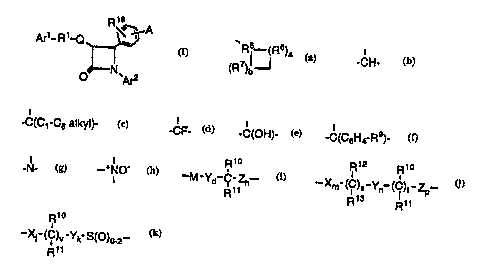Note: Descriptions are shown in the official language in which they were submitted.
CA 02207627 1997-06-12
WO 96!19450 PCT/ITS95/I6007
4-~(HETEROCYCLOALKYL OR HETEROAROMATIC)
SUBSTITUTED PHENYLI-2-AZETIDINONES USEFUL AS
HYPOLIPIDEMIC AGENTS
BACKGROUND OF THE INVENTION
The present invention relates to 4-[(heterocycloalkyl or
heteroaromatic)-substituted phenyl)-2-azetidinones useful as
hypocholesterolemic agents in the treatment and prevention of
atherosclerosis, and to the combination of a 4-[(heterocycloalkyl or
heteroaromatic)-substituted phenyl]-2-azetidinone of this invention and
a cholesterol biosynthesis inhibitor for the treatment and prevention of
atherosclerosis.
Atherosclerotic coronary heart disease represents the
major cause for death and cardiovascular morbidity in the western
world. Risk factors for atherosclerotic coronary heart disease include
hypertension, diabetes mellitus, family history, male gender, cigarette
smoke and serum cholesterol. A total cholesterol level in excess of 225-
250 mg/dl is associated with significant elevation of risk.
Cholesteryl esters are a major component of
atherosclerotic lesions and the major storage form of cholesterol in
arterial wall cells. Formation of cholesteryl esters is also a key step in
the intestinal absorption of dietary cholesterol.
A few azetidinone compounds have been reported as
being useful in lowering cholesterol and/or in inhibiting the formation of
cholesterol-containing lesions in mammalian arterial walls. U.S.
4,983,597 discloses N-sulfonyl-2-azetidinones as anticholesterolemic
agents and Ram, et al., in Indian J Chem. Sect. B 29B, 12 (1990), p.
1134-7, disclose ethyl 4-(2-oxoazetidin-4-yl)phenoxy-alkanoates as
CA 02207627 1997-06-12
WO 96/19450 . PCT/iJS95/16007
-2-
hypolipidemic agents. European Patent Publication 264,231 discloses
1-substituted-4-phenyl-3-(2-oxoalkylidene)-2-azetidinones as blood
platelet aggregation inhibitors. European Patent 199,630 and European
Patent Application 337,549 disclose elastase inhibitory substituted
azetidinones said to be useful in treating inflammatory conditions
resulting in tissue destruction which are associated with various disease
states, e.g. atherosclerosis. W093/02048 discloses substituted (i-
lactams useful as hypocholesterolemic agents.
In addition to regulation of dietary cholesterol, the
regulation of whole-body cholesterol homeostasis in humans and
animals involves modulation of cholesterol biosynthesis, bile acid
biosynthesis, and the catabolism of the cholesterol-containing plasma
lipoproteins. The liver is the major organ responsible for cholesterol
biosynthesis and catabolism and, for this reason, it is a prime
determinant of plasma cholesterol levels. The liver is the site of
synthesis and secretion of very low density lipoproteins (VLDL) which
are subsequently metabolized to low density lipoproteins (LDL) in the
circulation. LDL are the predominant cholesterol-carrying lipoproteins in
the plasma and an increase in their concentration is correlated with
increased atherosclerosis.
When cholesterol absorption in the intestines is reduced,
by whatever means, less cholesterol is delivered to the liver. The
consequence of this action is a decreased hepatic lipoprotein (VLDL)
production and an increase in the hepatic clearance of plasma
cholesterol, mostly as LDL. Thus, the net effect of an inhibition of
intestinal cholesterol absorption is a decrease in plasma cholesterol
levels.
The inhibition of cholesterol biosynthesis by 3-hydroxy-3-
methylglutaryl coenzyme A reductase (EC1.1.1.34) inhibitors has been
shown to be an effective way to reduce plasma cholesterol (Witzum,
Circulation, 80, 5 (1989), p. 1101-1114) and reduce atherosclerosis.
Combination therapy of an HMG CoA reductase inhibitor and a bile acid
sequestrant has been demonstrated to be more effective in human
hyperlipidemic patients than either agent in monotherapy (Illingworth,
Drugs , 36 (Suppl. 3) (1988), p. 63-71 ).
CA 02207627 1997-06-12
WO 96/19450 , PCT/US95/16007
-3-
SUMMARY OF THE INVENTION
Compounds of the present invention are represented by
the formula I
R~s
~~A
Are- R'-Q
I
O N ~Ar2
or a pharmaceutically acceptable salt thereof, wherein
A is selected from the group consisting of R2-substituted
heterocycloalkyl, R2-substituted heteroaryl, R2-substituted benzofused
heterocycloalkyl, and R2-substituted benzofused heteroaryl;
Are is aryl or R3-substituted aryl;
Ar2 is aryl or R4-substituted aryl;
Q is a bond or, with the 3-position ring carbon of the azetidinone,
'R5 (R6)a
forms the spiro group (R~)b ; and
R1 is selected from the group consisting of
-(CH2)Q-, wherein q is 2-6, provided that when Q forms a
spiro ring, q can also be zero or 1;
-(CH2)e-G-(CH2),-, wherein G is -O-, -C(O)-, phenylene,
-NR8- or -S(O)o_2-, a is 0-5 and r is 0-5, provided that the sum of a and r
is 1-6;
-(C2-C6 alkenylene)-; and
-(CH2)f-V-(CH2)g-, wherein V is C3-C6 cycloalkylene, f is 1-
5 and g is 0-5, provided that the sum of f and g is 1-6;
R5 is
I I
-CH-, -C(Ci-Cs alkyl)-, -CF-, -C(OH)-, -C(C6H4-Rs)-, -N-, or -+NO- ;
I
R6 and R~ are independently selected from the group consisting
0 of -CH2-, -CH(C~-C6 alkyl)-, -C(di-(C1-C6) alkyl), -CH=CH- and
-C(C1-C6 alkyl)=CH-; or R5 together with an adjacent R6, or R5 together
with an adjacent R~, form a -CH=CH- or a -CH=C(C~-C6 alkyl)- group;
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-4-
a and b are independently 0, 1, 2 or 3, provided both are not zero;
provided that when R6 is -CH=CH- or -C(Ci-Cs alkyl)=CH-, a is 1;
provided that when R~ is -CH=CH- or -C(C1-C6 alkyl)=CH-, b is 1;
provided that when a is 2 or 3, the R6's can be the same or different;
and provided that when b is 2 or 3, the Re's can be the same or different;
and when Q is a bond, R~ also can be:
Ryo R~2 Rio Rio
i
-M-Yd-C-Zh , -Xm-(C)S-Y~ (C)t-ZP or -Xj-(C)v-Yk S(O)o_2 ;
R»R~s R» Ryy
M is -O-, -S-, -S(O)- or -S(O)2-;
X, Y and Z are independently selected from the group consisting
of -CH2-, -CH(C~-C6 alkyl)- and -C(di-(C~-C6) alkyl);
R» and R~2 are independently selected from the group
consisting of -OR~4, -O(CO)R~4, -O(CO)OR~6 and -O(CO)NR~4R~5; R~ ~
and R13 are independently selected from the group consisting of
hydrogen, (C~-C6)alkyl and aryl; or Ri~ and R1~ together are =O, or R12
and R~3 together are =O;
dis l,2or3;
h is 0, 1, 2, 3 or 4;
s is 0 or 1; t is 0 or 1; m, n and p are independently 0-4; provided
that at least one of s and t is 1, and the sum of m, n, p, s and t is 1-6;
provided that when p is 0 and t is 1, the sum of m, s and n is 1-5; and
provided that when p is 0 and s is 1, the sum of m, t and n is 1-5;
vis0orl;
