Note: Descriptions are shown in the official language in which they were submitted.
CA 02208097 1997-06-18
WO96/19577 PCT~B95/00141
VS RIBOZYMES
Backaround o~ The Invention
This invention relates to rihozymes.
The following is a brief description o~ publications
concerning ribozymes, and in particular, VS ribozymes.
None are admitted to be the prior art to the pending
claims, and all are incorporated by re~erence herein.
Si~ basic varieties of naturally-occurring enzymatic
nucleic acids are known presently. Each can catalyze the
hydrolysis of RN7~ phosphodiester bonds in trans (and thus
can cleave other RNA molecules) under physiological
conditions. Table I summarizes some of the
cllaracteristics o~ these ribozymes.
In general, enzymatic nucleic acids act by ~irst
binding to a target RNA. Such binding occurs through the
target binding portion of a enzymatic nucleic acid which
is held in close proximity to an enz~natic portion of the
molecule that acts to cleave the target RNA. Thus, the
enzym~tic nucleic acid first recognizes and then binds a
target RNA through complementary base-pairing, and once
bound to the correct site, acts enzymatically to cut tho
target RNA. Strategic cleavage of such a target RNA will
destroy its ability to direct synthesis of an encoded
pro~ein. After an enzymatic nucleic acid has bound and
cleaved its RNA target, it is released from that RNA to
search for another target and can repeatedly bind and
cleave new'targets.
The enzymatic nature of a ribozyme is advantageous
over other technologies, such as antisense technology
(where a nucleic acid molecule generally simply binds to a
nucleic acid target to block its translation) since the
concentration o~ ribozyme necessary to affect a
SU~SrITUTE S~EET (RULE 263
CA 02208097 1997-06-18
PC~/IBg5/00141
W~6119577
therapeutic treatment is lower than that of an ~ntisense
oligonucleotide. This advantage reflects the ability of
the ribozyme to act enzymatically. Thus, a single
ribozyme molecule is able to cleave many molecules of
S target RNA. In addition, the ribozyme is a highly
specific inh.ibitor, with the specificity of inhibi~ion
depending not only on the base-pairing mechanisrn of
bindiny to the target RNA, but also on the mechanism of
target RNA cleavage. Single mismatches, or base-
sub.stitutions, near the site of cleavage can completelyeliminate catalytic activity o~ a ribozyme. Similar
mismatches in antisense molecules do not prevent their
action (Woolf et al., 1992 Proc. Natl. Acad. Sci. USA 89,
7305-7309). Thus, the specificity of action of a ribozyme
is greater than that of an antisense oligonucleotide
binding the same RNA site.
~ small number of RNAs isolated from a variety of
natural sources have been found to possess a self-cleavage
activity that is involved in processing multimeric
transcripts into monomers, apparently as part of the
replication cycle. Several different RNA sequences and
secondary structures appear to be capable of such
activity. These include the hammerhead, ~ound in several
plant viral satellite RN~s, a viroid RNA, and the
transcript of a nuclear satellite DNA of a newt (revi~wed
by Symons, 1992 Annu. Rev. Biochem. 61, 641); the hairpin
(or paper-clip) in the minus strand of the satellite of
tobacco ringspot virus and related viruses (Buzayan et
al., 1986 Nature 323, 3q9; Feldstein et al., l990 Proc.
30 ~atl. Acad. sci. USA. 87, 2623); the genornic and
antigenomic RNAs o~ hepatitis delta virus (HDV; Sharmeen
et al ., 1988 J. Virol . 62 , 2674 ; Kuo et al ., l988 ~.
Virol. 62, 4439; Perrotta and Been, 1991 Nature 350, 434);
and Varkud Satellite (VS) RNA in the mitochondria of
certain Neurospora isolates (Saville and Collins, 1990
Cell 61, 685).
SUBSTITUTE S~EET (RULE 26
_ _ _ _
CA 02208097 1997-06-18
W O 96119577 PCT~B95/00141
In their natural contexts the ribozymes mentioned
above, as well as others such as Group I (Cech, 1990 ~7nu.
~ev. Biochem. 59, 543 ) and Group II introns (~lichel et
al., 1989 Gene 82, 5), perform intramolecular sel~-
cleavag~ and, in some cases, ligation reactions.
Structure-function studies of Group I introns (Zaug alld
Cech, 1986 Science 231, ~70; Szostak, 1986 Nature 322, 83)
and later hammerhead (Uhlenbeck, 1987 ~at ure 3 2 8, 5 9 6 ),
hairpin ~Feldstein et al., 1990 supra; Hampel et ~1., 1990
Nucleic Acids Res. 18, 299), and HDV (Perrotta and Been,
19 9 2 Bl ochemi s try 3 1, 16; Branch and Robertson, 1991 Proc .
~at . Acad. sci . USA 88, 10163) ribozymes have been
facilitated by altering these RNAs to perEorm
intermolecular trans-cleavage reactions. In a ~rans-
1~ cleavage reaction one RNA, the substrate, contains the
site to be cleaved; a separate RNA, the ribozyme, provides
the sequences required to catalyze the cleavage. One
naturally-occurring trans-acting ribozyme has been
discovered: the RNA component of RNase P, which cleaves
pre-tRNA precursors in trans (Guerrier-Takada et al., 1983
Cell 35, 849). Trans-cleavage reactions of most riboz~nes
have been designed such that binding of the substrate
occurs via formation of multiple Watson-Crick base pairs
with the ribozyme. Non-Watson-Crick and tertiary
interactions are also involved in substrate bincling and
may be essential Eor proper binding ~Pyle et al., 1992
Nature 358, 123; Dib-Haij et al., 1993 Nucl. Acids Res.
21, 1797: Smith et al ., 1992 J. Biol . Chem. 267, 2429;
Guerrier-Takada and Altman, 1993 Biochemistry 32, 7152).
With hammerhead, hairpin and Group I riboz~nes it has been
~ound that very ~ew specific nucleotides in the substrate
are required for trans cleavage, provided that the
adjacent region(s) are complementary to the binding site
on the ribozyme. This property has allowed the
~ 35 engineering of ribozymes that can c].eave sequences other
than those recognized by the naturally-occurriny riboz~ne.
Some engineered ribozymes also ~unction in vivo in non-
native host cells, which has raised the possibility oE
SUBSTITUrE ~EET (RULE 26~
.
CA 02208097 1997-06-18
W O 96/19577 PCTnB95/00141
their use as therapeutic agents in dominant inherited
disorders and against retroviruses and RNA viruses
(reviewed by Castanotto et al., 1992 Critical Reviews i~
Eukaryotic Gene Expression 2, 331) .
SummarY Of The InventLon
This invention concerns novel catalytic nucleic acid
which performs the same type of RNA cleavage as
hammerhead, hairpin, and HDV ribozymes, leaving products
with 2',3' cyclic phosphate and 5' OH termini ~Saville and
~0 Collins, 1990 supra), but it is different in sec~uence,
secondary structure, choice of cleavage site, and
functional properties from trans-cleaving ribozymes kn,o-
~in the art (Collins and Olive, 1993 Biochemistry 32, 2795;
Guo et al., 1993 Mol. Biol., 232, 351).
This invention features the construction an(~ use of
enzymatic nucleic acid molecules, for example, those
derived from ~eurospora Varkud Satellite (VS) RNA, that
can catalyze a trans-cleavage reaction, wherein a separate
substrate RNA is cleaved at a specific target site. The
minimal substrate may form a stable hairpin stem-loop
base-paired structure (Fig. 6). Substrate recognition ~y
the catalytic nucleic acid involves multiple, including
tertiary interactions. The catalytic nucleic acid includes
an RNA target binding domain which interacts with
nucleotides of the target RNA (preferably with b~ses 3' of
the cleavage/ligation site), and an en~ymatic portion
twhich may include a part or all of the RNA s~lbstrate
binding portion) having the enzymatic activity. The
nucleic acid binds to the target RNA, preferably, w:ith
bases 3' of the cleavage/ligation site and causes cleavage
of the RNA substrate at that cleavage site. Thus, in one
aspect, the invention features a nucleic acid molecule
that catalyzes the cleavage of a separate double-stranded
~JA target molecule i.n a sequence-specific manner.
SUBSrITUTE S~EET (RULE 26)
CA 02208097 1997-06-18
WO96119577 PCTnB95/00141
By ~trans-cleavage" is meant that the ribozyme is able
to act in ~rans to cleave another RNA molecule whic11 is
not covalently linked to the ribozyme itself. Thus, the
ribozyme is not able to act on itself in an intramolecular
cle~vage reaction.
By "base-pair" is meant a nucleic acid that can fonn
hydrogen or other bond(s) with other RNA sequence by
either traditional Watson-Crick or other non-traditional
t~es (for example Hoogsteen type) of interactions.
The en2ymatic RN~ molecules o~ this invention can be
designed to cleave RNA (minimum length of between ~-20 nt)
having only a pre~erence for at least one nucleotide
immediately 5' to the cleavage site and the availability
of an adjacent 2' hydroxyl group for cleavage to occur.
Tho 2'-hydroxyl group is generally provided by the
substrate R~A molecule. Thus, these enzymatic RNA
molecules provide signi~icant in vitro and in vivo
activities which can be used for diagnostic and
therapeutic procedures.
Thus, in a first aspect, the invention features a
ribozyme able to cleave a separate substrate RNA molecule.
The ribozyme has three base paired regions generally in an
"I" con~iguration. The upper and lower based paired
regions of the proposed "I" include between abou~ lO and
~0 bases inclusive, of which at least about 50~ are paired
with each other. The connecting region of the proposed "I"
betweon said upper and lower base paired regions includes
between about 8 and 20 bases inclusive, of which at least
about 50% are paired.
~ 30 By ~ribozyme~ is meant any enzymatic nucleic acid
molecule, usually containing at least some
~ ribonucleotides, which is active to cleave an RNA mo].ecule
without ~orming a covalent bond with that substrate.
