Note: Descriptions are shown in the official language in which they were submitted.
~ WO96/18S76 ' 2 2 0 ~ ~ 2 ~ PCT/AU95/00698
TITLE
"ALUMINO-SILICATE DERIVATIVES"
FIELD OF THE INVENTION
THIS INVENTION relates to the formation of new
materials in the form of alumino-silicate derivatives
from 2:1 clay minerals as hereinafter described and
processes to form these new materials which are obtained
by the chemical modification of 2:1 clay minerals.
The derivatives of these layer minerals are
characterised by a predominance of tetrahedrally-
coordinated Al+3 which has resulted from the chemical
modification of octahedrally-coordinated Al~`3 in the
parent mineral. This atomic-scale transformation makes
available a higher number of exchangeable sites than
would be normally available in the original clay
structure.
BACKGROUND OF THE INVENTION
Two features of the new materials which may
result from the chemical modification of these 2:1 clay
minerals are an enhanced capacity to exchange cations
from solution (i.e. a cation exchange capacity) and/or
an increase in the available surface area when compared
with the properties of the initial starting mineral.
These two features are of considerable significance to
the cost-effective use of these derivative materials in
a wide range of applications for cation-exchange (e.g.
for removal of toxic metal ions from aqueous and non-
aqueous solutionsi removal of NH,+ from aqueous and non-
aqueous solutions, as detergent builders and as water
softeners), absorption (e.g. for the removal of gases
from the environment, for absorptlon of cations from
solutions), as agents for the controlled release of
desired cations into an environment and as substrates for
catalysis reactions in the modification of hydrocarbons
and other chemicals.
Clay minerals are part of the larger family of
minerals called phyllosilicates - or "layer" silicates.
WO96/18576 2 ~ ~ 6 PCT/AU95/00698
These clay minerals are typically characterised by two-
dimensional arrangements of tetrahedral and octahedral
sheets, each with specific elemental compositions and
crystallographic relationships which define the mineral
group. Thus, the tetrahedral sheet may have the
composition T2O~ (where T, the tetrahedral cation, is Si,
Al and/or Fe) and the octahedral sheet may commonly
contain cations such as Mg, Al and Fe, but may also
contain other elements such as Li, Ti, V, Cr, Mn, Co, Ni,
Cu and Zn (Brindley and Brown, Crystal Structures of Clay
Minerals and their x-ray identification, Editors G.W.
Brindley and G. Brown, Mineralogical Society, London,
1980). Each of these clay mineral groups can be further
classified into trioctahedral and dioctahedral varieties,
depending on the occupancy of the octahedra in the
respective sheet arrangement(s). Some specific mineral
species may show cation occupancies whicll are
intermediate between the two varieties. Nevertheless,
the relative arrangement of these tetrahedral and
octahedral sheets also defines the basic mineral groups
in that an assemblage which links one tetrahedral sheet
with an octahedral sheet is known as a 1:1 type mineral.
An assemblage which links two tetrahedral sheets with
one octahedral sheet is known as a 2:1 mineral. This
basic classification of mineral species, based upon the
crystallographic relationships of specific sub-units, is
well-known to those skilled in the art of clay mineralogy
and forms a basis for description of this invention.
The production of an amorphous derivative,
termed "kaolin amorphous derivative" (KAD), from kaolin
clays which are 1:1 alumino-silicates, has been described
in an earlier disclosure (W095/00441). We have now
surprisingly found that an amorphous derivative can also
be manufactured from 2:1 clays which include
montmorillonites and other members of the smectite group.
