Note: Descriptions are shown in the official language in which they were submitted.
02206~37 1997-0~-30
GR 94 P 3717 P ..LE,~t~
TE*~- T~ ,LhT~
Description
Fuel cell with ceramic-coated bipolar plates and its
production
The invention relates to a fuel cell having the
features mentioned in the preamble of claim
(DE-A-42 37 602), in particular a high-temperature fuel
cell having a solid electrolyte of ion-conducting oxide
(so-called solid oxide fuel cell, SOFC). The invention
also relates to a method for coating the metallically
conductive plates used in a cell of this type, as well as
to a method for producing this cell.
Many known fuel cells have a tubular structure.
When the fuel cell has a planar layer structure, an
energy density which is substantially higher than in the
former case is to be expected (according to current
experience, about 1 MW/m3). The energy generation is in
this case based on the controlled chemical conversion of
o~y~e~ ions and hydrogen into water, that is to say on
the so-called cold hydrogen-oxygen reaction, which
respectively takes place in an active chamber which is
divided by an active layer structure into two sub-
chambers lying above one another. A hydrogen-cont~; n; ng
gas (for example hydrogen), a gas mixture (H2jCo/Co2)
produced from collventional hydroc~hon fuels, or a
mixture (H2/CO/CH4~ produced by reforming natural gas,
flows through one of the sub-chambers, while an oxygen-
cont~;n;ng gas, for example oxygen or air, flows through
the other sub-chamber.
A high-temperature fuel cell of this type, as is
developed further by the invention, is described in
DE-A-42 37 602. The most important constituent of the
active layer structure is a layer of an electrolyte. The
electrolyte surface respectively adjoining the sub-
cha~hers lying above and below
.
02206~37 1997-0~-30
GR 94 P 3717 P - 2 -
is de~igned as an electrode whose electrode potentials
are tapped via contact segments of electrically con~nc-
tive plates which lie opposite the electrolyte surfaces.
By series connection of a plurality of active chambers of
this type, bounded above and below by electrically
conductive plates, the potential differences occurring
across the individual electrolyte layers can be added to
form considerable voltages.
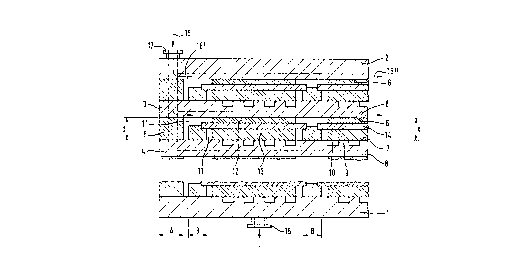
Figure 1 show~ the ba~ic structure of a ~n~ rich
~tructure of this type, covered by a metallically conduc-
tive base plate 1 and cover plate 2, which essentially
corresponds to the prior art and has the following
features:
- each pair of metallically conductive plates 3, 4,
arranged above one another, forms between them an
active chamber R surrounded by an outer assembly
region A, the active chamber being ~ubdivided by an
ion-conducting active layer structure (for example
a plate 14 made of the abovementioned solid electro-
lyte, and one electrode layer 12, 13 on each side of
the electrolyte plate) into two sub-chambers 11, 11'
which lie above one another and are closed off from
one another;
- this active ch~mber R is laterally closed off in
gas-tight fashion by insulating segments 5, the
insulating segments 5 being arranged in the outer
assembly region A and keeping the plates 3, 4 at a
distance d from one another; and
- the surface 6, 7 of these plates 3, 4 is in each
case profiled in the region of the active chamber R
and forms contact segments 8, 9, to which the active
layer structure is respectively applied by one of
the electrode faces 12, 13.
