Note: Descriptions are shown in the official language in which they were submitted.
CA 02202141 1997-04-08
~ ~3P~
CELLULOSE FIBRE
The present invention is concerned with a new cellulose fibre
and a process for the production of this fibre.
As an alternative to the viscose process, in recent years
there has been described a number of processes wherein
cellulose, without forming a derivative, is dissolved in an
organic solvent, a combination of an organic solvent and an
inorganic salt, or in aqueous saline solutions. Cellulose
fibres made from such solutions have received by BISFA (The
International Bureau for the Standardisation of man made
Fibres) the generic name Lyocell. As Lyocell, BISFA defines a
cellulose fibre obtained by a spinning process from an
organic solvent. By "organic solvent", sIsFA understands a
mixture of an organic chemical and water.
So far, however, only one process for the production of a
cellulose fibre of the Lyocell type has achieved industrial-
scale realization. In this process, in the following referred
to as amine-oxide process, a tertiary amine-oxide,
particularly N-methylmorpholine-N-oxide (NMMO), is used as a
solvent. Such a process is described for instance in US-A -
4,246,221 and provides fibres which exhibit a high tensile
strength, a high wet-modulus and a high loop strength.
A typical feature of the Lyocell fibres is their pronounced
tendency to fibrillate when wet. Fibrillation means the
breaking off of the fibre in longitudinal direction at
mechanical stress in a wet condition, so that the fibre gets
hairy, furry. The reason for fibrillation may be that the
fibres consist of fibrils which are arranged in the
longitudinal direction of the fibre axis and that there is
only little crosslinking between theseO
WO 92/14871 describes a process for the production of a fibre
having a reduced tendency to fibrillation. The reduced
tendency to fibrillation is attained by providing all the
CA 02202141 1997-04-OX
baths with which the fibre is contacted before the first
drying with a ~imllm pH value of 8,5.
Wo 92/07124 also describes a process for the production of a
fibre having a reduced tendency to fibrillation, according to
which the freshly spun, i.e.not dried, fibre is treated with
a polymer which can be cationized. As such a polymer, a
polymer having imidazole and azetidine groups is mentioned.
Additionally, there may be carried out a treatment with an
emulsifiable polymer, such as polyethylene or
polyvinylacetate, or a crosslinking with glyoxal.
In a lecture given by S. Mortimer at the CELLUCON conference
in 1993 in Lund, Sweden, it was mentioned that the tendency
to fibrillation rises as drawing is increased.
Moreover, the following methods to reduce the tendency to
fibrillation of Lyocell fibres have been published :
For instance, from WO 95/02082 of the applicant it is known
that fibrillation may be reduced by certain combinations of
spinning parameters.
Moreover, it is known that the fibrillation properties of
Lyocell fibres may be improved by chemical crosslinking.
Thus, e.g. EP-A - 0 538 977 describes crosslinking of Lyocell
fibres with chemical reagents able to react with cellulose in
a state before any drying, i.e. when the fibre is produced,
as well as in a dried state, i.e. substantially during the
textile finish of the plane fibre assemblies.
The crosslinking reagents exemplified in the above patent
application EP-A - 0 538 977 exhibit as groups capable of
crosslinking halogen-substituted, nitrogen-cont~;n;ng ring
structures able to react with the hydroxyl groups of the
cellulose in alkaline conditions. Moreover, compounds
comprising vinyl sulphone groups or their precursors are
described. These compounds substantially also react only when
CA 02202141 1997-04-08
alkali is added, or they require alkali as a neutralisation
reagent for cleaved acids.
A drawback consists in that when the halogenated, nitrogen-
cont~; n; ng rings or the vinyl sulphons and their precursor
substances respectively are reacted, salts are formed which
afterwards have to be washed out of the fibre. Moreover, also
excess residual chemicals not reacted with the cellulose have
to be washed out. This means that in a continuous fibre
production process, another post-treatment step is necessary,
causing further investment and operating costs and creating
additional problems with contaminated waste water.