The production of an amorphous derivative from these 2:1
clays is surprising insofar as the structure and
~ WO96/18576 ~ 2 0 ~ ~ 2 6 PCT/AU95/00698
chemistry of these minerals is markedly different to that
of the 1:1 kaolin group minerals. A unit layer of the
clays in the kaolin group consists of one octahedral
sheet and one tetrahedral sheet so that both sheets are
exposed to the interlayer space, a region which is
accessible to reacting species. However, a 2:1 clay
mineral comprises one octahedral sheet and two
tetrahedral sheets. The octahedral sheet, which contains
octahedrally co-ordinated aluminium, is sandwiched
between the tetrahedral sheets. The transformation of
this octahedral sheet is not readily predictable using
metal halides since the interlayer space is surrounded
by tetrahedral sheets. It i$ also relevant to point out
that the octahedral sheet in 2:1 clay minerals would not
be readily accessible to metal halides. It would be
assumed by those skilled in the art that reacting species
with 2:1 clay minerals would provide different products
to reaction products described in W095/00441 for these
reasons.
SUMMARY OF THE INVENTION
It is therefore an object of the invention to
provide the abovementioned modified 2:1 clay minerals
possessing the two features discussed above.
In one aspect, the invention provides modified
2 :1 clay minerals by a process using a metal halide which
may react with a 2:1 clay mineral or combination of 2:1
clay minerals to provide a modified 2:1 clay mineral
which includes the two features described above.
Examples of 2:1 clay minerals which may be
modified by the process(es) of the invention include
montmorillonite, illite, palygorskite and saponite. The
2 :1 layer clay mineral derivatives of the invention are
characterised by predominant tetrahedral Al+3 and for the
sake of convenience, are hereinafter termed "alumino-
silicate derivatives" or "ASDs". In the case of, e.g.
montmorillonite clays, the octahedral Al within the
parent (i.e. clay) has been transformed to tetrahedral
WO96/18576 ~ 2 ~ ~ ~ 2 6 PCTtAU95/00698 ~
co-ordination. Further elucidation of this ASD,
henceforth designated M-ASD, where M is the exchanged
cation obtained by the specific formation process, can
be obtained by conventional mineral characterisation
5techniques which demonstrate the following properties:-
(1) an "amorphous" nature (to X-ray
diffraction), i.e. without any apparent
long range order of the repeat units;
(2) an enhanced capacity to exchange cations
10(compared with the original starting
mineral) from solution;
(3) an increase in the available surface area
of the material (compared with the
original starting mineral) as measured by
15the conventional BET isotherm;
(4~ an enhanced capacity compared to the
original starting material to adsorb
anionic species or complex polyanions
from solution; and/or
20(5) an enhanced capacity compared with the
original starting material to absorb oil
and/or organic molecules.
In relation to property (2), this may be
exemplified by the ASDs of the invention having a cation
25exchange capacity of 20-900 milli-equivalents per 100 g
as measured by exchange of ammonium or metal cations from
an aqueous solution. Most preferably the cation exchange
capacity as measured by exchange of ammonium is between
about 300-450 milli-equivalents per 100 g.
30In relation to property (3), this may be
exemplified by the ASDs of the invention having a surface
area less than 400 m/g~~ as measured by the BET isotherm
which is higher than the 2:1 clay mineral starting
material. Most preferably the BET surface area is
35between 25 m'/g~' and 200 m2/g~'.
Properties (4) and (5) are demonstrated in
International Application No. PCT/AU95/OO~qg having the
WO96/18576 ~ 9 2 6 PCT/AU95/00698
same international filing date as the present
application.
One form of the ASD of the invention has the
chemical composition:-
MpAlqSi 2r ( OH)sxt-uH2o
where M is an exchangeable alkali metal cation, X is a
halide, 0.5 < p < 2.0, 1.0 < q < 2.2, 4.5 < r < 8.0, 1.0
' s < 3.0, 0.0 ' t < 1.0 and 0.0 ' u < 3Ø In one
specific form, the ASD may contain the element potassium,
such that M-K.
In the ASD referred to above, it is possible
to exchange, at least partly, the alkali metal cation
with any cation which is stable in aqueous solution.
