Note: Descriptions are shown in the official language in which they were submitted.
CA 02207451 1997-07-11
-1-
Expendable Infra-red Radiating Means
The present invention relates to a covert, expendable infra-red (IR) radiating
means and
in particular to a covert countermeasure or decoy flare capable of generating
an IR interference
cloud to divert an incoming missile equipped with an IR seeker system away
from its intended
target or to create a covert JR screen.
Known IR decoy flares conventionally comprise pyrotechnic compositions bound
together with an organic binder and pressed to form pellets. When an incoming
missile is
detected a pellet is ignited and launched from the target. The pellet burns
over its surface to
produce an intense infra-red source which can lure the infra-red seeker system
of the missile
away from the target.
However, advances in missile seeker systems and the development of
`intelligent' missile
systems have led to seeker systems which are designed to recognise the typical
characteristics of
a decoy flare and ignore it. Some advanced seeker systems are programmed with
a
characteristic infra-red signature of the intended target, the exhaust plume
of a jet aircraft for
example, and will ignore the almost point like radiating source of a
conventional flare.
There has therefore become a need for a countermeasure which radiates over a
large
area, either to appear more like the intended target to the missile system or
to act as a screen,
especially for larger or slower moving targets.
One known decoy flare which is capable of generating a large cloud emitting in
the
infra-red range is described in the patent US 4 624 186. This flare comprises
a casing containing
combustible flakes and an ignition expediting material. These combustible
flakes comprise a thin
base material, such as paper or metal foil, on to which is pressed a
phosphorus containing
incendiary paste. In use, the flare is launched into the air and the ignition
expediting material
creates a fireball which passes through the combustible flakes, igniting the
incendiary paste
which burns to emit IR radiation and spreading the flakes which float slowly
downward creating
the interference cloud.
One problem with this type of flare is that phosphorus has a characteristic IR
emission
spectrum which some `intelligent' seeker systems can be programmed to ignore.
Also, these
CA 02207451 1997-07-11
-2-
types of flares are quite expensive. Further this type of flare also radiates
in the visible and ultra-
violet (UV) regions and produces a large, visible smoke cloud. This has the
disadvantage of
revealing that a countermeasure has been deployed which can indicate that a
certain threat has
been detected.
Furthermore, some `intelligent' seeker systems use other radiation, for
example UV
emission, when deciding to ignore some IR sources and are therefore not
deflected by flares
emitting significant amounts of radiation the visible or UV regions. Also,
some missile
systems, for example ones often employed in ground based anti-aircraft
batteries, require
human operators to make an initial target acquisition for a particular missile
before the IR
seeker system of that missile guides it to its acquired target. This target
acquisition is done
visually and hence, particularly at night, illumination of the target by the
visible emission
from the decoy is undesirable.
One known type of covert flare uses Activated Metal Disks (AMDs). These are
disks of
metals made pyrophoric by a process described in US patent 4,895,609. The
disks are held in a
store which, on ejection from the target, ruptures to dispense the disks. As
the disks are
pyrophoric they ignite on contact with the air and burn to act as a decoy.
However this type of flare is expensive to produce and, due to the pyrophoric
nature of
the disks, has a relatively large delay before becoming effective as the disks
have to be ejected
and dispensed before ignition can occur. For some applications, e.g. for fast
moving vehicle
decoys, the narrow field of view of some modern seeker systems means that the
disks can be
outside the missiles effective vision by the time combustion is fully underway
and will not
therefore be effective as a decoy. Also, the mass of a flare of this type can
be significantly
greater than standard IR flares.
It is therefore an aim of the present invention to provide an JR radiator
which alleviates
at least some of the aforementioned problems.
