Note: Descriptions are shown in the official language in which they were submitted.
CA 02205670 1997-OS-16
MIXTURES FOR PIPELINE TRANSPORT OF GASES
Field of the Invention
This invention relates to the transfer by pipeline of mixtures which contain
methane or natural gas.
Backgiround of the Invention
As is well known, methane is the largest component of natural gas, and
a sually accounts for at least 95% by volume of what is known as "transmission
specification" natural gas. Other usual components are ethane (usually about
2%),
propane (usually about 0.5%), butanes, pentanes and possibly hexanes
(altogether
amounting to less than about 0.3%), with the balance being nitrogen and carbon
dioxide. In this disclosure, transmission specification natural gas will be
hereinafter
called "natural gas". For example, the natural gas as transmitted through the
pipelines
of TransCanada Pipeline Limited from Alberta, Canada to Ontario, Canada has
typically
the following percentage composition by volume:
Component Feed
Nitrogen 0.01270
Carbon Dioxide 0.00550
Methane 0.95400
Ethane 0.01970
Propane 0.00510
i-Butane 0.00170
n-Butane 0.00080
i-Pentane 0.00020
n-Pentane 0.00010
n-Hexane 0.00020
The relation between pressure, volume and temperature of a gas can be
expressed by the Ideal Gas Law, which is stated as PV = nRT, where:
P = pressure of gas
V = volume of gas
n = number of moles of the gas
CA 02205670 1997-OS-16
-2-
R = the universal gas constant (which, as is known, varies somewhat depending
on volume and temperature)
T = temperature of the gas.
If the equation is expressed in English units, the pressure is in pounds per
square inch
absolute (psia), the volume is in cubic feet, and temperature is in degrees R
(degrees
Fahrenheit plus 460).
The Ideal Gas Equation does not give exactly correct results in actual
practice, because gases are compressible. Gas molecules, when compressed, pack
more tightly together than would be predicted by the Ideal Gas Equation,
because of
intermolecular forces and molecular shape. To correct for this, an added term,
the
compressibility factor z, can be added to the Ideal Gas Equation. This is a
dimensionless factor which reflects the compressibility of the particular gas
being
measured, at the particular temperature and pressure conditions.
At atmospheric pressure or gage pressures of a few hundred pounds, the
compressibility factor is sufficiently close to 1.0 so that it can be ignored
for most gases,
and so that the Ideal Gas Law can be used without the added term z. However,
where
pressures of more than a few hundred pounds exist, the z term can be different
enough
from 1.0 so that it must be included in order for the Ideal Gas Equation to
give correct
results.
According to the van der Waals theorem, the deviation of a natural gas
from the Ideal Gas Law depends on how far the gas is from its critical
temperature and
critical pressure. Thus, the terms TR and PR (known as reduced temperature and
reduced pressure respectively) have been defined, where
TR = T
T
PR = P
P
where,
T = the temperature of the gas in degrees R
T~ = the critical temperature of the gas in degrees R
CA 02205670 1997-OS-16
- -3-
P = the pressure of the gas in psia
P° = the critical pressure of the gas in Asia
Critical pressures and critical temperatures for pure gases have been
calculated, and are available in most handbooks. Where a mixture of gases of
known
composition is available, a pseudo critical temperature and pseudo critical
pressure
which apply to the mixture can be obtained by using the averages of the
critical
temperatures and critical pressures of the pure gases in the mixture, weighted
according the percentage of each pure gas present.
Once a pseudo reduced temperature and pseudo reduced pressure are
known, the compressibility factor z can be found by use of standard charts.
One of
these is "Compressibility Factors for Natural Gases" by M.D. Standing and D.L.
Katz,
published in the Engiineering Data Book, Gas Processors Suppliers Association,
10th
edition (Tulsa, Oklahoma, U.S.A.) 1987.
When the compressibility factor z of methane is read from the charts, it
is found that the factor z is always less than 1.0 in normal temperature
ranges (i.e.
between about -40°F and 120°F) and that it decreases as 'the
pressure rises or the
temperature falls. Therefore, less energy need be used to pump a given volume
of
methane (measured at standard volume) at any given normal temperature than
would
be expected at that temperature if the methane were an ideal gas. This effect
is more
marked at higher pressures. Similarly, as the pressure is increased at a
constant
temperature, more methane (measured at standard volume) can be stored in a
given
vclume than would be predicted from the Ideal Gas Equation. "Standard volume"
is
volume measured at standard pressure and temperature (STP)).
