overboiling and spattering of the lime-water reaction mixture
which can be a hazard to the user or can contaminate the food.
The hot, milky, caustic residue is also considered to be a
hazardous substance in the United States and can only be
disposed of at special sites that accept and store hazardous
materials.
Statement of the Prior Art
There are many possible configurations for the container
such as a tray as disclosed in U.S. Patent No. 4,771,761. The
most popular form of heatable food container is a can-in-can
30 form of product in which the inner sealed can contains the
food and the outer annulus between the two cans contains two
W096/09503 PCT~S55~ 5
2 '200586
compartments separated by a pierceable membrane. One com-
partment contains the hydratable lime and the other contains
water.
U.S. Patent No. 4,501,259 to Apellaniz discloses a food
container in which the reactivity of the quicklime has been
reduced by calcining the quicklime at a temperature from
1,100 C to 1,400 C. Apellaniz utilizes an excess of water.
The amount of water is from 0.75 to 3.0 parts by weight per
part by weight of the quicklime. The excess water results in
a milky, alkaline, hot residue which can leak out the puncture
holes and become a hazard to the user. Apellaniz in a later
patent (4,748,035) teaches that slow reacting, overburnt lime
(calcined above 1150 C) can be used if 6-75 percent of a high
or medium-reactivity quicklime is added, preferably from 12 to
50 percent by weight of the high- or medium-reactivity soft-
burnt quicklime, i.e. quicklime burned at a temperature from
900 to 1,150 C to a porous condition. The grain size is
usually 2-5 mm. The overburnt limes exemplified were calcined
at 1,200 C for four hours. Again an excess amount of water
was used to provide the slaking reaction and the reaction
product is a hazardous, hot, alkaline liquid. The disposal of
the container with caustic liquid residue is an environmental
and safety concern.
A self heating food can product now being produced and
marketed in England and Europe uses a container design and
chemical fuel that is commercially acceptable. The chemical
reactants are water and quicklime, an industrial chemical that
is a reactive, but impure and inexpensive form of calcium
oxide (CaO). Solid calcium hydroxide and heat are produced as
shown in the following reaction:
CaO + H2O --> Ca(OH) 2 + 277 calories/gram of CaO
The reaction of normal commercial quicklime with water is
too rapid; the unreacted water boils and before sufficient
W096/09s03 2 2 U ~ 5 ~ 6 PCT~S95/05905
_.
heat.can pass through the wall of the inner food can and into
the food, 543 calories per gram of vaporized water can be lost
from the fuel mixture as steam. Extra fuel had to be added to
compensate for the loss. Since the annulus between the can of
food and the outer can must be vented during the heating reac-
tion, there was the danger that steam or hot lime milk, which
is caustic (pH = 12.5), would be expelled from the can and
injure the user. Also quicklime readily absorbs water vapor
and carbon dioxide from air. Therefore, the annulus of the
self heating can must be tightly sealed and the barrier bet-
ween the quicklime and the water must be very effective, or
the fuel will slowly lose its heat-producing capacity while
the can is in storage before use. Furthermore, the calcium
hydroxide that remains in the used, self-heating food can is
a moderately strong base that cannot be easily disposed of in
a safe manner.
In the self heating food can currently available commer-
cially in England and Europe, the quicklime reacts more slowly
with water because it is produced by calcining limestone at
1100 to 1300 C rather than the 900 C used for conventional
quicklime. Although it adds to the weight, a large excess of
water assures more uniform heating and helps conduct the heat
to the wall of the inner food can. A torus-shaped metal-foil
bag holds the water above the lime. It is pierced to initiate
the heating. The empty bag reduces the likelihood of lime-
milk spattering. The metal foil assures an adequate shelf-
life. However, this commercial product has extra weight due
to the excess water and also suffers from the hazard of the
hot calcium hydroxide residue.
Statement of the Invention
It has now been discovered in accordance with the inven-
tion that even-heating, long shelf-life, self-heatable food
containers can be provided in which the reaction product is
not hazardous. The fuel-water mixture of the invention heats
W096/09503 PCT~S95/05905
4 ~200586
the food in acceptable time in a safe and efficient manner.
The fuel of the invention generates heat in a controlled and
sustained manner for a period of time sufficient to warm the
food in the inner container without the hazards of flame,
boilover, explosion, alkalinity, or toxicity. The fuel of the
invention adds an acid to the fuel mixture that exothermically
reacts with lime or other base to produce heat and a neutral
(non-alkaline) residue.
