Note: Descriptions are shown in the official language in which they were submitted.
CA 02206626 1997-OS-30
- 1 -
USE OF CATALYTIC MEMBRANES AS INTERPHASE CONTACTORS FOR
MULTIPI3ASE REACTIONS
The present invention pertains to
improvements in the field of multiphase reactions.
More particularly, the invention relates to the use of
catalytic membranes as interphase contactors for gas-
liquid and liquid-liquid reactions.
Different kinds of membrane reactors have
been developed in which the membrane played different
roles. In biotechnology, ultrafiltration membranes may
be used to continuously withdraw the bioreaction
products and so enhance the conversion efficiency. In
this type of membrane reactor, the membrane only
retains the suspended enzymes (biological catalysts)
and allows the selective permeation of the products but
does not play any role in the bioreaction itself. In
the hydrogenation and dehydrogenation of organic
compounds, porous ceramic membranes have been used for
catalyst support and reaction product withdrawal. In
the latter case, the reaction actually takes place
inside the membrane which may be made of either noble
metal thin films or catalyst physically supported on a
porous inorganic material.
CA 02206626 1997-OS-30
- 2 -
On the other hand, zeolites are now commonly
used as molecular sieves or as catalysts. H.J.C. Te
Hennepe et al. have reported in J. Membrane Sci., Vol.
35, (1987), pp 39-55 and in Stud. Surf. Sci., Vol. 57,
(1991) pp. 289-296 that zeolite particles may be
incorporated into a polymeric matrix in order to
perform separation of various compounds, thus taking
advantage of the narrow pore size distribution of the
zeolites. The catalytic properties of zeolites have
been also extensively studied. In particular, M.
Taramasso et al. have described in US Patent No.
4,410,501 that zeolitic titanium silicalite is a very
efficient catalyst for the oxygenation of various
organic compounds with aqueous hydrogen peroxide.
Nevertheless, in a conventional batch reactor where the
catalyst particles are suspended, the reaction rates
are quite low because of the immiscibility of the
organic and aqueous phases which leads to a much lower
concentration of one of the reactants in the other one
phase. In such a reactor, the use of a co-solvent is
required to increase the organic reactant concentration
at the catalyst surface and therefore increase the
reaction rate. Using a co-solvent at the industrial
scale represents however serious technical and
environmental limitations for this chemical process.
CA 02206626 1997-OS-30
- 3 -
The oxyfunctionalization of n-hexane (n-C6H14)
is an example of partial oxidation reactions of organic
compounds that titanium silicalites allow to perform
with dilute aqueous (30 wt ~) solutions of hydrogen
peroxide (H202), which is considered as a clean and
safe oxidant. In the oxyfunctionalization of r~-hexane
with H202, two simultaneous reactions are actually
taking place (Eqs. 1 and 2) which produce 2- or
3-hexanol:
n-C6H14 +~2-C6H130H +~2-C6H120 (1)
n-C6H14 +~3-C6H130H +~3-C6H120 (2)
Each of these above reactions is followed by a
secondary reaction which produces 2- or 3-hexanone.
Also, hydrogen peroxide is decomposed into water and
oxygen (Eq. 3 ) .
H202~H20 + '~ 02 (3)
J.E. Gallot et al. have indicated in J.
Catal., Vol. 161, (1996) p. 789 that these
oxyfunctionalization reactions can be performed under
very mild conditions in a conventional reactor if an
appropriate catalyst is used. When performed with a
solid catalyst, the oxyfunctionalization is triphasic:
CA 02206626 1997-OS-30
- 4 -
there are two liquid phases which are the organic
(n-hexane) and the aqueous phase (H202 solution), and a
solid phase which is the catalyst. As mentioned above,
a co-solvent such as acetone, methanol or acetonitrile
must be used to increase the reaction rates and
selectivities.
