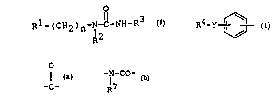Note: Descriptions are shown in the official language in which they were submitted.
~ WO96/10559 ~2 ~ ~ 9 8 ~ PCT1~5101982
DESCRIPTION
UREA DERI~ATIVES AND THEIR USEAS ACAT-INHIBITORS
TECHNICAL FIELD
This invention relates to new urea derivatives and
pharmaceutically acceptable salts thereof which are useful
as a medicament.
BACKGROUN~ ART
Some urea derivatives have been known as acyl-coA :
cholesterol acyltransferase en7yme (hereinafter, ACAT)
inhibitors, for example, in U.S. Pztent Nos. 4,473,579 and
4,623,662, EP Patent Application Publication NGS. 0354994,
0399a22 and 0512570 and PCT International Publication Nos.
WO 91/13871, WO 93/24458 and Wo 34/26738.
DISCLOSUR~ OF INVENTION
This invention relaies to new urea derivatives and
pharmaceutically acceptable salts thereof which have an
inhibitory activity against ACAT and an advantage of good
absorption into blood on oral administration, to processes
for the preparation thereof, to a pharmaceutical
composition comprising the same and to a method for the
prevertion and/or treatment of hypercholesterolemia,
hvperlipidemia, atherosclerosis or diseases caused thereby.
One object of this invention is to provide new and
useful urea derivatives and pharmaceutically acceptable
salts which possess an inhibitory activity against ACAT.
~nother object of this invention is to provide
processes for pre~aration of sald urea derivatives and
salts thereof.
A further object of this invenlion is to provide a
pharmaceutical composition comprising, as an active
ingredient, said urea derivatives and pharmaceutically
WO96/10~S9 2 ~ 0 ~ 9 8 ~ PCTl~95/01982 ~
acceptable salt thereof.
Still further object of this invention is to provide a
therapeutic method for the prevention and/or treatment of
hypercholesterolemia, hyperllpidemia, atherosclerosis or
diseases caused thereby in human beings or animals, using
said urea derivatives and pharmaceutically acceptable salts
thereof.
High levels of blood cholestercl and blood lipids are
conditions which are involved in the onset of
lG atherosclerosis.
It is well known that inhibition of ACAT-catalyzed
cholesterol esterification could lead to diminish
intestinal absorption of cholesterol as well as a decrease
in the intracellular accumulation of cholesterol esters ir
the intima of the arterial wall. Therefore, ACAT
inhibitors are useful for the prevention and/or treatment
of hypercholesterolemia, hyperlipidemia, atherosclerosis of
diseases caused thereby such as cardiac insufficiency (e.g.
angina pectoris, myocardial infarction, etc.),
cerebrovascular disturbance (e.g. cerebral infarction,
cerebrai apoplexy, etc.), arter'al aneurism, peripheral
vascular disease, xanthomas, restenosis after percutaneous
transluminal coronary angioplasty, or the like.
The object urea derivatives of this invention are new
and can be represented by the rollowing general formula
(I):
O
Rl-(CH2)n-N-C-NH-R3 (I~
R2
~ WO96tlOSS9 22 ~ ~ ~ 8 ~ PCT/JP95/01982
wherein
Rl is a group of the formula :
(in which
R4 is aryl which may have suitable substituent(s),
or heterocyclic group which may have
suitable substituent(s), and
Y is bond, lower alkylene, -S-, -O-, -C-, =CH-,
-CONH-, -N-CO-, (in which R7 is lower
R7 alkyl),
-NHSO~-, -SO2NH-, -SO2N~CO- or -CONHSO2-);
o~
thiazolyl, imidazolyl, pyrazolyl, pyridyl, hieny',
furyl, isoxazolyl or chromanyi, each of which may have
suitable substituent(s);
R2 is lower alkyl, lower alkoxy(lower)alkyl, cycloalkyl,
ar(lower)alkyl which may have suitable substituenl(s),
heterocyclic group or heterocyc'ic(lower)alkyl,
R3 is aryl which may have suitable substituent(s) or
heterocyclic group which may have suitable
s~bstituent(s), and
n is 0 or 1.
The object compound (I) of ~he present invention c~n
be prepared by the following processes.
WO96110559 ~2~ ~ 9 ~ ~ PCT/~S/01982 ~
Process (l)
Rl- (CH2 ) n~~JH
R2
(II)
or a salt thereo~
o=c--N-~.3
(III)
or a salt thereof
2 0 R 1 _ ( CH2 ) n -N-C -NH- R 3
R2
(I)
or a salt thereof
PrQcess (2)
30 Rl-(CH2)~-1H + H2N-R3
R-
~II) ~I'~)
o~ a salt thereof o~ a salt thereof
PCT/JP95101982
~ w096~05s9 ~2~
-- 5
formation of
ureido group
. 5
Rl- (CH2 ) n-N-C-NH-R3
R2
(I)
or a salt thereo'
Process (3)
Rl_(CH2~n~~_C_NH_Ra
(Ia)
or a salt thereof
oxidation
- -
~t ~ A n n s PCT/JP95/01982
W096/lOSS9 ~ L V U ~ 8 IY _
. - (CH2 ) ~-7-C-NH-R3
R-
(I~))
or a s~lt .hereof
1~
wherein
R1, R2, R3 and n are each as defined above,
Ra is pyridyl having two lower alkylthio and lower alkyl,
and
Rb is pyridyl having two lower alkylsulfonyl and lower
alkyli pyridyl having two lower alkylsulfinyl and
lower alkyl; or pyridyl having lower alkylsulfonyl,
lower alkylsulfinyl and lower alkyl.
The starting compound can be prepared by tne following
processes.
Process (A)
o
CH3-C ~ CN
(v)
PCI1JP95101982
~ W096/lOS59 22~
(~ CH-N
R5/ \R6
(VI~
N-CH=CH-~ ~3} CN
R6/
(VII)
or a salt thereof
H~N-NH
(VIII )
or a salt thereof
HN ~CN
(IXa)
o r a s al t thereo f
2 2 Q O 9 8 ~ PCT/~95/01982
W096/lOSS9
Process (B)
Rl - CN
(IX)
or a salt thereof
reduction
. Rl - CHO
(X)
or a salt thereo~
20 Process (C)
Ra - OH
(XI)
or a sal~ thereof
X~C~O
(XII)
or a sall thereo_
' PCT1~5/019X2
WO96110559
2 2 ~
R4-o ~ CHO
(Xaj
or a salt therecf
Process (~)
R4 - B(OH)2
(XIII)
or a salt thereof
X~C~iO
(XII)
or a salt thereof
R4~CHo
(Xb)
or a salt thereof
~3 ~ PCTt~5/01982
WO96/105S9
-- 10 --
Process (~)
R1 - CHO
(X)
or a salt thereof
i ) R2 -NH2
(XIV)
or a salt thereof
ii) reductlon
R1-CH2-NH
R2
(IIa)
or a salt thereof
wherein Rl, R2, and R4 are each as defined above,
R5 is lower alkoxy,
R6 is lower alkyl,
Ra ls aryl which may have sultable
substituent(s), and
X is a leaving group.
Suitable pharmaceutically acceptable salts of the
PCT1~5/01982
WO961105~9
~2~a~8 ~
object compound (I) are conven~ional non-to~ic salts and
may include a salt with a base or an acid addi~ion salt
such as a salt with ~n inorganic base, for example, an
alkali metal salt (e.g., sodium salt, potassium salt,
etc.), an alkaline earth metal salt ~e.g., calcium salt,
magnesium salt, etc.), an ammonium salt; a salt wlth ,-n
organic base, for example, an organic amine salt ~e.g.,
triethylamine salt, pyridine s~lt, picoline salt,
ethanolamine salt, triethanola~.ine sall, dicyclohexylamine
salt, N,N'-dibenzylethylenedia~ine salt, etc.);
an inorganic acid addition salt (e.g., hydrochloride,
hydrobromide, sulfate, phosphate, etc.ji an organic
carboxylic or sulfon~- acid addition salt (e.g., formale,
acetate, trifluoroacetate, maleate, tartrate, fumarate,
citrale, methanesulfonate, benzenesulfonate,
toluenesulfonate, etc.); a salt with a basic or acidic
amino acid (e.g., arginine, asp2rtic acid, glutamic acid,
etc.).
In the above and subsequen~ descriptions of the
present specification, suitable examples and illustratior.
cf the various definitions which the present invention
intends to include within the scope thereol^ are explained
in detail as fsllows.
The term "lower" is used to intend a group having l to
6, preferably l to 4, carbon atom(sJ, unless otherwise
provided.
The term "h gher" is used .o ~ntend a group having
-o 20 carbon 2toms, unless otherwise provided.
Suitable "lower alkyl" and "lower ~lkyl moiety'~ in ~he
terms "ar(lower)alkyl", "lower alkoxy(lower)al~yl" and
"heterocyclic(lower)alkyl" may include st~aight or branched
one having l to 6 carbon a~om(s), such as methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl,
W096/10559 ~ ~ 0 9 8 ~ PCTI~5/01982
12 -
pentyl, tert-pentyl, hexyl, and the like, and ir which more
pre~erable example may be C -C~ 21kyl.
Suitable "lower al~ylene" may ir.clude strzight o~
branched one such as methyiene, ethylene, trimethylene,
tetramethylene, pertamethylene, hexamethylene,
methylmethylene, ethylethylene, propylene, and the like, in
which more ~referable example may be C1-C~ alkylene and the
mos~ preferable one may be methylene.
Suit~ble "lower alkoxy" and "lower alkoxy moiety" in
0 the term "lower alkoxy(lower)alkyl" may include methoxy,
ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, t-butoxy,
pentyloxy, t-per.tyloxy, hexyloxy and the like.
Suitable "cycioalkyl" may include cy_lo(C3-C7)alkyl
(e.g., cyclopropyl, cyclopentyl, cyclohexyl, cycloheptyl,
etc.) and the like.
Suilable "aryl'~ and "aryl moiety" in the term
"ar(lower)alkyl" may include phenyl, naphthyl and the 'ike.
Suitable "halogen" may include fluorine, brcmire,
chlorine and iodine.
2C Suitable "leaving group" may include acid residue, anc
the like.
Suitable "acid residue" may include haiogen as
exemplified asove, and the li~e.
Suitable "heterocylic sroup" and 'Iheterocyclic moietyr'
2~ in the te-~ "heterocyclic(lower)alkyi" may irciude
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyciic group containing 1 to g nitrogen
atom(s), for example, pyrrolyl, pyrrolinyl, imidazolyl,
pyrazolyl, ~yridyl, dihydropyridyl, pyrimidinyl, pyrazinyl,
pyridazinyl, triazolyl (e.g., lH-1,2,4-tri2zolyi, 4H-1,2,4-
triazolyl, lH-1,2,3-triazolyl, 2H-1,2,3-t~iazolyl, etc.),
tetra7clyl (e.g., lH-tetrazolyl, /n-tetrazolyl, etc.),
etc.;
saturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromcnccycii_ g_oup cont~ining 1 to 4 nitrcgen
W096/lOS~9 PCTI~510198
- 13 -
atom(s), for example, pyrrolidinyl, imidazolidinyl,
piperidyl, piperazinyl, etc.;
unsaturated condensed heterocyclic group cor.taining 1
to 4 nitrogen atom(s), for example, indolyl, isoindolyl,
indolinyl, indolizinyl, benzimidazolyl, quinolyl,
isoquinolyl, indazolyl, benzotriazolyl, etc.i
unsaturated 3 to 8-membered (more preferably ~ or 6-
membered) heteromonocyclic grou~ containing 1 to 2 oxygen
atom(s) and 1 to 3 nitrogen a~om(s), for example, oxazolyl,
isoxazolyl, oxadia701yl (e.g., 1,2,4-oxadiazolyl, 1,3,4-
oxadiazolyl, 1,2,5-oxadiazolyl, etc.), etc.;
saturated 3 to 8-membered (more preferabiy 5 or 6-
membered) heteromonocyclic group containing 1 to 2 oxygen
atom(s) and 1 io 3 nitrogen atom(s), for example,
morpholinyl, sydnonyl, etc.i
unsaturated condensed heterocyclic group containing 1
to 2 oxygen atom(s) and 1 to 3 nilrogen atom(s), for
example, benzoxazolyl, benzoxadlazolyl, etc.;
unsaturated 3 to 8-memDered (more preferably 5 or 6-
membered) heteromonocyclic group _ontaining 1 to 2 sulfur
atom(s) and 1 to 3 nitrogen atcm(s), for example,
thiazolyl, isothiazolyl, thiad azolyi (e.g., 1,2,3-
thiadia701yl, 1,2,4-thiadiazolyl, 1,3,4-thiaaiazolyl,
1,2,5-thiadiazolyl, etc.), dihydrothiazinyl, etc.i
saturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic grolp conta~ning 1 to 2 su'fur
atom(s) and 1 to 3 nitrogen atom(s), for example,
thiazolidinyl, etc.i
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing 1 to ~ sulrur
atom(s), for example, thier.yl, dihydrodithiinyl,
dihydrodithionyl, etc.i
unsaturated condensed heterocyclic group con.ain-ng 1
tc 2 sulfur atom(s) and 1 to 3 nitro~en atom(s), for
example, benzothiazolyl, benzothiadiazolyl, e.c.;
~ 2 ~ PCT1~5/01982
W096/lOS59 ~ L
- 14 -
unsaturated 3 to 8-membered (more preferably 5 or c-
membered) heteromonocyclic group containing an oxygen atom,
for example, furyl, etc.;
saturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing an oxygen atom,for example, 4H-2,3,5,6-tetrahydropyraryl, etc.;
unsaturated condensed heterocyclic group containing 1
to 3 oxygen atom(s), for examGie, chro~.anyl, isochromanyl,
methylenedioxyphenyl, etc.;
unsaturated 3 to 8-membered (more preferably 5 or 6-
membered) heteromonocyclic group containing an oxygen atom
and 1 to 2 sulfur atom(s), for example, dihydrooxathiinyl,
etc.;
unsaturated condensed heterocyclic group containing 1
to 2 sulfur atom(s), for example, benzothienyl,
benzodithiinyl, etc.;
unsaturated condensed heterocyclic group containing an
oxygen atom and 1 to 2 sulfur atom(s), for example,
benzoxathiinyl, etc.; and the like.
Suitable "protected amino" may include acylamino or an
amino group substituted by a conventional protecting group
such as mono(or di or trijaryl(lower)alkyl, rGr example,
mono(or di or tri)phenyl(lower)alkyl (e.g., benzyl, Lrityi,
etc.) or the like.
Suitable "hydroxy protective group" in tne term
"protected hydroxy" may include acyl, mono(or di or
tri)phenyl(lower)alkyl which may have one or ~ore suitable
substituent(s) (e.g., benzyl, 4-methoxyben~yl, trityl,
etc.), trisubstituted silyl [e.g., tri(lower)alkylsilyl
(e.g., trimethylsilyl, t-butyldimethylsilyl, etc.), e.c.],
substituted (lower)alkyl (e.g., methoxymethyl,
ethoxymethyl, etc.), tetrahydropyranyl and the like.
Suitable "acyl" and "acyl moiety" in the term
"acylamino" may include
.
PCT/~5/01982
WO96110559
~ ~2~ 0~ ~ p
- 15 -
Carbamoyl; Thiocarbamoyl;
Aliphatic acyl such zs lower or higher alkanoy (e.g.,
formyl, acetyl, prop~noyl, butanoyl, 2-methylpropanoyl,
pentanoyl, 2,2-dimethylpropanoyl, hexanoyl, heptanoyl,
octanoyl, nonanoyl, decanoyl, undecanoyl, dodecanoyl,
Iridecanoyl, tetradecanoyl, pen~adecanoy , hexadecanoyi,
heptadecanoyl, octadecanoyl, nonadecanoyl, icosanoyl,
etc.);
lcwer o~ hlgher alkoxycarbonyl (e.g., methoxycarbonyi,
ethoxycarbGnyl, t-butoxycarbonyl, i~-pentyloxycarbonyl,
heptyloxycarbonyl, etc.);
lower or higher alkylsulfonyl (e.g., methylsul~onyl,
ethylsul~onyl, etc.);
lower or higher alkoxysulfonyl (e.s., methoxysulfonyl,
ethoxysulfonyl, etc.); cyclo(lcwer)alkylcarbonyl (e.g.,
cyclopentylcarbonyl, cyclohexylcarbonyl, etc.); or the
like.
Aromatic acyl such as
aroyl (e.g., benzoyl, toluoyl, naphthoyl, etc.);
ar(lower)alkanoyl [e.g., phenyl(lower)21kanoyl (e.g.,
phenylacetyl, phenylpropanoyl, phenylbutanoyl,
phenylisobutanoyl, phenylpentanoyl, phenylhexanoyl, etc.),
naphthyl(lower)alkanoyl (e.g., naphthylacetyl,
naphthylpropanoyl, naphthylbutanoyl, etc.), etc.];
ar(lower)alkenoyl [e.g., phenyl(lower)alkenoyl (e.g.,
phenylpropenoyl, phenylbutenoyl, phenyl~ethacryloyl,
phenylpentenoyl, phenylhexenoyl, etc.),
naphthyl(lower)alkenoyl (e.g., naphthylpropenoyl,
naphthylbutenoyl, etc.), etc.~;
ar(lower)alkoxycarbonyl ~e.g., phen~l(lower)alkoxycarbonyl
(e.g., benzyloxycarbonyl, etc.), etc.~;
arylcxycarbonyl (e.g., phenoxycarbonyl,
naphthyloxycarbonyl, etc.);
aryloxy(lower)alkanoyl (e.g., phenoxyacetyl,
phenoxypropionyl, etc.)i
W096/lOS59 ~ 8 1 PcTl~5lols82
- 16 -
arylglyoxyloyl (e.g., phenylglyoxyloyl, naphthylglyoxyloyl,
etc.);
arylsulfonyl (e.g.~, phenylsulfonyl, p-tolylsulfonyl, etc.);
or the like.
Suitable "substituent" in the ~erms"aryl which may
have suitable substituent(s)" and "a~(lower) 21 kyl which may
have suitable substituent(s)" may ir.clude lower alkyl as
exemplified above, lower alkoxy as exemplified above, lower
alkenyl, lower alkynyl, mono(or di or tri)halo(lower)alkyl
wherein halogen moiety and lower alkyl moie~y are each as
exemplified above, cyclo(lower)alkyl, cyclo(lower)alkenyl,
halogen as exemplified above, carbcxy, protected carboxy,
hydroxy, protected hydroxy, aryl as exemplified above,
ar(lower)alkyl wherein aryl moiety and lower alkyl moiety
are each as exemplified above, carboxy(lower)~lkyl wherein
lower alkyl moiety as exemplified above, protected
carboxy(lower)alkyl, nitro, amino, protected amino,
di(lower)alkylamino wnerein lower alkyl moiety i5 as
exempiified above, amino~lower)alkyl wherein lower alkyl
moiety is as exemplified above, protected
amino(lower)alkyl, hydroxy(lower)alkyL wherein lower alkyl
moiety is as exemplified above, protected
hydroxy(lower)alkyl, cyano, sulfo, sulfamoyl, carbamoyloxy,
mercapto, lower alkylthio wherein lower alkyl moiety is as
exemplified above, imino, protected amino as exemplified
above, heterocyclic g~oup which may have mono(cr di or
tri)ar(lower)alkyl wherein heterocyclic group, aryl moiety
and lower alkyl moiety are each as exemplified above, and
the like.
~ uitable "substituent" in the term "heterocyclic group
whicn may have sui.able substituen~(s)" may include lower
alkyl as exemplified above, lower alkoxy as exemplified
above, lower alkenyl, lower alkynyl, mono(or di or
tri)halo(lower)alkyl wherein halogen moiety and lower alkyl
~ WO96/10559 PCTI~95/01982
~2 ~ ~ ~ 8
- 17 -
m~olety are each as exemplif ea zbove, cyclo(lcwer)alkyl,
cyclo(lower)al.ken.yl, halogen as exemvlified abcve, ca~boxy,
~rotected c~rboxy, hydroxy, prorected hydroxy, as
exemplified above, ary as exem~l ied a~ove, mono~o~ ~ or
tri)ar~lower~alkyl wher^ir~ aryl moiP,y and ower â' kyl
moiety are each 2S exemplified above, carboY.y(lowe-)al~yl
wherein lower alkyl moiety as exemplified abovre, protected
carboxy(lower)alky', nitro, amino, protected amino,
di(]ower)aikylamino wherein low-- alkyl ~oiety is as
exemplified above, aminc(lower)aikyi wherein iower alkyi
moiely is ~s exemp'ifiev above, prolecte~ amir.o(lower?-
alkyl, hydrox~l(lower)al~yl wher~in lower alkyl moiely is CLS
exemplified above, vrotected rydroxy(lower)a~:Yyi, cyanc,
sulfo, sulf~.oyl, carbamoyioxy, mercapto, lower alkylthio
wherein lower alkyl moiety is as exemplified above, ower
alkylsulfinyl wnerein lower a kyl moieLy is as exempl fied
above, acyl as exemplified above, oxo, imino, and the like.
S~itable "suvsiituert" in the term "thiazolyl,
mida7Oiyl, pyrazolyl, pyridyl, t:nienyl, tury' or
isoxazolyl, each of which may rave suila21e subst t_er,(s)"
may include lower alkyl as exe~plified above, lower alkoxv
as exemplified above, iower alkenyl, lower a kynyl, mGno(or
di or trl)nalo;lower)alkyl wherein haloger moiety and ower
alkyl moie y ar^ each as exemplified above,
cyclo(lo~^rer)alkyl, cyclo(lower)alkenyl, halogen as
exemplified above, carboxy, proLected carboxy, hydroxy,
~rotected hydroxy, aryl as exemplified above, haloaryl
wherein halogen moiety and aryl moiety are each as
ex2m~1ified above, arylthio wherein aryl moiety s as
exe~pli ied above, heterocyclic grou~ as exem~ ified abovc,
~r(lower)alkyl wherein ary~ moiety and lower alkyl moie.y
are each as exempl fied above, carDoXY('ower~a kyl wherein
lower alk.yl moiety as exemplified above, protected
czr~oxy(lower)alkyl, nitro, amino, ~rotected ~m.ino,
dl(lower)alkylamino wherein lower alkyl moiety ls as
~ PCT/~5/01982
WO96/105S9 ~ ~ V ~ ~ ~ 4
- 18 -
exempilfied above, amino(lower)al~yl wherein lower alkyl
moiety is as exemplified above, protected
amino(lower)alkyl, hydroxy(lower)alkyl whereir lower alkyl
mciety is as exemplified above, prolected
hydroxy(lower)alkyl, cyano, sulfo, sulr^amoyl, carbamoyloxy,
mercapto, lower alkylthio wherein lower al}.yl moiety is as
exempliried above, imino, and thc like.
The processes for preparing the object and st~rting
compounds cf the present invention are explained in detail
in the following.
Process (1)
The compound (I) or a salt therecf can be ~repared by
reactirg the compound (II) Gr a salt thereof with the
compound (III) or a salt thereof.
This reaction is usually carried out in a solvent such
as water, alcohol (e.g., methanol, ethanol, etc.), benzene,
N,N-dimethylformamide, tetrahydrofuran, toluene, methylene
2C chloride, ethylene dic~loride, chloroform, dioxane, d e~hyl
ether or any other solvents which do no~ adversely affect
he reaction, or the mixture thereof.
The reaction temperature is not critical and the
reac~ion is usually carried o~t ~nder cool ng to warilling.
When the starting compound is in liquid, it can be
used also as a solvent.
Proces s ( ~ )
The ccmpound (I) or 2 salt thereo~ can be prepared by
subjecting the compound (II) or a sall thereof and 'he
compound (IV) or a salt thereof to formation reaction of
ureido group.
This reaction is carried out in the presence of
reagent which introduces carbonyi group such as phosgene
[e.g. , triphosgene, etc.], haloformate compound ~e.g.
W096/lOS59 PCTl~5l01982
-- 19 -- .
ethyl chloroformate, trichloromethyl chloroformate, phenyl
chloroformate, etc.], N,N'-carbonyldiimida-ole, metal
- carbonyl compounds [~.g. cobalt carbonyl, manganese
carbonyl, etc.], a comblnation of carbon monoxide and
5 catalvsts such as palladium chloride, etc., or the like.
This reaction is usually carried out in a solvent such
as water, alcohol (e.g., methanol, ethanol, etc.), benzene,
N,N-dimethylformamide, tetrahydrofuran, toluene, methylene
chloride, ethylene dichloride, chloroform, dioxane, diethyl
ether or any other solvents which do not adversely affect
the reaction, or the mixture thereof.
The reaction temperature is not critical and the
reaction is usually carried out under cooling to heating.
The reaction is usually carried out in tne presence of
an organic base such as tri(lower)alkylamine (e.g.,
trimethylamine, triethylamine, diisopropylethylamine,
etc.), or the like.
Process (3)
The compound (Ib) or a salt thereof can be prepared by
subjecting the compound (Ia) or a ;ai thereor to oxidation
reaction.
Oxidation is carried out in a conventional manner,
which is capable or oxidizing a sulfur atom to an oxidized
sulfur atom, and suitable oxidizing reagent may be oxygen
acid such as periodate (e.g. sodiu~ periodate, potassium
periodate, etc.), peroxy acid such las perbenzoic acid
(e.g., perbenzoic acid, m-chloroperbenzoic acid, etc.), and
the like.
The reaction is usually carrie~ out in a conventional
solvent such as water, alcohol, (e.~., methanol, ethanol,
isopropyl alcohol, etc.), tetrahydr~furan, dioxane,
dichloromethane, ethylene dichloride, chloroform, N,N-
dimethylformamide, N,N-dimethylacetamide, or anv other
organic solvent which does not adversely affect the
PCT/~5101982
W096/10559 220 ~9 8 ~
- 20 -
reaction.
Among these solvents, hydrophilic sclvents may be used
in a mixture with water.
The reaction`temperature is nct criticai and the
reaction is usually carried OUI under cooling to heating.
Process (A)- ~
~ he compound (VII) or a salt thereof can be prepared
by reacting the compound (V) ~-ith the compound (VIj.
The reaction can be carried out in the ~anner
disclosed in Preparation 2 or similar manners thereto.
Process (A)- ~
The compound (IXa) or a salt Ihereof can be prepared
by reacting the compound (VII) or a salt thereof with the
compound (VIII) or a salt thereof.
The reaction can be carried out in the manner
disclosed in Preparation 20 or similar manners thereto.
Process (B)
The compound (X) Gr a salt thereG_ can be preparcd b,
subjecting the compound (IX) or a salt thereof to reduction
reaction.
Reduction is carried out in a conventional manner,
including chemical reduction and catalytic reduction.
Suitable reducing reagents to be used ~ r chemical
reduction and hydrides (e.g., hydrogen iodide, hydrogen
sullide, lithium aluminum hydride, sodium borohydride,
sodium cyanoborohvdride, diisobutylaluminum hydride, elc.)~
a metal (e.g., tin, zinc, iron, etc.) or metallic compound
(e.g., chromium chloride, chromium acetate, etc.), and the
like.
Suitable catalysts to be used in catalytic reduction
are conventional ones such as ~latinum câtalysts (e.g.,
platinum plate, spongv platinum, platinum black, COlloidâl
WO~6110559 ~ 8 ~ PCT1~5101982
plalinum, platinum oxide, platinum wire, etc.), palla~ium
catalysts (e.g., spongy palladium, palladium blac~.,
~ palladium oxide, ~lladium on carbon, colloidal palladium,
palladium on barium 5ulfate, palladium on b?.rium carbonate,
etc.j, nickel catalysts (e.g., reduce~ nickel, nickel
oxide, Raney nickel, etc.), cobalt calalysts (e.g., reduced
cobalt, Raney cobalt, etc.), 'ron catalysts (e.g., reduced
iron, Raney iron, Ullma~ iror., etc.), and the like.
The reduction is usually carried out in the
conventional solvent such as water, alcohol ~e.g.,
methanol, ethanol, prop2nol, etc.), tetrahydrcfuran,
toluene, dichloromethane, dioxane, N,N-dimethylformamide,
N,N-dimethylacetamide or any other solvents which do not
adversely affect the reaction, or a mixture thereof.
The reduction is usually ca~ried out in the presence
of an organic acid or an inorganic acid (e.g., formic acid,
acetic acid, propionic acid, ~rifluoroacetic acid,
p-toluenesulfonic acid, hydrochloric acid, hydrobromic
acld, etc.).
2C Additionally, in case thal the above-mentioned acids
to be used in chemical reduction are in liuid, they ca.
also be used as a solvent.
Process (C)
The compound (Xa) or a sal~ thereof can be prepared by
reacting the compound (XI) or a salt thereof with Ihe
compcund (XII) or a salt therecf.
The reaction can be carried out in the manner
disclosed in Preparation 48 or similar manners thereto.
Process (D)
The compound (Xb) or a salt thereof can be pre~ared b~
reacting the compound (XIII) or a salt ~hereo with the
compound (XII) or a salt thereof.
The reaction can be carried out in the manner
WO96/10559 ~ ~ o ~ 9 ~ ~ PCT/~95/01982
- 22 -
disclosed in Preparation 38 or similar manners thereto.
.
Process (E)
The compound (IIa) or a salt thereof can be prepared
by reacting the compound (X) or a sal~ thereof with the
compound (XIV) or a salt thereof and then by subjecting the
resultant compound to reduction reaction.
Reduction is carried out in a conventional manner,
including chemical reduction and catalytic reduction.
Suitable reducing reagent to be used in chemical
reduction are hydrides (e.g., hydrogen iodide, hydrogen
sulfide, lithium aluminum hydride, sodium borohydride,
sodium cyanoborohydride, etc.) or a combination of a metal
(e.g., tin, zinc, iron, etc.) or metallic compound (e.g.,
chromium chloride, chromium acetate, etc.) and an organic
acid or an inorganic acid (e.g., rormic acid, acetic acid,
propionic acid, trifluoroacetic acid,
p-toluenesulfonic acid, hydrochlo~ic acid, hydrobromic
acid, etc.).
Suitable catalysts to be used in catalytic reductio~
are conventional ones such as platinum catalysts (e.g.,
platinum plate, spongy platinum, platinum black, colloidal
platinum, platinum oxide, platinum wire, etc.), palladium
catalysts (e.g., spongy palladium, palladium black,
palladium oxide, palladium on carbon, colloidal palladium,
palladium on barium sulfate, palladium on bari~-m carbonate,
etc.), nickel catalysts (e.g., reduced nickel, nickel
oxide, Raney nickel, etc.), cobalt catalysts (e.g., reduced
cobalt, Raney cobalt, etc.), iron catalysts (e.g., reduced
iron, Raney iron, Ullman iron, etc.), and the like.
The reduction is usually carried out in a conventional
solvent such as water, alcohol (e.g., methanol, ethanol,
propanol, etc.), tetrahydrofuran, toluene, dioxane,
N,N-dimethylformamide, N,N-dimethylacetamide or any other
solvents which do not adversely affect tne reaction, or a
WO9611~559 ~ PCT/~5/01982
mixture thereof.
Additionally, in case th2t tne above-mentioned acids
- to be used in chemical reduction are in li~uid, they can
also be used as a solvent.
r 5 Suitable salts of the object and starting compounds in
Processes (l)~(3) and (A)~(E) can be referred to the ones
as e~emplified for the compound (I).
The compounds obtained by the above processes can be
isolated and purified by a conventional method such as
pulverization, recrystallization, column chromatography,
reprecipitation, or the like.
It is to be noted that the compound ( T ) and the other
compounds may include one o~ more stereoisomer(s) such as
optical isomerls) and geometrical isomer(s) due to
asymmetric carbon atom(s) and double bond(s), and all cf
such isomers and mixture thereof are included within the
scope of this invention.
P~eferred embodiments of the object compound (I) are
as follows.
Rl is a group of the formula :
R4-Y
(in which
R4 is phenyl which may have l to 3 suitable
substituent(s) (more preferably substituent
selected from the group consisting of h~logen,
lower alkyl, dl(lower)alkylamino, protected am.~no
(more preferably acylamino;
most preferably lower alkylsulfonylamino), cyano,
heterocyclic group (more preferably .etra~olyl)
WO96/10559 ~ ~ a ~ 8 ~ PCTt~5/01982
- 2~
which may have ~ono(or di or tri)ar(lower)alky'
(more preferably mono(or d or
tri)phenyl(lower)alkyl; most pre~erably
triphenyl(lower)alkyl), hydroxy, protected
hydroxy (~.ore prererably lowen
alkoxy(lower)alkcxy) and mono(or di or
tri)halollower)alkyl (~ore pre~erably
trihalo(lower)alkyl)), [more preferably pheryl,
halophenyl, lower alkylphenyl,
di(lower)alkylaminophenyl, iower
alkylsulfonyl2m nophenyl, cyanophenyl,
tetra7Olylphenyl, (triphenyl(lower)-
alkyltetrazolyl)~henyl, trihalo(lower)-
alkylphenyl, phenyl having ~wo lowe~ alkyl and
hydroxy, or phenyl havlng two lower alkyl and
lower alkoxy(lower)alKoxy]; or heterocy_lic group
(more preferably thienyl, pyrazolyl, imidazolyl,
triazoiyl, pyridyl, pyrrolyl, tetrazolyl,
oxazolyl, thiazolyl, oxadiazolyl, piperazinyl,
thiazolidinyl or methylenedioxy~henyl) which may
have 1 to 3 (more preferabl~ one or two) suitable
substituent(s) (more ~re erably substituent
selected from the group consisting of lower
alkyl, mono(or di or tri)ar(lower)alkyl (more
preferably phenyl(lower)alkyl or
triphenyl(lower alkyl) and oxo) rmore preferably
thienyl; pyrazolyl which may have lower alkyl or
triphenyl(lower)alkyl; imidazolyi;
triazolyl which may have one cr two
substituent(s) selected from the group consisting
of lower alkyl and phenyl(lower)alkyl; pyridyl;
pyrrolyl; tetrazolyl which may have lower alkyl
or triphenyl(lower)alkyl; cxazolyl;
lower alkylthiazolyl; lower alkyloxadiazolyl;
lower alkylpiperazinyl; dioxothiazolidinyl; or
PCT/~5/01982
~ WO96/10559 ~2~0~ ~
- 25 -
methylenedioxyphenyl];
O
~ .
v is bond, Iower alkylene, -a-, -C-, -C-, =CH-,
-CONH-~ -N-CO- (in which R7 is lower alkyl),
-NHSO~- ~ -SO2NH- J -SO2NHCO- c r ~CONHSO2 ~ ); or
thiazolyl, imidazolyl, pyrazolyl, pyridyl, thienyi,
furyl, isoxazolyl or chromanyl, each of which may have
1 to 5 suitable substituent(s) (more preferably
substituent selected from the group consisting of
lower alkyl, hydroxy, protected hydroxy (more
preferably acyloxy), phenyl, halophenyl, phenylthlo
and pyrrolyl) [more preferably halophenylthlazclyl,
pherylimidazolyl, phenylpyrazolyl, phenylpyridyl,
phenylthiopyridyl, pyrrolylpyridyl, phenylthienyl,
phenylfuryl, phenylisoxazolyl or chromanyl h.-ving
lower alkyl and hydroxy];
R2 is 'ower alkyl, lower aikoxy(lower)alkyl,
cyclo(C3-C7)alkyl (more prefevably cyclopentyl,
cyclohexyl or cycloheptyl), phenyl(lower)alkyl which
may have l to 3 (more preferably one or two;
most preferably one) suitable substituent(s) (more
preferably substituent(s) selected from the group
consisting of halogen, lower alkoxy and di(lower
alkyl)amino) [more preferably phenyl(lower)alkyl,
halophenyl(lower)alkyl, lower alkoxyphenyl(lower)alkyl
or Gi (lower alkyl)amirophenyl(lower)aikyl],
tetrahydropyranyl or furyl(lowev)~lkyl, and
R3 is pheryl which may have l to 3 (more pveferab y two o~
three) suitable substituert(s) ~more preferabl~7
substituent selected fro~ the group consisting o~
lower alkyl and halogen) [more preferably di(or
tvi);lower alkyl)phenyl or trihalophenyl~;
pyridyl or pyrimidinyl, each or which may nave l to 3
PCT/~95/01982
W096/lOSS9 ~ 2~ ~ g 8 ~
- 26 -
(more pref2rably two or three) suitable substltuent(s)
(more preferablv substituent selected from the group
consisting of lower alkyl, lower alkylthio, halogen,
lower alkoxy; lower alkylsulrinyl and lower
alkylsulfonyl) [more preferably pyridyl having two
lower alkylthic and lower alkyl;
pyridyi having halogen, lower alkyl and lower
alkylthio; tri(lower alkyl)pyridyl; pyridyl having two
(lower)alkoxy a~.d lower alkyl; pyridyl having lower
alkoxy, lower alkyllhio and lower alkyl; pyridyl
having two lower alkylsulfinyl and lower alkyl;
pyridyl having two lower alkylsulfonyl and lower
alkyli pyridyl having iower alkylthio, lower alkoxy
and lower alkyl; pyridyl having lower alkylsulfinyl,
lower alkylsulfonyl and lower alkyl; pyridyl having
lower alkylthio, lower aikylsulfonyl and lower alkyl;
pyridyl having two halogen and lower alkyl;
di(lower)alkoxypyrimidinvl; or pyrimidinyl having two
lower alkyllhio and lower alkyl', and
n is O or l.
The object compounàs ~I) and pharmaceutically
acceptable salts thereof possess a strong inhibitory
activity against ACAT, and are useful for the preventicn
and/or treatment of hypercholesterolemia, hyperlipidemia,
atherosclerosis or diseases caused Ihereby.
In o~der to illustrate the usefulress of ~he object
compound (I), the pharmacological test data or the
reresentative compound of the compound ( T ) are shown _n
the followinG.
Test compound (a) :
l-Cycloheptyl-l-(4-phenoxyphenylmethyl)-3-(2,4,6-
35 trifluorophenyl)urea
WO96/10559 ~ ~ ~ 0 9 ~ ~ PCT/~5/01982
- 27 -
Test :
Acyl-CoA : cholesterol acyltransferase (ACAT)
inhibitory activity
Method :
ACAT activity was measured by the melhod of Heider et
al. described in Journal of Lipid Research, ~ol. 24, page
1127 (1983). The enzyme AC~T W2S prepared from the mucosal
microsome fraction of the small intestine of ~ale, 18-week
old ~apanese white rabbits which had been fed diet
containing 2~q- cholesterol for 8 weeks. The inhibitory
activity of test compound was calculated by measuring the
amount of the labeled cholesterol ester produced from
[14C]oleoyl-CoA and endogenous cholesterol as follows ~
[14C]Oleoyl-CoA and microsome were incubated with test
compound at 37C for 5 minutes. The reaction was stopped
by the addition of chloroform-methanol (2:1, V/V).
Cholesterol ester fraction in the chloroform-methanol
extracts was isolated by thin-layer chromatography and was
cour.ted their label.
Result :
Test Compound IC50 (M)
25 (a) 1.1 x 10-8
For therapeutic purpose, the compound (I) of the
present invention car be used in a form of pharmaceutical
prepar-tion containing one of said compounds, as ~r. ac.ive
ingredienl, in admixture with a pharmaceu~ic211y acceptable
carrier such as an organic or inorganic solid or li~uid
excipient suitable for oral, parenteral or external
(topical) administration, wherein more preferable one is
oral administration. The pharmaceutical preparations may
PCT/~95101982
WO96/10559 22~ ~ 9 8 ~ -
- ~8 -
be capsules, tablets, dragees, granules, suppositories,
solution, lotion, suspension, emulsion, ointmcrt, gel, cr
the like. If desired, there may be included in these
preparations, auxiliary substances, stabilizing agents,
wetting or emulsifying agents, bu fers and other commonly
used additives.
While the dosage of the compound (I) will vary
depending upon the age and condition of the patient, an
average single dose of about O.l mg, l mg, lO mg, 50 mg,
lOlO0 mg, 250 mg, 500 mg and lO00 mg of the compound (I) ~,ay
be effective for treating the above-mentioned diseases. In
general, amounts between O.l mg/body and about l,000
mg/body may be administered per day.
15The following Preparations and Examples are given for
the purpose of illustrating the present invention in more
detail
- to be cortirued on the nexl page -
PCT/~5/01982
W096/10559 ~ ~ ~ 0 ~
- 29 -
Prea~ation 1
To a solution of acetophenone (20 g) and dimethyl
t oxalate (23.6 g) in N,N-dimethylformamide (160 mlj was
added sodium hydride (60`~; oil suspension, 8 g) at 0-5C.
The mixture was stirred fcr one hou~ at room temperature,
then heated for 30 minutes at 50C. After cooling, to the
reaction mixture was added 2.4N-hydrochloric acid (70 ml)
and extracted with ethyl acetate. The organic layer was
washed with water, brine, dried over magnesium sulfate,
evaporated in vacuo. The residue was chromatographed on
silica gel (700 g, n-hexane - ethyl acetate (4:i to 1:1))
to give methyl 2,~-dioxo-4-phenylbutyrate (20.32 g).
IR (KBr) : 1732, 1622, 1601, 1574, 1444, 1269 cm 1
NMR (CDCl3, o) : 3.95 (3H, s), 7.10 (lH, s), 7.45-
7.68 (3H, m), 7.95-8.06 (2H, m), 15.0-15.5 (lH,
br)
APCI-MASS (m/z) : 207 (M+H+)
Pre~aration 2
The mixture of 3-acetylbenzonitrile (43.55 g) and N,N-
dimethylformamide dimethyl acetal (107.2 g) was stirred a
90C for 3 hours under nitrogen. The mixture was
concentrated in vacuo and diisopropyl ether (400 ml) was
added thereto. The red-brown precipitates were collected
by filtration, washed with diisopropyl ether and dried to
give 3-[(E)-3-dimethylaminopropenoyl]benzonitrile (48.62
g).
IR (KBr) : 3070, 2900, 2225, 1545, 1600, 1550 cm 1
NMR (DMSO-d6, ~) : 2.96 (3H, s), 3.17 (3H, s),
~ 30 5.93 !lH, d, J=12.1H7), 7.65 (lH, dd, J=7.7,
7.7Hz), 7.72 (lH, d, J=12.1Hz), 7.95 (lH, d,
- J=7.7Hz), 8.20 (lH, d, J=7.7Hz), 8.34 (lH, s)
APCI-MASS (m/z) : 201 (M+H+)
PCT/~5/01982
wo96/10559 22~ ~9 8 1
- 30 -
Pre~aration 3
To a solu~ion of N-(3-acetylbenzyl)-acetamid~ (9.56 g~
in 1,2-dimethoxyethane (150 ml) was added dropwise bromine
(7.99 g) at room temperature and the mixture was stirred at
the same temperature for 1.5 hours. The preci~itates were
dissolved by addition of ethar.ol (150 ml) and thioacetamide
(4.51 g) was dded tc the solution. The mixture was
refluxed fo~ 2.5 hours and e~Japorated in vacuo. The
residue was extracted by ethyl acetate and the organic
layer was washed with water and brine, dried over magnesium
sulfate and evaporated in vacuo. The residue was purified
by column chromatography on silica gel to g~ve N-[(2-
methylthiazol-4-yl)benzyl]-acetamide (8.24 g).
IR (KBr) : 3295, 3110, 3070, 293C, 1645, 1550 cm 1
NMR (DMSO-d6, o) : 1.89 (3H, s), 2.72 (3H, s),
4.29 (2H, d, J=5.9Hz), 7.2-7.9 (4H, m), 7.9G (lH,
s), 8.40 (lH, t, J=5.9Hz)
APCI-MASS (m/z) : 247 (M+H+)
.
~reparation 4
To a solutior of N-[3-(2-methylthia7O1-4-yl)benzylj-
aceta~ide (8.23 g) in ethanol (100 ml) was added conc.
nydrochloric acid (13.9 ml) and the mixture was refluxed
for 12 hours. The mixture was cooled to 5C and acetone
(100 ml) was added thereto slowly. The precipitates wer2
collected by filtration and washed with acetone, drie over
phosphorus pentoxide to give 3-(2-methylthiazol-4-yl)-
benzylaminehydrochloride (5.14 g).
IR (~Br) : 3090, 2915, 2840, 2635, 1605, 1575,
1510 cm~l
NMR (DMSO-d~, o) : 2.73 (3H, s), 4.07 (~H, ~Ba,
J=5.7Hz), 7.47 (2H, d, J=5.lHz), 7.9-8.0 (lH, m),
7.97 (lH, s), 8.14 (lH, s), 8.57 (2H, br s)
APCI-MASS (m/z) : 205 (M of free compound +H+)
PCT/~5/01982
~ W096/lOS59 2 ~ 8 ~ ~
- 31 -
Prepa~ation 5
To z suspension of methyl 4-[(~)-3-dimethyiamino-
propenoyl]benzoate (5.0 g) in methanoi ( 1 50 ml j was added
acetic acid (1.84 ml) and hydra7ine monohvdrate (1.56 ml).
After stirring for 10 hours at room te~perature, the
solvent was evaporated in vacuo. The residue W2S dissolveG
in ethyl acetate, washed with water and brine, dr'ed over
magnesium sulfate, evaporated in vacuo to give methyl 4-
(pyrazol-3-yl)benzoate (4.21 g).
IR (KBr~ : 2800-3500 (br), 1709, i610, 1537, 1439,
1414 cm~1
~MR (DMSO-d~, o) : 3.86 (3H, s), 6.85 (1~, d,
J=2.2Hz), 7.84 (lH, br s), 7.85-8.10 (4H, m),
13.IO (lH, br)
APCI-M~SS (m/z) : 203 (M+~+)
Preparation 6
To a solution of methyl 4- r (E)-3-dimethylamino-
propenoyl)benzoate (5.23 g) in acetic acid (50 ml) was
added methylhydrazine (1.31 ml). The mixture was stirre~
ror 3 hours at room tem~erature. To the solution W25 added
5N-sodium hydroxide solution in order to ~asify under ice
cooling and extracted with ethyl acetate. The crganic
layer was washed with saturated sodium bicarbonate
solution, water, brine, dried over magnesium sulfate,
evaporzted in vacuo. After chromatography on siiica gel
(eluting with dichloromethane-methanol (120:1)), methyl
4-(1-methylpyrazol-3-yl)benzoate (3.14 g) was isolated and
methyl 4-(1-methylpyrazol-5-yl)benzoate (i.63 g) was
obtained.
Methyl 4-(1-methylpyrazol-3-yi)benzoate:
I~ (KBr) : 3134, 2949, 1705, 1612, 1439, 1344,
1281 cm~1
N~R (CDCl3, o) : 3.52 (3H, s), 3.97 (3H, s), 6.61
(1~, d, J=2.2Hz), 7.41 (lH, d, J=2.2Hz), 7.82-
PCT/JP95/01982 ~
w096ll05s9 a 2 ~
7 . 93 (2H, m), 8 . 03-8 . 14 (2H, m)
APCI_MASS ~m/Z): 217 (M+~I+) r
Methyl 4-(1-methylpyrazol-5-yl)benzoate :
-~R (K3r) : 3035, 2960, 1718, 1614, 1464, 1425,
1286 Cm~1
NMR (CDCl3, o) : 3 . 93 (3H, S), 3 . 96 (3H, S), 6. 38
(lH, d, J=2 . OHZ), 7 . 46-7. 57 (2~, m), 7 . 54 (lr:, d,
J=2 . OHZ), 8.0 8-8 . 19 (2H, m)
APCI-MASS (m/z) : 217 (M'H+)
Preparation 7
To a solution of thiophenol (2.20 g) in metnanol ('0
ml) was added 28~, sodium methoxide-metha~ol solution ( 3 . 8 6
ml) arld the mixture was stirred at roo~ temperatu~e fcr 15
minutes. TG the mixture was added met~yl 6-
chlororicotinate (3.43 g) and the mixture was refluxed for
6.5 hours under nitrogen. The mixture was evaporated to
dryness and the residue was extracted with ethyl acetate.
The organic layer was washed with water and brine, dried
over magnesium sulfate and evaporated n vacuo. The
residue was purifieà by col~mn chromatography on s~lica gel
to give methyl 6-(phenylthio)nicotinate (5.13 g) as a
cryst~l.
IR (XBr) : 3070, 2950, 172C, 1585, 1553 cm l
NMR (CDCl3, o) : 3.91 (3H, s), 6.86 (lH, dd, J=8.5,
0.8Hz), 7.4-7.5 (3H, m), 7.55-7.7 (2H, m), 8.00
(lH, dd, J=8.5, 2.2Hz), 9.00 (lH, dd, J=2.2,
0.8~z)
~PCI-MASS (m/7) : 246 (M+H+)
Pre~aration 8
To a solution of aniline (8.~0 g) in ~yridine (100 ml~
was added portionwise 4-carboxybe~zenesulfonyl chloride
(17.65 g) at 5C and Ihe mixture was s-irred at 9CC for 6
.. . . . . .
PCTI~5/01982
~ WO96/10559 ~ 2 ~
- 33 -
hours under nitrogen. The mixture was poured lr.to a
mixture of ethyl acetate (300 ml), ice water (200 ml) and
conc. hydrochloric acid (150 ml). The precipitates were
formed and collected by filtration and washed with ethyl
acetate and diisopropyl ether and cried in vacuo over
phosphorus ~entoxide to give 4-(phenylsulfamoyl)benzoic
acid ~6.87 g) as white crystal. The filtrate was separated
and the organic layer was washed wi,h brine, dried over
magnesium sulfate and evaporated in vacuo. To the residue
was added diisopropyl ether and the second crop (6.57 g)
was obtained by filtration.
IR (K~r) : 3265, 2840, 2675, 2560, 1680, 1600,
1575 cm~l
NMR (DMSO-d6, o) : 7.0-7.2 (3H, m), 7.2-7.35 (2H,
m), 7.85 (2H, d, J=8.4Hz), 8.07 (2H, d, J=8.4Hz),
10.45 (lH, s)
Preparation 9
To a solution or ethyl 4-aminobenzoate (8.26 g) in
pyridine (25 ml) was added dropwise benzenesulfonyl
chloride (8.83 g) at 5C and the mlxture was stirred at
room temperature for 1 hour under nitrogen. The mixture
was poured lnto a mixture of ethyl acetate 1150 ml), ice
water (100 ml) and conc. hydrochloric acid (30 ml). he
precipitates were formed and collected by filtration,
washed with ethyl acetate ar.d diisopropyl ether and dried
in vacuo over phosphorus pentoxide to give ethyl ~-
(phenylsulfonylamino)benzoate (10.72 g~ 2S Z white crys~al.
The filtrate was separ2ted and the organic layer was washed
with brine, dried over magnesium sulfate and evaporated ir
vacuo. To the residue was added diiso_rop~l ether ar.d the
second crop (3.83 g) was obtained by filtratior.
IR (KBr) : 3230, 3070, 2990, 2940, 2880, 1695,
1610, 1510 cm~1
NM~ (DMSO-d6, o) : 1.27 (3H, t, =7.1:~z), 4.2~ ,2~,
PCT/~5/01982
WO96110559
~2~98~1 ~
- 34 -
q, J=7.lHz), 7.22 (2H, d, J=8.8Hz), 7.5-7.7 (3H,
m), 7.8-7.9 (4H, m), 10.86 (lH, s)
APCI-MASS (m/z) : 306 (M+H )
Pre~a~ation 10
To a stirred mixtu~e of bromine (50.2 ml) in
dichloromethane (1 ~) and anhydrous sodium carbonate (20~.8
g) was added a solution of 1-methylpvrazole (80 g! in
dichloromethane (100 ml) at G-5C. After stirring for one
hour under ice-cooling, the mixture was stirred for further
one hour at room temperature, then cooled. To the reaction
mixture water (1 ~) was added thereto. The dichloromethane
layer was separated and aqueous layer was extracted twice
with dichloromethane. The combined organic layer was
washed with water and brine, dried over magnesium sulfate
and evaporated under reduced pressure. The residue was
distilled in vacuo to afford 4-bromo-1-methylpyrazole
(150.6 g).
bp : 82C (20 mmHg)
IR (Neat) : 3100, 2930 cm 1
NMR (CDCl3, o) : 3.89 (3H, s), 7.38 (lH, s),
7.44 (lH, s)
APCI-MASS (m/z) : 161, 163 (M+H~)
~5 Pre~aration 11
To a solution of methyl 4-formylbenzoate (4.0 g) and
tosylmethyl isocyanide (5.0 g) in methanol (40 ml) was
added potass um carbonate (3.54 g). The mixture was
refluxed for 3.5 hours. After cooling, the reaction
mixture was diluted with ethyl acetate (300 ml), washed
with water and brine, d~ied over magnesium sulfate,
evaporated in vacuo. The residue was chromatographed or.
silica gel (100 g, eluting with n-hexane - ethyl acetate
(2:1 to 1:1) to give methyl 4-(oxazol-5-yl)benzoate (~.04
g).
f PCT/~5/01982
W096/lOS59 ~ ~ 0
- 35 -
IR (KBr) : 1726, 1614, 1275, 1109 cm 1
NMR (CDCl3, ~) : 3.94 (3H, s), 7.48 (lH, s),
7.68-7.78 ~2H, m), 7.9? (lH, s), 8.06-8.16 (2H,
m)
APCI-MASS (m/z) : 204 (M+H+)
Prepaxation 12
A solution of methyl 2,4-dioxo-4-phenylbutyrate (6 g)
and hydroxylaminehydrochloride (6.07 g) in methanol (120
ml) was refluxed for 4 hours. The solvent was re~oved in
vacuo. ~o the residue was added chloroform. The organic
solution was washed with water, brine, dried over magnesium
sulfate, evaporated in vacuo. The residue was
chromatographed on silica gel (150 g, n-hexane - ethyl
acetate (3:1)) to give 3-methoxycarbonyl-5-phenylisoxazole
(5.25 g).
