Note: Descriptions are shown in the official language in which they were submitted.
CA 02210224 1997-07-11
WO 96/21410 I'CT/GB96/00055
"SELF--ADHESIVE T.~MTN~E"
3 This invention relates to a laminate, and relates
4 particularly but not exclusively to a dressing which is
self-adherent to body surfaces around a wound.
7 In recent years, laminates for use as wound dressings
8 have become available which are based on plastics
9 films, commonly polyurethane films. The film may
contain or act as a carrier for substances which act as
11 bacteriostats or promote healing, for example, silver
12 salts or alginates.
13
14 Film dressings of this nature have considerable
advantages. Firstly, they can readily be produced in
16 transparent form and thus allow the condition of a
17 wound to be monitored without removing the dressing.
18 Secondly, they can be produced in micro-porous forms
19 which allow movement of water vapour to the exterior of
the dressing and movement of air to the interior of the
21 dressing while preventing movement of bacteria through
22 the dressing; this is of particular benefit in treating
23 exuding wounds such as burns.
24
To date, a disadvantage associated with known film
CA 02210224 1997-07-11
WO96/21410 PCT/GB96/00055
l dressings has been that they can be difficult to apply
2 to a wound. It is usual for a film dressing to have an
3 adhesive layer for application to the skin of a user,
4 the adhesive layer being initially covered by a release
paper. Once the release paper is removed from the
6 adhesive layer, the film is delicate and difficult to
7 handle. Stretching of the film can destroy its barrier
8 integrity, whilst a lack of tension during application
9 can lead to wrinkling. For these reasons, most film
dressings currently used are provided with a stiffening
ll edge or frame on the outer face, which stiffening must
12 be removed after the dressing has been applied. Known
13 arrangements of dressings can, however, be undesirably
14 complex to apply to a wound or other desired area.
16 According to a first aspect, the present invention
17 provides a laminate having an associated holding
18 portion, the laminate comprising a carrier sheet
19 sandwiched between a removable cover sheet and a
removable backing sheet, the laminate further
21 comprising a cover sheet tab attached to the cover
22 sheet and extending away from the carrier sheet, a
23 backing sheet tab attached to the backing sheet and
24 extending away from the carrier sheet and wherein the
holding portion is releasably connected to the carrier
26 sheet and is positioned so that it extends along a
27 substantially central portion of one edge of the
28 laminate.
29
The laminate may be provided as a dressing, for
31 example, a wound dressing, or a dressing intended to
32 release a medicament or therapeutic agent. In
33 particular the laminate may be suitable as a medium for
34 administration of an active ingredient in a controlled
release manner over a relatively long period of time
36 (eg several days to weeks). The active ingredient will
CA 022l0224 lgg7-o7-ll
WO96/21410 PCT/G~9G/00055
1 normally be suitable for transdermal a~; n; ~tration.
3 The cover sheet tab and backing sheet tab preferably
4 facilitate removal of the cover sheet and backing sheet
S respectively from the laminate to facilitate
6 application of the laminate to a wound or other area.
8 The holding portion is preferably attached to the
9 laminate at a substantially central portion of the edge
at which it is positioned and/or on each side of that
11 substantially central portion.
12
13 Preferably, the holding portion extends substantially
14 along one edge of the laminate. The holding portion
may be attached to the carrier sheet by means of one or
16 more lines of weakness which may be provided by
17 perforations. The holding portion may be a
18 continuation of the laminate.
19
The carrier sheet may be formed of a polymeric film
21 material. Preferably, the carrier sheet is
22 substantially porous to vapour and air but is
23 substantially impermeable to particles of dirt or other
24 contaminants, to micro-org~n; ~m~ and pathogens; the
carrier sheet may be comprised wholly or partially of
26 polyurethane or of compositions containing
27 polyurethane.
