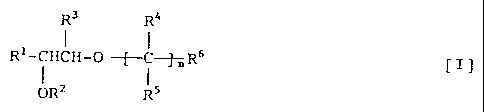Note: Descriptions are shown in the official language in which they were submitted.
T
~
2.~~
- 1 -
DESCRIPTION
AGENT FOR POTENTIATING NERVE GROWTH FACTOR ACTIVITY
CONTAINING 1,2-ETHANEDIOL DERIVATIVE OR SALT THEREOF
TECHNICAL FIELD
This invention relates to a 1,2-ethanediol
derivative or a salt thereof which potentiates the
activity of a nerve growth factor (referred to herein-
after as NGF).
BACKGROUND ART
It has been known that NGF acts as a survival
and maintenance as well as neurite outgrowth factor for
sympathetic neurons and sensory neurons in the peripheral
nervous system [Physiol. Rev., vol. 60, pages 1284-1335
(1980) and Ann. Rev. Biochem., vol. 51, pages 845-868
(1982)] and that high NGF levels were found in the
regions innervated by the magnocellular neurons
(hippocampus, neocortex, olfactory bulb), and in the
regions containing the cell bodies of these neurons
(septum, nucleus of the diagonal band of Broca, nucleus
basalise of Meynert) and that NGF acts as neurotrophic
factor for magnocellular cholinergic neurons [EMBO J.,
vol. 4, pages 1389-1393 (1985)].
NGF has also been drawing attention in terms of
relation to central nervous diseases such as senile
dementia of Alzheimer type [Science, vol. 232, page 1341
(1986)], Huntington's chorea [Neurosci. Lett., vol. 40,
22u2i() 32
- 2 -
No. 2, pages 161-164 (1992)], and to peripheral nervous
diseases such as various neuropathies {diabetic
neuropathy [Brain Res., vol. 634, pages 7-12 (1994)],
neuropathy caused by drugs (Brain Res., vol. 640, pages
195-204 (1994)] and the like}, Riley-Day syndrome
[Japanese J. of Clinical Medicine, vol. 50, No. 4, pages
178-183 (1992)], traumatic neuropathy [Pharmacol. Ther.,
vol. 65, No. 1, pages 1-16 (1995)], amyotrophic lateral
sclerosis (ALS) [Nature Medicine, vol. 1, No. 2, pages
168-172 (1995) and the like.
An attempt has been made to use NGF or a
NGF-like substance for the treatment of central and
peripheral nervous diseases described above [Brain and
Nerve, vol. 43, No. 12, pages 1101-1112 (1991) and the
like]. However, these substances are all proteins, and
when they are used as drugs, their stability,
antigenicity and the like will become a problem.
Therefore, a compound useful as a drug for potentiating
the NGF activity has been desired.
DISCLOSURE OF THE INVENTION
Under such circumstances, the present inventors
have made extensive research and have consequently found
that a 1,2-ethanediol derivative represented by the
following general formula [I] or a salt thereof
potentiates the NGF activity:
CA 02202032 2007-11-07
3
R3 R4
Rl-CHCH-0
0R2 RS
wherein R' represents a substituted or unsubstituted
phenyl, naphthyl, indanyl, indenyl, tetrahydronaphthylor
heterocyclic group; R 2 represents a hydrogen atom, a lower
alkyl group or a hydroxyl-protecting group; R3 represents
a hydrogen atom or a lower alkyl group; nR4's may be the
same as or different from one another and each represents
a hydrogen atom or a lower alkyl group; nR5's may be the
same as or different from one another and each represents
a hydrogen atom or a lower alkyl.group; R6 represents a
substituted or unsubstituted amino or nitrogen-containing
heterocyclic group or an ammonio group; and n represents
0 or an integer of 1 to 6.
In one preferred embodiment of the invention:
R1 represents a phenyl group which is optionally
substituted by a halogen atom, substituted or unsubstituted
amino, C1_6alkyl, aryl, ar-C1-9alkyl,
C1_6alkoxy, ar-C1-9alkoxy, aryloxy, carbamoyloxy,
C1-6alkylthio, C2-6alkenyl, C2-6alkenyloxy, ar-C1-4alkylthio,
ar-C1-6alkylsulfonyl, arylsulfonyl, C1-6alkylsulfonylamino,
arylsulfonylamino, protected amino, protected or
unprotected hydroxyl, nitro, oxo, or C1-4alkylenedioxy;
R 2 represents a hydrogen atom or a C1-6alkyl,
CA 02202032 2007-11-07
3a
C1_6acyl, tetrahydropyranyl, or ar-C1-6alkyl group;
nR4's may be the same as or different from one another and
each represents a hydrogen atom or a Cz-6alkyl group;
nR5's are hydrogen atoms;
R6 represents an amino or benzothienylmethylamino group,
which group is optionally substituted by at least onfa
substituent which at each occurrence is a halogen atiDm, a
protected or unprotected hydroxyl group, a protected or
unprotected carboxyl group, a protected or unprotected
amino group, a C1-6alkyl group which is unsubstituted or
substituted by a protected or unprotected hydroxyl group, a
halogen-substituted or unsubstituted aryl group, a halogen-
substituted or unsubstituted aroyl group, a C1-6alkoxy
group-substituted or unsubstituted C1-6alkoxy group, a halo-
C1_6alkyl group, a C1_6acyl group, an ar-C1-4alkyl group, an
ar-C2_4alkenyl group, a heterocyclic group, a heterocyclic
carbonyl group, an oxo group,. a C1_6alkylsulfonyl group or
an arylsulfonyl group.
In another preferred embodiment:
R1 is a phenyl, halogen-substituted phenyl,
C1_6alkylsubstituted phenyl or C1_9alkylsubstituted biphenyl
group;
R2 is a hydrogen atom;
nR9' s are hydrogen atoms;
nR51 s are hydrogen atoms;
CA 02202032 2007-11-07
3b
R6 is an amino group which is optionally substituted by at
least one substituent which at each occurrence is a halogen
atom, a protected or unprotected hydroxyl group, a
protected or unprotected carboxyl group, a protected or
unprotected amino group, a C1-6alkyl group which is
unsubstituted or substituted by a protected or unprotected
hydroxyl group, a halogen-substituted or unsubstituted aryl
group, a halogen-substituted or unsubstituted aroyl group,
a C1-6alkoxy group-substituted or unsubstituted C1_6alkoxy
group, a halo-C1_6alkyl group, a C1-6acyl group,
an ar-C1_4alkyl group, an ar-C2-4alkenyl group, a
heterocyclic group, a heterocyclic carbonyl group, an oxo
group, a C1-6alkylsulfonyl group or an arylsulfonyl group.
In yet another preferred embodiment:
R' is a naphthyl group which is optionally substituted by
a halogen atom, substituted or unsubstituted amino,
C1-6alkyl, aryl, ar-C1_9alkyl, C1-6alkoxy,
ar-C1-4alkoxy, aryloxy, carbamoyloxy, C1-6alkylthio,
C2_6alkenyl, C2_6alkenyloxy, ar-C1-9alkylthio,
ar-C1-6alkylsulfonyl, arylsulfonyl, C1-6alkylsulfonylamino,
arylsulfonylamino, protected amino, protected or
unprotected hydroxyl, nitro, oxo, or C1-4alkylenedioxy;
R2 is a hydrogen atom, or a C1_6alkyl,
C1_6acy1, tetrahydropyranyl, or ar-C1_6alkyl group;
CA 02202032 2007-11-07
3c
nR9`s may be the same as or different from one another and
each represents a hydrogen atom or a C1_6alkyl group;
nR5's are hydrogen atoms;
R6 is an ammonio group or an amino, pyrrolyl, piperazinyl,
imidazolyl, pyrazolyl, pyridyl, pyrimidyl, quinolyl,
quinolidinyl, tetrahydroquinolinyl, quinuclidinyl,
thiazolyl or thiadiazolyl group, which group is optionally
substituted by at least one substituent which at each
occurrence is a halogen atom, a protected or unprotected
hydroxyl group, a protected or unprotected carboxyl group,
a protected or unprotected amino group, a C1_6alkyl group
which is unsubstituted or substituted by a protected or
unprotected hydroxyl group, a halogen-substituted or
unsubstituted aryl group, a halogen-substituted or
unsubstituted aroyl group, a C1_6alkoxy group-substituted or
unsubstituted C1_6alkoxy group,a halo-C1_6alkyl group,
a C1_6acy1 group, an ar-C1_4alkyl group, an ar-C2-9alkenyl
group, a heterocyclic group, a heterocyclic carbonyl group,
an oxo group, a C1-6alkylsulfonyl group or an arylsulfonyl
group.
In a further preferred embodiment:
R' is a naphthyl group;
R2 is a hydrogen atom;
R3 is a hydrogen atom;
nR4' s are hydrogen atoms;
CA 02202032 2007-11-07
3d
R6 is an amino group which is optionally substituted by at
least one substituent which at each occurrence is a halogen
atom, a protected or unprotected hydroxyl group, a
protected or unprotected carboxyl group, a protected or
unprotected amino group, a C1_6alkyl group which is
unsubstituted or substituted by a protected or unprotected
hydroxyl group, a halogen-substituted or unsubstituted aryl
group, a halogen-substituted or unsubstituted aroyl group,
a C1-6alkoxy grou.p-substituted or unsubstituted C1_6alkoxy
group, a halo-C1_6alkyl group, a C1_6acy1 group, an
ar-C1_9alkyl group, an ar-C2_9alkenyl group, a heterocyclic
group, a heterocyclic carbonyl group, an oxo group,
a C1-6alkylsulfonyl group or an arylsulfonyl group.
In yet a further preferred embodiment:
R' is a heterocyclic group which is optionally substituted
by a halogen atom, substituted or unsubstituted amino,
C1_6alkyl, aryl, ar-C1_9alkyl, C1_6alkoxy, ar-C1_9alkoxy,
aryloxy, carbamoyloxy, C1_6alkylthio, C2_6alkenyl,
C2_6alkenyloxy, ar-C1_4alkylthio, ar-C1_6alkylsulfonyl,
arylsulfonyl, C1_6alkylsulfonylamino, arylsulfonylamino,
protected amino, protected or unprotected hydroxyl, nitro,
oxo, or C1-9alkylenedioxy.
Another embodiment of the invention is disclosed in
which:
CA 02202032 2007-11-07
3e
R' is a benzothienyl, benzofuranyl or 2,3-
dihydrobenzothienyl group which is unsubstituted or
substituted by a halogen atom, a C1_6alkyl group, a
C1-6alkoxy group or a phenyl group;
R2 is a hydrogen atom;
R3 is a hydrogen atom;
nR4rs and nR5's are hydrogen atoms;
R6 is an amino, pyrolidinyl, piperazinyl,
tetrahydropyridyl or morpholinyl group which is
unsubstituted or substituted by a C1_6alkyl group, or a
halogen-substituted or unsubstituted aroyl group.
More preferably:
R' is a benzothienyl group which is unsubstituted or
substituted by a halogen atom or a C1_6alkyl group;
R6 is an amino group which is unsubstituted or substituted
by a C1-6alkyl group; and
n is 2.
In a particular preferred embodiment:
R' is an unsubstituted or halogen-substituted
benzo[b]thiophen-5-yl group;
R6 is a di-C1_6alkyl amino group.