Such exchange cations include other alkali metal cations,
alkaline earth cations, transition metal cations,
lanthanide and actinide cations, heavy metal cations and
ammonium. While exchange does not proceed to completion
for all cations, there are many transition metal cations
(e.g. Mn2+, Cr3+, Co-+, Ni2+, Cu2+, Zn2+, Ag+), lanthanide
cations (e.g. La3+, Nd3+), heavy metal cations (e.g. Pb7+,
Cd2+, Hg'+) and the actinide uo2+ which do. For some
cations exchange is complete after three hours at room
temperature (e.g. Pb-+, Cu2+), while others require longer
times and temperatures up to 110C (e.g. Zn2+).
Preferably the cations NH~, Na+, K+, Li+, Rb+
or Cs+ is exchanged by Pb2+, Cu2+, Cd2+, Ni2+, Co'+, Cr3+,
Sr2+, Zn2+, Nd3+ or UO22+.
Such cation exchange essentially preserves the
XRD-amorphous character of the ASD. However, the
specific surface of the exchanged materials, while still
- higher than that of the starting 2:1 mineral, does
increase or decrease depending on the exchange cation.
For example, in the case of exchange of Cu+2
from an aqueous solution, a new material, termed Cu-ASD,
is formed and which, for example, shows a high surface
area as measured by the conventional BET isotherm. To
differentiate, in generic formulae, between new ASD
WO96/18576 ~ 2 ~ ~ 9 2 ~ PCT/AU95~693
materials formed directly via the transformation of a 2:1
clay mineral (as in Examples 1 and 2 below) and those ASD
materials formed by cation exchange of the directly
derived ASD, the following terminology is utilised in
this document:-
M-ASD denotes material directly formed
via the general processes described in
Examples 1 and 2
M~-ASD denotes material subsequently
formed via a cation exchange with M-ASD
material. Descriptions of this type of
material, and the methods used to obtain
same, are given in Examples 3 and 4.
Clearly partially formed ASDs in which two
cations occupy sites or in which multiple cations are
exchanged via a series of partial reactions are possible
forms of this new material.
One of the primary crystallographic methods to
define ASD material is powder X-ray diffraction (XRD).
In the case of powder XRD, the formation of M-ASD as a
primary component of the reaction is denoted by a loss
of sharp diffraction peaks corresponding to the original
starting mineral (e.g. Ca-montmorillonite) and a
corresponding increase in intensity of a broad "hump"
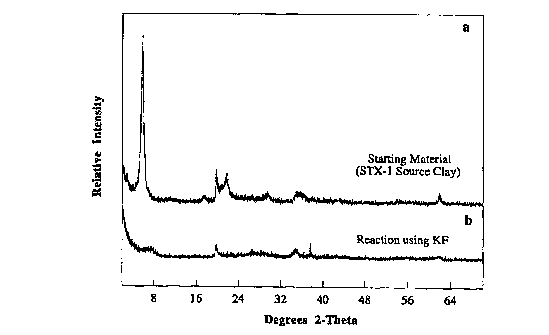
between 22 and 32 2~ using CuK~ radiation (see, for
example, FIG. 1b, 2b). An example of a typical XRD
pattern, for the starting montmorillonite (STx-1) and for
the respective M-ASD material formed by the process of
Example 1 is given in FIGS. 1a and 1b, respectively.
As is known by those skilled in the art,
montmorillonites such as STx-1 and SWy-1 contain
octahedrally-coordinated aluminium ions. This
crystallographic feature can be demonstrated by a number
of methods including recalculatlon of chemical analyses
as mineral formulae and assignment of aluminium atoms to
the octahedral sites in the montmorillonite structure.
