Note: Descriptions are shown in the official language in which they were submitted.
CA 02211412 1997-07-24
W 096/27704 PCT/~,GI~Cell
DRY ~T.~NTNG ~Y~l~ USTNG D~NSTFT~n ~R~ nT~T
~nn A sn~FACT~rr An TInNCT
F;~l~ o~ ~he Tnv~t; ~n
The invention pertains to a dry cleaning system utilizing
densified carbon dioxide and a surfactant adjunct. The
invention also pertains to a method of dry cleaning ~abrics
10 utilising densified carbon dioxide and a surfactant.
adjunct.
Rz~c!k~rolln~ of l-he Tnv~l~ n
Densified, particularly supercritical fluid, carbon dioxide
15 has been suggested as an alternative to halo-carbon
solvents used in conventional dry cleaning. For example, a
dry cleaning system in which chilled liquid carbon dioxide
is used to extract soils from fabrics is described in U.S.
4,012,194 issued to Maffei on March 15, 1977.
Supercritical fluid carbon dioxide provides a nontoxic,
inexpensive, recyclable and environmentally acceptable
solvent to remove soils in the dry cleaning process. The
solvent has been shown to be effective in removing nonpolar
25 stains such as motor oil, when combined with a viscous
cleaning solvent, particularly mineral oil or petrolatum as
described in US S/N 715,299, filed June 14, 1991, assigned
to The Clorox Company and corresponding to EP 518,653.
Supercritical fluid carbon dioxide has been combined with
30 other components, such as a source of hydrogen peroxide and
an organic bleach activator as described in US S/N 754,809,
filed September 4, 1991 and owned by The Clorox Company,
corresponding to EP 530,949.
35 The solvent power of densified carbon dioxide is low
relative to ordinary liquid solvents and the carbon dioxide
CA 02211412 1997-07-24
W 096/27704 PCTAEP96/00811
solvent alone is less effective on hydrophilic stains such
as grape juice, coffee and tea and on compound hydrophobic
stains such as lipstick and red candle wax, unless
surfactants and solvent modifiers are added.
A cleaning system combining particular anionic or nonionic
surface active agents with supercritical fluid CO2 is
described in DE 39 04 514 Al published August 23, 1990.
These anionic and nonionic agents, such as alkylbenzene
10 sulfates and sulfonates, ethoxylated alkyl phenols and
ethoxylated fatty alcohols, were particularly effective
when combined with a relatively large amount of water
(greater than or equal to 4~). The patented system appears
to combine the detergency mechanism of conventional agents
15 with the solvent power of supercritical fluid carbon
dioxide.
It has been observed that most commercially available
surfactants have little solubility in supercritical fluid
20 carbon dioxide as described in Consani, K.A., J. Sup .
Fluids, 1990 (3), pages 51-65. Moreover, it has been
observed that surfactants soluble in supercritical fluid
carbon dioxide become insoluble upon the addition of water.
No evidence for the formation of water-containing reversed
25 micelles with the surfactants was found. Consani supra.
Thus, the dry cleaning systems known in the art have merely
combined cleaning agents with various viscosities and
polarities with supercritical fluid CO2 generally with high
30 amounts of water as a cosolvent. The actives clean soils
as in conventional washing without any synergistic effect
with the CO2 solvent.
The formation of water-containing reversed micelles is
35 believed to be critical for the solubility and removal of
hydrophilic stains. Studies of the interaction of
, . . _ . _ . . . _ . . .. . . _ . . . . .. . . _ _ _ _ _ _
CA 02211412 1997-07-24
W O 96/~7704 ~ ; ~CT~hY~
~ .
sur~actants in supercritical carbon dioxide with water,
cosurfactants and cosolvents led to the conclusion that
most commercially available sur~actants are not designed
~or the formation of reversed micelles in supercritical
5 carbon dioxide as described in McFann, G., Dissertation,
University of Texas at Austin, pp. 216-306, 1993.
Therefore, the problem of developing an effective dry
cleaning system utilizing supercritical ~luid carbon
dioxide to clean a variety of consumer soils on fabrics has
10 remained unsolved until the present invention.
- :,
Sl1mm~ry of ~he Inv~nt;on
It is therefore an~?h~l~r~ of the present invention to
provide a dry cleaning system utilizing an environmentally
15 safe, nonpolar solvent ~Irh ~53 densified carbon dioxide,
which effectively removes a variety of soils on fabrics.
q,~
Another ~bjcc~ is the design of e~ective surfactants for
use in supercritical fluid carbon dioxide.
Another ~b3~c~ of the invention is to provide a dry
20 cleaning system o~ solvent, sur~actant, enzyme and bleach
for the total cleaning o~ fabrics using
densified/supercritical fluid carbon dioxide that gives
results equivalent to the cleaning demonstrated by
.-- ~ ~
-~- conventional dry cleaning solvents.
In one aspect of the present invention, the dry cleaning
system used for cleaning a variety o~ soiled fabrics
comprises densified carbon dioxide and C~ou~ 0.001~ to
C ~ 5~ of a surfactant in supercritical fluid carbon
30 dioxide. The surfactant has a supercritical ~luid C02-
philic functional moiety connected to a supercritical fluid
C02-phobic functional moiety. Preferred C0z-philic moieties
o~ the surfactant include halocarbons such as
fluorocarbons, chlorocarbons and mixed fluoro-
35 chlorocarbo~s, polysiloxanes, and branched polyal]cyleneoxides. The C02-phobic groups for the surfactant contain
hMEN~E~ SHEET
CA 02211412 1997-07-24
W O 96127704 ;~ ~ ', ~ T~s~
preferably polyalkylene oxides, carboxylates, C130 alkyl
sulfonates, carbohydrates, glycerates, phosphates, sulfates
and Cl30 hydrocarbons.
5 The dry cleaning system may also be designed to include a
modifier, such as water, or an organic solvent up to only
about 5~ by volume; enzymes up to about 10 wt.~ and a
bleaching agent such as a peracid.
10 In a second aspect o~ the invention, a method for dry
cleaning a variety of soiled fabrics is provided wherein a ~;
selected surfactant and optionally a modifier, an enzyme,
bleaching agent or mixtures thereof are combined and the
cloth is contacted with the mixture. Densified carbon~5 dioxide is introduce~ into a cl~aning ves~el which is then
.C 4-~3x1~3K~J, ~ ~-4_ 10~'')
pressurlzed ~rom about~700 psi~ to a~out~ ,000 psi~ and
heated to a range of about 20~C to about 100~C. Fresh
densified carbon dioxide is used to flush the cleaning
vessel.
Rrief DescriDtion of the Drawing
Figure l is a diagrammatic flow chart o~ the supercritical
fluid carbon dioxide dry cleaning process according to the
invention. ~-~~
Det~;le~ Descr;~t;o~ of Preferre~ ~mho~;m~nts
The invention provides a dry cleaning system which replaces
conventional solvents with densi~ied carbon dioxide in
combination with selected cleaning surfactants.
30 Optionally, modifiers, enzymes, bleaching agents and
mixtures thereof are combined with the solvent and
surfactant to provide a total cleaning system.
For purposes of the invention, the following definitions
35 are used:
'4P~/\J~ED S~lEEr
CA 02211412 1997-07-24
- W O 96 m 704 ' ~ 7 ' ' ' ~C~ o~a@8
'' 7
"Densified carbon dioxide" means carbon~dloO~d~$ ln a gas
form which is placed under pressures excee~lng abo ~ (700
psi) at about 20~C.
"Supercritical fluid carbon dioxide" means carbon dioxide
5 which is at~or above the critical temperature of 31~C and a
cr1tlcal ~LesJsure of~71 atmospheres) and which cannot be
condensed into a liquid phase despite the addition of
~urther pressure.
10 The term "densified carbon dioxide-philic" in reference to
surfactants ~Zn- wherein n and n~ are each independently l
to 50, means that the functional group, Rn~ is soluble in
~ 3 ~S ~ -6-~-q~ ~J~
carbon aloxide a~ pressures a~500-10,000 pSl and
~emperatures of 0-100~C to greater than 10 weight percent.
15 Preferably n and n are each independently 1-35. Such
~unctional groups (Rn~) include halocarbons, polysiloxanes
and branched polyalkylene oxides.
The term "densified carbon dioxide-phobic" in re~erence to
20 surfactants, RnZn, means that Zn-~ will have a solubility in
carbon dioxl~el~. ~Fessures ofj(500-10,000 psi)and
temperatures of 0-100~C of less than 10 weight percent.
The functional groups in Zn-~ include carboxylic acids,
~-~ phosphatyl esters, hydroxys, Cl30 alkyls or alkenyls,
25 polyalkylene oxides, branched polyalkylene oxides,
carboxylates, Cl30 alkyl sulfonates, phosphates,
glycerates, carbohydrates, nitrates, substituted or
unsubstituted aryls and sulfates.
30 The hydrocarbon and halocarbon containing surfactants
(i.e., ~Zn-, containing the C02-philic functional group,
Rn~~ and the CO2-phobic group, Z~,-) will have an HLB of
less than 15, preferably less than 13 and most preferably
less than 12.
A~,lc~cD Sh'EET
CA 02211412 1997-07-24
W 096/27704 PCT/~r~G
The polymeric siloxane containing surfactants, RT~Zn,r also
designated MD"D*"M, with M representing trimethylsiloxyl
end groups, Dx as a dimethyl~iloxyl backbone (CO2-philic
functional group) and D*y as one or more substituted
5 methylsiloxyl groups substituted with CO2~phobic R or R'
groups as described in the Detailed Description Section
will have a DXD*y ratio of greater than 0.5:1, preferably
greater than 0.7:1 and most preferably greater than 1:1.
10 The term "nonpolar stains~ refers to those which are at
least partially made by nonpolar organic compounds such as
oily soils, sebum and the like.
The term '~polar stains" is interchangeable with the term
15 "hydrophilic stains" and refers to stains such as grape
juice, coffee and tea.
The term "compound hydrophobic stains" refers to stains
such as lipstick and red candle wax.
The term "particulate soils" means soils containing
insoluble solid components such as silicates, carbon black,
etc.
25 Densified carbon dioxide, preferably supercritical fluid
carbon dioxide, is used in the inventive dry cleaning
system. It is noted that other densified molecules having
supercritical properties may also be employed alone or in
mixture. These molecules include methane, ethane, propane,
30 ammonia, butane, n-pentane, n-hexane, cyclohexane, n-
heptane, ethylene, propylene, methanol, ethanol,
isopropanol, benzene, toluene, p-xylene, sulfur dioxide,
chlorotrifluoromethane, trichlorofluoromethane,
perfluoropropane, chlorodifluoromethane, sulfur
35 hexafluoride and nitrous oxide.
CA 02211412 1997-07-24
W O 96~7704 ' ''- . .''~CT~EP~6/~
During the dry cleaning process, the temperature range is
between about 20~C and about 100~C, preferably 20~C to 60~C
and most preferably 30~C to about 60~C. T~e pressure
. L ~-~3~o3K~;J ~c~ qx103~J
5 dUrlng ~ ~anlng lS labou~(700 ~pSl~ ~O a30Ut~10~000 pS~
5 pre~erablyi~800 ps~ o c~OU~_/,000 pSi) and most preferably
S~XI~(80o psi) to abou ~6,00~ psi~
A "substituted methylsiloxyl group" is a methylsiloxyl
group substituted with a CO2-phobic group R or R . R or R
10 are each represented in the following formula:
= :
~ ~CH2) a (C6H4) b (A) d-- [ (L)e(A')~]n~ ')gZ(G)h
wherein a is 1-30, b is o-l, C6~4 is substituted or
15 unsubstituted with a C1_1Q alkyl or alkenyl and A, d, L, e,
A-, F, n L , g, Z, G and h are defined below, and mixtures
o~ R and R .
A "substituted aryl" is an aryl substituted with a Cl30
20 alkyl, alkenyl or hydroxyl, preferably a Cl20 alkyl or
alkenyl.
A "substituted carbohydrate~ is a carbohydrate substituted
with a Cl10 alkyl or alkenyl, preferably a Cls alkyl.
25 The terms ~'polyalkylene oxide", ~alkyl" and ~alkenyl~ each
contain a carbon chain which may be either straight or
branched unless otherwise stated.