In the case of an imp~rme~hle solid, the total
electrical conductivity Atotal can be divided into a
"metallic" conductivity Ael, which depends on the elec-
trons which are in the conduction band of the solid and
have a high mobilit:y which decreases with increasing
temperature, and an ion conductivity Aion~ the
'' CA 02206~37 1997-0~-30
}
GR 94 P 3717 P - 3 -
ba~is of which is ~he re~tricted mobility of ion~ in the
solid (in the ca~e of an oxide ~olid electrolyte: o2-)
and increa~es with increasing temperature:
Atotal = Ael ~ Aion
The partial conductivities Ael and Aion are described by
the concentration of the correspo~;ng charge carrier~
and by "transport number~" tel and tion which depend on
the value of the charge~ and their mobility within the
crystal ~tructure of the ~olid.
For fuel cell~, it is important for the oxygen
and the hydrogen ~upplied to the ~eparate ~ub-chamber~ to
be ionized by electron ~Y~h~nge with the corre~po~;ngly
charged electrode~ of the chamber~. During the formation
of H+, electron~ are respectively given up at the elec-
trode of the corre~po~;ng ~ub-ch~ber and are di~charged
via the corre~po~;ng plate before, on the other ~ide of
the plate, either being given up to form o2~ or being
tapped a~ a current in a load circuit connected to the
cell. In thi~ ca~e, through ion migration, the electro-
lyte layer allow~ the ion~ to combine and form H20.
It i~ therefore nece~~ary in term~ of de~ign forthe plate~ arranged between individual active chamber~ to
seal the~e chambers again~t diffu~ion of the reaction
ga~e~ and the ion~. In phy~ical term~, at lea~t the
plate~ 3, 4 which lie between the ba~e plate 1 and the
cover plate 2 and re~pectively ~eparate two neighboring
chamber~, mu~t have a high electron conductivity in order
to permit the formation of H+ in the ~ub-chamber of one
of the chamber~, and ~imultaneou~ly the formation of o2~
in the ~ub-chamber of the other chamber. They are there-
~ fore referred to a~ bipolar plate~ (BIP). For the
material of the electrolyte layer, the direct oppo~ite i~
true: it mu~t have a low electron conductivity, ~o that
the ionization potential i~ ~u~tained at the electrode~,
CA 02206~37 1997-0~-30
GR 94 P 3717 P - 4 -
but must permit the requisite migration of the ions.
Thu~, as regards the ratio of the transport numbers for
electrons and ions r which characterizes the mobility of
the electrons and ions at the operating temperature of
the fuel cell (600 to 1000~C), then this ratio must be
set greatly in favor of electrons for the BIP and greatly
in favor of the ions (in particular oxygen ions) for the
electrolyte layer.
As the electrolyte 14, use is normally made of
zirconium oxide (ZrO2), the linear coefficient of ~h~rr-l
expansion of which must be compatible with the linear
expansion of the BXPs 3, 4, in order to ensure that the
overall arrangement is stable and leaktight. In this
case, provision is made to divide the active layer
structure R into a plurality of units arranged next to
one another. The edge of the layer structure K, or of its
units, is in this case respectively held in an inner
assembly region B i.n such a way that the sub-ch~her 12
consists of a plurality of spaces which lie next to one
another and are each closed off in gas-tight fashion from
the sub-chamber 11.
The plates themselves may consist of an electri-
cally conductive ceramic which is particularly tailored
to the requirements of this field of application, of
steel with high shape stability or of an alloy in which,
for example, an oxide is dispersed. A particularly
suitable example of an oxide dispersion alloy (ODS alloy)
of this type is a chromium-based alloy cont~; n; ng 5% iron
and 1% yttrium oxide (Y2O3), the chromium-iron alloy
being essentially matched to the linear expansion of the
electrolyte, while the oxide dispersed therein serves
primarily to improve the corrosion properties of this
alloy.