Thus, the molecule generally lacks any nucleophilic
~ttac~ing group that is able to cause cleavage o~ the
SU~SI ITUTE ~EET (RULE 26)
CA 02208097 l997-06-l8
W O96/19577 PCTnB95/00141
substrate and form a covalent bond wi~h that substrate (at
least in a transient form).
A "separate RNA molecule" is one that is not
covalently bonded with the ribozyme, and may cont.ain non-
5 ribonucleotides within its length. It is pre~erably anaturally occurring RN~ molecule, such as a viral mRNA, or
pathogenic RNA molecule.
The proposed "I" configuration is shown generall~ in
the figures 5B through 8. This structure may cont:ain
other nucleic acid chains attached to dif~erent portions
of the ~I", but those in the art will recognize that it is
advantageous to have as few of these extra cl1ains as
possible so that secondary structure interactions are
reduced and so that the size of the molecule is maintained
as small as possible. The proposed "I~' has an "upper" and
'lower" region as describe above and these are connected
by an intermediate ("connecting') region. Togeth~r these
reyions provide enzymatic activity to the ribozyme. While
base pairing in these regions is important, those in the
art will recognize that other types of pairing
interactions, e.g., Hoogsteen pairing, are also useful in
this invention. These regions may, as noted, includo
unpaired regions at the ends of the paired regions, or
even within or intermediate these paired regions so long
as enzymatic activity is not eliminated. By 50~ base-
pairing is meant that along a length of the region at
least half of the bases in the region interact with other
bases to hold the ribozyme in the generally an "I" shape.
In pre~erred embodiments, there is at least 70 or even 80
base pairing, as is illustrated in the attached figures.
The proposed "I" configuration is meant to be a non-
limiting structure. Those with ordinary skill in the art
will rf-cognize modifications (insertions, deletions, base-
substitutions and/or chemical modifications) to the
proposed "I" structure can be readily generatef1 using
techniques known in the art. Additionally, structures
SUBSTITUTE S~EET (RULE 26~
CA 02208097 1997-06-18
WO96119577 PCT~B9~/00141
distinct from the proposed "I" con~iguration can be
readily generated by those skilled in the art and are
within the scope of this invention.
In other preferred embodiments, the "connecting"
region ~urther includes a single-strand region oE between
about 3 and 7 bases inclusive, e.g., the single-strand
region is adjacent the "upper" base-paired region as shown
in ~igures 6-8 the "upper" region includes a "left" and
~right" hand portion each between at least about 6 and 30
bases inclusive; and the "lower'~ region also includes a
~le~t" and "right" hand portion each between at least
about 6 and 30 bases inclusive. Such regions are
delineated by the "connecting" region noted above and as
showrl in the figures.
In yet other preferred embodiments, the "lower" region
and/or the "connecting" regions includes at least one
bulged nucleotide (e.g., A), that is an unpaired base,
wh~ch may be available for interaction with proteins; the
"upper" base-paired region includes bases unpaired with
other bases in the "upper" base paired region which are
available to base pair with a substrate RNA, e.g., as
shot~n in the figures 8 and 9, where the bases w11ich are
unpaired include at least 3 bases. In addition, the
substrate for the ribozyme has a base-paired region of at
least 2 base pairs, e.g., the substrate ha~s the sequence
3' G~NN ~' where cleavage by the ribozyme is between each
N (each N independently is any base; throughout the
document the term N or N' is independently any base or
base equivalent).
In fur~her preferred embodiments, the '~lower" base-
paired region has unpaired bases at its 5' end, available
to base pair with a substrate RN~; the ribozyme contacts
the RN~ substrate only 3' of the cleavage site; the RNA
substrate is a double-stranded RNA, and the nucleic acid
mol~cule is able to contact the double-strancled RN~
substrate only 3' of the cleavage site and cause cleavage
SU~STITUTE SI~EET (RULE 26)
_ _ . . . . _
CA 02208097 1997-06-18
W O96/19577 PCT~B95/00141
o~ the RNA substrate at the cleavage site; the RNA
substrate is a single-stranded RNA, and the ribozyme is
able to contact the single-stranded RN~ substrate only 3'
of the cleavage site and cause cleavage of the RNA
5 substrate at the cleavage site.
In a most preferred embodiment, ~he ribozyme is
derived from Neurospora VS RNA. That is, the ribozyme has
the essential bases of the VS RN~ molecule held together
in a suitable configuration as described above so that RNA
substrates can be cleaved at th~ cleavage site. Such
essential bases and configuration are determined as
described below; those in the art will recognize ~ha~ it
is now ro~tine to determine such parameters. One e~ample
of such a ribozyme is that having about 80 - 90~ the
sequence shown in the figures 5-8.
In other em~odiments, the ribozyme is enzymatically
active to cut an RNA duplex having at least two base-
pairs; the ribozyme is enzymatically active to cut 5' to
the sequence, 5'NAGNnGUCNm 3'(see ~ig. 6B), where eac:h N
is independently any nucleotide base, n and m are
independently an integer between 3 and 20 inclusive, and
the sequence forms at least two intramolecular base-pairs;
the RNA substrate binds the ribozyme at a site distant
from the cleavage site; the ribozyme is a circular
molecule, where the circuLar molecule contacts a separate
RN~ substrate and causes cleavage of the RNA substrate at
a cleavage site; and the ribozyme includes RNA.
In other aspects, the invention features a cell
including nucleic acid encoding the ribozyme above, an
expression~ vector having nucleic acid encoding this
ribozyme in a manner which allows expression of ~he
rlbozyme within a cell, and a cell including such an
expression vector. Other aspects also include an
expression vector where the ribozyme encoded by the vector
is capable of cleaving a separate RNA substrate nlolecule
SU{~STITUTE S~IEET (RULE 26~
CA 02208097 1997-06-18
WO9611g577 PCT~B95100141
selected from a group consistiny of viral RNA, messenger
E~NA, pathogenic RNA and cellular RNA.
In further related aspects, t11e invention features a
method for cleaving a single-stranded RN~ substrate at a
5 cleavage site by causing base-pairing of the RNA substrate
with a nucleic jacid molecule only 3~ of the cleavage site
(Figure 7). Such a method includes contactiny the RN~
substrate with a nucleic acid molecule having an RNA
substrate cleaving enzymatic activity which cleaves a
separate RNA substrate at a cleavage site. T~lis nuclei.c
acid molecule includes an RNA substrate binding portion,
which base pairs with the RNA substrate only 3~ of the
cleavage site, and an enzymatic portion (which may include
a part or all of the RN~ substrate binding portion) having
~he enzymatic activity. The nucleic acid molecule is able
to base pair with the RNA substrate only 3' of the
cleavage site, and causes cleavage of the RNA substrate at
the cleavage site. The contacting is per~ormed under
conditions in which the nucleic acid molecule causes
cleavage of the RNA substrate at the cleavage site.
In preferred embodiments of the above aspects, the
nucleic acid molecule is derived from Neurospora VS RNA;
the nucleic acid molecule is active to cleave 5~ to the
RN~ duplex substrate (Fig. 6) of sequence 5'-
AAGGGCGUCGUCGCCCCGA, or ~'-NNNNNNNNNNNNNNNNNNN, where
each N independently can be any specified nucleotide base,
where the sequence forms at least 2 base-pair duplex
structure; the nucleic acid molecule is RNA; the nucleic
acid is a mixture of ribo and deoxyribonucleotides; the
nucleic acid contains at least one nucleotide-containing
modificatilons of sugar, phosphate and/or base or
combinations thereof; the nucleic acid molecule may or
contain abasic and/or non-nucleotide substitutions; the
nucleic acid molecule contacts the target RNA sequence;
the nucleic acid molecule is circular; and the nucleic
acid molecule is active to cut a single-stranded ~IA lFig.
SU~SrITUTE SI~EET (RULE 26)
CA 02208097 1997-06-18
WO96/19577 PCTnB9SI~0141
7) 5' to the sequence AAGGGCG or NNNNNNN or ~AGGGCGUCGUC
or N~JNNNNNNNNNN where each N independently can be any
specified nucleotide base, where the sequence ~orms at
least 2 base-pairs with a complementary sequence in the 5'
region o~ the enzymatic nucleic acid moleculo, where the
substrate RNA has at least one nucleotide 5' of the
cleavage site.
By "derived" is meant that the enzymatic portion of
the proposed "I" ribozyme is essentially the se~uence
shown in Fig. SA and 6A.
In yet another preferred embodiment, the nucleic acid
molecule derived from Neurospora VS RNA contacts a
separate RNA duplex substrate molecule via base-paired
interactions (Fig. 8 and 9) and causes cleavage of the
duplex substrate RNA at the cleavage site. ~rhis
interaction improves the specificity of the RN~ cleavage
~eaction.
In another aspect, the invention features synthesis
and assembly of enzymatic nucleic acid in one or rnore
pieces, where the nucleic acid contacts a .separate
suhstrate RNA molecule and cleaves the substrate RNA at
the cleavage site.
In yet another aspect, the invention features a
circular nucleic acid molecule having an enzymatic
activity which cleaves a separate RN~ substrate at a
cleavage site. The circular nucleic acids can be
constructed using one of the methods described in the art
(e.g., Been et al., WO 93/14218; Puttaraju et al., 1993
Nucleic A,cids Res. 21, 4253, Blumenfeld et al., WO
93/05~.57).
Other features and advantages of the invention will be
apparent from the following description of the preferred
embodiments thereof, and from the claims.
S(~SrITUTE SffEET (RULE 26)
-
CA 02208097 1997-06-lX
PCT~B95100141
W O 96119577
1 1
Descri~tion of the Preferred Embodiments
The drawings will first brie~ly be described.
Dra~,Jinqs
Figure 1 is a diagrammatic representation o~ a
hammerhead ribozyme domain known in the art. Stem II can
be ~ 2 base-pair long, or can ev~n lack base pairs and
consist of a loop region.
