Note: Descriptions are shown in the official language in which they were submitted.
CA 02203526 2006-09-22
CATHETER
Technical Field
This invention relates to a disposable catheter for temporary insertion into
the
body, for example, through a venepuncture, and more specifically, to a
diagnostic elec-
trophysiology catheter which can diagnose underlying cardiac arrhythmias and
can treat
the diagnosed arrhythmias by stimulating the human heart with pacing,
cardioversion,
defibrillation, and/or radiofrequency ablation energies.
Background Art
The human heart is a complex muscular organ which is responsible for pumping
blood throughout the body. The heart contains four chambers, namely, the right
atrium,
the right ventricle, the left atrium, and the left ventricle. Blood enters the
right
chambers of the heart from veins all over the body, is pumped into the lungs
where it
becomes saturated with oxygen, and then is pumped into the left ventricle
where it is
pumped throughout the body.
To accomplish this task, the human heart has "pacemaker cells" which are
respon-
sible for the rhythmic contraction of the heart. These pacemaker cells are
made of
nerve bundles and fibers and consist of several nodes and branches. The atrial
contrac-
tions are governed by the sino-atrial node. Nerve tracts from the sino-atrial
node extend
to the atrial-ventricular node at the base of the right atrium. The sino-
atrial node and its
corresponding nerve tracts are responsible for rhythmic atrial contractions.
Large nerve
tracts extend from the atrial-ventricular node to the ventricular chambers of
the heart.
These tracts are called the right and left bundle branches. These nerve fibers
extend
1
W 0 97/07835 CA 0 2 2 0 3 5 2 6 19 9 7- 0 4- 2 3 PCTIUS96/13969
' throughout the ventricles and are responsible for synchronous ventricular
contractions.
Occasionally, abnormal heart rhythms or contractions can occur due to other
cells acting
as pacemaker cells or blocking the accessory conduction pathways. These
irregular
heart rhythms or arrhythmias can be lethal, resulting in strokes and cardiac
arrest. Ar-
rhythmias can originate in the atrium resulting in atrial tachyarrhythmias
and/or atrial
fibrillation which may be a precursor to strokes. Ventricular tachyarrhythmias
may
result in minimal blood pumping through the heart and may possibly lead to
cardiac
arrest or ventricular fibrillation, both which can be fatal.
Electrophysiological (EP) testing is typically used to diagnose arrhythmias in
a
patient and this testing involves the passing of several diagnostic EP
catheters into the
right side of the heart. These EP catheters typically include several sensing
rings and a
distal tip electrode for the delivery of low energy pacing pulses directly to
the heart.
The electrical signals of the heart are sensed with the ring electrodes and
once an aber-
rant pathway is determined, low energy pacing pulses are applied to the heart
tissue at
various intervals or frequencies in order to induce or start the arrhythmia.
Once the
arrhythmia is induced, it must be terminated and this termination of the
arrhythmia is
typically accomplished by delivering a high energy defibrillation shock across
the
patient's chest (transthoracic shocks) with paddles. A specific very high
voltage or
voltage gradient, namely, a therapeutic voltage, is required within the heart
to terminate
the induced arrhythmias. Since the thoracic cavity and the skin dissipate much
of the
applied voltage through resistance, the voltage delivered through a
transthoracic
defibrillator paddle is substantially greater than the necessary therapeutic
voltage in order
to compensate for the energy losses through the skin and the thoracic cavity
and to
ensure that the necessary therapeutic voltage does reach the heart.
Unfortunately, the
delivery of these external shocks are painful to the patient because these
external shocks
produce shin burns and, since these external shocks are not direct applied to
the heart, a
misalignment of the paddles may also result in insufficient voltage reaching
the heart
which may necessitate the need for yet additional, painful, external shocks.
Once the presence of arrhythmia has been determined, it can be treated and one
30 method of treating the arrhythmia is an arrhythmia control system. Early
arrhythmia
control systems employed two different sets of electrodes, one set to sense
the onset of
arrhythmia and a second set to treat the arrhythmia. The first set of
electrodes were
2
CA 02203526 2006-09-22
known as rate sensing electrodes while the second set of electrodes were known
as epi-
cardial patch electrodes. Both the rate sensing and epicardial patch
electrodes were
anchored directly onto the outer surface of the heart and both electrodes
required a
major surgery under general anesthesia in order to insert into a patient.
For this reason, epicardial patch electrodes were replaced with transvenous
cathe-
ters which combined the functions of both the rate sensing and epicardial
electrodes into
one catheter having a long tubular body and a plurality of electrodes on a
distal portion.
With a transvenous electrode, no major surgery with general anesthesia was
required.