Natural gas, like methane, shows z factor changes with pressure. Under
about 1000 psia the dominant variable in the power relationship is the
molecular weight
of the gas. At this pressure level, addition of further amounts of ethane or
propane
increases the molecular weight of the gas more rapidly than the z factor
decreases
Thus, there is an advantage to removing ethane and propane from the gas.
It is usual in the gas transportation and storage industry to try to strip out
higher hydrocarbons such as ethane, propane, butane and unsaturated
hydrocarbons
CA 02205670 1997-OS-16
-4-
from natural gas if the gas is to be transmitted through pipelines. This
leaves mostly
methane (with some traces of nitrogen and carbon dioxide) to be transported by
the gas
pipeline. The materials which are stripped out are then transported or stored
separately, often as liquids.
Summar)i of the Invention
It has now been found that, at pressures over 1,000 psia, it is
advantageous to add to natural gas an additive which is a C2 or C3 hydrocarbon
compound or a mixture of such additives. Above a lower limit (which varies
with the
additive being added and the pressure), this results in a smaller product of
the z factor
times the average molecular weight of the gas (hereinafter called the zMW
product ) than
would exist with methane alone, therefore leading to a decrease in the amount
of
power needed to pump the mixture or to compress it.
Detailed Description of the Invention
If ethane is the additive, enough ethane must be added to methane or
natural gas to give a gas composition having a minimum of about 26% ethane for
- operation at 1,000 psia and normal temperatures (-40°F to
+110°F). (All percentages
in this document are percentages by volume). Ethane can be added until just
before
the mixture separates into separate gas and liquid (which occurs at about 40%
ethane
for a pressure 1,000 psia and a temperature of about 35°F). To reduce
the danger of
liquefaction if there is inadvertent pressure drop, and to reduce temperature
extremes,
generally operation at 25-35% ethane and 35°F to +40°F is
preferred.
When the pressure is raised to 2,200 psia, the addition of enough ethane
to natural gas to give a gas composition having more than 6% ethane gives some
beneficial results. Thus, as pressure increases, in the range from 1,000 psia
to about
2,:?00 psia the beneficial results occur with less and less ethane. For the
most
beneficial results, however, an addition of enough ethane to give at least 15%
ethane
is preferred at pressures of 2,200 psia.
CA 02205670 1999-03-04
-5-
Thus, for ethane as an additive, an amount is added to give a gas which
has at least 26% ethane (but preferably 35% ethane) at 1,000 psia, and at
least 6%
ethane (but preferably 15% ethane) at 2,200 psia, with the minimum percentage
of
ethane decreasing smoothly with rise in pressure. Ethylene may be substituted
for all
or part of the ethane on a 1:1 volume basis. Where pressure fluctuates, as in
a gas
pipeline where the gas is compressed at compressor stations and becomes less
compressed as it flows between compressor stations, the pressure indicated is
the
maximum pressure to which the gas is compressed. In such a compression-
rarefaction
arrangement, it is preferred that the ratio between the most compressed and
the most
rarefied pressures of the gas not exceed 1.3:1.
C3 hydrocarbons alone can also be used as the additive. Minimum
useable percentage of the total gas mixture vary from a minimum of 5% at 1,000
Asia
to about 3% at 2,200 psia. Maximum amounts are those which will not cause
separation of a liquid phase at the temperature used. The C3 hydrocarbons may
be any
of propane, isopropane or propylene, separately or in admixture.
One or more C~ hydrocarbons may also be substituted, preferably on a
1:3.5 volume basis, for CZ hydrocarbons, but not to a point where they cause
separation
of a liquid phase at the pressure and temperature of operation. (A 1:3.5 basis
means
that each standard volume of C3 hydrocarbon replaces three and a half standard
volumes of C2 hydrocarbon.) Generally, the limitation that a liquid phase
should not be
formed means that not more than about 12% of C3 hydrocarbons should be present
at
1000 psia and 60°F, and lesser amounts should be used as the pressure
increases or
temperature decreases.
Two or more of the C2 or C3 additives can also be used. The use of two
or more additives has a synergistic effect in many cases, so that less than
the minimum
amount of each is needed than would be needed if only one were present, in
order to
produce a zMW product smaller than that of an equivalent standard volume of
natural
gas at the pressure and temperature involved.