It is well known to those skilled in mineralogy
WO96/18576 ~ 9 2 6 PCT/AU95/00698
that conversion of octahedral Al to tetrahedrally co-
ordinated Al results in positive charge deficit in the
tetrahedral framework. However, this charge deficit in
the anionic framework can be balanced by fixed or
exchangeable cations (Na, K, Ca, Mg, etc.) seated in the
available voids. This mechanism is precisely exemplified
by natural phases such as feldspar, feldspathoids and
zeolites, which contain varying proportions of
tetrahedrally co-ordinated Al (Klein and Hurlbutt, Jr.,
Manual of Mineralogy, after J.D. Dana, John Wiley & Sons,
New York, pp 446). These fundamental mineralogical
principles are employed in this disclosure to ascertain
the presence of tetrahedrally co-ordinated Al. It is
clear from the chemical composition data referred to
hereinafter that Al in ASD is tetrahedrally co-ordinated
and consequence charge deficit is balanced by K or Na
incorporated into the structure from the reactants. The
incorporated cations are largely exchangeable as shown
by cation exchange data referred to hereinafter which
further demonstrates the tetrahedrally co-ordinated
nature of Al in the disclosed material.
It is clear from the specific CEC data referred
to hereinafter that a high number of exchangeable sites
are available in the M-ASD. This is clear evidence that
octahedrally co-ordinated aluminium has been convereted
to tetrahedrally co-ordinated aluminium.
The above primary crystallographic and chemical
techniques define the atomic arrangements of the critical
elements in this new material and forms the basis of a
family of mineral derivatives which have been obtained
by the chemical reaction of 2:1 clay minerals. The
essential crystallographic features are:-
the transformation of long-range order to
an "amorphous" structure showing a broad
X-ray diffraction ';hump", or peak,
between 22 and 32 2~ using CuK~
radiation; and
WO96/18576 ~ 2 ~ 1 9 2 ~ PCT/AU95J~C9~ ~
the presence of primarily tetrahedrally-
coordinated aluminium.
Chemical analysis can be effected by a number
of means, but in this disclosure, the use of an electron
microprobe to quantify the amounts of elements with
atomic number greater than 11 (i.e. Na or higher) is
preferred. The presence of oxygen is determined
according to general principles for microanalysis of
minerals known to those skilled in the art. Depending
on the nature of the reactant (i.e. the metal halide) an
exchangeable cation, such as Na or K, will be present in
the alumino-silicate derivative.
Bul~ physical properties for these alumino-
silicate derivatives, such as BET surface area, cation
exchange capacity (CEC), oil absorption, degree of
basicity etc., are influenced by the nature of the
processing used to form the ASD. In another aspect of the
invention, this relationship shows that specific ASDs may
be more suited to one application (e.g. removal of trace
amounts of divalent cation) than another (e.g. absorption
of gases or o~ils) but that in relative comparison to the
clay mineral used to form the ASDs, each ASD has
properties more suited to the application than the clay.
The as-formed ASD, for example, via reaction
with KF, will contain a high percentage of K+ ions on the
exchangeable sites of this new material. As shown in
Examples 3 and 4, cations such as Cu+-, Li- or NH~ will
readily exchange with the K+ or Na+ of these exchangeable
sites in an M-ASD to form a Cu-rich, Li- or NH~+ -rich
derivative, respectively. In this instance, the Cu-ASD
shows a high value for available surface area which, with
suitable pre-treatment, enables use of this material, for
example, as a catalyst for dehydrogenation reactions of
organic compounds. Similarly, ammonium-exchanged ASD,
or NH~-ASD, has significant potential for use as a
fertiliser or nutrient-provider in the agricultural,
horticultural and feedstock industries. Alternatively,
~ WO96/18576 ~ Q ~ ~ PCT/AU9S/00698
M-ASD (where M=K or Na) may also be used in the
agricultural or horticultural industries to exchange
ammonium ion onto a stable substrate (e.g. to form NH~-
ASD) for later easy removal, or subsequent use.
Other uses of the ammoniuM-exchange capacity
of ASDs such as extraction of ammonium ion from
industrial effluent or from waste products are readily
envisaged by those skilled in the art.