j and k are independently 1-5, provided that the sum of j, k and v
is 1-5;
R2 is 1-3 substituents on the ring carbon atoms selected from the
group consisting of hydrogen, (Cy-C1 o)alkyl, (C2-C1 o)alkenyl,
(C2-C1 o)alkynyl, (C3-Cs)cycloalkyl, (C3-C6)cycloalkenyl, R~ ~-substituted
aryl, R17-substituted benzyl, R~~-substituted benzyloxy, R17-substituted
aryloxy, halogeno, -NR14R~5, NR~4R~5(C1-C6 alkylene)-,
NRl4RisC(O)(C1-C6 alkylene)-,-NHC(O)R16, OH, C~-C6 alkoxy,
-OC(O)R16, -COR~4, hydroxy(C1-C6)alkyl, (Cy-C6)alkoxy(C1-C6)alkyl,
N02, -S(O)o_2R~6, -SO2NR14R1s and -(C~-C6 alkylene)COOR~4; when
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-5-
R2 is a substituent on a heterocycloalkyl ring, R2 is as defined, or is =O
0
I CH2) ~-2
or 'o ; and, where R2 is a substituent on a substitutable ring
nitrogen, it is hydrogen, (C1-C6)alkyl, aryl, (Ci-C6)alkoxy, aryloxy, (Ci-
C6)alkylcarbonyl, arylcarbonyl, hydroxy, -(CH2)1_6CONRi8R18,
R~s'
N
or
(CH2)o-a
J is -O-, -NH-, -NRi8- or -CH2-;
R3 and R4 are independently selected from the group consisting
of 1-3 substituents independently selected from the group consisting of
(Ci-C6)alkyl, -OR14, -O(CO)R14, -O(CO)ORis, -O(CH2)1-50814,
-O(CO)NR14R15~ _NR14R15~ _NR14(CO)R15, -NR14(CO)ORis,
-NR14(CO)NR15Ri9, _NR14S02Ris, _COOR14, -CONR14R15, _COR14,
-S02NR14R15~ S(0)0-2R16~ _O(CH2)1-10-COOR14,
-O(CH2)1-IOCONR14R15, _(C1_C6 alkylene)-COOR14, -CH=CH-COOR14,
-CF3, -CN, -N02 and halogen;
R8 is hydrogen, (C1-C6)alkyl, aryl (C1-C6)alkyl, -C(O)R14 or
-COOR14;
R9 and Ri~ are independently 1-3 groups independently selected
from the group consisting of hydrogen, (Ci-C6)alkyl, (C1-C6)alkoxy,
-COOH, N02, -NR14R15, OH and halogeno;
814 and 815 are independently selected from the group
consisting of hydrogen, (C1-C6)alkyl, aryl and aryl-substituted (Ci-
Cs)alkyl;
Ris is (Ci-Cs)alkyl, aryl or R1~-substituted aryl;
Ri$ is hydrogen or (C1-C6)alkyl; and
Ri9 is hydrogen, hydroxy or (C1-C6)alkoxy.
"A" is preferably an R2-substituted, 6-membered
heterocycloalkyl ring containing 1 or 2 nitrogen atoms. Preferred
heterocycloalkyl rings are piperidinyl, piperazinyl and morpholinyl
groups. The ring "A" is preferably joined to the phenyl ring through a
ring nitrogen. Preferred R2 substituents are hydrogen and lower alkyl.
Ri 9 is preferably hydrogen.
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-6-
Ar2 is preferably phenyl or R4-phenyl, especially (4-R4)-
substituted phenyl. Preferred definitions of R4 are lower alkoxy,
especially methoxy, and halogeno, especially fluoro.
Ar1 is preferably phenyl or R3-substituted phenyl,
especially (4-R3)-substituted phenyl.
There are several preferred definitions for the -R1-Q-
combination of variables:
Q is a bond and R~ is lower alkylene, preferably propylene;
Q is a spiro group as defined above, wherein preferably R6 and
i i
R~ are each ethylene and R5 is -CH- or -C(OH)- ;
Rio
Q is a bond and R~ is -M-Yd-C-Zh wherein the variables
are chosen such that Ri is -O-CH2-CH(OH)-;
R~2 R1o
Q is a bond and R~ is _ Xm- (C)s-Y~ (C)t _ ZP wherein the
Rye
variables are chosen such that R~ is -CH(OH)-(CH2)2-; and
Rio
Q is a bond and R~ is -XI-(C)~-Yk-S(p)o_2 wherein the
R' ~
variables are chosen such that R1 is -CH(OH)-CH2-S(O)o_2-.
This invention also relates to the use of a compound of
formula I as a hypocholesterolemic agent in a mammal in need of such
treatment.
In another aspect, the invention relates to a pharmaceutical
composition comprising a substituted azetidinone of formula I in a
pharmaceutically acceptable carrier.
The present invention also relates to a method of reducing
plasma cholesterol levels, and to a method of treating or preventing
atherosclerosis, comprising administering to a mammal in need of such
treatment an effective amount of a combination of a 4[(heterocycloalkyl
or heteroaromatic)-substituted phenyl]-2-azetidinone cholesterol
CA 02207627 2005-04-20
_ 7 -
absorption inhibitor for combined use with a cholesterol
biosynthesis inhibitor (and, similarly, use of a
cholesterol biosynthesis inhibitor for combined use with a
4[(heterocycloalkyl or heteroaromatic)-substituted phenyl]-
2-azetidinone cholesterol absorption inhibitor) to treat or
prevent athersclerosis or to reduce plasma cholesterol
levels.
In yet another aspect, the invention relates to a
pharmaceutical composition comprising an effective amount
of a 4[(heterocycloalkyl or heteroaromatic)-substituted
phenyl]-2-azetidinone cholesterol absorption inhibitor, a
cholesterol biosynthesis inhibitor, and a pharmaceutically
acceptable carrier. In a final aspect, the invention
relates to a kit comprising in one container an effective
amount of a 4[(heterocycloalkyl or heteroaromatic)-
substituted phenyl]-2-azetidinone cholesterol absorption
inhibitor in a pharmaceutically acceptable carrier; and in
a separate container, an effective amount of a cholesterol
biosynthesis inhibitor in a pharmaceutically acceptable
carrier.
In yet a further aspect of the invention there is
a compound selected from:
3-[4-(4-methyl-1-piperazinyl)phenyl]-2,7-diphenyl-2-
azaspiro- [5, 3] nonan-1-one; and represented by the
formula:
A
wherein A and Ar2 and cis and trans isomers are
defined in the following table:
CA 02207627 2005-04-20
- 7a -
A A~ Relative Stereo-
Chemistry
4 - (CH30)-C6H4- trans
-N\ /N-CH2C6H5
~ 4 ( CH3 - C6H4 t rans
- 0 ) -
-N
-CH
3
C6H5 - trans
-N\
N ~
OCH3
/
~
~ C6H5 - trans
-
p 4 ( CH30 C6H4 t rans
- ) - -
N
-
O
O C6H5- tran
-N
A Ar'~ Relative Stereo-
chemistry
4 ( CH30 C6H4 t rans
- ) - -
-N 4- (CH30) C6H4- CiS
-
4 ( CH30 C6H4 t rans
-N - ) - -
CA 02207627 2005-04-20
- 7b -
-continued
/~ 4- (CH30) -C6H4- cis
-N S
4- (CH30) -C6H4- trans
-N S
° 4- (CH30) -C6H4- cis
-N
0
trans
° 4-(CH30)-C6H4-
-N
O
C6H5 - C7. S
-N\ /N-CH3
C6H5- trans
-N\ N-CH3
/~ 4 - ( CH30 ) - C6H4 - trans
-N NH
4- (CH30) -C6H4- trans
°~°
N-N H
A A~ Relative Stereo-
chemistry
° CH3 4 - ( CH3 O ) - C6H4 - t rans
N
DETAILED DESCRIPTION
As used herein, the term "alkyl" or "lower alkyl"
means straight or branched alkyl chains of 1 to 6 carbon
atoms and "alkoxy" similarly refers to alkoxy groups having
1 to 6 carbon atoms.
CA 02207627 2005-04-20
- 7C -
"Alkenyl" means straight or branched carbon
chains having one or more double bonds in the chain,
conjugated or unconjugated. Similarly, "alkynyl" means
straight or branched carbon chains having one or more
triple bonds in the chain. Where an alkyl, alkenyl or
alkynyl chain joins two other variables and is therefore
bivalent, the terms alkylene, alkenylene and alkynylene are
used.
"Cycloalkyl" means a saturated carbon ring of 3
to 6 carbon atoms, while "cycloalkylene" refers to a
corresponding bivalent ring, wherein the points of
attachment to other groups include all positional isomers.
