Note: Descriptions are shown in the official language in which they were submitted.
CA 02201419 2004-11-24
TITLE OF INVENTION
HUMAN T-CELL LYMPHOTROPIC VIRUS TYPE I ENVELOPE PROTEIN
AND HUMAN MONOCLONAL ANTIBODIES SPECIFIC THEREFOR
FIELD OF INVENTION
The present invention relates to the field of
immunology and is particularly concerned with Human T-cell
Lymphotropic Virus type I envelope proteins and human
monoclonal antibodies specific therefor.
BACKGROUND TO THE INVENTTON
Human T-cell Lymphotropic Virus type I (HTLV-I) was
the first human retrovirus to be associated with disease,
Adult T-cell Leukemia/Lymphoma (ATL; ref. 1, 2 - various
references are referred to in parenthesis to more fully
describe the state of the art to which this invention
pertains. Full bibliographic information for each citation
is found at the end of the specification, immediately
preceding the claims.) HTLV-I was later associated with
the disease, HLTV-I Associated Myelopathy/Tropical Spastic
Paraparesis (HAM/TSP; 3). Recently this virus has been
associated with arthropathy (ref. 4), uveitis (ref. 5) and
infective dermatitis (ref. 6). HTLV-I has been found in
almost every region of the world and it is estimated that
approximately 10 to 20 million people are infected (ref.
7) .
The envelope protein of HTLV-I is composed of an
external surface glycoprotein, gp46 and a noncovalently
associated transmembrane anchor protein, gp2l; both of
these are derived from a common precursor, gp63 (ref . 8) .
The gp46 HTLV-I envelope protein is one of the smallest
retroviral envelope proteins known and exhibits little
sequence variability (ref. 9, 10, 11). This genetic
stability may be a reflection of the limited coding
sequence and a need for structural conservation in order
to preserve its functionality. While a number of studies
2 .
have characterized the HTLV-I gp46 protein (refs. 12,
13), it has been difficult to heterologously express
recombinant envelope protein in large amounts for use in
biochemical and immunological studies. (refs. 40, 41,
42, 43, 44, 45) We have recently described the expression
of the entire HTLV-I envelope protein, gp63, in a
baculovirus expression system (ref. 14). Although the
recombinant protein was expressed in large amounts, it
was insoluble and the majority of protein was not
completely post-translationally processed. Following
successful solubilization of this protein, the soluble
and insoluble forms of gp63 have been used to generate
human T-cells lines in vitro (ref. 15) and high anti-
envelope antibody titres in rabbits (ref. 16).
Unfortunately , only non-neutralizing antibodies were
induced by the recombinant gp63 protein as either
insoluble inclusion bodies (ref. 14) or in its soluble
form. This baculovirus-expressed envelope protein thus
cannot be in the natural conformation that it is present
in the virus~.and thus is not optimal for vaccine or
diagnostic purposes.
Our previous study demonstrated that a recombinant
vaccinia virus (RW E3) containing the HTLV-I coding
region for gp46 alone, produced the conformationally
correct envelope surface protein, induced neutralizing
antibodies in mice (refs. 16, 17) and expressed envelope
protein at much higher levels that it did when gp21 was
concomitantly expressed in another construct RVV E1 (ref.
17). In this previous work, however, there was no
provision of an isolated and purified envelope protein of
Human T-cell Lymphotrophic Virus Type I (HTLV-I) devoid
of non-envelope proteins of HTLV-I having substantially
the same conformation as the envelope protein in native
HTLV-I, especially the Tox and pl2l proteins which have
demonstrated oncogenic potential.
3
It would be advantageous to provide a recombinantly-
produced, isolated and purified envelope protein of HTLV-
I which is devoid of other HTLV-I proteins and having
substantially the same conformation as the native protein
in high yields and methods of purification of such
proteins. Such proteins have use as antigens,
immunogenic preparations, including vaccines, as
components of diagnostic assays and for the generation of
diagnostic reagents. It would also be advantageous to
provide human monoclonal antibodies which are HTLV-I
envelope protein specific and substantially non-binding
to HTLV-I envelope protein in a denatured form.
SUMMARY OF INVENTION
In accordance with one aspect of the present
invention, there is provided an isolated and purified
envelope protein of Human T-cell Lymphotrophic Virus Type
I (HTLV-I) devoid of non-envelope proteins of HTLV-I
having substantially the same conformation as the
envelope protein in native HTLV-I. This envelope protein
is sometimes referred to herein as the "gp46 envelope
protein". The envelope protein is provided devoid of
non-envelope protein of HTLV-I by production by
recombinant means as described in more detail below.
The isolated and purified envelope protein generally
is provided in a glycosylated form with an apparent
molecular weight of about 47 to about 49 kDa, as
determined by sodium dodecyl sulfate gel electrophoresis
(SDS-PAGE). The protein provided herein may be in the
form of a mixture of two envelope proteins of HTLV-I
having an apparent molecular weight of about 47 kDa and
about 49 kda respectively.
The isolated and purified envelope protein provided
herein generally binds to a HTLV-I envelope protein-
specific human monoclonal antibodies which do not bind to
denatured envelope protein of HTLV-I, particularly
4
~monoclonal antibodies which recognize conformational
epitopes of the envelope protein of HTLV-I.
The recombinant procedure described herein produces
a mixture of proteins of varying molecular weights and
degrees of glycosylation. Accordingly, in another aspect
of the invention, there is provided a mixture of at least
two isolated and purified envelope proteins of HTLV-I
devoid of non-envelope proteins of HTLV-I having an
apparent molecular weight which is selected from about 39
kDa, about 43 kDa, about 45 kDa, about 47 kDa and about
49 kDa, as determined by SDS-PAGE, which may include a
mixture of all such envelope proteins.
The envelope proteins provided herein are also
recognized by antibodies specific for HTLV-II envelope
proteins. Accordingly, in another aspect of the present
invention, there is provided an isolated and purified
envelope protein of HTLV-I devoid of non-envelope
proteins of HTLV-I which is recognized by antibodies
specific for the envelope protein of Human T-cell
Lymphotrophic Virus Type II (HTLV-II).
The present invention also provides an immunogenic
composition comprising an immunoeffective amount of an
active component, which may be the novel envelope protein
provided herein, which may be formulated along with a
pharmaceutically acceptable carrier therefor. The
immunogenic composition may be formulated as a vaccine
for in vivo administration to a host.
The immunogenic composition may be formulated as a
microparticle, capsule, ISCOM or liposome preparation.
The immunogenic composition may be used in combination
with a targeting molecule for delivery to specific cells
of the immune system or to mucosal surfaces. Some
targeting molecules include strain B12 and fragments of
bacterial toxins, as described in WO 92/17167 (Biotech
Australia Pty. Ltd.), and monoclonal antibodies, as
described in U.S. Patent No. 5,194,254 (Barber et al).
CA 02201419 2004-11-24
The immunogenic compositions of the invention (including
vaccines) may further comprise at least one other
immunogenic or immunostimulating material and the
immunostimulating material may be at least one adjuvant.
s Suitable adjuvants for use in the present invention
include, (but are not limited to) aluminum phosphate,
aluminum hydroxide, QS21, Quil A, derivatives and
components thereof, ISCOM matrix, calcium phosphate,
calcium hydroxide, zinc hydroxide, a glycolipid analog,
to an octadecyl ester of an amino acid, a muramyl dipeptide
polyphosphazare, ISCOPRP, DC-chol, DDBA and a
lipoprotein and other adjuvants to induce a Thl
response. Advantageous combinations of adjuvants are
described in United States Patent No. 6,764,682.
In a further aspect of the invention, there is
provided a method of generating an immune response in a
host, comprising administering thereto an immuno-
effective amount of the immunogenic composition as
provided herein. The immune response may be a humoral
or a cell-mediated immune response. Hosts in which
protection against disease may be conferred include
primates including humans.
The present invention additionally provides a
method of producing antibodies specific for an envelope
2s protein of HTLV-I, comprising:
(a) administering the envelope protein provided
herein to at least one mouse to produce at least one
immunized mouse;
(b) removing B-lymphocytes from the at least one
3o immunized mouse;
(c) fusing the B-lymphocytes from the at least
one immunized mouse with myeloma cells, thereby
producing hybridomas;
6
(d) cloning the hybridomas;
(e) selecting clones which produce anti-envelope
protein antibody;
(f) culturing the anti-envelope protein antibody-
producing clones; and then
(g) isolating anti-envelope protein antibodies from
the cultures.
The present invention further provides a HTLV-I
envelope protein specific human monoclonal antibody which
is substantially non-binding to HTLV-I envelope protein
in a denatured form. Such monoclonal antibody generally
binds to the isolated and purified envelope protein which
provides the first aspect of this invention.
The~monoclonal antibody provided herein preferably
recognizes a conformational epitope of the envelope
protein of HTLV-I and is capable of neutralizing HTLV-I
syncytium formation. Such monoclonal antibody may be one
having the characteristics of a monoclonal antibody
produced by a hybridoma selected from the group
consisting of WAll/1F5, WA07/2F7, WA07/1G7, WAll/2E2,
WAll/2F3 and WA04/2B10.
The present invention provides, in an additional
aspect thereof, a, method for producing an immunogenic
composition comprising administering the immunogenic
composition provided herein to a first test host to
determine an amount and a frequency of administration
thereof to elicit a selected immune response against
HTLV-I; and formulating the immunogenic composition in a
form suitable for administration to a second host in
accordance with the determined amount and frequency of
administration. The second host may be a human.
The novel envelope protein provided herein is useful
in diagnostic procedures and kits for detecting
antibodies to retroviruse's, including HTLV-I, HTLV-II and
related primate T-cell lymphotrophic viruses ~(PTLVs),
such PTLV-L, and STLVs, such as STLVpan_p, STLVI, STLVII
7
and other primate retroviruses related to HTLV-II.
