Note: Descriptions are shown in the official language in which they were submitted.
CA 02207116 1997-06-06
ELECTROLYSIS APPARATUS FOR COMBUSTION ENGINES
Field of the Invention
The invention relates to a water electrolysis system for production of hydrogen and
oxygen gases for supply to a combustion engine.
5 Background of the Invention
Hydl ogen gas has been used as an engine fuel supplement for hydrocarbon fuels, such
as gasoline, to improve combustion and to reduce unwanted emissions. In most
applications, it is necessary to bring the source of hydrogen close to the engine into
which it is being supplied. The hazards of having a tank of hydrogen adjacent an10 engine are known and, therefore, systems have been developed to generate the
hydrogen as it is needed by use of an electrolysis chamber.
An electrolysis chamber for generation of hydrogen gas is taught in U.S. Patent
5,231,954 to Stowe. The chamber includes a housing having a pair of electrodes
therein at least partially submerged in an electrolyte solution. The electrodes are
15 connected to a source of electrical potential to generate hydrogen and oxygen gases
from the electrolyte solution in the chamber. The chamber is mounted in association
with an engine and any hydrogen gases generated are fed to the engine via a lineconnected to the air intake manifold.
In order to reduce the risk of explosion, the chamber has a friction fitted top cap which
20 provides for pressure release under conditions where hydrogen builds up within the
chamber. The top cap has an end wall and a cylindrical side wall extending therefrom.
The side wall fits over and extends down the sides of the chamber. To be removed,
this top cap requires substantial clearance above the chamber. Such clearance is often
unavailable in the engine area of most vehicles.
CA 02207116 1997-06-06
In addition, should the top cap described in the patent be released it is generally
incapable of reseating itself to seal the chamber. If the top cap blows off, the vehicle
operator can continue to operate the vehicle for a period of time without noticing that
the chamber is open. This results in the potential for spillage of the electrolyte and,
5 most importantly, in the operation of the vehicle without the benefits of hydrogen gas
fuel supplementation.
Summary of the Invention
An electrolysis system has been invented which has safety features which substantially
eliminate any risk of explosion by use of the chamber and facilitates efficient use of the
10 chamber. Some of the aspects of the inventive system include a chamber provided with
a pressure release cap which functions with very limited clearance suited to automotive
applications where engine compartment space is limited. The chamber is provided with
a blow-out area formed therein to release any explosion pressures which may build up
in the chamber. The blow-out area is located to reduce or substantially eliminate
15 expulsion of electrolyte in the event of explosion. The electrolysis chamber is of a
construction which avoids the release of shattered parts in the event of an explosion.
The inventive electrolysis system is provided with a gas supply shunting valve to vent
any unwanted gases to the atmosphere therethrough. The electrolysis system has apower supply interruption switch responsive to chamber over-pressure to disconnect
20 the electrolysis chamber from its power source in the event of over-pressure thereby
halting further unwanted production of hydrogen gas.
The electrolysis system has a lockout switch to disconnect the electrolysis chamber
from its power source when the combustion engine supplied with the combustion gases
produced by the electrolysis chamber is not consuming or drawing combustion gases.
25 The system is provided with an electrode arrangement suitable for increasing the
conversion efficiency of supplied electrical energy from a power source to hydrogen
CA 02207116 1997-06-06
and oxygen gas production, thereby reducing the production of waste heat throughohmic heating of the electrolyte solution and potential for overheating and boiling of the
electrolyte.
The chamber requires only minimum amounts of maintenance because of the nature
5 of the pressure release lid which is shaped to easily reseat itself in the event it is
dislodged by a pressure release. In another aspect of the invention, the potential for
spillage of the electrolyte is reduced by introducing baffles in the electrolyte chamber
which limit wave production in the liquid electrolyte thereby minimizing unwanted
spillage. In one manner of construction, electrolyte baffles are provided by selection
10 of the arrangement of electrodes. In yet another aspect, the electrode connection
terminals of the chamber are spaced above the fill level of the electrolyte to reduce the
chances of leakage of electrolyte via or about the connection terminals.
Thus, according to a broad aspect of the present invention there is provided an
electrolysis chamber having a housing defining an interior and an exterior, a pair of
15 electrodes disposed therein and connected to electrical connectors exposed at the
exterior of the housing and a hydrogen delivery port extending from the interior of the
housing to the exterior thereof, the improvement co,nprising a pressure releasable plug
formed to be inserted into an opening in the chamber, the plug being formèd with a
bottom and side walls, the side walls converging toward the bottom of the cap.