The production of propylene oxide form
propylene is a process of significant industrial
application. As reported in J. Catal, Vol. 140,
(1993), p. 1, M.G. Clerici and P. Ingallina have found
that microporous titanium-silicalite(TS-1) is an
effective epoxidation catalyst using hydrogen peroxide
as oxidant. They have also found that under mild
reaction conditions such as at ambient temperature and
with dilute methanol solutions of H202, quantitative
propylene conversion could be achieved to produce
propylene oxide. A solvent such as methanol has to be
used to enhance the olefin solubility in the aqueous
phase, and to improve epoxidation selectivity. Epoxide
selectivity was found to decrease with increasing
temperature and reaction time. For example, only 750
of propylene oxide selectivity was obtained at 323 K
after 2 hours of reaction. When the propylene
epoxidation is performed in a conventional
gas-liquid-solid slurry system, further separation is
also needed for catalyst recovery. The contact time
CA 02206626 1997-OS-30
- 5 -
and the reaction temperature are relatively difficult
to control in the semi-batch slurry reactor for the
exothermic epoxidation reaction.
It is therefore an object of the present
invention to overcome the above drawbacks and to
provide an improved method of conducting catalysed
multiphase reactions.
It is another object of the invention to
avoid the use of solvents in catalysed multiphase
reactions.
It is a further object of the invention to
improve the epoxidation selectivity in the gas-liquid
reaction of propylene epoxidation with aqueous H202.
According to one aspect of the invention,
there is provided a method of conducting a gas-liquid
reaction in the presence of a catalyst, which comprises
the steps of:
a) providing a catalytic membrane
comprising particles of the catalyst embedded in a
polymer matrix;
b) contacting one side of the catalytic
membrane with a first reagent in gaseous phase and the
CA 02206626 1997-OS-30
- 6 -
other side of the catalytic membrane with a second
reagent in liquid phase; and
c) allowing the first and second reagents
to permeate through the catalytic membrane and contact
the catalyst particles;
whereby reaction between the first and second reagents
occurs within the catalytic membrane in the presence of
the catalyst.
According to another aspect of the invention,
there is provided a method of conducting a liquid-
liquid reaction in the presence of a catalyst, which
comprises the steps of:
a) providing a catalytic membrane
comprising particles of the catalyst embedded in a
polymer matrix;
b) contacting one side of the catalytic
membrane with a first reagent in liquid phase and the
other side of the catalytic membrane with a second
reagent in liquid phase; and
c) allowing the first and second reagents
to permeate through the catalytic membrane and contact
the catalyst particles;
whereby reaction between the first and second reagents
occurs within the catalytic membrane in the presence of
the catalyst.
CA 02206626 1997-OS-30
The catalyst is preferably a zeolite, such as
a silicalite. The polymer constituting the matrix, on
the other hand, is preferably an elastomeric polymer.
When carrying out the epoxidation of propylene with
aqueous hydrogen peroxide or the oxygenation of various
organic compounds such as n-hexane with hydrogen
peroxide, use is advantageously made of a catalytic
membrane comprising particles of zeolitic titanium-
silicalite embedded in a matrix of pure or silane-
modified polydimethylsiloxane. Preferably, the
titanium-silicalite has a Ti/Ti + Si molar ratio of
about 0.019. Where the catalytic membrane comprises
pure polydimethylsiloxane, such a membrane preferably
comprises about 50 weighty of zeolitic titanium-
silicalite and about 50 weighto of
polydimethylsiloxane. On the other hand, where the
catalytic membrane comprises polydimethylsiloxane
modified with a silane modifier such as
trimethylacetoxysilane, triacetoxyvinylsilane and
trimethoxymethylsilane, such a membrane preferably
comprises about 50 weighty of zeolitic titanium
silicalite, about 40 weighto of polydimethylsiloxane
and about 10 weighto of silane modifier.
Trimethylacetoxysilane is particularly preferred as
silane modifier.
CA 02206626 1997-OS-30
Applicant has found quite surprisingly that
zeolitic titanium-silicalite/polydimethylsiloxane
membranes are highly permeable to hydrocarbons such as
propylene and n-hexane, following a diffusive
solubility flow. Hydrogen peroxide, on the other hand,
accesses the titanium-silicalite in the
polydimethylsiloxane matrix through the channels of the
zeolite. Such catalytic membranes are thus highly
effective in conducting the gas-liquid reaction of
propylene epoxydation with aqueous hydrogen. peroxide,
as well as the liquid-liquid reaction of n-hexane
oxyfunctionalization with aqueous hydrogen peroxide.
In the case of propylene epoxydation, selectivities
over 99~ can be reached depending on the gas permeation
flux through the membrane and the membrane thickness.
Modification of the polydimethylsiloxane with
silane modifiers has effects on the rates and
selectivities of the reactions which are comparable to
the effects of a co-solvent in a conventional slurry
reactor.
