Note: Descriptions are shown in the official language in which they were submitted.
CA 02210273 2006-03-13
28976-298
-1-
Herbicidal compositions based otN-isoprppyl-N-(4-fluomphenyl,,2(5-
trifluoromethyl-1,3,4-thiadiazol-2-AoMLacetamide
The invention relates to novel synergistic herbicidal combinations of a known
hetaryloxyacetamide with other known herbicides belonging to other classes
which can be used particularly advantageously for selective weed control in a
variety of crop plants.
The patents listed below describe hetaryloxyacetamides which are particularly
active against monocotyledonous weeds (= grass weeds), but additionally also
act against some dicotyledonous weeds. They are virtually exclusively soil-
acting
and have little foliar activity, and some of them are very selective in mono-
and
dicotyledonous crop plants such as cereals, maize, rice, soybeans and cotton
[cf for example EP-A 5. 501 (= US 4 509 971 and US 4 833 243); EP-A 18 497
(= US 4 645 525 and US 4 756 741); EP-A 29 171 (= US 4 408 055);
EP-A 100 044 (= US 4 549 899); EP-A 100 045 (= US
4 540 430); EP-A 161 602 (= US 4 784 682); EP-A 195 237 (= US 4 788 291);
DE-A 3 724 467; EP-A 348 734 (= US 4 988 380); EP-A 348 737,(= US 4 968
342 and 5 090 991); 1)E-A 4 113 421; and WO 91/06544].
In addition, DE 4 223 465 describes synergistic mixtures of
hetaryloxyacetamides with N-phenylureas, N-benzothiazolylureas,
2,6-dinitroanilines, s-triazines, as-triazinones, sulfonylureas,
imidazolinones,
pyridinecarboxamides and diphenyl ethers. However, none of the mixtures
described therein has as yet achieved any practical significance. A
disadvantage
of the mixtures described in DE 4 223 465 is that their action is not always
distinct in case of strong overgrowth by a variety of weeds.
CA 02210273 2006-03-13
28976-298
-2-
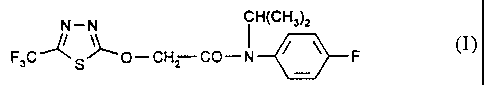
The present invention provides on the basis of N-isopropyl-
N-(4-fluorophenyl)(5-trifluoromethyl-1,3,4-thiadiazol-2-yloxy)acetamide of the
formula (I)
N - N CH(CH3)2
I
~
F3C S O-CH2 CO-N
novel synergistic herbicidal mixtures which are superior in their activity to
prior
art mixtures and additionally act against a broad spectrum of weeds.
This is achieved by combinations of known herbicides with the active compound
of the formula (I) surprisingly found, in biological tests, to show pronounced
synergistic effects with regard to activity against weeds and to be especially
advantageously useful as effective broad range combination products for the
selective
control of weeds - both of monocotyledonous and of dicotyledonous weeds by the
pre-
emergence 'and post-emergence method -- in monocotyledonous and dicotyledonous
crop
plants, such as, for example, maize, wheat, barley, rice, soybeans and
sunflowers,
allowing the effective control of a number of economically important (problem)
weeds
and grass weeds.
The present invention accordingly provides synergistic herbicidal
compositions,
characterized in that they comprise an effective amount of an active compound
combination of N-isopropyl-N-(4-fluorophenyl)(5-trifluoromethyl-1,3,4-
thiadiazol-2-yloxy)acetamide of the formula (I)
H(CH3)2
N-N CH(CH3)2
F3C' \S~O-CHZ CO-N (I)
and at least one herbicidally active compound of the formula (II) or (III)
(a)
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
-3-
ci o
0-- 0 ~ o O.C2H5
NO I i CH3
(II) fenoxaprop-ethyl
and/or
(b) 0 (III) clodinafop-propargyl
CI , F ~ O O
~ I ( i CH3
N O ~C
CH
The compound of the formula (I) is known from EP-A 348 737 and US
4 968 342.