At pressures over 1000 psig, CQ hydrocarbons do not contribute much to
the improvement of the zMW product. Thus C4 hydrocarbons are not additives
CA 02205670 1997-OS-16
-6-
contemplated by this invention. However C4 hydrocarbons which are already
present
in the natural gas need not be removed if they are present in insufficient
quantity to
liquify or to affect the zMW product very adversely. The presence of more than
1 % C4
hydrocarbon in the mixture is not preferred, however, as C4 hydrocarbons tend
to liquify
easily at pressures between 1,000 psia and 2,200 psia, and more than 1% C4
hydrocarbons give rise to increased danger that a liquid phase will separate
out. C4
hydrocarbons also have an unfavourable effect on the mixture's z factor at
pressures
just under 900 psia, so care should be taken that, during transport through a
pipeline,
that mixtures according to the invention which contain C4 hydrocarbons are not
allowed
to decompress to less than 900 psia, and preferably not to less than 1,000
psia.
The addition of amounts of additive below the lower limit (unless two
additives with a synergistic effect are used) actually increase the zMW
product over that
of methane or natural gas alone, and is thus detrimental. For example, when
the
pressure is 1,000 psia and the temperature is 35°F, mixtures of methane
and ethane
having less than about 26% ethane have a z factor greater than methane alone
(all
percentages are based on volumes at standard pressures and temperatures).
Adding
ethane to increase the percentage of ethane from 2% to, for example 12% at
this
pressure and temperature is therefore counterproductive, as it increases the
zMW
product and therefore increases the energy required to pump or compress for
storage
a given standard volume of gas. However, when more than 26% of ethane is
present,
the z factor falls so much that the zMW product fiends to lower values than
that of pure
methane. The z factor continues to get smaller with increased percentages of
ethane,
bringing with it a lower zMW product to the point where further increase of
ethane causes
separation of a liquid phase (at about 40% ethane at 1,000 psia and
35°F). Thus,
adding ethane to natural gas so that there is a mixture containing more than
26%
ethane at 1,000 psia and 35°F leads to increased packing of molecules
and a
decreased zMW product, hence decreased pumping costs and more ability to store
wii:hin a given volume. At 1,350 psia and 85°F, improved results over
methane alone
are obtained when only 17% of ethane is present in the mixture. Where the
pressure
is increased to 1,675 psia at 35°F, mixtures with 13% or more ethane,
and the balance
CA 02205670 1997-OS-16
-7-
methane give improved results over methane alone. At 2,140 psia and
35°F, the
improved effect is shown in mixtures of 6% ethane and the balance methane.
By the avoidance of liquid phase in this disclosure is meant the avoidance
of enough liquid to provide a coherent liquid phase in the pipeline at the
temperatures
and pressures used. Such a phase can create pipeline problems through pooling
in low
portions of the pipeline or forming liquid slugs which affect pumping
efficiency. A few
liquid droplets in the line however, can be tolerated.
The use of a hydrocarbon as additive has the additional advantage that
it permits transport of a mixture of methane or natural gas and the
hydrocarbon additive
in a single pipeline with less energy expenditure than if the two were
transported
sE~parately in separate pipelines.
Brief Description of the Drawings
The invention will be described further in association with the following
drawings in which:
Figures 1A to 1 E are plots of capacity gain in percent against the content
of C2 hydrocarbons in a mixture of methane and ethane. Each of the plots shows
the
results at a different pressure. Figure 1 F shows calculations of flow ratios
for various
mixtures of methane and ethane..
Figures 2A and 2B are plots of capacity gain versus temperature (in
degrees Fahrenheit) for pipelines at 800 psia and 1,675 psia respectively.
Figure 3 is a summary of pipeline horsepower requirements for various
gays mixes, using a 36" pipeline operating at a maximum operation pressure of
1,740
psia, an inlet temperature of 80°F and a ground temperature of
32°F.
Figure 4 is a plot of the horsepower requirements per thousand cubic feet
of gas showing different mixtures of ethane and methane, at different
pressures.
Detailed Description of the Embodiments Shown in the Drawing~~s
Dealing first with Figure 1, this shows, for various pressures and the
same temperature, the effect of the addition of ethane to methane. In each
case, the
CA 02205670 1999-03-04
_8_
z factor has been calculated for each percentage of ethane from 0 to 40%.
Then, the
lowest calculated z factor has been arbitrarily defined as 0% capacity gain.
Each of the
other results has been plotted as a percentage capacity gain with reference to
the 0%
capacity gain in order to prepare a curve. Curves developed in this way are
given in
Figures 1A to 1 E, for different pressures, with each curve representing the
situation for
one pressure. The temperature represented by each curve is 35°F.