20 In accordance with another broad aspect of the present invention there is provided an
electrolysis chamber having a housing defining an interior and an exterior, a pair of
electrodes disposed therein and having electrical connection exposed at the exterior
of the chamber and a hydrogen delivery port extending from the interior of the chamber
to the exterior thereof, the improvement comprising the housing formed at least in part
25 of acrylonitrile butadiene styrene resin.
In accordance with another broad aspect of the present invention there is provided an
electrolysis chamber having a housing defining an interior and an exterior, a pair of
CA 02207116 1997-06-06
electrodes disposed therein and having electrical connection exposed at the exterior
of the chamber and a hydrogen delivery port extending from the interior of the chamber
to the exterior thereof, the improvement comprising a pressure release device on the
housing burstable upon application of pressure thereon.
5 In accordance with another broad aspect of the present invention there is provided a
feed line and a valve in the feed line, the valve having a first outlet for diverting gas to
the air intake valve and a second outlet for diverting gas to a vent line for release to the
atmosphere, the valve being actuated to divert gases to the first outlet when the engine
of the vehicle is operating and being actuated to divert gases to the second outlet when
10 the vehicle is not operating.
In another aspect of the invention there is provided an electrolysis apparatus for
producing hydrogen and oxygen gases by electrolysis of water including: a housing
forming a chamber to contain a conductive solution of water and an electrolytic agent;
a first electrode and distal therefrom a second electrode disposed in said chamber each
15 configured to form a contact surface with the conductive solution contained in said
housing; a first terminal exterior to said housing electrically connected to said first
electrode a second terminal exterior to said housing electrically connected to said
second electrode; an opening formed in said housing for passage of electrolysis gases
therethrough located above the upper surface of any conductive solution contained in
20 said housing; the improvement comprising: at least one intermediate electrodedisposed in said chamber in contact with the conductive solution contained in said
housing, said intermediate electrode configured to substantially contour a selected
equipotential surface induced between said first and second electrodes.
In another aspect, the invention provides apparatus to control the supply of
25 supplementary fuel gases to a combustion engine comprising: an intake line for
connection to the air intake of a combustion engine to recover operating vacuum
therefrom; a supply line for connection to a source of supplementary fuel gases; means
for controlling the supply of gas obtained from the source of supplementary fuel gas in
CA 02207116 1997-06-06
response to a control signal control; means to produce said control signal in response
to a supplied vacuum; a summing junction in communication with control means andinterconnecting said intake line and said supply line, whereby operating vacuum
present in said intake line exceeding the supply of supplementary fuel gases present
5 in said supply line causes a net differential vacuum to be supplied to said control
means to produce a control signal increasing the supply of gas available from said
means for controlling the supply of gas and, conversely, operating vacuum present in
said intake line exceeded by the supply of supplementary fuel gases present in said
supply line causes a net differential pressure to be supplied to said control means to
10 produce control signalling decreasing the supply of gas available from said means for
controlling the supply of gas.
Another aspect of the invention provides apparatus to control the supply of
supplementary fuel gases to a combustion engine comprising: an intake line for
connection to the air intake of a combustion engine to recover operating vacuum
15 therefrom; a supply line for connection to a source of supplementary fuel gases, a
venting line providing a gas path communicating with ambient atmosphere valving
means interconnecting said intake line, supply line and venting line to direct a supply
of gas from said supply line to either said intake line or said venting line in response
to a control signal; control means to produce said control signal in response to20 operation of the combustion engine whereby any gas supplied to said supply line will
be directed to said intake line during engine operation and will be directed to said
venting line when the engine is not operating.
Brief Description of the Drawings
A further, detailed, description of the invention, briefly described above,
25 will follow by reference to the following drawings of specific embodiments of the
invention. These drawings depict only typical embodiments of the invention and are
therefore not to be considered limiting of its scope. In the drawings:
CA 02207116 1997-06-06
Figure 1 is a perspective view of a electrolytic chamber according to the present
invention;
Figure 2 is a sectional view along line 2-2 of Figure 1;
Figure 2a is an alternate embodiment of the explosion vent of Figure 2;
5 Figure 3 is sectional view along line 3-3 of Figure 1;
Figure 3a is a sectional view of an alternative shape of the electrolysis chamber; and
Figure 4 is a schematic view of a hydrogen generating system according to the present
invention.