28
29 The carrier sheet may comprise (for example be
impregnated or coated with) a medicament or a
31 therapeutic agent (for example, an antibiotic
~ 32 antiseptic, antibacterial, a bacteriostat), a substance
33 which is intended to promote healing (for example by
34 stimulation of cell growth) or by an absorbent. The
carrier sheet may be impregnated with a silver compound
36 or an alginate. The carrier sheet may be provided with
CA 02210224 1997-07-ll
PCT/GB9G/OOo~i!;
WO 96/21410
1 an absorbent island, for example an alginate pad, which
2 may cover the area to be treated.
4 A surface of the carrier sheet may be wholly or
partially coated with a pressure sensitive adhesive to
6 facilitate its attachment and retention to the area to
7 be treated. The pressure sensitive adhesive may be a
8 skin friendly acrylic adhesive.
The cover sheet tab may be provided by a tab secured to
11 the cover sheet. The cover sheet tab is preferably
12 positioned at the laminate adjacent to the holding
13 portion. The cover sheet tab may extend across the
14 width of the laminate and may be of a similar length to
the holding portion.
16
17 Positioning the cover sheet tab adjacent to the holding
18 portion may facilitate removal of the cover sheet from
19 the laminate in use.
21 Preferably, the holding portion can be held by the user
22 in one hand and the cover sheet tab is pulled away from
23 the holding portion (thus removing the cover sheet from
24 the laminate) by the user using his or her other hand.
26 The backing sheet tab may be provided as an extension
27 of the backing sheet; it may extend beyond a periphery
28 of the carrier layer of the laminate. The backing
29 sheet tab may extend substantially along one edge of
the laminate. Preferably, the backing sheet tab
31 extends along a side of the laminate which is opposite
32 the holding portion. The backing sheet tab may be
33 formed of a flexible tape, for example a polypropylene
34 tape; other self-adhesive pressure sensitive tapes are
also suitable.
36
CA 02210224 1997-07-11
WO96121410 PCT/GB96100055
1 The backing sheet may be secured to the laminate by
2 means of adhesion between the material of the backing
3 sheet and the material of the carrier sheet;
4 alternatively, it may be held on the carrier sheet by a
separate adhesive material.
7 The backing sheet is preferably affixed to the rear
8 face of the carrier sheet with an adhesiveness which is
9 less than the adhesiveness between the front face of
the carrier sheet and the cover sheet or, when the
11 laminate has been affixed to a surface, between the
12 carrier sheet and the surface (for example, where the
13 laminate is a dressing the surface will be the wound to
14 which it is applied).
16 The backing sheet may have instructions for use of the
17 laminate printed on it; it may have a pattern printed
18 on it.
19
One or more of the carrier sheet, the cover sheet and
21 the backing sheet may be translucent or substantially
22 transparent; this may facilitate visual alignment of
23 the laminate over a wound site when it is to be
24 applied. Where the carrier sheet is substantially
transparent, this may facilitate visual inspection of a
26 wound covered by the laminate, when the laminate is in
27 the form of a dressing, without necessitating removal
28 of the dressing from the wound.
29
The cover sheet tab and/or the backing sheet tab may be
31 coloured and/or have a pattern and/or indications
32 printed on it to identify the tab region to a user.
33
34 According to a second aspect, the present invention
provides a method of applying a laminate in accordance
36 with the first aspect of the invention, in which:
CA 02210224 1997-07-ll
WO96/21410 PCT/GB96/00055
1 a) a user grips the holding portion in one hand and
2 removes the cover sheet from the laminate by
3 holding the cover sheet tab and pulling the cover
4 sheet tab away from the holding portion to detach
the cover sheet from the carrier sheet;
6 b) the exposed surface of the carrier sheet is then
7 applied over a wound or other desired area using
8 the holding portion to grip and position the
g dressing;
c) the backing sheet is removed from the laminate by
11 gripping the backing sheet tab and pulling the
12 backing sheet tab away from the carrier layer to
13 detach the backing sheet from the carrier sheet;
14 and
d) the holding portion is then removed from the
16 positioned carrier sheet.