Looking at Figure 1A, it is seen that, for an 800 psia pipeline, the best
packing occurs when the line is filled with pure methane. As ethane is added,
the
capacity gain percent decreases until there is about 25% ethane in the line.
After this,
the capacity gain begins to increase again, but it does not reach the levels
obtained for
pure methane.
Figure 1 B shows the effect of addition to methane of ethane for a pipeline
at 1,150 psia. Here, the capacity gain steadily decreases from D% ethane to
about 12%
ethane, and then increases again. After approximately 25% ethane, the capacity
gain
is greater than occurred with no ethane at all.
Figure 1 C shows that this effect is even more pronounced when the
pipeline pressure is increased to 1,350 psia. The lowest capacity now occurs
at
approximately 7%, and mixtures with more than 17% ethane exhibit packing (and
hence, pipeline or storage capacity) gains unattainable with of natural gas or
methane
alone.
Figure 1D shows that at 1,675 psia, the lowest capacity occurs at about
5%, and anything over 12% ethane gives a better capacity gain than is
attainable with
natural gas or methane alone. For best results, however, at least 15% ethane
should
be present.
At 2,140 psia (Figure 1 E) the addition of about 4% ethane gives a benefit,
and the benefit steadily increases all the way up to the point at which the
ethane begins
to separate out in a liquid phase. For best results, however, at least 12%
ethane, and
preferably 15% ethane, should be present.
CA 02205670 1999-OS-21
_g_
Figure 1 F shows the effect of the z factor and its product with the average
molecular weight of the gas for gases having various amounts of methane and
ethane
(the ethane is shown in the table as "C2") The flow ratio (1lthe root of the
zMW product)
is plitted..
Thus, it will be seen that for pressures above about 1,000 psia better
packing, and hence lower Compression cost and pumping cost for transportation
occurs
when increased amounts of ethane are added over a minimum amount which
decreases with increasing pressure. At 1,150 psia (Figure 1 B) about 24%
ethane must
be added to get the same packing effect as the approximately 2% ethane in
normal
natural gas. If more than this amount is added, however, better packing occurs
with
each addition. As the pressure increases, successively less ethane need be
added to
give better packing (and hence lower transmission costs and compression costs)
than
with natural gas. Indeed, at .2,140 psia even about 4% ethane shows sonie
advantage,
although the advantage is of course greater as more ethane is added: v
, Figure 2 shows how the effect changes with .temperature. Even at 800
psia (Figure 2A) there is a capacity increase (i.e. better packing) as
temperature drops,
and the capacity gain is greater the more C2 that is present. However, the
effect is not
nearly as significant as ai: higher pressures. With higher pressure (Figure
2B) the
capacity gain is much greater with temperature, and the improvement in
capacity gain
becomes still greater as increased amounts of ethane are added. Generally,
therefore,
it is preferred to operate at a relatively low temperature, such as 70°
to -20°F. Higher
temperatures (e.g. up to about 120°F) can be tolerated, but detract
from the benefits
of the invention.
Figure 3 shows horsepower required for different gas mixes of ethane and
methane, through a pipeline at a maximum pressure of 1,740 psia and a minimum
pressure of 1,350 psia. Figures are for a pipeline of 36" in diameter and a
length of
1,785 miles, with pumping stations located every 56 miles. At a throughput of
2.0
million, standard cubic feet per day, a mixture of 98% methane and 2% ethane
(which
corresponds to ordinary natural gas) would require 812,579~horsepower.
However, the
same standard volume of gas, but containing 35% C2, can be moved with only
651,860
CA 02205670 1997-OS-16
-10-
horsepower, for a saving of over 150,000 horsepower. When the throughput is
raised
to 2.5 million standard cubic feet, natural gas containing 2% ethane cannot
practically
be transmitted, because the velocities and temperatures involved are too
great.
However, gas with 6% ethane can be transmitted, and gas with 35% ethane shows
a
saving of over, 500,000 horsepower over that which is used to transmit the gas
with 6%
ethane.