Further monoclonal antibodies specific for the envelope
protein are useful in diagnostic procedure and kits for
detecting the presence of HTLV-I protein.
Accordingly, a further aspect of the invention
provides a method of determining the presence in a
sample, of antibodies specifically reactive with an
envelope protein of HTLV-I, HTLV-II or related primate T-
cell lymphotrophic viruses (PTLVs), such PTLV-L, and
STLVs, such as STLVpan_p, STLVI, STLVII and other primate
retroviruses related to HTLV-II comprising the steps of:
(a) contacting the sample with the HTLV-I envelope
protein or proteins as provided herein to produce
complexes comprising the HTLV-I envelope protein and
any said antibodies present in the sample
specifically reactive therewith; and
(b) determining production of the complexes.
In a further aspect of the invention, there is
provided a method of determining the presence, in a
sample, of an envelope protein of HTLV-I,comprising the
steps of:
(a) immunizing a host with HTLV-I envelope protein
as provided herein, to produce antibodies specific
for the envelope protein;
(b) contacting the sample with the antibodies to
produce complexes comprising any envelope protein
present in the sample and said envelope protein
specific antibodies; and
(c) determining production of the complexes.
A further aspect of the invention provides a
diagnostic kit for determining the presence of antibodies
in a sample specifically reactive with an envelope
protein of HTLV-I, HTLV-II or related primate T-cell
lymphotrophic viruses (PTLVs), such PTLV-L, and STLVs,
such as STLVpBn_p, STLVI, STLVII and other primate
retroviruses related to HTLV-II, comprising:
8
(a) the envelope protein as provided herein;
(b) means for contacting the envelope protein with
the sample to produce complexes comprising the
envelope protein and any said antibodies present in
the sample; and
(c) means for, determining production of the
complexes.
The invention also provides a diagnostic kit for
detecting the presence, in a sample, of an envelope
protein of HTLV-I, comprising:
(a) an antibody specific for the novel envelope
protein as provided herein;
(b) means for contacting the antibody with the
sample to produce a complex comprising the envelope
protein and envelope protein-specific antibody; and
(c) means for determining production of the
complex.
In this application, the term "HTLV-I envelope
protein" is used to define a family of HTLV-I envelope
proteins generally having an apparent molecular weight of
from about 39 to about 49 kDa and includes proteins
having variations in their amino acid sequences : In this
application, a first protein is a "functional analog" of
a second protein if the first protein is immunologically
related to and/or has the same function as the second
protein. The functional analog may be, for example, a
fragment of the protein or a substitution, addition or
deletion mutant thereof. The invention also extends to
such functional analogs.
Advantages of the present invention include:
- an isolated and purified envelope protein of HTLV-I
produced recombinantly to be devoid of non-envelope
proteins of HTLV-I and having substantially the same
conformation as the envelope protein in native HTLV-I;
~a ~~ ~
9
- HTLV-I envelope protein specific human monoclonal
antibodies which are substantially non-binding to HTLV-I
envelope protein in non-denatured form; and
- a diagnostic kits and immunological reagents for
specific identification of hosts infected by HTLV-I,
HTLV-II and related primate T-cell lymophotrophic
viruses.
BRIEF DESCRIPTION OF DRAWINGS
The present invention will be further understood
from the following detailed description and Examples with
reference to the accompanying drawings in which:
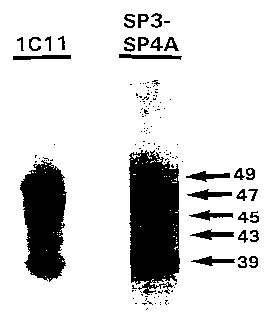
Figure 1 shows an immunoblot analysis of HTLV-I
envelope proteins produced in HeLa cells. The primary
antibody used: 1C11, anti-gp46 mouse Mab; SP3/SP4A, anti-
envelope peptide rabbit polyclonal sera;
Figure 2 shows the N-glycosylation of recombinant
HTLV-I envelope protein forms. Lysates of 2.5x106 [35S] -
cysteine-labelled cells were immunoprecipitated with
mouse monoclonal antibody 1011 and digested with endo H
or PNGase F. Untr., immunoprecipitate of untreated vTME-
46/vTF7-3 infected cell lysate; EndoH and PNGF,
immunoprecipitates of untreated vTME-46/vTF7-3 infected
cell lysate digested with endonuclease H and PNGase F
respectively; Tunc., immunoprecipitate of tunicamycin-
treated dual vaccinia infection lysate;
Figure 3 shows the effect of multiplicity of
infection upon envelope protein production by immunoblot
analysis HeLa cells (2.0 x 105 cells) infected with both
vTM-46/vTF7-3, RVV E3 alone or vTF7-3 alone, at various
virus multiplicities. HAM/TSP sera was used for
immunoblot detection;
Figure 4 shows a time course of HTLV-I envelope
protein production by immunoblot analysis of cell lysates
(1.2 x 105 cells) infected with recombinant vaccinia at
an MOI of 2 and harvested at the designated times. The
blot was incubated with HAM/TSP sera;
'~ ~~o ~~~ ~
Figure 5 shows the expression of HTLV-I envelope
protein in Human H9 versus HeLa cells. Immuno blot
analysis of H9 and HeLa cell lysates (2.0 x 105 cells)
co-infected with vTME-46/vTF7-3 at an moi of 4. The blot
5 was incubated with HAM/TSP sera;
Figure 6 shows reactivity of HTLV-I, HTLV-II and
STLVpan_psera with HTLV-I envelope protein. HeLa cell
lysates infected with vTME-46/vTF7-3 were immunoblotted
with normal human serum (NHS 1:500 dilution);
10 asymptomatic HTLV-I-infected patient sera (lanes 1 and 2,
patients A and B c~ 1:500); sera from HTLV-I infected
patients diagnosed with HAM/TSP (lane 3, patient C Q
1:10000; lane 4, patient D Q1:4000); HTLV-II-infected
patient sera (lane 5-8, patient E-H Q1:500 dilution);
STLVp~_p-infected pygmy chimp serum (lane 9, 1:100; lane
10, 1:200; lane 11, 1:400 dilution); normal pygmy
chimpanzee serum (NCS, 1:100 dilution);
Figure 7 shows the radioimmunoprecipitatin of
vaccinia-infected cell lysates with HTLV-I envelope
specific human monoclonal antibodies. [35S]-cysteine
labelled proteins from vTME-46/vTF7-3 infected HeLa cells
(lanes 3-10) were immunoprecipitated with equal IgG
concentrations (7.5~,g/ml] of HTLV-I-specific human
monoclonal antibodies (lane 4, WA11/1F5; lane 5,
WA07/2F7; lane 6, WA07/1G7; lane 7, WAll/2E2; lane 8,
WAll/2F3; and lane 9, WA04/2B10) ; or an anti-CMV isotype-
matched human monoclonal antibody (lane 3, 7.5 ~,g IgG/ml
R04); or polyclonal sera from HTLV-I infected patients
diagnoses with HAM/TSP (lane 10, patient C Q 1:20000;
lanes 11 and 12, patients D and I Q 1:4000).
Radiolabelled lysates from singly vTF7-3-infected HeLa
cells were not immunoprecipitated with polyclonal HTLV-I
infected patient C serum (lane 1 Q 1:4000) or human
monoclonal antibody, WA04/2B10 (lane 2, 7.5~,g/IgG/ml),
were negative controls; and
11
Figure 8 shows recombinant envelope proteins were
expressed by vTME-46/vTF7-3 infected HeLa cells in the
absence or presence of the glycosylation inhibitors
Brefeldin A and tunicamycin. Equivalent amounts of [35S] -
cysteine-labelled lysates (vTME-46/vTF7-3 infections:
panel A, untreated; panel B, Brefeldin A-treated; panel
C, tunicamycin-treated) were immunoprecipitated with the
same series of polyclonal sera and monoclonal antibodies .
Lane 1, 1011 mAb; lane 2, HAM/TSP patient C Q 1:20000;
lane 3, HAM/TSP patient D Q 1:4000; lane 4, WAll/1F5[7.5
~Cg/ml] ; lane 5, WA07/1G7 [7.5 ~.g/ml] ; lane 6, WA07/2F7
[7.5 ~.g/ml] ; lane 7, HTLV-II infected human sera Q 1:400;
lane 8, STLVgan_p infected chimp sera Q 1:200.
GENERAL DESCRIPTION OF INVENTION
As described above, the present invention provides
certain novel HTLV-I envelope proteins which are provided
devoid of non-envelope proteins of HTLV-I and with
substantially the same conformation as the envelope
protein in native HTLV-I. This novel protein may be
prepared using recombinant procedures, wherein the
protein is expressed from a suitable expression vector
and then isolated and purified.
In one specific embodiment of the invention, a
vaccinia/T7 polymerase system was used to express the
recombinant HTLV-I surface envelope protein in mammalian
cells. This strategy required the construction of a
recombinant vaccinia virus, vTME-46, encoding the HTLV-I
gp46 gene fragment under the control of the T7
bacteriophage promoter and terminator regulatory
elements. Co-infection with a second recombinant virus,
vTF7-3, encoding the T7 polymerase gene (ref. 23)
resulted in expression of the gp46 envelope protein.
Five differentially glycosylated forms of the
surface envelope protein were produced by vTME-46/vTF7
3-infected HeLa cells, having an apparent molecular
weight of about 39 kDa, about 43 kDa, about 45 kDa, about
~! ~2~'~~1 ~
12
47 kDa and about 49 kDa. N-glycosylation inhibition by
tunicamycin and N-glycan removal with endo H and PNGase
F revealed that the 39 kDa protein was the unglycosylated
form and the 49 kDa protein was the fully glycosylated
envelope protein. Each oligosaccharide on average
contributes approximately 2 kDa to the apparent molecular
mass of the protein (ref. 38); thus the observed 10 kDa
ladder of different recombinant envelope forms was
consistent with differential attachment of four
oligosaccharides. This result indicated that all four
potential N-glycosylation sites in gp46 (ref. 39) were
utilized for oligosaccharide modification in the
vaccinia/T7 polymerase system.