According to the present invention there is provided an expendable infra-red
radiating
means comprising a rupturable container, a plurality of decoy plates housed
within the
rupturable container and an ignition means for igniting the decoy plates
wherein
CA 02207451 1997-07-11
-3-
the decoy plates comprise a composition of a metal and an oxidant capable of
an
exothermic combustion reaction upon ignition, the composition being selected
such that the
combustion reaction produces negligible radiation in the visible and ultra
violet regions and
wherein following completion of the combustion reaction the decoy plate
comprises hot
metal emitting infra-red radiation.
In use the container is deployed into the air and the decoy plates are ignited
by the
ignition means. The container is then ruptured, for example by build up of
pressure within
the container, to dispense the decoy plates to form a cloud of IR radiation
sources.
Use of a composition of a metal and an oxidant capable of an exothermic
combustion reaction upon ignition for the decoy plates provides a relatively
inexpensive
flare capable of generating a cloud of material which is emitting strongly in
the IR range.
As the combustion reaction primarily produces heat, which may be stored in the
hot
metal which remains after the reaction has ended, the decoy plates, during and
after
combustion, produce negligible amounts of radiation in the visible or UV
regions. In
daylight, when the decoy plates are deployed as a screen or as a decoy flare,
the small
amount of visible radiation will not be seen against the background light.
Even at night, the
dispersal of the disks means that the glow of the plates in the visible region
will be virtually
undetectable at the ranges concerned. Therefore the countermeasure may be
deployed
covertly. Also a missile seeker system will not see any radiation in the UV
region which
would be characteristic of a decoy flare.
Metal present after the combustion of the decoy plate will be hot due to the
heat
generated during combustion and therefore will be emitting in the IR range but
will have
negligible visible or UV radiation. The decoy is therefore effective beyond
the duration of
combustion of the decoy plates and a decoy cloud having a relatively long
duration can be
produced without the need for slow burning compositions.
As a consequence the duration of the combustion reaction can actually be
reduced
and fast burning compositions can be chosen for their heat generation
properties. The
combustible composition also has a fast ignition time and the decoy plates can
be ignited
CA 02207451 1997-07-11
-4-
before dispersal ensuring that the plates become effective within the missiles
field of view.
Minimising the duration of the ignition of the decoy plates is also
advantageous because the
ignition reaction produces not only heat but visible and ultra-violet (UV)
emission.
A further advantage is that the IR emission spectrum of the decoy plate after
the
combustion reaction has finished will be characteristic of hot metal which is
to be expected
from a target, for example the hot metal parts of a tank engine or an aircraft
exhaust, and
will not include any components which the seeker system will recognise as
artificial and
characteristic of a decoy.
Also, the electrical conduction of the plates and any metal existing after
reaction
means that the cloud produced is formed of conducting elements which could
reflect radio
frequency (RF) signals and therefore act as a large RADAR reflective surface.
The cloud,
due to its conducting properties, could also both reflect any RF signals
generated by the
target thereby possibly confusing any systems which look for these signals or
alternatively
could scatter any incident RADAR pulses which may result in deflecting a
system which
uses active RADAR guidance.
In order to ensure that the decoy functions effectively after combustion the
composition of the decoy plates is preferably selected such that the mass of
hot metal
emitting infra-red radiation after combustion of a decoy plate is at least 10%
of the mass of
the decoy plate before combustion. For a decoy relying on hot metal remaining
after the
reaction, the amount of that metal remaining should be at least 10% of the
mass of the
decoy plate to ensure that the plates are acting efficiently.
Preferably the composition of the decoy plates comprises an excess of metal.
Ignition and combustion of a decoy plate produces heat due to the combustion
reaction between the metal and the oxidant. The excess metal does not undergo
reaction
and absorbs a lot of the heat generated during the reaction thereby resulting
in hot metal
remaining after the end of the reaction.
CA 02207451 1997-07-11
-5-
It will be clear to one skilled in the art that by varying the ratio of metal
to oxidant
present in the composition of the decoy plates, the amount of metal remaining
after the
reaction can be altered, as can the amount of heat generated.