In W0 94/24343 of the applicant, similar processes for
crosslinking Lyocell fibres to reduce fibrillation are
proposed describing the use of alkali buffers and an exposure
to electromagnetical waves as particularly advantageous.
Wo 94/20656 describes a reduction of the fibrillation of
Lyocell fibres by means of crosslinking using conventional
crosslinking chemicals usually employed to improve crease
angles of cellulose textiles, while a simultaneous reduction
of the dye absorption does not occur, when the crosslinking
is carried out in the simultaneous presence of flexible,
linear polymers. Substantially, conventional N-methylol
resins (cont~;ning a low formaldehyde level) and the usual
acidic catalysts are used. This method is described as
efficient for use on the dried as well as the never dried
fibre.
From US-A- 3,251,642 it is known that treating the fibres of
regenerated cellulose, such as viscose, or cotton,
particularly treating the textiles made from these fibres,
with sulphatoalkyl sulphonium salts leads to an improvement
of the recuperative capacity of the wet and dry crease angles
due to the crosslinking of the regenerated cellulose.
Therefore these salts are employed as so-called non-creasing
finishing agents. Furthermore, it is known that in strong
- CA 02202141 1997-04-08
alkaline conditions, the reaction of cotton with
sulphatoalkyl sulphonium salts, particularly the inner salt
of disodium-tris(~-sulphatoethyl)sulphonium (in the
following, this inner salt will be abbreviated by trisSS),
takes place already at room temperature.
Treatment of polymers cont~; n; ng reactive NH-, OH- or SH
groups with a sulphonium salt such as trisSS and a base is
described in the Belgian patent applications nos. 627 220 and
no. 640 713, as well as in the British patent applications
nos. 988,511, 1,047,323 and 1,059,568 and in US-A -
3,251,642. The crosslinking of cellulose textile materials
with trissS is described in US-A - 3,480,382 and in US-A -
3,542,503. Further, it is known from GB-A - 1,082,600 to use
trisSS in combination with dyes to crosslink cellulose during
a dyeing process.
In the literature cited above, dried substrates such as
fibres, textiles, films and paper are impregnated with
aqueous 5 - Z5~ solutions of the crosslinking agent and 4 -
35% aqueous solutions of alkali, either treating first with
the crosslinking agent and afterwards with alkali or
inversely treating first with alkali and thereafter with the
crosslinking agent.
When potentially alkali compounds such as alkali metal
bicarbonates are used, the crosslinking agent may be applied
with the alkali from a bath, and afterwards a treatment for
crosslinking may be carried out at an elevated temperature.
Moreover, between treatment with the crosslinking agent and
treatment with alkali, an intermediate drying may be carried
out. As alkali compounds, alkali metal hydroxides, alkali
metal carbonates and bicarbonates and quaternary ammonium
bases such as trimethylammonium hydroxide are used. As
reaction times, 10 minutes at room temperature and shorter
residence times at a higher temperature, e.g. 3 minutes at
150C, are indicated. A~ter the reaction, the alkali has to
be washed out of the substrate.
CA 02202141 1997-04-08
In the state of the art, no process is known whereby the
tendency to fibrillation of fibres produced according to the
amine-oxide process may be efficiently controlled. Thus it is
the object of the present invention to provide a process for
the production of Lyocell fibres whereby fibres having a
predetermined tendency ~o fibrillation may be produced.