While not wishing to be bound by theory, the
chemical transformation or conversion of a long range
order to a short range ordered structure as described
above may be represented by the following example in
which montmorillonite, with Al and Si in octahedral and
tetrahedral sites in the structure, respectively, is
reacted with an alkali metal halide where the cation is
Kf or an ammonium ion in an aqueous solution such that
excess halide (e.g. X~) is readily exchangeable with the
available hydroxyl groups (OH-) in the montmorillonite
structure. This exchange results in the formation of a
highly basic solution with higher concentration of OH-
ions compared with hydrogen ions which can cause
rearrangement of octahedrally co-ordinated aluminium
through the action of these OH ions on hydrogen-bonded
oxygen atoms. This rearrangement of aluminium co-
ordination results in primarily tetrahedrally co-
ordinated aluminium in this resultant stable material.
The reaction rate and preferred forms of these
alumino-silicate derivatives with desirable properties
will be dependent on the precise temperature of reaction
for a given period of time. In general, temperatures less
than 200C may be used and more preferably temperatures
are utilised between 50-200C. A suitable reaction time
may be between one minute and 100 hours and more
preferably a reaction time of less than 24 hours is
utilised. In concert with this rearrangement of co-
ordination of the aluminium atom(s), the presence of an
additional cation (from the reagent) causes the
WO96/18576 2 ~ 2 ~ PCTIAU95100698
1 0
disordered structure to be stabilised through
"attachment'l of the cation to an exchange site so formed
by this rearrangement. During the overall chemical
transformation, loss of aluminium (as well as minor
amounts or silicon) from the alumino-silicate structure
to the highly basic solution may occur. The preferred pH
of this highly basic solution, during and near the end
of the reaction, is generally >12, although reaction to
form the preferred ASD may occur for solutions with pH
>7Ø
As noted above, M-ASD may be produced by a
number of similar processes which involve the following
generic modifications to the parent mineral structure:-
attack by the reactant anion (e.g. F-,
Cl-) or cation (e.g. K+, Nar or Li+) so
that a proportion of the Al-O and/or Si-O
bonds within the mineral structure are
weakened or broken;
loss of long-range periodicity (sometimes
referred to as "crystallinity") in the
mineral structure so that the derivative
material resembles the original structure
only as a disordered (short-range
ordered) array of sub-units (e.g. SiO~
tetrahedra; AlO~ tetrahedra and newly-
formed "exchange sites" which may or may
not contain a cation);
loss of a proportion of aluminium atoms
(and/or a lesser amount of silicon atoms)
from the original parent mineral(s);
addition of the reactant cation (e.g. Na+
or K+) as well as a smaller proportion of
the reactant anion (e.g. F- or Cl-) to the
derivative material structure.
The following generic modifications to bulk
physical properties also occur with progress of any of
these processes for the formation of an M-ASD:-
~ WO96/1~576 2 2 ~ ~ 9 2 ~ PCT/AU95/00698
the reaction proceeds with an increase in
the viscosity of the reaction mixture to
a certain maximum level - determined by
the relative proportions and nature of
the initial reactants;
an increase in the "dispersability" of
individual particles formed during the
reaction process - this is assumed due,
in part, to a reduction in size of the
individual alumino-silicate particles -
compared with the dispersability and/or
size of the original starting mineral;
an increase in the bulk volume occupied
by a dried powder (i.e. a fluffy" or
less-compact powder) compared with the
volume occupied by the original starting
mineral.
Various combinations of reactant
concentrations, along with some product properties, are
given in Table 1. In all these combinations, water is
added to the reaction mix in various amounts. In Table
1, CEC for ammonium exchange using the method in Example
4, relative Cu+- exchange (Example 3) and BET surface
area of derivatives of STx-1 and SWy-1 are provided.
Comparison data for the starting 2:1 clay minerals are
also given in Table 1. Chemical compositions for
derivatives of STx-1 and SWy-1 are given in Table 2.
Specific examples of the formation of alumino-
silicate derivatives are given below.
