Note: Descriptions are shown in the official language in which they were submitted.
CA 02211848 1998-07-27
METHOD AND APPARATUS FOR PERFORMING PERITONEAL DIALYSIS
This invention relates to a new machine and method
for carrying out automated peritoneal dialysis (PD).
Background of the Invention
Unlike the extracorporeal system used in
hemodialysis (HD) to treat end stage renal disease
(ESRD), PD makes use of the internal peritoneal membrane
to purify the blood of ESRD patients. The two modalities
for carrying out PD are automated peritoneal dialysis
(APD) and the manual non-automated procedure of
continuous ambulatory peritoneal dialysis (CAPD).
According to the latter method, dialysis fluid is
exchanged from four to six times throughout the day,
every day. The fluid remains inside the patient for
about four hours between exchanges and for a much longer
period (10-12 hours) at night.
It has become conventional to refer to the basic
stages of the PD procedure as FILL, DWELL and DRAIN. In
the FILL stage, dialysate is instilled through a catheter
into the peritoneal cavity of a patient.
During the fixed time period known as the DWELL, the
dialysate draws soluble waste and excess fluid from blood
contained in numerous blood vessels of the peritoneal
membrane, by the operation of osmosis and diffusion.
Additionally, the dialysate re-balances the electrolyte
concentration and corrects for acidosis of the blood.
At the end of the DWELL, spent dialysate is removed
from the peritoneal cavity (DRAIN) and discarded. This
exchange action must be repeated several times over a
twenty-four hour period, as the body continuously
produces waste products.
~ . CA 02211848 1998-07-27
Compared with HD, PD is a very gentle modality, its
slow corrective action resembling that of the natural
kidney. It is operationally simple, eliminates the need
for venipunctures and has low operational costs. Because
the system is not an extracorporeal one, there is no need
for a high degree of heparinization, a factor which is
especially important in the case of diabetic patients.
However, to date HD has continued to dominate in the
treatment of ESRD patients. The following aspects of PD
may be contributing factors to this state of affairs:
- In PD, the peritoneal membrane is exposed to the
external environment every time a catheter is
connected or disconnected from the solution supply,
making infection (peritonitis) a significant
problem.
- Currently available commercial dialysate for PD
exhibits a low pH which is not truly compatible with
the biochemistry of the peritoneal membrane.
Consequently this bio-incompatibility is believed
to be one of the factors which eventually degrades
the performance of the membrane with time.
- The most popular osmotic agent used in PD dialysates
is glucose, Glucose can be absorbed by the body via
the peritoneum membrane. This can result in patient
obesity and its accompanying complications
Moreover, heat sterilization of the dialysate which
contains glucose produces harmful glucose by-
products.
- Current techniques of PD afford no ability to
monitor the pressure build-up in the peritoneum
during either Dwell or during the Fill sequence.
CA 02211848 1998-07-27
- Current PD solutions are of fixed composition and
cannot be systematically adjusted either in their
constituent parts or in the concentration of each
constituent during a treatment.
In a continuing effort to provide adequate PD
treatment for the varied population of ESRD pàtients,
clinicians have developed a number of different forms of
the APD modality of treatment. These include the APD
modalities of:
(i) Continuous Cycling Peritoneal Dialysis (CCPD),
a method of performing PD in which an automated
cycler performs 4 to 6 regular exchanges every
night.
(ii) Intermittent Peritoneal Dialysis (IPD), a
method of performing PD in hospitals or at home
with an automatic cycler two or three times a
week for a period of about eight to twenty
hours each time.
(iii) Nightly Peritoneal Dialysis (NPD), a method of
performing nightly peritoneal dialysis at home
for patients with high efficiency peritoneal
membranes. Such patients do not fare well with
long dialysate DWELL times.
(iv) Tidal Peritoneal Dialysis (TPD). This modality
utilizes an initial maximum dialysate fill
volume (usually three litres) and periodically,
during a long and continuous DWELL time, drains
a fraction of the fill volume (usually one-
third, the tidal volume) and re-infuses about a
similar amount, adjusting for ultrafiltration
(excess fluid removed from the patient's body
during kidney dialysis) into the patient.