Instead, a doctor inserted the distal portion of the catheter into a patient's
vein and then
torqued and pushed a substantial segment of the tubular body into the vein so
that the
distal portion of the catheter containing the electrodes was guided along the
vein and
precisely located within the patient's heart for sensing and then treating the
arrhythmia.
An example of a current transvenous catheter is U.S. Patent No.
5,005,587 for its teaching on an endocardial defibrillation and pacing
catheter.
This endocardial defibrillation and pacing catheter includes an elongated
hollow polyurethane tube with a proximal portion and a distal portion wherein
the
distal portion includes a distal tip electrode, a plurality of ring electrode,
and a surface
braid electrode. Although providing. good stiffness and biocompatability, the
polyurethane tube lacked sufficient translation between the rotational and
displacement
forces applied along the body of the polyurethane tube and the corresponding
rotational
and displacement forces resulting in the distal portion. Instead, only a small
fraction of
the rotational and displacement forces provided along the body of the
polyurethane tube
was translated to rotational and displacement forces at the distal portion.
The remain-
der, the majority of the rotational and displacement forces provided along the
body of
the polyurethane tube, merely caused an accumulation of stress in the
polyurethane tube.
If sufficient stress was accumulated, the polyurethane tube also tended to
buckle or
form helices and thereby not allowing for the distal portion to reach the
precise location
within the patient's heart.
Summary Of The Invention
With the foregoing in mind, it is an object of the present invention to
provide a
3
WO97/07835 CA 02203526 1997-04-23 PCTIUS96/13969
catheter which can-translate a substantial fraction of the rotational and
displacement
forces applied along the body of the catheter to rotational and displacement
forces at the
distal portion of the catheter.
It is another object of the present invention to provide a catheter which can
trans-
late a substantial fraction of the rotational and displacement forces applied
along the body of the catheter to rotational and displacement forces at the
distal portion of the
catheter and can also conduct electrical current between the proximal portion
and at least
one of the distal portion and the one or more exposed segments of the
catheter.
It is another object of the present invention to provide a catheter that is
flexible, is
of relatively small diameter, and can deliver current through one or more
exposed seg-
ments without the need for special connections, couplers, and/or separate
conductor
wires.
It is another object of the present invention to provide a flexible catheter
wherein
the one or more wires in the one or more exposed segments have a large
effective sur-
face area.
It is another object of the present invention to provide a transvenous
catheter pri-
marily for subcutaneous use which provides current to a precisely controlled
location.
It is yet another object of the present invention to provide a diagnostic EP
catheter
that can sense the heart, can apply pacing pulses to the heart, and can then
apply
cardioversion, defibrillation, and/or radiofrequency ablation energies to the
heart.
According to the invention, one or more of the foregoing objects are achieved
in a
catheter that includes a torque transmission assembly coupled to an elongate
body and an
electrical connector wherein the torque transmission assembly can transmit
torque and
electrical current along the elongate body.
In one exemplary embodiment, the elongate body includes a distal portion and
an
opposite proximal portion. The torque transmission assembly includes one or
more
wires extending along the elongate body from the proximal portion to the
distal portion
and one or more exposed segments along the elongate body. An electrical
connector is
coupled to the wires and these wires can conduct electrical current between
the electrical
connector and at least one of the distal portion and the one or more exposed
segments.
When inserted within a human body, these wires can also conduct electrical
current
between the electrical connector and the human body via the exposed wires in
the one or
4
WO 97/07835 CA 0 2 2 0 3 5 2 6 19 9 7- 0 4- 2 3 pCT/1JS96/13969
more exposed segments of the torque transmission assembly.
In another exemplary embodiment, the catheter includes a torque transmission
as-
sembly coupled to an elongate body wherein the elongate body includes a distal
portion
and an opposite proximal portion. The torque transmission assembly includes a
plurality
of first wires, a plurality of third wires, and one or more exposed segments
along the
elongate body. The plurality of third wires extends along the elongate body
and along
the third wires from the proximal portion to the distal portion. The plurality
of first
wires extends along the elongate body from the proximal portion to at least
one of the
distal portion and the one or more exposed segments. An electrical connector
is coupled
to the plurality of first wires and these first wires can conduct electrical
current between
the electrical connector and at least one of the distal portion and the one or
more ex-
posed segments. When inserted within a human body, the plurality of first
wires can
also conduct electrical current between the electrical connector and the human
body via
the exposed first wires in the one or more exposed segments.
In accordance with one aspect of the invention, the one or more wires of the
torque transmission assembly can be disposed along the elongate body in a
variety of
manners. For example, the one or more wires of the torque transmission
assembly may
be helically wound along the elongate body. Alternatively, the one or more
wires of the
torque transmission assembly may be braided along the elongate body.