The envelope glycoproteins expressed by the
mammalian system appeared to have both mannose-rich and
hybrid oligosaccharides attached, as determined by endo
H digestion. The glycosylation of the recombinant
envelope proteins resembled that of gp46 produced by HUT
102-HTLV-I infected human cells, which were also
sensitive to endo H digestion (ref. 35).
The dual infection system (vTME-46/vTF7-3) was
compared with the single yaccinia virus recombinant
system (RW E3) in which expression of gp46 was under
control of the vaccinia promoter P7.5. The efficiency of
the T7 polymerase/T7 promoter-dependent expression by the
dual vaccinia system was apparent in its ability to
express more total HTLV-I surface envelope protein with
faster kinetics and less extensive cytopathic destruction
of the host cells. The promoter of such enhanced
quantities of expression product and the availability of
the HTLV-I envelope protein specific human monoclonal
antibodies described herein enables, for the first time,
the isolation and purification of HTLV-I envelope protein
having substantially the same conformation. as the
envelope protein in HTLV-I.
~~ola~~
13
Optimal conditions for the vTME-46/vTF7-3 dual
expression system were determined to attain maximal HTLV-
I envelope production. Best yields of the recombinant
envelope protein were observed approximately 36 to 48
hours following dual infection. There was no advantage
in allowing the infection to progress further due to the
increased cytopathic effects and cell death mediated by
vaccinia virus. When the effect of virus multiplicities
was studied in the dual gp46 expression system, it
appeared that a multiplicity of about two pfu/cell
yielded maximal amounts of glycosylated envelope protein.
Higher multiplicities of infection of 4,7 and 10 did not
result in increased yields of the recombinant protein.
The requirement of low viral multiplicities in the system
employed herein is in contrast to what was observed in a
dual vaccinia system constructed to express beta-
galactosidase (ref. 14). which required viral
multiplicities of ten for maximal yields.
Having regard to the observation that recombinant
protein expression levels can vary between different cell
types, human H9 T-cells were compared to human HeLa
epithelial cells as hosts for dual vaccinia infection and
recombinant envelope protein expression. While H9 and
HeLa cells are very different in function and origin, the
apparent size of the glycosylated (49 and 47 kDa) and
unglycosylated (39 kDa) envelope protein forms produced
by the two cell types did not vary significantly. This
result suggested that similar post-translational
processing of the recombinant envelope protein occurs in
different cell types of endothelial (HeLa) and
lymphocytic (H9) origin. In terms of protein yields,
however, there was a marked difference between the two
cell lines. The vTME-46/vTF7-3-infected HeLa cells
produced 4-fold more envelope protein than the same
number of co-infected H9 cells by densitometer analysis.
~~n ~~~
14
Native folding of the recombinant HTLV-I envelope
proteins produced by the dual vaccinia system was shown
by their ability to bind several HTLV-I envelope-specific
human monoclonal antibodies. All HTLV-I-specific
antibodies tested were capable of neutralizing HTLV-I
syncytium formation. These human monoclonal antibodies
do not recognize linear epitopes of denatured HTLV-I
envelope protein as shown by their lack of binding to
viral lysate-based Western blots. Instead, the
monoclonal antibodies appear to bind to discontinuous,
conformational epitopes as determined by
radioimmunoprecipitation and immunofluorescence of HTLV-I
infected cell lines (ref. 44). The ability of these
conformation-dependent monoclonal antibodies to bind to
the recombinant HTLV-T envelope proteins produced in the
dual vaccinia system, indicates that the proteins are
being folded and maintained in a native conformation.
The most glycosylated recombinant envelope protein forms
(49 and 47 kDa) appear to be processed and folded
substantially in the manner of the envelope protein
expressed by HTLV-I infected human cells, since the
recombinant proteins were readily immunoprecipitated with
the full panel of conformation-dependent human monoclonal
antibodies as well as several polyclonal sera obtained
from HTLV-I-infected human patients.
Conformational integrity of recombinant envelope
protein was also indicated by the ability of the specific
human monoclonal antibodies WA07/1G7, WA07/2F7 and
WAll/2F3 to bind to dual vaccinia-infected HeLa cells as
detected by indirect immunofluorescence. The WA07/1G7
and WA07/2F7 monoclonal antibodies exhibited the most
avid neutralizing/syncytium inhibition properties of the
six monoclonal antibodies tested, inhibiting greater than
90~ of syncytium formation at concentrations less than 5
~,g IgGl/ml. Strong binding of these two monoclonal
antibodies to the recombinant HTLV-I envelope protein
z~o~~~
suggested that the protein contains conformational
epitopes that are significant in virus neutralization/
syncytium inhibition.
The absence or weak immunofluorescence exhibited by
5 the other human monoclonal antibodies may be due to their
weaker affinity for the native HTLV-I envelope protein as
suggested by the higher concentrations of these
antibodies required to attain 90% syncytium inhibition of
HTLV-I infected cells (23 to 90 ~,g IgGl/ml) . Weak
10 immunofluorescence demonstrated by some of the monoclonal
antibodies may also be the result of slight sequence
variation between the HTLV-I envelope sequence encoded by
the recombinant vaccinia virus and that of the viral
strain of the monoclonal antibody hybridoma source.
15 Significant differences in epitope topography due to
sequence variation of the two envelope proteins are
unlikely since all six monoclonal antibodies appeared to
be equally capable of immunoprecipitating the recombinant
envelope proteins at an IgGl concentration of 7.5 ~,g/ml-
below the 100% syncytium inhibition threshold of all six
monoclonal antibodies. More likely, the lack of antibody
binding during immunofluorescence analysis may result
from the expression of gp46 in the absence of gp21 by the
dual vaccinia system. Without this transmembrane anchor
protein, the orientation of the recombinant gp46 (rgp46)
on the membrane surface of vTME-46/vTF7-3 infected cells
may not be identical to native gp46 found on the membrane
of HTLV-I-infected cells. If the HTLV-I envelope protein
naturally exists as an oligomer on the surface of virions
and infected cells, it may be expected that the gp46
oligomeric complex has differentially exposed epitopes
compared to a monomeric form. Distinct differences in-
the conformation and accessibility of various epitopes of
HIV gp120 monomers and oligomers has been detected using
domain-specific monoclonal antibodies (refs. 46, 47).
The unavailability of specific epitopes would prevent the
16
binding of particular antibodies to surface-associated
rgp46; however, these epitopes would become available
once the recombinant envelope proteins were in solution,
as demonstrated by their immunoprecipitation by all six
human monoclonal antibodies.
The biochemical and conformational integrity of the
recombinant envelope proteins produced in the dual
vaccinia system suggests that they may prove to be an
effective vaccine candidate. The conserved epitopes
recognized by HTLV-I, HTLV-II and STLVpan_p sera suggest
that the recombinant envelope proteins may afford
protection against infection of various HTLV-I isolates,
independent of viral primary sequence and potentially
confer cross-protection against HTLV-II and related
primate T-cell lymphotrophic viruses (PTLVs), such PTLV-
L, and STLVs, such as STLVPan_p, STLVI, STLVII and other
primate retroviruses related.to HTLV-II.
Preferential precipitation of the most glycosylated
recombinant HTLV-I envelope forms (47 and 49 kDa) by the
various polyclonal human sera and' the conformational
dependent human monoclonal antibodies indicates that
proper glycosylation of the recombinant is important in
establishing the native conformation of the protein.
However, an optimal vaccine against HTLV-I may require
inclusion of all five differentially glycosylated forms
of the recombinant envelope protein since each form may
present different epitopes to the immune system.
The recombinant HTLV-I envelope proteins provided
herein and produced recombinantly as described herein are
also useful diagnostic reagents in light of the fact that
they were recognized by various HTLV-II-infected human
sera and serum from a Pan paniscus chimpanzee infected
with STLVpan_p (ref . 29) . The STLVpan_p did not react with
either HTLV-I gp46 viral antigen, HTLV-I envelope peptide
MTA-1, or HTLV-II peptide K55 found on HTLV-I 2.3 blot
strips. In addition, the envelope sequence could not be
~o ~~~ ~
17
amplified by PCR from this infected monkey cell DNA by
HTLV-I/STLV-I envelope-specific primers (ref. 29). The
observation that the recombinant HTLV-I envelope proteins
provided herein and produced in the vaccinia/T7
polymerase system, are recognized and immunoprecipitated
by sera from the distantly related STLVpan_P, indicates
that these proteins are useful in diagnosis of HTLV-I and
HTLV-II in human Pygmy tribes (Bambuti and Bakola) who
demonstrate an atypical HTLV-I and HTLV-II seropositivity
(refs. 36, 37). This diagnostic embodiment is
particularly useful to screen primates for human or
simian related retroviruses where organs are to be used
in xenotransplantation. In particular, HTLV-I ELISA kits
use envelope antigen deficient in glycoproteins. The
envelope glycoprotein of HTLV-I having eventually the
same conformation as the envelope protein in native HTLV-
I allows for the ability to recognize and detect
conformational sensitive antibodies.
Accordingly, the HTLV-I gp46 envelope protein has
been expressed in a vaccinia/T7 polymerase system. The
protein was produced at high levels in a properly
processed and folded form. Glycosylation of the
recombinant gp46 in this mammalian system occurs at all
four potential N-linked glycosylation sites and resembles
that produced by an HTLV-I infected cell. The biochemical
and structural homology with native gp46 suggests that
the recombinant envelope protein might be useful as a
vaccine in eliciting protective immune responses in vivo,
and possibly aid in identifying the cell surface receptor
utilized by HTLV-I during infection. In addition, this
protein may prove to be an instrumental diagnostic
reagent for the identification of novel human
retroviruses.