Figure 2a is a diagrammatic representation of the
hammerhead ribozyme domain known in the art; Fig-lre 2b is
a diagrammatic representation o~ the hammerhead ribozyme
as divided by Uhlenbeck (1987, Nature, 327, 596) into a
substrate and enzyme portion; Figure 2c is a similar
diagram showing the hammerhead divided by Haseloff and
Gerlach (198~, Nature , 334, 585) into two portions; and
Figure 2d is a similar diagram showing the hammerhead
divided by Je~fries and Symons (1989, Nucleic. Acids.
~es., 17, 1371) into two portions.
Figure 3 is a diagrammatic representation of the
general structure of a hairpin ribozyme. Helix 2 (H2) is
pro~ided with a least 4 base pairs (i.e., n is 1, 2, 3 or
~) and helix 5 can be optionally provided o~ length 2 or
more bases (preferably 3 - 20 bases, i.e., m is from 1 -
20 or more). Helix 2 and helix ~ may he covalently linked
by one or more bases (i.e., r is 2 1 base). Helix 1, 4 or
5 may also be extended by 2 or more base pairs (e.g., 4 -
20 base pairs) to stabilize the ribozyme structure, and
preferably is a protein binding site. In each instance,
each N and N' independently is any normal or modified base
and each dash represents a potential base-pairing
interaction. These nucleotides may be modified at the
sugar, base or phosphate. Complete base-pairing is not
required in the helices, but is preferred. Helix 1 and 4
can be of any size (i.e., o and p is each independently
from 0 to any number, e.g., 20) as long as some base-
pairing is maintained. Essential bases are shown as
SUBSrITUTE SHEET (RULE 26)
CA 02208097 1997-06-18
W Og6/19577 PCTnB95100141
specific bases in the structure, but those in the art will
recognize that one or more may be modi~ied chemically
(abasic, base, sugar and/or phosphate modifications) or
replaced with another base without signi~icant effect.
~ielix 4 can be formed from two separate molecules, i.e.,
without a connecting loop. The connecting ]oop when
present may be a ribonucleotide with or without
modifications to its base, sugar or phosphate. "q" :is 2
2 bases. The connecting loop can also be replaced with a
non-nucleotide linker molecule. H , refers to bases A, U
or C. Y refers to pyrimidine bases. " " refers to
a chemical bond.
Figure 4 is a representation of the general structure
of the hepatitis delta virus ribozyme domain known in the
art (Perrotta and Been, 1991 supra) .
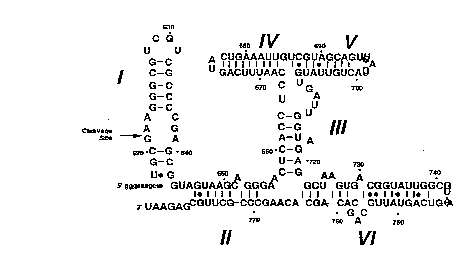
Figure 5 ~ is a representation of the general
structure of the self-cleaving Neurospora VS RNA domain.
B is a line diagram representing the "I" ribozyme motif.
Tho figure shows the "Upper" and the "Lower" base-paired
regions linked by the "connecting" region. IV (left) and
V ~right) shows the left and the right handed regions
ithin the "upper" region, respectively. II (left) and VI
(right) shows the left and the right handed regions within
the "lo~er" region, respectively).
Figure 6 is a diagrammatic representation of a trans-
cleaving VS RNA enzyme catalyzed cleavage of a double-
stranded duplex RNA . A) Stem I is an intramolecular he:Lix
formed within the substrate RN~. Stems II through VI are
in~ramolecular helices formed within the ribozyme. B)
schematic ~epresentation of minimal substrate sequerlce
requirement for cleavage by the "I" ribozyme. N,. refers
to any base. N' refers to any base that is complementary
to N. Y, refers to a pyrimidine.
Figure 7 is a diagrammatic representation o~ a trans-
cleaving vs RNA enzyme catalyzed cleavage of a sin~le-
SUBSrl~UTE S~EET (RULE 26)
CA 02208097 1997-06-18
WO96119577 PCT~B95/00141
13
stranded RNA. A) Stem I is an intermolecular helix formed
between the substrate RNA and the ribozyme. Stems II
through VI are intramolecular helices formed within the
ribozyme.B) An alternate strategy to facilitate cleavage
of a single-stranded R~A by the "I" ribozyme.
Figure 8 is a diagrammatic representation of the VS
self-cleaving RNA. Base-paired interactions between
nucleotides in the loop l (G630, U631 and C632) with
complementary nucleotides in loop 5 (C69g, A698 and G697)
10 is shown as bold lines.
Figure 9 is an enlarged view of the interaction
between loop l and loop V. A) shows base-pairincr of G630
with C699, U631 with A698 and C632 and G697. B) shows
bas~-paired interaction between nucleotides in loop l with
nucleotides in loop V, where N can be any base (e.g., A,
U, G, C) and N' can be any base that is complementary to
N.
By "complementary" is meant a nucleotide sequence that
can form hydrogen bond(s) with other nucleotide sequence
by either traditional Watson-Crick or other non-
traditional types (for example Hoogsteen type) of base-
paired interactions.
Figure lO shows the time course of double-.stranded
(ds) RNA cleavage by the VS RNA. A plot of fraction of
substrate RNA cleaved as a function of time is shown.
Figure ll shows the rate of RNA cleavage by the VS
ribozyme as a function of ribozyme concentration.
Figure 12 shows the effect of temperature variation on
~ the RNA cleavage reaction catalyzed by the vS ribo~yme.
Figure 13 shows the effect of pH on RNA cleavage
reaction catalyzed by the VS ribozyme.
SUBSrlTUTE SHEET ~RULE 26)
CA 02208097 l997-06-l8
W O96119577 PCT~B95/~0141
14
Figure 14 shows the effect of spermidine concentration
on the RNA cleavage reaction catalyzed by the VS ribozyme.
Figure 15 shows the ef~ect of Mg2~ concentration on
~ cleavage reaction catalyzed by the VS ribozy~e.
Figure 16 shows the kinetics of RNA cleavage reaction
catalyzed by the VS ribozyme. A) E~fect of ribozyme
concentration on the trans-cleavage reaction under optimum
reaction conditions. B) Effect of substrate R~A
concentration on the trans-cleavage reaction under opt:imum
reaction conditions.
Figure 17 shows enhancement of RN~ cleavage react:ion
catalyzed by the VS ribozyme. Numbers 0, 5, and 30 min
refers to the length of pre-incubation of VS RNA with 100
mM viomycin prior to the initiation of RNA catalysiC:. -
viomycin refers to RNA catalysis in the absence ofviomycin.
Figure 18 shows viomycin-dependent reduction in the
concentration of magnesium chloride required for
catalysis.
Tarqe~ sites
Targets for useful ribozymes can be deternlined as
disclosed in Draper et al . WO 93/23S69, Sullivan et al.,
wo 9~/02595 as well as by Draper et al., "~lethod and
reagent for treatment of arthritic conditions U.S.S.N.
08/152,487, filed 11/12/93, and hereby incorporated by
reference herein in totality. Rather than repeat the
guidance provided in those documents here, below are
provided specific examples, not limiting to those in the
art. Ribozymes to such targets are designed generally as
described in those applications and synthesized to be
tested in vitro and in vivo, as also described. Such
ribozymes can also be optimized and delivered as described
therein.
SUBSTITUTE S~EET tRULE 26)
CA 02208097 1997-06-18
WO9611gS77 PCTnB9Sl00141
Ribozyme activity can be optimized by chemically
synthesizing ribozymes with modifications that prevent
their degradation by serum ribonucleases, modifications
which enhance their efficacy in cells, and removal of
helix-containing bases to shorten RNA synthesis times and
reduce cllemical requirements. See e.g., Eckstein et al .,
International Publication No. WO 92/07065; Perrault 1990
et al ., Nature 344:565; Pieken et al., 1991 Sciellce
253:314; Usman and Cedergren, 1992 Trends in ~ioc}lem. Sci.
17:334; Usman et al ., International Publication No.
wo 93/15187; and Rossi e~- al ., International Publication
No. WO 91/03162, as well as Usman, N. et al. US Patent
~ppli cation 07/829,729, and Sproat, B. Europeall Patent
Application 9Z110298. 4 ;Chowrira and Burke, 1992 supra;
Chowrira et al., 1993 J Biol. Chem. 268, 19458, which
describe various chemical modifications that can be made
to the sugar moieties of enzymatic RNA molecules. All
these publications are hereby incorporated by reference
herein.
R7 bozymes are added directly, or can be complexed with
cationic lipids, packaged within liposomes, or otherwise
delivered to target cells. The RN~ or RNA complexes can be
locally administered to relevant tissues ex vivo, or in
vivo through injection, aerosol inhalation, infusion pump
or stent, with or without their incorporation in
biopolymers.
Sullivan, et al., supra, describes the general methods
for delivery of enzymatic R~A molecules. Ribozymes may be
administered to cells by a variety of methods known to
those familiar to the art, including, but not restricted
~ to, encaps~lation in liposomes, by iontophoresis, or by
incorporation into other vehicles, such as hydrogels,
cyclodextrins, biodeyradable nanocapsules, and bioadhesive
microspheres. For some indications, ribozymes may be
directly delivered ex vivo to cells or tissues with or
without the aforementioned vehicles. ~lternatively, the
SU~SI ITUTE ~lEET ~RULE 26)
CA 02208097 l997-06-l8
W O 96/19577 PCTnB95/00141
16
RN~/vehicle combination is locally dalivered by di1~ect
injection or by use o~ a catheter, infusion pump or stent.
Other routes of delivery include, but are not limited to,
intravascular, intramuscular, subcutaneous or joint
injection, aerosol inhalation, oral (tablet or pill ~orm),
topical, systemic, ocular, intraperitoneal and/or
intrathecal delivery. More detailed descriptions of
ribozyme delivery and administration are provided in
Sullivan, et al., supra and Draper, et al., supra which
have ~een incorporated by reference herein.
~ nother means o~ accumulating high concentrations of a
ribozyme(s) within cells is to incorporate the riboz~e-
enco~ing sequences into a DNA expression ~ector.
