Note: Descriptions are shown in the official language in which they were submitted.
1-20951/A CA 022100~4 1997-07-10
Process for trichro,lldlic dyeing or printing
The present invention relates to a process for dyeing or printing natural or synthetic
polyamide fibre materials by the trichromatic technique.
It is the object of this invention to provide a process for dyeing or printing natural or
synthetic polyamide fibre "laterials with dyes suitable for combination dyeing by the
lrichrollldlic techn:;ue.
It has now been found that this object can be achieved by the inventive process described
hereind~ler. The dyeings so obtained meet the above requ;, e, l ,enls and are distinguished in
particular by a level colour build-up, while at the same time having consistency of shade at
dirrefehl concenl,dlions as well as good co"lpalibilily.
Accordingly, this invention relates to a process for dyeing or printing natural or synthetic
polyamide fibre ,lldl~:rial by the trichromatic dyeing technique, which comprises using
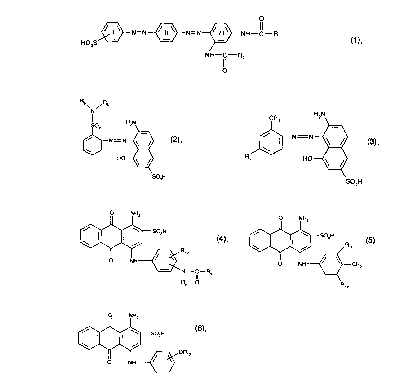
at least one yellow- or orange-dyeing dye of formula (1 )
,~N=N~N=N~NH--C--R
HO3S )~/ (1 )
o
wherein
R, and R2 are each independently of the other C1-C4alkyl or C,-C4alkoxy, each of which is
unsuhstituted or suhstituted by hydroxy, C,-C4alkoxy or halogen;
phenyl or phenoxy, each of which is unsubstituted or substih~ted by C,-C4alkyl, C1-C4alkoxy
or halogen; or
a radical of formula -N(R3)R4, wherein R3 and R4 are each independently of the other
hydrogen, C,-C4alkyl, or C2-C4alkanoyl which may be further suhstituted in the alkyl moiety
by hydroxy or C,-C4alkoxy; and
the benzene rings 1, ll and lll of the dyes of formula (1~ may be further substituted by C,-C4-
alkyl, C,-C4alkoxy, C2-C4alkanoylamino, ureido or halogen;
CA 02210054 1997-07-10
together with at least one red-dyeing dye of formulae (2) and (3)
~ / 6
N
~N=I'I~ (2),
S03H
wherein R5 is C,-C6alkyl, phenyl or cyclohexyl, and R6 is C1-C6alkyl, or
R5 and R6, together with the linking nitrogen atom, form an azepinyl ring
~ H~
R7 H0~
S03H
wherein R7 is hydrogen, halogen or C2-C4alkanoylamino;
and together with at least one of the blue-dyeing dyes of formulae (4), (5) and (6)
8 NH2
N--C--Rg
RE, o
wherein R8 is hydrogen or C,-C4alkyl, and
Rg is C1-C4alkyl or C~-C4alkoxy, each of which is unsubstituted or s~ ~hstituted by hydroxy, C~-
C4-alkoxy or halogen; or amino, and
R~o is hydrogen, C,-C4alkyl or C,-C4alkoxy
CA 022100~4 1997-07-10
O NH
~3~ R" (5)
~ NH~CH3
wl,erei,l R" is hydrogen or methyl, and R12 is a C2 4hydroxyalkylsulphamoyl radical
~ (6),
wherein R,3 is C,-C4alkyl.
By the Irich~llldlic techn-.que is meant the additive blending of suitably chosen yellow or
orange, red or blue-dyeing dyes with which each desired shade of the visible colour
spectrum may be ~-ljusted by appropridle choice of the quantity ratios.
