Note: Descriptions are shown in the official language in which they were submitted.
~WO 96/12182 . J 5 PCT/CA95100582
A METHOD AND INSTRUMENT FOR MEASURING DIFFERENTIAL
OXYGEN CONCENTRATION BETWEEN TWO FLOWING GAS STREAMS
FIELD OF INVENTION
The invention relates to a method and an instrument for measuring the
difference in concentration of 02 between two flowing gas streams, and use of
the
instrument to measure the rate of 02 production or consumption from living or
non-
living materials. The instrument differs from previous instruments designed
for this
purpose in that it can resolve 02 differentials of less than 5 ppm 02 in the
presence
of background p02 from 100 ppm to 100%, is compact, easily calibrated,
relatively
inexpensive to manufacture, and may be battery powered for use outside of the
laboratory environment. The primary use of the instrument is the measurement
of
respiratory 02 consumption by plants, animals and micro-organisms, but it may
also
be used for respiratory studies on humans, studies of 02-producing
photosynthetic
cells, and for monitoring small 02 concentration differentials relative to a
reference
gas in biotic or abiotic systems.
BACKGROUND OF INVENTION AND PRIOR ART
Oxygen is essential to the survival of all cells which respire aerobically,
and
measurement of 02 consumption rate by cells, is frequently used as an index of
the
cells' metabolic activity. C02 is produced during respiration, and in most
cells under
normal aerobic conditions, the ratio of C02 produced to 02 consumed is close
to
unity. This ratio is termed the respiratory quotient (RQ) and it is an
important
indicator of the cells' metabolic state. For example, RQ changes depending on
the
nature of the respiratory substrate used by the cells, and increases greatly
when the
cells are subjected to anaerobic stress. Measurements of respiratory OZ
consumption,
C02 production and RQ are used in studies ranging from human exercise
physiology
to bacterial degradation of organic waste materials.
1
SUBSTITUTE SN~ET (RULE ~6)
WO 96/12182 ' PCT/CA95/00582
Green plants also respire aerobically, but in the light their photosynthetic
activity results in a net production, rather than consumption, of 02.
Measurement .
of 02 production from plants is often used as an assay of photosynthetic
activity.
During photosynthesis, plants convert atmospheric C02 to organic carbon, and
water
is converted to 02 gas. The ratio of COZ consumed to 02 produced by the plant
is
termed the photosynthetic quotient (PQ) and, like the RQ, this value is an
important
indicator of the plant's metabolic condition.
The most common method of monitoring 02 consumption or production from
cells is to place an organism, tissue or cell suspension in a chamber
(cuvette)
containing a known concentration of 02, and to monitor changes in the 02
concentration within the cuvette with time. This can be done by either "closed
system" or "open system" gas exchange methods. The closed system method
involves
sealing the biological sample in the cuvette and then taking samples of the
air from
the cuvette at measured time intervals. Each air . sample is analyzed for 02
concentration, and the change of 02 concentration with time can be used to
estimate
the mean rate of 02 production or consumption between sampling periods. In its
simplest form, the closed system assay involves only an initial and a final
measurement of 02 concentration, and the difference in the amount of 02 in the
cuvette is divided by the incubation period to give the rate of 02 exchange
during the
assay period.
The closed system assay for measuring 02 exchange has several limitations,
the most important of which is that rates of 02 exchange cannot be measured in
real
time. Therefore, any changes in the rate of biological 02 exchange cannot be
observed as they occur. Also, unless the atmosphere of the cuvette is sampled
at
regular intervals, any short-term perturbations in the rate. cannot be
detected. Such
perturbations are likely to occur in a sealed cuvette since respiration rate
and
photosynthesis in many cells are affected by p0,, and p02 will change in the
cuvette
as 02 is either depleted or evolved. To overcome these problems, open gas
exchange '
2
SUBSTITUTE Sf-~EET (RULE 26~ _
.:
'T
systems have been developed to provide continuous measurements of 02 exchange
under either steady or non-steady state conditions.
Measurement of 02 exchange in an open gas exchange system involves placing
the biological,sample in a cuvette through which gas of known composition
flows at
a measured rate. The 02 concentration of the effluent gas from the cuvette is
monitored by an OZ analyzer, and the difference in Q concentration between the
input and effluent gases multiplied by the flow rate through the cuvette gives
a
measure of the rate of 02 exchange. If the OL analyzer used in the open system
is
itself a flow-through instrument, the OZ concentration in the effluent gas
stream can
be monitored continuously, and real-time measurements of OZ exchange can be
performed.
The most accurate method of measuring 02 exchange in an open flow gas
exchange system is to use a differential 02 analyzer. Such instruments
continuously
monitor the difference in 02 concentration between a reference gas stream and
a
stream of the same gas composition which has passed through a cuvette
containing
the material under study. The most sensitive differential OZ analyzers
currently
available contain either paramagnetic OZ sensors (e.g. the Oxygor~ 6N, Maihak
AG,
Hamburg, Germany) or zirconium oxide sensors (e.g. Model S-3A/II, Servomex
Company, MA 02062, USA). However, the sensitivities of these instruments are
limited. The Oxygor~ 6N can resolve a minimum OZ differential of only 100 ppm
02 (which equals 10 Pa at 1 Atm total pressure) when air (approx. 20.9 kPa 02
is used
as the reference gas. Under the same conditions, the Servomex S-3A/II has an
accuracy limit of only ~ 3pa OZ in differential mode. Therefore, neither
instrument
has the sensitivity required to measure the very small OZ differentials that
may occur
when the biological sample under study has a low metabolic rate. Also, both
types
of differential 02 analyzer are essentially laboratory-based instruments which
are not
readily adaptable for field use, each requiring AC power and stable
environmental
conditions for most accurate function. They also require calibration by
laboratory-
3
AMENDED SHEET
~2~~~~3 ~ ..
based calibration systems involving compressed gases and/or gas mixing
instruments
This adds to the considerable expense of the analyzers.
Since differential OZ analyzers are relatively insensitive, many researchers
use
closed gas exchange systems to study organisms with low rates of Oz exchange.
For
example, the Micro-Oxymax Respirometer~ developed by Columbus Instruments
International Corporation (Columbus, OH 43204, USA) is among the most
sensitive
closed system device currently available for measuring OZ exchange from living
organisms. This device is capable of measuring an 02 consumption rate of 0.2
~1
O~/ h over a 24 hour period. However, at this level of sensitivity, the user
can obtain
only a single average measurement of 02 exchange over the 24 h period, and is
unable
to observe respiratory dynamics during the measurement. Use of the Micro-
Oxymax
Respirometer~ is further limited in that the gas supplied to the cuvette
cannot have
a partial pressure of OZ (pO~ exceeding 30 kPa. Therefore, the instrument
cannot be
used in experiments which require elevation of POZ beyond this limit (e.g. the
measurement of 02-saturated respiration rates in cells with respiratory rates
limited
by 02 diffusion, or the measurement of photosynthesis at high rates of
photorespiration that occur at elevated p0~. Like commercially available
differential
02 analyzers analyzers, the Micro-Oxymax Respirometer~ is a laboratory-based
instrument that is.not readily adaptable for field use. Its purchase price is
also in the
same range as that of available differential 02 analyzers.