Figure 1 also shows a feed connector 15 for a gas
which is fed into the gas channels 10 that are perpen-
dicular to the plane of the drawing and is discharged via
a discharge connection (not shown). Correspo~;ngly, the
arrows 16, 16', 16'' also indicate that the other gas is
fed via the gas connection
CA 02206~37 l997-0~-30
GR 94 P 3717 P - 5 -
17 into the channel.s between the contact segments 8, and
thereby through the other sub-chambers of the active
chambers, before being discharged through a discharge
connector (not represented in Figure 1) on the other side
of the fuel cell. The water produced during the cold
hydrogen-oxyyell rea.ction is discharged from the fuel cell
through these gas flows, together with the residual
enthalpy of reaction which is not converted into electri-
cal energy.
If the bipolar plates 3, 4 are not insulated from
one another sufficiently by the segments 5, then internal
electrical losses occur which can greatly impair the
efficiency of the fuel cell. In order for internal
electrical losses of this type not to exceed 1 per
thousand, care must be made to provide sufficient insula-
tion in the segments 5 of the outer ass~mhly region A.
In addition, the fuel cell should have substan-
tially no leaks through which one of the reaction part-
ners can escape. It is customary to seal the fuel cell in
2 0 the assembly region. using a solder glass which is stable
at high temperatures, but for a customary layer thickness
of about 700 ~m, the leaktightness of such a wide
soldered gap raisec considerable difficulties.
In particular, it raises technical difficulties
25 of filling such wide soldering sites with soldering
material, without giving rise to internal stresses or
even microcracks which could lead to failure of the
oldering sites, an a~la~ating factor being that sealing
the soldering site requires a sintering process which is
usually associated with a reduction in volume.
It is also difficult for solder glass, which
fills a wide gap, to be sintered in such a way that the
soldering material does not start to flow and gain access
to regions of the active chamber in which it has a
3 5 disruptive effect.
A solder glass is generally an oxide powder
(usually white) which is m;Y~ with a binder (for example
an organic binder),
CA 02206~37 l997-0~-30
GR 94 P 3717 P - 6 -
in order to permit controlled application of the solder-
ing material. The soldering itself i~ then performed by
heating, in which case the organic binder escapes and the
rr~-;n;ng oxide is fused or sintered and thereby forms a
gas-imperr~-hle amorphous filler.
This ~intered amorphous filler has electron
conductivity which, although it decreases with increasing
temperature, cannot be ignored, especially if chromium
oxide diffuses from the bipolar plate into the solder
glass during the fusion or sintering process, for
example. During the sintering, it is also possible for
chromium oxide that has diffused in to be reduced and for
chromium boride or other compon~nts which impair the~
insulation to be formed.
Vapors which are produced when the binder is
burnt off or escapes may also be toxic and difficult to
dispose of. For this reason, care should be taken that
the amount of solder glass needed remains limited.
The escaping binder can also damage other sur-
faces in the active ch~her, in particular the sensitiveelectrode surfaces of the active layer structure.
It has therefore already been proposed, instead
of completely filling the gap width d with solder glass
of this type, to solder a correspon~; ng frame made of an
electrically insulating ceramic (for example "spinel",
MgAl204) into the soldering gap. Correspo~;ngly, the
assembly region according to the prior art is thus
characterized by a layer sequence: BIP/solder
glass/spinel/solder glass/BIP.
An oxide frame of this type, the height of which
should be only a few 100 ~m, nevertheless requires a
large outlay on production and careful treatment. It can
therefore
' CA 02206~37 l997-0~-30
GR 94 P 3717 P - 7 -
only be used in the laboratory, and not under economic
manufacturing cond;tions.
At the high operating temperatures, the ~urfaces
of the BIPs forming the gas ch~nnels on the cathode side
of the electrolyte layer are also particularly sensitive.
Indeed, oxygen corrosion may occur there in the alloy of
the BIPs, in particular the formation of chromium oxide.
For its part, thi~ chromium oxide may, through solid-
state diffusion, reach other components of the SOFC and
damage them. Similarly, hydrogen corrosion or carbon
corrosion may occur on the surface of the BIP on the
anode side in the gas ch~nnels, which may in the long
term lead to embrittlement and destruction of the cor-
respon~;ng contact segments.
