Note: Descriptions are shown in the official language in which they were submitted.
22u2J.~ i
-1-
SPECIFICATION
CONDENSED INDAN DERIVATIVES AND SALTS THEREOF
Technical Field
The present invention relates to novel
condensed indan derivatives and salts thereof. The
compounds of the invention have excellent antitumor
activity and are useful as antitumor agents.
Background Art ,
Conventionally known 11H-indeno[1,2-b]quinolin-
11-one derivatives which have a skeleton similar to that
of the compound of the present invention include, for
example, compounds disclosed in Pol. J. Chem., 55, 121-
128 (1981), Pol. J. Pharmacol. Pharm., 35 327-332 (1983),
ibid., 35 523-530 (1983) and ibid., 38 221-227 (1986).
Specifically stated, these documents disclose compounds
substituted at the 10-position by a lower alkylamino
group which may have a substituent, a phenylamino group,
etc. These documents, however, refer to only anti-
inflammatory and analgetic effects as pharmacological
activity of said compounds, and do not report or disclose
antitumor activity. Accordingly, the antitumor activity
of the condensed indan derivatives of the present
invention has not been found yet.
An object of the present invention is to
provide a compound which has excellent antitumor activity
22 ~2i~31
-2-
and is useful as a therapeutic agent for tumors. Another
object of the invention is to provide the use of said
compound or salts thereof as an antitumor agent, and a
method for treating tumors using said compound or a salt
thereof. A further object of the invention is to provide
a method for preparing said compound.
Disclosure of the Invention
The inventors of the present invention , .
conducted extensive research and found that condensed
indan derivatives have excellent antitumor activity and
are useful as antitumor agents. The present invention
has been accomplished based on this finding.
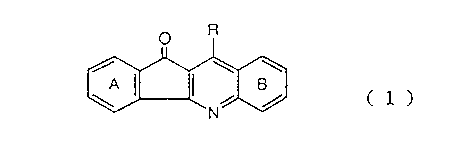
The present invention provides a condensed
indan derivative represented by the formula (1):
(1)
N _
R
/. \ \
i
wherein the ring A is an optionally substituted benzene
ring or a benzene ring which has lower alkylenedioxy
group(s), the ring B is an optionally substituted benzene
ring or a benzene ring which has lower alkylenedioxy
group(s), and R is a group -NR1R2, an optionally
substituted nitrogen-containing heterocyclic group, a
22U~~~~ i
-3-
group -OR3 or a group -SR4 (wherein R1 and R2 are the
same or different and each represent a hydrogen atom, a
phenyl group, an optionally substituted nitrogen-
containing heterocyclic group, or a lower alkyl group
which may be substituted by optionally substituted amino
group(s), lower alkoxy group(s), phenyl group(s),
nitrogen-containing heterocyclic groups) or hydroxyl
group(s), and R3 and R4 each represent a lower alkyl , ,
group which may be substituted by substituted amino
group(s)), provided that at least one of the rings A and
B is a substituted benzene ring and R is not -NHCH3, or a
salt thereof.
The compound of the present invention
represented by the formula (1) has excellent antitumor
activity and is useful for treating various tumors.
The present invention provides a composition
comprising an effective amount of the compound of the
formula (1) or a phamaceutically acceptable salt thereof
and a pharmaceutical carrier.
In particular, the present invention provides
an antitumor agent comprising an effective amount of the
compound of the formula (1) or a pharmaceutically
acceptable salt thereof and a pharmaceutical carrier.
The present invention further provides a method
for treating tumors in mammals comprising administering
~2~~~~i
-4-
to mammals an effective amount of the compound of the
formula (1) or a pharmaceutically acceptable salt
thereof.
The groups represented by R, R1, R2, R3 and R4
and other groups shown in the present specification are
described below more specifically.
Examples of the substituents in the benzene
rings represented by the rings A and B include a halogen.
atom, a lower alkyl group, a lower alkoxy group, a
hydroxyl group, a nitro group, an amino group, a lower
acyloxy group, a benzyloxy group, a lower acylamino
group, a cyano group, a carboxyl group, a lower
alkoxycarbonyl group, etc. Among them, a lower alkoxy
group and a hydroxyl group are preferred.
Said substituents may be at any positions of
each ring and are the same or different. Each of the
rings may have 1 to 4 substituents. Preferred positions
are the 2- and/or 3-positions in the case the ring A,_ and
7- and/or 8-positions in the case of the ring B.
Preferably, each of the rings A and B has 0 to 2
substituents.
Particularly preferred are compounds in which
one of the rings A and B has 1 or 2 substituents and the
other has no substituents.
Table 1 given later shows the structure of the
22~~U~ i
-5-
indeno[1,2-b]quinoline ring and the positions of the
substituents in the rings A and B.
Examples of the lower alkylenedioxy group for
use in the present invention include C1-4 alkylenedioxy
groups such as methylenedioxy, ethylenedioxy,
trimethylenedioxy and tetramethylenedioxy. The positions
of these substituents are preferably selected so as to
form a 1,2- or 2,3-substituted indeno[1,2-b]quinoline , ,
ring in the case of the ring A, while in the case of ring
B, the positions are selected so as to form a 7,8- or
8,9-substituted indeno[1,2-b]quinoline ring.
Examples of the halogen atom include fluorine,
chlorine, bromine and iodine.
The lower alkyl group for use in the present
invention includes, for example, straignt- or branched-
chain C1-6 alkyl groups such as methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl,
pentyl, hexyl, etc. _.
The lower alkoxy group includes, for example,
straight- or branched-chain C1-6 alkoxy groups such as
methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy,
isobutoxy, sec-butoxy, tert-butoxy, pentyloxy, hexyloxy,
etc.
The lower acyloxy group includes, for example,
straight- or branched-chain C1-6 acyloxy groups such as
2 _ l,
~u ~ i
~2 i~ ~,
-6-
formyloxy, acetoxy, propionyloxy, butyryloxy, 2-
methylpropionyloxy, pivaloyloxy, pentanoyloxy, 3-
methylbutyryloxy, hexanoyloxy, etc.
The lower acylamino group includes, for
example, straight- or branched-chain Cl-6 acylamino
groups such as formylamino, acetylamino, propionylamino,
butyrylamino, 2-methylpropionylamino, pivaloylamino,
pentanoylamino, 3-methylbutyrylamino, hexanoylamino, etc,.
The lower alkoxycarbonyl group includes, for
example, straight- or branched-chain C2-~ alkoxycarbonyl
groups such as methoxycarbonyl, ethoxycarbonyl, n
propoxycarbonyl, isopropoxycarbonyl, n-butoxycarbonyl,
isobutoxycarbonyl, sec-butoxycarbonyl, tert-
butoxycarbonyl, pentyloxycarbonyl, hexyloxycarbonyl, etc.
Examples of the substituents in the nitrogen-
containing heterocyclic group represented by R, R1 and R2
include a lower alkyl group, a lower alkyl group which
has a hydroxyl group, a phenyl group, a nitrogen- _
containing heterocyclic group, etc., among which a lower
alkyl group and a piperidino group are preferred.
As to the "optionally substituted amino
group(s)" in the definitions of R1 and R2 and "a lower
alkyl group which may be substituted by substituted amino
group(s)" represented by R3 and R4, examples of the
substituents in the substituted amino group include lower
22~203i
alkyl, lower cycloalkyl, di-lower alkylamino-alkyl,
hydroxy-lower alkyl, benzyloxycarbonyl, lower acyl, etc.
Among them, preferred is lower alkyl. The amino group
may be either mono- or di-substituted, but a di-
substituted amino group is preferred.
The lower alkyl group which has substituted
amino groups) includes, for example, mono- or di-
alkylaminoalkyl groups wherein the alkyl moiety has 1 to.
6 carbon atoms, such as methylaminomethyl,
ethylaminomethyl, methylaminoethyl, ethylaminoethyl,
dimethylaminomethyl, dimethylaminoethyl,
dimethylaminopropyl, diethylaminomethyl,
diethylaminoethyl, diethylaminopropyl, diethylaminobutyl,
diethylaminopenta-2-yl, dipropylaminoethyl,
dibutylaminoethyl, dibutylaminohexyl, etc.; alkyl groups
substituted by C2-6 acylamino group(s), such as N-
dimethylaminoethyl-N-methylaminoethyl, acetylaminoethyl,
acetylaminopropyl, propionylaminoethyl, _
propionylaminopropyl, pivaloylaminoethyl,
pivaloylaminopropyl, etc.; alkyl groups substituted by a
C3-6 cycloalkylamino group, such as
cyclopropylaminomethyl, cyclopentylaminomethyl,
cyclopentylaminoethyl, cyclohexylaminomethyl,
cyclohexylaminoethyl, etc.; alkyl groups substituted by a
C1_4 hydroxyalkylamino group, such as
22~2~~~
-8-
hydroxymethylaminomethyl, 2-hydroxyethylaminomethyl, 3-
hydroxypropylaminomethyl, hydroxymethylaminoethyl, 2-
hydroxyethylaminoethyl, 3-hydroxypropylaminoethyl, 4-
hydroxybutylaminoethyl, etc.; alkyl groups substituted by
a benzyloxycarbonylamino group, such as
benzyloxycarbonylaminomethyl,
benzyloxycarbonylaminoethyl, N-benzyloxycarbonyl-N-
methylaminoethyl, etc.; and the like. .