Collectively, the
wires of the torque transmission assembly ensure that a substantial fraction
of the rota-
tional and displacement forces provided along the elongate body are translated
to rota-
tional and displacement forces at the distal portion. This allows a doctor to
easily push
the distal portion of the catheter along a patient's vein and to precisely
locate the distal
portion within the patient's heart. The wires of the torque transmission
assembly also
ensure that electrical current can flow between the electrical connector and
at least one
of the distal portion and the one or more exposed segments and, when inserted
within
the body, between the electrical connector and the body via the exposed wires
in the
exposed segments. This allows the catheter to be used in conjunction with, for
example,
an arrhythmia control system to provide cardioversion, defibrillation, and/or
radiofrequency ablation energies to a precise location within a human heart.
An advantage of the catheter according to the present invention over those
present-
ly in use is the ability to use different types of assemblies on the distal
portion. For
5
CA 02203526 2006-09-22
example, in the exemplary embodiment, the catheter has secured to the distal
portion or
the elongate body a pacing and sensing assembly which can sense and pace
precise
locations within the human heart. Alternatively, in, another exemplary
embodiment, the
catheter could also have secured to the distal portion of the elongate body a
floating
inflatable and deflatable balloon apparatus. This ability to use different
types of as-
semblies in conjunction with the catheter enhances the catheter's versatility
because the
catheter can be used in many more different types of treatments than those
catheters
presently in use.
According to an aspect of the invention there is provided a catheter for
temporary
insertion into a patient, the catheter comprising:
an elongate body including a distal portion and an opposite proximal portion
and having an
open region;
a torque transmission assembly coupled to the elongate body to transmit torque
along the
elongate body, the torque transmission assembly including at least one first
wire extending
along the elongate body, at least one segment of the torque transmission
assembly exposing
the first wire at the open region of the elongate body, and the torque
transmission assembly
having sufficient torque properties for ensuring that a substantial fraction
of the rotational
and displacement forces provided along the elongate body are translated to
rotational and
displacement forces at the distal portion of the elongate body such that the
catheter can be
inserted into a patient and located within the patient without a stylet to
guide the catheter; and
an electrical connector coupled to the at least one first wire whereby the
first wire can
conduct electrical current between the electrical connector and the at least
one exposed
segment of the torque transmission assembly.
According to another aspect of the invention there is provided a catheter for
temporary insertion into a patient, the catheter comprising:
an elongate body including a distal portion and an opposite proximal portion
and having an
open region;
a torque transmission assembly coupled to the elongate body to transmit torque
along the
elongate body, the torque transmission assembly including a plurality of first
wires and a
plurality of third wires, at least one segment of the torque transmission
assembly exposing
the plurality of first wires at the open region of the elongate body, the
plurality of third wires
extending along the elongate body from the proximal portion toward the distal
portion and
the plurality of first wires extending along the elongate body from the
proximal portion
6
CA 02203526 2006-09-22
toward the distal portion, and the torque transmission assembly having
sufficient torque
properties for ensuring that a substantial fraction of the rotational and
displacement forces
provided along the elongate body are translated to rotational and displacement
forces at the
distal portion of the elongate body such that the catheter can be inserted
into a patient and
located within the patient without a stylet to guide the catheter; and
an electrical connector coupled to the plurality of first wires whereby the
plurality of first
wires can conduct electrical current between the electrical connector and the
at least one
exposed segment of the torque transmission assembly.
Other objects and advantages of this invention will become apparent from the
detailed description that follows. It should be understood, however, that the
detailed
description and specific embodiments are provided for illustration only since
various
additions and modifications within the spirit of the invention will become
apparent to
those skilled in the art from this disclosure.
Brief Description Of The DrawinLs
FIGURE 1 is a cutaway view of a human heart with a catheter according to the
present invention implanted therein;
FIGURE 2 is a side view of a catheter according to the present invention;
FIGURE 3A is an enlarged cross-sectional view in the direction of line 3A-3A
of
the catheter shown in FIGURE 2;
FIGURE 3B is an enlarged cross-sectional view in the direction of line 3B-3B
of
the catheter shown in FIGURE 2;
FIGURE 4 is a cross-sectional view in the direction of line 4-4 of the
catheter
shown in FIGURE 3B;
FIGURE 5 is a cross-sectional view in the direction of line 5-5 of the
catheter
shown in FIGURE 3B;
FIGURE 6 is an enlarged cross-sectional view in the direction of line 6-6 of
the
catheter shown in FIGURE 2;
FIGURE 7 is a side view of another catheter according to the present invention
with a plurality of exposed segments; and
FIGURE 8 is an enlarged side view of an exposed segment of a catheter
according
6a
W O 97/07835 CA 0 2 2 0 3 5 2 6 19 9 7- 0 4- 2 3 PC'I1US96/13969
to the present invention with an alternating plurality of insulated and
noninsulated wires.