Further features and advantages of the
invention will become more readily apparent from the
following description of preferred embodiments as
illustrated by way of examples in the accompanying
drawings, in which:
CA 02206626 1997-OS-30
- g -
Figure 1 is a schematic sectional view of a
catalytic membrane reactor for carrying out gas-liquid
and liquid-liquid reactions according to the method of
the invention;
Figure 2 is a graph showing the results of
water permeation tests;
Figure 3 is a graph showing the results of n-
hexane permeation tests;
Figure 4 is a graph showing the results of
n-hexane, 2-hexanol and 2-hexanone permeation tests;
Figure 5 is a graph showing gas permeation
behaviors:
Figures 6 is a graph showing gas permeation
as a function of time;
Figure 7 is a graph showing the permeation of
C3H6-N2 mixtures;
Figure 8 is a graph showing the individual
permeation flux of C3H6 and N2;
CA 02206626 1997-OS-30
- 10 -
Figure 9 is a graph showing the results of
H202 solution separation tests;
Figure 10 is a graph showing the results of
n-hexane/2-hexanol and n-hexane/2-hexanone solution
separation tests;
Figure 11 is a graph showing the effect of
H202 solution on water permeation flux;
Figure 12 is a graph showing the reaction
rates in the membrane reactor;
Figure 13a is a graph showing the catalytic
selectivities in a conventional reactor;
Figure 13b is a graph showing the catalytic
selectivities in the membrane reactor; illustrated in
Fig. 1;
Figure 14 is a graph showing the selectivity
of propylene oxide as a function of reaction time in a
conventional reactor;
Figure 15 shows the product distribution in a
conventional reactor;
CA 02206626 1997-OS-30
- 11 -
Figure 16 is a graph showing the temperature
effect on the propylene oxide selectivity as a function
of propylene permeation flux in the membrane reactor
illustrated in Fig. 1;
Figure 17 shows the product distribution in
the membrane reactor illustrated in Fig. 1; and
Figure 18 is a graph showing the selectivity
of propylene oxide as a function of propylene
conversion.
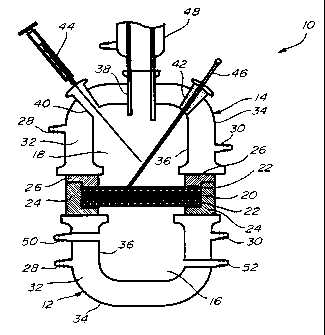
Referring to Fig. 1, there is illustrated a
membrane reactor which is generally designated by
reference numeral 10 and comprises two sections 12, 14
defining lower and upper compartments 16,18 separated
by a catalytic membrane 20. The membrane 20 is held
between two TEFLON (trade-mark) mesh supports 22. A
TEFLON sealing ring assembly having two 0-rings 24,26
is used for sealing the reactor. The lower and upper
sections 12, 14 are each provided with an inlet 28 and
an outlet 30 for the circulation of a thermostatic
fluid through the space 32 defined between the inner
and outer walls 34, 36 of each section. Openinqs 38,40
and 42 in the upper section 14 allow one to introduce a
liquid reagent into compartment 18, take liquid samples
using a seringe 44 and check the temperature inside the
CA 02206626 1997-OS-30
- 12 -
reactor with a thermometer 46. The upper section is
also provided with a condenser 48 having the lower and
thereof extending through the opening 38. the other
reagent which may be in either gas or liquid phase is
introduced into compartment 16 via the inlet 50. For
example, when conducting a liquid-liquid reaction such
as the oxyfunctionalization of n-hexane with hydrogen
peroxide, a batch feed of n-hexane is introduced into
the upper compartment 18 and aqueous hydrogen peroxide
(30 wt.~) is circulated through the lower compartment
16 via the inlet 50 and outlet 52, using a peristaltic
pump. When conducting a gas-liquid reaction such as
the epoxidation of propylene with hydrogen peroxide,
the hydrogen peroxide is introduced into the upper
compartment 18 and propylene is circulated through the
lower compartment 16 via the inlet 50 and outlet 52.
The following non-limiting examples
illustrate the invention.