It is clearly apparent to one skilled in the art,
that the various embodiments of the present invention
have many applications in the fields of vaccination,
zzot~~
18
diagnosis, treatment of viral infections and the
generation of immunological reagents. A further non-
limiting discussion of such uses is further presented
below.
1. Vaccine Preparation and Use
Immunogenic compositions, suitable to be used as
vaccines, may be prepared from the envelope protein of
HTLV-I, as well as analogs and fragments thereof, as
disclosed herein. The immunogenic composition elicits an
l0 immune response which produces antibodies, including
anti-envelope protein antibodies and antibodies that are
opsonizing or virus neutralizing.
Immunogenic compositions, including vaccines, may be
prepared as injectables, as liquid solutions or
emulsions. The envelope protein of HTLV-I may be mixed
with pharmaceutically acceptable excipients which are
compatible therewith. Such excipients may include,
water, saline, dextrose, glycerol, ethanol, and
combinations thereof.' The immunogenic compositions and
vaccines may further contain auxiliary substances, such
as wetting or emulsifying agents, pH buffering agents, or
adjuvants to enhance the effectiveness thereof.
Immunogenic compositions and vaccines may be administered
parenterally, by injection subcutaneously or
intramuscularly. Alternatively, the immunogenic
compositions formed according to the present invention,
may be formulated and delivered in a manner to evoke an
immune response at mucosal surfaces. Thus, the
immunogenic composition may be administered to mucosal
surfaces by, for example, the nasal or oral
(intragastric) routes. Alternatively, other modes of
administration including suppositories and oral
formulations may be desirable. For suppositories,
binders and carriers may include, for example,
polyalkalene glycols or triglycerides. Oral formulations
may include normally employed incipients such as, for
19
example, pharmaceutical grades of saccharine, cellulose
and magnesium carbonate. These compositions can take the
form of solutions, suspensions, tablets, pills, capsules,
sustained release formulations or powders and contain
about 1 to 95~ of the HTLV-I envelope protein. The
immunogenic preparations and vaccines are administered in
a manner compatible with the dosage formulation, and in
such amount as will be therapeutically effective,
protective and immunogenic. The quantity to be
administered depends on the subject to be treated,
including, for example, the capacity of the individual's
immune system to synthesize antibodies, and if needed, to
produce a cell-mediated immune response. Precise amounts
of active ii'~.gredient required to be administered depend
on the judgment of the practitioner. However, suitable
dosage ranges are readily determinable by one skilled in
the art and may be of the order of micrograms of the
lactoferrin receptor protein. Suitable regimes for
initial administration and booster doses are also
variable, but may include an initial administration
followed by subsequent administrations. The dosage may
also depend on the route of administration and will vary
according to the size of the host.
The concentration of the HTLV-I envelope protein in
an immunogenic composition according to the invention is
in general about 1 to 95~. A vaccine which contains
antigenic material of only one pathogen is a monovalent
vaccine. Vaccines which contain antigenic material of
several pathogens are combined vaccines and also belong
to the present invention. Such combined vaccines
contain, for example, material from various pathogens or
from various strains of the same pathogen, or from
combinations of various pathogens.
Immunogenicity can be significantly improved if the
antigens are co-administered with adjuvants, commonly
used as 0.05 to 0.1 percent solution in phosphate
20 ' 2
buffered saline. Adjuvants enhance the immunogenicity of
an antigen but are not necessarily immunogenic
themselves. Adjuvants may act, by retaining the antigen
locally near the site of administration to produce a
depot effect facilitating a slow, sustained release of
antigen to cells of the immune system. Adjuvants can
also attract cells of the immune system to an antigen
depot and stimulate such cells to elicit immune
responses.
Immunostimulatory agents or adjuvants have been used
for many years to improve the host immune responses to,
for example, vaccines. Intrinsic adjuvants, such as
lipopolysaccharides, normally are the components of the
killed or attenuated bacteria used as vaccines.
Extrinsic adjuvants are immunomodulators which are
typically non-covalently linked to antigens and are
formulated to enhance the host immune responses. Thus,
adjuvants have been identified that enhance the immune
response to antigens delivered parenterally. Some of
these adjuvants are toxic, however, and can cause
undesirable side-effects, making them unsuitable for use
in humans and many animals. Indeed, only aluminum
hydroxide and aluminum phosphate (collectively commonly
referred to as alum? are routinely used as adjuvants in
human and veterinary vaccines. The efficacy of alum in
increasing antibody responses to diphtheria and tetanus
toxoids is well established and a HBsAg vaccine. has been
adjuvanted with alum. While the usefulness of alum is
well established for some applications, it has
limitations. For example, alum is ineffective for
influenza vaccination and inconsistently elicits a cell
mediated immune response. The antibodies elicited by~
alum-adjuvanted antigens are mainly of the IgGl isotype
in the mouse, which may not be optimal for protection by
some vaccinal agents.
CA 02201419 2004-11-24
21
A wide range of extrinsic adjuvants can provoke
potent immune responses to antigens. These include
saponins complexed to membrane protein antigens (immune
stimulating complexes), pluronic polymers with mineral
oil, killed mycobacteria in mineral oil, Freund's
complete adjuvant, bacterial products, such as muramyl
dipeptide (MDP) and lipopolysaccharide (LPS), as well as
lipid A, and liposomes.
To efficiently induce humoral immune responses
io (HIR) and cell-mediated immunity (CMI), immunogens are
often emulsified in adjuvants. Many adjuvants are
toxic, inducing granulomas, acute and chronic
inflammations (Freund's complete adjuvant, FCA),
cytolysis (saponins and Pluronic polymers) and
pyrogenicity, arthritis and anterior uveitis (LPS and
MDP) . Although FCA is an excellent adjuvant and widely
used in research, it is not licensed for use in human or
veterinary vaccines because of its toxicity.
Desirable characteristics of ideal adjuvants
2o include:
(1) lack of toxicity;
(2) ability to stimulate a long-lasting immune
response;
(3) simplicity of manufacture and stability in long-
z5 term storage;
(4) ability to elicit both CMI and HIR to antigens
administered by various routes, if required;
(5) synergy with other adjuvants;
(6) capability of selectively interacting with
3o populations of antigen presenting cells (APC);
(7) ability to specifically elicit appropriate TH1 or
TH2 cell-specific immune responses; and
(8) ability to selectively increase appropriate
antibody isotype levels (for example, IgA) against
35 antigens.
U.S. Patent No. 4,855,283 granted to Lockhoff et al
on August 8, 1989 teaches glycolipid analogues including
CA 02201419 2004-11-24
22
N-glycosylamides, N-glycosylureas and N-
glycosylcarbamates, each of which is substituted in the
sugar residue by an amino acid, as immuno-modulators or
adjuvants. Thus, Lockhoff et al. (US Patent No.
4,855,283 and ref. 29) reported that N-glycolipid
analogs displaying structural similarities to the
naturally-occurring glycolipids, such as
glycosphingolipids and glycoglycerolipids, are capable
of eliciting strong immune responses in both herpes
io simplex virus vaccine and pseudorabies virus vaccine.
Some glycolipids have been synthesized from long chain
alkylamines and fatty acids that are linked directly
with the sugars through the anomeric carbon atom, to
mimic the functions of the naturally occurring lipid
i5 residues.
U.S. Patent No. 4,258,029 granted to Moloney
teaches that octadecyl tyrosine hydrochloride (OTH)
functioned as an adjuvant when complexed with tetanus
toxoid and formalin inactivated type I, II and III
2o poliomyelitis virus vaccine. Also, Nixon-George et al.
(ref. 30), reported that octadecyl esters of aromatic
amino acids complexed with a recombinant hepatitis B
surface antigen, enhanced the host immune responses
against hepatitis B virus.
2s Lipidation of synthetic peptides has also been used
to increase their immunogenicity. Thus, V~Iiesmuller
1989, describes a peptide with a sequence homologous to
a foot-and-mouth disease viral protein coupled to an
adjuvant tripalmityl-s-glyceryl-cysteinylserylserine,
3o being a synthetic analogous of the N-terminal part of
the lipoprotein from Gram negative bacteria.
Furthermore, Deres et al. 1989, reported in vivo priming
of virus-specific cytotoxic T lymphocytes with synthetic
lipopeptide vaccine which comprised of modified
35 synthetic peptides derived from influenza virus
nucleoprotein by linkage to a lipopeptide, N-palmityl-s-
[2, 3-bis (palmitylxy) - (2RS) -propyl- [R] -cysteine (TPC) .
CA 02201419 2004-11-24
23
2. Immunoassays
The envelope protein HTLV-I of the present invention
is useful as an immunogen for the generation of anti-
envelope protein antibodies, as an antigen in
immunoassays including enzyme-linked immunosorbent assays
(ELISA), RIAs and other non-enzyme linked antibody
binding assays or procedures known in the art for the
detection of antibodies. In ELISA assays, the envelope
protein is immobilized onto a selected surface, for
example, a surface capable of binding proteins such as
the wells of a polystyrene microtiter plate. After
washing to remove incompletely adsorbed envelope protein,
a nonspecific protein, such as a solution of bovine serum
albumin (BSA) that is known to be antigenically neutral
with regard to the test sample, may be bound to the
selected surface. This allows for blocking of
nonspecific adsorption sites on the immobilizing surface
and thus reduces the background caused by nonspecific
bindings of antisera onto the surface.