The invention discloses further preferred embociiments,
one in which:
CA 02202032 2007-11-07
3f
R1 represents a phenyl group which is optionally
substituted by a halogen atom, substituted or unsubstituted
amino, C1_6alkyl, aryl, ar-C1_4alkyl,
C1_6alkoxy, ar-C1_4alkoxy, aryloxy, carbamoyloxy,
C1_6alkylthio, C2_6alkenyl, C2_6alkenyloxy,
ar-C1_9alkylthio, ar-C1_6alkylsulfonyl, arylsulfonyl,
C1_6alkylsulfonylamino, arylsulfonylamino, protected amino,
protected or unprotected hydroxyl, nitro, oxo, or
C1_4alkylenedioxy;
R2 represents a hydrogen atom or a C1_6alkyl,
C1_6acy1, tetrahydropyranyl, or ar-C1_6alkyl group;
nR9rs may be the same as or different from one another and
each represents a hydrogen atom or a C1_6alkyl group;
nR5's are hydrogen atoms; and
R6 represents an amino or benzothienylmethylamino group,
which group is optionally substituted by at least one
substituent which at each occurrence is a halogen atom, a
protected or unprotected hydroxyl group, a protected or
unprotected carboxyl group, a protected or unprotected
amino group, a C1-6alkyl group which is unsubstituted or
substituted by a protected or unprotected hydroxyl group, a
halogen-substituted or unsubstituted aryl group, a halogen-
substituted or unsubstituted aroyl group, a C1_6alkoxy
group-substituted or unsubstituted C1-6alkoxy group, a halo-
C1_6alkyl group, a C1_6acy1 group, an ar-C1_4alkyl group,
CA 02202032 2007-11-07
3g
an ar-C2-4alkenyl group, a heterocyclic group, a
heterocyclic carbonyl group, an oxo group,
a C1-6alkylsulfonyl group or an arylsulfonyl group.
In another:
R' is a phenyl, halogen-substituted phenyl,
C1-6alkylsubstituted phenyl or C1_4alkylsubstituted biphenyl
group; ,
R2 is a hydrogen atom;
nR4' s are hydrogen atoms;
nR5' s are hydrogen atoms; and
R6 is an amino group which is optionally substituted by at
least one substituent which at each occurrence is a halogen
atom, a protected or unprotected hydroxyl group, a
protected or unprotected carboxyl group, a protected or
unprotected amino group, a C1-6alkyl group which is
unsubstituted or substituted by a protected or unprotected
hydroxyl group, a halogen-substituted or unsubstituted aryl
group, a halogen-substituted or unsubstituted aroyl group,
a C1-6alkoxy group-substituted or unsubstituted C1_6alkoxy
group, a halo-C1-6alkyl group, a C1-6acyl group,
an ar-C1-9alkyl group, an ar-C2_4alkenyl group, a
heterocyclic group, a heterocyclic carbonyl group, an oxo
group, a C1-6alkylsulfonyl group or an arylsulfonyl group.
And another:
CA 02202032 2007-11-07
3h
R' is a naphthyl group which is optionally substituted by
a halogen atom, substituted or unsubstituted amino,
C1-6alkyl, aryl, ar-C1_9alkyl, C1-6alkoxy, ar-C1-4alkoxy,
aryloxy, carbamoyloxy, C1-6alkylthio, C2-6alkenyl,
C2-6alkenyloxy, ar-C1-4alkylthio, ar-C1-6alkylsulfonyl,
arylsulfonyl, Cz-6alkylsulfonylamino, arylsulfonylamino,
protected amino, protected or unprotected hydroxyl, nitro,
oxo, or C1_9alkylenedioxy;
R2 is a hydrogen atom or a C1-6alky1, C1-6acyl,
tetrahydropyranyl, ar-C1-6alkyl group;
nR4's may be the same as or different from one another and
each represents a hydrogen atom or a C1_6alkyl group;
nR5's are hydrogen atoms;
R6 is an ammonio group or an amino, pyrrolyl, piperazinyl,
imidazolyl, pyrazolyl, pyridyl, pyrimidyl, quinolyl,
quinolidinyl, tetrahydroquinolinyl, quinuclidinyl,
thiazolyl or thiadiazolyl group, which group is optionally
substituted by at least one subsituent which at each
occurrence is a halogen atom, a protected or unprotected
hydroxyl group, a protected or unprotected carboxyl group,
a protected or unprotected amino group, a Cz-6alkyl group
which is unsubstituted or substituted by a protected or
unprotected hydroxyl group, a halogen-substituted or
unsubstituted aryl group, a halogen-substituted or
CA 02202032 2007-11-07
3i
unsubstituted aroyl group, a C1_6alkoxy group-substituted or
unsubstituted C1-6alkoxy group,a halo-C1-6alkyl group,
a C1-6acyl group, an ar-C1-4alkyl group, an ar-CZ-qalkenyl
group, a heterocyclic group, a heterocyclic carbonyl group,
an oxo group,a C1-6alkylsulfonyl group or an arylsulfonyl
group.
In yet another embodiment:
R' is a naphthyl group;
R2 is a hydrogen atom;
R3 is a hydrogen atom;
nR4' s are hydrogen atoms;
R6 is an amino group which is optionally substituted by at
least one substituent which at each occurrence is a halogen
atom, a protected or unprotected hydroxyl group, a
protected or unprotected carboxyl group, a protected or
unprotected amino group, a C1-6alkyl group which is
unsubstituted or substituted by a protected or unprotected
hydroxyl group, a halogen-substituted or unsubstituted aryl
group, a halogen-substituted or unsubstituted aroyl group,
a C1-6alkoxy group-substituted or unsubstituted C1_6al.koxy
group, a halo-C1-6alkyl group, a C1_6acy1 group,
an ar-C1_9alkyl group, an ar-C2-9alkenyl group,
a heterocyclic grbup, a heterocyclic carbonyl group, an oxo
group, a C1-6alkylsulfonyl group or an arylsulfonyl group.
And yet another:
CA 02202032 2007-11-07
3j
R' is a heterocyclic group which is optionally substituted
by a halogen atom, substituted or unsubstituted amino,
C1_6alkyl, aryl, ar-C1-9alkyl, C1-6alkoxy, ar-C1_qalkoxy,
aryloxy, carbamoyloxy, C1-6alkylthio, C2_6alkenyl,
C2_6alkenyloxy, ar-C1_4alkylthio, ar-C1-6alkylsulfonyl,
arylsulfonyl, C1-6alkylsulfonylamino, arylsulfonylamino,
protected amino, protected or unprotected hydroxyl, nitro,
oxo, or C1-4alkylenedioxy.
In still another embodiment of the invention:
R' is a benzothienyl, benzofuranyl or
2,3-dihydrobenzothienyl group which is unsubstituted or
substituted by a halogen atom, a C1_6alkyl group,
a C1-6alkoxy group or a phenyl group;
R2 is a hydrogen atom;
R3 is a hydrogen atom;
nR4' s and nR5' s are hydrogen atoms;
R6 is an amino, pyrolidinyl, piperazinyl,
tetrahydropyridyl or morpholinyl group which is
unsubstituted or substituted by a C1-6alkyl group, or a
halogen-substituted or unsubstituted aroyl group.
In a further preferred embodiment:
R' is a benzothienyl group which is unsubstituted or
substituted by a halogen atom or a C1-6alkyl group;
R6 is an amino group which is unsubstituted or substituted
by a C1_6alkyl group; and
CA 02202032 2007-11-07
3k
n is 2.
And more preferably:
R' is an unsubstituted or halogen-substituted
benzo[b]thiophen-5-yl group; and
R6 is a di-C1-6alkyl amino group.
The invention also discloses another compound useful
in the preparation of a medicament for potentiating nerve
growth factor activity for the treatment of a disease caused
by degeneration of the peripheral nervous system, the
compound being a 1,2-ethanediol derivative represented by
the following general formula or a pharmaceutically
acceptable salt thereof:
0Fi2a
1Rl~ CHCN2OCH2CN2-Rg'
S
wherein:
Rla at each occurrence is fluorine, chlorine, bromine,
iodine, C1-6alkyl, C1_6alkoxy or phenyl;
R2a represents a hydrogen atom or a C1-6alkyl,
C1-6acyl, tetrahydropyranyl, or ar-C1_6alkyl group;
R6a represents a C1_6alkylsubstituted amino group o,r a
pyrrolyl, pyrrolidinyl, piperidyl, piperazinyl, imidazolyl,
pyrazolyl, pyridyl, tetrahydropyridyl, pyrimidinyl,
CA 02202032 2007-11-07
31
morpholinyl, thiomorpholinyl, quinolyl, quinolidinyl,
tetrahydroquinolinyl, tetrahydroisoquinolinyl,
quinuclidinyl, thiazolyl, tetrazolyl, thiadiazolyl,
pyrrolinyl, imidazolinyl, imidazolidinyl, pyrazolinyl,
pyrazolidinyl, purinyl, indazolyl, furyl, thienyl,
benzothienyl, pyranyl, isobenzofuranyl, oxazolyl,
benzofuranyl, indolyl, benzimidazolyl, benzoxazolyl,
benzothiazolyl, quinoxalyl, dihydroquinoxalinyl,
2,3-dihydrobenzothienyl, 2,3-dihydrobenzopyrrolyl,
2,3-dihydro-4H-1-thianaphthyl, 2,3-dihydrobenzofuranyl,
benzo.[b]dioxanyl, imidazo[2,3-a]pyridyl,
benzo[b]piperazinyl, chromenyl, isothiazolyl, isoxazolyl,
thiadiazolyl, oxadiazolyl, pyridazinyl, isoindolyl or
isoquinolyl group; and
n represents an integer of 1 to 5.
Various preferred embodiments of this aspect of the
invention include embodiments in which:
each Rla is a C1-6alkyl group; and n is an integer of 1 to
5; or
Rla at each occurrence is fluorine, chlorine, bromine or
iodine; and n is an integer of 1 to 5; or
R" is a fluorine atom; and n is an integer of 1 to 5; or
Rla is a fluorine atom bonded to the 6-position; R`a is a
hydrogen atom; a C1-6alkylsubstituted amino group of R6a is a
diethylamino group; and n is an integer of 1; or
CA 02202032 2008-04-25
3m
Rla is a fluorine atom bonded to the 4-position; R2a is a
hydrogen atom; a C1_6alkyl-substituted amino group of R6a is a
diethylamino group; and n is an integer of. 1.
The 1,2-ethanediol derivative may be an optically active
compound.