Advantageously the composition of the decoy plates is selected such that a
reaction
product of the exothermic combustion reaction between the metal and the
oxidant is hot
metal emitting infra-red radiation.
By producing metal in the combustion reaction then the proportion of the decoy
plate which undergoes reaction can be high whilst retaining the advantages of
having metal
present after the reaction has finished. Thus the proportion of excess metal
could be
reduced, which could result in more heat being generated, whilst retaining the
same
proportion of metal present after reaction.
Alternatively production of a metal in the combustion reaction means that all
the
metal of the decoy plate could take part in the combustion reaction and there
would still be
hot metal at the end of the reaction. This could allow the metal of the plate
to be chosen
because of its properties as a fuel whereas the metal produced could be
selected for its
thermal properties, for instance thermal conductivity or melting point.
Metal may conveniently be produced by ensuring that the metal of the decoy
plate is
a first metal and the oxidant is an oxide of a second metal. Upon ignition the
first metal
and second metal oxide undergo a combustion reaction wherein the oxide of the
second
metal dissociates to produce the second metal and the first metal reacts with
the dissociated
oxygen to form an oxide of the first metal.
Suitable metal fuels liberate large amounts of heat in the combustion reaction
and
include aluminium, iron, calcium, titanium, silicon and boron although it will
be apparent to
one skilled in the art that other metals could be used. The oxidant must
obviously be such
that it will be reduced in the combustion reaction and liberate sufficient
heat and the skilled
worker could easily determine suitable oxidants for a given metal fuel. For
aluminium, for
example, suitable oxidants include ferric oxide, calcium oxide, tungsten
dioxide or trioxide,
manganese dioxide and sodium chlorate.
CA 02207451 1997-07-11
-6-
One advantageous composition of a decoy plate has iron as the metal and
potassium
perchlorate as the oxidant. This composition is inexpensive and reliable and
burns to
produce potassium chloride and oxides of iron. Preferably 82 to 88% by weight
of the
decoy plate is iron and 18 to 12% by weight of the decoy plate is potassium
perchlorate.
This ratio is optimised to give maximum thermal and electrical conduction
before, during
and after combustion, due to the excess iron.
An alternative composition in which metal is produced has aluminium as the
metal
and ferric oxide as the oxidant. This composition burns to produce iron and
aluminium
oxide. A nearly stoichiometric composition of aluminium and ferric oxide is
preferably
used in order to maximise efficiency.
A further composition which could be used, in which metal is produced, has
titanium as the metal and manganese dioxide as the oxidant. This composition
burns to
produce manganese and oxides of titanium. It will be apparent to one skilled
in the art
however that other such compositions could be used.
Preferably the decoy plates comprise a pressed composition of a particulate
metal
and particulate oxidant.
Pressing the composition to form the decoy plates offers an inexpensive and
simple
method of manufacture with reliable results. The size of the particles has an
effect on the
heat of the reaction and can be varied for different applications although it
will be apparent
to one skilled in the art to choose particle sizes that are not too large as
to give inefficient
burning or problems with ignition but that are not too small as to lead to
problems with
safety and uncontrollability with the plates being too sensitive. The pressing
loads will
likewise be such to ensure good metal to oxidant contact.
The decoy plate composition may advantageously further comprise a binder
material to improve the stability of the plates and to modify the thermal
characteristics of
the plates.
Advantageously the thicknesses of the decoy plates are adapted such that, in
use, at
least some of the decoy plates break up on dispersal from the rupturable
container. The
CA 02207451 1997-07-11
-7-
shock of ejection into the atmosphere from the rupturable container causes
thin plates to
break up on ejection. This results in a cloud having IR emitting pieces of
differing sizes.
The differing sized pieces would have different air resistances which would
aid in spreading
the cloud over a large area. Also the varying sized pieces, because of their
conductive
properties, could have a greater RADAR reflection potential.
Most usefully the decoy plates have grooves running across the surface.