IR (KBr) : 1728, 1570, 1448, 1250 cm~l
~MR (CDC13, o) : 4.01 (3H, s), 6.94 (lH, s),
7.a5-7.55 (3~, m), 7.75-7.88 (2H, m)
- 20 APCI-MASS (m/z) : 204 (MlH+)
Pre~aration 13
A solution of methyl 2,4-dioxo-4-phenylbutyrate (6 g)
and hydrazine, monohydrate (1.42 ml) in ethanol (48 ml) was
refluxed for 5 hours. The solvent was removed in vacuo.
The resulting solid was collected by filtration, washed
with diisopropyl ether to give 5-methoxylcarbonyl-3-
pyenylpyra7ole (3.0 g).
IR (KBr) : 250C-3400 (br), 1730, 1491, 1244 cm~
NMR (DMSO-d6, ~) : 3.83, 3.88 (total 3H, each s),
7.18-7.53 (aH~ m), 7.78-7.34 (2-i, m), 13.30-14.15
(lH, m)
APCI-MASS (m/z) : 203 (M+H+)
PCT/~5/01982
WO96/10559
2~0~8 ~ --
- 36 -
Preparation 14
A mixture of methyl 3-cyanobenzoate (8.0 g), sodium
azide (19.38 g) and ammonium chloride (15.95 g) in N,N-
dimethylformamlde (32 ml) was heated for 2.5 hours at
120C. The mixture was poured into ice water (300 ml) -
ethyl acetate (100 ml). Under ice cooling, to the solution
was added sodium nitrite (2C.5 g) then
6N-hydrochloric acid until pH was adjusted to 1-2. After
stirring for 30 minutes at room ~emperature, the mixture
was extracted with ethyl acetate - tetrahydrofuran, washed
with water and brine, dried over magnesium sulfate,
evaporated in vacuo to give methyl 3-(lH-tetrazol-5-
yl)benzoate (10.01 g).
IR (KBr) : 2300-3500 (br), 1705, 1684, 1618,
1562 cm~1
NMR (DMSO-d6,o) : 3.93 (3H, s), 7.78 (lH, dd,
J=7.9, 7.9Hz), 8.iO-8.20 (lH, m), 8.25-8.38 (lH,
m), 8.6G-8.70 (lH, m)
APCI-MASS (m/z) : 2G5 (~+H+)
2G
Preparation 15
To the solution of 4-bromobenzyl alcohol (4.85 g) and
3-tri-n-butylstannylthiophene (11.6 g) was added
tetrakis(triphenylphosphine)palladium(0) (0.9 g), then the
mlxture was heated for one hour at 140C. After cooling,
the resulting precipitate was collecled by filtration and
washed with n-hexane to give 4-(3-thienyl)benzyi alcohol
(2.67 g).
IR (KBr) : 3300 (br), 1425, 1200, 1045, 1014,
777 c~. 1
NMR (C~Cl3, o) : 1.72 (lH, t, J=5.9Hz), 4.72 (2H, d,
J=5.9Hz), 7.30-7.5C ~5H, m), 7.60 (2H, dd, J=6.4,
1.8Hz)
PCT/~5/01982
_ W096/lOS59
Pre~aration 16
The following compound was obtained according to a
similar manner to that or Preparation 15.
- 5 4-(2-Thienyl)benzyl alcohol
IR (KBr) : 3300 (br), 1427, 1213, 1047, 806 cm 1
NMR (CDCl3, ~) : 1.70 (iH, t, J=5.9~z), 4.71 (2H,
d, J=5.9Hz), 7.08 (lH, dd, J=5.i, 3.6Hz), 7.22-
7.42 (4H, m), 7.52-7.68 (2H, m)
Preparation 17
A mixture of ethyl 4-acetylbenzoate (iO g) and N,N-
dimethylformamide dimethyl acetal (41.8 ml) was heated fo-
18 hours at 85C. After cooling, the resulting solid was
collected by filtration, washed with diisopropyl ether tc
give methyl 4-[(E)-3-dimethylaminopropenoyl~benzoate
(10.44 g)
IR (KBr) : 1718, 1637, 1578, 1541, 1425 cm 1
NMR (DMSO-d6, o) : 2.94 (3H, s), 3.17 (3H, s),
3.88 (3H, s), 5.85 (lH, d, J=12.2Hz), 7.77 (lH,
d, J=12.2Hz), 7.90-8.05 (4H, mj
APCI-MASS (m/z) : 234 (M+H )
Preparation 18
To a suspension of lithium aluminum hydride (569 mg)
in tetrahydrofuran (120 ml) was added dropwise a solution
of 2-methoxycarbonyl-4-(pyrrol-1-yl)pyridine (3.03 g) at
5C and the mixture was stirred at room temperature for 3
hours. To the mixture were added sodium fluoride (2.52 g)
and water (811 mg) and the mixture was stirred at room
temperature for 30 minutes. The insoluble materials were
~ removed by filtration and washed with ~etrahydroluran. The
filtrate was evaporated in vacuo and the residue was
purified by column chromatography on silica gel to give [4-
(pyrrol-1-yl)pyridin-2-yl]methanol (1.14 g).
WO96/105~9 ~ 2 0 0 ~ ~ ~ PCT/~5/01982
- 38 -
IR (K~r) : 3190, 2955, 2845, 1595, 1575, 1500 cm~i
NMR (DMSO-d6, o) : 4.58 (2H, d, J=5.8Hz), 5.48 (lH,
t, J=5.8Hz), 6.35-6.4 (?H, m), 7.52 (lH, dd,
J=5.6, 2 4Hz), 6.55-6.6 (2H, m), 7.62 (lH, d,
J=1.9Hz), 8.47 (lH, d, J=5.6Hz)
~PCI-MASS (m/z) : 175 (~+H+)
Preparation 19
The following compounds were obtained according to a
similar manner to that of Pre~aration i8.
(1) 3-(Pyrazol-3-yl)benzyl alcohol
IR (Film) : 3245, 2930, 2880 cm~l
N~LR (DMSO-d6, o) : 4.52 (2H, d, J=5.6Hz), 5.29 (lH,
t, J=5.6Hz), 6.68 (lH, d, J=2.2Hz), 7.2-7.7 (4H,
m), 7.76 (lH, d, J=2.2Hz), 12.3 (lH, br s)
APCI-MASS (m/z) : 175 (M+H+)
(2) (6-Phenylpyridin-3-yl)methanol
IR (Film) : 3325, 2865, 1600, 1565, 1475 cm 1
NMR (CDCl3, o) : 4.74 (2H, s)~ 7 a-7 55 (3H, m),
7.7-7.85 (2H, m), 7.9-8.05 (2H, m), 8.62 (lH, d,
J=1.3Hz)
APCI-MASS (m/z) : 186 (M+H+)
(3) 4-(Benzoylamino)benzyl alcohol
IR (KBr) : 3320, 2840, 1655, 1595, 1545 cm~1
NMR (DMSO-d6, o) : 4.50 (2H, d, J=5.7Hz), 5.22 (lH,
t, J=5.7Hz), 7.05 (lH, d, J=7.6Hz), 7 ?9 (lH, d,
J=7.6Hz), 7.5-7.7 (4H, m), 7.77 (lH, s), 7.96
(2H, dd, J=7.6, 1.5Hz), 10.23 (lH, s)
APCI-MASS (m/z) : 228 (M+~+)
(4) 4-(Phenylsulfonylamino)benzyl alcohol
IR (Film) : 3515, 3265, 3060, 2935, 2875, 1705,
PCT/~S/01982
WO96/10559
- 39 -
1650, 1615, l_iS c~-1
NMR (DMSO-d6, o) : 4.36 ~2H, d, J=5.8Hz), 5.07 (lH,
t, J=5.8Hz), 7.02 (2~, d, J=8.6Hz), 7.15 (2H, d,
J=8.6Hz), 7.5-7.65 (3H, m), 7.7-7.8 (2H, mi,
10.21 (lH, s)
APCI-~SS (m/z) : 264 (M+~+)
(5) (6-Phenylthiopyridin-3-yl)methanol
IR (Film) : 3320, 2865, 1590, 1560 cm 1
NMR (CDCl3, o) : 2.46 and 2.71 (total lH, t,
J=5.6Hz), 4.64 and 4.72 (total 2H, d, J=5.6Hz),
6.88 and 7.31 (total lH, d, J=8 3Hz), 7.4-7.75
(6H, m), 8.3-8.4 (lH, m)
APCI-MASS (m/z) : 218 (M+H+)
(6) 4-(Oxazol-5-yl)benzyl alcohol
IR (KBr) : 3330 (br), 1510, 1491, 1041, 818 cm~
NM~ (CDCl3, o) : 4.74 (2H, s), 7.34 (lH, s),
7.35-7.50 (2H, m), 7.59-7.72 (2H, m), 7.91 (lH,
s)
APCI-MASS (m/z) : 176 lM+H+J
(7) (3-Phenylpyrazol-5-yl)methanol
IR (KBr) : 2500-3500 (br), 1471, 1360, 1030, 1001,
766 cm~1
NMR (DMSO-d6, o) : 4.38-4.58 (2H, m), 4.95-5.37
(lH, m), 6.52-6.66 (lH, m), 7.20-7.53 (3H, m)~
7.68-7.90 (2~, ~), 12.~8-'3.10 (1~, ~)
A.~CI-MASS (m/z) : 175 (M+H+)
(8) 4-(Pyrazol-3-yl)benzyl alcohol
IR (KBr) : 2500-3600 (br), 1522, 1456, 1419, 1032,
841, 762 c~~
N~R (DMSO-d6, o) : 4.51 (2H, d, J=5.7Hz), 5.07-5.27
(lH, m), 6.60-6.74 (lH, br s), 7.20-7.85 (5H, m),
PCT1~5/01982
W096/lOSS9 ~2~981 ~
- 40 -
12.82, 13.24 (total lH, each br s)
APCI-MASS (m/z) : 175 (M+H+)
(5) 4-(1-Methylpyrazol-5-yl)benzyl alcohol
IR (KBr) : 2500-3600 (br), 1495, 1460, 1425, 1385,
1273 cm~l
NMR (CDCl3, o) : 2.12 (lH, t, J=5.7Hz), 3.88 (3H,
s), 4.77 (2H, d, J=5.7Hz), 6.30 (lH, d, J=1.9Hz),
7.35-7.52 (4H, m), 7.51 (lH, a, J=1.9Hz)
APCI-MASS (m/z) : 189 (M+H+)
(10) 3-(lH-Tetrazol-5-yl)benzoyl alcohol
IR (KBr) : 2100-3600 (br), 1562, 1~85, 1419,
1219 cm 1
NMR (DMSO-d6, o) : 4.61 (2H, s), 5.20-5.60 (lH,
br), 7~48-7n65 (2H, m), 7.85-7.98 (lH, m), 8.05
(lH, s)
APCI-MASS (m/z) : 177 (M+H+)
Prepara~ion 20
To a solution of 3-~(E)-3-dimetnylaminopropenoyl~-
benzonitrile (48.5 g) in methanol (500 ~l) was added acetic
acid (21.82 g) followed by slow addition of hyàrazine
monohydrate (18.17 g) at room temperature and the mixture
was stirred at 17.5 hours al the same lemperature. The
mixture was evaporated to dryness and the residue was
extracted with ethyl acetate. The organ c layer was washed
with water and brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was crystallized and the
crystal was collected by filtralion, washed with
diisopropvl ether and dried to give 3-(pyrazol-3-
yl)benzonitrile (37.71 g).
IR (KBr) : 3190, 3075, 2840, 2760, 2230, 1560 cm~l
NMR (DMSO-d6, o) : 6.88 (lH, d, J=2.1Hz), 7.62 (lH,
dd, J=7.7, 7.7Hz), 7.75 (lH, d, J=7.7Hz), 7.83
WO9611~559~ PCT/~5/01982
- 41 -
(lH, br s), 8.16 (lH, d, J=7.7Hz), 8.24 (lH, s),
13.08 (lH, br)
r
Preparation 21
~ 5To a suspension of sodium hydride (~.0 g) in N,N-
dimethylformamide (100 ml) was added thiophenol (5.51 g),
and the mixture was stirred at room temperature for 15
minutes. To the mixture was added 4-fluorobenzonitrile
(6.66 g), and the mixture was stirred at 130C for 16 hours
under niLrogen. The mixture was poured into a mixture of
ethyl acetate and ice water and the separated crganic layer
was washed with water and brine, dried over magnesium
sulfate an~d evaporated in vacuo. The residue was purified
by colu~n ~hro~atography on silia gel to gi~v~e
4-(phenylthio)benzoritrile ~'~.24 g) as 2n oil.
IR (Film) : 3070, 2235, 1595, 1505 cm i
NMR (CDCl3, o) : 7.15-7.3 (2H, m), 7.65-7.8 (2H,
m), 7.4-7.6 (5H, m)
APCI-MASS (m/z) : 212 (M+H+)
Preparation ~2
To a suspension of 4-(phenylsulfamoyl)benzoic acid
(13.43 g) in 1,2-dichloroethane (130 ml) were added thionyl
chloride (11.52 g) and N,N-dimethylformamide (2 drops) and
the mixture was stirred at 100C for 2 hours, under
nitrogen. The resulting solution was evaporated in vacuo
and the residue was dissolved in dichloromethane (150 ml).
To this solution was added N,O-dimethylhydroxylamin2hydro-
chloride (5.19 g), followed by dropwise addition cf
triethylamine (9.80 g) at 5C. The mixture was stirred at
room temperature for 4 hours. Water was added thereto and
the separated organic layer was washed with br_ne, dried
over magnesium sulfate ar.d evaported in vacuo. The residue
was purified by column chromatography on silica gel to give
N-methyl-N-methoxy-4-(phenylsulfamoyl)benzamide (11.31 g)
.
PCT/~Sl01982
W096/lOS59 ? 2 O O 9 8 1 ~
- 42 -
as an oil.
IR (KBr) : 3150, 2950, 2905, 2890, 1625, 1600,
1570, 1495 cm~1
N~R (DMSO-dç, ~) : 3.24 (3H, s), 3.48 (3~, s), 7.0-
7.2 (3H, m), 7.2-7.3 (2H, m), 7.7-7.9 (4H, m),
10.38 (lH, s)
Preparation 23
To the solution of 4-fluorobenzonitrile (10 g) and
pyrazole (6.74 g) in N,N-dimethylformamide ~100 ml) was
added potassium carbonate (13.7 g). Then the mixture was
heated for 4 hours at 120C. After cooling, the reaction
mixture was diLuted with ethyl acetate (1 ~), washed with
water, brine, dried over magnesium sulfate and evaporated
in vacuo. The residue was chromatographed on silica gel
(400 g, eluting with n-hexane - ethyl acetate (3:1)) to
give 4-(pyrazol-1-yl)benzonitrile ~10.54 g).
IR (KBr) : 2226, 1608, 1529, 1394 cm~1
NMR ~CDCl3, ~) : 6.54 ~1~, dd, J=2.5, 1.8Hz), 7.70-
7.90 (5H, m), 8.00 (lH, d, J=2.5Hz)
APCI-MASS ~m/z) : 17C (M+H+)
Preparation 24
To the solution of 4-fluorobenzonitrile ~10 g) and
imidazole (6.74 g) in N,N-dimethylformamide (200 ~i) was
added potassium carbona~e (13.7 g). Then the mixture was
heated for 2 hours at 120C. After cooling, the reaction
mixture was ~iluted with ethyl acetate ~2 ~), washed with
water, brine, dried over magnesium sulfate and evaporated
in vacuo to give ~-~imidazol-1-yl)benzonitrile (10.34 sj.
IR (KBr) : 2225, 1608, 1520 cm 1
NMR (CDCl~, o) : 7.27 ~lH, s), 7.34 (lH, t,
J=1.2Hz), 7.46-7.60 (2H, m~, 7.75-7.83 ~2H, m),
7.95 (lH, s)
APCI-MPSS (m/z) : 170 (M+H+)
pCT/~5/01982
WO96110S59 ~ 9
- 43 -
Preparation 25
To a solution of ~.ethyl a-(1-methy~pyra2Ol-3-
- yl)benzoate (2.5 g) in dichlorome~hane (80 ml) was added
dropwise diisobutylaluminum hydride (1.02M toluene
~ 5 solution, 25.0 ml) at -60 ~ -50C. After stirring for 30
minutes at the same temperature, sodium fluoride (4.28 g)
and water (i.38 ml) was added thereto. The mixture was
warmed to room temperature cver 15 minutes and stirred for
one hour. Insoluble materials were removed by filtration.
The filtrate was evaporated in vacuo to give 4-(1-
methylpyrazol-3-yl)benzyl alcohol (1.74 g).
IR (KBr) : 2500-3650 (br), 1508, 1462, 1431, 1360,
1302 cm~1
NMR (CDC13, o) : 1.90 (lH, t, J=5.7Hz), 3.95 (3H,
s), 4.70 (2H, d, J=5.7Hz), 6.54 (1~, d, J=2.2Hz),
7.33-7.43 (3H, m), 7.74-7.84 (2H, m)
APCI-MA5S (m/7) : 189 (MtH+)
Preparation 26
2G To a solution of 4-bromo-1-methylpyrazole (1 g) in
ether (15 ml) was added dropwise n-butyllithium (1.63M in
hexane, 4.2 ml) keeping the temperature below -60C. After
stirring for 30 minutes, a solution of tri-n-butyltin
chlo~ide (1.85 ml) in ether (1.85 ml) was added thereto.
After stirring for one hour, the mixture was warmed to room
tem~erature over 30 minutes and stirred for one hour. The
reaction mixture was diluted with ether, washea with water
and brine, dried over magnesium sulfate, and evaporated
under reduced pressure to give 1-methyl-4-tri-(n-
butyl)stannylpyra701e (2.3 g).
lR (Neat) : 2930, 1504, 1460, 1120 cm ~
NMR (CDC13, o) : 0.75-1.70 (27H, m), 3.93 (3H, s),
7.23 (lH, s), 7.42 ~lH, s)
WO96110S59 22 ~ ~ 9 8 ~ PCT1~5/01982
- 44 -
Preparation 27
To a suspension of 5-bromc-2-furancarboxylic acid (10
g), N,O-dimethylhydroxylaminehydrochloride (5.1 gj and 1-
hydroxybenzotriazole (7.07 g) in dichloromethane (300 ml)
was added dropwise a solution of 1-(3-dimethylaminopropyl)-
3-ethylcarbodiimide (6.37 g) in dichloromethare (60 ml) at
room temperature. The resulting mixture was stirred at
room temperature for 18 hours. Water (180 ml) was added
thereto and the insoluble materials were removed by
filtration. The organic layer was separaled and washed
with brine, dried over magnesium sulfate, evapGrated in
vacuo. The residue was chromatographed on silica gel (350
g, eluting with ethyl acetate - n-hexane (1:1)) to give 5-
bromo-2-(N-methyl-N-melhoxycarbamoyl)furan (7.60 g).
IR (Neat) : 2974, 2937, 1649, 1566, 1477 cm~l
NMR (CDC13, o) : 3.34 (3H, s), 3.77 (3H, s), 6.45
(lH, d, J=3.5Hz), 7.G9 (lH, d, J=3.5Hz)
APCI-MASS (m/z) : 234, 236 (M+H+)
Preparation 28
To a mixture of 3-methylbiphenyl (5.0 g) and
N-bromosuccinimide (5.29 g) in tetrachloromethane (15G ml)
was added benzoyl peroxide (144 mg) and the mixture was
refluxed for 6 hours. The mixture was cooled, ar.d the
insoluble materials were filtered off. The fillrate was
evaporated in vacuo and the residue was purified by column
chromatography on silica gel to give crude 3-bromomethyl
biphenyl (6.59 g) as a yellow oil.
IR (Film) : 3030, 1600, 1575 cm 1
NMR (CDC13, o) : 4.56 (2H, s), 7.35-7.7 (9H, m)
Preparation 29
The following compounds were obtained according to a
similar manner to that of Preparation 28.
PCT/~5/01982
~ ~096/10~59 ~ 2 ~ ~ 9 ~ ~
(1) 4-Bro~omethylbenzophenone
IR (KBr) : 3050, 1650, 1605 cm 1
NMR (CDCl3, o) : 4.54 (2H, s), 7.4-7.85 (9H, m)
- 5 (2) a-(Pyriàin-3-yl)benzyl bromide
NMR (DMSO-d6, ~) : 6.10 (2H, s), 7.4-8.4 (6H, m),
8.9-9.3 (2H, m)
(3) 4-(Pyridin-2-yl)benzyl bromide
IR (Film) : 3050, 3010, 2385, 1735, 1585, 1565 cm 1
NMR (CDCl3, o) : 4.58 (2H, s), 7.2-8.1 (7H, m),
8.7-8.8 (lH, m)
Preparation 30
To a solution of 4-ethoxycarbonyl-2-(4-
chlorophenyl)thiazole (2.68 g) in a mixture of
tetrahydrofuran (40 ml) and ethanol (10 ml) was added
lithium borohydride (218 mg) at room temperature and the
mixture was stired at 50C for 1.5 hours. The mixture was
poured into a mixture of ethvl acetate and ice water, ard
the separated organic layer was washed w th water and
brine, dried over magnesium sulfate and evaporated in
vacuo. The residue crystalline solid was collected by
filtration to give [2-(4-chlorophenyl)~hiazol-4-yl]methanol
(1.43 g).
IR (KBr) : 3270, 3080, 292G, 2865, 1595, 1525,
1505 cm-1
NMR (DMSO-d6, o) : 4.63 (2H, d, J=5.8H7), 5.40 (lH,
t, J=5.8Hz), 7.51 (lH, s), 7.5-7.6 (2H, m), 7.9-
8.0 (2H, m)
APCI-MASS (m/ 7 ) 226 (M+H+)
-
Pre~aration 31
To a solution of methyl 6-chloronicotinate (6.86 g)
and dihydroxyphenyl borane (5.85 g) in 1,2-dimethoxyethane
W096tlOSS9 22 0 0 ~ 1 PCT/~5/01982
- 46 -
(150 ml) was added 2M sodium carbonate aaueous solution (48
ml), followed by tetrakis(triphenylphosphine)palladium(O)
(2.31 g) and the mixture was refluxed for 16 hours. The
mixture was poured into a mixture of ethyl acetate and ice
water, and the separated organic layer was washed with
water and brlne, dried over magnesium sulfate ar~d
evaporated in vacuo. The residue was purified by column
chromatograDhy on silica gel to give methyl 6-
phenylnicotinate (7.75 g) as a white crystal.
IR (~Br) : 3070, 3030, 2995, 2945, 2845, 1725,
1595, 1560 cm~l
NMR ~CDC13, o) : 3.98 (3H, s), 7.4-7.6 (3H, m),
7.82 (lH, dd, J=8.3, 0.9Hz), 8.0-8.1 (2H, m),
8.35 (lH, dd, J=8.3, 2.2Hz), 9.28 (lH, dd, J=2.2,
0.9Hz)
Preparation 32
The following compound was obtained according to a
similar manner to that of Preparation 31.
N-Metnyl-N-methoxy-4-~4-(dimethylamino)phenyl~-
benzamide
IR (KBr~ : 3255, 3000, 2815, 1605, 1540, 1505 cm~
NMR (CDC13, o) : 3.01 (6H, s), 3.38 (3H, s!, 3.60
(3H, s), 6.80 (2H, d, J=8.9Hz), 7.5-7.65 (4~, m),
7.74 (2H, dd, J=6.5, 1.9Hz)
A~CI-~SS (m/z) : 285 (M H+)
Preparation 33
~o a suspension of 4- (pyrrol-1-yl)benzoic acid (3.7~
g) and N,O-dimethylhydroxylaminehvdrochloride (1.95 g) in
dichloromethane (100 ml) was added dropwise a solution of
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (2.43 g) in
dichloromethane (15 ml) at room temperature. The resulting
solution was stirred at the same temperature for 18 hours.
..... . _ . . . .
n ~ PCT/~5/01982
WO96/10559 ~ ~ U U
~7 -
Water (60 ml) was added to the mixture, and the insoluble
materials were removed by filtration. The filtrate was
- separated, and the organic layer was washed with brine,
dried over magnesiùm sulfate and evaporated in vacuo. The
- 5 residue was purified by column chromatography on silica gel
to give 4-(pyrrol-1-yl)-N-methyl-N-methoxyber.zamide (2.12
g) as a white crystal.
IR (KBr) : 3130, 3045, 2375, 2935, 1640, 1610,
1580, 1525 c~-1
NMR (CDCl3, o) : 3.39 (3H, s), 3.58 (3H, s),
6.4-6.45 (2H, m), 7.15-7.2 (2H, m), 7.4-7.5 (2H,
m), 7.&-7.9 (2H, m)
APCI-MASS (m/z) : 231 (M+~+!
Preparation 34
To a suspension of 3-(pyrrol-1-yl)benzoic acid (5.62
g), N,O-dimethylhydroxylaminehydrochloride (2.93 g) and l-
hydroxybenzotriazole (4.05 g) in dichloromethane (150 ml)
was added dropwise a solution of 1-(3-dimethvlaminopropyl)-
3-ethylcarbodiimide (3.65 g) in dichloromethane (30 ml) at
room temperature. The resulting solution was s~irred at
room temperature for 20 hours. Water (100 ml) was added
thereto and the insoluble materials were removed by
filtrat~on. The filtrate was separated and the organic
layer was washed with brine, dried over magnesium sulfate
and evaporated in vacuo. The residue was ~uriried by
column chromatography on silica gel to give 3-(pyrrol-1-
yl)-N-methyl-N-methoxybenzamide (5.19 g) as a yellow oil.
IR (Film) : 3130, 2935, 1645, 1610, 1585, 1500 Cm~1
NMR (CDCl3, o) : 3.39 (3H, s), 3.57 (3H, s),
6.35-6.4 (2H, m), 7.1-7.15 (2H, m), 7.45-7.6 (3H,
r . m), 8.7-8.75 (lH, m)
APCI-~SS (m/z) : 231 (M+H+)
WO96/10559 ~2~ 09 8 1 PCT/~95101982
- 48 -
Prep~ration 35
The following compound was obtained according to 2
similar manner to that of Preparation 34.
[4-(N-Methyl-N-methoxy)carbamoylphenyl~-
dihydroxyborane
IR ~KBr) : 338G, 1610, 1545, 1510 cm~1
NMR (DMSO-d6, o) : 3.25 (3H, s), 3.53 (3H, s),
7 5-7.8 (4H, m!
APCI-MASS (m/z) : ~10 (Ml~+)
Pre~ration 36
To a suspension of lithium aluminum hydride (348 mg)
in tetrahydrofuran !30 ml) was added dropwise a solution of
4-(pyrrol-1-yl)-N-methyl-N-methoxybenz~mide (2.11 g) in
tetrahydrofuran ~40 ml) at 5C and the mixture was sti~red
at 5C for 1.5 hours. To the mixture were added sodium
fluoride (1.54 g) and water (495 mg), ard the ~ixture was
stlrred at room temperature for 30 minutes. The insoluble
materials were riltered off ~nd washed with
.etrahydrofuran. The filtrate was evaporated in vzcuo and
the residue was purified bv column chromatography o~ s, Iic2
gel to give 4-(pyrrol-1-yl)benzaldehyde (1.65 g).
IR (KBr) : 3130, 2800, 2745, 1690, 1605, 152G cm~
NMR (CDC13, o) : 6.35-6.45 (2H, m), 7.i5-7.25 (2H,
~), 7.5-7.6 (2H, m), 7.9-8.0 (2H, mj, 9 99 (I~
s )
A~PCI-MASS (m/z) : 172 (M+H+)
Preparation 37
The following compounds were obtained according to Q
similar manner to that of Preparation 36.
(1) 3-(Pyrrol-1-yl)benzaldehvde
IR (Film) : 3220, 1700, 1650, 1590, 1540, 1500 cm 1
. .
PCTt~5/01982
WO96/105Ss
~2 Q ~ g 8
- 49 -
NMR (CDC13, o) : 6.4-6.45 (2H, m), 7.1~-7.20 (2H,
m) ~ 7.55-7.8 (3H, m), 8.9-8.95 (1~, m), 10.06
(lH, s)
APCI-MASS (mi7) 172 (M+:~+)
(2) 4-(4-Dimethylaminopnenyl)benz21dehyde
IR (KBr) : 2895, 2810, 2725, 1695, 168C, 1595,
1540 cm~l
N~R (CDC13, o) : 3.03 (6H, s), 6.8-6.9 (2H, m),
7.55-7.65 (2H, m) ~ 7.65-7.75 (~H, ~), 7.85-7.95
(2H, m), 10.01 (lH, s)
APCI-MASS (m/z) : 226 (M+H+)
(3) 4-(Phenylsulfamoyl)benzaldehyde
IR (KBr) : 3260, 3055, 28~0, 1695, 1595 cm~l
NMR (DMSO-d6, o) : 7.0-7.15 (3H, m), 7.2-7.3 (2H,
m), 7.93 (2H, d, J=8.1Hz), 8.05 (2H, d, J=8.1~z),
10.04 (lH, s), 10.48 (lH, br s)
APCI-MASS (m/z) : 262 (M+H+)
(4) 2-Bromo-5-furaldehyde
IR (KBr) : 1670, 1464, 1377, 1271 cm 1
NMR (CDC13, o) : 6.57 (lr., d, J=3.6Xz), 7.1~ (lH,
d, J=3.~Hz), 9.54 (1~, s)
~reparation 38
To a suspension of 4-bromobenzaldehyde (1.85 g) and
[4-flurophenyl]dihydroxyborane ~1.40 g) in loluene (50 ml)
was added powdered potassium carbonate (2.07 g), rollowed
by addition of tetrakis(triphenylphosphine)palladium(0)
(578 mg) and the mixture was refluxed for 24 hours under
r~ltroger.. The mixture was poured into a mixture ol ethyl
acetate and ice water, and the se~arated organ - layer was
washed witn water and brine, dried over magnesium sulfate
and evaporated in vacuc. The residue was puri_ied by
.
WO96/10559 ~2 ~ O ~ ~ 1 PCT/~5/01982
- 50 -
column chromatography on silica gel ~c give 4-(4-
fluorophenyl)benzaldehyde (1.67 g) as a white crvstal.
IR (KBr) : 3055, 2855, 27J5/ 1705, 1600, 1565,
1520 cm~1
NMR (CDCl3, o) : 7.1-7.25 (2H, m), 7.55-7.7 (2H,
m), 7.71 (2H, d, J=8.2Hz), 7.95 (2H, d, J=8.~Hz),
10.06 (lH, s)
APCI-MASS (m/z) : 201 (M+H+)
Preparation 39
To a solution of 2-bromo-5-thiopnenecarbaldehyde (2 g)
and dihydroxyphenylborane (1.66 g) was added 2M sodium
carbonate solution (13.6 ml) and
tetrakis(triphenylphosphlne)palladium(0) !605 mg). The
mixture was heated for 5 hours at 80C. The reaction
mixture was poured into water, extracted with
dichloromethane. The organic layer was washed with water
and brine, dried over magnesium sulf te, evaporated in
vacuo. The residue was chromatographed on silic2 gel (100
g, eluting with n-hexane - ethyl aceta~e (5:1)) tc give 2-
phenyl-5-thiophenecarbaldehyde (1.80 g).
IR (KBr) : 1647, 1441, 1232, 754 cm~1
NMR (CDC13, o) : 7.33-7.50 (4H, m), 7.60-7.80 (3H,
m), 9.90 (lH, s)
APCI-MASS (m/z) : 189 (M+H+)
Preparation 40
The following compounds were obtained according to a
similar manner to that of Preparation 39.
(1) 2-Phenyl-5-furaldehyde
IR (Neat) : 1674, 1522, 1475, 1257 cm~1
NMR (CDC13, o) : 6.85 (lH, d, J=3.7Hz), 7.33 (lH,
d, J=3.7Hz), 7.37-7.53 (3H, m), 7.80-7.92 (2H,
m), 9.66 (lH, s)
PCT/~5/01982
WO96/10~59
- 51 -
~PCI-MASS (m/z) : 173 (~+H+)
(2) 4-Phenyl-2-thiophenecarbaldehyde
IR (KBrj : 1676, 1539, 1429, 1173, 760 cm 1
N~ (CDCl3, o) : 7.30-7.66 (5H, m) ~ 7.82-7.90 (lH,
m), 8.00-8.08 (1~, m), 9.98 (lH, d, J=1.2Hz)
FAB-~SS (m/ 7. ) 189 (MlH+)
(3) 4-(4-Methylphenyl)benzaldehyde
IR (KBr) : 3095, 3060, 2860, 2765, lo90, 1600,
1575, 1505 c~
NMR (CDCl3, o) : 2.42 (3H, s), 7.29 (2H, d,
J=10.4Hz), 7.55 (2H, dd, J=6.3, 1.8Hz), 7.74 (2H,
dd, J=6. 6, 1. 8Hz), 7.94 (2H, dd, J=6.6, 1.8Hz),
10.05 (lH, s)
APCI-MASS (m/z) : 197 (M+H+!
(4) 4-(4-Chlorophenyl)benzaldehyde
IR (KBr) : 3055, 2820, 2720, 1695, 1605 cm 1
NMR (CDC13, o) : 7.4-7.5 (2H, ~), 7.55-7.65 (2H,
m), 7.7-7.8 (2H, m), 7.9-8.0 (2H, m), 10.06 (i:H,
s)
APCI-MASS (m/z) : 217 (M+H+)
(5) 4-(4-Bromophenyl)benzaldehyde
IR (KBr) : 3050, 2820, ~725, 705, 1605, i575,
1555 cm~l
NMR (CDC13, o) : 7.45-7.55 (2H~ m), 7.5_-7.65 (2U,
m), 7.65-7.75 (2H, m), 7.35-8.05 (2H, m), 10.06
(lH, s)
APCI-M~SS (m/z) : 263, 261 (M+H+)
..
Pre~ation 41
To a solution of 4-carboxyben aldehyde (3.00 g3 and
triethylamine (2.23 g) in dichloromethane (50 ml) was added
WO96/10559 2 2 ~ ~ ~ 8 ~ PCT/~5/01982
dropwise isobutyl chloroformate (3.01 g) at 5C and the
mixture was stirred at 5C for 40 minutes. To thls
solution was added aniline (2.05 g) and the mixture was
stirred at room temperature for 15 hours. ~ater was added
to the mixture, and the separated organic layer was washed
with brine, dried over magnesium sulfate and evaporated in
vacuo. To the residue was added hexane:ethyl acetate (1:1
and the powder was collected by filtration to give 4-
(phenylcarbamoyl)benzaldehyde (2.24 g). The filtrate was
evaporated in vacuo and the resldue was purlfied by column
chromatography on silica gel to give the second crop (1.12
g)-
IR (KBr) : 3340, 3055, 2820, 2725, 1705, 1650,157', 1535 cm~l
NMR (DMSO-d6, o) : 7.13 (lH, t, J=7.3Hz), 7.3-7.45
(2H, m), 7.79 (2H, d, J=7.5Hz), 8.0-8.2 (4H, m),
10.12 (lH, s), 10.46 (lH, s)
APCI-M~SS (m/z) : 226 (M HT)
Preparation 42
To a solution of ethyl 4-aminobenzoate (3.30 g) in
pyridine (10 ml) was added dropwise benzoyl chloride
(3.09 g) at 5C, and the mixture was stirred at room
temperature for 1.6 hours. The mixture was poured into a
mixture of ethyl acetate, ice water and 6N hydrochloric
acid (40 ml), and the separated organic layer was washed
with water and brine, dried over magnesium sulfate and
evaporated in vacuo. The resicue was crystallized from
hexane and ethyl acetate (5:1), and the crystal was
collected bv filtration to give ethyl 4-(benzoylamino)-
benzoate (5.14 g~.
IR (KBr) : 3300, 3050, 2980, 1720, 1650, 1530 cm~l
NMR (DMSO-d6, o) : 1.34 (3H, t, J=7.1Hz), 4.34 (2H,
q, J=7.1Hz), 7.5-7.7 (5H, m), 7.g5-8.2 (3H, m),
8.45-8.5 (lH, m), 10.48 (lH, s)
~ PCT/~5/01982
WO96/10559 ~ 2
- 53 -
APCI-MASS (m/7) 270 (M+H+)
- Preparation 43
The following compound was obtained according to a
- 5 similar manner to tha~ of Preparation 41.
4-(2-Pyridylcarbamoyl)ben7aldehyde
IR (KBr) : 3230, 3180, 3115, 3035, 2810, 2725,
1710, 1675, 1585, 1540 cm~1
NMR (DMSO-d6, o) : 7.20 (lH, dd, J=6.8, 1.5Hz),
7.8-7.9 (lH, m) ~ 8.03 (2H, d, J=8.4Hz), 8.20 (2.i,
d, J=8.4Hz), 8.15-8.25 (1~, m), 8.4-8.45 (lH, m),
10.12 (lH, s), 11.06 (lH, s)
APCI-MASS (m/z) : 227 (M+H+)
Pre~aration 44
To a solution of [2-(4-chlorophenyl)thiazol-4-
yl]methanol (1.4~ g) in chloroform (80 ml) was added
activated manganese dioxide (5.48 g) and the mixture was
refluxed for 1.8 hours. The mixture was fil-~red and the
fil.rate was evaporated in vacuo tG give 4-formyl-2-(4-
chlorophenyl)thiazole (1.28 g).
IR (KBr) : 3110, 2840, 1695, 1595, 1575, 1500 cm
NMR (DMSO-d6, o) : 7.55-7.65 (2H, m) ~ 8.0-8.1 (2H,
m), 8.80 (lH, s), 3.99 (lH, s)
APCI-MASS (m/z) : 224 (M+H+)
'reparation 45
To a solution of (3-phenylpyrazol-5-yl)methanol (1.30
g) in acetone (130 ml) was added activated manganese
dioxide (6.5 g) and the mixture was refluxed 1.5 hours.
The mixture was filtered and the filtrate was evaporated in
vacuo to give 3-phenyl-5-formylpyrazole (1.16 g).
IR (KBr) : 2400-3500 (br), 1676, 1473, 1282,
1192 cm~l
~ ~ O ~ ~ ~ PCT/~5/01982
WO96/10559
- 54 -
NMR (DMSO-d6, o) : 7.20-7.56 (4H, m), 7.75-7.95
(2H, m), 9.93 (lH, s), 14.05-14.30 (lH, br)
APCI-MASS (m/z) : 173 (M+H+)
.
Preparation 46
The following compounds were obtained according to
similar manners to those of Preparations 44 and 45.
(1) 3-(Pyrazol-3-yl)benzaldehyde
IR (Film) : 3325, 2975, 2920, 2840, 27a5, 17C0,
1610, 1585 cm~î
NMR (DMSO-d6, o) : 6.84 (lH, d, J=2.0Hz), 7.6-8.2~
(4H, m), 8.36 ~lH, s), 10.07 (lH, s), 13.05 (lH,
br s)
APCI-MASS (m/z) : 173 (M+H+)
(2) 6-Phenyl-3-formylpyridine
IR (KBr) : 3060, 2835, 2785, 2740, 1695, 1590,
1560 cm~l
NMR (CDC13, o) : 7.25-7.4 (4H, m), 7.92 (lH, d,
J=8.3Hz), 8.05-8.15 (2H, m), 8.24 (lH, dd, J=8.3,
2.2Hz), 9.14 (lH, dd, J=2.2, 0.7Hz), 10.14 ~lH,
s )
APCI-MASS (m/z) : 184 (M+H+)
(3) 2-Formyl-4-(pyrrol-i-yl)pyridine
IR (KBr) : 3110, 2845, 1705, 1535 cm 1
NMR (DMSO-d6, o) : 6.35-6.4 (2H, m), 7.75-7.8 (2H,
m), 7.98 (lH, dd, J=5.2, 2.5Hz), 8.12 (lH, d,
J=2.2Hz), 8.80 (lH, d, J=5.5Hz), 10.0 (lH, s)
APCI-MASS (m/z) : 173 (M+H+)
(4) 6-Phenylthio-3-formylpyridine
IR (Film) : 3055, 2840, 2780, 1700, 1585, 1550 cm~
NMR (CDCl3, ~) : 6.94 and 7.4g (total lH, d,
pCT/~5/01982
WO96/10559 ~ 8
- 55 -
J=8.4Hz), 7.45-7.65 (6H, m), 7.89 and 8.14 (total
lH, dd, J=8.4, 2.2Hz), 8.82 and 8.87 (total lH,
- d, J=2.2Hz), 9.98 and 10.10 (total lH, s)
APCI-MASS (m/z) : 215 ~M+H+)
(5) 4-(Benzoylamino)benzaldehyde
IR (KBr) : 3305, 3055, 2840, 2735, 1715, 1660,
1645, 1540 cm~1
N~R (DMSO-d6, o) : 7.5-7.7 (5H, m), 7.95-8.15 (3H,
m), 8.40 (lH, s), 10.02 (lH, s), 10.54 (lH, s)
APCI-M~SS (m/z) : 226 (M+H+)
(6) a-(Phenylsulfonylamino)benzaldehyde
IR (KBr) : 3240, 3060, 2935, 2850, 2765, 1690,
1680, 1580, 1510 cm~1
NMR (DMSO-d6, o) : 7.29 (2H, d, J=8.6Hz), 7.55-7.7
(3H, m), 7.75-7.9 (4H, m), 9.81 (lH, s), 11.01
(lH, s)
APCI-MASS (m/z) : 262 (M+H-)
(7) 4-(3-Thienyl)benzaldehyde
IR (KBr) : 1689, 1601, 1211, 1167 cm~1
NMR (CDCl3, o) : 7.41-7.47 (2H, m), 7.62 (lH, t,
- J=2.1Hz), 7.7û-7.83 (~H, m~, 7.85-7.98 (2H, m~,
10.02 (lH, s)
APCI-MASS (m/z) : 189 (M+H+)
(8) 4-(2-Thienyl)benzaldehyde
IR (KBr) : 1699, 1601, 1213, 1170 cr.~1
NMR (CDC13, o) : 7.14 (lH, dd, J=5.1, 3.7Hz), 7.4û
(lH, dd, J=5.1, l.lHz), 7.47 (lH, dd, J=3.7,
l.lHz~, 7.70-7 82 (2H, m), 7.82-7.96 (2Y, m),
10.00 (iH, s)
APCI-MASS : 189 (M+H+)
.
WO96/10559 PCT/~5/01982
2 2 ~
- 56 -
(9) 4-(Pyrazol-3-yl)benzaldehyde
IR (Neatj : 2400-3700 (br), 1697, 1606, 12il, 1171,
837 cm~l
NMR (DMSO-d6, o) : 6.90 (lH, d, J=2.3Hz), 7.83 (lH,
br s), 7.85-8.12 (4H, m), 10.00 (lH, s), 13.13
(lH, br)
~PCI-MASS (m/z) : 173 (M+H )
(10) 4-(1-Methylpyrazol-3-yl)benzaldehyde
IR (KBr) : 1695, 1603, 1566, 1431, 1306 cm 1
NMR (CDC13, o) : 3.99 (3H, s), 6.64 (lH, d,
J=2.3Hz), 7.43 (lH, d, J=2.3H7), 7.86-8.03 (4H,
m), 10.01 (lH, s)
APCI-MASS (m/z) : 187 (M+H+,
(11) 4-(1-Methylpyrazol-5-yl)benzaldehyde
IR (KBr) : 1695, 1608, 1568, 1390, 1215, 1184 cm~
NMR (CDC13, o) : 3.95 (3H, s), 6.~1 (ln, d,
J=1.9Hz), 7.56 (lH, d, J=1.9Hz), 7.57-7.68 (2H,
m), 7.93-8.04 (2H, m), 10.08 (lH, s)
APCI-M~SS (m/z) : 187 (M+H+)
(12) 3-(lH-Tetrazol-5-yl)benzaldehyde
IR (KBr) : 2400-3500 (br), 1674, 1612, 1560, 1373,
1207 cm~l
NMR (DMSO-d~, o) : 7.86 (lH, dd, J=7.7, 7.7Hz),
8.08-8.20 (lH, m), 8.30-8.~2 (lH, .~..), 8.57 (lH,
dd, J=1.5, 1.5Hz), 10.13 (lH, s)
APCI-MASS (m/z) : 175 ~M+H+)
Preparation 47
To a suspension of 3-(pyrazol-3-yl)benzoritril_ (37.70
g) in formic acid (300 ml) was added a susper~sion of Rane~
Nickel (Trademark : NDT-90) in water (130 ml) and the
mixture was refluxed for 3.5 hours. The mixture was cooled
~ ~ PCT/~5/01982
WO96/10559 8
to room temperature and Raney N.ckel was removed b~
fillration and washed with formic acic (150 ml!. The
filtrate was evaporated to dryness and dichloromethane and
ice water were added to the residue. The mixture was
adjusted tc pH ca. 8.5 by addition of 5N sodium hydroxide
aaueous soiution. The insoluble materials were re~oved by
celite pad and the filtrate was separated. The organic
layer was washed with brine, dried over magnesium sulfate
and evaporated in vacuo. The residue was purilied by
cclumn chromatography on silica gel to give 3-(~yrazol-3-
yl)benzaldehyde (37.17 g).
IR (KBr) : 3190, 2975, 2840, 1690, 1605, 1585 cm 1
NMR (DMSO-d6, o) : 6.84 (lH, d, J=2.2H7), 7.65 (lH,
dd, J=7.6, 7.6Hz), 7.75-7.85 (2H, m), 7.84 (lH,
d, J=7.6Hz), 8.35 (lH, s), 10.07 (lH, s;, 13.C6
(lH, br s)
Preparation 48
To a solution of 4-fluorobenzaldehyde (2.48 g) and 4-
bromophenol (3.46 g) in N,N-dimethylace~amide (20 ml) was
added powdered potassium carbonate (2.76 g), and tne
mixture was refluxed for 17 hours. The mixture W2S poured
into a mixture of ethyl acetate and ice water, and the
separated organic layer was washed with water and brine,
dried over magnesium sulfate and evaporated in vacuG. The
residue was purified by colu~n chromatography on silica ael
to give 4-(4-bromophenoxy)benzaldehyde (1.51 g).
IR (~Br) : 3030, 2920, 2840, 2735, 1705, 16~0,
1560 cm 1
3C NMR (CDC13, o) : 6.95-7.1 (4H, ~), 7.45-7.55 (2H,
m), 7.8-7.9 (2H, m), 9.94 (lH, s)
APCI-MASS (m/z) : 279, 277 (M+H~)
Preparation 49
To a solution of 4-bromobenzaldehyde (4.96 g) and
PCT/~5/01982
Wo g6/10559 ~ 8 ~1 ~
4-fluorophenol (4.48 g) in N,~-di~ethylacetamide (25 m')
was added powdered potassium carbonaLe (5.53 g), and the
mixture was refluxed for 6 hou~s under nitrogen. The
mixture was poured into a mixture of ethyl acet2te ana
water, and the separated organic layer was -~ashed with
water and brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give 4-(4-
fluorophenoxy)benzaldehyde (1.92 g) as an crange oil.
IR (Film) : 3360, 3075, ~835, 2740, 1695, 16C0,
1585, 1495 c~,-1
NMR (CDCl3, o) : 6.7-6.8 (1~, m), 6.8~-6.95 ~lH,
m), 7.0-7.2 (4H, m), 7.8-7.9 (2H, m), 9.92 (lH,
s)
APCI-MASS (m/z) : 217 (M+H+)
Preparation 50
To a solution of 4-phenylthiobenzonitrile (12.23 g) in
toluene (200 ml) was added dropwise diisobutylaluminum
hydride (1.02M toluene solution) (114 ml) a~ -70C over 50
minutes and the mixture was stirred at -70C for 30
minutes. To the mixture were added sodium fluoride (13.45
g) and water (6.26 g), and the mixture was warmed to room
temperature. The insoluble materials were removed by
filtration and washed with toluene. The filtrate was
evaporated in vacuo and the residue was dissolveà in
tetrahydrofuran (50 ml). To this solution was added 6N
hydrochloric acid (19.3 ml) and the mixtu~e was stirred at
room temperature fo~ 1 hour. The mixture was extracted
with ethyl acetate, and the organic layer was washed with
water and brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was ~uriried by column
chromatography on cilica gel to give 4-
(phenylthio)benzaldehyde (9.83 g) as a vellow oil.
IR (Film) : 3055, 2830, 2745, 1695, 1595, 1560,
W096tlO559 PCTt~5/01982
g 8 ~1 .
- 59 -
1505 cm~1
NMR (CDCl3, o) : 7.15-7.3 (4H, m), 7.35-7.6 (3H,
m?, 7.65-7.75 (2H, m), 9.91 (lH, s)
APCI-MASS (m/z~ : 215 (M+H+)
Preparation 51
To a solution of 4-(pyrazol-1-yl)benzonitrile (5.0 g)
in dichloromethane (150 ml) was added dropwise
diisobutylaluminum hydride (1.02M tcluene solution, 58 ml)
keeping the temperature below -60C. After stirring for
one hour, sodium fluoride (9.95 g) and water (3.2 ml) were
added thereto. The reaction mixture was warmed to room
temperature over 30 minutes and stirred for 1.5 hours.
Insoluble material was removed by fll~ration= T:ne filtrate
was concentrated by evaporation in vacuo. The residue was
dissolved in tetrahydrofuran (25 ml). To he solution was
added lN-hydrochloric acid and stirred for one hour at room
temperature. To the mixture was added 5~-sodium hydroxide
solution (10 ml). The objective compound was extracted
with dichloromethane. The organic layer was washed with
water and brine, dried over magnesium sulfate, evaporated
ir. vacuo. The residue was chromatographed on silica ge'
(100 g, n-hexane - ethyl acetate (1:1)) to give 4-(pyrazol-
1-yl)benzaldehyde (4.36 g).
IR (KBr) : 1695, 1605, 1390, 1200 cm~1
NMR (CDCl3, o) : 6.54 (lH, dd, J=2.5, 1.8Hz), 7.79
(lH, d, J=1.5Hz), 7.85-8.10 (5H, m), 10.02 (lH,
s )
APCI-MASS (m/z) : 173 (M+H+)
Preparation 52
The followirg compound was obtained according to a
similar manner to that of Preparatio~ 51.
4-(Imidazol-l-yl)benzaldehyde
WO96/1055s PCT/~5101982
2200g8 1
- 60 -
IR (K3r) : 1686, 1606, 152~, 1313 cm 1
NMR (CDCl3, ~ : 7.15-8.10 (7H, ~J, 10.05 (lH, s)
APCI-MASS (m/z) : 173 (M+H+)
Preparation 53
To a solution ol methyl 5-phenyl-3-
isoxazolecarboxylate (4.73 g) in d~chloromethane (150 ml)
was added dropwise diisabutylaluminum hydride (1.02M
toluene solution 45.7 ml) at -7CC ~ -60C. After stirring
for one hour at the same temperature, sodium fluoride (7.83
g) and water (2.52 ml) were added thereto. ~he mixture was
warmed to room temperature over 30 minutes and stirred for
one hour. Insoluble materials were removed by filtration.
The filtrate was evaporated in vacuo. The residue was
chromatographed on silic2 gel (85 g, n-hexane - ethyl
acetate (3:1)) to give 5-phenyl-3-isoxazolecarbaldehyde
(1.94 g).
IR (KBr) : 3126, 1713, 1568, 145~, 1184 cm 1
NMR (CDCl3, o) : 6.90 (lH, s), 7.35-7.68 (3H, m),
7.75-7.92 (2H, m.), 10.20 (1~, s)
Preparation 54
To the solution of 4-bromobenzaldehyde (462 mg) and 1-
methyl-4-tri-n-butylstannylpyrazole (1.1 g) was added
tetra~is(triphenylphosphine)palladium(0) (87 mg). Then the
mixture was heated for 3 hours at 140C. After cooling,
the reaction mixture was diluted with toluene (6 ml). ~
aaueous solution (5 ml) of potassi~m fluoride ~1.7~ g) was
added to the mixture and stirred ror one hour. Insoluble
material was removed ~y ~iltration. The filtrate was
washed with water and brine, dried over magnes um sulfate,
and evaporated in V2CUG. ~he residue was chromatographed
on silica gel (4C g, eluting with n-hexane - ethyl acetate
(1:2)) to give 4-(1-methylpyrazol-4-yl)benzaldehyde (427.4
mg).
