Note: Descriptions are shown in the official language in which they were submitted.
CA 02209367 1997-07-03
FIELD OF THE INVENTION
The present invention pertains generally to surgical devices and
procedures. More particularly, the present invention pertains to devices and
methods for treatment of coronary ischemia resulting from stenotic occlusions
of the coronary blood supply. The present invention is particularly, but not
exclusively useful, for neovascularization of the myocardial tissue of a
patient.
BACKGROUND OF THE INVENTION
Many medical complications are created by the total or even partial
1o blockage of blood vessels of the body. For example, it is relatively common
for stenotic segments to accumulate in the arterial vessels which supply the
heart. Stenotic segments of this type may partially or fully occlude the
involved vessels and often result in decreased cardiac capacity or even
myocardial infarction. As a result, numerous methods and devices have been
developed to treat or remove blockages which occur within the internal
vessels of the body. One well known example of a treatment directed at
removal of stenotic occlusions of arterial vessels is the angioplasty
procedure. In general terms, angioplasty generally involves inflation of a
tubular balloon within the stenotic segments which occlude a particular
2o vessel. Inflation of the balloon dilates the stenotic segment and fully or
partially restores the flow of blood within the involved vessel.
Atherectomy is another procedure which has been developed to clear
stenotic segments from occluded vessels. In an atherectomy procedure, a
rotateable cutting tool is advanced through the stenotic segments which
1
CA 02209367 1997-07-03
occlude a particular vessel. The rotating cutter severs the material forming
the stenotic segment, and allows the severed stenotic material to be removed
by operation of a vacuum or other means. Atherectomy, like angioplasty, has
shown to be an efficacious procedure when used for its intended purpose.
Stenotic segments, however, can occur in tissue where neither
angioplasty nor traditional atherectomy techniques are efficacious, for
example the development of stenotic segments within those vessels that are
internal to the various organs of the body presents special problems which
may not be entirely addressed by traditional angioplasty and atherectomy
1o procedures. Specifically, it is not uncommon for stenotic segments to
accumulate within the internal vessels of the heart. Because these vessels
provide blood and oxygen to the myocardial tissue, occlusions which develop
within these internal vessels may present a serious risk to the health of the
involved patient. As indicated above, the size and location of many of these
15 vessels, makes treatment with traditional methods and devices, such as
angioplasty and atherectomy, difficult and generally ineffective, if not
impossible. In such instances neovascularization may be required.
Neovascularization is a third technique which, like angioplasty and
atherectomy, may be used to treat conditions resulting from blocked or
2o occluded arterial vessels. Functionally, neovascularization involves the
creation of new pathways for the flow of blood within the internal tissues of
an
organ. Typically, the neovascularization technique is performed by boring, or
cutting, new vessels within the internal tissues of an organ. Each new vessel
is connected to an existing vessel, allowing blood passing through the
25 existing vessel to pass through the new vessel to oxygenate and nourish
adjacent tissues. Generally, a neovascularization procedure, which may be
used singularly, or in conjunction with more traditional techniques, such as
2
CA 02209367 1997-07-03
angioplasty and atherectomy, is a highly effective technique for reducing the
harmful effects associated with occlusion of arterial vessels.
In light of the above, it is an object of the present invention to provide a
device and method for neovascularization of the cardiac muscle. Another
object of the present invention is to provide a device and method for
neovascularization which may be used in combination with traditional
techniques, such as angioplasty. Yet another object of the present invention
is to provide a device for neovascularization which is relatively simple to
manufacture, easy to use, and comparatively cost effective.
SUMMARY OF THE PREFERRED EMBODIMENTS
The present invention is a device and method for neovascularization of
the cardiac muscle. Structurally, the present invention includes a positioning
catheter formed with a deployment lumen and an inflation lumen. The
deployment lumen passes from the proximal end of the positioning catheter
and terminates in a specially formed orifice positioned near, and slightly
proximal to, the catheter's distal end. The orifice is directed radially
outward
and distally from the positioning catheter. The inflation lumen also passes
from the proximal end of the positioning catheter. Unlike the deployment
lumen, however, the inflation lumen connects with the catheter's distal end.