The invention makes it possible to seal the fuel
cell in the assembly region in gas-tight and electrically
insulating fashion, in a simple manner which can be
carried out economically on an industrial scale. In
particular, the required mechanical stability of the fuel
cell can be achieved in simple fashion in this case.
However, in addition, the invention makes it possible to
protect the fuel-cell surface exposed to the aggressive
operating gases from chemical attack by these gases.
The basis of the invention is to protect the
surface of a bipolar plate by an electrically insulating
coating, at least in the regions in which diffusion
processes may cause particular problems. For this protec-
tive layer, low electron mobility is thus required in
addition to low permeability for the reaction gases and
other neutral extraneous substances, while particularly
low ion mobility is not necessary, since an electric
field is a prerequisite for transport of such ions. The
same properties (low transport n~hers for electrons,
high transport numbers for ions, in particular oxygen
ions) are required in the selection of the
-
CA 02206~37 1997-0~-30
GR 94 P 3717 P - 8 -
electrolyte of the active layer structure, so that a
crystalline oxide, as is suitable for the electrolyte,
can also be used for the coating.
Thi~ is provided primarily in the a~sembly
regions of the fuel cell. However, a protective layer of
this type can equally well also, as a diffusion barrier
layer on the surfaces of the gas ch~nnels, prevent
aggressive operating gases from diffusing into the
material of the BIPs.
A firmly adhering and imp~rm~hle coating of this
type, made of electrically insulating crystalline
ceramic, may, for example, essentially consist of zirco-
nium oxide (ZrO2) which, in particular, is stabilized by
a stabilizing componçnt, for example CaO, Y203, MgO, CeO2,
etc. Al203 or the abovementioned spinel or combinations of
these components, may also be used as the coating
material. The coating itself may also be built up in
layers, for example with a thin bo~;ng layer and a
thicker cover layer. It may namely be possible for a
highly adhering material to be applied economically only
in thin layers, but for a material which is cheaper to
apply to have poorer adhesion to the BIP surface.
A suitable adhesion base is, for example,
NiCrAlY.
It is also sufficient for only the surface of one
of the two plates to be coated and the opposite surface
of the other plate to bear a less insulating material
which is used only for gas-type filling of the rem~;n;ng
gap in the assembly region.
The coating material may, for example, be applied
as a dispersion or colloid in a gel, which is sub-
sequently dried in air and burnt into the surface. A
screen printing process or wet powder spraying (WPS) is
also possible,
CA 02206~37 1997-0~-30
GR 94 P 3717 P - 9 -
the material which is applied being subsequently burnt in
in air and sintered.
It is like.wise possible, especially with very
thin layers, to produce a correspon~;ng coating by
chemical vapor deposition (CVD). Atmospheric plasma
spraying (APS) or flame spraying with high jet pressure
(for example 6 bar) and correspo~A;ngly high jet velocity
also make it possible to apply the abovementioned protec-
tive layers.
While the thickness of the coating is advanta-
geously between about 30 and 50 ~m in the region of the
gas channels, coating thicknesses of between about 30 and
200 ~m are advantageous in the ~uter assembly region.
While a solder glass contains grains of an amorphous
oxide and, during soldering, is sintered to give an
amorphous impprmeAhle mass which lacks insulation, the
coating comprises a dense crystalline ceramic in which
virtually no electrical cnnAllction takes place.
According to claim 1, and one aspect of the
invention, the insulating segments of the fuel element
are formed by a firmly adhering imperm~Ahle coating on
the surfaces of the two plates in the assembly region of
the fuel cell, the coating having an insulating effect
against electron conduction, and the rc~-;n;ng gap
between the coated ~urfaces being filled with an imperme-
able filler, in particular the abovementioned solder
glass. In this case, the coating advantageously consists
of a crystalline ceramic whose transport number for
electrons is substantially smaller (for example a factor
of 0.01 or less) than for ions (in particular oxygen
ions). An amorphous oxide (for example glas~) is particu-
larly suitable for the filler.