Exothermic chemical compositions are provided that are
comprised of one or more solid, particulate, basic reactants
such as oxides of alkaline earth metals that are reacted with
water and one or more solid, anhydrous, granular, acidic reac-
tants, such as acids or acid salts. The exothermic hydration
and neutralization reactions are initiated by contacting the
dry, particulate solids with water. The proportion and total
quantity of the alkaline and acidic reactants are selected so
that when completely reacted the residue will be a solid,
nonhazardous, essentially neutral salt of the cation of the
alkaline earth metal and the anion of the acid or acid salt.
By careful choice of quantity, particle size and/or acidic or
basic strength of the solid reactants, the reactivity of the
alkaline earth oxide and the amount of water used, a
controlled, two-stage heating process is produced that does
not require as much water. There is first a moderately rapid
hydration reaction that heats but does not overheat the water
and solid reactants in the annular chamber. There is a second
less rapid neutralization reaction that produces heat more
slowly to keep the reactants hot but not overheated as heat is
transferred from the annular chamber through the wall of the
inner container and into the food.
These and many other features and attendant advantages of
the invention will become apparent as the invention becomes
better understood by reference to the following detailed
description when considered in conjunction with the accom-
panying drawings.
W096/09503 ~a-58~ PCT~S95/05905
Brief Description of the Drawings
Figure 1 is a top plan view of a food container in accor-
- dance with the invention;
Figure 2 is a view in section taken along line 2-2 of
Figure 1;
Figure 3 is a set of curves of temperature change versus
elapsed time of food (simulated by water) within the inner
food can for four experiments in which the same amounts of
quicklime, water and oxalic acid were used as exothermic reac-
tants with equivalent amounts of quicklimes of different reac-
tivities or with equivalent amounts of mixtures of these
quicklimes;
Figure 4 is a set of curves of temperature change versus
elapsed time of the reactants in the annulus for the same four
experiments as in Figure 3;
Figure 5 is a set of curves of temperature change versus
elapsed time of fuel-water reactants between the annulus and
the cans and of food (simulated by water) within the inner
food can for a set of experiments in which appropriate amounts
of water, oxalic acid, and a single, moderately reactive
quicklime were used as exothermic fuel and a set of analogous
curves from a set of experiments in which lesser amounts of
water oxalic acid and equivalent amount of a mixture of two
quicklimes of different reactivities were used as the fuel-
water reactants in the annulus;
Figures 6 and 7 are sets of curves of temperature change
versus time of the annulus reactants and of food (simulated by
water) within the inner food can for four experiments in which
the same amounts of water and a quicklime of moderate reac-
tivity were used as reactants with an equivalent amount in
each experiment of the solid particulate acids: oxalic acid,
sulfamic acid, tartaric acid and citric acid; and
Figure 8 is a set of inner-can (food) and annulus
temperature-time curves for the annulus fuel-water mixtures of
Example 4.
WO g~ ~9~3 PCT~S9S/059OS
6 ~00586
Detailed Descri~tion of the Invention
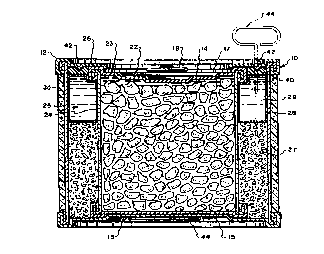
Referring now to Figures 1 and 2, a tightly closed
cylindrical can 10, contains the food to be heated before its
consumption. The food can 10 is placed in an outer container
12 having the shape of a cylindrical can. The outer container
12 has a closed bottom 13 and is open at its opposite end 14.
The outer container 12 iS coaxial with the food can 10, the
bottom 15 of which is centered on the bottom 13 of the outer
can 12 by means of a centering ring 16. The top 17 of the
food can 10 is provided with an opening device 18 on the lid
22 which closes the top of the can 10. This device 18 can
comprise a tongue 19 provided with an eyelet 20 and with a tab
21 that is attached to the lid 22. When the tongue 19 is
pulled by hand, the lid 22 of the food can 10 tears along a
circular line of small resistance shown by the thinned line 23
of the lid 22. The food can 10 may be opened by means of this
known device 18, without the need of using a special opening
tool.