"Halogeno" refers to fluorine, chlorine, bromine
or iodine radicals.
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-8-
"Aryl" means phenyl, naphthyl, indenyl, tetrahydronaphthyl
or indanyl. "Phenylene" means a bivalent phenyl group, including ortho,
mete and pare-substitution. R~ ~-benzyl and R~ ~-benzyloxy refer to
benzyl and benzyloxy radicals which are substituted on the phenyl ring.
"Heterocycloalkyl" means a 3- to 7-membered saturated -
ring containing a nitrogen atom, optionally containing one aditional
heteroatom selected from the group consisting of N, O and S(O)o_2, and
optionally containing a double bond between a ring nitrogen and an
adjacent carbon. The heterocycloalkyl ring is substituted on one or
more ring carbon or nitrogen atoms by a variable R2 as defined above.
"Benzofused heterocycloalkyl" refers to heterocycloalkyl groups as
defined wherein a benzene radical is joined to adjacent carbon atoms.
Typical heterocycloalkyl and benzofused heterocycloalkyl groups are
exemplified as shown:
O
(CH2) 1-2
O ~S~O)o-2 ~N-Z O
N NJ NJ NJ NJ N
'N 'N 'N ~T
-N\/ _O
N~ N O S ,~JN
\ / I ( \ ~ \
/ \ / /
N~ N Y N Q N~U)o-2 NJ-H
~N ~ ~N ~ N
i i p ~ g
NJ NJ
"Heteroaryl" means 5- to 6-membered aromatic rings
containing a nitrogen atom and optionall containing 1 to 3 additional
heteroatoms selected from the group consisting of N, S and O. The
heteroaryl ring is substituted on one or more ring carbon or nitrogen
atoms by a variable R2 as defined above. Benzofused heteroaryl refers
to radicals formed by the bonding of a benzene radical to adjacent
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-9-
carbon atoms on a heteroaryl ring; examples are indolyl, quinolyl,
quinazolinyl, quinoxalinyl, benzotriazolyl, indazolyl, benzoxazolyl,
benzothienyl and benzofuranyl. Typical heteroaryl groups are
exemplified as shown:
/ I ~NI ~N/ N'N/N 'N" ~NI
N N N N O-N O
~N I / I I W ~I w' ~~ N I Nw
s~ o-N ~ N l J
N ~J N N
N
N) ' ( O~ ~ ( S) ' ~ o N
~N ~N ~N ~ N
' N I ~ ~ N
I : ~ ~ I J 'N . I
N N a
IJ I N
. N NJ
The terms heterocycloalkyl and heteroaryl include all
positional isomers for a given heterocycloalkyl or heteroaryl group as
defined above, for example 2- piperdinyl, 3- piperidinyl or 3-piperidinyl,
and 2-pyridyl, 3-pyridyl and 4-pyridyl.
The above statements, wherein, for example, R~4~, R~5 and
R~ 9 are said to be independently selected from a group of substituents,
means that R~4, R~5 and R19 are independently selected, but also that
where an R14, R~5 or R~9 variable occurs more than once in a molecule,
those occurrences are independently selected (e.g., if R3 is -OR~4
wherein R~4 is hydrogen, R4 can be -OR~4 wherein R14 is lower alkyl).
Those skilled in the art will recognize that the size and nature of the
substituent(s) will affect the number of substituents which can be
present.
Compounds of the invention have at least one
asymmetrical carbon atom and therefore all isomers, including
diastereomers and rotational isomers are contemplated as being part of
this invention. The invention includes d and I isomers in both pure form
and in admixture, including racemic mixtures. Isomers can be prepared
using conventional techniques, either by reacting optically pure or
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-10-
optically enriched starting materials or by separating isomers of a
compound of formula I.
Those skilled in the art will appreciate that for some
compounds of formula I, one isomer will show greater pharmacological
activity than other isomers.
Compounds of the invention with an amino group can form
pharmaceutically acceptable salts with organic and inorganic acids.
Examples of suitable acids for salt formation are hydrochloric, sulfuric,
phosphoric, acetic, citric, oxalic, malonic, salicylic, malic, fumaric,
succinic, ascorbic, malefic, methanesulfonic and other mineral and
carboxylic acids well known to those in the art. The salt is prepared by
contacting the free base form with a sufficient amount of the desired acid
to produce a salt. The free base form may be regenerated by treating
the salt with a suitable dilute aqueous base solution such as dilute
aqueous sodium bicarbonate. The free base form dififers from its
respective salt form somewhat in certain physical properties, such as
solubility in polar solvents, but the salt is otherwise equivalent to its
respective free base forms for purposes of the invention.
Certain compounds of the invention are acidic (e.g., those
compounds which possess a carboxyl group). These compounds form
pharmaceutically acceptable salts with inorganic and organic bases.
Examples of such salts are the sodium, potassium, calcium, aluminum,
gold and silver salts. Also included are salts formed with
pharmaceutically acceptable amines such as ammonia, alkyl amines,
hydroxyalkylamines, N-methylglucamine and the like.
Cholesterol biosynthesis inhibitors for use in the
combination of the present invention include HMG CoA reductase
inhibitors such as lovastatin, pravastatin, fluvastatin, simvastatin and CI-
981; HMG CoA synthetase inhibitors, for example L-659,699 ((E,E-11-
[3'R-(hydroxy-methyl)-4'-oxo-2'R-oxetanyl]-3,5,7R-trimethyl-2,4-
undecadienoic acid); squalene synthesis inhibitors, for example ,
squalestatin 1; and squalene epoxidase inhibitors, for example, NB-598
((E)-N-ethyl-N-(6,6-dimethyl-2-hepten-4-ynyl)-3-[(3,3'-bithiophen-5- .
yl)methoxy]benzene-methanamine hydrochloride). Preferred HMG CoA
reductase inhibitors are lovastatin, pravastatin and simvastatin.
CA 02207627 2005-04-20
-11-
Compounds of formula I can be prepared by known
methods, for example WO 93/02048 describes the preparation of
compounds wherein -R1-Q- is alkytene, alkenylene or alkylene
interrupted by a hetero atom, phenylene or cyeloalkylene; WO
94/17038 describes the preparation of compaunds wherein Q is a
spirocyclic group; PCT/US94/10099 describes the preparation of
compounds wherein -R~-Q- is a hydroxy-substituted alkylene group;
PCTNS95/03196, filed March 22, 1995, describes compounds wherein
-R1-Q- is a hydroxy-substituted alkylene attached to the Are moiety
through an -O- or S(O)o..2- group; and U.S. Serial No. 08/342,197, filed
November 18, 1994, describes the preparation of comppunds wherein
-R1-Q- is a hydroxy-substituted aikytene group attached the the
azetidinone ring by a =S(O)o-z- group.
For example, compounds of formula I wherein Art-Rt-Q- is
phenylpropyl can be made according to the following procedures:
Method A:
1) ArzNH2
A ~ ~ CHO la
2) n-tr~rlamine,
Ph(CH~~COCf
II
A heterocyqloalkyl- or heteroaryhsubstituted benzaldehyde
of formula II is refluxed with an aniline derivative of forrrfula Ar~NH2 in
an inert solvent such as-toluene. n-~ributyiamir~e is added at reflux,
then 5-phenylvaleryi chloride (Ph(CMy4~OCl) is added and the mixture
refluxed. Conventional extraction -anl chromatographic techniques are
used to obtain the traps isomer of formula la.
ethod B:
1 ) ArzNH2
It la, Ib
2) Ph CH~4COCI ~ ~ c~ and traps)
-,.
CA 02207627 1997-06-12
WO 96/19450 PCT/iTS95/16007
-12-
A heterocycloalkyl- or heteroaryl-substituted benzaldehyde
of formula II is refluxed with an aniline derivative of formula Ar2NH2 in
an inert solvent such as toluene. Pyridine and Ph(CH2)4COCI are
added and the mixture is refluxed, or, alternatively, the toluene is
removed and pyridine serves as both the solvent and the base.
Conventional extraction and chromatographic techniques are used to
obtain a mixture of the cis and traps isomers of formula la and Ib.
r NH2
CONHNH2
/\
BrCN, NaHC03
N.A~ H20, dioxane
V O A~ Ic
and
o N.~~o
0
rNxN~ / \
V NJ ~N
/ ~
Triethylamine ~ I
O N,A~ Id
Compounds wherein R~9 is hydrogen and A is oxadiazolyl
can be prepared from the corresponding benzoic acid hydrazide of
formula V: an amino-substituted oxazolyl of formula Ic is prepared by
reacting a mixture of the hydrazide in water and dioxane at room
temperature with cyanogen bromide and NaHC03; a keto-substituted
oxazolyl of formula Id is prepared by reacting the hydrazide with 1,1'-
carbonyl diimidazole and a base such as triethylamine in an inert
solvent such as tetrahydrofuran.