C,-C4Alkyl R" R2, R3, R4, R8, Rg, R,O, R,3 and the C,-C4alkyl substituent of the benzene rings
1, 11 and 111 of the dyes of formula (1) are each independenlly of one another typically methyl,
ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl or isobutyl, preferably methyl or ethyl. R"
R2 and Rg defined as C,-C4alkyl can be further suhstituted by hydroxy, halogen, e.g. bromo
or, pfe~erably, chloro, or in particular, by C1-C4alkoxy, e.g. ethoxy or, preferably, methoxy.
The i"ell,oxymethyl radical may be mentioned as an exd",ple of corresponding substituted
r~l C~15,
CA 022100~4 1997-07-10
- 4 -
R5 and R6 defined as C1-C6alkyl are suitably e.g. methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl, tert-butyl or straight-chain or branched pentyl or hexyl. Methyl and ethyl
are pr~ .ad. Methyl is particularly pre~er,ed.
C1-C~"~oxy R1, R2, Rg, R~o and the C1-C4alkoxy s~hstituent of the benzene rings 1, ll and lll
of the dyes of formula (1 ) are each independently of one another typically methoxy, ethoxy,
propoxy, isopropoxy, butoxy or isobutoxy"~re~rably methoxy or, more prefarably, ethoxy.
R1, R2 and R9 defined as C1-C4alkoxy can be further suhstltl~ed by hydroxy, halogen, e.g.
bromo or"Gr~rt:r~bly, chloro or, in particular, by C1-C4alkoxy, such as methoxy or ethoxy.
The cor.asponding unsuhstitl~ted radicals are prt~ r,ed.
R3 and R4 defined as C2-C4alkanoyl are each independently of the other suitably e.g.
propionyl or, prer~.ably, acetyl. R3 and R4 defined as C2-C4alkanoyl can be further sub-
stituted by hydroxy or, preferably, C1-C4alkoxy, typically methoxy or ethoxy.
R1 and R2 defined as phenyl or phenoxy can be, in addition to the cor.esponding unsub-
stituted radicals, those radicals which are substituted by C1-C4alkyl, e.g. methyl, ethyl,
propyl, isopropyl, butyl, sec-butyl, tert-butyl or isobutyl, by C1-C4alkoxy, e.g. methoxy,
ethoxy, propoxy, isopropoxy, butoxy or isobutoxy, or by halogen, typically fluoro, bromo or,
prerarably, chloro. The cvr-aspondi"g phenyl radicals are prere,-ed.
I lalogen R7 and the halogen substituent of the benzene rings 1, ll and lll of the dyes of
formula (1) are typically bromo or, pr~erably, chloro.
C2-C4Alkanoylamino R7 and the C2-C4alkanoylamino suhstitl~ent of the benzene rings 1, ll
and lll of the dyes of formula (1) are preferably acetylamino.
R5 and R6, rvr",ir,g an azepinyl ring together with the linking nitrogen atom, are a radical of
formula --N~ -
A C2 4hydroxyalkylsulphamoyl radical R12 is preferably a radical of formula
CA 022100~4 1997-07-10
-SO2-NH-CH2-CH2-OH .
Further possiLle substituents of the benzene rings 1, ll and lll of the dyes of formula (1) are
preterdbly C,-C4alkyl, C,-C4alkoxy, ureido or halogen, more preferably C,-C4alkyl, C,-C4-
alkoxy or ureido and, most preferably, C,-C4alkyl or C,-C4alkoxy. Particularly pr~atably, the
benzene rings 1, ll and lll of the dyes of formula (1) carry no further s~hstitl~ents.
Particularly i"leresli,lg dyes of formula (1 ) are those of formula
Ho3S~N=N~3N=N ~NH--C--R, (1 a),
NH~ R2
wherein R, and R2 have the meanings given above.
R3is preferably hydrogen or C,-C4alkyl, more pr~arably hydrogen.
R4is pre~erably hydrogen, C,-C4alkyl, or C2-C4al';al-oyl which may be further substituted in
the alkyl moiety by C,-C4alkoxy, more pre~erdbly hydlogen, or C2-C4alkanoyl which may be
further substitllted in the alkyl moiety by C,-C4alkoxy. R4is particularly preferably hydrogen.