= The segments accordingly consist, for example, of
the layer sequence BIP/crystalline ceramic/amorphous
oxide/crystalline ceramic/BIP. In this case, it may be
sufficient to provide just the surface of one
- CA 02206~37 l997-0~-30
GR 94 P 3717 P - 10 -
of the two bipolar plates with the coatiny, which cor-
responds to the layer structure BIP/crystalline
ceramic/amorphous oxide/BIP.
The filler is, for example, a powder, in particu-
lar oxide powder (for example a solder glass) whichoriginally contain a binder and is consolidated and
virtually freed of binder by sintering. Thus, a sintered
or fused sheet is preferably used. Sheets of this type
are commercially available as solder glass green sheets
and are particularly suitable for inexpensive construc-
tion of the fuel cell according to the invention.
According to another aspect, the fuel cell
mentioned at the start is characterized, according to the
features of claim 9, in that a firmly adhering coating of
a crystalline ceramic which is impermeable and resistant
to corrosion by the reactive gas, and whose transport
nl~her for electrons is substantially smaller than for
oxygen ions, is applied to the surfaces of the plate at
least in the gas channels.
In order to produce the fuel cell according to
the invention, according to claim 19, the profiled plates
which are required as bipolar plates may first be provi-
ded and coated on the parts of their surface intended for
the coating. Likewise, the active layer structure
required for each active ch~mher is provided. A further
starting material is a sheet which consists of an amor-
phous oxide powder held together by a binder and whose
sheet thickness is greater than the distance between the
at least partly coated surfaces of the plates which is
30 intended for the finished fuel cell. Sheets of this type
can be manufactured in long webs which are subsequently
cut up into individual sheets (sheet sections) in cor-
respo~n~e with the assembly regions of the fuel cell,
that is to say their cross section corresponds approxi-
35 mately to the cross section of the fuel cell and theycontain cut-out windows
CA 02206~37 l997-0~-30
GR 94 P 3717 P - 11 -
into which the active layer structures fit. The coated
plates, the active layer ~tructure~ and the ~heet ~ec-
tion~ are then formed into a ~andwich ~tructure, in which
the ~heet sections are each placed between two plate~,
and the active layer ~tructure~ in the free ~pace~ of the
~heet ~ection~. The sandwich structure is then ~intered
until its height reache~ the intended height of the fuel
cell.
Thi~ and other features of the invention will be
explained in more detail with reference to a preferred
illu~trative embodiment and three further figure~.
FIG 1 shows a fuel cell, already de~cribed, which for
practical purpo~e~ i~ the prior art,
FIG 2 ~how~ the surface of a bipolar plate and it~
coating,
FIG 3 ~hows a cro~~ ~ection through a part of the
active ch~mher at the edge of the fuel cell
according to the preferred illustrative embodi-
ment, and~0 FIG 4 show~ the ~andwich structure of the parts shown
in Figure 3, before the final sintering.
The outer assembly region A, which represents the
side edge of the fuel element and in which the bipolar
plate~ are held at a mutual ~eparation d, ha~ already
been expl~;neA at the ~tart. Figure 2 ~hows the plan view
o~ a bipolar plate 30 of this type, which extends over
the cross section of the fuel element and, in this case,
is composed of a plurality of parts soldered together: in
the outer assembly region, the plate 30 contains a
thick~neA edge 32, into which a metal sheet or a
CA 02206~37 l997-0~-30
GR 94 P 3717 P - 12 -
metal platelet is inserted, Figure 2 showing four plate-
lets 32' lying next to one another.