The immobilizing surface is then contacted with a
sample, such as clinical or biological materials, to be
tested in a manner conducive to immune complex
(antigen/antibody) formation. This may include diluting
the sample with diluents, such as solutions of BSA,
bovine gamma globulin (BGG) and/or phosphate buffered
saline (PBS)/Tween'"". The sample is then allowed to
incubate for from about 2 to 4 hours, at temperatures
such as of the order of about 25° to 37°C. Following
incubation, the sample-contacted surface is washed to
remove non-immunocomplexed material. The washing
procedure may include washing with a solution, such as
PBS/Tween or a borate buffer. Following formation of
specific immunocomplexes between the test sample and the
bound envelope protein of HTLV-I, and subsequent washing,
the occurrence, and even amount, of immunocomplex
formation may be determined by subjecting the
0 1 4~ °t 9
24
immunocomplex to a second antibody having specificity for
the first antibody. If the test sample is of human
origin, the second antibody is an antibody having
specificity for human immunoglobulins and in general IgG.
To provide detecting means, the second antibody may have
an associated activity such as an enzymatic activity that
will generate, for example, a colour development upon
incubating with an appropriate chromogenic substrate.
Quantification may then be achieved by measuring the
degree of colour generation using, for example, a visible
spectra spectrophotometer.
The envelope protein of HTLV-I provided herein also
recognizes antibodies to HTLV-II and other T-cell
lymphotroptic retroviruses, such as STLVpan_P, STLVI,
STLVII and other primate retroviruses related to HTLV-II
and hence is useful for detection of. monoclonal
antibodies specific to such diseases in suitable
biological samples.
Bioloctical Deposits
Certain hybridomas produce human monoclonal
antibodies specific for HTLV-I envelope protein that
according to aspects ~of the present invention that are
described and referred to herein have been deposited with
the American Type Culture Collection (ATCC) located at
12301 Parklawn Drive, Rockville, Maryland, USA, 20852,
pursuant to the Budapest Treaty and prior to the filing
of this application. Samples of the deposited hybridomas
will become available to the public upon grant of a
patent based upon this United States patent application.
The invention described and claimed herein is not to be
limited in scope by the hybridomas deposited, since the
deposited embodiment is intended only as an illustration
of the invention. Any equivalent or similar hybridomas
that produce similar or equivalent antibodies as
described in this application are within the scope of the
invention.
~~ ~~1 9
Deposit Summary
Hybridomas ATCC Designation Date Deposited
WA07/1G7
WA07/2F7
5 WAl1/2F3
WA11/1F5
WA04/2B10
EXAMPLES
The above disclosure generally describes the present
10 invention. A more complete understanding can be obtained
by reference to the following specific Examples. These
Examples are described solely for purposes of
illustration and are not intended to limit the scope of
the invention. Changes in form and substitution of
15 equivalents are contemplated as circumstances may suggest
or render expedient. Although specific terms have been
employed herein, such terms are intended in a descriptive
sense and not for purposes of limitations.
Methods of molecular genetics, protein biochemistry,
20 and immunology used but not explicitly described in this
disclosure and these Examples are amply reported in the
scientific literature and are well within the ability of
those skilled in the art.
Example 1
25 This Example describes the construction of a
recombinant plasmid containing the gene encoding the
HTLV-I gp46 protein.
The HTLV-I envelope gene fragment encoding gp46 was
derived from the plasmid pMT-2 (provided by Dr. R. C.
Gallo, NCI/NIH; ref. 18). Two translational stop codons
were placed immediately downstream of the gp46 coding
region by insertion of a Kpnl-EcoRI oligonucleotide.
Plasmid pTME-46 was constructed by insertion of the 945
base pair (bp) envelope fragment (Ncol-Xmal) into the
expression vector, pTM-1, proviced by Dr. B. Moss (NIH,
NIAID, LVD; ref. 19).
26
Example 2
This Example describes the construction of
recombinant vaccinia virus.
Mouse thymidine kinase negative (TK-) L cells and
human HeLa cells (American Type Tissue Culture
Collection) were grown in Dulbecco modified Eagle medium
containing 10% fetal bovine serum (FBS; gibco-BRL) and
100 units/ml of penicillin G and streptomycin.
The recombinant expression vector, pTME-46, was used
as the vehicle for insertion of the HTLV-I envelope
glycoprotein gp46 gene fragment into the vaccinia virus
genome (strain J, provided by Dr. S. Dales, University of
Western Ontario; Ref. 20). The recombinant vaccinia
transfer~vector (pTME-46) was constructed by inserting
the HTLV-I envelope gp46 gene fragment immediately
downstream of the bacteriophage T7 promoter of pTM-1
(ref. 19). The presence of the encephalomyocarditis
virus (EMC) 5' untranslated region between the T7 promoter
and the gp46.gene promised efficient translation of the
uncapped envelope RNA (ref. 19).
Recombinant virus was prepared by infecting mouse
TK-L cells with wild-type vaccinia virus and transfecting
them with calcium phosphate-precipitated pTME-46 plasmid
DNA (refs. 21, 22). The cells were harvested and TK-
vaccinia virus was isolated by three successive plaque
assays on TK- cells in the presence of 5-
bromodeoxyuridine (BUdR) 25~.g/ml. Following southern
blot confirmation, large stocks of recombinant virus
(vTME-46) were prepared under nonselective conditions in
HeLa cells. The recombinant vaccinia virus expressing T7
RNA polymerise, vTF7-3, was obtained from Dr. T. Fuerst
and Dr. B. Moss (through the AIDS Research and Reference
Reagent Program of AIDS, NIAID, HIH; ref. 23). The
single recombinant vaccinia virus RW E3 was used as a
positive control and has been previously described in
detail (refs. 16, 17). RW E3 contains the identical
- CA 02201419 2004-11-24
27
gp46 gene fragment as vTME-46, but expression is
regulated by the vaccinia early/late promoter P7.5.
Example 3
This Example describes the analysis of recombinantly
produced gp46.
The isolated vTME-46 viral clone was capable of T7
promoter-controlled expression of HTLV-I gp46 upon co-
infection with the vaccinia virus vTF7-3 (ref . 23) , which
encodes the highly efficient bacteriophage T7 polymerase.
Western blot analysis of dually infected HeLa cells
revealed expression of five forms of the surface envelope
protein, ranging in molecular weight from about 39 to
about 49 kDa. Their HTLV-I envelope origin was shown by
their specific immunoreactivity to both anti-gp46 1C11
monoclonal antibody and polyclonal anti-SP3/SP4A envelope
peptide sera (Figure 1).
For immunoblotting, cell pellets were resuspended in
extraction buffer and 1mM PMSF. Following addition of an
equal volume of 2x Laemmli buffer, the samples were
boiled for 10 minutes and briefly sonicated. The
supernatants were electrophoresed and transferred from a
SDS/6M urea 12% polyacrylamide gel (ref . 29) to ImmobilonT""
P membrane (Millipore) . Blots were blocked in a solution
of 5% skim milk powder (ref. 30) at room temperature for
5 hours . Blots were incubated in the appropriate primary
antibody, diluted in blocking buffer, overnight at 4°C
with rocking. Primary antibodies include (i) 1C11, an
anti-gp46 mouse monoclonal antibody (ref. 28); (ii) anti-
SP3/4A rabbit polyclonal serum (SP3 and SP4A peptide
sequences were derived from gp46; (ref. 28); (iii) human
HTLV-I patient sera (HAM/TSP); (iv) human HTLV-II patient
sera (American Red Cross); (v) human "HTLV-indeterminate"
patient sera (Canadian Red Cross); and (vi) polyclonal
serum from a pygmy chimpanzee (Pan paniscus) infected
with STLVpan-p; (ref. 29). Blots were then washed in
blocking buffer and exposed to goat anti-mouse, goat
CA 02201419 2004-11-24
28
anti-rabbit or goat anti-human IgG antibodies (Abs)
conjugated to alkaline phosphatase or horse radish
peroxidase (Jackson ImmunoResearch Laboratories Inc.) at
a final dilution of 1:5000, for 30 minutes at room
temperature. The blot were washed and exposed to
substrate according to the manufacturer's instruction
(Blot Detection Kit for the alkaline phosphatase
conjugated 2°Abs or Enhanced Chemiluminescence for the
horse radish conjugated 2°Abs; Amersham International,
PLC ) .
To determine if the five variant forms of the
surface envelope protein resulted from differential
glycosylation of the protein, vTME-46/vTF7-3 infected
cell lysates were digested with endoglycosidase H (endo
H) and glycopeptidase F (PNGase F).
Culture supernatants were supplemented with 1 mM
ethylenediaminetetraacetic acid (EDTA) and 1 mM phenyl-
methyl-sulfonyl-fluoride. Cells were lysed in cold
extraction buffer (pH 7.6: 100mM NaCl, 10 mM sodium
phosphate, 1% Triton" X-100, 0.5% sodium deoxycholate,
0.1% SDS, 1 mM EDTA, and 1 mM PMSF). Supernatants and
cell lysates were pre-cleared for 18 hours at 4°C by
incubation with 60 ~.1 Protein G Plus/A Agarose (Oncogene
Science), which had been preincubated for 2 hour at 4°C
with normal sera of the appropriate species. The Protein
G Plus/A Agarose was pelleted and the resulting
supernatant was divided into 5 x 106 cell equivalents.
Immunoprecipitations were performed at 4°C for 18 hours,
in the presence of 40 ~1 of Protein G Plus/A agarose and
various HTLV-I specific antisera as specified. Anti-gp46
mouse monoclonal antibody 1C11 was provided by Dr. Tom
Palker (Duke University, North Carolina; ref. 28) as
tissue culture supernatant and diluted 1:4. HTLV-I
envelope-specific human monoclonal antibodies (HMAbs;
WAll/1F5; WA07/2F7; WA07/1G7; WAll/2E2; WAll/2F3; and
WA04/2B10) were added at a concentrated of 7.5 ~.g of
X20 ~°(
29
IgG/ml of suspension. Polyclonal human sera from HAM/TSP
patients were used at dilutions of 1:4,000 to 1:20,000,
as specified. Human sera from an HTLV-II positive
individual was used at a dilution of 1:400. Polyclonal
sera from a pygmy chimpanzee (Pan paniscus) infected with
STLVpan-p was provided by Dr. G. Franchini (NCI,
Maryland) and was diluted 1:200. Immune complexes were
washed 4 times with extraction buffer and then
resuspended in the appropriate buffer.