The limitations of available differential and closed system OZ analyzers
indicate the need for an 02 analyzer that can provide continuous measurements
of
very small (less than 1 Pa) OZ differentials between two. flowing gas streams.
Such
an analyzer should be able to measure OZ differentials between reference and
sample
gas streams containing any p02 up to 100 kPa, and should be sensitive enough
to
resolve a p02 difference of as little as 1 Pa between these gas streams
irrespective of
the P02 in the reference gas. The instrument should be inexpensive, easily
calibrated,
preferably by an internal calibration system, and readily adaptable for
4
AME1VDED SHEET
J
WO 96/12182 i J ~ PCT/CA95/00582
,..
field use. Also, the instrument should be readily adaptable for simultaneous
measurements of differential p02 and CO2, enabling the user to obtain a direct
' measure of the respiratory quotient or photosynthetic quotient of the
experimental
material.
OBJECT OF THE INVENTION
It is an object of the present invention to provide an inexpensive
differential
02 analyzer, adaptable for use in the laboratory or the field, that is capable
of
continuously measuring 02 differentials between flowing reference and sample
gas
streams containing any p02, and capable of resolving such differentials of
less than
1 Pa in a background p02 from 10 Pa to 100 kPa O2.
It is a further object of the present invention to provide a differential 02
analyzer which incorporates environmental sensors to monitor the conditions
under
.. which the analyzer is used, so that the data from these sensors may be used
to
automatically correct the output of the analyzer as environmental conditions
vary.
It is a further object of the present invention to provide a method by which
the said differential 02 analyzer may be calibrated by an integral calibration
system
that does not require the use of ancillary gas-mixing apparatus.
It is a further object of the present invention to provide a differential 02
analyzer incorporating, within a single instrument, a system to measure both
the
differential p02 and the differential pC02 between reference and sample gas
streams.
BRIEF STATEMENT OF INVENTION
By one aspect of this invention there is provided an apparatus for measuring
differential oxygen concentrations between two flowing gas streams,
comprising;
temperature-controlled housing means having first and second gas flow paths
therethrough, each said flow path having means to introduce a selected gas
stream;
means to control pressure and flow rate of said gas stream; means to generate
signals
s
SUBSTITUTE SHEET (RUL,E 26)
' E'
WO 96!12182 ''' PCTlCA95100582
~~~~9~3 .
representative of pressure and oxygen concentration differentials between said
gas
streams; and computer means to monitor said pressure, flow and temperature and
,
said signals representative of said differential pressure and oxygen
concentration so
as to measure and record differential oxygen concentration between said gas
streams. .
By another aspect of this invention there is provided a method for measuring
differential oxygen concentration between rivo flowing gas streams, at least
one of
which contains oxygen, comprising; passing each said gas stream at a selected
temperature, pressure and flow rate over a respective one of a pair of
electrically
interconnected oxygen sensors, so as to produce an output signal that is
proportional
to the differential oxygen concentration bet<veen said sensors.
BRIEF DESCRIPTION OF DRAWINGS
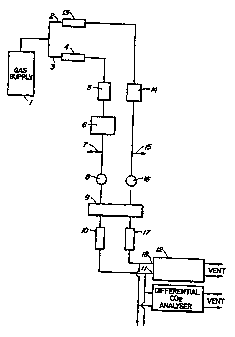
Figure 1 is a diagram of a typical open-flow gas exchange system incorporating
the differential 02 analyzer and showing how a differential C02 analyzer may
be
incorporated into the system to measure RQ or PQ.
Figure 2 shows an arrangement of ~ components within the differential 02
analyzer and the flow of gases through the instrument.
Figure 3 is an alternative arrangement of components, similar to Figure 2.
Figure 4 shows details of the sensor block that encloses the 02 sensors to be
used in the differential mode.
Figure 5 shows the electrical schematics for connecting reference and sample
02 sensors to provide a differential output voltage which can be balanced if
the
sensors differ in their output at a given p02, and the electrical circuit for
amplifying
the output signal from .the 02 sensors. ,
Figure 6 shows the mathematical relationship between the actual respiratory
quotient of an organism and the respiratory quotient measured from the outputs
of
the differential 02 analyzer and a differential CO, analyzer, when various p02
are
s
SUBSTITUTE SHEET (RULE 26)
WO 96!12182 J ~ PCT/CA95/00582
. ; va
supplied to the cuvette containing the organism and the organism depletes this
p02
by 10 kPa.
' Figure 7 shows the relationship between the actual respiratory or
photosynthetic quotient of ~ an organism and the extent to which the 02
exchange in
the sample gas stream measured by the differential 02 analyzer underestimates
or
overestimates the 02 exchange carried out by the organism.
Figure 8 shows the arrangement of 02 sensors in a double differential 02
analyzer designed for the simultaneous measurement of 02 and C02 exchange.
Figure 9 shows the relationship between the ratio of the sensor outputs in the
double differential 02 sensor and the actual respiratory quotient of an
organism when
the organism is supplied with 50 Pa C02 at various p02 in the sample gas
stream and
depletes this stream by 10 Pa 02.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
A Typical Open Circuit Gas Exchange System.
The differential 02 analyzer is designed for use in an open flow gas exchange
system of the type shown in Fig. 1. A supply of gas 1 (air or a mixed gas)
containing
02 is divided into a reference stream 2 and a sample stream 3. The flow rate
of the
sample stream is measured using a flow meter 4, and the gas passes through a
humidihcation system 5 before entering a cuvette 6 containing material which
either
produces or consumes 02. After passage through the cuvette, a proportion of
the gas
is vented to atmosphere 7, and the remainder is dried by being drawn, ~y~pump
8;
through a condenser in an ice water bath 9 and through a column of magnesium
perchlorate 10. The gas then enters the sample side 11 of the 02 analyzer 12.
The flow rate of the reference gas stream is measured by a flow meter 13 and
the gas then enters a humidifier 14 of the same type as that in the sample gas
stream.
a A proportion of the reference gas is vented to atmosphere 15, and the
remainder is
dried by being drawn, by a pump 16, through a condenser in an ice water bath 9
and
7
SUBSTITUTE SHEET (RULE 26)
WO 96!12182 ," . PCT/CA95100~82
~~~C~~~~
through a magnesium perchlorate column 17. The reference gas then enters the
reference side 18 of the OZ analyzer. After passage through the analyzer both
the ,
reference and sample gas streams are vented to atmosphere.
In an alternative embodiment, the differential 02 analyzer could be used to .
measure the 02 concentration of a gas collected in a specific environment,
rather
than the 02 concentration of a gas modified by passage through a cuvette
containing
living material. Such measurements would.be important to atmospheric
monitoring
in which information is required on diurnal, seasonal or regional fluctuations
in
atmospheric p02. In this application the reference gas used in the analyzer
would
be a bottled, or mixed, gas of known p02, and the sample gas would be pumped
from
a container directly to the dehumidifier of the gas exchange system before
entering
the sample side of the differential analyzer.