17
18 According to a third aspect, the present invention
19 provides a method of manufacturing a laminate in
accordance with the first aspect of the invention, said
21 method comprising the following steps:
22 a) providing a laminate having a carrier sheet
23 sandwiched between a cover sheet and a backing
24 sheet;
b) forming a backing sheet tab;
26 c) forming a cover sheet tab; and
27 d) providing said carrier sheet with an associated
28 holding portion.
29
Embodiments of the present invention will now be
31 described, by way of example only, with reference to
32 the accompanying drawings, of which:-
33
34 Fig. 1 is a plan view of a first embodiment of a
laminate in the form of a wound dressing embodying
36 the present invention, prior to use of the
CA 02210224 1997-07-11
PCT/~D3C~O&~
WO96/21410
1 dressing;
2 Fig. 2 is a cross-section of Fig. 1;
3 Fig. 3 is a cross-section of a second embodiment
4 of a laminate in the form of a wound dressing
embodying the present invention;
6 Fig. 4 is a cross-section of a third embodiment of
7 a laminate in the form of a wound dressing
8 embodying the present invention; and
g Fig. S is a cross-section of a fourth embodiment
of a wound dressing embodying the present
11 invention.
12
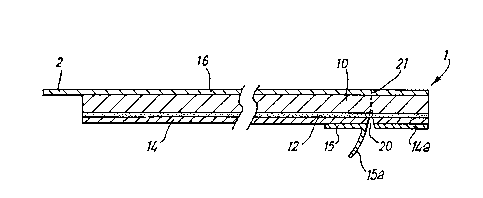
13 The laminate of the dressing 1 shown in Fig 1 comprises
14 a carrier sheet 10 in the form of a transparent and
permeable polyurethane film. A front face of the film
16 lo (the lower face as viewed in Fig 2) is coated with
17 an adhesive layer 12 which may be of any adhesive
18 material suitable for medical or veterinary use; in
19 this embodiment the material is a pressure-sensitive
solvent-based acrylic compound. The adhesive material
21 of the layer 12 and the polyurethane carrier sheet 10
22 may comprise a bacteriostatic silver compound such as a
23 soluble glass containing silver orthophosphate. The
24 layer 12 is covered by a cover sheet 14 which is a
transparent polymer sheet held in place by the adhesive
26 layer 12.
27
28 The right edge of the cover sheet 14 (as viewed in Fig
29 2) is provided on its outer edge with a holding portion
14a which is an extension of the laminate, releasably
31 connected to the carrier sheet 10. Immediately in from
~ 32 the holding portion 14a, the cover sheet 14 is also
33 provided with a pull tab 15 having a free-standing
34 extension flap 15a. The holding portion 14a extends
along a substantially central portion of the edge of
36 the dressing 1. The holding portion 14a may thus be
CA 02210224 1997-07-11
W096/21410 PCT/~ OQ~
1 held at the central portion of this edge to facilitate
2 handling of the dressing 1 and removal of the cover
3 sheet 14. In this embodiment, the holding portion 14a
4 extends along the entire length of the edge of the
dressing 1. The pull tab 15 extends across the entire
6 width of the dressing 1 adjacent to the holding portion
7 14a.
9 A rear face of the carrier sheet 10 (the upper face as
viewed in Fig 2) is covered by a backing sheet 16 which
11 adheres to the sheet 10 with an adhesiveness which is
12 lower than that between the sneet 10 and the layer 12.
13 The relatively lower tack between the rear face of the
14 carrier sheet 10 and the backing sheet 16 ensures that
when the dressing is applied, with the cover sheet 14
16 removed, from the front face of the sheet 10, to a
17 surface of a patient's body and the backing sheet 16 is
18 pulled, the backing sheet 16 separates from the sheet
19 10 while the relatively higher tack of the adhesive in
the layer 12 continues to hold the carrier sheet 10 on
21 to the body tissue of the patient. The backing sheet
22 16 is of a transparent polymer film, but other
23 materials such as siliconised paper may alternatively
24 be used for the backing sheet.