EXAMPLE 1: Synthesis Of Zeolitic Titanium-Siliaalite
Particles
The objective of the zeolite synthesis was to
obtain sufficiently small and monodispersed size
zeolite particles to allow easy incorporation of these
particles into a polymeric matrix. The titanium-
silicalite zeolite(TS-1) was synthesized according to a
procedure adapted from the procedure proposed by
CA 02206626 1997-OS-30
- 13 -
Thangaraj et al. Studies on the Synthesis of Titanium
silicalite, TS-l, Zeolite,~Vol. 12, (1992), p. 943-950.
In this synthesis, tetraethyl orthosilicate (TEOS) and
titanium (IV) tetrabutoxide (Ti(OBu)4) were used as the
sources of silicon and titanium respectively, whereas
tetrapropylammonium hydroxide (TPAOH) was used as the
template. A 20 wt~ aqueous solution of TPAOH was
prepared free from Na+ and K+ ions using the method
described by Furniss et al., Vogel's Textbook of
Practical Organic Chemistry Including Qualitative
Organic Analysis, Wiley, New York, 1978, p. 334. TPAOH
was added to the TEOS under stirring at room
temperature. The required quantity of Ti(OBu)4, which
was dissolved in dry isopropyl alcohol, was added
dropwise to the latter mixture under vigorous stirring.
The final mixture was stirred at 348-353 K for about 3
hours to remove the alcohol. The chemical composition
of the initial gels were: x Ti02:Si02:0.36TPA:35H20,
where x varied from 0.00 to 0.03. The crystallization
reaction was carried out at 443-448 K typically for 16-
20 hours. The solid obtained was filtered, washed with
distilled water, and dried at 373 K for 5 hours then it
was calcined in air at 825 K for 10 hours. Scanning
electron micrographs showed that these synthesis
conditions allowed to obtain uniform and cubic shape
zeolite crystals with a 0.5~,un average size.
CA 02206626 1997-OS-30
- 14 -
X-ray powder diffraction confirmed that the
produced solids have the MFI-type structure before and
after calcination. Atomic absorption analysis was
performed to confirm the Ti/Ti+Si ratio in the
titanium-silicalite catalysts. UV-visible spectroscopy
confirmed that all the titanium lies in the tetrahedral
coordination state.
EXAMPLE 2: Composite Membrane Preparation
The catalytic membranes were prepared by
embedding the zeolite particles into a polymeric
matrix. Polydimethylsiloxane (PDMS) was selected as
the polymeric matrix because of its high affinity for
hydrocarbons. In several preparations, some
hydrophilicity was also given to the polymeric matrix
by grafting different polar functional groups to the
PDMS using commercial silane modifiers. The list of
modifiers utilized is reported in Table 1 hereinbelow.
PDMS matrix was prepared from vinyl
methylpolysiloxane and polydimethyl hydrogen siloxane.
These two components were dissolved in toluene and
mixed with the titanium-silicalite powder. When
necessary, a silane modifier was also added to this
blend. The mass ratio of the two components was kept
constant at 9:1, whereas a zeolite content of 50 wt.~
CA 02206626 1997-OS-30
- 15 -
was chosen, as shown in Table 1. After vigorously
stirring this mixture for 4 hours, a stable suspension
was formed. This viscous suspension was then cast on a
TEFLON flat mold and left to dry at room temperature
for 10 hours to allow solvent evaporation. The
membrane was then kept at 358 K in a vacuum oven
overnight to ensure complete curing. The membrane was
cut into disks of 60 and 50 mm diameter for permeation
and reaction tests respectively. The membranes
prepared and tested are listed in Table 1. The
membrane thickness was measured both with a Palmer and
from scanning electron micrographs pictures of cross
sections of the membranes. For all M1 to M6 membranes,
the thickness was kept essentially constant at 250 +
l2um.
TABLE I
Membrane Matrix Catalyst Modifier wt~s
Mat./Cat./
Mod.
M1 PDMS None None 100:-
M2 PDMS Y None 50:50:-
M3 PDMS TS-1 Trimethylacetoxysilane 40:50:10
M4 PDMS TS-1 None 50:50:-
M5 PDMS TS-1 Triacetoxyvinylsilane 40:50:10
M6 PDMS TS-1 Trimethoxymethylsilane 40:50:10
Y: Si/A1=2.3, TS-l:Ti/Ti+Si= 0.019
CA 02206626 1997-OS-30
- 16 -
Permeation of pure water and n-hexane as well
as separation of aqueous H202 solution (30o wto) and of
2-hexanol and 2-hexanone solutions in n-hexane (5~ w/w)
were performed under pervaporation conditions. These
pervaporation experiments were performed at room
temperature with the catalytic membranes in a standard
tangential flow permeation cell which allows testing of
flat circular coupons of the membrane (15.90 cm2). The
feed side was under atmospheric pressure while the
pressure or the permeate side was kept at 0.6 Torr for
water permeation and for aqueous solution separation,
and at 20 Torr for organics permeation and separation.