Design of the Differential OZ Analyzer.
A block diagram of the differential 02 analyzer is shown in Fig. 2. A
proportion of the reference 21 and sample 22 gas streams, which have been
dried to
a similar extent by passage through the ice water bath and magnesium
perchlorate
columns, are drawn into the analyzer by pumps 23 and 24, respectively, which
work
against downstream flow restrictors 25 and 26. The remainder of the gases vent
to
atmosphere through by-passes 27 and 28. An 02 sensor 29 (Model KE-25, Figaro
USA Inc., Illinois, USA) in the reference gas by-pass line measures the
absolute OZ
concentration in the reference stream. Particulate matter is removed from both
gas
streams by glass wool filters 30 and 31 positioned adjacent to the flow
restrictors.
The reference stream is divided at a tee-piece 32, one branch entering Pump
23, and
the other entering solenoid 33. When solenoid 33 is in the "on" position, the
analyzer is in measurement mode and all of the reference gas passes through
pump
23, and through the reference side of the differential analyzer. In this
configuration,
the sample gas entering solenoid 33 is pumped through the sample side of the
analyzer. When solenoid 33 is in the "off" position, reference gas is pumped .
s
SUBSTITUTE SHEET (RULE 26)
WO 96/12182 PCTICA95l00582
through both the reference and sample sides of the analyzer, and the
instrument is
in zero mode. In either mode, the gases which pass through the reference 34
and
sample 35 02 sensors (Model KE-25 Figaro USA, Illinois, USA) first enter heat
exchangers 36 and 37, integrated into the temperature-controlled detector
block 38
in which the sensors are housed. For optimal operation, the temperature of the
block should be maintained above the highest ambient temperature to which the
instrument is likely to. be exposed, but its normal operating temperature is
40' C.
This temperature is monitored by a thermistor 39, or other temperature
sensitive
device, integrated into the sensor block.
The reference gas stream exits through a detachable flow restrictor 40 in the
form of a stainless steel tube with a small orifice. When both solenoid 41 and
42 are
in the "off' position, the sample stream exits through a detachable flow
restrictor 43
having physical properties similar to those of flow restrictor 40 located in
the
reference gas flow stream. Therefore, at the same gas flow rate in the
reference and
sample flow streams, the flow restrictors will provide similar backpressures
on the
differential 02 sensors and the differential pressure sensor 44 will read near
zero.
When solenoid 42 is activated, and solenoid 41 is off, the sample gas stream
is vented
directly to atmosphere at 45, resulting in a lower backpressure in the sample
gas
stream relative to that in the reference gas stream, the magnitude of which is
measured by the differential pressure sensor 44. By selecting flow restrictors
having
different characteristics, or by controlling the gas flow rate of the
reference and
.. sample streams, the user can regulate the magnitude of the pressure
differential
obtained when solenoid 41 is activated.
When solenoid 41 is activated and solenoid 42 is off, the sample gas stream
is vented through a flow restrictor 46 having a resistance to gas flow
approximately
twice that of flow restrictors 40 and 43. This results in an increase in the
differential
pressure in the sample gas stream relative to the reference gas stream, the
.magnitude
of which is measured by the differential pressure sensor 44.
s
SUBSTITUTE SHEET (RULE 26)
WO 96/12182 ~ ~ ~ ~ PCTlCA95/00582
~~~JU~3~~
The increases or decreases in differential pressure in the sample gas stream
created by the activation of solenoid 41 or solenoid 42 results in small
changes in the ,
02 concentration in the sample side of the analyzer which can be detected by
the
differential 02 sensor. This is the basis for the integrated calibration
system of the
analyser. To ensure that the changes in the backpressure on the sample gas
stream
has a minimal effect on the flow rate of the gas stream, it is recommended
that the
resistances of the flow restrictors 40, 43 and 46 are less than 15% of the
resistance
of the flow restrictors 25 and 26 which are located immediately downstream of
pumps 23 and 24 and filters 30 and 31.
During operation of the 02 analyzer, atmospheric pressure is monitored by an
absolute pressure sensor 47 which has its own port to the exterior of the
instrument.
Measurement of atmospheric pressure is essential for the calculation of 02
concentration in the reference gas stream.
In an alternative embodiment of the instrument (Fig. 3) the design of the
temperature-controlled sensor block 38 is identical to that shown in Fig. 2,
but the
flow path of the reference gas 21 and sample gas 22, through the instrument is
different. In the alternative embodiment both the reference gas and sample gas
are
drawn into the instrument by a single pump 48 located just before the point 49
at
which the gases vent from the instrument. A void volume 50 of approximately 10
cm3 is incorporated into the gas line just before the pump to dampen any
oscillations
in flow rate.
The flow of gases through the instrument is controlled by solenoid valves 51,
52 and 53 and the activation and deactivation of these differ depending~on the
use
of the instrument in calibration mode or measurement mode. To calibrate the
instrument zero conditions are first established by flushing reference gas
through
both the reference and sample side of the instrument. The normally closed
solenoid
51 is deactivated so that the sample gas 22 is blocked from entering the
sample side
of the analyzer and vents to atmosphere through by-pass 28. A sample of the
SUBSTITUTE SHEET (RULE 26)
~WO 96/12182 L ' ~~ j ~ PCT/CA95/00582
reference gas 21 enters a tee-piece 32 and is drawn through both the reference
and
sample sides of the instrument by pump 48. The remainder of the reference gas
passes through an absolute 02 sensor 29 and vents from the instrument at by-
pass 27.
In the sample side of the instrument, the reference gas enters a tee-piece 54
connected to solenoid valve 52. This valve is closed when deactivated and
diverts the
reference gas through a flow restrictor SS of the same type as those described
for the
embodiment of the instrument shown in Fig. 2. Having passed through the flow
restrictor S5, the gas enters a tee-piece 56 attached to a solenoid valve 53
which is
open when deactivated. When open, this valve allows the gas to pass without
further
resistance to the sample 02 gas sensor 35 in the temperature-controlled
detector
block 38. After passing through 02 sensor 35, the reference gas exits the
detector
block and is drawn by the pump through a flow restrictor 57 having a
resistance
approximately 20 times that of flow restrictor 55. After exiting flow
restrictor 58, the
reference gas in the sample side of the instrument is combined at tee-piece 59
with
gas that has passed through the reference side of the instrument, and the
mixed gas
is pumped from the instrument via the void volume 50. With solenoid valves 51,
52
and 53 in the configuration described above, a proportion of the reference gas
entering tee-piece 32 passes into the reference side of the instrument via a
needle
valve 61. The gas then enters the reference 02 sensor 34 in the detector block
38
and is drawn from the detector block through a flow restrictor 60 having a
similar
resistance to the flow restrictor 57 in the corresponding position in the
sample side
of the instrument. The reference gas is then combined at tee-piece 59 with the
reference gas that has passed through the sample side of the instrument, and
the
combined gases are pumped from the instrument at vent 49. The flow rate of gas
through the instrument is controlled by pump 48, and this is calibrated by
means of
a different pressure sensor 58 located between the input and output ports of
flow
restrictor 59. At high flow rate, the pressure differential across flow
restrictor 59 is
greater than at lower flow rates, and the relationship between pressure
differential
11
SUBSTITUTE SHEET (RULE 26)
WO 96/12182 ~ ~ ~,U '~ ~ ~ PCT/CA95/00582
and flow rate is stored in the instrument software so that the voltage output
of
differential pressure sensor 58 can be used to monitor and control the speed
of the
pump.