The process according to the invention for the production of
cellulose fibres according to the amine-oxide process,
wherein a solution of cellulose in an aqueous tertiary amine-
oxide is spun to fibres and the fibres are contacted with a
crosslinking agent, is characterized in that as a
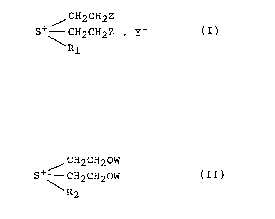
crosslinking agent a sulphonium compound of the formula (I)
CH2 CH2 Z
S ~ CH2CH2Z . Y (I)
Rl
wherein R1 is CH2CH2Z, alkyl, particularly C1-C4alkyl, aryl,
substituted alkyl or a hetero ring, Z is halogen, sulphato,
thiosulphato, phosphato or hydroxy, and Y- is an anion,
and/or a sulphonium compound of the formula (II)
/ CH2CH20W
S+ - CH2CH2OW (II)
R2
wherein OW is the residue of a polybasic acid in the form of
salt or acid and R2 is CH2CH20W, alkyl, particularly C1-
C4alkyl, aryl, substituted alkyl or a hetero ring,
is used.
Surprisingly, it has been shown that by treating fibres
produced according to the amine-oxide process with the above
sulphonium compounds, it becomes possible not only to reduce
the tendency to fibrillation of the fibres, but to
efficiently control it.
CA 02202141 1997-04-08
--6--
In the process according to the invention, an inner salt of
dialkali-tris-(~-sulphatoethyl)sulphonium, particularly the
inner salt of disodium-tris-(~-sulphatoethyl)sulphonium, is
preferably used as the sulphonium compound of formula (II).
According to the process according to the invention, the
fibres may be contacted with the crosslinking agent in a
never dried state as well as in a dried state. It is
preferred however to contact the fibres with the crosslinking
agent in a never dried state, e.g. when they are freshly
spun.
Preferably, the fibres are treated with a base after having
been contacted with the crosslinking agent. The fibres may be
dried before treatment with the base.
A particularly advantageous embodiment of the process
according to the invention consists in that the fibres are
treated with a base and simultaneously are contacted with the
crosslinking agent, base and crosslinking agent being present
in a mol ratio of substantially 3 : 1 (base:crosslinking
agent). Subsequently, the fibres are dried. It has been shown
that in this embodiment of the process according to the
invention, no washing of the fibres is required. This is
advantageous insofar as it allows to eliminate a step in the
production process.
The fibres are best contacted with an aqueous solution
containing trisSS in a concentration of from 0,3 to 25% by
mass, preferably 0,5 to 5% by mass. The base is contained in
the aqueous solution in 0,8 to 100 molar amounts, preferably
in 2,5 to 3,5 molar amounts, based on trisSS. As bases,
particularly alkali metal hydroxides, alkali metal carbonates
and bicarbonates, quaternary ammonium bases and polymer
amines are used. Further it was found that according to the
invention, also buffer solutions having a pH in the range of
from 5 to 12, preferably of from 7 to 9, may be employed.
CA 02202141 1997-04-08
Washing also will not be necessary when a potentially
alkaline compound such as a hydrogen carbonate is used as a
base.
The invention is also concerned with cellulose fibres having
a predetermined tendency to fibrillation which may be
obtained according to the process according to the invention.
Further, the invention is concerned with the use of a
sulphonium compound of the formula tI)
CH2 CH2 Z
S ~ CH2CH2Z . Y (I)
Rl
wherein R1, z and Y- have the meaning indicated above, and/or
a sulphonium compound of the formula (II)
CH2 CH20W
S+ - CH2CH2OW (II)
R2
wherein OW and R2 have the meaning indicated in Claim 1,
for treating fibres produced according to the amine-oxide
process and threads and textiles of fibres produced according
to the amine-oxide process.
The invention is further concerned with the use of an inner
salt of dialkali-tris(~-sulphatoethyl)sulphonium for treating
fibres produced according to the amine-oxide process and
threads and textiles of fibres produced according to the
amine-oxide process.
It is particularly appropriate to use an aqueous solution
containing a base and an inner salt of dialkali-tris(~-
sulphatoethyl)sulphonium, base and crosslinking agent being
CA 02202141 1997-04-08
present in a mol ratio of substantially 3:1
(base:crosslinking agent) for treating fibres produced
according to the amine-oxide process and threads and textiles
of fibres produced according to the amine-oxide process.