Preferably, the positioning catheter is formed from a resilient and flexible
material.
An inflatable balloon is attached to the distal end of the positioning
catheter. Generally, the balloon may be of any type which is adaptable for
inflation within the arterial vessels. Preferably, however, the balloon is an
inflatable angioplasty-type balloon. The balloon is connected in fluid
communication with the inflation lumen of the positioning catheter. As a
3
CA 02209367 1997-07-03
result, fluid may be passed through the inflation lumen to selectively
inflate,
or deflate, the balloon.
Alternatively, a. cylindrical sleeve may be attached to the distal end of
the positioning catheter in place of the inflatable balloon. The sleeve is
preferably formed from a wire mesh and has a distal end and a proximal end,
with the proximal end of the sleeve attached to the distal end of the
catheter.
An actuator wire is attached to the distal end of the sleeve and is passed
through the positioning catheter. Functionally, the actuator wire may be
withdrawn through the positioning catheter, forcing the distal end of the
1o cylindrical sleeve to move translationally in a proximal direction towards
the
distal end of the positioning catheter. The resulting compressive force
applied
to the cylindrical sleeve causes the sleeve to expand radially outward. Thus,
the cylindrical sleeve, like an inflatable balloon which is also usable for
the
present invention, provides a selectively expandable device which may be
used to anchor the distal end of the positioning catheter at a particular
location within a vessel.
The present invention also includes a cutting catheter. The cutting
catheter has a proximal end and a distal end, with a cutting element mounted
on the distal end which is pointed or otherwise sharpened for incising and
2o dilating the tissue of the cardiac muscle. Preferably, the cutting element
of
the present invention includes a plurality of cutting blades, each of which is
attached to the distal end of the cutting catheter. The blades are distributed
radially around the cutting catheter and aligned with the longitudinal axis of
the cutting catheter. The blades may be fixedly attached to the surface of the
positioning catheter or each blade may be retractable into the cutting
catheter. In cases where the blades are retractable, each blade is spring-
loaded, or otherwise biased, to preferentially move from a first position
where
4
CA 02209367 1997-07-03
the blade is substantially contained within the cutting catheter to a second
position where the blade extends from the surface of the cutting catheter.
The cutting catheter may also be formed to have a guidewire lumen
and the present invention may include a cutting guidewire which is insertable
through the guidewire lumen. Generally, a cutting guidewire of this type will
be formed from a resilient and flexible metal, such as stainless steel, and
have a sharpened distal end. The guidewire is insertable through the
guidewire lumen allowing the sharpened distal end of the guidewire to be
selectively extended from the distal end of the cutting catheter.
Operationally, the positioning catheter is first advanced into one of the
arteries which supplies blood to the cardiac muscle. The advancement of the
positioning catheter continues until the distal end of the positioning
catheter
is located within boundaries of the heart itself and the orifice of the
positioning catheter is located adjacent to the site where a new perfusion
channel is to be formed.
With the positioning catheter positioned at the proper location, fluid is
passed through the inflation lumen to expand the inflatable balloon. The
expanded balloon contacts the artery surrounding the positioning catheter,
anchoring the distal end of the positioning catheter at a fixed location
within
2o the artery. After the inflatable balloon has expanded to anchor the
positioning
catheter, the cutting catheter is inserted into the deployment lumen.
Insertion
of the cutting catheter into the deployment lumen causes each of the blades
to adopt the first position where the blade is substantially contained in the
cutting catheter. The cutting catheter is then advanced through the
deployment lumen and the advancement of the cutting catheter causes the
distal end of the cutting catheter to project from the orifice formed near the
positioning catheter's distal end. As the distal end of the cutting catheter
5
CA 02209367 1997-07-03
emerges from the orifice, the spring-loaded blades adopt the second position
where the blades extend from the surface of the cutting catheter. Further
advancement of the: cutting catheter forces the pointed distal end of the
cutting catheter to bore a channel through the myocardial tissue. The boring
of the channel is aided by the blades which incise the myocardial tissue to
accommodate the advancing cutting catheter.