Method D:
0
/ C02H / \
~NH2
_ - I
N,A~ EDCI, HOBT, NMM O N~Ar~ IX
CH2CI2
VIII
CA 02207627 1997-06-12
WO 96/19450 . PCT/US95/16007
-13-
CH3
IX H9~OAc)2
HOAc le
O Arz
Compounds of formula I wherein A is an oxazolyl group, e.g.,
compounds of formula le, can be prepared by reacting a benzoic acid of
formula VIII with propargylamine in the presence of well known coupling
reagents such as 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride, hydroxybenzotriazole and N-methylmorpholine to obtain
the corresponding N-3-propyne-benzamide of formula IX. The
compound of formula IX is then treated with a reagent such as mercury
acetate to obtain an oxazolyl-substituted compound of formula le.
Those skilled in the art will recognize that compounds of
formula I wherein Ar1-R~-Q- is other than phenylpropyl can be prepared
by procedures similar to Methods A-D, provided that when reactive
groups are present, such as in compounds wherein a hydroxy group is
present on the side chain, said reactive groups are suitably protected
during the reactions.
Also, compounds of formula I wherein Q is a spiro ring can
be made according to the following procedure:
~At2
N
If(A)
Arl-R1
~---~ A
Arl-R~~C02H ~) (COCI)2, CH2Ch
2) toluene, pyridine A
X Ar2-N ~ ~ A
XI N If(B)
-O
Are-R~
An acid of formula X can be converted to the corresponding acid
chloride, and can then be reacted with an imine of formula XI by
refluxing in a mixture of toluene and pyridine. The resultant crude
CA 02207627 1997-06-12
WO 96/19450 PCTlUS95I16007
-14-
mixture of diastereomers can be purified and separated using
techniques well known in the art.
Starting materials of formula Ila, wherein A is a nitrogen-
containing heterocycloalkyl or heteroaromatic group joined to the
phenyl ring through a ring nitrogen, can be prepared by known
methods, for example:
Rls
K2CO3
' N-H R~s ~ \ N- ~ ~ CHO
Ill F ~ ~ CHO IV Ila
A compound of formula III, wherein B and N form a heterocycloalkane or
a heteroaromatic moiety and R2 is as defined above, is combined with
K2C03 (anhydrous) and heated to obtain the corresponding nitrogen-
containing heterocycloalkyl- or heteroaryl-substituted benzaldehyde of
formula Ila.
Starting materials of formula V can be prepared by known
methods, for example:
CO2R20
_ 1) Ar2NH2 = \
R2402C ~ ~ CHO -
VI 2) Ph(CH2)4COCI I
-N VII
O Ar2
CONHNH2
/ \
NH2NH2(H20)
VII CH30H ~N' V
O Arz
An ester of formula VI, wherein R2~ is lower alkyl, e.g., methyl, is reacted
with an aniline derivative of formula Ar2NH2 followed by 5-phenylvaleryl
chloride as described above in Method A to obtain the benzoate of
formula VII. The benzoate is then refluxed with hydrazine hydrate to
obtain a hydrazide of formula V.
Starting materials of formula VIII are prepared by
deprotecting the corresponding ester of formula VII by conventional
methods, e.g., by treating with a base such as LiOH or NaOH. Starting
CA 02207627 1997-06-12
WO 96119450 PCT/US95/16007
-15-
materials of formulas III, VI and X are known in the art or can be
prepared by well known methods.
It will also be apparent to those skilled in the art, and by
reference to the following examples, that compounds of formula I can be
converted into different compounds of formula I by known methods. For
example, a compound of formula I wherein A is a (4-(4-benzylpiper-
aziny-1-yl) group can be converted to the corresponding (4-piperazin-1-
yl) compound by treatment with palladium and ammonium formate.
Reactive groups not involved in the above processes can
be protected during the reactions with conventional protecting groups
which can be removed by standard procedures after the reaction. The
following Table 1 shows some typical protecting groups:
Table 1
Group to be ~ Group to be Protected and
Protected Protecting Group
-COOH I -COOalkyl, -COObenzyl; COOphenyl
i NH ~ NCOalkyly NCObenzyl, ~ NCOphenyl
~NCH20CHZCH2Si(CH3)3, 'NC(O)OC(Cb~)3,
' C~ s
/N-benzyl, ~NSi(CH3)3, NSi-C(CH~
O CHs
-NH2 -N-
O ~ H3
-O H -O C H3, -pCH2pCH3,- OSi(CH3)3, - OS~ C(CH)3
CH3
or - OCHZphenyl
We have found that the compounds of this invention lower
serum lipid levels, in particular serum cholesterol levels. Compounds of
this invention have been found to inhibit the intestinal absorbtion of
cholesterol and to significantly reduce the formation of liver cholesteryl
esters in animal models. Thus, compounds of this invention are
hypocholesterolemic agents by virtue of their ability to inhibit the
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-16-
esterification and/or intestinal absorption of cholesterol; they are
therefore useful in the treatment and prevention of atherosclerosis in
mammals, in particular in humans.
In addition to the compound aspect, the present invention
therefore also relates to a method of lowering serum cholesterol levels,
which method comprises administering to a mammal in need of such
treatment a hypocholesterolemic effective amount of a compound of
formula I of this invention. The compound is preferably administered in
a pharmaceutically acceptable carrier suitable for oral administration.
The present invention also relates to a pharmaceutical
composition comprising a compound of formula I of this invention and a
pharmaceutically acceptable carrier. The compounds of formula I can
be administered in any conventional oral dosage form such as capsules,
tablets, powders, cachets, suspensions or solutions. The formulations
and pharmaceutical compositions can be prepared using conventional
pharmaceutically acceptable excipients and additives and
conventional techniques. Such pharmaceutically acceptable excipients
and additives include non-toxic compatible fillers, binders, disintegrants,
buffers, preservatives, anti-oxidants, lubricants, flavorings, thickeners,
coloring agents, emulsifiers and the like.
The daily hypocholesteremic dose of a compound of
formula I is about 0.1 to about 30 mg/kg of body weight per day,
preferably about 0.1 to about 15 mg/kg. For an average body weight of
70kg, the dosage level is therefore from about 5 to about 2000 mg of
drug per day, preferably about 5 to about 1000 mg, given in a single
dose or 2-4 divided doses. The exact dose, however, is determined by
the attending clinician and is dependent on the potency of the
compound administered, the age, weight, condition and response of the
patient.
For the combinations of this invention wherein the
substituted azetidinone is administered in combination with a
cholesterol biosynthesis inhibitor, the typical daily dose of the
cholesterol biosynthesis inhibitor is 0.1 to 80 mg/kg of mammalian
weight per day administered in single or divided dosages, usually once
or twice a day: for example, for HMG CoA reductase inhibitors, about 10
to about 40 mg per dose is given 1 to 2 times a day, giving a total daily
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-17-
dose of about 10 to 80 mg per day, and for the other cholesterol
y biosynthesis inhibitors, about 1 to 1000 mg per dose is given 1 to 2
times a day, giving a total daily dose of about 1 mg to about 2 g per day.
The exact dose of any component of the combination to be administered
is determined by the attending clinician and is dependent on the
potency of the compound administered, the age, weight, condition and
response of the patient.
Where the components of a combination are administered
separately, the number of doses of each component given per day may
not necessarily be the same, e.g. where one component may have a
greater duration of activity, and will therefore need to be administered
less frequently.
Since the present invention relates to the reduction of
plasma cholesterol levels by treatment with a combination of active
ingredients wherein said active ingredients may be administered
separately, the invention also relates to combining separate
pharmaceutical compositions in kit form. That is, a kit is contemplated
wherein two separate units are combined: a cholesterol biosynthesis
inhibitor pharmaceutical composition and a substituted azetidinone
absorption inhibitor pharmaceutical composition. The kit will preferably
include directions for the administration of the separate components.
The kit form is particularly advantageous when the separate
components must be administered in different dosage forms (e.g. oral
and parenteral) or are administered at different dosage intervals.