The permeate was collected in two successive liquid
nitrogen cold traps to ensure complete collection. The
H202 concentrations were measured by the potassium
permanganate titration technique and hexane, hexanol
and hexanone were analyzed by gas chromatography. The
molar permeation flux for a pure substance, F, and the
separation factor of the membrane for the component i
of a mixture, ai, are defined by the following
equations:
F = Mp
At
where
F is expressed in mol/(m2 h);
Mp is the amount of collected permeate (mol);
CA 02206626 1997-OS-30
- 17 -
A is the membrane surface area (m2);
t is the pervaporation time (h);
and
CC"1-C'~~feed
ai-
[C~1- Ci ), permeate
where Ci is the molar fraction of component i in the
solution.
Results of the standard pervaporation of
water tests are reported in Fig. 2. With the pure PDMS
membrane M1, a rather low flow value (0.22 mol/m2 h) is
observed and a higher pervaporation rate is obtained
with all composite membranes. This observation
suggests that the zeolite crystals provide a new
pathway for the diffusion of water. This may be
intraparticular diffusion of water with connections at
the contacts between particles. Membrane M3 which is
believed to display a better adhesion of the PDMS
matrix to the particles than membrane M4, yields a
higher pervaporation flow rate. Also the membrane M2
which contains a more hydrophilic zeolite Y with higher
micropore volume yields the highest permeation flux.
It is therefore suggested that water diffusion is by
the intraparticular pathway.
CA 02206626 1997-OS-30
- 18 -
Fig. 3 shows similar pervaporation tests for
n-hexane permeation through the same membranes. The
measured flow rates are much higher than for water and
the pure PDMS membrane M1 yields the highest flow rate.
This suggests that the mechanism of n-hexane transport
is different than the one of water and dominated by
diffusive solubility of n-hexane in the PDMS matrix.
The presence of the zeolite particles in the composite
membranes has only a minor effect on the n-hexane
diffusion rates.
Fig. 4 shows a comparison of permeation
fluxes observed in the standard pervaporation tests for
n-hexane, 2-hexanol and 2-hexanone over membrane M4.
Similar results are observed with the other membranes,
namely that of reduced permeation fluxes for 2-hexanol
and 2-hexanone compared to n-hexane.
In the measurement of gas permeation fluxes,
the propylene stream was introduced into the lower
compartment 16 of the membrane reactor 10 shown in Fig.
1, and permeated through the membrane 20 under a
moderate pressure. Prior to the permeation measurement,
the feed side was purged with the gas being tested for
1 h at atmospheric pressure to ensure a pure stream. In
order to keep similar flowing conditions as in the
epoxidation reaction tests, 15 g water was charged over
CA 02206626 1997-OS-30
- 19 -
the membrane during the permeation measurements.
Permeation of such inert gases as nitrogen and helium
was also measured in order to determine the permeation
regime through this kind of membranes.
The organophilic behavior of PDMS allows
hydrocarbons to be absorbed in its matrix due to the
presence of numerous methyl groups on the surface. To
illustrate this property. The single gas permeation
behavior of C3H6 and nitrogen through a 250~un titanium-
silicalite PDMS membrane at room temperature was
measured. Fig. 5 shows the permeation fluxes as a
function of pressure drop across the membrane. Two
straight lines representing the single gas permeations
of propylene and nitrogen were observed. The permeation
of C3H6 is much easier than that of nitrogen. This
behavior would be attributed to a diffusive solubility
flow mechanism for C3H6 permeation. Since the TS-
1/silicone rubber membrane is strongly organophilic,
C3Hg can be sorbed and then diffuse through the
membrane. The permeation flux may be expressed in terms
of Fick's law:
J= D(C1-C2)/~
where J is the permeation flux (mol/m2/s), D is the
diffusion coefficient (m2/s), ~ the membrane
CA 02206626 1997-OS-30
- 20 -
thickness and C the molar concentration inside the
membrane. The linear relationship between J and OP
(Fig. 4) allows one to further simplify the above
equation as follows:
J = Dk(P1-P2)/1= DkOP/1
where DP is the pressure drop (Pa). Classically, this
expression is correct in the region where Henry's law
holds. Thus, the constant k should be Henry coefficient
(mol/m3Pa) .
