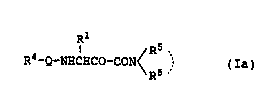Note: Descriptions are shown in the official language in which they were submitted.
O O ~ 6 4
WO96/16079 - 1 - PCT/~5tO238g
DESCRIPTION
~IPH~KETD~MnDEDE~VA~n~SASC~I~r~ L ~Hl~lIU~
Technical Field
This invention relates to a cathepsin L inhibitor
comprising an a-ketoamide derivative or a salt thereof as
an active ingredient, and use thereof.
Backqround Art
Osteoporosis is a pathologic state or disease
involving some symptom or risk due to quantitative bone
reduction exceeding a certain degree. Major symptoms are
spinal kyphosis, fractures of dorsolumbar bones, vertebral
centra, femoral necks, lower end of radius, ribs, upper end
of humerus, and others. In normal bone tissue, bone
destruction occurs continuously, but there is good balance
between bone formation and resorption; osteoblasts and
osteoclasts play key roles in bone formation and bone
resorption, respectively. Upon deterioration of this
balance, bone resorption surpasses bone formation,
resulting in quantitative bone reduction. Drugs suppres-
sing bone resorption are therefore expected to serve well
in preventing and treating osteoporosis. Traditionally,
bone resorption-suppressing agents, such as estrogens and
calcitonin have been used to treat osteoporosis. However,
these therapeutic agents fail to achieve satisfactory
effect in some cases, due to subject limitations or
uncertain efficacy. There is therefore need of a new
prophylactic/therapeutic method for accentuated bone
resorption.
It has recently been shown that cathepsin L, a
protease secreted by osteoclasts in the process of bone
resorption, is integrally involved in the decomposition of
collagen, a bone-supporting protein (FEBS Lett., Vol. 321,
p. 247, 1993). Presumably therefore, bone collagen
decomposition due to bone resorption can be prevented by
~ 2 2 ~ 0 ~ 6 4
WO96/16079 - 2 - PCT1~5/02389
.
inhibiting cathepsin L activity, a means useful in the
prevention and treatment of osteoporosis. Leupeptin,
antipain and epoxysuccinic acid derivatives (Japanese
Patent Unexamined Publication Nos. 304074/1990, 304075/1990
and 304085/1990) are known to exhibit cathepsin L inhibito-
ry action.
Traditionally, naturally occurring postostain has been
known as an ~-ketoamide derivative possessing protease
inhibitory activity, and various compounds have been
synthesized as ~-ketoamide derivatives from amino acids.
For example, PCT Int. Appl. WO 94/00095 discloses a-
ketoamide derivatives as calpain inhibitors, and PCT Int.
Appl. WO 92/12140 (Japanese Publication of translations of
International patent application No. 504547/1994) discloses
~-ketoamide derivatives as calpain, papain and cathepsin B
inhibitors.
However, no ~-ketoamide derivatives have been reported
as inhibiting cathepsin L. In addition, most synthetic ~-
ketoamide derivatives have been racemates at their ketone
~-position, because they are synthesized by converting the
corresponding amino acids to ~-keto esters by the Dakin-
West reaction of the carboxyl group of each amino acid.
Disclosure of Invention
The present inventors sought to develop a more
commonly applicable drug showing selective inhibition of
cathepsin L and direct action on the bone to suppress bone
resorption, and found that an ~-ketoamide derivative shows
potent cathepsin L inhibition and direct action on the bone
to excellently suppress bone resorption, and that it serves
well as a prophylactic/therapeutic agent for bone disease.
The inventors made further investigations based on this
finding, and developed the present invention.
Accordingly, the present invention relates to:
(1) A cathepsin L inhibitor comprising a compound of
the formula (Ia):
~ ~ O ~ 6 4
~ WO96/16079 ~ 3 ~ PCT/~5/02389
Rl
/R5 `
R4-Q-NHCHCo-CoN~ (Ia)
R
wherein Q represents a direct bond or l or 2 amino acid
residues that may be substituted; Rl represents a hydrogen
atom or a hydrocarbon or heterocyclic group that may be
substituted; R4 represents an acyl group or a carboxyl
group that may be esterified and R5 and R6 independently
represent a hydrogen atom or a hydrocarbon or heterocyclic
group that may be substituted or R5 and R6 may bind
together to form a ring; or a salt thereof,
(2) A cathepsin L inhibitor of item (l), wherein the
compound is one o the formula (I):
R3 R2 Rl
/ R5 ~
R4-(NHCHCo) n~ ( NHCHCO)m-NHCHCO-CON ~ (I)
R
wherein Rl, R2 and R3 independently represent a hydrogen
atom or a hydrocarbon or heterocyclic group that may be
substituted; R4 represents an acyl group or a carboxyl
group that may be esterified and R5 and R6 independently
represent a hydrogen atom or a hydrocarbon or heterocyclic
group that may be substituted or R5 and R6 may bind
together to form a ring; m and n independently represent 0
or l; or a salt thereof,
(3) A method for inhibiting a cathepsin L activity of
a m~mm~l which comprises administering to said mammal a
pharmaceutically effective amount of a compound of the
formula (Ia) in item (l),
(4) Use of a compound of the formula (Ia) in item (l)
for the manufacture of a medicament to be used as a
cathepsin L inhibitor,
(5) A compound of the formula (I')
WO 96tl6079 2 ~ ~ n ~ ~ 4 PCTIJP95/02389
IR3 IR2 IRl R5a ~
R4a- ( NHC~ECO ) n~ ( NHCHCO ) m-NHCHCO-CON ~ ' ( I ' )
\ R6a .
wherein Rl, R2 and R3 independently represent a hydrogen
atom or a hydrocarbon or heterocyclic group that may be
substituted; R4a is a group represented by the formula -
CORa or -SO2Rb wherein Ra and Rb are independently an
optionally substituted aryl or aromatic heterocyclic group;
R5a and R6a independently represent a straight-chain or
branched Cl_6 alkyl group which may be substituted with an
optionally substituted aryl or aromatic heterocyclic group
or an optionally esterified carboxyl group and m and n
independently represent 0 or 1; provided that where Ra is
an optionally substituted aromatic heterocyclic group, R5a
and R6a independently represent a straight-chain or
branched Cl_6 alkyl group which is substituted with an
optionally substituted aryl or aromatic heterocyclic group
or an optionally esterified carboxyl group; or a salt
thereof,
(6) A compound of item (5), wherein the aryl group for
Ra and Rb is naphthyl,
(7) A compound of item (5), wherein the aromatic
heterocyclic group for Ra and Rb is quinolyl,
(8) A compound of item (5), wherein one of R5a and R6a
is a hydrogen atom and the other is benzyl,
(9) A compound of item (5), wherein R' is a straight-
chain or branched Cl_6 alkyl group which is substituted
with a phenyl gorup,
(10) A compound of item (5), wherein R2 and R3 are
independently a straight-chain or branched Cl_6 alkyl
gorup,
(11) A compound of N-(quinoline-2-carbonyl)-L-
isoleucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide, or a salt thereof.
- =
2 2 0 0 ~ 6 4
WO96/1607s ~ 5 ~ PCT/~5/02389
(12) A compound of N-[N-(6-oxo-1,4,5,6-
tetrahydropyridazine-3-carbonyl)-L-leucyl]-(3s)-3-amino-2
oxo-4-phenylbutyric acid benzylamide, or a salt thereof,
(13) A compound of N-benzyloxycarbonyl-L-leucyl-L-
leucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid benzylamide,
or a salt thereof,
(14) A compound of N-(quinoline-2-carbonyl)-L-leucyl-
L-leucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide, or a salt thereof,
(15) A compound of the formula (I")
IR3 lR2 IRl R5b
R4b-(NHCHCo) n~ ( NHCHCO)m-NHCHCO-CON ~ . (I")
\ R6b .'
wherein Rl, R2 and R3, whether identical or not,
independently represent a hydrogen atom or a hydrocarbon
group or heterocyclic group that may be substituted; R4b is
represented by the formula -CORC wherein Rc is a straight-
chain or branched Cl_6 alkyl group which is substituted
with an optionally substituted aryl or aromatic
heterocyclic group; R5b and R6b independently represent a
straight-chain or branched Cl_6 alkyl group which is
substituted with an optionally substituted aryl group or an
esterified carboxyl group; and m and n independently
represent 0 or 1, or a salt thereof,
(16) A method of producing a compound of item (5)
which comprises subjecting a compound of the formula (II')
R3 Rl2 RIl~OH Rsa ~
R4a-(NHCHCo)n-(NHCHCo)m-NHCHCH-CoN < (II')
R6a
wherein Rl, R2 and R3 independently represent a hydrogen
atom or a hydrocarbon group or heterocyclic group that may
be substituted; R4a is a group represented by the formula -
CORa or -SO2Rb wherein Ra and Rb are independently an
wo 96/16079 a ~ ~ 6 4 6 - PCT/~5/02389 ~
optionally substituted aryl or aromatic heterocyclic group
and R5a and R6a independently represent a straight-chain or
branched Cl_6 alkyl group which may be substituted with an
optionally substituted aryl or aromatic heterocyclic group
or an optionally esterified carboxyl group; provided that
where Ra is an optionally substituted aromatic heterocyclic
group and R5a and R6a independently represent a straight-
chain or branched Cl_6 alkyl group which is substituted
with an optionally substituted aryl or aromatic
heterocyclic group or an optionally esterified carboxyl
group; or a salt thereof, to an oxidation reaction,
(17) A method of producing a compound of item (15)
which comprises subjecting a compound of the formula (II")
R3 R2 RlOH R5b ~
1 1 1 1 / ~.
R4b- ( NHCHCO ) n~ ( NHCHCO ) m-NHCHCH-CON C . ( I I ' ' )
\ R6b,
wherein Rl, R2 and R3 independently represent a hydrogen
atom or a hydrocarbon group or heterocyclic group that may
be substituted; R4b is a group represented by the ~ormula -
CORC wherein Rc is a straight-chain or branched Cl_6 alkyl
group which is substituted with an optionally substituted
aryl or aromatic heterocyclic group and R5b and R6b
independently represent a straight-chain or branched Cl_6
alkyl group which is substituted with an optionally
substituted aryl group or an esterified carboxyl group; or
a salt thereof, to an oxidation reaction,
(18) A composition which comprises a compound of item
(5), and
(19) A composition which comprises a compound of item
(15)-
The a-ketoamide derivative for the present invention,
synthesized by oxidizing a corresponding optically active
a-hydroxyamide derivative under easy DMSO oxidization
a o ~ 6 4
WO96/16079 ~ 7 ~ PcT/J19J,~2389
conditions, is an optically active isomer with the S-
configuration at ~ position.
With respect to the above formula (Ia), the amino acid
residue for the "l or 2 amino acid residues that may be
substituted," shown by Q, is exemplified by a-amino acids,
~-amino acids and r-amino acids, which are represented by
the respective formulas RCH(NH2)COOH, H2NCH2CHRCO2H and
H2NCH2CH2CHRCO2H (R represents a hydrogen atom or a
hydrocarbon group or heterocyclic group that may be
substituted), with preference given to ~-amino acids. When
Q is a dipeptide residue resulting from the binding of 2
amino acids and the 2 amino acids may be of the same type
or not, but the residue preferably consists of 2 ~-amino
acids. When the dipeptide residue consists of 2 amino
acids of the same type (e.g., amino acid residue consisting
of 2 ~-amino acids), the 2 amino acids may be identical or
not.
The "hydrocarbon group that may be substituted," shown
by R, is exemplified by the same hydrocarbon groups as
those exemplifying the "hydrocarbon group that may be
substituted," shown by Rl, R2 or R3 below.
The "heterocyclic group that may be substituted,"
shown by R, is exemplified by the same heterocyclic groups
as those exemplifying the "heterocyclic group that may be
substituted," shown by Rl, R2 or R3 below.
The substituent for the "hydrocarbon group or
heterocyclic group that may be substituted," shown by R, is
exemplified by the same substituents as those for the
"hydrocarbon group or heterocyclic group that may be
substituted," shown by Rl, R2 or R3 below.
The above-described a-amino acid residue is
exemplified by glycine and natural or non-natural L- or D-
~-amino acids. Such amino acids include glycine, and ~-L-
amino acids or ~-D-amino acids (e.g., ~-L- or ~-D-alanine,
valine, leucine, isoleucine, serine, threonine, asparagine,
glutamine, aspartic acid, glutamic acid, lysine, arginine,
wo 96/16079 2 ~ ~ n ~ 6 4 8 - PCT/~5/02389
cysteine, methionine, phenylalanine, tyrosine, tryptophan,
histidine, proline), with preference given to glycine and
a-L-alanine, valine, leucine, isoleucine, methionine,
phenylalanine, tyrosine, tryptophan etc.
The "1 or 2 amino acid residues that may be
substituted," shown by Q, may have 1 to 3 substituents at
any possible positions. Such substituents are exemplified
by the same substituents for the "hydrocarbon group or
heterocyclic group that may be substituted," shown by Rl,
R2 or R3 below.
Q is preferably a group represented by formula (Ia-Q):
R3 R2
- ( NHCHCO ) n~ ( NHCHCO ) m~ ( Ia--Q)
wherein R2 and R3, whether identical or not, independently
represent a hydrogen atom or a hydrocarbon group or
heterocyclic group that may be substituted; m and n,
whether identical or not, independently represent 0 or 1.
With respect to the above formulas (I) and (Ia-Q), the
"hydrocarbon group that may be substituted," shown by Rl,
R2 or R3, is exempli~ied by saturated or unsaturated
aliphatic hydrocarbon chain groups, saturated or
unsaturated alicyclic hydrocarbon groups and aryl groups.
Such saturated aliphatic hydrocarbon groups include
straight or branched saturated aliphatic hydrocarbon groups
having 1 to 10 carbon atoms (e.g., Cl_lo alkyl groups such
as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, tert.-
pentyl, hexyl, isohexyl, heptyl and octyl), with preference
given to straight or branched saturated aliphatic
hydrocarbon groups having 1 to 6 carbon atoms.
Such unsaturated aliphatic hydrocarbon groups include
straight or branched unsaturated aliphatic hydrocarbon
groups having 2 to 10 carbon atoms (e.g., C2_10 alkenyl
2 ~ O O ~ 6 4
wo96ll6o7s ~ 9 ~ PCT/J~3~'~2~9
groups such as ethenyl/ l-propenyl, 2-propenyl, l-butenyl,
2-butenyl, 3-butenyl, 2-methyl-1-propenyl, l-pentenyl, 2-
pentenyl, 3-pentenyl, 4-pentenyl, 3-methyl-2-butenyl, 1-
hexenyl, 3-hexenyl, 2,4-hexadienyl, 5-hexenyl, l-heptenyl
and l-octenyl; C2_l0 alkinyl groups such as ethynyl, 1-
propinyl, 2-propinyl, l-butinyl, 2-butinyl, 3-butinyl, 1-
pentinyl, 2-pentinyl, 3-pentinyl, 4-pentinyl, l-hexinyl, 3-
hexinyl, 2,4-hexadinyl, 5-hexinyl, l-heptinyl and 1-
octinyl), with preference given to straight or branched
unsaturated aliphatic hydrocarbon groups having 2 to 6
carbon atoms.
Such saturated alicyclic hydrocarbon groups include
saturated alicyclic hydrocarbon groups having 3 to 12
carbon atoms (e.g., C3_l2 cycloalkyl groups such as
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl, cyclooctyl, bicyclo[2.2.1]heptyl,
bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl, bicyclo[3.3.1]nonyl,
bicyclo~4.2.1]nonyl and bicyclo[4.3.1]decyl), with
preference given to saturated alicyclic hydrocarbon groups
having 3 to 6 carbon atoms.
Such unsaturated alicyclic hydrocarbon groups include
unsaturated alicyclic hydrocarbon groups having 5 to 12
carbon atoms (e.g., C5_12 cycloalkenyl groups such as 1-
cyclopentenyl, 2-cyclopentenyl, 3-cyclopentenyl, 1-
cyclohexenyl, 2-cyclohexenyl, 3-cyclohexenyl, 1-
cycloheptenyl, 2-cycloheptenyl, 3-cycloheptenyl, 2,4-
cycloheptadienyl, 2-cyclopenten-1-yl, 3-cyclopenten-1-yl,
2-cyclohexen-1-yl and 3-cyclohexen-1-yl; C5-12
cycloalkadienyl groups such as 2,4-cyclopentadien-1-yl,
2,4-cyclohexadien-1-yl and 2,5-cyclohexadien-1-yl).
The "hydrocarbon group that may be substituted," shown
by Rl, R2 or R3, may be a saturated aliphatic hydrocarbon
group having 1 to 8 carbon atoms and substituted with one
of the above saturated or unsaturated alicyclic hydrocarbon
groups (e.g., C3-7 cycloalkyl-cl-8 alkyls or Cs_7
W~96116Q79 ~ ~ 0 ~ ~ 6 4 - 1 o - PCT/Jr ~3J`~ 9
cycloalkenyl-Cl_g alkyls, such as cyclopropylmethyl,
cyclopropylethyl, cyclobutylmethyl, cyclopentylmethyl, 2-
cyclopentenylmethyl, 3-cyclopentenylmethyl,
cyclohexylmethyl, 2-cyclohexenylmethyl, 3-
cyclohexenylmethyl, cyclohexylethyl, cyclohexylpropyl,cycloheptylmethyl and cycloheptylethyl), or the like.
Aryl groups include monocyclic or condensed polycyclic
aromatic hydrocarbon ring groups having 6 to 14 carbon
atoms. Such aromatic hydrocarbon ring groups include
phenyl, tolyl, xylyl, biphenyl, 1- or 2-naphthyl, 1-, 2- or
9-anthryl, 1-, 2-, 3-, 4- or 9-phenanthryl, 1-, 2-, 4-, 5-
or 6-azulenyl and acenaphthylenyl, with preference given to
C6_l0 aryl such as phenyl, l-naphthyl, 2-naphthyl etc.
The "hydrocarbon group that may be substituted," shown
by Rl, R2 or R3, may have 1 to 3 optionally chosen
substituents at any possible positions. Such substituents
include aryl groups that may be substituted, cycloalkyl or
cycloalkenyl groups that may be substituted, heterocyclic
groups that may be substituted, carboxyl groups that may be
esterified, carbamoyl groups that may be substituted, amino
groups that may be substituted, hydroxyl groups that may be
substituted, thiol groups that may be substituted, halogens
(e.g., fluorine, chlorine, bromine, iodine) and phosphono
groups that may be substituted.