R3 and R4 are very particularly preferably hydrogen.
R, and R2 are preferably each independently of the other C,-C4alkyl or C,-C4alkoxy which is
unsubstituted or substituted by hydroxy, C,-C4alkoxy or halogen; or a radical of formula
-N(R3)R4, wherein R3 and R4 have the meanings and prefened meanings given above. R3is
praferably hydrogen, and R4is pr~rably hydrogen or C2-C4alkanoyl which is further sub-
stituted in the alkyl moiety by C,-C4alkoxy and, particularly preferably, hydrogen.
R, and R2 are preferably each independently of the other C,-C4alkoxy; C,-C4alkyl which is
unsuhstit~lted or substituted by C,-C4alkoxy; or a radical of formula -N(R3)R4, wherein R3
and R4 have the meanings and prefer,ed meanings given above. R3is preferably hydrogen,
CA 022100~4 1997-07-10
and R4 is preferably hydrogen or C2-C4alkanoyl which is further sl~hstituted in the alkyl
moiety by C,-C4alkoxy and, particularly preferably, hydrogen.
R1 is particularly preferably C1-C4alkyl or C1-C4alkoxy, most preferably C1-C4alkoxy.
R1 is very particularly preferably methyl, ethyl, methoxy or ethoxy and, most preferably,
methoxy or ethoxy.
R2 is particularly prt:~rdbly C1-C4alkyl or amino and, most pr~erably, methyl, ethyl or
amino.
Particularly i"teresling dyes are those of formula (1), preferably those of formula (1 a),
wherein R1 is C1-Cialkyl or C1-C4alkoxy, prefe,dbly C1-C4alkoxy, and R2 is C1-C4alkyl or
amino.
Very particularly inlere:iling dyes are those of formula (1), preferably those of formula (1 a),
wherein R1 is methyl, ethyl, methoxy or ethoxy, prererably nletlloxy or ethoxy, and R2 is
methyl, ethyl or amino, preferably methyl or amino.
The sulfo group in the dyes of formula (1 ) and, in particular, in the dyes of formula (1 a) is
pre~erably bound in para-positon to the azo group.
In a pl~rer,ed embodiment of the process of this invention, the yellow- or orange-dyeing
dyes are a dye mixture consisting of
at least one yellow- or orange-dyeing dye of formula (1), and
at least one yellow- or orange-dyeing dye of formula (7)
NO2
HO35~NH~N=N~o--X (7)~
wherein
CA 022100~4 1997-07-10
R,4 is hydrogen, C1-C4alkyl, C,-C4alkoxy or halogen, and
X is hydrogen or C,-C4alkyl. In this case, the dyes of formula (1 a) are particularly interesting.
R,4 is preferably hydrogen, C,-C4alkyl, in particular methyl, or C,-C4alkoxy, preferably
thoxy. R,4 is particularly prererably hydrogen or C,-C4alkyl, in particular hydrogen or
methyl. R,4 is preferably bound in ortho-position to the azo group.
X is ple~erably hydrogen or methyl and, more preferably, hydrogen.
Those dyes of formula (7) are pr~:~rred, wherein R,4 is hydrogen or C,-C4alkyl, in particular
hydrogen or methyl, and X is hydrogen or methyl, preferdbly hydrogen.
The yellow- or orange-dyeing dyes der formula (7) are pre~erably a mixture of the dyes of
formulae
NO
~ 2 (8) and
HO3S~NH~N N~3OH
NO2
HO3S ~NH ~N N~OH (9),
wherein R,4 has the meanings and pre~er.ed meanings cited above. R,4 is preferably C,-
C4alkyl, more prer~r~bly methyl. R,4 is pr~r~rably bound in ortho-position to the azo group.
R5 is preferably phenyl or cyclohexyl, more preferably cyclohexyl.
R6 is preferably methyl or ethyl, more preferably methyl.
Rs and R6, together with the linking nitrogen atom, can form an azepinyl ring.
CA 022100~4 1997-07-10
R7 is preferably hydrogen, chloro or acetylamino, more preferably acetylamino.