The slight permeation of N2 might be caused
by diffusion through the PDMS matrix. From the
comparison of permeation fluxes between C3H6 and N2,
one can exclude the contribution of molecular diffusion
(Knudsen diffusion) through the TS-1/PDMS composite
membrane. A parallel permeation measurement with helium
(Fig. 6) gave almost the same permeation flux as that
with N2, indicating that the minor diffusion observed
with permanent gases is non selective.
It should be mentioned that permeation fluxes
of C3H6, He or N2 could easily and rapidly reach a
steady state value as long as the back pressure got
stable. Fig. 6 shows such permeation behavior with
time. The experiments also revealed that all these
permeations were completely reversible once the driving
force was removed from the system, and reproducible.
CA 02206626 1997-OS-30
- 21 -
To further understand the gas permeation
behavior through TS-1/PDMS membranes, the permeation of
C3H6-N2 mixtures was studied. The permeation gas
composition was analyzed using GC by taking samples
directly from the small bubbles over the watex layer.
Results of the permeation flux measured at room
temperature and 156 kPa inlet pressure are presented in
Fig. 7 as a function of the inlet mole fraction of
propylene. It can be seen that the permeation flux of
the mixtures were below the diagonal of the permeation
flux-inlet mole fraction plot. There is clearly a
permeation coupling effect. The permeation of C3H6 was
affected by the presence of N2. With increasing inlet
C3H6 mole fraction, the permeation flux increased
gradually. Up to 83.48 of C3H6, the permeation flux is
even less than half of that in the case of pure
propylene permeation. From the plot of outlet versus
inlet mole fractions (Fig. 7), only a small enrichment
of C3H6 could be seen at the outlet. The product of the
outlet mole fraction and the permeation flux gave
individual permeation fluxes for both C3H6 and N2,
which are shown in Fig. 8. Interestingly, different
permeation behaviors of C3H6 and N2 were revealed. In
the case of C3H6, the permeation flux increased
gradually with the mole fraction in the inlet. However,
over a wide range of the inlet mole fraction, the
CA 02206626 1997-OS-30
- 22 -
permeation flux of N2 remained constant, and equal to
the value of the permeation flux for the pure N2 under
the same total inlet pressure.
The incorporation of TS-1 particles in the
PDMS matrix also allows the access of H20 or H202 into
the microchannels of the zeolite, which was confirmed
by the pervaporation measurements. Table II shows the
H20 permeation flux through a series of membranes M4
having different membrane thicknesses. The permeation
flux decreased with the increase of the thickness. The
access of H202 into the TS-1 channels, although small,
allows eventually the epoxidation to take place inside
the catalytic membrane.
TABLE II
Permeation flux,
Membrane Thickness, mm g/m2h
M4 200 8.45
M4 250 5.80
M4 350 3.51
Fig. 9 is a report of the H20/H202 separation
factors obtained with a 30% H202 solution. The
permeation fluxes expressed in g/m2h are similar to the
values observed with pure water but the permeate is
CA 02206626 1997-OS-30
- 23 -
less rich in H202 than the feed solution. This
difference is maximum with the pure PDMS membrane Ml.
Values of the separation factors obtained
with 5~ solutions of 2-hexanol and 2-hexanone in n-
hexane are reported in Fig. 10. Here also the
separation factors n-hexane/2-hexanol and n-
hexane/2-hexanone are significantly higher than one,
reflecting the higher affinity of PDMS for n-hexane
than for the two oxygenates.
EXAMPLE 4: Effect Of H 02 on Membrane Permeation
Properties
In order to detect any possible modification
of the membranes due to its prolonged contact with the
H202 solution, the following permeation tests were
performed. The pervaporation of water at room
temperature was conducted continuously for a period of
12 hours followed by a 12 hours contacting of the
membrane with the 30g H202 solution. This procedure was
repeated for six consecutive days. The results of water
permeation fluxes are reported in Fig. 11 and compared
to the fluxes measured during a continuous 6 days water
pervaporation test. It is seen from Fig. 11 that the
contact with the H202 solution did not affect
significantly the permeation rates. This suggests that
CA 02206626 1997-OS-30
- 24 -
the composite membrane is stable in the H202 300
solution.