The lower alkyl group which has lower alkoxy
groups) includes, for example, straignt- or branched-
chain C1-6 alkyl groups substituted by a C1-6 alkoxy
group, such as methoxymethyl, ethoxymethyl,
propoxymethyl, methoxyethyl, ethoxyethyl, methoxypropyl,
etc.
The lower alkyl group which has phenyl groups)
includes, for example, straignt- or branched-chain C1-4
alkyl group containing 1 to 3 phenyl groups, such as
benzyl, phenetyl, 2-phenetyl, phenylpropyl, benzhydry~
and trityl.
The nitrogen-containing heterocyclic group
represented by R, R1 and R2 is preferably 5- or 6-
membered monocyclic heterocyclic group which has 1 to 4
nitrogen atoms and 0 or 1 oxygen or sulfur atom.
Examples of said group include pyrrolyl, oxazolyl,
thiazolyl, isoxazolyl, isothiazolyl, pyrazolyl,
22;:)~ 3 i
_9-
imidazolyl, oxadiazolyl, thiadiazolyl, triazolyl,
oxatriazolyl, thiatriazolyl, tetrazolyl, pyridyl,
pyridazinyl, pyrimidinyl, pyradinyl, pyrrolidinyl,
pyrrolinyl, imidazolidinyl, imidazolinyl, pyrazolidinyl,
pyrazolinyl, piperidyl, piperidino, piperadinyl,
morpholinyl, morpholino, etc. Among them, preferred are
5- or 6-membered monocyclic heterocyclic groups which
have 1 to 3 nitrogen atoms and 0 or 1 oxygen atom. , .
Particularly preferred are pyridyl, pyrrolidinyl,
piperidyl, piperidino, piperadinyl, morpholinyl,
morpholino and 1,2,4-triazolyl, and more preferred are
pyrrolidinyl, piperidino and piperadinyl.
The optionally substituted nitrogen-containing
heterocyclic group includes, for example, 4-
methylpiperadinyl, 4-ethylpiperadinyl, 4-
phenylpiperadinyl, 4-methylpiperidino, 4-ethylpiperidino,
4-piperidinopiperidino, etc.
The lower alkyl group which has nitrogen-
containing heterocyclic groups) represented by R1 and R2
includes, for example, straight- or branched-chain C1-6
alkyl groups containing a nitrogen-containing
heterocyclic group, such as 2-pyridylmethyl, 3-
pyridylmethyl, 4-pyridylmethyl, 2-pyridylethyl, 3-
pyridylethyl, 4-pyridylethyl, pyrrolidinylmethyl,
pyrrolidinylethyl, piperidinomethyl, piperidinoethyl,
22u~~J3 r
-10-
piperadinylmethyl, piperadinylethyl, morpholinomethyl,
morpholinoethyl, etc. Among them, preferred are
pyrrolidinylmethyl and pyrrolidinylethyl.
The lower alkyl group which has hydroxyl
groups) includes, for example, straight- or branched-
chain C1-6 alkyl groups containing 1 or 2 hydroxyl
groups, such as hydroxymethyl, 1-hydroxyethyl, 2-
hydroxyethyl, 2-hydroxypropyl, 3-hydroxypropyl, 2,3-
dihydroxypropyl, 4-hydroxybutyl, 2,3-dihydroxybutyl, 5-
hydroxypentyl, 2,3-dihydroxypentyl, 6-hydroxyhexyl, 2,3-
dihydroxyhexyl, etc.
The salts of the compound of the present
invention are not limited specifically insofar as they
are pharmaceutically acceptable, and include, for
example, salts of organic acids such as formic acid,
acetic acid, propionic acid, trifluoroacetic acid,
tartaric acid, malic acid, malefic acid, fumaric acid,
succinic acid, oxalic acid, methanesulfonic acid, p-
toluenesulfonic acid, etc. and salts of inorganic acids
such as hydrochloric acid, hydrobromic acid, sulfuric
acid, phosphoric acid, etc.
In the compound of the formula (1), the ring A
is preferably an unsubstituted or hydroxyl- or lower
alkoxy-substituted benzene ring, more preferably an
unsubstituted or hydroxyl-substituted benzene ring.
22U2U3i
-11-
The ring B is preferably an unsubstituted or
hydroxyl- or lower alkoxy-substituted benzene ring, more
preferably an unsubstituted or hydroxyl-substituted
benzene ring.
R is preferably a group -NR1R2 or -OR3,
particularly -NR1R2.
In the group -NR1R2, R1 and R2 are the same or
different, and preferably, they each represent a hydrogen
atom or a lower alkyl group which may be substituted by
substituted amino groups) or nitrogen-containing
heterocyclic group(s). More preferably, R1 and RZ each
represent a hydrogen atom or a lower alkyl group which
may be substituted by a di-lower alkyl-substituted amino
group or a pyrrodinyl group, and still more preferably,
R1 is a lower alkyl group substituted by a dimethylamino
group, a diethylamino group or a pyrrolidinyl group and
R2 is a hydrogen atom.
R3 and R4 are preferably a lower alkyl groin
which may be substituted by a di-lower alkyl-substituted
amino group.
Preferred species of the compound of the
present invention are those of the formula (1) wherein
the ring A is an unsubstituted or hydroxyl- or lower
alkoxy-substituted benzene ring, the ring B is an
unsubstituted or hydroxyl- or lower alkoxy-substituted
220~~j~
-12-
benzene ring (provided that at least one of the rings A
and B is a substituted benzene ring), R is
-NR1R2 (wherein R1 and R2 are the same or different and
each represent a hydrogen atom or a lower alkyl group
which may be substituted by a substituted amino group or
a nitrogen-containing heterocyclic group) other than
-NHCH3, or -OR3 (wherein R3 is a lower alkyl group which
may be substituted by a di-lower alkyl-substituted amino.
group).
More preferred species are compounds of the
formula (1) wherein the ring A is an unsubstituted or
hydroxyl-substituted benzene ring, the ring B is an
unsubstituted or hydroxyl-substituted benzene ring
(provided that at least one of the rings A and B is a
substituted benzene ring), R is -NR1R2 (wherein R1 and R2
are the same or different and each represent a hydrogen
atom, or a lower alkyl group which may be substituted by
a di-lower alkyl-substituted amino group or a _
pyrrolidinyl group) other than -NHCH3, and in particular,
R1 is a lower alkyl group substituted by a dimethylamino
group, a diethylamino group or a pyrrolidinyl group, and
R2 is a hydrogen atom).
As used herein "lower alkyl group which may be
substituted by a di-lower alkyl-substituted amino group
or a pyrrolidinyl group" means "a lower alkyl group which
22 ~2U5 i
-13-
may be substituted by a di-lower alkyl-substituted amino
group, or a lower alkyl group which may be substituted by
a pyrrolidinyl group".
The compound of the present invention
represented by the formula (1) can be produced, for
example, according to the following reaction scheme 1.
<Reaction Scheme 1>
O X O R
Process A /
B A ~ I g
R H
N N
~2) ~1)
wherein the rings A and B and R are as defined above, X
is a halogen atom and RH is an amine [NH(R1)(R2) or a
nitrogen-containing heterocyclic compound which may have
substituent(s)], alcohol (R30H) or thiol (ROSH) (wherein
R1, R2 R3 and R4 are as defined above).
(Process A]
The 10-halogenoindeno[1,2-b]quinolin-11-one
derivative of the formula (2) is aminated, alkoxylated or
thioxylated using the amine represented by RH [NH(R1)(R2)
or a nitrogen-containing heterocyclic compound which may
have substituent(s)], alcohol (R30H) or thiol (ROSH),
respectively, without using solvents or in an appropriate
solvent, whereby the compound of the present invention
22~2?3j
-14-
represented by the formula (1) is obtained.
For the amination, sodium hydride, tert-
butoxypotassium, sodium hydroxide, potassium hydroxide,
potassium carbonate, sodium carbonate, sodium
bicarbonate, triethylamine, etc. may be employed without
using solvents or in an appropriate solvent. For
alkoxylation, the alcohol may be used as such or as an
alcoholate prepared by adding sodium, sodium hydride,
tert-butoxypotassium, etc. in an appropriate solvent.