The "aryl group that may be substituted" is
exemplified by C6_l4 aryl such as phenyl, naphthyl,
anthryl, phenanthryl and acenaphthylenyl, with preference
given to phenyl, l-naphthyl and 2-naphthyl. Said aryl may
have 1 to 2 optionally chosen substituents at any possible
positions, these substituents including hydroxy, alkoxy
groups that may be substituted (e.g., Cl_3 alkoxys such as
methoxy, ethoxy and propoxy), halogen atoms (e.g.,
fluorine, chlorine, bromine, iodine) and alkyl groups that
may be substituted (e.g., Cl_3 alkyls such as methyl, ethyl
and propyl). These alkoxy groups and alkyl groups may have
1 or 2 optionally chosen substituents at any possible
~ ~ û O ~ 6 4
WO 96/16079 ~ 11 ~ PCT/Jr95~ 2389
positions, these substituents including phosphono groups
that may be substituted (e.g., dimethoxyphosphoryl, di-
ethoxyphosphoryl).
The "cycloalkyl group that may be substituted" is
exemplified by C3-7 cycloalkyl groups such as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl and cycloheptyl. The
kinds and number of substituents for the cycloalkyl group
that may be substituted are the same as those of the
substituents for the above-described aryl group that may be
substituted.
The "cycloalkenyl group that may be substituted" is
exemplified by C3-6 cycloalkenyl groups such as
cyclopropenyl, cyclobutenyl, cyclopentenyl and
cyclohexenyl. The kinds and number of substituents for the
cycloalkenyl group that may be substituted are the same as
those of the substituents for the above-described aryl
group that may be substituted.
The "heterocyclic group that may be substituted" is
exemplified by aromatic heterocyclic groups having at least
l hetero atom selected from atoms of oxygen, sulfur and
nitrogen as a ring-constituting atom (ring atom), and
saturated or unsaturated non-aromatic heterocyclic groups
(aliphatic heterocyclic groups), with preference given to
aromatic heterocyclic groups. The aromatic heterocyclic
group is exemplified by 5- to 7-membered aromatic
heterocyclic groups containing 1 atom of sulfur, nitrogen
or oxygen, 5- to 6-membered aromatic heterocyclic groups
containing 2 to 4 atoms of nitrogen and 5- or 6-membered
aromatic heterocyclic groups containing l or 2 atoms of
nitrogen and l atom of sulfur or oxygen. These aromatic
heterocyclic groups may have condensed with a 6-membered
ring containing 2 or fewer atoms of nitrogen, a benzene
ring or a 5-membered ring containing 1 atom of sulfur.
Such aromatic heterocyclic groups include aromatic
monocyclic heterocyclic groups (e.g., furyl, thienyl,
pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl,
-
W096/16079 ~ 9 ~ 4 - 12 - PCT/~5102389
imidazolyl, pyrazolyl, 1,2,3-oxadiazolyl, 1,2,4-
oxadiazolyl, 1,3,4-oxadiazolyl, furazanyl, 1,2,3-
thiadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl,
1,2,3-triazolyl, 1,2,4-triazolyl, tetrazolyl, pyridyl,
pyridazinyl, pyrimidinyl, pyrazinyl, triazinyl) and
aromatic condensed heterocyclic groups (e.g., benzofuranyl,
isobenzofuranyl, benzo[b]thienyl, indolyl, isoindolyl, lH-
indazolyl, benzimidazolyl, benzoxazolyl, 1,2-
benzisoxazolyl, benzothiazolyl, 1,2-benzisothiazolyl, lH-
benzotriazolyl, quinolyl, isoquinolyl, cinnolinyl,quinazolinyl, quinoxalinyl, phthalazinyl, naphthyridinyl,
purinyl, pteridinyl, carbazolyl, ~-carbolinyl, ~-
carbolinyl, y-carbolinyl, acridinyl, phenoxazinyl,
phenothiazinyl, phenazinyl, phenoxathiinyl, thianthrenyl,
phenathridinyl, phenathrolinyl, indolizinyl, pyrrolo[l,2-
b]pyridazinyl, pyrazolo[l,5-a]pyridyl, imidazo[l,2-
a]pyridyl, imidazo[l,5-a]pyridyl, imidazo[l,2-
b]pyridazinyl, imidazo[l,2-a]pyrimidinyl, 1,2,4-
triazolo[4,3-a]pyridyl and 1,2,4-triazolo[4,3-
b]pyridazinyl), with preference given to quinolyl,isoquinolyl, furyl, thienyl, indolyl, isoindolyl,
imidazolyl, pyrazinyl, pyridyl, pyrimidinyl etc. The non-
aromatic heterocyclic group is exemplified by 5- to 7-
membered non-aromatic heterocyclic groups containing 1 atom
of sulfur, nitrogen or oxygen, and 4- to 7-membered non-
aromatic heterocyclic groups containing 1 atom of nitrogen
and 3 or fewer atoms selected from nitrogen, oxygen and
sulfur. Such non-aromatic heterocyclic groups include
oxylanyl, azetidinyl, oxetanyl, thietanyl, pyrrolidinyl,
tetrahydrofuryl, thiolanyl, piperizyl, tetrahydropyranyl,
morpholinyl, thiomorpholinyl and piperazinyl. The
substituent for said heterocyclic group that may be
substituted is exemplified by alkyl groups having 1 to 3
carbon atoms (e.g., methyl, ethyl, propyl).
The carboxyl groups that may be esterified include -
COOH, (lower(Cl_6)alkoxy)carbonyls (e.g., methoxycarbonyl,
~ ~009B4
WO96/16079 - 13 - PCT/~5/02389
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl, tert.-butoxycarbonyl, sec.-butoxycarbonyl,
pentyloxycarbonyl, isopentyloxycarbonyl,
neopentyloxycarbonyl, tert.-pentyloxycarbonyl) and (C6_l0
aryloxy)carbonyls (e.g., phenoxycarbonyl, 1-
naphthoxycarbonyl), (C7-13 aralkyloxy)carbonyls (e.g.,
benzyloxycarbonyl), with preference given to the carboxyl
group, methoxycarbonyl and ethoxycarbonyl.
The substituent for said carbamoyl group that may be
substituted is exemplified by lower (Cl_6) alkyls (e.g.,
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, hexyl,
isohexyl), C3-6 cycloalkyl groups (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
(e.g., phenyl, l-naphthyl, 2-naphthyl) and C7-13 aralkyl
groups (e.g., benzyl, phenethyl); 1 or 2 of these
substituents, whether identical or not, may be present.
The substituent for said amino group that may be
substituted is exemplified by lower (Cl_6) alkyls (e.g.,
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, hexyl,
isohexyl), C3-6 cycloalkyl groups (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
(e.g., phenyl, l-naphthyl, 2-naphthyl) and C7-l3 aralkyl
groups (e.g., benzyl, phenethyl); 1 or 2 of these
substituents, whether identical or not, may be present.
The substituent for said hydroxyl group that may be
substituted is exemplified by lower (Cl_6) alkyls (e.g.,
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, hexyl,
isohexyl), C3-6 cycloalkyl groups (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
(e.g., phenyl, l-naphthyl, 2-naphthyl) and C7-13 aralkyl
groups (e.g., benzyl, phenethyl).
The substituent for said thiol group that may be
substituted is exemplified by lower (Cl_6) alkyls (e.g.,
-
WO96/16079 ~ 14 - PCT/~5/02389
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, hexyl,
isohexyl), C3-6 cycloalkyl groups (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
(e.g., phenyl, l-naphthyl, 2-naphthyl) and C7_l3 aralkyl
groups (e.g., benzyl, phenethyl).
The substituent for said phosphono group that may be
substituted is exemplified by lower (Cl_6) alkyls (e.g.,
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, hexyl,
isohexyl), lower (Cl_6) alkoxys (e.g., methoxy, ethoxy,
propoxy, isopropoxy, butoxy, isobutoxy, sec.-butoxy, tert.-
butoxy, pentyloxy, isopentyloxy, neopentyloxy, hexyloxy,
isohexyloxy). Said phosphons groups include phosphoryl,
dimethoxyphosphoryl, diethoxyphosphoryl,
dipropoxyphosphoryl, diisopropoxyphosphcryl,
ethylenedioxyphosphoryl, trimethylenedioxyphosphoryl and
tetramethylenedioxyphosphoryl.
When the "hydrocarbon group that may be substituted,"
shown by Rl, R2 or R3, is an alicyclic hydrocarbon group or
an aryl group, the substituent may be an aliphatic
hydrocarbon group that may be substituted. Such aliphatic
hydrocarbon groups include the same saturated or
unsaturated (preferably saturated) aliphatic hydrocarbon
groups as those exemplifying the "hydrocarbon group that
may be substituted," shown by Rl, R2 or R3 above, with
preference given to alkyl groups (e.g., Cl_3 alkyls such as
methyl, ethyl and propyl). The aliphatic hydrocarbon group
may have l or 2 optionally chosen substituents at any
possible positions, these substituents including phosphono
groups that may be substituted (e.g., phosphoryl,
dimethoxyphosphoryl, diethoxyphosphoryl).
The "heterocyclic group that may be substituted,"
shown by Rl, R2 or R3, is exemplified by aromatic
heterocyclic groups having at least 1 hetero atom selected
from atoms of oxygen, sulfur and nitrogen as a ring-
~ 2 ~û O 9 6 4
WO96/16079 - 15 - PCT/~5lo238s
constituting atom (ring atom), and saturated or unsaturated
non-aromatic heterocyclic groups (aliphatic heterocyclic
groups), with preference given to aromatic heterocyclic
groups.
Such aromatic heterocyclic groups are exemplified by
5- to 7-membered heterocyclic groups containing 1 atom of
sulfur, nitrogen or oxygen, 5- to 6-membered heterocyclic
groups containing 2 to 4 atoms of nitrogen and 5- or 6-
membered aromatic heterocyclic groups containing 1 or 2
atoms of nitrogen and 1 atom of sulfur or oxygen. These
aromatic heterocyclic groups may have condensed with a 6-
membered ring containing 2 or fewer atoms of nitrogen, a
benzene ring or a 5-membered ring containing 1 atom of
sulfur. Such aromatic heterocyclic groups include aromatic
monocyclic heterocyclic groups (e.g., furyl, thienyl,
pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl,
imidazolyl, pyrazolyl, 1,2,3-oxadiazolyl, 1,2,4-
oxadiazolyl, 1,3,4-oxadiazolyl, furazanyl, 1,2,3-
thiadiazolyl, 1,2,4-thiadiazolyl, 1,3,4-thiadiazolyl,
1,2,3-triazolyl, 1,2,4-triazolyl, tetrazolyl, 2-, 3- or 4-
pyridyl, 3- or 4-pyridazinyl, 2-, 4-, 5- or 6-pyrimidinyl,
2-pyrazinyl, triazinyl) and aromatic condensed heterocyclic
groups (e.g., benzofuranyl, isobenzofuranyl,
benzo[b]thienyl, indolyl, isoindolyl, lH-indazolyl,
benzimidazolyl, benzoxazolyl, 1,2-benzisoxazolyl,
benzothiazolyl, 1,2-benzisothiazolyl, lH-benzotriazolyl,
quinolyl, isoquinolyl, cinnolinyl, quinazolinyl,
quinoxalinyl, phthalazinyl, naphthyridinyl, purinyl,
pteridinyl, carbazolyl, ~-carbolinyl, ~-carbolinyl, y-
carbolinyl, acridinyl, phenoxazinyl, phenothiazinyl,phenazinyl, phenoxathiinyl, thianthrenyl, phenathridinyl,
phenathrolinyl, indolizinyl, pyrrolo[l,2-b]pyridazinyl,
pyrazolo[l,5-a]pyridyl, imidazo[l,2-a]pyridyl, imidazo[l,5-
a]pyridyl, imidazo[l,2-b]pyridazinyl, imidazo[l,2-
3S a]pyrimidinyl, 1,2,4-triazolo[4,3-a]pyridyl, 1,2,4-
triazolo[4,3-b]pyridazinyl), with preference given to
; 2 2 0 ~ ~ ~ 4 - 16 - PCTI~5/0~89 ~
quinolyl, iso~uinolyl, furyl, thienyl, imidazolyl, indolyl,
isoindolyl, pyrazinyl, pyridyl, pyrimidinyl etc.
Such non-aromatic heterocyclic groups are exemplified
by 5- to 7-membered non-aromatic heterocyclic groups
containing 1 atom of sulfur, nitrogen or oxygen, and 4- to
7-membered non-aromatic heterocyclic groups containing 1
atom of nitrogen and 3 or fewer atoms selected from
nitrogen, oxygen and sulfur (e.g., oxylanyl, azetidinyl,
oxetanyl, thietanyl, pyrrolidinyl, tetrahydrofuryl,
thiolanyl, piperidyl, tetrahydropyranyl, morpholinyl,
thiomorpholinyl, piperazinyl, homopiperidyl, pyrrolinyl,
imidazolidinyl). These non-aromatic heterocyclic groups
may condense with a benzene ring, a 6-membered ring
containing 2 or fewer atoms of nitrogen, or a 5-membered
ring containing 1 atom of sulfur. Specifically, such
condensed non-aromatic heterocyclic groups include
chromanyl, isochromanyl, indolinyl, isoindolinyl,
thiochromanyl and isothiochromanyl.
The "heterocyclic group that may be substituted,"
shown by Rl, R2 or R3, may have 1 to 3 optionally chosen
substituents at any possible positions. Such substituents
include aryl groups that may be substituted, cycloalkyl or
cycloalkenyl groups that may be substituted, heterocyclic
groups that may be substituted, carboxyl groups that may be
esterified, carbamoyl groups that may be substituted, amino
groups that may be substituted, hydroxyl groups that may be
substituted, thiol groups that may be substituted, halogens
(e.g., fluorine, chlorine, bromine, iodine), phosphono
groups that may be substituted, and aliphatic hydrocarbon
groups that may be substituted.
Said aryl groups that may be substituted, cycloalkyl
or cycloalkenyl groups that may be substituted,
heterocyclic groups that may be substituted, carboxyl
groups that may be esterified, carbamoyl groups that may be
substituted, amino groups that may be substituted, hydroxyl
groups that may be substituted, thiol groups that may be
- 17 _ 2 ~ G O 9 6 4
WO96/16079 PCT/~95102389
substituted, halogens (e.g., fluorine, chlorine, bromine,
iodine), phosphono groups that may be substituted, and
aliphatic hydrocarbon groups that may be substituted are
exemplified by the same substituents as those for the
"hydrocarbon group that may be substituted," shown by Rl,
R2 or R3 above
The "hydrocarbon group that may be substituted," shown
by Rl, R2 or R3 above, is exemplified by alkyl groups,
preferably Cl_10 alkyls, with greater preference given to
linear or branched lower alkyls having 1 to 6 carbon atoms
(e.g., methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
sec.-butyl, tert.-butyl, pentyl, isopentyl, neopentyl,
tert.-pentyl, hexyl, isohexyl, 4-methylpentyl, 1,1-
dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, 2-
ethylbutyl). Preferable substituents for the "hydrocarbon
group that may be substituted" are aryls that may be
substituted (preferably phenyl etc.) and heterocyclic
groups that may be substituted.
Rl is preferably an alkyl group substituted with an
optionally substituted aryl or heterocyclic group.
More preferably, Rl is an alkyl group substituted with
an aryl or heterocyclic group. Said alkyl substituted with
an aryl is exemplified by groups resulting from binding of
a monocyclic or condensed polycyclic aromatic hydrocarbon
group having 6 to 14 carbon atoms (e.g., phenyl, naphthyl,
anthryl, phenanthryl, acenaphthylenyl) and a lower alkyl
having 1 to 6 carbon atoms (preferably Cl_4 alkyl) (e.g.,
benzyl, 2-phenylethyl, 3-phenylpropyl, 2-phenylpropyl, 1-
phenylpropyl, a-naphthylmethyl, ~-naphthylethyl, ~-
naphthylmethyl, ~-naphthylethyl). Said alkyl substituted
with a heterocyclic group is exemplified by groups
resulting from binding of an aromatic heterocyclic group
and a lower alkyl having 1 to 6 carbon atoms (preferably
Cl_4 alkyl group). Such aromatic heterocyclic groups
include 2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 2-pyridyl,
3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-
WO96/16079 - 18 - PCT1~5/02389 ~
2 ~ 4
pyrimidinyl, 6-pyrimidinyl, 3-pyridazinyl, 4-pyridazinyl,
2-pyrazinyl, 2-pyrrolyl, 3-pyrrolyl, 2-imidazolyl, 4-
imidazolyl, 5-imidazolyl, 3-pyrazolyl, 4-pyrazolyl,
isothiazolyl, isoxazolyl, 2-thiazolyl, 4-thiazolyl, 5-
thiazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, 1,2,4-
triazol-3-yl, 1,2,3-triazol-4-yl, tetrazol-5-yl,
benzimidazol-2-yl, indol-3-yl, lH-indazolyl,
benz[b]furanyl, isobenzofuranyl, benz[b]thienyl, lH-
pyrrolo~2,3-b]pyrazin-2-yl, lH-pyrrolo[2,3-b~pyridin-6-yl,
1~ lH-imidazo[4,5-b]pyridin-2-yl, lH-imidazo[4,5-c]pyridin-2-
yl and lH-imidazo[4,5-b]pyrazin-2-yl, with preference given
to 2-pyridyl, 3-pyridyl, 4-pyridyl, 4-imidazolyl, 2-
thienyl, 2-furyl, indol-3-yl etc.
Rl is more preferably a straight-chain or branched Cl_
6 alkyl group which is substituted with phenyl.
More preferably, R2 and R3 are independently a
straight-chain or branched Cl_6 alkyl.
Rl is preferably indol-3-ylmethyl, benzyl, methyl,
isopropyl, l-naphthylmethyl, or the like.
R2 and R3, whether identical or not, are preferably
sec.-butyl, benzyl, isobutyl, isopropyl, or the like.
With respect to the combination of Rl and R2, it is
preferable that Rl be indol-3-ylmethyl, benzyl, methyl,
isopropyl or l-naphthylmethyl and R2 be sec.-butyl, benzyl,
isobutyl or isopropyl.
With respect to the combination of Rl, R2 and R3, it
is preferable that Rl be indol-3-ylmethyl, benzyl, methyl,
isopropyl or l-naphthylmethyl, R2 be sec.-butyl, benzyl,
isobutyl or isopropyl, and R3 be sec.-butyl, benzyl,
isobutyl or isopropyl.