CA 02211848 1998-07-27
A number of examples of more or less "automated"
peritoneal dialysis machines are to be found in the art.
Examples are afforded by U.S. Patent No. 4,096,859
(Agarwal et al.); U.S. Patent No. 5,141,492 (Dadson et
al.); U.S. Patent No. 5,324,422 (Colleran et al.); and
U.S. Patent No. 5,438,510 (Bryant et al.). These have
proven to be unsatisfactory in various respects in
addressing clinical concerns and in effectively
implementing PD modalities such as those described above.
General Description of the Invention
Applicant's overall objective was to provide an
automated peritoneal dialysis machine capable of fully
"customizing" the composition of dialysate delivered to a
patient to meet his or her immediate physiological needs
and, to that same end, capable of monitoring the
effectiveness of treatment during the treatment process
and use this diagnostic information to optimise the
customisation process.
It is a particular object of the invention to
provide an automated peritoneal dialysis apparatus as
aforesaid, including means for metering solutions of
osmotic agent, electrolytes and other desired dialysate
components from separate solution containers into mixing
chamber means for combination, in desired proportions, to
provide the desired dialysis fluid and for delivering a
selected quantity of said dialysis fluid to the
peritoneal cavity of a patient.
It is likewise an object of the present invention to
provide automated peritoneal dialysis apparatus as
aforesaid, wherein said means for metering dialysate
components into the mixing chamber and delivering
dialysis fluid to the patient includes means for
withdrawing spent dialysis fluid from the patient.
- - - - - -
CA 02211848 1998-07-27
It is a still further object of the invention to
provide automated peritoneal dialysis apparatus as
aforesaid, wherein the means for delivery of fresh
dialysis fluid to a patient and for removing spent
dialysis fluid from the patient includes means for
monitoring interperitoneal pressure and electronic
control means responsive to the signal of said pressure
monitoring means, for controlling the rates of infusion
of fluid into the patient and for the removal of fluid
from the patient and, for the customisation of the
dialysate.
Brief Description of the Drawings
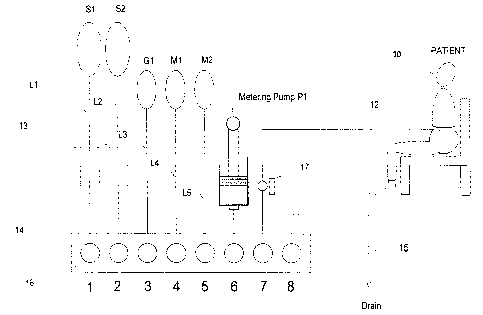
Figure 1 is a schematic representation of the first
"basic" APD machine according to the present invention.
Figure 2 schematically illustrates one of the liquid
input/output ports and a portion of the occluding
mechanism in the machine at Figure 1.
Figure 3 shows an exploded view of the occlusion
mechanism for an automated peritoneal dialysis machine
according to the present invention.
Figure 4 is a graphical representation of the
variation of intraperitoneal pressure versus time during
the cycles of an apparatus according to the present
invention.
Figure 5 is a graphical representation of the volume
of fluid removed to stabilize pressure as a function of
time during the DWELL period of a dialysis cycle, as
measured using APD apparatus according to the present
invention.
CA 02211848 1998-07-27
Figure 6 is a schematic illustration of a second
embodiment of APD machine according to the present
invention.
Figure 7 is a schematic illustration of a second
embodiment of APD machine according to the present
invention.
Figure 8 is a preferred version of the apparatus
embodiment of Figure 7, in which components are
structurally integrated into a compact cartridge.
Figure 9 is a schematic illustration of a fourth
embodiment of apparatus according to the present
lnvent lon .
Detailed Description of the Invention
A "basic" layout of components of apparatus
according to the present invention is illustrated
schematically in Figure 1. The apparatus is connected to
the peritoneal cavity of patient 10 by means of a patient
tubing line 12, through which fresh fluid is infused and
spent fluid is withdrawn.
An essential component of apparatus according to the
present invention is an occlusion manifold 14, the hollow
interior communication channel 16 of which is in
communication with all of the fluid input lines to
containers of selected dialysate solution components and
to output tubing lines to the catheter and to drain.