Detailed Description of the Preferred Embodiments
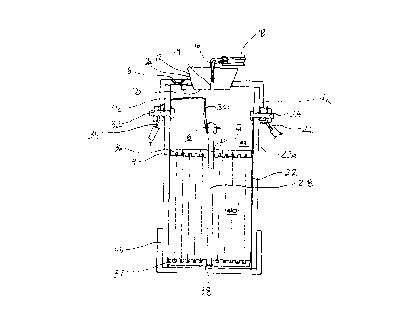
10 Referring to Figures 1 and 2, a electrolytic chamber 2 is shown. When in use, the
chamber generates hydrogen and oxygen gases.
The various features of the invention for release of internal pressure, for avoiding
spillage and leakage of electrolytic solution and for enhancing operation of thechamber, as will be described, need not all be present in the same chamber or
15 electrolysis system, as the presence of one or more of the features may not be required
for the application to which the chamber is to be put. Alternately, the various aspects
can all be present in the chamber or the system at all times, but be only used as
needed.
Chamber 2 includes a housing 4 formed to contain an electrolyte solution 6. The
20 chamber can be cylindrical as shown or any other shape suitable for its intended use.
To facilitate construction, housing 4 preferably has a top 4a and a bottom 4b sealably
CA 02207116 1997-06-06
secured as by suitable adhesives to a cylindrical side wall 4c. Housing 4 is formed
from any chemically and electrically inert material. Preferably, housing 4 is formed from
the material known as acrylonitrile butadiene styrene resin (ABS) because of itsresistance to chemicals such as the electrolyte solution and its ability to withstand large
5 temperature fluctuations without degradation. In addition, ABS plastic is not brittle and
during a chamber failure wherein there is a build up of internal pressure, the chamber
formed using ABS will tend to crack rather than shatter.
Housing 4 has a pressure release section 8 which is burstable upon application of
pressure, such as internal pressure, thereon. Section 8 of the housing has a reduced
10 thickness T relative to the thickness of the balance of the housing. This section can be
formed during the molding or extrusion process or can be milled out after formation of
the housing. Section 8 can be integral with the housing or, alternately, be an inset
piece of material, such as is shown by way of example in Figure 2a. In the alternative
configuration of Figure 2a, a vent port 8a is covered by a displaceable cover 8b which
15 is urged into sealing contact with the top 4a by means of a biasing element 8c such as
a spring. In the preferred embodiment of Figure 2, pressure release section 8 has a
lower strength than the material used in the formation of the remainder of the housing.
Section 8 is selected to burst when a selected amount of pressure, such as caused by
an explosion of the combustible gases within the chamber, is applied thereto. To burst
20 section 8, the amount of pressure is selected to be greater than that pressure which is
exerted toward the inside of the chamber when the chamber is under vacuum duringuse.
A port 10 is formed through the housing at an upper portion thereof for introduction of
water, electrolytes and/or electrolytic solution. Port 10 has removably inserted therein
25 a plug 12 for sealing the port. Preferably, plug 12 is only frictionally engaged in the
port and can be removed by application of a force to pull or push the plug out of the
port. Preferably, plug 12 has side walls 12a which converge toward the bottom 12b of
the plug (i.e. the end which is inserted into the port) and the port is preferably
positioned on the top of the housing, as determined by the intended mounting position
CA 02207116 1997-06-06
of the chamber. Such a plug and port arrangement facilitates the release of internal
pressure and greatly reduces the risk of explosion which was encountered in previous
systems since, it will be appreciated, that due to the converging side walls anymovement of the plug out of the port will immediately break the seal between the plug
and the housing. In addition, the shape of the plug permits it to easily reseat itself
should it be pushed out of sealing position, but remain loosely, in the port. To further
facilitate reseating, the plug is preferably formed to be generally conical in shape.
Preferably, side walls 12a of plug 12 are coated with a resilient material, such as
rubber, to facilitate sealing against the edges of port 10. Alternately, plug 12 can be
formed at least in part of a resilient material. In a preferred embodiment, plug 12 is
formed from a rubber stopper.