R8 is preferably hydrogen.
Rg is pr~ferably methyl, ethyl; methyl or ethyl which are substitl~ted by methoxy or ethoxy, or
amino, more preferably methyl, ethyl or methoxymethyl and, most preferdbly, ethyl.
R~o is preferably hydrogen, methyl or methoxy, particularly prefer~bly hydrogen.
R11 is preferably methyl.
R,3 is preferably methyl or ethyl, particularly preferably methyl.
The red-dyeing dyes of formula (2) are pr~ferably those, wherein Rs is phenyl or cyclohexyl,
and R6 is methyl or ethyl, or wherein R5 and R6, together with the linking nitrogen atom, form
an azepinyl rlng.
The red-dyeing dyes of formula (2) are particularly preferably those, wherein R5 is cyclo-
hexyl and R6 is methyl, or wherein R5 and R6, together with the linking nitrogen atom, form
an azepinyl ring and, more preferably those, wherein R5 is cyclohexyl and R6 is methyl.
Red-dyeing dyes are preferably those of formula (2), which have the meanings andprefe"ed meanings cited above.
Blue-dyeing dyes of formula (4) are preferably those, wherein the radical of formula
-N(R8)-O-Rg is bound in meta- or para-position, preferably in meta-position, to the amino
group. In this case, R8 is preferdbly hydrogen, and Rg is methyl, ethyl, or methyl or ethyl
which is substituted by methoxy or ethoxy, or amino, more preferably methyl, ethyl or meth-
oxymethyl and, most prererably, ethyl. In this case, R~o is preferably hydrogen, methyl or
methoxy, particularly preferably hydrogen.
The blue-dyeing dyes of formula (5) are preferably those, wherein R,2 is a radical of formula
-SO2-NH-CH2-CH2-OH. In this case, R" is preferably hydrogen.
CA 022l00~4 l997-07-lO
The blue-dyeing dyes of formula (6) are preferdbly those, wherein R13 is methyl or ethyl
and, particularly preferably, methyl.
The blue-dyeing dyes preferably used for the inventive process are the dye of formula (4) or
a mixture of the dye of formula (4) with at least one dye of formulae (5) and (6). In another
prerer,ed embodiment of the inventive process, interesting blue-dyeing dyes are a mixture
of the dye of formula (5) with a dye of formula (6). Mixtures of the dye of formula (4) with a
dye of formula (5) are particularly i"leresling. In this case, those dyes of formulae (4), (5)
and (6) are particularly prel~r,ed, wherein R8 is hydrogen, Rg is methyl, ethyl, methoxy-
methyl or amino, R,0 is hydrogen, methyl or methoxy, R,2 is a radical of formula -SO2-NH-
CH2-CH2-OH, and R13 is methyl or ethyl.
A pre~er-ed embodiment of the inventive process is that, wherein
the red-dyeing dye is a dye of formula (2), wherein Rs is cyclohexyl and R6 is methyl, or Rs
and R6, together with the linking nitrogen atom, form an azepinyl ring, and
the blue-dyeing dye is a dye of formula (4) or, prererably, a mixture of the dye of formula (4)
with at least one dye of formulae (5) and (6), or a mixture of the dye of formula (5) with a
dye of formula (6), wherein R8 is hydrogen, Rg is methyl, ethyl, methoxymethyl or amino, R~o
is hydrogen, methyl or methoxy, R,2 is a radical of formula -SO2-NH-CH2-CH2-OH, and R,3 is
methyl or ethyl. In this case, the yellow- or orange-dyeing dyes of formula (1 ) or the mix-
tures of the dyes of formulae (1) and (7) have the meanings and prerer,ed meanings cited
above. The pr~fer-ed mixtures are those consi~Ling of at least one yellow- or orange-dyeing
dye of formula (1 a), wherein R, and R2 are each independently of the other C,-C4alkoxy;
unsl~hstituted or C,-C4alkoxy-sllhstituted C,-C4alkyl; or amino, and
at least one yellow- or orange-dyeing dye of formula (7), wherein R,4 is hydrogen or C,-C4-
alkyl, and X is hydrogen. The yellow- or orange-dyeing dyes of formula (7) are preferably a
mixture of the dyes of formulae (8) and (9), wherein R,4 is methyl. The red-dyeing dye is
p~rt ~dbly a dye of formula (2), wherein Rs is cyclohexyl, and R6 is methyl.