The freshly spun, never dried fibre strand or the never dried
fleeze (staple fibre) may first be impregnated with a
solution of trisSS and after squeezing to a moisture content
of 100 to 150% be impregnated with the base solution. The
order of the trea~ment may also be reversed. After a
residence time of approximately lo minutes at room
temperature, it may be washed and dried.
Furthermore it is possible to first impregnate the fibre with
a solution of trisSS and after squeezing to a moisture
content of 100 to 150~ dry the fibre and carry out the
treatment with the base immediately afterwards or later.
Moreover, the process according to the invention may be
carried out in a single step, treating the ~ibre
simultaneously with the solution of trisSS and small amounts
of base in a single-skep process. Surprisingly, it has been
found that for the single-step process not only potentially
alkaline compounds such as alkali metal bicarbonates may be
used, but also alkali in an appropriately low concentration
may be used.
After the single-step process, a temperature treatment of the
impregnated fibre is necessary. The temperature treatment may
be attained by drying at temperatures of at least 60C. When
the mol ratio of base to trisSS is not higher than 3 : 1, a
subsequent washing will not be necessary.
It has been shown that when the above sulphonium compounds
are used, an efficient reduction or even elimination of
fibrillation may be obtained by means of the reaction
conditions (concentration of crosslinking agent, amount and
type of the bases used).
CA 02202141 1997-04-08
It has also been shown that the treatment with the
crosslinking agent according to the invention may be carried
out before, simultaneously with or after a dyeing process.
For instance, the indicated sulphonium compounds may be added
to an alkaline dyeing bath.
By means of the following Examples, the invention will be
explained in more detail. For the production of trisSs,
reference to the Belgian patent applications nos. 627 204 and
no. 620 775 is made.
Evaluation of fibrillation
To evaluate the tendency to fibrillation, 8 fibres each
having a length of 20 mm were put into a 20 ml test bottle
with 4 ml of water and shaken during 9 hours at stage 12 in a
laboratoLy r"echanical shaker OL ~he RO-iû t-ype, lllade by the
company Gerhardt, Bonn (Germany). Afterwards, the
fibrillation behaviour of the fibres was evaluated under the
microscope by means of counting the fibrils per û,276 mm of
fibre length.
According to this test, a conventional cellulose fibre of the
Lyocell type exhibits approximately 5û fibrils per û,276 mm
of fibre length. A conventional cellulose fibre of the Modal
type which has no tendency to fibrillation as is known was
used as a comparative Example exhibited 1 to 2 fibrils.
Example 1 - procedure A
1 g of freshly spun, not yet dried Lyocell fibres as a staple
fleeze were impregnated with 100 ml of an aqueous solution of
trisSS for 3 minutes at room temperature, squeezed to a water
content of 140% and afterwards impregnated with lOû ml of an
aqueous sodium hydroxide, i.e. an aqueous solution of sodium
hydroxide, for 10 minutes at room temperature. Afterwards the
. CA 02202141 1997-04-08
--10--
alkali was washed out of the fibres by means of 3% acetic
acid and water, and the fibres were dried over night at 60C.
Thereafter, the tendency to fibrillation of the fibres was
analyzed according to the test described above. The results
are shown below in Table 1.
Example 1 - procedure B
1 g of freshly spun, not yet dried Lyocell fibres as a staple
fleeze were impregnated with 100 ml o~ an a~ueous solution of
trisSS for 3 minutes at room temperature, dried for 2 hours
at 60C and afterwards impregnated with 100 ml of an aqueous
sodium hydroxide for 10 minutes at room temperature.
Afterwards the alkali was washed out of the fibres by means
of 3% acetic acid and water, and the fibres were dried over
night at 60C.
Thereafter, the tendency to fibrillation of the fibres was
analyzed according to the test described above. The results
are shown below in Table 1.