For radiolabelling cells were labelled with [35S]L-
cysteine (0.125 mCi/5 x 106 cells; NEN) for 5 hours in
cysteine-free media appropriate for the cell-type
utilized, supplemented with 2% dialyzed FBS. When
appropriate, 2 ~g/ml of tunicamycin was added to the
washing and incubated media.
Following immunoprecipitation with mouse monoclonal
antibody 1C11, immune complexes were washed four times in
extraction buffer. Protein equivalents of 2.5 x 106
cells were then digested with endoglycosidase H (endo H)
or glycopeptidase F (PNGase F) at 37°C for 20 hours.
EndoH digestion was performed in the presence of 25 mM
sodium acetate, pH 5.0, 1 mM PMSF and 12 mU endoH
(Boehringer Mannheim). For PNGase F digestion, the
immune complexes were incubated 25 mM sodium phosphate,
pH 7.0, 1% Nonidet P-40, 1 mM EDTA, 1 mM PMSF and 1.5U of
PNGase F (Boehringer Mannheim). Digestion products were
compared to immunoprecipitates obtained from vTME-
46/vTF7-3 infected cells treated with 2 ~.g/ml tunicamycin
for 8 hours prior to radiolabelling.
The endo H enzyme is only able to remove glycan
branches of mannose-rich or hybrid glycoproteins but not
those of complex glycoproteins (ref. 32) while PNGase F
will remove all N-linked oligosaccharides independent of
complexity (ref. 33). Digestion products were compared
to immunoprecipitates obtained from vTME-46/vTF7-3
~o ~~~ 9
infected cells treated with the N-glycosylation
inhibitor, turiicamycin.
As shown in Figure 2, immunoprecipitation of normal
infected cell lysates with anti-gp46 monoclonal antibody
5 1C11 yielded the five variant forms of the surface
envelope protein, 39-49 kDa. Complete Endo H digestion
of the normal infected cell lysates produced only the 39
kDa protein form. Digestion with PNGase F repeatedly
produced both the 43 and 39 kDa forms. Resistance of the
10 43 kDa protein form to PNGase F digestion suggests that
one glycosylation site may be located in a region of the
protein that is not readily susceptible/accessible to
PNGase F. As expected, dual infection in the presence of
tunicamycin yielded only the 39 kDa protein from upon
15 radioimmunoprecipitation. No protein forms of molecular
weight less than 39 kDa were visualized suggesting that
the 39 kDa form~represents the completely unglycosylated
envelope protein. The 10 kDa shift in molecular mass
observed following endoglycosidase digestion or
20 tunicamycin treatment is consistent with the loss of four
oligosaccharrides and, suggested that all four potential
N-linked glycosylation sites of the recombinant envelope
protein are utilized for glycan addition (ref. 48). The
envelope glycoproteins expressed by this mammalian system
25 have both mannose-rich and hybrid oligosaccharides
attached, as determined by endo H digestion.
Samples were resuspended in an equal volume of 2x
Laemmli sample buffer, boiled for 10 minutes and analyzed
by SDS-PAGE using 12~ polyacrylamide SDS/6M urea gels.
30 Example 4
This Example describes the optimization of
expression of HTLV-I envelope proteins.
Since the T7 RNA polymerase and the HTLV-I gp46
envelope genes are carried on different viruses, we
determined the amount of each virus needed for optimal
expression. HeLa cells were co-infected with recombinant
31
viruses vTF7-3 and vTME-46 at a range of multiplicities
of infection. Upon western blot analysis, it appeared
that a multiplicity of 2 pfu/cell appeared to yield
maximal amounts of glycosylated envelope protein (Figure
3). Higher mois of 4, 7 and 10 pfu/cell did not result
in increased yields of the recombinant protein forms.
The dual infection system (vTME-46/vTF7-3) was
compared with a single vaccinia virus recombinant system
(RW E3 ) in which expression of gp46 was under control of
the vaccinia promoter P7.5. This vaccinia promoter P7.5,
controlling gp46 expression in RW E3, was the same as
that regulating expression of the T7 polymerase gene in
recombinant virus vTF7-3. The high efficiency of T7
polymerase/T7 promoter-dependent expression was observed
when vTME-46/vTF7-3 infection at a moi of 2 (Figure 3,
lane 2) expressed twice as much total envelope protein
(total of all the variously glycosylated forms) as
compared by densitometry to that produced by single
vaccinia RW E3 infection at a moi of 4 pfu/cell (Figure
3, lane 1).
To further determine the conditions for optimal
expression, a time course of HTLV-I envelope expression
in infected HeLa cells was examined. Cells dually
infected with vTME-46/vTF7-3 or singly infected with RW
E3 were harvested at various times and the proteins were
analyzed by immunoblotting with sera from an HTLV-I
infected patient (Figure 4) . Maximal yields of the HTLV-
I surface envelope forms by the dual vaccinia/T7
polymerase system appear to occur between 36 and 48 hours
post infection (p.i.). Already at 12 hours p.i., the
vTME-46/vTF7-3 infection was capable of producing more
total HTLV-I envelope protein than the single vaccinia
system, RW E3 , was able to produce at 24 hours post-
infection.
Human H9 T-cells were compared to human HeLa
epithelial cells as hosts for dual vaccinia virus
~~o~~~~
32
infection and recombinant envelope protein expression.
Both cell lines were infected with the same viral stocks,
at the same multiplicities and harvested at the same
time. Each infection was examined by both western blot
analysis and immunofluorescence for envelope protein
expression.
Western blot analysis revealed that HeLa cells
produced 4-fold more envelope protein than the same
number of H9 cells (Figure 5). The five different
envelope forms (39-49 kDa) are seen upon dual vaccinia
infection of HeLa cells (Figure 5, lane 2) while infected
H9 cells express only the two most glycosylated envelope
forms, 47 kDa and 49 kDa (Figure 5, lane 1) and trace
amounts of the 39 kDa protein. The difference in
expression levels was also apparent upon
immunofluorescence analysis of the two infected cell
lines. Strong immunofluorescence ~of >90% of the HeLa
cells in the monolayer was observed, in contrast to
moderate immunofluorescence of only 30% of the H9 cells.
Higher virus multiplicities of 8 and 10 pfu/cell did not
appear to increase the amount of gp46 expression by H9
cells or the percentage of vaccinia-infected cells.
Single infections with vTME-46 suggested that this
recombinant virus is restricted in its infectivity of H9
cells, but not of HeLa cells.
For indirect immunofluorescence of dual recombinant
vaccinia-infected cells, human H9 T-cells were infected
at a multiplicity of infection (moi) of 4 for 1 hour at
room temperature. The free viral inoculum was removed
and the infection was allowed to progress for 24 hours.
The cells were washed three times in phosphate buffered
saline (PBS) containing 2% FBS and resuspended at a final
concentration of 8 x 106 cells/ml. Each spot of a 24-
spot teflon-coated super-cured slide (HTC) received 3 ~,l
of cell suspension. HeLa cells were infected in
suspension at an moi of 4 for 1 hour at room temperature.
33
The viral supernatant was removed and the cells were
resuspended at a final concentration of 1.6 x 105
cells/ml. Three hundred ~,l of cells suspension was
plated per well in permanox 6-chamber slides (Nunc) and
incubated for 24 hours. Following air-drying, all slides
were fixed in room temperature acetone for 10 minutes.
Uninfected cells and those cells infected with only one
recombinant virus. vTF7-3, were included on each slide
as negative controls. slides were rinsed twice in PBS
(pH 7.2) supplemented with 2% FCS. Non-specific binding
was blocked by incubating the washed cells in a 4~
solution of skim milk powder in PBS (Blotto) for 1 hour
at room temperature. Cells were further incubated 30
minutes at 37°C in 1:20 dilutions of various primary sera
(i) HTLV-I envelope-specific human monoclonal antibodies
at 40 ~.g/ml (WA11/1F5; WA07/2F7; WA07/1G7; WA11/2E2;
WA11/2F3; and WA04/2B10;44); (ii) Human monoclonal
antibody 0.5-alpha specific for gp46 (ref. 30); (iii)
human HTLV-I-infected patient sera (HAM/TSP). After
washing, cells were incubated for 30 minutes at 37°C with
goat anti-human IgG fluorescein isothiocyanate conjugated
antibody (GAH-FITC, Jackson ImmunoResearch) diluted in
blocking solution. Rinsed cells were viewed on an
Olympus BH-2 fluorescence microscope with a fluorescein
filter.
Example 5
This Example illustrates that epitopes of HTLV-I
gp46 is shared with HTLV-II and STLVpan-p~
A panel of HTLV-I positive sera (confirmed by ELISA
and PCR) from both asymptomatic and HAM/TSP patients was
screened for reactivity to the recombinant envelope
proteins produced in the vTME-46/vTF7-3 infected HeLa
cell system (Figure 6, lane 1-4). Despite different
clinical status, all HTLV-I positive sera recognized each
of the five glycosylated recombinant envelope proteins
upon western blot analysis. Figure 6 contains a
_.
34
representative panel of asymptomatic HTLV-I patient sera
( lanes 1 and 2 ) and HA~I/TSP patient sera ( lanes 3 and 4 ) .
Western blot reactivity of HTLV-II sera with the
recombinant envelope proteins produced by the dual
vaccinia/T7 polymerase system was also tested. All HTLV
II positive samples tested had been previously confirmed
by ELISA and PCR to be HTLV-I negative/HTLV-II positive
by the American Red Cross . Upon western blotting, all 11
samples of HTLV-II-infected patient serum tested
recognized one or more of the recombinant HTLV-I envelope
protein forms. Seroreactivity to the 47 kDa envelope
protein was consistently observed in all the HTLV-II
positive samples. Figure 6 contains a panel of four
representative HTLV-II human sera which demonstrated
heterologous reactivity patterns to the various envelope
forms (lanes 5-8). Some HTLV-II individuals exhibited
seroreactivity patterns identical to sera from
individuals infected with HTLV-I (Figure 6, compare lanes
5 and 6 to lanes 1 to 4).