S-~f~c~nt Adjl~nct
30 A surfactant which is effective for use in a densified
carbon dioxide dry cleaning system requires the combination
of densified carbon dioxide-philic functional groups with
densified carbon dioxide-phobic functional groups (see
definitions above). The resulting compound may form
35 re~ersed micelles with the CO2-philic functional groups
extending into a continuous phase and the COz-phcbic
A;~ c,~t ~ ET
. CA 02211412 1997-07-24
W O 96127704 . ~ ~C.T~o'~
~unctional groups directed toward the center of the
micelle.
The surfactant is present in an amount o~ ~rom 0.001 to 10
wt.~, preferably 0.01 to 5 wt.~.
The CO2-philic moieties o~ the sur~actants are groups
exhibiting low Hildebrand solubility parameters, as
described in Grant, D. J. W. et al. "Solubility sehavior of
Organic Compounds", Techniques of Chemistry Series, J.
10 Wiley & Sons, NY (lg90) pp. 46-55 which describes the
Hildebrand solubility equation, herein incorporated by
re~erence. These CO2-philic moieties also exhibit low
polarizability and some electron donating capability
allowing them to be solubilized easily in densi~ied ~luid
15 carbon dioxide.
As defined above the CO2-philic functional groups are
soluble in densified carbon dioxide to greater than 10
~3 ~s~6~ q wei ~t percent, pre~erably greater than 15 weight percent,
~u ~L ~Le~ssures o~(500-10,000 psi) and temperatures of 0-100~C.
Pre~erred densi~ied CO2-philic functional groups include
halocarbons (such as fluoro-, chloro- and fluoro-
chlorocarbons), polysiloxanes and branched polyalkylene
25 oxides.
The CO2-phobic portion o~ the surfactant molecule is
obtained either by a hydrophilic or a hydrophobic
functional group which is less than 10 weight percent
30 soluble in densified CO2, preferably less than 5 wt. ~, at
( 3-4 5 h~ G~-9~l~3 ~ ~ ~
a ~ressures of~500-10,000 psi)and temperatures of 0-100~C.
Examples of moieties contained in the CO2-phobic groups
include polyalkylene oxides, carboxylates, branched
acrylate esters, ~1-30 hydrocarbons, aryls which are
35 unsubstituted or substituted, sulfonates, glycerates,
phosphates, sulfates and carbohydrates. Especially
CA 02211412 1997-07-24
W O 96127704 PCT/~r,~i(J0311
preferred CO2-phobic groups include C220 straight chain or
branched alkyls, polyal]~ylene oxides, glycerates,
carboxylates, phosphates, sulfates and carbohydrates.
5 The CO2-philic and CO2-phobic groups may be directly
connected or linked together via a linkage group. Such
groups include ester, keto, ether, amide, amlne, thio,
alkyl, alkenyl, fluoroalkyl or fluoroalkenyl.
10 Surfactants which are useful in the invention may be
selected from four groups of compounds. The first group o~
compounds has the following formula:
[(CX3(CX2)a(CH2)b)c(A)d- [(L)e- (Al)~]n- (L~)g]oZ(G)h (I)
wherein X is F, Cl, Br, I and mixtures thereof,
preferably F and Cl;
a is 1 - 30, preferably 1-25, most preferably 5-20;
b is 0 - 5, preferably 0 - 3;
c is 1 - 5, preferably 1 - 3;
A and A' are each independently a linking moiety
representing an ester, a keto, an ether, a thio, an amido,
an amino, a Cl4 fluoroalkyl, a Cl4 fluoroalkenyl, a
branched or straight chain polyalkylene oxide, a phosphato,
a sulfonyl, a sulfate, an ammonium and mixtures thereof;
d is 0 or 1;
L and L' are each independently a Cl30 straight
ch~;ned or branched alkyl or alkenyl or an aryl which is
unsubstituted or substituted and mixtures thereof;
e is 0-3;
f is 0 or 1;
n is 0-10, preferably 0-5, most preferably 0-3;
g is 0-3;
o is 0-5, preferably 0-3;
Z is a hydrogen, a carboxylic acid, a hydroxy, a
35 phosphato, a phosphato ester, a sulfonyl, a sulfonate, a
sulfate, a branched or straight-chained polyalkylene oxide,
CA 02211412 1997-07-24
W 096/27704 PCTAEP96/00811
a nitryl, a glyceryl, an aryl unsubstituted or substituted
with a Cl30 alkyl or alkenyl, (pre~erably C125 alkyl), a
carbohydrate unsubstituted or substituted with. a Cl10 alkyl
or alkenyl (preferably a Cls alkyl) or an ammonium;
5 G is an anion or cation such as H+, Na+, ~i+, K+, NH4+ Ca+2,
Mg+2; Cl-, Br~, I-, mesylate, or tosylatei and h is 0-3,
preferably 0-2.
- Preferred compounds within the scope of the formula I
10 include those having linking moieties A and A which are
each independently an ester, an ether, a thio, a
polyalkylene oxide, an amido, an ammonium and mixtures
thereof;
L and L are each independently a C125 straight chain
15 or branched alkyl or unsubstituted aryl; and Z is a
hydrogen, carboxylic acid, hydroxyl, a phosphato, a
sulfonyl, a sulfate, an ~mmo~;um, a polyalkylene oxide, or
a carbohydrate, preferably unsubstituted. G groups which
are preferred include H+, Li+, Na+, NH+4, Cl-, ~r~ and
20 tosylate.
Most preferred compounds within the scope of formula I
include those compounds wherein A and A' are each
independently an ester, ether, an amido, a polyoxyalkylene
25 oxide and mixtures thereof; L and L' are each independently
a C120 straight chain or branched alkyl or an unsubstituted
aryl; Z is a hydrogen, a phosphato, a sulfonyl, a
carboxylic acid, a sulfate, a polyalkylene oxide and
mixtures thereof; and
G is H+, Na+ or NH4+.
Non-limiting examples of compounds within the scope of
~ormula I include the following:
CA 02211412 1997-07-24
W 096/27704 PCT~EP96/00811
11
Perhalogenated Surfactants
CF3 (CF2) ,CH2CH2C (O) OX CF3 (CF2) ,CH2CH2S (CH2) mC (O) OG
CF3 ( CF2),LCH2C (O) OX CF3 ( CF2) ,CH2S ( CH2) mC ( O) OG
CF3 (CF2) ,C (O) OX CF3 (CF2) ,S (CH2) nC (O) OG
CF3 ( CF2) ,CH2CH2C (O ) O ( CH2)",CH3
CF, (CF2) ,CH2C (O) O (CH2) mCH3
CF3 (CF2) ,C (O) O (CH2) mCH3
CF3 (CF2) ,CH2CH2OP (O) (OH) 2
CF3 (CF2) ,CH2OP (O) (OH) z
CF3 (CF2) ,OP (O) (OH) 2
[CF3 (CF2) ,CH2CH2O] 2P (O) (OH)
[CF3 (CF2) ,CH2O] 2P (O) (OH)
[CF3 (CF2) ,~] 2P (O) (OH)
CFI ( CF2 ) ,CH2cH2s03G
CF3 ( CF2) ,CH2SO3G
CF3 ( CF2) ,SO3G
CF3 (CF2) ,CH2CH2C (O) (CH2) mCH3
CF3 ( CF2) ,CH2C (O ) ( CH2) ~CH3
CF3 (CF2),.C (O~ (CH2) mCH3
CF3 ( CF2) ,CH2CHzO ( CH2) ,CH3 a = 1- 3 0
CF3 (CF2) ,CH2O (CH2) mCH3 a ' = 1-20
CF3 ( CF2) ,O ( CH2)I"CH3 m = 1- 3 0
p = 1-50
CF3 (CF2) ,CH2CH2C (O) N [(CH2)mCH3]2 G = H', Na', K', NH.,',
CF3 ( CF2) ,CH2C ( O) N [ ( CH2)"~CH3] 2 Mg~2 Ca~2 etc
CF3 (CF2) ,C (O) N [(CH2)mCH3]2
CA 02211412 1997-07-24
W 096/27704 PCT/~r~G
Perhalogenated Surfactants (cont.)
CF3 (CF2) ,CH2CH2C (O) OCH2CH2 [OCH2CH (CH3) ] pOH
CF3 (CF2) aCH2C (O) OCH2CH2 [OCH2CH (CH3) ] pOH
CF3 (CF2) ,~C (O) OCH2CH2 [OCH2CH ~CH3) ] pOH
CF3 (CF2) ,CH2CH2C (O) OCH2CH2 [OCH2CH2] pOH
CF3 (CF2) ,CH2C (O) OCHsCH2 [OCH2CH2] pOH
CF3 (CF2) aC (O) OCH2CH2 [OCH2CH2] pOH
CF3 (CF2) ,CH2CH2C (O) OCH2CH2OCH2CH (OH) CH2OH
CF, (CF2) ,CH2C (O) OCH2CH2OCH2CH (OH) CH2OH
CF3 (CF2) ,C (O) OCH2CH2OCH2CH (OH) CH2OH
CF3 (CF2) ,CH2CH20 (CH2) ,.C (O) O (CH2) ",CH3
CF3 (CF2) ,CH2O (CH2) ,.C (O) O (CH2) ,CH3
CF3 (CF2) ,O (CH2) ,.C (O) O (CH2) mCH3
CF3 (CF2) ,CH2CH2S (CH2) ,.C (O) O (CH2) ,CH3
CF3 ~CF2) ,CH2S (CH2) ,.C (O) O (CH2) ~CH3
CF3 (CF2) ,S (CH2) ,.C (O) O (CH2) mCH3
CF3 (CF2) ,CH2CH2O (CH2) ,. (OCH2CH2) pOH
CF3 (CF2) .CH2O (CH2) ,. (OCH2CH2) pOH
CF3 ( CF2 ) ,O ( CH2 ) ,. ( OCH2CH2 ) pOH
CF3 (CF2) ,CH2CH2O (CH2) ,. (OCH2CH (CH3) ) pOH a = 1-30
CF3 ( CF2 ) ,CH2O ( CH2 ) ,, ( OCH2CH ( CH3 ) ) pOH a ' = 1- 2 0
CF3 (CF2) ,O (CH2) ,. (OCH2CH (CH3) ) pOH m = 1-30
p = 1-50
CF3 (CF2) ,CH2CH2C (O) O (CH2) .. (OCH2CH2) pOH G = H~, Na+, Kt,CF3 (CF2) ,CH2C (O) O (CH2) ,. (OCH2CH2) pOH NH"', Mg~2, Ca~2, etc .
CF3 (CF2) .C (O) O (CH2) ,, (OCH2CH2) pOH
CA 02211412 1997-07-24
W 096/27704 PCTA~P96/00811
Perhalogenated Sur~actants (cont.)
CF, (CF2) ,CH2CH2C (O) O (CH2) ,. (OCH2CH (CH3) )I,OH
CF3 (CF2) ~CH2C (O) O (CH2)," (OCH2CH (CH3) ) pOH
CF3 (CF2) ,C (O) O (CHl) ,. (OCH2CH (CH3) ) pOH
a = 1-30
a ' = 1-20
m = 1-30
p - 1-50
G - Ht, Na~, K~, NH"',Mg'2, Ca'2, etc.
Perhalogenated Surfactants (cont.)
CF3 (CF2) ,CH2CH2OCH2CH2OCH2CH (OH) CH2OH
CF3 (CF2) ,CH2OCH2CH2OCH2CH (OH) CH2OH
CF3 (CF2) ,OCH2CH2OCH2CH (OH) CH2OH
[CF3 (CF2) ,CH2CH2C (O) OCH2] 2N (CH2) mCOOX
[CF3 ( CF2) ,CH2C ( O) OCH2] 2N ( CH2) ,COOX
[CF3 ( CF2) ,C (O) OCH2] 2N ( CH2)I,,COOX
[CF3 (CF2) ,CH2CH2C (O) OCH2] 2CH (CH2) mCOOX
[CF3 (CF2) ,CH2C (O) OCH2] 2CH (CH2) mCOOX
[CF3 ( CF2) ,C (O ) OCH2] 2CH ( CH2) mCOOX
[CF3 (CF2) ,CH2CH2S (CH2) ..C (O) N [ (CH2) mCH3] 2
[CF3 (CF2) ~CH2S (CH2) ,.C (O) N t (CH2)mCH3]2
[CF3 (CF2) ,S (CH2) ,,C (O) N [ (CH2) mCH3] 2
CF3 (CF2) ,CH2CH2O (CHl) ,,C (O) N [(CH2)mCH3]2
CF3 (CF2) ~CH20 (CH2) ,.C (O) N [ (CH2).~CH3] 2
CF3 (CF2) ~O (CH2) ,.C (O) N [(CH2)mCH3]2
CA 022ll4l2 l997-07-24
W 096/27704 PCT/~6l~J8
14
Perhalogenated Surfactants (cont.)