In the arrangement shown in Figure 1, eight separate
input or output connection ports into channel 16 of
cartridge 14 are shown, numbered 1 to 8. Containers
(solution bags) S1, S2 carry sterile PD solutions of two
different electrolyte compositions and are connected to
cartridge inputs 1 and 2 by tubing lines L1 and L2,
CA 02211848 1998-07-27
respectively. In-line heaters 13 are provided, as is
conventional in PD apparatus, to warm the sterile PD
solutions to body temperature. Containers S1 and S2 could
alternatively carry standard PD solutions (glucose or
similar as the osmotic agent).
Input 3 of manifold 14 is connected by line L~ to a
container G1 of highly concentrated sterile osmotic agent
(glucose solution or other known osmotic agent).
Container bags M1 and M2 connected by lines L4 and L5,
respectively, to the corresponding manifold inputs could
contain different medications or additives to improve the
clinical value of the solutions in S1 and S2. Apparatus
according to the present invention includes a precise
metering pump P1 whose operation is described in more
detail below. Aforementioned patient line 12 is
connected to input 7, while a drain line 15 is connected
to port 8.
For registering and monitoring the interperitoneal
pressure from time to time during the course of
treatment, a pressure transducer means 17 is preferably
included, the signal from which is monitored by
electronic control means for the apparatus (not shown).
A preferred arrangement for the occlusion mechanism
of occlusion manifold 14 is illustrated in Figures 2 and
8. Figure 2 schematically illustrates one of the tubing
connecting ports 18 onto which an input tubing line L
fits. Port 18 communicates with interior communication
channel 16 of occlusion manifold 14.
To ports 18 there correspond flexible sealing
diaphragms 20, each positioned in the wall of manifold 14
opposite the wall through which corresponding port 18
enters channel 16, and electronically controlled plungers
22.
-
CA 022ll848 l998-07-27
-- 8
Figure 3 shows an exploded view an assembly of
plungers 22 and 22c, springs 22a and 22b and motors 23
for assembly of an occlusion mechanism of the manifold of
automated PD apparatus according to the present
invention. In assembly, plungers 22 and plunger springs
22b are first inserted into manifold 14c. Cams 23a
attached to individual small rotary motors 23 are
inserted into the manifold, so that the plungers are
retained inside the manifold by the cams and ride
directly on the cams. The small springs 22a and
corresponding plunger heads 22c are inserted from the top
into respective plungers 22 through the manifold. All
the motors 23 are mounted on a motor mounting plate 24.
Two screws 24a are used to secure motor mounting plate 24
to manifold 14c.
Each motor 23 rotates its associated cam 23a and
corresponding plunger 22c follows the cam for up or down
movement. The respective up and down positions of the
individual plungers 22 can be sensed electronically and a
signal sent to the microprocessor means for stopping
motors at up or down plunger positions as appropriate.
The tubing connecting ports 18 terminating inside channel
16 of manifold 14 align with plungers 22c. The "up"
position of a plunger has the effect of occluding the
cartridge port to which it corresponds, while the "down"
position opens the port. Fluid flows may accordingly be
controlled as discussed below.
It is contemplated that the machine will be
controlled by microprocessor means (not shown), having
stored memory for on-line monitoring of information and
for programming of set operational parameters. A
removable memory card can also be incorporated to ensure
easy collection and transfer of treatment data for the
patients. Optionally, an interactive voice interface and
visual and audio alarm systems can be incorporated to
- - - - - - - - -
CA 02211848 1998-07-27
simplify the diagnosis of problems during and after PD
treatments.
The microprocessor means is programmed for receiving
signals from various sensors and for producing output
control signals for controlling the metering pump P1 and
plungers 22 through an electromechanical means such as
the motor/cam arrangement discussed above.
During dialysis, the desired filling fluid volumes
of each cycle are programmed into the microprocessor.
The ratios of the corresponding medications and/or
additives are also entered. Initializing the machine
operation, all input and output ports of the cartridge 14
are closed by their respective plungers. These plungers
are controlled individually by their respective motors.