Eight additional sera were obtained from the
Canadian Red Cross that had been previously identified as
"HTLV-indeterminate" by analysis with the diagnostic
Biotechnology HTLV Blot 2.3 strips. Interestingly, all
eight "indeterminate" serum samples (1000 exhibited
western blot reactivity to one or more of the recombinant
HTLV-I protein forms produced by the dual vaccinia/T7
polymerase system. Binding to the 47 kDa protein was
observed in 7/8 cases (data not shown). Thus, the
recombinant envelope proteins allowed identification of
HTLV infected individuals that was not previously
possible. No reactivity to any HTLV-I envelope proteins
was observed in the normal human serum samples tested
thus far (example Figure 6).
When sera from a pygmy chimp infected with a novel
STLVpan_p strain (ref . 29) was screened by western blot,
predominant reactivity was observed to the fully
35
glycosylated 49 kDa protein and the unglycosylated 39 kDa
HTLV-I envelope proteins (Figure 6, lanes 1 to 11). Low
level reactivity of the STLVpan_p sera to the intermediate
glycosylated forms was observed following longer film
exposures of the blot. In addition to recognition of the
recombinant proteins in a denatured form, the sera from
the STLVPa"_p infected chimpanzee and HTLV-I infected and
HTLV-II infected humans were also capable of their
immunoprecipitation. The two most glycosylated forms of
the envelope protein (47 and 49 kDa) were preferentially
bound and precipitated by all the clinical samples
tested. Dr. Giri and colleagues reported that the
STLVPan_P did not react with either HTLV-I gp46 viral
antigen, HTLV-I envelope peptide MTA-l, or HTLV-II
peptide K55 found on HTLV-I 2.3 blot strips (Cellular
Products). In addition, envelope sequence could not be
amplified by PCR from this infected monkey cell DNA by
HTLV-I/STLV-I envelope-specific primers (ref. 29). The
recombinant HTLV-I envelope proteins, produced here are
recognized and immunoprecipitated by sera form the
distantly related STLVpan_p, and these proteins may also be
useful in diagnosis of HTLV-I and HTLV-II infection in
human Pygmy tribes (Bambuti and Bakola) who demonstrate
an atypical HTLV-I and HTLV-II seropositivity (refs. 36,
37) as well as to other more distantly related human
retroviruses.
Example 6
This Example describes the generation of human
monoclonal antibodies specific for HTLV-I gp46.
B lymphocytes from a HAM/TSP patient were isolated,
activated with Epstein-Barr virus (EBV), and fused to
mouse-human heteromyeloma cell lines by electrofusion, as
previously described (refs. 24, 25, 26). Briefly, B cell
enriched populations were prepared by rosetting out T
cells with 2-aminoethylsiothioronium bromide hydrobromide
treated sheep red blood cells. B lymphocytes were
36
activated a 104 cells/well with 10-305 v/v supernatant
from the B95-8 marmoset line as a source of EBV, and when
proliferating and yellowing the supernatant, were
screened for IgG anti-HTLV-I activity by indirect
immunofluorescence assay with MT-2 cells fixed on a slide
(ref. 27) . Hybridomas were produced, by fusing cells from
reactive wells to heteromyeloma fusion partners by
electrofusion using an alternating current of lMHz 6V AC,
and then screened for initial activity in the same manner
l0 as the EBV activated B cells, cloned to stabilize and
ensure monoclonality, and supernatant produced for the
further characterization. The WA07 hybrids were produced
fusing 1.5 x 106 EBV activated B cells to K6H6-B5
(courtesy of R Levy, Stanford University) in iso-osmolar
fusion medium 300L3) with 3 pulses of 15 microseconds at
1.75 kV/cm DC. WA4 2B10 was produced fusing 1.5 x 105
EBV activated B cells to H73C11 in hypo-osmolar fusion
medium (100L3} with 1 pulse of 10 seconds at 1.25 kV/cm
DC.
This procedure produced human monoclonal antibodies
specific for the HTLV-I envelope protein, designated
WAll/1F5, WA07/2F7, WA07/1G7, WAll/2E2, WAll/2F3 and
WA04/2B10 and the characteristics of these are shown in
Table I below.
37
TABLE I
B cells from a HAM/TSP patient were EBV activated and
fused by electrofusion with mouse-human heteromycloma
cells. Specific HMAbs were identified and characterized
by immunofluorescence assay (IFA) with fixed cells or
with live cell assays (LCA) with HTLV-I or HTLV-II
infected cells, by Western blots, by RIPA and by
syncytium inhibition assays (SIA).
IFA IFA LCA LCA WESTERN
ANTI$QI)Y I II I II BLOT RIPA SIA
WA07/1G7 + + + + 0 gp68 ++
WA07/2F7 + + +, 0 gp68 ++
WA07/2F9 + + + + 0 gp68 +
WAll/2F3 + +/- + +/- 0 gp68 +
WAl1/2E2 + +/- + +/- 0 gp68 +
WAll/1F5 + 0 + +/- 0 gp68 +
WA04/2B10 + 0 + 0 0 gp68 ++
WA07/2B10 + 0 + 0 gp46 gp68 0
WA08/2E9 + 0 + 0 0 0 0
WA07/lE4 + + 0 0 gp21;p21E 0 0
WA11/2C2 + 0 0 0 0 0
The data collectively indicates HMAbs o
HTLV-I t the
transmembrane protein, one three
nonconformational
and
conformational epit opes of the envelope rotein.
p
~Q~~~
38
SUMMARY OF THE DISCLOSURE
In summary of this disclosure, the present invention
provides novel isolated and purified envelope protein of
Human T-cell Lymphotrophic Virus Type I (HTLV-I) devoid
of non-envelope proteins of HTLV-I having substantially
the same conformation as the envelope protein in native
HTLV-I and methods of purification of such envelope
proteins. Also provided are human monoclonal antibodies
specific for conformational epitopes of the HTLV-I
envelope protein which are substantially non-binding to
HTLV-I envelope protein in a denatured form.
Modifications are possible within the scope of the
invention.
39
REFERENCES
1. Robert-Guroff, M., Nakao, Y., Notake, K. Ito, Y.,
Sliski, A., and R.C. Gallo. 1982. Natural antibodies
to human retrovirus HTLV in a cluster of Japanese
patients with adult T cell leukemia. Science. 215:975-
978.
2. Yoshida, M., Seiki, M., Yamaguchi, K., and K.
Takatsuki. 1984. Monoclonal integration of human T-
cell leukemia provirus in all primary tumours of adult
T-cell leukemia suggests causative role of human T-
cell leukemia virus in the disease. Proc. Natl. Acad.
Sci. USA. 81:2534-2537.
3. Osame, M., Usuku, K., Izumo, S., Ijichi, N., Amitani,
H., Igata, A., Matsumoto, M., and M. Tara. 1986.
HTLV-I Associated Myelopathy, a new clinical entity.
Lancet. 1:1031-1032.
4. Ijichi, S., Matsuda, T., Maruyama, I., Izumihara, T.,
Kojima, K., Niimura, T., Maruyama, Y., Sonoda, S.,
Yoshida, A., and M. Osame. 1990. Arthritis in human
T lymphotropic virus type (HTLV-I) carrier. Ann.
Rheum. Dis. 49:718-721.
5. Mochizuki, M., Watanabe, T., Yamaguchi, K., Takatsuki,
K., Shirao, M., Nakashima, S., Mori, S., Araki, S.,
and N. Miyata. 1992. HTLV-I uveitis: a distinct
clinical entity caused by HTLV-I. Jpn. J. of Cancer
Res. 83:236-239.
6. Lagrenade, L., Hanchard, B., Fletcher, V., Cranston,
B., and W. Blattner. 1990. Infective dermatitis of
Jamaican children: a marker for HTLV-I infection.
Lancet. 336:1345-1347.
7. De The, G., and R. Bonford. 1993. An HTLV-I vaccine:
why, how and for whom? AIDS Res. Hum. Retroviruses.
9:381-386.
8. Lillehoj, E., Tal, C., Nguyen, A., and S. Alexander.
1989. Characterization of env and tax encoded
polypeptides of human T-cell leukemia virus type I.
Clin. Biotechn. 1:27-41.
9. De, B., Lairmore, M., Gri.ffis, K., Williams, L.,
Villinger, F., Quinn, T., Brown, C., Nzilambi,
Sugimoto, M., Araki, S., and T. Folks. 1991.
Comparative analysis of nucleotide sequences of the
partial envelope gene (5' domain) among human T-
lymphotropic virus type I (HTLV-I) isolates.
Virology. 182:413-419.
i
~a ~~~ ~
J
10. Kinoshita, T., Tsujimoto, A., and K. Shimotohno. 1991.
Sequence variations in LTR and env regions of HTLV-I
do not discriminate between the virus from patients
with HTLV-I associated myelopathy and adult t-cell
leukemia. Int. J. Cancer. 47:491-495.
11. Komurian, F., Pelloguin, F., and G. De The. 1991. In
vivo genetic variability of human T-cell leukemia
virus type I depends more upon geography than upon
pathologies. J. Virol. 65:'3770-3778.
12. Pique, C., Tursz, T., and M.-C. Dokhelar. 1990.
Mutations introduced along the HTLV-I envelope gene
result in a non-functional protein: a basis for
envelope conservatino? EMBO J. 9:4243-4248.
13. Pique, C., Pham, D., Tursz, T., and M.-C. Dokhelar.
1992. Human T-cell Leukemia Virus Type I envelope
protein maturation process: Requirements for syncytium
formation. J. Virol. 66:906-913.