With reference gas flowing in both the reference and sample sides of the
instrument, the differential 02 concentration between 02 sensors 34 and 35
will be
zero only if there is no differential pressure between the gases in each
sensor. The
differential pressure sensor 44 positioned between the reference and sample
gas
streams monitors the pressure differential between the reference and sample
sides
of the instrument, and the needle valve 61 in the reference line can be
adjusted to
set this pressure differential to zero.
When zero conditions have been established, the differential 02 analyser may
be calibrated by varying the pressure of the reference gas in the sample side
of the
instrument. To increase the pressure of gas in the sample 02 sensor relative
to that
in the reference sensor, and thereby increase p0, in the sample sensor,
solenoid 52
is activated. When activated solenoid 52 is in the open position, and allows
the
reference gas to flow without restriction to the sample 02 sensor. Under these
conditions the differential pressure sensor 44 reads a pressure differential
which is
related to the 02 concentration differential between the sample and reference
OZ
sensors.
To reduce the pressure in the sample gas line relative to that in the
reference
gas Line, and thereby reduce the 02 concentration'in sample sensor 35,
solenoid 52
is deactivated (closed) and solenoid 53 is activated (also closed). With
solenoid 52
and 53 both in the closed position, the reference gas must flow through flow
restrictor 55, and an additional flow restrictor 62 with the same resistance
before
entering sample 02 sensor 35. By doubling the resistance in the sample side of
the
instrument the gas pressure in the sample O, sensor is decreased. The
differential
pressure sensor 44 monitors the resulting pressure differential and the
differential 02
analyser produces a signal proportional to the differential 02 concentration
between
12
SUBSTITUTE~SH~EET (RULE 26)
WO 96/12182 ~- . j ,5 ~ PCT/CA95/00582
the two sensors. The calibration procedure therefore involves establishing a
zero
condition, then performing a two point calibration involving both higher and
lower
p02 conditions in the sample 02 sensor relative to the reference 02 sensor.
a Measurement of the differential pressure between the sensors allows
calculation of
the differential p02 values that these pressure changes cause.
In measurement mode, solenoid 51 is activated (opened) to allow sample gas
to flow through the sample side of the instrument. Solenoid 52 is closed and
solenoid 53 is open so that the sample gas passes only through flow restrictor
55.
Under these conditions there will be no differential pressure between the
reference
and sample gas streams, since the resistance of flow restrictor 55 was
balanced by
adjustment of needle valve 61 in the reference gas stream during the
calibration
procedure. Therefore, any signal generated from the differential 02 analyser
will be
due only to the differential p02 between the reference and sample 02 sensors,
and
not influenced by pressure effects.
In either embodiment of the instrument, the differential OZ analyzer may be
calibrated and operated using computer control. The outputs of the absolute 02
sensor, the reference and sample 02 sensors, the block temperature thermistor,
the
absolute pressure sensor and the differential pressure sensor undergo analog
to
digital conversion and the digital values are stored for use in subsequent
calculations.
The speed of pumps 23 and 24, (Fig. 2) or pump 48 in the alternative
embodiment
(Fig. 3) and the block temperature are controlled by an analog output from a
digital
to analogue converter, or by digital control of a voltage divider supplying
power to
the pumps. In addition, the positions of solenoids 33, 41, and 42 (Fig. 2) or
solenoids
51, 52 and 53 in the alternative embodiment of the instrument (Fig. 3), are
digitally
controlled. The calibration procedure for the embodiment of the differential
02
analyser shown in Fig. 2 is described in detail below, as are the mathematical
analyses required for calibration and use of both embodiments of the
instrument.
13
SUBSTITUTE SHEET (RULE 26)
WO 96!12182 ~ ~,'~ ~~;~;~ ~ ~.5 PCTICA95/00582
Design of the Sensor Block
The design of the temperature-controlled sensor block (38; Fig. 2 and Fig. 3)
is shown in Fig. 4. The reference 34 and sample 35 02 sensors are located in
identical housings 62 and 63, respectively; constructed of a heat-conducting
material,
such as aluminum or stainless steel. The two housings are joined at a centre
plate
64. The reference and sample sensors are oriented in their housings so that
their
lead anodes 65 and 66 are located towards the base of the instrument. The
sensing
heads 67 and 68 of the 02 sensors are held tightly in position by the body of
their
respective housings, while the remaining part of the sensors fit into air-
filled cavities
69 and 70 within the housings. The air in these cavities is continous with the
ambient atmosphere within the instrument via ports 71 and 72. Other ports 73
and
74 through the sensor housings into the air-filled cavities 69 and 70 allow
passage of
the electrical connections 75 between the sensors. The entire sensor block,
consisting
of the two joined housings, is wrapped in heating foil (not shown), the
temperature
of which is controlled by a power supply. A resistive temperature device 76,
submerged in a heat transfer gel in a cavity 77 within the centre plate of the
sensor
block, continuously monitors block temperature during use.
Reference and sample gas enter the sensor block by ports 78 and 79,
respectively. The gases then pass through heat exchangers 80 and 81 consisting
of
circular gas channels within the sensor housings which direct the gases by a
tortuous
route to the sensing heads 67 and 68 of their respective 02 analysers 34 and
35. The
gases reach the temperature of the sensor block during passage through the
heat
exchangers, and after passing across the 02 sensors they leave the sensor
block by a
direct route through ports 82 and 83.
The pre-amplifer 84 (and see Fig. 5) which conditions the signal from the
differential 02 analyser is temperature sensitive and is therefore maintained
at a
constant temperature by incorporation within the sensor block.
14
SUBSTITUTE SHEET (RULE 26j
CA 02200953 2005-06-27
The Differential OZ Analyzer: Design and Principle of Operation.
(a) Electrical Configuration of the Differential OZ Sensor.
The reference and sample OZ sensors (preferred embodiment is Model KE-25,
Figaro USA Inc., Illinois, USA) used within the differential 0 2 analyzer
operate on the
principle of a lead-oxygen battery. Each contains a lead anode, an oxygen
cathode (which
is made of gold) and a weak acid electrolyte. Oz passing across the surface of
the sensor
diffuses through a TEFLON' FEP membrane and is reduced electrochemically at
the gold
electrode. As supplied from the manufacturer, each sensor contained a resistor
and a
thermistor connected in series (with a total resistance of about 1.2 kOhms)
between the
anode and the cathode so that the voltage differential is proportional to the
OZ
concentration at the teflon membrane despite variations in temperature.