F enoxaprop- ethyl is ethyl 2-[4-(6-chlorobenzoxazol-2-yl)oxyphenoxy]pro-
pionate, described in DE 2 640 730.
Clodinafop-propargyl is propinyl (R)-2-[4-[(5-chloro-3-fluoropyridin-2-yl)-
oxy]phenoxy]propionate, disclosed in EP-A 248 968.
Hetaryloxyacetamides including the active compound of the formula (I) are
particularly active against monocotyledonous weeds (= grass weeds), but
additionally also act against some dicotyledonous weeds.
The active compounds (II) and (III) mentioned can be used for the selective
control of a broad spectrum of grass weeds in economically important crop
plants such as, for example, cereals, maize, soybeans, cotton, beet and rice.
However, their activity against certain harmful monocotyledons is not always
satisfactory. Important problem weeds, such as, for example, Apera spica-
venti,
are often not sufficiently controlled.
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
-4-
Surprisingly, it has now been found that the active compound combinations of
the hetaryloxyacetamide of the formula (I) and the active compounds (II)
and/or (III) defined above have a particularly high activity and can be used
selectively in many crop plants.
Surprisingly, the herbicidal activity of the active compound combinations
according to the invention is considerably higher than the sum of the
activities
of the individual active compounds.
This means that not merely a complementation of action is present, but a true
synergistic effect, which was unforeseeable. The novel active compound
combinations are well tolerated by many crop plants, and even weeds which are
otherwise difficult to control, such as Apera spica-venti, are controlled well
by
the novel active compound combinations. The novel active compound
combinations are therefore a useful addition to the range of the selective
herbicides.
The active compound combinations according to the invention can be used for
example in connection with the following plants:
Dicotyledonous crop 12lants of the genera: Gossypium, Glycine, Beta, Daucus,
Phaseolus, Pisum, Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon,
Arachis, Brassica, Lactuca, Cucumis and Cucurbita.
Monocotyledonous weeds of the Wnera= Echinochloa, Setaria, Panicum,
Digitaria, Phleum, Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena,
Cyperus, Sorghum, Agropyron, Cynodon, Monochoria, F imbristylis, Sagittaria,
Eleocharis, Scirpus, Paspalum, Ischaemum, Sphenoclea, Dactyloctenium,
Agrostis, Alopecurus and Apera.
Monocotyledonous crop plants of the genera: Oryza, Zea, Triticum, Hordeum,
Avena, Secale, Sorghum, Panicum, Saccharum, Ananas, Asparagus and Allium.
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
-5-
However, the use of the active compound combinations according to the
invention is in no way restricted to these genera, but also extends in the
same
manner to other plants.
The synergistic effect of the active compound combinations according to the
invention is especially pronounced at specific concentration ratios. However,
the weight ratios of the active compounds in the active compound combinations
can be varied within relatively wide ranges. In general, 0.001 to 1000 parts
by
weight, preferably 0.01 to 100 parts by weight, especially preferably 0.1 to
30
parts by weight, of active compound of the formula (II) and/or (III) are used
per part by weight of active compound of the formula (I).
The active compounds or active compound combinations can be converted into
the customary formulations, such as solutions, emulsions, wettable powders,
suspensions, powders, dusts, pastes, soluble powders, granules, suspoemulsion
concentrates, natural and synthetic . materials impregnated with active
compound, and microencapsulations in polymeric substances.
These formulations are produced in a known manner, for example by mixing
the active compounds with extenders, that is liquid solvents and/or solid
carriers, optionally with the use of surfactants, that is emulsifiers and/or
dispersants and/or foam-formers.