Descrintion of Preferred Embodiment(s)
FIGURE 1 is a cutaway view of the human heart in which a catheter according to
the teachings of the present invention has been implanted and used in
conjunction with a
control unit such as an external arrhythmia control unit 2. The external
arrhythmia
control unit 2 includes sensing and detecting circuitry, as well as
cardioverter, defibrilla-
tor, and ablation circuitry, the outputs of which are all coupled to a
catheter 4 of the
present invention. The' external arrhythmia control unit 2 senses an
arrhythmic condition
of the heart, and in response thereto, emits cardioverting, defibrillating,
and/or ablating
energies to the heart through the implanted catheter 4 according to the
teachings of the
present invention. The arrhythmia control unit 2 includes a plurality of
receptacles 6
and, coupled to the arrhythmia unit 2, via a plurality of electrical
connectors 8 is the
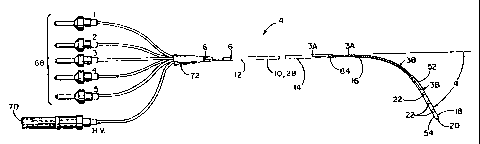
catheter 4 of the present invention. The catheter 4 includes an elongate body
10 which
has a proximal portion 12, an opposite distal portion 14, and at least one
exposed seg-
ment 16. A pacing and sensing assembly 18 may be attached to the distal
portion 14 of
the elongate body 10 and includes a tip electrode 20 and a plurality of ring
electrodes
22.
The catheter can be inserted intravenously through a variety of venipuncture
sites
including the femoral, the sub-clavian, the jugular, and the brachial vein
because of its'
very small diameter. For example, the catheter 4 shown in FIGURES 1-6 has a
diame-
ter of 6 French (F) or less and is typically inserted intravenously through a
venipuncture
site in the arm or leg. (The French scale is the scale used to denote the
sizes of cathe-
ters, each French scale being approximately equivalent to 0.33 millimeters in
diameter.)
After insertion into a vein, rotational and displacement forces provided along
the elon-
gate body 10 push the distal portion 14 of the catheter 4 and the pacing and
sensing
assembly 18 through the vein and into the heart. One or more radio-opaque
marker
bands 84 located near each exposed segment 16 then allows for the exact
placement of
the catheter 4 within the heart. For example, as illustrated in FIGURE 1, the
exposed
segment 16 can be positioned within the right ventricle 24 of the heart and
the tip and
ring electrodes 20, 22 can be positioned in the right ventricle apex 26 of the
heart. It
7
WC 97/07835 CA 02203526 1997-04-23 PCT/US96/13969
should also be appreciated that FIGURE 1 is only a single embodiment
illustrating a
specific use for the catheter of the present invention and that the catheter
can be used for
a broad range of different procedures and treatments, depending on the
specifications of
the catheter and the nature of the assembly secured to the distal portion.
In one of the most simple embodiments of the present invention, a catheter
accord-
ing to the teachings of this invention includes a torque transmission assembly
coupled to
an elongate body wherein the torque transmission assembly can transmit torque
along the
elongate body. The elongate body includes a distal portion and an opposite
proximal
portion. The torque transmission assembly includes one or more wires extending
along
the elongate body and one or more exposed segments along the elongate body.
The
torque transmission assembly ensures that a substantial fraction of the
rotational and
displacement forces provided along the elongate body are translated to
rotational and
displacement forces at the distal portion. An electrical connector is coupled
to the wires
and these wires can conduct electrical current between the electrical
connector and at
least one of the distal portion and the one or more exposed segments.
In other embodiments of the present invention, the torque transmission
assembly of
a catheter according to the teachings of this invention may include a
plurality of wires
extending along the elongate body from the proximal portion to the distal
portion; a
plurality of wires and a plurality of different insulation layers, both
extending along the
elongate body from the proximal portion to the distal portion; a plurality of
wires, each
plurality of wires being disposed though an insulation layer, and a plurality
of different
insulation layers, both the plurality of wires and the plurality of insulation
layers extend-
ing along the elongate body from the proximal portion to the distal portion;
and/or a
plurality of different wires, each wire in the plurality of wires being bare,
partially
insulated, or completely insulated, and a plurality of different insulation
layers, both the
plurality of wires and insulation layers extending along the elongate body
from the prox-
imal portion to the distal portion. In each embodiment, the torque
transmission assem-
bly of a catheter according to the teachings of this invention ensures that
(1) a substantial
fraction of the rotational and displacement forces provided along the elongate
body are
translated to rotational and displacement forces at the distal portion and
that (2) electrical
current can be conducted between the proximal portion and at least one of the
distal
portion and the one or more exposed segments in the elongate body.