A closed, annular chamber 25 is formed between the outer
container 12 and the side wall 24 of the food can 10. The
chamber 25 is closed on the side adjacent to the top end 17 of
the food can 10, by an annular strip or ring 26 that is made
of an easily pierceable material, such as thin metal sheet or
a plastic sheet such as polyethylene or polyester. One edge
of the annular ring 26 is crimped to the free edge (opposite
the closed bottom 13) of the container 12, whereas the other
edge of the annular strip 26 is also crimped to the end of the
side wall 24 of the inner food can 10 that is adjacent to its
top end 17. The annular strip 26 tightly closes the annular
chamber 25 that contains the reactants necessary for the
exothermic reaction used for heating the contents of food can
10. An annular plastic ring 40, or a ring of cardboard, metal
or other material coated with plastic or an elastomer may be
disposed below the annular strip 26 to further ensure sealing
of the annular chamber 25.
W096t09503 22 0 0 5 8 6 PCT/U~ 5~05
-
.The particles 27 of base and acid reactants are placed in
the lower part of the annular chamber 25. A torus shaped bag
28 is disposed in the upper portion of the annular chamber 25
above the particles 27. The bag 28 can be made of a water-
vapor impermeable, very flexible plastic such as, for example,
polyethylene. The flexible bag 28 contains water 29 under
slight pressure. When a pointed tool, such as a spike 44, is
pushed through thinned metal ports 42 or apertures in the
metal ring 26 and the underlying plastic ring 40, the bag is
pierced, the water is expelled from the bag 28 and flows and
distributes quickly, by gravity, into the solid reactant par-
ticles 27 located under the bag 28 and initiates the exother-
mic reaction uniformly across the body of the particles 27.
The outer face of the outer container 12 can be provided with
a heat-insulating layer 30 such as polystyrene foam and can be
decorated with the product label. The spike tool 44 can be
attached to the bottom or top of the can-in-can product.
The exothermic fuel compositions of the present invention
are comprised of solid, particulate, alkaline, earth oxide
reactants and solid, particulate, anhydrous acid reactants.
The use of alkaline earth hydroxides or alkali oxides
instead of alkaline earth oxides would produce less heat on a
molar basis, so more reactants would be required. Acids
having high solubility in water could be dissolved in the
water contained in the bag. Acids that are not anhydrous,
granular solids would be quite strong and extremely corrosive
if they were sufficiently soluble in water to avoid excessive
weight. A failure of the bag containing the fuel would rapid-
ly corrode the inner and outer cans and become a serious
hazard to the user. Also, when solutions of the strong acids
are heated, noxious or objectionable vapors would be released.
Excess weight or bulk of the fuel is also a prime con-
sideration. Therefore, alkaline earth oxide and anhydrous
acid reactants with lower equivalent weights are more ad-
vantageous, i.e., magnesium oxide or calcium oxide would be
W096/09503 PCT~S~5~^~59~5
8 ~'2~()5~6
favored over strontium oxide or barium oxide. Solid acids
such as oxalic, sulfamic, citric and tartaric would be favored
over benzoic, trichloroacetic, gluconic and succinic.
Because the alkaline earth oxides are all quite hygros-
copic, the solid acids must be anhydrous, i.e., they must not
carry waters of hydration or absorbed moisture. When a
alkaline earth oxides is mixed with a hydrated solid acid, the
alkaline earth oxide would be slowly dehydrated, slowly
liberating heat. A significant portion of the heating
capacity of the fuel would be lost while the can was in
storage awaiting use.
Some water must be added to initiate the reaction but
since water is a byproduct of the neutralization reaction,
less water would be required than for an acid-base fuel com-
position than one that uses alkaline earth oxide alone. Also,
the weight of the total solid acid-base reactants required to
produce the same amount of heat is less than the weight of a
comparable alkaline earth oxide exothermic fuel. For example,
slaked quicklime produces 277 calories per gram of solid fuel.
However, a mixture of equivalent amounts of calcium oxide and
oxalic acid produces 340 calories per gram of solid fuel.
CaO + H2C2O4 --~ CaC2O4H2O + 340 calories/gram of solid fuel
Thus it can be seen that the acid-base fuel of the inven-
tion would not only produce innocuous calcium oxalate rather
than hazardous calcium hydroxide, it would also produce more
heat on a weight basis. Therefore, less fuel by weight could
be used to produce the same heating effect.
