Note: Descriptions are shown in the official language in which they were submitted.
~ CA 02204906 1997-05-08
NEUROTROPIIIN ANTAGONISTS
FIELD OF THE INVI~ON
The present invention relates to n~ulolluphin antagonists. In particular, the present
5 invention relates to nelllvLr~l)hin-derived peptides which inhibit or reduce lln-iesir?~ble
neulvllophin activity.
BACKGROUND OF THE INVENTION
A family of stmct~l~lly and functionally related n~ulu~uphic factors exist which are
10 collectively known as nt;urulluphins. The family of nt;;uluLrophins include the nerve growth
factor (NGF), brain-derived neuloll~hic factor (BDNF), neur~ ~hin-3 (NT-3), neurollu~hin-4
(NT-4) neurotrophin-5 (NT-5) and n~ulullophin-6 (NT-6).
The neurotrophins exhibit simila;r structural collf~ ion~, incln-ling three surface J3-
15 hairpin loops, a B-strand, an int~rn~l reverse turn region, and N- and C- t~rmini With respect
to sequence simil~riti~s~ the n~ur~ ~hins share a~pr~xi---~t~ly 50% amino acid identity. The
neuloll~hins are also functionally simil~r in that they each exhibit low af~mity binding to a
receptûr known as the "p75 nerve grûwth factor receptûr~ or p75NGFR. Each n~ulotl~phin also
exhibits binding to a receptor of the tyrûsine kinase (trk) family which is of higher affinity than
20 the binding to the p75 receptor. This interaction is believed to be related to neuron survival,
but is also involved with neuron dirfele~ lion inclll~in~ process form~tion The Trk receptor-
neurotrophin interaction has been found to be more selective than n~;ur~ ~hin int~r~cti~m with
the p75NGFR receptor. In particular, NGF binds only a trk receptor known as the TrkA receptor,
while BDNF, Nl-4 and NT-5 exhibit exclusive binding to a Trkl3 receptor. NT-3 is less
25 selective and, although it binds primarily with a TrkC receptor, it also exhibits some binding to
the TrkA and TrkB receptors abanez et al., EMBO J. 1993, 12:2281).
CA 02204906 1997-05-08
The neurotrophins function primarily to promote survival of certain classes of peripheral
and central neurons both during development and following neuronal damage. NG~, in
particular, is involved with the development of neurons in the peripheral nervous system and
supports neuronal survival, as well as enh~nrin~ and ..~ the dirre~ Pcl state of
5 neurons. However, in some neurological disease states, the nt;ulollu~hins may also support
ina~pl~opliate neurite oulgçvwlll thereby f~ci1it~tin~ the progression of a disease condition. For
example, neulu~ruph~ns promote the undesirable spluulillg of hippocampal "mossy fibres". Such
inal?~ro~?liate sprouting of mossy fibres is a common accomp~niment of epilepsy in hllm~n~.
In other pathological states, such as ~17hPimer's disease, aberrant process growth, known as
10 dystrophic neurite formation, is a strong correlate of disease severity.
Thus, although the n~;ur~ s are ess~nti~l for the normal development and growth
of neurons, they may be detrimenf~l under certain circnm~t~n~es. In such in~t~n~eS~ ligands
capable of inhihiting or redllçin~ selected n~uluLr~hin-mediated activities would be desirable
15 therapellti~lly to treat neurode~,elleld~ e disease and repair of nervous system injury.
SI~MMAR~ OF 1~ INVENTION
It is an object of the present invention to provide peptides capable of inhihitin~, or at
least reducing, lln~lesir~ble n~;ululluphin-me~ t~cl activity.
Accordingly, in one of its aspects the present invention provides bicyclic nt;u~ ~hin-
derived peptides, or functional equivalents thereof, which inhibit a nt;ulol-~o~hin-m~li~te~l
activity.
Another aspect of the present invention provides a peptide comprising amino acids from
positions 58-68 and 108-110 of a n~ulolru~hin, or a ffin~tion~l equivalent thereof, wherein the
amino acid from position 58 is covalently bound to the amino acid from position 108 and the
amino acid from position 68 is covalently bound to the amino acid at position 110 to form a
bicyclic structure.
CA 02204906 1997-05-08
In another aspect of the present invention, a composition is provided which incllldes a
carrier and a peptide compri~in~ amino acids from positions 58-68 and 108-110 of a
n~;;u~ hin, or a fimct1on~l equivalent thereof, wherein the amino acid from position 58 is
covalently bound to the amino acid from position 108 and the amino acid from position 68 is
5 covalently bound to the amino acid at position 110 to form a bicyclic structure.
In a further aspect of the present invention, there is provided a method for inhibiting a
n~;uro~ phin-mediated activity compri~ing the step of exposing neurons to a composition as
described above incl~l(1in~ a bicyclic peptide in combination with a suitable carrier.
A further aspect of the present invention provides a method for inhibiting neurotrphin-
me~ t~d activity in a m~mm~l compri~ing the step of ~lmini~t~ring to said m~mm~l a
therapeutically effective amount of a co~ osiLion which includes a bicyclic neuloLl~hin-derived
peptide in combination with a ph~rm~e~lti~l carrier.
These and other aspects of the present invention will be described in detail by reference
to the following figures in which:
BRIEF REFERENCE TO l~ DRAWINGS:
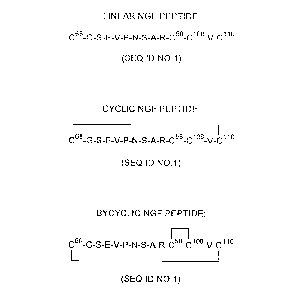
20 Figure 1 illustrates generally the structure of a nt;ulullophin;
Figure 2 ill~lstr~t~s linear, cyclic and bicyclic peptides prepared from the 68-58/108-110 region
of NGF;
Figure 3 graphically ilhlstr~tes the effect of the bicyclic NGF peptide of Fig. 2 on neurite
~U~grOW~I; and
25 Figure 4 illllstr~tes the effects of the peptides of Fig. 2 on kin~lling-induced s~i~ur~;s.
DETAILED DESCRIPI~ON OF THE INVENTION:
The present invention relates to bicyclic neulvl.o~ -derived peptides, or functional
equivalents thereof, which inhibit a n~ur~llopl~ill-me~liat~d activity.