For use in the differential OZ analyser, two individual sensors were modified
and placed in an electrical circuit, as shown in Fig. S, such that the output
of the circuit
was proportional to the differential OZ concentration between the two sensors.
The
modification and features of the differential sensors include:
(i) The connection between the internal resistors of each sensor was severed
and the wire leads connected in parallel across a fixed 800 ohm resistor 85,
and a 200
ohm potentiometer 86, as shown in Fig. 5.
(ii) The sensor with the larger current output at a given p02 was placed in
the
circuit such that the anode was connected to the wiper on the 200 ohm
potentiometer.
Typically, the current produced by the reference sensor was approximately
lSp,A at
40°C and 20 kPa O2, while that of the sample sensor was 20~A under the
same
conditions.
(iii) When gas streams having the same p02 and atmospheric pressure were
presented to both sensors, the potentiometer 86 was adjusted so that the
differential
output of the circuit was zero volts.
~~ PCT/CA95/00582
WO 96/12182
(iv) Increases or decreases in the p0~ at the sample sensor altered the
current flow through the fixed resistor 85 and potentiometer 86, which in turn
altered
the output voltage differential.
(b) Amplification of differential voltage.
The voltage output of the differential 02 sensor is inherently linear with the
differential p02 between the reference and sample gas streams, but the
voltages
produced are very small. For example, a p02 differential of 10 Pa (equivalent
to ca.
100 ppm OZ at 1 atmosphere pressure) between the reference and analysis
sensors
produces a voltage differential of only 12.5 ~V. A low noise ~V amplifier,
such as
that shown in Fig. 5 is required to boost the signal into a measurable range.
First, the positive and negative outputs from the sensor circuit are DC
filtered
by passage through two tantalum 5 ~cF capacitors 87 and 88 arranged back to
back
in series. The negative output is connected to ground, and the positive output
enters
a TLC1150 operational amplifier 89 configured as a non-inverting amplifier.
This
has a voltage gain factor of 6800 which is achieved with temperature stable
precision
resistors. The input resistor 90 has a resistance of 1 Ohm, and the feedback
resistor
91 has a resistance of 6.8 kOhm. Two 1.5 ~F tantalum capacitors 92 and 93
arranged
back to back in series with each other, and in parallel with the 6.8 kOhm
resistor
prevent amplification of high frequency noise. The outputs from the TLC1150
operational amplifier enter a 1 Hz low pass filter 94 to further reduce noise.
The
outputs from the low pass filter can be connected to an analogue recording
device,
such as a chart recorder, or to an analogue to digital converter for data
logging by
a computer (not shown).
Calibration of the Differential Oxygen Analyzer
Calibration of any differential 02 analyzer requires that a reference gas with
a known p02 be passed through the reference 02 sensor while gases containing
known p02s, different from that in the reference gas, are passed through the
sample
sensor. To check linearity of the analyzer response, at Ieast a three point
calibration
16
SUBSTITUTE Sf~EET (RULE 26)
WO 96/12182 ~ L ~ ~ r~ ,~ 5 PCT/CA95/00582
should be carried out. This should include p02 values above and below that in
the
reference gas if positive and negative O, differentials are Lo be measured
during use
of the analyzer. Also, the calibration should be performed to encompass the
range
of p02 differentials that the instrument is required to measure. Standard
gases used
in calibrations may be mixed in the laboratory using high precision gas mixing
pumps
such as Wosthoff pumps, or by mixing gases using electronic mass flow
controllers.
Both of these options are very expensive. Alternatively, standard gases may be
purchased from a gas supply company, but this too is very expensive in the
long term.
It should also be noted that these current methods of calibration require the
use of
laboratory-based ancillary equipment. In contrast, the embodiments of the
differential 02 analyzer shown in Fig. 2 and Fig. 3 have an integrated
calibration
routine that does not involve gas mixing, and which does not require the use
of
additional laboratory facilities. Dry air is used as the calibration gas, and
the same
air is passed through the reference and sample O~ sensors by deactivation of
solenoid
33 (Fig. 2) or solenoid 51 (Fig. 3), but the p02 of the air in the sample gas
stream
is varied by applying different gas pressures on the sample 02 sensor. To
calculate
differentials of p02 resulting from pressure differentials between the
reference and
sample sensors requires measurement of the environmental conditions in which
the
instrument is used. Calibration of the environmental sensors within the
analyzer, and
use of the data they provide to calculate 02 differentials is described below.
(a) Measurement of atmospheric pressure.
For accurate calibration of the differential 02 analyzer, the absolute partial
pressure of 02 in the air entering the instrument must be known in units of
kPa.
This p02 will vary depending on the ambient atmospheric pressure, since air at
higher pressure contains a greater number of molecules of 02 per unit volume
than
air at lower pressure (e.g. air containing 20% 02 has a p02 of 24 kPa at an
atmospheric pressure of 120 kPa, but a p0, of 20 kPa at 100 kPa atmospheric
pressure). The analyzer therefore incorporates an absolute pressure sensor
(47; Fig.
SUBSTITUTE SH~ET (RULE 26)
WO 96112182 ~ ~ PCTICA95/00582
2) to measure ambient atmospheric pressure. The voltage output of this
absolute
pressure sensor itself must be converted to units of kPa pressure, which
requires .
calibration of the sensor using a Hg manometer. This is done during assembly
of the
analyzer, but the calibration may be modified by the user through software.
A two point calibration procedure for the absolute pressure sensor is used in
which the voltage output is measured under ambient pressure conditions, and
when
zero pressure is, established by attaching the sensor to a vacuum pump.
Ambient
pressure is measured in units of mm Hg by a mercury manometer, and this value
is
converted to units of kPa taking into account the effects bf ambient
temperature on
the relative expansion of Hg and glass within the barometer, and latitude-
dependent
gravitational force on the mm Hg reading. Ambient temperature is read from the
instrument thermistor within the analyzer (39: Fig. 2). The temperature
correction
factor is stored as a linear regression in software, and the gravitational
correction
factor as a third order polynomial. These correction factors avow for an
accurate
conversion of mm Hg to kPa irrespective of the temperature or geographical
location
in which the instrument is used. The relationship between kPa and voltage
output
of the absolute pressure sensor is stored as a linear regression in software.
The
absolute pressure of the air within the reference sensor is the sum of the
ambient
pressure plus the back pressure exerted on the sensor by the flow restrictor
on the
reference gas outlet port (40; Fig. 2). This backpressure is measured during
calibration of reference gas flow rate as described in (d) below.
(b) Calibration of the absolute Cf~2 sensor.