With respect to formula (I), the "acyl group" shown by
R4 is exemplified by acyl groups derived from carbamic
acids that may be substituted, thiocarbamic acids that may
be substituted, carboxylic acids that may be substituted,
sulfinic acids that may be substituted, sulfonic acids that
may be substituted, etc., specifically those represented by
Z2~096~
WO96/16079 - 19 - PCT/~95/02389
the respective general formulas -CoNHR7, -CSNHR8, -COR9,
SOR10 SO2Rll (R7 R8 R9, R10 and Rll, whether identical
or not, independently represent a hydrogen atom or a
hydrocarbon group or heterocyclic group that may be
substituted) etc.
The "hydrocarbon group that may be substituted," shown
by R7, R8, R9, R10 or Rll, is exemplified by the same
hydrocarbon groups as those exemplifying the "hydrocarbon
group that may be substituted," shown by Rl, R2 or R3
lo abOVe.
The "hydrocarbon group that may be substituted for,"
shown by R7, R8, R9, R10 or Rll, may have 1 to 3 optionally
chosen substituents at any possible positions, these
substituents being exemplified by the same substituents as
those defined for the "hydrocarbon group that may be
substituted," shown by Rl, R2 or R3 above.
The "heterocyclic group that may be substituted,"
shown by R7, R8, R9, R10 or Rll, is exemplified by the same
heterocyclic groups as those for the "heterocyclic group
that may be substituted," shown by Rl, R2 or R3 above.
The "heterocyclic group that may be substituted,"
shown by R7, R8, R9, R10 or Rll, may have 1 to 3 optionally
chosen substituents at any possible positions, these
substituents being exemplified by the same substituents as
those defined for the "heterocyclic group that may be
substituted," shown by Rl, R2 or R3 above.
The "acyl group" shown by R4 iS exemplified by
aliphatic acyl groups such as alkanoyl groups (e.g., (lower
Cl_6 alkyl)carbonyl groups such as formyl, acetyl,
propionyl, butyryl, isobutyryl, valeryl, isovaleryl,
pivaloyl and hexanoyl), alkenoyl groups (e.g., (lower C2_6
alkenyl)carbonyl groups such as acryloyl, methacryloyl,
crotonoyl and isocrotonoyl), cycloalkanecarbonyl groups
(e.g., (C3-6 cycloalkyl)carbonyl groups such as
cyclopropanecarbonyl, cyclobutanecarbonyl,
cyclopentanecarbonyl and cyclohexanecarbonyl), ( C3_7
WO96/16079 - 20 - PCTI~5/02389
6 4
cycloalkenyl)carbonyl groups (e.g., cyclopropenylcarbonyl,
cyclobutenylcarbonyl, cyclopentenylcarbonyl,
cyclohexenylcarbonyl) and alkanesulfonyl groups (e.g.,
(lower Cl_6 alkyl)sulfonyl groups such as mesyl,
ethanesulfonyl and propanesulfonyl); aromatic acyl groups
such as aroyl groups (e.g., (C6_10 aryl)carbonyl groups
such as benzoyl, p-toluoyl, l-naphthoyl and 2-naphthoyl),
arylalkanoyl groups (e.g., (Cl_6 alkyl)carbonyl groups
substituted with C6_10 aryl groups, such as phenylacetyl,
phenylpropionyl, hydroatropoyl and phenylbutyryl),
arylalkenoyl groups (e.g., (C2_6 alkenyl)carbonyl groups
substituted with C6_l0 aryl groups, such as cinnamoyl and
atropoyl) and C6_l0 arylsulfonyl groups (e.g.,
benzenesulfonyl group, p-toluenesulfonyl group); and
aromatic acyl groups such as aromatic heterocyclic carbonyl
groups (e.g., furoyl, thenoyl, nicotinoyl, isonicotinoyl,
pyrrolcarbonyl, oxazolcarbonyl, imidazolcarbonyl and
pyrazolcarbonyl), aromatic heterocyclic alkanoyl groups
(e.g., (Cl_6 alkyl)carbonyl groups substituted with
aromatic heterocyclic groups, such as thienylacetyl,
thienylpropanoyl, furylacetyl, thiazolylacetyl, 1,2,4-
thiadiazolylacetyl and pyridylacetyl).
Of the above-mentioned acyl groups for R4, those
represented by the formula -COR9 or -SO2Rll are preferable.
The groups of the formula -COR9 are preferably
represented by the formula -coRa (Ra is an optionally
substituted aryl or aromatic heterocyclic group) or the
formula -CORC (Rc is a straight-chain or branched Cl_6
alkyl group which is substituted with an optionally
substituted aryl or aromatic heterocyclic group).
The "optionally substituted aryl group" shown by Ra is
exemplified by C6-10 aryl such as phenyl, l-naphthyl, 2-
naphthyl.
The "optionally substituted aromatic heterocyclic
group" shown by Ra is exemplified by quinolyl, isoquinolyl,
2 ~ ~ Q ~ 6
~ WO96/16079 - 21 - PCTI~5/02389
.
furyl, thienyl, indolyl, isoindolyl, pyrazinyl and pyridyl,
with preference given to quinolyl, isoquinolyl.
The "optionally substituted aryl or aromatic
heterocyclic group" shown by Ra may have one or more
optionally chosen substituents at any possible position,
these substituents being exemplified by Cl_3 alkyls (e.g.
methyl, ethyl, propyl), hydroxy, Cl-3 alkoxys (e.g.
methoxy, ethoxy, propoxy) and halogens (e.g. fluorine,
chlorine, bromine).
The "straight-chain or branched Cl_6 alkyl group"
shown by Rc includes methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, sec.-butyl, tert.-butyl, pentyl,
isopentyl, neopentyl, tert.-pentyl, hexyl, isohexyl.
The "straight-chain or branched Cl_6 alkyl group"
shown by Rc has one or more (preferably one or two)
substituents such as an optionally substituted aryl or
aromatic heterocyclic group. Said optionally substituted
aryl or aromatic heterocyclic group is exemplified by the
same "optionally substituted aryl or aromatic heterocyclic
group" as defined in Ra.
Rc is more preferably a straight-chain or branched Cl_
4 alkyl group which has one or two optionally substituted
aryl (preferably phenyl) groups.
The group of the formula -SO2Rll is preferably
represented by the formula -so2Rb (Rb is an optionally
substituted aryl or aromatic heterocyclic group).
The "optionally substituted aryl or aromatic
heterocyclic group" shown by Rb is exemplified by the same
"optionally substituted aryl or aromatic heterocyclic
group" as defined in Ra.
Rb is more preferably an optionally substituted aryl
group.
The "aryl group that may be substituted," shown by Rb,
is exemplified by C6_l4 aryl group such as phenyl, 1-
naphthyl and 2-naphthyl. Said aryl group may have 1 or 2
optionally chosen substituents at any possible positions,
=
WO96/16079 ~ 22 - PCT1~5/02389
these substituents including alkyl groups (e.g., Cl-3
alkyls such as methyl, ethyl and propyl).
The "carboxyl group that may be esterified" shown by
R4 is exemplified by the groups represented by the general
formula -COORl2 (Rl2 represents a hydrogen atom, a Cl-6
alkyl, a C2_6 alkenyl, a C6_l0 aralkyl, or the like). For
example, groups resulting from binding of a carboxyl group
and an alkyl group having l to 6 carbon atoms include Cl_6
alkoxycarbonyls (e.g., methoxycarbonyl, ethoxycarbonyl,
propoxycarbonyl, isopropoxycarbonyl, butoxycarbonyl,
isobutoxycarbonyl, sec.-butoxycarbonyl, tert.-
butoxycarbonyl, pentyloxycarbonyl, hexyloxycarbonyl);
groups resulting from binding of a carboxyl group and an
alkenyl group having 2 to 6 carbon atoms include C2-6
alkenyloxycarbonyls (e.g., allyloxycarbonyl,
crotyloxycarbonyl, 2-pentenyloxycarbonyl, 3-
hexenyloxycarbonyl); the groups resulting from binding of a
carboxyl group and an aralkyl group having 7 to lO carbon
atoms include C7-lo aralkyloxycarbonyls (e.g., benzyloxy-
carbonyl, phenethyloxycarbonyl).
With respect to the above formula (I), the
"hydrocarbon group that may be substituted," shown by R5 or
R6, is exemplified by saturated or unsaturated aliphatic
chain hydrocarbon groups, saturated or unsaturated
alicyclic hydrocarbon groups and aryl groups.
Such saturated aliphatic hydrocarbon groups include
straight or branched saturated aliphatic hydrocarbon groups
having l to lO carbon atoms (e.g., Cl_lO alkyl groups such
as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, tert.-
pentyl, hexyl, isohexyl, heptyl and octyl), with preference
given to straight or branched saturated aliphatic
hydrocarbon groups having l to 6 carbon atoms.
Such unsaturated aliphatic hydrocarbon groups include
straight or branched unsaturated aliphatic hydrocarbon
groups having 2 to lO carbon atoms (e.g., C2_l0 alkenyl
~ 2200964
WOg6/16079 - 23 - PcT/Jls~ as
groups such as ethenyl, l-propenyl, 2-propenyl, l-butenyl,
2-butenyl, 3-butenyl, 2-methyl-1-propenyl, l-pentenyl, 2-
pentenyl, 3-pentenyl, 4-pentenyl, 3-methyl-2-butenyl, 1-
hexenyl, 3-hexenyl, 2,4-hexadienyl, 5-hexenyl, l-heptenyl
5 and l-octenyl; C2-10 alkynyl groups such as ethynyl, 1-
propynyl, 2-propynyl, l-butynyl, 2-butynyl, 3-butynyl, 1-
pentynyl, 2-pentynyl, 3-pentynyl, 4-pentynyl, l-hexynyl, 3-
hexynyl, 2,4-hexadynyl, 5-hexynyl/ l-heptynyl and 1-
octynyl), with preference given to straight or branched
10 unsaturated aliphatic hydrocarbon groups having 2 to 6
carbon atoms.
Such saturated alicyclic hydrocarbon groups include
saturated alicyclic hydrocarbon groups having 3 to 12
carbon atoms (e.g., C3_12 cycloalkyl group such as
15 cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl, cyclooctyl, bicyclo~2.2.1]heptyl,
bicyclo[2.2.2]octyl, bicyclo[3.2.1]octyl,
bicyclo[3.2.2]nonyl, bicyclo[3.3.1]nonyl,
bicyclo[4.2.1]nonyl and bicyclo[4.3.1]decyl), with
20 preference given to saturated alicyclic hydrocarbon groups
having 3 to 6 carbon atoms.
Such unsaturated alicyclic hydrocarbon groups include
unsaturated alicyclic hydrocarbon groups having 5 to 12
carbon atoms (e.g., C5_l2 cycloalkenyl groups such as 1-
25 cyclopentenyl, 2-cyclopentenyl, 3-cyclopentenyl, 1-
cyclohexenyl, 2-cyclohexenyl, 3-cyclohexenyl, 1-
cycloheptenyl, 2-cycloheptenyl, 3-cycloheptenyl, 2,4-
cycloheptadienyl, 2-cyclopenten-1-yl, 3-cyclopenten-1-yl,
2-cyclohexen-1-yl and 3-cyclohexen-1-yl; C5-12
30 cycloalkadienyl groups such as 2,4-cyclopentadien-1-yl,
2,4-cyclohexadien-1-yl and 2,5-cyclohexadien-1-yl).
The "hydrocarbon group that may be substituted," shown
A by R5 or R6, may be a saturated aliphatic hydrocarbon group
having l to 8 carbon atoms and substituted with one of the
35 above saturated or unsaturated alicyclic hydrocarbon groups
(e.g., C3-7 cycloalkyl-cl-8 alkyls or C5-7 CYcloalkenyl-cl-8
W096/l6079 ~ 2 ~ 6 4 - 24 - PCT/J15~ 389
alkyls, such as cyclopropylmethyl, cyclopropylethyl,
cyclobutylmethyl, cyclopentylmethyl, 2-cyclopentenylmethyl,
3-cyclopentenylmethyl, cyclohexylmethyl, 2-
cyclohexenylmethyl, 3-cyclohexenylmethyl, cyclohexylethyl,
cyclohexylpropyl, cycloheptylmethyl and cycloheptylethyl),
or the like.
Aryl groups include monocyclic or condensed polycyclic
aromatic hydrocarbon ring groups having 6 to 14 carbon
atoms. Such aromatic hydrocarbon ring groups include
phenyl, tolyl, xylyl, biphenyl, 1- or 2-naphthyl, 1-, 2- or
9-anthryl, 1-, 2-, 3-, 4- or 9-phenanthryl, 1-, 2-, 4-, 5-
or 6-azulenyl and acenaphthylenyl, with preference given to
C6_10 aryl such as phenyl, l-naphthyl and 2-naphthyl.
The "hydrocarbon group that may be substituted," shown
by R5 or R6, may have 1 to 3 optionally chosen substituents
at any possible positions. Such substituents include aryl
groups that may be substituted, cycloalkyl or cycloalkenyl
groups that may be substituted, heterocyclic groups that
may be substituted, carboxyl groups that may be esterified,
carbamoyl groups that may be substituted, amino groups that
may be substituted, hydroxyl groups that may be
substituted, thiol groups that may be substituted for,
halogens (e.g., fluorine, chlorine, bromine, iodine) and
phosphono groups that may be substituted.
The "aryl group that may be substituted" is
exemplified by C6_14 aryl such as phenyl, naphthyl,
anthryl, phenanthryl and acenaphthylenyl, with preference
given to phenyl, l-naphthyl and 2-naphthyl. Said aryl may
have 1 to 2 optionally chosen substituents at any possible
positions, these substituents including hydroxy, alkoxy
groups that may be substituted (e.g., Cl-3 alkoxys such as
methoxy, ethoxy and propoxy), halogen atoms (e.g.,
fluorine, chlorine, bromine, iodine) and alkyl groups that
may be substituted (e.g., Cl_3 alkyls such as methyl, ethyl
and propyl). These alkoxy groups and alkyl groups may each
have 1 to 2 optionally chosen substituents at any possible
2 ~ ~ 0 9 6 ~
WO96/16079 - - 25 - PCT/~5/02389
positions, these substituents including phosphono groups
that may be substituted (e.g., dimethoxyphosphoryl,
diethoxyphosphoryl).
The "cycloalkyl group that may be substituted" is
exemplified by C3-7 cycloalkyl groups such as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl and cycloheptyl. The
kinds and number of substituents for the cycloalkyl group
that may be substituted are the same as those of the
substituents for the above-described aryl group that may be
substituted.
The "cycloalkenyl group that may be substituted" is
exemplified by C3-6 cycloalkenyl groups such as
cyclopropenyl, cyclobutenyl, cyclopentenyl and
cyclohexenyl. The kinds and number of substituents for the
cycloalkenyl group that may be substituted are the same as
those of the substituents for the above-described aryl
group that may be substituted.
The "heterocyclic group that may be substituted" is
exemplified by aromatic heterocyclic groups having at least
1 hetero atom selected from atoms of oxygen, sulfur and
nitrogen as a ring-constituting atom (ring atom), and
saturated or unsaturated non-aromatic heterocyclic groups
(aliphatic heterocyclic groups), with preference given to
aromatic heterocyclic groups. The aromatic heterocyclic
group is exemplified by 5- to 7-membered aromatic
heterocyclic groups containing 1 atom of sulfur, nitrogen
or oxygen, 5- to 6-membered aromatic heterocyclic groups
containing 2 to 4 atoms of nitrogen and 5- or 6-membered
aromatic heterocyclic groups containing 1 to 2 atoms of
nitrogen and 1 atom of sulfur or oxygen. These aromatic
heterocyclic groups may be condensed with a 6-membered ring
containing 2 or fewer atoms of nitrogen, a benzene ring or
a 5-membered ring containing 1 atom of sulfur. Such
aromatic heterocyclic groups include aromatic monocyclic
heterocyclic groups (e.g., furyl, thienyl, pyrrolyl,
oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, imidazolyl,
-
WO96/16079 ~ 6 4 26 - PCT/~5/02389
pyrazolyl, 1,2,3-oxadiazolyl r 1,2,4-oxadiazolyl, 1,3,4-
oxadiazolyl, furazanyl, 1,2,3-thiadiazolyl, 1,2,4-
thiadiazolyl, 1,3,4-thiadiazolyl, 1,2,3-triazolyl, 1,Z,4-
triazolyl, tetrazolyl, pyridyl, pyridazinyl, pyrimidinyl,
pyrazinyl, triazinyl) and aromatic condensed heterocyclic
groups (e.g., benzofuranyl, isobenzofuranyl,
benzo[b]thienyl, indolyl, isoindolyl, lH-indazolyl,
benzimidazolyl, benzoxazolyl, 1,2-benzisoxazolyl,
benzothiazolyl, 1,2-benzisothiazolyl, lH-benzotriazolyl,
quinolyl, isoquinolyl, cinnolinyl, quinazolinyl,
quinoxalinyl, phthalazinyl, naphthyridinyl, purinyl,
pteridinyl, carbazolyl, ~-carbolinyl, ~-carbolinyl, y-
carbolinyl, acridinyl, phenoxazinyl, pheno~hiazinyl,
phenazinyl, phenoxathiinyl, thianthrenyl, phenathridinyl,
phenathrolinyl, indolizinyl, pyrrolo[l,2-b]pyridazinyl,
pyrazolo[l,5-a]pyridyl, imidazo[l,2-a]pyridyl, imidazo[l,5-
a]pyridyl, imidazo[l,2-b]pyridazinyl, imidazo[l,2-
a]pyrimidinyl, 1,2,4-triazolo[4,3-a]pyridyl, 1,2,4-
triazolo[4,3-b]pyridazinyl), with preference given to
quinolyl, isoquinolyl, furyl, thienyl, indolyl, isoindolyl,
pyrazinyl, pyridyl, pyrimidinyl etc. The non-aromatic
heterocyclic group is exemplified by 5- to 7-membered non-
aromatic heterocyclic groups containing l atom of sulfur,
nitrogen or oxygen, and 4- to 7-membered non-aromatic
heterocyclic groups containing 1 atom of nitrogen and 3 or
fewer atoms selected from nitrogen, oxygen and sulfur.
Such non-aromatic heterocyclic groups include oxylanyl,
azetidinyl, oxetanyl, thietanyl, pyrrolidinyl,
tetrahydrofuryl, thiolanyl, piperizyl, tetrahydropyranyl,
morpholinyl, thiomorpholinyl and piperazinyl. The
substituent for said heterocyclic group that may be
substituted is exemplified by alkyl groups having 1 to 3
carbon atoms (e.g., methyl, ethyl, propyl).