Grooves
aid in fracturing of plates, especially after the combustion reaction between
the metal and
the oxidant, and may either compliment or be an alternative to thin plates.
Grooves give
more control over the sizes of pieces produced, which could be tailored to the
wavelengths
of the likely RADAR signals. The addition of grooves to the decoy plates also
speeds the
ignition times of the plates by allowing hot ignition gases to pass along the
grooves
The decoy plate composition may also be adapted such that at least some of the
hot
metal produced by the combustion of a decoy plate is molten. By selecting the
composition
of the decoy plates such that the hot metal produced has a melting point lower
than the
temperature reached by the exothermic combustion reaction then at least some
of that
metal produced will be molten.
Molten metal will radiate in the IR region and will quickly cool enough to
solidify
forming various shapes, from droplets to misshapen plates depending upon the
proportion
of metal which was molten initially. These shapes produced will be more
effective at
scattering incident RADAR radiation and will have a greater range of
reflective
characteristics.
Conveniently the decoy plates are interlayered with combustible cloth
material. The
cloth can act as a spacer to reduce the weight of the decoy and can aid in
dispersal of the
decoy plates by reducing the tendency of the plates to stick to one another.
Also,
combustible cloth will contribute to the effectiveness of the decoy and can be
chosen to
produce negligible visible or UV radiation.
Further advantages and embodiments of the invention will now be described by
way
of example only with reference to the accompanying drawings in which:
CA 02207451 1997-07-11
-8-
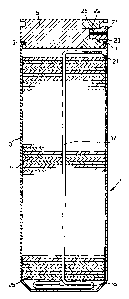
Figure 1 shows a sectional view of a decoy flare according to the present
invention,
Figure 2 shows a decoy plate suitable for use in the flare shown in figure 1,
Figure 3 shows a plot of relative intensity of infra-red radiation against
time
for ignition of a typical decoy plate suitable for use in a flare according to
the present invention,
Referring now to figure 1, a rupturable container, generally indicated 1, has
a cylindrical
casing 3 with an open end, the open end being sealed by a lid 5. The lid 5 is
held in place as
the edges 7 of the open end of the casing 3 are slightly crimped into a groove
9 in the lid 5.
A plurality of decoy plates 11 are stacked in the casing 3.
One type of decoy plate suitable for use in this flare is shown in figure 2.
The decoy
plate is in the form of a disk 11 provided with a central hole 13. The disk 11
has a diameter
of about 45mm and a thickness of 0.6mm with the central hole 13 having a
diameter of
6mm. Grooves 15 extend radially from the central hole 13 to the edge of the
disk, the
grooves being about 1 mm wide and 0.4mm deep.
In one particular embodiment the disk 11 is formed from a particulate
composition
of 86% by weight iron (Fe) and 14% by weight potassium perchlorate (KC1O4)
pressed
together under a load of about 100MPa. The iron particles are around 5-15 m in
size and
the potassium perchlorate particles are greater than 454m in size. Upon
ignition the iron
and potassium perchlorate undergo a combustion reaction to produce oxides of
iron and
potassium chloride the principle reaction being;
3Fe + KC1O4 --> KCl + Fe3O4 + Heat (1)
It can be seen that as the atomic weight of iron is about 56 and that of
potassium
perchlorate is about 90 then the stoichiometric mixture would have about 65%
by weight
CA 02207451 1997-07-11
...................
-9-
iron with 35% by weight of potassium perchlorate. It is apparent therefore
that
approximately 60% by weight of the disk is present as excess iron. However, in
use the
production of other iron oxides would occur and the reaction would be
complemented by
oxygen in the atmosphere thus the actual amount of excess iron would be lower
than this.
The typical temperature reached by such a disk would be around 1000 with a
burn rate of
about l0cm/s.