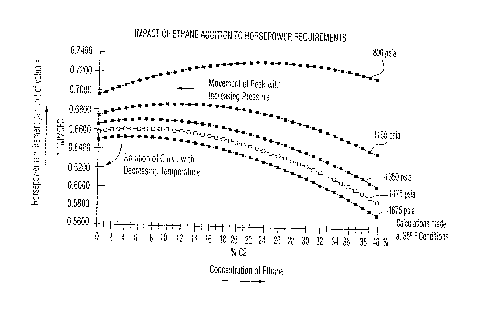
Figure 4 shows the effect on horsepower requirements per million cubic
feet of gas being pumped through the same pipeline as used in Figure 3 when
the
pipeline gas contains different concentrations of ethane at 35°F.
Figure 4 also shows the negative effect of adding ethane to a typical
pipeline running at about 800 psia pressure and 35°F. Required power
for pumping
increases until the mix contains 26% ethane and then decreases for higher
concentrations approaching the liquid phase limits. However, the decrease is
not
sufficient so that, by the concentration where liquefaction occurs (about 40%)
there is
any saving of horsepower over pumping ordinary natural gas. This energy hill
however
peaks at decreasing concentrations of ethane as operational pressure
increases, e.g.,
14% at 1,150 psia, 8% at 1,350 psia, 6% at 1,475 psia. This is due to the rate
of
decrease in the value of the z factor overcoming the rate of increase in
density.
As noted, at 800 psia, increasing the amount of ethane even up to 40%
of the mixture does not produce a saving of horsepower. At 1,150 psia,
however, an
in~;rease of ethane to over about 24% causes a horsepower saving when compared
to
the values at 2% ethane (which is approximately the amount of ethane in most
natural
gas). At 1,350 Asia, a decrease in horsepower occurs with mixtures containing
more
than about 14% ethane. As the pressure increases, the horsepower saving occurs
with still less added ethane. As can be seen from Figure 4, the peak
horsepower
requirement shifts to the left of the graph and becomes smaller as pressure
increases.
However, reading Figure 4 with Figure 2A and 2B shows that, as temperature
decreases, the curve tends to rotate to the right. Thus, as temperature
decreases, less
addition of ethane is necessary to obtain the desired additional packing and
hence
decreased compression and pumping energy requirements. The result is lower
CA 02205670 1997-OS-16
-11-
compressive power requirements for equivalent volumes of higher B.T.U. gas
mixes
than can be achieved with conventional pipeline specification natural gas.
Higher
pipeline throughput are able to be economically achieved with these enhanced
gas
mixes with corresponding increases in horsepower.
Similar effects are seen when ethylene is used in substitution for all or part
of the ethane.
When propane, isop,ropane or propylene, or mixtures of any of these
gases, are substituted for all or part of the ethane, the effects are even
more
pronounced than with ethane, and occur with a smaller addition of each of the
compounds mentioned than was necessary with ethane as noted previously in this
disclosure.
The preferred composition of the resulting gas is as follows:
COMPONENT MAXIMUM VALUE MINIMUM VALUE
METHANE . 92% VOLUME 68% VOLUME
(ETHANE AND/Or 35% VOLUME 6% VOLUME
ETHYLENE
(PROPANE AND/OR 12% VOLUME 0% VOLUME
OTHER C3
(BUTANES AND OTHER Not required , but 0% VOLUME
COMPONENTS OF THE amount present in
NATURAL GAS original natural gas
(up to
about 1 %)can be
tolerated if it does
not
cause separation of
a
liquid phase at the
pressure and
tem erature used
CA 02205670 1997-OS-16
-12-
NITROGEN Not required, but 0% VOLUME
amount
present in the original
natural gas can be
tolerated if below
2% by
volume
CARBON DIOXIDE Not required, but 0% VOLUME
amount
present in the original
natural gas can be
tolerated if below
1 % by
volume.
COMPONENTS TOTAL
100%
TEMPERATURE 120F -20F
PRESSURE 2160 PSIA 1150 PSIA
Compositions with hydrocarbon additives outside these ranges generally
are of little economic benefit, or approach 'the limits at which iv~ro-phase
or liquid
behaviour can be seen. Therefore, if mixtures outside these parameters are
used, care
should be taken to avoid conditions which might cause liquid to fall out of
the mixture.
Liquid is generally to be avoided, as it may pool in low areas of a pipeline
and be
difficult to remove. The preferential liquefaction of some components will
also cause
the composition of the gaseous phase of the mixture to change, thereby
changing the
z factor and hence the compressibility of the gaseous phase.
The foregoing has illustrated certain specific embodiments of the
invention, but other embodiments will of course be evident to those skilled in
the art.
Therefore it is intended that the scope of the invention not be limited by the
embodiments described, but rather by the scope of the appended claims.