CA 02204906 1997-05-OX
As it is used herein, the term "n~;ul~lruphin" refers to n~u~ uphic factors that are
structurally homologous to NGF, i.e. include three surface B-hairpin loops, a B-strand, an
intern~l reverse turn region, and N- and C- termini as illllst~ted in Fig. 1, and which promote
at least one of neuron survival and neuron dirrel~llL;ation, as delP....i~-ed using assays of
5 conventional design such as the in vitro assay ex~mplified herein and described by Riopelle et
al. in the Can. J. of Phys. and Pharm., 1982, 60:707. M~mm~ n nerve growth factor (NGF),
brain-derived neu.otruphic factor (BDNF), neul~,ll~hin-3 (NT-3), n~ur~ phin-4 (NT-4) and
neurotrophin-5 (NT-S) are examples of n~ulvll~hins.
10. The term "n~ul~ll~hin-derived" refers to peptides comprising an amino acid sequence
native to a given ~ n neulvlr~phin.
"Functional equivalents" of neulotlophin-derived peptides in accol~lce with the present
invention are peptides which differ from a n~uru~l~hin-derived peptide, by deletion,
15 replacement or modification of one or more of its amino acids, but which retains the activity of
the n~ulolrophin-derived peptide, i.e. is capable of inhibiting a neulolL~,phin-mediated activity.
Functional equivalents of a neul~L.~hin-derived peptide in accordance with the present invention
may comprise, for example, conservative amino acid replacements of native amino acids, e.g.
an amino acid of the n~ulotlophin-derived peptide may be replaced with an amino acid having
20 a similar charge such as replacement of an arginine residue with a lysine residue. ~llt~,. .I ~lively,
the n~ur~tlophin-derived peptide may include derivatized intPrn~l or termin~l amino acids, as
discussed in more detail herein, to yield a peptide which retains the activity of the ne~lr~ o~
derived peptide.
"N~urolr~pl~ -mediated activity" is a biological activity that is normally promoted, either
directly or indirectly, in the presence of a nt;ul~ulr~phin. N~ulol~phi~l-mediated a~;livilies
include, for example, n~;ul~ L)hin binding to the p75NGPR receptor or n~;uloll~phin binding to
one of the trk receptors, neuron survival, neuron dirrt;l~ lion incln-ling neuron process
formation and neurite ~uLgrJwlll, and biochPmic~l changes such as enzyme in(l~lction A
biological activity that is mediated by a particular n~;ul~ lr~hin, e.g. NGF, is referred to herein
--4--
CA 02204906 1997-05-08
by reference to that neurotrophin, e.g. NGF-m~li~ted activity. To de(~ e the ability of a
bicyclic peptide, or a functional equivalent thereof, to inhibit a nt;uloll~phin-m~Ai~te l activity,
conventional i vitro and m vivo assays can be used. Por example, a receptor fflI~ity cross-
linking assay, such as the assay described in herein in PY~mple 2, can be used to assess the
5 extent to which a bicyclic peptide inhibits nw~ )phin/receptor binding. Peptide inhibition of
neurite survival and ~ulglow~h can be det~rmin~cl using the i vitro assay described by Riopelle
et al. in the Can. J. of Phys. and Pharm., 1982, 60:707, exemplified herein in ~x~mplP 3, or
using the in vivo kindling expe~ment described in Example 4.
The term "bicyclic" is used herein to refer to a peptide in which there exists two ring
closures. The ring closures are formed by covalent linkages between amino acids in the peptide.
covalent linkage between two non-adjacent a-m~no acids con~tit ltes a ring closure, as does a
second covalent linkage between a pair of ?~dj~c~nt amino acids which are already linked by a
covalent peptide linkage. The covalent linkages forming the ring closures may be amide
linkages, i.e. the linkage formed between a free amino on one amino acid and a free carboxyl
of a second amino acid, or linkages formed between the side chains or "R" groups of amino
acids in the peptides. Thus, bicyclic peptides in accordance with the present invention may be
"true" bicyclic peptides, i.e. peptides cyclized by the formation of a peptide bond between the
N-terminlls and the C-tçrmimls of the peptide, or they may be "depsi-bicyclic" peptides, i.e.
peptides in which the termin~l amino acids are covalently linked through their side chain moities.
In one aspect, the bicyclic peptide is con.ci.~tent with the int~rn~l reverse turn region of
the selected neurotrophin. The reverse turn region of a n~ulvllophin extends from the amino
acid at position 58 to the amino acid at position 68, and incllldes also the region e~tPnding from
the amino acid at position 108 to the amino acid at position 110, as illustrated in Figure 1. The
"reverse turn" results from the dual linkage occurring in this region. The dual linkage in~ln-lçs
a ~lrst covalent linkage l~lween the amino acid at position 58 and the amino acid at position 108,
and a second covalent linkage between the amino acid at position 68 and the amino acid at
position 110.
--5--
CA 02204906 1997-05-08
-
Depsi-bicyclic peptides in accordance with the present invention result from the formation
of covalent linkages between the side chains of the amino acids from positions 58, 68, 108 and
110. Preferably, the amino acid residues from these positions have side chains that will readily
react to form such covalent linkages. For example, cysteine residues are particularly suitable
5 amino acids for this pulpose since the free thiol R groups of cysteine residues readily oxidize
to form covalent ~liculfi~le bridges. ~ ;vely~ the R groups of the amino acids in these
positions can be derivatized to yield groups, such as free thiol groups, which will readily react
to form the desired covalent linkages. In another alternative, amino acids from positions 58 and
108, and positions 68 and 110, can be selected to have R groups, or derivatized to yield R
10 groups, which will form amide linkages. Thus, for example, an amide linkage can be formed
between the amino acids from positions 58 and 108 if the amino acid at one of these positions
yields a free amino group, while the amino acid at the other position yields a free carboxyl
group. Examples of amino acids which yield a free amino group s~lit~hle for the formation of
an amide bond are lysine, A~ArAgine and pl~ ,.,inP. Examples of amino acids which yield a
15 free carboxyl group suitable for the formation of an amide bond are gl~lt~mic acid and aspartic
acid.
In the case of depsi-bicyc]ic peptides, it will be appreciated that the N- and C- termini
remain as free amino and free carboxyl resi~lnPs, respectively, since it is the side chains of the
20 terminal amino acids which are involved in the covalent cycli~ing linkage. The free terminA1
amino and carboxyl groups may also be derivatized or altered without affecting the activity of
the peptide as an inhibitor of a neulolru~hin-mediated activity. For example, the termini may
be derivatized to include a non-peptidic blocl~ng group that will pL~ve ll pu~ei~ial degr~fil~n
at the N- and C- tPrmin~l ends from occurring. Such non-peptidic grûups include protecting
25 groups such as those conveMtion~lly used in the art of peptide synthesis which wiU not adversely
affect the in vitro and in vivo uses of the bicyclic peptide. For example, suitable non-peptidic
N-termin~l blocking groups can be introduced by aLkylation or acylation of the N-tel.llilll~s.