PCT/~95/01982
Wo96tlO559
- 61 - -
IR (KBr) : 1693, 1605, 1169, 831 cm~1
NMR (CDCl3, o) : 3.98 (3H, s), 7.57-7.67 (2H, m),
7.73 (1~:, s), 7.85 (lH, s), 7.80-7.9~ (2H, m),
9.98 (lH; s)
- 5 APCI-MASS (m/z) : 187 (M+H+)
Preparation 55
To a solution o~ oxalyl chloride (1.5 ml) in
d chloromethane (30 ml) was added a solution of dimethyl
sulroxide (1.83 ml) in dichloromethane (4 ml) keeping the
temperature below -60C. After 20 minutes, 4-(oxazol-5-
yl)benzyl alcohol (2.5 g) in dichloromethane (25 ml) and
dimethyl sulfoxide (2 ml) was added dropwise at the same
temperature then stirred for one hour. To the mixture was
added triethylamine (8 ml) and stirred for 30 minutes. The
reaction mixture was warmed to room te~erature over 30
minutes. After stirring for one hour, the mixture was
diluted with ethyl acetate, washed with water and brine,
dried over magnesium sulfate, eva~orated in vacuo. The
residue was chromatographed on silica gel (80 g, eiuting
with n-hexane - ethyl acetate (1:1)) to give 4-(cxazol-5-
yl)benzaldehyde (2.20 g).
IR (KBr) : 1693, 1610, 1211, llll, 829 cm~1
NMR (CDC13, o) : 7.54 (lH, s), 7.75-8.05 (4H, m),
8.00 (1~, s), 10.03 (lH, s)
~PCI-MASS (m/z) : 17a (M+H+j
Preparation 56
To the solution o 3-(lH-tetrazol-5-yl)ben7aldehyde
(1.0 g) in pyrldine (15 ml) was added
triphenylchloromethane (1.76 g) at 0~5C. The mixture was
stirred for 4 hours at room temperature. The reaction
mixture was poured into water and extracted with elhyl
acetate. The organic layer was washed with dil.
hydrochloric acid, water, brine, dried over magnesium
WO96/10559 PCrJ~/01982
~2~ 09 8`~
- 62 -
sulfate, evaporated in vacuo to give 3-(1-trityl-lH-
tetrazol-5-yl)benzaldehyde (2.51 g).
IR (KBr) : 1699, 1516, 1491, 1446, 1201 cm~l
NMR (DMSO-d6, o) : 7.05-7.20 and 7.38-7.53 (15H,
m), 7.80 (lH, dd, J=7.7, 7.7Hz), 8.05-8.14 (lH,
m), 8.30-8.40 (lH, m), 8.50-8.55 ~lH, m), 10.12
(lH, s)
Preparation 57
The mixture of 4-formylbiphenyl (3.64 g) and
cycloheptylamine (2.49 g) was heated at 120C for 6 hours
under nitrogen. The mixture was cooled to room temperature
and dissolved in ethanol (30 ml). To the solution was
added carefully sodium borohydride (757 mg), and the
mixture was stirred at room temperature for 1 hour. The
mixture was evaporated in vacuo and the residue was
extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give N-(4-
biphenylylmethyl)cycloheptylamine (5.24 g) as a yellow oil.
IR (Film) : 3030, 2920, 2850 cm 1
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.5-2.7 (lH,
m), 3.72 (2H, s), 7.3-7.7 (9H, m)
APCI-MASS (m/z) : 280 (M+H+)
Preparatio~ 58
The suspension of 4-[4-(dimethylamino)phenyl]-
benzaldehyde (640 mg) and cycloheptylamine (643 mg) in
toluene (3 ml) was stirred at 1~0C for 5 hours under
nitrogen. The mixture was evaporated to dryness and
dissolved in ethanol (20 ml). To this solution was added
sodium borohydride (107 mg) and the mixture was stirred at
room temperature for 1 hour. The mixture was evaporated to
dryness and the residue was extracted with dichloromethane.
PCT/~5/01982
W096/lOS59
- 63 -
The organic layer was washed with brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give N-
cycloheptyl a-[4-(dimethylamino)phenyl]benzylamine (945
mg).
IR (KBr) : 3275, 3025, 2920, 2850, 2805, 1610,
1535, 1505 cm~1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.65-2.85 (lH,
m), 2.99 (6H, s), 5.79 (2H, s), 6.80 (2H, d,
J=8.8Hz), 7.34 (2H, d, J=8.8Hz), 7.45-7.65 (4H,
m)
APCI-MASS (m/z) : 323 (M+H+)
Preparation 59
The mixture of 4-phenoxybenzaldehyde (1.98 g) and
benzylamine (1.61 g) was stirred at 120C for 4 hours under
nitrogen. The mixture was cooled to room temperature and
dissolved in ethanol (20 ml). To this solution was added
sodium borohydride (378 mg) and the mixture was stirred at
room temperature for 1 hour. The mixture was evaporated to
dryness and the residue was extracted with dichloromethare.
The organic layer was washed with brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give N-
benzyl-4-phenoxybenzylamine (2.07 g).
IR (Film) : 3035, 2915, 2820, 1680, 1505 cm 1
N~R (CDC13, o) : 3.78 (2H, s), 3.8~ (2H, s), 6.9-
7.4 (14H, m)
APCI-MASS (m/z) : 290 (M+H+)
Preparation 60
The mixture of 4-phenoxybenzaldehyde (1.98 g) and
furfurylamine (1.61 g) was stirred at 120C for 4 hours
under nitrogen. The mixture was cooled to room temperature
and dissolved in ethanol (20 ml). To this solution was
PCT1~5/01982
W096/10559 2 2
- 64 -
added sodium borohydride (378 mg) and the mixture was
stirred at room temperature for 1 hour. The mixture was
evaporated to dryness and the residue was extracted with
dichloromethane. The organic layer was washed with brine,
dried over magnesium sulfate and evaporated in vacuo. The
residue was puri~ied by column chromatography on silica gel
- to give N-furfuryl-4-phenoxybenzylamine (2.51 g).
IR (Film) : 3060, 3035, 2920, 2830, 159G, 1505 cm 1
NMR (CDC13, o) : 3.76 (2H, s), 3.80 (2H, s),
6.15-6.2 (lH, m), 6.3-6.35 (lH, m), 6.9-7.4 (lOH,
m)
APCI-MASS (m/z) : 280 (M+H+)
Pxeparation 61
The following compounds were obtaine~ according to
similar manne~s to those of Preparation 57, 58, 59 and 60.
(1) N-(2-Biphenylylmethyl)-cycloheptylamine
IR (Film) : 3060, 3020, 2935, 2910, 2850, 1460 cm~
NMR (CDCl3, o~ : 1.2-1.8 (12H, m), 2.4-2.6 (lH, m),
3.71 (2H, s), 7.2-7.5 (9H, m)
APCI-MASS (m/z) : 280 (M+H+)
(2) N-Cycloheptyl-4-phenoxybenzylamine
IR (Film) : 3030, 2920, 2850, 1590, 1505 cm~1
NMR (CDC13, o) : 1.4-2.0 (12H, m), 2.6-2.8 (lH,
m)~ 3.75 (2H, s), 6.9-7.4 (9H, m)
APCI-MASS (m/z) : 296 (M+H+)
(3) N-Cyclohexyl-3-phenoxybenzylamine
IR (Film) : 3035, 2925, 2850, 1585 cm 1
NMR (CDCl3, o) : 1.3-2.0 (12H, m), 2.6-2.8 (lH, m),
3.75 (2H, s), 6.8-7.4 (9H, m)
APCI-MASS (m/z) : 296 (M+H~)
PCT/~5/01982
WO96/10559 ~ 2 ~ ~ 9 ~ 1 .
- 65 -
(4) N-Cycloheptyl-[2-(4-chlorophenyl)thiazol-4-
yl]methylamine
IR (KBr) : 2930, 2850, 1595 cm 1
NMR (DMSO-d6; o) : 1.3-2.2 (12H, m), 2.6-2.8 (lH,
m), 3.84 (2H, s), 7.49 (lH, s), 7.5-7.6 (2H, m),
7.9-8.0 (2H, m)
APCI-MASS (m/z) : 321 (M+H+)
(5) N-Cycloheptyl-(2-phenylimidazol-5-yl)methylamine
IR (KBr) : 3080, 2925, 2855, 1575 cm 1
NMR (DMSO-d6, o) : 1.3-l.9 (12H, m), 2.6-2.75 (lH,
m), 3.62 (2H, br s), 6.82 and 7.06 (total lH, br
s), 7.25-7.45 (3H, m), 7.8-7.95 (2H, m), 12.28
(lH, br)
APCI-MASS (m/z) : 270 (M+H+)
(6) N-Cycloheptyl-4-(pyrrol-1-yl)benzylamine
IR (Film) : 2925, 2850, 1610, 1525 cm 1
NMR (CDCl3, o) : 1.3-2.0 (12H, m), 2.6-2.8 (lH, m),
3.79 (2H, s), 6.3-6.4 (2H, m), 7.0-7.1 (2H, m),
7.3-7.45 (4H, m)
APCI-MASS (m/z) : 269 (M+H+)
(7) N-Cycloheptyl-3-(pyrrol-1-yl)benzylamine
IR (Film) : 2925, 2850, 1610, 1595, 1545, 1500 cm 1
NMR (CDCl3, o) : 1.4-1.95 (12H, m), 2.6-2.8 (lH,
m), 6.30-6.35 (2H, m), 7.10-7.15 (2H, m), 7.15-
7.45 (4H, m)
APCI-MASS (m/z) : 269 (M+H+)
(8) N-Cycloheptyl-[4-(pyrrol-1-yl)pyridin-2-yl]methylamine
IR (Film) : 3305, 3135, 3100, 2925, 2855, 1600,
1575 cm-1
NMR (DMSO-d6, o) : 1.3-2.0 (12H, m), 2.55-2.7 (lH,
m), 3.82 (2H, s), 6.35-6.4 (2H, m), 6.5-6.55 (2H,
2 ~ PCT/~5101982
WO96/10559
- 66 -
m), 6.55-6.6 (lH, m), 6.65-6.7 (lH, d, J=2.2Hz),
8.47 (lH, d, J=5.6Hz)
APCI-MASS (m/z) : 282 (M+H+)
(9) N-Cycloheptyl(6-phenylpyridin-3-yl)methylamine
IR (Film) : 3030, 2910, 2850, 1560 cm 1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.6-2.8 (lH, m),
3.83 (2H, s), 7.3-7.5 (2H, m), 7.65-7.8 (2H, m),
7.95-8.05 (2H, m), 8.61 (lH, s)
APCI-MASS (m/z) : 281 (M+H+)
(10) N-Cycloheptyl-3-(pyrazol-3-yl)benzylamine
IR (Film) : 3210, 2915, 2850, 1610, 1540 cm 1
NMR (DMSO-d6 o) : 1.3-1.9 (12H, m), 2.5-2.7 (lH,
m), 3.72 (2H, s), 6.68 (lH, d, J=2.1Hz), 7.15-7.8
(5H, m)
APCI-MASS (m/z) : 270 (M+Ht)
(11) N-Cycloheptyl-4-(4-fluorophenyl)benzylamine
IR (Film) : 2925, 2855, 1500 cm 1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.65-2.85 (lH,
m), 3.81 (2H, s), 7.05-7.2 (2H, m), 7.35-7.6 (6H,
m)
APCI-MASS (m/z) : 298 (M+H+)
(12) N-Cycloheptyl-4-(4-chlorophenyl)benzylamine
IR (KBr) : 3030, 2925, 2855, 1485 cm~1
NMR (CDCl3, o) : 1.35-2.0 (12H, m), 2.6-2.8 (lH,
m), 3.82 (2H, s), 7.4-7.6 (8H, m)
30 APCI-MASS (m/z) : 314 (M+H+)
(13) N-Cycloheptyl-4-(4-bromophenyl)benzylamine
IR (KBr) : 3035, 2925, 2855, 1480 cm~1
NMR (CDCl3, o) : 1.3-2.0 (12H, m), 2.6-2.8 (lH, m),
3.81 (2H, s), 7.35-7.65 (8H, m)
WO96/105S9 PCTt~5/01982
,~ ~2~0~8~ ~
- 67 -
APCI-MASS (m/z) : 360, 358 (M+H+)
(14) N-Cycloheptyl-4-(4-methylphenyl)benzylamine
IR (Film) : 3025, 2910, 2855, 1500 cm 1
- 5 NMR (CDCl3, o) : 1.3-2.0 (12H, m), 2.39 (3H, s),
2.65-2.8 (lH, m), 3.81 (2H, s), 7.24 (2H, d,
J=7.6Hz), 7.37 (2H, d, J=8.3Hz), 7.4-7.6 (4H, m)
~PCI-MASS (m/z) : 294 (M+H+)
(15) N-Cycloheptyl-4-(4-bromophenoxy)benzylamine
IR (Film) : 3030, 2925, 2850, 1585, 1505, 1480 cm 1
NMR (CDCl3, o) : 1.3-2.0 (12H, m), 2.6-2.8 (lH, m),
3.75 (2H, s), 6.8-7.0 (4H, m), 7.25-7.5 (4H, m)
APCI-MASS (m/z) : 376, 374 (M+H+)
(16) N-Cycloheptyl-4-(4-phenylthio)benzylamine
IR (Film) : 2920, 2850, 1510 cm~l
NMR (CDC13, o) : 1.3-2.0 (12H, m), 2.55-2.75 (lH,
m), 3.75 (2H, s), 7.2-7.5 (9H, m)
APCI-MASS (m/z) : 312 (M+H+)
(17) N-Cycloheptyl-(6-phenylthiopyridin-3-yl)methylamine
IR (Film) : 3305, 2925, 2850, 1700, 1585, 1560 cm~
NMR (CDC13, o) : 1.3-2.0 (12H, m), 2.55-2.75 (lH,
m), 3.71 (2H, s), 6.87 (lH, d, J=8.2Hz), 7.4-7.7
(6H, m), 8.35-8.4 (lH, m)
APCI-MASS (m/z) : 313 (M+H+)
(18) N-Cycloheptyl-4-(4-benzoylamino)benzylamine
IR (Film) : 3265, 3150, 3070, 2925, 2850, 1645,
1615, 1595, 1555 c~-l
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.55-2.7 (lH,
m), 3.69 (2H, s), 7.07 (lH, d, J=7.7Hz), 7.27
(lH, t, J=7.7Hz), 7.5-7.8 (5H, m), 7.9-8.0 (2H,
m), 10.22 (lH, s)
WO96/10559 22 ~ O ~ ~ ~ PCT1~5/01982
- 68 -
APCI-MASS (m/z) : 323 (M+H+)
(19) N-Cycloheptyl-4-(2-pyridylcarbamoyl)benzylamine
IR (KBr) : 3305, 2925, 2855, 1680, 1610, 1580,
1535, 1505 c~-l
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.5-2.7 (lH,
m), 3.76 (2H, s), 7.17 (lH, dd, J=6.3, 4.9Hz),
7.45 (2H, d, J=8.2Hz), 7.98 (2H, d, J=8.2Hz),
7.8-7.9 (lH, m), 8.19 (lH, d, J=8.4Hz),
8.35-8.4 (lH, m), 10.70 (lH, s~
APCI-MASS (m/z) : 324 (M+H+)
(20) N-Cycloheptyl-4-(4-fluorophenoxy)benzylamine
IR (Film) : 2925, 2855, 1505 cm~l
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.65-2.8 (lH, m),
3.75 (2H, s), 6.85-7.1 (6H, m), 7.2-7.35 (2H, m)
APCI-MASS (m/z) : 314 (M+H+)
(21) N-Cycloheptyl-4-(phenylsulfamoyl)benzylamine
NMR (DMSO-d6, o) : 1.2-1.8 (12H, m), 2.5-2.6 (lH,
m), 3.70 (2H, s), 7.0-7.15 (3H, m), 7.15-7.25
(2H, m), 7.47 (2H, d, J=8.3Hz), 7.68 (2H, d,
J=8.3Hz), 10.23 (lH, s)
APCI-MASS (m/z) : 359 (M+H+)
(22) N-Cycloheptyl-4-(3-thienyl)benzyiamine
IR (KBr) : 2924, 1458, 1201, 775 cm~l
NMR (CDCl3, ~) : 1.30-1.98 (12H, m), 2.62-2.78 (lH,
m), 3.80 (2H, s), 7.30-7.47 (5H, m), 7.50-7.60
(2H, m)
APCI-MASS (m/z) : 286 (M+H+)
(23) N-Cycloheptyl-4-(2-thienyl)benzylamine
IR (Neat) : 2924, 1502, 1458, 1101, 810 cm 1
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.62-2.78 (lH,
PCT/~5/01982
W096/10559
~ 0~ 8
- 69 -
m), 3.79 (2H, s), 7.07 (lH, dd, J=5.1, 3.6Hz),
7.22-7.40 (4H, m), 7.50-7.65 (2H, m)
f APCI-MASS (m/z) : 286 (M+H+)
(24) N-Cycloheptyl-4-(pyrazol-1-yl)benzylamine
IR (Neat) : 2927, 1610, 1525, 1460, 1394 cm 1
NMR (CDCl3, o) : 1.30-1.95 (12H, m), 2.60-2.78 (lH,
m), 3.81 (2H, s), 6.46 (lH, t, J=2.1Hz), 7.36-
7.46 (2H, m), 7.58-7.68 (2H, m), 7.71 (lH, d,
J=1.6Hz), 7.91 (lH, d, J=2.lHz)
APCI-MASS (m/z) : 270 (M+H+)
(25) N-Cycloheptyl-4-(imidazol-1-yl)benzylamine
IR (Neat) : 2922, 1522, 1303, 1057 cm 1
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.60-2.80 (lH,
m), 3.83 (2H, s), 7.20 (lH, d, J=l.OHz), 7.27
(lH, d, J=l.OHz), 7.30-7.50 (4H, m), 7.84 (lH, s)
APCI-MASS (m/z) : 270 (M+H+)
(26) N-Cycloheptyl-4-(1-methylpyrazol-4-yl)benzylamine
IR (KBr) : 3277, 2924, 1572, 1443, 1194, 802 cm l
NMR (CDCl3, ~) : 1.30-2.20 (12H, m), 2.62-2.80 (lH,
m), 3.78 (2H, s), 3.94 (3H, s), 7.27-7.47 (4H,
m), 7.58 (lH, s), 7.74 (lH, s)
APCI-MASS (m/7) : 284 (M+H+)
(27) N-Cycloheptyl-2-(2-phenylthiophen-5-yl)methylamine
IR (Neat) : 2924, 1599, 1462, 754 Gm-l
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.66-2.85 (lH,
30 m), 3.98 (2H, s), 6.87 (lH, d, J=3.6Hz), 7.15
(lH, d, J=3.6Hz), 7.18-7.45 (3H, m), 7.52-7.64
- (2H, m)
APCI-MASS (m/z) : 286 (M+H+)
(28) N-Cycloheptyl-4-(oxazol-5-yl)benzylamine
~ ~ ~ O ~ 8 ~ PCT/~5/01982
WO96/10559
- 70 -
IR (KBr) : 2924, 1510, 1485, 1103, 822 cm 1
NMR (CDC13, o) : 1.30-1.98 ~12H, m), 2.60-2.80 (lH,
m), 3.81 ~2H, s), 7.33 (lH, s), 7.33-7.46 (2H,
m), 7.55-7.69 (2H, m), 7.90 (lH, s)
APCI-MASS (m/z) : 271 (M+H+)
(29) N-Cycloheptyl-(2-phenylfuran-5-yl)methylamine
IR (Neat) : 2924, 1545, 1456, 1020, 760 cm 1
NMR (CDCl3, o) : 1.30-1.95 (12H, m), 2.64-2.80 (lH,
m), 3.84 (2H, s), 6.24 (lH, d, J=3.3Hz), 6.57
(lH, d, J=3.3Hz), 7.17-7.45 (3H, m), 7.58-7.7
(2H, m)
APCI-MPSS (m/z) : 270 (M+H+)
(30) N-Cycloheptyl-(5-phenylisoxazol-3-yl)methylamine
IR (Neat) : 2926, 2854, 1616, 1574, 1456, 1113,
766 cm~l
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.65-2.82 (lH,
m), 3.90 (2H, s), 6.53 (lH, s), 7.34-7.53 (3H,
m), 7.70-7.86 (2H, m)
APC T -MAS S (m/z) : 271 (M+H+)
(31) N-Cycloheptyl-(3-phenylpyrazol-5-yl)methylamine
IR (Neat) : 2300-3600 (br), 1570, 1460, 1358,
1026 cm~l
NMR (CDCl3, ~) : 1.30-1.98 (12H, m), 2.65-2.82 (lH,
m), 3.92 (2H, s), 6.46 (lH, s), 7.20-7.50 (3H,
m), 7.64-7.80 (2H, m)
APCI-M~SS (m/z) : 270 (MtH+)
(32) N-Cycloheptyl-(4-phenylthiophen-2-yl)methylamine
IR (Neat) : 2924, 2852, 1502, 1458, 1367, 841,
735 cm~l
NMR (CDCl3, o) : 1.32-1.98 (12H, m), 2.70-2.88 (lH,
m), 4.01 (2H, s), 7.19-7.62 (7H, m)
.. . . . ..
PCT/~5/01982
WO96/10559
2 2 0 (~ 9 8 11
APCI-MASS (m/z) : 286 (M+H+)
(33) N-Cycloheptyl-4 (pyrazol-3-yl)benzylamine
IR (Neat) : 2300-3600 (br), 1514, 1456, 1350,
1205 cm~l
NMR (DMSO-d6, o) : 1.20-1.93 (12H, m), 2.50-~.72
(lH, m), 3.73 (2H, s), 6.~7 (lH, d, J=1.9Hz),
7.30-7.90 (5H, m), 12.70-13.40 (lH, br)
APCI-MASS (m/z) : 270 (M+HT)
(34) N-Cycloheptyl-4-(1-methylpyrazol-3-yl)benzylamine
IR (KBr) : 2922, 2852, 1510, 1462, 1429, 1358,
1234 cm~l
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.61-2.78 (lH,
m), 3.80 (2H, s), 3.95 (3H, s), 6.52 (lH, d,
J=2.2Hz), 7.29-7.40 (3H, m), 7.70-7.80 (2H, m)
APCI-MASS (m/z) : 284 (M+H+)
(35) N-Cycloheptyl-4-(1-methylpyrazol-5-yl)benzylamine
IR (Neat) : 2924, 2854, 1493, 1462, 1385, 1273 cm 1
N~R (CDCl3, o) : 1.32-1.98 (12H, m), 2.62-2.81 (lH,
m), 3.83 (2H, s), 3.89 (3H, s), 6.29 (lH, d,
J=1.9Hz), 7.33-7.46 (4H, m), 7.51 (lH, d,
J=1.9Hz)
APCI-MASS (m/z) : 284 (M+H+)
(36) N-Cycloheptyl-3-(1-trltyl-lH-tetraZol-5-yl)benzylamine
IR (KBr) : 2922, 2852, 1697, 1515, 1452, 750,
698 cm~l
30 NMR (CDCl3, o) : 1.30-1.95 (12H, m), 2.62-2.78 (lH,
m), 3.83 (2H, s), 7.10-7.50 (17H, m), 7.96-8.12
(2H, m)
FAB-MASS (m/z) : 514 (M+H+)
(37) N-Cycloheptyl-4-(phenylcarbamoyl)benzylamine
WO96/105S9 ~ 2 ~ ~ ~ 8 1 PCT1~5101982
- 72 -
IR tKBr) : 3475, 3345, 3055, 2925, 2850, 1645,
1600, 1525, 1505 cm~1
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.5-2.65 (lH,
m), 3.78 (2H, s), 7.09 (lH, t, J=7.3Hz), 7.35
(2H, s), 7.48 (2H, d, J=8.2~z), 7.78 (2H, d,
J=7.5H ), 7.90 (2H, d, J=8.2Hz), 10.20 (lH, s)
APCI-MASS (m/z) : 323 (M+H+)
(38) N-Cycloheptyl-4-(phenylsulfonylamino)benzylamlne
IR (KBr) : 3130, 3015, 2930, 2855, 1610, 1570,
1505 cm-1
NMR (DMSO-d6, o) : 1.2-1.8 (12H, m), 2.5-2.6 (lH,
m), 3.58 (2H, s), 6.99 (2H, d, J=8.5Hz), 7.16
(2H, d, J=8.5Hz), 7.45-7.6 (3H, m), 7.65-7.75
(2H, m)
APCI-MASS (m/z) : 359 (M+H+)
Preparation 62
The mixture of 4-formyl-2-(4-chlorophenyl)thiazole
(2.24 g) and benzylamine (2.14 g) was stirred at 120C
under nitrogen for 5 hours. The mixture WGS cooled to room
temperature and dissolved in ethanol (30 ml). To this
solution was added sodium borohydride (378 mg) and the
mixture was stirred at room temperature for 1.1 hou~s. The
mixture was evaporated to dryness and the residue was
extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography to give N-benzyl-[2-(4-chlorophenyl)thia,ol-
4-yl]methylamine (3.22 g).
IR (Film) : 3060, 3030, 2915, 2835, 1495 cm 1
NMR (DMSO-d6, o) : 2.64 (lH, br s), 3.67 (2H, s),
3.78 (2H, s), 7.2-7.4 (5H, m), 7.52 (lH, s), 7.5-
7.6 (2H, m), 7.9-8.0 (2H, m)
APCI-MASS (m/z) : 315 (M+H+)
Wo96/lOS59 PCT/~5/01982
~ 8
Preparation 63
The mixture of 3-bromomethylbiphenyl (6.58 5) and
cycloheptylamine (6.03 g) was stirred at 120C for 3.5
hours under nitrogen. The mixture was cooled to room
- 5 temperature, and the mixture of dichlorometnane and water
were added thereto. The separated organic layer was washed
with brine, dried over magnesium sulfate and evaporated in
vacuo. The residue was purified by column chromatography
on silica gel to give N-(3-biphenylylmethyl)-
lQ cycloheptylamine (4.49 g) as an orange oil.
IR (Film) : 3060, 3030, 2920, 2850, 1460 cm 1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.7-2.85 (lH,
m), 3.85 (2H, s), 7.3-7.7 (9H, m)
APCI-MASS (m/z) : 280 (M+H+)
Preparation 64
The following compounds were obtained according to
similar manners to those of Preparations 62 and 63.
(i) N-Cycloheptyl-4-(pyridin-3-yl)benzylamine
NMR (CDC13, o) : 1.3-1.9 (12H, m), 2.9-3.05 (lH,
m), 7.3-7.6 (5H, m), 7.8-7.9 (lH, m), 8.5-8.6
(lH, m), 8.8-8.85 (lH, m)
APCI-MASS (m/z) : 281 (M+H+)
(2) N-Cycloheptyl-4-(pyridin-2-yl)benZylamine
IR (Film) : 3050, 3005, 2920, 2850, 1585, 1565 cm 1
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.6-2.7 (lH,
m), 3.74 (2H, s), 7.25-7.5 (3H, m), 7.8-8.1 (4H,
m), 8.6-8.7 (lH, m)
APCI-MASS (m/z) : 281 (M+H+)
(3) N-Cycloheptyl-4-(4-benzoyl)benzylamine
IR (Film) : 3050, 2925, 2850, 1655, 1605 cm~-
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.6-2.8 (lH, m~,
PCT1~5/01982
WO96/10559
22~811 ~ ~
- 74 -
3.87 (2H, s), 7.4-7.65 (5H, m), 7.75-7.9 (4H, m)
APCI-MASS (m/z) : 308 (M+H+)
Preparation 65
To a solution of 3-(2-methvlthiazol-4-yl)-
benzylamine-hydrochloride (2.41 g) in 2 mixture of
dichloromethane (30 ml) and water (10 ml) was added 5~T
sodium hydroxide aqueous solution and adjusted to pH 9~10.
The separated organic layer was washed with brine, dried
over magnesium sulfate and evaporated in vacuo. To the
residual oil was added cyclohepianone (1.68 g) and the
mixture was stirred at 120C under nitrogen. The mixture
was cooled to room temperature and dissolved in ethanol (30
ml). To this solution was added sodium borohydride (378
mg) and the mixture was stirred at room temperature for 2. 5
hours. The mixture was evaporated to dryness and the
residue was extracted with dichloromethane. The organic
layer was washed with brine, dried over magnesium sulfate
and evaporated in vacuo. The residue was purified by
column chromatogaphy on silica gel to give N-cycloheptyl-3-
(2-methylthiazol-4-yl)benzylamine (2.07 g) as a yellow oil.
IR (Film) : 3380, 2915, 2855, 1455 cm 1
NMR (CDCl3, o) : 1.30-2.0 (12H, m), 2.7-2.85 (lH,
m), 2.76 (3H, s), 3.82 (2H, s), 7.32 (lH, s),
7.25-7.4 (2H, m), 7.75-7.9 (2H, m)
APCI-MASS (m/z) : 301 (M+H+)
Preparation 66
To a suspension of N-cycloheptyl-4-(4-
benzoyl)benzylamine (1.87 g) in ethylene glycol (lO ml)
were added potassium hydroxide (511 mg) and hydrazine
monohydrate (1.95 g), and the mixture was stirred at 150C
for 5 hours and at 200C for 4 hours. The mixture was
poured into a mixture of dichloromethane and ice water, and
the separated organic layer was washed with water and
Wo96tlO559 PCT/~5/01982
2 ~
brine, dried over magnesium sulfate and evaporated in
vacuo. The residue was purified by column chromatography
on silica gel to give N-cyclohepty~-4-(4-benzyl)benzylamine
(1.29 g) as an orànge oil.
IR (Film) : 3025, 2905, 2850, 1510 cm~1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.6-2.8 (lH, m),
3.74 (2H, s), 3.96 (2H, s), 7.1-7.4 (9H, m)
APCI-MASS (m/z) : 294 (M+H+)
Preparation 67
To a solution of 3-(pyrazol-3-yl)benzaldehyde (4.33 g)
in pyridine (20 ml) was added trityl chloride (7.71 g)
under ice cooling. The mixture was stirred for 30 minutes,
and then warmed to room temperature. After stirring for 3
hours at the same temperature, the reaction mixture was
poured into ice aqueous hydrochloric acid, extracted with
ethyl acetate. The organic layer was washed with water and
brine, dried over magnesium sulfate, and evaporated in
vacuo. The residue was purified by column chromatography
on silica gel (eluting with n-hexane - ethyl acetate (2:1))
to give 3-(1-tritylpyrazol-3-yl)benzaldehyde (9.26 g).
IR (KBr) : 3477, 3060, 3030, 1697, 1601, 1491,
1444 cm-1
NMR (DMSO-d6, o) : 6.93 (lH, d, J=2.5Hz), 7.1-7.5
(16H, m), 7.63 (lH, dd, J=7.7, 7.7Hz), 7.85 (lH,
d, J=7.7Hz), 8.08 (lH, d, J=7.7Hz), 8.25 (lH, s),
10.04 (lH, s)
Preparation 68
The mixture of 3-(1-tritylpyrazol-3-yl)benzaldehyde
(15.31 g) and benzylamine (7.91 g) was stirred at 120C for
5 hours under nitrogen. The mixture was cooled to room
temperature and dissolved in ethanol (120 ml). To this
solution was added carefully sodium borohydride (1.40 g) at
room temperature and the mixture was stirred for 2 hours.
WO96/10559 ~ ~ n ~ 9 ~ 1 PCT/~S/01982
- 76 -
The mixture was concentrated in vacuo and to the residue
were added dichloromethane and ice water. Thé separated
organic layer was washed with brine, dried over magnesium
sulfa~e and evaporated in vacuo. The residue was purified
S by column chromatography on silica gel to give N-ben7yl-3-
(1-tritylpyrazol-3-yl)benzylamine (12.18 g) as an amorphous
solid.
IR (KBr) : 3059, 3028, 539, 1493 cm 1
~R (DMSO-d6, o) : 3 ~5-3.75 (4H, m), 6.77 (lH, d,
lG ~=2.5Hz), 7.05-7.45 (18H, m), 7.55-7.75 (2H, m)
Preparation 69
To a suspension of N-ben~yl-3-(1-tritylpyrazol-3-
yl)benzylamine (8.60 g) in anisole (17.2 ml) was added
trifluoroacetic acid (34.4 ml) at room temperature and the
mixture was stirred at 80C ~cr 3.5 hours. The mixture was
concenirated in vacuo and the residue was pulverized with
diisopropyl ether. The powder was collected by filtration,
washed with diisopropyl ether and dried in vacuo to give N-
benzyl-3-(pyrazol-3-yl)benzvlamine bis(trifluoroacetate)
(7.35 g)-
IR (KBr) : 3059, 3005, 1669, 1510, 1489 cm 1
NMR (DMSO-d6, o) : 4.2-4.3 (4H, m), 6.70-6.75 (lH,
m), 7.1-7.6 (7H, m), 7.75-8.0 ~3H, m)
Preparation 70
To a solution of 2,4-dichloro-6-methyl-3-nitropyridine
(30.33 g) in acetonitrile (100 ml) was added dropwise
sodium methoxide (28~q methanol solution) (85.1 ml) at 5C,
and the mixture was stirred at 80C ~or 6 hours. The
mixture was cooled and poured into a mixture OL ethyl
acetate and ice water. The separated organic layer was
washed with water and brine, dried over magnesium sulfate
and evaporated in vacuo. The residue was purified by
column chromatography on silica gel to give 2,4-dimethoxy-
, . . .. . _
WO96/10559 PCT/~5/01982
6-methyl-3-nitropyridine (28.21 g) as a pale yellow
crystal.
IR (KBr) : 3093, 3035, 3005, 2960, 2868, 1601, 1581,
i531 cm~1
NMR (DMSO-d6, o) : 2.44 (3H, s), 3.92 and 3.94 (6H,
s x 2), 6.97 (lH, s)
APCI-MASS (m/z) : 199 (M+H+)
Preparation 71
To a solution of 2,4-dimethoxy-6-methyl-3-
nitropyridine (28.1 g) in 1,4-dioxane (200 ml) and methanol
(100 ml) was added 10~- palladium on carbon (14 g) under
nitrogen and the mixture was hydrogenated under atmospheric
pressure for 4.5 hours. Palladium on carbon was filtered
off and the filtrate was evaporated in vacuo. The residue
was purified by column chromatography on silica gel to give
3-amino-2,4-dimethoxy-6-methylpyridine (23.41 g) as an
orange oil.
IR (Film) : 3458, 3373, 2945, 2856, 1605 cm 1
NMR (DMSO-d6, o) : 2.26 (3H, s), 3.79 and 3.82 (6H,
s x 2), 3.96 (2H, br s), 6.52 (lH, s)
APCI-MASS (m/z) : 169 (M+H+)
Preparation 72
To a solution of 3-amino-2,4-bis(methylthio)-6-
methylpyridine (7.90 g) in dichloromethane (160 ml) was
added N,N-dimethylaniline ~5.73 g) at 5C, followed by
dropwise addition of phenyl chloroformate (6.78 g). The
mixture was warmed to room temperature and stirred at 4
hours. To the mixture were added ice water (60 ml) and 6N
hydrochloric acid (10 ml), and the separated organic layer
was washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was crystallized, and the
crystal was collected by filtration, washed with
diisopropyl ether and dried in vacuo to give 3-
-
WO96/10559 ~2 0 0 9 8 ~ PCTt~5101982 ~
- 78 -
phenoxycarbonylamino-2,4-bis(methylthio)-6-methylpyridine
(10.46 g).
IR (KBr) : 3410, 3228, 3196, 3145, 3003, 2926, i732,
1591, 1556, 1537 cm~1
NMR (DMSO-d6, o) : 2.45 ~6H, s), 2.46 (3H, s), 6.94
(1~, s), 7.0-7.5 (5H, m), 9.48 (lH, br s)
~PCI-MASS (m/z) : 321 (M~H+)
Preparation 73
To a solution of 2,4,6-tri~luoroaniline (883 mg) and
N,N-dimethylaniline (0.91 ml) in methylene chloride (18 ml)
was added phenyl chloroformate (0.83 ml) and the mixture
was stirred at room temperature for 4 hours. The reaction
mixture was washed with lN-hydrochloric acid (three times),
water, aqueous sodium bicarbonate, water, and brine. The
organic layer was dried over magnesium sul~ate and
evaporated in vacuo. The resulting solid was collected and
washed with n-hexane to give phenyl N-(2,4,6-
trifluorophenyl)carbamate (1.46 g).
I~ (KBr) : 3253, 1749, 1722, 1538, 1240, 1200 cm~1
NMR (CDCl3, o) : 6.26 (lH, br s), 6.70-6.86 (2H, m~,
7.10-7.46 (5H, m)
APCI-MASS (m/~) : 268 (M+H+)
Preparation 74
To a solution of 3-amino-2,4-dimethoxy-6-
methylpyridine (23.40 g) in dichloromethane (200 ml) was
added N,N-dimethylaniline (20.23 g), followed by dropwise
addition of phenyl chloroformate (23.94 g) at 5C. The
mixture was warmed to room temperature and stirred ~or 3
hours. The resulting precipitates were collected by
filtration, washed with dichloromethane and diisopropyl
ether, and dried in vacuo to give 2,4-dimethoxy-6-methyl-3-
phenoxycarbonylaminopyridine (21.95 g) as a white crystal.
I~ (KBr) : 3408, 3251, 3147, 30c4, 2983, 2947, 286C,
. , , , _ .
WO96/10559 PCT/~/01982
~ ~2~ 0~ 8
- 79 -
1713, 1593, 1497 cm~1
NMR (DMSO-d6, o) : 2.38 (3H, s), 3.85 (6H, s),
6.72 (lH, s), 7.05-7.3 (3H, m), 7.35-7.45 (2H,
m), 8.83 (lH, br)
5APCI-MASS (m/z) : 289 (M+H+)
Preparation 75
A mixture of 4-(4-bromophenoxy)berzaldehyde (10.0 g)
and benzylamine (5.~2 g) was stirred al 120C for 4 hours.
After cooling to room temperature, the resulting solid was
suspended in ethanol (150 ml). To the suspension was added
carefully sodium borohydride (1.36 g), and the mixture was
stirred at room temperature for 2 hours. The mixture was
evaporated in vacuo and the residue was extracted with
methylene chloride. The organic layer was washed with
water, brine, dried over magnesium sulfate, and evaporated
in vacuo. The residue was purified by column
chromatography on silica gel (250 g, eluting with methylene
chloride - methanol (20:1!) to give N-benzyl-4-(4-
bromophenoxy)benzylamine (11.51 g) as a pale yellow oil.
IR (Neat) : 3061, 3028, 2700-3000 (br), 1608, 1583,
1504, 148i, 1240 cm~'
NMR (CDCl3, o) : 3.79 (2H, s), 3.82 (2H, s),
6.80-7.00 (4H, m), 7.20-7.50 (9H, m)
APCI-MASS (m/z) : 368, 370 (M+H+)
Preparation 76
The mixture of 3-(1-tritylpyrazol-3-yl)benzaldehyde
(9.18 g) and cycloheptylamine (3.75 g) was stirred at 12CC
for 4 hours. The mixture was cooled to room temperature
and dissolved in ethanol (120 ~Ll). To this solution was
added sodium borohydride (836 mg) and the mixture was
stirred at room temperature for 2 hou~s- The mixture was
evaporated in vacuo and extracted with methylene chloride.
The organic layer was washed with water and brine, dried
~ 2 0 ~ ~ 1 PCT~5~01982
WO96/10559
- 80 -
over magnesium sulfate and evaporated in vacuo. The
resldue was purified by column chromatography on silica gel
(eluting with methylene chloride - methanol (40:1 to 10:1))
to give N-cycloheptyl-3-(1-tritylpyrazol-3-yl)ben~ylamine
(7.92 g).
NMR (DMSO-d6, ~) : 1.20-1.90 (12H, m), 2.50-2.70 (lH,
m), 3.69 (2H, s), 6.77 (lH, d, J=2.5Hz), 7.05-
7.50 (12H, m), 7.55-7.65 (lH, m), 7.71 (lH, s)
APCI-MASS (m/z) : 512 (M+H+)
Preparation 77
To a suspension of 3-amino-2,4,6-
trimethylpyridine hydrochloride (5.18 g) in 1,2-
dichloroethane (120 ml) was added diisopropylethylamine
(19.39 g) at room temperature, followed by addition of
phenyl chloroformate (7.05 g). The mixture W2S refluxed
for 10 hours under nitrogen. The mixture was cooled and
poured into ice water. The separated organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give 3-
phenoxycarbonylamino-2,4,6-trimethyl pyridine as a crude
orange oil (3.17 g).
IR (KBr) : 3275, 2924, 1740, 1713, 1605, 1550 cm 1
NMR (DMSO-d6, o) : 2.22 (3H, s), 2.39 (6H, s), 7.01
(lH, s), 7.2-7.6 (5H, m), 9.42 (lH, brs)
APCI-MASS (m/z) : 257 (M+H+)
Preparation 78
To a suspension of 4-chloro-6-methyl-2-methylthio-3-
nitropyridine (16.0 g) in a mixture of 1,4-dioxane (200 ml)
and methanol (50 ml) was added Raney-N ckel (NDT-90;
trademark: Kawaken fine chemicals) (ca. 30 g) under
nitrogen, and the mixture was hydrogenated under
atomospheric pressure for 3 hours. Raney-Nickel was
PCT/~5/01982
WO96/1ossg ~ 8 ~ ~
- 81 -
filtered off and the filtrate was eva~orated in v2cuo. The
residue was purified by coLumn chromato5raphy on sillca gel
to give 3-amino-2-chloro-6-methyl-~-~ethylthio~yridine
(12.86 g) as an orange oll.
IR (film) : 34~4, 3322, 2922, 1707, loO~, 1570,
1529 cm
NMR (DMSO-d6, ~ 9 (3~, s), 31 ~3~, s), 4.93
(2H, brs), 6.98 (lH, ~)
A~CI-~M~SS (m/z) : 191, 189 'M+H+)
Preparation 79
To a solution of 3-amino-4-chloro-6-methyl-2-
methylthiopyridine (12.75 g) in dichloromethane (~00 ml)
was added N,N-dimethylaniline (6.00 g) at 5~C, followed by
dropwise addition of phenvlchloroformate (7.il g). The
mixture was warmed to room temperature and stirred at the
same temperature for 4 hours. The ~ixture was washed with
dilute hydrochloric acid and brine, dried over magnesium
sulfate and evaporated in vacuo. The residue was
triturated with diisopropyl ether ard collected by
filtration, washed with dilsopropyl ether and dried in
vacuo under phosphorus pentoxide tc give 2-chloro-6-methyl-
4-methylthio-3-phenoxycarbonylamlnopyridine (9.58 g).
IR (KBr) : 3194, 2924, 1751, 1579, 1514, 1489 cm 1
NMR (DMSO-d6, o) : 2.29 (3H, s), 2.50 (3H, s),
6.7-6.85 (3H, m), 6.98 (lH, s), 7.1-7.25 (2H, m),
9.35 (lH, brs)
Preparation 80
To a solution of 3,5-di-te~t-bu~yl-a-hydroxyphenol
(9.65 g) and imidazole (3.55 g) in N,N-dimethy'formamide
(80 ml) WâS added tert-butyldimethylsilyl chloride (6.54 g!
at 5C, and the mixture was stirred at room tem~erature for
3 hours. The mixture was poured into a mixture of ethyl
acetate and ice water, and the separated organic layer was
WO96/lOS59 ~2 0 ~ PCT/~5101982
wasned with w~ter and brine, dried over r.agnesium sulfate
and evaporated in vacuo. The residue was purified by
column chromclogra~hy on sillca gel to give l-tert-
butyldimethylsilyloxy-3,5-di-tert-butyl-4-hyd-oxyben7ene
(13.91 g) as a ~Jhite solid.
R (K3r) : 3651, 2958, 2325, 2858, 1601, 1~70 cm
(CDCl3, o) : 0.18 (6h, S) ~ O. 91 (9H, s),
1.41 ~18H, s), 6.73 (2H, s)
A~CI-M~SS (m/z) : 336 (M+)
Preparaiion 81
To a suspension of sodium hydride (60~ oil dispersion)
(1.65 g) in N,N-dimethylformamide (100 ml) was added
dropwise a solution of 1-tert-butyldimethylsilyloxy-3,5-di-
tert-butyl--hydroxybenzene (13.89 g) i~ N,N-
dimethylformamide (70 ml) al 5C, and the mixture was
stirred at the same temperatu~e for 1 hcur. Tc the
resulting solution was added chloromethvi methyl ethe~
(4.99 g) at 5~C and the mixture was stirred at room
2C lemperature for 5 hours. The mixture was poured into a
mixture of ethyl acetate znd ice wa~er, and the separated
organic layer was washed wi~h water and brine, dried o~Jer
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give 1-
tert-butyldimethylsilyloxy-3,5-di-tert-butyl-4-methoxy
methoxybenzene (13.49 g) as a yellow solid.
IR (KBr) : 2962, 2929, 2897, 2860, 1597 ~m 1
N~R (CDCl3, o) : 0.19 (6H, s), C.98 (9H, s), 1.41
(18H, s), 3.62 (3H, s), 4.86 (2H, s), 6.72 (2H,
s)
~ECI-M~SS (m/z) : 3~1 (M+~.+)
Pre~ar~tion 82
To a soiution o~ 1-tert-bu-yldimeth~lsilyloxy-3,5-di-
tert-butyl-4-methoxymethoxybenzene (13.42 g) in
... . . . . . .
096/10559 ~ PCT/~StO1982
- 83 -
tetrahydrofuran (20 ml) was added l.OM solution of
tetrabutylammonium fluoride (38.8 ml) a' room temperature
and the mixture was stirred at the same temperature for 2
hours. The mixture was poured into a mixture of ethyl
acetate and ice water and the separated organic layer was
washed with water and brine, drled over magnesium sulfate
- and evaporated in vacuo. The residue was purified by
column chromatography on silica gel to give 3,5-di-tert-
butyl-4-methoxymethoxyphenol (9.43 g) as a yellow crystal.
IR (KBr) : 3369, 3012, 2958, 2910, 2870, 2779, 1610,
1589 cm~1
NMR (CDCl3, o) : 1.42 (18H, s), 3.63 (3H, s),
4.87 (2H, s), 6.74 (2H, s)
Preparation 83
To a solution of 1-(4-fluorophenoxy)-4-nitrobenzene
(22.9 g) in ethyl acetate (200 ml) was added 10- palladium-
carbon (50 wet) (9.16 g), and the mixture was hydrogenated
under atmospheric pressure at room temperature for 3 hours.
~alladium on carbon was filtered orf and washed with
tetrahydrofuran. The filtrate was evaporated in vacuo and
the residue was purified by column chromatography on silica
gel to give 4-(4-fluorophenoxy)aniline (18.27 g) as a red
powder.
IR (KBr) : 3450, 3395, 3325, 3230, 3070 3045, 3020,
1635, 1490 cm~1
NMR (CDCl3, o) : 6.65-6.75 (2H, m), 6.8-7.G5 (6H, m)
APCI-MASS (m/z) : 204 (M+H+)
Preparation 84
To a solution of 3-(4-fluorophenoxy)benzyl alcohol
(3.97 g) in chloroform (50 ml) was added activated
manganese dioxide (15.82 g) and the mixture was refluxed
for 4.5 hours. Manganese dioxide was filtered of and the
filtrate was evaporated in vacuo to give crude 3-(4-
WO96110559 2 ~ PCT/~9~/01982
- 84 -
fluorophenoxy)benzaldehyde (3.81 g) as a yellow oil.
IR (Film) : 3074, 2837, 2819, 2731, 170~, 1585, 1502,
1481, 1450 cm~1
NMR (DMSO-d6, o) : 7.1-7.45 (6H, m), 7.55-7.75 (2H,
m), 9.98 (lHj s)
Preparation 85
To a suspension of lithium aluminum hydride (5.69 g)
in tetrahydrofuran (300 ml) was added dropwise a solution
of 4-(4-fluorophenoxy)benzonitrile (21.32 g) in
tetrahydrofuran (200 ml) at 5C, and the mixture was
stirred at room temperature for 4 hours. To the mixture
was added sodium fluoride (16.80 g), followed by dropwise
addition of cold water (5.al g) and the mixture was stirred
at room temperature for 45 minutes. The insoluble
materials were filtered off and washed with
tetrahydrofuran. The filtrate was evaporated in vacuo and
the residue was purified by column chromatography on silica
gel to give 4-(4-fluorophenoxy)benzylamine (21.39 g) as a
yellow oil.
IR (KBr) : 3352, 3269, 3043, 2864, 1645, 1606,
1495 cm~1
NMR (DMSO-d6, o) : 3.69 (2H, s), 6.9-7.4 (8H, m)
APCI-MASS (m/z) :201 (M+H+-NH3)
Preparation 86
To a solution of phenyl chloroformate (31.2 g) in 1,2-
dichloroethane (250 ml) was added dropwise a solution of
3-amino-2,4,6-trimethylpyridine (22.62 g) in 1,2-
dichloroethane (120 ml) at 100C, and ihe mixture was
refluxed for 1 hour under nitrogen. The mixture was cooled
~o room temperature and added dropwise a mixture of ethyl
acetate (2 ~) and tetrahydrofuran (1 ~). The precipitates
were collected by filtration, washed with ethyl acetate and
diisopropyl ether and dried in vacuo over phosphorus
WO96/l0559 ~ PCT/nW5/0l982
- 85 -
pentoxide to give 2,4,6-trimethyl-3-
phenoxycarbonylaminopyridine-hydrochloride (48.84 g).
IR (KBr) : 3413, 1741, 1645, 1541, 1483 cm~1
NMR (DMSO-d6j o) : 2.49 (3H, s), 2.69 (6H, s),
7.2-7.5 ~5H, m), 7.65-7.75 (lH, m), 9.63 and
10.20 (total lH, br s)
APCI-MASS (m/z) : 257 (M+H+-HCl)
Preparation 87
To a solution of 5-amino-4,6-bis(methylthio)-2-
methylpyrimidine (4.10 g) in dichloromethane (80 ml) was
added N,N-dimethylaniline (2.96 g) at 5C, followed by
dropwise addition of phenyl chloroformate (3.51 g). The
mixture was stirred at room temperature for 2 hours under
nitrogen. The mixture was washed with dilute hydrochloric
acid and brine, dried over magnesium sulfate and evaporated
in vacuo. The residue was triturated with diisopropyl
ether collected by filtration, washed with diisopropyl
ether and dried in vacuo to give 4,6-bis(methylthio)-2-
methyl-5-phenoxycarbonylaminopyrimidine (5.74 g).
IR (KBr) : 3217, 3005, 2924, 1711, 1595, 1485 c~ 1
NMR (DMSO-d6, o) : 2.49 (6H, s), 2.59 (3H, s)~
7.0-7.5 (5H, m), 9.27 and 9.68 (total lH, s)
~PCI-MASS (m/z) : 322 (M+H+)
Preparation 88
To a solution of 2-(3-bromophenyl)-1,3-dioxolane
(20.42 g) and triisopropoxyborane (25.14 g) in
tetrahydrofuran (350 ml) was added dropwise n-butyllithium
(1.70M hexane solution, 78.8 ml) at -72C over 2-hours
under nitrogen. The mixture was warmed to room temperature
and stirred for 21 hours. The mixture was poured into a
mixture of ethyl acetate and dilute hydrochloric acid and
the separated organic layer was washed with water and
brine, dried over ~agnesium sulfate and evaporated in
WO96/105S9 ~ ~ n O 9 8 ~ PCT/~5/Olg82
- &6 -
vacuo. The residue was purlfied by colu~n chromatography
on silica gel to give crude dih~droxy-(3-~ormyipnenyl)-
borane (14.83 g). -
IR (KBr) : 3354, 284C, 1678, 1603, 158' cm~1
NMR (DMSO-d5, oi : 7.55-7.7 (l~, m), 7.8-8.'5 (2h,
~), 8.33 (2~, s), 1~.03 (1~, s;
Prep2ration 89
~o a suspension of a-bromo-1-tritylpyrczole (18.96 g)
and crude di;nydroxy-~3-fo~mylphenyl)borane (14.6 g) in
toluene (400 ml) were added ~owdered potassi~m carbonate
(10.10 g) and tetrakis(triphenylphos~hine)~aliadit1m(C)
~.81 g), and the mixlure was refluxed for 6 hours. The
mixture was poured into a mixture of ethyl acelate and ice
water, and the separaled organic layer was washed with
brine dried over magnesi~m sulfate and evaporated in
vacuo. The residue was pur fied by columr chromatography
on silica gel LO give 3-('-tritylpyrazol-4-yl)benzaldehyde
(2.6~ g) as a yellow solid.
2C IR (KBr) : 3C57, 3024, 2812, 2727, 1699, 1503,
1585 cm~î
~R (D~SO-d6, o) : 7.0-7.15 (5~, m), 7.35-7.5 (lC~,
m), 7.7-7.85 (2H, m~, 7.97 (1~, d, J=7.7H7), 8.13
(1~, d, J=7.7Hz~, 8.31 (1~, s), 10.13 (lH, s)
Preparation 90
~o a solution of 3-bromobenzaldehyde (1.25 g) and
1-methyl-4-tri-n-butylstanniopyrazole (3.0 g) was added
tetra~is(triphçnylphosphine)palladium() (234 mg). Then
the mixture was heated for 4 hours at 140C. After
cooling, the reaction mixture was diluted with toluene (16
ml). ~ aqueous solution (14 ml) of ~otassium fluoride
(~.7 g) was added to the mixture and stirred fo~ one hour.