CH2C (O) O (CF2) ~CF3
~ CH (SO3G) C (O) (CF2) ,CF3
CH (SO,G) C (O) OCH2CH2 (CF2) ~CF3
CH2C (O) OCH2CH2 (CF2) ,CF3
CH2C (O) OCH2 (CF2) ,CF,
CH (SO3G) C (O) OCH2 (CF2) ,CF3
CF3 (CF2) ,CH2CH2O (CH2) m ~3 SO3G
CF3 ( CF2 ) ,CH2O ( CH2 ) ~ 03G
CF3 ( CF2 ) ,O ( CH2 ) ~ ~ SOlG
CH20CH2CH2 (CF2) ,CF3
~0
O~H
CH2OCH2 ( CFz ~ ,CF3 CH2O ( CF2 ) ,CF3
J_O J o
OH OH OH OH
a = 1-30
a' = 1-20
m = 1-30
G = H+, Na+, K', Li ', Ca+2, Mg+2, NH4+, etc .
CA 022ll4l2 l997-07-24
W O 96/27704 PCT~EFS''-Ell
Perhalogenated Surfactants (cont.)
CF3~CF2),CH2CH2C(O)(CH2)mN(CH3)3G
~ CF3(CF2),CH2C(O)(CH2),N(CH3)3G
CF3(cF2)~c(o)(cH2)mN(CH3)3G
CClF2(CClF).CH2CH2C(O)OX
CClF2(CClF),CH2C(O)OX
CClF2(CClF),C(O)OX
CClF2(CClF),CH2CH2C(O)O(CH2)~CH3
CclF2(cclF)~cH2c(o)o(cHa)~cH3
CClF2(CClF),C(O)O(CH2)mCH3
CClF2(CClF),CH2CH20P(O)(OH) 2
CClF2(CClF),CH20P(O)(OH) 2
CClF2(CClF),OP(O)(OH) 2
[CClF2(CClF),CH2CH20]2P(O)(OH)
[CClF2(CClF),CH20]2P(O)(OH)
[CClF2(CClF),0]2P(O)(OH)
CClF2(CClF),CH2CH2SO3G
CClF2(CClF),CH2SO3G
CClF2(CClF),SO3G
CClF2(cclF)~cH2cH2c(o)(cH2)mcH3
CclF2(cclF)~cH2c(o)(cH2)mcH3
CClF2(CClF),C(O)(CH2)mCH3
CClF2(CClF),CH2CH2S(CH2)~.C(O)O(CH2)mCH3
CClF2(CClF),CH2S(CH2),.C(O)O(CH2)mCH3
CClF2(CClF),S(CH2),.C(O)O(CH2)mCH3
CA 02211412 1997-07-24
PCT~E~9G
W 096/27704
Perhalogenated Surfactants (cont.)
CClF2(CClF),CH2CH2O(CH2),,(OCH2CH2)pOH
CClF2 (CClF) ,CH2O (CH2) ,. (OCH2CH2) pOH
CClF2 (CClF) ,O (CH2) ,. (OCH2CH2) pOH
CClF2 (CClF) ,CH2CH2O (CH2) ,, (OCH2CH (CH3) ) pOH
CClF2(CClF),CH2O(CH2),.(OCH2CH(CH3) ) pOH
CClF2(CClF),O(CH2),.(OCH2CH(CH3) ) pOH
CClF2(CClF),CH2CH2C(O)(CH2)mN(CH3)3G
CClF2 (CClF) ,CH2C (O) (CH2) mN (CH3) 3G
CClF2(CClF),C (O) (CH2)mN( CH3 ) 3G
CClF2 (CClF) ,CH2CH2O (CH2) mCH3
CClF2 (CClF) ,CH2O (CH2) mCH3
CClF2(CClF),O(CH2)mCH3
CClF2(CClF),CH2CH2C(O)N [ (CH2) mCH3] 2
CClF2 (CClF) ,CH2C (O) N [ (CH2) mCH3] 2
CClF2 (CClF) ,C (O) N t (CH2)mCH3]2
a = 1-30
a' = 1-20
m = 1-30
p = 1-50
G = H', Na', K~, NH~, Mg~2, Ca~2, Cl-,. Br~, -OTs, -OMs, etc.
CA 02211412 1997-07-24
W 096/27704 PCTAEP~'~a~ll
Compounds of formula I are prepared by any conventional
preparation method known in the art such as the one
described in March, J., "Advanced Organic Chemistry", J.
Wiley ~ Sons, NY (1985).
Commercially available fluorinated compounds include
compounds supplied as the Zonyl~ series by Dupont.
The second group of surfactants useful in the dry cleaning
10 system are those compounds having a polyalkylene moiety and
having a formula (II).
R R
[H-[-CH-CH-O-]i-(A) d-[~L),-(A ) f]n~ (L )g]oZ(G) h (II)
wherein R and R each represent a hydrogen, a Cl5
straight chained or branched alkyl or alkylene oxide and
mixtures thereof;
i is 1 to 50, preferably 1 to 30, and
A, A , d, L, L , e f, n, g, o, Z, G and h are as
defined above.
Preferably R and R are each independently a hydrogen,
a Cl3 alkyl, or alkylene oxide and mixtures thereof.
Most preferably R and R are each independently a
hydrogen, C13 alkyl and mixtures thereof. Non-limiting
examples of compounds within the scope of formula II are:
CA 02211412 1997-07-24
W 096/27704 PcT/~G
18
Polypropylene Glycol Surfactants
HO (CH2CH (CH3) ~) l (CH2CH20) ~H
HO (CH (CH3) (CH20) 1 (CH2CH20) ,H
HO (CH2CH (CH3) ~) l (CH2CH20); (CH2CH (CH3) O) "H
HO (CH (CH3) CH20) 1 (CH2CH20) ~ (CH2CH (CH,) O) "H
HO (CH2CH (CH3) O) ~ (cH2cH2o); (CH2 (CH3) CH2~) lCH
HO (CH (CH3) CH20) ~ (CH2CH20) ~ (CH2 (CH3) CH20) "H
HO (CH2CH20) i (CH2CH (CH3) O); (CH2CH20) ,~H
HO (CH2CH20) 1 (CH (CH3) CH20) ~ (CH2CH20) "H
HO (CH (CH3) CH20) lC (O) (CH2) mCH3
HO (CHzCH (CH,) O) lC (O) (CH2) mCH3
HO ( CH ( CH3 ) CH20 ) i ( CH2 ) mCH3
HO ( CH2CH ( CH3 ) ~ ) 1 ( CH2 ) mCH3
HO (CH (CH3) CH2O) lC (O) O (CH2) mCH3
HO ( CH2CH ( CH3 ) O ) lC ( O ) O ( CH2 ) ~CH3
HO (CH (CH3) CH2O) lC (O) N ~ (CH2) mCH3] 2
HO ( CH2CH ( CH3 ) O ) ~ C ( O ) N [ ( CH2 ) mCH3 ] 2
HO (CH (CH3) CH2o) ~C (O) (CH2) mCOOG
HO ( CH2CH ( CH3 ) O ) lC ( O ) ( CH2 ) ,"COOG
HO ( CH ( CH3 ) CH20 ) 1 ( CH2 ) mCOOG
HO (CH2CH (CH3) ~) 1 (CH2) mCOOG
HO (CH (CH3) CH20) lC (O) O (CH2) mCOOG
HO (CH2CH (CH3) O) lC (O) O (CH2) mCOOG
HO (CH (CH3) CH2O) lC (O) N [ (CH2) mCOOG] 2
HO (CH2CH (CH3) O) iC (O) N [(CH2)mC~OG] 2
HO ( CH ( CH3 ) CH20 ) lC ( O ) ( CH2 ) ~nSO3G
HO (CH2CH (CH3) O) lC (O) (CH2) mSO3G
HO ( CH ( CH3 ) CH2o) 1 ( CH2) mSO3G
HO ( CH2CH ( CH3 ) ~) i ( CH2) ~SO3G
CA 02211412 1997-07-24
W O 96/27704 PCTAEPg''~_Ull
19
Polypropylene Glycol Surfactants (cont.)
HO (CH (CH3) CH20) ~C (O) CH2CHzOCH2CH (OH) CH20H
HO (CH2CH (CH3) O) ~C (O) CH2CH20CH2CH (OH) CH20H
HO (CH (CH3) CH20) ~.CH2CH20CH2CH (OH) CH20H
HO (CH2CH (CH3) O) lCH2CH20CHzCH (OH) CH20H
HO (CH (CH3) CH20) iC (O) (CH2) ,,N (CH3) 3G
HO ( CH2CH ( CH3 ) O ) ~C ( O ) ( CH2 ) ",N ( CH3 ) 3G
HO (CH (CH3) CH20) i (CH2) mN (CH3) 3G
HO ( CH2 CH ( CH3 ) ~ ) 1 ( CH2 ) mN ( CH3 ) 3G
HO (CH (CH3) CH20) lC (O) O (CH2) ",N (CH3) 3G
HO (CH2CH (CH3) O) ~C (O) O (CH2) ."N (CH3) 3G
SO3G
HO ( CH ( CH3 ) CH20 ) lC ( O ) ( CH2 ) ~" ~ j~~
SO3G
HO ( CH2CH ( CH3 ) O ) ~ C ( O ) ( CH2 ) m ~~~~~~~
COOG
HO (CH (CH3) CH20) lC (O) (CH2) ", ~
COOG
HO (CH2CH (CH3) O) ~C (O) (CH2) " _~~~
CH2C (O) O (CH (CH3) CH20) ~H
CH2 (SO3G) C (O) O (CH2CH (CH3) O) lH
CH2C (O) O (CH2CH (CH3) O) lH
CH2 (S03G) C (O) O (CH (CH3) CH20) lH
fH2C (O) N [ (CH (CH3) CH20) lH] 2 i = 1-50
CH2 (SO3G) C (O) N[(CH2CH(CH3) O) lH] 2 i = 1-50
fH2C (O) N [ (CH2CH (CH3) O) lH] k = 1-50
CH2 (SO3G) C (O) N[(CH (CH3) CH20) iH] 2 m = 1-30
G= H+, Na+, K+, NH,,+, Ca+2, Mg+2, Cl-, Br~, -OTs, -OMs, etc .
CA 02211412 1997-07-24
W 096127704 PCT/~r~Gl~~
.
Polypropylene Glycol Surfactants (cont.)
CH2(OCH(CH3)CH2)~OH
~~~ .
~ OH O~
oQ OH
CH2(OCH2CH(CH3))~OH
,Lo
~ OH O~
i = 1-50
j = 1-50
k = 1-50
m = 1-30
G= H', Na', K+,NH~, Ca~2, Mg'2, Cl-, Br~, -OTs, -OMs, etc.
CA 02211412 1997-07-24
W 096127704 PCTAE~95'~C~ll
Compounds of formula II may be prepared as is known in the
art and as described in March et al., Supra.
5 Examples of commercially available compoùnds of formula II
may be obtained as the Pluronic series from BASF, Inc.
A third group of surfactants useful in the invention
contain a fluorinated oxide moiety and the compounds have a
10 formula:
[(CX3(XO)r (T) 8) C (A) d- [ (L)e-(A ) f ~ ] n (L )g]oZ(G) h ( III)
wherein XO is a halogenated alkylene oxide having Cl6
15 straight or branched halocarbons, preferably Cl3,
r is 1-50, preferably 1-25, most preferably 5-20,
T is a straight chained or branched haloalkyl or
haloaryl,
s is o to 5, preferably 0-3,
X, A, A', c, d, L, L , e, f, n, g, o, Z, G and h are
as defined above.