An opening for p~ss~ge of electrolysis gases is provide by means of gas delivery port
14 found at the upper portion of the chamber and is present to provide an exit for the
hydrogen and oxygen gases produced during the electrolysis process. In a preferred
embodiment, port 14 is formed through plug 12. As may be understood, the port 14 can
alternately be formed through any suitable opening provided in housing 4. A connector
16 is provided at port 14 for connection to a delivery line 18 at the time of installation
for use. Preferably, as shown, connector 16 is removable from the port for replacement
or repair.
Referring also to Figure 3, electrodes 22, 23 and 28 are provided within chamber 2.
The electrode material is selected from any suitable electric conductor which will not
chemically react with the electrolytic solution either when electrically energized or not.
A suitable material for construction of electrodes 22, 23, and 28 is stainless steel.
While it will be understood that electrode 28 may be configured as a cathode andelectrode 22 as an anode, or polarity of each may be reversed without changing the
principles of operation, for the purpose of illustration, the central electrode 28 has been
configured as an anode while outer electrode 22 is configured as a cathode. Preferably
electrode 22 is positioned to rest against the interior surface of housing 4 consequently
CA 02207116 1997-06-06
making it cylindrical in shape to correspond with the cross-sectional dimension of the
chamber.
An extension 22a of the cathode extends up the inside of the housing 4 for electrical
connection to a power supply terminal 24, which is conveniently provided by a bolt.
5 Bolt 24 passes through an aperture in the housing and is electrically connected to a
wire 26 when installed for use. Wire 26 extends to a negative ground pole of a battery
or a vehicle ground structure, as will be described in more detail with reference to
Figure 4. Centrally located in the electrolyte solution 6 is anode 28. Anode 28 may be
constructed from any suitable electrical conductor which does not react with the10 electrolyte solution and is preferably a cylinder and may conveniently be a rod formed
of stainless steel in common with cathode 22 and intermediate electrodes 23. Anode
28 is supported by a conductor bracket 30 which provides for electrical connection
between anode 28 and power supply terminal 32 which is a bolt extending through an
aperture in the housing. Bolt 32 is electrically connected at one end to bracket 30 and,
15 when installed, to wire 34 at its opposite end. Wire 34 is ultimately in electrical contact
with the positive pole of a battery, as will be described in more detail with reference to
Figures 4 and 5.
Anode 28 is further maintained in position concentrically within cathode 22 by plates
36, 37. Plates 36, 37 are formed of a non-conductive material such as, for example,
20 an ultra high molecular weight polyethylene (UHMW polymeric resin). Anode 28 is
positioned in centrally located apertures 38, 39 in the plates. Upper plate 36 acts to
divide the chamber into an electrolyte supply region 42 and an electrolysis region 40,
containing the electrodes 22, 23 and 28 submersed in the electrolytic solution 6. A
plurality of apertures 41 are formed in plate 36 for passage of the electrolysis25 generated gases from area 40 to area 42 where the gases will bubble up and flow
toward gas delivery port 14.
CA 02207116 1997-06-06
- 10-
A plurality of intermediate electrodes 23 are disposed between the powered cathode
22 and anode 28. The shape of these electrodes conforms to the equipotential lines
of the electric field induced in electrolyte solution 6 when power is applied to the
cathode 22 and anode 28. As most clearly seen in Figure 3, the intermediate
5 electrodes 23 are formed into cylinders to conform with the circular cross-sectional
shape of the electrolysis chamber 4 and are positioned between anode 28 and cathode
22. Each electrode is constructed from suitable chemically inert electrically conductive
material, such as stainless steel, which has been, for example, rolled and welded along
a seam (not shown). The number of intermediate electrodes 23 is selected to provide
10 approximately 2 volts across each cell formed by the gap in spacing between each
electrode. A 2 volt difference is preferable to reduce the ohmic heating of the
electrolyte solution bounded by adjacent electrode by the current passing therethrough
as the ele~;t,."noli~/e force or voltage required for electrolysis of water is approximately
1.5 volts. Thus for a 12 volt vehicle, a group of 5 intermediate electrodes 23 may be
15 provided. For other operating voltages, a differing number of electrodes are provided
to achieve like effect. While the electrodes are depicted in Figures 2 and 3 as being
equic~islanlly spaced, it will be understood that the actual physical placement or spacing
of the intermediate electrodes 23 will be such as to create approximately a 2 volt
differential between adjacent electrodes.