A very particularly pr~re-,ed embodiment of the inventive process is that, wherein
the red-dyeing dye is a dye of formula (2), wherein R5 is cyclohexyl, and R6 is methyl,
CA 022l00~4 l997-07-lO
- 10 -
the blue-dyeing dyes are a mixture of the dye of formula (4) with a dye of formula (5),
wherein R8 is hydrogen, Rg is methyl, ethyl, methoxymethyl or amino, R1o is hydrogen,
methyl or methoxy, and R,2 is a radical of formula -SO2-NH-CH2-CH2-OH, and
the yellow- or orange-dyeing dyes are a dye mixture consisting of at least one dye of
formula (1a), wherein R1 is C1-C4alkyl or, preferably, C1-C4alkoxy, and R2 is C1-C4alkyl or
amino, a dye of formula (8) and a dye of formula (9), wherein R14 is methyl.
This invention also relates to dye mixtures coi"prising at least one yellow- or orange-dyeing
dye of formula (1 ) together with at least one yellow- or orange-dyeing dye of formula (7).
The mixtures of the dyes of formula (1 ) and (7) and also the dyes of formulae (1 ) and (7)
have the meanings and prerer-ed meanings cited above.
The dye mixtures of the dyes of formulae (1 ) and (7) can be prepared, for example, by
mixing the individual dyes, typically in suitable mills, such as ball mills and pin mills, and
also in kneaders or mixers.
The dye mixtures can also be prepared e.g. by spray-drying the aqueous dye mixtures.
The dye mixtures preferably comprise 5 to 95 % by weight, more preferably 20 to 95 % by
weight and, particularly prererably, 40 to 95 % by weight, of a dye of formula (1 ), based on
the total amount of the dyes of formulae (1 ) and (7). The dye mixtures particularly prererdbly
comprise 50 to 90 % by weight, more preferably 60 to 90 % by weight, of a dye of formula
(1), based on the total amount of the dyes of formulae (1 ) and (7) .
This invention also relates to dyes of formula (4),
wherein R8 is hydrogen or C1-C4alkyl, and
Rg is C1-C4alkyl or C1-C4alkoxy, each of which is substituted by hydroxy or C1-C4alkoxy, and
R-o is C,-C4alkyl or C,-C4alkoxy.
The dyes of formulae (1), (2), (3), (4), (5), (6) and (7) employed in the novel process for
trichron,alic dyeing or printing are known or may be prepared in general analogy to known
dyes.
CA 022100~4 1997-07-10
- 11 -
The dyes of formula (1), for example, can be obtained by dia~ulising an amine of formula
HO3S~N=N~NH2 (10),
coupling the diazonium compounds so obtained to a coupling component of formula
~NH2
~ (11)
NH--r--R2
and reacting the reaction product so obtained with a compound introducing the radical of
formula
(12),
--~, R1
wherein R, and R2 have the meanings given for formula (1).
The dia~ulis~lion of the compound of formula (10) is ~r~cted in a manner known per se,
typically with a nitrite, for example with an alkali metal nitrite such as sodium nitrite, and in a
medium containing mineral acid, conven-Ehlly hydrochloric acid, in the temperature range
from e.g. -5 to 40~C, prefer~bly from 0 to 1 0~C.
The coupling of the coupling component of formula (11 ) is carried out in a manner known
per se in the acid, neutral or weakly alkaline pH range, typically at a pH from 3 to 7, and in
the temperature range from e.g. -5 to 30~C, preferably from O to 25~C.