Non-limiting examples of halogenated oxide containing
compounds include:
CA 02211412 1997-07-24
W 096/27704 PCT/~l3
Perhaloether Surfactants
CF3 ( CF2CF20) r (CH2CH20) tH
CF3 (CF2CF20) r (CH2CH (CH3) O) tH
CF3 ( CF2CF (CF3 ) ~) r ( CH2CH2~) tH
CF3 (CF2CF (CF3) O) r (CH2CH (CH3) O) tH
CF3 (CF2CF20) rP (O) (OH) 2
CF3 (CF2CF20) rCF2P (O) (OH) 2
CF3 (CF2CF20) rCF (CF3) P (O) (OH) 2
[CF3 (cF2cF2o) r] 2P (O) (OH)
[CF3 (cF2cF2o) rcF2] 2P (O) (OH)
[CF3 (CF2CF20) rCF (CF3) ] 2P (O) (OH)
CF3 (CF2CF (CF3) O) rP (O) (OH) 2
CF3 (CF2CF (CF3) O) rCF2P (O) (OH) 2
CF3 (CF2CF (CF3) O) rCF (CF3) P (O) (OH) 2
[CF3 (CF2CF (CF~) O) r] 2P (O) (OH)
[CF3 (CF2CF (CF3) O) rCF2] 2P (O) (OH)
[CF3 (CF2CF (CF3) ~) rCF (CF3) ] 2P (O) (OH)
CF3 (CF2CF20) rC (O) OG
CF3 (CF2CF20) rCF2C (O) OG
CF3 (CF2CF20) rCF (CF3) C (O) OG
CF3 (CF2CF (CF3) O) rC (O) OG
CF3 (CF2CF (CF3) O) rCF2C (O) OG
CF3 (CF2CF (CF3) O) rCF (CF3) C (O) OG
CF3 (CF2CF20) rC (O) O (CH2) mCH3
CF3 (CF2CF20) rCF2C (O) O (CH2) mCH3
CF3 (CF2CF20) rCF (CF3) C (O) O (CH2)mCH3
CF3 (CF2CF (CF3) O) rC (O) O (CH2) mCH3
CF3 ( CF2CF ( CF3 ) ~ ) rcF2c ( O ) O ( CH2 ) mCH3
CF3 (CF2CF (CF3) O) rcF (CF3) C (O) O (CH2) mCH3
CA 02211412 1997-07-24
W 096/27704 pcT/~r
Perhaloether Surfactants (cont.)
CF3 (CF2CF20) nC (O) OCH2CHlOCH2CH (OH) CH20H
CF3 (CF2CF20) ~CF2C (O) OCH2CH20CH2CH (OH) CH20H
CF3 (CF2CF (CF3) O) nC (O) OCH2CH20CH2CH (OH) CH20H
CF3 ( CF2CF20 ) rC ( O ) N [ ( CH2 ) ",CH3] 2
CF3 (CF2CF20) rCF2C (O) N [ (CHl) ~CH3] 2
CF3 (CF2CF20) rCF (CF3) C (O) N t (CH2) mCH3] 2
CF3 (CF2CF (CF3) O) rC (O) N [ (CH2) ,,CH3] 2
CF3 (CF2CF (CF3) O) rCF2C (O) N [ ~CH2) ,CH3] 2
CF3 (CF2CF (CF3) O) rCF (CF3) C (O) N [ (CH2) ~CH3] 2
CF3 ( CF2CF20 ) r~ ( CH2 ) ~CH3
CF3 (CF2CF20) rCF2~ (CH2) mCH3
CF3 ( CF2CF20 ) rCF ( CF3 ) O ( CH~ ) mCH3
CF3 (CF2CF (CF3) ~) r~ (CH2) ~CH3
CF3 (CF2CF (CF3) O) rCF20 (CH2) mCH3
CF3 (CF2CF (CF3) O) rcF (CF3) O (CH2) ,I,CH3
CF3 (CF2CF20) rC (O) O (CH2) "SO3G
CF3 (CF2CF20) rCF2C (O) O (CH2) ~S~3G
CF3 (CF2CF20) rCF (CF3) C (O) O (CH2) mSO3G
CF3 (CF2CF (CF3) O) rC (O) O (CH2) mSO3G
CF3 (CF2CF (CF3) O) rCF2C (O) O (CH2) ,~,SO3G
CF3 ( CF2CF ( CF3 ) O) rCF ( CF3 ) C (O) O ( CH2 ) mSO3G
CF3 (CF2CF20) rC (O) O (CH2) mCO3G
CF3 (CF2CF20) rCF2C (O) O (C~I2) mCO3G
CF3 ( CF2CF20 ) rCF ( CF3 ) C ( O ) O ( CH2 ) mCO3G
CF3 (CF2CF (CF3) O) rC (O) O (CH2) mCO3G
CF3 (CF2CF (CF3) O) rcF2c (O) O (CH2) ~C03G
CF3 (CF2CF (CF3) O) rcF (CF3) C (O) O (CH2) ~CO3G
CA 02211412 1997-07-24
W 096/27704 PCT/~G/00811
24
Perhaloether Surfactants (cont.)
CF~ (CF2CF2O) rC (O) (CH2) mCH3
CF3 ( CF2CF2O ) rCF2C ( O ) ( CH2 ) ,CH3
CF3 ( CF2CF2O ) rCF ( CF3 ) C ( O ) ( CH2 ) mCH3
CF3 ( CF2CF ( CF3 ) O ) rC ( O ) ( CH2 ) mCH3
CF3 (CF2CF (CF3) ~) rCF2C (O) (CH2) ",CH3
CF3 ( CF2CF ( CF3 ) O ) rCF ( CF3 ) C ( O ) ( CH2 ) ,CH3
CF3 (CF2CF2O) rC (O) (CH2) mN (CH3) 3G
CF3 (CF2CF20) rCF2C (O) (CH2) ,"N (CH3) 3G
CF3 ( CF2CF2O ) rCF ( CF3 ) C ( O ) ( CH2 ) mN ( CH3 ) 3G
CF3 (CF2CF (CF3) O) rC (O) (CH2) mN (CH3) 3G
CF3 ( CF2 CF ( CF, ) O ) rCF2 C ( O ) ( CH2 ) ~N ( CH3 ) 3G
CF3 (CF2CF (CF3) O) rCF (CF3) C (O) (CH2) mN (CH3) 3G
r = 1-30
t = 1-40
m = 1-30
G = H~, Na', K', Li', NH~+, Ca~2, Mg~2, Cl-, Br~, -3Ts, -OMs,
etc.
CA 02211412 1997-07-24
W 096/27704 PCT/~G
Perhaloether Surfactants (cont.)
CF3(CF2CF20)rC(O)O(cH2) m ~ SO3G
CF3 (CF2CF20) rCF2C (O) O (CH2) ~> SO3G
CF3 (CF2CF20) rCF (CF3) C (O) O (CH2) m~ SO3G
CF3 (CF2CF (CF3) O) rC (O) O (CH2) ,, ~ SO3G
CF3(CF2 CF ( CF3 ) O ) rCF2 C ( O ) O ( CH2 ) m ~ SO3G
CF3 (CF2CF (CF3) O) rCF (CF3) C (O) O (CH2), ~ SO3G
CF3 ( CF2CF20 ) r ( CH2 ) m ~> SO3G
CF3 (CF2CF20) rCF2~ (CH2)m ~ SO3G
CF3 ( CF2CF20 ) rCF ( CF3 ) O ( CH2 ) m _~) SO3G
CF3 ( CF2CF20) rCF ( CF3 ) O ( CH2) m ~ SO3G
CF3 ( CF2CF ( CF3 ) ~ ) rCF20 ( CH2 ) m ~ SO3G
CF3 ( CF2CF ( CF3 ) ~ ) rCF ( CF3 ) O ( CH2 ) m_~> SO3G
CA 02211412 1997-07-24
PCT/~l3G
W 096/27704
26
Perhaloeter Sur~actants (cont.)
CH20C ( O ) ( CF2CF2~ ) rCF3
CH (SO3G) OC (O) (CF2CF2O) rCF3
CH20C (O) (CF2CF20) rCF3
CH (SO3G) OC (O) CF2 (CF2CF2O) rCF3
CH2OC (O) CF (CF3) (CF2CF2O) rCF3 r = 1-30
CH (SO3G) OC (O) CF (CF3) (CF2CF2O) rCF3
CH2OC ( O ) ( CF2CF ( CF3 ) O ) rCF3 m = 1- 3 0
CH (SO3G) OC (O) (CF2CF2 (CF3) O) rCF3
CH2OC (O) CF2 (CF2CF (CF3) O) rCF3
CH (SO3G) OC (O) CF2 (CF2CF (CF3) O) rCF3
CH2OC (O) CF (CF3) CF2CF (CF3) O) rCF3
CH (SO3G) OC (O) CF (CF3) (CF2CF (CF3) O) rCF3
G = H~, Na', Li ,NH~', Ca~2, Mg'2, Cl-, Br~, -OTs, -OMs, etc.
CA 02211412 1997-07-24
W O 96/27704 PCT~EPg6/00811
27
Perhaloether Surfactants (cont.)
CclF2(cclFcclFO)r(cH2cH2o~tH
CClF2(CClFCClFO)r(CH2CH(CH3)0)tH
CClF2(CClFCF(CClF2)~r(CH2CH2)0)tH
CClF2tCClFCF(CClF2)0rtCH2CH(CH3)0)tH
CClF2(CClFCClFO)rP(O)(OH~ 2
CClF2(CClFCClFO)rCF2P(O)(OH) 2
CClF2(CClFCClFO)rCF(CF3)P(O)(OH)2
[CClF2(CClFCClFO) r] 2P (O) (OH)
[CClF2(CClFCClFO)rCF2]2P(O) (OH)
[cclF2(cclFcclFO)rcF(cF3)]2P(o)(OH)
CClF2(CClFCF(CClF2)0)rP(O)(OH) 2
CclF2(cclFcF(cclF2)o)rcF2p(o) (OH) 2
CClF2(CClFCF(CClF2)0)rCF(CF3)P(O)(OH)2
[CClF2(CClFCF(CClF~)O)r]2P(O)(OH)
[CClF2(CClFCF(CClF2)0)rCH2]2P(O)(OH)
[cclF2(cclFcF(cclF2)o)rcH2]2P(o)(OH)
CClF2(CClFCClFO)rC(O)OG
CClF2(CClFCClFO)r(CH2)C(O)OG
CClF2(CClFCClFO)r(CH(CF3)C(O)OG
CClF2(CClFCF(CClF2)0)rC(O)OG
CClF2(CClFCF(CClF2)0)rCF2C(O)OG
CClF2(CClFCF(CClF2)0)rCF(CF3)C(O)OG
r = 1-30
t = 1-40
G = H', Na~, Li~, K', NH~', Mg'2, Ca'2
C1-, Br~, -OTs, -OMs, etc.
CA 02211412 1997-07-24
W 096/27704 PCTAEP96/00811
28
Examples of commercially available compounds within the
scope of formula III include those compounds supplied under
the Krytox~ series by DuPont having a formula:
Il
CF3(CFCF2O)XCFCO-NH4+
CF3 CF3
wherein x is 1-50.
Other compounds within the scope of formula III are made as
known in the art and described in March et al., Supra.
The fourth group of surfactants useful in the invention
include siloxanes containing surfactants of formula IV
MDxD*yM (IV)
wherein M is a trimethylsiloxyl end group, Dx is a
20 dimethylsiloxyl backbone which is CO2-philic and D~y is one
or more methylsiloxyl groups which are substituted with a
CO2-phobic R or R' group,
wherein R and R each independently have the following
formula:
(CH2) ~ (C6H4) ~ (A) d- [ (L) e~~ (A ) f~] n~ (L )gZ(G) h
wherein a is 1-30, preferably 1-25, most preferably 1-
20,
b is 0 or 1,
C6H4 is unsubstituted or substituted with a C110 alkyl
or alkenyl, and
A, A', d, L, e, f, n, L , g, Z, G and h are as defined
above and mixtures of R and R' thereof.
- ~ ~= =
CA 02211412 1997-07-24
W 096/27704 PCTAEP~G~ Lll
29
The DX:D*y ratio of the siloxane cont~; n; ng surfactants
should be greater than 0.5:1, preferably greater than 0.7:1
and most preferably greater than 1:1.
5 The siloxane compounds should have a molecular weight
ranging from 100 to 100,000, preferably 200 to 50,000, most
preferably 500 to 35,000.