Examples of suitable N-tP~nin~l blocldng groups include Cl-Cs br~n~ hell or llnhr~nched aLkyl
groups, acyl groups such as formyl and acetyl groups, as well as substituted forms thereof.
30 Amino acid analogues lacl~ng the amino functionality are also useffil to block the N-t~l ."i""s.
-6-
CA 02204906 1997-05-08
-
Suitable non-peptidic C-t~rmin~l blocl~g groups, in which the carboxyl group of the C-tt ~ ls
may be either incorporated or not, include esters, ketones or amides. Ester or ketone-forming
aD~yl groups, particularly lower aLI~yl groups such as methyl, ethyl and propyl, and amide-
forming amino groups such as pl~na-~y amines (-N E2), and mono- and di-aLkyla"~ o groups such
5 as methylamino, ethylamino, dimethylamino, di~lllylalllino, methylethylamino and the like are
examples of C-tP....i~.~l blocking groups. Amino acid analogues lacking the call~o~yl
functionality are also useful C-lP~ in~l blocking groups such as ~gm~tin~. Further, it will be
appreciated that the free amino and carboxyl groups at the termin~ can be removed altogether
from the bicyclic peptide to yield des~minc and descarboxylated forms thereof without affect on
peptide activity.
True bicyclic peptides are also peptides in accordance with the present invention. Such
peptides result from the formation of a peptide linkage between the N-l~, -,,ill~1 amino group of
the amino acid from position 68 and the C-t~rmin~l carboxyl group of the amino acid from
position 110.
Bicyclic peptides in accordance with the present invention may be derived from any
m~mm~ n neuro~l~hin due to the highly homologous nature of n~ur(~ phins among dirrt;;,c;l,~
species with regard to both conformation and amino acid sequence. In particular, the amino acid
residues of neulolluphins at positions 58, 68 and 108-110 are conserved across species and have
been found to play an important role in peptide co~ ion- The amino acid residues in
positions 59-67 do not appear to be important for peptide conform~tion, and further have not
been found to participate in NGF interactions (Drinkwater et al., J. Biol. Chem. 1993,
268(31):23202). The bicyclic peptides of the present invention, thus, may be derived from the
58-68/108-110 amino acid region of, for e~:~mple, human, mouse or rat NGF. Likewise, the
bicyclic peptide may be derived from the 58-68/108-110 region of any m~mm~ n BDNF or
N~r-3. The following general formula (1) defines the bicyclic peptides:
AA68-Xx c58 Cl08-V-A~ll0 (SEQ ID NO:1) (I)
30 wherein
--7--
-
~ CA 02204906 1997-05-08
A A68iS selected from cysteine, the des~mino form thereof, and an N-termin~l~y
blocked cysteine;
~ 0 is selected from cysteine, descarboxylate cysteine, and a C-tennin~lly
blocked cysteine; and
XX represents a peptide c~mpri.~in~ from 1-10 amino acid residues.
In one aspect the bicyclic peptide has an amino acid sequence represented by formula
~):
AA68-G-S-Xl-V-P-N-X2-X3-~-Cs8-CI~8-V-AAll~ (SEQ ID NO:2) ~)
wherein
A A68 and AAIlO are as de~med above;
X, is an acidic amino acid;
X2 is seIected from the group con~i~tin~ of a non-polar amino acid and an
unch~rged polar amino acid; and
X3is selected from the group con.~i.cting of an ~cidic amino acid and a non-polar
amino acid.
In this regard, acidic amino acid refers to an amino acid which is negatively charged at pH 6.0,
a non-polar amino acid refers to an amino acid having a non-polar side-chain such as alanine,
leucine, methionine and proline, and an uncharged polar amino acid refers to an amino acid
having an uncharged polar side-chain such as glycine, serine, cysteine and ~ ginP.
In another aspect the bicyclic peptide has an amino acid sequence represented generally
by formula (II):
AA68 G S Xl V-P-N-X2-X3-R-C58-Cl~8-V-AAIl~ (SEQ ID NO:2)
wherein
A A68 and AAl10 are as defined above;
Xl is s~l~cted from glnt~mic acid and ;~.~p~r~ic acid;
X2 is selected from proline and serine; and
X3is selecte~l from aspartic acid and ~ ninP
-8-
,
CA 02204906 1997-05-08
~ .
Speci~lc peptide sequences in accordance with the present invention derived from mouse
and human neuloll~hins in~ e:
NGFm C68-G-S-E-v p N s A R c5s Clos v cllo (SEQ ID NO:3)
NGFh~ C68 G s D v p N p D R_c5s_clos_v cllo (SEQ ID NO:4)
BDNFm C68 G E K T y c M p N C58 cl08 v cllo (SEQ ID NO:5)
BDNFh~ C68 G E K T y G M p N c58 Cl08 v cllo (S~Q ID NO:6)
NT-3 C68-GN-K-v-p-R-A-E-K-c58-clo8-v-cllo (SEQ ID NO:7?
NT-3hum c68 G N K_v p R_A E_K c58 cl08 v cll0 (SEQ ID NO:7)
In a specific embodiment of the present invention, a bicyclic peptide derived from the
58-68/108-110 region of mouse NGF, as ill--st~ted in Figure 2, was p~ d and found to
1~ inhibit rat NGF-mediated activity. In particular, and as set out in detail in the specific examples
herein, the bicyclic peptide inhibited cross-linking of NGF to both the p75NGPR receptor and the
trkA receptor, and inhibited NGF-m~i~teA neurite ou~growl~l as c~etermin~d both in vitro and
in vivo.
The bicyclic peptides of the present invention may be readily pr~p~d by standard, well-
20 est~bli~hed solid-phase peptide synthesis (SPPS) as described by Stewart et al. in Solid Phase
Peptide Synthesis, 2nd PAition, 1984, Pierce Ch~mic~l Company, Rockfor, Illinois; and as
described by Bodanszky and Bodanszky in The P~ctice of Peptide Synthesis, 1984, Springer-
Verlag, New York. At the outset, a suitably protected amino acid residue is attached through
its carboxyl group to a derivatized, insoluble polymeric support, such as cross-linked poly~lyr~,le
25 or polyamide resin. "Suitably protected" refers to the presence of protecting groups on both the
o~-amino group of the amino acid, and on any side chain functional groups. Side chain
protecting groups are generally stable to the solvents, reagents and reaction conditions used
throughout the synthesis, and are removable under conditions which will not affect the final
peptide product. Stepwise synthesis of the oligopeptide is carried out by the removal of the N-
30 protecting group from the initial amino acid, and cuu~ g thereto of the carboxyl end of the next
g
CA 02204906 1997-OF7-08
amino acid in the sequence of the desired peptide. This amino acid is also suitably protected.