Transcription of the ribozyme sequences are driven ~rom a
promoter ~or eukaryotic RNA polymerase I (pol I), RNA
polymerase II (pol II), or RNA polymerase III (pol III).
Transcripts from pol II or pol III promoters will be
expressed at high levels in all cells; the levels of a
given pol II promoter in a given cell type will depencl on
the nature of the gene regulatory sequences (enhancers,
silencers, etc. ) present nearby. Prokaryotic RNA
polymerase promoters are also used, providing ~hat the
prokaryotic RNA polymerase enzyme is expressed in the
appropriate cells (Elroy-Stein, O. and Moss, B., 1990,
25 Proc. Natl. Acad. sci. U S A, 87, 6743-7; Gao, X. and
Huang, L., 1993, Nucleic Acids Res., 21, 2867-72; Lieber,
A., et al., 1993, Methods Enzymol., 217, 47-66; Zhou, Y.,
et al., 1990, Mol . Cell . Biol ., 10, 4529-37). Several
investigators have demonstrated that ribozymes expressed
from such promoters can function in mammalian cells (e.g.
Kashani-Sapet, M., et al .,, 1992 , Antisense Res. Dev. 2,
3-15: Ojwang, J. O., et al. ., 1992, Proc. Natl. Acad.
sci. U S A 89, 10802-6; Chen, C. J., et al.,, 1992,
Nucleic Acids ~es., 20, 4581-9; Yu, M., et al., 1993,
35 Proc. Natl. Acad. Sci. U S A, 90, 6340-4; L~Huillier, P.
J., et al., 1992, EMBO ~., 11, 4~11-8; Lisziewicz, J., et
al., 1993, Proc. Natl. Acad. Sci. U. S. A., 90, 8000-
SUBSTITUTE ~IEET (RULE 26)
CA 02208097 1997-06-18
W O 96119577 PCTnB95/00141
4)).The activity o~ such ribozymes can b~ augmented by
their release from the primary transcript by a second
ribozyme (Draper et al., PCT W093/23569, and Sullivan et
al., PCT W094/02~95, both hereby incorporated in their
totality by re~erence herein; Ohkawa et al., 1992 Nucleic
Acids Symp. Ser., 27, 15-6; Taira et al., 1991, Nucleic
Acids Res., 19, 5125-30, Ventura et al., 1993 Nucleic
Acids Res ., 21, 3249-55; Chowrira et al., 1994 ~. Biol .
Cl~em. 269, 25~56). The above ribozyme transcription units
tO can be incorporated into a variety of vec~ors for
introduction into mammalian cells, including but not
restricted to, plasmid DNA vectors, viral DNA vectors
(such as adenovirus or adeno-associated vectors), or viral
R~A vectors (such as retroviral and alpha virus vectors).
In a preferred embodiment of the invention, a
transcription unit expressing an ~ ribozyme tha~ cleaves
target RNA is inserted into a plasmid DMA vector or an
adenovirus or adeno-associated DNA viral or retroviral
vector. Viral vec~ors have been used to transfer genes to
the lung and these vectors lead to transient gene
expression (Zabner et al., 1993 Cell 75, 207; Carter, 1992
Curr. Opi . ~iotech. 3, 533) and both vectors lead to
~ransient gene expression. The adenovirus vector is
delivered as recombinant adenoviral particles. DNA may be
delivered alono or complexed with vehicles (as described
for RNA above).The DNA, DNA~vehicle complexes, or the
recombinant adenovirus particles are locally administered
to the site o~ treatment, e.g., through the use of an
injection catheter, stent or infusion pump or are directly
added to cells or tissues ex vivo.
In another aspect of the invention, ribozymes that
cleave target molecules are expressed from transcription
units inserted into ~NA or RNA vectors. The recombinant
vectors are preferably DNA plasmids or viral vectors.
Ribozyme expressing viral vectors could be constructed
based on, but not limited to, adeno-associated virus,
SVBSTITUTE S~IEET (RULE 26)
CA 02208097 1997-06-lX
W O96/19577 PCT~B95/~0141
18
retrovirus, adenovirus, or alphavirus. Preferably, the
recombinant vectors capable of e~pressing the ribozymes
are locally delivered as described above, and persist in
target cells. Alternatively, viral vectors may be used
that provide for transient expression of ribozymes. Such
vectors might be repeatedly administered as necessary.
Once expressed, the ribozymes cleave the target mRNA.
Delivery of ribozyme expressing vectors could be systemic,
such as by intravenous or intramuscular administration, by
administration to target cells ex-planted from the patient
followed by reintroduction into the patient, or by any
other means that would allow for introduction into the
desired target cell.
Thus, ribozymes of the present invention that cleave
target m~NA and thereby inhibit and/or reduce taxget
activity have many potential therapeutic uses, and there
are reasonable modes of delivering the riboz~les in a
number of the possible indications. Developmollt of an
effective ribozyme that inhibits specific ~unction are
described in the art.
By "inhibit" is meant that the activity or level of
target RNA is reduced below that observed in the absence
of the ribozyme, and preferably is below that level
observed in the presence of an inactive RNA molecule c~ble
to bind to the same site on the RN~, but unable to cleave
that R~A.
By "vectors" is meant any nucleic acid- and/or viral-
based technique used to deliver a desired nucleic acid.
Exam~les
The following materials and methods were usecl in the
following examples:
SllBSTITUTE StlEET (RULE 26)
-
CA 02208097 1997-06-18
W O96/l9S77 PCT~B95/00141
1 9
Clones and site-~irected muta~enesis.
Clone G11 has been described previously (Guo et al.,
; 19g3 J. Mol. Biol. 232, 351) and contains bases 617 to 881
of VS RNA in vector pTZ19R. Mutations were made in clone
5 G11 or DG11 (from which the ScaI, AvaI, AcyI sites in the
vector had been destroyed to facilitate future subcloning
by retaining only a uni~ue site for each enzyme within thi
vs sequence). Substitutions on the 5' or 3' side o~ a
helix were made by oligonucleotide directed mutagenesis
(Kunkel et al., 1987 in Me~ods Enzymol. eds. Wu and
Grossman, vol. 154, pp. 367, Academic Press, San Diego,
CA.); compensatory mutants were also made this way unless
a unique restriction site separated the 5' and 3'
mutations, in which case recombinant DNA techniaues were
us~d to combine the two mutations into a single clone.
Usually two separate isolates of each mutant were
identified and sequenced from the T7 promoter to the SspI
site which was the 3' end of the run-off transcripts used
to measure cleavage rates.
Measurement of self-cleavaae rates:
RNAs were synthesized by T7 transcription from plasmid
templatos linearized with SspI (VS RNA nt 783). Uncleaved
precursor RNAs were obtained ~rom wild type an~ active
mutctnts using decreased magnesium concentrations during
transcription (Collins and Olive, 1993 ~iochemistry 32,
2795). Transcription reactions were extracted once each
with phenol/Chloro~orm.Isoamyl Alcohol (CIA) and once with
CIA and precipitated with ethanol. RNAs (approximately 50
n~) were dissolved in water, preincubated at 37~C, and
mi~ed with one fifth v~lume o~ 5X buffer (final
concentxations: 50 mM Tris-HCl pH 8.0, 50 mM KCl, 2 n~
spermidine, 10 mM MgCl2). Aliquots were removed at
various times, the precursor and product RNAs separated hy
electrophoresis and quantitated using a PhosphorImager as
described previously (Collins and Olive, supra) . First-
SUBSTITUTE S~EET (RULE 26)
CA 02208097 1997-06-18
W O96/19577 PCTnB95/~0141
2~
order self-cleavage rates were determined from the s].opes
of plots of ~raction of uncleaved RNA versus time.
Site-Directed muta~enesis:
~pplicant constructed site-directed base substi~ution
mutants that would be predicted to disrupt helices by
changing one or more bases on the 5' or 3' side of
predicted helices. Compensatory mutations that would
restore a helix, but using a different base pair, were
also constructed. Self-cleavage rates were measured for
wild-type, the 5' and 3' mutants, and the compensatory
mutant, denoted 5'3'. The data for representative mutants
are shown in Table 2.
DNA tem~lates and svnthesis of RNAs:
Fragments of VS DNA were cloned into vectors E~TZl~,R or
l9R (Pharmacia). Clone Gll (see Guo et al., 1993 supra)
contains VS nts 617 to 881 numbered as in Saville and
Collins (199~ Cell 61, 685); the cleavage site is between
nucleotides G620 and A621. substrate RNAs were
transcribed (see below) ~rom Gll or its derivatives which
had been linearized at the AvaI site (nucleotide 639) or
the SspI site (nucleotide 783) to make RN~s designated
Gll/Ava and Gll/Ssp, respectively. These RNAs begin with
nine vector nts (5'gggaaagcu; see Figure 5) fo]lowed by VS
sequence. A site-directed mutant of Gll, clone 621U which
contains a single A to U substitution immediately
~ollowing the self-cleavage site, was also used.
Clone A-3 contains VS sequences downstream of the AvaI
site (nts. 640-881) in a derivative oE pTZ19R that lacks
the XbaI and SphI sites in the multiple cloning site
(constructed for reasons unrelated to the project
described here). Transcripts of clone A-3 digested with
SspI (VS nucleotide 783) begin with 9 vector nucleotides
(5'GGGAAAGCU) ~ollowed by 144 nucleotides of VS RNA; this
RNA is designated the Ava ribozyme, or Rz.
SU~SI ITUTE StlEET (RULE 26)
CA 02208097 1997-06-18
WO96119577 PCTnB95/00141
RNAs were prepared by in vi~ro Bacteriophage T7 RNA
polymerase transcription ~rom linearized plasmid DN~s.
Trar~scription reactions (usually 300 ~l) containe~ lO to
20 ~Ig o~ appropriately linearized template, l ~ o~ each
MTP (Pharmacia), 5 m~l dithiothreitol, lX T7 polymerase
bu~fer (Bethesda Research Laboratories: 40 mM Tris-HCl pH
8.0; 8 mM MgC12; 25 n~I NaCl; 2 mM spermidine-HCl~), 300 U
RNAguard (Pharmacia), lS0 to 200 Units T7 RNA polymerase
(Bethesda Research Laboratories) for 2 hrs at 37~C.