At any time during advancement of the cutting catheter, the cutting
guidewire may be advanced through the guidewire lumen of the cutting
catheter. Advancement of the cutting guidewire selectively extends the
sharpened distal end of the guidewire from the distal end of the cutting
catheter boring a path, or pilot hole, for subsequent advancement of the
cutting catheter. The process of alternately advancing the cutting guidewire
and cutting catheter may be repeated until one or more channels through the
myocardial tissue have reached the desired depth.
i5 Once the cutting catheter has been fully advanced, the cutting catheter
may be removed from the patient and removed from the positioning catheter.
In some cases it will be preferable to position a vascular stent at the
junction
between the involved artery and the newly created perfusion channel. In such
cases a self-expanding stent may be advanced through the deployment
lumen to be emitted at the orifice formed near the positioning catheter's
distal
end. As the stent leaves the orifice, it may be expanded to support the newly
formed perfusion channel.
BRIEF DESCRIPTION OF THE DRAWINGS
The novel features of this invention, as well as the invention itself, both
as to its structure and its operation, will be best understood from the
accompanying drawings, taken in conjunction with the accompanying
6
CA 02209367 1997-07-03
description, in which similar reference characters refer to similar parts, and
in
which:
Figure 1 is an :isometric view of the neovascularization device of the
present invention;
Figure 2 is a cross-sectional view of the distal portion of the
positioning catheter of the present invention as seen along the line 2 - 2 in
Figure 1 with the cutting catheter withdrawn and held within the positioning
catheter;
Figure 3 is a cross-sectional view of the positioning catheter of the
present invention, as shown in Figure 2,.with the inflatable balloon shown in
an expanded configuration and the cutting catheter advanced to project from
the positioning catheter;
Figure 4 is a cross-sectional view of the distal portion of the cutting
catheter as seen along the line 4-4 in Figure 1 with the blades shown in a
retracted position;
Figure 5 is a cross-sectional view of the cutting catheter as shown in
Figure 4 with the blades now shown in an extended position;
Figure 6 is a plan view of the distal portion of an alternate embodiment
for the cutting catheter of the present invention;
Figure 7 is a cross-sectional view of the distal portion of an alternate
embodiment of the positioning catheter of the present invention as would be
seen along a line corresponding to the line 2 - 2 in Figure 1;
Figure 8 is a cross-sectional view of an alternate embodiment of the
positioning catheter of the present invention, as shown in Figure 7, with the
cylindrical sleeve shown in an expanded configuration and the cutting
catheter advanced to project from the positioning catheter; and
7
CA 02209367 1997-07-03
Figure 9 is a plan view of the neovascularization device shown
operationally positioned within a cardiac vessel after establishment of an
exemplary perfusion channel.
DESCRIPTION OF THE PREFERRED EMBODIMENT
The present invention is a device and method for neovascularization of
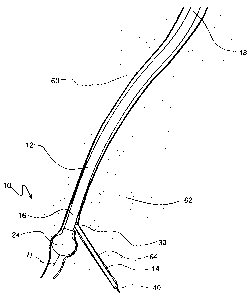
the cardiac muscle. Referring initially to Figure 1, the device of the present
invention is shown and generally designated 10. In general terms, it may be
seen that the device 10 includes a positioning guidewire 11, a positioning
catheter 12 and a cutting catheter 14. Structurally, the positioning guidewire
io 11 extends through a positioning guidewire lumen 15 in the positioning
catheter 12.
The positioning catheter 12 is formed to have a cylindrical or otherwise
elongated shape and has a distal end 16 and a proximal end 18. Preferably,
the positioning catheter 12 is formed from a flexible and somewhat stiff
material. The cutting catheter 14 is also formed to have a cylindrical or
otherwise elongated shape and has a sharpened or pointed distal end 20.