Thiol for the thioxylation may be used as such or as a
thiolate prepared by adding sodium hydride, sodium
hydroxide, potassium carbonate, triethylamine, etc.
Examples of the solvent include alcohols such
as methanol, ethanol, propanol, tert-butanol, etc.,
dimethylformamide, dimethylacetoamide, pyridine, toluene,
benzene, acetonitrile, tetrahydrofuran, water, etc.
These solvents can be used singly or as a mixture of two
or more. -
For the reaction, it is suitable to use the
amine, alcohol or thiol in a proportion of 0.1 to 100
moles, preferably 1 to 10 moles per mole of the compound
of the formula (2). The reaction advantageously proceeds
at a reaction temperature of 0 to 200°C, preferably 20 to
150°C, for a reaction period of 0.1 to 100 hours,
preferably 0.5 to 60 hours.
~2uz~:~ i
-15-
When the compound of the formula (1) produced
according to the reaction scheme 1 has lower alkoxy
groups) or benzyloxy groups) in the ring A or B, the
substituent can be converted into hydroxyl groups) when
necessary, for example by the reaction with a inorganic
acid such as hydrobromic acid, hydroiodic acid,
hydrochloric acid, sulfuric acid or the like without
using solvents or in an appropriate solvent. Examples.o~
the solvent include acetic acid, water, etc., which can
be used singly or as a mixture of two or more. For the
reaction, the hydrobromic acid, hydroiodic acid,
hydrochloric acid, sulfuric acid or the like is used in a
proportion of 1 to 100 parts (v/w), preferably 5 to 100
parts (v/w), per part of the compound of the formula (1)
substituted by lower alkoxy groups) or benzyloxy
group(s). The reaction advantageously proceeds at a
reaction temperature of 0 to 200°C, preferably 50 to
150°C, for a reaction period of 0.1 to 100 hours, _
preferably 0.5 to 60 hours.
When the compound of the formula (1) produced
according to the reaction scheme 1 has hydroxyl groups)
in the ring A or B, the substituent can be converted into
alkoxyl, benzyloxy or acyloxy groups) by alkylation,
benzylation or acylation, respectively, if necessary.
The alkylation or benzylation is carried out by
~2~2U31
-16-
reacting the compound with an alkylating agent or a
benzylating agent, respectively, in an appropriate
solvent in the presence of a base. Examples of the
solvent include dimethoxyethane, dioxane,
tetrahydrofuran, dimethylformamide, acetone, etc.
Examples of the base are potassium carbonate, sodium
carbonate, potassium hydroxide, etc. The alkylating
agent is, for example, halide, sulfuric acid ester or , .
sulfonic acid ester of optionally substituted alkane, and
the like. Examples of the benzylating agent include
benzyl halide and the like. The proportions of the base
and alkylating or benzylating agent in the reaction
system are 1 to 5 moles of the base and 1 to 5 moles of
the alkylation or benzylating agent per mole of the
hydroxyl group. The reaction advantageously proceeds at
a reaction temperature of 0 to 80°C for a reaction period
of 0.1 to 24 hours, preferably 0.5 to 10 hours.
The acylation is carried out by reacting th._e
compound with a desired carboxylic acid or a reactive
derivative thereof. When a reactive derivative is used,
the reaction is carried out in an appropriate solvent,
and a suitable base may be added to accelerate the
reaction, although dependent on the kind of the reactive
derivative and the starting phenolic derivative. Such
reactive derivative includes, for example, acid
22C~2u5 i
anhydrides, acid anhydride mixtures, acid halides and the
like. Examples of the solvent include chloroform,
dichloromethane, dioxane, tetrahydrofuran,
dimethylformamide, pyridine, etc. Examples of the base
include sodium bicarbonate, potassium bicarbonate,
triethylamine, pyridine, 4-dimethylaminopyridine, etc.
The proportions of the base and acylating agent in the
reaction system are 1 to 5 moles of the former and 1 to, 5
moles of the latter per mole of the hydroxyl group. The
reaction advantageously proceeds at a reaction
temperature of 0 to 50°C for a reaction period of 0.1 to
24 hours, preferably 0.5 to 3 hours.
The compound of the present invention produced
by the above reaction can be made into a salt by a
conventional process, for example by reacting the
compound in an appropriate solvent with an organic acid
such as formic acid, acetic acid, propionic acid,
trifluoroacetic acid, tartaric acid, malic acid, male_ic
acid, fumaric acid, succinic acid, oxalic acid,
methanesulfonic acid, p-toluenesulfonic acid or the like,
or an inorganic acid such as hydrochloric acid,
hydrobromic acid, sulfuric acid, phosphoric acid or the
like, as mentioned above. Examples of the solvent
include water, methanol, ethanol, dichloromethane,
tetrahydrofuran, etc. The reaction temperature is
2202u~ ~
-18-
preferably 0 to 50°C.
The 10-halogeno-11H-indeno[1,2-b]quinolin-11-
one derivative of the formula (2) used as the starting
material in the reaction scheme 1 can be produced, for
example by the process described in Pol. J. Chem., 55
121-128 (1981). Specifically stated, said compound can
be produced according to the following reaction scheme 2,
for instance.
<Reaction Scheme 2>
O O
COORS Process B COOH Process C
BI I ~ I I
N / N /
AI H A(
(3) (4)
x
O x
' COX process D
N~ y . A I I ~ B
A1 ' N '_
(S) (2)
wherein the rings A and B and X are as defined above, and
R5 is a lower alkyl group.
[Process B]
The compound of the formula (3) is hydrolyzed
with a basic compound usually in an appropriate solvent
to produce the carboxylic acid of the formula (4).
z~ u~~~ i
-19-
The compound of the formula (3) can be produced
by the process described in Berichte 18 2632 (1885) using
the benzoyl halide compound (ring A) and the aniline
derivative (ring B) as starting materials.
The solvent is not limited specifically insofar
as it does not participate in the reaction. Examples are
alcohols such as methanol, ethanol, propanol, etc.,
ethers such as dioxane, tetrahydrofuran, dimethoxyethane;
etc., water, and the like. These solvents can be used
singly or as a mixture of two or more. Examples of the
basic compound include hydroxides of alkali metals such
as sodium hydroxide, potassium hydroxide, etc. and
hydroxides of alikali earth metals such as barium
hydroxide, etc.
For the reaction, the basic compound is used in
a proportion of 1 to 10 moles per mole of the compound of
the formula (3). The reaction advantageously proceeds at
a reaction temperature of 0 to 100°C, preferably 50 t_o
100°C for a reaction period of 0.1 to 100 hours,
preferably 1 to 50 hours.
[Process C]
The compound of the formula (4) obtained by the
process B is reacted with a halogenating agent usually
without using solvents or, when necessary, in an inert
solvent to produce the compound of the formula (5).
2202U3i
-20-
The inert solvent for use in the reaction is
not specifically limited insofar as it does not
participated in the reaction. Examples are chloroform,
benzene, toluene, xylene, etc. Examples of the
halogenating agent include thionyl chloride, thionyl
bromide, phosphorus oxychloride, phosphorus trichloride,
phosphorus tribromide, phosphorus pentachloride,
phosphorus pentabromide, etc. For accelerating the , -
reaction, pyridine, dimethylformamide or the like may be
added.
For the reaction, the halogenating agent is
preferably used in a proportion of 1 to 100 moles per
mole of the compound of the formula (4). The reaction
advantageously proceeds at a reaction temperature of 0 to
200°C, preferably 50 to 150°C, for a reaction period of
0.5 to 100 hours, preferably 0.5 to 10 hours.
The compound of the formula (5) obtained by the
reaction can be isolated and purified when necessary,_ but
can be used for the subsequent process without being
purified.
[Process DJ
The compound of the formula (5) obtained by the
process C is reacted with protonic acid or Lewis acid
without using solvents or, when necessary, in an inert
solvent to produce the compound of the formula (2).
2202~3i
-21-
The inert solvent for use in the reaction is
not limited specifically insofar as it does not
participate in the reaction. Examples are nitrobenzene,
xylene, dichloromethane, carbon tetrachloride, etc.
Examples of protonic acid are sulfuric acid, phosphoric
acid, polyphosphoric acid, hydrogen bromide, etc., and
examples of Lewis acid are aluminum chloride, tin
chloride, iron chloride, etc. .
When protonic acid is used, the proportion
thereof is 1 to 1000 parts (v/w), preferably 3 to 100
parts (v/w) per part of the compound of the formula (5),
whereas when Lewis acid is used, the proportion thereof
is 1 to 100 moles, preferably 1 to 10 moles per mole of
the compound of the formula (5). The reaction
advantageously proceeds at a reaction temperature of 0 to
200°C, preferably 20 to 150°C, for a reaction period of
0.5 to 50 hours, preferably 0.5 to 20 hours.