20 With concentric cylindrical electrodes, varying physical spacings are required to
maintain a uniform electromotive force differential between adjacent electrodes
increasing the complexity of the electrolyte chamber in both construction and operation.
For cylindrical electrolysis chambers, each cell, being the electrolyte and surrounding
operative electrode pair, has a unique electrolyte volume and electrode surface area
25 resulting in variations in gas production efficiencies and operating parameters. A
uniform result for each cell in the electrolysis chamber apparatus may be obtained by
employing a chamber in the shape of a box having a rectangular cross-section as
shown in Figure 3a. With such a chamber shape, the equipotential surfaces induced
in the electrolyte when electrical potential is applied to the cathode 22 and anode 28
30 are flat surfaces enabling the intermediate electrodes 23 to be flat and equidistantly
CA 02207116 1997-06-06
spaced from one another resulting in substantially uniform construction and operating
parameters for the electrolysis chamber 4.
The spacing of the i"lerrrlediate electrodes 23 can be achieved in any suitable way, for
example, by plates 36, 37 which have formed therein a plurality of grooves into which
5 intermediate electrodes 23 are fitted as shown most clearly in cross section in Figure
2. The grooves maintain the positioning of the electrodes relative to each other and to
the anode and cathode. The intermediate electrodes 23 serve a number of useful
purposes. First, the electrodes act as baffles to substantially damp any wave action in
the liquid within the chamber. This reduces the likelihood that the electrolyte solution
10 6 will splash around in the chamber. Where the chamber is cylindrical in shape the
damping action will be effective regardless of the direction in which the chamber is
moved. Additionally, the intermediate electrodes increase the electrode surface area
for the generation of electrolysis gases, as well as reduce the electromotive force being
applied to the cell to a value most efficacious for water electrolysis. This provides a
15 more efficient chamber with higher gas generation capabilities and lower operating
temperatures than a chamber of similar size having therein only the cathode and the
anode electrodes. By having lower operating temperatures, the unwanted production
of heat and potentially steam from the electrolytic solution is avoided.
Referring to Figure 4, electrolysis chamber 2 generates hydrogen and oxygen gases
20 to supplement the fuel supply of a combustion engine (not shown), such as a
h~dloca,bon fueled internal combustion engine employed to supply motive power to an
automobile. Common vehicle gasoline or diesel engines have an air intake system
supplying a mixture of fuel and air to be combusted within the engine. The air intake
system is maintained under vacuum during operation of the engine.
25 A vehicle also includes a battery 60 as a source of electrical potential. The battery has
a positive pole 60a and a negative ground pole 60b. In accordance with the invention,
a chamber 2 is mounted in the vehicle compartment housing the engine. Power supply
wire 26 from cathode 22 is grounded, for example by contact with the vehicle frame.
CA 02207116 1997-06-06
Power supply wire 34 runs from contact with anode 28 to a control power relay switch
62. From relay switch 62, power wire 64 runs through an overcurrent protection device
66, such as a circuit breaker, fusible link or fuse to positive pole 60a of battery 60.
Relay switch 62 controls the supply of electrical energy to the electrolysis chamber 2.
5 Overcurrent protector 66 prevents overcurrent damage to the components caused by
a malfunction, such as a short circuit. To control and prevent unwanted generation of
the explosive oxygen hydrogen gas mixture, the control relay switch 62 is configured
in such a manner as to ensure that no electrical power will be supplied to electrolysis
chamber 2 unless the vehicle engine is both switched on and running. This is
10 controlled in the following manner.
Power relay control wire 68 controls the activation of power relay 62 depending on
control signalling received via vacuum switch 70. Vacuum switch 70 is a normally open
switch which is closed, making electrical contact with ignition line 72, when vacuum is
supplied to tubing 71.1gnition line 72 is powered from the vehicle ignition key system
15 becoming powered when the vehicle ignition switch is turned ON. A fuse 75 is provided
for safety. The vacuum to operate the vacuum switch 70 is obtained from the air intake
system of the vehicle engine communicated by intake supply line 73 to which tubing 71
is connected via T-connector 80. As will be readily understood, the vehicle engine will
only generate a vacuum when it is running and the presence of vacuum switch 70
20 ensures that hydrogen and oxygen combustible gas production will only occur when the
engine is running. Thus when the engine has stalled or the ignition switch is, for any
reason, ON but the engine is not running, no combustible gas production will occur.