Such carboxyls that may be esterified include -COOH,
(lower Cl_6 alkoxy)carbonyls (e.g., methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
2 ~ ~ G 9 6 4
WO96tl6079 - 27 - PCT/Jl~r,~2~9
butoxycarbonyl, tert.-butoxycarbonyl, sec.-butoxycarbonyl,
pentyloxycarbonyl, isopentyloxycarbonyl,
neopentyloxycarbonyl, tert.-pentyloxycarbonyl) and (C6_l0
aryloxy)carbonyls (e.g., phenoxycarbonyl, 1-
~ 5 naphthoxycarbonyl), (C7-l3 aralkyloxy)carbonyls (e.g.,
benzyloxycarbonyl), (e.g., with preference given to the
carboxyl group, methoxycarbonyl and ethoxycarbonyl.
The substituent for said carbamoyl group that may be
substituted is exemplified by lower (Cl_6) alkyls that may
be substituted (e.g., methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, sec.-butyl, tert.-butyl, pentyl,
isopentyl, neopentyl, hexyl, isohexyl), C3-6 cycloalkyl
groups that may be substituted (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
that may be substituted (e.g., phenyl, l-naphthyl, 2-
naphthyl) and C7-13 aralkyl groups that may be substituted
(e.g., benzyl, phenethyl); 1 or 2 of these substituents,
whether identical or not, may be present. The substituent
for said lower (Cl_6) alkyl that may be substituted and C3-6
cycloalkyl group that may be substituted is exemplified by
carboxyl groups that may be esterified, aromatic
heterocyclic groups (e.g., furyl, thienyl, indolyl,
isoindolyl, pyrazinyl, pyridyl, pyrimidinyl, imidazolyl),
amino groups, hydroxyl groups and phenyl groups. The
substituent for said aryl group that may be substituted and
aralkyl group that may be substituted is exemplified by
halogen atoms (e.g., fluorine, chlorine, bromine, iodine)
and carboxyl groups that may be esterified. Also, 2
substituents on the nitrogen atom may cooperate with the
nitrogen atom to form a cyclic amino group, such cyclic
amino groups including l-azetidinyl, l-pyrrolidinyl,
piperidino, morpholino and l-piperazinyl.
The substituent for said amino group that may be
substituted is exemplified by lower (Cl_6) alkyls that may
be substituted for (e.g., methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, sec.-butyl, tert.-butyl, pentyl,
-
WO96/16079 ~ ~ ~ Q ~ 6 4 - 28 - PCT/~5102389
isopentyl, neopentyl, hexyl, isohexyl), C3-6 cycloalkyl
groups that may be substituted (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
that may be substituted (e.g., phenyl, l-naphthyl, 2-
naphthyl) and C7-l3 aralkyl groups that may be substituted
(e.g., benzyl, phenethyl); l or 2 of these substituents,
whether identical or not, may be present. The substituent
for said lower (Cl_6) alkyl that may be substituted and C3-6
cycloalkyl group that may be substituted is exemplified by
carboxyl groups that may be esterified, aromatic
heterocyclic groups (e.g., furyl, thienyl, indolyl,
isoindolyl, pyrazinyl, pyridyl, pyrimidyl, imidazolyl),
amino groups, hydroxyl groups and phenyl groups. The
substituent for said aryl group that may be substituted and
aralkyl group that may be substituted is exemplified by
halogen atoms (e.g., fluorine, chlorine, bromine, iodine)
and carboxyl groups that may be esterified. Also, 2
substituents on the nitrogen atom may cooperate with the
nitrogen atom to form a cyclic amino group, such cyclic
amino groups including l-azetidinyl, 1-pyrrolidinyl,
piperidino, morpholino and l-piperazinyl.
The substituent for said hydroxyl group that may be
substituted is exemplified by lower (Cl_6) alkyls that may
be substituted (e.g., methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, sec.-butyl, tert.-butyl, pentyl,
isopentyl, neopentyl, hexyl, isohexyl), C3-6 cycloalkyl
groups that may be substituted (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
that may be substituted (e.g., phenyl, l-naphthyl, 2-
naphthyl) and C7-l3 aralkyl groups that may be substituted
(e.g., benzyl, phenethyl).
The substituent for said thiol group that may be
substituted is exemplified by lower (Cl_6) alkyls that may
be substituted (e.g., methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, sec.-butyl, tert.-butyl, pentyl,
isopentyl, neopentyl, hexyl, isohexyl), C3-6 cycloalkyl
~ ~ ~ 0 9 6 4
wo96ll6o7s - 29 - PCT/~5tO2389
groups that may be substituted (e.g., cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl), C6_l0 aryl groups
that may be substituted for (e.g., phenyl, l-naphthyl, 2-
naphthyl) and C7_l3 aralkyl groups that may be substituted
(e.g., benzyl, phenethyl).
The substituent for said phosphono group that may be
substituted is exemplified by lower (Cl_6) alkyls (e.g.,
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, hexyl,
isohexyl), lower (Cl_6) alkoxys (e.g., methoxy, ethoxy,
propoxy, isopropoxy, butoxy, isobutoxy, sec.-butoxy, tert.-
butoxy, pentyloxy, isopentyloxy, neopentyloxy, hexyloxy,
isohexyloxy). Said phosphono groups include phosphoryl,
dimethoxyphosphoryl, diethoxyphosphoryl,
dipropoxyphosphoryl, diisopropoxyphosphoryl,
ethylenedioxyphosphoryl, trimethylenedioxyphosphoryl and
tetramethylenedioxyphosphoryl.
When the "hydrocarbon group that may be substituted,"
shown by R5 or R6, is an alicyclic hydrocarbon group or an
aryl group, the substituent may be an aliphatic hydrocarbon
group that may be substituted. Said aliphatic hydrocarbon
group is exemplified by the same saturated or unsaturated
(preferably saturated) aliphatic hydrocarbon groups as
those exemplifying the "hydrocarbon group that may be
substituted," shown by R5 or R6 above, with preference
given to alkyl groups (e.g., Cl-3 alkyls such as methyl,
ethyl and propyl). The aliphatic hydrocarbon group may
have 1 or 2 optionally chosen substituents at any possible
positions, these substituents including phosphono groups
that may be substituted (e.g., phosphoryl, dimethoxyphos-
phoryl, diethoxyphosphoryl).
The "heterocyclic group that may be substituted,"
shown by R5 or R6, is exemplified by aromatic heterocyclic
groups having at least 1 hetero atom selected from atoms of
oxygen, sulfur and nitrogen as a ring-constituting atom
(ring atom), and saturated or unsaturated non-aromatic
WO~6/16079 ~ ~ Q ~ ~ ~ 4 PCT/J~ U~9
heterocyclic groups (aliphatic heterocyclic groups), with
preference given to aromatic heterocyclic groups.
Such aromatic heterocyclic groups are exemplified by
5- to 7-membered heterocyclic groups containing 1 atom of
sulfur, nitrogen or oxygen, 5- to 6-membered heterocyclic
groups containing 2 to 4 atoms of nitrogen and 5- or 6-
membered aromatic heterocyclic groups containing 1 or 2
atoms of nitrogen and 1 atom of sulfur or oxygen. These
aromatic heterocyclic groups may have condensed with a 6-
membered ring containing 2 or fewer atoms of nitrogen, abenzene ring or a 5-membered ring containing 1 atom of
sulfur. Such aromatic heterocyclic groups include aromatic
monocyclic heterocyclic groups (e.g., furyl, thienyl,
pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl,
imidazolyl, pyrazolyl, 1,2,3-oxadiazolyl, 1,2,4-
oxadiazolyl, 1,3,4-oxadiazolyl, furazanyl, 1,2,3-
thiadiazolyl, 1,2,4-thiadiazolyi, 1,3,4-thiadiazolyl,
1,2,3-triazolyl, 1,2,4-triazolyl, tetrazo~yl, 2-, 3- or 4-
pyridyl, 3- or 4-pyridazinyl, 2-, 4-, 5- or 6-pyrimidinyl,
2-pyrazinyl, triazinyl) and aromatic condensed heterocyclic
groups (e.g., benzofuranyl, isobenzofuranyl,
benzo[b]thienyl, indolyl, isoindolyl, lH-indazolyl,
benzimidazolyl, benzoxazolyl, 1,2-benzisoxazolyl,
benzothiazolyl, 1,2-benzisothiazolyl, lH-benzotriazolyl,
quinolyl, isoquinolyl, cinnolinyl, quinazolinyl,
quinoxalinyl, phthalazinyl, naphthyridinyl, purinyl,
pteridinyl, carbazolyl, a-carbolinyl, ~-carbolinyl, y-
carbolinyl, acridinyl, phenoxazinyl, phenothiazinyl,
phenazinyl, phenoxathiinyl, thianthrenyl, phenathridinyl,
phenathrolinyl, indolizinyl, pyrrolo[l,2-b]pyridazinyl,
pyrazolo[l,5-a]pyridyl, imidazo[l,2-a]pyridyl, imidazo[l,5-
a]pyridyl, imidazo[l,2-b]pyridazinyl, imidazo[l,2-
a]pyrimidinyl, 1,2,4-triazolo[4,3-a]pyridyl, 1,2,4-
triazolo[4,3-b]pyridazinyl), with preference given to
quinolyl, isoquinolyl, furyl, thienyl, indolyl, isoindolyl,
pyrazinyl, pyridyl, pyrimidinyl etc.
2 20 Q ~ 6 4
WO 96116079 ~ 31 -- PCT/Jr~,J~2389
Such non-aromatic heterocyclic groups are exemplified
by 5- to 7-membered non-aromatic heterocyclic groups
containing 1 atom of sulfur, nitrogen or oxygen, and 4 - to
7-membered non-aromatic heterocyclic groups containing 1
atom of nitrogen and 3 or fewer atoms selected from
nitrogen, oxygen and sulfur (e.g., oxylanyl, azetidinyl,
oxetanyl, thietanyl, pyrrolidinyl, tetrahydrofuryl,
thiolanyl, piperizyl, tetrahydropyranyl, morpholinyl,
thiomorpholinyl, piperazinyl, homopiperidyl, pyrrolinyl,
imidazolidinyl). These non-aromatic heterocyclic groups
may be condensed with a benzene ring, a 6-membered ring
containing 2 or fewer atoms of nitrogen, or a 5-membered
ring containing 1 atom of sulfur. Specifically, such
condensed non-aromatic heterocyclic groups include
chromanyl, isochromanyl, indolinyl, isoindolinyl,
thiochromanyl and isothiochromanyl.
The "heterocyclic group that may be substituted,"
shown by R5 or R6, may have 1 to 3 optionally chosen
substituents at any possible positions. Such substituents
include aryl groups that may be substituted, cycloalkyl or
cycloalkenyl groups that may be substituted, heterocyclic
groups that may be substituted, carboxyl groups that may be
esterified, carbamoyl groups that may be substituted, amino
groups that may be substituted, hydroxyl groups that may be
substituted, thiol groups that may be substituted, halogens
(e.g., fluorine, chlorine, bromine, iodine), phosphono
groups that may be substituted, and aliphatic hydrocarbon
groups that may be substituted.
Said aryl groups that may be substituted, cycloalkyl
or cycloalkenyl groups that may be substituted,
heterocyclic groups that may be substituted for, carboxyl
groups that may be esterified, carbamoyl groups that may be
substituted, amino groups that may be substituted for,
hydroxyl groups that may be substituted, thiol groups that
may be substituted, halogens (e.g., fluorine, chlorine,
bromine, iodine), phosphono groups that may be substituted,
W096/16079 ~ 6 4 PCT/~5/02389
and aliphatic hydrocarbon groups that may be substituted
are exemplified by the same substituents as those for the
"hydrocarbon group that may be substituted," shown by R5
and ~6 above.
R5 and R6, in cooperation with the adjoining nitrogen
atom, may bind together to form a heterocyclic ring that
may contain l more hetero atom (e.g., oxygen, nitrogen,
sulfur), such heterocyclic groups including l-azetidinyl,
l-pyrrolidinyl, piperidino, morpholino, l-piperazinyl and
l-homopiperazinyl. These heterocyclic groups may each have
1 or 2 substituents at any possible positions, these
substituents including alkyls (e.g., Cl-3 alkyls), carboxyl
groups and hydroxyl groups.
The "hydrocarbon group that may be substituted," shown
by R5 or R6 above, is exemplified by alkyl groups,
preferably Cl_lo alkyls, with greater preference given to
straight or branched lower alkyls having l to 6 carbon
atoms (e.g., methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec.-butyl, tert.-butyl, pentyl, isopentyl,
neopentyl, tert.-pentyl, hexyl, isohexyl, 4-methylpentyl,
l,1-dimethylbutyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, 2-
ethylbutyl) etc. Preferable substituents for the
"hydrocarbon group that may be substituted" are aryls that
may be substituted (preferably phenyl etc.), heterocyclic
groups that may be substituted and carboxyl groups that may
be esterified.
More preferably, the "hydrocarbon group that may be
substituted," shown by R5 and R6, is an alkyl group
substituted with an aryl or heterocyclic group. Said alkyl
substituted with an aryl is exemplified by groups resulting
from binding of a monocyclic or condensed polycyclic
aromatic hydrocarbon group having 6 to 14 carbon atoms
(e.g., phenyl, naphthyl, anthryl, phenanthryl,
acenaphthylenyl) and a lower alkyl having 1 to 6 carbon
atoms (preferably Cl_4 alkyl) (e.g., benzyl, 2-phenylethyl,
3-phenylpropyl, 2-phenylpropyl, l-phenylpropyl, ~-
2 2 0 0 9 6 b~
WO96/16079 ~ 33 ~ PCT/~5102389
naphthylmethyl, ~-naphthylethyl, ~-naphthylmethyl, ~-
naphthylethyl). Said alkyl substituted with a heterocyclic
group is exemplified by groups resulting from binding of an
aromatic heterocyclic group and a lower alkyl having l to 6
carbon atoms (preferably Cl-4 alkyl group). Such aromatic
heterocyclic groups include 2-furyl, 3-furyl, 2-thienyl, 3-
thienyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-
pyrimidinyl, 5-pyrimidinyl, 6-pyrimidinyl, 3-pyridazinyl,
4-pyridazinyl, 2-pyrazinyl, 2-pyrrolyl, 3-pyrrolyl, 2-
imidazolyl, 4-imidazolyl, 5-imidazolyl, 3-pyrazolyl, 4-
pyrazolyl, isothiazolyl, isoxazolyl, 2-thiazolyl, 4-
thiazolyl, 5-thiazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl,
l,2,4-triazol-3-yl, l,2,3-triazol-4-yl, tetrazol-5-yl,
benzimidazol-2-yl, indol-3-yl, lH-indazolyl,
benz[b]furanyl, isobenzofuranyl, benz[b]thienyl, lH-
pyrrolo[2,3-b]pyrazin-2-yl, lH-pyrrolo[2,3-b]pyridin-6-yl,
lH-imidazo[4,5-b]pyridin-2-yl, lH-imidazo[4,5-c]pyridin-2-
yl and lH-imidazo[4,5-b]pyrazin-2-yl, with preference given
to 2-furyl, 3-furyl, 2-thienyl, 3-thienyl, 2-pyridyl, 3-
pyridyl, 4-pyridyl and 4-imidazolyl etc.
With respect to the combination of R5 and R6, it is
preferable that one be a hydrogen atom, and the other be
sec.-butyl, benzyl, isobutyl or isopropyl, with preference
given to benzyl.
When one of R5 and R6 is a hydrogen atom, the other is
exemplified by a group represented by formula (Ia-aa):
Rd
~CH-Re (Ia-aa
- wherein Rd represents a hydrogen atom or a hydrocarbon
group or heterocyclic group that may be substituted; Re
represents a carboxyl group that may be esterified or a
carbamoyl group that may be substituted.
With respect to formula (Ia-aa), the "hydrocarbon
group that may be substituted," shown by Rd, is exemplified
WO96tl6079 ~ 6 4 - 34 PCT/J15~ 89
by the same hydrocarbon groups as those for the
"hydrocarbon group that may be substituted," shown by Rl,
R2 or R3 above.
The "hydrocarbon group that may be substituted," shown
by Rd, may have 1 to 3 optionally chosen substituents at
any possible positions, these substituents being
exemplified by the same substituents as those for the
"hydrocarbon group that may be substituted," shown by Rl,
R2 or R3 above.
With respect to formula (Ia-aa), the "heterocyclic
group that may be substituted," shown by Rd, is exemplified
by the same heterocyclic groups exemplifying the
"heterocyclic group that may be substituted," shown by Rl,
R2 or R3 above.
The "heterocyclic group that may be substituted,"
shown by Rd, may have 1 to 3 optionally chosen substituents
at any possible positions, these substituents being
exemplified by the same substituents as those for the
"heterocyclic group that may be substituted," shown by Rl,
R2 or R3 above
The "hydrocarbon group that may be substituted for,"
shown by Rd, is exemplified by alkyl groups, preferably
Cl_lo alkyl, with greater preference given to linear or
branched lower alkyls having 1 to 6 carbon atoms (e.g.,
methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec.-
butyl, tert.-butyl, pentyl, isopentyl, neopentyl, tert.-
pentyl, hexyl, isohexyl, 4-methylpentyl, l,1-dimethylbutyl,
2,2-dimethylbutyl, 3,3-dimethylbutyl, 2-ethylbutyl).
Preferable substituents for the "hydrocarbon group that may
be substituted" are aryls that may be substituted
(preferably phenyl etc.), heterocyclic rings that may be
substituted, and carboxyl groups that may be esterified.
With respect to formula (Ia-aa), the "carboxyl group
that may be esterified" shown by Re, is exemplified by
carboxy, (lower Cl_6 alkoxy)carbonyls (e.g.,
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
2 ~ O 0 9 ~ 4
WO96/1607s - 35 - PCT1~S/02389
isopropoxycarbonyl, butoxycarbonyl, tert.-butoxycarbonyl,
sec.-butoxycarbonyl, pentyloxycarbonyl,
isopentyloxycarbonyl, neopentyloxycarbonyl, tert.-
pentyloxycarbonyl) and (C6_l0 aryloxy)carbonyls (e.g.,
phenoxycarbonyl, l-naphthoxycarbonyl), (C7-l3
aralkyloxy)carbonyls (e.g.,benzyloxycarbonyl), with
preference given to the carboxyl group, methoxycarbonyl and
ethoxycarbonyl.