The compounds of the present invention and the
other compounds obtained above can be isolated and
purified by conventionally known processes for isolation
and purification, such as concentration, extraction with
a solvent, filteration, recrystallization, various types
of chromatography, etc.
When the compound of the formula (1) of the
invention is used as an agent for treating malignant
22~2'i~31
-22-
tumors of mammals including humans (subjects), various
pharmaceutical dosage forms can be employed in accordance
with the purposes. Specifically stated, the dosage forms
include, for example, oral preparations such as tablets,
coated tablets, pills, powders, granules, capsules,
solutions, suspensions and emulsions, etc., and non-oral
preparations such as injections, suppositories, ointments
and plasters. These preparations are formulated in a , -
manner conventionally known in the art.
Carriers which are usable for the formulation
of tablets includes, for example, excipients such as
lactose, sucrose, sodium chloride, glucose, urea, starch,
calcium carbonate, kaolin, crystalline cellulose, silicic
acid, etc.; binders such as simple syrup, glucose
solution, starch solution, gelatin solution,
carboxymethyl cellulose, shellac, methyl cellulose,
potassium phosphate, polyvinylpyrrolidone, etc.,
disintegrators such as dried starch, sodium alginate,._
agar powder, laminaran powder, sodium hydrogen carbonate,
calcium carbonate, polyoxyethylene sorbitan fatty acid
esters, sodium lauryl sulfate, stearic acid
monoglyceride, starch, lactose, etc., disintegration
preventing agents such as sucrose, stearic acid, cacao
butter, hydrogenated oils, etc., absorption promoting
agents such as quaternary ammonium base, sodium lauryl
22J~~.~ i
-23-
sulfate, etc., humectants such as glycerin, starch, etc.,
adsorbents such as starch, lactose, kaolin, bentonite,
colloidal silicic acid, etc., lubricants such as purified
talc, stearic acid salts, boric acid powder, polyethylene
glycol, etc., and the like. The tablets may be further
processed into coated tablets using an ordinary coating
film, for example sugar coated tablets, gelatin coated
tablets, film coated tablets, etc., and may be double-,
layered tablets, multilayered tablets and the like.
For the formulation of pills, usable carriers
include, for example, excipients such as glucose,
lactose, starch, cacao butter, hardened vegetable oils,
kaolin, talc, etc., binders such as gum arabic powder,
tragacanth powder, gelatin, etc., disintegrators such as
laminaran, agar, etc., and the like.
Capsules are usually prepared by mixing the
compound of the present invention with the above-
mentioned various carriers and enclosing the mixture into
hardened gelatin capsules, soft capsules or the like.
For the formulation of suppositories, carriers
can be used which include, for example, polyethylene
glycol, cacao butter, higher alcohols, esters of higher
alcohols, gelatin, semisynthetic glyceride, etc.
For the formulation of injections, the
solutions, emulsions and suspensions are preferably
2202U3i
-24-
sterilized and isotonic with blood. For producing these
forms, diluents can be employed which include, for
example, water, ethyl alcohol, macrogol, propylene
glycol, ethoxylated isostearyl alcohol, polyoxylated
isostearyl alcohol, polyoxyethylene sorbitan fatty acid
esters, etc. In this procedure, sodium chloride, glucose
or glycerin may be incorporated into the pharmaceutical
preparation in an amount sufficient to provide isotonic -
solutions. Conventional solubilizing agents, buffers,
soothing agents and the like may be also added.
Ointments can be prepared in a usual manner by
adding, to the compound of the invention, conventionally
used bases, stabilizers, lubricants, preservative, etc.
when necessary. Examples of useful bases are liquid
paraffin, white petrolatum, bleached beeswax, paraffin,
etc. Usable preservatives include methyl para-
hydroxybenzoate, ethyl para-hydroxybenzoate, propyl para-
hydroxybenzoate, etc. --
Plasters can be prepared in a usual manner by
applying said ointments, pastes, creams, gels, etc. to an
ordinary support. Examples of the suppports include
woven fabrics or non-woven fabrics composed of cotton,
staple fibers, chemical fibers or the like; films or
foamed sheets made from non-rigid polyvinyl chloride,
polyethylene, polyurethane or the like; etc.
~2J~JS i
-25-
When required, the above preparations may
contain pharmaceutically acceptable additives, such as
coloring agents, preservatives, perfumes, flavors,
sweeteners, etc., other medicaments and the like.
The amount of the compound of the invention to
be incorporated in the pharmaceutical preparation is not
specifically limited and can be selected from a wide
range. A suitable amount is in the range of 1 to 70~ by.
weight based on the pharmaceutical preparation.
The mode of administration is not specifically
limited and is suitably determined according to the
dosage form, patients' age, sex and other factors, the
severity of diseases, etc. For example, tablets, pills,
solutions, suspensions, emulsions, granules and capsules
are orally administered. Injections are intravenously
administered singly or in mixture with conventional
parenteral fluids such as glucose, amino acid, etc. or
may be administered intramuscularly, intracutaneously_,
subcutaneously or intraperitoneally. Suppositories are
applied into the rectum. Ointments are applied to the
skin, mucous membrane in the buccal cavity, or the like.
The amount of the compound of the invention in
each dosage unit of the above forms depends on the
conditions of the patient to be treated, the particular
dosage form selected and other factors. Generally,
22~~?:~ i
-26-
preferred amounts per dosage unit are about 1 to about
1,000 mg for oral preparations, about 0.1 to about 500 mg
for injections, and about 5 to about 1,000 mg for
suppositories. The daily dosage of the pharmaceutical
preparation in any of the forms mentioned above is also
dependent on the patients' condition, body weight, age,
sex and other factors, but it is generally recommendable
to administer about 0.1 to about 5,000 mg, preferably , -
about 1 to about 1,000 mg, per day for an adult patient,
either in a single dose or in 2 to 4 divided doses.
The malignant tumors to be treated with the
pharmaceutical preparation containing the compound of the
invention are not specifically limited and include, for
example, cancers of the head and neck, esophagus,
stomach, colon, rectum, liver, gall bladder-bile duct,
pancreas, lung, breast, ovary, urinary bladder, prostate,
testis, cervix uteri, skin and other parts, osteosarcoma
or sarcoma of soft part, malignant lymphoma, leukemia.,
cerebral tumor, etc.
Best Mode for Carrying out the Invention
Reference Examples, Examples and
Pharmacological Test Example are given below to
illustrate the present invention in further detail.
Reference Example 1
Synthesis of 1,4-dihydro-2-(4-methoxyphenyl)-4-
~2f~~~.~:~ i
-27-
oxo-3-quinolinecarboxylic acid
A mixture of 7.0 g (21.6 mmol) of 1,4-dihydro-
4-(4-methoxyphenyl)-4-oxo-3-quinolinecarboxylic acid
ethyl ester, 17.5 ml of ethanol, 52.5 ml of water and 6.1
g (108 mmol) of potassium hydroxide was refluxed with
heating for 10 hours. After completion of the reaction,
the reaction mixture was made acid with 25 ml of 6N
hydrochloric acid and filtered to collect precipitated ,
crystals. The obtained crystals were washed with
ethanol-diethylether, giving 6.0 g of the title compound
(yield: 93.9 ~).
m.p.. >300°C
1H-NMR (DMSO-d6) 8:
12.87 (lH.s), 8.30 (1H, d, J=8Hz), 7.91-7.82
(2H, m), 7.58 (1H, d-d-d, J=8, 7, 2Hz), 7.49
(2H, d, J=9Hz), 7.07 (2H, d, J=9Hz), 3.85 (3H,
s)
IR(KBr)cm 1. _
2930, 1688, 1634, 1608, 1517, 1507, 1472, 1447,
1257
Reference Example 2
Synthesis of 10-chloro-2-methoxy-11H-
indeno[1,2-b]quinolin-11-one
A mixture of 5.5 g (18.6 mmol) of 1,4-dihydro-
2-(4-methoxyphenyl)-4-oxo-3-quinolinecarboxylic acid
2202~~ i
_28_
obtained in Reference Example 1 and 55 ml (0.59 mol) of
phosphorous oxychloride was refluxed with heating for 5
hours in the presence of a catalytic amount of N,N-
dimethylformamide. After completion of the reaction, the
reaction mixture was evaporated to dryness, and the
residue was washed several times with n-hexane. 55 g of
polyphosphoric acid was added to the washed residue, and
the mixture was stirred for 2 hours with heating at
130°C. After completion of the reaction, the reaction
mixture, while hot, was added to ice water, and
precipitated crystals were collected by filtration. The
obtained crystals were washed with water and ethanol and
dissolved in a large amount of chloroform. The solution
was filtered to remove insoluble matter and evaporated to
dryness and recrystallized from toluene, giving 2.7 g of
the title compound (yield: 49.00 .
m.p.. 216-218°C
1H-NMR (DMSO-d6) 8: _
8.23 (1H, d-d, J=8, 1Hz), 8.02 (1H, d-d, J=8,
1Hz), 7.90-7.84 (2H, m), 7.68 (1H, d-d-d, J=8,
7, 1Hz), 7.29 (1H, d-d, J=8, 3Hz), 7.24 (1H, d,
J=2Hz), 3.90 (3H, s)
IR(KBr)cm 1.