8
W097/07835 CA 02203526 1997-04-23 PCTIUS96/13969
The torque transmission assembly of a catheter according to the present
invention
may have one or more exposed segments along the elongate body. As will be
discussed
more fully below, FIGURES 2-6 illustrate an embodiment of the present
invention
wherein the torque transmission assembly includes only one exposed segment 16
along
the elongated body. Alternatively, the torque transmission assembly may
include a
plurality of exposed segments 16, as illustrated in FIGURE 7.
An embodiment of a catheter 4 according to the teachings of this invention is
shown generally in FIGURES 2-6 where like numerals designate previously
described
elements. In this embodiment, the catheter 4 includes a torque transmission
assembly 28
coupled to the elongate body 10 wherein the torque transmission assembly can
transmit
torque along.the elongate body. The elongate body 10 includes a distal portion
12, an
opposite proximal portion 14, and one or more insulation layers while the
torque trans-
mission assembly 28 includes a plurality of different wires, each plurality of
wires pref-
erably being disposed through an insulation layer.
As best shown in FIGURES 3A and 4, the torque transmission assembly 28 of the
present invention includes a central region 30 that has a plurality of second
wires 32
extending from beyond the distal portion 14 to beyond the proximal portion 12.
A
second insulation layer 34 is disposed over the plurality of second wires 32
and along
the elongate body 10 from the distal portion 14 to the proximal portion 12 to
provide
electrical and environmental isolation for the plurality of second wires 32. A
plurality
of third wires 36 are disposed over the second insulation layer 34 and are
disposed along
the elongate body 10 from the distal portion 14 to the proximal portion 12.
The plurali-
ty of third wires 36 are preferably extruded through a fifth insulation layer
38 according
to any known fabrication process and the fifth insulation layer 38 extends
from the distal
portion 14 to the proximal portion 12. A first insulation layer 40 is disposed
over the
plurality of third wires 36 and along the elongate body 10 from the distal
portion 14 to
the proximal portion 12 to provide electrical and environmental isolation for
the plurality
of third wires 36. A plurality of first wires 42 are disposed over the first
insulation
layer 40 and are extruded through a fourth insulation layer 44 according to
any known
fabrication process. The plurality of first wires 42 are preferably disposed
along the
elongate body 10 from the proximal portion 12 to the distal portion 14 but may
be dis-
posed along a lesser length of the elongate body, i.e., from the proximal
portion 12 to
9
CA 02203526 1997-04-23
WO 97/07835 PCT/US96/13969
the exposed segment 16. The fourth insulation layer 44 extends from the distal
portion
14 to the proximal portion 12 but with the exception of the exposed segment 16
of the
torque transmission assembly 28 where one or more of the plurality of first
wires 42 are
bare and exposed to the outside environment. A third insulation layer 46 is
disposed
over the plurality of frrst wires 42 and along the elongate body 10 from the
distal por-
tion 14 to the proximal portion 12 with the exception of the exposed segment
16 of
torque transmission assembly 28 which exposes the one or more of the plurality
of first
wires 42 to the outside environment. The third insulation layer 46 also
provides the
catheter 4 with electrical and environmental isolation from the outside
environment.
In accordance with one or more aspects of the invention, the plurality of
different
wires of the torque transfer assembly can be disposed along the elongate body
in a vari-
ety of manners. For example, the plurality of different wires of the torque
transmission
assembly may be braided along the elongate body, as will be explained more
fully be-
low. Alternatively or additionally, as will be explained more fully below, the
plurality
of different wires of the torque transmission assembly may be helically wound
along the
elongate,body. Whether braided or helically wound, the plurality of wires of
the torque
transmission assembly ensures that a substantial fraction of the rotational
and displace-
ment forces provided along the elongate body are translated to rotational and
displace-
ment forces at the distal portion. This allows a doctor to easily push the
distal portion
of the catheter through a patient's vein and to precisely locate the distal
portion within
the patient's heart. The plurality of different wires of the torque
transmission assembly
also ensures that electrical current can flow between the electrical connector
and at least
one of the distal portion or the one or more exposed segments and, when
inserted within
the body, between the electrical connector and the body via the exposed wires
in the ex-
posed segments of the torque transmission assembly. This allows the catheter
to be used
in conjunction with, of example, an arrhythmia control system to provide
cardioversion,
defibrillation, and/or radiofrequency ablation energies to a precise location
within a
human heart.