The carboxyl of the incoming amino acid can be activated to react with the N-tPi.llillllc of the
support-bound amino acid by formation into a reactive group such as formation into a
carbodiimide, a symmetric acid anhydride or an "active ester" group such as
5 hydroxybenzotriazole or pe~ lorophenyl esters.
Examples of solid phase peptide synthesis methods include the BOC method which
utilizes tert-butyloxycarbonyl as the cY-amino protecting group, and the FMOC method which
utilizes 9-fluorenylmethyloxycarbonyl to protect the o~-amino of the amino acid residues, both
10 methods of which are well-known by those of skill in the art.
Incorporation of N- and/or C- protecting groups can also be achieved using protocols
conventional to solid phase peptide synthesis methods. For incolporation of C-IP~ i,.i..~l
protecting groups, for example, synthesis of the desired peptide is typically performed using,
15 as solid phase, a supporting resin that has been c~hP.mi~lly modi~led so that cleavage from the
resin results in a peptide having the desired C-t~rmin~l protecting group. To provide peptides
in which the C-~PI j~;llll,C bears a ~ aly amino protecting group, for in~tzln~e, synthesis is
performed using a p-methylbenzhydrylamine (MBHA) resin so that, when peptide synthesis is
completed, tre~tlnent with hyd~ lori~ acid releases the desired C-tPrmin~lly ~mi~l~te~ peptide.
20 Similarly, incorporation of an N-m~lhyl~..me protecting group at the C-lP~ S is achieved
using N-methylaminoethyl-derivatized DVB resin, which upon HF tre~tment releases peptide
bearing an N-methyl~mi(l~te~ C-t~ $. Protection of the C-te. ."i"~,s by estPrifi(~tion can also
be achieved using conventional procedures. I~is entails use of resin/blocking group combination
that permits release of side-chain protected peptide from the resin, to allow for subsequent
25 reaction with the desired alcohol, to form the ester function. F~OC protecting groups, in
combination with DVB resin derivatized with m~thc~xyaL~oxybenzyl alcohol or equivalent linker,
can be used for this purpose, with cleavage from the support being effected by TE~A in
(1icholoromethane. ~terifi~tion of the suitably activated c~l,-,;Lyl function e.g. with DCC, can
then proceed by ~1flition of the desired alcohol, followed by deprotection and isolation of the
30 esterified peptide product.
-10-
-
CA 02204906 1997-05-08
Incorporation of N~ 1 protecting groups can be achieved while the syntlle~i7ecl
peptide is still attached to the resin, for instance by treatment with s11itz~h1e anhydride and nitrile.
To incorporate an acetyl protecting group at the N~ .llilllls, for instance, the resin-coupled
peptide can be treated with 20% acetic anhydride in acetQnitri1e. The N-protected peptide
5 product can then be cleaved from the resin, deprotected and subsequently isolated.
Recombinant techniques, well-est~bli~h~ in the art, can also be used to ~r~;palc; peptides
in accordance with the present invention. DNA encoding the desired peptide is pl~i~d and
inserted into an appropriate expression vector. The vector is used to transfect a suitable host
10 organism for production of the peptide. Conventional techniques are then used to culture the
host and to isolate the peptide product from the cell culture media.
To ensure that the peptide obtained from either ch~mi~l or biological synthetic
techniques is the desired peptide, analysis of the peptide composition should be carried out.
15 Such amino acid composition analysis may be con-lucted using high resolution mass spectrometry
to determine the molecular weight of the peptide. ~lt~rn~t;vely, the amino acid content of the
peptide can be confirmed by hydrolyzing the peptide in aqueous acid, and sep~,~fi~ ide~ yiilg
and quantifying the components of the nlL~lul~ using HPLC, or an amino acid analyzer. Protein
sequenators, which sequentially degrade the peptide and identify the amino acids in order, may
20 also be used to ~letermine tlefinite1y the sequence of the peptide.
Having confirmed the identity of the peptide in linear form, it is then cyclized to form
an active bicyclic peptide in accordance with the present invention. Many techniques are
available for ap~lvplidl~ly cyclizing the peptide, and the protocol used will depend on the type
25 of linkages used to form the bicyclic product. As out1ined above, there are numerous covalent
linkages which are suitable to cyclize the peptide inc111-1in~, for ~x~mI)1e, side chain linkages
such as tlisl11fide linkages and peptide or amide linkages. In one embodiment of the present
invention, ~ slllfid~ linkages were used to form a bicyclic peptide as i11nst~ted in l~ig. 2. The
int~rn~1 cysteine residues of the linear peptide were first protected in order to conduct the
30 cyclization reactions in a stepwise fashion. The protected peptide was then air ~-xi~1i7ed to allow
-11-
CA 02204906 1997-05-08
a ~lis~ {1e linkage to form between the termin~l cysteine residues. FoIlowing this cyclization
reaction, the protecting groups were removed from the intern~l cysteine residues, and the peptide
was again subjected to oxidiz~ng con(lition~ to allow a disulfide linkage to form between the
intern~l cysteine residues thereby resulting in the bicyclic peptide.
Prior to its use to inhibit ncu~ hin-mediated activity, the bicyclic peptide is purified
to remove cont~min~ntS which may adversely affect its activity. In this regard, it will be
appreciated that strict standards of purity, such as those required for ph~ cel~tic~l compounds,
may not be required for use of the present compounds in vitro. On the other hand, if a
10 compound according to the present invention is to be used in a ph~rm~ce~lti~l sense, it must be
purified so as to meet the standards set out by the a~r~ idl~ regulatory agencies. Any one of
a number of conventional pllrific~tioll procedures may be used to attain the required level of
purity inclnflin~, for example, reversed-phase high-pressure liquid chromatography ~HPLC)
using an aLkylated silica column such as C4-, C8- or Clg- silica. A gradient mobile phase of
1~ increasing organic content is generally used to achieve purifi~tion, for example, acclo,,;l~;le in
an aqueous buffer, usually co"l;~ ing a small amount of triflLuoroacetic acid. Ion-e~ch~n~e
chromatography can also be used to separate peptides based on their charge.