In an alternative embodiment the disk 11 comprises a pressed particulate
composition of aluminium (Al) and ferric oxide (Fe2O3). Upon ignition of this
mixture the
aluminium and ferric oxide undergo a combustion reaction to produce iron and
aluminium
oxide, the principle reaction being;
2A1 + Fe2O3 -4 2Fe + A1203 + Heat (2)
Here it can be seen that the reaction produces iron thus ignition of the disk
11 results in the
production of red hot iron. The disk 11 has a nearly stoichiometric ratio of
aluminium to
ferric oxide with 30% by weight of the disk being aluminium.
In a third embodiment the disk 11 could comprise a pressed particulate
composition
of titanium (Ti) and manganese dioxide (Mn02) which, on ignition undergoes a
combustion
reaction produce manganese and oxides of titanium, one of the principle
reactions being;
Ti + Mn02 --+ Mn + TiO2 + Heat (3)
It will, of course, be apparent to one skilled in the art that other suitable
metal fuels
and oxidants could be used.
Referring back to figure 1 the plates 11 are stacked with an ignition cord 17
running
from adjacent the lid 5 through the centre of the decoy plates 11 to form a
coil 19 at the
closed end of the casing 3. The ignition cord has its primed end 21 located
adjacent an
ignition transfer means 23 in the lid 5. A piston 25, such as a millboard,
plastic or
CA 02207451 1997-07-11
-10-
aluminium disk, for example, having a diameter equal to or just less than that
of the interior
of the casing, is located between the ignition cord 21 and the stack of decoy
plates 11.
For certain applications the decoy plates can be stacked interlayered with a
combustible cloth (not shown) in order to reduce the tendency for the ignited
plates to stick
to one another and to reduce the amount of plates in the stack and therefore
the weight of
the decoy.
In use pyrotechnic mixture 25 is ignited by, for example, a standard
electrical igniter
(not shown), and the rupturable container is deployed into the air. Once the
rupturable
container is clear of its housing spring 27 is released allowing the ignition
stimulus to travel
down tube 29 to ignite delay 23. Delay 23 allows the flare to move away from
its housing
before igniting primed end 21 of the ignition cord 17. Should the rupturable
container
become jammed spring 27 prevents propagation of the ignition stimulus thus
preventing the
decoy flare from igniting inside its housing.
Ignition of the primed end 21 of the ignition cord 17 causes the cord to
quickly
combust, igniting the decoy plates 11 as the fireball passes down the cord.
The gasses
generated from this combustion and ignition of the decoy plates 11 causes a
build up of
pressure in the casing which is enough to the eject the lid 5. The first few
decoy plates 11
will probably fall out of the casing. Meanwhile the combustion of the ignition
cord coil 19
produces a large amount of gas which drives the piston 25 to eject the ignited
decoy plates
11.
Generally the dispersion of the decoy plates 11 may be altered by choosing an
ignition train which generates more or less gas. A useful ignition cord may
be, for example,
a magnesium/Viton/Teflon (MTV) ignition cord which generates useful quantities
of gas to
disperse the plates over a large area.
The typical variation in intensity of the total IR radiation emission of a
decoy plate
not having a grooved surface is shown in figure 3. The plate ignited was a
0.5mm thick
disk of 86% iron and 14% potassium perchlorate having a diameter of 47mm.
It can be seen from the plot that even without grooves in the surface the disk
shows
a very fast rise time from ignition to maximum intensity, ensuring that the
decoy starts
CA 02207451 1997-07-11
-11-
operating within the field of view of the missile. The peak intensity drops
relatively rapidly
but the intensity stays moderately high for a long duration due to the
radiating metal
present after reaction. Thus the flare offers a fast response coupled with a
long duration
and is suitable for use with fast or slow targets. Also, whilst the initial
ignition may trigger
a countermeasures device and cause the missile to ignore its guidance system
for a short
time, when the guidance comes back on line the decoy will still be radiating
and acting as a
decoy but without any of the telltale characteristics of known decoys.