Radioacti~e transcrlpts were prepared as above except an
additional 30 mCi of ~a-32P] GTP (or, ~or speci~ic
experiments, ATP or UTP) was added. Samples were
subsequently treated with DNase I (Pharmacia; S U/~g DNA
template) ~or 15 minutes, then EDT~ was added to lO ~I.
RN~s were extracted with phenol: chloro~orm: isoamyl
alcohol, chloroform:isoamyl alcohol (CIA) and ethanol
precipitated in the presence of 0.3 M sodium acetate, pH
5.2.
Precipitated RNAs were dissolved in water and two
volumes o~ sequencing dye (95% ~ormamide, 0.5X T~E, 0.1%
xylene cyanol, 0.1% bromphenol blue), heated at 75~C for 3
min, and fractionated by electrophoresis on denaturing
polyacrylamide gels (40:1 acrylamide:bis-acrylamide) of
appropriate concentration containing 8.3 M urea and lX TBE
(135 m~I Tris, 45 mM boric acid, 2.5 mM EDTA). RNAs were
visualized either by autoradioyraphy or UV shadowing.
B~nds o~ interest were excised, eluted overnight at 4~C in
water and filtered to remove residual polyacrylamide.
RNAs were precipitated with ethanol in the presence of 0.3
M sodium acetate and dissolved in water. Concentrations
were determined spectrophotometrically, assuming l ~D260
to correspond to an ~NA concentration of 40 mg/ml.
End-labelinq o~ RNAs:
RNAs were labeled at 5' termini using T4
polynucleotide kinase and ~g_32p] ATP or at 3' termini
using T4 RNA ligase and S~[32p~ pCp. End-labeled RNAs
SllBSTlTUTE SHEET (RULE 26)
CA 02208097 1997-06-18
W O 96/19577 PCT~B95/~)0141
Z2
were fractionated on denaturing polyacrylamide gels and
detected by autoradiography.
In order to remove 5' triphosphates prior to 5' end
labeling, some RNAs were treated with 1 U calf intest:inal
alkaline phosphatase (Boehringer ~Iannheim) in a lO ~Ll
reaction containing 50 mM Tris-HCl pH 8.0, O.l ~ EDT,~ at
55~C for 30 min. Reactions were termi.nated by e~traction
with phenol:CIA and CIA.
Txans-cleavaqe reactions:
Trans-cleavage of substrate RNA (S) by the Ava
ribozyme (Rz) was carried out following pre-incu}~ation of
gel-purified S and Rz in the appropriate lX reaction
solution for 2 min. Reactions were initiated by addition
of ribozyme to substrate in a final volumè of 20 l~l. In a
typical reaction, lO aliquots of l.5 ~ll were removed at
specified times, terminated by addition of 13.5 ~Ll of stop
mix (70% formamide, 7 mM EDTA, 0.4x TBE, 0.07~ xylene
cyanol, and 0.07~ bromphenol blue) and stored on ice.
Samples were fractionated by electrophoresis on denaturing
20~ po].yacrylamide gels.
The effects of temperature, pH, MgCl2 and spermidine-
(HCl)3 on the trans-cleavage reaction were analyzed by
incubating equimolar concentrations of Rz and S (0.05 ~M
each) in solutions described in the figure legends.
final study of the effects of MgCl2 under otherwise
"optimized" conditions was performed at 30~C, 50 m~I Tris-
HCl pH 8.0, 2 mM spermidine, 25 mM KCl.
Experiments to establish single-turnover conditions
(Fi~. 10) ~ere performed at 30~C in 50 mM Tris-HCl pH 7.l,
25 mM MgCl2, 25 mM KCl, 2 mM spermidine. Analyses of the
effect of pH under single turnover conditions (l~ig. 13)
were performed as above, except the concentrations of Rz
and S were 5 ~IM and 0.13 ~M, respectively. 50 mM Tris-I~Cl
was used for pHs 7.1 to 8.9; 16.5 mM PIPES/44 m~irrris was
used for pH 6.
SU~STITUTE SHEET (RU~E 26)
-
CA 02208097 1997-06-18
W O96119577 PCTAB95/00141
23
Amounts of substrate and products were quantitated
using a PhosphorImager and ImageQuant version 3.0
software (Molecular Dynamics, Sunnyvale, CA, USA) .
Estimates o~ initial cleavage rates were derived from
plots of fraction of substrate cleaved vs. time using
Grafit software tErithacus Software Ltd, Staines, U.KJ.
Up to 9O~ o~ the substrate could be cleaved in ~0 minutes
at approximately equimolar concentration of ribozyme, with
the curve indicating the presence o~ approximately 10~
unr~active starting material. Curves were not adjusted to
lOO~ completion, and the nature of the unreactive
substrate has not been characterized further.
Exam~le 1: Mutational analvsis of the sel~-cleavina V5
RNA
t5 As a starting point ~or structure precliction,
applicant used the MFOLD program of Zuker and
collaborators (Zuker, 19 89 Sci ence 244, 48) to obtain five
ma~or families o~ thermodynamically reasonable models ~or
the minimal self-cleaving RNA. The models di~fered in the
number or length of helices and/or the predicted pairing
partners for a given region of the sequence, ancl ranged
from the structure predicted to be most stable to sub-
optimal foldings 10% less stable than the lowest free
energy structure. Structures witllin this range o~ ~re~
energy have been found to predict the majority of helices
in other RNAs (Jaegar et al., 1989 Proc. Natl. Acad. sci.
USA ~6, 7706). These various structural models were tested
by making use of site-directed mutagenesis.
Of the various models evaluated, that shown in Fig. 5A
was the most consistent with the data from the cleavage
activity of all of the mutants. In general, mutal:ions on
the 5' or 3' side of predicted helixes II througll VI
inactivated the ribozyme or decreased activity well below
that o~ the wild type sequence. Compellsatory
3~ substitutions that restored a helix, but with a diE~erent
base sequence, restored activity usually to that of wild
SUBSrlTUTE StlEET (RULE 26~
_ _ _ , . _ .
CA 02208097 1997-06-18
W O 96/19577 PCTnB95100141
type or greater, but always to a level at leas~ gxeater
than that of the individual 5' or 3' mutants. These data
sho~,Jed that regions of each of these helices perform :roles
that are not se~uence-specific but are presumably involved
in proper folding o~ the RNA.
In some cases, mutations on the S' and 3' side did not
reduce activity to the same extent. For example, mutant
Va5~ shows essentially no activity, but Va3' retains more
than hal~ the activity of wil~ type. It may be that: the
particular substitutions chosen did not disrupt the helix
e~ually well or that one of the bases makes a specific
contribution to local or tertiary structure (Cech, 1988
Gene 73, 259).
At some positions activity could not be restored by
the compensatory substitutions attempted, even though
restoration was possible at other positions in the same
helix. This was especially common at predicted base pairs
adjacent to natural disruptions in a helix, such as the
unpaired adenosines at positions 652 and 718 (Table 2;
ZO e.g., positions IIc and IIIc). Mutant G653C showed no
actitJity, as did each of the three substitutions at: the
predicted complementary position C771; the double mutant
G653C:C771G showed some restoration of activity, but ~Jas
still 10-fold slower than wild type (mutant IIc).
Similarly, the A661:U717 pair immediately above unpaired
A718 could not be replaced by a U:~ (mutant IIIc), even
though the next pair, C662:G716, could be substituted by a
G:C (rnutant IIIb). Deletion of either unpaired adenosine
also decreased activity, severely so in the case of A652.
These observations suggest that specific local struct:ures
may be espbcially important in these areas, or that some
of these bases may be involved in alternative and/or
additional interactions.
The structure and sequence re~uirements of ~Ielix ~
appear to be more complex than implied by the model in
Fig. 5. Although several base substit:utions decreased
SUBSTITUTE ~HEET (RULE 26)
CA 02208097 1997-06-18
PCT~B95/00141
WO g611~577
2~
activity severely (e.g., mutants Ia5', Ic3~), other
mutations that might be expected to have an equally
disruptive effect on the helix (mutants Ia3', Ib3', Ic5')
decreased activity only slightly. We have not ~ound any
positions at which the compensatory substitution.s that we
have tried restored activity much above the level of the
individual mutants. This may result in part ~rom the stem
o~ ~ive G-C pairs, possibly extended by non-Watson-Crick
interactions, which would be predicted to be very stable.
This existence and stability o~ helix I is sup~orted by
chemical structure probing and difficulties in sequencing
this region. Taken together, these observations suggest
that certain bases in helix I may be involved in
alternative secondary structures or tertiary interactions
that are crucial for activity.
Based on the above data, applicant has constructed a
model for the secondary structure of the VS self-cleaving
R~A, which contains the minimal contiguous region o f VS
R~ required for self-cleavage. In five of the six
holices pxoposed in the model, site directed base
substitution mutations that disrupt the helix decrease or
eliminate activity. Compensatory substitutions restore
activity, usually to wild type level or even greater.
These data provide strong support Eor a sequence-
independent, presumably structural, role for portions ofthese five helices.
Several observations suggest that the formation o~ the
active structure is more complicated than implied above.
While site directed mutants of helices II through VI
indicate that portions of these helices play a sequence-
independen~ structural role, mutants in helix I show a
more complex pattern. ~iutations at certain positions in
helix I in~ctivated the ribozyme but compensatory
substitutions did not restore activity. Furthermore,
there is evidence from site-directed mutagenesis and
compensating substitutions for a tertiary interaction tllat
SUB5TITUl E SHEET (RULE 263
CA 02208097 1997-06-18
W O96/19577 PCT~B95/(~0141
26
requires the unwinding of at least the top base pair in
helix I (G628:C632), to allow an interaction with loop V
(see Figure 8 and 9). Taken together, these observations
suggest that a substantial con~ormational change may c~ccur
in helix I under native conditions. The model predicts
that VS RNA contains some structural features foun~ or
predicted in other RNAs. The GU~ tetraloop capping heli~
VI is an example of a GNRA loop that is common in rRNAs
(Woese et al ., 1990 Proc. natl . Acad. sci . USA 87, 8467)
and contains internal hydrogen bond and stac]cing
interactions that stabilize the loop structure (Heus and
Pardi, 1991 Science 253, 191; Santa-Lucia et al., 1992
Science 256, 217).