Bicyclic peptides of the present invention are useful to inhibit or reduce undesirable
20 neur~Lr~hin activity both in vitro and in vivo. Thus, in another aspect of the invention, a
composition comprising an effective amount of a n~ulolrophin-derived bicyclic peptide and a
suitable carrier is provided. By "suitable carrier" is meant a carrier which a-lmi~s with the
selected bicyclic peptide to yield a composition s~litab1e for the appliç~tion for which it is to be
used. By "effective amount" is meant an amount of bicyclic peptide snfficient to inhibit an
25 undesired ncuroLl-)phin-mediated activity by about 50 % as det~rmined using assays of
convention~l design such as those described herein in the specific examples.
l~e present bicyclic peptides have use as media supplements to ~lcvell~ undesirable
n~ulo~n~L~hin-me~i~te~ activity of neuron cells i .vitro. For example, pl.,~ y sensory neurons
30 require NGF for survival in cell culture; however, NGF also inflnences neuron dirrclcnLidLion,
CA 02204906 1997-OF7-OX
notablv process formation and t~ulgl~t~wLh, which are ~lntle~ ble for the use of plimaly sensory
neurons in cell culture. Thus, to ~lt;st;l~/e neuron survival in vitro while inhihiting cell
differentiation, NGF is added to the cell culture media along with a bicyclic peptide. For
addition to the cell culture, the bicyclic peptide is first combined with a carrier which will not
5 adversely affect the growth of the cells in culture. Such c~rriers will include, for example,
physiologicallv acceptable fluids such as water or any other fluid suitable for ~d~iition to the cell
culture. ~1tt~.rn~tively, the peptide can be combined with media s~lit~hle for cultllrin~ neuronal
cells prior to being added to the cell culture. To be effective to prevent neuron ,lilrele~ ion,
the concentration of the peptide in the cell culture will be in the range of from about 1-500 ~M,
10 and preferably from about l-lO0 ~4M. The optimal concentr~tion of bicyclic peptide for use in
preventing neuron dirrelellLialion in cell culture will, of course, vary in each independent case,
and will depend on the extent of inhibition desired as well as the type of neuronal cells involved.
Compositions for in vivo ~timini~str~tion~ e.g. for treating neurological conditions such
15 as epilepsy or Alzheimer's disease, are also contemplated. Such compositions comprise a
therapeutically effective amount of a bicvclic peptide together with a l?h~rm~- eutically acceptable
carrier. In this context, the term "ph~rm~t-elltit~ tl1y acceptable" means acceptable for use in the
pharmaceutical and veterinary arts, i.e. non-toxic and not adversely affecting the activity of the
bicyclic peptide. The term "therapeutically effective amount" means an amount of the compound
20 sllfflcient to reduce lln-lesir~hle nt;ulol~~t~hin-medi~ted activity, as d~t~rmined using assays of
conventional design such as the assays described herein in the specific ex~mpl~s, in an inflictecl
individual without causing adverse effects.
Pharmaceutically acceptable carriers useful to prepare compositions for in yivo
25 ~rlmini~tr~tion include convention~l carriers generally selected for combination with peptide-
based drugs such as t~i1nent~, ex~ ipi(~nt~ and the like. Reference may be made to "Remington's
ph~rm~eelltical Sciences", 17th Ed., Mack Publishing Co~ ally, Easton, Penn., 1985, for
guidance on drug forrmll~tions generàlly. As will be appreciated, the ph~rm~ce~ltie~l carriers
used to ~ c compositions in accordance with the present invention will depend on the
30 ~mini~t~hle form to be used to treat the inflicted individual.
-13-
CA 02204906 1997-05-08
According to one embodiment of the invention, the compounds are formulated for
admini~tration by injection intraventricularly, and are accordingly provided as aqueous solutions
in sterile and pyrogen-free form and optional1y buffered or made isotonic. Thus, the compounds
may be ~(lminictered in distilled water or, more desirably, in saline or 5% dextrose solution.
5 Water solubility of these and other co-llp-~unds of the invention may be enh~nced, if desired, by
incorporating into the composition a solubility çnh~ncer, such as cely~ thylammonium
bromide or chloride. Lyoprotectants, such as m~nnitQl, sucrose or lactose and buffer systems,
such as acetate, citrate and phosphate may also be in~ dçd in the formulation, as may buLking
agents such as serum albumin.
Allelllalively~ the compounds of the present invention may be form~ t~ for
~1mini~tration by routes other than injection. For ~x~mp1e, oral dosage forms, such as tablets,
capsules and the like, form~ ted in accordance with standard ph~ eel1ti- ~l p ~ti~e, may be
employed.
For use in treating individuals with a neurological condition, precise dosage sizes of a
ph~rm~cel1tit~1 composition a~l)ro~llate for tr~ nt can readily be est~b1i~h~d in appl.~plial~ly
controlled tri~ls, and will correspond to an amount of bicyclic peptide that reduces lln~le~ hle
neurotrophin-mediated activity without causing intolerable side effects to the individual being
20 treated. It is anticipated that an effective tre~tment regimen for p~fient~ will involve the
intraventricular atlmini~tr~tinn of dosages which achieve a level of peptide in the spinal fluid of
the individual being treated of about 1-500 ,uM. It will be appre~i~te~ of course, that the
dosage sizes required to attain this in vivo conct ntr~tion will vary according to the route of
~1mini~tr~tion, the frequency of a(lmini~tration, on the individual being treated and on the
25 neurological condition being treated.
-14-
-
CA 02204906 1997-05-08
Speci~lc embodiments of the present invention are described in more detail in the
following examples which are not to be construed as 1imitin~.
5 ~xample 1 - Synthesis of NGP Bicyclic Peptide
The bicyclic peptide illllstr~t~d in Fig. 2 was ~r~paL~d by first synth~si~in~ the linear
form thereof using the solid phase synthesis method. An automated synffl~si7er, e.g. Applied
Biosystems 430A, was used with a Wang resin (available from NovaBiochem). All amino acid
side chains were protected with Mtr (4-methoxy-2,3,6-trimethyl-benzene-sulfonyl) groups, with
the exception of the cysteines from positions 58 and 108 which were protected with ACM
(acetamidomethyl) groups. A TFA-cleavage (1-2 hrs) from the resin yielded the linear peptide
retaining only the ACM protecting groups.
To cyclize the peptide, the two free thiol groups at positions 68 and 110 were then
15 covalently linked. The peptide was dissolved in 0.1 M ammonium bica-l~o~ e buffer, pH 8.3,
at a concentration of 0.1 mg/mL. The reaction ll~i~Llulc; was stirred at room temperature, and
the progression of the reaction was monitored by E~PLC. The HPLC solvent, comprising
solvent A of water with 0.1 % l~A and solvent B of acetonitrile with 0.1% ~FA, was run on
a gradient from solvent A to solvent B at 1% per minute. On completion of the reaction, the
20 mono-cyclized product was isolated by lyophilization and purified by HPLC.