With respect to formula (Ia-aa), the substituent for
the "carbamoyl group that may be substituted," shown by Re,
is exemplified by lower (Cl_6) alkyls that may be
substituted (e.g., methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec.-butyl, tert.-butyl, pentyl, isopentyl,
neopentyl, hexyl, isohexyl), C3-6 cycloalkyl groups that
may be substituted (e.g., cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl), C6_l0 aryl groups that may be
substituted (e.g., phenyl, l-naphthyl, 2-naphthyl) and C7-
13 aralkyl groups that may be substituted (e.g., benzyl,
phenethyl); l or 2 of these substituents, whether identical
or not, may be present. The substituent for said lower
(Cl_6) alkyl that may be substituted and C3-6 cycloalkyl
group that may be substituted is exemplified by carboxyl
groups that may be esterified, aromatic heterocyclic groups
(e.g., furyl, thienyl, indolyl, isoindolyl, pyrazinyl,
pyridyl, pyrimidinyl, imidazolyl), amino groups, hydroxyl
groups and phenyl groups. The substituent for said aryl
group that may be substituted and aralkyl group that may be
substituted is exemplified by halogen atoms (e.g.,
fluorine, chlorine, bromine, iodine) and carboxyl groups
that may be esterified. Also, 2 substituents on the
nitrogen atom may cooperate with the nitrogen atom to form
a cyclic amino group, such cyclic amino groups including l-
azetidinyl, l-pyrrolidinyl, piperidino, morpholino and l-
piperazinyl.
WO96/16079 ~ ~ O O ~ ~ ~ 6 PCT1~5/02389
With respect to the combination of m and n in general
formula (I), it is preferable that i) m be l and n be l,
ii) m be l and n be 0, or iii) m be 0 and n be 0.
In the present invention, the salt of the compound of
general formula (Ia), (I), (I') or (I") is preferably a
physiologically acceptable salt, exemplified by salts with
inorganic bases, salts with organic bases, salts with
inorganic acids, salts with organic acids and salts with
basic or acidic amino acids. Preferable salts with
inorganic bases include alkali metal salts such as sodium
salt and potassium salt; alkaline earth metal salts such as
calcium salt and magnesium salt; and aluminum salt.
Preferable salts with organic bases include ammonium salts
and salts with trimethylamine, triethylamine, pyridine,
picoline, ethanolamine, diethanolamine, triethanolamine,
dicyclohexylamine and N,N'-dibenzylethylenediamine.
Preferable salts with inorganic acids include salts with
hydrochloric acid, hydrobromic acid, nitric acid, sulfuric
acid and phosphoric acid. Preferable salts with organic
acids include salts with formic acid, acetic acid,
trifluoroacetic acid, fumaric acid, oxalic acid, tartaric
acid, maleic acid, citric acid, succinic acid, malic acid,
methanesulfonic acid, benzenesulfonic acid and p-
toluenesulfonic acid. Preferable salts with basic amino
acids include salts with arginine, lysine and ornithine.
Preferable salts with acidic amino acids include salts with
aspartic acid and glutamic acid.
Production methods for the compound (I), (Ia), (I') or
(I") of the present invention are hereinafter described in
detail.
When the starting compound used in each of the
reactions for synthesizing the below-described desired
compounds, the starting compound has an amino group,
carboxyl group or hydroxyl group as a substituent, these
substituents may have a protective group in common use in
peptide chemistry etc.; the desired compound can be
2 2 0 Q ~ 6 4
WO96/16079 ~ 37 - PCT/~5/02389
obtained by removing, as appropriate, the protective group
after completion of the reaction.
Amino-protecting groups include, for example, formyl,
Cl_6 alkylcarbonyl groups te-g-, acetyl, ethylcarbonyl),
phenylcarbonyl group, Cl_6 alkyl-oxycarbonyl groups (e.g.,
methoxycarbonyl, ethoxycarbonyl), phenyloxycarbonyl group,
C7_l0 aralkyl-carbonyl group (e.g., benzylcarbonyl), trityl
group, phthaloyl group and N,N-dimethylaminomethylene
group, which are optionally substituted. Examples of the
substituents which these groups optionally have, include
halogen atoms (e.g., fluorine, chlorine, bromine, iodine),
Cl_6 alkyl-carbonyl groups (e.g., methylcarbonyl,
ethylcarbonyl, butylcarbonyl) and nitro groups, the number
of substituents being l to 3.
Examples of the carboxyl-protecting groups include Cl_
6 alkyl groups (e.g., methyl, ethyl, n-propyl, isopropyl,
n-butyl, tert.-butyl), phenyl, trityl and silyl, which are
optionally substituted. Examples of the substituents which
these groups optionally have, include halogen atoms (e.g.,
fluorine, chlorine, bromine, iodine), formyl, Cl_6 alkyl-
carbonyl groups (e.g., acetyl, ethylcarbonyl,
butylcarbonyl) and nitro groups, the number of substituents
being l to 3.
Examples of the hydroxyl-protecting groups include Cl_
6 alkyl groups (e.g., methyl, ethyl, n-propyl, isopropyl,
n-butyl, tert.-butyl), phenyl, C7-1o aralkyl groups (e.g.,
benzyl), formyl, Cl_6 alkyl-carbonyl groups (e.g., acetyl,
ethylcarbonyl), phenyloxycarbonyl, benzoyl, C7-lo aralkyl-
carbonyl groups (e.g., benzylcarbonyl), pyranyl, furanyl
and silyl, which are optionally substituted. Examples of
substituents which these groups optionally have, include
halogen atoms (e.g., fluorine, chlorine, bromine, iodine),
Cl_6 alkyl groups (e.g., methyl, ethyl, n-propyl), phenyl,
C7_10 aralkyl groups (e.g., benzyl) and nitro groups, the
number of substituents being l to 4.
-
-
WO96/16079 ~ 6 4 38 PCT/Jl~3l'~h9
Protecting groups can be removed by commonly known
methods or modifications thereof, including treatments with
acids, bases, reducing agents, ultraviolet rays, hydrazine,
phenylhydrazine, sodium N-methyldithiocarbamate,
tetrabutylammonium fluoride, palladium acetate etc.
Compound (Ia) can be produced by subjecting to an
oxidation reaction described in detail below a compound
represented by general formula (IIa):
Rl lo, H
R4-Q-NHcHcH-CoNR5R6 (IIa)
wherein Q represents a direct bond or l or 2 amino acid
residues that may be substituted; Rl represents a hydrogen
atom or a hydrocarbon group or heterocyclic group that may
be substituted; R4 represents a carboxyl group that may be
esterified or an acyl group; R5 and R6, whether identical
or not, independently represent a hydrogen atom or a
hydrocarbon group or heterocyclic group that may be
substituted; R5 and R6 may bind together to form a ring.
Compound (I) can be produced by subjecting to an
oxidation reaction described in detail below a compound
represented by general formula (II):
Rl3 Rl2 RIllOH
R4-( NHCHCO ) n~ ( NHCHCO ) m-NHCHCH-CoNR5R6 ( I I )
wherein Rl, R2 and R3, whether identical or not,
. independently represent a hydrogen atom or a hydrocarbon
group or heterocyclic group that may be substituted for; R4
represents a carboxyl group that may be esterified or an
acyl group; R5 and R6, whether identical or not,
independently represent a hydrogen atom or a hydrocarbon
group or heterocyclic group which may be substituted for;
R5 and R6 may bind together to form a ring; m and n,
2 2 ~ 0 9 6
W096/16079 ~ 39 ~ PCT/~95tO2389
whether identical or notj independently represent O or 1.
Compound (I') and (I") can be produced by the same manner
as mentioned above in production of compounds (I) and (Ia).
Method A
R3 R2 RlOH
I I
R4-(NHCHCo) n~ ( NHCHCo)m-NHCHCH-CoNR5R6
R3 R2 R
R4-(NHCHCo) n~ ( NHCHCo)m-NHCHCo-CoNR5R6
(I)
In the above formulas, the symbols have the same
definitions as those shown above.
This oxidation is carried out by a known oxidizing
reaction. Such reactions include chromic acid oxidations
such as Jones' oxidation, using chromium oxide-sulfuric
acid-pyridine, Collins oxidation, using chromium oxide-
pyridine complex, oxidation with pyridinium chlorochromate
(PCC) and oxidation with pyridinium dichromate (PDC);
oxidation with activated DMSO, described in detail for
method B below; and oxidation with oxoammonium salt.
In the case of an optically active configuration, this
oxidation is advantageously carried out by activated
dimethyl sulfoxide (DMSO) oxidation. Activated DMSO
oxidation is carried out in a solvent in the presence of
both DMSO and an electrophilic reagent. This solvent is
exemplified by ethers such as ethyl ether, isopropyl ether,
tetrahydrofuran and dioxane, aromatic hydrocarbons such as
benzene, toluene and xylene, halogenated hydrocarbons such
as chloroform and dichloromethane, N,N-dimethylformamide
(DMF), pyridine and dimethyl sulfoxide, chosen as
appropriate according to the kind of electrophilic reagent.
Methods of activated DMSO oxidation include the
dicyclohexylcarbodiimide (DCC) method, the acetic anhydride
method, the phosphorus pentoxide method, the chlorine
method, the sulfur trioxide-pyridine method, the
WO96/16079 2 ~ O ~ 40 - PCTI~S/02389
ketenimine-enamine method and the mercury (II) acetate
method, named according to the electrophilic reagent used.
This oxidation is advantageously carried out by a
modification of the dicyclohexylcarbodiimide (DCC) method,
in which oxidation is achieved using the pyridine salt of
trifluoroacetic acid as a catalyst and l-ethyl-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (WSCD HCl)
as a DMSO activator reagent. This reaction can also be
carried out with dimethyl sulfoxide as a solvent. The
amount of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
hydrochloride (WSCD HCl) used is 1 to 10 mol, preferably 2
to 5 mol per mol of compound (II). The amount of the
pyridine salt of trifluoroacetic acid used is 0.1 to 2 mol
per mol of compound (II). Reaction temperature is -70 to
80C, preferably -20 to 40C, reaction time being 0.5 to 10
hours.
Ketoamide derivatives (Ia) and (I) thus obtained may
be isolated and purified by known means of separation and
purification such as concentration, concentration under
reduced pressure, solvent extraction, crystallization,
recrystallization, redissolution and chromatography.
Compound (II) for the present invention can, for
example, be produced as follows:
Method B
R3 R2 RloH
R4 - ( N~('F~CO ) n~ ( NHCHCO ) m~OH ~ H2NCHCH-CoNR5R6 ~ ( I I )
(III) (IV)
In the above formulas, the symbols have the same
definitions as those shown above.
-
2 2 0 ~ 9 6
WO96116079 - 41 - PCT/J155~ag
In this method, compound (III), its derivative
reactive at the carboxyl group thereof, or a salt thereof
is reacted with compound (IV), its derivative reactive at
the amino group thereof, or a salt thereof to yield
compound (II). Preferable derivatives of compound (IV)
reactive at the amino group thereof include Schiff's base
type imino or enamine form tautomeric isomers resulting
from reaction of compound (IV) and a carbonyl compound such
as aldehyde or ketone; silyl derivatives resulting from
reaction of compound (IV) and a silyl compound such as
bis(trimethylsilyl)acetamide, mono(trimethylsilyl)acetamide
or bis(trimethylsilyl)urea; and derivatives resulting from
reaction of compound (IV) and phosphorus trichloride or
phosgene. Preferable salts of compound (IV) and its
reactive derivatives are exemplified by the same acid
adduct salts as specified for compound (I) above.
Preferable derivatives of compound (III) reactive at
the carboxyl group thereof include acid halides, acid
anhydrides, activated amides and activated esters. Other
preferable reactive derivatives include acid chlorides;
acid azides; mixed acid anhydrides such as those with a
substituted phosphoric acid such as dialkylphosphoric acid,
phenylphosphoric acid, diphenylphosphoric acid,
dibenzylphosphoric acid or halogenated phosphoric acid, or
with dialkylphosphorous acid, sulfurous acid, thiosulfuric
acid or sulfuric acid, or with a sulfonic acid such as
methanesulfonic acid, or with an aliphatic carboxylic acid,
such as acetic acid, propionic acid, butyric acid,
isobutyropivalic acid, pentanoic acid, isopentanoic acid or
trichloroacetic acid, or with an aromatic carboxylic acid
such as benzoic acid; symmetric acid anhydrides; activated
amides with imidazole, 4-substitutional imidazole,
dimethylpyrazole, triazole or tetrazole; activated esters
such as cyanomethyl ester, methoxymethyl ester,
dimethyliminomethyl ester, vinyl ester, propargyl ester, p-
nitrophenyl ester, trichlorophenyl ester, pentachlorophenyl
W096~l6079 7 ~ ~ o ~ 6 4 - 42 - PCT/~5/0238s
ester, mesylphenyl ester, phenylazophenyl ester, phenylthio
ester, p-nitrophenyl ester, p-cresylthio ester,
carboxymethylthio ester, pyranyl ester, pyridyl ester,
piperidyl ester and 8-quinolylthio ester; and esters with
N-hydroxy compounds such as N,N-dimethylhydroxylamine, 1-
hydroxy-2-(lH)-pyridone, N-hydroxysuccinimide, N-
hydroxyphthalimide and l-hydroxy-lH-benzotriazole. These
reactive derivatives can be optionally chosen according to
the kind of compound (III) used. Preferable salts of
reactive derivatives of compound (III) include salts with
bases, exemplified by alkali metal salts such as sodium
salt and potassium salt, alkaline earth metal salts such as
calcium salt and magnesium salt, ammonium salt, and organic
base salts such as trimethylamine salt, triethylamine salt,
pyridine salt, picoline salt, dicyclohexylamine salt and
N,N-dibenzylethylenediamine salt. This reaction is
normally carried out in an ordinary solvent such as water,
an alcohol such as methanol or ethanol, acetone, dioxane,
acetonitrile, chloroform, methylene chloride, ethylene
chloride, tetrahydrofuran, ethyl acetate, N,N-
dimethylformamide or pyridine, but can be carried out in
any other organic solvent, as long as it does not interfere
with the reaction. These ordinary solvents may be used in
mixture with water.
When compound (III) is used in the form of free acid
or salt thereof, this reaction is preferably carried out in
the presence of an ordinary condensing agent such as N,N'-
dicyclohexylcarbodiimide, N-cyclohexyl-N'-
morpholinoethylcarbodiimide, N-cyclohexyl-N'-(4-
diethylaminocyclohexyl)carbodiimide, N,N'-
diethylcarbodiimide, N,N'-diisopropylcarbodiimide, N-ethyl-
N'-(3-dimethylaminopropyl)carbodiimide, N,N'-carbonylbis(2-
methylimidazole), pentamethyleneketene-N-cyclohexylimine,
diphenylketene-N-cyclohexylimine, ethoxyacetylene, 1-
alkoxy-l-chloroethylene, trialkyl phosphite, ethyl
polyphosphate, isopropyl polyphosphate, phosphorus
``` 2 ~ O O ~ 6
WO96/16079 43 PCT1~5/02389
oxychloride, diphenylphosphorylazide, thionyl chloride,
oxalyl chloride, a lower alkyl haloformate such as ethyl
chloroformate or isopropyl chloroformate,
triphenylphosphine, 2-ethyl-7-hydroxybenzisoxazolium salt,
2-ethyl-5-(m-sulfophenyl)isoxazolium hydroxide
intramolecular salt, N-hydroxybenzotriazole, l-(p-
chlorobenzenesulfonyloxy)-6-chloro-lH-benzotriazole, or
what is called Wilsmeier reagent as prepared by reaction of
N,N'-dimethylformamide and thionyl chloride, phosgene,
trichloromethyl chloroformate, phosphorus oxychloride, or
the like. This reaction may also be carried out in the
presence of an inorganic or organic base such as alkali
metal hydrogen carbonate tri(lower)alkylamine, pyridine, N-
(lower)-alkylmorpholine or N,N-di(lower)alkylbenzylamine.
Although reaction temperature is not subject to limitation,
this reaction is normally carried out under cooling to
heating conditions.
Starting material compound (III) for method B is
produced by methods C through J as follows:
Method C
R3 R2
R4--NHCHCOOH+ H2NCHCOOL
(V) (VI)
R3 R2
~ Protective group removal
R4-NHCHCo-NHCHCooL ~ (III)
(VII)
In the above formulas, L represents a carboxy-protecting
group; the other symbols have the same definitions as those
given above.
The carboxy-protecting group for L is exemplified by
protecting groups in common use in the field of peptide
synthesis, such as ester derivatives.
In this method, compound (V), its derivative reactive
at the carboxyl group thereof, or a salt thereof is reacted
W096/lG079 ~ 2 0 o 9 6 4 PCT/Jr5~J~&9 ~
with compound (VI), its derivative reactive at the amino
group thereof, or a salt thereof to yield compound (VII),
which is then subjected to a deprotecting reaction to
remove the carboxy-protecting group to yield compound
(III). The reaction of compound (V), its derivative
reactive at the carboxyl group thereof, or a salt thereof
and compound (VI), its derivative reactive at the amino
group thereof, or a salt thereof is carried out in the same
manner as method B.
The deprotecting reaction of compound (VII) to remove
its carboxy-protecting group can be achieved by any common
method of carboxy-protecting group removing reaction, such
as deprotection by hydrolysis, reduction or Lewis acid.
When the carboxy-protecting group is an ester, it can be
removed by hydrolysis or Lewis acid, preferably in the
presence of a base or acid. Preferable bases include
inorganic bases such as alkali metal hydroxides (e.g.,
sodium hydroxide, potassium hydroxide), alkaline earth
metal hydroxides (e.g., magnesium hydroxide, calcium
hydroxide), alkali metal carbonates (e.g., sodium
carbonate, potassium carbonate), alkaline earth metal
carbonates (e.g., magnesium carbonate, calcium carbonate),
alkali metal bicarbonates (e.g., sodium bicarbonate,
potassium bicarbonate), alkali metal acetates (e.g., sodium
acetate, potassium acetate), alkaline earth metal
phosphates (e.g., magnesium phosphate, calcium phosphate),
alkali metal hydrogen phosphates (e.g., disodium hydrogen
phosphate, dipotassium hydrogen phosphate), and organic
bases such as trialkylamines (e.g., trimethylamine,
triethylamine), picoline, N-methylpyrrolidine, N-
methylmorpholine, 1,5-diazabicyclo[4.3.0]non-5-ene, 1,4-
diazabicyclo[2.2.2]non-5-ene and 1,8-diazabicyclo[5.4.0]-7-
undecene. Hydrolysis using a base is often carried out in
water or a hydrophilic organic solvent or mixture thereof.