If water is used as an extender, organic solvents can, for example, also be
used
as auxiliary solvents. Liquid solvents which are mainly suitable are:
aromatics
such as xylene, toluene or alkylnaphthalenes, chlorinated aromatics and
chlorinated aliphatic hydrocarbons such as chlorobenzenes, chloroethylenes or
methylene chloride, aliphatic hydrocarbons such as cyclohexane or paraffins,
for example petroleum fractions, mineral and vegetable oils, alcohols such as
butanol or glycol as well as their ethers and esters, ketones such as acetone,
methyl ethyl ketone, methyl isobutyl ketone or cyclohexanone, strongly polar
solvents such as dimethylformamide and dimethyl sulphoxide, and water.
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
-6-
Suitable solid carriers are: for example ammonium salts and ground natural
minerals such as kaolins, clays, talc, chalk, quartz, attapulgite,
montmorillonite
or diatomaceous earth, and ground synthetic minerals such as finely divided
silica, alumina and silicates; suitable solid carriers for granules are: for
example
crushed and fractionated natural rocks such as calcite, marble, pumice,
sepiolite
and dolomite, or else synthetic granules of inorganic and organic meals, and
granules of organic material such as sawdust, coconut shells, maize cobs and
tobacco stalks; suitable emulsifiers and/or foam formers are: for example non-
ionic and anionic emulsifiers, such as polyoxyethylene fatty acid esters,
polyoxyethylene fatty alcohol ethers, for example alkylaryl polyglycol ethers,
alkylsulfonates, alkyl sulfates, arylsulfonates and protein hydrolyzates;
suitable
dispersants are: for example lignin-sulphite waste liquors and
methylcellulose.
Adhesives such as carboxymethylcellulose and natural and synthetic polymers
in the form of powders, granules or latices such as gum arabic, polyvinyl
alcohol and polyvinyl acetate, or else natural phospholipids such as cephalins
and lecithins, and synthetic phospholipids can be used in the formulations.
Further additives can be mineral and vegetable oils.
It is possible to use colorants such as inorganic pigments, for example iron
oxide, titanium oxide and Prussian Blue, and organic dyestuffs such as
alizarin
dyestuffs, azo dyestuffs and metal phthalocyanine dyestuffs, and trace
nutrients
such as salts of iron, manganese, boron, copper, cobalt, molybdenum and zinc.
The formulations generally comprise between 0.1 and 95. percent by weight of
active compound, preferably between 0.5 and 90%.
In general, the active compound combinations according to the invention are
applied in the form of ready mixes. However, the active compounds in the
active compound combinations can also be formulated iridividually and mixed
upon application, that is to say applied in the form of tank mixes.
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
- 7-
The new active compound combinations as such or in the form of their
formulations can also be used as mixtures with further known herbicides,
finished formulations or tank mixes again being possible. Mixtures with other
known active compounds such as fungicides, insecticides, acaricides,
nematicides, bird repellants, growth promoters, plant nutrients and soil
conditioners, are also possible. Furthermore, it may be advantageous for
specific
purposes, in particular when using the post-emergence method, to incorporate
mineral or vegetable oils tolerated by plants (for example "Oleo Dupont 11E",
which is commercially available) or ammonium salts such as, for example,
ammonium sulfate or ammonium thiocyanate, as further additives in the
formulations.
The novel active compound combinations according to the invention can be
used as such, in the form of their formulations or in the use forms prepared
therefrom by further dilution, such as ready-to-use solutions, suspensions,
emulsions, powders, pastes and granules. They are used in the customary
manner, for example by watering, spraying, atomizing, dusting or spreading.
The rates of application of the active compound combinations according to the
invention can be varied within a certain range; they depend, inter alia, on
the
weather and on the condition of the soil. In general, the rates of application
are
between 0.01 and 10 kg per ha, preferably between 0.03 and 5 kg per ha, in
particular between 0.05 and 3.0 kg per ha.
The active compound combinations according to the invention can be applied
before
and after the emergence of the plants, i.e. by the pre-emergence and post-
emergence
method.
The good herbicidal activity of the novel active compound combinations is
evident from the examples below. While the individual active compounds show
weaknesses in their herbicidal activity, the combinations all exhibit very
efficient control of weeds, and this control exceeds a simple sum of the
activities.