The mono-cyclized product (0.1 mmol) was then cyclized at the ACM protected sites.
The product was dissolved in 1.5 mL of methanol. I~is solution was added dropwise to 2.5 mL
of methanol cont~ining 63.5 mg of iodine over 30 minutes with stirring at room temperature.
25 l~e stirring was contim-ed (approx. 3 hrs) while the progression of the reaction was drl~ d
using HPLC. Upon completion of the cyclization, the reaction was qlle~chPd by the addition
of solid zinc powder (1-2 mg). The mixlul~ was diluted with 10 mL of water, f~tered and
lyophilized. I~e bicyclic product was purified using HPLC, and its structure was confirmed by
standard methods.
-15-
CA 02204906 1997-05-08
Example 2 - Af~mity Cross-T inkin~ Experiments
The ability of the peptides derived from the 68-58/108-110 region of NGF to antagonize
N GF intP~ctiQn with the p75 and trkA receptors was detçrmined The peptides tested were
bicyclic ~BC) 68-58/108-110, cyclic (C) 68-58/108-110, and l~lear (L) 68-58/108-110, each of
which are illustrated in Figure 2.
PC12 rat pheochromocytoma cells (ATCC CRL 1721) were independently incubated in
RPMI (GIBCO)/10% fetal calf serum (GIBCO) with 20 ,uM and 200 ,uM solutions of NGF
peptide, prepared as described in detail in Example 1, in the presence of l25I-NGP (isolated from
mouse subm~xill~ry- gland as described in Mobley et al., 1976, Biochemistry, 15:1543) for 2
hours at 4~C. Control PC12 cells were incub~tP~l in RPMI/10% fetal calf serum in the presence
of l2sI-NGF only (no NGF peptide).
For trkA cross-lin~ng, bis-(sulfosuccinimidyl)suberate (Pierce) was added to theincubation ~ Lulc to a final conePnt~tion of 0.4 mM (in 20 ,ul), and i~ b;ll~l for 20 min ~t
25~C. For cross-linking to p75NGFR, N-hydroxysulfosuccinimi~le (Pierce) was added to the
incubation mixture to a final con~ent~ti~ n of 2 mM and l-ethyl-3-(3-
dimethylaminopropyl)carbodiimide (Pierce) was added to a fmal con(~Mtr~tion of S mM (in 20
,ul), and incubated for 3() min at 25~C. On comr1Ption of the cross-linking reaction, the cells
were washed three times in HKR buffer (Gibco) with BSA at 4~C to remove excess free
radiolabelled ligand and reagents.
The cells were solubilized in 1 ml of lysis buffer cont~inin~ 10 mM tris-HCl, pH 7.4,
150 mM NaCl, 10~ glycerol, 1% NP-40, 0.01 mg/ml apr~ , 0.5 mM orthov~n~te, 0.5
~bl/ml leupeptin, and 2 mM phenyl-methyl-sulrolly111-.ori(1e at 4 ~C for 15 min. C1~1h~1~r debris
was removed by ce~ r~g~tion TrkA antibody (supplied by W. Mobley, Unive~ y of
California, San Francisco) or p75 antibody (suppli~d by E. Shooter, Sl~lrol l U., California)
were added to the supe~ to a final concentration of 20 ,ug/ml, and incubated at 4~C for 2
hrs. with constant mixing. Protein A Sepharose (Sigma) pre-equilibrated in lysis buffer was
added (50 ,bl of a 50% solution) to the TrkA sample and incubated at 4~C for 2 hrs. For the
-16-
CA 02204906 1997-05-08
.,
p75 sample, pre-equilibrated goat anti-mouse agarose (Sigma) was added (50 ~bl of a 50%
solution) and incubated.
The imm~lnoprecipitated proteins were then washed with lysis buffer and eluted from the
5 affimity gels using reducing SDS sample buffer, and solubilized in the redllçin~ buffer for 10 min
at 95~C. Samples were separated on a modified T~emmli discontinuous acrylamide gel system
(Laemmli, Nature, 1970, 227:680) using 4% SDS PAGE staking gel and a ~-iient urea
polyacrylamide separating gel rang~ng from 4.5 % acrylamide/18 % urea to 7.5 % acrylamide/48 %
urea. The gels were fixed and processed for 7l~ltor~-liography using -70~C exposure with Kodak
10 XAR rllm and manual processing.
The following results were obtained by observing the density of bands (the less the
density, the greater the antagonism) appearing on the autoradiog~ms:
PEPTID~ Tnhi~ '- Of p75 Inhibition of TrkA
lnteraction Tnt~r~.ti~m
1. BC 20 ~M 1. + I 1. ++
2. BC 200 ,uM 2. + + 2 2. + +
C 200 ,IIM no inhil~:~in....... no illLI, ~ nn
L 200 ,~4M no inhih:~inn no i~hibition
"+" indicates less than 50% inhih ~ nn
Z ~ + + ~ dicates greater than 50% inhih;tinn
Thus, as can be seen from the tabulated results, only the bicyclic peptide was capable of
inhibiting NGF inter~ction at the p75 and TrkA receptors.
25 Example 3 - Inhibition of Neurite Oul~rowlll
Eight-day chick embryo dorsal root ganglia (DRG) were freed of meninges and removed
aseptically. The DRG were kept at 4~C at all times. ~anglia from six embryos (40-50 per
embryo) were washed in Ca2+- and Mg2+- free Gey's balanced salt solution (Gibco) and exposed
to 0.01% tIypsin (WorthingtQn) in the same solution for 10 min at 37~C. A half-volume of
30 phosphate-buffered Gey's balanced salt solution was added for a further 5 min at 37~C and the
reaction was then stopped with one-third volume of Ham's F12 medium (Gibco) CO~ g 5 %
-17-
CA 02204906 1997-OF7-08
fetal calf serum (Gibco). The ganglia were then ~ PC1 using a 5 mL narrow-tip pipette to
a single cell suspension. Following fîltration through 37-,um nylon mesh (Small Parts Inc.,
Miami, FL) in a millipore chamber to remove clumps, the cell suspension was washed through
a 500-,ul FCS undercut (700 x g for 5 min at 4~C) and resuspended in 4 mL of Ham's F12
5 medium plus 5 % FCS. The cell suspension was then preplated on a 100-mm FIacon culture dish
and incubated for 45-60 min at 37~C in a 5% CO2 hllmi~lified atmosphere. CelIs enriched in
neurons were decanted for the bioassay, since non-neuronal cells of DRG preferentially stick to
the culture substrate.