1720, 1616, 1604, 1583, 1567, 1513, 1493, 1365,
1287, 1240, 797, 760
2202031
-29
Reference Example 3
Synthesis of 10-chloro-8-methoxy-11H-
indeno[1,2-b]quinolin-11-one
A mixture of 3.5 g (10.8 mmol) of 1,4-dihydro-
6-methoxy-4-oxo-2-phenyl-3-quinolinecarboxylic acid ethyl
ester, 4.5 g (21.6 mmol) of phosphorus pentachloride and
100 ml of benzene was stirred at room temperature for 1
hour and then refluxed with heating for 2 hours. After, -
completion of the reaction, the reaction mixture was
evaporated to dryness, and the residue was crystallized
from n-hexane. The obtained crystals were collected by
filteration and dried under reduced pressure, giving 3.2
g of 4-chloro-6-methoxy-2-phenyl-3-quinolinecarboxylic
acid chloride (yield: 89.20 . 2.7 g (8.1 mmol) of the
obtained acid chloride was added to 27 g of
polyphosphoric acid heated to 90°C, and the mixture was
stirred for 4 hours with heating at 130°C. After
completion of the reaction, the reaction mixture, while
hot, was added to ice water, and precipitated crystals
were collected by filteration. The obtained crystals
were dissolved in chloroform, and the solution was
filtered to remove insoluble matter. The chloroform was
distilled off, and the residue was recrystallized from
benzene, giving 0.8 g of the title compound (yield:
33.40 .
2202031
-30-
m.p.. 232-234°C
1H-NMR (CDC13) 8:
8.04 (1H, d, J=8Hz), 8.02 (1H, d, J=9Hz), 7.84
(1H, d, J=7Hz), 7.68 (1H, d-d-d, J=8, 7, 1Hz),
7.62 (1H, d, J=3Hz), 7.51 (1H, d-d-d, J=8, 7,
1Hz), 7.44 (1H, d-d, J=9, 3Hz), 4.00 (3H, s)
IR(KBr)cm 1.
1718, 1621, 1614, 1514, 1239, 847, 831, 761,,
724
Reference Example 4
Synthesis of 10-chloro-8-hydroxy-11H-
indeno[1,2-b]quinolin-11-one
A mixture of 500 mg (1.5 mmol) of 4-chloro-6-
methoxy-2-phenyl-3-quinolinecarboxylic acid chloride
obtained in Reference Example 3 and 10 ml of sulfuric
acid was stirred for 3 hours with heating at 130°C.
After completion of the reaction, the reaction mixture,
while hot, was added to ice water, and precipitated _
crystals were collected by filteration. The obtained
crystals were air-dried and dissolved in hot acetone, and
the solution was filtered to remove insoluble matter.
The acetone was distilled off, and the residue was
recrystallized from benzene, giving 220 mg of the title
compound (yield: 51.90 .
m.p.. 253°C
22 02031
-31-
1H-NMR (DMSO-d6) 8:
10.60 (1H, s), 7.97 (1H, d, J=9Hz), 7.96 (1H,
d, J=7Hz), 7.80-7.74 (2H, m), 7.59 (1H, d-d-d,
J=8, 7, 1Hz), 7.53 (1H, d, J=3Hz), 7.44 (1H, d-
d, J=9, 3Hz)
IR(KBr)cm 1.
2600, 2361, 1723, 1615, 1575, 1528, 1452, 1374,
1256, 1237, 1189, 859, 825, 761, 726
Reference Example 5
The following compounds were synthesized using
the compounds obtained in Reference Examples 1-4.
* 10-Chloro-3-methoxy-11H-indeno[1,2-b]quinolin-11-one
m.p.. 218-221°C
1H-NMR (CDC13) 8:
8.36 (1H, d-d, J=9, 2Hz), 8.11 (1H, d-d, J=8,
1Hz), 7.84-7.78 (2H, m), 7.63 (1H, d-d-d, J=9,
8, 1Hz), 7.56 (1H, d, J=2Hz), 7.02 (1H, d-d,
J=8, 2Hz), 4.01 (3H, s)
IR(KBr)cm 1.
1706, 1617, 1597, 1587, 1567, 1483, 1339, 1276,
1256, 1233, 1091, 773
* 10-Chloro-1-methoxy-11H-indeno[1,2-b]quinolin-11-one
m.p.. 230-232°C
1H-NMR (CDC13) 6:
8.37 (1H, d-d, J=8, 2Hz), 8.12 (1H, d-d, J=8,
22 i~2C.~ 1
-32-
1Hz), 7.81(1H, d-d-d, J=9, 8, 2Hz), 7.73-7.60
(3H, m), 7.05(1H, d, J=8Hz), 4.04 (3H, s)
IR(KBr)cm 1.
1711, 1617, 1594, 1567, 1484, 1442, 1367, 1276,
1193, 1174, 1054, 1037, 948, 838, 761, 740
*10-Chloro-7-methoxy-11H-indeno[1,2-b]quinolin-11-one
m.p.. 212-214°C
1H-NMR (CDC13) 6:
8.23 (1H, d, J=9Hz), 8.05 (1H, d-d, J=8, 1Hz),
7.84 (1H, d-d, J=7, 1Hz), 7.68 (1H, d-d-d, J=8,
8, 1Hz), 7.53 (1H, d-d-d, J=8, 7, 1Hz), 7.47
(1H, d, J=3Hz), 7.24 (1H, d-d, J=9, 3Hz), 4.01
(3H, s)
IR(KBr)cm 1.
1714, 1612, 1584, 1573, 1504, 1349, 1225, 1137,
933, 728
Example 1
Synthesis of 10-(((dimethylamino)ethyl)amirro)-
2-methoxy-11H-indeno[1,2-b]quinolin-11-one
dihydrochloride (Compound 1)
A mixture of 1.2 g (4.1 mmol) of 10-chloro-2-
methoxy-11H-indeno[1,2-b]quinolin-11-one obtained in
Reference Example 2, 1.1 g (14.2 mmol) of N,N-
dimethylethylenediamine and 20 ml of ethanol was refluxed
with heating for 3 hours. After completion of the
22J2J3 i
-33-
reaction, the reaction mixture was exsiccated under
reduced pressure. Water was added to the residue, and
the mixture was subjected to extraction with chloroform.
The chloroform layer was dried with magnesium sulfate and
distilled off. The residue was purified by the use of
silica gel column chromatography (developing solvent;
chloroform:ethanol = 20:1 (v/v)), giving 1.1 g of 10-
(((dimethylamino)ethyl)amino)-2-methoxy-11H-indeno[1,2-,b}
quinolin-11-one (yield: 78.00 . 550 mg (1.58 mmo1) of
the obtained compound was dissolved in 10 ml of a mixture
of tetrahydrofuran and methanol (1:1 (v/v)). The
solution was made acid with 1.5 ml of 4N hydrochloric
acid/dioxane. The acid solution was exsiccated under
reduced pressure, and the residue was washed with diethyl
ether, giving 645 mg of the title compound (yield:96.9 ~,
yield calculated based on the compound of Reference
Example 2: 75.60 . The property values are shown in
Table 1. _
Example 2
Synthesis of 10-(((dimethylamino)ethyl)amino)-
2-hydroxy-11H-indeno[1,2-b]quinolin-11-one
dihydrochloride (Compound 2)
15 ml of 47~ aqueous hydrogen bromide was added
to a solution of 550 mg (1.58mmo1) of 10-
(((dimethylamino)ethyl)amino)-2-methoxy-11H-indeno[1,2-
~~ ~~~131
J
-34-
b]quinolin-11-one in 20 ml of acetic acid, and the
mixture was refluxed with heating. Forty eight hours
later, 10 ml of 47~ aqueous hydrogen bromide was added to
the reaction mixture, followed by reflux with heating for
22 hours. After completion of the reaction, the reaction
mixture was exsiccated under reduced pressure. Water was
added to the residue, and the mixture was made weakly
basic with aqueous ammonia. Saturated saline solution, ,
was added, followed by extraction with tetrahydrofurane.