Following are examples of preparing compounds of
formula I. The stereochemistry listed is relative stereochemistry unless
otherwise noted. The terms cis and trans refer to the relative
orientations at the (3-lactam 3- and 4-positions.
Preparation 1
4-l4-Senzyla~perazin-1-~~)b n~~ldehvdg
Heat a mixture of 4-benzylpiperazine (5.0 mL, 28.8 mmol),
4-flurobenzaldehyde (3.1 mL, 28.8 mmol)) and anhydrous K2C03 (5.96g,
43.1 mmol)) in DMF (50 mL) to 150 °C overnight. Cool the mixture to
room temperature, partition between water and ether (Et20), and extract
with Et20. Combine the Et20 extracts, wash with water and brine, dry
over anhydrous Na2S04 and concentrate in vacuo to obtain 7.91 g (98%)
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-18-
of 4-(4-benzylpiperazin-1-yl)benzaldehyde as a yellow solid of sufficient
purity to be employed in Example 1 without further purification.
1 H NMR(400 MHz, CDCI3): 9.77(1 H, s), 7.74(2H, d, J=8.8 Hz), 7.32(5H,
m), 6.90(2H, d, J=8.8 Hz), 3.58(2H, s), 3.41 (4H, m), 2.61 (4H, m).
Preparation 2
4-[1-(4-Methoxvo-~ henyly-3-(3-ohenylorooyly-2-oxo-4-azetidinyl]benzoic
acid hydrazide
Ste~i : Reflux a solution of methyl 4-formylbenzoate (5.23g, 31.9 mmol)
and p-anisidine in toluene (50 mL) overnight with azeotropic removal of
water via a Dean-Stark trap. Add n-tributylamine (22.8 mL, 95.6 mmol),
followed by 5-phenylvaleryl chloride (47.8 mL, 47.8 mmol, 1 M in toluene)
and reflux overnight. Cool the reaction to room temperature, quench with
1M HCI and stir 15 min. Dilute the reaction mixture with ethyl acetate
(EtOAc), wash with 1 M HCI, water and brine, dry over Na2S04 and
concentrate. Dissolve the resulting residue in tetrahydrofuran (THF),
dilute with an equal volume of CH30H, add NaBH4 (1.22g, 32 mmol) and
stir for 30 min. Add 1 M HCI, dilute with EtOAc and wash with 1 M HCI,
water and brine. Dry over Na2S04, filter and concentrate on enough
silica gel to obtain a free-flowing powder. Load the powder onto a
chromatography column packed with silica and 30% EtOAc/hexanes and
elute with the same solvent to obtain 12.2g (92%) of methyl 4-[1-(4-
methoxyphenyl)-3-(3-phenylpropyl)-2-oxo-4-azetidinyl~benzoate as a
1211 trans/cis mixture. 1 H NMR (400 MHz, CDCI3, trans isomer) 8.05(2H,
d, J=8.2 Hz), 7.39(2H, d, J=8.2 Hz), 7.28(3H, m), 7.17(6H, m), 6.77(2H, d,
J=6.9 Hz), 4.65(1 H, d, J=2.1 Hz), 3.91 (3H, s), 3.73(3H, s), 3.09(1 H, m),
2.65(2H, m), 1.97(1 H, m), 1.82(3H, m). Diagnostic C-4 proton for cis
diastereomer 5.18(J=5.7 Hz). MS(EI): 429(M+,6), 269(13), 149(100).
Stp,~ 2: Reflux a mixture of the product of Step 1 (7.5g, 17.5 mmol, 12/1
trans/cis mixture) and hydrazine hydrate (4.7 mL, 87.3 mmol) in CH30H
(40 mL) overnight, monitoring the reaction by TLC (30% EtOAc/hexanes)
and adding additional hydrazine and refluxing further as necessary.
Evaporate most of the solvent in vacuo and partition the resultant residue
between water and EtOAc. Wash with water and brine, dry over Na2S04
and concentrate onto silica gel to obtain a free-flowing powder. Load the
powder onto a chromatography column packed with silica and EtOAc.
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-19-
Elute with EtOAc to obtain 3.8g (50%) of the title compound as a 6/1
trans/cis mixture. ~ H NMR (400 MHz, CDCI3, trans isomer) 7.74(2H, d,
J=8.2 Hz), 7.39(2H, d, J=8.2 Hz), 7.28(2H, m), 7.16(5H, m), 6.76(2H, d,
J=9.1 Hz), 4.63(1 H, d, J=2.1 Hz), 3.73(3H, s), 3.06(1 H, m), 2.65(2H, m),
1.98(1 H, m), 1.85(3H, m). Diagnostic C-4 proton for cis diastereomer
5.16(J=5.6 Hz). MS(EI): 429(M+,74), 249(100), 149(35). HRMS
calculated for C2gH27NgOg: 429.2052; found 429.2052
Preparation 3
4-f 1-(4-Metho~p henyl)-3-i(3-I henylp roprl 2 oxo 4
girl]'benzoic acid
Add 2N NaOH (39 mL, 78 mmol) to a room temperature
solution of the product of Preparation 2, Step 1 (6.7g, 15.6 mmol, 12/1
ranc%is mixture) in 50% THF/CH30H (200 mL). Stir the mixture
overnight, evaporate most of the solvent in vacuo and partition the
residue between 3N HCI and EtOAc. Extract with EtOAc, combine the
extracts, wash with water and brine , dry over Na2S04 and concentrate
to obtain 6.87g (approx. 100%) of the title compound as a 11/1 trans/cis
mixture. 1 H NMR (300 MHz, CDC13, trans isomer) 8.17(2H, d, J=8.3 Hz),
7.50(2H, d, J=8.3 Hz), 7.35(3H, m), 7.25(4H, m), 6.85(2H, d, J=9.0 Hz),
4.74(1 H, d, J=2.2 Hz), 3.81 (3H, s), 3.13(1 H, m), 2.73(2H, m), 1.92(4H, m).
Diagnostic C-4 proton for cis diastereomer 5.27(J=5.7 Hz). Elemental
analysis for C26H25N04: calculated C=75.15, H=6.06, N=3.37; found
C=74.79, H=6.18, N=3.58.
Example 1
trans-1-(4-Methoxy hen~~~3-(3-~ hen~rl~ocvll
4-14-l4-~ylpperazin~l ray henyl]-2-azptidinone
Reflux a solution of the product of Preparation 1 (7.89g,
28.1 mmol) and 4-methoxyaniline (3.47g, 28.1 mmol) in toluene (100
mL) with azeotropic removal of water via a Dean-Stark trap. Monitor the
progress of the reaction by NMR. When the reaction is complete, add n-
tributylamine (20.1 mL, 84,4 mmol) at reflux, then add 5-phenylvaleryl
chloride (42.2 mL, 42.2 mmol, 1 M in toluene) and reflux the mixture
overnight. Again, monitor the progress of the reaction by NMR, and if it
indicates that a considerable amount of imine remains, add addtional 5-
phenylvaleryl chloride (1.5-2.0 eq, 1 M in toluene) and n-tributylamine
(2.2-3.0 eq) and continue to reflux; repeat this process as needed until
CA 02207627 1997-06-12
WO 96/19450 . PCT/IJS95/16007
-20-
no further reaction progress is evident by NMR. Cool the mixture to
room temperature, partition between NH4CI (sat.) and EtOAc, and
extract with EtOAc. Combine the extracts, wash with NH4CI (sat.), water
and brine, dry over anhydrous Na2S04 and concentrate to a brown oil.
Chromatograph on silica gel, eluting with 33% EtOAc/hexanes, to obtain
4.14g (27%) of an amber oil. Recrystallize from EtOAc/hexanes to
obtain 0.67g of the title compound. M.p. 139-140~C; 1 H NMR:
(400MHz, CDC13): 7.33(4H, m), 7.26(4H, m), 7.17(6H, m), 6.87(2H, d,
J=8.6 Hz), 6.75(2H, d, J=8.9 Hz), 4.51 (1 H, d, J=2.12 Hz), 3.72(3H, s),
3.58(2H, m), 3.21 (4H, s), 3.07(1 H, m), 2.61 (6H, m), 1.94(1 H, m),
1.82(3H, m); MS: (CI): 546 (M+, 47), 397(24), 150(100), 91 (33).