At lea~t one surface of the thickened edge is,
according to the invention, coated with a crystalline
ceramic (already mentioned at the start) which insulate~
substantially against electron conduction and has a
thickness of about 150 ~m. The ceramic bears an imperme-
able filler. Longit~l~;n~l grooves 31 can be seen in the
windows of the platelets 32' and are connected below the
platelets 32' to supply ch~nn~ls 33 and discharge chan-
nels 34 and form gas rh~nnels on one of the ~ides of the
plate 30, while correspo~;ng transverse grooves form gas
channels on the other side (not visible in Figure 2) of
the plate 30 which are co~nected to supply chAnnels and
discharge channels 36, 37. In this case, one of the gas
connections 38 for these ch~nn~ls 36, 37 and the trans-
verse grooves can also be seen, while the correspon~;ng
term;n~ls which are arranged above and below on the fuel
element for the channels 33, 34 is [sic] not visible.
The segments 39 ext~n~;ng between the longitudi-
nal grooves or ga ch~nnels 31 are not coated on their
upper side. Instead, as contact segments, they form the
electrically conductive contact for one of the electrodes
of the active layer structure. Nevertheless, the surfaces
of the longitl~;n~l grooves or gas channels 31 likewise
bear a coating of t:he crystalline ceramic, the thickness
of which is about 30 ~m. The material of the plate 30 is
essentially a chromium base alloy with 5% iron content.
Figure 3 shows a cross section through the edge
of the finished fuel cell. The bipolar plate 30 as well
as the correspo~; ng underlying bipolar plate 30' can be
seen here. An oxygen-contA;n;ng reaction gas is fed via
the gas supply ch~nnels 33 to the longit-l~;n~l yLo~ve~ or
gas ch~nnels 31, while a hydrogen-/carbon-cont~;n;ng
reaction gas flows through the correspo~;ng transverse
grooves 31'.
CA 02206~37 lgg7-o~-3o
GR 94 P 3717 P - 13 -
The surface~ of the two bipolar plates 30, 30'
are provided with the coating 41, 41' of crystalline
ceramic, only the segments 42 existing between the gas
channels not being coated on their outer side. The active
structure 43, the essential part (layer 44) of which is
formed by a ceramic solid-ion [sic] conducting oxide, for
example zirconium oxide (ZrO2, stabilized by the addition
of Y2O3), is applied to these outer sides. As is customary
in the prior art, one electrode layer (cathode 45, anode
46) is arranged on both sides of this electrolyte layer
44, a functional layer 47, 48 being additionally provided
in each case for balancing height differences and surface
irregularities on these electrodes. The functional layer
48 may, for example, be a network-like metal-ceramic (so-
called cermet) which in this case is based on nickel.
A decisive property of this functional layer isthat it has a very good conductivity for electrons, while
the conductivity for ions plays a subordinate role. In
contrast to the material of the electrolyte and of the
coating 41, 41', the transport numbers for electrons are
large, but as small as possible for ions.
In DE-A-42 37 602, which is mentioned at the
start, and whose content up to the coating of the BIP is
to be ascribed to the disclosure of the present inven-
tion, it is proposed to spray the functional coating ontothe surface of the BIP as well, that is to say onto the
surfaces of the gas channels.
The bipolar plates 30, 30' are held by a segment
50 which extends in a ring around the edge of the fuel
cell, at a distance d (approximately 700 ~m). This
segment seals the sides of the fuel cell in ga~-tight
fashion and insulates the BIPs 30 and 30'. To this end,
the surface of the BIP 30 i~ coated according to the
invention with a protective layer of stabilized ZrO2
(thickness d' of t;his protective layer about 100 ~m),
which provides a ~irmly adhering leaktight insulation
layer. A
CA 02206~37 1997-0~-30
GR 94 P 3717 P - 14 -
correspo~;ng protective layer 52 (thickness d'' about
150 ~m) is also located on the surface of the BIP 30'.