Preferable acids include organic acids (e.g., formic acid,
acetic acid) and inorganic acids (e.g., hydrochloric acid,
2 ~ O ~ ~ 6
WO96/16079 PCT/~5/02389
hydrobromic acid, sulfuric acid). This hydrolysis is
normally carried out in an organic solvent, water or a
mixture thereof. Reaction temperature, not subject to
limitation, is chosen as appropriate, according to the kind
of carboxy-protecting group and method of deprotection.
Deprotection using a Lewis acid is achieved by reacting
compound (VII) or a salt thereof with a Lewis acid such as
a boron trihalide (e.g., boron trichloride, boron
trifluoride), a titanium tetrahalide (e.g., titanium
tetrachloride, titanium tetrabromide), an aluminum halide
(e.g., aluminum chloride, aluminum bromide) or a
trihaloacetic acid (e.g., trichloroacetic acid,
trifluoroacetic acid). This deprotecting reaction is
preferably carried out in the presence of a cation
capturing agent (e.g., anisole, phenol) and normally
carried out in a solvent which does not interfere with the
reaction, such as a nitroalkane (e.g., nitromethane,
nitroethane), an alkylene halide (e.g., methylene chloride,
ethylene chloride), diethyl ether or carbon disulfide.
These solvents may be used in mixture.
Deprotection by reduction is preferably applied to
removing the protecting groups such as esters of haloalkyls
(e.g., 2-iodoethyl, 2,2,2-trichloroethyl) and esters of
aralkyls (e.g., benzyl). Methods of reduction for this
deprotecting reaction include reduction with a combination
of a metal (e.g., zinc, zinc amalgam) or a chromium
compound salt (e.g., primary chromium chloride, primary
chromium acetate) and an organic or inorganic acid (e.g.,
acetic acid, propionic acid, hydrochloric acid), and
ordinary catalytic reduction in the presence of an ordinary
metal catalyst (e.g., palladium-carbon, Raney nickel).
Although reaction temperature is not subject to limitation,
this reaction is normally carried out under cooling, room
temperature or heating conditions.
Method D
WO 96/16079 ~ 3 6 4 PCT/JP95/02389
~R3(R2)
H2NCHCOOL + R9-COOH
(VIII ) ( IX)
R3(R2) R3(R2)
I Protective group removal
5R9CO-NHCHCOOL ~ R9CO-NHCHCOOH
(X) (III-l)
In the above formulas, the symbols have the same defini-
tions as those shown above.
In this method, compound (IX), its derivative reactive
at the carboxyl group thereof, or a salt thereof is reacted
with compound (VIII), its derivative reactive at the amino
group thereof, or a salt thereof to yield compound (X),
which is then subjected to a deprotecting reaction to
remove its carboxy-protecting group to yield compound (III-
1). This method is carried out in the same manner as
method C.
Method E
0 (VIII) + Rll-SO2Cl
(XI)
R3(R2) R3(R2)
I Protective group removal
RllS02-NHCHCOOL ~ RllS02--NHCHCOOH
(XII) (III-2)
In the above formulas, the symbols have the same defini-
tions as those shown above.
In this method, compound (XI) or a salt thereof is
reacted with compound (VIII) or a salt thereof to yield
compound (XII), which is then subjected to a deprotecting
reaction to remove its carboxy-protecting group to yield
compound (III-2). The reaction of compounds (VIII) and
(XI) is carried out in an appropriate solvent. This
solvent is exemplified by aromatic hydrocarbons such as
benzene, toluene and xylene~ ethers such as dioxane,
tetrahydrofuran and dimethoxyethane, alcohols such as
2 2 ~ ~ 9 6
WO96/16079 47 PCT/JP95/02389
methanol, ethanol and propanol, ethyl acetate,
acetonitrile, pyridine, N,N-dimethylformamide, dimethyl
sulfoxide, chloroform, dichloromethane, 1,2-dichloroethane,
1,1,2,2-tetrachloroethane, acetone, 2-butanone and mixtures
thereof. The reaction of compounds (VIII) and (XI) is
carried out in the presence of an appropriate base,
exemplified by alkali metal salts such as sodium hydroxide,
potassium hydroxide, potassium carbonate, sodium carbonate
and sodium hydrogen carbonate, amines such as pyridine,
triethylamine and N,N-dimethylaniline, sodium hydride and
potassium hydride. The amount of these bases used is
preferably about 1 to 5 mol per mol of compound (VIII).
The reaction is normally carried out at -20 to 150C,
preferably about -10 to 100C. Compound (XII) thus
obtained is subjected to a deprotecting reaction to yield
compound (III-2). This deprotection is carried out in the
same manner as the deprotecting reaction in method C.
Method F
IR3(R2)
H2NCHCOOH + (XI) ~ (III-2)
In the above formulas, the symbols have the same defini-
tions as those shown above.
In this method, compound (XIII) or a salt thereof is
reacted with compound (XI) or a salt thereof to yield
compound (III-2). This sulfonylation is normally carried
out under what is called Schotten Baumann's conditions, in
which amino acid derivative (XIII), prepared as a sodium
salt in an aqueous solution, is reacted with compound (XI)
and then acidified.
W0961l6079 2 ~ O 0 9 ~ 4 - 48 - PCT1~95102389
Method G
R3(R2)
(XIII) + R9-COCl ~ R9Co-NHCHCooH
(XIV) (III-l)
In the above formulas, the symbols have the same defini-
tions as those shown above.
In this method, compound (XIII) or a salt thereof is
reacted with compound (XIV) or a salt thereof to yield
compound (III-l). This acylation is carried out in the
same manner as method F.
Method H
IR3(R )
(XIII) + Rl2-OCOCl ~ (III-4)
In the above formulas, the symbols have the same defini-
tions as those shown above.
In this method, compound (XIII) or a salt thereof is
reacted with compound (XV) or a salt thereof to yield
compound (III-4). This method is carried out in the same
manner as method G.
Method I
(VIII) + R7-NCo
(XVI)
R3(R2) R3(R2)
I Protective group removal
R7NHCo-NHCHCooL ~ R7NHCo-NHCHCooH
(XVII) (III-5)
In the above formulas, the symbols have the same defini-
tions as those shown above.
In this method, compound (VIII) or a salt thereof is
reacted with compound (XVI) to yield compound (XVII), which
is then subjected to a deprotecting reaction to remove its
carboxy-protecting group to yield compound (III-5). The
2 ~ O O 9 ~ ~
WO96tl6079 49 PcT/~155J~&9
reaction of compound (VIII) or a salt thereof and compound
(XVI) is carried out in an appropriate solvent. This
solvent is exemplified by aromatic hydrocarbons such as
benzene, toluene and xylene, ethers such as dioxane,
tetrahydrofuran and dimethoxyethane, ethyl acetate,
acetonitrile, pyridine, N,N-dimethylformamide, dimethyl
sulfoxide, chloroform, dichloromethane,.l,2-dichloroethane,
1,1,2,2-tetrachloroethane, acetone, 2-butanone and mixtures
thereof. The amount of compound (XVI) used is preferably
about 1 to 5 mol per mol of compound (VIII). The reaction
is normally carried out at -20 to 150C, preferably about
-10 to 100C. Compound (XVII) thus obtained is subjected
to a deprotecting reaction to yield compound (III-5). This
deprotection is carried out in the same manner as the
deprotection in method C.
Method J
(VIII) + R-NCS
(XVIII)
R3(R2) R2(R3)
I Protective group removal
R8NHCS--NHCHCOOL ~ R3NHCS-NHCHCooH
(XIX) (III-6)
In the above formulas, the symbols have the same defini-
tions as those shown above.
In this method, compound (VIII) or a salt thereof is
reacted with compound (XVIII) to yield compound (XIX),
which is then subjected to a deprotecting reaction to
remove its carboxy-protecting group to yield compound (III-
6). This reaction is carried out in the same manner asmethod I.
Starting material compound (II) for method A can also
be produced as follows:
Method K
WO96/16079 ~ ~ ~ O ~ 6 4 PCT/~5102389
R3 R2 RlOH
l l
M-(NHCHCO)n~(NHCHCO)m-OH + H2NCHCH-COOL
(XX) (XXI)
R3 R2 RloH Protective
1 1 1 I group removal
M-(NHCHCO)n~(NHCHCO)m-NHCHCH-COOL
(XXII)
R3 R2 RlOH
l l
M-(NHCHCO)n~(NHCHCO)m-NHCHCH-COOH
(XXIII)
R3 R2 RloH Protective
I l l I group removal
M-(NHCHCO) n~ ( NHCHCo)m-NHcHcH-coNHR5R6
(XXIV)
~3 R
l l
H-(N~C~CO) n~ ( NHCHCo)m-NHCHCH-CoNHR5R6 ~ (II)
(XXV)
In the above formulas, M represents an amino-protecting
group; the other symbols have the same definitions as those
shown above.
The amino-protecting group for M is exemplified by
protecting groups in common use in the field of peptide
synthesis, such as oxycarbonyl derivatives, with preference
given to benzyloxycarbonyl.
In this method, compound (XX), its derivative reactive
at the carboxyl group thereof, or a salt thereof is reacted
with compound (XXI), its derivative reactive at the amino
group thereof, or a salt thereof to yield compound (XXII),
which is then subjected to a deprotecting reaction to
remove its carboxyl-protecting group to yield compound
(XXIII). The reaction of compound (XX), its derivative
reactive at the carboxyl group thereof, or a salt thereof
and compound (XXI), its derivative reactive at the amino
group thereof, or a salt thereof is carried out in the same
manner as method B. The carboxyl-protecting group-removing
reaction of compound (XXII) can be carried out by the
method described for method C above. Compound (XXIII), its
~ 2 ~ ~ 9 ~ 4
WO96/16079 - 5l - PCT/~5/02389
derivative reactive at the carboxyl group thereof, or a
salt thereof is reacted with an amine derivative or a salt
thereof to yield compound (XXIV), which is then subjected
to a deprotecting reaction to remove its amino-protecting
group to yield compound (XXV). The reaction of compound
(XXIII), its derivative reactive at the carboxyl group
thereof, or a salt thereof and an amine derivative or a
salt thereof is carried out in the same manner as method B.
In the amino-protecting group-removing reaction of compound
(XXIV), the amino-protecting group can be removed by any
commonly used method of reaction to remove the amino-
protecting group. For example, the benzyloxycarbonyl group
is removed by catalytic reduction in the presence of a
commonly used metal catalyst (e.g., palladium-carbon, Raney
lS nickel). Reaction temperature is not subject to
limitation; the reaction is normally carried out under
cooling, room temperature or heating conditions. Compound
(XXV) is then acylated in the same manner as the reaction
of compounds (VIII) and (IX) in method D or the reaction of
compounds (XIII) and (XIV) in method G, sulfonylated in the
same manner as the reaction of compounds (VIII) and (XI) in
method E, oxycarbonylated in the same manner as the
reaction of compounds (VIII) and (XV) in method H,
carbamoylated in the same manner as the reaction of
compounds (VIII) and (XVI) in method I, and then
thiocarbamoylated in the same manner as the reaction of
compounds (VIII) and (XVIII) in method J, to yield compound
(II).
In the present invention, the compound of general
formula (I), (Ia), (I') or (I") can be administered orally
or non-orally, as formulated at an effective dose with a
physiologically acceptable carrier, in the form of solid
preparations such as tablets, capsules, granules and
powders, or liquid preparations such as syrups and
injectable preparations.
WO96/16079 2 ~ ~ ~ 9 ~ 4 PCT/~5/02389 ~
Pharmaceutically acceptable carriers are various
organic or inorganic carrier substances in common use as
pharmaceutical materials, including excipients, lubricants,
binders and disintegrating agents for solid preparations,
and solvents, dissolution aids, suspending agents,
isotonizing agents, buffers and soothing agents for liquid
preparations. Other pharmaceutical additives such as
preservatives, antioxidants, coloring agents and sweetening
agents may be used as necessary.
Preferable excipients include lactose, sucrose, D-
mannitol, starch, crystalline cellulose and light silicic
anhydride.
Preferable lubricants include magnesium stearate,
calcium stearate, talc and colloidal silica.
Preferable binders include crystalline cellulose,
sucrose, D-mannitol, dextrin, hydroxypropyl cellulose,
hydroxypropylmethyl cellulose and polyvinylpyrrolidone.
Preferable disintegrating agents include starch,
carboxymethyl cellulose, carboxymethyl cellulose calcium,
cross carmelose sodium and carboxymethyl starch sodium.
Preferable solvents include water for injection,
alcohol, propylene glycol, macrogol, sesame oil and corn
oil.
Preferable dissolution aids include polyethylene
glycol, propylene glycol, D-mannitol, benzyl benzoate,
ethanol, tris-aminomethane, cholesterol, triethanolamine,
sodium carbonate and sodium citrate.
Preferable suspending agents include surfactants such
as stearyltriethanolamine, sodium lauryl sulfate,
laurylaminopropionic acid, lecithin, benzalkonium chloride,
benzethonium chloride and monostearic glycerol; and
hydrophilic polymers such as polyvinyl alcohol,
polyvinylpyrrolidone, carboxymethyl cellulose sodium,
methyl cellulose, hydroxymethyl cellulose, hydroxyethyl
cellulose and hydroxypropyl cellulose.
2 ~ 0 ~ ~ ~ 4
WO96/16079 53 PCT1~5/02389
Preferable isotonizing agents include sodium chloride,
glycerol and D-mannitol.
Preferable buffers include buffer solutions of
phosphates, acetates, carbonates and citrates.
Preferable soothing agents include benzyl alcohol.
Preferable preservatives include p-oxybenzoic acid
esters, chlorobutanol, benzyl alcohol, phenethyl alcohol,
dehydroacetic acid and sorbic acid.
Preferable antioxidants include sulfites and ascorbic
acid.
The compound represented by general formula (I), (Ia),
(I') or (I") or a salt thereof can be orally or non-orally
used by inhalation, rectal injection or local
administration. It can be used as a pharmaceutical
composition or preparation (e.g., powders, granules,
tablets, pills, capsules, injectable preparations, syrups,
emulsions, elixirs, suspensions, solutions), which may
contain one or more inventive compounds with
pharmaceutically acceptable carriers (e.g., adjutants,
excipients, shaping agents and/or diluents).
Pharmaceutical compositions can be prepared as
pharmaceutical preparations by ordinary methods. In the
present specification, "non-oral" includes subcutaneous
injection, intravenous injection, intramuscular injection,
intraperitoneal injection and drip infusion. Injectable
preparations, e.g., aqueous or oily suspensions for aseptic
injection, can be prepared by methods known in relevant
fields, using an appropriate dispersing agent or wetting
agent and a suspending agent. The aseptic injectable
preparation thus obtained may be an aseptically injectable
solution or suspension in a diluent or solvent which
permits non-toxic non-oral administration, such as an
aqueous solution. Acceptable vehicles or solvents include
water, Ringer's solution and isotonic saline. It is also
possible to use aseptic non-volatile oils in common use as
solvents or suspending media. For this purpose any non-
-
WO96/16~79 ~ ~ ~ 0 ~ 6 4 - 54 - PCT/~5/02389
volatile oil or fatty acid can be used, including natural,
synthetic or semi-synthetic fatty oils or acids, and
natural, synthetic or semi-synthetic mono- or di- or tri-
glycerides.
Suppositories for rectal administration may be
produced as a mixture of the drug and an appropriate non-
irritative shaping agent, such as cacao butter or
polyethylene glycol, which is solid at normal temperatures
and liquid at intestinal temperatures and melts and
releases the drug in the rectum.
Solid dosage forms for oral administration include the
above-mentioned forms such as powders, granules, tablets,
pills and capsules. In these dosage forms, the active
ingredient compound may be mixed with at least one additive
such as sucrose, lactose, cellulose sugar, mannitol,
maltitol, dextran, starch, agar, alginate, chitin,
chitosan, pectin, gum tragacanth, gum arabic, gelatin,
collagen, casein, albumin, synthetic or semi-synthetic
polymer or glyceride. Such dosage forms may contain
additional additives as usual, including inert diluents,
lubricants such as magnesium stearate, preservatives such
as paraben and sorbic acid, antioxidants such as ascorbic
acid, ~-tocopherol and cysteine ! disintegrating agents,
binders, thickening agents, buffers, sweeteners, flavoring
agents and perfumes. Tablets and pills may be produced
with enteric coating. Liquid dosage forms for oral
administration include pharmaceutically acceptable
emulsions, syrups, elixirs, suspensions and solutions,
which may contain inert diluents, such as water, in common
use in relevant fields.
The dose for a particular patient is determined
according to age, body weight, general health status, sex,
dietary status, administration time, method of
administration, excretion rate, drug combination, severity
of the illness being treated and other factors.
2 ~ ~ ~ 9 6 4
WO96/16079 PCT/J195~a23~9
Since the compound represented by general formula (I),
(Ia), (I') or (I") or a salt thereof possesses potent
cathepsin L inhibitory activity, it excellently suppresses
bone resorption, and can be safely used at low toxicity.
The compounds (Ia), (I), (I') or (I") of the present
invention can therefore be advantageously used to prevent
or treat osteoporosis in m~mm~l S ( e.g., mice, rats,
rabbits, dogs, cats, bovines, swines, humans).
When the compound (Ia), (I), (I') or (I") of the
present invention or a salt thereof is used as a
prophylactic/therapeutic agent for osteoporosis, its daily
dose for an adult (50 kg), varying depending on the patient
condition, body weight, type of compound, route of
administration and other factors, is normally about l to
500 mg, preferably about lO to 500 mg for oral
administration, and about l to 300 mg, preferably about 5
to lO0 mg for non-oral administration.
Best Mode for Carryinq out the Invention
The actions of compounds (Ia), (I), (I') or (I") are
hereinafter described by means of the following
experimental examples.