The invention also discloses a nerve growth factor
activity-potentiating pharmaceutical composition for the
treatment of a disease caused by degeneraton of the peripheral
nervous sytem, comprising a pharmaceutically acceptable diluent
or carrier and a 1,2-ethanediol derivative represented by the
following general formula or a pharmaceutically acceptable salt
thereof:
OR2'
\a~;
CHCH2OCH~H2-Rsa 15 S wherein:
Rla at each occurrence is fluorine, chlorine, bromine, iodine,
C1_6alkyl, C1_6alkoxy or phenyl;
R 2a represents a hydrogen atom or a C1_6alkyl, C1_6acyl,
tetrahydropyranyl, or ar-C1_6alkyl group;
R6a represents a C1_6alkylsubstituted amino group or a
pyrrolyl, pyrrolidinyl, piperidyl, piperazinyl, imidazolyl,
pyrazolyl, pyridyl, tetrahydropyridyl, pyrimidinyl,
morpholinyl, thiomorpholinyl, quinolyl, quinolidinyl,
tetrahydroquinolinyl, tetrahydroisoquinolinyl, quinuclidinyl,
thiazolyl, tetrazolyl, thiadiazolyl,
CA 02202032 2008-04-25
3n
pyrrolinyl, imidazolinyl, imidazolidinyl, pyrazolinyl,
pyrazolidinyl, purinyl, indazolyl, furyl, thienyl,
benzothienyl, pyranyl, isobenzofuranyl, oxazolyl,
benzofuranyl, indolyl; benzimidazolyl, benzoxazolyl,
benzothiazolyl, quinoxalyl, dihydroquinoxalinyl, 2,3-
dihydrobenzothienyl, 2,3-dihydrobenzopyrrolyl, 2,3-dihydro-
4H-1-thianaphthyl, 2,3-dihydrobenzofuranyl,
benzo[b]dioxanyl, imidazo[2,3-a]pyridyl,
benzo[b]piperazinyl, chromenyl, isothiazolyl, isoxazolyl,
thiadiazolyl, oxadiazolyl, pyridazinyl, isoindolyl or
isoquinolyl group; and
n represents an integer of 1 to 5.
In preferred embodiments of the pharmaceutical
composition:
Rla is a C1-6alkyl group; and n is an integer of 1 to 5; or
Rla at each occurrence is fluorine, chlorine, bromine or
iodine; and n is an integer of 1 to 5; or
Rla is a fluorine atom; and n is an integer of 1 to 5; or
Rla is a fluorine atom bonded to the 6-position; R 2a is a
hydrogen atom; a C1-6alkyl substituted amino group of R6a is
a diethylamino group; and n is an integer of 1; or
Rla is a fluorine atom bonded to the 4-position; R 2a is a
hydrogen atom; a C1-6alkyl-substituted amino group of R6a is
a diethylamino group; and n is an integer of 1.
CA 02202032 2008-04-25
3o
The 1,2-ethanediol derivative in the pharmaceutical
composition may also be an optically active compound.
The invention also discloses a 1,2-ethanediol
derivative represented by the following general formula or
a pharmaceutic.ally acceptable salt thereof:
O R2'
(R taIn
CHCH20CH2q{2_Rsa
wherein nRla's may be the same as or different from one
another and each represents a fluorine, chlorine, bromine
or iodine atom, C1_6alkyl, C1_6alkoxy or phenyl group; R2a
represents a hydrogen atom; R6a represents a C1-6alkyl-
substituted amino, piperidyl or morpholinyl group; and n
represents an integer of 1 to 5.
In preferred embodiments of the invention:
each R" is a C1_6alkyl group; and n is an integer of 1 to
5; or
Rla at each occurrence is fluorine, chlorine, bromine or
iodine; and n is an integer of 1 to 5; or
Rla is a fluorine atom; and n is an integer of 1 to 5; or
CA 02202032 2008-04-25
3p
Rla is a fluorine atom bonded to 6-position; R2a is a
hydrogen atom; a C1_6alkyl-substituted amino group of R6a is
a diethylamino group; and n is an integer of 1; or
Rla is a fluorine atom bonded to 4-position; R2a is a
hydrogen atom; a C1_6alkyl-substituted amino group of R6a is
a diethylamino group; and n is an integer of 1.
The 1,2-ethanediol derivative may be an optically
active compound as well.
This invention is explained in detail below.
In the present specification, unless otherwise
specified, the terms used herein have the following
meanings.
The term "halogen atom" means fluorine atom, chlorine
atom, bromine atom or iodine atom. The term "lower alkyl
group" means a C1-6 alkyl group such as methyl, ethyl, n-
propyl, isopropyl, n-butyl, isobutyl, tert-butyl, pentyl,
hexyl or the like. The term "lower alkenyl group" means a
C2_6 alkenyl group such as vinyl, propenyl, butenyl,
pentenyl, hexenyl or the like. The term "lower alkenyloxy
group" means a C2-6 alkenyl-0-
22U2032
- 4 -
group. The term "cycloalkyl group" means a C3_6cycloalkyl
group such as cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl or the like. The term "lower alkoxy group"
means a C1_6 alkyl-O- group. The term "lower alkylthio
group" means a C1_6 alkyl-S- group. The term "halo-lower
alkyl group" means a halogen-C1_6 alkyl group. The term
"aryl group" means a phenyl, naphthyl, indanyl or indenyl
group. The term "aryloxy group" means an aryl-0- group.
The term "ar-lower alkyl group" means an ar-C1_4 alkyl
group such as benzyl, diphenylmethyl, trityl, phenethyl
or the like. The term "ar-lower alkoxy group" means an
ar-C1_4 alkyl-O- group. The term "ar-lower alkylthio
group" means an ar-C1_4 alkyl-S- group. The term "ar-
lower alkenyl group" means an ar-C2_4 alkenyl group. The
term "lower alkylenedioxy group" means a C1_4alkylenedioxy
group such as methylenedioxy, ethylenedioxy or the like.
The term "lower acyl group" means a C1_6 acyl group such
as formyl, acetyl, butyryl or the like. The term "aroyl
group" means an aryl-CO- group. The term "lower alkyl-
sulfonyl group" means a C1_6 alkyl-S02 group. The term
"ar-lower alkylsulfonyl group" means an ar-C1_6 alkyl-S02-
group. The term "arylsulfonyl group" means an aryl-S02-
group. The term "arylsulfonylamino group" means an aryl-
SOZNHZ- group. The term "lower alkylsulfonylamino group"
means a C1_6 alkyl-SOZNH- group. The term "di-lower alkyl
amino group" means a(C1_6alkyl)ZN- group such as dimethyl-
amino, diethylamino or the like. The term "ammonio
22U2032
- 5 -
group" means a tri-lower alkylammonio group such as
trimethylammonio, triethylammonio or the like. The term
"nitrogen-containing heterocyclic group" means a hetero-
cyclic group of a 5-membered or 6-membered ring, fused
ring or bridged ring containing at least one nitrogen
atom as hetero atom forming the ring which may further
contain at least one oxygen or sulfur atom such as
pyrrolyl, pyrrolidinyl, piperidyl, piperazinyl,
imidazolyl, pyrazolyl, pyridyl, tetrahydropyridyl,
pyrimidinyl, morpholinyl, thiomorpholinyl, quinolyl,
quinolidinyl, tetrahydroquinolinyl, tetrahydro-
isoquinolinyl, quinuclidinyl, thiazolyl, tetrazolyl,
thiadiazolyl, pyrrolinyl, imidazolinyl, imidazolidinyl,
pyrazolinyl, pyrazolidinyl, purinyl, indazolyl or the
like. The term "heterocyclic group" means the above-
mentioned nitrogen-containing heterocyclic group or a
5-membered or 6-membered ring, a fused ring or a bridged
ring containing at least one hetero atom selected from
the group consisting of nitrogen, oxygen or sulfur atom
which may contain at least one oxygen or sulfur atom as
hetero atom forming the ring such as furyl, thienyl,
benzothienyl, pyranyl, isobenzofuranyl, oxazolyl, benzo-
furanyl, indolyl, benzimidazolyl, benzoxazolyl, benzo-
thiazolyl, quinoxalyl, dihydroquinoxalinyl, 2,3-dihydro-
benzothienyl, 2,3-dihydrobenzopyrrolyl, 2,3-dihydro-4H-1-
thianaphthyl, 2,3-dihydrobenzofuranyl, benzo[b]dioxanyl,
imidazo[2,3-a]pyridyl, benzo[b]piperazinyl, chromenyl,
isothiazolyl, isoxazolyl, thiadiazolyl, oxadiazolyl,
22 02032
- 6 -
pyridazinyl, isoindolyl, isoquinolyl or the like. The
term "heterocyclic carbonyl group" means a heterocyclic-
CO- group.
The substituents of the phenyl, naphthyl,
indanyl, indenyl, tetrahydronaphthyl and heterocyclic
groups in R' include, for example, halogen atoms;
substituted or unsubstituted amino, lower alkyl, aryl,
ar-lower alkyl, lower alkoxy, ar-lower alkoxy, aryloxy,
carbamoyloxy, lower alkylthio, lower alkenyl, lower
alkenyloxy, ar-lower alkylthio, ar-lower alkylsulfonyl,
arylsulfonyl, lower alkylsulfonylmaino, arylsulfonylamino
and heterocyclic groups; protected amino groups;
protected or unprotected hydroxyl groups; nitro group;
oxo group; lower alkylenedioxy groups and the like.
The substituents of the lower alkyl, aryl,
ar-lower alkyl, lower alkoxy, ar-lower alkoxy, aryloxy,
carbamoyloxy, lower alkylthio, lower alkenyl, lower
alkenyloxy, ar-lower alkylthio, ar-lower alkylsulfonyl,
arylsulfonyl, lower alkylsulfonylmaino, arylsulfonylamino
and heterocyclic groups in the substituent of the phenyl,
naphthyl, indanyl, indenyl, tetrahydronaphthyl and
heterocyclic groups of R1; and the substituents of the
nitrogen-containing heterocyclic group in R6 include
halogen atoms, protected or unprotected hydroxyl groups,
protected or unprotected carboxyl groups, protected or
unprotected amino groups, lower alkyl groups which is
unsubstituted or substituted by a protected or
unprotected hydroxyl group, halogen-substituted or
22 02 ;-)32
- 7 -
unsubstituted aryl groups, halogen-substituted or
unsubstituted aroyl groups, lower alkoxy group-
substituted or unsubstituted lower alkoxy groups, halo-
lower alkyl groups, lower acyl groups, ar-lower alkyl
groups, ar-lower alkenyl groups, heterocyclic groups,
heterocyclic carbonyl groups, oxo group, lower alkyl-
sulfonyl groups and arylsulfonyl groups and these may be
substituted by at least one of these substituents.
The substituents of the amino group in R1 and
the substituted amino group in R6 include protected or
unprotected hydroxyl groups, lower alkyl groups which are
unsubstituted or substituted by a protected or
unprotected hydroxyl or carboxyl group, cycloalkyl
groups, aryl groups, lower acyl groups, ar-lower alkyl
groups, heterocyclic groups, oxo group-substituted or
unsubstituted heterocyclic carbonyl groups, adamantyl
group, lower alkylsulfonyl groups and arylsulfonyl
groups, and these may be substituted by at least one of
these substituents.
Hydroxyl-protecting group of R2 and the
protective group of hydroxyl, carboxyl and amino groups
included in the substituents include usual hydroxyl-,
carboxyl- and amino-protecting groups mentioned in
"Protective Groups in Organic Synthesis" by Theodra W.
Greene (1981), published by John Wiley & Sons, Inc. and
in particular, as the hydroxyl-protecting group, there
are mentioned lower alkyl groups, lower acyl groups,
tetrahydropyranyl group and substituted or unsubstituted
2202032
- 8 -
ar-lower alkyl groups such as benzyl.
The salt of the 1,2-ethanediol derivative
represented by the general formula [I] may be any
pharmaceutically acceptable salt and includes salts with
mineral acids such as hydrochloric acid, hydrobromic
acid, sulfuric acid, phosphoric acid and the like; salts
with carboxylic acids such as formic acid, acetic acid,
oxalic acid, fumaric acid, maleic acid, malic acid,
tartaric acid, aspartic acid and the like; salts with
sulfonic acids, such as methanesulfonic acid, benzene-
sulfonic acid, p-toluenesulfonic acid, naphthalene-
sulfonic acid and the like; salts with alkali metals such
as sodium, potassium and the like; etc.