The r~mA;n;ng space between the two protective layers 51
and 52 is, according to the invention, filled in the
region of this segment 50 (outer a~sembly region) with a
fused or sintered solder glass which, in correspo~nce
with the composition of the solder glass grains, forms a
network structure of amorphous oxide.
This layer structure of the segment 50 prevents
a current carried by electrons in the conduction band of
the coating material. At the same time, during soldering,
the protective layers prevent ions or other materials,
which may increase the conductivity of the solder glass
layer, from being able to diffuse from the plates 30, 30'
into the segment. The solder glass layer 53 therefore has
a low electrical conductivity, even at the operating
temperature.
As shown by Figure 3, the edge of the active
layer structure 43 is fused into a layer 54, protruding
from the outer assembly region A and it~ filler (solder
glass 53) into the inner assembly region B, and is held
on the surface of the BIP 30'. This also ensures that the
active chamber, formed by the plates 30, 30' and the
~egments 50, is divided into an upper chAmher 49, con-
nected to the gas channels 31, and a lower chamber 49',
connected to the chAnnels 31', these chambers being
closed off in gas-tight fashion from one another and only
being in ion-conducting connection with one another via
the electrolyte.
During the end stage of the manufacture of this
fuel cell, the sandwich structure shown in Figure 4 is
stacked and is then just exposed to the temperatures
which lead to solidification of the filler (53, 54 in
Figure 3). This sandwich structure consists of the lower
BIP 30', with the protective layers 41' and 52, as well
as two sheets 53, 54, lying above one another, which are
cut from commercial, so-called solder glass green sheets.
In these solder glass green sheets, the correspnn~;ng
.
CA 02206~37 l997-0~-30
GR 94 P 3717 P - 15 -
solder glass powder is bound by means of an organic
binder which escapes at the soldering temperatures (800
to 1000~C). The thickness of these sheets i~ chosen in
such a way that the distance do between the coated sur-
faces of the plates 30, 30' is greater than the amountintended for the finished fuel cell; this makes it
possible, during a subsequent soldering process, for a
volume reduction to take place in the soldering material
as well, it is also being possible for the height of the
solder layer to be reduced in favor of a greater width.
The two solder glass green sheets 53 and 54
contain cuts, forming windows, into which the individual
layers of the act:ive layer structure, and thus, in
particular, the functional layers 47, 48 as well as the
electrolyte layer 44 with the two electrode layers 45 and
46, can be fitted. The upper BIP 30 is applied after
this. In this way, as many coated BIPS and green glass
solder sheets are st~cke~ on one another as are needed
for the finished fuel cell to contain the desired number
of active chambers lying above one another. The sandwich
structure produced in this way is then soldered by
correspo~; ng heat-treatment.
By virtue of the invention, the amount of solder
glass needed, and therefore of the binder contA;ne~
therein, which generally includes toxic constituents, is
reduced overall. The width of the gap to be filled with
solder is reduced by the width of the two protective
layers, as a result of which both the leaktightness and
the strength of the assembly point is improved, and the
danger of excess solder flowing into regions of the fuel
cell where it may cause functional impairment, is sub-
stantially less or even completely avoided. Since the
= material (for example stabilized ZrO2) of the protective
layer adheres well to a high-density crystal structure in
which virtually no dissolving and diffusion of inter-
fering extraneous atoms (in particular chromium oxide)
takes place, neither can the solder glass take up
extraneous materials from the material of the BIP.
CA 02206~37 l997-0~-30
GR 94 P 3717 P - 16 -
Test~ with the fuel cell according to the inven-
tion have shown that the leaktightness and insulation of
the assembly region i8 at least as good as with conven-
tional fuel cells which contain a ceramic frame soldered
with a high degree of outlay carefully between the
surfaces of the plates in the assembly region.
The outlay for producing and processing the
individual component~ is in this case reduced to a
tolerable level, so that industrial production of the
fuel cell is simplified.