14. Arp, J., Ford, C., Palker, T., King, E., and G.
Dekaban. 1993. Expression and immunogenicity of the
entire human T-cell leukaemia virus type I envelope
protein produced in a baculovirus system. J. Gen.
Virol. 74:211-222.
15. Manta, F., Li Pira, G., Fenoglio, D., Valle, M.T.,
Kunkl, A., Ferraris, A., Mortara, L., Balderas, R.,
Arp, J., Baccala, R., Dekaban, G., Dalgleish, A.G.,
and A.N. Theofilopoulos. 1995. Recognition of HTLV-I
envelope protein by human CD4+ T cell lines generated
from HTLV-I seronegative individuals. Blood. 85:1547-
1554.
0
16. Ford, C., Arp, J., King, E., Dekaban, G.A. and T.
Palker. 1991. Expression and immunogenicity of HTLV-I
envelope proteins by recombinant vaccinia virus,
p.253-258. In R.M. Chanock, et al. (eds.), Vaccines
91: Modern Approaches to New Vaccines Cold Spring
Harbour Laboratory, New York.
17. Ford, C.M., Arp, J., Palker, T.J. King, E.E., and G.A.
Dekaban. 1992. Characterizatino of the antibody
response to three different versions of the HTLV-I
envelope protein expressed by recombinant vaccinia
viruses: Induction of neutralizing antibody. Virology.
191:448-453.
18. Ratner, L., Josephs, S., Starcich B., Hahn, B., Shaw,
G., Gallo, R. and F. wong-Staal. 1985. Nucleotide
sequence analysis of a variant Human T-cell Leukemia
Virus (HTLV-Ib) provirus with a deletion in pX-I. J.
Virol. 54:781-790.
~za~~m
41
19. Elroy-Stein, O., Fuerst, T., and B. Moss. 1989. Cap-
independent translation of mRNA conferred by
encephalomyocarditis virus 5' sequence improves the
performance of the vaccinia virus/bacteriophage T7
hybrid expression system. Proc. Natl. Acad. Sci. USA.
86:6126-6130.
20. Dales, S., and L. Siminovitch. 1961. The development
of vaccinia virus in Earle's L strain cells as
examined by electron microscopy, J. Biophys. Biochem.
Cytol. 10:475-503.
21. Mackett, M., Smith, G., and B. Moss. 1984. General
method for the production and selection of infectious
vaccinia virus recombinants expressing foreign genes.
J. Virol. 49:857-864.
22. Mackett, M., Smith, G., and B. Moss. 1985. The
construction and characterization of vaccinia virus
recombinants expressing foreign genes, p. 191-211: In
D. Rickwood and B.D. Hames (ed.), DNA cloning, vol. 2.
IRL Press, Washington D.C.
23. Fuerst, T., Niles, E., Studier, F., and B. Moss. 1986.
Eukaryotic transient-expression system based on
recombinant vaccinia virus that synthesizes
bacteriophage T7 RNA polymerase. Proc. Natl. Acad.
Sci. USA. 83:8122-8126.
~24. Foung, S. and S. Perkins. 1989. Electric field-induced
cell fusion and human monoclonal antibodies. J.
Immunol. Methods. 116:117-122.
25. Perkins, S., Zimmermann, U. and S. Foung. 1991.
Parameters to enhance hybridoma formation with hypo-
osmolar electrofusion. Hum. Antibod. Hybridomas.
2:155-159.
26. Perkins, S. and S. Foung. 1995. Stabilizing antibody
secretino of human Epstein-Barr virus activated B.
lymphocytes with hybridoma formatino by electrofusion.
In Nickoloff, J. (ed.), Protocols for electroporation
and electrofusion of plant and animal cells, in press.
Humana Press, New Jersey.
27. Hayden, D.B., Baker, N.R., Percival, M.P., and P.B.
Beckwith. 1986. Modification of the Photosystem II
light-harvesting chlorophyll a/b protein complex in
maize during chill-induced photoinhibition. Biochim.
Biophys. Acta. 851:86-92.
28. Palker, T., Tanner, M., Scearce, R., Streilein, R.,
Clark, M. and B. Haynes. 1989. Mapping of immunogenic
regions of human T-cell leukemia type I (HTLV-I) gp46
~20~~~~
42
and gp21 envelope glycoproteins with env-encoded
synthetic peptides and a monoclonal antibody to gp46.
J. Immunol. 142:971-978.
29. Giri, A., Markham, P., Digilio, L., Hurteau, G.,
Gallo, R., and G. Franchini. 1994. Isolation of a
novel simian T-cell lymphotropic virus from Pan
paniscus that is distantly related to the human T-cell
leukemia/lymphotropic virus types I and II. J. Virol.
68:8392-8395.
30. Matsushita, S., Robert-Guroff, M., Trepel, J.,
cossman, J., Mitsuya, H., and S. Broder. 1986. Human
monoclonal antibody directed against an envelope
glycoprotein of human T-cell leukemia virus type I.
Proc. Natl. Acad. Sci. USA. 83:2672-2676.
31. Hayden, D.B., Baker, N.R., Percival, M.P., and P.B.
Beckwith. 1986. Modification of the Photosystem II
light-harvesting chlorophyll a/b protein complex in
maize during chill-induced photoinhibition. Biochim.
Biophys. Acta. 851:86-92.
32. Trimble, R. and g. Maley. 1984. Optimizing hydrolysis
of N-linked high-mannose oligosaccharides by endo-
beta-N-acetyl-glucosaminidase H. Analyt. Biochem.
141:515-522.
33. Plummer, T., Jr., Elder, J., Alexander S., Phelan, A.,
and A. Tarentino. 1984. Demonstration of peptide: N-
glycosidase F activity in endo-beta-N-
acetylglucoaminidase F preparations. J. Biol. Chem.
259:10700-10704.
34. Rowe, J., Perkins, S., Bradshaw, P., Song, G-Y, S.
Fuong. 1994. Neutralizing HTLV-human monoclonal
antibodies (HMAbs) to conformational epitopes. AIDS
Res. Hum. Retroviruses. 10:509.
35. Lee, T., Coligan, J., Homma, T., McLane, M.,
Tachibana, N., and M. Essex. 1984. Human T-cell
leukemia virus-associated membrane antigens: identity
of the major antigens recognized after virus
infection. Proc. Natl. Acad. Sci. USA. 81:3856-3860.
36. Geyer, H., Holschbach, D., Hunsmann, G., and J.
Schneider. 1988. Carbohydrates of human
immunodeficiency virus: structures of oligosaccharides
linked to the envelope glycoproteins 120. J. Biol.
Chem. 263:11760-11767.
37. Goubau, P., Liu, H., DeLange, G. Vandamme, A. and J.
Desmyter. 1993. HTLV-II seroprevalence in pygmies
,:
43
across Africa since 1970. AIDS Res. Hum.
Retroviruses. 9:709-713.
38. Kornfeld, R., and S. Kornfeld. 1985. Assembly of
asparagine-linked oligosaccharides. Ann. Rev. Biochem.
54:631-664.
39. Seiki, M., Hattori, S., Hirayama, Y., and M. Yoshida.
1983. Human adult T-cell leukemia virus: Complete
nucleotide sequence of the provirus genome integrated
in leukemia cell DNA. Proc. Natl. Acad. Sci. USA.
80:3618-3622.
40. Chen, Y., Lee, T., Samuel, K., Okayama, A., Tachibana,
N., Miyoshi, I., Papas, T., and M. Essex. 1989.
Antibody reactivity to different regions of human T-
cell leukemia virus type I gp61 in infected people.
Journal of Virology. 63:4952-4957.
41. Kiyokawa, T., Yoshikura, H., Hattori, S., Seiki, M.,
and Yoshida, M. 1984. Envelope proteins of human T-
cell leukemia virus: Expression in Escherischia coli
and its application to studies of env gene functions.
Proceeding of the National Academy of Science, U.S.A.
81:6202-6206.
42. Kuga, T., Hattori, S., Yoshida, M., and T. Taniguchi.
1986. Expression of human t-cell leukemia virus type
I envelope protein in Saccharmyces cerevisiae. Gene.
44:337-340.
43. Nyunoya, H., Ogura, T., Kikuchi, M., Iwamoto, H.,
Yamashita, K., Maekawa, M., Takebe, Y., Miyamura, K.,
Yamazaki, S., and Shimotohno, K. 1990. Expression of
HTLV-I envelope protein of hydrophobic amino-terminal
peptide of baculovirus polyhedrin in insect cells and
its application for serological assays. AIDS Research
and Human Retroviruses. 6:1311-1321.
44. Samuel, K., Lautenberger, J., Jorcyk, C., Josephs, S.,
along-Staal, F., and T. Papas. 1984. Diagnostic
potential for human malignancies of bacterially
produced HTLV-I envelope glycoprotein. Science.
226:1094-1097.
45. Vile, R., Schulz, T., Danos, O., Collins, M., and R.
Weiss. 1991. A Murine cell line producing HTLV-I
pseudotype virions carrying a selectable marker gene.
Virology. 180:420-424.
46. Sattentau, Q. and J. Moore. 1995. HIV-1
neutralization is determined by epitope exposure on
the gp120 oligomer. J. Exp. Med. 182:185-196.
f
44
47. Stamatatos, L. and C. Cheng-Mayer. 1995. Structural
modulations of the envelope gp120 glycoprotein of
human immunodeficiency virus type 1 upon
oligomerization and differential V3 loop epitope
exposure of isolates displaying distinct tropism upon
virion-soluble receptor binding. J. Virol. 69:6191-
6198.
48. Seiki, M., Hattori, S., Hirayama, Y., and M. Yoshida.
1983. Human adult T-cell leukemia virus: Complete
nucleotide sequence of the provirus genome integrated
in leukemia cell DNA. Proc. Natl. Acad. Sci. USA.
80:3618-3622.