When the 1,2-ethanediol derivative represented
by the general formula [I] or its salt has isomers (for
example, optical isomers, geometrical isomers, tautomers
and the like), this invention includes all these isomers,
and also include hydrates, solvates and all crystal forms
of the above compound or its salt.
The 1,2-ethanediol derivative represented by
the general formula [I] or its salt can be formed into a
preparation such as tablet, capsule, powder, granules,
fine granules, pill, suspension, emulsion, solution,
syrup, injection or the like using a pharmaceutically
acceptable preparation adjuvant such as excipient,
carrier, diluent or the like in a conventional manner,
and the resulting preparation can be administered orally
or parenterally. The administration route, dosage and
2 2 0 2 0 - 9 -
number of administrations may be adequately varied
depending upon the age, weight and symptom of a patient
and in the case of oral administration, the dosage is
usually 0.01 to 500 mg/day per adult and this may be
administered in one to several portions.
An explanation is made below of a process for
producing the 1,2-ethanediol derivative represented by
the general formula [I] or its salt.
The 1,2-ethandiol derivative represented by the
general formula [I] or its salt can be produced by the
methods described in JP-A-3-47,158; JP-A-3-197,422; JP-A-
3-232,830; and JP-A-4-95,070 and the like or methods
known per se or in appropriate combinations thereof, for
example, according to each of the following production
processes.
22Li 2US2
- 10 -
R'-CHO
[II]
R3 R4
X1Mg-CH-O
[III] RS
R3 R4 R3 R4
R6H [V] I I * I I
[ I] F- Rl-CHCH-O -C~ nX2 R1-CHCH-0 --t--C-)-.--R6
ORZ R5 OR2 RS
[IV] [Ia]
[oxidation] R6H [V]
R3 R4 R3 R4
[reduction]
R1-C-CH-O R'-CHCH-O nX2
IO RS OH R5
[VI] [IVa]
wherein Rl, R2, R3, R4, R5, R6 and n have the same meanings
as described above, * represents an asymmetric carbon and
X1 and X2 represent halogen atoms.
Production Process 1
(1) A compound of the general formula [IV] or its
salt can be produced by reacting a compound of the
general formula [II] with a compound of the general
formula [III].
In this reaction, any solvent may be used as
far as it does not adversely affect the reaction, and the
2202032
- 11 -
solvent includes ethers such as diethyl ether, tetra-
hydrofuran, dioxane and the like; aromatic hydrocarbons
such as benzene, toluene and the like; etc. These
solvents may be used alone or in admixture of two or
more.
In the above reaction, the amount of the
compound of the general formula [III] used is 0.8 to 100
moles, preferably 0.8 to 10 moles, per mole of the
compound of the general formula [II].
Said reaction may be carried out usually at a
temperature of -78 C to +100 C, preferably -78 C to
+50 C, for a period of 5 minutes to 24 hours.
The compound of the general formula [IV] or its
salt thus obtained may be used in the subsequent reaction
as it is without being isolated.
Incidentally, the compound of the general
formula [III] used here can be produced by a method known
per se, for example, the method described in Bull. Soc.
Chim. Fr., 1967(5), pages 1533-1540.
(2) The compound of the general formula [I] or its
salt can be produced by reacting the compound of the
general formula [IV] or its salt with a compound of the
general formula [V] or its salt in the presence or
absence of a catalyst and in the presence or absence of a
base.
In this reaction, any solvent may be used as
far as it does not adversely affect the reaction, and the
solvent includes halogenated hydrocarbons such as
JL
220
- 12 -
methylene chloride, chloroform and the like; ethers such
as tetrahydrofuran, dioxane and the like; alcohols such
as ethanol, propanol, butanol and the like; nitriles such
as acetonitrile and the like; amides such as N,N-
dimethylformamide and the like; water; etc. These
solvents may be used alone or in admixture of two or
more.
The optionally used catalyst includes, for
example, potassium iodide, sodium iodide and the like.
The amount of the catalyst used is 0.1 to 1
mole per mole of the compound of the general formula [IV]
or its salt.
The optionally used base includes organic and
inorganic bases such as triethylamine, diisopropylethyl-
amine, 1,8-diazobicyclo[5.4.0]undec-7-ene (DBU),
pyridine, potassium tert-butoxide, sodium carbonate,
potassium carbonate, sodium hydride and the like. Also,
the compound of the general formula [V] or its salt can
be used as the base.
The amount of the compound of the general
formula [V] or its salt or the base used is at least one
mole, preferably 1 to 20 moles, per mole of the compound
of the general formula [IV] or its salt.
Said reaction may be carried out usually at a
temperature of 10 C to 150 C, preferably 20 C to 100 C,
for a period of 10 minutes to 20 hours.
The compounds or bases used in each of the
above production processes can also be used as solvents
~2~~-* tj 3~
- 13 -
depending upon their properties.
When the compounds of the general formulas
[II], [III], [IV] and [V] in the above-mentioned produc-
tion processes have isomers (for example, optical
isomers, geometrical isomers, tautomers and the like),
all of them can be used, and also, they can be used in
the form of hydrates and solvates and in all crystal
forms.
When the compounds of the general formulas
[II], [III], [IV] and [V] have a hydroxyl group, an amino
group or a carboxyl group, said hydroxyl, amino or
carboxyl group may previously be protected with a conven-
tional protective group and, after the reaction, the
protective group can, if necessary, be removed in a
manner known per se.
Production Process 2
(1) A compound represented by the general formula
[VI] or its salt can be produced by oxidizing the
compound represented by the general formula [IV] or its
salt by a conventional method as described in "Modern
Synthetic Reactions", Second Edition by Herbert 0. House
(1972) published by W.A. Benjamin, Inc., or the like.
Any solvent may be used in this reaction as far
as it does not adversely affect the reaction, and the
solvent includes ethers such as diethyl ether, tetra-
hydrofuran, dioxane and the like; aromatic hydrocarbons
such as benzene, toluene and the like; etc. These
r/ i 11 ~ J ~ ~
- 14 -
solvents may be used alone or in admixture of two or
more.
The compound represented by the general formula
[VI] or its salt thus obtained can be used in the
subsequent reaction as it is without being isolated.
(2) A compound represented by the general formula
[IVa] or its salt can be produced by reducing the
compound represented by the general formula [VI] or its
salt in the presence or absence of a catalyst and in the
presence or absence of a base by a method known per se,
for example, the method described in Tetrahedron Letters,
vol. 33, No. 29, page 4102, or the like.
(3) The compound of the general formula [I] or its
salt can be produced by reacting the compound represented
by the general formula [IVa] or its salt with the
compound represented by the general formula [V] in the
presence or absence of a catalyst and in the presence or
absence of a base in the same manner as in the Production
Process 1 (2) mentioned above.
The 1,2-ethanediol derivative represented by
the general formula [I] or its salt thus obtained can be
purified and isolated by a conventional method such as
extraction, crystallization, distillation, column
chromatography or the like. Also, the 1,2-ethanediol
derivative represented by the general formula [I] or its
salt can be converted to another 1,2-ethanediol
derivative or its salt by an adequate combination of
known methods per se such as oxidation reaction,
CA 02202032 2004-09-15
- 15 -
reduction reaction, addition reaction, acylation
reaction, alkylation reaction, sulfonylation reaction,
deacylation reaction, substitution reaction, dehydration
reaction, hydrolysis reaction and the like.
Reference Examples and Production Examples are
shown below for more specifically explaining the
processes for producing the compounds of this invention.
In the following Reference Examples and Produc-
tion Examples, the mixing ratio of the solvents is all
indicated in volume basis. In column chromatographic
purification, Silica gel (70-230 meshes, mfd. by Merck &
Co., Inc.) is used as a support. In moderate pressure
column chromatographic purification, LC Sorb SP-A-Si
Tm
(mfd. by Chemco) are used as a support.
The compound of the general formula [II], a
starting material for producing the compound of the
present invention., is known per se. Otherwise, it can be
prepared by a method known per se or an adequate combina-
tion of methods known per se in accordance with the
following Reference Examples.
Reference Example 1
(1) In 250 ml of water is suspended 25.0 g of
3-fluoro-4-methylaniline, and 34.7 ml of conc. hydro-
chloric acid is added to the suspension. Thereafter, the
resulting mixture is cooled to 5 C. To this mixture is
dropwise added a solution of 15.2 g of sodium nitrite in
20 ml of water at a temperature of 5 C to 10 C over one
22U2U5~
- 16 -
hour. The reaction mixture thus obtained is dropwise
added to a solution of 64.0 g of potassium 0-ethyl
dithiocarbonate in 200 ml of water at a temperature of
50 C to 60 C over one hour. The resulting reaction
mixture is cooled to room temperature, and thereafter,
300 ml of ethyl acetate is added thereto. Thereafter,
the resulting organic layer is separated, washed with a
saturated saline solution and then dried over anhydrous
magnesium sulfate. Then, the solvent is removed by
distillation under reduced pressure, to obtain brown,
oily 0-ethyl S-(3-fluoro-4-methyl)phenyl dithiocarbonate.
(2) To a solution of O-ethyl S-(3-fluoro-4-methyl)-
phenyl dithiocarbonate in 150 ml of methanol is added
22.4 g of potassium hydroxide at room temperature under a
nitrogen atmosphere. The resulting mixture is stirred at
room temperature for five hours. Thereafter, 34.8 ml of
bromoacetaldehyde diethyl acetal is added to the thus
stirred mixture. The resulting mixture is refluxed for
six hours and then cooled. Thereafter, insolubles are
removed from the thus cooled mixture by filtration. The
resulting filtrate is concentrated under reduced
pressure. To the residue obtained are added 300 ml of
water and 150 ml of ethyl acetate, and the resulting
organic layer is separated, washed with a saturated
saline solution and dried over anhydrous magnesium
sulfate. Thereafter, the solvent is removed from the
thus dried layer by distillation under reduced pressure.
The residue thus obtained is purified by a column
2 2 0 2
- 17 -
chromatography [eluent: hexane : ethyl acetate =
: 1], to obtain 43.6 g of oily 1,1-diethoxy-2-(3-
fluoro-4-methylphenylthio)ethane.
NMR (CDC13) S value: 1.19 (6H, t, J=7.OHz), 2.22
5 (3H, d, J=2.OHz), 3.09 (2H, d, J=5.4Hz), 3.58 (2H, q,
J=7.OHz), 3.63 (2H, q, J=7.OHz), 4.63 (1H, t, J=5.4Hz),
6.9-7.3 (3H, m)
(3) To a solution of 43.6 g of 1,1-diethoxy-2-(3-
fluoro-4-methylphenylthio)ethane in 400 ml of toluene is
10 added 80 ml of 85% phosphoric acid. The resulting
solution is refluxed for 2.5 hours using an azeotropic
dehydration apparatus. After cooling, to the thus cooled
reaction mixture are added 600 ml of water and 200 ml of
ethyl acetate, and the resulting organic layer is
separated, washed successively with water and a saturated
saline solution and then dried over anhydrous magnesium
sulfate. Thereafter, the solvent is removed from the
thus dried layer by distillation under reduced pressure.