The absolute 02 sensor in the reference gas stream (35; Fig. 2) is calibrated
during assembly of the analyzer, but the calibration may be altered in
software by the
user. A two point calibration is made in which the voltage output of the
sensor is
measured at 100% 02 and 0% 02 (pure N2). Percentage 02 at the pressure
maintained within the reference 02 sensor is converted to kPa 02 as described
in (a)
above. A linear regression of absolute 02 sensor voltage output against kPa 02
is
~s
SUBSTITUTE SHEET (RULE 26)
WO 96/12182 ~ ~ ~ ~, ~, '~ ~ PCT/CA95/00~82
stored in software and used to convert voltage output to kPa 02 at any input
p02 and
pressure.
(c) Calibration of differential pressure sensor.
The differential pressure sensor is calibrated by a three point calibration in
which zero, positive and negative pressure differentials are applied between
the
reference and analysis 02 sensors. The absolute pressure differentials are
measured
by a water manometer placed between the reference and sample gas streams, and
the
pressure units are converted from mm H20 to Pa taking into account the
gravitational correction factor due to latitude as described in (b) above for
conversion of mm Hg to kPa. A temperature correction factor is not included in
this
conversion of units since it is assumed that glass and water have similar
temperature
dependent coefficients of expansion. A linear regression is run of
differential
pressure (Pa) against the voltage output of the differential pressure sensor,
which
allows differential pressures to be estimated within the range of pressures
used in the
calibration routine.
In the embodiment of the differential 02 analyser shown in Fig. 2,
differential
pressures between the sample and reference 02 sensors are achieved by
diverting the
sample gas stream through different flow restrictors before it vents from the
instrument. The reference gas stream always vents through a fixed resistance
(40;
Fig. 2) and, under normal operating conditions for the analyzer, solenoids 41
and 42
(Fig. 2) are set in the off position so that the sample gas vents through a
similar
resistance to that in the reference line. This produces a zero pressure
differential
between the reference and analysis 02 sensors, and the voltage output of the
differential pressure sensor reflects this zero. To obtain a positive pressure
differential between the sample and reference sensors, solenoid 33 (Fig. 2) is
activated so that the sample gas flows through restrictor 26 with a greater
back
pressure than that in the reference line. To obtain a negative pressure
differential
19
SUBSTITUTE SHEET (RULE 26)
WO96/12182 ~,~.~:.~'r~ ~ ~ PCT/CA95/00582
between the sample and reference sensors, solenoid 41 (Fig. 2) is deactivated
and
solenoid 42 (Fig. 2) is activated so that the sample gas vents directly to
atmosphere.
The range of backpressures over which the differential pressure sensor is
calibrated may be varied by changing the flow restrictors through which the
reference
and sample gas vent. Since these restrictors consist of detachable tubes with
different bore sizes, they are easily replaced by tubes having either narrower
or wider
bores. Alternatively, tubes of the same bore size, but differing in length,
can be used
to vary back pressure.
The way in which differential pressures are achieved between the reference
and sample 02 sensors in the alternative embodiment of the instrument shown in
Fig.
3 has already been described above. The range of pressures over which this
embodiment of the analyser may be calibrated can be changed by changing the
internal flow restrictors 55 and 56 for restrictors of greater or less
resistance.
(d) Calibration of pump speed.
In the embodiment of the differential 02 analyser shown in Fig. 2, the back
pressure exerted on the reference and sample 02 sensors is a function of the
rate at
which the reference (23; Fig. 2) and analysis (24; Fig. 2) pumps sample their
respective gases, as well as the magnitude of the flow restrictors through
which the
gas streams vent to atmosphere. Therefore, it is necessary to set the pump
speeds
to achieve a zero pressure differential between the sensors when the two gas
streams
vent through restrictors of similar resistance. To achieve this at a given
flow rate it
is only necessary to calibrate the reference-gas pump while the sample gas
pump is
inactive. Under these conditions, atmospheric pressure will be maintained in
the
sample gas line while a positive pressure will occur in the reference gas
line. The
magnitude of the pressure differential between the two gas lines will be
determined
by the speed of the reference pump and by the back pressure exerted by the
resistor
(40; Fig. 2) on the reference gas vent. By varying the pump speed and
attaching a
zero resistance flow measuring device to the reference gas vent, the
relationship
SUBSTITUTE SHEET (RULE 26)
WO 96112182 ~ ~ PCTICA95100582
between flow rate of the reference gas and the pressure differential between
the
reference and sample gas lines can be determined. A look-up table can be
established in the analyzer software which relates flow rates and pressure
differentials
for different resistors in the reference stream.
To attain a desired flow rate through the instrument, zero voltage is supplied
to the sample pump while voltage supply to the reference pump is increased
until the
pressure differential corresponding to the desired flow rate of the reference
gas is ,
attained. Voltage supply to the sample pump is then increased until a zero
pressure
differential is obtained between the reference and sample gas streams.
This calibration procedure also allows the total pressure of gas in the
reference and analysis sensors to be measured. At a given flow rate with a
given
resistance in the reference line, the Look-up table in the analyzer software
will show
the amount by which the pressure in the 02 sensors exceeds atmospheric
pressure.
This excess pressure can then be added to the atmospheric pressure, measured
as in
(a) above, and be used to calibrate the differential 02 analyzer.
Calibration and control of pump speed in the alternative embodiment of the
differential 02 analyser shown in Fig. 3 has already been described above.
(e) Calculation of differential pO2 from differential pressure between the
reference
and analysis 02 sensors.
Steps (a) to (d) in the calibration routine allow measurement of the total
pressure (TP; kPA) of gas in the 02 sensors, the absolute p02 (AO; kPa) of the
gas
in the reference sensor and the pressure differential (DP; Pa) between the
reference
and sample sensors. Differential p02 (DO; Pa) between the two sensors at a
given
pressure differential can then be calculated as:
DO = AO . (DP / TP)
Measurement of DO and the voltage output from the differential analyzer
when positive, negative and zero pressure differentials are applied between
the
reference and analysis sensors, allows a three point calibration of the
analyzer to be
2~
SUBSTITUTE SHEET RULE 26~
WO 96/12182 PCTICA95100582
performed. This calibration is characterised by a linear regression that is
stored in
the analyzer software. However, this regression is only valid at the sensor
temperature at which the calibration took place since the outputs of the
sensors are
temperature sensitive. The relationship between sensor output and temperature
is
described by a third order polynomial which is stored in the analyzer
software. This
allows values of DO derived from the linear regression to be corrected for
changes
in temperature of the sensor block. This temperature is set by the user and
monitored continuously by a thermistor (39; Fig. 2) embedded within the block
during calibration and use of the analyzer. To obtain the most stable output
from
the analyzer, the block temperature must be set at above ambient temperature.
A
block temperature of 40 ° C is recommended for use of the analyzer in
the laboratory,
but a somewhat higher block temperature may be necessary if the instrument is
exposed to a high heat load in the field.
Calibration checks during analyzer use.
The calibration of the differential 02 analyzer can be checked at any time
simply by changing the configuration of solenoids 41 and 42 (Fig. 2), or
solenoids 52
and 53 in the alternative embodiment of the instrument (Fig. 3), to create a
known
pressure differential between the reference and analysis 02 sensors
corresponding to
a known 02 differential. This procedure can be implemented even when the
instrument is being used to measure O~ differentials in measurement mode.