26 The laminate of the cover sheet 14, the carrier sheet
27 10 and the backing sheet 16 as described above is
28 available in manufactured strip form from, for example,
29 the Rexham division of Bowater plc.
31 One edge of the dressing 1 (the left edge as viewed in
32 Fig 2) is cut away through the cover sheet 14, the
33 adhesive layer 12 and the carrier sheet 10, but leaving
34 the backing sheet 16 intact across its original width.
This partially cut-away edge of the dressing 1 forms a
36 backing sheet tab 2. The backing sheet tab 2 is an
CA 022l0224 lgg7-o7-ll
WO96/21410 PCT/GB96/00055
1 extension of the backing sheet 16 and extends from the
2 backing sheet away from the carrier sheet 10. The
3 backing sheet tab 2 extends substantially along one
.,
4 edge of the dressing 1 opposite the edge provided with
the holding portion 14a.
7 A score line 20 is formed through the cover sheet 14
8 adjacent and parallel to the edge of the dressing 1
g between the holding tab 14a and the pull tab 15
(avoiding the pull tab extension flap 15a). A line of
11 perforations 21 is formed completely through the
12 dressing 1 below the score line 20 from the same
13 (lower) face of the dressing 1 as the score line 20 is
14 formed. It is to be noted that the relative
thicknesses of the various layers shown in the
16 accompanying drawings are not necessarily to scale.
17
18 In use, the dressing is supplied sterile in a sealed
19 pouch (not shown). At the time and place of use of the
dressing 1, the pouch is opened and the dressing 1 is
21 removed from the pouch. The dressing is grasped by the
22 holding portion 14a, and bent around the score line 20,
23 which raises an adjacent edge of the main portion of
24 the cover sheet 14 under the tab 15. The extension
flap 15a is gripped by the user who can then peel the
26 cover sheet 14 away from the adhesive layer 12 while
27 holding the tab 14a on the opposite edge.
28
29 Once the cover sheet 14 has been removed, the dressing
1 is then applied over the wound or other application
31 site and pressed into place on the body tissue around
32 the wound. During this operation, the backing sheet 16
33 maintains the carrier sheet 10 in a dimensionally
34 stable and readily handled condition, allowing the
dressing to be secured to the body tissue around the
36 wound or other application site without creasing or
CA 022l0224 1997-07-ll
WO96/21410 PCTIGB96/00055
1 stretching of the carrier sheet 10.
3 Thereafter, the backing sheet tab 2 is gripped by the
4 user and is used to peel the backing sheet 16 away from
the carrier sheet 10.
7 The holding portion 14a is then removed from the film
8 10 by tearing along the perforations 21 after
g application of the main portion of the dressing 1 to
the patient, the overlying edge of the carrier sheet 10
11 subsequently being smoothed down onto the body tissue
12 of the patient.
13
14 The carrier sheet 10 conforms well to the underlying
tissue surface because the dressing 1 is flexible in
16 two directions, ie length and width. The act of
17 peeling the cover sheet 14 away from the carrier sheet
18 10 automatically takes the user's hands away from the
19 face of the dressing 1 which will be applied to the
patient's tissue, thus avoiding potentially
21 contaminating contact to the wound. As an alternative
22 to removing the holding portion 14a by tearing along
23 the perforations 21, this edge of the dressing 1 could
24 be cut off with scissors after application of the main
portion of the dressing 1 to the patient.
26
27 The dressing 1 may be readily manufactured. It is
28 known to make film dressing material in bulk by
29 laminating polyurethane film with adhesive and release
layers. A dressing of the present invention can be
31 made by removing a strip of the carrier sheet 10,
32 adhesive layer 12 and cover sheet 14 along one edge of
33 the dressing 1 to form the backing sheet tab 2, cutting
34 the score line 20 and perforations 21 (for example y
using a rotary knife) and cutting the resulting strip
36 across its width at suitable intervals to form
CA 022l0224 lgg7-07-ll
WO96/21410 PCT/GB96/00055
l individual dressings.