EXAMPLES
Example 1: Formation of M--ASD via reaction of Ca-
mon tmori l l on i t e wi th me t a l h a l i d e
10 g of Source Clay montmorillonite from Texas
(Sample No. STx-1; van Olphen and Fripiat, 1979, Data
handbook for clay materials and other non-metallic
minerals, Pergamon Press, Oxford, 342pp.) is thoroughly
mixed with 50 g of potassium fluoride (KF) and 20 mls of
WO96/18576 PCTIAU95/00698 ~
220 ~Q2~ --
12
distilled water in a beaker and heated at 80C for five
hours. The resulting slurry is washed with water until
any excess potassium fluoride is removed. The powder is
then dried and subjected to a series of characterisation
tests which include powder X-ray diffraction (FIG. 3),
NH4+/Cu+2exchange and BET surface area measurements. Data
from these characterisation techniques indicate that the
material has an atomic arrangement (i.e. crystallographic
features) as defined above.
ExamPle 2: Formation of M-ASD from Na-
mon tmori l l oni te cl ay
20 g of Source Clay montmorillonite from
Wyoming (Sample No. SWy-1; van Olphen and Fripiat, 1979,
Data handbook for clay materials and other non-metallic
minerals, Pergamon Press Oxford, 342pp.) is thoroughly
mixed with 60 g of potassium fluoride (KF) and 50 mls of
distilled water in a beaker and heated at 80C for five
hours. The resulting slurry is washed with water until
any excess potassium fluride is removed. The powder is
then dried and subjected to a series of characterisation
tests which include powder X-ray diffraction (FIG. 4),
cation exchange (for Cu+2 and NH~+ foIlowing Examples 3
and 4 below) and BET surface area measurements. Data from
these characterisation techniques indicate that the
material has an atomic arrangement (i.e. crystallographic
features) as defined above. The BET surface area and CEC
(NH4+) values for M-ASD formed by this method are 97 m'/g
and 108 meq/100 g, respectively. For uptake of Cu+2
cations, M-ASD formed by this method extracted, after a
period of 16 hours at room temperature, 106 ppm of Cu are
taken from a 200 ppm solution prepared via the method
described in Example 3.
Example 3: Uptake of Cu+- from an aqueous sol ution
using M-ASD and forma tion of M,-ASD
75 mg of M-ASD, obtained by the general process
defined in Examples 1 and 2, is placed in a 0.1 M NaNO~
solution containing 200 ppm Cu+2 at pH ~5.6 and shaken
WO96/18576 ~ 2 6 PCT/AU95/00698
13
overnight for a period of approximately sixteen hours and
held at room temperature (~25C) during this time. The
sample was centrifuged and an aliquol of the supernatant
solution was analysed for remaining Cut2. In this
experiment, the concentration of Cu+2 remaining in the
aqueous solution is 52.8 ~g/ml (or 52.8 ppm). This
result indicates that, in this specific case, the M-ASD
produced by the process described in Examples 1 and 2
will remove 74% of the Cu+' cations in a 200 ppm Cu+2
solution in a period of approximately 16 hours. This
example presents one method used for assessing the
relative capacity of these new materials for exchange of
Cu+2 cations.
Table 1 lists, for various classes of
processing conditions used in these reactions, the
proportion of Cu+2 removed from a standard solution by a
defined amount of M-ASD under the above standard
conditions. Entries in this table which do not designate
reaction conditions, the Cu+2 exchange data refer to the
capacity of the original starting material (e.g.
montmorillonite STx-1) for exchange of Cu+2ions. Values
for remaining Cu+~ which are less than 100 ~g/ml are
reasonably considered commercially-viable materials for
the exchange of divalent cations. In general, this
tabulation of Cu+2 exchange capacity is considered a
guide to the relative exchange capacity for each M-ASD
for a wide range of cations including Al+3, Mgt2, Ca+2,
Fe+2, Cr+3, Mn+2, Ni+2, Co+2, Ag+, Zn+-, Sr+', Nd+3, Hgt7,
Cd+2, Pb+' and UO2+'.
The material formed upon exchange with Cu+',
designated Cu-ASD, is itself a new material which has
similar structural properties to the generically-
designated M-ASD except for the replacement of K (and/or
Na) on the exchange site with Cu. This material has high
surface area values, in some cases, considerably higher
than that recorded for the original M-ASD material before
Cu +2 exchange.