Therefore, during an experiment, the user can increase or reduce the 02
differential
between the 02 sensors and check whether or not the change in the analyzer
output
matches that predicted by the current calibration. This comparison could be
made
by the system software and the user could be warned of significant deviations
from
the set calibration.
Use of the Differential Oxygen Analyzer
The differential 02 analyzer is compact and can be operated either by DC or
AC power. Its calibration requires only a supply of air and can be performed
without -
22
SUBSTITUTE Sh~EET (RULE 26)
WO 96!12182 ~ ; ~ j PCT/CA95/00582
expense at any time. The air should be completely dried by passage through
magnesium perchlorate or other drying agent before it enters the instrument.
The
analyzer continuously monitors the temperature and pressure of the environment
in
which it is being used, and the calibration corrects for any fluctuations in
these
conditions. Therefore, the analyzer is ideally suited for use in the
laboratory or the
field, unlike other differential 02 analyzers that require stable
environmental
conditions within a laboratory environment.
Trials of the analyzer have shown that the integral calibration method
produces results which are very similar to those obtained from calibrations
using
Wosthoff precision gas mixing pumps to produce different partial pressures of
02 in
N2. The resolution and stability of the instrument is such that a differential
p02 of
0.2 Pa can be measured with 20 kPa 02 flowing through the reference 02 sensor.
This represents a 15 to SO fold greater sensitivity than prior art
differential 02
analyzers. Also, the output of the analyzer is constant for a given 02
differential
irrespective of the absolute p02 of the reference gas stream.
Effect of Respiratory Quotient and Photosynthetic Quotient on Oxygen
Consumption
and Depletion Rates Measured by Differential Oxygen Analysis.
The accuracy with which the differential 02 analyzer can measure rates of 02
exchange from an organism depends on whether or not 02 consumption or
production is coupled with the consumption or production of any other gas.
Typically, during aerobic respiration under atmospheric conditions, the rate
of
respiratory 02 uptake by cells is equal to the rate of respiratory C02
production. In
this case, the respiratory quotient (RQ = unit C02 produced per unit 02
consumed)
is unity. However, RQ may be less than unity under aerobic conditions, and can
be
significantly greater than unity as C02 evolution increases relative to 02
consumption
under anaerobic conditions. RQ is also dependent on the nature of the
substrate
consumed in respiration. In most higher photosynthetic cells, C02 fixation is
associated with 02 consumption and the photosynthetic quotient (PQ = unit C02
23
SUBSTITUTE SHEET (RULE 26)
WO 96/12182 ~ ~ PCT/CA95/00582
consumed per unit 02 produced) may be greater or lesser than unity depending
on
environmental conditions.
The differential 02 content measured between the reference and sample gas
streams represents actual rates of 02 evolution or consumption by organisms,
cells
or tissues only if these rates are balanced exactly by equal rates of
consumption and
evolution, respectively, of another gas. That is, the total pressure. of gases
exiting the
cuvette must equal the total pressure of gases in the reference gas stream.
The
errors which occur when 02 exchange is not balanced exactly by the exchange of
another gas are best shown by examples.
Example 1. Measurement of 02 depletion by an organism having a respiratory
quotient of 2Ø
Assume that gas at an atmospheric pressure of 100kPa, containing 20kPa 02
in a balance of N2 is supplied to a cuvette at 500 mL/min containing an
organism
which depletes the supplied p02 by 10 Pa. At an RQ of 2.0, the organism would
add
2 Pa of C02 to the supplied gas for every 1 Pa of 02 consumed. Therefore, the
gas
exiting the cuvette 6 (Fig. 1) would have a total pressure of 100 010Pa,
containing 19
990 Pa 02, 20 Pa C02 and 80 000 Pa N2. The majority of gas exiting the cuvette
is
vented to atmosphere via vent 7, and since the total pressure of gas exiting
the
cuvette is greater than that entering the cuvette, the flow rate of vented gas
is greater
than if the input and output gases had equal pressures.
The sample gas stream entering the analyzer is at atmospheric pressure (100
000 Pa), the proportion of 02 in this gas being the same as the proportion
exiting the
cuvette i.e 19 990 parts in 100 010. The p02 of the gas entering the analyzer
would
therefore be 19988 Pa, and the 02 analyzer would measure an apparent OZ
depletion
of 12 Pa even though the cell depleted the supplied gas mixture by only 10 Pa.
The
rate of OZ consumption estimated from this measurement would exceed the actual
rate by 20%, and the estimated RQ value would be 1.67 compared to the actual
value of 2Ø
24
SUBSTITUTE SHEET (RULE 26)
r r
W O 96112182 ~ ~- ~ l~ v ,J .5 PCT/CA95/00582
Example 2. Measurement of OZ consumption by an organism having a respiratory
quotient of 0.2.
Assume that gas at an atmospheric pressure of 100kPa, containing 20kPa 02
in a balance of N2 is supplied to a cuvette at 500 mL/min containing an
organism
which depletes the supplied p02 by 10 Pa. At an RQ of 0.2, the cell would add
0.2
lcPa of C02 to the supplied gas for every 1 Pa of 02 consumed. Therefore, the
gas
exiting the cuvette 6 (Fig. 1) would have a total pressure of 99992. Pa,
containing 19
990 Pa 02, 2 Pa C02 and 80 000 Pa N2. The majority of gas exiting the cuvette
is
vented to atmosphere via vent 7, and since the total pressure of gas exiting
the
cuvette is lower than that entering the cuvette, the flow rate of vented gas
is less than
if the input and output gases had equal pressures.
The sample gas stream entering the analyzer is at atmospheric pressure
(100kPa), the proportion of 02 in this gas being the same as the proportion
exiting
the cuvette i.e 19 990 parts in 99 992. The p0, of the gas entering the
analyzer
would therefore be 19 991 and the sample 02 sensor would measure an apparent
02
depletion of 9 Pa 02 though the cell depleted the supplied gas mixture by 10
Pa.
The rate of 02 consumption estimated from this measurement would therefore be
90% of the actual rate, and the estimated RQ value would be 0.22 compared to
the
actual value of 0.20.
The type of errors caused by the occurrence of RQ values different from 1.0,
as illustrated by the above examples, would also occur in estimating rates of
02
consumption by photosynthetic cells with photosynthetic quotients different
from 1Ø
To correct for the errors illustrated in examples 1 and 2 above, the amount
of C02 produced or consumed by the organism, tissue or cells within the
cuvette
must be measured simultaneously with measurements of differential 02. This may
be achieved by incorporating a differential C02 analyzer into the gas exchange
system
in parallel with the differential 02 analyzer as shown in Fig. 1. Output from
the
differential C02 analyzer may be read by the differential oxygen analyzer
which then
SUBSTITUTE SHEET RULE 26)~
WO 96/12182 ~ ; ~ 5 PCT/CA95/00582
calculates a measured RQ or PQ value. The software of the differential 02
analyzer
incorporates the mathematical relationships between measured and actual
respiratory
and photosynthetic quotients, and these are used to correct for errors in
measured
02 differentials.