In Table 1, the concentration of trisSS is indicated in g/l.
The concentration of the aqueous sodium hydroxide is
indicated in g of NaOH/l.
Table 1
Example trisSS Aqueous sodium Procedure ~umber of
no. concentration hydroxide fibrils
concentration
1 - - - 50
2 1 0,16 A 50
3 50 11,6 A 20
4 50 11,6 B 10
2,22 B 45
6 10 4,44 B 45
7 10 44,4 B 17
8 10 88,8 B 6
CA 02202141 1997-04-08
Examples 5 to 8 show that as the aqueous sodium hydroxide
concentration increases, the number of fibrils decreases,
i.e. that the fibre produced has a reduced tendency to
fibrillation. Thus, when trisSS is used according to the
invention, it is possible to control the tendency to
fibrillation of the Lyocell fibre via the alkali
concentration. Naturally, when bigger amounts of alkali are
employed it is necessary to wash out the alkali of the
fibres.
Identical results were obtained with a fibre strand.
Example 2 - procedures C1, C2, D1 and D2
1 g of freshly spun, not yet dried Lyocell fibres as a staple
fleeze were impregnated with 100 ml of an aqueous solution of
trisSS and a base (TBAH (tetrabutylammonium hydroxide), NaOH
or KHCO3) for 5 minutes at room temperature and squeezed to a
water content of 140%. Afterwards, the fibre was either;
dried at 60C over night, washed and dried again (=
procedure C1); or
dried at 60C over night and not washed (= procedure
C2); or
dried at 100C for 10 minutes, washed and dried again
(procedure D1); or
dried at 100C for 10 minutes and not washed
(=procedure D2).
Then the tendency to fibrillation of the fibres was analyzed
according to the test described above. The results are shown
below in Table 2. In Table 2, the trisSS concentration is
indicated in g/l. The concentration of the base is indicated
in g/l.
Table 2
Example trisSS Base Procedure Number of
no. concentration concentration fibrils
1 - - - 50
9 15 0,34 TBAH D1 50
CA 02202141 1997-04-08
-
-12-
150,34 TBAH D2 50
11 1525,7 TBAH Cl 20
12 1525,7 TBAH C2 20
13 1525,7 TBAH D1 20
14 1525,7 TBAH D2 25
154,5 NaOH Cloccasional
16 154,5 NaOH C2 0
17 154,4 NaOH Cloccasional
18 15 4,4 NaOH C2occasional
19 15 3,5 NaOH C1 33
3,5 NaOH C2 35
21 15 3,5 NaOH Dl 32
22 15 3,5 NaOH D2 30
23 10 2,7 NaOH Dl 0
24 10 2,7 NaOH D2 0
79,0 KHCO3 C1 20
26 15 79,0 KHCO3 C2 35
27 15 79,0 KHC03 Dl 20
28 15 79,0 KHCO3 D2 35
From Examples 9 to 24, the influence of the base
concentration on the reduction of fibrillation can be seen.
Already at a mol ratio of base to crosslinking agent of 3 :
1, fibrillation is eliminated when NaOH is used as the alkali
component (see Examples 23, 24), and it is not necessary to
use higher amounts of NaOH (see Examples 15-18).
At a mol ratio of 2,7 : 1 (Examples 10-22), fibrillation is
reduced by 40%.
When TBAOH is used in a ratio of 3 : 1 to the crosslinking
agent, fibrillation is reduced by 50%.
Moreover, it can be seen from the Examples that when reduced
amounts of alkali (e.g. 2,5 to 3,5 mol of base per 1 mol of
crosslinking agent) are used, washing out is not necessary.
The same results were obtained with a fibre strand.
CA 02202141 1997-04-08
-13-
Furthermore, it has been shown that the treatment of the
fibres with the above sulphonium compounds according to the
invention actually has no negative effect on its textile
parameters such as strength, elongation etc.