EXAMPLES 5: Catalytic Tests
A) Oxyfunetionalization of n-hexane with H202
The catalytic reaction tests were performed
at 328 K and under atmospheric pressure. The
oxyfunctionalization of n-hexane was carried out by
using 10 g of n-hexane and 250 ml of H202 solution
(30 wt~). The membrane area was 10.75 cm2. The mass of
titanium-silicalite catalyst embedded in the membrane
varied form 120 to 170 mg. Gas chromatographic analysis
of samples taken from the upper compartment allowed to
follow the evolution of the primary and secondary
products concentrations in the organic phase. The H202
concentration in the aqueous phase was measured by
iodometric titration. The volume of oxygen generated
was measured as a function of time using the gas
collector.
Reaction rates obtained with the catalytic
membrane are reported in Fig. 12. It is seen that the
experiment performed with membrane M4 yields a rate
value comparable to the one of the standard test TS-1
in a conventional reactor. This is a remarkable result
indicating that the use of the catalytic membrane does
not involve a decrease in rate per unit mass of
CA 02206626 1997-OS-30
- 25 -
catalyst. Thus, the membrane does not impose a rate
limiting mass transfer of the reactant. Moreover,
using a modifier (membranes M3 and M5) yields a change
in reaction rate which is correlated with the change in
water permeation flow rates (see Fig. 2). It is
therefore believed that the observed change in reaction
rate with the modification of the PDMS matrix is
associated with differences in the transfer rate of the
H202 reactant. A higher rate would correspond to a
higher local concentration of H202 at the catalyst
surface. It is interesting to note that using membrane
M3 corresponds to an increased rate compared to
membrane M4 which is about half the increase
corresponding to the optimum use of methanol as a
co-solvent in the conventional reactor. It is thus
clear that the proper choice of the components of the
catalytic membrane allows this membrane to play a role
similar to the one of a co-solvent.
In Fig. 13, the product distributions are
represented as the molar ratios:
alcohol and
ketone = 2 - hexanol + 3 - hexanol
2 - hexanone + 3 - hexanone
2-
3- -_ 2 - hexanol + 2 - hexanone
3 - hexanol + 3 - hexanone
CA 02206626 1997-OS-30
- 26 -
It is seen from the comparison of Figs 13a
and 13b that the catalytic membrane affects more
dramatically the alcohol/ketone ratio. This ratio
reflects the relative rate of the secondary oxidation
of the alcohols to ketones compared to the rate of the
primary oxidation. It is believed that the rate of
secondary oxidation is lowered in the membrane due to
the rapid transfer of the alcohols out of the membrane.
This is in line with the high values observed for the
hexane/hexanol separation factors which reflects a
lower affinity of the PDMS for the oxygenated products.
The results in Fig. 13 demonstrate that not only the
reaction rates but also the selectivities may be
controlled through the proper choice of the membrane
components.
B) Epoxidation of propylene with X02
Prior to the use in the catalytic membrane
reactor 10 shown in Fig. l, the intrinsic catalytic
activity of TS-1 towards the propylene epoxidation was
examined in a conventional three-phase bubble-slurry
reactor containing suspended catalyst particles. The
selectivity of propylene oxide was studied at various
temperatures as a function of reaction time as shown in
Fig. 14. Low reaction temperatures were found to favor
the selectivity to propylene oxide. Besides propylene
oxide as the main product, acetone, glycerol, allyl
CA 02206626 1997-OS-30
- 27 -
alcohol, propylene glycol and glycidol were also
detected as by-products. The liquid product
distribution is shown in Fig. 15. At 277 K, the
selectivity to propylene oxide was near 75~ and kept
almost constant as a function of time. Increasing
temperature significantly reduced the selectivity of
propylene oxide, but increased that to acetone. At
323 K, the selectivity of acetone was 57.3 after lh of
reaction, and increased over 75~ after 2h of reaction.