Silicones may be prepared by any conventional method such
10 as the method described in Hardman, B. "Silicones" the
~.ncycl ope~;a of Poly~er Science ~n~ ~ng; ~eer; ng, V. 15, 2nd
Ed., J. Wiley and Sons, NY, NY (1989).
Examples of commercially available siloxane containing
15 compounds which may be used in the invention are those
supplied under the ABIL series by Goldschmidt.
Suitable siloxane compounds within the scope of ~ormula IV
are compounds of formula V:
~ . ~ -- --.
CH3 ICH3 CH3
(CH3)3-Si-O Si-O- Si-O Si-O- Si-(CH3)3 (V)
CH3 R R
the ratio of x:y-and y' is greater than 0.5:1,
preferably greater than 0.7:1 and most preferably greater
30 than 1:1, and
R and R are as defined above.
Preferred CO2-phobic groups represented by R and R include
those moieties of the following formula:
(CH2)a(C6H4)~(A) d- [ (L)e-(A~)~~]~(L~)gZ(G) h
CA 02211412 1997-07-24
W 096/27704 PCTnEP96100811
- wherein a is 1-20,
b is 0,
C6H4 is unsubstituted,
A, A', d, L, e, f, n, g, Z, G and h are as defined
above, and mixtures of R and R'.
Non-limiting examples of polydimethylsiloxane surfactants
substituted with CO2-phobic R or R groups are:
Polydimethylsiloxane Surfactants
C~ H3 ,CH3 ~CH3
(CH3) 3--Si-o Si--O--~;i--O Si--O_Si- ~CH3) 3 (V)
CH3 R R
X y Y
x = 1-300; y = 1-100; y' = 1-100
R or R = (CH2),CH,
( CH2 ) ,CH3 = CH ( CH2 ) ,CH3
( CH2 ) ,O ( CH2 ) ,CH3
( CH2 ) ,S ( CH2 ) ,CH3
= (cH2)~N[(cH2)~cH3] 2
CA 02211412 1997-07-24
W O 96/27704 PCT~EF~'~ 811
31
Polydimethylsiloxane Surfactants (cont.)
R or R = (CH2),C(O)O(CH~),CH3
= (CH2),C(O)(CH2),CH3
(CH,),C(O)N[(CH2),CH3] 2
~ (CH2),(CH3)
R or R = (CH2),
(CH2),
= (CH2)~CH=CH(CH2)m
a = 1-30
m = 1-30
CA 02211412 1997-07-24
W 096/27704 PCTnEP96/00811
Polydimethylsiloxane Surfactants (cont.)
CH3 /CH3 ICH3
(CH3) 3--Si-O Si--O--Si--O Si--O _Si- (CH3) 3 (V)
CH3 R R'
" _ ~ y~ y ~
x = 1-300; y = 1-100; y' = 1-100
R or R = (CH2) ~ (CH2CH2O) pH
(CH2) ~ (CH2CH2O) yCH3
( CH~ ), ( CH2CH2O ) y ( CH2 ) mCH3
(CH2) ~ (CH2CH (CH3) O) pH
( CH2 ) ~ ( CH2CH ( CH3 ) O ) pCH3
( CH2 ), ( CH2CH ( CH3 ) O ) p ( CH2 ) mCH3
( CH2 ) . COOG
( CH2 ) .SO3G
(CH2) ,OP (O) (OG) 2
[ (CH2) ,0] P (O) (O (CH2)l"CH3) (OG)
( CH2 ) "0 ( CH2 ) mCOOG
( CH2 ) ,S ( CH2 ) mCOOG
= (CH2)~N[(CH2)mC~~G] 2
( CH2 ) ,0 ( CH2 ) mSO3G
( CHZ ) .S ( CH2 ) mSO3G
(CH2)~,N[ (CH2)mS03G] 2
= (CH2)~O(CH2)mOP(O)(oG)2 a = 1-30; m = 0-30
= (CH2) ~S (CH2) m~P (O) (OG) 2 P = 0-50i P ~ 50
G = H+, Na+, K+,
NH~+,
( CH2 ) "0 ( CH2 ) mN ( CH3 ) 3G Mg+2 Ca+2 Cl- Br~
( CH2 ) ,O ( CH2 ) mN ( CH3 ) 3G -OTS, -OMs, etc .
CA 02211412 1997-07-24
W O 96/27704 PCT~EP9''~
Polydimethylsiloxane Surfactants (cont.)
R or R = (CH,),(CH2CH20)pH
= (cH2)~(ocH2cH2o)p(ocH2cH(CH3))p-OH
= (cH2)~(ocH2cH2o)p(OcH(CH3)cH2)p~OH
= (CH~)~(OCH2CH2O)p(CH2)~COOG
(CH2), (OCH2CH20) p (CH2) mS~3G
R = (CH2)~ ~ COOG
= (CH~)~ ~ SO3G
= (CH2)~O(CH2)~ ~ COOG
= (CH2)~S(CH2)~ ~ COOG
(CH2)~0(CH2)m ~--503G
~ /'=\
- (CHz)~S(cH2)m ~ 03G
CH20 (CH2) nCH3
~0~
HO ~ OH 0~
OH
CA 02211412 1997-07-24
W O 96/27704 : - : ''p~T~5P~5/00;2~1
34
F~n zyrn e B
Enzymes may additionally be added to the dry cleaning
system of the invention to improve stain removal~ Such
enzymes include proteases (e.g., Alcalase, Savinase and
5 Esperase from Novo Industries A/S); amylases (e.g.,
Termamyl from No~o Industries A/S); lipases (e.g~,
Lipolase ~rom Novo Industries A/S); and oxidases. The
enzyme should be added to the cleaning drum in an amount
from 0.001~ to 10~, preferably O.01% to 5% The type o~
10 soil dictates the choice of enzyme used in the system. The
enzymes should be delivered in a conventional manner, such =~
as by preparing an enzyme solution, typically o~ 1~ by
volume (i.e., 3 mls enzyme in bu~fered water or solvent).
15 Mo~;~;ers
In a pre~erred embodiment, a modi~ier such as water, or a
useful organic solvent may be added with the stained cloth
in the cleaning drum in a small volume. Pre~erred amounts
of modifier should be 0.0~ to about 10~ by volume, more
20 preferably 0.0% to about 5% by volume, most preferably 0.0%
to about 3~. Preferred solvents include water, ethanol,
acetone, hexane, methanol, glycols, acetonitrile, Cl10
alcohols and Cs1s hydrocarbons. Especially preferred g~
solvents include water, ethanol and methanol.
Per~c;d Precursors
Organic peracids which are stable in storage and which
solubilize in densified carbon dioxide are effective at
bleaching stains in the dry cleaning system. The selected
30 organic pera~id should be solub~e in carbon dioxide to
~ 3.~5 ~ 6~ qxlO3~ ~J
greater than O.~)Ul w~ s a~ pressures ofl(SOO-10,000 psi) and
temperatures of 0-100~C. The peracid compound should be
present in an amount of about 0.01~ to about 5~, preferably
0.1% to arbout 3%.
~IE~DE~ S~7
CA 02211412 1997-07-24
W 096/27704 PCT~E~
The organic peroxyacids usable in the present invention can
contain either one or two peroxy groups and can be either
aliphatic or aromatic. When the organic peroxyacid i8
aliphatic, the unsubstituted acid has the general formula:
O
HO~O~C~(CH2)n~y
where Y can be, for example, H, CH3, CH2Cl, COOH, or COOOH;
10 and n is an integer from 1 to 20.
When the organic peroxy acid is aromatic, the
unsubstituted acid has the general formula:
UO
HO-O-C-C6H4-Y
wherein Y is hydrogen, alkyl, alkylhalogen, halogen, or
COOH or COOOH.
CA 02211412 1997-07-24
W 096/27704 PCTi~G~'~C~ll
Typical monoperoxyacids useful herein include alkyl
peroxyacids and aryl peroxyacids such as:
(i) peroxybenzoic acid and ring-substituted
peroxybenzoic acid, e.g. peroxy-a-naphthoic acid;
(ii) aliphatic, substituted aliphatic and arylalkyl
monoperoxy acids, e.g. peroxylauric acid, peroxystearic
acid, and N,N-phthaloylaminoperoxycaproic acid tPAP)i and
(iii) amidoperoxy acids, e.g. monononylamide of
either peroxysuccinic acid (NAPSA) or of peroxyadipic acid
10 (NAPAA).
Typical diperoxy acids useful herein include alkyl diperoxy
acids and aryldiperoxy acids, such as:
(iii) 1,12-diperoxydodecanedioic acid;
(iv) 1,9-diperoxyazelaic acid;
(v) diperoxybrassylic acid; diperoxysebacic acid
and diperoxyisophthalic acid;
(vi) 2-decyldiperoxybutane-1,4-dioic acid;
(vii) 4,4'-sulfonylbisperoxybenzoic acid; and
(viii) N,N~-terephthaloyl-di(6-aminoperoxycaproic
acid) (TPCAP).
Particularly preferred peroxy acids include PAP, TP~AP,
haloperbenzoic acid and peracetic acid.
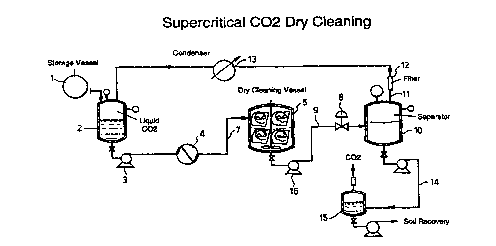
D~y Cl ~n; n~ Proce s
A process of dry cleaning using densified carbon dioxide as
the cleaning fluid is schematically represented in Figure
1. A cleaning vessel 5, preferably a rotatable drum,
30 receives soiled fabrics as well as the selected surfactant,
modifier, enzyme, peracid and mixtures thereof. The
cleaning vessel may also be referred to as an autoclave,
particularly as described in the examples below.
35 Densified carbon dioxide, such as supercritical fluid
carbon dioxide, is introduced into the cleaning vessel from
.. . , . , . ~
. CA 02211412 1997-07-24
W 096127704 ~ . ~. P~Tn~P9~/008il
~ "
37
a storage vessel l. Since much of the CO2 cleaning fluid
is recycled within the system, any losses during the dry
cleaning process are made up t~rough a CO2 liquid supply
vessel ~. The CO fluid is pu~ped into the cleaning vessel
~4-~4 an~ 6~9 ~1O3~J
5 by a pump ~ at ~es~L~s ranging between ~00 and l0,000
~-52 ~ ~1'4x1~3K~J ~
ps~, pre~era~ly~ to 5000 ps~. The CO2 fluid is heated
to its supercritical range of about 20~C to about 60~C by a
heat exchanger 4.
lQ During operation, the densified CO2 is transferred ~rom the
t_,. 5upply vessel 2 to the cleaning vessel 5 through line 7 ~or
a dry cleaning cycle of between about lS to about 30
minutes. Before or during the cleaning cycle, surfactants,
modi~iers, enzymes, peracid and mixtures thereof as
15 discussed above are introduced into the cleaning vessel,
pre~erably through a line and pump system connected to the
cleaning vessel.
At the end of the dry cleaning cycle, dirty CO2, soil and
20 spent cleaning agents are trans~erred through an expansion
valve 6, a heat exchanger 8 by way of a line 9 into a ~lash
drum lO. In the ~lash drum pressures are reduced to
5- 5~ ~103 i~ ), pS,) (~ ;~C103./~lJ~J
Detween a~out~800~and about)~l,0~0 ~ psi) and to a
~- . temperature of about 20~C to about 60~C. Gaseous CO2 is
25 separated ~rom the soil and spent agents and transferred
via line ll through a filter 12 and condenser 13 to be
recycled back to the supply vessel 2. The spent agents and
residue CO2 are trans~erred via line 14 to an atmospheric
tank 15, where the rPm~; n; ng C02 iS vented to the
30 atmosphere.
Other processes known in the art may be used in the claimed
dry cleaning system such as those described in Dewees et
al., US Patent No. 5,267,455, owned by The Clorox Company,
35 herein incorporated by reference.