The residue thus obtained is purified by a column
chromatography [eluent: hexane], to obtain 15.9 g of a
colorless, solid mixture of 4-fluoro-5-methyl-benzo-
[b]thiophene and 6-fluoro-5-methyl-benzo[b]thiophene.
(4) To a solution of 15.9 g of a mixture of 4-
fluoro-5-methyl-benzo[b]thiophene and 6-fluoro-5-methyl-
benzo[b]thiophene in 160 ml of carbon tetrachloride are
added 17 g of N-bromosuccinimide and 0.31 g of 2,2'-azo-
bisisobutylonitrile. The resulting mixture is refluxed
for two hours. After cooling, insolubles are removed
- 18 - 22020311
from the thus cooled mixture by filtration, and the
filtrate obtained is concentrated under reduced pressure.
The residue obtained is suspended in 75 ml of acetic acid
and 75 ml of water, and 26.8 g of hexamethylenetetramine
is added thereto, after which the resulting mixture is
refluxed for two hours. After cooling, 150 ml of water
and 200 ml of ethyl acetate are added to the thus cooled
mixture, and the resulting organic layer is separated,
washed successively with water, an aqueous saturated
sodium carbonate solution and a saturated saline solution
and then dried over anhydrous magnesium sulfate.
Thereafter, the solvent is removed from the thus dried
layer by distillation under reduced pressure. The
residue thus obtained is purified by a moderate pressure
column chromatography [eluent: hexane : ethyl acetate =
15 : 1], to obtain 1.71 g of 4-fluorobenzo[b]thiophene-5-
carbaldehyde and 5.82 g of 6-fluorobenzo[b]thiophene-5-
carbaldehyde.
Physical properties of each of the compounds
are as follows:
= 4-Fluorobenzo[b]thiophene-5-carbaldehyde
IR (KBr) cm 1: 1684
NMR (CDC13) S value: 7.5-8.1 (4H, m), 10.55 (1H, s)
= 6-Fluorobenzo[b]thiophene-5-carbaldehyde
IR (KBr) cml: 1684
NMR (CDC13) 6 value: 7.3-7.6 (2H, m), 7.67 (1H, d,
J=10.3Hz), 8.34 (1H, d, J=6.4Hz), 10.46 (1H, s)
In the same manner, the following compounds are
- 19 - 22 02032
obtained:
= 7-Fluorobenzo[b]thiophene-5-carbaldehyde
IR (KBr) cm-1: 1678
NMR (CDC13) S value: 7.2-7.8 (3H, m), 8.16 (1H, s),
10.60 (1H, s)
= 4-Bromobenzo[b]thiophene-5-carbaldehyde
IR (KBr) cm-1: 1674
NMR (CDC13) 8 value: 7.1-8.0 (4H, m), 10.54 (1H, s)
= 6-Bromobenzo[b]thiophene-5-carbaldehyde
IR (KBr) cm-1: 1681
NMR (CDC13) S value: 7.3-7.6 (2H, m), 8.18 (1H, s),
8.41 (1H, s), 10.51 (1H, s)
= 4-Chlorobenzo[b]thiophene-5-carbaldehyde
IR ( KBr ) cm 1: 1678
NMR (CDC13) S value: 7.3-8.2 (4H, m), 10.66 (1H, s)
= 6-Chlorobenzo[b]thiophene-5-carbaldehyde
IR ( KBr ) cm 1: 1678
NMR (CDC13) S value: 7.2-7.7 (2H, m), 7.98 (1H, s),
8.42 (1H, s), 10.60 (1H, s)
Reference Example 2
(1) In a solution of 5 g of benzo[b]thiophene-5-
carbaldehyde in 100 ml of benzene are added 20 ml of
ethylene glycol and a catalytic amount of p-toluene-
sulfonic acid, and the resulting mixture is refluxed for
two hours using an azeotropic dehydration apparatus.
After cooling, 200 ml of water and 100 ml of ethyl
acetate are added to the thus cooled mixture, and the
22O2052
- 20 -
resulting organic layer is separated, washed successively
with water and a saturated saline solution and dried over
anhydrous magnesium sulfate. Thereafter, the solvent is
removed from the thus dried layer by distillation under
reduced pressure. The residue obtained is purified by a
moderate pressure column chromatography [eluent: hexane
: ethyl acetate = 10 : 1], to obtain 6.12 g of colorless,
solid 5-(1,3-dioxoran-2-yl)benzo[b]thiophene.
NMR (CDC13) S value: 3.9-4.3 (4H, m), 5.94 (1H, s),
7.2-7.6 (3H, m), 7.7-8.1 (2H, m)
(2) A solution of 1.5 g of 5-(1,3-dioxoran-2-yl)-
benzo[b]thiophene in 15 ml of tetrahydrofuran is cooled
to -40 C, at which temperature 4.55 ml of 1.6 M hexane
solution of n-butyllithium is then dropwise added to the
solution. The temperature of the resulting reaction
mixture is elevated to -10 C, and then lowered to -40 C
again, at which 0.45 ml of methyl iodide is added to the
thus re-cooled reaction mixture. The temperature of the
resulting mixture is elevated to room temperature. 30
Milliliters of water and 30 ml of ethyl acetate are added
thereto, and the resulting organic layer is separated,
washed successively with water and a saturated saline
solution and dried over anhydrous magnesium sulfate.
Thereafter, the solvent is removed from the thus dried
layer by distillation under reduced pressure. The
residue obtained is purified by a moderate pressure
column chromatography [eluent: hexane : ethyl acetate =
10 : 1], to obtain 1.45 g of colorless, solid 2-methyl-5-
- 21 - 2202
(1,3-dioxoran-2-yl)benzo[b]thiophene.
NMR (CDC13) S value: 2.55 (3H, s), 3.9-4.3 (4H, m),
5.89 (1H, s), 6.9-8.0 (4H, m)
(3) A catalytic amount of p-toluenesulfonic acid is
added to a solution of 1.5 g of 2-methyl-5-(1,3-dioxoran-
2-yl)benzo[b]thiophene in 20 ml of acetone at room
temperature, at which temperature the resulting mixture
is stirred for 30 minutes. After the reaction, the
solvent is removed from the reaction mixture by
distillation under reduced pressure. The residue
obtained are added 20 ml of water and 20 ml of ethyl
acetate, after which the resulting organic layer is
separated, washed successively with water and a saturated
saline solution and then dried over anhydrous magnesium
sulfate. The solvent is thereafter removed from the thus
dried layer by distillation under reduced pressure, to
obtain 1.15 g of colorless, solid 2-methylbenzo[b]-
thiophene-5-carbaldehyde.
IR (KBr) cm 1: 1694
NMR (CDC13) S value: 2.60 (3H, s), 5.89 (1H, s),
7.0-8.4 (4H, m), 10.10 (1H, s)
Reference Example 3
(1) To a solution of 3 g of benzo[b]thiophene-5-
carbaldehyde in 30 ml of acetic acid is dropwise added
1.43 ml of bromine with ice-cooling. The temperature of
the reaction mixture is elevated to room temperature, at
which temperature the mixture is stirred for three hours.
22U2~/~
- 22 -
After the reaction, 50 ml of water and 50 ml of ethyl
acetate are added to the thus stirred reaction mixture,
and the resulting organic layer was separated, washed
successively with water, a saturated aqueous sodium
hydrogencarbonate solution and a saturated saline
solution and then dried over anhydrous magnesium sulfate.
Thereafter, the solvent is removed from the thus dried
layer by distillation under reduced pressure. The
residue obtained is purified by a moderate pressure
column chromatography [eluent: hexane : ethyl acetate =
: 1], to obtain 4.2 g of 3-bromobenzo[b]thiophene-5-
carbaldehyde.
NMR (CDC13) S value: 7.5-8.4 (4H, m), 10.19 (1H, s)
(2) The same procedure as in Reference Example 2
15 (1) is repeated, except that 3-bromobenzo[b]thiophene-5-
carbaldehyde is substituted for the benzo[b]thiophene-5-
carbaldehyde, to obtain 3-bromo-5-(1,3-dioxoran-2-yl)-
benzo[b]thiophene.
NMR (CDC13) S value: 3.9-4.2 (4H, m), 5.93 (1H, s),
20 7.3-8.0 (4H, m)
(3) The same procedure as in Reference Example 2
(2) is repeated, except that 3-bromo-5-(1,3-dioxoran-2-
yl)benzo[b]thiophene is substituted for the 5-(1,3-di-
oxoran-2-yl)benzo[b]thiophene and diethyl ether is
substituted for the tetrahydrofuran, to obtain 3-methyl-
benzo[b]thiophene-5-carbaldehyde.
NMR (CDC13) S value: 2.51 (3H, s), 7.0-8.4 (4H, m),
10.15 (1H, s)
22 020 32
- 23 -
Reference Example 4
The same procedure as in Reference Example 2
(2) and Reference Example 2 (3) is repeated, except that
N-fluorobenzenesulfonimide is substituted for the methyl
iodide to obtain 2-fluorobenzo[b]thiophene-5-carbaldehyde
from 5-(1,3-dioxoran-2-yl)benzo[b]thiophene.
NMR (CDC13) 8 value: 6.84 (1H, d J=2.OHz), 7.6-8.4
(3H, m), 10.09 (1H, s)
Reference Example 5
The same procedure as in Reference Example 2
(2) and Reference Example 2 (3) is repeated, except that
3-bromo-5-(1,3-dioxoran-2-yl)benzo[b]thiophene is
substituted for the 5-(1,3-dioxoran-2-yl)benzo[b]-
thiophene; diethyl ether is substituted for the tetra-
hydrofuran; and N-fluorobenzenesulfonimide is substituted
for the methyl iodide, to obtain 3-fluorobenzo[b]-
thiophene-5-carbaldehyde from 3-bromo-5-(1,3-dioxoran-2-
yl)benzo[b]thiophene.
NMR (CDC13) 6 value: 6.99 (1H, d, J=2.OHz), 7.7-8.4
(3H, m), 10.14 (1H, s)
Reference Example 6
(1) To a solution of 2.0 g of 5-(1,3-dioxoran-2-
yl)benzo[b]thiophene in 15 ml of tetrahydrofuran is added
dropwise 6.06 ml of a 1.6 M hexane solution of
n-butyllithium at -78 C. The temperature of the reaction
mixture was elevated to -10 C and thereafter lowered
22 372
- 24 -
again to -78 C, after which 1.55 g of bromine is added to
the thus re-cooled reaction mixture. The temperature of
the resulting mixture is elevated to room temperature,
and thereafter, 30 ml of water and 30 ml of ethyl acetate
are added thereto. The resulting organic layer is
separated, washed successively with water and a saturated
saline solution, and then dried over anhydrous magnesium
sulfate. Thereafter, the solvent is removed from the
thus dried layer by distillation under reduced pressure.
The residue obtained is purified by a moderate pressure
column chromatography [elueant: toluene], to obtain 2.45
g of colorless, solid 2-bromo-5-(1,3-dioxoran-2-yl)benzo-
[b]thiophene.
NMR (CDC13) S value: 3.9-4.3 (4H, m), 5.89 (1H, s),
7.2-8.0 (4H, m)
(2) To a solution of 2.5 g of 2-bromo-5-(1,3-
dioxoran-2-yl)benzo[b]thiophene in 50 ml of toluene are
added 6.44 g of phenyltri-n-butyltin and 0.05 g of tetra-
kis(triphenylphosphine)palladium (0), and the resulting
mixture is refluxed under a nitrogen atmosphere for five
hours. The reaction mixture is cooled to room temper-
ature. Thereafter, 30 ml of water and 30 ml of ethyl
acetate are added to the thus cooled reaction mixture.