The results indicated that a long contact time
unfavored the epoxidation selectivity in the
conventional reactor. The formation of propylene oxide
is a primary reaction, followed by secondary reactions
forming acetone or propylene glycol. The parallel
propylene oxidation to allyl alcohol is obviously,
slower and favored by an increase in temperature.
The membrane reactor shown in Fig. 1 was used
for the epoxidation tests. 15 g of aqueous hydrogen
peroxide (30 wt~) was charged into the upper
compartment 18 of the reactor and propylene was fed
into the lower compartment 16 under a constant pressure
of 163.4 kPa. The reaction temperature was controlled
by circulating a thermostatic fluid in the space 32
between the inner and outer walls 34,36 of each section
of the reactor. The gas effluent over the liquid phase
was passed through the condenser 48 at 275 K and then
CA 02206626 1997-OS-30
- 28 -
through a bubble flow-meter (not shown) for the
measurement of the outlet flow-rate. Since in this
membrane reactor the membrane 20 separates the two
compartments 16 and 18 of the reactor, this membrane
may be considered as an interphase contactor. Moreover,
since the propylene gas is bubbling through, the
membrane 20 may be viewed as a low flow gas
distributor.
Both gas and liquid products were analyzed
during permeation and reaction measurements. The
liquid product aliquots were taken from the liquid
solution by using the syringe 44 (15 ~L) at fixed time
intervals, and analyzed using a gas chromatograph. A 3-
m column of 5$ bentone-34+5~DIDP, was used for the
analysis of the organic components with an FID detector
at 343 K. Individual components in the liquid product
were identified by their mass spectrum. The gas sample
was taken from the small bubbles generated during the
permeation and reaction over the liquid solution. The
propylene conversion was given as the ratio of
propylene consumed and that fed, and the selectivity
was calculated from the distribution of liquid products
based on a carbon balance.
Fig. 16 shows the temperature effect on the
propylene oxide selectivity as a function of propylene
CA 02206626 1997-OS-30
- 29 -
permeation flux. Similar to the case in the
conventional reactor, increasing temperature resulted
in a decreased selectivity to propylene oxide. It must
however be noted that unlike the case of the
conventional reactor, high selectivities can be reached
at room temperature. Fig. 16 also indicates that
propylene oxide selectivity increases with the
propylene permeation flux. Indeed a high permeation
flux results in a short contact time of C3H6 with the
TS-1/PDMS membrane, which favors the epoxide
selectivity. It is interesting to note that the
membrane acts here as a plug-flow reactor which allows
to suppress the secondary reactions compared to a batch
reactor. Moreover, in the case of the conventional
bubble-slurry reactor, both the dissolved propylene and
propylene oxide remain in contact with the H202
solution and the TS-l catalyst during all the test.
This is not the case with the membrane reactor where
propylene oxide is dissolved in the H202 solution but
is essentially not contacting the catalyst any more.
Thus the use of a catalytic membrane as interphase
contactor is beneficial in all situations where
secondary reactions are to be suppressed. The liquid
product distribution is shown in Fig. 17. In this
figure, the selectivity to propylene oxide increased
with the reaction time as the experiments were
conducted as the propylene flow-rate was increased with
CA 02206626 2004-07-30
- 30 -
time. After 4 hours, the propylene permeation flux was
stable and equal to 5 mol/m2s. The selectivity to
propylene oxide was much higher than that in the
conventional reactor (see. Fig. 15), and this may still
be improved by optimizing the propylene permeation
flux. Moreover, the selectivity to acetone was
significantly suppressed in the case of the membrane
reactor. Less of the other by-products was produced in
the membrane reactor as well due to the short contact
time of reactants with the catalyst. Generally,
increasing the propylene permeation flux results in a
high epoxidation selectivity while the conversion is
however decreased, as shown in Fig. 18 for several
membranes. Selectivities over 99~ can be reached with
thicker membranes at relatively low propylene
conversion depending on the permeation flux. It was
found that a 338 pm thick membrane allowed a conversion
of 290 of a 0.8 SCCM flow-rate with essentially 100$
selectivity to propylene oxide.
. The cumulative propylene conversion was also
calculated from material balance of organic compounds
in both the liquid phase solution and the gaseous
propylene stream. The relative agreement of material
balances between the liquid solution and the gaseous
stream (within a few percents) indicated that the
CA 02206626 1997-OS-30
- 31 -
permeated propylene through the membrane was
continuously converted to propylene oxide.