The secondary structure of VS RNA is different from
the hammerhead and hairpin ribozymes in that, although a
short helix upstream of the site of cleavage could form in
VS RNA, it is not required for activity (Guo et al., 1993
supra) as it is in these two ribozymes (Foster and Symons,
1987 Cell 50, 9;Berzal-Herranz et al ., 1993 EM~O. ~. 12 ,
2567). Also, VS RNA does not contain the set of bases
known to be important for activity of hammerhead (Symon,
1992 Ann. ~ev. Biochem. 61, 641) or hairpin (Berzal-
Herranz et al., supra) ribozymes. Like vS RNA, the HDV
ribozyme (Been, 1994 TI~S 19, 251) re~uires only a single
nucleotide upstream of the cleavage site, and a GC-rich
helix is found downstream of the cleavage site in both
ribozymes. Beyond these similarities, however, the
secondary structures have nothing in common.
ExamDle 2: Trans-cleavaae reaction catalvzed bv the VS
RNA.
The trans-reaction described below was constru~ted
using various restriction fragments of VS DN~ cloned ln a
T7 promoter vector to construct pairs of non-overlapping
regions of VS ~JA. One member of each pair, the substrate
(S), contained the expected cleavage site, following
nucleotide G620 (numbered as in Saville and Collir-s, 1990
SUBSTITUTE SltEET (RULE 26~
-
CA 02208097 1997-06-lX
W O96/19577 PCTnB95/00141
27
supra): the other, the enzyme or ribozyme ~RzJ, contained
the remainder of the VS sequence, terminating a~ the SspI
site at nucleotide 783. In preliminary experimerlts these
transcripts were mi.xed at approximately 1:1 ratio and
incubated under conditions known to support self-cleavage
(Collins and Olive, 1993 supra). Most combinatioIIs showed
little or no cleavage; however, almost complete cleavage
of a 32 nucleotide substrate RNA that terminates at the
~aI site (nucleotide 639) was observed during a one hour
inctlbation with a ribozyme that begins at the AvaI site
and ends at the Ssp~ site ~nucleotide 783), no cleavage
was observed in the absence of ribozyme. Tho
elec~rophoretic mobility of the two cleavage products were
appro~imately those expected for cleavage a~ter nucleotide
620, which is the site o~ intramolecular self-cleavage or
VS RNA. Applicant chose to examine this trans-cleavage
reaction in further detail.
Exam~le 3- Trans-cleavaqe Qccurs at the same si~e as sel~-
cleavaae
To determine the precise site of cleavage, Gll~Ava
substrate, Pl and P2 were labeled at their 5' ends and
sequenced by partial enzymatic digestion using RNases Tl
or U2. Cleavage products o~ a mutant substrate containing
a single base substitution 3' o~ the cleavage site (A621U)
were also characterized to resolve possible ambiguities
due to anomalous migration of some bands. Because the
substrate and Pl are identical in sequence from the 5' end
to the cleavage site, all R~ase sequenciny bands
comigrated, as expected. Full length P1 comigrated with
the 13 nucleotide RNase Tl fragment o~ Gll/Ava that
terminates ,at G620, which is the site of intramolec~llar
self-cleavage in VS RNA. Also the 3' end of P1 was found
to be guanosine 2'3' cyclic phosphate, indicating that
both the location and chemical pathway of trans cleavage
are the same as in the self-cleavage reaction.
SU~STITUTE S~EET (RULE 26)
CA 02208097 1997-06-18
W O96119577 PCT~B95/~0141
28
As expected ~rom the finding of a cyclic phosphate at
the 3' end of Pl, a 5' hydroxyl group was found at the 5'
end o~ P2, as evidenced by its end-labeling by [g_32p] ATP
and T4 polynucleotide kinase without prior phosphatase
5 treatment. Alkaline hydrolysis ladders of 5' end-labeled b
P2 contained only 18 of the expec~ed 19 bands. This is
the result of a compression artifact involving the
formation of a very stable stem-loop structure in the
longer RNAs; this is described in detail below.
10 Nonetheless, the 5' terminal nucleotides of P2 derived
from cleavage of G11/Ava S and the A621U mutant were A and
U, respectively, confirming that cleavage occurred between
nts 620 and 621, as in the self-cleavage reaction.
~xam~le 4: ~linimal lenath of the substxate RNA
To determine the minimal se~uence required downstream
of the cleavage site, applicant used essentially the
approach described by Forster and Symons (1987 su~ra). 5'
end-labeled G11/Ssp RNA was partially hydro]yzed by
treatment at high pH, then incubated with or without the
20 ribozyme. Incubation in the absence of ribozyme confirmed
applicant's previous finding that full length G11/SSD RNA
and deletion derivatives lacking ten or fewer nucleotides
at the 3' end can self-cleave (Guo et al., 1993 supra) .
Incubation with the ribozyme resulted in the
25 disappearance, or at least decrease in intensity, of bands
corresponding to RNAs terminating at nucleotide 639 or
longer. A few RNAs were not cleaved to completion under
these conditions, indicating that they are relatively
poorer substrates. The minimal length substrate
30 terminates at residue 639, which by coincidence
correspondslprecisely to the RNA used in Fig. 6, which was
synthesized by runoff transcription o~ a template
linearized at the AvaI site. Thus only 19 nucleotides
downstream of the cleavage site are required for trans-
35 cleavage by the Ava ribozyme.
SUBSTITUTE S~EET (RULE 26)
CA 02208097 1997-06-18
PCT~B95/00141
WO961195~7
29
A parallel experiment using 3' end-labeled RNA showed
t~lat only a single nucleotide upstream o~ the cleavage
site is required for trans-cleavage. Taken together with
the results from 5' end labeled RN~, these data show that
~- 5 the minimum contiguous region of the native RNA required
for ~rans-cleavage consists of one nucleotide upstream o~
the cleavage site and l9 nucleotides downstream.
Exam~le S: The minimzl substrate RNA consists mostlv of a
hair~in 10Q~
RNA structure prediction using the MFO~D program of
Zuker and collaborators (Zuker, 1989 supra) suggests that
the most thermodynamically reasonable structure of the
s~Ibstrate RNA would be the hairpin-containing structure
drawn in Fig. 6. During the characterization of the trans-
cleavage products applicant noted several observations
that were consistent with such a structure. P2 migrated
faster than expected relative to size markers for a l9
nucleotide RNA, suggesting that it contained a structure
that was not fully denatured even in a gel containing 8.3
M urea. Certain guanosine (523-625, 627 and 633) and
adenosine (621 and 622) residues in S and P2 were cleaved
weakly or not at all by RNases Tl and~or U2, even though
sequencing reactions were performed under putatively
denaturing conditions of 50~C, 1 mM EDTA and 7 M urea.
Only 18 of the expected l9 bands were observed in the 5'
end-labeled partial alkaline hydrolysis products of P2.
~xamvle 6: Biochemical Characteristics of the Trans-
5leavaae Reaction Conditions:
Applicant has investigated the effects of several
variables t~at would be expected to affect RNA s~ructure
- and that have been found to affect the cleavage rates of
other ribozymes. An equimolar ratio of S and Rz (0.05 ~IM
each) for most initial investigations was used; more
detailed analysis specifically under either steady-state
or single-turnover conditions is described below.
SU~STITUTE ~EET ~RUL~ 26)
CA 02208097 1997-06-18
W O 96/19577 PCTnB95/00141
Cleavage rate increased with temperature unti:L an
optimum was reached around 30~C, and then decreased
sharply above 40~C (Fig. 12). No reaction was observed in
the absence o~ a divalent cation, and reaction rate
increased with increasing MgCl2, reaching a maximum around
100 mM, when magnesium was the only cation present. To
determine whether some of the MgC12 was acting simply as a
structural counterion, the e~fects of spermidine (Fig.
14), NaC1, and KCl were investigated in the presence of a
subsaturating concentration of MgC12 (10 n~i). In the
presence of 10 mM MgC12, spermidine at 1 mM or greater
enhanced the rate of cleavage nearly 10-fold compared to
the same reaction without spermidine (Fig. 14). Low
concentrations of KCl (< 100 mM) also stimulated the
reaction rate up to about 10 fold. Perhaps surprisingly,
NaCl had almost no effect. These observations are ~imilar
to the e~fects of cations observed previously on the :rate
of self-cleavage of vs RNA (Collins and Olive, 1993
supra).
The rate of reaction showed only a small pH
dependence: the nearly 100-fold increase in the hydroxide
concentration between pH 7.1 and 8.9 resulted in only a 2-
fold increase in rate (Fig. 13). The effect o~ pH
specifically under single turnover conditions is clescribed
below.
~ inally, the e~fect of MgCl2 was re-assayed under
"optimized" reaction conditions containing 50 mM Tris, pH
8.0, 2 mM spermidine, 25 m~I KCl, and incubated at 30~C
(Fig. 15). Under these conditions 10 mM MgCl2 allowed the
same rate of cleavage as a reaction containing 70 mM MgCl2
under suboptimal conditions. Thus, the combined effects
o~ temperature, pH, and cations other than mc~gnesium
enhanced cleavage substantially. However, no reaction was
observed in the absence of MgC12, indicating that neit:her
spermidine nor KCl can replace magnesium in cleavage.
SU~SfITUTE SH~ET (RULE 26)
CA 02208097 1997-06-18
W O 96/19577 PCTnB95/00141
Ef~ects of ~I under sinale turnover conditions:
The trans-cleavage reaction rate showed only a small
- pH dependence at equimolar concentrations of ribozyme and
substrate ~Fig. 13). However, these experiments wore
per~ormed at subsaturating concentrations of MyCl~ and
they were probably not under single turnover conditions.