Using appropriate starting materials in a procedure similar
to that described above, compounds shown in the following table can be
prepared, wherein A and Ar are as defined in the table:
CA 02207627 1997-06-12
WO 96!19450 PCT/US95/16007
-21-
.: ~ __ _
M _ ~ N ~ o ~ o
r t_n d. O r w. r W
_N O ~_ :- ~2 N ~ M
r
0 r ~ ~ ..
(O O O M O O ~ ~
~f r r ~ r M d' 00 N
..
O
M M
IL M r ul ~ U C'C0~ U!
r (p
N N r N ~ O N
N N ~ N N ~ (~ COr~
t~ t~
M -U C ~ -a ~ N
U V ~p U c4 p U c~4
r' V ~. V w
..: _
E
r E r N w.
O ~ ~ v r ~ r r
~ l_I7
.-s d'
CV ~ = y
.~v _r I~
M ~ 1~ E = (O N ~ E
CG M Z E r tr ~~ M
N ..: r ~ E °' = i
r CO
N N '. _
N M Z ~ E N
M ~ ~ r
OD N ~ CO ~ O
E r D ' M r E
~ i
-p .o Z E U ,a ~ co
Z N Z Z Z Z ~ CO
M W r ~_ E r
O O LO .s ~ O op N
v.. (O ~t E r ~ CO C9 ~ E
U ~ ~"~ ~r
c o ao 0
r
CO N ~ r
O O r C~
T' CV O
m
Q a a a s
.a
v
z
U .~
M
U ~ ~ Z
U
Z
Z Cz)
CZ)
U
w a m r T
CA 02207627 1997-06-12
WO 96/19450 PCT/LJS95/16007
-22-
o ~ N o
T ~ v O
v.. N O r
N ~_ N
CO D tf O O C9
h O ~ O_ O
M N ~ C~ N
et ~ O N O
M ~ ~ N N
_~ ~
C~9 U ~ N U
U -v .o U v .o
~d
~_ czo E Z
N ~ = tt ~ N .-:
NO et a°°o E E
E p ~ N ~ Z_ Z
= r~ ~ Z = O_ et
O
O p .N = ..ri O Ch m
M = v ~ ~ E E
ca
.- .-: .r c~~ -
N 'a v~ 1~ N N ~ Z Z
T
_.. N
N ~ O (O O
M " ~ M v r
U ~ nM., ~ U ~ m c~i r-
E U a ~ ~ E
= Z Z ~ _ = I Z = Z
f0 N ~rj m ~ .N.~ N ~ N
N tn st t0
N E ~ ~ CO ~ e~ N
I~ CO
(~ r
N
t
s a
a
O
~z O
~O
CzJ z
z
w
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-23-
Example 2
4-l4-lMornholin-1-yl)yyr~)~j3-~p~y~~ ronvll
1-(4-methoxvphen~~)-2-azetidone
Reflux a solution of 4-(morpholin-1-yl)benzaldehyde
(8.94g, 46.8 mmol) and 4-methoxyaniline (5.76g, 46.8 mmol) in toluene
(250 mL) with azeotropic removal of water via a Dean-Stark trap for 10h
and cool to room temperature. Collect the resultant precipitate via
vacuum filtration, wash with hexanes and dry under vacuum to give
10.28 (74%) of N-[4-(morpholin-1-yl)benzidene)-4-methoxyaniline.
Dissolve the product in toluene (75 mL). Add pyridine (1.43 mL, 17.5
mmol) followed by 5-phenylvaleryl chloride (15.2 mL, 15.2 mmol, 1 M in
toluene) at room temperature. Warm the mixture to reflux, and reflux
overnight. Monitor the progress of the reaction as described in Example
1. Cool the mixture to room temperature, diluted with EtOAc and wash
with 0.1 M NaOH, 1 M HCI, water and brine, dry over anhydrous Na2S04
and concentrate. Chromatograph the residue on silica gel, eluting with
40% EtOAc/hexanes to obtain 2.71 g (77%) of the title compound as a
1/1 mixture of cis and trans isomers. Additional chromatography
provides pure cis and traps isomers:
2A) traps isomer: ~ H NMR (200MHz, CDCI3): 7.20(9H, m),
6.92(2H, d, J=8.7 Hz), 6.76(2H, d, J=9.0 Hz), 4.54(1 H, d, J=2.2 Hz),
3.87(4H, m), 3.73(3H, s), 3.17(4H, m), 3.07(1 H, m), 2.64(2H, m),
1.83(4H, m); HRMS: C29H32N2O3 calf. 456.2413, obsvd. 456.2426;
MS: (CI):457(M+, 100), 307(27), 150(47).
2B) cis isomer: ~H NMR (200MHz, CDC13): 7.21(7H, m),
6.91 (4H, m), 6.77(2H, d, J=9.1 Hz), 5.08(1 H, d, J=5.6 Hz), 3.88(4H, m),
3.74(3H, s), 3.52(1 H, m), 3.35(1 H, m), 3.20(3H, m), 2.41 (2H, m),
1.61 (2H,m), 1.26(2H, m); HRMS: C29H32N2O2 calf. 456.2413, obsvd.
456.2420.
Using appropriate starting materials in a procedure similar
to that described above, compounds shown in the following table can be
prepared, wherein A and Ar are as defined in the table:
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-24-
_ ~: .,
t- .. o
(n~ _ ~ __ cN" n ci
~ C~ + N r
O ~ O _
~ O 1 ~ I~ O
~t
.rr
:-:tn O = O
M N r-
~ CO~N V N T
N
O O CAD N ~ t~
1 N N O N N
~ N
~ ~ N ~
M
O -V C O) C
M 'V
U v ~ N ,o
U v
I Z _ _ N _M ,.: N _M
O ~ E h (h ~ = LO
O = = CO I
cM Z r:
~ c~
Z M f'~ N C~ ~ N O
'D = 00
Z T M r'
O Z N ~ N ~ E
M ~ .-..-. N II
~
Q _
v Z ~ c0 a N ~ 'Q Z
Z E ~ cfl E cD ~ ~ ~C
= ~ Ch T = r N 1~ r
N
O N
O O Is
Z
C~'~
U ~ ri r U i c$._U ~ cvi
p - __~ p -~ __ p
U ' = E U 'v E ~ U -d ~
Z Z ~? I Z Z Z Z I = = E
~2 N ~ ' ~ ~ ~ ~ v Z
~ Z
'~'
T
O r ~ _ O is T (OO 1~ CO
'a N M .M,i~C ('0r ~ (O M
r
r. N
r ~ T
r ~ T
O ~ r
~. ~ T
T r
N
L a a a
Q
Q C~ C~
0
L1JN D N
N
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-25-
o ~ °° o ° " -" _ ~
r r ° ~ tC) ~ ° M
r
~2 N ~2 N r~ ~ N O ~ N
~I ~/
_
_N ~ N r ~ C~ N ~ ~ 0~0 i~ ~ O
.. ~ ..i ,._
Ch ~ M O ~ ....
UN Ucow UcMO°°v~jc°~Ir ~oo~
N r O
.r CO .r M r .r f9 N '. C9 ~ .r M r i~
I I 1 I 1
'D
r ~
Zzv ~ _'.c~ =c~ N== N E
0 o cri N ri ~''~ ~ ~ " oD
~ j E p°~ °
M CvD ° ~ ~ n v ~p ° ~ cG M
~.: 00
~ r ~ _ _ ~ ~ r E N M ~ ~ 01
r O M = = Z .-:
= Ci tn ~ = h ~ _ ~p N r V N
n Z ~ 11 ch Z
0~0 CO ~ r ~ .~ ~' ~ N et ~ ~ E ~ N
cG f~ ~ N ~ CO CNp i~ 'C =_
~ N (= r ~ ~ r ~ _.,
~ v v ~ Z i E = (V
r ~ ~ r d; N
r N
~O M = ~ M = (p r
N ~ ~ ~ r N N
N -p ~, N ~ t0 ~ N ~ N .~ N
= 1~ I~ _ = 00 n = ~ ~ _ ~ = 1~
M V N (~'9 N v r ~ r N ~~,~ CO ~ ~ M = N
~: ~ ~ v _
p ~ _~ p C°~. ° ~ V t~? ~ V ~ c'~ r V ~ E
cG _~ D ef~ D ~ ~: D
N N ~ N N ~ N ~ ~ U ~ E E U ~: _N E
~t C'~ ~ C'~
° ~ ~ ~ ° ~ ~ N ° ~ M b o~D i~ t0 °
~ CO
r ~ ~ ~ ~ v ~ ~ ~ ~D N r ~ ~ ~ r
1 r
n SCI ((~ O r 1
1 st ~, t~ N °
r r T T r r f
T
r
t
0.
a a
v ~'r
O ~ O ~ U U
CJ C~
N N N N N
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-26-
Example 3
1-(4-Methoxvohenyll-(3-nhenyrlnrooyrl)-4-(Imidazoyrl-1-yrll~mhenvl
2-azetidone
Heat a mixture of 4-(1-imidazoyl)benzyaldehyde (3.79g,
22 mmol) and 4-methoxyaniline (2.718, 22 mmol) in CH30H (200 mL) to
reflux for a short time. Allow the solution to cool to room temperature
and stand overnight. Collect the resultant precipitate by vacuum
filtration and dry to obtain 6.1g (100%) of N-[4-(imidazoyl-1-yl)-
benzidene]-4-methoxyaniline. Treat the product with pyridine, n-
tributylamine and 5-phenylvaleryl chloride in the manner described in
Example 2, then extract and chromatograph in a manner similar to
Example 2 obtain the title compound as a cis/trans ratio of 1/1.17.