To introduce the radical of formula (12) it is possible to use compounds of formula
CA 022l00~4 l997-07-lO
(13),
Hal--~ R1
wherein hal is halogen, such as chloro, bromo or iodo, preferably chloro. Illustrative
examples of compounds of formula (13) are acetyl chloride, propionyl chloride, methoxy-
acetyl chloride and ethyl chlorocarbonate. Further examples of compounds introducing the
radical of formula (12) are acetic anhydride and propionic anhydride.
The introduction of the radical of the above-mentioned formula (12) can be carried out, for
example, in dipolar aprotic solvents, typically dimethyl~ur",a"lide or dimethylsulfoxide, or in
water or, preferably, in pyridine, in the temperature range from e.g. 10 to 80~C"~re~erably
from 10 to 50~C.
The dyes of formula (7) may be obtained e.g. in accordance with the processes known from
US-A-4 060 383 and GB-A-1 454 475.
The dyes employed in the novel process for trichromatic dyeing or printing are either in the
form of their free sulfonic acids, or preferably, in the form of their salts.
Examples of suitable salts are the alkali metal salts, alkaline earth metal salts or ammonium
salts, or the salts of an organic amine. Typical examples are the sodium, lithium, potassium
or ammonium salts or the salt of mono-, di- or triethanolamine.
The dyes employed in the process of this invention may contain further auxiliaries, such as
sodium chloride or dextrin.
The lrichrolllalic dyeing or printing process of this invention is susceptible of applicz lion to
conventional dyeing or printing methods. In addition to containing water and the dyes, the
dye liquors or printing pastes may contain further auxiliaries, for example wetting agents,
antifoams, levelling agents or agents which influence the property of the textile materials,
e.g. softeners, flameproofing additives, or dirt, water and oil repellents, as well as water
softeners and natural or synthetic thickeners, e.g. alginates and cellulose ethers.
CA 022l00~4 l997-07-lO
- 13-
The novel process for trichrornalic dyeing or printing is also suit~hle for dyeing from short
liquors, e.g. in continuous dyeing or in batchwise or continuous foam dyeing processes.
The amounts in which the individual dyes are used in the dye liquors or printing pastes can
vary within wide limits, depending of the desired tinctorial strength. It has been found that
advantageous amounts are usually in the range from 0.01 to 15 % by weight, preferably
from 0.01 to 10 % by weight, based on the goods to be dyed or the printing paste.
Dyeing is ,cr~t~rdbly carried out at a pH in the range from 3 to 7, more pr~rerdbly from 4 to
7. The liquor ratio can be chosen from a wide range, typically from 1:5 to 1:50, prererably
from 1:5 to 1:30. Dyeing is preferably carried out in the temperature range from 70 to
1 1 0~C, more prererdbly from 80 to 1 05~C.
In trichromatic dyeing or printing, the dyes employed in the process of this invention are
distinguished by level colour build-up, good exhaustion properties, good consislency of
shade even at dirrerenl concenl,dlions, good fastness properties and solubility and, in-
particular, by very good co",palibility.
The novel process for l,ichro",dlic dyeing or printing is suitable for dyeing or printing natural
polyamide material, e.g. wool, and also, in particular, synthetic polamide material, e.g. poly-
amide 6 or polyamide 66, and is suitable for dyeing or printing wool and synthetic polyamide
blends and yarns.
This textile ",aterial may be in any form of presentation, e.g. in the form of fibre, yarn,
wovens or knits, and in particular, of carpets.
The dyeings obtained are level and have good allround fastness properties, in particular
good fastness to rubbing, wet treatment, wet rubbing and light.
In the r." .~;.,g Examples, parts are by weight and temperatures are given in degrees
Celsius. The relationship between parts by weight and parts by volume is the same as that
between the gram and the cubic centimetre.
CA 022l00~4 l997-07-lO
- 14-
Example 1: 1 part of a levelling agent (based on the condensate of a higher aliphatic amine
and ethylene oxide) is added to 2000 parts of demineralised water at room temperature.