AMENDED SHEET
CA 02211412 1997-07-24
W O 96/27704 ~ : P~lf~GiO~1
38
~b&fsl~d 3, S ~ d l~ ~I b ~
The following examples¦will more fully illustrate the
embodiments of the invention. All parts, percentages and
proportions referred to herein and in appended claims are
by weight unless otherwise indicated. The de~inition and
5 examples are intended to illustrate and not limit the scope
of the invention. ~aY~pl~S ~r~be~ , 4 o~ 9 ~ ¦ Z
~Q ~Cv~ C~ m ~v'C
~x~le 1
Hydrocarbon and fluorocarbon containing surfactants useful
lo in the invention must exhibit a hydrophilic/lipophilic
balance of less than 15. This example describes the .
calculation of HLB values for various surfactants to
determine their effecti~eness in supercritical carbon
dioxide. This calculation for various hydrocarbon and
15 fluorocarbon surfactants is reported in the literaturel and
is represented by the following equation:
HLB = 7 + ~(hydrophilic group numbers) -
~(lipophilic group numbers)
The hydrophilic and lipophilic group numbers have been
assigned to a number of common surfactant functionalities
including hydrophilic groups such as carboxylates, sulfates
and ethoxylates and lipophilic groups such as -CH2, CF2 and ~v
25 PPG's.l These group numbers for the functional groups in
surfactants were utilized to calculate the HLB number for
the following hydrocarbon or fluorocarbon surfactant:
AM~NDED SHEET
CA 022ll4l2 l997-07-24
W 096l27704 PCT~Er~C
39
Sll~f~ct~nt Tr~ m~ E~
l CF3 (CF2) ~CH2H2O (CH2CH2O) rN Zonyl FSN2 2.1
2 CF3 (CF2 ) ~CH2CH2O (CH2CH20) l2HZonyl FSo3 3.4
3 CF3 (CF) "CH2CH2C tO) O (CH2) loCH3 ------ 4 . 6
4 CF3 (CF2) l2CH2CH2C (O) O (CH2) sCH3 7 .1
CF3 (CF2) ~CH2CHzC ~O) ONa ------ 17 . 3
6 CF3 (CF2) l2CH2C~2C (O) ONa ------ 13 . 8
7 CF3 (CF2)~CH2CH2SO3Na Zonyl TBS~ 9 . 2
8 CF3 (CF2) l2CH2CH2SO3Na 5 . 7
9 HO (C~2CH2O) 3 (CH (CH3) C~20) 30 (CH2CH20) 3H Pluronic 3.o
10 HO (CH2CH2O) 2 (CH (CH3) CH2O) 16 (C~2CH20) 2H Pluronic 4.5
L3l6
11 HO (CH2CH2O) 8 (CH (CH3) CH2O) 30 (CH2CH2O) ~H Pluronic 7 . 0
L627
12 (CH2CH2O) 7 (CH (CH3) CH2O) 2~ (CH2CH20) 7HPluronic 12 . O
~43~
13 HO (CH (CH3) CH2O) ll (CH2CH2O) g (CH2CH (CH3) O) l2H Pluronic 8.0
17~29
14 Polyethylene glycol surfactant (PEG) Akyporox NP 19. 2
12 0 0 V10
15 PEG 100- Laurate 19.1
16 Linear alkyl benzene sulfonate 20 . 0
17 Sodium lauryl sulfate 40.0
18 Sodium Cocoyl Sarcosinate 27 . O
Attwood, D.; Florence, A. T. "Surfactant Systems: Their
chemistry, pharmacy and biology.", Chapman and Hall, NY,
1983, pp. 472-474.
2-4 Supplied by Dupont.
5-9 Supplied by BASF.
o Supplied by Chem-Y GmbH of Germany.
The conventional surfactants (Nos. 14-18) exhibit an HLB
value of greater than 15 and are not effective as dry
cleaning components in the invention.
CA 02211412 1997-07-24
W O 96/27704 ' ~ ' ; ~CT ~ 6j~0~11
~ple 2
Supercritical fluid carbon dioxide only as a cleaning
medium was used to dry clean several hydrophobic stains on
cotton and wool fabrics.
The stained fabrics were prepared ~y taking a two inch by
three inch cloth and applying the stain directly to the
cloths. The cloths were allowed to dry.
The stained fabrics were then placed in a 300 ml autoclave
having a gas compressor and an extraction system. The ~_
stained cloth was hung ~rom the bottom of the autoclave's
overhead stirrer using a copper wire to promote good
agitation during washing and extraction. After placing the
cloth i~ ~he autoclave and sealing it, liquid CO2 at a tank
5 ~ ~ ~103~'r~ ~--
pressul~ ufl(850 psi)was allowed into the system and was
heated to reach a temperature of about 40~C to 45~C. When
the desired temperature was ~eached i3n ~e autoclave, the
pressure inside the autoclave ~d~ lllCreaSea~(4, 000 pSi) by
pumping in more CO2 with a gas compressor. The stirrer was
then turned on for 15 minutes to mimic a wash cycle. At
the completion of the wash cycle, 20 cubic feet of fresh
CO2 were passed through the system to mimic a rinse cycle.
The pressure of the autoclave was then released to
atmospheric pressure and the cleaned cloths were removed
from the autoclave. To measure the extent of cleaning, the
cloths were placed in a Reflectometer- supplied by
Colorguard. The R scale, which measures darkness ~rom
black to white, was used to determine stain removal.
Cleaning results were reported as the percent stain removal
according to the ~ollowing calculation:
~ stain removal = st~in remove~ = cle~ne~ cloth re~ing -
st~ine~ cloth re~;ng x 100~
~ stain applied unstained cloth
reading - stained cloth reading
~HS a~
CA 02211412 1997-07-24
W096/27704 PCT~6/00811
41
The cleaning results fox the cotton and wool cloths dry
cleaned with supercritical ~luid carbon dioxide alone are
in Table 1 below.
T~hle 1
Dry Cl~n; n~ Resul~s on Several Hydrophobic Stains
Using Supercritical Carbon Dioxide Only A~ Cle~n;n~ Medium
Stain Cloth % Stain Remo~al
Ragu spaghetti Cotton 95
sauce
Sebum Wool 99
Olive Oil with Wool 97
Blue Dye
Lipstick Wool *
The results confirm what was known in the art: that
hydrophobic stains are substantially removed with
supercritical ~luid carbon dioxide alone. However, the
lipstick stain, which is a compound hydrophobic stain with
pigment particulates, was removed only to the extent of its
waxy components. The colored portion of the stain fully
rem~;ned.
~ e 3
The hydrophilic stain, grape juice, was dry cleaned using
supercritical fluid carbon dioxide, a polydimethylsiloxane
surfactant, water as a modifier and mixtures thereof
according to the invention.
Two inch by three inch polyester cloths were cut and
stained with concentrated grape juice which was diluted
1:10 with water. The grape juice stain was then dried and
was approximately 2 wt.~ and 7 wt.~ grape juice stain after
drying. The cloths were then placed in the autoclave as
~ CA 02211412 1997-07-24
W O 961Z7704 . , ~ P~T~ n6/~08~1
described in E~ample 2, except these experiments were run
~ 4l ~o3Kr~J
at a press~re o~l(6,000 ps~.
Two di~erent polydimethylsiloxane sur~actants were used
alone or in combination with O.S ml of water and
supercritical fluid car~on dioxide. The control was
supercritical fluid carbon dioxide alone.
The water was added directly to the bottom of the autoclave
and noti on the stain itself and the surfactant was applied
directly to the stain on the cloth. A~ter the wash and
rinse cycles, cleaning results were evaluated and the
results are reported in Table 2 below.
T~hle 2
Dry Cleaning Results on Grape Juice Stains ~sing
Supercritical Carbon Dioxide and Polydimethylsiloxane
Surfac tant
' stain~ Cloth-r-~, Surfacta~t ~._Modlfler: %-.stal~.
~ Remo~al
2~ grape Polyester None None 18
~ ulce
2~ grape Polyester O.2 g ABIL None O
juice 88184l (darker)
7~ grape Polyester None 0.5 ml 21
~ulce water
7~ grape Polyester O.2 g ABIL O.5 ml 49
juice 88184 water
7~ grape Polyester O.2 g ABIL O.5 ml 51
f juice 88512 water
A~,N~ S~'t~
CA 02211412 1997-07-24
W 0~6/27704 PCTnEP96/008
43
A polydimethylsiloxane having a molecular weight of
13,200 and 5~ of its siloxyl group substituted with a 86/14
ethylene oxide/propylene oxide chain supplied by
Goldschmidt of Virginia.
2 A polydimethylsiloxane having a molecular weight of 7,100
and 14~ of its siloxyl yroup substituted with a 75/25
ethylene oxide/propylene oxide chain also supplied by
Goldschmidt.
It was observed that the combination of water as a modifier
with the selected polydimethylsiloxane surfactants improved
dry cleaning results in supercritical fluid carbon dioxide.
In fact, none of the three components alone removed
substantially any of the grape juice stain.
~mple 4
As a comparison with the prior art, a conventional alkane
surfactant was used alone or in combination with a modifier
and supercritical CO2 to dry clean the hydrophilic stain,
grape juice, on polyester, as described in Example 3 above.
The surfactant, linear alkylbenzene sulfonate is a solid
and has an HLB value of 20. The LAS was added to the
bottom of the autoclave with varying amounts of water. The
following cleaning results were observed and are reported
in Table 3 below.
CA 02211412 1997-07-24
W 096127704 PCTAEF7G~'~CUll
T~h~e 3
Dry Cl~; n~ ResultQ on Grape Juice Stain~ Using
Supercritical Carbon Dioxide and T-; ~e~ Al]cylbenzene
Sulfonate Surfactant (hAS)
Stain Cloth Surfactant Modifier ~ Stain
: Remo~al
.
2~ grape Polyester None None 18
juice
7~ grape Polyester 0.25 g LAS 0.5 ml 0
juice water(darker)
7~ grape Polyester 0.25 g LAS 6.0 ml 75
juice water
2~ grape Polyester 0.12 g LAS 6.0 ml 84
juice water
2~ grape Polyester 0.12 g LAS 0.5 ml Stain
juice water moved on
cloth
It was observed that LAS was only effective in a larger
amount of water (6 ml). When the modifier was reduced from
6 ml to 0.5 ml, the stain only wicked up the cloth and was
not removed.
It i8 noted that DE 3904514 describes dry cleaning using
supercritical fluid carbon dioxide in combination with a
conventional surfactant. The publication exemplifies
cleaning results with LAS. The experimental conditions in
the examples state that the stained cloth has only minimal
contact with supercritical fluid carbon dioxide, namely a
10 minute rinse only. It appears that the cleaning obtained
with LAS and the large amount of water is similar to spot
CA 02211412 1997-07-24
W 096/27704 PCTI~r~G~'~ ell
or wet cleaning, since the cloth rP~;n~ wet at the end of
the process. There appears to be little to m;n;m~l
influence of the supercritical fluid carbon dioxide on spot
removal under these conditions.
~dditionally, in a dry cleaning process, the use of LAS
with supercritical fluid carbon dioxide would not be
possible with water-sensitive fabrics such as silks and
wools since such large amounts of water are necessary.
~ le 5
A hydrophilic stain, namely grape juice, was dry cleaned
using polydimethylsiloxane surfactants with water and
supercritical fluid carbon dioxide according to the
invention.
Polyester cloths were stained with 7~ grape juice stain as
described in Example 3 above. Two different
polydimethylsiloxane surfactants were used with varying
amounts of water and supercritical fluid carbon dioxide.
In comparison, LAS, the conventional surfactant, used with
the same amounts of water was used to remove the grape
juice stains. The cleaning results for the two types of
surfactants are reported in Table 4 below.
CA 02211412 1997-07-24
W 096/27704 PCTAEF9''~-~11
46
T~hle 4
Dry Cle~n;~ Re~ult~ on Grape Juice Stai3ls Using
Supercritical Carbon Dioxide and Surfactants with Increased
Water Levels
Stain Cloth Surfactant Modifier % Stain
. ~ Removal
7~ grape Polyester 0.25 g.6.0 ml 75
juice LAS water
7~ grape Polyester0.25 g. 0.5 ml o
juice LAS water(darker)
7~ grape Polyester 0.2 g ABIL 6.0 ml 41
juice 88184l water
7~ grape Polyester 0.2 g ABIL 0.5 ml 49
juice 88184 water
7~ grape Polyester 0.2 g ABIL 6.0 ml 43
juice 88184 water
7~ grape Polyester 0.2 g ABIL 0.5 ml 51
juice 88512 water
A polydimethylsiloxane having a molecular weight of
13,200 and 5~ of its siloxyl group substituted with a 86/14
ethylene oxide/propylene oxide chain supplied by
Goldschmidt.