When plunger 22c moves upwards (i.e., toward the
manifold), it pushes against flexible diaphragm 20,
closing the outlet of port tube 18 within channel 16 of
the manifold, precluding entry or withdrawal of fluid
from the chamber by way of that port. Moving the plunger
downward leaves the tube outlet in communication with the
chamber, so that fluid may flow freely in or out of the
chamber and communicate with any other outlets which are
also open at that time.
Referring specifically to Figure 1, the
microprocessor electronic control means is programmed so
that port #8 (to drain line 15) and port #6 (to the
metering pump pumpline) are opened. In sequence, the
plungers corresponding to input/output ports numbers 1,
2, 3, 4 and 5 are activated thus opening and closing
input ports 1,2,3,4 and 5 at predetermined times. During
the opening period, metering pump P1 operates to draw
fluid in from respective container bags and to flush them
out to the drain. Patient line 12 is flushed by opening
either #1 or #2, opening #6 and drawing fluid out of
-
CA 02211848 1998-07-27
- 10 -
either S1 or S2, then closing #1 or #2, opening #7 and
infusing the fluid into patient line 12.
For efficient operation in drawing calibrated
volumes of fluid from container bags and infusing the
withdrawn fluid to target locations, whether the
container bags and the target locations are vertically
above or below the machine, it is essential that metering
pump P1 provide positive displacement of fluid and have a
known volumetric displacement. One way this has been
arranged in practice has been to have a variable
volumetric displacement mechanism included in the
metering pump P1. Variable displacement was achieved
with controlled linear translation of a volume
displacement member. This type of motion was achieved
by coupling a worm gear to the output driveshaft of an
electric motor. The controlled rotary motion of the
electric motor is then smoothly translated into a
controlled linear motion which in turn will adjust the
volumetric displacement of the pump.
A common example of this type of metering pump is a
syringe pump with controlled linear translation of the
plunger in the barrel of the syringe. The linear motion
(volume displacement) of a metering syringe pump was
calibrated in the following manner. The internal shaft
on the electric motor was digitally encoded. Its rotary
position was optically sensed thus generating a set of
electrical pulses whose number were directly proportional
to linear displacement of the worm gear. One particular
configuration which was used in this way gave a fluid
displacement of 20cc for each 2.15 inches of linear
travel of the worm gear. The lead screw of the worm gear
had a lead of 0.12 inches and was driven through a gear
box (gear ratio 81:1). The encoder of the motor produced
512 pulses/revolution. The microprocessor control means,
through linkage to the motor shaft encoder, could track
each pulse generated by the encoder. In principle this
CA 02211848 1998-07-27
- 11 -
metering system had a sensitivity of 30 x 10-9 litres.
This is at least 3 orders of magnitude more precise than
required for APD apparatus according to the invention to
achieve its metering objectives. Naturally those skilled
in the art could construct a number of variations of this
particular device.
During FILL mode if solution S1, say, is selected,
ports numbers 1 and 6 would be opened and the metering
pump activated to draw the correct amount of fluid from
container bag S1. That done, port #1 closes and desired
additives G1 selected by opening port #3, drawing the
correct volume into P1, then closing port #3. Continuing
in this way, incremental additions can be made of fluids
from M1 and M2 into the pump. Then, to infuse the metered
fluid composition into the patient, port #7 opens and the
metering pump causes the fluid to be injected into the
peritoneal cavity of the patient, while the machine
monitors the volume of fluid instilled into the patient.
This injection procedure is repeated several times
until the correct total amount of dialysis fluid has been
delivered or some other predetermined state is achieved.
A graphical example of such a predetermined state is
shown in Fig 4 at point P2. During the FILL mode, the
intraperitoneal pressure will increase slowly from To to
T1 and in proportion to the filled volume. There is an
inflective increase in pressure at the maximum fill
volume attained at time beyond T1 and corresponding
pressure P2. The machine will be programmed to remove
enough fluid to back off the pressure from the maximum P2
to a safe and controlled pressure level P1. This would
be the steady state pressure for the monitoring process.
The official DwELL period then begins at T2.