Thereafter, insolubles are removed from the resulting
mixture by filtration. Thereafter, the resulting organic
layer is separated, washed successively with water and a
saturated saline solution and then dried over anhydrous
magnesium sulfate. Thereafter, the solvent is removed
- 25 -
from the thus dried layer by distillation under reduced
pressure. The residue obtained is purified by a column
chromatography [eluent: hexane : ethyl acetate =
20 : 1], to obtain 1.20 g of colorless, solid 2-phenyl-5-
(1,3-dioxoran-2-yl)benzo[b]thiophene.
(3) In the same manner as in Reference Example 2
(3), 2-phenylbenzo[b]thiophene-5-carbaldehyde is obtained
from 2-phenyl-5-(1,3-dioxoran-2-yl)benzo[b]thiophene.
IR (KBr) cm 1: 1692
NMR (CDC13) S value: 7.2-8.4 (9H, m), 10.13 (1H, s)
In the same manner, the following compound is
obtained:
= 3-Phenylbenzo[b]thiophene-5-carbaldehyde
IR (KBr) cm 1: 1686
NMR (CDC13) 8 value: 7.3-8.1 (8H, m), 8.37 (1H, m),
10.09 (1H, s)
Reference Example 7
(1) The same procedure as in Reference Example 1
(1) and Reference Example 1 (2) is repeated, except that
methyl 4-amino-2-methylbenzoate is substituted for the
3-fluoro-4-methylaniline, to obtain methyl 4-(2,2-
diethoxyethylthio)-2-methylbenzoate.
NMR (CDC13) S value: 1.20 (6H, t, J=7.OHz), 2.57
(3H, s), 3.18 (2H, d, J=5.4Hz), 3.3-3.8 (4H, m), 3.86
(3H, s), 4.67 (1H, t, J=5.4Hz), 7.0-7.3 (2H, m), 7.7-7.9
(1H, m)
(2) The same procedure as in Reference Example 1
22(~2u.~2
- 26 -
(3) is repeated, except that methyl 4-(2,2-diethoxy-
ethylthio)-2-methylbenzoate is substituted for the 1,1-
diethoxy-2-(3-fluoro-4-methylphenylthio)ethane, to obtain
a mixture of methyl 4-methylbenzo[b]thiophene-5-
carboxylate and methyl 6-methylbenzo[b]thiophene-5-
carboxylate.
(3) In 20 ml of tetrahydrofuran is suspended 0.37 g
of lithium aluminum hydride. Thereafter, a solution of 2
g of a mixture of methyl 4-methylbenzo[b]thiophene-5-
carboxylate and methyl 6-methylbenzo[b]thiophene-5-
carboxylate in 20 ml of tetrahydrofuran is dropwise added
to the suspension. The temperature of the resulting
suspension is elevated to room temperature, and 30 ml of
water and 30 ml of ethyl acetate are added thereto, and
the resulting mixture is filtered. The resulting organic
layer is separated, washed successively with water and a
saturated saline solution and then dried over anhydrous
magnesium sulfate. The solvent is removed from the thus
dried layer by distillation under reduced pressure. The
residue obtained is purified by a column chromatography
[eluent: hexane : ethyl acetate = 10 : 1], to obtain 1.7
g of a mixture of 4-methylbenzo[b]thiophene-5-methanol
and 6-methylbenzo[b]thiophene-5-methanol as colorless
solid.
(4) In 17 ml of chloroform is dissolved 1.7 g of a
mixture of 4-methylbenzo[b]thiophene-5-methanol and
6-methylbenzo[b]thiophene-5-methanol, and 4.1 g of
manganese dioxide is added thereto at room temperature.
- 27 - 22020132
Thereafter, the resulting mixture is refluxed for one
hour. After the reaction, insolubles are removed from
the resulting reaction mixture by filtration and the
filtrate obtained is concentrated under reduced pressure.
The residue obtained is purified by a moderate pressure
column chromatography [eluent: hexane : toluene =
1 : 1], to obtain 0.65 g of 4-methylbenzo[b]thiophene-5-
carbaldehyde as colorless solid and 0.48 g of 6-methyl-
benzo[b]thiophene-5-carbaldehyde as colorless solid.
Physical properties of each of the compounds
are as follows:
= 4-Methylbenzo[b]thiophene-5-carbaldehyde
IR ( KBr ) cm 1: 1673
NMR (CDC13) S value: 2.95 (3H, s), 7.5-7.9 (4H, m),
10.50 (1H, s)
= 6-Methylbenzo[b]thiophene-5-carbaldehyde
IR (KBr) cm 1: 1696
NMR (CDC13) 8 value: 2.77 (3H, s), 7.2-7.6 (2H, m),
7.75 (1H, s), 8.26 (1H, s), 10.35 (1H, s)
Reference Example 8
In the same manner as in Reference Example 6,
6-methoxybenzo[b]thiophene-5-carbaldehyde is obtained
from 4-amino-2-methoxybenzoic acid.
NMR (CDC13) S value: 3.99 (3H, s), 7.33 (2H, m),
7.42 (1H, s), 8.28 (1H, s), 10.56 (1H, s)
- 28
Reference Example 9
(1) To a solution of 2.04 g of diisopropylamine in
30 ml of tetrahydrofuran is dropwise added 9.19 ml of 1.6
M n-hexane solution of n-butyllithium at -20 C. At the
same temperature, the resulting mixture is stirred for
one hour and then cooled to -70 C, at which temperature a
solution of 3 g of 3,5-difluorobromobenzene in 10 ml of
tetrahydrofuran is dropwise added thereto over 30
minutes. The temperature of the resulting reaction
mixture is elevated to -40 C and then lowered again to
-70 C, at which temperature 0.97 ml of methyl iodide is
then added thereto. The temperature of the resulting
mixture is elevated to room temperature, and 80 ml of
water and 80 ml of ethyl acetate are then added thereto,
after which the resulting organic layer is separated,
washed successively with water and a saturated saline
solution and then dried over anhydrous magnesium sulfate.
Thereafter, the solvent is removed from the thus dried
layer by distillation under reduced pressure. The
residue obtained is purified by a column chromatography
[eluent: hexane : ethyl acetate = 10 : 1], to obtain
1.98 g of colorless, oily 4-bromo-2,6-difluorotoluene.
NMR (CDC13) S value: 2.23 (3H, t, J=1.5Hz), 7.00
(2H, d, J=6.3Hz)
(2) To a solution of 1.98 g of 4-bromo-2,6-
difluorotoluene in 20 ml of tetrahydrofuran is dropwise
added 5.7 ml of 1.6 M n-hexane solution of n-butyllithium
at -70 C. The resulting mixture is then stirred at the
- 29 -
same temperature for one hour. Then, a solution of 2.88
g of 2,2,2',2'-tetraethoxydiethyl disulfide in 5 ml of
tetrahydrofuran is dropwise added to the thus stirred
reaction mixture at the same temperature. The
temperature of the resulting mixture is elevated to room
temperature and 80 ml of water and 80 ml of ethyl acetate
are added thereto. The resulting organic layer is
separated, washed successively with water and a saturated
saline solution and then dried over anhydrous magnesium
sulfate. Thereafter, the solvent is removed from the
thus dried layer by distillation under reduced pressure.
The residue obtained is purified by a column
chromatography [eluent: hexane : ethyl acetate =
: 1], to obtain 2.1 g of colorless, oily 1,1-diethoxy-
15 2-(3,5-difluoro-4-methylphenylthio)ethane.
NMR (CDC13) S value: 1.20 (3H, t, J=7.OHz), 1.23
(3H, t, J=7.2Hz), 2.1 (3H, t, J=1.7Hz), 3.10 (2H, d,
J=5.6Hz), 3.4-3.9 (4H, m), 4.64 (1H, t, J=5.6Hz), 6.87
(2H, d, J=7.6Hz)
20 (3) The same procedure as in Reference Example 1
(3) is repeated, except that 1,1-diethoxy-2-(3,5-
difluoro-4-methylphenylthio)ethane is substituted for the
1,1-diethoxy-2-(3-fluoro-4-methylphenylthio)ethane, to
obtain 4,6-difluoro-5-methylbenzo[b]thiophene.
NMR (CDC13) S value: 2.31 (3H, t, J=1.9Hz), 7.2-7.5
(3H, m)
(4) To a solution of 0.48 g of 4,6-difluoro-5-
methylbenzo[b]thiophene in 5 ml of carbon tetrachloride
2 2 0 - 30 -
are added 0.92 g of N-bromosuccinimide and 0.01 g of
2,2'-azobisisobutylonitrile, and the resulting mixture is
refluxed for 16 hours. After cooling, insolubles are
removed from the thus cooled mixture by filtration, and
the filtrate obtained is concentrated under reduced
pressure. The residue obtained is purified by a column
chromatography [eluent: hexane], to obtain 0.18 g of
colorless, solid 5-bromomethyl-4,6-diflu-
orobenzo[b]thiophene.
NMR (CDC13) S value: 4.67 (2H, t, J=1.2Hz), 7.2-7.5
(3H, m)
(5) To a solution of 0.18 g of 5-bromomethyl-4,6-
in 5 ml of N,N-dimethyl-
difluorobenzo[b]thiophene
formamide is added 0.23 g of potassium acetate, and the
resulting mixture is stirred at 60 C for one hour. The
temperature of the thus stirred mixture is lowered to
room temperature, and 10 ml of water and 10 ml of ethyl
acetate are added thereto. Thereafter, the resulting
organic layer is separated, washed successively with
water and a saturated saline solution and then dried over
anhydrous magnesium sulfate. Thereafter, the solvent is
removed from the thus dried layer by distillation under
reduced pressure, to obtain 0.14 g of colorless, oily
5-acetoxymethyl-4,6-difluorobenzo[b]thiophene.
NMR (CDC13) S value: 2.07 (3H, s), 5.31 (2H, t,
J=1.2Hz), 7.3-7.6 (3H, m)
(6) To a solution of 0.14 g of 5-acetoxymethyl-4,6-
difluorobenzo[b]thiophene in 5 ml of methanol is added
22 0C0~%
- 31 -
0.03 g of potassium hydroxide at room temperature, and
thereafter, the resulting mixture is stirred at the same
temperature for 30 minutes. After the reaction, 10 ml of
water and 10 ml of ethyl acetate are added to the thus
stirred reaction mixture. The resulting organic layer is
separated, washed successively with water and a saturated
saline solution, and then dried over anhydrous magnesium
sulfate. Thereafter, the solvent is removed from the
thus dried layer by distillation under reduced pressure
to obtain 0.12 g of colorless, oily 5-hydroxymethyl-4,6-
difluorobenzo[b]thiophene.