InsoluDle material was filtered off. Tne filtrate was
washed with water and brine, dried o~er magnesium sulf~te,
., . , . . . . _ . .. . . . . . .
~ PCT/~S/01982
W096/lOS59 ~ ~ ~ 0
- 87 -
and evaporated in vacuo. The residue was chromatographed
on silica gel (60 g, eluting with n-hexane - éthyl acetate
2)) to give 3-(1-methylpyrazol-4-yl)benzaldehyde (978.1
mg)-
IR (Neat) : 2943, 2818, 1686, 1608, 1230, 1174 cm~
NMR (CDCl3, o) : 3.98 (3H, s), 7.47-7.58 (lH, m),
7.65-7.78 (3H, m), 7.83 (lH, s), 7.93-7.98 (lH,
m), 10.04 (lH, s)
APCI-MASS (m/z) : 187 (M+H+)
Preparation 91
To a solution of 3-[(E)-3-dimethylaminopropenoyl]-
benzonitrile (8 g) in acetic acid (80 ml) was added
methylhydrazine (2.23 ml). The mixture was stirred for 3.5
hours at room temperature. To the solution was added 5N-
sodium hydroxide aqueous solution in order to basify under
ice cooling and extracted with ethyl acetate. The organic
layer was washed with saturated sodium bicarbonate
solution, water, brine, dried over magnesium sulfate,
evaporated in vacuo. After chromatography on silica gel
(eluting with dichloromethane-methanol), 3-(1-
methylpyrazol-3-yl)benzonitrile (4.45 g) and 3-(1-
methylpyrazol-5-yl)benzonitrile (2.09 g) were obtained.
3-(1-Methylpyrazol-3-yl)benzonitrile;
mp : 97-98C
IR (KBr) : 3115, 2935, 2220, 1602, 1471, 1352,
1246 cm~1
NMR (CDCl3, o) : 3.97 (3H, s), 6.56 (lH, d, J=2.3H7),
7.37-7.60 (3H, m), 7.95-8.10 (2H, m)
APCI-MASS (m/z) : 184 (M+H+)
-
3-(1-Methylpyra7O1-5-yl)benzonitrile;
mp : 95-97C
IR (~Br) : 3066, 2951, 2231, 1475, 1416, 1335,
PCT/~5/01982
W096/10559 ~ ~ ~ 9 8 1
- 88 -
1236 cm1
NMR (CDCl3, o) : 3.92 (3H, s), 6.37 (lH; d, J=1.5Hz),
7 50-7.75 (5H, m)-
APCI-MASS (m~z) : 184 (M~H+)
Preparation 92
A mixture of 3-(bromoacetyl)benzonitrile (38.2 g) and
formamide (190 ml) was heated for 30 minutes at 185C and
cooled to room temperature. The ~ixture was poured into
saturated sodium bicarbonate solution (400 ml) and
extracted with ethyl acetate (1.8 e) . The organic layer
was washed with water and brine, dried over magnesium
sulfate. After evaporation to 200 ml, t~e resulting
~recipitate was collected by filtration, washed with ethyl
acetate - isopropyl ether (2:1) to give 3-(imidazol 4-
yl)benzonitrile (13.3 g).
mp : 190-191C
IR (KBr) : 2250-3240 (br), 2224, 1606, 1477, 1333,
1070, 970, 824, 789 cm~1
NMR (DMSO-d6, o) : 7.50-7.68 (2H, m), ,.70-7.87 (2H,
m), 8.05-8.20 (2H, m), 12.3~ (lH, br)
APCI-MASS (m/z) : 170 (M+H+)
Preparation 93
To a solution of methyl 4-formylbenzoate (5.0 g) ir
ethanol (50 ml) was added sodium borohydride (576 mg)
carefully at 0-5C and stirred for 30 minutes. The mixture
was poured into water and extracted with dichloromethane.
The organic layer was washed with water and brine, dried
over magnesium sulfate, evaporated in vacuo to give methyl
4-hydroxymethylbenzoate (5.06 g).
IR (KBr) : 2750-3670 (br), 1722, 1614, 1437, 1286,
1111, 1047, 1016, 756 c~-1
NMR (CDCl3, o) : 1.89 (lH, t, J=5.9Hz), 3.92 (3H, s),
4.77 (2H, d, J=5.9Hz), 7.37-7.50 (2H, m), 7.97-
~ WO96/lU559 a2 ~ ~ Q 8 1 rCDn~s~0~98~
- 89 -
8.10 (2H, m)
APCI-MASS (m/z) : 167 (M+H+)
.i .
Preparation 94
To a solution of methyl 4-hydroxymethylbenzoate (5.0
g) and imidazole (4.1 g) in N,N-dimethylformamide (25 ml)
was added tert-butyldimethylsilyl chloride (4.77 g)
carefully at 0-5C and stirred for 2 hours at room
temperature. The reaction mixture was poured into O.lN
hydrochloric acid (100 ml) and extracted with ethyl
acetate. The organic layer was washed with water and
brine, dried over magnesium sulfate, evaporated in vacuo to
give methyl 4-(tert-butyldimethylsilyloxymethyl)benzoa~e
(8.43 g).
IR (Neat) : 2954, 2859, 1724, 1464, 1281, 1107,
841 cm~l
NMR (CDCl3, o) : 0.11 (6H, s), 0.95 (9H, s), 3.91
(3H, s), 4.79 (2H, s), 7.34-7.44 (2H, m), 7.95-
8.05 (2H, m)
A~CI-MASS (m/z) : 281 (M+H+)
Preparation 95
A mixture of methyl 4-(tert-butyldimethylsilyloxy-
methyl)benzoate (1.0 g) and hydrazine monohydrate (0.87 ml)
in ethanol (0.8 ml) was refluxed for one hour. After
cooling to room temperature, the reaction mixture was
poured into water and extracted with ethyl acetate. The
organic layer was washed with water and brine, dried over
magnesium sulfate, evaporated in vacuo to give [4-(te~t-
butyldimethylsilyloxymethyl)benzoyl]hydrazine (1.0 g).
mp : 83-85C
IR (KBr) : 3273 (br), 2954, 2858, 1662, 1599, 1539,
1335, 1254, 1093, 841 cm-i
NMR (DMSO-d6, o) : 0.08 (6H, s), 0.91 (9H, s), 4.47
(2H, s), 4.75 (2H, s), 7.30-7.40 (2H, m), 7.75-
PCT/~95/01982
WO96/105~9 2 2 Q ~
- 90 -
7.85 (2H, m), 9.72 (lH, s)
APCI-MASS (m/z) : 281 (M+H+)
Preparation 96
To a mixture of [4-(tert-butyldimethylsilyloxymethyl)-
benzoyl]hydrazine (8.0 g) and ethyl
acetimidate-hydrochloride (4.24 g) in ethanol (160 ml) was
added triethylamine (4.8 ml) at room temperature and
stirred for 30 minutes. The reaction mixture was
evaporated in vacuo. Then the residue was dissolved in
ethyl acetate (120 ml), washed with water and brine. The
organic layer was dried over magnesium sulfate, evaporated
in vacuo. And the residue was heated for 10 minutes ~t
200C, cooled to room temperature, chromatographed on
silica gel (200 g, eluting with n-hexane - ethyl acetate
(2:1)) to give 2-[4-(tert-butyldimethylsilyloxymethyl)-
phenyl]-5-methyl-1,3,4-oxadiazole (6.35 g).
mp : 62-65C
IR (KBr) : 2956, 2933, 2897, 2860, 1576, 1502, 1257,
1086, 843 cm~1
NMR (DMSO-d6, o) : 0.10 (6H, s), 0.92 (9X, s), 2.58
(3H, s), a 80 (2H, s), 7.45-7.55 (2H, m), 7.90-
8.00 (2H, m)
APCI-M~SS (m/z) : 305 (M+H+)
Preparation 97
To a solution of 2-[4-(tert-butyldimethylsilyloxy-
methyl)phenyl]-5-methyl-1,3,4-oxadiazole (2.0 g) in
methanol (20 ml) was added lN hydrochloric acid (13 ml)
dropwise at 0-5C and stirred for cne hour. The reaction
mixture was recooled to 0-5C, and sodium bicarbonate (1.15
g) was added thereto carefully. The mixture was extracted
with dichloromethane, washed with water and brine, dried
over magnesium sulfate, evaporated in vacuo. The resulting
precipitate was collected by filtration and washed with
~ WO96/10559 2 2 ~ ~ ~ 8 ~ PCTl~5/01982
- 91 -
n-hexane to give ~-(4-hydroxymethylphenyl)-5-methyl-(1,3,4-
oxadiazole (0.75 g).
I~ (KBr) : 3323 (br), 2877, 2819, -1614, 1576, 1416,
1~57, 1053, 833, 729 c~-1
NMR (DMSO-d6, o) : 2.58 (3H, s), 4.59 (2~, d,
J=5.4Hz), 5.35 (lH, t, J=5.4~z), 7.47-7.57 (2~;,
~), 7.87-7.97 (2H, m)
APCI-MASS (m/z) : 191 (M+H+)
Preparation 98
A mixlure of 2-[4-(tert-butyldimethylsilyloxymethyl)-
phenyl]-5-methyl-1,3,4-oxadiazole ~5.C5 g) and benzylamine
(18 ml) was heated fo~ 2 drops at 150C. After cooling to
room temperature, the mixture was chromatographed on silica
gel (250 g, eluting with dichloromethane-methanol (20:1))
to give 4-benzyl-3-[4-(tert-butyldimethylsilyloxymethyl)-
phenyl]-5-methyl-GH-1,2,4-triazole (5.04 g).
mp : 90-91C
IR (KBr) : 2953, 2929, 2885, 2854, 1524, 1460, 1431,
1255, 1101, 1003, 835 c~-1
NMR (CDCl3, o) : 0.08 (6H, s), 0.94 (9H, s), 2.37
(3H, s), 4.77 ~2H, s), 5.16 (2H, s), 6.90-7.03
(2H, m), 7.27-7.55 (7H, m)
APCI-MASS (m/z) : 394 (M+H+)
?reoaration 99
Tc the solution of N-cycloheptyl-4-(4-benzyl-5-methyl-
4~-1,2,4-t~iazol-3-yl)benzylamine (500 mg) in methanol (25
ml) was added Palladium Black (500 mg) and formic acid
(1. 25 ml). The mixture was stirred fo~ 4.5 hours at the
room temperature. Palladium Black was removed by
r iltration. The filtrate was basified with lN sodium
hydroxide aqueous solution under ice cooling and evaporated
in vacuo to dryness. The residue was diluted with
dichloromethane-methanoi (5:1), dried over magnesium
WO96/10559 PCT/~/01982
220098 1
- 92 -
sulfate, evaporated in vacuo. Afler chromatogra~hy or
silica gel (15 g, eluting with dichloro.~ethané-methanol
(4:1)) N-cycloheptyl-4-(5-methyl-4H-1,2,4-t~iazol-3-
yl)benzylamine (21g.6 mg) was isolated.
IR (KBrj : ~500-3700 (br,, 2926, 2854, 1564, 1458,
1099 cm~l
NMR (CDC13, o) : 1.30-2.00 (12H, m), 2.48 (3H, s),
2.65-2.80 (lH, m), 3.83 ~2H, s), 4.60-5.15 (~H,
br), 7.30-7.40 (2H, m), 7.90-8.00 (2H, m)
APCI-M~SS (m/z) : 285 (M+H~)
PreparatiQn 100
To the solution of 3-(lH-tetrazol-5-yl)benzaldehyde
(600 mg) in N,N-dimethylformamide (6 ml) was added sodium
hydride (60C- oil suspension, 138 mg) at 0-5C. A~ter
stirring for 15 minutes, to the mixture was added methyl
iodide (0.43 ml). The solution was s.irred for 3 hours at
room temperature, then stirred for 30 minutes at 40C. The
reaction mixture was ~oured into water and extracted with
ethyl aceta~e, washed with wate~ and brine, dried over
magnesium sul~ate, evaporated in vacuo. After
chromatography on silica gel (25 g, eluting with n-hexane -
ethyl acetate (1:1), 3-(2-methyl-2H-tetrazol-5-
yl)benzaldehyde (510.7 mg) and 3-(1-methyl-lH-tetrazol-5-
yl)benzaldehyde (81.6 mg) was obtained.
3-~2-Methyl-2H-tetrazol-5-yl)benzaldehvde
mp : 98-99C
IR (KBr) : 3072, 2839, 1691, 1587, 1520, 1443 cm 1
NMR (DMSO-d6, o) : 4 a7 (3H, s), 7.81 (lH, dd, J=7.7,
7.7Hz), 8.05-8.10 (lH, m~, 8.33-8.40 (lH, m)
8.55-8.58 (lH, m) ~ 10.14 (lH, s)
APCI-MASS (m/ z ) 189 (M+H )
3-(1-Methyl-lH-tetrazol-5-yl)benzaldehyde
PCT/~5/01982
WO96/105S9
-- 2 2 ~
- 93 -
IR (KBr) : 1699, 1608, 1535, 1450, 1394 cm 1
NMR (DMSO-d6, o) : 4.22 (3H, s), 7.87 (iH, dd, J=7.7,
7.7Hz), 8.13-8.25 (2H, m), 8.38-8.ao (lH, m),
10.14 (lH, s)
APCI-MASS (m/z) : 189 (M+H+)
Preparation 101
To the solution of 4-fluorobenz21dehyde (3.0 g) and
lH-1,2,4-triazole (2.0 g) in N,N-dimethylformamide (30 ml)
was added potassium carbonate (4.0 g). Then the mixture
was heated for one hour at 120C. After cooling, the
reaction mixture was diluted with ethyl acetate (300 ml),
washed with water, brine, dried over magnesium sulfate and
evaporated in vacuo. The resulting solia was collected and
washed with diisopropyl ether to give 4-(lH-1,2,4-triazol-
1-yl)benzaldehyde (1.95 g).
mp : 147-148C
IR (KBr) : 3130, 2856, 1709, 1603, 1518, 1441,
1275 G~ 1
NMR (CDCl3, o) : 7.88-8.01 (2H, m), 8.01-8.14 (2H,
m), 8.16 (lH, s), 8.70 (lH, s), 10.07 (lH, s)
APCI-MASS (m/z) : 174 (M+H+)
~reparation 102
To a solution of 4-fluorobenzaldehyde (5.0 g) and lH-
1,2,3-tria7O1e (3.33 g) in N,N-dimethylformamide (50 ml)
w2s added potasslum carbonate (6.68 g). Then the mixture
was heated for one hour at 120C. Afte~ cooling, the
reaction mixture was diluted with ethyi acetate (300 ml),
washed with water, brine, dried over magnesium sulfate and
evaporated to about 50 ml in vacuo- The resulting
~ precipitate was collectea by filtration, washed with n-
hexane to give 4-(lH-1~2~3-tria7Ol-l-y~l)ben7âldehyde (3.44
g). The mother li~uid was evaporated to about 10 ml in
vacuo. The resulting precipitate was also collected in Ihe
PCT/~5/01982
Wo 96lloss9 2 ~ Q ~
- 94 -
similar procedure as mentioned above to give 4-(2H-1,2,3-
triazol-2-yl)benzaldehyde (297 mg).
4-(lH-1,2,3-Triazol-l-yl)benzaldehyde
IR (KBr) : 3138, 3116, 2845, i695, 16G3, 1516, 1419,
1389 cm~1
N~R (CDCl3, o) : 7.91 (lH, s), 7.93-8.11 (4P, m),
8.12 (lH, s), 10.09 (lH, s)
APCI-MASS (m/z) : 174 (M+H+~
4-(2H-1,2,3-Triazol-2-yl)benzaldehyde
IR (KBr) : 3114, 3084, 2715, 1699, 1603, 1508, 1408,
1383 cm~1
NMR (CDC13, o) . 7.89 (2H, s), 7.95-8.06 (2H! m),
8.23-8.33 (2H, m), 10.06 (lH, s)
APCI-MASS (m/z) : 174 (M+H+)
Preparation 103
To a solution of 4-fluorobenzaldehyde (6.21 g) in
N,N-dimethylformamide (100 ml) were added
ll-methylpiperazine (6.01 g) and powdered potassium
carbonate (8.29 g), and the mixture was stirred at 150C
for 4.5 hours under nitrogen. The mixture was poured into
a mixture of ethyl acetate and ice water, and the separated
organic layer was washed with water and brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give 4-
~4-methylpiperazin-1-yl)benzaldehyde (5.31 g) as a yellow
solid.
IR (KBr) : 2935, 2840, 2790, 2750, 1690, 1600, 156G,
1520 cm~1
NMR (DMSO-d6, o) : 2.22 (3H, s), 2.4-2.5 (4H, m),
3.35-3.45 (4H, m), 7.04 (2H, d, J=8.8Hz), 7.70 ~ ~
(2H, d, J=8.8Hz), 9.71 (lH, s)
WO96/10559 PCT/~5/01982
- 95 -
Preparation 104
To a solution of 4-bromoaniline (6.88 g) in pyridine
(20 ml) was added dr~pwise methanesulfonyl chloride (4.58
g) at 5C and the mixture was stirred at 5C for 1.5 hours
and at room temperature for 1.5 hours. The mixture was
poured into a mixture of ethyl acetate and dilute
hydrochloric acid and the insoluble materials were filtered
off. The filtrate was separated and the organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was crystallized and the
crystal was collected by filtration, washed with
diisopropyl ether and dried to give 4-bromo-N-
methylsulfonylaniline (8.30 g).
IR (KBr) : 3290, 1490 cm 1
NMR (DMSO-d6, o) : 3.00 (3H, s), 7.16 (2H, d,
J=8.7Hz), 7.52 (2H, d, J=8.7Hz), 9.92 (lH, br)
Pre~aration 105
To a suspension of N-methyl-N-methoxy-4-
sulfamoylbenzamide (3.53 g) and benzoic acid (1.95 g) in
dichloromethane (lO0 ml) were added 4-dimethylaminopyridine
(1.96 g) and 1-(3-dimethylaminopropyl)-3-ethyl
carbodiimidehydrochloride (3.07 g) at room temperature and
the mixture was stirred at the same temperature for 18
hours. The mixture was washed with water and brine, dried
over magnesium sulfate and evaporated in vacuo. The
residue was purified by column chromatography on silica gel
to give N-methyl-N-methoxy 4-(N-benzoylsulfamoyl)benzamide
(1.35 g).
IR (KBr) : 3072, 2970, 2937, 1649, 1597, 1560,
1544 cm~1
NMR (DMSO-d6, o) : 3.27 (3H, s), 3.55 (3H, s), 7.35-
7.5 (3H, m), 7.68 (2H, d, J=8.2Hz), 7.85-7.95
(2H, m), 7.96 (2H, d, J=8.2Hz)
APCI-MASS (m/z) : 349 (M+H+)
WO96/10559 2 2 0 0 ~ 8 ~ PCTt~5/01982
- 96 -
?re~aration 106
To a suspension of 4-cyanobenzaldehyde (26.23 g) was
added carefully sodium borohydride (3.78 g) at room
temperature, and the mixture was stirred at the same
temperature for 2 hours. The mixture was eva~orated in
vacuo and the residue was extracted with dichloromethane.
The organic layer was washed with brine, dried over
magnesium sulfate and evaporated in vacuo to give crude
4-cyanobenzyl alcohol (24.97 g) as an oil.
IR (Film) : 3419, 2916, 2875, 2229, 1610 cm 1
NMR (CDCl3, o) : 2.07 (lH, br), 4.79 (2H, br s), 7.48
(2H, d, J=8.lHz), 7.65 (2H, d, J=8.lHz)
APC I -MAS S ~ m/ z ): 1 3 4 (M+H~ )
Preparation 107
~ o a solutior of 4-cyanobenzyl alcohol (24.96 g) in
N,N-dimethylformamide (100 ml) were added imidazole (16.0
g~ and tert-butyldimethylsilyl chloride (31.0 g) at room
temperature and the mixture was stirred for ~ hours. The
mixture was poured intc a mixture of ethvl acetate and ice
water, and the separated organic layer was washed with
water and brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give 4-(tert-
butyldimethylsilyloxymethyl)benzonitrile (40.78 g) as an
oll .
IR (Film) : 2954, 2429, 2885, 2858, 2229, 1610 cm~
NMR (CDCl3, o) : 0.11 (6H, s), 0.95 (9H, s), 4.79
(2H, s), 7.43 (2H, d, J=8.3Hz), 7.63 (2H, d,
J=8.3Hz)
APCI-MASS (m/z) : 248 (M+H+)
~reparation 108
To a solution of n-butyllithium (1.71M hexane
solution, 58.5 ml) in diethyl ether (150 ml) was added
W096/lOSSs PCTt~5/01982
9 8 ~ .
- 97 -
dropwise 3-bromoDyridine (15.8 g) at 5C, and the mixture
W25 5 ~irred at 5C for an hour. The mixture was cooled tc
- -6CC and a solution of 4-(tert-butyldimethylsiiyl-
oxymethyl)benzonitrile (13.79 g) in diethyl ether (80 ml)
was added dropwise over 1.2 nou~s under ni_roger. The
mixture was graduall~ warmed ~o room tem~erâture and
stirred at the same temperature for additional 2 hours.
The mixture was poured into a mixture of ethyl aceta.e and
dilute hydrochloric acid, and the separated organic layer
was washed with water and brine, dri2d over r. gnesium
sulfate and evaporated in vacuo. The residue was purified
by column chromatography on sillca 5el to give 3-[(4-te~t-
butyldimethylsilyloxymethyl)benzoyl]pyridine (~.96 a) as a
red oll.
IR (Film) : 3034, 2954, 2930, 2885, 2856, 1~60, 16a8,
158~, 1537 c~ 1
L~MR (CDCl3, o) : C.16 (~, s), 0.99 (9n, s), 4.87
(2H, s), 7.50 (2~, d, J=7.6Hz), 7.83 (2H, d,
J=7.6~z), 7.4-7.~ , m), 8.1-8.2 (lH, m), 8.8-
8.9 (1~., m), 8.99 (lH, d, J=2.1~z)
~CI-MASS (m/7) :328 (M H+)
Preparation 109
To a suspension cf 3-[(4-tert-butyldimethylsilyloxy-
methyl)benzoyl]Dyridine (~.94 g) in ethylene glycol (40 ml)
were added potassium hydroxide (1.27 g) and hydra7ine
hydrate (4.84 g) and the mixture was stirred at 150C for 2
hours and at 200C for 4 hours. The mixture was poured
into a mixture of ethyl acetate and ice water, and the
separated crganic layer was washed with water and brir.e
dried over magnesiwm sulfate and evaporated n vacuo. The
residue was purified by column chroma-o~raphy on silica gel
to give 4-(3-~yridylmethyl)benzyl alcohol ~1.2~ g) 25 cn
orange oll.
_R (Film) : 3323, 3030, 2920, 2868, 15/9, 1549,
.
WO96110559 2200Y8 1 1~cT/Jr9sl~l982
-- 98 --
'514 cm~1
~MR (CDC13, o) : 1. 85 (lH, ~r), 3.97 (2~, c)~ a . 67
(2H, s), 7.15-7.~ (6~, ~), 8.45-8.5a i2~, m)
A CI-~ASS (m/7) 20C (M 4: j
Preparatio~ 110
To a solullon of 4-(3-pyridylmet;~yl)benzyl alcohol
(1.26 g) in chlorofor~ (3~ m.1) was activated mang2nese
dioxide (5.50 g) and the mix'ure was refluxed for 2 nours.
Manganese dio~ide was re~oved off and t~e Liltr~te was
evaporated in vacuo and the resldue was purified bv cclumn
chromatography on silica gel ~o give 4-(3-pyridylmethyl)-
benzaldehyde (1.09 g) as an orange c_l.
TR (~ilm) : 3029, 2989, 2910, 2831, 2738, i697, '599,
15~0 c~ -
NMR (CDC13, o) : ~.07 !2~, s), 7.2~ (lUl, dd, J=7.8,
4.8~), 7.35 (2H, d, J=8.lHz), 7.47 (lH, dd,
J=7.8, 1.4Hz), 7.83 (~H, d, J=8.lHz), 8.49 (lH,
d, J=1.4H7), 8.5~ , s), 9.99 (1~:, s)
APCI-MASS (m!7) : lg8 (M U+)
Pre~aration 111
To a solution of 1-ethoxycarbonyl-4-diethylphosphono-
1,4-dihydropyridine (34.71 g) in tetrahydrofuran (200 ml~
was added dropwise n-butyllithium (1.71M kexane solution,
70.2 ml) at -60C over 30 minutes under nitrcger, and the
mixture was stirred at -60C for 40 minutes. To this
solution was added dropwise a solution of 4-cyanobenzyl
bromide (27.aO g) in tetrahydrofuran (80 ml) 2t -50C and
the mixture was gradually warmed to room temperature and
st rred fcr 21 hours. The mixture was poured into a
~ixture of ethyl acetate and dilute hydrochloric acid, and
the separated orgar.ic layer was washed with wate and
brine, dried over magnesium sulfate and evaporated in
vacuo. The residue was purified by column chromatography on
WO961105S9 PCT/~5/01982
~2~ ~9 ~ ~ s
_ 99 _
silica gel to give 1-ethoxycarbonyl-4-(4-cyanobenzyl)-4-
diethylphos~hono-1,4-dihydropyridine (47.10 g) cs a crude
- red oi'.
IR (Film) : 3053, 2831, 2933, 29G3, 22~7, 1728, 1683,
1626, 1606 cm~1
N~R (CDCl3, o) : 1 -1.4 (9~, ~), 3.06 (2H, d,
J=7.6Hz), 4.1-4.3 (6H, m), 4.7-4.9 (2H, m),
7.65-7.9 (2H, ~)/ 7.22 (2h, ~, J=8.2:~z), ,._5
(2H, d, J=8.2Hz)
APCI-~SS (m/z) : 405 (M+HT)
Pre~aration 112
To a solution of 1-ethoxycarbonyl-4-(4-cyanobenzyl)-4-
diethyl~hos~hono-1,4-dihydropyridine (42.l0 g) ir
dichloromethane (350 ml) was added d~o~wise
diisobutylaluminum hydride (l.OlM toluene solu~ion, 515 ml)
at -oOC over 55 minutes and the mixture was stirred 2t -
60C for 1.5 hours. The mixture was gradually warmed to
5._ and stirred at 5C for 1.5 hours. To the mixture were
added sodium fluoride (87.34 g) and water (28.~1 g) ard tne
mixtur2 was stirred at room temperature fGr an hou- Th~
insoluble mater~als were filtered of_ and washed witn
dichloromethane. The filtrate was eva~orated in vacuo an
the residue was dissolved in tetrahydrofuran (2C0 ml). To
this solution was added 6N hydrochlcric acid (3Q ml) and
the mixture was stirred at room temperature ror 3 hours.
The mix~ure was adjusted to pH ca. 8 bv addition or 5~
sodium hvdroxide and extracted with dichloromethane. The
orgaric layer was washed with water and brine, dried over
magnesium sulfate and eva~orated in vacuG. The residue waC
purified by _olumn chromatog--aphy on silica gel to CiV2
~ 4-(4-~yridylmethyl)benzaldehyde (4.62 g) as a red o l.
I~ (Film) : 3381, 3053, 3030, 2924, 2831, 2738, 16g7,
1606, 1576 cm~L
~R (CDCl3, o) : 4.08 (2-~, s), 7.1-7.2 (,H, m~,
2 2 0 0 9 8 1 PCT/~5101982
WO96/10559 _ ~
-- 100 --
7.3-7.45 (2H, m), 7.8-7.9 (2H, m), 8.5-8.6 (2H,
m), 10.00 (lH, s)
Preparation 113
To a solution of pyrazole (1.67 g) in
N,N-dimethylformamide (30 ml) was added sodium hydride (60c
oil suspension, 950 mg) at 0-5C. After stirring for 30
minutes, to the mixture was added a solution of
4-bromomethylbenzonitrile (4.0 g) in N,N-dimethylformamide
(10 ml) dropwise under ice cooling, and the mixture was
stirred for two hours at room tem~erature. The reaction
mixture was diluted with ethyl acetate (240 ml), washed
with water and brine, dried over magnesium sulfate,
evaporated in vacuo. The residue was chromatographed on
silica gel (100 g, eluting with n-hexane - ethyl acetate
(1:1)) to give 4-(pyrazol-1-ylmethyl)benzonitrile (3.49 g).
mp : 80-81C
IR (KBr) : 3055, 2958, 2229, 1610, 1510, 1446, 1392,
1275 cm~l
NMR (CDCl3, o) : 5.39 (2H, s), 6.33 (lH, dd, J=2.1,
2.lHz) ! 7.18-7.30 (2H, m), 7.44 (lH, d, J=2.lHz),
7.55-7.70 (3H, m)
APCI-MASS (m/z) : 184 (M+H+)
Preparation 114
To a solution of imidazole (1.67 g) in
N,N-dimethylformamide (30 ml) was added sodium hydride (60
oil suspension, 950 mg) at 0-5C. After stirring for 30
minutes, to the mixture was added a solution of
4-bromomethylbenzonitrile (4.0 g) in N,N-dimethylformamide
(iO ml) dropwise under ice cooling, and the mixture was
stirred for two hours at room temperature. The reaction
mixture was diluted with ethyl acetate (240 ml), washed
with water and brine, dried over magnesium sulfate,
evaporated in vacuo. The residue was chromatographed on
PCT/~S/01982
W096/lOS59
~200~8
-- 101 --
silica gel (100 g, eluting with dichloromethane - methanol
(15:1!) to give 4-(imidazol-1-ylmethyl)benzonitrile (3.26
g)
IR (KBr) : 3095, 3057, 2229, 1608, 151G, 1425, 1236,
1074, 731 cm~l
NMR (CDC13, o) : 5.21 (2H, s), 6.90 (lH, s), 7.14
(lH, s), 7.15-7.27 (2H, m), 7.57 (lH, s), 7.60-
7.72 (2H, m)
APCI-MASS (m/z) : 184 (M+H+)
Preparation 115
To a suspension of 3,5-di-tert-butyl-4-hydroxybenzoic
acid (lO.O g) in methanoi (100 ml) were added sodium
hydroxide (1.6 g) and water (6 ml) and the mixture was
stirred at room temperature for 35 minutes. The mixture
was evaporated in vacuo and dried thoroughly. The sodium
salt was suspended in petroleum ether (60 ml), and thionyl
chloride (30.93 g) was added thereto and the mixture was
stirred at room temperature for 16 hours. The mixture was
- 20 evaporated in vacuo and the residue was redissolved _n
etroleum ether (200 ml). The insoluble materials were
filtered off and the filtraie was evaporated in vacuo to
give 3,5-di-tert-butyl-4-hydroxybenzoyl chloride (9.81 a
as a yellow solid.
IR (KBr) : 3554, 2974, 2956, 1736, 1597, 1574 cm~-
Preparation 116
To a solution of sodi~m azide (4.61 g) in wate-- (3G
ml) was added dropwise a solution of 3,5-di-tert-butyl-4-
3C hydroxybenzoyl chloride (12.72 g) in tetrahydrofuran (60
ml) at 5C over 30 minutes, and the mixture W2S stirred a.
~ 5C for 1.5 hours. The mixture was extracted with ethyl
acetate, and the organic layer was washed with brine, dried
over magnesium sulfate and evaporated in vacuo To the
residue was added n-hexane (60 ml) and the insoluble
Wog6/losss 2~ 0 ~ 9 P~T/J~S/01982
- 102 -
materials were filtered off. The filtrate was evaporated
in vacuo to give 3,5-di-tert-butyl-4-hydroxybenzoyl azide
(1.49 g) as a yellow solid.
IR (KBr) : 3593, 2966, 2912, 2873, 2141, 1668,
1599 cm~
Preparation 117
A suspension of 3,5-di-tert-butyl-4-hydroxybenzoyl
azide (1.49 g) in benzene (30 ml) was refluxed for an hour
under nitrogen. To the mixture was added tert-butanol
(4.01 g) and the mixture was refluxed for 3 hours. The
mixture was evaporated in vacuo and the residue was
purified by column chromatography on silica gel to give
N-tert-butoxycarbonyl-3,5-di-tert-butyl-4-hydroxyaniline
(1.17 g) as a white solid.
IR (KBr) : 3647, 3331, 2958, 2913, 2873, 1693, 1606,
1547 cm~1
NMR (DMSO-d6, o) : 1.34 (18H, s), 1.44 (9H, s), 6.60
(lH, s), 7.22 (2H, s), 8.84 (lH, br s)
Dreparation 118
To a solution of N-tert-butoxycarbonyl-3,5-di-tert-
butyl-4-hydroxyaniline (3.97 g) in ethyl acetate (60 ml)
and ethanol (15 ml) was added 4N hydrochlo~ic acid in ethyl
acetate (30.8 ml) and the mixture was stirred at room
temperature for 24 hours. The mixture was evaporated in
vacuo and the residue was triturated with diisopropyi
ether. The powder was collected by filtration, washed wi~h
diisopropyl ether and dried in vacuo to give 3,5-di-tert-
butyl-4-hydroxyaniline-hydrochloride (2.85 g).
IR (KBr) : 2966, 2912, 2873, 2590, 1581, 1512 cm~
NMR (DMSO-d6, o) : 1.38 (18~, s), 7.12 (2H, s),
7.34 (lH, s), 9.83 (2H, br s)
PCT1~5101982
WO96/10559 C U 0 9
- 103 -
Preparation 119
To a suspension of 4-formylbenzoic acid (1.65 g), 3,5-
di-tert-butyl-4-hydroxyaniline-hydrochloride (2.83 g) and
1-hydroxybenzotriazole (1.49 g) in dichloromethane (60 ml)
was added 3-(3-dimethylaminopropyl)-1-ethylcarbodiimide
(1.71 g) at room temperature and the resulting solution was
stirred at the same temperature for 20 hours. The mixture
was washed with water and brine, dried over magnesium
sulfate and evaporated in vacuo. The residue was purified
by column chromatography on silica gel to give 4-[(3,5-di-
tert-butyl-4-hydroxyphenyl)carbamoyl]benzaldehyde (2.53 g).
IR (KBr) : 3624, 3286, 2958, 2912, 2872, 1703, 1645,
1606, 1547 cm~1
NMR (DMSO-d6, o) : 1.43 (18H, s), 6.83 (2H, d,
J=5.1Hz), 7.09 (2H, d, J=5.1Hz), 7.12 (2H, s),
10.07 (lH, s), 10.19 (lH, s)
APCI-MASS (m/z) : 354 (M+H+)
Preparation 120
To a suspension of 4-formylbenzoic acid (7.5 g) in
dichloromethane (25 ml) were added thionyl chloride (11.9
g) and N,N-dimethylformamide (365 mg) at room temperature,
and the mixture was refluxed for 4 hours under nitrogen.
The mixture was evaporated in vacuo and dried in vacuo to
give crude 4-formylbenzoyl chloride (8.53 g) as a white
powder.
IR (KBr) : 3066, 2856, 1745, 169î, 1576, 1504 cm~
Preparation 121
To a solution of 4-fluoroaniline (5.0 g) and
triethylamine (6.07 g) in dichloromethane (60 ml) was added
~ portionwise 4-formylbenzoyl chloride (8.53 g) at 5C and
the mixture was stirred at room temperature for 2 hours.
The mixture was washed with water and brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
WO96110559 2Z ~ 0 9 8 ~ PCT/~5/01982
- 104 -
crystallized from hexane - ethyl acetate (3:1) and
collected by filtration, washed with hexane - ethyl acetate
(3:1) and dried in vacuo to give 4-[N-(4-fluorophenyl)-
carbamoyl]benzaldehyde (4.58 g).
IR (KBr) : 3356, 2872, 1703, 1651, 1606, 1537,
1514 cm~l
NMR (DMSO-d6, o) : 7.15-7.3 (2H, m), 7.8-7.9 ~2H, m),
8.06 (2H, d, J=8.4Hz), 8.14 (2H, d, J=8.4Hz),
10.12 (lH, s), 10.53 (lH, br s)
APCI-MASS (m/ 7 ) 244 ~M+H+)
Preparation 122
To a susPension of sodium hydride (60~ oil dispersion,
464 mg) in N,N-dimethylformamlde (50 ml) was added dropwise
a solution of 4-[N-(4-fluorophenyl)carbamoyl]benzaldehyde
(2.63 g) in N,N-dimethylformamide (40 ml) at 5C under
nitrogen, and the mixture was stirred at room temperature
for an hour. To the mixture was added methyl iodide ~3.29
g), and the mixture was stirred at room temperature for 3
hours. The mixture was poured into a mixture of ethyl
acetate and ice water. The separateà organic layer was
washed with water and brine, dried over magnesium sulfate
and evaporated in vacuo. The residue W2S purified by
column chromatography on silica gel to give 4-[N-(4-
fluorophenyl)-N-methylcarbamoyl]~enZaldehyde (2.24 g) as an
orange oil.
IR (Film) : 3068, 2981, 2939, 2839, 2737, 1703, 1639,
1608, 1571, 1510 cm~1
NMR (DMSO-d6, o) : 3.37 (3H, s), 7.05-7.2 (2H, m),
7.25-7.35 (2H, m), 7.46 (2H, d, J-8.1Hz), 7.77
(2H, d, J=8.lHz), 9.~3 (lH, s)
APCI-MASS (m/z) : 258 (M+H+)
Preparation 123
To a solution of 1,4-bis~hydroxymethyl)benzene (25.7^
. , . _ . . . . .
WO96/10559 PCTt~5/01982
~ 8 ~ .
- 105 -
g) in N,N-dimethylformamide (300 ml? were added imidazole
(15.21 g) and tert-butyldimethylsilyl chloridé (28.06 g) at
room temperature, and the mixture was stirred for 10 hours.
The mixture was poured into a mixture of ethyl acetate and
ice water, and the separated organic layer was washed with
water and brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give 4-(tert-
butyldimethylsilyloxymethyl)benzyl alcohol (27.89 g) as an
oil.
IR (Film) : 3352, 2954, 2931, 2887, 2858, 1541, 1514,
1466 cm~1
NMR (DMSO-d6, o) : 0.07 (6H, s), 0.90 (9H, s), 4.47
(2H, d, J=5.7Hz), 4.68 (2H, s), 5.10 (lH, t,
J=5.7Hz), 7.2-7.3 (4H, m)
Preparation 124
To a solution of 4-(tert-butyldimethylsilyloxymethyl)-
benzyl alcohol (27.86 g) in chloroform (300 ml) was added
activated manganese dioxide (47.98 g) and the mixture was
refluxed for 3.5 hours. Manganese dioxide was filtered off
and the filtrale was evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give 4-
(tert-butyldimethylsilyloxymethyl)benzaldehyde (26.86 g) as
a pale yellow oil.
IR (Film) : 2955, 2931, 2889, 2858, 2731, 1703
1608, 1578, 1541 cm~1
NMR (DMSO-d6, o) : 0.10 (6H, s), 0.92 (9H, s), 4.82
(2H, s), 7.53 (2H, d, J=8.2Hz), 7.89 (2H, d,
J=8.2Hz), 9.99 (lH, s)
Preparation 125
To a solution of N-cycloheptyl-4-(tert-
butyldimethylsilyloxymethyl)benzylamine (53.74 g) in
methanol (250 ml) was added dropwise conc. hydrochloric
W096/10~59 ~ ~ O O 9 8 1 PCT/~5/01982 ~
- 106 -
acid (38.6 ml) at 5C, and the mixture was stirred at room
temperature for 3 hours. The mixture was evaporated in
vacuo and the residue was pulverized with tetrahydrofuran
and ethyl acetate. The powder was collected by filtration,
washed with ethyl acetate and tetrahydrofuran and ethyl
acetate (1:1), and dried in vacuo under phosphorus
pentoxide to give N-cycloheptyl-4-
hydroxymethylbenzylaminehydrochloride (37.92 g).
IR (KBr) : 3294, 2927, 2858, 2791, 1578, 1541, 1514,
1456 cm~1
NMR (DMSO-d6, o) : 1.4-2.2 (12H, m), 3.05-3.25 (lH,
m), 4.12 (2H, s), 4.52 (2H, d, J=5.7Hz), 5.27
(lH, t, J=5.7Hz), 7.36 (2H, d, J=8.OHz), 7.48
(2H, d, J=8.0Hz), 8.7-8.9 (lH, br)
15APCI-MASS (m/z) : 234 (M+H+-HCl)
Preparation 126
To a suspension of N-cycloheptyl-4-
hydroxymethylbenzylaminehydrochloride (37.9 g) in
chloroform (400 ml) were added activated manganese dioxide
(oO.86 g) and~triethylamine (14.21 g), and the mlxture was
refluxed for 4 hours. Manganese dioxide was filtered off
and the filtrate was washed with water and brine, dried
over magnesium sulfate and evaporated in vacuo. The
residue was purified by column chromatography on silica gel
to give N-cycloheptyl-4-formylbenzylamine (18.27 g) as a
yellow oil.
IR (Film) : 3051, 2924, 2854, 2731, 1701, 1606, 1576,
1468 cm~1
30NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.0-2.2 (lH,
br), 2.5-2.7 (lH, m), 3.77 (2H, s), 7.56 (2H, d,
J=8.lHz), 7.85 (2H, d, J=8.lHz), 9.97 (lH, s)
APCI-MASS (m/z) : ~32 (M+H+)
WO96/105~9 PCT/~5/01982
~20~8
- 107 -
Preparation 127
To a solution of N-cycloheptyl-4-formylbenzylamine
(18.26 g) in ethanol (200 ml) were added thiazolidin 2,4-
dione (9.25 g) and piperidine (6.72 g), and the mixture was
refluxed for 17 hours. The mixture was cooled to 5C and
the precipitates were collected by filtration, washed with
ethanol and diisopropyl ether and dried in vacuo to give
N-cycloheptyl-4-[(2,4-dioxothiazolidin-5-ylidene)methyl~-
benzylamine (9.61 g) as a yellow crystal. The flltrate was
evaporated in vacuo and the residue was purified by column
chromatography on silica gel to give the second crop (4.13
g).
IR (KBr) : 3429, 3024, 2929, 2858, 1684, 1622, 1576,
1547, 1458 cm~l
NMR (DMSO-d6, o) : 1.3-2.2 (12H, m), 3.05-3.25 (lH,
m), 4.12 (2H, s), 7.35 (lH, s), 7.52 (2H, d,
J=8.5Hz), 7.58 (2H, d, J=8.5Hz)
APCI-MASS (m/z) : 331 (M+H+)
Preparation 128
To a suspension of N-cycloheptyl-4-[(2,4-
dioxothiazolin-5-ylidene)methyl]benzylamine (13.61 g) in
tetrahydrofuran (300 ml) and methanol (300 ml) was added 5
sodium-amalgam. (56.8 g~, and the m.ixture ~as stirred at
room temperature for 24 hours. The insoluble materials
were removed by filtration on celite and the filtrate was
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give N-cycloheptyl-4-[(2,4-
dioxothiazolidin-5-yl)methyl]benzylamine (5.84 g) as a
yellow solid.
IR (KBr) : 3028, 2933, 2862, 2764, 1674, 1630, 1581,
1460 cm~l
NMR (DMSO-d6, o) : 1.3-2.2 (12H, m), 2.9-3.1 (lH, m),
2.95 (lH, dd, J=13.8, 9.3Hz), 3.36 (lH, dd,
J=9.3, 4.0Hz), 4.54 (lH, dd, J=9.3, 4.0Hz), 7.25
W096/lOS59 22 0 0 9 8 1 PCT/~5/01982
- 108 -
(lH, d, J=8.lHz), 7.38 (lH, d, J=8.lHz)
APCI-MASS (m/z) : 333 (M+H+)
Preparation 129
To a solution of 4-fluorobenzaldehyde (20.11 g) and 4-
chlorophenol (25.0 g) in N,N-dimethylformamide (250 ml) was
added powdered potassium carbonate (26.81 g), and the
mixture was stirred at 150C under nitrogen for 7 hours.
The mixture was cooled and poured into a mixture of ethyl
acetate and water. The separated organic layer was washed
with water and brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give 4-(4-
chlorophenoxy)benzaldehyde (32.49 g) as a yellow oil.
IR (Film) : 3070, 2985, 2830, 2740, 1735, 1695, 1605,
1580, 1485 cm~1
NMR (CDCl3, o) : 7.0-7.15 (4H, m), 7.35-7.45 (2H, m),
7.8-7.9 (2H, m), 9.93 (lH, s)
APCI-MASS (m/z) : 235, 233 (M+H+)
Pre~aration 130
To a solution of 4-fluorobenzaldehyde (5 g) and 3-
fluorophenol (5.42 g) in N,N-dimethylformamide (50 ml) was
added potassium carbonate (6.68 g). Then the mixture was
heated for 3.5 hours at 150C. After cooling, the reaction
mixture was diluted with ethyl acetate (300 ml), washed
with water, brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was chromatographed on
silica gel (200 g, eluting with n-hexane - ethyl acetate
(10:1)) to give 4-(3-fluorophenoxy)benzaldehyde (8.67 g).
IR (Neat) : 3072, 2831, 2738, 1697, 1587, 1483 cm~
NMR (CDCl3, o) : 6.75-7.00 (3H, m), 7.05-7.18 (2H,
m), 7.28-7.42 (lH, m), 7.82-7.95 (2H, m), 9.95
(lH, s)
APCI-MASS (m/z) : 217 (M+H+)
~ WO96/10559 ~2 ~ O PCT/~5101982
-- 109 --
Preparation 131
To a solution of 4-fluorobenzaldehyde (3 g) and
4-trifluoromethylphenol (4.7 g) in N,N-dimethylformamide
(30 ml) was added potassium carbonate (4.0 g). Then the
mixture was heated for 5 hours at 150C. After cooling,
the reaction mixture was diluted with ethyl acetate (300
ml), washed with water, brine, dried over magnesium sulfate
- and evaporated in vacuo. The residue was chromatographed
on silica gel (200 g, eluting with n-hexane - ethyl acetate
(15:1)) to give 4-(4-trifluoromethylphenoxy)benzaldehyde
(982.1 mg).
IR (Neat) : 3074, 2831, 2738, 1701, 1587, 1502 cm 1
N~R (CDC13, o) : 7.05-7.25 (4H, m), 7.60-7.75 (2H,
m), 7.85-7.98 (2H, m), 9.96 (lH, s)
FAB-MASS (m/z) : 267 (M+H+)
Preparation 132
To a solution of 4-fluorobenzaldehyde (3 g) and 3,4-
methylenedioxyphenol (4 g) in N,N-dimethylformamide (30 ml)
was added potassium carbonate (4 g). Then the mixture was
heated for 2 hours at 150C. After cooling, the reaction
mixture was diluted with ethyl acetate (200 ml), washed
with water, brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was chromatographed cn
silica gel (120 g, eluting with n-hexane - ethyl acetate
(5:1)) to give 4-(3,4-methylenedioxyphenoxy)benzaldehyde
(2.67 g).
m~ : 65-66C
IR (KBr) : 1691, 1600, i481, 1227 cm~l
NMR (CDC13, o) : 6.02 (2H, s), 6.50-6.65 (2H, m),
6.82 (lH, d, J=8.3Hz), 6.96-7.07 (2r', m), 7.78-
7.83 (2H, m), 9.91 (lH, s)
APCI-MASS (m/z) : 243 (M+H+)
WO96/lOSS9 2 ~ ~ O g 8 ~ PCTt~5/01982
-- 110 --
Preparation 133
To a solution of 4-fluorobenzaldehyde (2.48 g) and
3,5-di-tert-butyl-4-methoxymethoxyphenol (5.33 g) in N,~--
dimethylformamide (40 ml) was added powdered potassium
carbonate (2.76 g), and the mixture was stirred at 150C
for 6 hours under nitrogen. The mixture was poured into a
mixture of ethyl acetate and ice water, and the separated
organic layer was washed with water and brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give 4-
(3,5-di-tert-butyl-4-methoxymethoxyphenoxy)benzaldehyde
(4.03 g) as an orange oil.
IR (Film) : 2960, 2872, 2740, 2693, 1581, 1504 c~ 1
NMR (CDCl3, o) : 1.43 (18H, s), 3.66 (3H, s), 4.94
(2H, s), 6.99 (2H, s), 7.02 (2H, d, J=8.8Hz),
7.83 (2H, d, J=8.8Hz), 9.92 (lH, s)
APCI-MASS (m/z) : 371 (M+H+)
Preparation 134
To a solution of 4-fluoronitrobenzene ~14.11 g) and 4-
fluorophenol (12.33 g) in N,N-dimethylformamide (150 ml)
was added powdered potassium carbonate (15.20 g), and the
mixture was stirred at 100C for 4.5 hours under nitrogen.
The mixture was poured into a mixture of ethyl acetate and
ice water and the separated organic layer was washed with
water and brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was crystallized and the
crystal was collected by filtration and washed with hexane
and dried to give 4-(4-fluorophenoxy)nitrobenzene (22.96 g)
as a yellow crystal.
IR (KBr) : 3110, 3075, 2925, 2835, 1585, 1510 c~~-
NMR ~CDCl3, ~) : 6.95-7.2 (6H, m), 8.15-8.3 (2H, m)
APCI-MA5S (m/z) : 234 (M+H+)
WO96/10559 PCT/~5/01982
~ 09 8 ~
Preparation 135
To a suspension of 3-hydroxybenzyl alcohol (12.41 g)
and 1-chloro-4-fluoro~enzene (19.58 g) in 1,3-dimethyl-2-
imidazolidinone (40 ml) were added powdered potassium
carbonate (8.29 g), cuprous chloride (198 mg) and
8-hydroxy~uinoline (290 mg) at room temperature, and the
mixture was stirred at 150C for 8 hours. The mixture was
poured into a mixture of-ethyl acetate and ice water and
the separated organi- layer was washed with water and
brine, dried over magnesium sulfate and evaporated in
vacuo. The residue was purified by column chromatography
on silica gel to give 3-(4-fluorophenoxy)benzyl alcohol
(3.98 g) as a yellow oil.
IR (Film) : 3352, 3074, 2931, 2875, 1610, 1585, 1502,
1448 cm~1
NMR (DMSO-d6, o) : 4.47 (2H, d, J=5.6Hz), 5.22 (lH,
t, J=5.6Hz), 6.8-7. r (8H, m)
Pre~aration 136
To a solution of 4-fluorobenzonitrile (50.0 g) and 4-
fluorophenol (50.93 g) in N,N-dimethylformamide (400 ml)
was added powdered potassium carbonate (62.75 g), and the
mixture was stirred at 150C for 6 hours. The mixture was
cooled to 5C and poured into ice water (2.5 Q). The
precipitates were collected by filtrztion, washed with
water and dried in vacuo to give 4-(4-fluorophenoxy)-
benzon trile (87.56 g).
IR (KBr) : 3188, 3076, 2220, 1649, 1608, 1483 cm~1
NMR (DMSO-d6, o) : 7.05-7.15 (2H, m), 7.2-7.45 (4H,
m), 7.8-7.9 (2H, m)
APCI-M~SS (m/z) : 214 (M+H+)
Preparation 137
To a stirred suspension of 3-acetylbenzonitrile (25.4
g) in ethyl ether - 1,4-dioxane (10:1, 275 ml) was added
WO96/105S9 2 ~ 8 ~ PCTI~S/01982
- 112 -
bromine (9 ml) dropwise at room temperature. Arter 40
minutes,to the mixture was added sodium bicarbonate (15 g)
in water (200 ml) at 0-5C, and extracted with ethyl
acetate. The organic layer was separated and washed with
saturated sodium bicarbonate solution, water and brine,
dried over magnesium sulfate, evaporated in vacuo to give
3-(bromoacetyl)benzonitrile (39.2 g).
IR (KBr~ : 3103, 3068, 2941, 2229, 1707, 1599,
1429, 1279, 1223, 1149 cm~1
NMR (CDCl3, o) : 4.42 (2H, s), 7.66 (lH, dd, J=8.1,
8.1Hz), 7.85-7.95 (lH, m), 8.18-8.32 (2H, m)
Preparation 138
A mixture of 3-(pyrazol-3-yl)benzaldehyde (56.0 g) and
benzylamine (42.6 ml) in toluene (560 ml) was refluxed for
5 hours. The reaction mixture was cooled to room
temperature, and evaporated in vacuo. The residue was
suspended in ethanol (840 ml) and sodium borohydride (12.3
g) was added carefully under ice cooling. Then the mixture
was stirred for one hour at 50C. After additional
stirring for 2 hours at room temperature, the reaction
mixture was evaporated in vacuo. To the residue was added
water (300 ml), and extracted with dichloromethane. The
organic layer was washed with water and brine, dried over
magnesium sulfate, evaporated in vacuo. The residue was
chromatogra~hed on silica gel (1.5 kg, eluting with
dichloromethane - methanol (10:1)) to give N-benzyl-3-
(pyrazol-3-yl)benzylamine (71.8 g).
mp : 82-83C
IR (KBr) : 2290-3310 (br), 1606, 1543, 1441,
1354 c~ 1
NMR (DMSO-d6, o) : 3.71 (2H, s), 3.7~ (2H, s), 6.68
(lH, d, J=2.lHz), 7.15-7.42 (7H, m), 7.50-7.90
(3H, m), 12.85, 13.22 (total lH, each br)
APCI-MASS (m/z) : ~64 (M+H+)
PCT/~5/01982
_ WO96/10559
~ ~2~ 09 8 ~ ~
- 113 -
Preparation 139
To a solution of 4-(4-fluorophenoxy)ariline (~.03 g)
and cyclohe~tanone (1.35 g) in ethanol (40 ml) we~e added
simul~aneousiy a solution of sodium cyanoborohydride (314
mg) in ethanol (30 mlj and a solution of acetic acid (60
mg) in ethanol ;lG ml) over 1 hour at room temperature.