10The inside wells of 96-well Falcon microculture plates were coated with polylysine (0.1
mg/mL) (Sigma) for 4 h at 37~C (the outside wells were f~ed with ~ till~d water to provide
humi~lity) and, following a rinse with tissue culture media, 100 ,uL of neuron-rich cell
suspension was added to each well at 105 cells/mL. Ninety (90) ,~L of N~F solution (~r~
in tissue culture media) was then added to each well to a final concentration of 0.25 ng/mL NGF
15per well. Ten (10) ,uL of bicyclic 68-58/108-110 peptide solution, i.e. tissue culture media
admixed with bicyclic peptide prepared as described in Example 1, was then added to test wells
in duplicate to yield wells cont~ining 0 ,uM, 25 ,uM, 100 ,uM and 250 ~M peptide. For control
assays, 10 ,uL of Ham's F12 medium was added to duplicate NGF-cont~inin~ wells. The plates
were covered and incubated in the dark for 24-30 hrs. at 37~C in a 55~ CO2 hllmit1ified
20 atmosphere.
The bioassays were read using a Leitz Diavert microscope with phase optics. To afford
adequate optics, the meni~c~is effect of each well was removed by filling the well with a
balanced salt solution until a flat, air-f~ed int~rf~e was achieved at the top of the well. At
25 least 100 neurons per well were counted, and the assay was scored as the ratio of cells bearing
neurites greater than one ceU ~ m~ter to total viable (phase-bright) cells.
The results of this assay are illns~r~ted in Fig. 3. In this expçriment the IC50, i.e. the
concentration of bicyclic peptide required to inhibit neurite growth on 50% of the cells, was
30 calculated to be 250 ~uM.
-18-
~ CA 02204906 1997-05-08
Example 4 - Effect of Peptide on Neuron ~urvival
Cells enriched for sensory neurons were prepared from ED8 chick DRG as describedabove. The cells were plated at a density of 800-1000 cells per well in Terasaki plates treated
with poly-D-lysine and l~minin in tissue culture m~ m cont~ining 1 ng/ml NGF and 68-
58/108-110 depsibicyclic peptide in the amounts shown below. Following a 20-22 hr. incubation
at 37~C, 5~ CO2, the cells were fixed in 4% form~k1~P.hyde in PBS and cells on the tissue
culture surface were counted as a percentage of total cells.
Additives % Viable Cells
0 78.2 +1.1
methanol (1. 8 ~ 66.3 + 5.0
peptide (5 nM) 70.4 + 3.4
peptide (50 nM) 67.2 + 1.2
peptide (500 nM~ 86.5 + 4.6
15 peptide (5 ~ M) 79.5 _ 2.4
peptide (20 ,uM)2 79.5 +1.4
I highest fillal c~,..c~,~tld~ion used as a vehicle for peptide
2 This concentration is twice the IC5" for neurite growth i~
As can be seen from the results of this assay, the depsibicyclic peptide had no ~ignifi~ ~nf
effect on NGF-mediated survival at the concentrations tested.
Example 5 - Effect of Neul~t~L)hin-Derived Peptide on Kin~lling
Kinflling is a phenomenon in which repeated low-illL~llsily (subconvulsive) electrical
stim~ tion of fo~ areas leads to a progressive and perm~nPnt ~mp1ific~tion of seizure
activity, and is thus, widely accepted as a model for human temporal lobe epilepsy. The effect
of the present n~urotr~hin-derived peptides on kin-lling was cletermined as follows.
Male Long-Evans hooded rats (300-400 g) were used. The ~nim~l~ were housed
individually, m~int~ined on an ad lib feeding s-hedlllP and kept on a 12 h onll2 h of f light
cycle.
-19-
~ CA 02204906 1997-05-08
The rats were ;~n~stllPti~ed with O.l ml per lO0 g body weight of lO0 mg/ml ketarnine
hydrochloride (~ogar/STE', Inc., London, Canada) and 0.05 ml per lO0 g body weight of 20
mg/ml xylocaine 2% hydrochloride (Astra, Mi.~ s~llg~, Canada), and then placed in a
stereotaxic holder. The rats were imp1~nt~1 llnil~terAlly with a bipolar twisted, teflon-coated,
stainless steel electrode with an exposed tip (wire ~i~meter l9o~bm) in the right a,lly~,dala at
stereotaxic coord~nates of 3.3 mm caudal and 8.0 mm lateral to bregma and 8.5 mm ventral to
the brain surface (selected from Paxinos and Watson, 1982, "The rat brain in stereotaxic
coordinates", ~c;~lemic Press, Sydney). Following implantation of the electrode, a cannula
(Alzet brain infusion kit, Alza Corp.) was implanted in the lateral ventricle, 5 mm below the
skull surface, at 0.6 mm caudal to bregma and 1.3 mm lateral to the midline. It was firmly
attached to the skull with dental cement and anchored with three st~in1Pss steel screws. An
osmotic pump (Alzet model 2002, flow speed 0.5 ~ul/h, effective m~im~11y for 14 days) was
connected to the ~nn~ via polyethylene tubing and placed subcutaneously in the neck area.
Histological ex~.";~ n of lateral ventricle sections was done to con~lrm that the c~nn~ was
correctly placed. Forty-five (45) ,uM of peptide, in a physiologically acceptable buffer, was
delivered throughout the duration of the experimP~t to each test animal. There were five groups
of test ~nim~l.s, 5 ~nim~l~ per peptide test group, lO ~nim~1~ in the negative control group and
12 ~nim~1s in the positive control group. Each test group was zl~lministered one peptide selected
from the linear, cyclic and bicyclic 68-58/108-llO peptides. The negative control group was
infused with control serum IgGs, and the positive control group was infused with lO0 ~g/day
of anti-NGF antibody. The anti-NGF antibody was obtained from sheep ;~ ~~ with 0.5
mg of 2.5S NGF (prepared from male mouse salivary glands according to the method of Mobley
et al., supra) intr~-1Prrn~11y in complete Freund's adjuvant initially, and in incomplete adjuvant
every 4 weeks thereafter. Blood was collected lO days after each booster injection. Serum was
prepared by clotting the blood at room temperature followed by centrifugation at 1,500 g for 30
min., heat inactivation at 56~(~ for 30 min. and stPrili~tion using o.~ ,um filters (Nalgene).