In herbicides, a synergistic effect is always present when the herbicidal
activity
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
- 8-
of the active compound combination exceeds that of the active compounds
applied individually.
The expected activity for a given combination of two herbicides can be
calculated as follows (cf. Colby, S. R.; "Calculating synergistic and
antagonistic
responses of herbicide combinations", Weeds 15, pages 20-22, 1967):
If X=% damage by herbicide A (active compound of group 1) at
the rate of application of p kg/ha and
Y=% damage by herbicide B (active compound of group 2) at
the rate of application of q kg/ha and
E the expected damage caused by herbicides A and B at a
rate of application of p and q kg/ha,
then E= X+ Y_ (x.Y
100
If the actual damage exceeds the calculated value, the combination is super-
additive with regard to its activity, i.e. it shows a synergistic effect.
The examples below reveal that the herbicidal activity of the active compound
combinations according to the invention found exceeds the calculated value,
i.e.
that the novel active compound combinations have a synergistic action.
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
-9-
Use Examples
To prepare the active compound preparations required for the tests, suitable
amounts of a water-dispersible powder formulation (WP) of the
hetaryloxyacetamide of the formula (I) and a commercially available
formulation of the compounds (II) and/or (III), respectively, are weighed out
and diluted with water to the desired concentration; by mixing, various
combinations of the two active compounds were prepared.
A) Post-emergence tests/ greenhouse
Test plants which have a height of 5 to 15 cm are sprayed with the active
compound preparations in such a way as to apply the particular amounts
of active compound desired per unit area. The concentration of the spray
liquor is chosen such that the particular amounts of active compound
desired are applied in 500 1 of water per ha. After the treatment, the test
plants are kept in the greenhouse under controlled conditions
(temperature, atmospheric humidity, light) until the evaluation. After
three weeks, the degree of damage to the plants is rated in % damage in
comparison to the development of untreated control plants.
The figures denote:
0% = no action/damage (like untreated control)
100% = total destruction
Active compounds, application rates and results are listed in the tables
below.
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
- 10-
Table 1 Herbicidal activity of (I), clodinafop-propargyl and tank mixes of
(I) and clodinafop-propargyl against Apera spica-venti by the
post-emergence method
Preparation Application rate herbicidal activity in %
g of a.i./ha Apera spica-venti
found calc.
(I) 60 40
(I) 30 40
(I) 15 20
Clodinafop-propargyl 30 10
Clodinafop-propargyl 15 0
(I)+ 60
80 46
Clodinafop-propargyl + 30
(I) + 30
70 46
Clodinafop-propargyl + 30
(I) + 15
50 28
Clodinafop-propargyl + 30
(I) + 60
60 40
Clodinafop-propargyl + 15
(I) + 30
50 40
Clodinafop-propargyl + 15
(I) + 15
20
Clodinafop-propargyl + 15
CA 02210273 1997-07-14
Le A 31 773-Foreign countries
-I1-
Table 2 Herbicidal activity of (I), fenoxaprop-ethyl and tank mixes of (I)
and fenoxaprop-ethyl against Apera spica-venti by the post-
emergence method
Preparation Application rate herbicidal activity in
gofa.i./ha %
Apera spica-venti
found calc.
(1) 60 40
F enoxaprop-ethyl 30 20
F enoxaprop-ethyl 15 0
(I) + 60
60 52
F en oxaprop- ethyl + 30
(I) + 60
50 40
Fenoxaprop-ethyl + 15
Notes to Tables 1 and 2:
found = activity or damage (in percent) found;
calc. = activity or damage (in percent) calculated using the COLBY
formula above
a.i. = active ingredient
The compounds fenoxaprop-ethyl (II) und clodinafop-
propargyl (III) have been used in form of the following
commercially available formulations:
(II) as (R)RALON 060 EW (emulsion-in-water) (AgrEvo);
(III) as (R) TOPIK 240 EC (emulsion concentrate)(Novartis).