Turning to the DwELL period, all ports of the
manifold are closed and variations in the interperitoneal
pressure are monitored by the microprocessor from signals
CA 022ll848 l998-07-27
- 12 -
transmitted by in-line pressure transducer 17. Any
ultrafiltration which occurs (drawing fluid from the body
of the patient into the peritoneal cavity) will
necessarily result in an increase in intraperitoneal
pressure which, on detection, signals the control means
to open ports #s 6 and 7. Pump P1 will then suck back
sufficient fluid, namely, the-excess amount, from the
patient's peritoneal cavity until the steady state
pressure level (P1) has been restored. The volume which
has been so removed during the DWELL period is recorded by
the computer as "ultrafiltration" with respect to the
time it was taken. Whenever the pressure reaches P2, the
pump is activated to reduce the fluid volume enough to
drop the pressure back to the steady state P1. This
volume Vf iS recorded with respect to time t2 (the length
will be dependent on osmotic pressure of the fluid). This
action is operated as often as it may be necessary.
This process of restoring steady state pressure and
recording the cumulative volume of fluid removed as a
function of time in order to do so, is carried out
automatically throughout the DWELL period and the measure
of cumulative ultrafiltration (UF) is recorded. A
graphical measurement of intraperitoneal pressure versus
time affords valuable diagnostic information. When the
pressure does not change from its steady state value for
a predetermined period of time, it may be inferred that
the dialysis fluid is no longer performing its optimal
clinical function. At such a time, T3 (Fig 4), the fluid
can be safely drained out of the patient without waste of
further time. An onset of a pressure drop, however,
would indicate that the patient is absorbing fluid from
the peritoneal cavity which could indicate that the
patient is absorbing glucose from the dialysate, or that
dialysate is leaking into extra-abdominal tissues. These
undesirable clinical conditions are avoided by arranging
the control logic of the machine to automatically drain
CA 02211848 1998-07-27
out all spent dialysate volume from the patient when such
pressure drop occurs.
During the DRAIN mode ports #6 and #7 are opened. The
metering pump P1 draws the spent fluid from the patient
and into the syringe. This volume is measured as it is
being withdrawn. When the syringe is full, port #7 closes
and port #8 opens. The pump P1 reverses its direction and
pushes the waste fluid from the syringe through the drain
line and into a receptacle for spent dialysate. This is
operated until all the fluid is drained out or the
pressure registers negative, or until the end of the set
DRAIN time. The final UF is then determined by the
machine.
This completes one dialysis cycle. The above
procedure is repeated as many times as required until the
desired amount of treatment is obtained.
Another important characteristic of this invention
is its ability to make decisions based on real-time
physiological needs of a patient or provide previously
unattainable clinical information. A graphical
representation of the on-line monitoring of volume of
fluid removed in order to stabilize pressure at a steady
state, as a function of time during the DWELL period of
one dialysis cycle, is provided in Figure 5 and is but
one such example of new clinical information. The
invention will allow the normal set DWELL time (T2 to T3 )
to be rationally adjusted. At maximum UF volume, Vml the
dialysis fluid has reached equilibrium with the plasma in
the peritoneal membrane. Therefore anytime beyond Tx,
would be treatment time wasted. A clinician could either
program the machine to automatically drain the patient of
the spent fluid and introduce fresh fluid for better
dialysis or use the information to set more effective
DWELL time for the next treatment. Alternatively if the
set DWELL time terminates at the rising phase of the
CA 022ll848 l998-07-27
- 14 -
ultrafiltration curve, then the dialysate is not being
utilized properly. These are examples of the ability of
the apparatus to automatically make decisions based on
real-time physiological needs of a patient. This graph
also reflects real time solute and fluid transport rates
of the peritoneal membrane for any given dialysate
formulation. That is, the greater the efficiency of the
peritoneal membrane the greater the initial slope of the
ultrafiltration curve and or the faster time Tx is
achieved. For the first time clinicians will be able to
quantify the transport characteristics of the membrane
on-line and use this information to directly control the
machine or allow the machine to make the necessary
adjustments automatically.