In the embodiment of the catheter 4 illustrated in FIGURE 3A and 3B, the
torque
transmission assembly 28 includes the plurality of first wires 42 and the
plurality of third
wires 36 which are both helically wound along the elongate body 10 with a
pitch angle
between 1 degree and 179 degrees. In this application, the pitch angle means
the angle
W0 97/07835 CA 0 2 2 0 3 5 2 6 19 9 7- 0 4- 2 3 pCT/US96/13969
which is formed between the elongate body 10 and each wire in the plurality of
different
wires as the wire is being wound along the elongate body 10. Generally, a
pitch angle
of between 1 degree and 90 degrees is known in the art as a counter-clockwise
helical
rotation while a pitch angle of between 91 degrees and 179 degrees is known in
the art
as a clockwise helical rotation. The plurality of first wires 42 are comprised
of between
1 to 100 individual wires and, more preferably, between 20 to 60 individual
wires, with
each wire having a diameter in the range between 0.0005 inch to 0.020 inch
and, more
preferably, between 0.002 inch to 0.010 inch. The plurality of third wires 36
are also
comprised of between 1 to 100 wires and, more preferably, between 20 to 60
individual
wires, with each wire having a diameter in the range between 0.0005 inch and
0.020
inch and, more preferably, between 0.002 inch to 0.010 inch. In the embodiment
illustrated in FIGURES 2-6, the plurality of first wires 42 and the plurality
of third
wires 36 complete one revolution around the elongate body 10 in approximately
between
0.23 inch to 0.95 inch. The number of wires, the pitch angles, the diameter,
and the
material of the wires in the plurality of wires largely determines the torque
translation
characteristics of the catheter. Accordingly, by varying these criteria above,
a catheter
can be fabricated according to the teachings of this invention to have any one
of a wide
range of torque transmission characteristics.
In the embodiment illustrated in FIGURES 2-6, the plurality of first wires 42
and
the plurality of third wires 36 are typically any metal, polymer, composition,
or alloy
that is sufficiently conductive, has sufficient fatigue strength (flexibility)
and mechanical
strength (tensile and elongation properties), and is sufficiently
biocompatible and biost-
able in the human body to transmit torque and electrical current along the
elongate body
30. For example, in the catheter 4 shown in FIGURES 2-6, the plurality of
first wires
are preferably a titanium alloy but can also be titanium, platinum, and/or
platinum alloy.
The plurality of third wires may have many of the same characteristics as the
plurality of
first wires. However, in the embodiment shown in FIGURES 2-6, the plurality of
third
wires 36 are not coupled to an electrical connector so they may be less
conductive than
the.plurality of first wires 42. The plurality of the third wires 36 are
preferably stainless
steel. Typically, the plurality of first wires 42 and the plurality of third
wires 36 are
helically wound in the same direction, but can also be wound in opposite
directions.
The plurality of third wires 36 can also be separated into a plurality of
fourth wires 48
11
WO 97/07835 CA 02203526 1997-04-23
PCT/US96/13969
and a plurality of fifth wires 50 which can be wound in the same or opposite
directions
in different radii.
As an alternative or in addition to extruding the plurality of wires through
an
insulation layer, one or more of the individual wires in the plurality of
different wires of
the torque transmission assembly can be selectively and even partially
insulated along its' length depending on the configuration of the catheter.
For example, in an embodi-
ment of a catheter similar to the one illustrated in FIGURES 2-6, each wire in
the plu-
rality of first wires can be partially insulated dependant on the number of
exposed seg-
ments in the torque transmission assembly and the energy density required in
each ex-
posed segment. For example, the plurality of first wires may be divided into a
number
of groups which correspond directly with the number of exposed segments in the
torque
transmission assembly. Each group includes a number of first wires and each
group is
associated with a specific exposed segment. Each individual wire in each group
is insu-
lated from the proximal portion to the distal portion including all the
exposed segments
in the torque transmission assembly except for the associated exposed segment.
In the
exposed segment, the individual wires of the group are uninsulated or bare. If
a linear
decreasing energy density in the exposed segments from the proximal portion to
the
distal portion is preferred, then each group of first wires may have
approximately an
equal number of first wires. Alternatively, if a constant energy density
throughout all
the exposed segment in the catheter is preferred, then the group associated
with the ex-
posed segment closest to the proximal portion may have the fewest number of
first
wires, the groups associated with the exposed segments that are progressively
farther
away from the proximal portion may have progressively more first wires, and
the group
associated with the exposed segment farthest from the proximal portion may
have the
most of first wires. Similarly, one or more of the individual wires in the
plurality of
third wires can be either insulated or uninsulated dependant, for example, on
the number
of electrical connections that an assembly attached to the distal portion of
the elongate
body requires. If the assembly has a large plurality of signal, power, or
control lines
and/or does not have the plurality of second wires, then some or all of the
plurality of
third wires can be insulated and can extend beyond the distal portion so as to
provide the
necessary number of electrical connections between the assembly and any
apparatus
located beyond the proximal portion of the catheter.