Figure 6 shows the relationship between measured and actual respiratory
quotient at various p02 in the gas supplied to the organism in the cuvette
when the
organism is depleting 10 Pa 02 from this gas stream. An identical relationship
occurs
between values of measured and actual PQ when a photosynthetic organism is
adding
lOPa to the sample gas stream passing through the cuvette. The amount of 02
added
or removed from the sample gas stream affects these relationships, but the
maximum
difference that occurs between values measured at a p02 differential of 1 Pa
and
those measured at a p0~ differential of S00 Pa is only 1.2%. Since the
differential
oxygen analyzer is designed predominantly for measuring very low (below 500
Pa)
02 differentials, the error involved in assuming no effect of 02 differential
magnitude
on the relationship between actual and measured respiratory and photosynthetic
quotients is negligible.
The relationship illustrated in Fig. 6 allows actual RQ or PQ values to be
determined from measured RQ and PQ values. The analyzer software uses these
actual RQ and PQ values to calculate the extent to which the measured 02
differential overestimates or underestimates the actual 02 differential
between the
p02 in the reference gas stream and in the sample gas stream exiting from the
cuvette. The relationship between the measured and actual 02 differentials
with
respect to RQ and PQ at different p02 in the supplied gas stream is shown in
Fig.
7. This figure shows the relationship between these parameters for an actual
02
differential of 10 Pa between the reference and sample gas streams. However,
the
changes that occur in this relationship as O, differentials are increased
between 1
and S00 Pa are negligible.
26
SUBSTITUTE SE+EET (RULE 26)
WO 96/12182 ~ ~ ~, ~ ~~ j ~ PCT/CA95/00582
Use of a Double Differential Oxygen Analyzer for Simultaneous Measurement of
Differential p02 and Differential pCO~.
An alternative embodiment of the differential 02 analyzer described above
may be used to measure differential pOi and differential pC02 simultaneously
in the
same instrument. A schematic of such an instrument is shown in Fig. 8. The
instrument has the same general design as that shown in Fig. 2, except that it
incorporates two sensor blocks 95 and 96 each containing a reference and
sample 02
sensor configured in differential mode. The reference (21) and sample (22)
gases
pass through the reference (97) and sample (98) 02 sensors, respectively, in
the first
block, and on exiting the sensors are scrubbed of any COZ they may contain by
passage through columns (99 and 100) containing soda lime or similar C02
absorbing
material. The scrubbed reference and sample gases then enter the second sensor
block 96 and pass through the second reference (101) and sample (102) OZ
sensors,
respectively.
The removal of C02 between the first and second sensor housings causes the
p02 of the reference and sample gases to increase so that the differential p02
measured between the 02 sensors in the first housing differs from the
differential p02
measured between the OZ sensors in the second housing. For example, consider a
situation in which an organism with a RQ of 2.0 is supplied with a gas stream
containing 20 000 Pa 02, SO Pa C02 and 79 950 N2 at an atmospheric pressure of
100kPa. If the organism's respiratory activity depletes the supplied p02 by 10
Pa, the
gas exiting the cuvette will contain 19 990 Pa OZ, 70 Pa C02 and 79 950 N2.
However, since the gas is vented to atmosphere (at a pressure of 100 kPa), the
composition of gas entering the first sample 02 sensor will be 19 988 Pa 02,
70 Pa
C02 and 79 942 Pa N2, and the differential p02 between the sample and
reference
02 sensor will be 12 Pa O~. After COZ is scrubbed from the sample gas stream,
the
proportion of 02 in the sample gas will rise from I9 988/100 000 to 19 988/99
930,
and the p02 of the sample gas will increase to 20002 Pa. After C02 is scrubbed
from
27
SUBSTITUTE SHEET (RULE 26)
WO 96/12182 ~ ~ ~ ~ y i):5 PCT/CA95/00582
the reference gas the proportion of O, in the gas will rise from 20 000/100
000 to 20
000/99 950, and the p02 in the reference gas will increase to 20 0010 Pa.
Therefore,
the 02 differential between the 02 sensors in the second housing will be 8 Pa,
and
the ratio of the first differential sensor output to the second differential
sensor output
will be 12/8 = 1.5
The ratio of the outputs of the two differential sensors depends on the actual
RQ or PQ of the organism, tissue or cells in the cuvette, and the p02 and pC02
of
the gas supplied to the cuvette. The extent to which the biological sample
changes
the p02 of the supplied gas also has an effect on the ratio, but this is
negligible over
the range of 02 differentials that the instrument is designed to measure.
Figure 9
shows an example of the relationship among the ratio of the sensor outputs,
the
actual RQ of the biological sample and the p02 in the supplied gas stream when
50
Pa C02 is present in the supplied gas and the sample depletes the p0, in the
sample
gas stream by 10 Pa 02. A similar relationship exists bet<veen the ratio of
the sensor
outputs and the actual PQ of a photosynthetic organism when this organism is
supplied with gases of similar composition and adds 10 Pa 02 to the sample gas
stream. The software of the double differential analyzer incorporates the
relationships between these parameters at various supplied p02 and pC02, and
is
therefore able to calculate the actual RQ or PQ of the biological material in
the
cuvette from the ratio of the differential sensor outputs. Having calculated
the actual
RQ or PQ, the extent to which the 02 differential measured by the first
differential
sensor over or underestimates the 02 uptake or production by the material in
the
cuvette can be calculated in software using the relationship shown in Figs. 7.
From
this, the true 02 uptake or production by the material in the cuvette i~
calculated,
and the RQ or PQ determined from the ratio of the outputs of the two
differential
sensors in the instrument is used to calculate uptake or production of C02.
The
double differential 02 analyzer therefore provides simultaneous measurements
of 02
28
SUBSTITUTE SHEET (RULE 26)
WO 96/121H2 L ~ j ~ PCT/CA95/00582
depletion or production, C02 depletion or production and measurements of RQ or
PQ.
It should be noted that alternative embodiments of the double differential 02
analyzer shown in Fig. 8 may be used to measure the uptake or production rate
of
any gas that is produced or consumed by an organism, tissue or cell that is
also
producing or consuming 02. The instrument need only be modified by changing
the
C02 scrubbers between the two sensor housings for scrubbers which remove the
gas
in question. Removal of this gas by the scrubbers will have the same effect as
removing C02 i.e. there will be a change in the differential p02 between the
reference and sample gas streams after the gas is scrubbed. The ratio of the
differential 02 sensor outputs can then be used to calculate the ratio of 02
exchange
to the ratio of exchange of the other gas (equivalent to RQ and PQ), and this
ratio
can then be used to calculate both actual OZ exchange and exchange of the
other gas
in the same manner as that described for measurement of C02 exchange described
above.
29
SUBSTITUTE SHEET (RULE 26)