The mixture was s~irred a. room temperature for additional
1.2 hours. The mixture was evaporated in vacuo and the
residue was poured into a mixture of ethyl acetate and
water and adjuste~ to pH 8 by addition of 5N sodium
hydroxide aqueous solution. The separated organic layer
was washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on s~lica gel to give N-cycloheptyl-a-(4-
fluorophenoxy)aniline (2.11 g) âS a red oil.
IR (Film) : 3405, 2925, 2855, 1735, 161C, 1495 cm 1
NMR (CDCl3, o) : 1.4-2.15 (12H, m), 3.3-3.5 (lH, m),
6.4-6.6 (2H, m), 6.75-7.05 (6H, m)
APCI-~SS (m/z) : 3GC (M+H~)
Preparation 140
The mixture of 4-(4-fluorophenoxy)benzaldehvde (1.73
g) and benzylamine (1.29 g) was stirred a~ 120C for 4
hours under nitrogen. The mixture was cooled to room
25 temperature and dissGlved in ethanol (40 ml). TG this
solution was added carefully sodium borohydride (303 mg)
~nd the mixture was stirred at room temperature for 2
hours. The mixture W2S evaporated in vacuo ard the residue
was extracted with dichloromethane. The orgaric iayer was
washed with brine, dried over ~agnesiu~.L sulfa~e and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to sive N-benzyl-[~-( d-
fluorophenoxy)]benzyla~.ine (1.78 g) as a yellow oil.
IR (Film) : 3062, 3028, 2916, 282', 1605, 1497 cm 1
NMR (CDCl3, o) : 3.78 (2~, s), 3.82 (2r~, s), 6.9-7.1
WO96/10559 PCT/~5/01982
~ 2 ~ ~ ~ 8 ~ - --
- 114 -
(6H, m), 7.2-7.4 (7H, m)
APCI-MASS (m/z) : 308 (M+H+)
Preparation 141
The mixture of 4-(4-~luorophenoxy)benzaldehyde (1.73
g) and pentylamine (1.40 g) was stirred at 120C for
hours under nitrogen The mixture was cooled to room
temperature and dissolved in ethanol (40 ml). To this
solution was added carefully sodium borohydride ~303 mg),
and the mixture was stirred at room temperature for 2
hours. The mixture was evaporated in vacuo and the residue
was extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give N-pentyl-4-(4-
fluorophenoxy)benzylamine (1.72 g) as a yellow oil.
IR (Film) : 3051, 2956, 2929, 2858, 2818, 1610,
1498 cm~l
NMR (CDCl3, o) : 0.89 (3H, t, J=6.~Hz), 1.2-1.4 (4H,
m), 1.5-1.7 (2H, m), 2.63 (2H, ~, J=7.1H~), 3.76
(2H, s), ~.9-7.1 (6H, m), 7.28 (2H, d, J=9.lHz)
~PCI-MASS (m/z) : 288 (M+H+)
Preparatio~ 142
The mixture of 4-(4-fluorophenoxy)benzaldehyde (2.16
g) and cyclohexylamine (1.49 g) was stirred at 120C for 4
hours under nitrogen. The mixture was cooled to room
te~perature and dissolved in ethanol (40 ml). To this
solution was added carefully sodium borohydride (378 mg),
and the mixture was stirred at room tem~erature for 2
hours. The mixture was evaporated in vacuo and the residue
was extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium suifate and
evaporated in vacuo. The residue was purified by colu~n
chromatography on silica gel to give N-cyclohexyl-4-(4-
, . . . . _ . . . . . ... .
PCT/~5/01982
W096/10559
~Z0~98 ~
- 115 -
fluorophenoxy)benzylamine (3.06 g) as a yellow Gil .
IR (Film) : 3034, 2929, 2852, 1608, 1497 cm 1
NMR (CDCl3, o) : 1.0-1.4 and 1.5-2.0 (lOH, m),
t 5 2.4-2.6 (lH, m), 3.78 (2H, s), 6.9-7.1 (~H, m),
7.28 (2H, d, J=8.4Hz)
APCI-MASS (m/z) : 300 (M+H+)
Preparation 143
The mixture of 4-(4-fluorophenoxy)benzaldehyde (~.16
g) and cyclopentylamine (1.28 g) was stirred at 120C for 4
hours under nitrogen. The mixture was cooled to room
temperature and dissolved in ethanol (40 ml). To this
solution was added carefully sodlum borohydride (378 mg)
and the mixture was stirred at room temperature for 2
hours. The mixture was evaporated in vacuo and the residue
was extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give N-cyclopentyl 4-~4-
fluorophenoxy)benzylamine (2.67 g) as a yellow oil.
IR (~ilm) : 3032, 2953, 2868, 2815, 1608, 1500 cm 1
NMR (CDC13, o) : 1.3-2.0 (8H, m), 3.05-3.25 (lH, m),
3.74 (2H, s), 6.9-7.1 (6H, m), 7.27 (2H, d,
J=8.4Hz)
APCI-MASS (m/z) : 286 (M+H+)
Prepar~tion 144
The mixture of 4-(4-fluorophenoxy)benzylamine (4.35 g)
a~d 2,3,5,6-tetrahydro-4H-pyran-4-one (2.40 g) was stirred
at 120C for 4 hours under nitrogen. The mixture was
cooled to room temperature and dissolved in ethanol (8C
ml). To this solution was added carefully sodium
borohydride (757 mg) and the mixture was stirred at room
temperature for 2 hours. The mixture was evaporated in
W096/lOS59 22 ~ ~ ~ 8 ~ PCT~5101982 ~
- 116 -
vacuo and the residue was extracted with dich1oromethane.
The organic layer was washed with brine, dried over
magnesium sulfate and eva~orated in vacuc. The residue was
purified by column chromatogra~hv on silica gel to give N-
2,3,5,6-tetrahydro-4H-pyran-4-yl)-4-(g-
fluorophenoxy)benzylamine (5.15 g) as an orange oil.
IR (Film) : 2927, 2845, 1498, 1464 cm 1
NMR (CDC13, o) : 1.4-1.7 (4H, m), 3.3-4.0 (4H, m),
3.80 (2H, s), 6.8-7.1 (6H, m), 7.2-7.4 (2H, m)
APCI-MASS (m/z) : 302 (M+H+)
Preparation 145
The mixture of 4-(4-fluorophenoxy)benzaldehyde (3.24
g) and phenethylamine (2.73 g) was stirred at 120C for 4
hours under nitrogen. The mixture was cooled to room
temperature and dissolved in ethanol (60 ml). To this
solution was added carefully sodium borohydride (567 mg),
and the mixture was stirred at room temperature or 2
hours. The mixture was evaporated in vacuo and the residue
was extracted with dichloromethane. The organic layer was
washed with ~rine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica geL to give N-(2-phenethyl)-4-(4-
~luorophenoxy)benzylamine (4.73 g) as a vellow oil.
IR (~ilm) : 3061, 3028, 2927, 2821, 1608, 1497,
1454 cm~1
NMR (CDC13, o) : 1.47 (lX, br s), 2.75-3.0 (4H, m),
3.77 (2H, s), 6.85-7.1 (6H, m), 7.15-7.35 (7H, m)
APCI-MASS (m/z) : 322 (M+H+)
3D
Preparation 146
The mixture of 4-(4-fluorophenoxy)benzaldehyde (4.32
g) and 2-ethoxyethylamine (3.57 g) w~s stirred at 120C for
g hours under nitrogen. The mixture was cooled to room
temperature and dissolved in ethanol (80 ml). To this
. . .. . _ _
W096/1055s PCT/~5/01982
$ ~
- 117 -
solution was added carefully sodium oorohydride ~757 mg),
and the mixture was stirred at room temperature for 2
hours. The mixture was evaporated in vacuo and the residue
was extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give N-(2-ethoxyethyl)-4-
(4-fluorophenoxy)benzylamine (5.50 g) as a yellow oil.
IR (Film) : 3053, 2976, 2929, 2866, 1608, 1498,
1456 cm~1
NMR (CDC13, o) : 1.20 (3H, t, J=7.OHz), 2.8-2.9 (2H,
m), 3.45-3.6 (4H, m), 3.78 (2H, s), 6.9-7.1 (6H,
m), 7.25-7.35 (2H, m)
APCI-MASS (m/z) : 290 (M+H+)
Preparation 147
The mixture of 3-(pyrazol-3-yl)benzaldehyde (1.27 g)
and benzylamine (1.19 g) was stirred at 120C for 4 hours
under nitrogen. The mixture was cooled to room temperature
and dissolved in ethanol (40 ml). To this solution was
added carefully sodium borohydride (280 mg), and the
mixture was stirred at room temperature for 2 hours. The
mixture was evaporated in vacuo and the residue was
extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give N-benzyl-3-(pyrazol-3-
yl)benzylamine (1.22 g) as an oil.
IR (Film) : 3169, 3062, 3026, 2916, 2839, 1606, 1589,
1537, 1495 cm~1
NMR (DMSO-d6, o) : 3.70 (2H, s), 3.7 (2H, s), 6.69
(lH, d, J=2.lHz), 7.2-7.5 (7H, m), 7.7-7.9 (3H,
s)
APCI-MASS (m/z) : 264 (M+H+)
W096/10559 ~ PCT/~95/01982
- 118 -
Preparation 148
The mixture of 3-(pyrazol-3-yl)benzaldehyde (1.72 g)
and cyclohexylamine (1.49 g) was stirred a. 120C for 4
hours under nitrogen. The mixture was cooled to room
temperature and dissolved in ethanol (30 ml). To this
solution was added carefully sodium borohydride (378 mg)
and the mixture was stirred at room temperature for 3
hours. The mixture was evaporated in vacuo and the residue
was extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give-N-cyclohexyl-3-
(pyrazol-3-yl)benzylamine (1.15 g).
IR (KBr) : 3246, 3118, 3041, 2924, 2854, 1608,
1558 cm~1
NMR (DMSO-d6, o) : 1.0-2.0 (lOH, m), 2.4-2.6 (lH, m),
3.88 (2H, s), 6.70 (lH, br s), 7.25-7.45 ~2H, m),
7.6-7.9 (3H, m), 12.90 (lH, br s)
APCI-MASS (m/z) : 256 (M+H+)
Preparation 149
The mixture of 3-(pyrazol-3-yl)benzaldehyde (1.72 g)
and cyclopentylamine (1.70 g) was stirred at 120C for 4
hours under nitrogen. The mixture was cooled to room
temperature and dissolved in ethanol (40 ml). To this
solution was added carefully sodium borohydride ~378 mg),
and the mixture was stirred at room temperature for 3
hours. The mixture was evaporated in vacuo and the residue
was extracted with dichloromethane. The organic layer was
washed with brine, dried over magnesium sulfate and
evaporated in vacuo. The residue was purified by column
chromatography on silica gel to give N-cyclopentyl-3-
(pyrazol-3-yl)benzylamine (1.26 g).
IR (Film) : 3265, 1610, 1589 cm~1
NMR (DMSO-d6, o) : 1.3-1.9 (8H, m), 3.05-3.25 (lH,
WO96/10559 PCT/~5/01982
22~%~
-- 119 --
m), 3.78 (2H, s), 6.68 (lH, s), 7.2-7.4 (2H, m),
7.6-7.9 (3H, m), 12.88 (lH, br)
APCI-MASS (m/z) : 242 (M+H+)
Prep~ration 150
A mixture of 3-(1-tritylpyrazol-3-yl)benzaldehyde
(1.72 g) and 4-fluorobenzylamine (0.57 ml) was stlrred at
120C for 4 hours. The mixture was cooled to room
temperature and dissolved in ethanol (26 ml). To the
mixture was added sodium borohydride (158 mg) and the
reaction mixture was stirred at 50C for 2 hours. The
mixture was poured into water, extracted with
dichloromethane. The organic layer was washed with water
and brine, dried over magnesium sulfate, evaporated in
vacuo. The residue was chromatographed on silica gel (50
g, eluting with dichloromethane - methanol (50:1)) to give
N-(4-fluorobenzyl)-3-(1-tritylpyrazol-3-yl)benzylamine
(1.40 g).
IR (Neat) : 3059, 2827, 1603, 1506, 1446, 1219 cm 1
NMR (CDCl3, o) : 3.79 (2H, s), 3.82 (2H, s), 6.58
(lH, d, J=2.5Hz), 6.90-7.05 (2H, m), 7.10-7.45
(20H, m), 7.65-7.83 (2H, m)
FAB-MASS (m/z) : 524 (M+H+)
Preparation 151
A mixture of 3-(pyrazol-3-yl)benzaldehyde (1.0 g) and
4-methoxybenzylamine (0.91 ml) was heated for 3 hours at
120C. After cooling to room temperature, the mixture was
dissolved in ethanol (20 ml). To the solution was added
sodium borohydride (220 mg) and stirred for two hours at
ambient temperature. The reaction mixture was poured into
J water and extracted with dichloromethane, washed with water
and brine, dried over magnesiu~ sulfate- The solvent was
removed in vacuo and the residue was chromatographed on
silica gel (50 g, eluting with dichloromethane - methanol
WO96110S59 22 ~ ~ ~ 8 1 PCTl~5/01982
- 120 -
(10:1)) to give N-(4-methoxybenzyl)-3-(pyrazol-3-
yl)benzylamine (1.17 g).
IR (Film) : 2370-3680 (br), 1610, 1512, 1248,
1036 cm~1 t
S NMR (CDC13, o) : 3.77 ~2H, s), 3.79 (3H, s), 3.84
(2H, s), 6.60 (lH, d, J=2.2Hz), 6.80-6.92 (2H,
m), 7.17-7.41 (4H, m), 7.54-7.66 (2~, m), 7.85
(lH, s)
APCI-MASS (m/z) : 294 ~M+H+)
Preparation 152
A mixture of 3-(pyrazol-3-yl)benzaldehyde (1.0 g) and
4-~luorobenzylamine (0.8 ml) was heated for 4 hours at
120C. After cooling to room temperature, the mixture was
dissolved in ethanol ~20 ml). To the solution was added
sodium borohydride (220 mg) and stirred for two hours at
ambient temperature. The reaction mixture was poured into
water and extracted with dichloromethane, washed with water
and brine, dried over magnesium sulfate. The solvent was
removed in vacuo and the residue was chromatographed on
silica gel (50 g, eluting wlth dichloromethane - methanol
(10:1)) to give N-(4-fluorobenzyl)-3-(pyrazol-3-
yl)benzylamine (1.28 g).
IR (Film) : 2370-3680 (br), 1605, i508, 1220,
1095 cm~1
NMR (CDCl3, o) : 3.79 (2H, s), 3.84 (2H, s), 6.61
(lH, d, J=2.3Hz), 6.90-7.10 (2~, m), 7.18-7.45
(4H, m), 7.52-7.70 (2H, m), 7.75 (lH, s)
APCI-MASS (m/z) : 282 (M H+) v
Preparation 153
A mixture of 3-(pyrazol-3-yl)benzaldehyde (1.~ g), a-
(dimethylamino)benzylamine-dihydrochloride (1.87 g) and
triethylamine (11.7 ml) in toluene (30 ml) W25 refluxed ~o~
5 hours. An insoluble material was removed by ~iltration
PCT/~5/01982
wo g6~l0s59 2 2 ~ ~ ~ 8 ~ J
- 121 -
and evaporated in vacuo. The residue was dissolved in
ethanol (18 ml). To the solution was added sodium
borohydride (264 mg) and stirred for two hours at ambient
temperature. The reaction mixture was poured into water
and extracted with dichloromethane, washed with water and
brine, dried over magnesium sulfate. The solvent was
removed in vacuo and the residue was chromatographed on
silica gel (50 g, eluting with dichloromethane - methanol
(8:1)) to give N-[4-(dimethylamino)benzyl]-3-(pyrazol-3-
yl)benzylamine (1.68 g).
IR (Film) : 2330-3700 (br), 1614, 1524, 1446, 1350,
80~, 766 cm~1
NMR (CDCl3, ~) : 2.93 (6H, s), 3.75 (2H, s), 3.84
(2H, s), 6.59 (lH, d, J=2.2Hz), 6.65-6.75 (2H,
m), 7.15-7.40 (4H, m), 7.55-7.66 (2H, m), 7.76
(lH, s)
APCI-MASS (m/z) : 440 (M+Me2N ~ CH2)
Preparation 154
The following compounds were obtained according to a
similar manner to that of Preparation 57, 58, 59, 60, 62,
75, 76, 138, 140, 141, 142, 143, 144, 145, 146, 147, 148,
149, 150, 151, 152 or 153.
(1) N-Cycloheptyl-4-(4-chlorophenoxy)benzylamine
IR (Film) : 3035, 2925, 2855, 1610, 1590, 1505,
1485 cm~1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.6-2.8 (lH, m),
3.76 (2H, s), 6.9-7.05 (4H, m), 7.25-7.4 (4H, m)
APCI-MASS (m/z) : 332, 330 (M+H+)
(2) N-Cycloheptyl-4-(3-fluorophenoxy)ben7ylamine
_ _ .. . . . . .. . . . . .
PCT1~5/01982
WO96110S59
~a~9~8 ~ ~
- 122 -
IR (Neat) : 2926, 2854, 1599, 1483, 1269, 1213 cm 1
NMR (CDCl3, o) : 1.30-2.10 (12H, m), 2.62-2.80 (iH,
m), 3.77 (2H, s), 6.62-6.85 (3H, m), 6.93-7.05
(2H, m), 7.18-7.40 (3H, m)
APCI-MASS (m/z) : 314 (M+H+)
(3) N-Cycloheptyl-4-(4-triflloromethyl~henoxy)benzylamine
IR (Neat) : 2g26, 2854, 1601, 1504, 1462, 1327 cm 1
NMR (CDCl3, o) : 1.30-2.00 (12H, m), 2.65-2.80 (lH,
m), 3.78 (2H, s), 6.95-7.10 (4H, m), 7.30-7.40
(2H, m), 7.50-7.62 (2H, m)
APCI-MASS (m/z) : 364 (M+H+)
(4) N-Cycloheptyl-4-(3,4-methylenedioxyphenoxy)benzylamine
IR (Neat) : 2924, 2854, 1606, 1502, 1481, 1354 cm 1
NMR (CDC13, o) : 1.30-1.95 (12H, m), 2.60-2.75 (lH,
m), 3.74 (2H, s), 5.97 (2H, s), 6.47 (lH, dd,
J=8.4, 2.4Hz), 6.56 (lH, d, J=~.4H ), 6.75 (lH,
d, J=8.4Hz), 6.85-6.56 (2H, m), 7.20-7.31 (2H, m)
APCI-MASS (m/z) : 340 (M+H+)
(5) N-Cycloheptyl-4-(3,5-di-tert-butyl-4-
methoxymethoxyphenoxy)benzylamine
IR (Film) : 2920, 2860, 1587 cm 1
NMR (CDCl3, o) : 1.40 and 1.42 (total 18H, s),
1.4-2.2 (14H, m), 2.8-2.95 (lH, m), 3.62 and 3.64
(total 3H, s), 4.87 and 4.92 (total 2H, s), 6.92
(2H, s), 6.85-6.95 (2H, m), 7.4-7.5 (2H, m)
APCI-MASS (m/z) : 468 (M+H+) v
(6) N-Cycloheptyl-3-(4-fluorophenoXy)benzylamine
IR (Film) : 3062, 2926, 2854, 1608, 1583, 1502,
1446 cm~1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.6-~.8 (LH~ m~,
3.75 (2~, s), 6.8-7.3 (8~, ~)
PCT/~5/01982
WO96/10559
8 ~
- 123 -
APCI-MASS (m/zJ : 314 (M+~+)
(7) N-Cycloheptyl-3-(1-tritylpyraZol-4-yl)benzyla~ine
NMR (DMSO-d6,` o) : 1.2-i.9 (12H, m), 2.6-2.7 (lH, m),
r 5 3.70 (2H, s), 7.1-7.6 (19H, m), 7.76 (lH, s), 8.06 (lH, s)
APCI-MASS (m/z) : 512 (M~H+)
(8) N-Cycloheptyl-3-(l-methylpyraZOl-4-yl)be~zylamine
IR (Neat) : 2926, 2852, 1610, 1460, 1410, 1230 cm 1
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.63-2.80 (lH,
m), 3.79 (2H, s), 3.94 (3H, s), 7.13-7.48 (4H,
m), 7.63 (lH, s), 7.76 (lH, s)
APCI-MASS (m/z) : 284 (M+H+)
(9) N-Cycloheptyl-3-(1-methylpyrazol-3-yl)benzylamine
IR (Neat) : 3400 (br), 2924, 2854, 1610, 1462, 1354,
1242 cm~l
NMR (CDCl3, o) : 1.30-2.00 (12H, m), 2.64-2.8û (1~,
m), 3.83 (2H, s), 3.95 (3H, s), 6.56 (lH, d,
J=2.2Hz), 7.25-7.40 (3H, m), 7.60-7.78 (2;i, mi
APCI-MASS (m/z) : 284 (M+H+)
(10) N-Cycloheptyl-3-(1-methylpyraZol-5-yl)benzylamine
IR (Neat) : 2924, 2854, 1608, 1462, i385, 1275 cm i
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.62-2.80 (lH,
m), 3.83 (2H, s), 3.90 (3H, s), 6.31 (lH, d,
J=1.8Hz), 7.25-7.48 (4H, m), 7.51 (lH, d,
J-1.8Hz)
APCI-MASS (m/z) : 284 (M+H+)
(11) N-Cycloheptyl-3-(imidazol-4-yl)benzylamine
IR (Film) : 2300-3600 (br), 2924, 2854, 1610,
1460 cm~l
NMR (DMSO-d~, o) : 1.2û-1.95 (12H, m), 2.55-2.75 (lH,
WO96/10559 ~ 2 ~ ~ 9 8 ~ PCTl~5/01982
- 124 -
m), 3.73 (2H, s), 7.05-7.80 (6H, m), 12.00-12.25
(lH, br)
APCI-~ASS (m/z) : 270 (M+H+)
(12) N-Cycloheptyl-4-(5-methyl-1,3,4-oxadia7O1-3-
yl)benzylamine
IR (KBr) : 3442, 3292, 3211, 2920, 2852, 1689, 1576,
1502, 1450 cm~1
NMR (CDCl3, o) : 1.30-2.40 (12H, m), 2.61 (3H, s),
2.63-2.80 (lH, m), 3.87 (2H, s), 7.45-7.54 (2H,
m), 7.93-8.05 (2H, m)
APCI-MASS (m/z) : 286 (M+H+)
(13) N-Cycloheptyl-4-(4-benzyl-5-methyl-4H-1,2,4-triazol-3-
yl)benzylamine
IR (Neat) : 3298, 2924, 2852, 1612, 1527, 1458,
1358 cm~1
NMR (CDCl3, o) : 1.30-1.93 (12H, m), 2.38 (3H, s),
2.60-2.77 (lH, m), 3.81 (2H, s~, 5.16 (2H, s),
6.90-7.05 (2H, m), 7.27-7.55 (7H, m)
APCI-MASS (m/z) : 375 (M+H+)
(14) N-Cycloheptyl-3-(2-methyl-2H-tetrazol-5-yl)benzylamine
IR (Neat) : 2924, 2854, 1520, 1462, 1365 cm 1
NMR (CDCl3, ~) : 1.30-1.98 (12H, m), 2.65-2.80 (lH,
m), 3.86 (2H, s), 4.40 (3H, s), 7.40-7.48 (2H,
m), 7.95-8.05 (lH, m), 8.09 (lH, s)
APCI-MASS (m/z) : 286 (M+H+)
(15) N-Cyclohe~tyl-3~ met~yl-lH-tet~azol-5-yl)benzylamine
IR (Neat) : 2924, 2854, 1533, 1452, 1292 cm~1
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.65-2.80 (lH,
m), 3.88 (2H, s), 4.18 (3H, s)~ 7.4r-7.65 (3H,
m), 7.75 (lH, s)
APCI-MASS (m/7) : 286 (M+H+)
, . . . . . . .
~ 8 ~ PCT/~5/01982
W096/lOS59
- 125 -
('6) N-Cycloheptyl-4-(lH-1,2,4-tria701-1-yl)benzylamine
mp : 53-54C
IR (KBr) : 3101, 2922, 2852, 1518, 1460, 1277, 1147,
g84 cm~i
NMR (CDC13, o) : 1.30-2.00 (12h-, m), 2.60-2.80 (1~,
m), 3.84 (2~, s), 7.40-7.55 (2H, m), 7.55-7.70
(2H, m), 8.10 (lH, s), 8.54 (lH, s)
APCI-M~SS (m/z) : 271 (M H+)
(17) N-Cycloheptyl-4-(lH-1,2,3-triazol-1-yl)benzylamine
mp : 78-79C
IR (KBr) : 3319, 3124, 2920, 2852, 1520, 1230, 1101,
1041 cm~1
NMR (CDC13, o) : 1.30-2.00 (12H, m), 2.63-2.&0 (lH,
m), 3.87 (2H, s), 7.45-7.57 (2H, m), 7.64-7.76
(2H, m), 7.85 (lH, s), 7.98 (lH, s)
APCI-MASS (m/z) : 271 (M+X+)
(18) N-Cycloheptyl-4-(2H-1,2,3-tria701-2-vl)benzylamine
IR (Neat) : 2926, 2854, 1608, 1514, 146C, 1412, 1381,
1259, 951, 824 cm-1
NMR (DMSO-d6, o) : 1.20-1.90 ~12H, m), 2.50-2.7G (lH,
m), 3.74 (2H, s), 7.45-7.55 (2H, m), 7.90-8.CO
(2H, m), 8.10 (2H, s)
APCI-MASS (m/~) : 271 (M+H+)
~19~ N-Cycloheptyl-(4-methvlpiperazin-'-yl)ben7ylamine
IR (Film) : 2925, 2850, 2795, 1615, 1515 cm 1
NMR (D~SO-d6, o) : 1.3-1.9 ,12H, m), 2.21 (3H, s),
2.4-2.5 (4H, m), 3.1-3.2 (4H, m), 3.2-3.45 (lH,
m), 6.85 (2H, d, J=8.5Hz), 7.15 (2H, d, J=8.5H7)
APCI-~SS (m/-) : 302 (M+H+)
(20) N-Cycloheptyl-4-(4-methylsulfonylaminophenyl)-
benzylamine
WO96/10559 2 2 ~ PCT/~95/01982
- 126 -
IR (KBr) : 3020, 2930, 2855, 1605, 1495 cm l
NMR (DMSO-d6, o) : 1.3-2.0 (12H, m), 2.5-2.7 (lH, m),
3.01 (3H, s), 3.72 (2H, s), 7.27 (2H, d,
J=8.5Hz), 7.39 (2H, d, J=8.5Hz), 7.57 (2H, d,
J=8.2Hz), 7.63 (2H, d, J=8.2Hz)
APCI-MASS (m/z) : 373(M+H+)
(21) N-Cycloheptyl-4-(N-benzoylsulfamoyl)benzylamine
IR (KBr) : 3477, 3057, 2927, 2858, 1599, 1545 cm~1
NMR (DMSO-d6, o) : 1.3-2.2 (12H, m), 3.1-3.3 (lH, m),
4.17 (2H, s), 7.2-7.45 (5H, m), 7.4-7.5 (2H, m),
7.75-7.9 (2H, m), 8.4-8.7 (lH, br)
APCI-MASS (m/z) : 387 (M+H+)
(22) N-Cycloheptyl-4-(N-phenylsulfonylcarbamoyl)benzylamine
IR (KBr) : 3091, 2929, 2858, 1647, 1601, 1537 cm 1
NMR (DMSO-d6, o) : 1.35-2.2 (12H, m), 3.1-3.3 (lH,
m), 4.11 (2H, s), 7.35-7.5 (5H, m), 7.8-7.9 (2H,
m), 7.93 (2H, d, J=8.lHz)
APCI-MASS (m/z) : 387 (M+H+)
(23) N-Cycloheptyl-4-(3-pyridylmethyl)benzylamine
IR (Film) : 3304, 3026, 2924, 2852, 1574, 1512 cm 1
NMR (CDCl3, o) : 1.4-2.2 (12H, m), 2.6-2.8 (lH, m),
3.75 (2H, s), 3.95 (2H, s), 7.1-7.5 (6H, m), 8.45
(lH, dd, J=4.8, 1.8Hz), 8.49 (lH, d, J=1.8Hz)
APCI-MASS (m/z) : 295 (M+H+)
(24) N-Cycloheptyl-4-(4-pyridylmethyl)benzylamine
IR (Film) : 3323, 3022, 2924, 2852, 1599 cm 1
NMR (CDCl3, o) : 1.3-2.1 (12H, m), 2.6-2.8 (lH, m),
3.77 (2H, s), 3.94 (2H, s), 7.09 (lH, dd, J=4.5,
1.6Hz), 7.12 (2H, d, J=9.4Hz), 7.29 (lH, d,
J=9.4Hz), 8.48 (2H, dd, J=4.5, 1.6Hz)
APCI-MASS (m/z) : 295 (M+H+)
W096/10559 2 2 ~ PCTl~95/01982
- 127 -
(25) N-Cycloheptyl-4-(pyrazol-1-ylmethyl)benzylamine
IR (Neat) : 2924, 2854, 1514, 1458, 1090, 750 cm 1
NMR (CDCl3, o) : 1.30-1.98 (12H, m), 2.56-2.77 (lH,
m), 3.76 (2H, s), 5.30 (2H, s), 6.27 (lH, dd,
J=2.OHz), 7.10-7.40 (5H, m), 7.54 (lH, d,
J=2.OHz)
APCI-MASS (m/z) : 284 (M+H+)
(26) N-Cycloheptyl-4-(imidazol-1-ylmethyl)benzylamine
IO IR (Neat) : 3280 (br), 2924, 2854, 1506, 1458, 1230,
1107, 1076 cm~1
NMR (CDCl3, o) : 1.20-1.95 (12H, m), 2.60-2.78 (lH,
m), 3.76 (2H, s), 5.10 (2H, s), 6.90 (lH, s),
7.00-7.40 (5H, m), 7.54 (lH, s)
APCI-MASS (m/z) : 284 (M+H+)
(27) N-Cycloheptyl-(6-hydroxy-2,5,7,8-tetramethylchroman-2-
yl)methylamine
NMR (DMSO-d6, o) : 1.17 (3H, s), 1.3-1.9 (4H, m),
1.97 (3H, s), 2.01 (3H, s), 2.04 (3H, s), 2.5-2.7
(3H, m), 7.39 (lH, s)
APCI-MASS (m/z) : 332 (M+H+)
(28) N=Cycloheptyl-4-[N-(3,5-di-tert-butyl-4-
hydroxyphenyl)carbamoyl]benzylamine
IR (KBr) : 3639, 3304, 2926, 2858, 1643, 1606, 1547 cm~
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 1.39 (18H, s),
2.5-2.7 (lH, m), 3.77 (2H, s), 6.78 (lH, s), 7.45
(2H, d, J=8.2Hz), 7.88 (2H, d, J=8.2Hz), 7.58
(2H, s), 9.87 (lH, s)
APCI-MASS (m/z) : 451 (MTH+)
..
(29) N-Cycloheptyl-4-[N-(4-fluorophenyl)carbamoyl]-
benzylamine
IR (KBr) : 3354, 2927, 2854, 1651, 1612, 1529,
WO~6110559 ~2ao 9~ 1 PCT/~5/01982
- 128 -
1512 cm~1
N~R (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.5-2.65 (lH,
m~, 3.77 (2H, s~, 7.1-7.3 (2~, m~, 7.75-7.85
(2H, m~, 7.47 (2H, d, J=8.2H7), 7.89 (2H, d,
J=8.2Hz), 10.22 (lH, s)
~CI-MASS (m~z) : 341 (M+~+~
(30~ N-Cycloheptyl-4-EN-(4-fluorophenyl)-N-
methylcarbamoyl~benzylamine
IR (KBr~ : 3475, 3187, 3120, 3024, 292~, 2853, 1643,
1597, 1541, i500 cm~1
NMR (DMSO-d6, o~ : 1.3-1.9 (12H, m~, ~.4-2.6 (lH, m),
3.33 (3H, s~, 3.60 (2H, s), 7.05-7.3 (8H, m~
APCI-MASS (m/z~ : 355 (M~H+)
(31~ N-Cycloheptyl-4-(tert-~u~yldimethylsilyl~xymethyl)-
benzylamine
IR (Film) : 2927, 2850, 1514, 1464 cm~l
l~MR (DMSO-d6, o~ : 0.08 (6H, s), 0.89 (9H, s), 1.3-
1.9 (12H, m), 2.5-2.65 (lH, m), 3.67 (2H, s),
4.67 (2H, s), 7.22 (2H, d, J=8.3H ), 7.28 (2H, d,
J=8.3Hz)
APCI-MASS (m/z~ : 348 (M+H+)
(32) N-Benzyl-3-phenoxybenzylamine
I~ (Film) : 3062, 3030, 2829, 1583, 1487, 145~ cm 1
NMR (DMSO-d6, o) : 2.63 (lH, br s), 3.6a (2H, s),
3.66 (2H, s~, 6.8-7.45 (14H, m)
APCI-MASS (m/z) : 29Q (M+H+)
(33) N-Benzyl-3-(4-fluorophenoxy)ben7ylamine
IR (Film) : 3062, 3030, 2916, 2829, 1608, 1584,
1500, 1450 c~~l
NMR (CDCl3, o/ : 3.78 (2H, s), 3.79 (2H, s), 6.8-7.4
(13H, m)
PCT/JP95/01982
W096/lOSS9
2 2 0 ~
- 129 -
APCI-MASS (m/z) : 308 (M+H+)
(34) N-Benzyl-3-(1-Methylpyrazol-3-yl)behzylamine
IR ~Neat) : 3313, 3028, 2935, 1608, 1498, 1452, 1356,
1242 cm~1
NMR (CDCl3, o) : 3.83 (2H, s), 3.85 (2H, s), 3.95
(3H, s), 6.55 (lH, d, J=2.3Hz), 7.18-7.42 (8H,
m), 7.64-7.73 (lH, m), 7.77(lH, s)
APCI-MASS (m/z) : 278 (M+H+)
(35) N-Benzyl-3-(1-methylpyrazol-5-yl)benzylamine
IR (Neat) : 3310, 3026, 283C, 1606, 1454, 1387,
1275 cm~1
NMR (CDCl3, o) : 3.84 (2H, s), 3.87 (2H, s), 3.89
(3H, s), 6.31 (lH, d, J=1.9Hz), 7.20-7.q5 (9H,
m), 7.51 (lH, d, J=1.9Hz)
APCI-~SS (m/z) : 278 (M+H+)
~36) N-Benzyl-4-(l-methylpyrazol-3-yl)ben7yiamine
IR (Neat) : 3310, 3028, 2937, 2820, i504, 1454,
1430 c~ -
NMR (CDCl3, o) : 3.81 (2H, s), 3.83 (2n, s), 3.95
(3H, s), 6.53 (lH, d, J=2.3Hz), 7.18-7.43 (8H,
m), 7.70-7.80 (2H, m)
APCI-MASS (m/z) : 278 (M+H )
(37) N-Benzyl-4-(1-methylpyraZol-5-yl)benZVlamine
IR (Neat) : 3305, 3026, 2820, 143, 1454, '385,
1275 cm-1
NMR (CDCl3, o) : 3.85 (2H, s), 3.87 !2H, s), 3.89
(3H, s), 6.30 (lH, d, J=i.9Hz), 7.20-7.50 (9H,
m), 7.51 (lH, d, J=1.9Hz)
APCI-MASS (m/z) : 278 (M+H+)
(38) N-Benzyi-4-(pyrazol-3-yl)benzylamine
~ 9 8 1 PCT/~95/01982
WO96/10559
- 130 -
IR (Neat) : 2250-3680 (br), 1514, 1495, 1454, 1350 cm 1
NMR (DMSO-d6, o) : 3.69 (4H, s), 6.67 (lH, d,
-J=2.1Hz), 7.15-7.50 (7H, m), 7.60-7.90 (3H, m),
12.81, 13.20 (total lH, each br)
APCI-MASS (m/z) : 264 (M+H+)
(39) N-Benzyl-4-(1-methylpyrazol-4-yl)benzylamine
mp : 90-91C
IR (KBr) : 3300, 3020, 2914, 2854, 1570, 1473, 1452,
1194, 1097 cm~l
NMR (CDCl3, o) : 3.81 (2H, s), 3.82 (2H, s), 3.94
(3H, s), 7.20-7.50 (9H, m), 7.60 (lH, s), 7.75
(lH, s)
APCI-MASS (m/z) : 278 (M+H+)
(40) N-Benzyl-3-(imidazol-4-yl)benzylamine
IR (Neat) : 2200-3560 (br), 1608, 1491, 1454 cm~l
NMR (DMSO-d6, o) : 3.72 (4H, s), 7.10-7.40 (7H, m),
7.41-7.80 (4H, m)
20APCI-MASS (m/z) : 264 (M+H+)
(41) N-Benzyl-3-(2-methyl-2H-tetrazol-5-yl)benzylamine
IR (Neat) : 3028, 2825, 1520, 1452, 1363, 804 cm~
NMR (CDC13, o) : 3.84 (2H, s), 3.89 (2H, s), 4.40
25(3H, s), 7o20~7.52 (7H, m), 7.96-8.07 (lH, m),
8.12 (lH, s)
APCI-MASS (m/z) : 280 (M+H+)
.
(42) N-Benzyl-3~ methylpyrazol-4-yl)benzylamine
IR (Neat) : 3305, 3028, 2935, 2827, 1610, 1450, 1363,
1230 cm~l
NMR (CDCl3, o) : 3.84 (4H, s), 3.94 (3H, s), 7.13-
7.40 (8H, m), 7.45 (lH, s), 7.62 (lH, s), 7.77
(lH, s)
APCI-MASS (m/z) : 278 (M+H+)
WO96/10559 PCT/~5/01982
2 ~ Q ~
- 131 -
(43) N-(4-Methoxybenzyl)-4-(4-fluorOphenoxy)benzylamine
IR (Neat) : 3001, 2903, 2833, 1610, 1500, 1460, 1248,
21 cm~i
NMR (CDCl3, o) : 3.75 (2~;, s), 3.76 (2H, s), 3.80
(3H, s), 6.82-7.10 (8H, m), 7.20-7.35 (4~i, ~)
APCI-MASS (m/z) : 338 (M+H+)
~reparalion i55
The following compound was obtained accord ng to a
0 si~iiar manner tc that or Preparation 31~ 38, 39 or 89.
4-(1-Tritylpvrazol-4-yl)ioluene
NMR (DMSO-d6, o) : 2.27 (3~, s), 7.1-7.5 (i9~, m),
7.73 (1~, c), 8.04 (lH, s)
Preparation 156
The f~llowing compounds were obtained according to c
similar manner to that of Prepar2tion 28.
2G (1) 4-(1-Tritylpyrazol-4-yl)benzyl bromide
NMR (DMSO-d6, o) : 4.70 and 4.77 (total 2H, s),
7.Q-7.8 (21H, m)
(2) 3-3enzoylbenzyl bromide
IR (Film) : 3059, 3028, 1686, 1599 cm 1
NMR (CDCl3, o) : 4.53 (2H, s), 7.35-7.9 (9H, m)
APCI-MASS (m/7) : 277, 275 (M H+)
Preparation 157
The follGwing compounds were obtained accorcing to a
similar manner to that c- Preparalion 63.
(1) ~-Cycloheptyl-4-(1-trityl~yraZOl-4-Yl)benzylamine
IR (Film) : 3057, 3028, 2918, 2852, 1641, 1605,
1566 cm~'
PCT/~5/01982
WO96/10559
~ 2 ~
- 132 -
NMR (DMSO-d6, ~) : 1.3-2.0 (12H, m), 2.55-2.75 (lH,
m~, 3.68 and 3.75 (total 2H, s), 7.05-7.25 (5H,
m), 7.3-8.1 (16H, m~
APCI-MASS ~m~z~ : 512 (M+H~)
(2~ N-Cycloheptyl-4-(2-cyanophenyl)ben7ylamine
IR (Film) : 3060, 3030, 2910, 2855, 2~25, 1597,
1480 cm~1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.65-2.85 (lH, m),
3.85 (2H, s), 7.4-7.8 (8H, m)
APCI-MASS (m/z) : 305 (M+H+)
(3) N-Cycloheptyl-4-~2-(1-trityl-lH-tetrazol-5-
yl)phenyl]benzylamine
IR (KBr) : 3058, 3026, 2924, 2854, 1603, 1493,
1446 c~ 1
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.6-2.75 (lH,
m), 3.68 (2H, s), 6.8-6.95 (5X, m), 7.01 (2H, d,
J=7.9Hz), 7.20 (2H, d, J=7.9Hz), 7.3-7.8 (14H, m)
2C FAB-MASS ~m/z) : 590 (M+~+)
~4) N-Cycloheptyl-3-benzoylbenzylamine
IR (Film) : 3059, 2g27, 2855, 1653, 1599, 1580 cm~
NMR (CDCl3, c) : 1.3-2.0 (12H, m), 2.6-2.8 (lH, m),
3.85 (2H" s), 7.3-7.8 (9H, m)
APCI-MASS (m/z) : 308 (M+H~)
Preparation 158
The following compounds were obtained according to a
similar manner to that of Preparation 50 or 51.
(1) 3-(1-Methylpyrazcl-3-yl)ben~aldehyde
IR (Neat) : 2941, 2829, 2730, 1695, 1606, 1585, 1439,
1242 c~ 1
NMR (CDC13, ~) : 3.98 (3H, s), 6.62 (lH, d, J=2.2H~),
WO96/10559 22~ 1 PCT/~95/01982
- 133 -
7.42 (lH, d, J=2.2Hz), 7.51-7.62 (lH, m), 7.77-
7.86 (lH, m), 8.05-8.13 (lH, m), 8.25-8.32 (lH,
m), 10.07 (lH, s)
APCI-MASS (m/z) : 187 (M+H+)
(2) 3-(1-Methylpyrazol-5-yl)benzaldehyde
mp : 72-74C
IR (KBr) : 3041, 2831, 2733, 1697, 1573, 1462, 1377 cm~
NMR (CDCl3, ~) : 3.94 (3H, s), 6.39 (lH, d, J=1.4Hz),
7.56 (lH, d, J=1.4Hz), 7.58-7.74 (2H, m), 7.89-
7.97 (2H, m), 10.09 (lH, s)
APCI-MASS (m/z) : 187 (M+H+)
(3) 4-(Pyrazol-l-yl)benzaldehvde
mp : 53-55C
IR (KBr) : 3109, 2833, 2744, 1693, 1608, 1394, 1213,
760 cm~l
NMR (CDCl3, ~) : 5.43 (2H, s), 6.34 (lH, dd, J=2.1,
2.1Hz), 7.25-7.35 (2H, m), 7.45 (lH, d, J=2.1Hz),
7.59 (lH, d, J=2.1Hz), 7.80-7:9C (2H, m), 9.99
(lH, s)
APCI-MASS (m/z) : 187 (M+H+)
(4) 4-(Imidazol-l-ylmethyl)benzaldehyde
IR (Neat) : 2600-3600 (br), 1695, 15G6, 1232, 1076,
818, 737 cm~l
NMR (CDCl3, o) : 5.22 (2H, s), 6.85-7.95 (7H, m),
10.01 (lH, s)
APC I-MASS (m/z) : 187 (M+H+)
Preparation 159
The following compound was obtained according to a
similar manner to that of Preparation 47.
3-(Imidazol-4-yl)benzaldehyde
PCTI~S/01982
WO96/10559 ~ 9 8 ~ . ~
- 134 -
mp : 135-138C
IR (KBr) : 2080-3390 (br), 1691, 1606, 147g, 1327,
1186, 1066, 978, 781 cm~
NMR (DMSO-d6, o) : 7.59 (lH, dd, J=7.6, 7.6Hz), 7.67-
7.80 (3H, m), 8.05-8.15 (lH, m), 8.31 (lH, s),
10.04 ~lH, s), 12.30 (lH, br)
APCI-MASS (m/z) : 173 (M+H+)
Preparation 160
The following compounds were obtained according to a
similar manner to that of Preparation 44, 45, 84, 110, 124
or 126.
(1) 4-(5-Methyl-1,3,4-oxadiazol-2-yl)benzaldehyde
IR (KBr) : 2829, 1701, 1610, 1590, 1550, 1421 cm~
NMR (CDCl3, o) : 2.66 (3H, s), 7.96-8.07 (2H, m),
8.15-8.26 (2H, m), 10.10 (lH, s)
APCI-MASS (m/z) : 189 (M+H+)
(2) 4-(4-Benzyl-5-methyl-4H-1,2,4-triazol-3-
yl)benzaldehyde
IR (KBr) : 3450 (br), 1689, 1608, 157~, 1531,
1207 cm~1
NMR (CDCl3, o) : 2.44 (3H, s), 5.22 (2H, s), 6.93-
7.07 (2H, m), 7.30-7.47 (3H, m), 7.70-7.80 (2H,
m), 7.90-8.00 (2H, m), 10.05 (lH, s)
APCI-M~SS (m/z) : 278 (M+H+)
Preparation 161
The following compound was obtained according to a
similar manner to that of Preparation 97.
4-Benzyl-2-(4-hydroxymethyl)phenyl-5-methyl-4H-1,2,4-
tria7O1e
mp : 118-121C
_
-
2 2 0 0 9 8 ~ pCT/~5/01982
W096/lOS59
- 135 -
IR (KBr) : 2600-3650 (br), 1535, 1487, 1425, 1363,
1039, 854, 739 cm~l
- NMR (CDC13, o) : 2.36 (3H, s), 3.10-3.25 (lH, m),
4.65-4.77 (2H, m), 5.14 (2H, s), 6.90-7.03 (2H,
r 5 m), 7.25-7.50 (7H, m)
APCI-MASS (m/z) : 280 (M+H+)
preparation 162
The following compound was obtained according to a
similar manner to that of Preparation 31.
N-Methyl-N-methoxy-4-[4-(methylsulfonylamino)phenyl]-
benzamide
IR (KBr) : 3210, 2935, 1630, 1608, 1525 cm 1
NMR (DMSO-d6, o) : 3.04 (3H, s), 3.28 (3H, s)j 3.58
(3H, s), 7.32 (2H, d, J=8.6Hz), 7.6-7.8 (6H, m),
9.91 (lH, s)
Preparation 163
The following compounds were obtained according to a
similar manner to that of Preparation 36.
(1) 4-(4-Methylsulfonylaminophenyl)benzaldehyde
IR (KBr) : 3290, 2995, 2840, 2745, 1695, 1600, 1525,
1500 cm~l
NMR (DMSO-d6, o) : 3.06 (3H, s), 7.33 (2H, d,
J=8.5Hz), 7.78 (2H, d, J=8.5Hz), 7.89 (2H, d,
J=8.2Hz), 7.98 (2H, d, J=8.2Hz), 9.98 (lH, br s),
10.04 (lH, s)
APCI-MASS (m/z) : 276 (M+H+)
(2) 4-(N-Benzoylsulfamoyl)benzaldehyde
IR (KBr) : 3381, 3057, 2883, 1697, 1599, 1560 cm~l
NMR (DMSO-d6, o) : 7.3-7.5 (3H, m), 7.9-8.0 (2H, m),
7.44 (2H, d, J=8.3Hz), 8.00 (2H, d, J=8.3Hz),
PCT/~5/01982
W096/lOS59 ~ ~ 9 8
- 136 -
10.03 (lH, s)
APCI-MASS (m/z) : 290 (M+H+)
Preparation 164
5The following compounds were obtained according to a
similar manner to that of Preparation 33 or 34.
(1) 4-(N-Methyl-N-methoxysulfamoyl)benzamide
IR (KBr) : 3292, 3201, 3111, 2979, 2943, 1605, 1562,
1504 cm~1
NMR (DMSO-d6, o) : 3.28 (3H, s), 3.54 (3H, s), 7.49
(2H, br s), 7.74 (2H, d, J=8.4Hz), 7.88 (2H, d,
J=8.4Hz)
APCI-~ASS (m/z) : 245 (M+H+~
(2) N-Methyl-N-methoxy-6-hydroxy-2,5,7,8-
tetramethylchroman-2-carboxamide
IR (KBr) : 3479, 2983, 2935, 2870, 1655 cm 1
NMR (DMSO-d6, o) : 1.49 (3H, s), 1.97 (3H, s), 2.05
20(6H, s~, 1.6-1.75 (lH, m), 2.4-2.6 (3H, m), 3.34
(3H, s), 3.57 (3H, s), 7.48 (lH, s)
APCI-MASS (m/z) : 294 (M+H+)
Prepara~io~ 165
25The following compound was obtained according to a
similar manner to that of Preparation 105.
4-(N-Phe~ylsulfonylcarbamoyl)benzaldehyde
IR (KBr) : 3185, 3155, 3105, 2935, 2850, 17gO, 1695,
301645, 1605, 1565, 1550 cm~1
NMR (DMSO-d6, o) : 6.95 (2H, d, J=7.5Hz), 7.35-7.45
(2H, m) ~ 7.75-7.9 (3H, m), 8.20 (2H, d, J=7.5Hz),
10.02 (lH, s)
APCI-MASS (m/7) 290 (M+H+)
~ WO96/10559 2 2 ~ O ~ 8 ~ PCT/~5101982
- 137 -
Preparation 166
The following compound was obtained according to a
similar manner to that of Preparation 66.
N-Cycloheptyl-3-benzylbenzylamine
IR (Film) : 3059, 3026, 2926, 2852, 1601, 1495 cm 1
NMR (CDCl3, o) : 1.3-2.0 (12H, m), 2.6-2.8 (lH, m),
3.74 (2H, s), 3.97 (2H, s), 7.0-7.5 (9H, m)
APCI-MASS (m/z) : 294 (M+H+)
0
Preparatio~ 167
The following compound was obtained according to a
similar manner to that of Preparation 36.
2-Formyl-6-hydroxy-2,5,7,8-tetramethylchromane
IR (KBr) : 3541, 2981, 2933, 2872, 2833, 2727,
1732 cm~1
NMR (DMSO-d6, o) : 1.66 (3H, s), 1.7-1.9 (lH, m),
2.2-2.65 (3H, m), 1.97 (3H, s), 2.07 (3H, s),
2.08 (3H, s), 7.55 (lH, s), 9.53 (lH, s)
APCI-MASS (m/z) : 244 (M+H+)
Preparation 168
To a solution of 2-chloro-6-methyl-4-methylthio-3-
nitropyridine (13.25 g) in methanol (150 ml) was added 28-
sodium methoxide in methanol (23.4 ml), and the mixture was
refluxed for 7 hours under nitrogen. The mixture was
cooled and the precipitates were collected by filtration,
washed with methanol and diisopropyl ether and dried under
phosphorus pentoxide to give 2-methoxy-6-methyl-4-
methylthio-3-nitropyridine (10.29 g) as a yellow powder.
IR (KBr) : 3024, 2997, 2951, 2924, 2856, 1587, 1541,
1495, 1452 cm~1
NMR (DMSO-d6, o) : 2.46 (3H, s), 2.57 (3H, s),
3.94 (3H, s), 7.07 (lH, s)
WO96/105S9 ~ ~ 0 0 ~ ~ 7
- 138 -
APCI-MASS (m/z) : 215 (M+H+)
.
Preparation 169
To a solution of 2,4-dichloro-6-methyl-3-nitropyridine
(41.40 g) in methanol (400 ml) was added dropwise a 28
solution of sodium methoxide in methanol (38.6 ml), and the
mixture was stirred at 60C for an hour under nitrogen.
The mixture was evaporated in vacuo and the residue was
extracted with ethyl acetate. The organic layer was washed
with brine, dried over magnesium sulfate and evaporated in
vacuo. The residue was purified by column chromatography
on silica gel to give 2-chloro-4-methoxy-6-methyl-3-
nitropyridine (30.43 g) as a pale yellow crystal.
IR (KBr) : 3088, 2987, 2953, 2883, 1601, 1552, 1524,
1471 cm~1
NMR (DMSO-d6, o) : 2.51 (3H, s), 4.01 (3H, s),
7.42 (lH, s)
Preparation 170
To a solution of 2-chloro-4-methoxy-6-methyl-3-
nitropyridine (30.42 g) in methanol (300 ml) was added
dropwise a solution of sodium methanethiolate (12.63 g) in
methanol (200 ml) at room temperature and the mixture was
stirred at 50C for 4 hours under nitrogen. The mixture
was evaporated in vacuo and the residue was extracted with
ethyl acetate. The organic layer was washed with brine,
dried over magnesium sulfate and evaporated in vacuo. The
residue was purified by column chromatography on silica gel
to give 4-methoxy-2-methylthio-6-methyl-3-nitropyridine
(30.23 g) as a yellow powder.