This bath is then adjusted to pH 6 with 4 parts of sodium dihydrogen phosphate and
0.6 part of disodium hydrogen phosphale. To this bath is then added a mixture of 0.016 part
of a dye which, in the form of the free acid, corresponds to the compound of formula
NO2
HO3S~NH ~3N: N ~OH (101),
H3C
0.016 part of a dye which, in the form of the free acid, corresponds to the compound of
formula
NO2
HO3S~NH~N=N ~OH (102),
0.128 part of a dye which, in the form of the free acid, cor-esponds to the compound of
formula
HO3S~N=N~N=N~NH--C--O-CH3
~/ (103),
NH--ICl--CH3
o
0.09 part of a dye which, in the form of the free acid, corresponds to the compound of
formula
CA 022l0054 l997-07-lO
- 15-
N--CH3
(1 04),
' ~2 H2N
[~N~
SO3H
0.033 part of a dye which, in the form of the free acid, co,lesponds to the compound of
formula
O NH2
~"SO3H (105),
h~
O HN~,=~ 1~l
NH--C--CH2CH3
and
0.098 part of a dye which, in the form of the free acid, cor.esponds to the compound of
formula
O NH2
~,SO3H
~CH3 (106).
O HN~CH3
SO2NHCH2CH2CH
100 parts of polyamide 66 fibre r"aleriai (Helanca tricot) are put into the resultant dye
solution and the dyebath is heated over 45 minutes to c. 96~C. The dyebath is kept at this
temperature for 45 to 60 minutes and then cooled to 70~C. The dyed goods are removed
CA 022l00~4 l997-07-lO
- 16-
from the dyebath, rinsed with water and then dried, giving a fabric dyed in a beige-brown
shade. --
Examples 2 and 3: The procedure of Example 1 is repeated, but replacing the dye of
formula (103) with an equimolar amount of one of the dyes listed in the following Table 1,
column 2, to give a polyamide fabric likewise dyed in a beige-brown shade.
Table 1
Ex. Dye
Ho3S~N=N~3N=N~NH--C--O-CH2CH3
NH--C--CH3
o
Ho3S~N=N~3N=N~NH--C--CH3
NH--C--NH2
O
Examples 4 to 6: The procedure of Example 1 is repeated, but replacing the dye of formula
(104) with an equimolar amount of one of the dyes listed in the following Table 2, column 2,
to give a polyamide fabric likewise dyed in a beige-brown shade.
CA 02210054 1997-07-10
Table 2
Ex. Dye
N CH2CH3
4 ~~2 H2N
~3 r~
HO~
SO3H
~ ~2 H2N
>=~
[~N=N~
HO~
SO3H
CF3 H2N
~< >~
6 CH3~ HN~N=N~
O HO~
SO3H
Examples 7 and 8: The procedure of Example 1 is repeated, but replacing the dye of
formula (106) with an equimolar amount of one of the dyes listed in the following Table 3
column 2, to give a polyamide fabric likewise dyed in a beige-brown shade.
CA 022l0054 l997-07-lO
- 18-
Table 3
Ex. Dye
O NH2
~SO3H
O HN~CH3
SO2NHCH2CH2CH
O NH2
~,SO~,H
O HN~CH3
NHCOCH3
Exdlllpl~s 9 bis 13: The procedure of Example 1 is repeated, but replacing the dye of
formula (105) with an equimolar amount of the dyes listed in the following Table 4, column
2, to give a polyamide fabric likewise dyed in a beige-brown shade.
CA 02210054 1997-07-10
- 19-
Table 4
Ex. Dye
O NH2
~SO3H
O HN~
NHCOCH3
O NH2
0 ~SO3H
O HN~
NHCONH2
1 1
O HN~
NHCOCH20CH3
CA 02210054 1997-07-10
- 20 -
O NH2
O HN ~
NHCOCH3
O NH2
13 ~SO3H
O HN~3OCH3
Examples 14 to 26: The procedure of Examples 1 to 13 is repeated, but replacing the dyes
of formulae (101) and (102) with 0.032 part of the dye of formula (101), to give a polyamide
fabric likewise dyed in a beige-brown shade.