1 H RMS: C2gH27N3O2 calf. 437.2103, obsvd. 437.2096;
MS: (CI): 438(M+, 86), 289(67), 161 (38), 150(100).
Example 4
4- [4-(2-Pyrrimidyrlll~herazin-1-yllc~hen~rl)-3-(3-~enyrt r~Opyrl)-~4
methoxy~henyl)-2-azetidone
Reflux a mixture of 4-(2-pyrimidyl)piperazinyl]
benzaldehyde (5.628, 21 mmol) and 4-methoxyaniline (2.58g, 21 mmol)
in toluene (250 mL) with azeotropic removal of water via a Dean-Stark
trap. Monitor progress of the reaction by 1H NMR. After 3 days, at 85%
completion, cool the mixture to room temperature, concentrate and
recrystallize the residue from EtOAc and hexanes to obtain 6.6g (85%)
of N-[4-(morpholin-1-yl)benzidene]-4-methoxyaniline as a yellow solid.
Dissolve the product in pyridine (80 mL), add 5-phenylvaleryl chloride
(11 mL, 11 mmol, 1 M in toluene) at room temperature and reflux
overnight. Monitor the reaction as described in previous examples.
Remove most of the pyridine by distillation, cool the solution to room
temperature, partition between EtOAc and water, wash with water and
brine, concentrate and chromatograph on silica gel to provide 1.02g
(36%) of the title compound as a 1/1 cis/trans mixture. Additional silica
gel chromatography provides pure cis and trans isomers:
4A: 1H NMR (400MHz, CDC13): pertinent signals: 5.08 and 4.54 (1H, d,
J1=5.7 Hz, cis, J2 =2.1 Hz, traps C-4); MS: (CI): 534(M+, 18), 385(17),
150(100), 125(35), 91 (58).
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-27-
4B: M.p. 126-127~C; (400MHz, CDCIg): 8.34(2H, d, J=4.6 Hz, 7.21 (9H,
m), 6.93(2H, d, J=8.6 Hz), 6.76(2H, d, J=8.6 Hz), 6.53(1H, t, J=4.8 Hz),
4.54(1 H, d, J=2.2 Hz), 3.97(4H, m), 3.73(3H, s), 3.26(4H, m), 3.07(1 H,
m), 2.64(2H, m), 1.97(1 H, m), 1.83(3H, m); (CI): 534(M+, 33), 384(26),
150(100), 124(16), 91 (29).
Example 5
rt-ans-1-l4-Methoxyahenyl)-3-r(3-I hen~rlpropyrl) 4~4 pnera7in 1
)ltp~.Srl-2-azeti inone
Add ammonium formate (3.Og, 48 mmol) to a refluxing
suspension of the product of Example 1 (3.Og, 5.53 mmol) and 10%
Pd/C (0.7g) in CH30H (20 mL). React for 4 h, monitoring reaction
progress by TLC (eluting with 30% EtOAc/hexanes). Filter the reaction
mixture through celite and wash the filter cake well with CH30H.
Concentrate the filtrate and partition the resulting residue between brine
and EtOAc, and extract with EtOAc. Combine the extracts, wash with
brine, dry over anhydrous Na2S04 and concentrate. Chromatograph
the resulting residue on silica gel, eluting with 10% MeOH/CH2C12 to
obtain a crude product. Recrystallize to obtain the pure title compound.
M.p. 204-206~C; 1 H NMR (400MHz, CDC13): 7.33(4H, m), 7.26(4H, m),
7.17(5H, m), 6.89(2H, d, J=8.6 Hz), 6.77(2H, d, J=9.0 Hz), 4.54(1 H, d,
J=2.1 Hz), 3.73(3H, s), 3.50(4H, m), 3.37(4H, m), 3.05(1 H, m), 2.64(2H,
m), 1.95(1 H, m), 1.84(3H, m); MS: (CI): 456(M+, 100), 306(25), 150(17).
Example 6
5-f4-f1-l4-Methoxvohen~rl~(3-phenylpy) 2 oxo 4 azetidinyl]~ 1 3 4
9xadiazol-2-am ine
Stir a mixture of the product of Preparation 2 (0.59g, 1.36
mmol, 6/1 trans mixture), NaHC03 (0.12g, 1.42 mmol), water (2.5 mL)
and dioxane (3.5 mL) for 5 min. at room temperature. Add BrCN (0.15g,
1.42 mmol), stir for 4 h., and filter. Wash the filter cake with water and
dry in vacuo overnight to obtain 0.50g (81 %) of the title compound as a
8/1 trans/cis mixture. M.p. 207-210°C. 1 H NMR (400 MHz, CDC13, trans
isomer) 7.90(2H, d, J=8.5 Hz), 7.42(2H, d, J=8.3 Hz), 7.28(3H, m),
7.17(4H, m), 6.77(2H, d, J=9.1 Hz), 5.56(2H, bs), 4.64(1 H, d, J= 2.2 Hz),
3.73(3H, s), 3.10(1 H, m), 2.66(2H, m), 1.97(1 H, m), 1.85(3H, m).
Diagnostic C-4 proton for cis diastereomer 5.17(J=5.6 Hz). MS(EI):
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-28-
454(M+,55), 362(46), 305(100), 149(94). HRMS calculated for
C26H26N4~3: 454.2005; found 454.2012.
Example 7
5-[4-[1-(4-Methoxvnhen~)-)-3-,(3-pl~nvloro~rl)-2-oxo-4-4
azetidinKllnhen~l-1.3.4-oxadiazol-21,3H~-one
Add 1,1'-carbonyl diimidazole (0.228, 1.36 mmol) to a O~C
solution of the product of Preparation 2 (0.39g, 0.91 mmol, 6/1 trans/cis
mixture) and triethylamine (0.25 mL, 1.82 mmol) in THF (5 mL) and
allow the mixture to warm to room temperature overnight. Concentrate
the mixture in vacuo and dissolve the residue in EtOAc. Wash with 1 M
HCI, saturated NaHC03, water and brine, dry over Na2S04 and
concentrate on silica to obtain a free-flowing powder. Load the powder
onto a chromatography column packed with silica gel and 40%
EtOAc/hexanes and elute with the same solvent to obtain 0.383g (93%)
of the title compound as a 9/1 trans/cis mixture. 1 H NMR (400 MHz,
CDC13, trans isomer) 7.85(2H, d, J=7.9 Hz), 7.44(2H, d, J=7.9 Hz),
7.27(2H, m), 7.17(5H, m), 6.78(2H, d, J=8.8 Hz), 4.66(1 H, s), 3.73(3H, s),
3.10(1 H, m), 2.66(2H, m), 1.99(1 H, m), 1.86(3H, m). Diagnostic C-4
proton for cis diastereomer 5.18(J=5.4 Hz). MS(EI): 455(M+,94),
306(49), 295(81 ), 149(100). HRMS calculated for C27H25N304:
455.1845; found 455.1849.