IR (KBr) : 3066, 2997, 2956, 2933, 2858, 1585, 1549,
1514, 1466 cm~1
NMR (DMSO-d6, o) : 2.51 (3H, s), 2.53 (3H, s),
3.95 (3H, s), 7.11 (lH, s)
APCI-MA5S (m/z) : 215 (M+H~)
PCT/~5/01982
WO96/105S9 ~ ~ 0 ~ ~ 8
- 139 -
Pre~aration 171
To a suspension of 4-methoxy-2-methylthio-6-methyl-3-
nitropyridine (30.15 g) in ethanol (300 ml) was added conc.
hydrochloric acid (58.6 ml), and the mixture was refluxed
for 10 hours. The mixture was cooled to 5C and the
precipitates were collected by filtr2tion, washed with
ethanol and diiso~ropyl ether, and dried in vacuo under
phosphorus pentoxide to give 4-hydroxy-2-methylthio-6-
~ethyl-3-nitropyridine (19.79 g) as a vellow powder.
IR (KBr) : 2989, 2920, 2783, 1551, 1518 cm 1
NMR (DMSO-d6, o) : 2.39 (3H, s), 2.50 (3H, s),
6.62 (lH, s)
Preparation 172
To a suspension of 4-hydroxy-2-methylthio-6-methyl-3-
nitropyridine (30.65 g) in phosphorus oxychloride (140.8 g)
was stirred at 100C for 10 hours. The mixture was poured
into a mixture of ethyl acetate and water, and neutralized
by addition of 5N sodium hydroxide aqueous solution. The
insoluble materials were filtered off, and the filtrate W2S
separated. The organic layer was washed with brine, dried
over ~agnesium sulfate and evaporated in vacuo. The
residue was purified by column chromatography on silica gel
to give 4-chloro-2-methylthio-6-methyl-3-nitropyridine
(il.87 g) as a yellow powder.
IR (KBr) : 3103, 3053, 2933, 1560, 1518 cm 1
NMR (DMSO-d6, o) : 2.55 (3~, s), 2.59 (3H, s),
7.55 (lH, s)
APCI-MASS (m/z) : 221, 219 (M+H+)
Pre~aration 173
To a solution of 2,4-dichloro-6-methyl-3-nitropyridine
(4.14 g) in 1,4-dioxane (50 ml) and methanol ~50 ml) was
added Raney Nickel (NDT-90, purchased from Kawaken Fine
Chemicals) (ca. 2 g), and the mixture was hydrogenated for
WO96/10559 Z ~ ~ 8 1 PCT1~5/01982
- 140 -
4 hours under atmospheric pressure. Raney Nickel was
filtered off and washed with methanol, and the filtrate was
evaporated in vacuo. The residue was purified by column
chromatography on si~ica gel tc give 3-amino-2,4-dichloro- y
6-methylpyridine (3.53 g) as a yellow oil .
IR (Film) : 3479, 3385, 3221, 3188, 29~4, 1616, 1576,
1543, 1471 cm~1
NMR (DMSO-d6, o) : 2.28 (3~, s), 5.52 (2H, br s),
7.23 (1~, s)
APCI-MASS (m/z) : 181, 179, 177 (M+H+)
.
Preparation 174
To a solution of 3-amino-2,4-dichloro-6-methylpyridine
(3.51 g) in dichloromethane (5C ml) was added N,N-
dimethylaniline (2.88 g) at 5C, followed by dropwise
addition of phenyl chloroformate (3.41 g), and the mixture
was stirred at room temperature for 3.5 hours. The ~ixture
was washed with dilute hydrochloric acid and brine, dried
over magnesium sulfate and evaporated in vacuo. The
2C residue was crystallized from diisopropyl ether and the
crystal was collected by filtration, washed Wit~l
diisopropyl ether and dried in V2CUO to give 2,4-dichloro-
6-methyl-3-phenoxycarbonylamino~yridine (1.96 g).
IR (KBr) : 3282, 3244, 3i84, 30i3, 1718, 1637, 1608,
1524, 1491 cm~1
NMR (DMSO-d6, o) : 2.27 (3~, s), 7.1-7.5 (5H, m),
7.65 (lH, s~, 10.10 ~lH, br s)
APCI-M~SS (m/z) : 301, 299, 297 (M+H+)
Preparation 175
A mixture of 3-(pyra~ol-3-yl)benzaldehyde (1.0 g) and
~-methoxybenzylamine (0.91 ml) was heated for 4 hours at
120C. After cooling to room temperature, the mixture was
dissolved in ethanol (20 ml). To the solution was added
sodium borohydride (220 mg) and stirred for two hours at
WO96/10559 2 ~ PCTl~5/01982
- 141 -
ambient temperature. The reaction mixture was poured into
water and extracted with dichloromethane, washed with water
and brine, dried over magnesium sulfate. The solvent was
removed in vacuo and the residue was chromatographed on
silica gel (50 g, eluting with dichloromethane - methanol
(lD:1)) to give N-(2-methoxybenzyl)-3-(pyrazol-3-
y')benzylamine (1.06 g).
IR (Film) : 2400-3600 (br), 1603, 1493, 1462,
1244 cm~1
NMR (CDC13, o) : 3.81 (3H, s), 3.84 (2H, s), 3.85
(2H, s), 6.59 (lH, d, J=2.2Hz), 6.80-6.98 (2H,
m), 7.17-7.50 (4H, m), 7.53-7.67 (2H, m), 7.79
(lH, s)
A~PCI-M~SS (m/z~ : 294 (M+H+)
Preparalion 176
A mixture of 3-(pyrazol-3-yl)benzaldehyde (1.0 g) and
3-methoxybenzylamine (0.91 ml) was heated for 4 hours at
120C. After cooling to room temperature, the mixture W25
dissolved in ethanol (20 ml). To the solution was added
sodium borohydride (220 mg), and stirred ~or two hours at
ambient temperature. The reaction mixture was poured into
water and extracted with dichloromethane, washed with water
and b~ ne, dried oveL magnesl~-- sull~ate. The soivent was
removed in vacuo and the residue was chromatographed on
silica gel (50 g, eluting with dichloromethane - methancl
~15:1)) to give N-(3-methoxybenzyl)-3-(~yrazol-3-
yl)benzylamine (1.24 g).
IR (Neat) : 2370-3680 (br), 1603, 1487, 1439, 1263,
1157, 1045 cm-1
NMR (CDCl3, o) : 3.80 (3H, s), 3.81 (2H, s), 3.85
(2H, s), 6.61 (lH, d, J=2.2H7), 6.7--6.85 (lH,
m), 6.86-6.97 (2H, m), 7.19-7.43 (3-, m), 7.55-
7.68 (2H, m), 7.76 (lH, s)
APCI-MASS (m/z) : 29a (M+H+)
WO96/10559 PCT/~5101982 ~
220098 1
-- i&~
Preparation 177
A mixture of 4-(4-fiuorcphencxy)benzaldehyde (1.~ g)
and 3-~heryl~ropyla~ine (1. 5 ~..l) W25 heated for ~ hou-s at
120C. Arter coollng ~o room temperature, the mixture was
dissolved in ethancl (30 ~l). To the solution W2S added
sodium borohydride (2O~ mg) and stirred for two hours a.
ambient temperature. The reaction mixture was poured _nlo
water and extracted with dichloromethane, washed with water
~nd brine, dried over magnesium sulfate. The solven_ W2S
removed in vacuo and the residue was chromatographeQ O~l
silica gel (50 g, eluting with dichloromethane - meth-nol
(15:1)) to aive N-(3-phenylpropyl)-4-(4-fluorophenoxy)-
berzylamine (1.92 g).
IR (Neat) : 3028, 29~3, 2856, 2818, 1606, 1497, 1454,
1250, 1211 cm~l
~MR (CDCl3, ~) : 1.85 (2H, qn, J=7.4~z), 2.55-2.75
(4H, m), 3.75 (2H, s), 6.83-7.1G (6H, ~.), 7.10-
7.36 (I~J ~)
APCT-M~SS (m/z) : 336 (~+~ )
Preparation 178
A mixture of 3-(pyrazol-3-yl)benzaldehyde (1.0 gj and
phenethylamine (0.875 ml) was heated for 4 nours at 120C.
After cooling to room temperature, the mixture was
dissolved in ethancl (20 ml). To the solution was added
sodium borohydride (22C mg) and stirred for two hours -t
ambient tem3erature. ~he reaction mixture was poured into
water and extracted wlth dichloromethane, washed wi~h wa.er
and brine, driea over magnesium sulfate. The solver_ w~s
removed in vacuo and the residue was chromatographed on
silica gel (50 g, elutlng with dichloromethane - methanol
(i5:1 to 10:1)) to give N-(2-phenylethyl)-3-(pvrazo'-3-
y7)benzylamine (1.27 g).
I~ (Neat) : ~30C-3700 (br), loOo, 1495, 1452, 1354,
1097 c~-1
,
WO96/10559 a 2 0 ~ PCT/~5/01982
- 143 -
NMR (CDCl3, o) : 2.76-3.00 (4H, m), 3.86 (2H, s),
6.59 (lH, d, J=2.2H7), 7.10-7.43 (7H, m), 7.53-
7.68 (2H, m), 7.86 (lH, s)
APCI-MASS (miz) : 278 (M+H+)
Preparation 179
A mixture of 4-(4-fluorophenoxy)benzaldehyde (1.5 g)
and ~S)-1-phenylethylamine (1.08 mi) was heated for 4 hours
at 120C. After cooling to room temperature, the mixture
was dissolved in ethanol (30 ml). To the solution was
added sodium borohydride (262 mg) and stirred for two hours
at ambient temperature. The reaction mixture was poured
into water and extracted with dichloromethane, washed with
water and brine, dried over magnesium sulfate. The solvent
was removed in vacuo and the residue was chromatographed on
silica gel (5C g, eluting with n-hexane - ethyl acetate
(4:1 to 2:1) to give N-[(S)-1-phenylethyl]-4-(4-
fluorophenoxy)benzylamine (2.23 g).
IR (Neat) : 3028, 2966, 2831, 1606, 1498, 1452, 125Q,
1213 cm~1
~R (CDCl3, o) : 1.38 (3H, d, J=6.6Hz), 3.56 (lH, d,
J=13.1Hz), 3.63 (lH, d, J=13.1Hz), 3.82 (lH, q,
J=6.6Hz), 6.83-7.12 (6H, m), 7.15-7.43 (7H, m)
APCT-MASS (m/z) : 322 (M+H+)
[a]D9 : -31.2 (C=1.05, CHCl3)
~reparation 180
A mixture of 4-(4-fluorophenoxy)benzaldehyde (1.5 g)
and (R)-1-phenylethylamine (1.08 ml) was heated for 4 hours
at 120C. After cooling to room temperature, the mixture
was dissolved in ethanol (30 ml). To the solution was
added sodium borohydride (262 mg) and stirred for two nours
at ambient temperature. The reaction mixture was poured
into water and extracted with dichloromethane, washed with
water and brine, dried over magnesium sulfale. The solvent
PCT/~5/01982
WO96/10559 2~ 0~ 8 1
- 144 -
was removed in vacuo and the residue was chromatographed on
silica gel (50 g, eluting with n-hexane - ethyl acetate
(4:1 to 2:1) to give N-[(R)-1-phenylethyl]-4-(4-
fluorophenoxy)benzylamine (2.12 g).
IR (Neat) : 3028, 2966, 2831, 1606, 1498, 1452, 1250,
1213 G~ 1
NMR (CDCl3, o) : 1.37 (3H, d, J=5.6Hz), 3.56 (lH,d,
J=13.1Hz), 3.63 (lH, d, J=13.lHz), 3.81 (lH, a,
J=6.6Hz), 6.83-7.12 (6H, m), 7.15-7.42 (7H, m)
APCI-MASS (m/z) : 322 (M+H+)
[~]30 : +31.7 (C=1.02, CHC13)
P~eparation 181
The following compounds were obtained according to a
similar manner to that of Preparation 71, 78 or 173.
(1~ 3-Amino-2-methoxy-6-methyl-6-methylthiopyridine
IR (Film) : 3444, 3352, 2984, 2947, 2922, 2860, 1585,
1559, 1462 cm~1
NMR (DMSO-d6, o) : 2.26 (3H, s), 2.43 (3H, s), 3.84
~3H, s), 4.39 (2H, br s), 6.64 (lH, s)
APCI-MASS (m/z) : 185 (M+H+)
(2) 3-Amino-4-chloro-2-methylthio-6-methylpyridine
IR (KBr) : 3417, 3300, 3207, 2322, 1618, 1558 cm 1
NMR (DMSO-d6, o) : 2.31 (3H, s), 2.51 (3H, s), 4.36
(2H, br s)~ 6.96 (lH, s j
APCI-MASS (m/z) : 191, 189 (M+H+)
Preparation 182
The following compounds were cbtained according to a
simiiar manner to that of Preparation 74 or 79.
(') 2-Methoxy-6-methyl-4-methylthio-3-
phenoxycarbonylaminopyridine
PCT/~5/01982
WO96/1055s ~ ~ 0 Q Q
- 145 -
IR (KBr) : 3217, 1740, '700, 1649, 1541, 1518 cm
NMR (DMSO-d6, o) : 2.39 (3~, s), 2.45 (3H, s), 3.86
(3H, s), 6.81 (lH, s), 7.0-7.5 (5H, m), 8.76 and
9.17 (total lH, br s)
APCI-MASS (m/z) : 305 (M+~+)
(2) 4-Chloro-~-methylthio-6-methyl-3-
phenoxycarbonyliminopyridine
IR (KBr) : 3207, 3026, 3001, 2926, 1724, 1597, 1554,
1524, 1489 cm~1
NMR (DMSO-d6, o) : 2.48 (3H, s), 2.51 (3HL~ s), 7.Q-
7.5 (6H, m), 9.37 and 9.77 (total lH, br s)
APCI-MASS (m/z) : 311, 309 (M+~+)
Preparation 183
The following compound was obtained according to 2
similar manner to that of Example 7, 8, 9, 10, 13, 14, 15,
16 or 17.
1-[4-(4-Fluorophenoxy)ben~yl]-3-[2,4-bis(methylthio)-
c-methylpyridin-3-yl]urea
IR (KBr) : 3305, 3107, 2924, 1633, 1574, 1498 cm 1
NMR (DMSO-d6, o) : 2.39 (6H, s), 2.44 (3H, s), 4.22
(2H, d, J=5.8Hz), o.53-6.7 (lH, br), 6.86 (lH,
s), 6.9-7.4 (8H, m), 7.54 (lH, br s)
APCI-MASS (m/z) : 444 ~M+H+)
W096/lOS59 ~ 2 ~ O 9 8 ~ PCT/JP95/01982
- 146 -
~xample 1
To a solution of N-(4-biphenylylmethyl)-
cycloheptylamine (559 mg) in dichloromethane (10 ml) was
added 2,4,6-trimet~ylphenylisocyanate (322 mg), and the
mixture was stirred at room temperature for 1.3 hours under
nitrogen. The mixture was evaporated in vacuo and the
crystalline compound was collected by filtration using
hexane:ethyl acetate (5:1) to give 1-(4-biphenylylmethyl)-
1-cycloheptyl-3-(2,4,6-trimethylphenyl)urea (710 mg).
IR (KBr) : 3320, 2920, 2855, 1625, 1505 cm~1
NMR (DMSO-d6, o) : 1.5-1.8 (12H, m), 2 00 (6H, s),
2.20 (3H, s), 4.4-4.55 (lH, m), 4.55 (2H, s),
5.48 (lH, s), 6.79 (2H, s), 7.3-7.65 (9H, m)
APCI-~Sg (m/z) : 441 (MIH+)
~x~m~le 2
To a solution o N-(4-biphenylylmethyl)-
cyclGheptylamine (559 mg) in dichloromethane (10 ml) was
added 2,6-diisopropylphenylisocyanate (406 mg), and the
mixture was stirred at room temperature for 1.1 hours. The
mixture was evaporated in vacuo and the residue was
purified by column chromatogra~hy on silica gel to give
1-(4-biphenylylmethyl)-1-cycloheptyl-3-(2,6-
diisopropylphenyl)urea (885 mg) as a crystal.
IR (KBr) : 3415, 3340, 3060, 3030, 2960, 2930,
2865, 1625, 1500 cm~1
NMR (CDCl3, o) : 0.9-1.3 (lQH, m), 1.5-1.8 (12H,
m), 1.95-2.1 (2H, m), 2.8-3.0 (2~, m), 4.4-4.6
(lH, m), 4.56 (2H, s), 5.47 (lH, s), 7.0-7.65
(12H, m)
APCI-MASS (m/~) : 483 (MTH+
Ex~mple 3
To a solution of 2-amino-4,6-dimethoxypyrimidine (465
mg) and triphosgene (297 mg) in 1,2-dichloroethane ~20 ml)
.. . , . ~
PCT/~5/01982
WO96/10559 2 ~
- 147 -
W25 added triethylamine (304 mg) and the mixturé was
refluxed for 1.8 hours. The mixture was cooled to room
temperature and a solution of N-(4-biphenylylmethvl)-
cycloheptylamine (559 mg) in 1,2-dichloroethane (10 ml) was
added thereto. After being stirred at room temperature for
3.1 hours, the mixture was poured into water and the
separated organic layer was washed with brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give 1-
(4-biphenylylmethyl)-1-cycloheptyl-3-(4,6-
dimethoxypyrimidin-2-yl)urea (205 mg).
IR (KBr) : 3390, 3225, 2925, 2855, 1685, 1600,
1525 cm~1
NMR (CDCl3, o) : 1.4-2.1 (12H, m), 3.86 (6H, s),
4.25-4.45 (lH, m), 4.58 (2H, s), 6.88 (lH, s),
7.3-7.6 (9H, m)
APC I -MAS S ( m/z) : 461 (M+H+)
Example 4
To a solution of 2,4,6-trifluoroaniline (441 mg) and
triphosgene (297 mg) in dichloromethane (lC ml~ was added
riethylamine (304 mg) at 5C and the mixture was refluxed
for 2 hours under nitrogen. The mixture was cooled to rcom
temperature and a solution of N-(4-biphenylylmethyl?-
cycloheptylamine (559 mg) in dichloromethane (3 ml) was
added. The mixture was stirred at room temperature for 1.2
hours and evaporated in vacuo. The residue was purified by
cGlumn chromatography on silica gel to give l-(4-
biphenylylmethyl)-1-cycloheptyl-3-(2,4,6-
~rimethylphenyl)urea (752 mg).
IR (KBr) : 3285, 2930, 2860, 1635, 1520 cm 1
NMR (CDCl3, o) : 1.45-2.15 (12H, m), 4.3-4.45 (lH,
m), 4.59 (2H, s), 5.58 (lH, s), 6.55-6.7 ~2H, m),
7.3-7.65 (9H, m)
APCI-MASS (m/z) : 453 (M+H+)
PCT/~5/01982
W096/10559 a ~ ~ 9 ~ 8
- 148 -
F~xample 5
The ~ollowing compounds were obtained according to
similar manners to those of Examples 1, 2, 3 and 4.
(1) 1-Cycloheptyl-1-(4-phenoxyphenylmethyl)-3-(2,6-
diisopropylphenyl)urea
IR (KBr) : 3415, 3360, 2960, 2925, 2865, 1645,
1590 cm~l
NMR (CDCl3, o) : 0.9-1.35 (12H, m), 1.4-2.1 (12H,
m), 2.8-3.C (2H, m), 4.35-4.5 (lH, m), 4.50 (2H,
s), 5.46 (lH, s), 6.95-7.45 (12H, m)
APCI-MASS (m/z) : 499 (M+H+)
(2) 1-(3-Biphenylylmethyl)-1-cycloheptyl-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3325, 2925, 2855, 1625, 1505 cm 1
NMR (CDC13, o) : 1.4-2.1 (12H, m), 1.97 (6H, s),
2.20 (3H, s), 4.2-4.4 (lH, m), 4.57 (2H, s), 5.49
(lH, s), 6.78 (2H, s), 7.3-7.7 (9H, m)
APCI-MASS (m/z) : 441 (M H+)
(3) 1-(2-Biphenylylmethyl)-1-cycloheptyl-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3285, 2970, 2930, 2860, 1635, 1520 cm 1
NMR (CDC13, o) : 1.4-2.0 (12H, m), 1.96 (6H, s),
2.21 (3H, s), 4.25-4.4 (lH, m), 4.37 (2H, s),
5.30 (lH, s), 6.80 (2H, s), 7.2-7.7 (9H, m)
APCI-M~SS (m/z) : 441 (M~H+)
(4) 1-Cyclohe tyl-1-(4-phenoxyphenylmethyl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3295, 2920, 2855, 1620, 1590, 1510,
1490 cm~1
NMR (CDCl3, o) : 1.4-1.8 (12H, m), 2.00 (6H, s),
2.22 (3H, s), 4.35-4.5 (lH, m), 4.48 (2H, s),
WO96/10559 ~ ~ Q n g ~ PCT/~5/01982
- 149 -
5.47 (lH, s), 6.81 (2H, s), 7.0-7.4 (9H, m)
APCI-M~SS (m/z) : 457 (M+H+)
(5) 1-Cycioheptyl-1-(3-phenoxyphenylmethyl)-3-~2,4,6-
trimethylphenyl)urea
IR (KBr) : 3310, 2925, 2855, 1625, 1605, 1585,
- 1510 cm~l
NMR (CDC13, o) : 1.4-1.8 (12H, m), 2.00 ~6H, s),
2.21 (3H, s), 4.25-4.45 (lH, m), 4.47 (2H, s),
5.44 (lH, s), 6.80 (2H, s), 6.85-7.4 (9H, m)
APCI-MASS (m/z) : 457 (M+H+)
(6) 1-Cycloheptyl-1-[4-(pyridin-2-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 341C, 3320, 2920, 2855, 1625, 1585,
1560, 1505 cm~l
NMR (CDC13, o) : 1.4-1.8 (12H, m), 2.03 (6H, s),
2.20 (3H, s), 4.3-4.5 (lH, m), 4.58 (2H, s), 5 ag
(lH, s), 6.80 (2H, s), 7.2-7.3 (lH, m), 7.51 (2H,
d, J=8.3Hz), 7.7-7.85 (2H, m), 8.02 (2H, d,
J=8.3Hz), 8.7-8.75 (lH, m)
APCI-MASS (m/z) : 442 (M+H+)
(7) 1-Cycloheptyl-1-[4-(pyridin-3-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3315, 2920, 2855, 1645, 1510 cm 1
NMR (CDC13, o) : 1.4-1.9 (12H, m), 2.02 (6H, s),
2.21 (3H, s), 4.35-4.5 (lH,m), 4.58 (2H, s), 5.48
(lH, s), 6.80 (2H, s), 7.39 (lH, dd, J=7.9,
4.9Hz), 7.3-7.7 (4H, ~), 7.86 (lH, dt, J=8.2,
1.8Hz), 8.60 (lH, d, J=3.6Hz),
8.83 (lH, s)
APCI-MASS (m/z) : 442 (M+.H+)
(8) 1-Cycloheptyl-1-[[2-(4-chlorophenyl)thiazol-4-
PCT/~5/01982
WO96/10559
- 150 -
yl]methyl]-3-~2,4,6-trlmethylphenyl)urea
IR (KBr) : 3300, 2920, 2855, 1645, 1610, 1495 cm ;
NMR (CDC13, o) : 1.5-2.0 (12H, m), 2.13 (6H, s),
2.24 (3H, s), 4.2-4.4 (lH, m), 4.61 (2H, s), 6.85
(2H, s), 7.18 (lH, s), 7.24 (lH, s),
7.35-7.45 (2H, m), 7.8-7.9 (2H, m)
APCI-MASS (m/z) : 483 (M+H+)
(9) l-Cycloheptyl-1-[(2-phenylimidazol-5-yl)methyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3100, 2925, 2855, 1620, 1570 cm 1
NMR (DMSO-d6, o) : 1.35-1.8 (12H, m), 2.06 (6H, s),
2.21 (3H, s), 4.05-4.2 (lH, m), 4.36 (2H, s),
6.83 (2H, s), 7.23 (lH, s), 7.3-7.5 (3H, m), 7.8-
7.9 (2H, m), 8.68 (lH, s), 12.55 (lH, s)
APCI-MASS (m/z) : 431 (M+H+)
(10) 1-Cycloheptyl-1-[4-(pyrrol-1-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3310, 2920, 2855, 1625, 1525, 1510 cm~
NMR (CDCl3, ~) : 1.4-2.05 (12H, m), 2.01 (6H, s),
2.21 (3H, s), 4.3-4.5 (lH, m), 4.53 (2H, s), 5.46
(lH, s), 6.3-6.4 (2H, m), 6.80 (2H, s), 7.05-7.15
(2H, m), 7.35-7.5 (4H, m)
APCI-MASS (m/z) : 430 (M~H+)
(11) 1-Cycloheptyl-1-[3-(pyrrol-1-yl)benzyl]-3-(2,4,6-
tri~ethylphenyl)urea
IR (KBr) : 3320, 2920, 2855, 1625, 1610, i505 cm~
NMR (CDCl3, o) : 1.45-2.05 ~12H, m), 2.01 (6H, s),
2.21 (3H, s), 4.3-4.5 (lH, m), 4.56 (2H, s), 5.47
(lH, s), 6.35-6.4 (2H, m), 6.80 (2H, s), 7.05-
7.10 (2H, m), 7.25-7.5 (4H, m)
APCI-MASS (m/z) : 430 (M+H+)
WO96/10559 2 2 q ~ 9 ~ 1 PCT/~5/01982
- 15i -
(12) 1-Cycloheptyl-1-[[4-~pyrrol-1-yl)pyridin-2-yl]methyl]-
3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3220, 2920, 1645, 1605, 1575, 1500 cm 1
N-MR (DMSO-d6, o) : 1.4-1.8 (12H, m), 2.13 (6H, s),
- 5 2.21 (3H, s), 4.1-4.3 (lH, m), 4.56 (2H, s),
6.35-6.4 (2H, m), 6.84 (2H, s), 6.5-6.55 (2H, m),
6.55-6.65 (2H, m), 8.50 (lH, br s), 8.51 (lH, d,
J=5~6Hz)
APCI-MASS (m/z) : 431 (M+H+)
(13) 1-Cycloheptyl-1-[(6-phenylpyridin-3-yl)methyl~-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3315, 2920, 2855, 1630, 1560, 1515 cm 1
NMR (CDCl3, o) : 1.4-2.05 (12H, m), 2.09 (6H, s),
2.23 (3H, s), 4.1-4.3 (lH, m), 4.60 (2H, s), 5.53
(lH, s), 6.83 (2H, s), 7.35-7.55 (3H, m), 7.7-7.9
(2H, m), 7.95-8.05 (2H, m), 8.70 (lH, s)
APCI-MASS (m/z) : 442 (M~H+)
(14) 1-Cycloheptyl-1-[3-(2-methylthiazol-4-yl)benzyl~-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3360, 2925, 2855, 162Q, 1505 cm -
N-MR (CDCl3, o) : 1.4-2.05 (12H, m), 1.98 (6H, s),
2.20 (3H, s), 2.78 (3H, s), 4.4-4.55 (lH, m),
4.57 (2~, s), 5.49 (l~l~ s), 6.78 (2~, s), 7.33
(lH, s), 7.35-7.5 (2H, m), 7.79 (lH, d, J=7.1Hz),
7.93 (lH, s)
APCI-M~.SS (m/z) : 462 (M+H )
(15) 1-Cycloheptyl-1-[3-(pyrazol-3-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3405, 3210, 2925, 2855, 1640, 1610,
1500 cm-l
N-MR (CDCl3, o) : 1.4-1.9 (12H, m), 2.08 (6H, s),
2.20 (3H, s), 4.1-4.25 (lH, m~, 4.54 (2H, s),
W096/10559 2 ~ ~ O ~ 8 ~ PCTtJP95/01982
- 152 -
6.63 (lH, s), 6.82 (2H, s), 7.2-7.8 (6H, m),
12.86 (lH, s)
APCI-MASS (m/z) : 431 (M+H+)
(16) 1-Benzyl-1-(4-phenoxybenzyl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3310, 3030, 2915, 1630, 1590, 1505 cm~
NMR (CDCl3, o) : 2.01 (6H, s), 2.22 (3H, s), 4.63
(2H, s), 5.64 (lH, s), 6.82 (2H, s~, 7.0-7.4
(14H, m)
APCI-MASS (m/z) : 451 (M+H+)
(17) 1-Furfuryl-1-(4-phenoxybenzyl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3280, 3030, 2975, 2915, 1625, 1595,
1530, 1505 cm~
~MR (CDCl3, o) : 2.10 (6H, s), 2.25 (3H, s), 4.55
(2H, s), 4.61 (2H, s), 6.03 (lH, s), 6.25-6.3
(lH, m), 6.35-6.4 (lH, m), 6.86 (2H, s), 6.95-
7.45 (lOH, m)
APCI-MASS (m/z) : 441 (M+H+)
(18) 1-Cycloheptyl-1-[4-(4-chlorophenyl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3400, 3300, 2925, 2855, 1655, 1625,
1505 cm~1
NMR (CDC13, o) : 1.5-2.05 (12H, m), 2.01 (6H, s),
2.21 (3H, s), 4.3-4.5 (lH, m), 4.55 (2H, s), 5.46
(lH, s), 6.80 (2H, s), 7.4-7.65 (8H, m)
APCI-MASS (m/z) : 476 (M+H+3
(19) 1-Cycloheptyl-1-[4-(4-fluorophenyl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3400, 3300, 2925, 2855, 1655, 1625,
1490 cm~l
~ ~ A ~ ~ ~ ~ PCT/~5/01982
WO g6110559 ,~
- 153 -
~MR (CDC13 o) : 1.5-2.15 (;2H, ~), 2.01 (6H, s),
2.21 (3H, s), 4.4-4.6 (lH, m), 4.55 (2H, s), 5.47
(lH, s), 6.80 (2H, s), 7.05-7.2 (2H, ~), 7.45-7.6
(6H, m)
~PCI-MASS (m/7) 459 (M+U~)
(20) 1-Cycloheptyl-1-[4-(4-bro~ophenvl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3400, 3300, 2920, 2855, 1655, 1625,
1505 c~,-l
NMR (CDCl3, ~) : 1.5-2.05 (12H, m), 2.01 (6H, s),
2.21 (3H, s), 4.35-4.55 (2H, s), J.46 (lH, s),
6.80 (2H, s), 7.45-7.o (8H, m)
APCI-MASS (m/z) : 521 (M+H+)
(21) 1-Cycloheptyl-1-[4-(4-methylphenyl)benzyl]-3-~2,4,6-
trimethyl~henyl)urea
IR (KBr) : 3400, 3310, 3020, 2920, 2855, 1660,
1625, 1500 cm~l
NMR (CDC13, o) : 1.4-2.1 (12H, m), 1.93 (6H, s),
2.20 (3H, s), 2.40 (3H, s), 4.35-4.55 (lH, m),
4.54 (2H, s), 5.48 (lH, s), 6.79 (2H, s), 7.25
(2H, d, J=7.9Hz), 7.4-7.5 (4H, m), 7.59 (2H, d,
J=8.3Hz)
A~PCI-MASS (m/z) : 455 (M+H+)
(22) 1-Cycloheptyl-1-[4-(4-dimethylaminophenyl)benzyl]-3-
(2,4,6-trimethylphenyl)ure
IR (KBr) : 3405, 3325, 2920, 2855, 2805, 1650,
1610, 1535, 1500 cm~l
.~MR (CDCl3, o) : 1.5-2.2 (12H, m), 1.98 (6H, s),
2.20 (3H, s), 3.00 (6H, s), 4.4-4.6 (lr, m), 4.52
(2H, s), 5.50 (lH, s), 7.4-7.65 (8H, m)
APC I-.~ASS (m/z) : 484 (M+:H+)
W096/lOS59 PCTt~5101982
- 154 -
(23) l-Cycloheptyl-1-[4-(4-bromophenoxy)benzyl~-3-(2,4,6-
trimethylphenyl)urea
IR (K3r) : 3410, 3325, 2920, 2855, 1635, 1585,
1`505 cm~l
NMR (CDCl3, o) : 1.5-2.1 (12H, m), 2.01 (6H, s),
2.22 (3H, s), 4.35-4.55 (lH, m!, 4.49 (2H, s),
5.46 (lH, s), 6.81 (2H, s), 6.85-7.05 (4H, m),
7.3-7.5 (4H, m)
APCI-MASS (m/z) : 537 (M+H+)
(24) 1-Cycloheptyl-1-(4-benzoylbenzyl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3325, 2920, 2855, 1655, 1605, 1505 cm~
NMR (CDCl3, o) : 1.4-2.05 (12H, m), 2.06 (6H, s),
2.22 (3H, s), 4~2-4~a (lH, m), 4.61 (2H, s), 5.45
(lH, s), 6.82 (2H, s), 7.5-7.7 (5H, m), 7.75-7.9
(4H, m)
ADCI-MASS (m/z) : 469 (M+Ht)
~25) 1-Cycloheptyl-1-(4-benzylbenzyl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3305, 3025, 2920, 2855, 16~5, 1505 cm -
NMR (CDCl3, o) : 1.5-2.05 (12H, m), 1.93 (6H, s),
2.21 (3H, s), 3.97 (2H, s), 4.35-4.55 (lH, m),
4.46 (2H, s), 5.42 (lH, s), 6.78 (2H, s), 7.1-7.4
(9H, m)
APCI-MASS (m/z) : 455 (MtH+)
(26) 1-Cyclohe~tyl-1-(4-phenylthiobenzyl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3315, 2920, 1630, 1610, 1505 cm~l
NMR (CDCl3, o) : 1.4-2.05 (12H, m), 2.CO (6H, s),
2.22 (3H, s), 4.3-4.5 (lH, m), 4.48 (2H, s), 5.42
(lH, s), 6.81 (2H, s~, 7.07 (lH, t, J=8.6Hz),
7.25-7.45 (8H, m)
WO96/10559 ~ PCT/~5/01982
- 155 -
APCI-MASS (m/z) : 473 (M+H+)
(~7) 1-Cycloheptyl-1-[(6-phenyl~hiOpyridin-3-yl)methyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3310, 2925, 2855, 1630, '585, 1510 cm l
NMR (CDCl3, o) : 1.5-2.05 (12H, m), 2.05 (6H, s),
2.23 (3H, s), 4.05-4.2 (lH, m), 4.47 (2H, s),
5.49 (lH, s), 6.84 (2U, s), 6.90 ~lH, d,
J=8.3Hz), 7.4-7.65 (6H, mj~ 8.43 (lH, à, J=1.8Hz)
APCI-~SS (m/z) 474 (M+H~)
(28) 1-Cycloheptyl-1-(4-benzoylaminobenzyl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3350, 3055, 2920, 2855, 1655, 1610,
1550 cm~1
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.0O (6H, s),
2.19 (3H, s), 4.1-4.3 (lH, m), 4.51 (2H, s), 6.81
(2H, s), 7.05 (lH, d, J=7.7Hz), 7.~9 (lH, d,
J=7.7Hz), 7.40 (lH, s), 7.5-7.7 (4H, m) / 7.77
(lH, s), 7.9-8.0 (2H, m), 10.26 (lH, s)
APCI-MASS (m/z) : 484 (M+H+)
(29) 1-Cycloheptyl-1-[4-(phenylcarbamoyl)benzyl)-3-(2,4,6-
trimethylphenyl)urea
lR (KBr) : 3425, 3300, 2920, 2860, 1670, 1635,
1600, 1540 cm~l
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.11 (6H, s),
2.21 (3H, s), 4.1-4.3 (lH, m), 4.58j (2H, s), 6.85
(2H, s), 7.13 (lH, t, J=7.3Hz), 7.3-7.5 (4H, m),
7.65 (lH, s), 7.77 (2H, d, J=7.6Hz)l, 7.93 (2H, d,
J=8.2Hz), 10.17 (lH, s)
APCI-MASS (m/z) : 484 (M+H+)
(30) 1-cycloheptyl-l-[4-(2-pyridylcarbamoyl)ben7yl]-3
(2,4,6-trimethylphenyl)urea
pCT/~5/01982
W096/lOS59 2~ 8 1 ~
- 150 -
IR (KBr) : 3335, 292C, 2855, 1675, 1635, 1610,
1580, 1525, 15C5 cm~l
NMR (DMSO-d6, ~) : 1.4-1.9 (12H, m), 2.10 (6H, s),
2.21 (3H, s), 4.1-4.3 (lH, m), 4.57 (2H, s), 6.84
(2H, s), 7.16 (lH, dd, J=6.g, 5.8Hz), 7.42 (2H,
d, J=8.2Hz)~ 7.63 (lH, br s), 7.8-7.9 (lH, m),
8.00 (2H, d, J=8.2Hz), 8.19 (lH, d, J=8.4Hz),
8.35-8.45 (lH, m), 10.71 (lH, s)
APCI-MASS (m/z) : 485 (M+H+)
(31) l-Cycloheptyl-l-[4-(4-fluorophenoxy)ben7yl~-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3305, 2920, 2855, 1030, 1500 cm 1
NMR (CDCl3, ~) : 1.5-2.1 (12H, m), 2.00 (6H, s),
2.22 (3H, s), 4.3-4.5 (lH, m), 4.76 (2H, s), 5.~7
(lH, s), 6.82 (2H, s), 6.g-7.1 (6H, m), 7.36 (2H,
d, J=8.5Hz)
APCI-MASS (m/z) : 475 (M+H+)
- 20 (32) 1-Cycloheptyl-1-[4-~phenylsulfamoyl)ben yl]-3-(2,4,6- trimethylphenyl)urea
IR ~KBr) : 3395, 313C, 2925, 2860, 1635, 1600,
1500 cm~l
NMR (DMSO-d6, o) : 1.3-1.8 (12H, m), 2.0_ (6H, s),
2.20 (3Y~, s), 4.05-4.25 ~lH, m), 4.50 ~2H, s),
6.81 ~2H, s), 7.0-7.15 (3H, m), 7.15-7.3 (2H, m),
7.42 (2H, d, J=8.3Hz), 7.57 (lH, br s,, 7.70 ~2H,
d, J=8.3Hz), 10.23 ~lH, s)
APCI-MASS (m/z) : 520 ~M+H+)
(33) 1-Cycloheptyl-1-[4-(phenylsulfonylamino)~enzyll-3-
~2,4,6-trimethylphenyl)urea
IR (KBr) : 3410, 3110, 2925, 2860, 1630, 1510 cm~
NMR (DMSO-d6, ~) : 1.3-1.8 (12H, m), l.9g ~6H, s),
2.20 ~3H, s), 4.0-4.2 (lH, m), 4.3/ (-H, s), 6.81
.... .. .
~ F f n ~ PCT/~5/01982
~ WO96/10559 ~ 2 ~
- 157 -
(2H, s), 7.01 (2H, d, J=8.3Hz), 7.15 (2H, d,
J=8.3Hz), 7.37 (lH, br s), 7.5-7.65 (3H, m), 7.7-
7.8 (2H, m), 10.22 (lH, br s)
APCI-MASS (m~z) : 520 (M+H+)
(34) 1-Cycloheptyl-1-[4-(3-thienyl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3320, 2920, 1624, 150g, 1252, 775 cm 1
N~R (CDCl3, o) : 1.40-2.10 (12H, m), 2.00 (6H, s),
2.20 (3H, s), 4.35-4.55 (lH, m), 4.53 (2H, s),
5.47 (lH, s), 6.79 (2H, s), 7.34-7.50 (5H, m),
7.55-7.66 (2H, m)
APCI-MASS (m/z) : 447 (M+H+)
(35) 1-Cycloheptyl-1-[4-(2-thienyl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3319, 2922, 1624, 1504, 1253, 849 cm 1
NMR (CDC13, o) : 1.40-2.10 (12H, m), 2.01 (6H, s),
2.20 (3H, s), 4.35-4.55 (lH, m), 4.52 (2H, s),
5.46 (lH, s), 6.79 (2H, s), 7.09 (lH, dd, J=5.1,
3.6Hz), 7.25-7.35 (2H, m), 7.36-7.46 (2H, m),
7.58-7.68 (2H, m)
APCI-MASS (m/z) : 447 (M+H+)
(36) 1-Cycloheptyl-1-[4-(pyrazol-1-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3325, 2922, 1628, 1504, 1394 cm 1
NMR (CDCl3, o) : 1.40-2.08 (12H, m), 2.04 (6H, s),
2.21 (3H, s), 4.28-4.48 (lH, m), 4.55 (2H, s),
5.48 (lH, s), 6.48 (lH, t, J=2.3Hz), 6.81 (2H,
s), 7.42-7.54 (2H, m), 7.65-7.78 (3H, m), 7.92
(lH, d, J=2.3Hz)
APCI-MASS (m/z) : 431 (M+H+)
(37) 1-Cycloheptyl-1-[4-(imidazol-1-vl)benzyl]-3-(2,4,6-
PCT1~5101982
WO96110SS9 a~ ~ 9 8 1 ~
- 158 -
trimethylphenyl)urea
IR (KBr) : 3310, 2922, 1637, 1520, 1305 cm 1
NMR (CDCl3, o) : 1.38-2.10 (12H, ~.~, 2.05 (6H, s~,
2.22 (3H, s), 4.20-4.40 (lH, m), 4.57 (2H, s),
5.47 (lH, s), 6.83 (2H, s), 7.21 (lH, s), 7.28
(lH, s), 7.33-7.44 (2H, m), 7.45-7.57 (2H, m),
7.85 (lH, s)
APCI-MASS (m/z) : 431 (M+H+)
(38) 1-Cycloheptyl-1-[4-(1-methylpyrazol-4-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3321, 2922, 1628, 1504, 1209, 955 cm 1
NMR (CDCl3, o) : 1.38-2.08 (12H, m), 1.99 (6H, s),
2.20 (3H, s), 3.95 (3H, s), 4.35-4.55 (lH, m),
4.50 (2H, s), 5.47 (lH, s), 6.79 (2H, s), 7.32-
7.53 (4H, m), 7.61 (lH, s), 7.75 (lH, s)
APCI-MASS (m/z) : 445 (M+H+)
(39) 1-Cycloheptyl-1-[(2-phenylthiophen-5-yl)methyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3329, 2922, 1624, 1510, 758 cm 1
NMR (CDCl3, o) : 1.42-2.15 (12H, m), 2.04 (6H, s),
2.22 (3H, s), 4.25-4.43 (lH, m), 4.63 (2H, s),
5.82 (lH, s), 6.81 (2H, s), 7.02 (lH, d,
J=3.6Hz), 7.16 (lH, d, J=3.6Hz), 7.22-7.43 (3H,
m), 7.50-7.61 (2H, m)
APCI-~S (m/z) : 447 (M+H+)
(40) 1-Cycloheptyl-1-[4-(oxazol-5-Yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3302, 2922, 1624, 1508, 1105, 941 cm~l
N~MR (CDCl3, o) : 1.38-2.08 (12H, ~), 2.03 (6H, s),
2.21 (3H, s), 4.30-4.50 (lH, ~), 4.55 (2H, s),
5.45 (lH, s), 6.81 (2H, s), 7.36 (lH, s), 7.42-
7.53 (2H, m), 7.63-7.74 (2H, m), 7.92 (lH, s)
WO96/10559 a 2 ~ PCTt~5/0l982
- 159 -
APCI-MASS (m/z) : 432 (M+H+)
(41) 1-Cycloheptyl-1-[(2-phenylfura~-5-yl)methyl]-3-(2,4,6-
trimelhylphenyl)urea
IR (KBr) : 3340, 2g20, 1628, 1508, 76~ cm~l
NMR (CDC13, o) : 1.40-2.15 (12H, m), ~ 09 (6H, s),
2.23 (3H, s), 4.22-4.41 (lH, m), 4.53 (2H, s),
5.93 (lH, s), 6.41 (lH, d, J=3.3Hz), 6.62 (lH, d,
J=3.3Hz), 6.83 (2H, s), 7.20-7.43 (3H, m), 7.57-
7.67 (2~, m)
APCI-MASS (m/z) : 431 (M+H+)
(42) 1-Cycloheptyl-1-[(5-phenylisoxazol-3-yl)methyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3326, 2924, 1630, 1512, 766 cm~l
NMR (CDCl3, o) : 1.40-2.10 (12H, m), 2.14 (6H, s),
2.24 (3H, s), 4.05-4.25 (lH, m), 4.56 (2H, s),
6.14 (lH, s), 6.63 (lH, s), 6.86 (2H, s), 7.40-
7.53 (3H, m), 7.70-7.82 (2H, m)
~CI-l~ASS (m/z) : 432 (M+H+)
(43) 1-Cycloheptyl-1-[(3-phenylpyra 701- 5-yl)methyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 2700-3600 (br), 2924, 1633, 1508, 1250,
1201 cm~l
NMR (CDCl~, o) : 1.35-2.10 (12H, m), 2.12 (6H, s),
2.23 (3H, s), 3.92-4.12 (lH, m), 4.47 (2H, s),
6.24 (lH, br s~, 6.50 (lH, s), 6.84 (2H, s),
7.25-7.46 (3H, m), 7.62-7.75 (2H, m)
APCI-MASS (m/z) : 431 (M+H+)
(44) 1-Cycloheptyl-1-[(4-phenylthiopher-2-yl)methyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3315, 2922, 2854, 1628, 1508, i377,
1308 cm~l
WO96/10559 ~ 8 ~ PCT/~5/01982
- 160 -
N~R (CDC13, o) : 1.40-2.13 (i2H, m), 2.03 (6H, s),
2.21 (3H, s), 4.26-4.45 (lH, m), 4.66 (2H, s),
5.82 (lH, s), 6.81 (2H, s), 7.21-7.4~ (5H, m),
7.50-7.6G (2H, m!
APCI-MASS (m/z) : 447 (~I+H+)
(45) 1-Cycloheptyl-1-[4-(pyrazol-3-yl)benzyl~-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 2800-3500 (br), 2924, 2856, 1645, 1504,
1240 cm-l
NMR (CDC13, ~) : 1.38-2.10 (12H, m), 2.00 (6H, s),
2.19 (3H, s), 4.35-4.55 (lH, m), 4 5A (2H, s),
5.51 (lH, s), 6.60 (ln, d, J=2.3Hz), 6.78 (2H,
s), 7.42-7.53 (2H, m), 7.55 (lH, d, J=2.3Hz),
7.73-7.83 (2H, m)
APCI-MASS (m/z) : 431 (M+H+)
(46) 1-Cycloheptyl-1-[4-(1-methylpyrazol-3-yl)benzyl~-3-
(2,4,5-trimethylphenyl)urea
IR (KBr) : 3406, 3331, 2924, 2856, 1647, 1502,
1236, 849, 758 cm~l
NMR (CDC13, o) : 1.38-2.08 (12H, m), 2.00 (6H, s),
2.20 (3H, s), 3.96 (3H, s), 4.35-4.55 (lH, m), a 52
(2H, s), 5.50 (lH, s), 6.54 (lH, d, J=2.3Hz), 6.78
(2H, s), 7.35-7.47 (3H, m), 7.77-7.87 (2H, m)
APCI-M~5S (m/z, : 445 (M+~+)
(47) 1-Cycloheptyl-1-[4-(1-methylpyrazol-5-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3296, 2922, 2854, 1528, 1506, 1385 cm -
NMR (CDC13, o) : 1.38-2.10 (12r, m!, 2.02 (6H, s),
2.~1 (3H, s), 3.89 (3H, s), 4.3 -4.50 (lH, m),
4.57 (2H, s), 5.45 (lH, s), 6.30 (lH, d,
J=1.9Hz), 6.81 (2H, s), 7.33-7.56 (5r., m),
APCI-MASS (m/7) : 445 (M+X+)
W096tlO559 ~ ~ U ~ PCT/~5/01982
- 161 -
(48) l-Cycloheptyl-1-[3-(1-trityl-lH-tetra701-5-yl)benzyl]-
3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3340, 2924, 2856, 1649, 1495, 1448,
i240 cm~1
NMR (CDCl3, o) : 1.38-2.10 (12H, m), 1.96 (6H, s),
2.20 (3H, s), 4.30-4.50 (lH, m), 4.57 (2H, s),
5.42 (lH, s), 6.77 (2H, s), 7.08-7.57 (17H, m),
8.05-8.18 (2H, m)
(49) 1-Cycloheptyl-1-[4-phenoxybenzyl-3-(4,6-
dimethoxypyrimidin-2-yl)]urea
IR (KBr) : 3390, 2925, 2860, 1685, 1595 cm 1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 3.87 (6H, s),
4.2-4.4 (lH, m), 4.51 (2H, s), 5.66 (lH, s), 6.87
(lH, s), 6.95-7.4 (9H, m)
APCI-MASS (m/z) : 477 (M+H+)
(50) 1-Cyclohepiyl-1-(4-phenylbenzyl)-3-[2,4-
bis(methylthio)-6-methylpyridi~-3-yl]urea
IR (KBr) : 3360, 2925, 2855, 166C, 1565 cm 1
NMR (CDCl3, o) : 1.45-2.1 (12H, ~.~, 2.36 (3H, s),
2.45 (3H, s), 2.46 (3H, s), 4.3-4.5 (lH, m), 4.62
(2H, s), 5.52 (lH, s), 6.59 (lH, s), 7.3-7.7 (9H,
m)
APCI-MASS (m/z) : 506 (M+H+)
(51) 1-(3-Phenylbenzyl)-1-cycioheptyl-3-(2,4,6-
~rifluorophenyl)urea
IR (KBr) : 3285, 2925, 2860, 1635, 1610, 1520 cm 1
NMR (CDCl3, o) : 1.4-2.05 (12H, m), 4.3-4.5 (lH,
m), 4.62 (2H, s), 5.60 (lH, s), 6.55-6.7 (2H, m),
7.3-7.65 (9H, m)
APCI-MASS (m/z) : 453 (M+H+)
(52) 1-(2-Phenylbenzyl)-1-cycloheptyl-3-(2,4,6-
W096/lOS59 2 ~ 8 ~ PCT/~5/01982
- 162 -
trifluorophenyl)urea
IR (KBr) : 3415, 3320, 3060, 3020, 2920, 2855,
1625, 1575 cm~l
NMR (CDCl3, oj : 1.4-2.0 (12H, m), 4.2-4.35 (lH,
m), 4.40 (2H, s), 5.50 (lH, s), 6.55-6.75 (2H,
m), 7.25-7.6 (9H, m)
APCI-MASS (m/z) : 453 (M+H+)
(53) 1-Cycloheptyl-1-(4-phenoxybenzyl)-3-(2,4,6-
trifluorophenyl)urea
IR (KBr) : 3285, 2925, 2860, 1635, 1590, 1520 cm 1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 4.2-4.4 (lH, m),
4.51 (2H, s), 5.58 (lH, s), 6.6-6.75 ~2H, m),
7.0-7.4 (9H, m)
APCI-MASS (m/z) : 469 ~M+H+)
(54) 1-Cycloheptyl-1-(3-phenoxybenzyl)-3-(2,4,6-
trifluorophenyl)ure2
IR (KBr) : 3280, 2930, 2860, 1635, 1615, 1585,
1520 c~ 1
~MR (CDC13, o) : 1.4-2.0 (12H, m), 4.2-4.4 (lH, m),
4.50 (2H, s), 5.55 (lH, s), 6.6-6.75 (2H, m),
6.9-7.4 (9H, m)
APCI-MASS (m/z) : 469 (M+H+)
(55) 1-Cycloheptyl-1-[4-(pyridin-2-yl)benzyl]-3-(2,4,6-
trifluorophenyl)urea
IR (KBr) : 3285, 2925, 2860, 1635, 161C, 152C cm~
NMR (CDCl3, o) : 1.4-2.1 (12H, m), 4.25-4.4 (lH,
m), 4.61 (2H, s), 5.59 (lH, s), 6.6-6.75 (2H, m),
7.2-7.3 (lH, m), 7.47 (2H, d, J=8.4H_), 7.7-7.8
(2H, m), 8.02 (2H, d, J=8.4Hz), 8.65-8.75 (lH, m`
APCI-MASS (m/z) : 454 (M+H+)
(56) l-Benzyl-l-[[2-(4-chlorophenyl)thiazol-4-yl]methvl]-3
WO96/10559 ~ ~ Q ~ PCT/~5/01982
- 163 -
(2,4,6-trifluorophenyl)urea
IR (KBr) : 3270, 3050, 1665, 1640, 1615, 1520 c~. 1
NMR (CDCl3, o) ; 4.54 (2H, s), 4.64 ~2H, s),
6.65-6.8`(2H, m), 7.3-7.4 (5H, m), 7.34 (lH, s),
7.4-7.5 (2H, m), 7.85-7.95 (2H, m)
APCI-MASS (m/~) : 488 (M+H+)
(57) 1-(Cycloheptyl-1-[4-(pyrrol-1-yl)benzylj-3-(2,4,6-
trifluorophenyl)urea
IR (KBr) : 3285, 2925, 2860, 1635, 1610, 1520 cm~
~MR (CDCl3, ~) : 1.4-2.05 (12H, m), 4.2-4.4 (lH,
m), 4.56 (2H, s), 5.57 (lH, s), 6.3-6.4 (2H, m),
6.55-6.7 (2H, m), 7.05-7.15 (2H, m), 7.41 (4H, s)
APCI-MASS (m/z) : 442 (M+H+)
(58) 1-Cycloheptyl-1-[4-(3-thienyl)benzyl]-3-(2,4,6-
trifluorophenyl)urea
IR (KBr) : 3300, 2927, 1637, 1518, 1120, 777 cm 1
NMR (CDCl3, o) : 1.40-2.08 (12H, m), 4.27-4.47 (lH,
m), 4.56 (2H, s), 5.58 (lH, s), 6.58-6.73 (2H,
m), 7.30-7.50 (5H, m), 7.57-7.70 (2H, m)
APCI-MASS (m/z) : 459 (M+H~)
(59) 1-Cycloheptyl-1-[4-(2-thienyl)benzyl]-3-(2,4,6-
trifluorophenyl)urea
IR (KBr) : 3300, 2930, 1635, 1520, 1120 cm~1
NMR (CDCl3, ~) : 1.38-2.08 (12H, m), 4.25-4.45 (lH,
m), 4.55 (2H, s), 5.57 (lH, s), 6.55-6.72 (2H,
m), 7.09 (lH, dd, J=5.1, 3.6Hz), 7.22-7.42 (4H,
m), 7.57-7.70 (2H, m)
APCI-MASS (m/z) : 459 (M+H+)
~x~le 6
To a stirred suspension of l-cycloheptyl-1-[3-(1-
trityl-lH-tetrazol-5-yl)benzyl]-3-(2,4,6-
=
WO96/10559 ~ 8 1 PCTl~51019~2
- 164 -
trimethyl~henyl)urea (1.46 g) in methanol (14 ml) was added
conc. hydrochloric acid (0.722 ml). The mixture was
stirred for one hour at room tem~erature. Insolubie white
solid was collected by filtralion, washed with methanol
(x2), water (x3) to give 1-cycloheptyl-1-[3-(lH-tetrazol-5-
yl)benzyl]-3-(2,4,6-trimethylphenyl~urea (0.79 g).