NMR (CDC13) S value: 4.88 (2H, bs), 7.2-7.6 (3H, m)
(7) To a solution of 0.22 ml of oxalyl chloride in
10 ml of methylene chloride is added 0.35 ml of dimethyl
sulfoxide at -78 C. Then, to the resulting solution is
added dropwise a solution of 0.20 g of 5-hydroxymethyl-
4,6-difluorobenzo[b]thiophene in 3 ml of methylene
chloride. At the same temperature, the resulting mixture
is stirred for one hour, and then, 0.70 ml of
triethylamine is added thereto. The temperature of the
resulting mixture is elevated to room temperature, and
thereafter, 10 ml of water and 10 ml of ethyl acetate are
added thereto, after which the resulting organic layer is
separated, washed successively with water and a saturated
saline solution and then dried over anhydrous magnesium
sulfate. Then, the solvent is removed from the thus
dried layer by distillation under reduced pressure. The
residue obtained is purified by a column chromatography
22 0 2--0 52
- 32 -
[eluent: hexane], to obtain 0.20 g of colorless, solid
4,6-difluorobenzo[b]thiophene-5-carbaldehyde.
IR (KBr) cm-1: 1696
NMR (CDC13) S value: 7.4-7.6 (3H, m), 10.49 (1H, s)
Production Example 1
(1) To a solution of 1.6 g of 6-fluorobenzo[b]thio-
phene-5-carbaldehyde in 30 ml of tetrahydrofuran is
dropwise added 10 ml of 1.6 M tetrahydrofuran solution of
2-chloroethoxymethylmagnesium chloride at -30 C over ten
minutes, and thereafter, the resulting mixture is stirred
while ice-cooling for one hour. Subsequently, the thus
stirred reaction mixture is introduced into a mixture of
50 ml of ice water, 50 ml of ethyl acetate and 2 g of
ammonium chloride, and the resulting mixture is adjusted
to pH 2 with 6 N hydrochloric acid. Thereafter, the thus
pH-adjusted mixture is stirred at the same temperature
for five minutes. Subsequently, the thus stirred
reaction mixture is adjusted to pH 6 with a saturated
aqueous sodium hydrogencarbonate solution. Thereafter,
the resulting organic layer is separated, washed
successively with water and a saturated saline solution,
and then dried over anhydrous magnesium sulfate. The
solvent is then removed from the thus dried layer by
distillation under reduced pressure. The residue
obtained is purified by a column chromatography [eluent:
toluene : ethyl acetate = 4 : 1], to obtain 1.3 g of oily
2-(2-chloroethoxy)-1-(6-fluorobenzo[b]thiophen-5-
- 33 - 2202032
yl)ethanol.
(2) A mixture of 0.61 g of 2-(2-chloroethoxy)-1-(6-
fluorobenzo[b]thiophen-5-yl)ethanol, 3 ml of 50% aqueous
diethylamine solution, 0.45 mg of potassium iodide and 20
ml of ethanol is refluxed for three hours. Subsequently,
3 ml of 50% aqueous diethylamine solution is added to the
thus refluxed reaction mixture, and the resulting
reaction mixture is further refluxed for three hours.
The solvent is removed from the thus refluxed mixture by
distillation under reduced pressure. To the residue
obtained are added 30 ml of ethyl acetate and 30 ml of
water, after which the resulting mixture is adjusted to
pH 1.5 with 6 N hydrochloric acid. Thereafter, the
aqueous layer is separated and washed with 10 ml of ethyl
acetate. 30 Milliliters of ethyl acetate is then added
to the thus washed layer, after which the resulting
mixture is adjusted to pH 10.5 with potassium carbonate.
The organic layer is separated again, washed successively
with 10 ml of water and 10 ml of a saturated saline
solution, and then dried over anhydrous magnesium
sulfate. The solvent is then removed from the thus dried
layer by distillation under reduced pressure. The
residue obtained is dissolved in 6 ml of ethanol. To the
resulting solution are added 0.6 ml of 5 N dried
hydrochloric acid-ethanol solution and 6 ml of diethyl
ether. The mixture thus obtained is stirred at room
temperature for one hour. The crystals precipitated are
collected by filtration and washed with 2 ml of a liquid
2202032
- 34 -
mixture of diethyl ether and ethanol (1 : 1) and then
dried, to obtain 0.28 g of 2-[2-(N,N-diethylamino)-
ethoxy]-1-(6-fluorobenzo[b]thiophen-5-yl)ethanol
hydrochloride.
Melting point: 125-126 C
NMR (DMSO-d6) S value: 1.18 (6H, t, J=7.3Hz),
2.9-4.0 (10H, m), 5.0-5.4 (1H, m), 5.6-5.8 (1H, m),
7.4-8.2 (4H, m)
In the same manner, the following compounds are
obtained:
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(4-fluorobenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 127-128 C
NMR (DMSO-d6) S value: 1.18 (6H, t, J=7.3Hz),
2.9-4.1 (10H, m), 5.1-5.4 (1H, m), 5.6-5.8 (1H, m),
7.4-8.0 (4H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(7-fluorobenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 119-120 C
NMR (DMSO-d6) S value: 1.15 (6H, t, J=7.3Hz),
2.8-4.0 (10H, m), 4.7-5.1 (1H, m), 5.6-5.9 (1H, m),
7.1-8.0 (4H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(2-fluorobenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 130-131 C
NMR (DMSO-d6) S value: 1.17 (6H, t, J=7.3Hz),
2.8-4.0 (10H, m), 4.86 (1H, m), 5.6-5.9 (1H, m), 7.1-8.0
(4H, m)
22J2~~~3~
- 35 -
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(3-fluorobenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 106-107 C
NMR (DMSO-d6) S value: 1.17 (6H, t, J=7.3Hz),
2.8-4.0 (10H, m), 4.8-5.2 (1H, m), 5.4-6.0 (1H, m),
7.4-8.2 (4H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(2-methylbenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 136-137 C
NMR (DMSO-d6) 8 value: 1.18 (6H, t, J=7.3Hz), 2.54
(3H, s), 2.8-4.0 (10H, m), 4.7-5.0 (1H, m), 5.3-5.8 (1H,
m), 7.0-8.0 (4H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(3-methylbenzo[b]-
thiophen-5-yl)ethanol 1/2 fumarate
Melting point: 137-138 C
NMR (DMSO-d6) 6 value: 0.99 (6H, t, J=7.3Hz),
2.3-3.1 (9H, m), 3.4-3.8 (4H, m), 4.2-5.1 (3H, m), 6.53
(1H, s), 7.2-8.0 (4H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(4-methylbenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 189-190 C
NMR (DMSO-d6) S value: 1.17 (6H, t, J=7.2Hz), 2.57
(3H, s), 2.8-4.0 (10H, m), 4.9-5.3 (1H, m), 5.4-5.7 (1H,
m), 7.2-8.0 (4H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(6-methylbenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 144-145 C
NMR (DMSO-d6) 6 value: 1.17 (6H, t, J=7.2Hz), 2.41
- 36 - 2C.U2032
(3H, s), 2.7-4.1 (10H, m), 4.8-5.3 (1H, m), 5.4-5.8 (1H,
m), 7.2-8.1 (4H, m)
= 1-(4-Chlorobenzo[b]thiophen-5-yl)-2-[2-(N,N-dimethyl-
amino)ethoxy]ethanol hydrochloride
Melting point: 148-149 C
NMR (DMSO-d6) S value: 1.17 (6H, t, J=7.2Hz),
2.8-4.1 (10H, m), 5.1-5.4 (1H, m), 5.7-6.0 (1H, m),
7.3-8.2 (4H, m)
= 1-(6-Chlorobenzo[b]thiophen-5-yl)-2-[2-(N,N-diethyl-
amino)ethoxy]ethanol hydrochloride
Melting point: 140-141 C
NMR (DMSO-d6) S value: 1.17 (6H, t, J=7.2Hz),
2.6-4.1 (10H, m), 5.0-5.4 (1H, m), 5.7-6.1 (1H, m),
7.3-8.2 (4H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(2-phenylbenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 131-135 C
NMR (DMSO-d6) S value: 1.18 (6H, t, J=7.1Hz), 2.8-4.2
(10H, m), 4.7-5.1 (1H, m), 7.2-8.1 (9H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(3-phenylbenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 158-160 C
NMR (DMSO-d6) S value: 1.14 (6H, t, J=7.2Hz),
2.8-4.2 (10H, m), 4.7-5.1 (1H, m), 7.2-8.2 (9H, m)
= 2-[2-(N,N-Diethylamino)ethoxy]-1-(6-methoxybenzo[b]-
thiophen-5-yl)ethanol hydrochloride
Melting point: 161-162 C
NMR (DMSO-d6), 8 value: 1.19 (6H, t, J=7.3Hz),
2202032
- 37 -
2.8-4.2 (10H, m), 3.87 (3H, s), 5.1-5.3 (1H, m), 7.2-7.6
(3H, m), 7.92 (1H, s)
= 1-(4,6-Difluorobenzo[b]thiophen-5-yl)-2-[2-(N,N-
diethylamino)ethoxy]ethanol 1/2 fumarate
Melting point: 135-136 C
NMR (DMSO-d6) S value: 0.92 (6H, t, J=7.OHz),
2.4-2.9 (6H, m), 3.4-3.9 (4H, m), 5.1-5.5 (3H, m), 6.50
(1H, s), 7.4-7.9 (3H, m)
= 1-(4-Bromobenzo[b]thiophen-5-yl)-2-[2-(N,N-dimethyl-
amino)ethoxy]ethanol hydrochloride
Melting point: 150-151 C
NMR (CDC13) S value: 1.35 (6H, t, J=7.5Hz), 2.8-4.2
(10H, m), 5.2-5.6 (2H, m), 7.3-7.4 (4H, m)
= 1-(6-Bromobenzo[b]thiophen-5-yl)-2-[2-(N,N-diethyl-
amino)ethoxy]ethanol hydrochloride
Melting point: 153-154 C
NMR (CDC13) S value: 1.41 (6H, t, J=7.5Hz), 2.6-4.2
(10H, m), 5.3-5.6 (2H, m), 7.2-7.5 (2H, m), 7.99 (1H, s),
8.17 (1H, s)
= 2-[2-(N,N-Di-n-propylamino)ethoxy]-1-(6-fluorobenzo-
[b]thiophen-5-yl)ethanol
Melting point: 143-144 C
NMR (CDC13) S value: 0.95 (6H, t, J=7.OHz), 1.4-2.2
(4H, m), 2.8-3.4 (6H, m), 3.5-4.2 (4H, m), 5.1-5.5 (2H,
m), 7.1-7.6 (3H, m), 8.0-8.2 (1H, s)
= 1-(6-Fluorobenzo[b]thiophen-5-yl)-2-(1-piperazinyl)-
ethanol
Melting point: 172-174 C
l{J-,
- 38 -
NMR (CDC13) 8 value: 1.4-2.4 (6H, m), 2.6-4.2 (11H,
m), 5.2-5.4 (1H, m), 7.1-7.6 (3H, m), 7.9-8.2 (1H, m)
= 1-(6-Fluorobenzo[b]thiophen-5-yl)-2-(l-morpholinyl)-
ethanol
Melting point: 198-200 C
NMR (DMSO-d6) 8 value: 2.6-4.8 (15H, m), 4.9-5.3
(1H, m), 7.4-8.2 (4H, m)
Production Example 2
(1) To a solution of 8.73 ml of oxalyl chloride in
90 ml of methylene chloride is dropwise added 14.2 ml of
dimethyl sulfoxide at -70 C over 30 minutes. The
solution is stirred at the same temperature for ten
minutes, after which a solution of 11 g of 2-(2-
chloroethoxy)-1-(6-fluorobenzo[b]thiophen-5-yl)ethanol in
90 ml of methylene chloride is dropwise added thereto at
the same temperature over 30 minutes. The resulting
mixture is stirred at the same temperature for 30
minutes, and then, 50.2 ml of triethylamine is dropwise
added thereto. The temperature of the resulting mixture
is elevated to room temperature, and thereafter, 200 ml
of diethyl ether is added thereto. Then, insolubles are
removed from the diethyl ether-added mixture by filtra-
tion. Then, the solvent is removed from the resulting
filtrate by distillation under reduced pressure. To the
residue obtained are added 200 ml of water and 200 ml of
ethyl acetate, and then, the pH is adjusted to 1 with 1 N
hydrochloric acid. Thereafter, the resulting organic
22u20 32
- 39 -
layer is separated, washed successively with water and a
saturated saline solution and then dried over anhydrous
magnesium sulfate. The solvent is removed from the thus
dried layer by distillation under reduced pressure. To
the residue obtained is added 50 ml of diethyl ether,
after which insolubles are collected by filtration, to
obtain 9.5 g of colorless, solid 2-(2-chloroethoxy)-1-(6-
fluorobenzo[b]thiophen-5-yl)ethanone.