Example 8
2-[4-[ 1-(4-m ethoxy~hen,~rl)T3-(3-~~~~yl)-2-oxo-4-
azetidinyllnhen~l-4-meth~oxazole
Stem: Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (0.89g, 4.64 mmol) to a room temperature solution of the
product of Preparation 3 (1.61 g, 3.86 mmol, 11/1 trans/cis mixture),
propargylamine (0.318 mL, 4.64 mmol), hydroxybenzotriazole (0.625g,
4.64 mmol), and N-methylmorpholine (0.85 mL, 7.72 mmol) in CH2C12
(15 mL) and stir overnight. Dilute the mixture with CH2C12, wash with
water, dry over Na2S04 and concentrate onto silica. Load the silica
onto a chromatography column packed with silica and 50% EtOAc/
hexanes. Elute with the same solvent to obtain 1.24g (71 %) of N-3-
propyne-4-[1-(4-methoxyphenyl)-3-(3-phenylpropyl)-2-oxo-4-
azetidinyl]benzamide as a 12/1 trans/cis mixture. MS (CI): 453(M+1,
100), 304(57), 150(92).
CA 02207627 1997-06-12
WO 96119450 PCT/US95/16007
-29-
Combine the product of Step 1 (1.21g, 2.67 mmol, 12/1 trans/cis
mixture) and mercury acetate (0.05g, 0.16 mmol) in acetic acid (10 mL)
and reflux for 3 h. Cool the reaction mixture to room temperature and
concentrate in vacuo. Partition the resultant residue between saturated
K2C03 and EtOAc, then extract with EtOAc. Combine the extracts, wash
with water and brine, then dry over Na2S04 and concentrate onto silica.
Load the silica onto a chromatography column packed with silica and
30% EtOAc/ hexanes. Elute with 30-40% EtOAc/hexanes to obtain
0.74g (61%) of the title compound as a 6/1 trans/cis mixture. 1 H NMR
(400 MHz, CDCI3, trans isomer) 7.99(2H, d, J=8.4 Hz), 7.40(2H, d, J=8.5
Hz), 7.28(2H, m), 7.18(5H, m), 6.83(1 H, d, J=1.2 Hz), 6.77(2H, d, J=9.0
Hz), 4.63(1 H, d, J= 2.2 Hz), 3.73(3H, s), 3.12(1 H, m), 2.66(2H, m),
2.39(3H, s), 1.99(1 H, m), 1.86(3H, m). Diagnostic C-4 proton for cis
diastereomer 5.16(J=5.5 Hz). MS(EI): 452(M+,48), 303(100), 198(26).
HRMS calculated for C29H28N2O4: 452.2100; found 452.2089.
Example 9
~-I4-l4-Methvl-1-p erazinyly heny~- 7-did henyl 2
azas~pirQ[~3_]nonan-1-one
Add oxalyl chloride (0.8 mL, 9.46 mmol) to a refluxing
solution of 4-phenylcyclohexanecarboxylic acid in CH2C12 (10 mL).
After 2 h, cool to room temperature and evaporate the solvent in vacuo.
Dissolve the resultant residue in toluene, add to a refluxing solution of
N-4-[4-methyl-1-piperazinylJbenzylideneaniline (1.2g, 4.3 mmol) in a
mixture of toluene (15 mL) and pyridine (5 mL), and reflux overnight.
Pour the reaction mixture into water, extract with CH2C12, combine the
extracts and evaporate the solvent. Chromatograph the resultant
residue on silica, eluting with 10% CH30H/CH2C12 to obtain crude title
compound as a mixture of diastereomers. Purify the mixture by
preparative silica TLC, eluting twice with 10% CH30H/EtOAc to obtain
the title compound as a mixture of diastereomers. Separate the
diastereomers by preparative silica TLC, eluting three times with 10%
CH30H/EtOAc to obtain diastereomers A and B with a combined yield of
0.19g (9%).
Diastereomer A: M.p. 215-217~C. HRMS: calculated for C3~ H~N30
(M+~): 466.2858; found 466.2861. iH NMR (400 MHz, CDC13)
7.21 (11 H, m), 7.03(1 H, m), 6.93(2H, d, J=8.8 Hz), 4.88(1 H, s), 3.25(3H,
r
CA 02207627 1997-06-12
WO 96/19450 . PCTIUS95/16007
-30-
m), 2.63(3H, m), 2.52(2H, m), 2.38(3H, s), 2.13(3H, m), 2.1-1.8(5H, m),
0.89(1 H, m). ,
Diastereomer B: M.p. 166-168°C. HRMS: calculated for C31 H36NgO
(M+~): 466.2858; found 466.2857. 1H NMR (400 MHz, CDCI3) 7.24(9H,
m), 7.12(2H, d, J=8.6 Hz), 7.04(1 H, m), 6.90(2H, d, J=8.6 Hz), 4.67(1 H,
s), 3.27(4H, bs), 2.64(4H, bs), 2.41 (4H, m), 2.8-1.7(7H, m), 0.98(1 H, m).
The following formulations exemplify some of the dosage
forms of this invention. In each, the term "active compound" designates
a compound of formula I.
EXAMPLE A
,f~,. Inaredi n m tablet m tablet
1 Active Compound 100 500
2 Lactose USP 122 113
3 Corn Starch, Food Grade, as a 10% 30 40
paste in Purified Water
4 Corn Starch, Food Grade 45 40
5 Magnesium Stearate ~ 7
Total 300 700
Method of Manufacture
Mix Item Nos. 1 and 2 in suitable mixer
for 10-15 minutes.
Granulate the mixture with Item No.
3. Mill the damp granules through a
coarse screen (e.g., 1/4", 0.63 cm) if Dry the damp granules.
necessary.
Screen the dried granules if necessary
and mix with Item No. 4 and mix
for 10-15 minutes. Add Item No. 5 and
mix for 1-3 minutes. Compress
the mixture to appropriate size and weight
on a suitable tablet machine.
EXAMPLE B
l
Ca so
es m I r~r.g/tablet
I~,. In re~dient
1 Active Compound 100 500
2 Lactose USP ~ 106 123
3 Corn Starch, Food Grade 40 70
4 Magnesium Stearate NF 4_ 7
Total 250 700
CA 02207627 1997-06-12
WO 96/19450 PCT/US95/16007
-31-
~/lethod of Manufa .turn
Mix Item Nos. 1, 2 and 3 in a suitable blender for 10-15
minutes. Add Item No. 4 and mix for 1-3 minutes. Fill the mixture into
suitable two-piece hard gelatin capsules on a suitable encapsulating
machine.
Representative formulations comprising a cholesterol
biosynthesis inhibitor are well known in the art. It is contemplated that
where the two active ingredients are administered as a single
composition, the dosage forms disclosed above for substituted
azetidinone compounds may readily be modified using the knowledge
of one skilled in the art.
The ' v'v activity of the compounds of formula I can be
determined by the following procedure.
In Vivo Acsay of Hyoolipidemic Agents Using the Hyr~ erli~ idemic
Hamster
Hamsters are separated into groups of six and given a
controlled cholesterol diet (Purina Chow #5001 containing 0.5%
cholesterol) for seven days. Diet consumption is monitored to determine
dietary cholesterol exposure in the presence of test compounds. The
animals are dosed with the test compound once daily beginning with the
initiation of diet. Dosing is by oral gavage of 0.2mL of corn oil alone
(control group) or solution (or suspension) of test compound in corn oil.
All animals moribund or in poor physical condition are euthanized.
After seven days, the animals are anesthetized by IM injection of
ketamine and sacrificed by decapitation. Blood is collected into
vacutainer tubes containing EDTA for plasma lipid analysis and the liver
excised for tissue lipid analysis. Lipid analysis is conducted as per
published procedures (Schnitzer-Polokoff, R., et al, Comp. Biochem.
Physiol., 99A, 4 (1991 ), p. 665-670) and data is reported as percent
reduction of lipid versus control.
Using the hamster 'n viv test procedures substantially as
described above, the following data were obtained for representative
compounds. Compounds are referred to in the following table by the
corresponding example numbers. Data is reported as percent change
CA 02207627 1997-06-12
WO 96/19450 PCT/US95116007
-32-
versus control, therefore, negative numbers indicate a positive lipid-
lowering effect. Reductions in both serum cholesterol and cholesterol
esters were measured, but the measure of reduction of cholesterol
esters is recognized as the more reliable indication of activity.
Reduction
Ex. Cholest. Dose
# Esters m /k
2A -81 50
2C -64 50
9B -31 10
For racemic compounds of formula I or active
diastereomers or enantiomers of compounds of formula I, compounds
administered at a dosage of 50 mg/kg show a range of 0 to -96%
reduction in cholesterol esters, while compounds administered at a
dosage of 10-30 mg/kg show a range of 0 to -31 % reduction in
cholesterol esters.