IgG was puri~led from serum by dirre~ tial precipitation using caprilic acid followed by
ammonium sulfate (M~KinnPy and Parlcinson, 1987, J. Tmm11nol. Methods, 96:271). NGF-
specific antibody was further purifiP~d using affinity chrom~togrAI)hy on 2.5S NGF coupled to
CN-Br sephàrose 4B (Ph~nn~ci~). -
-20-
~ CA 02204906 1997-05-08
' .-
Following a three-day recovery, the kin~llin~ sfim~ tions were started. The ~nim~1~
received a one-second train of one-mi11icecond pulses at a frequency of 60 Hz and a pulse
intensity of 200-400 ,uA. These pulses were sl1fficient to t~igger an epil~ iro~ after ~ h~r~e
(AD) following each stimulation. Each animal was stim~ ted in this fashion twice a day over
5 a period of l l days. Progression of kinl1lin~ was monitored behaviorally and
electrophysiologically by recording the behavioral seizure stages and the d11~tion and m~nit~1de
of afterdischarges. Fully lrindled ~nim~1~ exhibited three consecutive stage-5 sei~ures (Racine,
1972, Electroencephalogr. Clin. Neurophysiol., 32:281).
The number of stimulations to reach stage-5 seizures for control rats and rats receiving
the linear, cyclic and bicyclic peptides is i~ st~ted graphically in Fig. 4. The results ill~-str~t~
that the bicyclic peptide has a potency which is approximately equal to that of the anti-NGF IgG
in delaying the onset of kindling in comparison to the control serum IgG, linear peptide and
cyclic peptide.
CA 02204906 l997-05-08
,
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: Riopelle, Richard J.
(ii) TITLE OF lNV~N'l'lON: NEUROTROPHIN ANTAGONISTS
(iii) NU.MBER OF SEQUENCES: 7
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Ridout & Maybee
(B) STREET: 2300 Richmond-Adelaide Centre, lO1 Richmond
Street West
(C) CITY: Toronto
(D) STATE: Ontario
(E) COUNTRY: Canada
(F) ZIP: M5H 2J7
(v) COMPUTER R~ADABLE FORM:
(A~ MEDIUM TYPE: Floppy disk
(B. COMPUTER: IBM PC compatible
(C OPERATING SYSTEM: PC-DOS/MS-DOS
(Dl SOFTWARE: PatentIn Release #1.0, Ver~ion #1.25
(Vi) CU~R~'l' APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE-
(c) CLASSIFICATION:
(ix) TELEcoM~nNTcATIoN INFORMATION:
(A) TELEPHONE: (416) 868-1482
(B) TELEFAX: (416) 362-0823
(2) INFORMATION FOR SEQ ID NO:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 6 amino acids
(B) TYPE: amino acid
(C) sTR~NnRnNFss single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION: 1
(D) OTHER INFORMATION: /note= "Xaa at pos.1 is Cys,
desamino Cys or N-term~ n~ 1 ly blocked Cys"
(ix) FEATURE:
(A) NAME/KEY: Modi~ied-site
(B) LOCATION: 2
(D) OTHER INFORMATION: /note= "Xaa at pos.2 represents
from 1-10 amino acid residues"
CA 02204906 l997-05-08
(ix) FEATURE:
~ (A) NAME/KEY: Modi~ied-site
(B) LOCATION: 6
(D) OTHER INFORMATION: /note= "Xaa at pos. 6 is Cys,
decarboxylated Cys or N-term;n~lly blocked Cys
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:l:
Xaa Xaa Cys Cys Val Xaa
(2) INFORMATION FOR SEQ ID NO:2:
(i) SBQUENCE CHARA~TR~T.STICS:
(A: LEN-GTH: 14 amino acids
(B~ TYPE: amino acid
(C STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
( ix) FEATURB:
(A) NAME/KEY: Modified-site
(B) ~OCATION: 4
(D) OTHER INFORMATION: /note= ~Xaa at pos.4 is an acidic
amino acid~
(ix) FEATURE:
(A) N~ME/KEY: Modified-site
(B) LOCATION: 1
(D) OTHER INFORMATIOh-: /note= "Xaa at pos.1 is Cys,
~s~m;n~ Cys or N-terr;n~71y blocked Cys"
(ix) FEATURE:
(A) NAME/KBY: Modified-site
(B) LOCATION: 8
(D) OTHER lN~OKM~TION: /note= "Xaa at pos.8 is a non-polar
amino acid or an uncharged polar amino acid~
(ix) FEATURE:
(A) NAME/KEY: Modified-site
(B) LOCATION: 9
(D) OTHER lN~o~I~TIoN: /note= "Xaa at pos.9 is an acidic
amino acid or a non-polar am.ino acid"
(ix) FBATURB:
(A) NAMB/KEY: Modi~ied-site
(B) LOCATION: 14
(D) OTHER l~O~I~TION: /note= "Xaa at pos.14 is Cys,
descarboxylated Cys or a C-term~n~-ly blocked Cys"
(xi) SEQUENCE DESCRIPTION: SBQ ID NO:2:
Xaa Gly Ser Xaa Val Pro Asn Xaa Xaa Arg Cys Cys Val Xaa
1 5 10
-23-
CA 02204906 l997-05-08
(2) INFORMATION FOR SEQ ID NO:3:
(i) SEQUENCE CHARACTERISTICS:
~A) LENGTH: 14 amino acids
(B) TYPB: amino acid
(C) STRANDEDNESS: ~ingle
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE DESCRIPTION: SEQ ID ~0:3:
Cys Gly Ser Glu Val Pro Asn Ser Ala Arg Cys Cys Val Cys
1 5 10
(2) INFORMATION FOR SEQ ID NO:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUE~CE DESCRIPTION: SEQ ID NO:4:
Cys Gly Ser Asp Val Pro Asn Pro Asp Arg Cys Cys Val Cys
1 5 10
(2) INFORMATION FOR SEQ ID NO:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(c) STR~NDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: ; nt~rn~l
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:5:
Cys Gly Glu Lys Thr Tyr Cys Met Pro Asn Cys Cys Val Cys
1 5 10
(2) INFORMATION FOR SEQ ID NO:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 amino acids
-24-
CA 02204906 l997-05-08
(B) TYPE: amino acid
(C) STRANDEDNESS: single
' (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE DESCRIPTION: SEQ ID ~0:6:
Cys Gly Glu Lys Thr Tyr Gly Met Pro Asn Cys Cys Val Cys
1 5 10
(2) INFORMATIO~ FOR SEQ ID NO:7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:7:
Cys Gly Asn Lys Val Pro Arg Ala Glu Lys Cys Cys ~al Cys
1 5 10