Consequently it was possible that some step in the
reaction other than the actual cleavage step itself may
have been the rate limiting step, thereby maslcing the
effect of increased hydroxide ion concentration. To
investigate this possibility, single turnover conditions
were established empirically under optimized reaction
conditions-by measuring the initial rates o~ trans-
cleavage of 0.13 mM substrate by increasing concentrations
of ribozyme. The initial rate of cleavage increased with
ribozyme concentration up to about 2.5 rn~I, and
subsequently leveled off, suggesting that the reaction was
approaching single turnover conditions (Fig. 11). The
cleavage rate as a function of concentration o~ ~gC12 was
~0 re-investigated using 0.13 ~M S and 5 ~I Rz and Eound to
be essentially the same shape as in Fig. 15; a
concentration of 25 mM MgC12 was chosen to ensure that
magnesium was not limiting. Trans-cleavage reactions
using 0.13 ~ substrate and 5 ~IM ribozyme over a range of
pH showed only a minor enhancement in reaction rate.
Steadv-state reaction kinetics:
To determine if the Ava ribozyme i5 capable of
multiple turnover, Rz was incubated with approximately a
20-fold molar excess o~ S (Fig. 16). If each ribozyme
molecule cleaved only a single substrate, a maximum of
1~20th of ~ could be cleaved. In contrast, we observed
that cloavage continued at a constant rate until about 40%
of S was cleaved, and then decreased slowly as the
concentration of available uncleaved S decreased. This
indicated that the Ava ribozyme behaved like a true
enzyme, in that it was capable oE multiple ro~lnds oE
SUBSTITUTE SHEET (RULE 26~
CA 02208097 1997-06-18
WO 961195M PCT~B9S/0~141
cleavage. Also, as expected of an enzyme, the initial
rate of cleavage was directly proportional to the
concentration of the ribozyme under conditions of
substrate excess (Fig. 16).
The trans-cleavage reaction exhibits a saturat:ion
curve with respect to substrate concentration that is
typical of Michaelis-~enten kinetics (Fig. 16B). ~ KM of
0.13 ~LM and kCat of 0.7 min~l were obtained ~rom these
data. These values have been observed to vary by aboul~ a
factor of about two when experiments were repeated with
different batches of ribozyme over a period of two years.
Applicant has modified the natural intramolecu].ar
self-cleavage reaction of VS RNA by constructing a
ribozyme containing 144 nucleotides of VS R~A that is
capable of an intermolecular trans-cleavage reaction.
This ribozyme acts as a true enzyme in cleavi.ng a 32
nucleotide substrate RNA. In the presence of excess
substrate, the initial rate of cleavage is proport:ional to
ribozyme concentration, and a single ribozyme molecule c:an
cleave multiple substrate molecules. The ribozyme is
specific in cleaving a single phosphodiester bvnd, the
same one as cleaved in the natural self-cleavage reaction.
The trans-cleavage reaction exhibits Michaelis-Menten
kinetics, with Km ~ 0.13 ~IM and kCat ~ 0.7 min~~-. Fedor
and Uhlenbeck (1990 P~oc. Natl. Acad. Sci. USA 87, 168)
have noted that KCat values in the range of 1 min~l and Km
values in the nanomolar range are characteristic of many
diverse ribozymes.
The shortest contiguous region of VS RNA that
3Q functions as a substrate for the ribozyme described here
contains a single nucleotide upstream of the cleavage site
and lg nucleotides downstream. Applicants previous
characterization of the intramolecular self-cleavage
reaction also showed that only a single nucleotide is
required upstream of the cleavage site IGuo et al., 1993);
in this respect, VS is similar to HDV ribozymes which also
SU~3STITUTE SI~EET (RULE 26)
CA 02208097 1997-06-18
WO96119577 PCTnB95/00141
33
require only a sinyle upstream nucleotide for self- or
trans-cleavage (Been, 1994 supra) . The substrate consists
mostly o~ a stem-loop structure ~lanked by three
nucleotides on the 5' and 3' ends, some of which may be
involved in non-Watson-Crick structure (Fig. 6). This
conclusion is based on minimum free energy predic~ions,
aberrant electrophoretic mobility and the pa~tern o~
accessibility to single-strand-specific nucleases.
Disruption o~ some base pairs in the stem by certain
single base substitutions has little or'no effect on self-
cleavage. However, at some positions the identity of one
of the bases in a particular pair is critical: even when
the compensating substitution is made in the complementary
position to restore the helix, cleavage is not restored.
1~ Applicant believes that specific bases at specific
positions are more important than simply the presence o~ a
stem-loop s~ructure.
The stem-loop structure of the vs substrate RNA leaves
no long regions available for Watson-Crick pairing with
ZO the ribozyme. The secondary structure of the minimal
self-cleaving VS RNA has been determined and a working
model for the structure of the ribozyme has been proposed
(Fig. 5). The ribozyme has no long (i.e., more ~han 5
nucleotides) single-stranded regions. This is in contrast
to most trans-acting ribozymes derived from hammerhead,
hairpin, HDV and Group I intron RNAs, which have been
designed to lnteract with single-stranded regions of their
substrates via formation of one or two intermolecular
helices flanking the site to be cleaved.
In addition to base-pairing, tertiary interactions are
known or suspected to contribute to substrate bil1ding oE
several ribozymes (Pyle et al., 1992 Nature 350, 628). In
~act, tertiary interactions alone are sufficient to allow
very weak (KM ~O.l ~LM) but specific binding of the Pl
stem-loop of a Group I intron to its catalytic core
(Doudna and Szostak, 1989 Nature 339, 519). RNase ~ also
SU~SrITUTE SHEET (RULE 26~
CA 02208097 1997-06-18
W O96/19577 PCT~B9S/1~0141
34
recognizes substrates that contain substantial secondary
structure and have very limited potential ~or Watson-Criclc
pairi.ng with the ribozyme (Guerrier-Takada and Altman,
1993 ~iochemistry 32, 7152).
we noted in our previous characterization of the VS
RNA se].~-cleavage reaction that the cleavaye rate was
essentially unaffected by pH (Collins and Olive, 1993
supra). Consistent with this observation, the trans-
cleavage reaction described here also showed little, if
any, pH dependence, even when examined under single
turnover conditions. These observations differ from
results examining the rate of the chemical cleavage step
o~ hammerhead ribozymes (Dahm et al., 1993 ~iochemistry
3~, 13040), RN~se P (Guerrier-Takada et al., 1986
Biocl~emistry 25, 1509; Smith and Pace, 1993 ~iochemistry
32, 5~.73; Beebe and Fierke, 1994 Biochemistry 33, 10294)
and Totrah~nena Group I intron (Herschlag et al., 1993
~iochemistry 32, 8312). For these ribozymes, the rate of
the cleavage step was found to increase with increasing
pH. Failure to observe pH dependence in VS RNA could mean
that OH- is not involved in the cleavage reaction, that
the reaction proceeds via a novel mechanism or, more
likely, that the vS trans reaction is not limited by the
rate of the chemical cleavage step under these conditions.
but rather by some step that precedes actual cleavage.
One interesting candidate for such a rate-limiting
step would be a conformational change in the substrate
and/or ribozyme following binding. At saturating ribozyme
concentration, the pseudo-first-order rate constant ~or
trans-cleavage of S (~0.6 min~1) is about 10-fold higher
than the rate of sel~-cleavage of G11 RNA under similar
conditions (Collins and Olive, 1993 supra). Since we
envision that the trans-cleavage reaction recreates
essentially the same RNA conformation as in the self-
3~ cleavage reaction, the higher rate suggests that thecleavable conformation may be more easily attained whell S
SUBSTITUTE SffEET ~RULE 26)
CA 02208097 1997-06-18
W O96/19577 PCTnB95/00141
(stem-loop I; Fig. 5) is not constrained by covalent
attachment to the ribozyme core. In support of this idea,
we have also found that rate of self-cleavage of ~11 RNA
can be increased several fold by increasing the distance
r 5 between stem-loop I and the ribozyme core. These
observations are consistent with the idea of at least one
confoxmational change involving the substrate stem-loop
occurring during the reaction.
The temperature optimum of the trans-cleavage reaction
10 is substantially lower than for the self-cleavage reaction
(30~C vs -45~C) and activity drops off much more sharply
a~ higher temperatures (Collins and Olive, 1993 supra) .
The retention of activity at higher temperature.s in the
self-cleavage reaction indicates that the active site o~
15 the ribozyme does not begin to denature until at least
45~C. The lower optimum temperature of the trans-cleavage
reaction may reflect decreased ~inding of the substrate at
higher temperatures.
The observation that the VS ribozyme can recognize a
20 substrate that contains a stable secondary structure may
be useful from the perspective of ribozyme engineering.
Among the limitations to modifyin~ hammerhead, hairpin or
Group I intron ribozymes to cleave non-native target RN~s
is the requirement that the target site be in a single-
25 stranded region to allow recognition via base pairing with
the ribozyme. Because the cleavage site for the VS
ribozyme is adjacent to a stable secondary structure, the
VS ribozyme may have unique properties that can be adapted
to cleaving certain RNAs that are not accessible to the
30 action of other ribozymes.
~xam~le 7: Antibiotic-mediated enhancement of RNA
~ Cleava~e reaction catalvzed bv the VS ribozvme
Several examples of inhibition of the function of a
ribozyme or RNA-protein interaction have sho~n tllat
3~ certain antibiotics can interact specifically with RN~
SUBSTITUTE St~EET (RULE 26)
CA 02208097 1997-06-18
W O96/19577 PCTnB95/00141
(Yarus, 1988 Science 240, 1751; Schroeder et al., 1993
Science 260, 1443). Small peptide antibiotics like
viomycin ~las been shown to inhibit reactions of certain
R~ and RN~-protein complexes (Liou and Tana]ca, .l976 BBr~C
71, 477; Wank et al., 1993 J. Mol. ~iol. 236, lOO.l).