A further example of the capability of apparatus
according to the invention in providing previously
unattainable clinical information and/or intelligent use
of such information by the APD machine is as follows: In
clinical PD applications, the characteristics of the
peritoneal membrane with respect to its active surface
area, and permeability (solute and fluid transport) are
all variable and mostly unknown for any given patient.
Hence methods have been developed to quantify peritoneal
membrane performance. However, these methods are
complex, indirect and none of them are on-line analytical
procedures. Two methods used for assessing membrane
performance are (a) the peritoneal Membrane Mass Transfer
Area Coefficient (MTAC) and (b) the Peritoneal
Equilibration Test (PET). . The later (PET), determines
the ratio of dialysate-to-plasma (D/P) of a given solute
and is the one most commonly used to assess patients. At
best this is performed once a month. Currently it is
impossible to obtain data to perform PET at various
stages of the DWELL period during treatment. If this time
dependent data could be obtained it could lead to a
better clinical understanding of the different types of
ultrafiltration failures. Combining the unique ability
-
CA 02211848 1998-07-27
of apparatus according to the present invention to secure
a sample of the fluid during DWELL at known correlated
points on the ultrafiltration curve clinicians will be
better able to properly evaluate the PD treatment in
vivo. This represents a major advance in the art of PD
treatment. A related clinical advantage is that
clinicians will immediately be able to correlate changes
in ultrafiltration curve with the type of medication or
additive used during a treatment cycle.
It will be appreciated from the foregoing that the
pressure monitoring activities used to control the UF
using a machine according to the present invention makes
it possible to perform a true tidal peritoneal dialysis.
By maintaining the pressure at its initial fill pressure
P1 we can infer that the actual volume of fluid in the
cavity is the same as the initial fill volume. This
volume is known. For the first time an APD machine will
be able to use the actual volume of fluid in the
peritoneal cavity and not a pre-estimated amount to
determine the actual tidal withdrawal and refill volumes.
This is a major improvement in the art.
Moreover, additional detectors and sensors may be
included in the system and their signals taken into
account to a programme microprocessor or diagnostic and
therapeutic advantage. For example, a turbidimeter
including a light source and light detector monitoring
the clarity of the effluent during DRAIN can give early
detection of the onset of infection. If patient line 12
is passed between such a light source and light detector,
it will be possible to detect whether or not the
patient's effluent is cloudy during DRAIN, owing to an
onset of peritonitis (production of enhanced level of
light-scattering white blood cells brought about by
infection). The detector will transmit this information
to the microprocessor and audio and visual alarms may be
initiated, the machine triggered to empty metering pump
CA 022ll848 l998-07-27
- 16 -
P1 and a sample of cloudy effluent collected for further
analysis.
The machine could therefore be programmed to make
important decisions as to the infusion pattern on the
basis of signals from sensors reflecting the composition
and pressure of fluid in the peritoneal cavity.
Alternative Embodiments of Apparatus
Although the "basic" machine shown in Figure 1
employs a metering pump to draw and deliver the
apportioned dialysate components, the arrangement could
be used without a metering pump at all, but employing a
weighing system and gravity for the discharge of sterile
fluids and medications from container bags supported
vertically above the patient, with a weigh bag located
below the patient for determining the volume.
Equally as valid, although the basic machine is
designed to customize the dialysate from a plurality of
solution bags, the machine can be used in a non
customisation mode, i.e., with dialysate pre-mixed in
each of one or more container bags. Each port can be
connected to a dialysate of fixed formulation. The
described diagnostic power of the apparatus can then be
used to select which port is connected to the patient
line (12) to FILL the patient, determine the DWELL period,
and drain the patient of that selected formulation using
the metering pump.
A second embodiment of a PD apparatus according to
the present invention is illustrated schematically in
Figure 6. The same reference numerals are used in Figure
6 and in the below-discussed Figures 7 and 8 to identify
parts of the apparatus which are entirely analogous and
co-functional with like-numbered components of the basic
machine shown in Figure 1.