The tetrahydrofuran layer was dried with magnesium
sulfate and distilled off, and the residue was purified
by the use of silica gel column chromatography
(developing solvent; chloroform: ethanol = 10:1 (v/v)).
The purified product was dissolved in tetrahydrofuran,
and the solution was made acid with an excess amount of
4N hydrochloric acid/dioxane and exsiccated under reduced
pressure. The residue was washed with diethyl ether,
giving 366 mg of title compound (yield: 56.90 . The "
property values are shown in Table 1.
Exam~,le 3
Synthesis of 10-(((dimethylamino)ethyl)thio)-8-
hydroxy-11H-indeno[1,2-b]quinolin-11-one (Compound 10)
A mixture of 400 mg (1.4 mmol) of 10-chloro-8-
hydroxy-11H-indeno[1,2-b]quinolin-11-one, 629 mg (8.5
mmol) of lithium carbonate, 963 mg (5.7 mmol) of 2-
22~2J~i
-35-
diethylaminoethanethiol hydrochloride and 8 ml of N,N-
dimethylformamide was stirred for 5 hours with heating at
90°C. After completion of the reaction, the reaction
mixture was added to water, and precipitated crystals
were collected by filteration. The crystals were
recrystallized from ethanol, giving 310 mg of the title
compound (yield: 57.70 . The property values are shown
in Table 1.
Example 4
Synthesis of 10-(((diethylamino)ethoxy)-8-
hydroxy-11H-indeno[1,2-b]quinolin-11-one hydrochloride
(Compound 11)
250 mg (6.3 mmol) of sodium hydride was added
to a mixture of 1.77 g (15.1 mmol) of diethylaminoethanol
and 20 ml of toluene with ice-cooling. The reaction
mixture was returned to room temperature, stirred for 30
minutes and heated to 50°C. To the reaction mixture was
added 800 mg (2.8 mmol) of 10-chloro-8-hydroxy-11H-
indeno[1,2-b)quinolin-11-one, and the mixture was stirred
at 60°C for 3 hours. After completion of the reaction,
saturated aqueous ammonium acetate was added to the
reaction mixture, and the solvent was distilled off.
Saturated aqueous sodium chloride was added to the
residue, and the mixture was subjected to extraction with
tetrahydrofuran. The tetrahydrofuran layer was dried
~2~~~3i
-36-
with magnesium sulfate and distilled off, and the residue
was purified by the use of silica gel column
chromatography (developing solvent; chloroform:ethanol =
10:1 (v/v)). The purified product was dissolved in 10 ml
of tetrahydrofuran, and 1 ml of 4N hydrochloric
acid/dioxane was added. After concentration under
reduced pressure, the obtained residue was crystallized
from diethyl ether, giving 203 mg of the title compound .
(yield: 17.90 . The property values are shown in Table
1.
Examples 5-16
Compounds 3 to 9 and 12 to 16 shown in Table 1
were prepared from the corresponding starting materials
by the procedure similar to that of Examples 1 to 4.
22U2i~3 r
-37-
1 M O GD O CD C~7 l17 M f~N
CD
g ~-~ c0 c0 M t~ ~ O 00I~COc0
U f~ CO V' M N (~ C'MN I
f~ cD ..-~.....-r
.-n .~ .--n .--mr~ .-.
L _ _ . _ _ . . _ __ _
G~ O O GO ~~ ~ N O 07 c0MO tc~
X O C' (~ M O CO CO l~cDO L
:
J
I~ GD t17 C' N cD 1!7C'M I~
N I~
G~ N ~~ ~ ~ ~.. M ~. .-.~..-.,
''3
n
n _ ~ . n _ N _
vI 'O N .'~'
Vl
. L I w ~. _
~ p 'O 00 ~ X v1 'C1N E7
N G~ N
.. vi . _ _ t~ M L II -
S
L S S GO N ~ p ~~ S CO . u7
tI~
.D ~ ~~ II . _ _ ~ 11
M II
Q) '..~ S 'p ~rGO N '-7
a ~ M M _ . _ .~ cDII
_
-CJ .-, t17 O~ 'O ~ -y. N ~~~ I~
_ M TJ tJ
I ~ . . 1 n O ~ . _N V'
O M GO c~ T7 N ~ '--~GO'OS
. S
V7 tt7 _ .:: n CD . O IGOd'
CO
g . . S N A ~ O GO . 'Q
a a; ... .-. .--~ - . .~._c0. 00
. _ c~
N N v 07 S ~A GO N SI)n OO
CC . T .'i: CO 11 . ~~-~H
N
n 07 CO CD "~ r-~ c0~ _ N -
~ N .
z N II II . _ GO V1 IIN. i
n
_ 1 L '~ '~ h 'C L. '~O7'ON .
e!' . V1
~1 . . I . n G L _ .I ~ n
-. _ ~p ~p . 'p _ ~ 'Qt~'CM A
V' 6
,r . . n . . -
rr ~ -. N .= ...... ..... S
. S
yr ... .-, x yr .~n.~c~
.~ n N ..-~ N
~r N ~ N ~ M ~r ~r v I 'r
C' a0 C' . u7 ~ O~ l~~.c~cD
Z O O
(p , c~ N CO M N , t!~ ~'c0CO...
t1~ u7
O II . II II.
.-. CO c0 ~~ -~ c0-7t~I~
c~ "~ M 07 M
n
G ~.-~ C
f~ I O C 1 O
C' A .'. cD .-
A
0. N U ~ N O
M d Vf O G7 ul -
A C' ''~ O . cD O
'C
N ~ 0. N ~ 0.
r
CG
O
N c7 .~ ~ ca
cm n
E-~ c~
_
_..
m
cC V O
G~ O I
I N
N
N N
m U m
V N U V
Z Z N
N N
U U
N N
U U
S
z z
I
v
0
n
0
-. N
a
- 22 ~2~3 i
I GO V'M CVCDI~ GOU7l!7U7tf~O N GOtf!
Q cD GOO t~.-,GO O N I~cDtt7O ~'Ot1~
U CD IIJ~ V'M ~ t~-COt17V'l~ l~ CD~C)t~
~ .--m.~..r.....-.~ -r~..-,.-. C~l.--n.-r
i-... __ . _ . _ _ _ _ _ __
~
Ca O Wit'M O ~ CD O N N ~ O O C NGV
M
:G N -~M c0cDcD O V'O O c~ u'J.--~t~-CD
CD
CO cD~ ~'C N I~cDcDLCJN O l~-tf7M
f~
N .r.~,-r~ .-r N .--~.--n.--n~ M .-n.-,.-.
~ ~ ~ . ~
_ N N A ~ N
~ x x _ _ VI
N ~ N
_ IIN 't7_ '~II
CO GO ~ ~ _ 1.'QM ~ _ Y..'~
1 il ..LfJU7 ~ p I ~ N ~ p -
.. ~p ~_~ 'pO _ v1..'BCDx v7 _ ~p
_ __ , x ~-.x . OoIn L-
~O 'D -p~ ~ ~'Cp p .--n,_,. II .O
~ IN rr ~ .,~ ._-~M ~7 _ ~ .-_n
x 'ax ~ . M x 07
x GO~~
p .-. .-~N n N .-.C'.-r_ 'p .-~tI7cDA
I ~ rT'.._ N N . ~ . M ~ _ a . ~-~
O ~ ~~C~, x N L~CO. A ~ CO CO
C/7 M ~_ N N M l~. CD M L~N
c-c- _ _ _ x ~ . _
G7 GJ GOII. CO~ Q7~ _ N I~ 07 ~ .O ~
'7n IIN N ~ ~ CO N ~Lf7
N
ca _ ~_ ~ --,x _ x A cc. . A. x ,
~ .'p _ GD ~ 07_ V'N ~ Q7.M t!7 -
z ~ ~I x w n ~nn x cn a xa -
c.-~~rd~ r..~o~_ --,
I ~...V1'>'N i ""'! _ _
x G tI ~ 'C- p
n ,Q _ ~~
_ p'~N . a~ _ 'OQ)..A _ '~CDA 'fl
x _. V'x x _ CD~ x . cD
xx . .--~x . N x ..,x ,x x
-,~ ~ M ~ _-W~ x N ~ ~ hN cD
~p ~~rI v CD~ I N ~ LI7'Ji
t17CO N c0LCJ V'-~d'..c0 V' tI7V'CD
C
. .-c~tI~c0t!7 . t~GOd'1d' . cDc0C'
CO
O . . O . . O
II
.~ c0I~c~cDN ~ c0n ~o ~ GOl~C'
M N
-
~ ~
C C ~ C
o U U I o M I o
A,_ A ... ~
0. CD O.~ cD M O N N O -'
cD U- tf~ N U ~ j U ...