IR (KBr) : 3359, 2400-3300 (br), 1595, 1512, 1456,
1257 cm~l
NMR (DMSO-d6, o) : 1.35-1.90 (12H., m), 2.06 (6H,
s), 2.20 (3H, s), 4.14-4.34 (lH, m), 4.59 (2H,
s), 6.82 (2H, s), 7.46-7.66 (2H, m), 7.80-7.9G
(lH, m), 8.00-8.08 (lH, m)
APCI-MASS (m/z) : 433 (M+H+)
Example 7
To a solution of N-cycloheptyl-4-(4-
rluorophenoxy)~benzylamine (2.51 g) in toluene (100 ml) were
added 3-phenoxycarbonylamino-2,4-bis(methylthio)-6-
methylpyridine (2.56 g) and triethylamine (2.43 g) and the
mixture was refluxed for 4 hours under nitrogen. The
mixture was cooled and poured into a mixture o ethyl
acetate and water. The separated organic layer was washed
with brine, dried over magnesium sulfate and evaporated in
vacuo. The residue was purified by column chromatographv
on silica gel to give 1-cycloheptyl-1-[4-(~-
fluorophenoxy)benzyl]-3-[2,4-bis(methylthio)-6-
methylpyridin-3-yl~urea (3.89 g).
T~ (KBr) : 3379, 3080, 3055, 2324, 2856, 1651, 1568,
1529, 1497 cm~1
NMR (DMSO-d6, o) : 1.4-2.0 (12H, m), 2.39 (6H, s),
2.44 (3H, s), 4.0-4.2 (lH, m), 4.45 (2H, s), 6.86
(lH, s), 6.93 (2H, d, J=8.5H7), 7.0-7.1 (2H, m),
7.15-7.3 (2H, m), 7.36 (2H, d, J=8.5H-), 7.83
(lH, br s)
APCI-MASS (m/z) : 54Q (M+H )
WO96/10559 ~ 2 ~ PCT/~5/01982
- 165 -
Fxample 8
To a solution of N-cycloheptyl-4-(4-
fluorophenoxy)benzylamine (1.57 g) in toluene (150 ml) were
added 2,4-dimethoxy-~-methyl-3-phenoxycarbonylaminopyridine
(1.44 g) and trlethylamine (i.52 g), and the mixture was
refluxed for 3 hours under nitrogen. The ~ixture was
poured into a mixture of ethyi acetate and ice water, and
the separated organic layer was washed with brine, dried
over magnesium sulfate and evaporated in vacuo. The
residue was purified by colu~n chromatography on silica sel
to give l-cycloheptyl-1-[4-(4-fluorophenoxy)benzyl]-3-(2,4-
dimethoxy-6-methylpyridin-3-yl)urea (1.83 g).
IR (KBr) : 3388, 3062, 2927, 2856, 1668, 1599,
1498 cm~1
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.35 (3H, s),
3.67 and 3.77 (6H, s x 2), 4.0-4.2 (lH, m), 4.43
(2H, s), 6.63 (lH, s~, 6.95-7.4 (8~, m)
APCI-MASS (m/z) : 496 (M+H+)
Fxample 9
To a suspension of N-benzyl-3-(pyrazol-3-
yl)benzylamine bis(trifluoroacetate) (2.46 g) ~n toluene
(80 ml) were added 2,4-bis(methylthio)-6-methyl-3-
phenoxycarbonylaminopyridine (1.60 g) and triethylamine
(2.53 g), and the mixture was refluxed for 4.5 hours under
nitrogen. The mixture was cooied and poured into a mixture
of ethyl acetate and ice water. The separated organic
layer was washed with brine, dried over magnesium sulfate
and evaporated ln vacuo. The residue was purified by
30 colu~n chromatography on silica gel to give l-benzyl-1-[3-
(pyrazol-3-yl)benzyl]-3-[2,4-bis~methylthio)-6-
methylpyridin-3-yl]urea (831 mg).
IR (KBr) : 3238, 3061, 3028, 2959, 2924, 2870, ;641,
1564, 1495 cm~1
NMR (DMSO-d6, o) : 2.42 (6H, s), 2.46 (3H, s), 4.49
W096/lOS59 ~ ~ ~ O g PCTl~5/01982
- 166 -
(4H, br s), 6.6-6.7 (lH, m), 6.90 (lH, s~, 7.2-
7.8 (lOH, m), 8.29 (lH, br s), 12.88 (lH, br s)
A~CI-MASS (m/z) : 49C (M+H+)
Example 10
The mixture of ~T-cycloheptyl-3-(1-tritylpyrazol-3-yl)-
benzylamine (14.63 g) and phenyl N-(2,4,6-
trifluorophenyl)carbamate (7.64 g) and triethylamine (~0
ml) in toluene (360 ml) was stirred at 100C for one hour.
After cooling to room temperature, the reaction mixture was
washed with water, aqueous sodium bicarbonate, water and
brine, dried over magnesium sulfate, and evaporated in
vacuo. The residue was purified by column chromatography
on silica gel (700 g, eluting with n-hexane - ethyl acetate
(4:1 to 3:1)) to give 1-cycloheptyl-1-~3-(1-tritylpyrazol-
3-yl)benzyl]-3-(2,4,6-trifluorophenyl)urea (13.6 g).
IR (KBr) : 2900-3600 (br~, 2927, 2858, 1635, i607,
1520, 1446 cm~1
NMR (CDCl3, o) : 1.35-2.1C (12H, m), 4.26-4.48 (lH,
m), 4.55 (2H, s), 5.57 (lH, s), 6.52-6.70 (3H,
m), 6.7S-6.97 (2H, m!, 7.10-7.45 (16H, m),
7.68-7.80 (2H, m)
.~xample 11
The following compounds were cbtained according tO
similar manners to those of Ex~mples 7, 8, 9 and 10.
(1) 1-Cyclohexyl-1-[4-(4-fluoro~henoxy)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
IR (KBr) : 3377, 3084, 3057, 2927, 2856, 1653, 1566,
~533, 1497 c~. -
NM~ (DMSQ-d6, o) : 1.3-1.8 (lOH, m), 2.39 (6H, s),
2.45 (3H, s), 3.85-4.05 (lH, m), 4.47 (2H, s),
6.86 (lH, s), 6.93 (2H, d, J=8.5Hz), 6.95-7.05
(2H, m), 7.35 (2H, d, J=8.5Hz), 7.88 (lH, s)
.
PCT/JP95/01982
~ WO96/10559 k2~ ~
- 167 -
APCI-MASS (m/z) : 526 (M+H+)
(2) 1-Benzyl-1-~4-(4-fluorophenoxy)benzyl]-3-[2,4-
bis(methyl~hio)-6-methylpyridin-3-yl)urea
IR (KBr) : 3307, 3062, 3029, 2939, 2922, 1735, 1660,
1564, 1497 cm~l
NMR (DMSO-d6 ~) : 2.42 (6H, s), 2.46 (3H, s), 4.43
(2H, s), 4.46 (2H, s), 6.89 (lH, s), 6.9-7.4
(13H, m), 8.26 (lH, s)
A~CI-MASS (m/z) : 526 (M+H~)
(3) 1-Cycloheptyl-1-(4-phenoxybenzyl)-3-[2,a-
bis(methylthio)-6-methylpyridin-3-yl]urea
IR (KBr) : 3371, 2922, 2856, 1653, 1485, 1219 cm~
NMR (CDC13, o) : 1.35-2.10 (12H, m), 2.36 (3H, s),
2.45 (3H, s), 2.46 (3H, s), 4.22-4.42 (lH, m),
4.55 (2H, s), 5.49 (lH, s), 6.59 (lH, s), 6.95-
7.15 (5H, m), 7.24-7.46 (4H, m)
APCI-MASS (m/z) : 522 (M+~+)
(4) 1-Cycloheptyl-1-[4-(4-bromophenoxy)benzyl]-3-[2,4-
- bis(methylthio)-6-methylpyridin-3-yl]ure2
IR (KBr) : 3377, 2924, 2852, 1668, 1481, 1238 cm 1
NMR (CDC13, o) : 1.40-2.10 (12H, m), 2.37 (3H, s),
2.46 (3H, s), 2.47 (3H, s), 4.25-4.40 (lH, m),
4.55 (2H, s), 5.47 (lH, s), 6.60 (lH, s), 6.80-
7.08 (4H, m), 7.35-7.50 (4H, m)
APCI-MASS (m/z) : 600, 602 (M+H )
(5) 1-Benzyl-1-[4-(4-bromophenoxy)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]ure2
IR (KBr) : 3200-3700 (br), 2922, 1662, 1564, 1481,
1236 cm~l
NMR (CDCl3, o) : 2.39 (3H, s), 2.47 (3H, s), 2.49
(3H, s), 4.61 (2H, s), 4.63 (2H, s), 5.68 (lH,
W096/lOSS9 PCT/~5/01982
~0~8 ~ ~
- 168 -
s), 6.62 (lH, s), 6.82-7.05 (4H, m), 7.25-7.50
(9H, m)
APCI-~S (m/z) : 594, 596 (M+~:+)
(6j 1-Cycloheptyl-1-[4-(4-bromophenoxy)benzyl]-3-[2,4-
dimethoxy-6-methylpyridin-3-yl]urea
IR (KBr) : 3100-3700 (br), 2925, 2856, 1668, 1597,
1504, 1481, 1240 cm~1
~R (CDCl3, o) : 1.40-2.10 (12H, m), 2.38 (3H, s),
iO 3.79 (3H, s), 3.83 (3H, s), 4.25-4.40 (lH, m),
4.52 (2H, s), 5.43 (lH, s), 6.36 (1~, s), 6.82-
7.06 (4H, m), 7.32-7.50 (4H, m)
APCI-MASS (m/z) : 568, 570 (M+~+)
(7) 1-Benzyl-1-[4-(4-bromophenoxy)benzyl]-3-[2,4-
dimethoxy-6-methylpyridin-3-yl]urea
I~ (KBr) : 3200-3400 (br), 2997, 1637, 1595, 1506,
1365 cm~l
NMR (CDCl3, o) : 2.39 (3H, s), 3.80 (3~, s), 3.85
(3H, s), 4.60 (4H, s), 5.6~ (lH, s), 6.38 (lH,
s), 6.80-7.05 (4H, m), 7.22-7.50 (8~, m)
APCI-MASS (m/z) : 562, 564 (M+H+)
Example 12
To a mixture of 1-cycloheptyl-1-~3-(1-lri,ylpyrazol-3-
yl)benzyl]-3-(2,4,6-trifluorophenyl)urea (17.6 g) and
anisole (35 ml) was added trifluoroacetic acid (70 ml).
The mixture was stirred at 60C for 3 hours anà cooled to
room temperature. The excess trifluoroacetic acid was
removed in vacuo. To the residue was added water and ethyl
ace_at2. The mixture was basi~ied with 5N-sodium hydroxide
under ice cooling and extracted with ethyl acetate. The
organic layer was washed with water, and brine, dried over
magnesium sulfate and evaporated in vacuo. Tne residue was
purified by coLumn chromatography on silica gel (530 g,
WO96/10559 ~2Q ~ PCT/~5/01982
- 169 -
eluting with n-hexane - ethyl acetate (2:1 to 1:2)) to give
1-cycloheptyl-1-[3-(pyrazol-3-yl)benzy']-3-(2,4,6-
trifluorophenyl)urea (10.71 g).
IR (KBr) : 3500-2600 (br), 2927, 2858, i635, 1520,
1448, 1248, 1120 cm~1
NMR (CDCl3, o) : 1.30-2.10 (12H, m), 4.26-4.46 (lH,
m), 4.59 (2H, s), 5.63 (lH, s), 6.53-6.73 (3H,
m), 7.30-7.50 (2H, m), 7.63 (lH, d, J=2.3Hz),
7.65-7.80 (2H, m)
iO APCI-MASS (m/z) : 443 (M+H )
~xamle 13
To a solution of N-cycloheptyl-4-(4-fluorophenoxy)-
benzylamine (1.57 g) in toluene (100 ml) were added 3-
phenoxycarbonylamino-2,4,6-trimethylpyridine (2.56 g) and
triethylamine (1.52 g), and the mixture was refluxed ror 3
hours under nitrogen. The mixture was cooled and pourea
into a mixture of ethyl acetate and water. ~he separated
organic layer was washed with brine, dried ove~ magnesium
sulfate and evaporated in vacuo. The residue was purified
by column chromatography on silica gel to give 1-
cycloheptyl-1-L4-(4-fluorophenoxy)benzyl]-3-(2,4,6-
trimethylpyridin-3-yl)urea (1.83 g).
IR (KBr) : 3313, 2924, 2856, 1630, 1603, 1497 cm 1
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.06 (3H, s),
2.24 (3H, s), 4.05-4.25 (lH, m), 4.48 (2H, s),
6.98 (1~., s), 6.9-7.1 (4H, m), 7.2-7.4 (4H, m),
7.66 (lH, s)
APCI-M~SS (m/z) : 476 (M+H+)
~xample 14
To a solution of N-cycloheptyl-4-(4-fluorophenoxy)-
benzylamine (2.51 g) in toluene (120 ml) were added 4-
chloro-6-methyl-2-methylthio-3-phenoxycarbonylaminopyridine
(2.47 g) and triethylamine (2.43 g) at room temperature and
W096/10559 ~ ~ ~ 9 8 1 PCTl~5/01982
- 170 -
the mixture was refluxed for 2.5 hours under nitrogen. The
mixture was poured into a mixture of ethyl acetate and ice
water and the separated organic layer was washed with
brine, dried over magnesium sul ate and evaporate~ in
vacuo. The residue was purified by column chrom~tography
on silica gel to give 1-cycloheptyl-1-[4-(4-fllorophenoxy)-
benzyl]-3-(2-chloro-6-methyl-4-methylthiopyridin-3-yl)urea
(2.76 g).
IR (K3r) : 3371, 3299, 2924, 2852, 1655, 1576,
1500 cm~l
NMR (DMSO-d6, o) : 1.3-1.8 (12H, m), 2.43 (6H, s),
4.0-4.2 (lH, m), 4.46 ~2H, s), 6.9-7.5 (9H, m),
8.07 (lH, br s)
~xample 15
To a solution of N-benzyl-3-(pyrazol-3-yl)benzylamine
(54.0 g) and triethylamine (143 ml) in toluene (1.35 ~) was
added 2,4-bis(methvlthio)-3-phenoxycarbonylamino-6-
methylpyridine (62.4 g) at room tem~erature ard stirred for
24 hours. The resulting preci~itale was collected by
~iltration and recrystallized from dichloromethane -
~ethanol - n-hexane to give 1-benzyl-1-~3-(pyra701-3-
yl)benzyl]-3-[2,4-bis(methylthio)-6-methylpyridin-3-yl]urea
(51.0 g).
mp : 2Q9-210~C
IR (KBr) : 3392, 3246, 2918, 1549, 1489, 1228,
1093 cm-1
NMR (DMSO-d6, o) : 2.42 (6H, s), 2.47 (3H, s), 4.49
(4H, s), 6.66 (lH, br s), 6.90 (lH, s), 7.18-7.90
(lOH, m), 8.30 (lH, s), 12.89, 13.30 (total lH,
each b~)
APCI-MPSS (m/~) : 490 (M+~+)
~xample 16
To a solution of N-benzyl-~4-~4-bromophenoxy)benzyl]-
a~a ~ 8 ~ pCTl~5/01982
WO96110559
- 171 - -
amine (1.84 g) and 2,4,6-trimethylphenyl-3-
phenoxycarbonylaminopyridine (2.20 g) in N,N-
dimethylformamide (5C ml) was added triethylamine (2.53 g),
and the mixture was stirred at 15QC for 3 hours under
nitrogen. The mixture was cooled and ethyl acetate (150
ml) was added thereto. The insoluble materials were
filtered off, and the filtrate was washed with water and
brine, dried over-magnesium sulfate and evaporated in
vacuo. The residue was purified by column chromatography
on silica gel to give 1-benzyl-1-[4-(4-bromophenoxy)-
benzyl]-3-(2,4,6-trimethylpyridin-3-yl)urea (2.51 g).
IR (KBr) : 3406, 3313, 2929, 2856, 1714, 1632, 1572,
1495 cm~1
NMR (DMSO-d6, o) : 2.08 (3H, s), 2.26 (3H, s), 2.35
(3H, s), 4.53 (2H, s), 4.57 (2H, s), 6.95-7.15
(5H, m), 7.3-7.6 (9H, m), 8.05 (lH, br s)
APCI-MASS (m/z) : 531 (M+H+)
Fxample 17
To a solution of N-cycloheptyl-4-(4-fluorophenoxy)-
benzylamine (1.25 g) in toluene (80 ml) were added 4,6-
bis(methylthio)-2-methyl-5-phenoxycarbonylaminopyrimidine
(1.29 g) and triethylamine (1.21 g), and the mixture was
refluxed for 2 hours under nitrogen. The mixtu~e was
poured into a mixture of ethyl acetate and ice water, and
the separated organic layer was washed with brine, dried
over magnesium sulfate and evaporated in vacuo. The
residue was purified by column chromatography on silica gel
to give 1-cycloheptyl-1-[4-(4-fluorophenoxy)ben 7yl ] - 3-~4,6-
bis(methylthio)-2-methylpyrimidir-5-yl]urea (1.33 g).
IR (KBr) : 3255, 2926, 2856, 1653, 1522, 1497 cm 1
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.43 (6H, s),
2.56 (3H, s), 3.95-4.1 (lH, m), 4.46 (2H, s),
6.9-7.4 (8H, m), 8.00 (lH, br s~
APCI-MASS (m/z) : 529 (M+H+)
PCTl~95/01982
WO96110559
- 172 -
Example 18
To a solution of 1-cycloheptyl-1-[4-(3,5-di-tert-
butyl-4-methoxymethoxyphenoxy)]benzyl-3-(2,4,6-
trimethylphenyl)urea (860 mg) in methanol (8.6 ml) was
added conc. hydrochloric acid (0-91 ml), and the mixture
was stirred at room temperature for 2 hours and at 40C for
3.5 hours. The mixture was poured into a mixture of ethyl
acetate and ice water, and neutralized by additior of
saturated sodium bicarbonate aqueous solution. The
separated organic layer was washed with water and brine,
dried over magnesium sulfate and evaporated ir vacuo. The
residue was purified by column chromatography on silica gel
to give 1-cycloheptyl-1-[4-(3,5-di-tert-butyl-4-
hydroxyphenoxy)benzyl]-3-(2,4,6-trimethylphenyl)urea (495
mg).
IR (KBr) : 3639, 3404, 3323, 295O, 2923, 2860, 1651,
1593, 1504 cm~1
NMR (CDCl3, o) : 1.41 (18H, s), i.5-2.1 (12H, m),
1.98 (6H, s), 2.22 (3H, s), 4.25-4.4 !lH, m),
4.45 (2H, s), 5.03 (lH, s), 6.80 (2H, s), o.86
(2H, s), 6.93 (2H, d, J=8.5Hz~, 7.3_ (2H, d,
J=8.5Hz)
APCI-MASS (m/z) : 585 (M+H+)
Example 19
To a solution of 1-cycloheptyl-1-[4-(4-
fluorophenoxy)benzyl]-3-[2,4-bis(methylthio)-6-
methylpyridin-3-yl]urea (22.11 g) in dichloromethane (150
ml) W2S added dropwise a solution of m--hloroperbenzoic
ac-d (26.51 g) in dichloromethane (600 mg) at room
temperature over 2 hours. The mixture was stirred at room
temperature for 23 hours. The precipitates were removed by
ril~ration and the filtrate was washed with dilute sodium
bicarbonate aqueous soiution and brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
WO96/10559 2 2 ~ ~ ~ 8 ~ PCT/~5/01982
- 173 -
pu~ified by column chromatography on silica gel to give 1-
cycioheptyl-1-[4-(4-fluorophenoxy)benzyl]-3-[2,4-
bis(methylsulfonyl)-6-methylpyridin-3-yl]urea (20.42 g).
IR (KBr) : 3361, 3074, 3041, 3016, 2927, 2860, 1740,
1664, 1500, 1325, 1153, 1128 cm~1
NMR (CDCl3, o) : 1.5-2.2 (12H, mj, 2.66 (3H, s), 3.19
(3H, s), 3.30 (3H, s~, 4.55 (2H, s), 6.95-7.05
(6H, m), 7.34 (2H, d, J-8.6Hz) 7.26 (lH, s), 7.85
(lH, s)
~CI-MASS (m/z) : 604 (M+H+)
.
Example 20
To a solution of 1-cycloheptyl-1-[4-(4-
fluo~ophenoxy)benzyl]-3-[2,4-bis(methylthio)-6-
methylpyridin-3-yl]urea (4.75 g) in dichloromethane (50 ml)
was added dropwise a solution of m-chloroperbenzoic acid
(3.96 g) in dichloromethane (80 ml) at room temperature.
The mixture was stirred at room temperature for 20 hours.
The mixture was washed with dilute sodium bicarbonate
aq~eous solution and brine, dried over magnesium sulfate
and evaporated in vacuo. The residue was purified by
column chromatography on silica gel to give 1-cycloheptyl-
1-[4-(4-fluorophenoxy)benzyl]-3-[2,4-bis(methylsulfinyl)-6-
methylpyridin-3-yl]urea (2.15 g).
IR (KBr) : 3251, 2927, 2858, 173~, 1651, 1498, 1055,
1036 cm~1
NMR (CDCl3, o) : 1.4-2.0 (12H, m), 2.59 (3H, s), 2.82
and 2.94 (total 3H, s), 2.98 (3H, s), 4.0-4.2
(2H, m), 4.51 (2H, br s), 6.9-7.1 (7H, m), 7.25-
7.35 (2H, m), 7.77-7.79 (total lH, s)
APCI-MASS (m/z) : 572 (M+H+)
Example 21
To a suspension of 1-cycloheptyl-1-[3-(1-
tri~ylpyrazol-4-yl)benzyl]-3-(2,4,6-trimethylphenyl)l~re2
~ ~ n PCT/~5/01982
W096/lOS59 ~ ~ U as 8 1
- 174 -
(800 mg) in anisole (2 ml) was adaed trifluoroacetlc acid
(6 ml) and the mixture was stirred at 100C for 2 hours.
The mixture was evaporated in vacuo and poured into a
mixture of ethyl acetate and water and adjusted to pH ca 9
by addition of sodium hydroxide aqueous solution. The
separated organic layer was washed with brine, dried over
magnesium sulfate and evaporated in vacuo. The residue was
purified by column chromatography on silica gel to give 1-
cycloheptyl~ 3-(pyrazol-4-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea (102 mg).
IR (KBr) : 340G, 3207, 2926, 2856, 1635, 1608,
1510 cm~l
N~R (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.08 (6H, s),
2.20 (3H, s), 4.1-4.3 (lH, m), 4.51 (2H, s), 6.83
(2H, s), 7.1-7.5 (5H, m), 7.8g (lH, s), 8.11 (lH,
s), 12.95 (lH, br s)
APCI-MASS (m/z) : 431 (M+H+)
~x~ple 22
The following compounds were obtained according to a
similar manner to that of Example 1, 2, 3 or 4.
(1) 1-Cycloheptyl-1-~4-(4-chlorophenoxy)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3410, 2920, 2850, 1660, 1590, 1505,
1485 cm~l
NMR (CDCl3, o) : 1.5-2.1 (12H, m), 2.00 (6H, s), 2.22
(3H, s), 4.3-4.45 (lH, m), 4.48 (2H, s), 5.6-5.8
(lH, br~, 6.81 (2H, s), 6.92 (2H, d, J=8.5Hz),
7.00 (2H, d, J=8.5Hz), 7.28 (2H, d, J=8.4Hz),
7.38 (2H, d, J=8.4Hz)
(2) 1-Cycloheptyl-1-[4-(3-fluorophenoXy)benzyl~-3-(2,4,6-
trimethylphenyl)ure~
mp : 127-128C
PCT1~95/01982
~WO96110559 22a 1~9 8 1
- 175 -
IR (KBr) : 29Z4, 2856, 1624, 1605, 1506, 1485 cm~
NMR (CDCl3, o) : 1.35-2.10 (12H, m), 2.01 (6H, s),
2.22 (3H, s), 4.30-4.50 (1~, m), 4.50 (2H, s),
5.46 (lH, s), 6.60-6.88 (3H, m), 6.79 (2H, s),
7.00-7.10 (2H, m), 7.20-7.35 (lH, m), 7.36-7.47
(2H, m)
APCI-MASS (m/z) : 475 (M+H )
(3) 1-Cycloheptyl-1-[4-(4-trifluoromethylphenoxy)benzyi~-
3-(2,4,6-trimethylphenyl)urea
mp : 146-147C
IR (KBr) : 2924, 2856, 1628, 1504, 1327, 1246 cm 1
NMR (CDCl3, o) : 1.40-2.10 (12H, m), 2.03 (6H, s),
2.23 (3H, s), 4.30-4.~G (lH, m), 4.51 (2H, s),
5.47 (lH, s), 6.83 (2H, s), 6.95-7.13 (4H, m),
7.35-7.50 (2H, m), 7.53-7.65 (2H, m~
APCI-MASS (m/z) : 525 (M+H+)
(4) 1-Cycloheptyl-1-[4-(3,4-metrylenediGxyphenoxy)benzyl]-
3-(2,4,6-trimethylphenyl)urea
mp : 125-126C
IR (KBri : 3323, 2922, 2854, 1628, 1506, 1481 cm~
NMR (CDCl3, o) : 1.38-2.10 (12H, m), 1.99 (6H, s),
2.22 (3H, s), 4.33-4.50 (lH, m), 4.46 (2H, s),
5.46 (lH, s), 5.98 (2H, s), 6.47 (lH, dd, J=8.3,
2.4Hz), 6.56 (lH, d, J=2.4Hz), 6.76 (lH, d,
J=8.3Hz), 6.81 (2H, s), 6.90-7.00 (2H, m), 7.28-
7.38 (2H, m)
APCI-MASS (m/z) : 501 (M+H+)
(5) i-Cycloheptyl-l- E 4-(3,5-di-tert-butyl-4-
methoxymethoxyphenoxy)]benzyl-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3406, 3323, 2956, 2924, 2862, 1641, 1589,
1504 cm
PCT/~5/01982
W096/lOS59 2~ V O 9 8 ~ ~
- 176 -
NMR (CDCl3, o) : 1.41 (18H, s), 1.4-2.2 (14H, m),
1.99 (6H, s), 2.22 (3H, s), 3.62 and 3.65 (total
3H, s), 4.3-4.5 (lH, m), 4.46 (2H, s), 4.86 and
4.92 (total 2H, s), 6.80 (2H, s), 6.35-7.1 (4H,
m), 7.4-7.5 (2H, m)
(6) 1-Cycloheptyl-1-[4-(4-fluorophenoxy)phenyl]-3-(2,4,6-
tri~ethylphenyl)urea
IR (KBr) : 3425, 2925, 2860, 1670, 1610, 1500 cm 1
NMR (C3Cl3, o) : 1.3-1.7 and 1.9-2.1 (12H, m), 2.12
(6H, s), 2.22 (3H, s), 4.45-4.65 (lH, m), 5.30
(lH, br s), 6.82 (2H, s), 7.0-7.3 t8H, m)
APCI-~ASS ~m/z) : 461 (~+H+)
(7) 1-Benzyl-1-[4-(4-fluorophenoxy)benzyl~-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3307, 3062, 3030, 2918, 1633, 1608, 1510,
1497 cm
~MR (CDCl3, ~) : 2.00 (6H, s), 2.22 (3H, s), 4.62
2G (4H, s), 5.68 (lH, s), 6.82 (2H, s), 6.9-7.1 (6H,
m), 7.3-7.45 (7H, m)
APCI-MASS (m/z) : 469 (M+H+)
(8) 1-Pentyl-1-[4-(4-fluorophenoxy)benzyl]-3-(2,4,6-
tri~ethylphenyl)urea
IR (KBr) : 3292, 2958, 2920, 2856, 1632, 1608,
1498 cm~1
~R (CDCi3, o) : 0.90 (3H, t, J=6.3Hz), 1.25-1.45
(4H, m), 1.6-1.8 (2H, m~, 2.09 (6H, s), 2.30 (3H,
s), 3.39 (2H, t, J=7.4Hz), 4.55 (2H, s), 5.74
(lH, br s), 6.84 (2H, s), 6.9-7.1 (6H, m), 7.30
(2H, d, J=8.4Hz)
APCI-MASS (m/z) : 449 (M+H+)
(9) 1-Cyclohexyl-1-r4-(4-fluorophenoxy)benzyl]-3-(2,4,6-
PCT/~5/01982
~ W096/10~59 ~ 2 ~
- 177 -
trimethylphenyl)urea
IR (KBr) : 3296, 2958, 2922, 2890, i624, 1520,
1487 cm~1
NMR (CDC13, o) : 1.3-2.0 (lOH, m), ;.99 (6H, s), 2.22
- 5 (3H, s), g.25-4.45 (lH, m), 4 47 (2H, s), 5.54
(lH, br s), 6.81 (2H, s), 6.9-7.1 i6H, m), 7.35
(2H, d, J=8.5Hz)
APCI-MASS (m/z) : 461 (M+H+)
.
(lQ) 1-Cyclopentyl-1-[4-(4-fluorophenoxy)benzyl]-3-(2,4,6-
trlmethylphenyl)urea
IR (KBr) : 3400, 3304, 3074, 2933, 2850, 1657, 1608,
1495 cm~1
NMR (CDCl3, o) : 1.5-1.8 and 2.0-2.15 (8H, m), 2.00
(6H, s), 2.22 (3H, s), 4.47 (2H, s), 4.7-4.9 (lH,
m), 5.35 (lH, br s), 6.82 (2H, s), 6.9-7.1 (6H,
m), 7.33 (2H, d, J=8.5Hz)
APCI-MASS (m/z) : 447 (M+H+)
(11) 1-Cycloheptyl-1-[4-(4-fluorophenoxv)benzyl]-3-(2,4,6-
trifluorophenyl)urea
IR (KBr) : 3284, 2929, 2858, 1633, 1612, 1518,
1497 cm~l
NMR (CDCl3, o) : 1.4-2.1 (12H, m), 4.25-4.45 (lH,
m), 4.50 (2H, s), 5.58 (lH, s), 6.55-6.7 (2H, m),
6.9-7.1 (6H, m), 7.25-7.4 (2H, m)
APCI-~SS (m/z) : 487 (M+H+)
(12) 1-Benzyl-1-r3-(pyrazol-3-yl)benzyl]-3-(2,4,6-
~ 30 trimethylphenyl)urea
IR (KBr) : 3404, 3207, 3060, 3029, 2967, 2918, 2858,
1635, 1608, 1510 cm~1
NMR (DMSO-d6, o) : 2.09 (6H, s), 2.21 (3H, s), 4.57
(2H, s), 6.0-6.05 (lH, m), 6.84 (2H, s), 7.2-7.5
(7H, m), 7.65-7.8 (3H, m), 7.87 (lH, s), 12.89
PCT/~510198~ _
W096/10559 22 ~ ~ ~ 8 ~
- 178 -
(lH, br)
APCI-MASS (m/z) : 425 (M+H+)
(13) i-Benzyl-1-[3-(pyrazol-3-yl~benzyl]-3-(2,4,6-
tvifluorophenyl)urea
IR (KBr) : 3246, 1637, 1522 cm 1
NMR (DMSO-d6, o) : 4.54 (4H, s), 6.65 (lH, br s),
7.2-7.5 (4H, m), 7.7-7.9 (3H, m), 8.47 (lH, br
s), 12.90 and 13.34 (total lH, br s)
1 0 APCI -MASS (m/z) : 437 (M+H+)
(14) 1-Cycloheptyl-1-~3-(pyrazol-3-yl)benzyl]-3-(2,4,6-
trifluorophenyl)urea
IR (KBr) : 3226, 3062, 2927, 2858, 1635, 1612,
1518 cm~1
NMR (DMSO-d6, ~) : 1.4-1.9 (12H, m), 4.0-4.2 (lH, m),
4.55 (2H, s), 6.63 (lH, d, J=1.9Hz), 7.15-7.5
(4H, m), 7.6-7.8 (3H, m), 8.10 (lH, br s)
APCI-MASS (m/z) : 444 (M+H+~
(15) 1-Cyclohexyl-1-[3-(pyrazol-3-yl)benzyl~-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3226, 2929, 2856, 1635, 1608, 1510 cm~
NMR (DMSO-d6, o) : 1.3-1.8 (lOH, m), 2.08 (6H, s),
2.20 (3H, s), 4.0-4.2 (lH, m), 4.57 (2H, s), 6.62
(lH, br s), 6.83 (2H, s), 7.2-7.45 (2H, m), 7.55-
7.85 (3H, m), 12.86 (lH, br s)
APCI-MASS (m/z) : 417 (M+H+)
(16) 1-Cyclopentyl-1-[3-(pyrazol-3-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3188, 2956, 2870, i635, 1608, 1510 cm -
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.08 (6H, s),
2.20 (3H, s), 4.45-4.6 (lH, m), 4.56 (2H, s),
6.63 (lH, br s), 6.83 (2H, s), 7.15-7.45 (2H, m),
~ ~ ~ PCT1~5/01982
~ WO96110559 ~ 2 ~
- 179 -
7.55-7.85 (5H, m), 12.87 (lH, br s
APCI-MASS (m/z) : 403 ~M+Ht)
.
(17) 1-Cycloheptyl-1-[3-(1-tritylpyrazol-4-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3408, 3323, 3059, 303C, 2924, 2856, 1645,
1608, 1562 cm~1
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.00 (6H, s),
2.20 (3H, s), 4.0-4.2 (lH, m), 4.48 (2H, s), 6.80
(2H, s), 7.1-7.5 (19H, m), 7.70 (1:~:, s), 8.02
(lH, s)
APCI-~ASS (m/z) : 673 (M+H+)
(i8) 1-Cycloheptyl-1-[4-(1-tritylpyrazol-4-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3406, 3323, 3057, 3030, 2924, 2854, 1640,
1568 cm~1
NMR (DMSO-d6, o) : 1.40-2.0 (12H, m), 2.08 (6H, sj,
2.20 (3~, s), 4.05-4.25 (lH, m), 4.47 (2H, s),
6.83 (2H, s), 7.05-7.15 ~5H, m), 7.25 (2H, d,
J=8.2Hz), 7.3-7.4 (llH, m), 7.45 (2H, d,
J=8.2Hz), 7.78 (lH, s), 8.07 ;'H, s)
APCI-MASS (m/z) : 673 (Mt~+)
(19) 1-Cycloheptyl-1-[3-(1-methylpyrazo~-4-yl)benzyl~-3-
(2,4,6-trimethyl~henyl)urea
IR (KBr) : 3408, 2924, 2856, 1637, 161C, 1497,
1234 cm 1
~MR (CDCl3, ~) : 1.38-2.10 (12H, m), 1.98 (6H, s),
~ 30 2.20 (3H, s), 3.95 (3H, s), 4.36-4.56 (iH, m),
4.52 (2H, s), 5.48 (;H, s), 6.78 (2H, s), 7.20-
7.52 (4H, m), 7.62 !lH, s), 7.75 (1~, s)
APCI-MASS (m/z) : 445 (M~H+)
(20) 1-Cycloheptyl-1-[3-(1-methyl~yraZol-3-yl)benzyl]-3-
W096/lOS59 ~ 8 ~ PCT/~5/01982
- 80 -
(2,4,6-trimethylphenyl)~rea
~p : 142-143C
1~ lYBr) : 3346, 2924, 2854, 163G, 1502, 1246 c~-
~R (CDC13, o) : 1.35-2.1G (12H, m), 1.9- (6~, s),
2.19 (3H, s), 3.95 (3H, s), ~.38-4.58 (ln, m),
4.55 (2n, s), 5.49 (i-, s), 6.33 (lH, d,
J=2.2H7), 6 77 ~2H, si, ?.30-7.50 (3T, ~), 7.65-
7.88 (2H, ~)
APCI-~SS (m/z) : 445 (M+i+)
(21) 1-Cycloheptyl-1-[3-(1-~ethylDyrazol-5-yl)benzyi]-3-
(2,4,6-trimetnylphenyi)ure~
mp : 171-172C
I~ (KB~) : 3307, 2924, 2855, 1620, 1506, 1254 cm 1
l~R (CDCl3, o) : 1.38-2.10 (12:~, m), 2.CC (6H, s),
2.21 (3H, s), 3.89 (3~, s), 4.30-4.50 (ln, m),
4.57 (2X, s), 5.46 (lH, s), 5.29 (1~, d,
J=1.9Hz), 6.80 (2H, s), 7.25-7.56 (5.I, m)
APCI-MASS (m/z) : 445 (M 'i+)
2~
(22) 1-Cvcloheptyi-1-'3-(imidazol-4-yl)benzyl]-3-(2,4,6-
trimethylphenyl)ure2
IR (KBr) : 3140 (br), 2924, 2856, 1635, 1608, iag7 cm~-
NMR (DMSO-d6, o) : 1.25-1.90 (12H, m), 2.07 (6k, s,,
2.20 (3H, s), 4.07-~.27 (lH, ~), 4. 52 (2H, s),
6.82 (2H, s), 7.08-7.80 (7~;, m), 12.13, 12.53
~total lH, each br)
APCI-~SS (m/z) : 431 (M+H+)
(23) 1-Cvcloheptyl-1-[4-(5-methyl-1,3,4-oxadiazol-2-
yl)benzyl~-3-(2,4,6-trimethylphenyl)ure~
~Lp : 123 124 C
IR (KBr) : 3319, 2924, 2856, 1622, 1500, 1248 cm~
~M~ ,'C3C13, ~ .35-2.10 (12~, ~), 2.0~ (o.-, s),
2.22 (3H, s), 2.62 (3H, s), 4.20-4.40 (l~, m),
.. . . . . . . . . . . . .
PCT/~5/01982
~ w096/los~9 ~2~98 ~
- 181 -
4.58 (2H, s), 5.58 (lH, s), 6.81 (2H, s), 7.49-
7.59 (2H, m), 7.98-8.08 (2H, m)
APCI-MASS (m/z) : 447 (M+H+)
(24) 1-Cycloheptyl-l-[4-(5-methyl-4H-1,2,4-triazol-3-
yl)benzyl]-3-(2,4,6-trimethylphenyl)urea
mp : 142-145C
IR (KBr) : 2600-3700 (br), 2924, 2856, 1633, 1608,
1558, 1504, 1238 cm~1
NMR (CDCl3, o) : 1.38-2.15 (12H, m), 1.90 (6H, s),
2.27 (3H, s), 2.16 (3H, s), 4.37-4.57 (lH, m),
4.58 (2H, s), 5.59 (lH, s), 6.71 (2H, s), 7.45-
7.57 (2H, m), 8.05-8.17 (2H, m)
APCI-MASS (m/z) : 446 (M+H+)
(25) 1-Cycloheptyl-1-[4-(4-benzyl-5-methyl-4H-1,2,4-
triazol-3-yl)benzyl]-3-(2,4,6-trimethylphenyl)urea
mp : 193-194C
IR (KBr) : 3296, 2924, 2856, 1626, 1506, 1252,
847 cm~1
NMR (CDCl3, o) : 1.35-2.05 (12H, m), 2.00 (6H, s),
2.21 (3H, s), 2.39 (3H, s), 4.20-4.40 (lH, m),
4.55 (2H, s), 5.15 (2H, s), 5.43 (lH, s), 6.80
(2H, s), 6.90-7.05 (2H, m), 7.30-7.60 (7H, m)
APCI-MASS (m/z) : 536 (M+H+)
(26) 1-Cycloheptyl-1-[3-(2-methyl-2H-tetra2O1-5-yl)benzyl]-
3-(2,4,6-trimethylphenyl)urea
mp : 175-176C
~ 30 IR (KBr) : 3327, 2922, 2856, 1628, 1500, 1255 cm~1
NMR (DMSO-d6, o) : 1.30-l.90 (12H, m), 2.09 (6H, s),
2.20 (3H, s), 4.12-4.30 (lH, m), 4.42 (3H, s),
4.59 (2H, s), 6.83 (2H, s), 7.40-7.65 (3H, m),
7.85-7.95 (lH, m), 8.06 (lH, s)
APCI-MASS (m/z) : 447 (M+H+)
.
PCT/JP95/01982
W096/lOSS9
- 182 -
(27) 1-Cycloheptyl-1-[3-(1-methyl-lH-tetrazol-5-yl)benzyl~-
3-(2,4,6-trimethylphenyl)urea
mp : 171-173C
IR (KBr) : 3323, 2924, 2854, 1626, 1502, 1444,
1254 cm~
NMR (DMSO-d6, ~) : 1.40-1.90 (12H, m), 2.06 (6H, s),
2.20 (3H, s), 4.16 (3H, s), 4.10-4.28 (lH, m),
4.59 (2H, s), 6.83 (2H, s), 7.54-7.80 (5H, m)
APCI-MASS (m/z) : 447 (M+H+)
(28) 1-Cycloheptyl-1-[4-(1,2,4-lH-triazol-1-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3310, 2924, 2856, 1639, 1518, 1277,
1147 cm~1
NMR (CDC13, ~) : 1.40-2.10 (12H, m), 2.07 (6H, s),
2.22 (3H, s), 4.20-4.40 (lH, m), 4.58 (2H, s),
5.49 (lH, s), 6.82 (2H, s), 7.50-7.60 (2H, m),
7.64-7.74 (2H, m), 8.11 (lH, s), 8.55 (lH, s)
APCI-MASS (m/z) : 432 (M+H+)
(29) 1-Cycloheptyl-l-[4-(1,2,3-lH-triazol-1-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3331, 2924, 2856, 1637, 1498, 1319, 1234,
1034 cm~1
NMR (CDCl3, o) : 1.40-2.10 (12H, m), 2.07 (6H, s),
2.22 (3H, s), 4.20-4.38 (lH, m), 4.60 (2H, s),
5.55 (lH, s), 6.83 (2H, s), 7.52-7.61 (2H, m),
7.70-7.80 (2H, m), 7.86 (lH, s), 8.00 (lH, s)
APCI-MASS (m/z) : 432 (M+H+)
(30) l-Cycloheptyl-l-[4-(2H-1,2,3-triazol-2-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
mp : 157-158C
IR (KBr) : 3311, 2924, 2856, 1626, 1512, 1255, 955,
847 cm~1
-
A A ~ ~ PCT/~5/01982
WO96/10559 ~ ~ V U ~ 8
- 183 -
NMR (CDC13, o) : 1.40-2.10 (12H, m), 2.04 (6H, s),
2.21 (3H, s), 4.25-4.45 (lH, m), 4.57 (2H, s),
5.51 (lH, s), 6.81 (2H, s), 7.48-7.58 (2H, m),
7.82 (2H, s), 8.04-8.14 (2H, m)
J , 5 APCI-MASS (m/z) : 432 (M+H+)
(31) 1-Cycloheptyl-1-[4-(4-methylpiperazin-1-yl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3390, 3335, 2925, 2855, 2795, 2360, 1645,
101610, 1515 cm~l
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.05 (6H, s),
2.20 (6H, s), 2.4-2.5 (4H, m), 3.05-3.15 (4H, m),
4.0-4.2 (lH, m), 4.39 (2H, s), 6.82 (2H, s), 6.88
(2H, d, J=8.5Hz), 7.16 (2H, d, J=8.5Hz), 7.34
15 (lH, br s)
APCI-MASS (m/z) : 463 (M+H+)
(32) 1-Cycloheptyl-1-[4-(4-methylsulfonylaminophenyl)-
benzyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3400, 3340, 2975, 2925, 2860, 1640,
1500 cm-l
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.08 (6H, s),
2.20 (3H, s), 3.01 (3H, s), 4.1-4.3 (lH, m), 4.53
(2H, s), 6.83 (2H, s), 7.27 (2H, d, J=8.4Hz),
7.37 (2H, d, J=8.4Hz), 7.53 (lH, br s), 7.55-7.7
(4H, m), 9.82 (lH, s)
APCI-MASS (m/z) : 534 (M+H+)
(33) 1-Cycloheptyl-1-[4-[2-(1-trityl-lH-tetrazol-5-
~ 30yl)phenyl]benzyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3407, 3058, 3026, 2924, 2856, 1647, 1608,
1493 cm~
NMR (DMSO-d6, o) : 1.4-1.8 (12H, m), 2.04 (6H, s),
2.20 (3H, s), 4.05-4.~5 (lH, m), 4.48 (2H, s),
6.83 (2H, s), 7.04 (2H, d, J=7.9Hz), 7.23 (2H, d,
PCTI~5/01982
W096/105S9 ~ ~ O ~ ~ %
- 184 -
J=7.9Hz), 7.5-7.8 (5H, m)
FAB-MASS (m/z) : 751 (M+H+)
(34) 1-Cycloheptyl-1-~4-(N-benzoylsulfamoyl)benzyl]-3-
(2,4,6-trimethylphenyl)urea
IR (KBr) : 3915, 3361, 2924, 2858, 1632, 1593,
1549 cm~1
NMR (DMSO-d6, o) : 1.4-2.0 (12H, m), 2.07 (6H, s),
2.20 (3H, s), 4.1-4.3 (lH, m), 4.51 (2H, s), 6.81
(2H, s), 7.2-7.4 (5H, m), 7.53 (lH, br s), 7.76
(2H, d, J=8.OHz), 7.88 (2H, d, J=8.OHz)
~PCI-MASS (m/z) : 548 (M+H+)
(35) 1-Cycloheptyl-1-~4-(N-phenylsulfonylcarbamoyl~benzyl]-
3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3380, 3290, 3055, 2920, 2855, 1690, 1625,
1610, 1505 cm~1
NMR (DMSO-d6, ~) : 1.3-1.8 (12H, m), 2.07 (6H, s),
2.21 (3H, s), 4.1-4.25 (lH, m), 4.53 (2H, s),
6.83 (2H, s), 7.38 (2H, d, J=8.2Hz), 7.65-7.8
(4H, m), 7.82 (2H, d, J=8.2H~), 8.00 (2H, d,
J=6.7Hz)
APCI-MASS (m/z) : 548 (M+H+)
(36) 1-Cycloheptyl-1-[4-(3-pyridylmethyl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3412, 3304, 3028, 292Q, 2854, 1626,
1502 cm~1
NMR (CDCl3, o) : 1.4-2.1 (12H, m), 1.95 (6H, s), 2.21
(3H, s), 3.98 (2H, s), 4.35-4.55 (lH, m), 4.48
(2H, s), 5.42 (lH, s), 6.79 (2H, s), 7.19 (2H, d,
J=7.7Hz), 7.15-7.25 (lH, m), 7.35 (2-, d,
J=7.7Hz), 7.4-7.5 (lH, m), 8.4-8.5 (2H, m)
APCI-MASS (m/z) : 456 (M+H+)
- 35
PCTt~5101982
Wo96lloss9 ~ 2 0 g ~ ~
- 1&~ -
(37) 1-Cycloheptyl-1-[4-(4-~y~idylmethyl)benzyll-3-(2,~,6-
trimethyi~henyl~urea
IR (KBr) : 3408, 3304, 3324, 2922, 2856, 1632, 1605,
1512 cm~1
r, N~R (CDC13, o) : 1.4-2.~ (12H, ~), 1.35 (6H, s), 2.21
(3;i, s), 3.96 (2H, s,, 4.35-4.5 (lH, m), 4.48
(2~, s), ~.42 (lH, s,, 6.79 (2~, s), 7.0~ (2H,
dd, J=6.0, 1.6~z), 7.20 (2~, d, J=8.1Hz), 7.37
(2H, d, J=8.1Hz), 8.49 (2H, dd, J=6.0, 1.6Hz~
~PCI-MASS (m/z) : 456 (M-~+)
(38) 1-Cyclohe~tyl-1-(3-benzyl~en~yl)-3-(2,4,6-
trimethylphenyl)urea
IR (KBr~: 3223, 3025, 2322, 2854, 1626, 1506 cm 1
N~ (CD~13, o) : 1.4-2.G (i2H, ~.~), 1.95 (6H, s), 2.21
(3H, s), 3.97 (2H, s!, 4.46 !2H~ s), 4.3-4.5 (1H,
m), 5.42 (lH, s), 6.7g (2H, s), 7.1-7.35 (gu~
APCI-~SS (m/z) : 455 (M+H+)
(39) '-Cycloheptyl-1-[4-(pyra~ol-1-ylmethyl)benzyl]-3-
(2,4,6-trimethyl~henyl)urea
.p : 150-151C
I~ (KBr) : 3307, 2922, 2856, 1O28, 1508, 1250,
750 cm-1
NMR (CDCl3, o) : 1.38-2.05 (12H, m), 1.97 (6H, s),
2.21 (3H, s), 4.30-4.45 (lH, ~.), 4.49 (2~, s),
5.32 (2H, s), 5.39 (l:i, s), 6.28 (lH, dd, J=2.0,
2.0H~,, 6.79 (2H, s), 7.1~-7.28 (2H, m), 7.32-
7.42 (3H, m), 7.55 (1:~l, d, J=2.0Hz)
APCI-MASS ~(m/z) : 445 (M+H+)
(40) 1-Cycloheptyl-1-~4-(imida~Cl-1-ylmerhyl)benzyl'-3-
(2,4,6-trimethylphenyl)ure~
I~ ~KBr) : 3329 ~br)j 292', 2856, 1637, 1504, 1234,
8ag, 735 cm 1
WO96/10559 2 ~ O ~ 9 8 ~ PCT/~95/01982 ~
- 186 -
NMR (CDC13, o) : 1.35-2.05 (12H, m), 1.99 (6H, s),
2.21 (3H, s), 4.25-4.45 (lH, m), 4.51 (2H, s),
5.12 (2H, s), 5.40 (lH, s), 6.80 (2H, s), 6.89
(lH, s), 7.10 (lH, s), 7.13-7.23 (2H, m), 7.35-
7.45 (2H, m), 7.61 (lH, s)
APCI-MASS (m/z) : 445 (M+H+)
(41) 1-Cycloheptyl-1-[(6-hydroxy-2,5,7,8-tetramethyl-
chroman-2-yl)methyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3313, 2924, 2858, 1740, 1643, 1610,
1510 cm~l
NMR (DMSO-d6, o) : 1.15 (3H, s), 1.3-2.1 (16H, m),
2.55-2.65 (lH, m), 1.92 (3H, s), 1.99 (3H, s),
2.02 !6H, s); 2 03 !3Hi s)j 2.21 (3H, s), 3.53
(2H, br s), 6.83 (2H, s), 7.44 (lH, br s)
APCI-MASS (m/z) : 493 (M+H+)
(42) 1-Cycloheptyl-1-[4-[N-(3,5-di-tert-butyl-4-
hydroxyphenyl)carbamoyl]benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3639, 3417, 3321, 2951, 2924, 2860, 1643,
1610, 1502 cm~l
NMR (DMSO-d6, o) : 1.39 (18H, s), 1.4-1.9 (12H, m),
2.10 (6H, s), 2.21 (3H, s), 4.1-4.3 (lH, m), 4.57
(2H, s), 6.78 (lH, s), 6.85 (2H, s), 7.41 (2H, d,
J=8.3Hz), 7.90 (2H, d, J=8.3Hz), 7.44 (2H, s),
7.59 (lH, br s), 9.87 (lH, br s)
APCI-MASS (m/z) : 612 (M+H+)
(43) 1-Cycloheptyl-1-[4-[N-(4-fluorophenyl)carbamoyl]-
benzyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3280, 2926, 2856, 1643, 161Q, 1549,
1508 cm~l
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.11 (6H, s),
2.21 (3H, s), 4.1-4.3 (lH, m), 4.57 (2H, s), 6.85
PCTl~95/01982
~ WO96/10559 ~2~ ~ 9 ~ ~ 1
- 187 -
(2H, s), 7.15-7.3 ~2H, m), 7.43 (2H, d, J=8.2Hz),
7.64 (lH, br s), 7.75-7.85 (2H, m), 7.90 (2H, d,
J=8.2Hz), 10.22 (lH, s)
APCI-MASS (miz) : 502 (M+H+)
(44) 1-Cycloheptyl-1-[4-[N-(4-fluorophenyl)-N-
methylcarbamoyl]benzyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3321, 2951, 2923, 2860, 1638, 1606 cm 1
NMR (DMSO-d6, o) : 1.4-1.8 (12H, m), 2.01 (6H, s),
2.20 (3H, s), 3.30 (3H, s), 4.0-4.2 (lH, m), 4.42
(2H, s), 6.82 (2H, s), 7.05-7.3 (8H, m), 7.47
(lH, br s)
APCI-MASS (m/z) : 516 (M+H+)
(45) 1-Cycloheptyl-1-[4-[(2,4-dioxothiazolidin-5-
yl)methyl]benzyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 2931, 2858, 2765, 1753, 1709, 1689, 1606,
1632, 1564, 1535, 1502, 1481 cm~l
NMR (DMSO-d6, o) : 1.4-2.1 (12H, m),2.05 (6H, s),
2.20 (3H, s), 3.0-3.2 (lH, m), 3.3-3.45 (lH, m),
4.0-4.2 (lH, m), 4.47 (2H, s), 4.85-5.0 (lH, m),
6.82 (2H, s), 7.19 (2H, d, J=8.2Hz), 7.25 (2H, d,
J=8.2Hz), 7.44 (lH, br s), 12.3 (lH, br)
APCI-MASS (m/z) : 494 (M+H+)
(46) 1-Cycloheptyl-1-[4-[(2,4-dioxothiazolidin-5-
ylidene)methyl]benzyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3410, 3122, 2924, 2958, 2758, 1743, 1707,
1603, 1504 cm -
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.08 (6H, s),
2.21 (3H, s), 4.1-4.3 (lH, m), 4.54 (2H, s), 6.84
(2H, s), 7.44 (2H, d, J=8.3Hz), 7.56 (2H, d,
J=8.3Hz), 7.61 (lH, br s), 7.77 (lH, s), 12.60
(lH, br)
APCI-MASS (m/z) : 492 (M+H+)
PCT/~5101982
W096/lOS59 ~ ~ 9 8 ~ ~
- 188 -
(47) 1-Cycloheptyl-1-[4-( -cy~nophenyl)ben7v1]-3-(2,4,6-
trimethylphenyl)ure2
IR (KBr) : 3410, 3330, 2925, 2855, 2225, 1540, 161C,
15CO cm~
NMR (C3Cl3, o) : 1.5-1.8 (12H, m), 2.02 (6H, s), 2.21
(3H, s), 4.35-4.55 (1~, ~.), 4.58 (2~, s), 5.49
(lH, s), 6.8C ~2H, s), 7.4-7.8 (8
APCI-l~ASS (m/z) : 466 (MlH~)
Fxample 23
The following compounds we~e obtained ac~Grding to ~
similar manner to that of Exam~le 7, 8, 9, 10, 13, 14, 15,
lZ cr 17.