Experimental Example l
Determination of human cathepsin L inhibitory activity
Purified recombinant human cathepsin L was diluted
with a diluent [0.1% Brij 35 (produced by Sigma
Corporation)] to a concentration of l ~g/ml. To l ~l of
this enzyme dilution, 46 ~l of the diluent, 2 ~l of O.l M
DTT and 25 ~l of an activator/buffer (340 mM sodium
acetate, 60 mM acetic acid, 4 mM disodium EDTA, pH 5.5)
were added. To this mixture, a l ~l sample, previously
diluted to 10-2 M with dimethyl sulfoxide (DMSO), and 25 ~l
of 20 ~M Z-Phe-Arg-NHMec (enzyme substrate solution) were
added, followed by incubation at 30C for lO minutes, after
which lO0 ~l of a reaction stopper (lO0 mM sodium
- - -
WO96/16079 2 ~ O ~ 9 ~ 4 - 56 - PCT/~5102389 ~
monochloroacetate, 30 mM sodium acetate, pH 4.3) was added.
This reaction was carried out on a 96-well fluoroplate
(produced by Labo Systems).
After the reaction was stopped, the fluorescence
intensity of free aminomethylcoumarin was determined at a
wavelength of 450 nm (excitation wavelength = 365 nm),
using a fluorometer FCA tproduced by saxter). For control,
1 ~1 of sample-free DMSO was added instead; the
fluorometric value obtained from this control reaction was
taken as 100% activity. When the residual activity was not
higher than 10%, the sample solution was further diluted
and then assayed for residual activity in the same
procedure as above to obtain the IC50 value. The results
are shown in Table 1.
Table 1
Compound Cathepsin L Inhibitory
(Example No.) Activity [IC50 Value (M)]
1 . 1 X 10-8
7 1.1 x 10-8
9 7.9 x 10-8
13 3.4 x 10-8
14 8.1 x 10-8
1.8 x 10-9
16 2.3 x 10-9
19 0.4 x 10-9
21 0.36 x 10-9
23 3.6 x 10-9
26 1.7 x 10-8
Experimental Example 2
Bone resorption-suppressing action
Bone resorption was measured by the method of Raisz
[Journal of Clinical Investigation, 44, 103-116 (1965)].
Specifically, one Sprague-Dawley rat at 18 days of
22~64
96/16079 PCT/~5/02389
gestation was given 50 ~Ci of 45Ca (calcium isotope, in
CaCl2 solution) by subcutaneous injection. On the
following day, the animal was laparotomized and fetal rats
aseptically removed. Both forearm bones (radius and ulna)
were cut from the body of each fetus under an anatomical
microscope, and connective tissue and cartilage were
removed to the maximum possible extent, to prepare bone
culture samples. Each bone fragment was pre-cultured at
37C for 24 hours in 0.6 ml of a medium (Fitton-Jackson
modification, GIBCO Laboratories, the United States)
prepared by adding bovine serum albumin (final
concentration 2 mg/ml), after which it was transferred to
the same medium as above but containing each compound
(final concentration l0 ~M) and cultured for two more days.
45Ca radioactivity in the medium and 45Ca radioactivity in
the bone were then measured; the percent ratio of 45Ca
released from the bone to the medium was calculated using
the following equation:
Percent ratio of 45Ca released from bone to medium
= (45Ca count in the medium)
x 100
(45Ca count in the medium) + (45Ca count in the bone)
For control, bone fractions from fetuses of the same
litter were cultured for two days in the absence of the
test compound. The mean + standard deviation for the
values from 5 bone fragments in each group were calculated,
and their percent ratios to the control were calculated.
The results are shown in Table 2.
WO96/160792 2 ~ 4 - 58 - PCT/~95/02389
Table 2
Bone Resorption-
Compound suppressing Action
(Example No.)145Ca Release Rate
(Percent to Control)]
2 77
3 82
12 67
14 77
l9 61
26 60
The present invention is hereinafter described in more
detail by means of, but is not limited to, the following
reference examples and working examples. Optical rotation
was determined at 20 to 25C. The room temperature ranged
from about 15C to about 25C.
In the following reference examples and working
examples, the component amino acids used are of the L-
configuration, unless otherwise stated. When shown by
abbreviations, their notation is in accordance with the
IUPAC (International Union of Pure and Applied Chemistry)-
IUB (International Union of Biochemistry) Biochemical
Nomenclature, e.g., Gly for glycine, Leu for leucine and
Ile for isoleucine.
Example l
N-Benzyloxycarbonyl-L-isoleucyl-(2R~3s)-3-amino-2
hydroxy-4-phenylbutyric acid benzylamide (5.0 g) was
dissolved in a mixed solvent of dimethyl sulfoxide (DMSO)
(20 ml) and toluene (60 ml). To this solution, l-ethyl-3-
(3-dimethylaminopropyl)carbodiimide hydrochloride
(WSCD-HCl) (5.4 g) and pyridinium trifluoroacetate (1.6 g)
were added at room temperature. After l hour of stirring,
the reaction mixture was poured over ice water and
2 2 G ~ ~ ~ 4
WO96116079 59 PCT1~951~2~9
extracted with ethyl acetate. The organic layer was washed
by sequential additions of an aqueous citric acid solution,
water, aqueous NaHCO3 and saline and dried (MgSO4). After
the solvent was distilled off under reduced pressure, the
resulting light yellow solid was washed with ethyl acetate-
hexane to yield a white solid of N-benzyloxycarbonyl-L-
isoleucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide (4.15 g, 83%).
Melting point: 201-202C
[~]D = -33.5 (c 0.35, MeOH) (20C)
Elemental analysis (for C31H35N3Os)
Calculated: C, 70.30; H, 6.66; N, 7.93
Found : C, 70.12; H, 6.48; N, 8.12
Example 2
The same procedure as in Example 1 was followed to
yield N-[(3S)-3-dibenzylacetylamino-2-oxo-4-phenylbutyryl]-
L-leucine methyl ester.
Melting point: 120-121C
[a]D = -7.5 (c 0.48, MeOH) (20C)
Example 3
The same procedure as in Example 1 was followed to
yield N-[(3S)-3-dibenzylacetylamino-2-oxo-4-phenylbutyryl]-
L-leucine benzylamide.
Melting point: 172-174C
[a]Hg = +37.5 (c 0.31, DMSO) (20C)
Example 4
The same procedure as in Example 1 was followed to
yield (3s)-3-dibenzylacetylamino-2-oxo-4-phenylbutyric acid
benzylamide.
Melting point: 157-158C
[a]D = -24.6 (c 0.50, MeOH)
Example 5
` The same procedure as in Example 1 was followed to
yield N-[(3S)-3-dibenzylacetylamino-2-oxo-4-phenylbutyryl]-
~-methyl-L-aspartic acid benzylamide.
Melting point: 178-179C
W096,l6079 2 ~ 6 4 - 60 - PCT/~5/02389 ~
~]Hg = +54.4 (c 0.60, DMSO) (20C)
Example 6
N-[(3S)-3-dibenzylacetylamino-2-oxo-4-phenylbutyryl]-
~-methyl-L-aspartic acid benzylamide (0.30 g) was dissolved
in a mixed solvent of tetrahydrofuran (THF) (6 ml) and
methanol (4 ml). To this solution, an a~ueous solution of
potassium hydroxide (65 mg) was added under ice cooling
conditions. After stirring under ice cooling conditions
for 5 hours, the reaction mixture was acidified with 1 N
hydrochloric acid and extracted with ethyl acetate. After
the organic layer was dried ~MgSO4), the solvent was
distilled off under reduced pressure. The resulting light
yellow oily substance crystallized from ethyl acetate-
hexane to yield a-white crystal of N-[(3S)-3-
dibenzylacetylamino-2-oxo-4-phenylbutyryl]-L-aspartic acid
benzylamide (0.18 g, 61%).
Melting point: 178-179C
[~]Hg = +54.4 (c 0.60, DMSO) (20C)
Example 7
The same procedure as in Example 1 was followed to
yield a colorless crystal of N-(l-naphthalenesulfonyl)-L-
isoleucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide.
Melting point: 183-185C
[a]D = +28.5 (c 0.35, DMSO) (20C)
Example 8
The same procedure as in Example 1 was followed to
yield a white solid of N-benzyloxycarbonyl-L-isoleucyl-
(3S)-3-amino-2-oxo-4-phenylbutyric acid 2-
pyridylmethylamide.
Melting point: 164-165C
[~]~g = +31.5 (c 0.60, DMSO) (20C)
Example 9
The same procedure as in Example 1 was followed to
yield a white solid of N-benzyloxycarbonyl-~-isoleucyl-
7 2 ~
~ wos6/16079 ~ 61 - PCT/~5/02389
.
(3S)-3-amino-2-oxo-4-phenylbutyric acid 4-
pyridylmethylamide.
Melting point: 204-205C
[a]D = -1.4 (c 0.74, DMSO) (20C)
Example 10
The same procedure as in Example 1 was followed to
yield a light yellow solid of N-benzyloxycarbonyl-L-
isoleucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid p-
diethylphosphonomethylphenylamide.
Melting point: 139-140C
[~]D = - 6.4 (c 0.88, DMSO) (20C)
Example 11
The same procedure as in Example 1 was followed to
yield a white solid of N-benzyloxycarbonyl-L-isoleucyl-
(3S)-3-amino-2-oxo-4-phenylbutyric acid isobutylamide.
Melting point: 215-216C
[a]D = +9.9 (c 1.00, DMSO) (2boC)
Example 12
The same procedure as in Example 1 was followed to
yield a white solid of N-benzyloxycarbonyl-L-isoleucyl-
(3S)-3-amino-2-oxo-4-phenylbutyric acid 3-(2-oxopyrrolidin-
l-yl)propylamide.
Melting point: 268-269C
[a]D = +2.5 (c 0.93, DMSO) (20C)
Example 13
The same procedure as in Example 1 was followed to
yield a white crystal of N-(quinoline-2-carbonyl)-L-
isoleucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide.
Melting point: 181-182C
[a]D = +34.5 (c 0.79, DMSO) (20C)
Example 14
The same procedure as in Example 1 was followed to
yield a white solid of N-(p-diethylphosphonomethyl-
cinnamoyl)-L-isoleucyl-(3S)-3-amino-2-oxo-4-phenylbutyric
acid benzylamide.
-
W096/16079 2 2 a o ~ 6 4 - 62 - PCT/~95/02389
Melting point: 207-209C
[a]D = +22.7 (c 0.893, DMSO) (20C)
Example 15
The same procedure as in Example 1 was followed to
yield a colorless crystal of N-(l-naphthalenesulfonyl)-L-
phenylalanyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide.
Melting point: 189-191C
[a]D = +11.9 (c 0.87, DMSO) (20C)
Example 16
The same procedure as in Example 1 was followed to
yield a colorless crystal of N-(l-naphthalenesulfonyl)-L-
isoleucyl-(3S)-3-amino-2-oxo-4-phenylbutyric acid 4-
pyridylmethylamide.
Melting point: 162-163C
[a]D = +16.9 (c 0.38, DMSO) (20C)
Examples 17-27
By substantially the same procedure as in Example 1,
compounds shown in Table 3 were produced.
2 2 ~ 4
WO96/16079 ~ 63 -- PCT/JI55l(~23&9
Table 3
No of (mocp) ation Optical
solvent [a]D
17 CONHCH2Ph 239-241 DMF-H2O +10;6(c=
Cbz-L-Leu-L-Phe-CONH~o 169-171 - 10.3(c=
18 (S) 0.79,DMSO)
1 0 Oq 178- - 3)
19HN`N~J`co-L-Leu-L-phe-coNHcH2ph + 48.7(c =
0.74,DMSO)
201-Nap-L-Leu-L-Phe-CONHCH2Ph 172-173 - + 5.7(c =
0.85,DMSO)
IO 199-200 - +11.1(C=
21 HN~3` 0.38,DMSO)
o CO-L-Leu-L-Phe-CONHCH2?h
~,Ph O
2 0 222-Qnl-L-Leu-L-Phe-CONH ~CH2~T NH
O
212- - + 37.0(c =
2132) 0.84,DMSO)
23 ~ OCH3
HN~lco-L-Leu-L-phe-coNHcH2 ~-OCH3
179- -6.5(c =
1802) 0.78,DMSO)
161-162 - -153.7(c=
24 OCH3 0.68,CH30H)
1-Nap-L-Leu-L-phe-coNHcH2 ~ -OCH3
W096,l6079 - 2 ~ O ~ 9 ~ 4 - 64 - PCT1JP95/02389
No. of Recrystali- Optical
~,y~mrle (C) zation Rotation
solvent [a]D
Cbz-~Leu-L-Leu-L-ph~-coNHcH2ph 200-201 - +6.9(c=
0.53,DMSO)
207-208 - + 49.6(c =
26 2-Qnl-L-Leu-~Leu-L-Phe-CONHCH2P l 0.75,DMSO)
0~ 165- ~ -53.2(c=
27 `~J`co-L-Ile-L-phe-coNHcH2ph 0.67,DMSO)
1) 1/4hydrate, 2) 1/2hydrate, 3) [a] Hg value
Val=valine, Phe=phenylalanine, Leu=leucine, Ile=isoleucine,
5 Ph=phenyl, Cbz=benzyloxycarbonyl, l-Nap=l-
naphthalenesulfonyl, 2-Qnl=quinoline-2-carbonyl, DMF=N,N-
dimethylformamide, DMSO=dimethyl sulfoxide
Reference Example 1
N-benzyloxycarbonyl-L-isoleucyl-(2R,3S)-3-amino-2-
hydroxy-4-phenylbutyric acid benzylamide (4.7 g) and 5% Pd-
C (2. 5 g) were added to a mixed solvent of tetrahydrofuran
(THF) (20 ml) and methanol (20 ml), followed by stirring at
room temperature for 1 hour in a hydrogen atmosphere.
5 After the catalyst was filtered off, the filtrate was
concentrated under reduced pressure. The resulting residue
was dissolved in N,N-dimethylformamide (DMF) (30 ml). To
this solution, a-naphthalenesulfonyl chloride (2.1 g) and
N,N-dimethylaminopyridine (1.2 g) were added under ice
cooling conditions. After 15 hours of stirring under ice
cooling conditions, the reaction mixture was poured over
ice-water and extracted with ethyl acetate. The organic
layer was washed by sequential additions of an aqueous
citric acid solution, water, an aqueous NaHCO3 solution and
saline and dried (MgSO4). After the solvent was distilled
~ 2 ~
WO96tl6079 - 65 - PCT/~5/02389
off under reduced pressure, the resulting residue was
washed with ethyl acetate to yield a colorless crystal of
N-(l-naphthalenesulfonyl)-L-isoleucyl-(2R,3S)-3-amino-2-
hydroxy-4-phenylbutyric acid benzylamide (4.75 g).
Melting point: 178-179C
[a]D = +5.7 (c 0.52, DMSO) (20C)
Elemental analysis (for C33H37N305S)
Calculated: C, 67.44; H, 6.35; N, 7.15
Found : C, 67.22; H, 6.45; N, 7.15
Reference Example 2
The same procedure as in Reference Example 1 was
followed to yield a colorless crystal of N-(l-
naphthalenesulfonyl)-L-phenylalanyl-(2R~3s)-3-amino-2
hydroxy-4-phenylbutyric acid benzylamide.
Melting point: 210-211C
[~]D = - 40.1 (c 0.76, DMSO) (20C)
Reference Example 3
The same procedure as in Reference Example 1 was
followed to yield a colorless crystal of N-(l-
naphthalenesulfonyl)-L-isoleucyl-(2R,3S)-3-amino-2-hydroxy-
4-phenylbutyric acid 4-pyridylmethylamide.
Melting point: 210-211C
[~]D = -3.2 (c 0.21, DMSO) (20C)
Reference Example 4
N-Benzyloxycarbonyl-L-isoleucyl-(2R,3S)-3-amino-2-
hydroxy-4-phenylbutyric acid benzylamide (4.5 g) and 5% Pd-
C (2.2 g) were added to a mixed solvent of tetrahydrofuran
(THF) (30 ml) and methanol (30 ml), followed by stirring at
room temperature for 2 hours in a hydrogen atmosphere.
After the catalyst was filtered off, the filtrate was
concentrated under reduced pressure. The resulting residue
and quinoline-2-carboxylic acid (1.54 g) were dissolved in
N,N-dimethylformamide (DMF) (40 ml). To this solution, 1-
hydroxybenzotriazole (HOBt) (1.43 g) and 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (WSCD HCl)
(1.79 g) were added under ice cooling conditions. After
WO96tl6079 ~ ~ ~ 0 9 ~ 4 - 66 - PCT/JI5S~ 9
stirring at room temperature for 16 hours, the reaction
mixture was poured over ice-water and extracted with ethyl
acetate. The organic layer was washed by sequential
additions of an aqueous citric acid solution, water, an
aqueous Na~CO3 solution and saline and dried (MgSO4).
After the solvent was distilled off under reduced pressure,
the resulting residue was subjected to silica gel column
chromatography and eluted with ethyl acetate-hexane (4:1)
to yield a colorless crystal of N-(quinoline-2-carbonyl)-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid
benzylamide (4.2 g).
Melting point: 157-158C
[¢]D = +2.6 (c 0.87, MeOH) (20C)
Elemental analysis (for C33H36N4O4)
Calculated: C, 71.72; H, 6.57; N, 10.14
Found : C, 71.68; H, 6.58; N, 10.05
Reference Example 5
The same procedure as in Reference Example 4 was
followed to yield a light yellow crystal of N-(4-
diethylphosphonomethylcinnamoyl)-L-isoleucyl-(2R~3s)-3
amino-2-hydroxy-4-phenylbutyric acid benzylamide.
Melting point: 153-154C
~]D = - 14-8 (C 0.76, MeOH) (20C)
Reference Example 6
To a solution of N-benzyloxycarbonyl-(2R,3S)-3-amino-
2-hydroxy-4-phenylbutyric acid (3.0 g) and L-isoleucine
methyl ester hydrochloride (1.74 g) in N,N-
dimethylformamide (DMF), triethylamine (1.4 ml), 1-
hydroxybenzotriazole (HOBt) (1.54 g) and 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide hydrochloride (WSCD HCl)
(1.92 g) were added under ice cooling conditions. After
stirring at room temperature for 16 hours, the reaction
mixture was poured over ice-water and extracted with ethyl
acetate. The organic layer was washed by sequential
additions of an aqueous citric acid solution, water, an
aqueous NaHCO3 solution and saline and dried (MgSO4).
2 ~ 4
WO96/16079 - 67 - PCT/~5/02389
After the solvent was distilled off under reduced pressure,
the resulting residue was subjected to silica gel column
chromatography and eluted with ethyl acetate-hexane (2:1)
to yield a colorless crystal of N-[(2R,3S)-2-
benzyloxycarbonylamino-3-hydroxy-4-phenylbutyryl]-L-
isoleucine methyl ester (2.3 g).