N OV7 N N N VJ N O VJ
A ~p '~O M 'QO
p, ~ 0. N 0.
~
3E ~E
(' O ~N
O N ~ O
... V I~- CD
m
x x
V O
x ..... x o I _
I ~ __
x x
~a o 0
R.' I W . x
M M
N N N N
~
~ ~ ... ~ .-. ~~ U
m M U m U
x S x x x
U U N U N U N
'r ~r
z, z z . z
N N N N
x x s x
U U U U
n N N n
x x x x
U U U U
x x
z z z z,
I I
0
a M I V'
A M
0
v
-39- 2 2 0 2 J 3 i
. . ._ . _ __. _ ._ ._
I O u700 GOM ~ u7 . GO -O t~C'd'
M GPI CV
H wY'~ CD V'CD ~~ GV O GOO ~-M CSI
GO ~-~ t~
O7
U Q1 c0~L'7V'N L~ M cD tf7W ~'CV
V' cD 7
V~
n N .-.~ ,--n..~ ~.. ~ ~ ~..._..-.r.~ C~l
r. .~
.-.
_ _ _ _ . . . _ __ ._ _ CO
_ .
0.7 Q N M (~O M O O COCb O GOM l~07GO
CD O t
'rC O cDO~ CDCOu7 u7 u7O N OcD C'V'
O7 M
l~
M (DLC7V'M N d' M C' ~4 CD1.C7C'M G~l
~' ~D N
~7
CYO M .-..r ..._.r.-.. M ~. .-. N .-..~ ~rrr.-.
.-. .-, M
,...., 07
_ _ . n
N _ t~ ~ H
S - H It H
~
a~ O _ .-.
H
II cD_ n N T - SO N
-
.. -~ . _ N _ S C' -~ N
T1 S
_ L~~ S ~ l~~N . ~ I ~~I~ncJ'
I
'Q I N (~VI II n N 'D II7I N C'
'Q CD
~
u~S _ _ ~~ H S Nc0S
cD
~'
m S cDc' cDS _ . . t~ _, . .O t~
= N M
Z7 ~ . _ IIcp w 0 t~ II ~ ~ f~. _
~ .
~p ''~ _ 1 N '~ ~ N 1t~l~
I yr C' '
O M II _ In S O ~ _ N __ II
1l7 n
v7 r... '~ .-.~,. .-. O ~ O nN :7_ "'7
O ~p H
.
n _ I ~ . 'd~ . H- ~ _ _
n
'1 GO N '~ 'ON .~'~ . t~ .C~ f~N "O
CD t~ S
N
I C' M cD I N
S
....TI Z _ . . I ~ . NGO _M Zt
_ M
n N ~ c0 N Q) n ~II n
n
.
N t~S ~ N N Q) C ~ N CO~ NO .Y
N CO
1 II~ (~S - S . S ~D- il~ '
T II
cD '~~ a1l~ n O M ~ ~ .'L7M'~~ M
M '7
II - 11 11 N II II t~I 11- V'
""f''?Lf7M "7 V ~7 . . '7 1'C "7'QGO '
~'7 T7 .
. 1 . _ ~ p . ~ ~ _ MI .I . ~ -
. I
.r 'Ot~ _ ~ H . 'p tn8 'C t~'G 'G'Gc0
'O ~ v1
~ ...
S . N S ~ S S S S ....I~S SS
S
r.,.-.n T N N ~ ~- M ~' ~ n :7
-~ -~
H M ~ ~ CD ~r~ ~r .~ ~'rH ~r
v ~
~
O C'_ _ a Q) c0 CO~ M ~I~ Ntt~.,
C' J ~ cD
I~
M 00S G1M t~ . M ~ O ~ NLCDC'~~S Q)
~ N
N 11. . O . . . . . .. N
. .
CO t~~ ~~N ~' ~ ~~ M M GO NI~ L~G~
f~ GO ~
I~ M
n
S~ C 5--~
t1~ I M
O
C' H M cD
.-.
4 N U O N N
.-i
v I l
-
N tI~O O
vJ
H ~ N 'fl N ~fJ
O
N v N N
p,
O. , .
...
7- cD c0 cp
m m
O O V U
I I O O
z c~ c~ I I
R
m _ , ~ -
N
N N ul
ef ~~ U N V
V S
U U N ~ N
Z I
N
N N Z
U
U U
N N
U U n
N N m Z
V V U I
z z z
i I
a
z n 1 ~ O
o n
0
v
- 22U2~J3 i
I O O ~ O M O N d' O 07GOIn O O C' co
M ~ ~
A CD CDGOCD Q) N ~ M GOIn O O M I~
M t~ N M
.-.
U M M Lf)~'d' M M I~ CD1l)M N I~ CDt!7 M
CD I~ CO
n N N r,.--~.r .~ .-~..-,r,.-.rr N r.r~
.~ ..., .-~
_ _ _ _ _ _ _ _ . _ _ _
_
G7 O O M u707 C' O Q~M c0I~ O cpf~ I~
d' O M t~ cD f
X I~ I~N CD tf7 'C'M COcDN t~ N t~ cD
V' GO '~' M O If7
O
07 u7u7d' d' cD cDu~d'M M cDt!7 '~'
M M N N I~ I~
I~-
C N N .~.~'-, .-.n N .--..~~ ..-. M .~.~ .-r
.~ .-n .-r .~ .~
n
_ N N n N
'Xr"'... - A x
A ~ ~ n . LCD
IIII N ~ n II
~ N _ _
N
N _ . GO ~ 'O n . n
1,"
.. ~ ,...,.. II V'1 _ v1 ~ A -
GO
N ~~ I~'L7~ t- A . 'O
II
L~ x 'x _ . _ ~ p . x
_ ' N ~-~
n N CD 'a I~~ .' . ,'~:N x
~'
,n I~ ~ ~ I ~ ~'.. .' d'~ CD
N :'
'C7
'>J I ~ ~ N x O ~ II
I CO CDGO ~--~CO~~~'1A ~ CO~(' c~
--~ x
_
O L~ . . ~ , d' d" GO. CO
. ~
n
V7 . l~ N O O t~. a.sx M . C' .
N ~
_ s o r-_ ~r . ~ N
m
a co _ . . _ x ~. o I _
N ~
II n n CO n . N N ~ d'n
. n ,
pC, ~ ~7 N N N ~ ~ M CO CON
Q1 N
A . ..~"..x .. x A GO. . . ~ A
II x
z _ '~ l~I~ n Q). ..~M n L~I~ .,
"7 I~ .
1 Z" IIII VI IIx II V1 il x
I n
_
M .fl ~ ~7 i. '~N l.(7.. c. , ~7 N
.0 ~~ v1
~ 1
I n p n
. >-.
.-.n ...Q' ., 't7I~. A _ v7~O ~O.
-p f-' p
-p
x . u7n x . ~ ..
_Y.. x x ~ x . N x ~ x x
~.." ~
.-. N d~ ~ ~~I~x N ~ .-.._..~
..-n cD .~ M
I ~ ~ v CO ~ I M ~ M ~ ~ I
~ ~ ~
p O N O N - N CO c0N O
N Q7
07 M d' . Q)CDQ7l~ . O)~C7 ~C7
tn M ~'
M
O . . II. O
.
(~ M N ~ I~I~'~ ~ L~t' M
t~ M CO
t~ ~
CO CO Q) n
1
M ~
' ~ E3
U N 0
N ~' o
I I o I ...
v
M C' CO In a-,
d
c0 N M O
'L7
N ~, N '
~ O.
'D ~ - ~ ~
Q) c0
O .. .
t~ O
u7 .~ cD
x x x x
O O O O
p I I I I
(1,'CO ~ M M
.
C~ x ~ x S
O
N ~
N N N (,] N
~","' U
n n .T' ~
N x
m to . m
W N
'Y" 1"
M
N N N U
.
U U U
.-,
~r G
z z N
x
N N N
.
C~ x x ~ U
U U U N
N N N ~..
x x U
U V U
V7 O .G
I I I
c
o O I ~ N
3 ~ O '~ ~'
A ..r
o
ti
-41- 22~2~31
I cD Q'--m!7~ N M M N O
N Q~
9 N cDc0~ c0C' O C' c0 u7 u7
t~
U I~ t1)V'C N N t~ ~I cD u~ N
-r .--n.~.--n~ .--n.-~M .--.rr .--n
~
.~
1. . _ . _ _ _
C7 O a7I~p CO~ O C' -v O tf~ GO
LfJ CV
X --~M MCDCO~I7CD M cD N CO ~
cD CO
CD cDtl7C'M N M CD N M tIJ M
C
PC N _..a.~.._.._~~ M --n -- M .-. .-.