12
W097/07835 CA 02203526 1997-04-23 PCTIUS96/13969
Alternatively, according to the teaching of this invention, the plurality of
wires
disposed within the exposed segments of the torque transmission assembly may
include
various sequences of insulated wires and non-insulated wires. For example, in
the em-
bodiment illustrated in FIGURE 8, the plurality of wires of the torque
transmission
assembly disposed in the exposed segment includes a first group 84 with a
clockwise
helical rotation and a second group 86 with a counter clockwise helical
rotation disposed
around the first group 84 wherein the first group 84 and the second group 86
both may
include an alternating plurality of insulated wires 88 and a plurality of non-
insulated
wires 90. Alternatively, the first group 84 and the second group 86 may also
include
any sequence of insulated and non-insulated wires. The plurality of insulated
wires 88
can be used for providing current to other exposed segments or to the assembly
secured
to the distal portion of the catheter. Similarly, the plurality of non-
insulated wires 90
can be used for the sensing of the heart, the delivery of energy to the heart,
or both the
sensing of and the delivery of energy to the heart. For example, if the
plurality of non-
insulated wires includes six individual non-insulated wires, then four of
these wires
could be used for the delivery of ablation energy to the heart while the
remaining two
wires could be used as sensing electrodes where one of the remaining wires is
of a first
polarity and the remaining wire is of opposite second polarity. As can be seen
from the
various embodiments, the plurality of wires in the torque transmission
assembly accord-
ing to the teaching of this invention may be used for the sensing of the
heart, the deliv-
ery of energy to the heart, both the sensing of and the transmission of energy
to the
heart, and/or the transmission of energy or signals to other exposed segments
and/or to
the assembly attached to the distal portion of the catheter.
An advantage of a catheter according to the teaching of the present invention
over
those presently in use is the ability to use different types of assemblies on
the distal
portion of the elongate portion. For example, a catheter according to the
teaching of
this invention could have secured to the distal portion a floating inflatable
and deflatable
balloon apparatus. Alternatively, as shown in FIGURE 2, the catheter 4 can
have se-
cured to the distal portion 14 of the elongate body 10 a pacing and sensing
assembly 18
which can sense the heart and apply pacing energy to precise locations within
the human
heart. The pacing and sensing assembly 18 has a proximal portion 52 and an
opposite
distal portion 54 and includes a ring electrode 20 disposed on the distal end
of the distal
13
W 0 97107835 CA 0 2 2 0 3 5 2 6 19 9 7- 0 4- 2 3 PCT/US96/13969
portion 54 and a plurality of ring electrodes 22 disposed in a positional
relationship with
the tip electrode 20. As best shown in FIGURE 3B, the proximal portion 52 of
the
pacing and sensing assembly 18 is secured to the distal portion 10 of the
catheter 4 by
the addition of medical adhesives to, the securing of, and the heat sealing
between a
cylindrical lip 56 of the pacing and sensing assembly 18 into a corresponding
aperture
58 formed between the third insulation layer 42 and the first insulation layer
40 of the
elongate body 10.
As best shown in FIGURES 3B and 5, the pacing and sensing assembly 18 in-
cludes a central region 62 that has the plurality of second wires 32 extending
from the
distal portion 54 to beyond the proximal portion 52, as will be explained more
fully
below. A first insulation layer 64 is disposed over the plurality of second
wires 32 from
the distal portion 54 to the proximal portion 52 and a second insulation layer
66 is dis-
posed over the first insulation layer 64 from the distal portion 54 to the
proximal portion
52. Collectively, the first and second insulation layers 64, 66 provide
electrical and
environmental isolation for the plurality of second wires 32 within the
central region 62
of the pacing and sensing assembly 18 from the outside environment. The
insulation
layers in the pacing and sensing assembly 18 and the elongate body 10 are
chosen from
any material that is biostable, biocompatible, flexible, inert, and is a good
insulator.
For example, the insulation layers in the catheter 4 shown in FIGURES 2-6 are
a
polyurethane. When attached to an arrhythmia control unit 2, the electrical
connectors
68, the plurality of second wires 68, and the tip and ring electrodes 20, 22
can sense the
heart for the onset of arrhythmia and can deliver pace energy to the heart to
induce
arrhythmia. Typically, as best shown in FIGURE 2, the distal portion 14 of the
catheter
4 and the pacing and sensing assembly 18 may also be pre-shaped into a curve
which
facilitates the placement of the distal portion 14 and the pacing and sensing
assembly 18
into the heart and/or within the vessels.