While it will be understood that tubing 71 can be directly connected to the engine
manifold to obtain a vacuum supply directly from the engine, the preferred construction
25 is to employ a T-connector 80 which bridges engine intake supply line 73 and the
hydrogen oxygen supply line 75. This provides added safety by preventing the
undesirable escape of the combustible hydrogen oxygen electrolysis production gases
into the engine compartment of the vehicle thereby avoiding potential explosion risks.
When the rate of production of electrolysis gases delivered by supply line 75 exceeds
CA 02207116 1997-06-06
the rate of consumption of those gases through the engine vacuum present in the
engine intake supply line 73, the excess production gases will "flood" into the vacuum
tubing 71 thereby causing vacuum switch 70 to open, thereby, interrupting the power
supplied to the electrolysis chamber 2 halting further gas production. In this way,
5 excessive gas production or system overheating and steam generation within the electrolysis chamber 2 will be prevented.
The electrolyte solution can be any suitable solution of water and electrolytic agent
permitting current to move through the solution between the electrodes 22 and 28. An
efficacious electrolytic agent will not react during or be affected by the water10 electrolysis process to thereby become expended, decomposed or depleted during the
water electrolysis process. The electrolytic agent must not be so volatile as to be
removed from solution along with the evolved gases; and, because hydrogen-ion
conce"l, alions are being rapidly perturbed at the electrodes during the water
electrolysis process, the electrolytic agent should have a strong resistance to pH
15 changes. In one embodiment, the electrolyte solution is made of distilled water and the
electrolytic agent is effective quantities of potassium hydroxide (KOH), generally about
10 g KOH per 1 .2L of water. During operation, electrolytic agent concentrations in the
water will vary.
The electrolyte solution is added through port 10 to the chamber. Once it is added, it
20 is only necessary to add distilled make-up water on an occasional basis to maintain the
unit in operation. Adding make-up water may be accomplished by removing plug 12
and pouring in the make-up water and thereafter replacing plug 12. Make-up watershould be distilled water to avoid contamination of the electrolytic solution with the
dissolved salts and other minerals and contan,inants present in water that is not
25 distilled. The initial charge of electrolytic agent will last indefinitely under normal
conditions.
Electrical current to the system is actuated by turning the ignition switch key to start the
engine. The current flowing from anode 28 to cathode 22 causes the electrolysis of the
CA 02207116 1997-06-06
- 14-
water in the electrolyte solution and accordingly the generation of hydrogen and oxygen
gas. The operation of the engine causes a vacuum to be set up in the air intake system
of the engine. This vacuum draws the plug 12 down into port 10 to seal the port. Any
generated gases from the chamber are drawn through supply line 18, to air intake valve
5 58, wherein they are mixed with the air and burned with the fuel.
Hydrogen and oxygen are generated as long as the engine is running and vacuum isapplied to vacuum switch 70. When either the vehicle ignition key 81 is turned to the
off position, or the supply of vacuum to vacuum switch 70 falls below a preselected
threshold amount (because either the engine stalls or stops, or gas or steam production
10 delivered over line 75 exceeds engine vacuum) power to the electrolysis chamber is
cut-off by power relay 62 in response to interruption of the control signal provided to
the relay by wire 68 resulting in cessation of generation of hydrogen and oxygen gases.
Electrolysis gases produced within chamber 2 are carried along supply line 18 to gas
flow control valve 78 which is a directional valve connecting supply line 18 to supply
line 75 when control wire 68 is energized (the ignition 81 is on and vacuum switch 70
is receiving vacuum) allowing produced gases to be directed to engine supply line 73.
Any gases which, through a system failure, are generated while the engine is turned
off or remain in electrolysis chamber 2 following engine shut off causes control line 68
to lose power thereby causing solenoid valve 78 to couple supply line 18 to the vent
20 line 82 to expel the excess/surplus gases harmlessly into the ambient atmosphere. In
the unlikely event that solenoid valve 78 fails or ignition of the hydrogen and oxygen
gases occurs within electrolysis chamber 2, any accumulated gases or explosive
pressures will be released by pushing out plug 12 or bursting area 8.
25 It will be apparent that many changes may be made to the illustrative embodiments,
while falling within the scope of the invention and it is intended that all such changes
be covered by the claims appended hereto.