Preferably, the cutting catheter 14 is formed from a flexible and somewhat
resilient material. A series of blades 22, of which blade 22a and 22b are
representative, are mounted radially around the pointed distal end 20 of the
cutting catheter 14. Figure 1 also shows that an inflatable balloon 24 is
mounted to the distal end 16 of the positioning catheter 12.
The structural details of the present invention may be better
appreciated by reference to Figures 2 and 3 where it may be seen that the
positioning catheter 12 is formed to surround an inflation lumen 26 and a
deployment lumen 28. The inflation lumen 26 passes between the distal end
16 and the proximal end 18 (shown in Figure 1 ) of the positioning catheter
8
CA 02209367 1997-07-03
12. At the distal end 16 of the positioning catheter 12, the inflation lumen
26
is connected in fluid communication to an inflatable balloon 24. As a result,
fluid may be passed through the inflation lumen 26 from a pressurized fluid
source (not shown) to selectively inflate the balloon 24. Inflation of this
nature
may be appreciated by comparison of Figure 2, where the balloon 24 is
shown in an uninflated state, and Figure 3, where the balloon 24 has been
partially inflated.
As also shown in Figures 2 and 3, the deployment lumen 28 passes
between the proximal end 18 of the positioning catheter 12 and a specially
1o formed orifice 30. The orifice 30 is positioned near the distal end 16 of
the
positioning catheter 12 and oriented radially outward and distally from the
positioning catheter 12. Importantly, as seen in Figures 2 and 3, the cutting
catheter 14 may be advanced through the deployment lumen 28. Continued
advancement of the cutting catheter 14 in this fashion results in the
projection
of the pointed distal end 20 of the cutting catheter 14 from the orifice 30.
Advancement of this nature may be appreciated by comparison of Figures 2
and 3. In more detail, it may be seen in Figure 2 that the cutting catheter 14
is
fully contained within the deployment lumen 28. In Figure 3, however, the
cutting catheter 14 has been advanced to project the distal end 20 of the
2o cutting catheter 14 from the orifice 30. The shape and orientation of the
orifice 30 directs the cutting catheter 14 in a general direction which is
radially outward and distally forward from the positioning catheter 12. It may
be appreciated that the cutting catheter- 14 may be advanced more or less
than the advancement shown in Figure 3. In this fashion, the distal end 20 of
the cutting catheter 14 may be projected a variable and selectable distance
from the positioning catheter 12. Importantly, projection of the cutting
catheter
14 from the positioning catheter 12 may be followed by subject withdrawal of
9
CA 02209367 1997-07-03
the cutting catheter 14 into the deployment lumen 28 of the positioning
catheter 12.
The structural; details of the cutting catheter 14 may be better
appreciated by reference to Figures 4 and 5. More specifically, it may be
seen that the distal end 20 of the cutting catheter 14 is preferably formed to
surround a hollow chamber 32. A spring carrier 34 is positioned inside the
hollow chamber 32 and forms the mounting point for each of the blades 22.
The spring carrier 34 is attached to a projection 36 which is attached to the
cutting catheter 14. Functionally, the combination of the chamber 32, spring
i0 carrier 34 and projection 36 allows each of the blades 22 to move between a
first position, shown in Figure 4, where the blade 22 is substantially
contained
within the chamber 32 and a second position, shown in Figure 5, where the
blade 22 projects radially from the surface of the cutting catheter 14.
Additionally, the spring carrier 34 is formed from a resilient material which
biases the blades 22 to preferentially adopt the second or extended position.
In this fashion, the blades 22 may be compressively retracted into the
chamber 32, as shown in Figure 4, to allow the cutting catheter 14 to advance
through the deployment lumen 28. When the distal end 20 of the cutting
catheter 14 is advanced to project from the orifice 30, however, the blades 22
2o expand to adopt the second, or extended position of Figure 5. Importantly,
each blade 22 is formed to include a sloping rear shoulder 38. The sloping
rear shoulder 38 is shaped and dimensioned to engage the orifice 30 when
the cutting catheter 14 is withdrawn into the deployment lumen 28. The
engagement between the sloping rear shoulder 38 and the orifice 30 applies
a force to each blade 22 causing the device to adopt the first position, shown
in Figure 4, where the blade 22 is substantially contained within the chamber
32.