(2) To a solution of 4.5 g of 2-(2-chloroethoxy)-1-
(6-fluorobenzo[b]thiophen-5-yl)ethanone in 45 ml of
tetrahydrofuran is added 0.46 g of (R)-5,5-diphenyl-2-
methyl-3,4-propano-1,3,2-oxazaborolidine at -10 C, and
thereafter, 9.9 ml of 1 M borane solution of tetrahydro-
furan is dropwise added thereto. The temperature of the
resulting mixture is elevated to room temperature, and
the mixture is stirred at the same temperature for 1.5
hours, after which 100 ml of water and 100 ml of ethyl
acetate are added thereto. The resulting organic layer
is separated, washed successively with water and a
saturated saline solution and then dried over anhydrous
magnesium sulfate. The solvent is then removed from the
thus dried layer by distillation under reduced pressure.
The residue obtained is purified by a column chromato-
graphy [eluent: toluene : ethyl acetate = 10 : 1], to
obtain 4.5 g of oily (+)-2-(2-chloroethoxy)-1-(6-fluoro-
benzo[b]thiophen-5-yl)ethanol.
(3) In the same manner as in Production Example 1
(2), (+)-1-(6-fluorobenzo[b]thiophen-5-yl)-2-[2-(N,N-di-
- 40 - 22 02032
ethylamino)ethoxy]ethanol hydrochloride is obtained from
(+)-2-(2-chloroethoxy)-1-(6-fluorobenzo[b]thiophen-5-yl)-
ethanol.
Melting point: 138-139 C
[a]D +40.8 (C = 1.40, CH3OH)
In the same manner, the following compound is
obtained:
= (-)-1-(6-Fluorobenzo[b]thiophen-5-yl)-2-[2-(N,N-
diethylamino)ethoxy]ethanol hydrochloride
Melting point: 138-139 C
[a]D -40.3 (C = 1.13, CH3OH)
An explanation is made below of the NGF
activity-potentiating effect of the 1,2-ethanediol
derivative represented by the general formula [I] or its
salt.
[Nervous process-elongating activity]
Test compound
As test compounds, there are used the compounds
disclosed in JP-A-3-47,158; JP-A-3-232,830 and JP-A-4-
95,070 and the compounds obtained in Production Examples
1 and 2 which are shown in Tables 1 to 6. The melting
points of the compounds other than those obtained in
'Production Examples 1 and 2 are also shown in Table 7.
Incidentally, the compounds were dissolved in water or
dimethyl sulfoxide.
22 0 20 32
- 41 -
Table 1
No. Compound
OH
0 iC2H5
1 c ~~~ N H C
s C2H5
OH
2 / 0\~~N~C2H5
HCI
C2H5
OH
0 CH3
3 N HC
CH3
OH
0 ~CH3
4 , N\ . H C I
S CH3
OH
0 iC3H7
N~ HC
C3H7
OH
ON,CzHs
6 TIIIIIi S ~ I
HC I OCH3
22U2J52
- 42 -
Table 2
No. Compound
OH
Q~~ ~C2H5
N HC1
CH
F 2 s
F OH
/C2H5
8 / N HC I
S C2H5
OH
CH3
9 Q N H C I
S ( CH3
OH
/ 0N0 H C I
S
OH
11 0NN-CH3
2HCI
O H 0
0 ~\ - 0
12 N N
=HCI 0
~20 2 0 2
- 43 -
Table 3
No. Compound
OH CF3
13 0
S
OH
0 ,/~
14 c o \~ / HC I
S N
C2H5
OH
15 Q N ~CH3
/ / ~~~ \ H C I
Q CH3
OH
16 NH2 1/ 2 CO2H
HO2C
OH
17 Q /c2H5
/ \/\ N H C I
0 C2H5
OH
18 0N N
0
2HCI
'J
- 44 22 02U.~2
-
Table 4
No. Compound
OH
19 0-'~ NC2H5
=HCI
C2H5
OH
CH
20 0\/\ N 3
~
HCI
CH3
OH
21 H 3 C IOAI," 0N3
HCI
CH3
OH
0 CH3
2 2 ~ I \~\ N H C I
\
F CH3
OH
0 N,CH3
23 CH3
H3 HCI
a C
OH
0 \/\
24 H \ ~ \
.HCI
22 02U32
- 45 -
Table 5
No. Compound
CH3 OH
0 C2H5
2 5 . N H C
S C2H5
CI OH
2 6 N H C
S C2H5
~ ~ OH
27 0~~ C2H5
N HC I
S C2H5
OH
0/C2H5
28 N H C I
S OCH3 C2H5
OH
29 ON/C2H5
HCI
S F c 2H5
OH
0 NC2H5
H C
S ~ H C2H5
- 46 - 220 2,('J,32
Table 6
No. Compound
Br OH
31 0"~~ zs
N HCI
CH
S 2 5
OH
0 CzHS
32 N HC I
S \ r \C2H5
B
OH
33 0 N = H C I
F
OH
34 0 N0 H C I
S
F
F OH
35 0N'02H5 1/ 2 CO2H
~ 2 H 5
I
C I
S F HO2C
OH H
0 N
36 2HCI
N
I
H
OH
0
37 HC I
S \ N
1ZL,~JS~
- 47 -
Table 7
Compound No. Melting point
( C)
1 120-120.5
2 119.5-120.5
3 191.5-192.5
4 180-180.5
134-137.5
6 143.5-145
9 207.5-210
166.5-167.5
11 232-234
12 191.5-193
13 199-202
14 163-169
168-169.5
16 170-173
17 109-110
18 234-234.5
19 155.5-157
184-185
21 165-166
22 171-172
23 223-225
24 157-161
22U2~J52
- 48 -
Test cell
PC12 cell [rat adrenal medullary xanthoma (NGF-
responding cell)]
Test medium
RPMI 1640 (mfd. by Nissui pharmaceutical Co.,
Ltd.) supplemented with 10% heat-inactivated (56 C, 30
min.) horse serum (Summit Biotechnology Inc.), 5% heat-
inactivated (56 C, 30 min.) fetal calf serum (mfd. by
Gibco Inc.) and 60 g/ml of Kanamycine sulfate is used.
Test method
PC12 cells are adjusted at a density of 8x103
cells/ml with the above culture medium and plated out
into 6-well plate (mfd. by Falcon Inc.) in a portion of 2
ml/well. Subsequently,.2.5S-NGF (mfd. by Wako Inc.)
dissolved in 0.1% bovine serum albumin/phosphate-buffered
saline solution, is added at a final concentration of 100
ng/ml and the test compounds are added at final
concentration of 10-5M at the same time. Cells are
incubated at 37 C in a humidified incubator with 5% COZ
atmosphere. On the 5th day after the treatment of the
test compounds, cells from three randomly chosen phase
contrast microscope fields are counted. The proportion
of cells bearing neutrites longer than cell body to the
other cells is determined. Besides, the proportion of
the control group free from the test compound is taken as
100%. The results are shown in Table 8.
r.
220
- 49 -
Table 8
Compound Concentration of Nervous process
No. test compound elongation
added (M) activity (~)
1 10-5 136
2 10-5 129
3 10-5 123
4 10-5 117
10-5 121
6 10-5 113
7 10-5 125
8 10-5 130
9 10-5 123
10-5 131
11 10-5 112
12 10-5 124
13 10'5 118
14 10-5 126
10-5 116
16 10-5 123
17 10-5 113
18 10-5 121
19 10-5 112
10-5 124
21 10-5 114
22 10-5 112
23 10-5 112
22 020)2
- 50 -
Table 8 (Cont'd)
Compound Concentration of Nervous process
No. test compound elongation
added (M) activity (%)
24 10-5 110
25 10'5 111
26 10-5 112
27 10-6 125
28 10'S 127
29 10-5 116
30 10-5 124
31 10'5 111
32 10-5 112
33 10-6 111
34 10-5 116
35 10-5 110
36 10-5 124
37 10-5 117
Best Mode for Carrying out the Invention
Preparation Example 1 (Tablet)
Tablets each containing 50 mg of 2-[2-(N,N-di-
ethylamino)ethoxy]-1-(benzo[b]thiophen-5-yl)ethanol
hydrochloride (Compound No. 1) are prepared by the
following method using the following recipe:
CA 02202032 2004-09-15
- 51 -
Per one tablet:
Compound No. 1 compound 50 mg
Milk sugar 20 mg
TM
Kollidon CL (mfd. by BASF) 15 mg (1)
Corn starch 30 mg
TM
Avicel (mfd. by Asahi Chemical) 50 mg
Polyvinylpyrrolidone K-90 5 mg
Light silicic acid anhydride 18 mg (2)
Magnesium stearate 2 mg
Total 175 mg
A mixture of the components (1) is kneaded with
8% aqueous solution of polyvinylpyrrolidone K-90, and
dried at 60 C, and thereafter, mixed with the components
(2), after which the resulting mixture is tableted into
circular tablets each having a diameter of 8 mm and a
weight of 175 mg.
Preparation Example 2 (Capsule)
Capsules each containing 50 mg of 2-[2-(N,N-
diethylamino)ethoxy]-1-(benzo[b]thiophen-5-yl)ethanol
hydrochloride (Compound No. 1) are prepared by the
following method using the following recipe:
Per one capsule:
Compound No. 1 compound 50 mg
Milk sugar 20 mg (1)
Corn starch 53 mg
TM
Kollidon CL (mfd. by BASF) 2 mg
CA 02202032 2006-09-05
- 52 -
Polyvinylpyrrolidone K-90 5 mg
Avicel PH302 (mfd. by Asahi Chemical) 18 mg (2)
Magnesium stearate 2 mg
Total 150 mg
A mixture of the components (1) is kneaded with
8% aqueous solution of polyvinylpyrrolidone K-90, and the
resulting mixture is dried at 60 C and then mixed with
the components (2). No. 3 gelatine capsules are filled
with the resulting mixture in a proportion of 150 mg per.
one capsule to obtain capsules.
Utilizability in Industry
The 1,2-ethanediol derivative represented by
the general formula [Ij or its salt has a NGF activity-
potentiating effect, and is useful as a remedy for
various diseases caused by degeneration of the peripheral
nervous system such as senile dementia of Alzheimer type,
Huntington's chorea, various neuropathies, Riley-Day
syndrome, traumatic nerve injury, amyotrophic lateral
sclerosis (ALS) and the like.