3 An example of a bulk film dressing material consists of
4 a lamination of:-
~.
6 sPETl/PUf/PSA/sPET2
8 where:-
9 sPETl and sPET2 = siliconised polyethylene
terephthalate having different
ll silicone linearisations;
12
13 PUf = polyurethane film;
14 PSA = pressure sensitive adhesive.
16 The PUf (polyurethane film) may be impregnated with a
17 silver-releasing product, for example, a bacteriostat.
18
l9 A grid pattern (not illustrated) may be printed onto
the backing sheet 16 for monitoring wound size. After
2l application of the carrier sheet lO but prior to
22 removal of the backing sheet 16, the outline of the
23 wound can be traced onto the backing sheet. The area
24 of the wound can subsequently be calculated from the
outline on the grid, which is also suitable to be
26 retained as a record as it is not cont~;n~ted by
27 contact with the wound, having been spaced from the
28 wound by the intervening polyurethane carrier sheet lO.
29
Instructions for use of the dressing can be printed on
31 the tabs 2, 14a and 15, as can advertisements and/or
~ 32 other messages.
33
34 Islands of alginate or other absorbent materials can be
pre-located in the centre of the dressing l as an aid
36 to healing.
CA 022l0224 lgg7-o7-ll
WO96121410 PCT/GB96100055
1 The embodiments shown in Fig 3, Fig 4 and Fig 5 are
2 generally similar to that shown in Fig 1 and Fig 2.
3 For clarity, the layers of the laminate forming the
4 dressing are shown separated although, in practice,
adjacent layers are in contact.
7 In the embodiment of Fig 3, the backing sheet tab 2 is
8 extended by a distance x away from the carrier sheet 10
9 with respect to the previously described embodiment.
Increasing the distance that the backing sheet tab 2
11 extends away from the carrier sheet 10 may permit the
12 user to bend the tab 2 towards the body of the carrier
13 sheet 10 before beginning to peel the backing sheet 16
14 from the carrier sheet 10. This may make the backing
sheet 16 easier to peel away without lifting the
16 carrier sheet 10 from the surface to which it has been
17 applied.
18
19 In the embodiment of Fig 4, the backing sheet tab 2 is
formed from a tab member bend around, and secured to,
21 an extension 16a of the backing sheet 16. In this
22 embodiment, the tab 2 may be provided by a piece of
23 tape, for example, a polypropylene tape which may be
24 self adhesive for attachment to the backing sheet
extension 16a.
26
27 In the embodiment of Fig 5, the backing sheet tab 2 is
28 attached to a surface of the backing sheet extension
29 16a by means of a layer of adhesive 3. The tab 2 may
be provided in the form of a tape, for example, of
31 polypropylene.
32
33 As an alternative to a wound dressing, the invention
34 may be utilised as a transdermal medicament delivery
system, for example as nicotine supply patches, or, as
36 a non-medical application in the electronics industry.
CA 02210224 1997-07-11
WO96/21410 PCT/GB96/00055
13
1 The above described embodiments of the present
2 invention provide a dressing having a number of
3 advantages:
5 - the dressing is easy to apply;
6 - the dressing is protected against stretching
7 during application;
8 - the dressing is protected against creasing during
9 application;
10 - the dressing is not touched by the user during
11 application, reducing problems of contamination;
12 - the amount of waste material is reduced;
13 - the dressing conforms well to uneven surfaces as
14 it is flexible in two directions, ie length and
width;
16 - manufacture is simple and a wide range of sizes
17 can readily be produced; for example, the depth of
18 the score line once set provides accurately
19 repeatable performance;
20 - the whole of the dressing is transparent thus
21 allowing the user to see exactly where it is being
22 applied.