CA 02209367 1997-07-03
Functionally, the cutting catheter 14 of Figures 4 and 5 provides a
combined incisor/dilator which is specifically adapted to be advanceable
through deployment )umen 28. It may be appreciated, however, that other
embodiments are possible for the cutting catheter 14. For example, in Figure
6 an alternate embodiment for the cutting catheter 14 is shown and
designated 14'. It may be seen that cutting catheter 14' is formed with a
pointed distal end 20' which is similar to the pointed distal end 20 utilized
by
cutting catheter 14. Cutting catheter 14' also includes a series of blades
22',
of which blades 22a' and 22b' are representative. In the case of cutting
catheter 14', however, blades 22' are fixed to distal end 20' and are not
retractable, as was the case with blades 22 of cutting catheter 14. Instead,
blades 22' are shaped and dimensioned to project from distal end 20' but not
to exceed the width of cutting catheter 14'. In this way cutting catheter 14'
may be advanced through deployment lumen 28 without danger of contact
between blades 22' and deployment lumen 28.
Referring again to Figures 2 and 3, it may be seen that the present
invention also includes a cutting guidewire 40. The cutting guidewire 40 has a
sharpened distal end 42 and is formed from a resilient and flexible material,
such as stainless steel. As shown in Figures 4 and 5, the cutting catheter 14
is formed to include a guidewire lumen 44 through which the cutting
guidewire 40 may be inserted allowing the distal end 42 of the cutting
guidewire 40 to be selectively extended from the distal end 20 of the cutting
catheter 14.
Alternate embodiments are also possible for the positioning catheter
12 of the present invention. Once such embodiment is shown in Figures 7
and 8 where an alternate embodiment for positioning catheter 12 is shown
and designated 12'. In general terms, it may be seen that positioning catheter
11
CA 02209367 1997-07-03
12' includes many of the structural elements of positioning catheter 12. In
the
case of positioning catheter 12', however, inflatable balloon 24 is omitted.
Instead, it may be seen that a cylindrical sleeve 46 is attached to the distal
end 16' of positioning catheter 12'. Cylindrical sleeve 46 is preferably
formed
from a wire mesh and has a distal end 48 and a proximal end 50. The
proximal end 50 of cylindrical sleeve 46 is attached to the distal end 16' of
positioning catheter 12'. A grommet 52, is attached to the distal end 48 of
cylindrical sleeve 46. Preferably, the grommet 52 is formed to allow for the
passage of fluid through the cylindrical sleeve 46. For example, in the case
of
the grommet 52 shown in Figures 7 and 8, there are a series of holes, or
ports 54, through which fluid may pass.
Continuing with Figures 7 and 8, it may be seen that the alternate
embodiment for the positioning catheter 12' is formed to include an actuator
lumen 56 in place of the inflation lumen 26 of positioning catheter 14.
Additionally, it may be seen that an actuator wire 58 passes through the
actuator lumen 56 and connects to the grommet 52. The positioning
guidewire 11' extends through the positioning guidewire lumen 15' in the
actuato r wi re 58.