The invention also contemplates fuel compositions formed
by mixing bases of different reactivity with acids of dif-
ferent reactivity. This offers a method for advantageous
control of the rate at which heat is generated from the fuel.
As discussed above, less fuel is needed if less of the heat is
lost due to water vaporization which occurs to a much greater
W096/09503 22 0 ~ ~ 8 6 PCT~S95/05905
~ 9
extent if the water in the annulus boils. However, it is
advantageous to rapidly heat the contents of the annulus to
near the boiling temperature of water so that heat will be
transferred more rapidly to the food due to a greater dif-
ference in temperature between the annulus and the food.
Thus, a fuel composition with two heat generation rates would
be advantageous. A first higher heat generation rate is sus-
tained only long enough to raise the temperature of the water
and fuel mixture to near, but not above, the boiling point of
water. A second lower heat generation rate to maintain the
temperature of the food in the inner can by a second heat rate
that is nearly equal to the rate at which heat is lost from
the system.
Different reactivities (heat generation rates) of the
reactants of the fuel composition can be provided by a mixture
of different alkaline earth oxides. This is because the base
strengths of the alkaline earth oxides decrease in the order
of increasing molecular weight, and base strength affects
acid-base reactivity. Another means to provide two-step heat
generation would be to use two quicklimes of different reac-
tivities caused by the different temperature at which they
were calcined in their production from limestone.
A self heating can similar to the assembly shown in
Figures 1 and 2 was used for a series of experiments. In
these experiments, 370 grams of water in the inner (food) can
was used to simulate food. An experiment with a thick chili
instead of water established that the heat capacity and vis-
cosity of water was sufficiently similar to food to permit
water to be used as a stand-in.
EXAMPLE 1
In each of the following four experiments, 65 grams of
lime, 110 grams of oxalic acid, and 155 grams of fuel water
were used. The annulus of the inner can contained 370 grams
of water.
W096/09503 PCT~S551~5~0~
'`2~
Sample No. Lime TyPe/Amount in Grams
Slowly Reactive / 65
26 Unslakable, Dead Burned / 65
27 Unslakable / 20
Slowly Reactive / 45
28 Very Reactive / 20
Slowly Reactive / 45
The line in Sample 25 is similar to the line used in the
commercial product currently available in England and Europe.
Figures 3 and 4 demonstrate the advantages of using mix-
tures of two quicklimes of different reactivity in combination
with an acidic reactant such as oxalic acid. The annulus
temperature curves are shown in Figure 4 and the food can
temperature curves are shown in Figure 3.
From Figure 4 it is clear that Sample 25 with a fuel
mixture of all slowly reactive quicklime and oxalic acid, and
Sample 28 with a fuel mixture of very reactive and slowly
reactive quicklimes and oxalic acid produced acceptable
heating of the contents of the inner can. The experiments
with unslakable lime and oxalic acid Samples 26 and 27 were
unsatisfactory.
The shapes of the curves in Figure 3 reveal the impor-
tance of a fuel composition with two different heat generation
rates. Curves 25 and 28, demonstrate a more rapid rise in
annulus temperature and a more delayed decrease in annulus
temperature. In Sample 28 the annulus temperature was above
200O F for a period longer than four minutes (curve 28).
During this period the food can temperature was increasing in
the range of 90 to 130 degrees Fahrenheit. The sustained
temperature difference of llo to 70 degrees Fahrenheit between
the annulus and the food can provided the driving force for
sustained heat transfer. Clearly, the more advantageous fuel
composition is the one that maximizes this integral of time
and annulus-food temperature difference. The curves of Figure
3 show a decreasing value of the annulus time-temperature
W09~,~5~0~ 2 2 0 0 ~ B ~ PCT~S95/05905
11
integral in the order 280, 250, 270 and 260.
By maximizing this integral, the heat generation process
is more controlled. Therefore, less fuel water will be needed
for such control.
The following experiment (Sample 29) was conducted to
optimize the fuel mixture.
Sample No. Lime Type/Amount, q Oxalic Acid q Water, q
29 Very Reactive/ 30 95 115
This is a total fuel weight of 270 grams or 18 percent
less weight than the single-quicklime fuel composition that
produced curves 250 and 25i. The results of the two ex-
periments are compared in Figure 5. The curves for the more
optimum fuel composition are identified as 290 and 29i. It is
noted that the annulus time-temperature integral for curve 290
is much larger than this integral for curve 250. This is why
less fuel produced a higher final food temperature.