Each one of the plurality of second wires 32 electrically connects one of the
tip
and ring electrodes 20,22 in the pacing and sensing assembly 18 to a
corresponding one
of a plurality of connectors. For example, in the embodiment of the catheter 4
shown in
FIGURES 2-6, there are five electrodes in the pacing and sensing assembly 18
and
correspondingly five electrical connectors 68. Physically, the tip and ring
electrodes 20,
22 are electrically connected to the electrical connectors 68 by the plurality
of second
14
W O 97/07835 CA 0 2 2 0 3 5 2 6 19 9 7- 0 4- 2 3 pC'I1US96/13969
wires 32 which are disposed in the central regions 30, 62 of both the pacing
and sensing
assembly 18 and the elongate body 10. Typically, as best shown in FIGURES 4
and 5,
each of the plurality of second wires 32 is insulated to electrically isolate
each second
wire from the remaining second wires. According to the teachings of this
invention,
each group of first wires associated with each exposed segment in the torque
transmis-
sion assembly may be electrically connected to a high voltage connector. For
example,
in the embodiment of the catheter shown in FIGURES 2-6, there is only one
exposed
segment 16 and correspondingly only one high voltage connector 70. When
attached to
an arrhythmia control unit 2, the high voltage connector 70 and the plurality
of first
wires 42 in the exposed segment 16, along with another defibrillation
electrode (not
shown), provide the cardioversion, the defibrillation, and/or the
radiofrequency ablation
energies to the precise locations within the human heart.
As best shown in FIGURE 3B, the catheter 4 includes a crimp 60 which is dis-
posed over the last plurality of helical winds that the plurality of first
wires 42 and the
plurality of third wires 36 make at the distal portion 14. The crimp 60
ensures that the
helically wound plurality of first wires 42 and the helically wound plurality
of third
wires 36 at the distal =portion 14 will not subsequently become unwound.
Similarly, as
best shown in FIGURES 2 and 6, the catheter 4 includes a cover 72 disposed
over the
proximal end of proximal portion 12 of the elongate body 10. The cover 72
includes a
tubular portion 74 and a conical portion 76 which are separated by an annular
ring 78.
The proximal portion 12 of the elongate body 10 is disposed in tubular portion
74 so
that the proximal end abuts the annular ring 78. Since the tubular portion 74
has a
smaller diameter than that of the elongate body 10, this smaller diameter
secures the
cover 72 to the elongate body 10 while the annular ring 78 ensures that the
helically
wound plurality of first wires 42 and the helically wound plurality of third
wires 36 will
not become subsequently unwound. The annular ring 78 includes a central
aperture 80
through which the plurality of second wires 32 and the plurality of first
wires 42 are
disposed to electrically connect the tip electrode 20, the plurality of ring
electrodes 22,
and the exposed segment 16 to their respective electrical connectors 68, 70.
Once
through the central aperture 80 and into the conical portion 76, the plurality
of first
wires 42 are typically pressed or twisted together and also have an insulation
layer 82
disposed over their collective outer surface to electrically and physically
insulate these
CA 02203526 2006-09-22
wires from the outside environment.
Although the plurality of wires in the embodiment illustrated in FIGURES 2-6
were helically wound, a catheter according to the teaching of this invention
can have the
plurality of wires in the torque transmission assembly disposed along the
elongate body
in any manner. One practical manner is to braid (braided design) the plurality
of wires
along the elongate body while another practical manner is to weave (woven
design) the
plurality of wires along the elongate body. Typically a standard braid
according to this
invention consists of multiple wire groups. For example, a 1 x 16 braided
design has 1
wire for 16 groups, all interwoven together. Alternatively, a standard woven
design
according to this invention consists of multiple, multiple wire groups. For
example, a 4
x 16 woven design represents 16 groups of 4 wires. Both the braided design and
the
woven design are interwoven along a single diameter of the elongate body with
individu-
al wires or groups of wires undulating over and under individual or groups of
wires.
Typically, the woven design has fewer and smoother undulations because of the
greater
number of wires in the group whereas the braided design has more and sharper
undula-
tions because of the fewer number of wires in the groups. The number of wires
in each
group, the pitch angles of each group, the diameter of the wires in each
group, and the
material of the wires in each group largely determine the torque translation
characteris-
tics of the resulting catheter. Accordingly, by varying these criteria, a
catheter can be
fabricated according, to the teachings of this invention to have any one of a
wide range
of torque transmission characteristics.
16