2 A polydimethylsiloxane having a molecular weight of 7,100
and 14~ of its siloxyl group substituted with a 75/25
ethylene oxide/propylene oxide chain also supplied by
Goldschmidt.
CA 02211412 1997-07-24
W o 96J27704 ~ ~Cl~EP~/O;a8~I
47
It was observed that the modi~ied polydimethylsiloxane
surfactants according to the invention are more effective
in the presence of less water (0.5 ml vs. 6.0 ml) as
cleaning was reduced from 50~ to 40~ when the water levels
were increased. The opposite effect was observed with LAS,
as stain removal increased ~rom 0~ to 75~ as the water
levels were increased to 6.0 ml. Thus, the claimed siloxane
surfactants provide better cleaning results with less water
which is bene~icial for water sensitive fabrics.
le 6
_
Polydimethylsiloxanes having varying molecular weights and
alkyl substituted moieties were tested as surfactants with
supercritical fluid carbon dioxide in the inventive dry
cleaning process. Various types of stained cloths were
tested under the dry cleaning conditions described in
Example 2 above.
A compound hydrophobic stain, red candle wax, was placed on
both cotton fabrics as follows. A candle was lit and
approximately 40 drops of melted wax were placed on each
cloth so that a circular pattern was achieved. The cloths
. = ~
-~ were then allowed to dry and the crusty excess wax layer
was scraped off the top and bottom of each stain so that
only a flat waxy colored stain was left.
Red candle wax was placed on the wool cloth by
predissolving the red candle in hexane and then pipetting
an amount of the hexane solution onto the fabric. The
~abric was dried and the resulting fabric contained about
10 wt.% stain.
As stated abov~, the pressure of therautoclave during the
. ~ 4~ /03~ ~~
washlny cycle was~(6000 ps~ at a temperature of 40~C with a
AMENDEI~ s~'r-'~
CA 02211412 1997-07-24
W 096/27704 PCTAEP96/008II
48
15 minute cycle. Twenty cubic feet of supercritical fluid
carbon dioxide was used for the rinse cycle.
Five types of modified polydimethylsiloxanes ha~ing formula
v:
~ .~ ., _ .
~H3 fH3 CH3
(CH3)3-Si-o Si-o _ Si-O _ Si-O - Si - (CH3)3 (V)
CH3 R R
~ J ~
X y y.
wherein x:y and y' ratio is 2 0.5:1 and R and R' are each
independently a straight or branched Cl30 alkyl chain were
prepared. The compound formula is represented as MDX
D*~M(Cz) wherein M represents the trimethylsiloxyl end
groups, Dx represents the dimethylsiloxane backbone (CO2-
philic), D*y represents the substituted methylsiloxyl group
(CO2-phobic) and (Cz) represents the carbon length of the
alkyl chain of R.
Molecular weights of the siloxanes ranged from 1,100 to
31,000. The polydimethylsiloxanes straight chain alkyl
group ranged from C8 to Cl8 carbons. The red wax stained
cloths were cleaned and the cleaning results were observed
and are reported in Table 5 below. No modifier was used.
CA 02211412 1997-07-24
W096/27704 PCT~P96/00811
49
T~hle 5
Red Candle Wax Stains Dry Cleaned with Modified
PolydimethylsiloY~es A~d Supercritical C~ho~ Dioxide
Stain Cloth Surfactant. % Stain
(0.2 g) ~- v~l
... . . . ~ , . . . . . .. ~
Red candleCotton None 13
wax
Red candleCottonMD10oD*2M(cl8) 20
wax
Red candleCottonMD40oD*8M(c8) 38
wax
Red candleCottonMD153D*lsM(cl2) 60
wax
Red candleCottonMD27oD*13M(Cl2) 64
wax
Red candleCottonMD124D*11M(Cl2) 59
wax
Red candle Wool None 33
wax
Red candle WoolMD153D*1.5M(Cl2) 54
wax
A copolymer of polydimethylsiloxane and a stearyl
substituted silicon monomer having a molecular weight of
8,200 and prepared as described in Hardman, B., "Silicones"
The Encyclopedia of Polymer Science and Engineering, v. 15,
2nd ed., J. Wiley and Sons, NY, NY (1989).
CA 02211412 1997-07-24
W O 96/27704 PCTAEP96/00811
2 A copolymer of polydimethylsiloxane and an octyl
substituted hydrocarbon silicon monomer having a molecular
weight of 31,000 and prepared as described in I~ardman
Sl~pr~.
3 A copolymer of polydimethylsiloxane and a lauric
substituted hydrocarbon silicon monomer having a molecular
weight of 1,500 and prepared as described in Hardman,
Sl~pr~ .
4 A copolymer of polydimethylsiloxane and a lauric
substituted hydrocarbon silicon monomer having a molecular
weight of 2,450 and prepared as described in Hardman,
.~t~pr~ .
5 A copolymer of polydimethylsiloxane and a lauric
substituted hydrocarbon silicon monomer having a molecular
weight of 1,170 and prepared as described in Hardman,
sl~r~.
It was observed that the modified polydimethylsiloxanes in
combination with supercritical fluid carbon dioxide
significantly improved removal of a compound hydrophobic
stain from both cotton and wool fabrics over the use of CO2
alone. It was also observed that the lower molecular
weight silicone surfactant5 (e-g-, MDl2.4D1.1*M(C12);
MD153D*1sM(Cl2); and MD270 D*11M(C12)) are more effective at
stain removal than the silicone surfactants having higher
molecular weights (e.g., MDlooD*2M(Cl8) and MD400D*8M(C8))
regardless of chain length of the alkyl moiety. Especially
beneficial were lower molecular weight silicones with chain
lengths of C1014.
CA 02211412 1997-07-24
- W 0 96/27704 ~ C~yE~6~0,~81
51
le 7
.,
A glycerated siloxane surfactant having a formula MD~*yM
wherein D*y is substituted by -(CH2)30C~2CX(OH) CH20H was used
to dry clean a grape juice stain on a polyester cloth under
the dry cleaning conditions described in Example 2 above.
About 0.2 gram o~ the sur~actant was combined with 0.5 ml.
water. The glycerated siloxane is a polydimethylsiloxane
with a glycerol side chain having a molecular weight o~ 870
and prepared as described in Hardman, Su~r~.
.
It was observed that the glycerated siloxane removed 33~ of
the grape juice stain.
~am~le 8
Various fluorinated sur~actants, either alone or with
water, were used with supercritical fluid carbon dioxide to
clean several types of stained fabric under the dry
cleaning conditions described in Example 2.
I O ~ ~J_~
Specifically, the pressure in the autoclave was~(4000 psi)
and the temperature was 40~C to 45~C.
_'~ .
Cotton stained with red candle wax and polyester stained
with grape juice were cleaned with the ~luorinated
surfactants and the following cleaning results were
observed as reported in Table 6 below.
.
c,~'~C~
CA 02211412 1997-07-24
W 096/27704 PCTAEP9G,'~C~ll
52
T~hle 6
Stains Dry Cleaned with Fluorinated Surfactants
and Supercritical Fluid Carbon Dioxide
Sta'ln~ - Clot~.-~ ~.Sùrfactant. Modifier ~ Stain
Red candle Cotton None None 13
wax
Red candle Cotton 0.6 g None 70
wax Krytox~
2~ grape PolyesterNone None 18
juice
2~ grape Polyester~0.25 g 0.5 ml 11
juice FSA2 water
2~ grape Polyester0.2 g FSO- 1.0 ml 43
juice 10 o3 water
2~ grape Polyester0.2 g FSN4 1.0 ml 48
juice water
2~ grape Polyester ~0.2 g FSA 1.0 ml 9
juice water
l A fluorinated polyether ammonium carboxylate supplied as
Krytox~ surfactant by DuPont, Inc. of Delaware.
2 A fluorinated nonionic having a lithium carboxylate salt
supplied under the Zonyl surfactant series by DuPont, Inc.
of Delaware.
3 A fluorinated nonionic surfactant supplied under the
Zonyl surfactant series by DuPont, Inc. of Delaware.
CA 02211412 1997-07-24
W O 96~27704 ; . PCl~E~61Q~811
.
53
4 A fluorinated nonionic surfactant supplied under the
Zonyl sur~actant series by DuPont, Inc., of Delaware.
It was observed that all of the fluorinated surfactants
equalled or improved dry cleaning of the tested stains over
the use of supercritical fluid carbon dioxide alone. It
was further observed that the ~luorinated nonionic
surfactants (FSO-lO0 and FSN) were more effective than the
fluorinated nonionic having a lithium carboxylate salt
(FSA).
~x~le 9
Various bleaching peracids were combined with supercritical
fluid carbon dioxide to dry clean stained fabrics.
The bleaching peracids tested include m-chloroperbenzoic
acid (m-CPBA), p-nitroperbenzoic acid (p-NPBA) and 6-
phthalimidoperoxy hexanoic acid (PAP) in an amount of about
0.2 to 0.5 grams each. Cotton stained with red candle wax
was cleaned as described in Example 5. The wash cycle o~
~ 4x103~
the dry cleaning Sys~1.1 was run at~(6000 psi)and 45~C as
described in Example 2. The coffee stains were applied to
F .:-
~~ polyester and wool cloths.
At the end of the cleaning cycle, the stained cloths were
evaluated and the results are reported below in Table 7.
~ ~t'''-~'
.
CA 02211412 1997-07-24
W O 96127704 - .~CT~EP96/O~
54
T~hle 7
Stains Dry Clea~ed with B1~h; ng Peracids
and Supercritical Fluid Carbon Dioxide
Stain Cloth Surfact. Modi~ier % Stain
~ ; ; Remo~al
Red Cotton None None 13
candle
wax
Red Cotton 0.5 g m- None 94
candle CPBA
wax
Red Cotton 0.11 g None 72
candle p-NPBA2
wax
Red Cotton 0.26 g None 50
candle PAP3
wax
Coffee Polyester 0.5 g m- None45
CPBA
Coffee Wool None None O
1 m-chloroperbenzoic acid having a solubility of )0.15 g at
f3~ 3~ 9oo psi), at 45~C, in 59.8 g CO2 and supplied by Aldrich
Chemical Co.
2 p-nitroperbenzoic acid having a solubility of )0.05 g at
~3-1x)O ~ ~1900 ps~, at 45~C, in 59.8 g CO2 and supplied by Aldrich
Chemical Co.
_t,t~ t--l~t~_, i
CA 02211412 1997-07-24
W O 96/Z7701 ~ ~ ~CT~EF~6/O~B~l
3.~xl~l<~6-phthalimidoperoxy hexanoic acid having a solubility of
0.05g a~2,000 ps~, at 45~C, in 59.8 g CO2 supplied by
Ausimont.
The results show that the three peroxides tested
signi~icantly improved stain removal on the two types of
stains cleaned over supercritical fluid carbon dioxide
alone
~t,~C,iTQ~ 1 e 10
-, Protease enzyme was used in supercritical carbon dioxide to
_ . ,
clean spinach stains from cotton cloth. Three (3) mls of
protease enzyme (Savinase supplied by Novo, Inc.) was added
to buffered water to ~orm a 1~ solution and then added to
each cloth. The cloths were then washed and rinsed as
described in Example 2 above. The cleaning results
observed and calclllated are as shown in Table 8 below:
T~hle 8
Stain~ Drycleaned with Savina~e in Supercritical
Carbon Dioxide
,--" Stain Cloth Enzyme Modifier % Stain
Solution Removal
Spinach cotton none none 6.9
Spinach cotton Savinase none 26.5
These results show enhanced cleaning of the spinach stain
over supercritical carbon dioxide alone when the enzyme is
added to the system.
~,t~ ,C~ t~ ~ r
CA 02211412 1997-07-24
W 096/27704 PCT~6100811
56
~Y~ e 1 1
Lipolase enzyme (1% enzyme solution of 3 mls in buffered
wear) was used in supercritical carbon dioxide to clean red
c~ndle wax stains from rayon cloth. The procedure used was
identical to that of Example 10. The results are
summarized in Table 9 below.