The use of different solid, particulate acids in acid-
base fuel compositions is illustrated in the following ex-
periments, each utilizing 65 grams of slowly reactive quick-
lime, along with an approximately equivalent amount of solid
acid and 100 grams of fuel water.
Sample No. Acid, q
Sulfamic
31 Tartaric
32 Citric
33 Oxalic
It is clear from the curves in Figures 6 and 7 that the
reactivity of mixtures of lime and an acid decreases in the
order sulfamic, oxalic, tartaric, and citric, the approximate
order of the strength of these acids. Mixtures of these or
other acids or acidic salts with mixtures of different
alkaline earth oxides or with mixtures of quicklimes of dif-
ferent reactivity provide even better control of the shape of
W096/09s03 PCT~S9S/0590S
12 22~5~J6
the time-temperature curves (larger annulus time-temperature
integrals) of the self heating food can than could be obtained
by using a single acid or acid salt in the fuel mixture.
In all the fuel mixtures, the end product will be neutral
salts of the alkaline earth salts and the acids or acid salts.
These salts have no hazardous or toxic properties. Of course,
the exact acid-mixture/base-mixture composition that would
give maximum control with adequate heating and minimal fuel
weight would depend on the heat-transfer and heat-capacity of
the food in the can, on the exact design of the self-heating
food can and in the case of the use of quicklimes, on the
specific reactivities of the industrial grade quicklime
materials available for inclusion in the product.
It has been discovered in accordance with the invention
that the temperature at which calcium carbonate is calcined,
though important, is not the only parameter controlling the
reactivity of overburnt lime. The reactivity of the overburnt
lime is also influenced by the type of kiln used to calcine
the limestone and also the retention time of the limestone in
the kiln. The physical nature of the limestone also influen-
ces the properties of the calcined product. Calcite limes-
tones have a rhombohedral crystal structure and are soft,
having a Moh hardness of about 3 and a specific gravity of
about 2.72 g/cm3. Aragonite limestones are more dense
(specific gravity of about 2.94 g/cm3), are harder (3.5 to 4.0
Mohs), and have an ortho-rhombic structure.
Rotary kilns and parallel flow regenerative kilns usually
produce soft burned, highly reactive limes. Rotary kilns with
a small feed size generally produce a somewhat harder burned
lime while counterflow, shaft kilns produce the hardest burned
limes.
The hardest burned limes have the highest compressive
strength and the best resistance to abrasion during handling
and storage. Therefore, there will be less physical change in
the quicklime fuel when hard burned limes are packaged in the
wo 96/0g503 2 2 0 ~ 5 ~ 6 PCT~5~S~5~5
,_ .
13
annular chamber.
Samples of quicklimes overburned at temperatures above
1,200C had the following properties:
- TABLE 1
SAMPLE SOURCE CALCINATION PROPERTIES
TEMPERATURE
F C
1 Exshaw, Soft,
Alberta 2329 1276 Chalky
2 Pavillion, Hard,
B. C. 2350 1288 Dense
3 Exshaw, Soft,
Alberta 2542 1394 Chalky
4 Faulkner, Hard,
Manitoba 2600 1427 Dense
Exshaw, Soft,
Alberta 2621 1438 Chalky
All the samples as received were acorn-to-walnut size
particles. The Exshaw materials are soft, chalky, more porous
and had a lower density. They crumbled when hit with a ham-
mer. The harder, denser Pavillion and Faulkner materials
shattered when hit with a hammer. All materials were crushed
to "Grape-nuts" sized particles about 1-4 mm in diameter.
The anhydrous, granular solid acid can be added to the
quicklime in an amount up to an equivalent proportion with
respect to the quicklime, usually from 10~ to 50~ by weight of
the mixture.
W096/09503 PCT~S95/05905
~2()0S8~
14
EXAMP~LE 2
A fuel mixture was prepared from 43 grams of the quick-
lime of Sample 3 from Table 1, an equivalent portion (75
grams) of oxalic acid and 137 grams of water. The water-to-
lime ratio was 3.18 and the water-to-fuel ratio was 0.86. The
total weight of reactants was 255 grams. The post-test an-
nular chamber contained solid calcium oxalate and some excess
water. The pH of the slurry was near neutral. Calcium
oxalate is not toxic nor hazardous. The excess water can
readily be eliminated by reducing the amount of water in the
annular bag. As shown in Figure 8, the heating performance
again closely followed that of the lime-water mixture of the
commercial product (155g of slowly reactive lime, 100g water,
255g of total reactant).