~ pplicant has found that certain peptide antibiotics
( e . g., viomycin) enhance RN~ cleavage reactions catalyzed
by the VS ribozyme. ~ntibiotics decrease, at least by an
order of magnitude, the concentration of metal ions
rQ~uired for ribozyme activity. Additionally, viomycin
facilitates inter-molecular interactions between VS RNA
molecules.
Referring to Fig. 17, vS RN~ are pre-incubated ~ith
100 mM viomycin ~or 0, 1, 15 and 30 min prior to adding
~ho reaction buffer (40 mM Tris-HCl pH 8.0;50 m~l~CCl and
10 m~ MgC12). The reaction is carried out at 37~C and
aliquots are taken out at regular intervals of time and
the reaction is stopped by adding an equal volumQ of
formamide stop buffer. The reaction products are reso]ved
on denaturing polyacrylamide gels. A plot o~ ~raction of
substrate cleaved as a function of time is plotted. The
fraction of RNA cleavage increased with an increase in the
time of preincubation. The antibiotic-mediated
enhancement in rates of cleavage is observed in solutions
that already contains optimal concentrations of magnesium
and KCl.
Referring to Figure 18, antibiotic-mediated lowering
of the re~uirement of divalent cation (Mg2+) is discussed.
RMA cleavage reaction catalyzed by the VS ribozyme is
assayed u~der varying concentrations of magnesium
chloride. VS RNA are pre-incubated with 75 mM viomycin
for 30 min in tl~e presence of 4~ mM Tris-HCl. Reaction
was initiated at 37~C by adding varying concentrations of
llgC12. A plot of rate (min~l) as a function of time is
shown. The presence of viomycin appears to signiEicantly
lowQr the requirement of MgCl2 in the reaction.
SUBSl ITUTE S~EET (RULE 26)
CA 02208097 1997-06-18
W O 96/19577 PCT~B95/00141
37
Sequences listed in Figures 6-9 are meant to be non-
limiting Those skilled in the art will recognize that
variants (base-substitutions, deletions, insertions,
mutati-ons, chemical modifications) of the VS ribozyme can
be readily generated using techniques known in the art,
and are within the scope of the present invention.
Diaanostic uses
Ribozymes of this invention may be used as diagnostic
tools to examine genetic drift and mutations within
diseased cells, or to detect specific RNA molecules, such
as virus RNA. The close relationship between ribozyme
activi.ty and the structure of the target RNA allows the
detection of mutations in any region of the molecule which
alters the base-pairing and three-dimensional structure of
the target RN~. By using multiple ribozymes described in
this invention, one may map nucleotide changes which are
important to RNA structure and function in vi tro, as well
as in cells and tissues. Cleavage of target R~As with
ribozymes may be used to inhibit gene expression and
define the role (essentially) of speci~ied gene products
in the progression of disease. In this manner, other
genotic targets may be defined as important mediators of
the disease. These experiments will lead to better
treatment o~ the disease progression by affording the
possibility o~ combinational therapies (e.g., multiple
ribozymes targeted to different genes, ribozymes coupled
~ith known small molecule inhibitors, or intermittent
treatment with combinations of ribozymes and/or other
chemical or biological molecules). Other in vitro uses of
ribozymes of this invention are well known in the art, and
include det~ction of the presence of mRMA associa~ed with
a related condition. Such RN~ is detected by determining
the presence of a cleavage product after treatment with a
ribozyme using standard methodology.
In a specific example, ribozymes which can cleave only
wild-type or mutant forms of the target RMA are used for
SUBSrITUTE SHEET (RULE 263
CA 02208097 1997-06-18
W O 96/19577 PCTnB95/1~0141
38
the assay. The first ribozyme is used to identify wild-
type RNA present in the sample and the second ribozyme
will be used to identify mutant RNA ln the sample. As
reaction controls, synthetic substrates of both wild-type
and mutant RNA will be cleaved by both ribozymes to
demonstrate the relative ribozyme efficiencies in the
reactions and the absence of cleavage of the "non-
targeted" RNA species. The cleavage products from the
synthetic substrates will also serve to generate size
markers for the analysis of wild-type and mutant RNAs in
the sample population. Thus each analysis will require
two riboz~nes, two substrates and one unknown sam~le which
will be combined into six reactions. The presence of
cleavage products will be determined using an RNAse
protection assay so that full-length and cleavaye
fragments of each RNA can be analyzed in one lane of a
polyacrylamide gel. It is not absolutely required to
quantify the results to gain insight into the expres;sion
of mutant RNAs and putative risk of the desired phenotypic
changes in target cells. The expression of mRMA whose
protein product is implicated in the development of the
phenotype is adequate to establish risk. If probes of
comparable specific activity are used for both
transcripts, then a qualitative comparison of RN.~ levels
will be adequate and will decrease the cost of tl1e initial
diagnosis. Higher mutant form to wild-type ratios will ~e
correlated with higher risk whether RNA levels are
compared qualitatively or quantitatively.
SU~STITUTE ~EET (RULE 26)
CA 02208097 l997-06-l8
W Og6119577 PCTnB95/00141
39
TABLE I
~ Characteristics of Ribozymes
Group I Introns
Size: -200 to >1000 nucleotides.
Requires a U in the target sequence immediately :~' of the cleavage
site.
Binds 4-6 nucleotides at 5' side of cleav2ge site.
Over 75 known members of this class. Found in Tetrahymen2
thermophila rRNA, fungal mitochondria, chloropiasts, phage T4,
blue-green algae, and others.
RNAseP F~NA (M1 RNA)
Size: ~290 to 400 nucleotides.
RNA portion oF a ribonucleoprotein enzyme. Cleaves tRNA
precursors to form mature tRNA.
Roughly 10 known members of this group all are bacterial in origin.
Hammerhead Ribozym~
Siz~: -13 to 40 nucl20tides.
Re~uires the target sequence UH immediately ~' of the cleavage
sit2.
Binds a variable number nucleotides on both sides of the cleavage
site.
14 known members of this class. Found in a number of plant
pathogens (virusoids) that use RNA as the in,ectious agent (rigures
1 and 2)
Hairpin Ribozyme
Size: -aO nucleotides.
Requires the target sequence GUC immedialely 3' Gf the cle-vaae
sit2.
Binds 4-6 nucleotides at 5' side of the cleavase site and a v_riable
number to the 3' side of the cleavage site.
Only 3 known member of this class. Found in three plant pa~hogen
(satellite RNAs of the tobacco ringspot virus, ~rabis mosaic virus
and chicory yellow mottle virus) which uses RNA as tne infeclious
agent (Figure 3).
Hepatitis Delta Virus (HDV) Ri~ozyme
Size: ~0 - 6~ nucleotides (at present).
Cleavage of target RNAs recently demonstrated.
Sequence requirements not fully determined.
Binding sites and structural requirements not fully delermined,
although no sequences 5' of cleavage site are required.
Only 1 known member of this class. Found in humar, HDV ('igure
4).
Ne~rospora VS RNA Ribozyme
Size: -144 nucleotides
SU~STITUTE SHEET (RULE 26)
CA 02208097 1997-06-18
W O 96/19577 PCT~B95N0141
Found in Neurospora VS RNA (Figure ~A).
SUBSTITUTE S~EET (RULE 26
CA 02208097 1997-06-18
W O 9611gS77 41 1~-111b5S~14l
~ Ta~le II. Effect of base-substitutions on th~ rate of
self-cleavage of the VS
RNA
Helix 8ase- k
Substitution
-- G11 wildtype 1.002
la 5' G624CtG625C 0.02
la 3' C634G/C635G 0.64
la 5'3' <0.01
Ib 5' C626G 1.21
Ib 3' G633C 0.74
Ib 5'3' 0.31
Ic 5' G627C 0.64
Ic 3' C632G c0.01
Ic 5'3' <0.01
lla 5' G650C 0.12
lla 3' C773G 0.29
lla 5'3' 1.27
llb 5' G655C <0.01
ilb 3' C769G 0.18
llb 5'3' 1.32
llc 5' G653C <0.01 .
Ilc 3' C771G cO.0-i
llc 5'3' 0.09
Illa 5' U659A ~0.01
Illa 3' A720U 0.05
Illa 5'3' 1.19
Illb 5' C662G 0.23
- Illb 3' G716C 0.21
Illb 5'3' 0.94
Illc5' A661U/C662G 0.06
Illc3' G716C/U717A 0.02
Illc 5'3' 0.08
SUB~ITUTE ~iEET[RUEE263
CA 02208097 1997-06-18
W O 96/19577 PCTnB95/aO141
42
. .
Illd 5' C665G0.01
Illd 3' G711C F3
Ilid 5'3' 0.01
IV 5'U670A/C672G 0.~4
IV 3'G678C/A681U <0.01
IV 5'3' 0.88
Va 5'A690U/C692G 0.07
Va 3'G704C/U706A 0.78
Va 5'3' 1.48
Vb4 5' U695G0.06
Vb4 3' A701C0.04
Vb4 5'3' 1.67
Vc 5'A693U/G694C ND5
Vc 3'C702G/U703A ND5
Vc 5'3' 0.31
Vla 5'G722C/C723G c0.01
Vla 3'G762C/C763G <0.01
Vla 5'3' 075
Vlb 5'G727C/U728A <0.01
Vlb 3'A759U/C760G <0.01
Vlb 5 3 0 94
Vlc 5'A735U/U737A 0.25
Vlc 3'A748U/U750A 0.~8
Vlc 5'3' 1.15
652~A <0.01
A652G ~0.01
. . 718~A 0.15
rate constant of the mutant divided by the rate constant of wild-
type G 1 1 .
Z the rate constant for the G11 varied from ~ 0.06 to 0.08 min~l .
3 cleavage rate not measured accuratley, but similar to wild type.
4 these mutants were made in a variant of G11 that contained r, o
different base pairs in helix V ( mutant Vc). Rates are normalized
using mutant Vc as the revelant wild type.
5 cleav2ge rate not determined.
SUBSTITUrE S~EET (RULE 26)