T~hle 9
Stains Dry Cleaned with Lipolase in Supercritical
Carbon Dioxide
Stain Cloth Enzyme Modifier % Stain
Solution Removal
Red Candle rayon none none 51
Wax
Red Candle rayon Lipolase none 60
Wax
Red Candle cotton none. none 13
Wax
Red Candle cotton Lipolase none 64
Wax
The results in Table 9 show enhanced cleaning of the red
candle wax stain when lipolase is used in conjunction with
supercritical carbon dioxide, on both rayon and cotton
cloths.
~m~le ~2
Amylase enzyme (1~ enzyme solution of 3 mls enzyme in
buffered water) was used to dryclean starch/azure blue
CA 02211412 1997-07-24
W ~ 96127704 ~ - pc~E~6/eG
57
stains on wool cloth in supercritical carbon dioxide. The
blue dye is added to make the starch stain visible so that
~ its removal may be detected by the reflecto~eter. The
drycleaning procedure used was identical to that of example
lO, and the results are presented in Table 10 below.
T~hle 1O
Dry Cl~n;ng o:~ Starch/Azure Blue Dye Stai~ on Wool Using
Amylase i~ Supercritical Car~on Dioxide
Staln Cloth E~zyme Modi~ier % Stai~
Solution Removal
Starch/Az wool none none cloth gets
ure Blue darker
Starch/Az wool Termamyl none 25.6
ure Blue
The results in Table 10 show that the Termamyl enzyme is
effective at cleaning the starch stain from wool cloth in
, . supercritical carbon dioxide.
~ le 13
Dry cleaning of grape juice stain was conducted on cloths
other than polyester fabric. The experiments on rayon and
silk cloth were conducted using the same procedure as in
Example 3, using cloths with 23w~t~%, grape ju~e~s6txali~ns~p~
with water as a modifier at pressures of~(6000 psi) an~;(40 0
psi)as noted in Table 11.
CA 02211412 1997-07-24
W O 96/27704 PCTJ~l~
58
T~hle 1l
Dry C~ n;ng of Grape Juice Stain~3 on Rayon and Silk Using
Supercritical Carbon Dioxide and Polydimethylsiloxane
Surfactant
Stain Cloth Pressure Surfactant Modifier % Stain
Remo~al
Grape rayon 6000 psi none 0.5 ml2.4
Juice water
Grape rayon 6000 psi 0.2g Abil 0.5 ml 75. 5
Juice 88184 water
Grape silk 6000 psi none0.5 ml 2.0
Juice water
Grape silk 6000 psi 0.2g Abil 0.5 ml 30.4
Juice 88184 water
Grape silk 4000 psi noneo. 5 ml 3.9
Juice water
Grape silk 4000 psi 0.2g Abil 0.5 ml 27.5
Juice 88184 water
These results show significantly enhanced cleaning of the
grape juice stain on rayon and silk when the
polydimethylsiloxane surfactant Abil 88184 is added to the
supercritical carbon dioxide dry cleaning system.
~m~le 14
Dry cleaning of red candle wax stains was conducted on
several different types of fabric, using an alkyl
modified polydimethylsiloxane surfactant, MD153D15M ~C12),
having a molecular weight of 1475 g/mole. The surfactant
was synthesized as described in Hardman, Sl~pr~. The dry
CA 02211412 1997-07-24
W O 96/27704 PCT~EF~C~C811
59
cleaning procedure used was the same as that used in
example 5, and the cleaning results are presented in the
~ollowing table.
Ts~hle 1~ ,
Dry Cle~n;~ of Red C:~ndle Wax Stains on Various Fabrics
Using an Alkyl-Modified Polydimethyl~iloxane Surfactant in
Supercritical Carbon Dioxide
Stain Cloth Surfactant % Stain
Remo~al
Red Candle cotton none 13 . O
Wax
Red Candle cotton o . 2 - O . 3g 52 . 9
Wax MDls 3D l.sM
(Cl2)
Red Candle wool none 36 . O
Wax
Red Candle wool O . 2 - O . 3g 51. 6
Wax MDls 3D 1 sM
(Cl2)
Red Candle silk none 61. 3
Wax
Red Candle silk O . 2-0 . 3g 77 . 3
Wax MDl5 3D l sM
(Cl2)
Red Candle rayon none 51. 2
Wax
Red Candle rayon O . 2-0 . 3g 50 .1
Wax MDl5 3D 1 sM
(Cl2)
CA 022ll4l2 l997-07-24
W 096/~7704 PCT/~Jr~ ~
The dry cleaning results ~how significantly enhanced
cleaning of the red candle wax stain on all fabrics except
for rayon, which shows no cleaning enhancement from
addition of the su~factant. The cleaning results for the
silk cloth are especially high, giving a cloth which looks
very clean to the eye.
.~A~pl e 15
Dry cleaning of grape juice on polyester cloth and of red
candle wax on cotton cloth was investigated at different
pressures to determine the effect of the pressure of
supercritical carbon dioxide on the cleaning effectiveness
of the system. The dry cleaning procedures used were the
same as those used in examples 3 and 6 except for the
variations in pressure, and the results are presented in
the following table.
T~h1e 13
Dry Cle~n~n~ of Grape Juice and Red Candle Wax Stains at
Different Pressure~
Stain Cloth Pressure Surfactant Modifier % Stain
Removal
Red Candle cotton 6000 psi MDls3DlsM none 52.9
Wax tC12)
Red Candle cotton 3000 psi MDis3D l5M none 51.0
Wax (Cl2)
Red Candle cotton 2000 psi MDls3DlsM none 39.3
Wax (Cl2)
Grape ~uice polyester 6000 psi Abil 88184 0.5 ml 61.0
water
Grape Juice polyester 4000 psi Abil 88184 0.5 ml 55.4
water
Grape Juice polyester 3000 psi Ahil 88184 0.5 ml 33.8
water
CA 02211412 1997-07-24
,~ - W O 96/27704 PCTJE~96/QC811
61
The results presented in the table~show that the cleaning
~ 20-7q~l~ n3~(~j
o~ red candle wax stal~s ~ inlshes between~3000 and 2000
psi~ while the cleaning of grape juice stains diminishes
~between~(4000 and 3000 psi).
G q.~d 20-~x10~
le 16
Further dry cleaning experiments were conducted on
polyester stained with grape juice using other ethylene
oxide/propylene oxide modi~ied polydimethylsiloxane
surfactants. The cleaning efficacy of these surfactants
was compared to that o~ the Abil 88184 surfactant, whose
cleaning results are presented in example 3. The dry
cleaning procedure used was that same as that in example 2.
Water (0.5 ml) was applied to the stained cloth before each
experiment was conducted. The results are presented in the
~ollowing table.
...
..... ~
c~ iC~
CA 02211412 1997-07-24
W 096127704 PCT/t~
T~hle 14
Dry Cl~n;n~ of Grape Juice on Polyester in Supercritical
Carbon Dioxide and Polydimethylsiloxane Surfactant~
Stain Cloth Surfactant Pressure % Stain
Removal
Grape polyester Abil 6000 psi 60.6
Juice 88184l
Grape polyester Abil 4000 psi 55.4
Juice 88184l
Grape polyester Abil 88782 4000 psi 38.6
Juice
Grape polyester Abil 88483 4000 psi 41.5
Juice
Grape polyester MDl27D1M 6000 psi 41.4
Juice EOlo
Grape polyester MD20D2M 6000 psi 43.7
Juice Eolo5
lA polydimethylsiloxane having a molecular weight of 13,200
and 5~ of its siloxyl groups substituted with a 86:14
ethylene oxide/propylene oxide chain. Supplied by
Goldschmidt.
2A polydimethylsiloxane having a molecular weight of 674
and having one siloxyl group substituted with a 100
ethylene oxide chain. Supplied by Goldschmidt.
3A polydimethylsiloxane having a molecular weight of 901
and having one siloxyl group substituted with a 8.5:4.5
ethylene oxide/propylene oxide chain. Supplied by
Goldschmidt.
CA 02211412 1997-07-24
~ I W O 961Z7704 : .................................................. PC~ '/0~8~1
._ ., ~ . .......................................... .......
- 63
~A polydimethylsiloxane having a molecular weight o~ 1660
~ and 6.4~ of its siloxyl groups substituted with a 100~
ethylene oxide chain. Synthesized according to Hardman,
Supr~.
~A polydimethylsiloxane having a molecular weight of 2760
and 8.3~ of its siloxyl groups substituted with a 100~
ethylene oxide chain. Synthesized according to Hardman,
Su~ra.
The dry cleaning results in the table show that all of the
.
~ ~ surfactants tested are ef~ective at removing ~he grape
juice stain from the polyester cloth, although the Abil
G 3~ 88184 is slightly better, even when the pressure is reduced
to ~000 psi). A dry cleaning run with no surfactant cleans
only 21~ o~ the grape juice stain.
~xam~le 17
The following tables show dry cleaning results on grape
juice stains made on polyester cloth where the stained
cloths were prepared by dipping the entire cloth in the
staining solution. The cloths are prepared with 2 wt.
... .
~~ stain, and otherwise, the drycleaning procedure is
identical to that of Example 3, including the use of 0.5 ml
water on each cloth prior to cleaning.
AM~5~E~ St~r
CA 02211412 1997-07-24
W 096127704 PCT~r~lU~811
64
T~hle 15
Dry Cle~n~ n~ of Dipped Grape Juice Stain~ Using Modi~ied
Polydimethylsiloxane ~urfactants in Supercritical Carbon
Dioxide
Stain Cloth Surfactant Pressure % Stain
Removal
Grape polyester Abil 6000 psi 50.2
Juice 881841
Grape polyester MD20D~2M 6000 pSi 48.0
Juice EOlo2
Grape polyester MD20D~2M 3000 psi 30.9
Juice EOlo2
Grape polyester MD20D2M 4000 psi 46.1
Juice EOlo2
Grape polyester MDlz7DlM 4000 psi 51.5
Juice Eolo3
A polydimethylsiloxane having a molecular weight of 13,200
and 5~ of its siloxyl groups substituted with a 86:14
ethylene oxide/propylene oxide chain. Supplied by
Goldschmidt.
2A polydimethylsiloxane having a molecular weight of 2760
and 8.3~ of its siloxyl groups substituted with a 100~
ethylene oxide chain. Synthesized according to Hardman
Sl~pr~.
3A polydimethylsiloxane having a molecular weight of 1660
and 6.4~ of its siloxyl groups substituted with a 100~
ethylene oxide chain. Synthesized according to Hardman
~l~pr~.
CA 02211412 1997-07-24
W O 961~7~04 . . ' PC7~rr~o~i8II
The dry cleaning results presented in this table show that
the synthesized surfactants (entries 2 and 3) are just as
e~fective at cleaning as Abil ~8184 In addition, the new
s~ ~actants are just as e~ectlv ~ (4000 psi) as they are
at~C6000 psi), although their cleaning ability ~l m; n; shes
~ ~ Zo ~~xlo~
som~wllaL dL~UL~U pS~!.
~ le 18
These experiments comprised the cleaning of both red candle
wax and grape juice stains simultaneously in the high
~ pressure autoclave. One o~ each stained cloth was used with
its respective surfactant and modi~ier (i.e. water added to
the grape juice stained cloth). The grape juice stained
cloth was prepared by the dipping method.pDry cleaning was
conducted as described in example 2 dllCi ~, at~(6000 psi~ and
43-45~C, and the results are presented in the following
table.
T~hle 16
~ixed Cloth Dry Cleaning in Supercritical Carbon Dioxide
ClothtStain Surfactant % Stain
--;
Removal
Red Wax/Cotton 0.5g Krytox~ 0.2g 77.2
Grape MDl2,DlM EOlo 45 9
Juice/Polyester
Red Wax/Cotton 0.5g Krytox~ 71.0
Grape 0.2g Abil 88184 29.8
Juice/Polyester
Red Wax/Cotton 0.2g MDls3Dl5M Cl2 50-4
Grape 0.2g MDl2~,DlM EOlo 52-8
Juice/Polyester
hMEN~ED Sh~ET
CA 02211412 1997-07-24
W 096127704 P ~ AEF~6/~BlI
66
The results in the table show that the surfactants provide
compatible amounts of cleaning of both stains, except for
the combination of Krytox(R) with Abil 88184, (entry 2),
where the effectiveness of the Abil 88184 at cleaning the
grape juice is ~im;n;shed. The cleaning ability of the
Krytox on red candle wax is actually enhanced somewhat in
combination with polydimethylsiloxane surfactants.