(1) 1-Cycloheptyl-1-[4-(4'-chlo~ophenoxy)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-y'~ure~
IR (~Br) : 3371, 2924, 2856, 1662, 1589, 156q, 506,
1485 c~-l
NMR (DMSO-d6, o) : 1.35-1.9 (12H, m), 2.39 (6X, s),
2.4g (3H, s), 4.0-4.2 (lH, m), 4.46 (2H, s), 6.86
(lH, s), 6.95-7.1 (4H, m), 7.35-7.5 (9H, m), 7.84
(lY, b~ s)
APCI-M~SS (m/z) : 556 (M~Ht)
(2) 1-Cycloheptyl-1-[9-(4-fluorophenoxy)benzyl]-3-[2, G-
bis(methylthio)-6-methylpyridin-3-yl]ure~
IR (KBr) : 3313, 2955, 2924, 2872, 1655, 1564,
1497 cm~1
NMR (DMSO-d6, o) : 1.4-1.9 (8H, m), 2.39 (6H, s),
3C 2.44 (3H, s), 4.3-4.5 (lH, m), 4.47 (2H, s), 6.8Z
(lH, s), 6.9-7.1 (4H, m), 7.15-7.35 (4:~, m), , ~7
(lH, s)
APCT-M~S (m/z) : 512 (~+:~ )
(3) 1-Cycloheptyl-1-(3-phenoxybenzyl)-3-~2,4-
WO96110559 ~ ~ 0 ~ Q ~ 1 PCT/~5/01982
- i83 -
bis(methylthio)-6-methylpy--idin-3-yll~rea
(KBr): 3294, 292a, 2~, 174C, 1635, ;502,
1483 c~. -
N~IR (DMSO-a6, o` : 1.3-~.3 (12H, ~), 2.32 (6H, s`,
2.43 (3H, s;, 4.0-4.2 (1~, m), 4.47 (2H, s), 6.83
(lH, s), 6.9-7.45 ;3H, .~), 7.84 (l~, br s)
~PCI-M SS (m/ 7 ) 522 (M-H+)
(4) 1-Cycloheptyl-1-[3-(4-fluorophenoxy)benzyl]-3-[2,4-
~is~methylthio)-6-~ethylpyridin-3-yl~urea
Br) : 3332, 3066, ~32O, 2856, 1664, i608, 1564,
1497 _m~1
N~R (CDCl3, o) : 1.45-2.05 (12X, m), 2.34 (3H, s),
2.45 (6H, s), 4.15-4.4 (lH, ~), 4.54 (2H, s),
5.46 (lH, s), 6.58 (lX, s), 6.85-7.4 (8~
(5) 1-(4-Dimethyla~inobenzyl)-1-[3-(pyrazol-3-yl)benzyl~-
3-(2,4,6-trifluorophenyl)u_ea
TR (KBr) : 2600-3650 (br), 1635, 1614, la22, 1448,
1352 c~ ~
NMR (DMSO-d6, o) : 2.83 ~oH, s), 4.38 (2H., s), 4.47
(2H, s), 6.55-6.77 (3H, ~), 7.C8-7.83 (9H, ~),
8.39 (l~, s), 12.8~, 13.33 (IOL~1 lH, eac~ br)
APCI-MASS (m/z) : a80 (M+~+)
2~
(6) 1-(2,3,5,6-Tetrahydro-4H-pyran-4-yl)-I-[4-(4-
fluoro?henoxy)benzvl]-3-[2,4-bis(methylthio)-o-
metnylpyridin-3-yl]urea
IR (KBr) : 3294, 3064, 2956, 2926, 2848, i655 1562,
1497 c~. 1
~R (DMSO-c6, o) : 1.5'-i.85 (4H, m), 2.4C (6H, s),
~ 2.45 (3H, s), 3.3-3.5 (2H, ~), 3.8-3.9 (2H, ~
4.1-4.3 (lH, ~), 4.51 (2H, s), 6.8~ (lH, sj, -o.3-
7.4 (8H, m), 7.98 (lH, br s)35
PCT/~5101982
WO96/105S9 ~20 ~ 9 8 1
-- 190 -- ,
(7) 1-(2-Phenylethyl)-1-[4-(4-fluorophenoxy)benzyl]-3-
[2,4-bis(methylthio)-6-methylpyridin-3-yl]urea
IR (KBr) : 329g, 3062, 3026, 2924, 1655, 1562,
1497 cm~l
NMR (CDC13, ~) : 2.40 (3H, s), 2.48 (3H, s), 2.51
(3H, s), 3.01 (2H, t, J=7.8Hz), 3.61 (2H, t,
J=7.8Hz), 4.43 (2H, s), 5.65 (lH, br s), 6.64
(lH, s), 6.9-7.1 (6H, m), 7.2-7.35 (7H, m)
APCI-MASS (m/z) : 548 (M+H+)
(8) 1-(2-Ethoxyethyl)-1-[4-(4-fluorophenoxy)benzyl]-3-
[2,4-bis(methylthio)-6-methylpyridin-3-yl~urea
IR (K~3r) : 3298, 3063, 2976, 2926, 2881, 2856, 1664,
1562, 1495 cm~l
NMR (DMSO-d6, o) : 1.12 (3H, t, J=6.9Hz), 2.40 (6H,
s), 2.45 (3H, s), 3.46 (2H, q, J=6.9Hz), 3.4-3.65
(4H, m), 4.54 (2H, s), 6.87 (lH, s), 6.93-7.4
(8H, m), 7.9 (lH, br s)
APCI-MASS (m/z) : 516 (M+H+)
(9) 1-Benzyl-1-(3-phenoxybenzyl)-3-[2,4-bis(methylthio)-6-
methylpyridin-3-yl]urea
IR (KBr) : 3404, 3032, 2997, 2922, 1668, 1610, 1562,
1500, 1452 cm~l
NMR (DMSO-d6, ~) : 2.35 (6H, s), 2.43 (3H, s), 4.44
(2H, s), 4.47 (2H, s), 6.86 (lH, s), 6.9-7.45
(14H, m), 8.24 (lH, br s)
APCI-MASS (m/z) : 516 (M+H+)
(10) 1-Benzyl-1-[3-(4-fluorophenoXy)benZyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
IR (KBr) : 3298, 3062, 3028, 2922, 1662, 1564,
1498 cm~l
NMR (CDC13, o) : 2.36 (3H, s), 2.46 (6H, s), 4.61
(2H, s), 4.62 (2H, s), 5.66 (lH, s), 6.85-7.4
PCT/~5/01982
~W096110559 ~ ) g. ~ 1
-- 191 --
(13H, m)
APCI-MASS (m/z) : 534 ~M+H+)
(11) 1-Cycloheptyi-1-[3-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
IR (KBr) : 3211, 3061, 2924, 2856, 1643, 1564, 1531,
1485 cm~1
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.39 (6H, s),
2.45 (3H, s), 4.0-4.2 (lH, m), 4.52 (2H, s), 6.6-
6.7 (lH, m), 6.86 (lH, s), 7.2-7.9 (6H, m), 12.85
(lH, br s)
APCI-MASS (m/z) : 496 (M+H~)
(12) 1-Benzyl-1-~3-(1-methylpyrazol=3=yl)benzyi]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 165-166C
IR (KBr) : 3280, 2922, 1643, 1562, 1500, 1435 cm 1
NMR (CDCl3, o) : 2.36 (3H, s), 2.46 (6H, s), 3.95
(3H, s), 4.66 (4H, s), 5 70 (lH, s), 6.57 (lH, d,
J=2.3Hz), 6.61 (lH, s), 7.22-7.45 (8H, m), 7.72-
7.80 (2H, m)
FAB-MASS (m/z) : 504 (M+H+)
(13) 1-Benzyl-1-[3-(1-methylpyrazol-5-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
IR (KBr) : 3280, 2922, 1649, 1562, 1500, 1431,
1390 cm~1
NMR (CDC13, ~) : 2.35 (3H, s), 2.45 (3H, s), 2.46
(3H, s), 3.88 (3H, s), 4.64 (2H, s), 4.71 (2H,
s), 5.70 (lH, s), 6.32 (lH, d, J=1.9Hz), 6.61
(lH, s), 7.20-7.55 (lOH, m)
FAB-MASS (m/z) : 504 (M+H+)
(14) 1-Benzyl-1-[4-(1-methylpyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
~ Q ~ ~ PCT/~5/0198~ _
W096/10559 ~ ~ ~ v
- 192 -
IR (KBr) : 3305, 2922, 1659, i564, 1489, 1338,
1227 cm~l
NMR (CDC13, o) : 2.38 (3H, s!, 2.47 (3H, s), 2.49
(3H, s), 3.96 (3H, s), 4.63 (4H, s), 5.71 (lH,
s), 6.54 (lH, d, J=2.3Hz), 6.62 (lH, s), 7.25-
7.47 (8H, m), 7.75-7.85 (2H, ~)
APCI-MASS (m/z) : 504 ~M+H+)
(15) '-Ben~yl-l-[4-(1-methylpyrazol-5-yl)benzyl]-3-[2,9-
bis(methylthio)-6-methylpyridin-3-yliurea
IR (KBr) : 3286, 2922, 1657, 1562, 1495, 1389 c~. 1
NMLR (CDCl3, o) : 2.40 (3H, s), 2.47 (3H, s), 2.43
(3H, s), 3.90 (3H, s), 4.66 (2H, s), 4.63 (2H,
s), 5.71 (lH, s), 6.31 (lH, d, J=1.9H~), 6.63
(lH, s), 7.25-7.51 (9H, m), 7.52 (lH, d, J=l.9~z)
APCI-MASS (m/z) : 504 (M+H+)
(16) 1-Benzyl-l-[4-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
m : 150-152C
IR (KBr) : 3400, 3215, 29~2, 1649, 1560, 1487,
1228 cm~l
NMR (DMSO-d6, o) : 2.44 (6H, s), 2.47 (3H, s), 4.~6
(4H, s), 6.72 (lH, s), 6.90 (lH, s), 7.22-7.9C
(lOH, m), 8.30 (lH, s), 12.87, 13.27 (total lH,
each br)
APCI-MASS (m/z) : 490 (M+H+)
(17) l-Cycloheptyl-l-l4-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 174-175C
IR (KBr) : 2690-3700 (br), 2924, 285S, 163/,
1564, 148~, i3~0, 1207, 804 c~-l
NMR (DMSO-d6, o) : 1.30-1.90 (12~, m), 2.41 (6H, s~,
2.45 (3H, s), 3.95-4.15 (lH, m), 4.4g (2H, s),
...... . . .....
W096/lOS~9 ~ PCT/~5/01982
- 193 -
6.67 (lH, br s), 6.86 (lH, s), 7.32-7.93 (6H, m),
12.80, 13.19 (total lH, each br)
APCI-MASS (mJz) : 496 (M+~+)
(18j 1-(4-Methoxybenzyl)-1-[3-(pyrazol-3-yl)benzyl~-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 170-173~C
IR (KBr) : 3394, 3250, 3101, 2920, 1664, 1562, 1483,
1223 cm~l
NMR (DMSO-d6, o) : 2.42 (6H, s), 2.47 (3H, s), 3.75
(3H, s), 4.41 (2H, s), 4.45 (2H, s), 6.67 (lH, br
s), 6.88-7.03 (3H, m), 7.13-7.90 (7H, m), 8.27
(lH, s), 12.89, 13.30 (total lH, each br)
APCI-MASS (m/z) : 520 (M-H+)
(19) 1-(4-Fluorobenzyl)-1-[3-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 166-168C
IR (KBr) : 3390, 3257, 2920, 1653, 1562, 1489,
1227 cm~l
NMR (DMSO-d6, o) : 2.42 (6H, s), 2.46 (3H, s), 4.4,
(2H, s), 4.49 (2H, s), 6.66 (lH, d, J=2.OHz),
6.90 (lH, s), 7.12-7.45 (6H, m), 7.60-7.90 (3H,
m), 8.30 (lH, s), 12.89, 13.30 (total lH, each
br)
APCI-MASS (m/z) : 508 (M+H+)
(20) l-(4-Dimethylaminobenzyl)-1-[3-(pyrazol-3-yl)benzyl]-
3-[2,4-bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 185-188C
IR (KBr) : 3236, 2922, 1633, 1612, 1524, 1487, 1338,
1219 cm~l
NMR (DMSO-d6, o) : 2.42 (sH, s), 2.47 (3H, s), 2.89
(6H, s), 4.35 (2H, br s), 4.42 (2H, br s), 6.60-
6.76 (3H, m), 6.90 (lH, s), 7.10-7.90 (7H, m),
WO96110559 ~2 0 ~ ~ PCT/~S/01982
- 194 -
8.23 (lH, s), 12.89, 13.30 (total lH, each br)
APCI-MASS (m/z) : 533 (M+H+)
(21) 1-Benzyl-1-[4-(1-methylpyrazol-4-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 224-225C
IR (KBr) : 3217, 2922, 1655, 1566, 1498, 1456, 1228,
806 cm~l
NMR (DMSO-d6, o) : 2.43 (6H, s), 2.47 (3H, s), 3.86
(3H, s), 4.30-4.50 (4H, m), 6.90 (lH, s), 7.20-
7.40 (7H, m), 7.50-7.60 (2H, m), 7.86 (lH, s),
8.13 (lH, s), 8.28 (lH, s)
APCI-MASS (m/z) : 504 (M+H+)
(22) 1-Cycloheptyl-1-[4-(1-methylpyrazol-4-yl)benzyl]-3-
[2,4-bis(methylthio)-6-methylpyridin-3-yl~urea
mp : 247-248C
I~ (KBr) : 3188, 2922, 2854, 1641, 1564, 1491,
1213 cm~l
NMR (DMSQ-d6, o) : 1.30-1.90 (12H, m), 2.40 (6H, s),
2.45 (3H, s), 3.85 (3H, s), 3.90-4.15 (lH, m),
4.45 (2H, s), 6.86 (lH, s), 7.28-7.38 (2H, m),
7.43-7.54 (2H, m), 7.83 (lH, s), 7.85 (lH, br s),
8.10 (lH, s)
APCI-MASS (m/z) : 510 (M~H+)
(23) 1-Benzyl-l-~3-(imidazol-4-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 134-136C r
IR (KBr): 2690-3700 (br), 1637, 1562, 1490, 1228 cm 1
NMR (DMSO-d6, o) : 2.43 (6H, s), 2.47 (3H, s), 4.47
(4H, s), 6.90 (lH, s), 7.10-7.75 (llH, m), 8.28
(lH, s), 12.17, 12.55 (total lH, each br)
APCI-MASS (m/z) : 490 (M~~+)
~ ~ ~ ~ ~ ~ ~ pCTl~5/01982
WO96/lOS59 ~ ~ U ~J
- 195 -
(24) 1-Benzyl-1-[3-(2-methyl-2H-tetrazol-5-yl)benzyl]-3-
[2,4-bis(methylthio)-6-methylpyridin-3-yl]urea
-~ IR (KBr) : 3290, 2922, 1655, 1562, 1493, 1227, 970,
~ 806 cm~l
NMR (CDCl3, o) : 2.39 (3H, s), 2.47 (3H, s), 2.48
(3H, s), 4.40 (3H, s), 4.67 (2H, s), 4.72 (2H,
s), 5.72 (lH, s), 6.62 (lH, s), 7.25-7.58 (7H,
m), 8.01-8.18 (2H, m)
APCI-MASS (m/z) : 506 (M+H+)
(25) 1-Cycloheptyl-1-[4-[(2,4-dioxothiazolidin-5-
yl)methyl]benzyl]-3-[2,4-bis(méthylthio)-6-
methylpyridin-3-yl]urea
IR (KBr) : 2924, 2860, 2769, 1753, 1701, 1603,
1506 cm~l
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.40 (6H, s),
2.45 (3H, s), 3.07 (lH, dd, J=14.0, 9.4Hz), 3.35
(lH, dd, J=14.0, 4.3Hz), 3.95-4.15 (lH, m), 4.45
(2H, s), 4.90 (lH, dd, J=9.4, 4.3Hz), 6.86 (lH,
s), 7.17 (2H, d, J=8.lHz), 7.30 (2H, d, J=8.lHz),
7.86 (lH, br s), 12.04 (lH, br)
APCI-MASS (m/z) : 559 (M+H+)
(26) 1-Cycloheptyl-1-[4-[(2,4-dioxothiazolidin-5-
ylidene)methyl]benzyl]-3-[2,4-bis(methylthio)-6-
methylpyridin-3-yl]urea
IR (KBr) : 3406, 3124, 2926, 2856, 2765, 1757, 1711,
1635, 1599, 1487 cm~l
NMR (DMSO-d6, o) : 1.3-1.9 (12H, m), 2.40 (6H, s),
2.45 (3H, s), 4.0-4.2 (lH, m), 4.52 (2H, br s),
6.86 (lH, s), 7.48 (2H, d, J=8.6Hz), 7.54 (2H, d,
J=8.6Hz), 7.77 (lH, s), 7.96 (lH, br s), 12.59
(lH, br)
APCI-MASS (m/z) : 557 (M+H+)
PCT/~5/01982
W096/10559 ~ ~ ~
- 196 -
(27) 1-3enzyl-1-[4-(4-fluorophenoxy)ben yl,-3-(2,4,6-
trimethylpvridin-3-yl)urea
IR (KBr) : 3294, 3030, 2922, 1632, 1605, 1498 cm 1
NMR (DMSO-d6, o) : 2.08 (3H, s~, 2.26 (3H, s), ~.35
~3H, s~, 4.52 (2H, s), 4.56 (2H, s), 6.95-7.45
(14H, m), 8.02 !lH, b~ s)
PDCI-M~SS (m/z) : 476 (~lH )
.
(28) 1-Cyclohexy~-1-[4-(4-fluorophenoxv)benzylj-3-(2,4,6-
lG trimethylpyridin-3-yl)urea
IR (K3r) : 3406, 3313, 2929, 2856, 1714, 1632, 1605,
1572, 1495 cm~l
N~R (DMSO-d6, o) : 1.0-i.9 (lOH, ~), 2.07 (3H, s),
2.24 (3H, s), 2.34 (3H, s), 3.95-4.15 (lH, m),
4.51 (2H, s), 6.95-7.4 (&H, ~), 7.70 (lH, s)
APCI-MASS (m/z) : 462 (M+H+)
(2g) 1-Cycloheptyl-1-[4-(4-bromophenoxy)benzyl~-3-(2,4,6-
trimethylpyridin-3-yl)urea
IR (KBr) : 3310, 1632, 1504, 1483, 1238 cm 1
NMR (CDC13, o) : 1.38-2.05 (12H, m), ~ C4 (3H, s),
2.20 (3H, s), 2.42 (3H, s), .30-4.5G ~iH, m)~
4.50 (2H, s), 5.49 (lH, s), 6.82 (lH, s), 6.83-
6.93 (2H, m), 6.98-7.08 (2H, m), 7.32-7.4& (4H,
m)
APCI-MASS (m/z) : 536, 538 (M+~+)
(30) 1-Benzyl-1-r3-(pyrazol-3-yl)benzyl]-3-(2,4,6-
trimethylpyridir~-3-yl)urea
IR (KBr) : 3236, 2924, 1645, 1564, 1493 c~ -
NMR (DMSO-d6, ~) : 2.10 (3H, s), 2.28 13H, s), 2.35
(3H, s), 4.59 (4H, s), 6.6-6.7 (lH, m.;, 6.94 (lr"
s), 7.2-7.8 (lOH, m), 3.0~ (lH, br s), 12.89 (ln,
b~)
APCI-MASS (m/z) : 426 (M+H+)
WO96/10559 ~2 Q ~ PCT/~5/01982
- 197 -
(31) 1-Cycloheptyl-1-[3-(1-tritylpyrazol-3-yl)benzyl]-3-
(2,4,6-trimethylpyridin-3-yl)urea
IR (KBr) : 3404, 3313, 3059, 3028, 2924, 2856, 1720,
1650, 1605, 1500, 1481 cm~1
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 1.96 (3H, s),
2.19 (3H, s), 2.33 (3H, s), 4.1-4.3 (lH, m), 4.54
(2H, s), 6.71 (lH, d, J=~.5Hz), 6.85 (lH, s),
7.1-7.8 (20H, m)
FAB-MASS (m/z) : 674 (M+H+)
(32) 1-Benzyl-1-[4-(4-fluorophenoxy)benzyl]-3-[4,6-
bis(methylthio)-2-methylpyridin-5-yl]urea
IR (KBr) : 3275, 3062, 3030, 292~, 1637, 1535,
1479 cm~1
NMR (DMSO-d6, o) : 2.46 (6H, s), 2.58 (3H, s), 4.44
(2H, s), 4.48 (2H, s), 6.95-7.4 (13H, m), 8.39
(lH, br s)
APCI-MASS (m/z) : 535 (M+H+)
(33) 1-Cycloheptyl-1-[4-(4-bromophenoxy)benzyl]-3-[4,6-
bis(methylthio)-2-methylpyrimidin-5-yl]urea
mp : 173-175C
IR (KBr) : 3375, 2926, 2852, 1668, 1583, 1479, 1238,
810 cm~1
NMR (CDCl3, o) : 1.38-2.10 (12H, m), 2.48 (6H, s),
2.59 (3H, s), 4.20-4.42 (lH, m), 4.54 (2H, s),
5.40 (lH, s), 6.85-6.93 (2H, m), 7.00-7.10 (2H,
m), 7.34-7.50 (4H, m)
APCI-MASS (m/z) : 601, 603 (M+H+)
(34) 1-Benzyl-1-[3-(4-fluorophenoxy)benzyl]-3-[4,6-
bis(methylthio)-2-methylpyrimidin-5-yl]urea
IR (KBr) : 3271, 3059, 3030, 2926, 2789, 2735, 2605,
1639, 1585, 1533, 1508 cm~1
NMR (CDCl3, o) : 2.46 (6H, s), 2.58 (3H, s), 4.61
PCTJ~5/01982
W096110559 ~ ~ O ~
- 198 -
(4H, br s), 5.58 (lH, s), 6.8-7.4 (13H, m)
APCI-MASS (m/z) : 535 (M+H+)
(35) 1-Cycloheptyl-1-[3-(pyrazol-3-yl)benzyl]-3-[4,6-
bis(methylthio)-2-methylpyrimidin-5-yl]urea
mp : 164-165C
IR (KBr) : 3194, 2926, 2856, 1633, 1518, 1419, 12g6,
812 cm~l
NMR (DMSO-d6, o) : 1.30-1.90 (12H, m), 2.43 (6H, s),
2.57 (3H, s), 3.95-4.15 (lH, m), 4.53 (2H, s),
6.65 (lH, s), 7.15-7.90 (5H, m), 8.07 (lH, s),
12.86, 13.30 (total iH, each br)
APCI-MASS (m/z) : 497 (M+H+)
(36) 1-Benzyl-1-[3-(pyrazol-3-yl)benzyl]-3-[4,6-
bis(methylthio)-2-methylpyrimidin-5-yl]urea
mp : 212-213C
IR (KBr) : 3388, 3265, 2924, 1653, 1524, 1487, 1390,
1356, 1298, 1228 cm~l
NMR (DMSO-d6, o) : 2.46 (6H, s), 2.58 (3H, s), 4.50
(4H, s), 6.60-6.70 (lH, m), 7.15-7.85 (lOH, m),
8.45 (lH, s), 12.89, 13.32 (total lH, each br s)
APCI-MASS (m/z) : 491 (M+H+)
(37) 1-Benzyl-1-[4-(4'-fluorop~enoxy)benzyl]-3-[2,4-
dimethoxy-6-methylpyridin-3-yl]urea
IR (KBr) : 3394, 3315, 3062, 2945, 2858, 1660, 1597,
1497 cm~1
NMR (DMSO-d6, o) : 1.99 (3H, s), 3.80 (3H, s), 3.81
(3H, s), 4.41 (2H, s), 4.45 (2H, s), 6.67 (lH,
s), 6.95-7.45 (13H, m)
APCI-MASS (m/z) : 502 (M+H+)
(38) 1-Cycloheptyl-1-~3-(pyrazol-3-yl)benzyl]-3-[2,4-
dimethoxy-6-methylpyridin-3-yl]urea
~ WO96/10559 22 0 ~ ~ 8 ~ PCT/~5101982
. g ci _
TR (KBr~ : 3373, 32C7, 3u55, 2926, 2856, 1651, 1557,
1502 c~; ~
- NM~ (DMSO-do, ~) : 1.3-l.g ~12~, r), 2.35 (3~, s),
3.75 (3~, `Sj ~ 3.76 (6H, s~, ~.0-4. (_~, ~..,, -.5C
_ (2H, s), 6.55 ( r, s3, c.o-6.65 (lH, ~i), 7.1-7.8
(_H, m,~) ~ i2.85 (lH, ~- s;
APC_-~SS (m/ 7 ~ : g 6a ~M+,~
(39) '-~enzyl- - r3- ( ~ -tri~lpyrazoi-3-v ~enzyll-3-(2, OE-
dimethoxy-6-metn~ipyridir-3-yl)urea
I~ (K~r) : 3990, 306O~ 3032, 25~C, 2533, 1'7~, 163-,
1512, 1497 cm.. -
NMR (3MSO-d~, o) : 2.36 (3H, s), 3.72 (3H, s), 3.74
(3~, s`, 4.46 (4~, br s!, 6.6~ (lH, s~, c.73
'5 d, J=2.5Hz), 7. -7.7 (~4H, ~.)
(40) 1-(4-Fluoroberzyl)-i-[3-(1-rritylpvra7OI-3-y )ben7yl'-
3-(2,4,6-trifluo~ophenylj~lrea
I~ (~Br) : 3294, 1637, 1608, 1518, la46, 1225 cm~
~ (CDCl3, ~) : 4.58 (2H, s), 4.6G (2H, s), 5.75
(lH, s~, 6.54 ('H, d, J=2.5Hz3, 6.57-7.15 (6H,
m,) ~ 7.13-7.43 (18H, ~,, 7.65-7.8G (2H, ~.)
(41) l-Cycloheptyl-1-(4-phenyl~en7yl)-3-(2,4,6-
~i~ethylpyridin-3-yl)urea
) : 3402, 3023, 232', 2854, 17~9, 166C, '6C~,
1566, 1493 cm~l
~R (DMSO-d~ : ;.4-1.9 (12H, m), 2.09 (3H, s),
2.27 (3H, s), 2.34 (3H, s), 4.05-4.25 ~lH, m),
~C 4.55 (2H, s), 6.~3 (lHt S) r 7.~-7.8 (9H, m)
A?CI-MASS (m/z) : 442 (M+H+)
Example 24
The following compounds we~e obt~ined according tG a
W096rlO559 ~ ~ g 8 ~ PCr/JPg5/01982 ~
- 200 -
similar manner to that of Example 6, 12 or 21.
(1) 1-Cycloheptyl-1-[4-(pyrazol-4-yl)benzyl]-3-(2,4,6-
trimethylphenyl)urea
IR (KBr) : 3184, 2926, 2856, 1630, 1650, 1510 cm~
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.09 (6H, s),
2.21 (3H, s), 4.05-4.25 (lH, m), 4.48 (2H, s),
6.83 (2H, s), 7.28 (2H, d, J=8.2Hz), 7.50 (lH, br
s), 7.56 (2H, d, J=8.2~z), 7.87 (2H, s)
(2) 1-Cycloheptyl-1-[3-(pyrazol-3-yl)benzyl~-3-[2,4-
dimethoxy-6-methylpyridin-3-yl)urea
IR (KBr) : 3406, 3228, 3062, 3026, 2974, 1676, 1653,
1597, 1508 cm~l
NMR (DMSO-d6, o) : 2.37 (3H, s), 3.79 (3H, s), 3.80
(3H, s), 4.47 (4H, s), 6.65 (lH, d, J=2.7Hz),
6.66 (lH, s), 7.2-7.5 (7H, m), 7.65-7.8 (4H, m)
APCI-MASS (m/z) : 458 (M+H+)
(3) 1-Cycloheptyl-1-[3-(pyrazol-3-yl)benzyl]-3-(2,4,6-
trimethylpyridin-3-yl)urea
IR (KBr) : 3400, 3224, 3055, 2929, 2856, 1714, 1633,
1568, 1500 cm~l
NMR (DMSO-d6, o) : 1.4-1.9 (12H, m), 2.09 (3H, s),
2.26 (3H, s), 2.34 (3H, s), 4.05-4.25 (lH, m),
4.56 (2H, s), 6.6-6.7 (lH, m), 6.91 (lH, s), 7.2-
7.5 (2H, m), 7.6-7.9 (3H, m), 12.85 (lH, br s)
APCI-MASS (m/z) : 432 (M+H+)
(4) 1-(4-Fluorobenzyl)-1-[3-(pyrazol-3-yl)benzyl]-3-
(2,4,6-trifluorophenyl)urea
mp : 204-206C
IR (KBr) : 3413, 3066, 1664, 1610, 1520, 1223 cm~l
NMR (DMSO-d6, o) : 4.51 (2H, s), 4.55 (2H, s), 6.65
(lH, d, J=2.3Hz), 7.10-7.50 (9H, m), 7.55-7.90
PCT/~5/~1982
WO96/10559
Q Q ~, ~
- 201 -
(2H, m), 8.46 (lH, s), 12.89, 13.30 (total lH,
each br)
_ APCI-MASS (m/z) : 455 (M+H~)
_ 5 (5) 1-Cycloheptyl-1-[4-[2-(lH-tetrazol-5-
yl)phenyl]benzyl]-3-(2,4,6-trimethylphenyl)urea
IR (KBr) : 3408, 3310, 2924, 28~6, 1620, 1605,
1506 cm~1
NMR (DMSO-d6, o) : 1.4-1.8 (12H, m), 2.04 (6H, s),
2.20 (3H, s), 4.05-4.25 (lH, m), 4.48 (2H, s),
6.83 (2H, s), 7.04 (2H, d, J=7.9Hz), 7.23 (2H, d,
J=7.9Hz), 7.5-7.8 (5H, m)
FAB-MASS (m/z) : 509 (M+H+)
~xample 25
To a solution of 1-cycloheptyl-1-[4-(4-
fluorophenoxy)benzyl]-3-[2,4-bis(methylsulfonyl)-6-
methylpyridin-3-yl]urea (3.04 g) in methanol (100 ml) was
added sodium methanethiolate (315 mg) and the mixture was
stirred at 50C for an hour under nitrogen. The mixture
was cooled to 5C and the precipitates were collected by
filtration, washed with methanol and diisopropyl ether and
dried in vacuo to give 1-cycloheptyl-1-[4-(4-
fluorophenoxy)benzyl~-3-(2-methylsulfonyl-4-methylthio-6-
methylpyridin-3-yl)urea (1.35 g) as a crystal.
IR (KBr) : 3377, 3072, 2926, 2858, 1657, 1572, 1498,
1473 cm~1
NMR ~CDCl3, o) : 1 5-2.1 (12H, m), 2.44 (3H, s), 2.54
(3H, s), 3.23 (3H, s), 4.1-4.3 (lH, m), 4.55 (2H,
30 s), 6.98 (lH, s), 6.9-7.1 (6H, m), 7.35 (lH, d,
J=8.6Hz)
Fxample 26
To a stirred solution of l-benzyl-1-[3-(pyrazol-3-
yl)benzyl]-3-[2,4-bis(methylthio)-6-methylpyridin-3-yl]urea
PCT/JP95/01982
W096/10559
- 202 -
(1 g) in dichloromethane (8 ml) was added a solution cf m-
chloroperbenzoic acid (1.32 g) in dichloromethane (26 ml)
at 0-5C. After stirring for one hour at room temperature,
the mixture was washed with saturated sodium bicarbonate
aqueous solution, water and brine, dried over magnesium
sul~ate, evaporated in vacuo. The residue was
chromatographed on silica gel to give 1-benzyl~ 3-
(pyrazol-3-yl)benzyl]-3-~2,4-bis(methylsulfonyl)-6-
methylpyridin-3-yl~urea (183 0 mg) and 1-benzyl-1-~3-
(pyrazol-3-yl)benzyl~-3-~2,4-bis(methylsulfinyl)-6-
methylpyridin-3-yl]urea (235.6 mg).
l-Benzyl~ 3-(pyrazol-3-yl)benzyl~-3-~2,4-
bis(methylsulfonyl)-6-methylpyridin-3-yl]urea
IR (KBr) : 3344, 2924, 1655, 1493, 1313, 1238,
1136 cm~l
NMR (DMSO-d6, o) : 2.70 (3H, s), 3.32 (6H, s), 4.52
(4H, br s), 6.75 (lH, br s), 7.20-7.85 (lOH, m),
8 13 (lH, s), 8.66 (lH, s), 12.87, 13.22 (total
lH, each br)
APCI-MASS (m/z) : 554 (M+H+)
l-Benzyl-1-~3-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylsulfinyl)-6-methylpyridin-3-yl]urea
IR (KBr) : 3217, 2922, 1651, 1495, 1236, 1038,
960 cm~1
NMR (DMSO-d6, ~) : 2.60-2.80 (9H, m), 4.42-4.75 (4H,
m), 6.71 ~lH, br s), 7.15-7.85 (llH, m), 8.84,
8.96 (total lH, each s), 12.93, 13.35 (total lH,
each br)
APCI-MASS (m/z) : 522 (M+H+)
~x~le 27
To a solution of N-cycloheptyl-4-(4-
fluorophenoxy)benzylamine (1.57 g) in toluene ~40 ml) we~e
WO96110~59 ~ 8 ~ PCT/JP95/01982
- 203 -
added 2,4-dichloro-6-methyl-3-phenoxycarbonylaminopyridine
(1.49 g) and triethylamine (1.52 g), and the mixture was
stirred at 100C for 3.5 hours. The mixture was poured
into a mixture of èthyl acetate and ice water, and the
separated organic layer was washed with brine, dried over
magnesium sul~ate and evaporated in ~-acuo. The residue was
purified by column chromatography on silica gel to give 1-
cycloheptyl-1-[4-(4-fluorophenoxy)benzyl]-3-(2,4-dichloro-
6-methylpyridin-3-yl)urea (916 mg).
IR (KBr) : 3365, 3275, 3062, 2927, 2858, 1653, 1581,
1543, 1497 cm~l
NMR (CDCl3, o) : 1.5-2.1 (12H, m), 2.47 (3H, s), 4.2-
4.4 (lH, m), 4.53 (2H, s), 5.89 (lH, s), 6.9-7.1
(6H, m), 7.14 (lH, s), 7.36 (2H, d, J=8.7Hz)
APCI-MASS (m/z) : 520, 518, 517 (M+H+)
Example 28
The following compounds were obtained according to a
similar manner to that of Example 7, 8, 9, 10, 13, 14, 15,
16, 17 or 27.
(1) 1-Cycloheptyl-1-[4-(4-fluorophenoxy)benzyl]-3-[(2-
methoxy-4-methylthio-6-methyl)pyridin-3-yl]urea
IR (KBr) : 3371, 3064, 2926, 2856, 1666, 1585,
1498 cm~l
NMR (CDCl3, o) : 1.5-2.1 (12H, m), 2.38 (6H, s), 3.79
(3H, s), 4.2-4.4 (lH, m), 4.52 (2H, s), 5.66 (lH,
br s), 6.53 (lH, s), 6.9-7.1 (6H, m), 7.35 (lH,
d, J=8.7Hz)
APCI-MASS (m/z) : 524 (M+H+)
(2) 1-Benzyl-1-[4-(4-fluorophenoxy)benzyl]-3-(2-chloro-4-
methylthio-6-methylpyridin-3-yl)urea
IR (KBr) : 3294, 3061, 3030, 2924, 1651, 1576,
1497 cm~1
PCT/~95/01982
WO96/10559 ~2~ 0 9 ~ 1 -
- 204 -
NMR (CDCl3, o) : 2.42 (3H, s), 2.47 (3H, s), 4.61
(2H, s), 4.63 (2H, s), 5.96 (lH, s), 6.82 (lH,
s), 6.9-7.1 (6H, m), 7.25-7.45 (7H, m)
(3) 1-Benzyl-1-[3-(1-methylpyrazol-4-yl)benzyl3-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 137-138C
IR (KBr) : 3255, 2922, 1651, 1562, 1493, 1228,
982 cm~l
NMR (DMSO-d6, o) : 2.42 (6H, s), 2.47 (3H, s), 3.87
(3H, s), 4.66 (2H, br s), 4.48 (2H, br s), 6.90
(lH, s), 7.13 (lH, d, J=7.4Hz), 7.20-7.56 ~8H,
m), 7.81 (lH, s), 8.06 (lH, s), 8.29 (lH, s)
APCI-MASS (m/z) : 504 (M+H+)
(4) 1-Cycloheptyl-1-[3-(l-methylpyrazol-4-yl~benzyl]-3-
[2,4-bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 197-198C
IR (KBr) : 3290, 2924, 2854, 1653, 1485, 1227 cm 1
NMR (DMSO-d6, o) : 1.25-1.90 (12H, m), 2.40 (6H, s),
2.45 (3H, sJ, 3.87 (3H, s), 3.98-4.17 (lH, m),
4.48 (2H, br s), 6.87 (lH, s), 7.15 (lH, d,
J=7.5Hz), 7.27 (lH, dd, J=7.5, 7.5Hz), 7.38 (lH,
d, J=7.5Hz), 7.52 (lH, s), 7.80 (lH, s), 7.90
(lH, br s), 8.04 (lH, s)
APCI-MASS (m/z) : 510 (M~H~)
(5) 1-(2-Methoxybenzyl)-1-[3-(~yrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl~urea
IR (KBr) : 3220, 2922, 1649, 1562, i491, 1240 cm 1
NMR (DMSO-d6, o) : 2.41 (6H, s), 2.46 (3H, s), 3.73
(3H, s), 4.44 (2H, br s), 4.53 (2H, br s), 6.67
(lH, br s), 6.88 (lH, s), 6.90-7.05 (2H, m~,
7.15-7.90 (7H, m), 8.19 (lH, br s), 12.89, 13.30
(total lH, each br)
~ PCT/~5/01982
W096/10559
- 205 -
APCI-MASS (m/z) : 520 (M+H+)
(6) 1-(3-Methoxybenzyl)-1-[3-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 165-166C
IR (KBr) : 3400, 3248, 3099, 2926, 1664, 1483, 1225,
1049 cm~1
NMR (DMSO-d6, o) : 2.41 (6H, s), 2.46 (3H, s), 3.75
(3H, s), 4.46 (2H, br s), 4.50 (2H, br s), 6.58-
6.72 (lH, m), 6.74-6.95 (4H, m), 7.15-7.85 (6H,
m), 8.28 (lH, s), 12.87, 13.29 (total lH, each
br)
APCI-MASS (m/z) : 520 (M+H+)
(7) 1-Benzyl-1-[3-(pyrazol-3-yl)benzyl]-3-[2-chloro-4-
methylthio-6-methylpyridin-3-yl]urea
IR (KBr) : 3230, 2922, 1647, 1576, 1497, 1338, 1279,
1232 cm~l
NMR (DMSO-d6, o) : 2.45 (6H, s), 4.51 (4H, br s),
6.57-6.70 (lH, m), 7.16 (lH, s), 7.17-7.85 (lOH,
m), 8.52 (1~, s), 12.89, 13.31 (total lH, each
br)
APCI-MASS (m/z) : 478, 480 (M+H+)
(8) 1-(4-Methoxybenzyl)-1-[4-(4-fluorophenoxy)benzyl]-3-
[2,4-bis(methylthio)-6-methylpyridin-3-yl]urea
mp : 130-131C
IR (KBr) : 3404, 2995, 2924, 2833, 1674,1610, 1562,
1493, 1250, 1211 cm-1
NMR (CDCl3, o) : 2.39 (3H, s), 2.49 (3H, s), 2.5i
(3H, s), 3.81 (3H, s), 4.56 (2H, s), 4.58 (2H,
- s), 5.72 (lH, s), 6.64 (lH, s), 6.85-7.12 (8H,
m), 7.20-7.38 (4H, m)
APCI-MASS (m/z) : 564 (M+H+)
PCT/~S/01982
W096/lOS59 22~ 0 9 8 1
- 2C~ -
(9) 1-Benzyl-1-[4-(4-fluorophenoxy)ben7yll-3-(2,4-
dichloro-6-methylpyridin-3-yl)urea
IR (~Br) : 3302, 3066, 3032, 2924, 1639, 1581, 1543,
1497 cm~
N'MR (CDCl3, o) : 2.48 (3.-, sj, 4.63 (2H, s), 4.54
(2H, s), 6.C5 (lH, br s), 5.9-,.4 (14~;, m),
APCI-MASS (~/z) : 514, 512, 510 (M+H )
(10) 1-~3-Phenylpropyl)-1-[4-(4-fluorophenoxy)benzyl]-3-
[2,4-bis(mLethylthio)-6-methylpyridin-3-yl]urea
IR (KBr) : 3290, 2922, 1645, 1562, 1497, 121i,
1093 cm-1
NMR (CDCl3, o) : 1.92-2.13 r2H, m), 2.38 (3H, s),
2.48 (3~, s), 2.49 (3H, s), 2.68 (2H, t,
J=7.7Hz), 3.39 (2H, t, J=7.6Hz), 4.57 (2H, s),
5.57 ~lH, s), 6.63 (lH, s), 6.87-7.10 (6H, m~,
7.10-7.37 (7H, m)
APCI-MASS (m/z) : 562 (M+'-'+)
~11) 1-(2-Phenylethyl)-1-[3-(pyrazo1-3-yl)benzyl]-_-[2,4-
bis(methylthio)-6-methylpyridir.-3-yl]urea
IR (KBr) : 3209 (br), 2922, 1647, 1562, i49', 1338,
1238 cm~1
NMR (DMSO-d6, o) : 2.42 (6H, s), 2.47 (3H, s), 2.80-
2.98 (2H, m), 3.35-3.54 (2H, m), 4.44 (2H, s),
6.65 (lH, br s), 6.90 (lr, 5), 7.10-7.45 (7h-, m),
7.45-7.83 (3H, m), 8.13 (lH, s), ;2.87, 13.3G
(total lH, each br)
A'~CT-M~SS (m/z) : 504 (M+.i )
(12) l-[(s)-l-phenylethyl]-l-r4-(4-fiuorophenoxv)ben7yll-3
[2,4-bis(methylthio)-6-methylpyrid~r.-3-yl]urea
IR (KBr) : 3373, 3310, 2978, 2924, 166C, 1562, 1497,
1246, 1211 c~ 1
NMR (CDCl3, o) : 1.63 (3~, d, J=7.' 7~7 j, /, 37 ~3--, 5),
~ WO 96/105S9 ~ Q ~ ~ PFT/~5/0l982
- 2u7 -
2.46 ~3~, s~ 7 (3., s), ~.~7 (_U, C,
J=17.2Hz), 4.55 ~l:i, d, J=17.2~z), 5.53 (iH, s;,
5.7'-_.92 (i~:, m;, 6.~0 (;H, s, 6.88-7.'^ 'cU,
m), 7.22-7._C l7-i m`
APCl-~MPSS (m~ 5 ~ 8 ; ~
l a~ 30: -6 .0 ~C = 0/ , CnC ~ 3)
(13) '~ [ i~) -1-Phe~yletnyl~-l- r4- (4-flucrcpr~noxy)beL~zyi3-3-
2,4 -bis (methyi thio j - 6-mer~yl~y ~ - 3-yl, ureG
' 0 R (~B~, : 3369, 33G5, 2378, 2924, 1O59, 562, 14Y7,
1246,
NMR (CDCl3, o) : i .63 (3H, ~, J-7. :~z ~ .37 '3r, s3,
2.46 (3H, s), ~.47 (3., s), ~. 7 (1~, c,
J=17.2Hz), '-.50 ( ~, d, J=l7.2H7), _.5~ (lH, s),
5.75-_.92 (lH, ~), 6.60 (lr, S; ~ o.88-7.10 (6H;
m), 7.22-7.5~ (7H, ~)
~PCL_.~SS (m/z) : 548 (~+1 )
l~]3 : T 62.2 ( ~ = 1.0 /, C ~ C 13)
2 Q ( i 4) '-Cyclohe~.yl-1-r4-!a-_'uoro~henoxy)be~zyl - 3 -,a-
chio-o-2-metrLylthlc-6-m2thyl~yridir-3-y~l)urea
(~B~): 3371, 3275, 3062, 2926, 2856, 1~53, 156~,
1498 cm- '
NM~ (CDCl3, ~ 4-2 1 (l~H, m), 2.44 (3H, s), 2.47
(3H, SJ, 4.25-4.45 \lH, r), 5.6- (2:H, s, 6.8Y
(lH, s), 6.9-7.1 (6H, m), 7.37 '2H, ~, J=8.6~z)
APC~-~SS (~./7) 530, 5~8 (M+~ )
*(1_) -~er-yl-1-[4-!4-r^luo-o~henoxy)benzy']-3-(4-c~~l oro-2- m2rhylihio-6-methylpyridir-3-yl) l~re~
_~ (KBr): 3275, 306 , 303S, 2924, 1645, 1560,
1497 c~ i
(CDCl~, o) : 2.46 (3H, s), 2.49 (3H, s), ~.6
(2H, s~, 4.63 (2H, s), 5. &0 (1 Y, br s), 6.9-7.
(7H, ~), 7.25-7. a (7~, ~.)
.
WO96/10559 2 ~ 8 ~ PCT/~5/01982
- 208 -
APCI-MASS (m/z) : 524, 522 (M+H+)
(16) 1-Cycloheptyl-l-[g-(4-bromophenoxy)benzyl]-3-[2-
chloro-4-methylthio-6-methylpyridin-3-yl]urea
mp : 105-107C
IR (KBr) : 3379, 2926, 2854, 1668, 1579, 1483,
1238 cm~l
NMR (CDCl3, o) : 1.38-2.08 (12H, m), 2.41 (3H, s),
2.48 (3H, s), 4.20-4.40 (lH, m), 4.54 (2H, s),
5.76 (lH, s), 6.82 (lH, s), 6.82-6.93 (2H, m),
iO 6.95-7.08 (2H, m), 7.32-7.50 (4H, m)
APCI-MASS (m/z) : 588, 590, 592 (M+~+)
(17) 1-Benzyl-1-[4-(4-bromophenoxy)benzyl]-3-[2-chloro-a-
methylthio-6-methylpyridin-3-yl]urea
IR (KBr) : 3280, 3030, 2920, 1651, 1578, 1504, 1435,
1236, 804 cm-l
NMR (CDC13, o) : 2.43 (3H, s), 2.49 (3H, s), 4.63
(2H, s), 4.64 (2H, s), 5.93 (lH, s), 6.84 (lH,
s), 6.84-6.94 (2H, m), 6.94-7.07 (2H, m), 7.22-
7.50 (9H, m)
APCI-MASS (m/z) : 582, 58a, 586 (M+H+)
(18) 1-Cycloheptyl-1-[3-(pyrazol-3-yl)benzyl]-3-[2-chloro-
4-methylthio-6-methylpyridin-3-yl]urea
~p : 165-166C
IR (KBr) : 3205, 2926, 2856, 1624, 1572, 1491,
804 cm-l
NMR (DMSO-d6, o) : 1.30-1.90 (12H, m), 2.43 (6H, s),
4.00-4.18 (lH, m), 4.53 (2H, br s), 6.55-6.67
(lH, m), 7.12 (lH, s), 7.20-7.83 (5H, m), 8.11
(lH, br s), 12.85, 13.28 (total lH, each br s)
APCI-MASS (m/z) : 484, 486 (M+H+)
Example 29
The following compound can be obtained by treating 1-
benzyl-1-[3-(pyrazol-3-yl)benzyl]-3-[2,4-bis(methylthio)-6-
WO96/105~9 ~2 ~ Q 9 8 1 PCT/~5/01982
- 209 -
methylpyridin-3-yl]urea with hydrochloric acid or
hydrochloride in a conventional manner.
1-3enzyl-1-[3-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methyl~yridin-3-yl]ureahydrochloride
Example 30
The rollowing compound can be obtained by treating 1-
benzyl-1-~3-(pyrazol-3-yl)benzyl.-3-[2,4-bis(methylthio)-6-
methylpyridin-3-yl]urea with sulfuric acid in a
conventional manner.
1-3enzyl-l-[3-(pyrazol-3-yl)benzyl]-3-[2,4-
bis(methylthio)-6-methylpyridin-3-yl]ureasulfate
Example 31
The following compound was obtained according to a
similar manner to that of Example 19.
1-Benzyl-1-[4-(4-fluorophenoxy)benzyl]-3-[2,4-
bis(methylsulfonyl)-6-methylpyridin-3-yl~urea
IR (KBr) : 3348, 3066, 3030, 2927, 1734, 1668, 1610,
1583, 1497 cm~l
NMR (C~Cl3, o) : 2.67 (3H, s), 3.20 (3H, s), 3.32
(3H, s), 4.6-4.7 (4H, m), 6.9-7.1 (6H, m), 7.3-
7.5 (2H, m), 7.62 (lH, br s), 7.88 (lH, ~!
APCI-MASS (m/z) : 598 (M+H+)
Example 32
The following compound was obtained according to a
similar manner to that of Example 29.
1-Cycloheptyl-1-[4-(4-fluorophenoxy)benzyl]-3-(2,4,6-
trimethylpyridin-3-yl)urea hydrochloride
mp: 176-178C
NMR (DMSO-d6, o) : 1.35-1.9 (12H, m), 2.32 (3H, s),
2.52(3H, s), 2.65 (3H, s), 4.1-4.3 (lH, m), 4.53
(2H, s), 6.95-7.4 (8H, m), 7-61 (lH, s), 8.30(1H,
br s)