WO96/18576 PCT/AU9S~ 9~ ~
220 ~2~
14
ExamPle 4: Exchange of NHJf from an aqueous solution
using M-ASD and formation of M,-ASD.
Determination of CEC for various cations
(e.g. Na+ and LI+).
0.5 g of M-ASD formed by modification of clay
minerals using the methods noted above is placed in a
centrifuge bottle and 30 ml of 1 M NH~Cl is added and
allowed to equilibrate overnight. The sample is
centrifuged and the supernatant is removed. A fresh
amount of 30 ml 1 M NH~Cl is added and the sample is
shaken for 2 hours. This procedure of centrifuging,
removal of supernatant and addition of 30 ml 1 M NH~Cl is
repeated three times. Any entrained NH~Cl is removed by
washing with ethanol. At this point, the remaining
material is an exchanged ASD, such as NH~ASD. To
determine a CEC value for the specific M-ASD material,
a further 3Q ml of 1 M NH~Cl is added to the washed
sample and allowed to equilibrate overnight. The
supernatant is then collected after centrifugation and
a further 30 ml of 1 M KCl solution is added and shaken
for two hours. This procedure of centrifuging, removal
of supernatant and addition of KCl is repeated three
times. Finally, distilled water is added to make up 100
ml of solution and the amount of NH~ present is measured
by ion-selective electrode. This procedure follows that
given by Miller et al., 1975, Soil Sci. Amer. Proc. 39
372-373, for the determination of cation exchange
capacity and similar procedures are used for CEC
determination for other cations such as Na+ and Li+. All
CEC values tabulated for a range of M-ASDs have been
determined by this basic procedure. The results of these
experiments are expressed in milli-equivalents of NH~+
exchanged per gram and are listed in Table 1.
W096/18576 ~ 2 ~ ~ 9 2 6 PCT/AU95~ C9
TABLES
ABLE 1 Summary of Process Conditions and Product (M-
ASD) properties
Sample MaterialsRatioTemp.Time Cu* Surface CEC
No. (C) (hrs)ppm Area (meq/100
m2lg 9)
STx-1Raw material 163.6 84 60
STx-4STx-1 :KF:H20 20:60:5080 3 44 128 132
STx-5STx-1 :KF:H20 20:80:6080 3 78 80
STx-6STx-1 :KF:H20 10:50:2080 5 66 80
SWy-1Raw material 147.5 32 96
SWy-4SWy-1 :KF:H20 20:60:5080 3 94 97 108
ABLE 2 Averaged microprobe analyses for derivatives
of montmorillonites
Element wt % STx-1 Derivative SWy-1 Derivative.
oxide
Na,O 0.03 1.20
K,O 10.47 6.72
MgO 5.07 4.36
CaO 2.47 1.35
Al,03 20.36 19.81
SiO, 49.72 48.28
Fe,O~ 0.84 4.72
Total 88.96 89.44
WO96/18576 = = - PCT/AU95/00698 ~
~2~ 1926
- 16
LEGENDS
TABLES
* Cu concentration in ppm remaining in solution from
an initial value of 200 ppm. See Example 3.
FIG. 1
Powder XRD patterns for (a) starting material Texas
montmorillonite (STx-1) before reaction, and (b) product
formed after reaction using KF (Example 1). For FIG. 1,
detailed enlargements of the region between 20 and 35
2~ are given in FIG. 2.
FIG. 2
Higher scale enlargements of powder XRD traces shown in
FIG. 1 demonstrating the region between 20 and 35 2~.
For FIG. 2d, corresponding to sample number STx-5 in
Table 1, the presence of a broad "hump" between 22 and
32 2~ is readily observed.
FIG. 3
Powder XRD trace for product obtained by reaction of Ca-
montmorillonite with KF (Sample No. STx-6 in Table 1).
FIG. 4
Powder XRD trace for product obtained by reactions of Na-
montmorillonite with KF (Sample No. SWy-4, Table 1).