Importantly, the actuator wire 58 is movable in translation within the
actuator lumen 56. As a result, the actuator wire 58 may be manipulated to
cause the grommet 52 to move translationally in line with the longitudinal
axis
of the positioning catheter 12'. Translational movement of the grommet 52 is
accompanied, of course, by an equivalent translational movement of the
distal end 48 of the cylindrical sleeve 46. In this fashion, the actuator wire
58
may be manipulated to move the distal end 48 of the cylindrical sleeve 46
translationally towards, or translationally away from, the distal end 16 of
the
positioning catheter 12. Movement of this type may be visualized by
12
CA 02209367 1997-07-03
comparison of Figure 7 and Figure 8. In particular, it may be seen in Figure 8
that cylindrical sleeve 46 has a shorter overall length than cylindrical
sleeve
46 shown in Figure 7.;
Comparison of Figures 7 and 8 also shows that the decrease in
overall length of the cylindrical sleeve 46, as shown in Figure 8, is
accompanied by a corresponding increase in the overall width of the
cylindrical sleeve 46. Alternatively stated, it may be appreciated that the
translational movement of the distal end 48 of the cylindrical sleeve 46
towards the distal end 16 of the positioning catheter 12 has compressively
1o expanded the cylindrical sleeve 46 of Figure 8. In this fashion, the
actuator
wire 58 may be manipulated to selectively expand the cylindrical sleeve 46.
OPERATION
Operation of the present invention, as best appreciated by reference to
Figure 9, begins with insertion of the positioning guidewire 11 into an
arterial
vessel 60. Generally, the particular arterial vessel 60 chosen will be one
that
terminates within the myocardial tissue 62 and will generally be connected to
a number of smaller vessels (not shown) some of which may be partially or
fully occluded. Next, the positioning catheter 12 is inserted into the
arterial
vessel 60 over the positioning guidewire 11. The insertion, or advancement,
of the positioning catheter 12 will continue until the distal end 16 and
orifice
of the positioning catheter 12 ace adjacent to a target area where a
perfusion channel is to be established.
Once the positioning catheter 12 is properly positioned, fluid is
supplied under pressure through the inflation lumen 26 to inflate the balloon
25 24. For the purposes of the present invention, numerous devices (not shown)
may be adapted to function as a source of fluid pressure. For example, many
13
CA 02209367 1997-07-03
types of fluid pumps and syringes may be utilized. Regardless of the type of
device which is used to supply fluid pressure through the inflation lumen 26,
the resulting expansion of the inflating balloon 24 functions to anchor the
distal end 16 of the positioning catheter in the vessel 60.
Once the positioning catheter 12 has been anchored using the
inflatable balloon 24, the cutting catheter 14 may be advanced through the
deployment lumen 28. As discussed previously, advancement of the cutting
catheter 14 through the deployment lumen 28 causes the distal end 20 of the
cutting catheter 14 to be projected from the orifice 30 of the positioning
catheter 12. As the cutting catheter 14 is projected from the orifice 30, the
distal end 20 of the cutting catheter 14 cuts a perfusion channel 64 in the
myocardial tissue 62 surrounding the vessel 60. The cutting of the perfusion
channel 64 is aided by the blades 22 which incise the myocardial tissue 62,
and by the pointed shape of the cutting catheter 14 which dilates the
myocardial tissue 62. Once the perfusion channel 64 has been established,
the cutting catheter 14 may be withdrawn from the deployment lumen 28.
Advancement of the cutting catheter 14 through the myocardial tissue
62 may be facilitated by use of the cutting guidewire 40. In more detail, it
may
be appreciated that by selectively extending the cutting guidewire 40 from the
2o cutting catheter 14, a pilot hole may be established through the myocardial
tissue 62. The cutting catheter 14 may then be advanced over the cutting
guidewire 40 to enlarge the pilot hole into the perfusion channel 64. The
process of advancing the cutting guidewire 40 followed by advancing the
cutting catheter 14 over the cutting guidewire 40 may be repeated until the
perfusion channel 64 or perfusion channels have reached the desire depth.
In some cases it may be desirable to deploy a stent, or other
prosthesis, to support the newly formed perfusion channel 64. In such cases,
14
CA 02209367 1997-07-03
the stent (not shown) may be advanced through the deployment lumen 28
and emitted through the orifice 30 to be position by any method well known in
the pertinent art. .
While the particular neovascularization catheter as herein shown and
disclosed in detail is fully capable of obtaining the objects and providing
the
advantages herein before stated, it is to be understood that it is merely
illustrative of the presently preferred embodiments of the invention and that
no limitations are intended to the details of construction or design herein
shown other than as described in the appended claims.
15