EXAMPLE 3
The experiment of Example 2 was repeated by reducing the
water in the annular bag to 120 grams. The equivalent mixture
of lime and oxalic acid were increased to maintain the total
reactants at 255 grams. The results are also shown in Figure
8. The water simulating food in the inner container was
heated in a shorter time to a higher temperature (about
160F). The temperature was sustained above 140C for at
least 20 minutes. There was no excess water in the annulus
after the test.
EXAMPLE 4
The experiment of Example 3 was repeated substituting 43
grams of the Exshaw-2621 quicklime of Sample 5 for the Exshaw-
2542 quicklime of Sample 3. The heating rate was only very
slightly below that of Example 3 and is considered to be
satisfactory for a commercial product.
A series of experiments were conducted to test com-
positions sealed into a can-in-can prototype. Oxalic acid and
limestone were blended together and placed in the bottom of
W096/09503 20~ ~i6 PCT~S95/05905
the annulus. A foil bag of water was added. A cardboard ring
was placed on top of the water bag. Four holes were pierced
through an annular ring. The annular ring was sealed to the
outer can with J.B. weld compound. The inner can contained 1
- 5 pound of a meat stew.
EXAMPLE 5
The can-in-can in this experiment contained a mixture of
45 grams of Exshaw 2621 limestone and 25 grams of Faulkner
limestone. The foil water bag contained 100 grams of water.
The cardboard seal was pierced through the 4 apertures in the
annular ring and the lid removed f rom the inner can.
Time Comment
30 sec. First sound of escaping steam
1 min. Small amount of steam escapes
2 min., 30 sec. More steam
3 min. Steam stops
3 min., 30 sec. Food 125 F
4 min. Stir
6 min. Food 150 F
10 min. Food 180 F
13 min. Food 180 F
15 min. Food 185 F
20 min. Food 165-170 F
26 min. Food still hot
35 min. Food still hot
Though noise and steam level were acceptable, a hazardous
calcium hydroxide residue remained in the annular chamber
which could leak out the four holes if the can was inverted.
EXAMPLE 6
Example 5 was repeated except that 80 grams of oxalic
acid dehydrate was added to the mixture of overburnt limes-
tones. The food was not heated as long as in Example 5 ~ut no
hazardous residue was produced.
An experiment was then conducted in which the amount of
water was decreased and the amount of oxalic acid was
increased.
W096/09503 PCT~S9S/05905
16 220~S~
EXAMPLE 7
The amount of water was decreased to 80 grams and the
amount of oxalic acid dehydrate was increased to 110 grams.
Though it was expected that the reaction would not
proceed or would proceed less vigorously, the reaction was
very vigorous with gurgling and production of substantial
amounts of vapor-steam and white material bubbled out of one
of the pierced holes. The food in the inner container did not
get hot enough to eat.
EXAMPLE 8
Example 7 was repeated using 50 grams of water. The
results were substantially identical.
EXAMPLE 9
Example 7 was repeated utilizing 25 grams of water. The
results were substantially the same.
EXAMPLE 10
The amount of water was reduced to 12 grams with the
following results:
Time Comment
4 min., 30 sec. First evolution of steam
6 min., 30 sec. Sizzling sound
7 min., 30 sec. No more steam or gurgling, Food 175 F
9 min., 30 sec. Food 175 F
11 min., 30 sec. Food 170 F
25 min. Food 160 F
Surprisingly, the food was warm enough to eat in 10
minutes and stayed at eating temperature for 25 minutes. The
reaction was moderate. There was no vigorous evolution of
steam or vapor and the final salt residue is not hazardous.
The lower amount of water permits use of a smaller can and a
product weighing substantially less.
W096/09S03 ~5 ~6 PCTtUS95tO5905
-
17
~In the preferred composition of the invention, the weight
of water based on acid-base fuel is no more than 20 parts per
100 parts of acid-base and preferably from 3 parts to 12 parts
of water. The acid is preferably present in an amount at
5least equal in weight to the base and usually from a 10~ ex-
cess to 60~ excess, preferably from about 15~ to 50~ excess.
It is to be realized that only preferred embodiments of
the invention have been described and that numerous
substitutions, modifications and alterations are permissible
10without departing from the spirit and scope of the invention
as defined in the following claims.