Melting point: 120-121C
[a]D = -58.5 (c 0.78, MeOH) ~20C)
Elemental analysis (for C25H32N26)
Calculated: C, 65.77; H, 7.06; N, 6.14
Found : C, 65.78; H, 6.95; N, 6.08
Reference Example 7
The same procedure as in Reference Example 4 was
followed to yield a colorless crystal of N-[(2R,3S)-2-
dibenzylacetylamino-3-hydroxy-4-phenylbutyryl]-L-isoleucine
methyl ester.
Melting point: 130-131C
[~]D = -47.8 (c 0.63, MeOH) (20C)
Reference Example 8
The same procedure as in Reference Example 4 was
followed to yield a colorless needle of (2R,3S)-2-
dibenzylacetylamino-3-hydroxy-4-phenylbutyric acid methyl
ester.
Melting point: 107-108C
[~]D = -84.8 (c 0.53, MeOH) (20C)
Reference Example 9
To a solution of (2R,3S~-2-dibenzylacetylamino-3-
hydroxy-4-phenylbutyric acid methyl ester (4.3 g) in a
mixed solvent of tetrahydrofuran (THF) (20 ml) and methanol
(10 ml), an aqueous solution of potassium hydroxide (1.2 g)
was added dropwise under ice cooling conditions. After
stirring under ice cooling conditions for 6 hours, the
reaction mixture was acidified with 1 N hydrochloric acid
and extracted with ethyl acetate. The organic layer was
washed with saline and dried (MgSO4). After the solvent
was distilled off under reduced pressure, the resulting
W096/16079 2 2 G O ~ 6 4 - 68 - PCT/J155~'~23~9
white solid was recrystallized from ethyl acetate-hexane to
yield a colorless crystal of (2R,3S)-2-dibenzylacetylamino-
3-hydroxy-4-phenylbutyric acid (3.0 g).
Melting point: 128-130C
~a]D = -60.3 (c 0.38, CHC13) (20C)
Elemental analysis (for C26H27No4-l/4H2o)
Calculated: C, 74.00; H, 6.57; N, 3.32
Found : C, 74.09; H, 6.50; N, 3.17
Reference Example 10
The same procedure as in Reference Example 6 was
followed to yield a colorless crystal of (2R,3S)-2-
dibenzylacetylamino-3-hydroxy-4-phenylbutyric acid
benzylamide.
Melting point: 148-149C
[a]D = -15.7 (c 0.99, MeOH) (20C)
Reference Example 11
The same procedure as in Reference Example 9 was
followed to yield a colorless crystal of N-[(2R,3S)-2-
dibenzylacetylamino-3-hydroxy-4-phenylbutyryl]-L-
isoleuclne.
Melting point: 93-94C
[a]D = -35.9 (c 0.65, DMSO) (20C)
Reference Example 12
The same procedure as in Reference Example 6 was
followed to yield a colorless crystal of N-~(2R,3S)-2-
dibenzylacetylamino-3-hydroxy-4-phenylbutyryl]-L-isoleucine
benzylamide.
Melting point: 156-158C
[a]D = -49.5 (c 0.61, MeOH) (20C)
Reference Example 13
The same procedure as in Reference Example 4 was
followed to yield a colorless crystal of N-
benzyloxycarbonyl-L-isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-
phenylbutyric acid methyl ester.
Melting point: 128-130C
[ajD = -77.1 (c 0.36, DMSO) (20C)
2 2 ~ a ~ ~ 4
W096/1607s PCT/~5/02389
Reference Example 14
The same procedure as in Reference Example 9 was
followed to yield a white solid of N-benzyloxycarbonyl-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid.
Melting point: 180-181C
[~]D = -64.3 (c 0.43, DMSO) (20C)
Reference Example 15
The same procedure as in Reference Example 6 was
followed to yield a white solid of N-benzyloxycarbonyl-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid
benzylamide.
Melting point: 178-179C
[~]D = -21.8 (c 0.52, MeOH) (20C)
Reference Example 16
The same procedure as in Reference Example 6 was
followed to yield a white solid of N-benzyloxycarbonyl-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid 2-
pyridylmethylamide.
Melting point: 156-157C
[~]D = -22.2 (c 0.47, DMSO) (20C)
Reference Example 17
The same procedure as in Reference Example 6 was
followed to yield a white solid of N-benzyloxycarbonyl-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid 4-
pyridylmethylamide.
Melting point: 223-225C
[~]D = - 21.5 (c 0.60, DMSO) (20C)
Reference Example 18
The same procedure as in Reference Example 6 was
30- followed to yield a white solid of N-benzyloxycarbOnyl-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid 4-
diethylphosphonomethylphenylamide.
Melting point: 145-146C
[~]D = -31.1 (c 0.89, DMSO) (20C)
Reference Example 19
WO96/16079 ~ ~ ~ ~ ~ 6 4 PCT/~5/02389
The same procedure as in Reference Example 6 was
followed to yield a white solid of N-benzyloxycarbonyl-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid
isobutylamide.
Melting point: 185-186C
[a]D = -40.7 (c 0.57, DMSO) (20C)
Reference Example 20
The same procedure as in Reference Example 6 was
followed to yield a white solid of N-benzyloxycarbonyl-L-
isoleucyl-(2R,3S)-3-amino-2-hydroxy-4-phenylbutyric acid 3-
(2-oxopyrrolidin-1-yl)propylamide.
Melting point: 153-154C
~]D = -35.2 (c 0.59, MeOH) (20C)
Reference Example 21
L-Phenylalaninol (15.1 g) and triethylamine (14.7 ml)
were added to a mixed solvent of dichloromethane (50 ml)
and tetrahydrofuran (THF) (50 ml). To this suspension,
ethyl chloroformate (9.5 ml) was added dro-pwsie under ice
cooling conditions. After stirring at room temperature for
1 hour, the reaction mixture was concentrated. The residue
was diluted with ethyl acetate and washed with water and
saline, and dried (MgSO4). After the solvent was distilled
off under reduced pressure, the resulting residue was
dissolved in a mixed solvent of dimethyl sulfoxide (DMSO)
(100 ml) and dichloromethane (50 ml). To this solution,
triethylamine (35 ml) was added and cooled with ice. After
a solution of sulfur trioxide-pyridine complex (40 g) in
dimethyl sulfoxide (DMSO) (160 ml) was added dropwise, the
reaction mixture was stirred at the same temperature for 2
hours. The reaction mixture was poured over ice-water and
extracted with ethyl acetate. The organic layer was washed
by sequential additions of an aqueous citric acid solution,
water and an aqueous NaHCO3 solution, and dried (MgSO4).
After the solvent was distilled off under reduced pressure,
the resulting yellow oily substance was added to a mixture
of dichloromethane (300 ml) and water (300 ml). Sodium
- 1
wo96ll6o7s 7 PCT/J~ 23~9
cyanide (5.3 g), acetic anhydride (10.3 ml) and
benzyltributylammonium chloride (7.5 g) were then added,
followed by vigorous stirring at room temperature for 5
hours. The organic layer was separated, washed with brine
and dried (MgSO4). After the solvent was distilled off
under reduced pressure, the resulting yellow oily substance
was subjected to silica gel column chromatography and
eluted with ethyl acetate-hexane (1:2) to yield a light
yellow oily substance of a diastereomeric mixture (R:S =
5:2) of (2S)-l-cyano-2-ethoxycarbonylamino-3-phenylpropyl
acetate (19.9 g).
lH-NMR (~ ppm in CDC13): 1.21 & 1.26 (3H, each t, J=7.0
Hz), 2.13 & 2.17 (3H, each s), 2.8-3.2 (2H, m), 4.0-4.2
(2H, m), 4.2-4.5 (lH, m), 4.7-5.0 (lH, m), 5.38 & 5.40 (lH,
each d, J=5.8 Hz), 7.1-7.4 (5H, m)
Reference Example 22
(2S)-l-cyano-2-ethoxycarbonylamino-3-phenylpropyl
acetate (30 g) was added to 6 N hydrochloric acid (140 ml),
followed by refluxing for 3 days. After the reaction
mixture was concentrated, the residue was added to a 2~
hydrochloric acid solution in methanol (200 ml), followed
by refluxing for 2 days. After the solvent was distilled
off under reduced pressure, the resulting residue was
dissolved in water. After being washed with ethyl acetate,
this aqueous solution was turned basic with NaHCO3. The
water layer was twice extracted with chloroform; the
resulting organic layers were combined and dried (MgSO4).
After the solvent was distilled off under reduced pressure,
the resulting brown solid was recrystallized from ethyl
acetate-hexane to yield a colorless needle of (3S,2R)-3-
amino-2-hydroxy-4-phenylbutyric acid methyl ester (9.0 g).
Melting point: 108-109C
[~]D = -34.2 (c 0.60, MeOH) (20C)
Reference Examples 23-31
By substantially the same procedure as in Reference
Example 4, compounds shown in Table 4 were produced.
Wo 96/16079 ; 2 2 0 0 ~ 6 4 -- 7 2 -- PCT/Jl 95,~23~9
Table 4
R4-Q-NH~CONH-R5
OH
NRoEof. R4-Q- R~ (mocp) t~;D
10 23 (C3H7)2CH-L-Val- -CH2Ph 203- -30.3(c=
2041) 0.81,DMSO)
0~ 198-199 -27.1(c=
24 ~lcO-L-Leu- -CH2Ph 0.69,DMSO)
O 178- -13.1(c=
15 25 HNJ~C -CH2Ph 1792) 0.78,DMSO)
o ~H O-L-Leu-
26 Cbz-L-Leu- ~,Ph O 228- -32.5(c=
~CH2~T NH 2301) 0.57,DMSO)
~,Ph O 164- +3.1(c=
27 2-Qnl-L-Leu- ~CH2~ NH 1651) 0.78,DMSO)
O
~ OCH3 206- -51.9(c=
28 HN~lCO-L-Leu- --CH2~CH3 2071) 0.51,CH30H)
29 Cbz-L-Leu-L-Leu- -CH2Ph 215- -32.2(c=
2172) 0.83,DMSO)
2-Qnl-L-Leu-L-Leu- -CH2Ph 127-128 + 14.8(c=
0.58,CH30H)
31 O~ 209-210 -15.2(c=
CO-L-Ile- -CH2Ph 0.45,DMSO)
-
~ 2 ~ Q ~ ~ 4
096/16079
PCT/~5/02389
1) 1/2hydrate, 2) 1/4hydrate
Val=valine, Leu=leucine, Ile=isoleucine,
DMSO=dimethylsulfoxide, Ph=phenyl, Cbz=benzyloxycarbonyl,
2-Qnl=quinoline-2-carbonyl
Reference Example 32
To a solution of (3S,2R)-3-amino-2-hydroxy-4-
phenylbutyric acid methyl ester (3.3 g) and N-
benzyloxycarbonyl-L-valine (4.2 g) in N,N-dimethylformamide
(DMF) (40 ml), l-hydroxybenzotriazole (HOBt) (2.7 g) and 1-
ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
(WSCDHCl) (3.4 g) were added under ice cooling conditions.
After stirring at room temperature for 18 hours, the
reaction mixture was poured over ice-water and extracted
with ethyl acetate. The ethyl acetate layer was washed by
sequential additions of water, an aqueous citric acid
solution, water, an aqueous NaHC03 solution and saline and
dried (MgSO4). After the solvent was distilled off under
reduced pressure, the resulting solid was filtered and
washed with ethyl acetate-hexane to yield (2R,3S)-3-[N-[N-
benzyloxycarbonyl-L-valyl]amino]-2-hydroxy-4-phenylbutyric
acid methyl ester.
Melting point: 131-132C
[~]D = - 98.3 (c=0.33, CH30H).
Reference Examples 33-37
By substantially the same procedure as in Reference
Example 32, compounds shown in Table 5 were produced.
W096/16079 ~ ~ ~ O ~ 6 4 PCT1~5/02389
Table 5
R4-Q-NH~CO-A
OH
Optical
NRoEoxf R4-Q- A m.p.(c) Rotation
tCr]D
33 Cbz-~Val--NHCH2Ph 171 - 172 -21.0(c=
0.87,CH30H)
34 Cbz-L-Leu- -OCH3 107 - 108 -101.5(c=
0.94,CH30H)
Cbz-L-Leu-
l 5 -52.8(c =
-NH ~O 201 - 202 0.77,DMSO)
36 Cbz-L-Leu--NHCH2Ph 173 - 174 -27.7(c=
2 0 0.78,DMSO)
37 Cbz-L-Leu- OCH3 -27.1(c=
-NHCH2 ~OCH3 142-143 0.80,CH30H)
Val=valine, Leu=leucine, Ph=phenyl, Cbz=benzyloxycarbonyl,
DMSO=dimethyl sulfoxide
Reference Examples 38 and 39
By substantially the same procedure as in Reference
Example 9, compounds shown in Table 6 were produced.
,
2 2 0 Q ~ ~ 4
W096/16079 PCT/J155~ 89
Table 6
R4-Q-NH~OOH
OH
No. of R4-Q- m.p.Optical Rotation
R. Ex. (C) [a]D
38 Cbz-L-Val- 188-189-63.7(c=0.87,CH30H)
39 Cbz-~Leu- 141-142-89.6(c=0.8~,CH30H)
Val=valine, Leu=leucine, Cbz=benzyloxycarbonyl,
DMSO=dimethyl sulfoxide
Reference Examples 40 and 41
By substantially the same procedure as in Reference
Example 1, compounds shown in Table 7 were produced.
Table 7
~S ~
02-~Leu-NH ~ ONH-R~
OH
No. of R5 m.p. Optical Rotation
R.Ex. (C) [a3D
benzyl 181-182 -49.0(c=0.74, dimethyl
sulfoxide )
413,4-dimethoxybenzyl 102-1031) -135.1(c=0.62,methanol)
1) 1/2hydrate
Reference Example 42
To a solution of N-benzyloxycarbonyl-L-phenylalaninol
(2.0 g), triphenylphosphine (Ph3P) (1.9 g), hydantoin (0.71
35 g) and tetrahydrofuran (THF) (50 ml), diethyl
azodicarboxylate (DEAD) (1.24 g) was added dropwise under
-
Wo96/l6079~ 2 2 0 0 ~ ~ 4 - 76 - PCT/~5/02389
ice cooling conditions. After stirring at room temperature
for 16 hours, the reaction mixture was poured over ethyl
acetate and washed by sequential additions of water, an
aqueous NaHC03 solution and brine, and dried (MgS04).
After the solvent was distilled off under reduced pressure,
the resulting residue was subjected to silica gel column
chromatography and eluted with ethyl acetate-hexane (3:1,
v/v) to yield l-[(2S)-2-(N-benzyloxycarbonylamino)-3-
phenylpropyl]hydantoin (1.15 g, 44%).
Melting point: 159-160C
~a]D = +15 (c=0.79, CH30H).
Preparation Examples
A cathepsin L inhibitor comprising inventive compound
(I) or (Ia) or a salt thereof as an active ingredient can,
for example, be produced with the following formulations:
1. Capsules
(1) N-(p-diethylphosphonomethylcinnamoyl)-
L-isoleucyl-(3S)-3-amino-2-oxo-4-
phenylbutyric acid benzylamide 10 mg
(2) Lactose 90 mg
(3) Microcrystalline cellulose 70 mg
(4) Magnesium stearate 10 mg
Total 180 mg per capsule
Components (1), (2) and (3) and a half portion of component(4) are mixed and granulated. To these granules, the
remaining portion of component (4) is added, and the whole
mixture is packed in a gelatin capsule.
2. Tablets
(1) N-benzyloxycarbonyl-L-isoleucyl-
(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide 10 mg
(2) Lactose 35 mg
(3) Corn starch 150 mg
35 (4) Microcrystalline cellulose 30 mg
(5) Magnesium stearate 5 mg
2 2 ~ 4
WO96/16079 77 PCT1~5/02389
Total 230 mg per tablet
Components (1), (2) and (3), a two-third portion of
component (4) and a half portion of component (5) are mixed
and granulated. To these granules, the remaining portions
of components (4) and (5) are added, and the whole mixture
is tableted by compressive tableting.
3. Injectable preparation
(1) N-(quinoline-2-carbonyl)-L-
isoleucyl-(3S)-3-amino-2-oxo-4-
phenylbutyric acid benzylamide 10 mg
(2) Inositol 100 mg
(3) Benzyl alcohol 20 mg
Total 130 mg per ampule
Components (1), (2) and (3) are dissolved in distilled
water for in]ection to a final quantity of 2 ml, and the
solution is packed in an ampule. The entire procedure is
performed aseptically.
4. Capsules
(1) N-(l-naphthalenesulfonyl)-L-isoleucyl-
(3S)-3-amino-2-oxo-4-phenylbutyric acid
4-pyridylmethylamide 10 mg
(2) Lactose 90 mg
(3) Microcrystalline cellulose 70 mg
25 (4) Magnesium stearate 10 mg
Total 180 mg per capsule
Components (1), (2) and (3) and a half portion of component
(4) are mixed and granulated. To these granules, the
remaining portion of component (4) is added, and the whole
mixture is packed in a gelatin capsule.
5. Tablets
(1) N-(l-naphthalenesulfonyl)-L-phenylalanyl-
(3S)-3-amino-2-oxo-4-phenylbutyric acid
benzylamide 10 mg
(2) Lactose 35 mg
WO96/16079 ~ 9 6 4 78
PCT/Jl551~3~9
(3) Corn starch 150 mg
(4) Microcrystalline cellulose 30 mg
(5) Magnesium stearate 5 mg
Total 230 mg per tablet
Components (1), (2) and (3), a two-third portion of
component 4 and a half portion of component (5) are mixed
and granulated. To these granules, the remaining portions
of components (4) and (5) are added, and the whole mixture
is tableted by compressive tableting.
6. Injectable preparation
(1) N-benzyloxycarbonyl-L-isoleucyl-(3S)-
3-amino-2-oxo-4-phenylbutyric acid
isobutylamide 10 mg
(2) Inositol 100 mg
15 (3) Benzyl alcohol 20 mg
Total 130 mg per ampule
Components (1), (2) and (3) are dissolved in distilled
water for injection to a final quantity of 2 ml, and the
solution is packed in an ampule. The entire procedure is
performed aseptically.
Industrial Applicability
Since the compound represented by general formula (I),
(Ia), (I') or (I") or a salt thereof possesses potent
cathepsin L inhibitory activity, it excellently suppresses
bone resorption,;and it can therefore be advantageously
used to prevent or treat osteoporosis in mammals.