--n .--.
_ n . .
_ A M n
.
s .. _ 6 c0 _ _ N
'v
N
H ~ - -~..N - L~-N I N
.:,
CO ~ nC' .' ~' '
~-~ 'fl
-
'O ~ u7 II N N~ ~ O I O
~
N CO '~ (~ 1.'M ~ II 'Q
I~ II
.o ~ ~ _ . MC' v1 '--~ v1
C' _ "'7
_
' t!7 M 'a t~ t-..~. _ i..
n
I O L~ ~ . _ I ~M ~ 'fl ~
C~ '~ vJ
O I O)n ~ t~ III _ ~ I
' i..
cn co ~ _o~a ~ c- -mn _. z a~ x
_ v ~
I CD~ ~ . _~ --~~--~ C~
~ ~' .
O N . NM ~ CO L~ 'Q
N
.-.c~.r 'C O IM (~ M (~ tI7
~~ N
4~ M_ ~ _ T7 O c~ ~ t~
CA '--'
GO . _n CO~ CO n . _ GD
n ~
Z ~ ~N ~ N N ~~ GO I~ ~
00 N N 00
I _ H IIX . z _ II ~H H . '
x
r. n . ~sM M Lf~n G7 ~. _ _ . h .
'-f V~ N
" V7 x _II II ~/1II 00x n n Z"' ~ " -
_ 11
1-.N 'Q~~_ '~ ~. ~ M~' VJ N M _ vJ
'O "'~ .
p ~.I. .-. .o . ..-. r...z .~ c. -
1 .~ ~-.
. M 'GT7H 'C - 'a c~t~ .O 00 O
'C b N A
x c~ ~. co n co
s
_ _ .-.x _. ~ ~ . - z
s x .:~
wr t'.-~.--~~'M ~ ~. nM ~ . r . d'
.-r M N
c0 I yrw r v Q1 ~ NI ~ Q 1 fwr
~ ~
CO O'JM~ M M I~ CO Sc~ CO . GO CD
O M ~
t~tneTO'JO7 . 07 MO~ 07 x GO cD
cD O'1 CO ~
0 . .. . O .. II.. . .--..II .
.
-W ~ I~c~M N ~-W~ ~7M O N
t~ N ~
t~
'7
d'
n
S~ C S-~
cn 1 co
a
V' a N
U ~~
CL N O N
~
I O v 1
~-
M cD O tt7
v7
C' N 'O N
O
N v N
p
'fl 3E 32 32
-. u7 ~ oo a~
d . . .
.- O cD
T u7 t~
cn
S Z
U O O
O 1 I
I
p
,.
m
...... U ~ U Z
U "'"' i "
.., U . _
r ..
I N I N
U
Z . .
Z
c >
U
Z ~
Z ,
,
i I Z
I
0
o M
s ..., -.
U
-42- 22 (3 2 ~ 5 i
I cD ~!'7 O CD M CV cD CO O tn O N tl)
H ~D ~-~ O ca M M V' CV M ~ N ~C7 V'
U GD CD ~f7 ~' V' N ~~ t~ Q) CD tfJ ~' N
n .~ .~ .-. ,-, ._. .--n .-r N .~ .~ .r .-r
4 . . . . . . . .
Oa O M N GO U7 t~ l~ M O O7 CO ~ G)
:G I~ CV Q7 t~ u7 M cD CV ~-~ Q~ f~ cD cD
-' L~ cD tl7 C 'a' M ~ O C' CD tf7 d' M
G: N .-. .-. .~ .-. r. .-. .~ M
n .,
N N
S . H
. (~ n O
n II . rn II S - -
N "7 '>O _ . ~ M n ~
I S n _ ~ V7 r/7 .
Q) 'a 'Q M v1 'L7 CO L. I-.
II . ~ _ M ~ G n
"') S _ . ~ CO S. ~ . . . V7
._» n n ._» O CD ~-~ (~ S S c.
'O ~ N N ~ . ~ ~ I CD C' ~
.. . l~ S S I~ M ~ rr Q)
S CD ~~ ~ O) M Q7 ~C7 . N N .:.
n L~ L~ N
I~ l~ I~ cD ~ N C~ L~ N
c~ M _ _ H S N -..
--. O . r c1 . . _ . . N N
U . n II II n .:: n n ~ n . .
p CO N ~7 ~7 N N N N N N CO
V - . S ~ S S S S II H H . '
v . f~ 'O 'CJ M CO (~ I~ C!J t~ '~ - _
G~ ~ II I I II 07 II II II II S S ~ ~' -
v1 'Wp -p ~7 . ~7 ~ '-7 '-i b M N N -
Z s-. . I I . M . - I ~ ~ L- -
I ~ '~ '>7 'Q '>7 . ~ H 'Q '>7 '>7 n cD .a
... . n . tf7 CO '
- .='SSSS NSS ._...-. . .S
S N N ~~ M ~-~ N
~ v ~ ~ M yr yr yr wr 1 I
M u7 M GO _ C' cD c~ c0 I~ QW ~ O~
CD O tI~ C' M ~ t1) 07 Q~ CD N Lf7 O C'
II . . . . . . . .
CO t~ c~ c~ t~ 'W N ~ I~ L~ I~ M N
n
S-~ C Sr-~
Q7 I O L~
cD
0. U O ~ N
1
l~ N N v1 C~
H ~ N 'fl O cD
-. ~ C1 N
'Q aE 3E
-. O cA
.
- O M
y. I~ M
M
S
U O O
4~ O i I
I
n
_.
C S S S
n
S
ZJ
U
n
S
_=
z=
N
v
C
o CO I
3 ~ .~ (Q
O
U
2~ (~2~5 i
-43-
Pharmacological Test Example 1
Cytocidal Effect
A 96-well plate was innoculated with 2 x 103
cells/well of P 388 mouse leukemia cell line. The
compound of the present invention was dissoved in
purified water or dimethylsulfoxide, and the solution was
diluted with medium to various concentrations and placed
into respective wells and incubated. After three day's, ,
incubation, the plate was fixed with glutaraldehyde andJ
stained with crystal violet for cytometry.
The cytocidal effect of each compound was
expressed as the concentration at which the cell count is
50~ less than that of the control (IC50). The results
are shown in Table 2.
Table 2
Compound No. IC50(~g/ml)
1 3.4 x 10 1
2 1.5 x 10 1
3-a 1.5 x 10 1
4 2.5 x 10 1
5 5.2 x 10 2
7-a 1.6 x 10 1
10-a 4.2 x 10 1
11 9.6 x 10 2
12 1 . 6 x 10 1
22Ci2~3i
-44-
13 3.7 x 10 1
14 2.2 x 10 1
16-a 4.3 x 10 1
Preparation Example 1
Capsules
According to the following formula, capsuls
were parepared by a conventional process. r
Compound 1 200 mg
Lactose 30 mg
Corn starch 50 mg
Crystalline cellulose 10 mg
Maq~nesium stearate 3 ma
Per capsule 293 mg
Preparation Example 2
Tablets
According to the following formula, tablets
were parepared by a conventional process.
Compound 2 100 mg
Lactose 47 mg
Corn starch 50 mg
Crystalline cellulose 50 mg
Hydroxypropyl cellulose 15 mg
Talc 2 mg
Magnesium stearate 2 mg
22 C~2~3 r
-45-
Ethyl cellulose 30 mg
Unsaturated fatty acid glyceride 2 mg
Titanium dioxide 2 ma
Per tablet 300 mg
Preparation Example 3
Granules
According to the following formula, granules
were parepared by a conventional process. , ,
Compound 3-a 200 mg
Mannitol 540 mg
Corn starch 100 mg
Crystalline cellulose 100 mg
Hydroxypropyl cellulose 50 mg
Talc 10 ma
Per packet 1000 mg
Preparation Example 4
Fine granules
According to the following formula, fine
granules were prepared by a conventional process.
Compound 5 200 mg
Mannitol 520 mg
Corn starch 100 mg
Crystalline cellulose 100 mg
Hydroxypropyl cellulose 70 mg
Talc 10 ma
22~2~i
-46-
Per packet 1000 mg
Preparation Example 5
Injection
According to the following formula, an
injection was parepared by a conventional process.
Compound 7-a 100 mg
Distilled water for injections cr.s.
Per ample 2 ml , ,
Preparation Example 6
Suppositories
According to the following formula,
suppositories were prepared by a conventional process.
Compound 11 200 mg
Witepsol S-55 l.~uu mg
(a mixture of mono-, di- and
triglycerides of saturated fatty
acids from lauric acid to stearic
acid product of Dynamite Nobel)
Per suppository 1500 mg