CA 02211848 1998-07-27
The second preferred embodiment as shown in Fig 6.
depends on pump P1 for achieving compact arrangement and
ease of clinical use and uniqueness in portability. The
cartridge is divided into two distinct chambers 14a and
14b. The first chamber houses ports #1, #2, #3, #4, and
#5. The second chamber houses ports #6, #7, and #8. The
ports #4 and #7 are now connected to the metering pump P1
via one way valves V1 and V2 for fluid inflow and outflow
respectively. The bulk sterile neutral fluid S (no
osmotic agent), is connected to port #1 through heater
13. The osmotic agent G1 (i.e. glucose etc.) is at port
#2, and the medication M1 is at #3. The inflow to the
patient is at port #6 and the outflow from the patient is
at port #5. Port #5 and #6 are joined to the patient line
via the pressure transducer means 17.
In operation all the ports are initially closed.
During FILL mode #1, #4, #6, and #7 are opened. The fresh
fluid is drawn in by P1 from S through the heater,
through the valve V1. At a predetermined volume #1
closes, #2 opens and the correct amount of the osmotic
fluid is also drawn into the metering pump. The port #2
then closes and #3 opens to allow the withdrawal of the
desired volume of the medication M1 into P1. Then #3 is
closed. Reversing direction the contents of P1 is
discharged into the patient via V2, pressure transducer
17 and the patient line 12. The above procedure is
repeated several times until the correct dosage volume is
delivered to the patient. By having unidirectional valves
V1 and V2, the frequency of closing and opening of ports
#4, #5, #6, and #7 is reduced.
During DRAIN mode only plungers #4, #5, #7, and #8
are opened. The spent fluid from the patient is drawn
into P1 through the patient line, the pressure transducer
17, through #5, #4 and Valve V1. The syringe P1 measures
the drawn in fluid accordingly. The feedback of the
-- -- -- ---- --
CA 022ll848 l998-07-27
- 18 -
pressure transducer regulates the rate of fluid drawn in
by P1 from the patient. When full, P1 is emptied through
V2, plungers #7 and #8 to the final drain. All the
previously mentioned activities are equally applicable to
this version. Plungers #4 and #7 are not strictly
necessary, because, with plungers #s 1, 2, 3, 5, 6 and 8
all closed, the metering pump is isolated and there is no
movement of fluid.
A machine according to this second embodiment could
be operated from a normal table top or a short stand,
with the solutions and medications located conveniently
below the main machine. The machine could even be
operated at the floor level. The positive displacement
pump P1 ensures that the efficiency of the delivery
system does not depend upon the relative vertical
positions of the solutions, the patients and/or the final
drain, as is the case in gravity-fed cyclers. This
therefore makes the new machine universal for patients on
normal beds, hospital beds or lying on floor mats.
A third preferred version is as shown in Figure 7.
The component arrangements are similar to the first
version shown in Figure 1 (the Basic Machine), discussed
above. The sterile PD solution (electrolytes only) is at
port #1. The Osmotic agents (G1, G2) are at ports #2 and
#3. The medication M1, is at port #4. The Patient line 12
is controlled by port #5. And port #6 controls the drain
line 15. The metering pump P1 communicates directly with
the whole occlusion chamber. And the fluid flowing in and
out of metering pump P1 passes through a pressure
transducer chamber 17' that communicates with pressure
transducer 17. All the tubing lines communicate with
occlusion manifold 14. Pressure in the lines, and hence
in connected bags, or in the patient and in the drain
line are all monitored by opening the appropriate port.
Using this arrangement, the pressure readings could be
used to detect other important conditions in addition to
-
CA 02211848 1998-07-27
- 19 -
all those already discussed under the previous versions;
the detection of these, (a) empty solution bags (S, G1, G2
& M1) (b) obstructions in the drain line and (c)
obstructions in the patient line.
A "compact cartridge" version of the system of
Figure 7 is as shown in Figure 8. The occlusion block
14, the heater and metering pump P1 are all integrated
into a single compact cartridge. The heating chamber is
divided into two sections: the initial heater chamber
13a that houses the incoming cold solution, and the
corrugated heater section 13b that directs the fluid path
to ensure proper heating of the solution. The output of
the heater is attached to port #1.
A preferred embodiment of the new APD apparatus is
as shown in Figure 9. The operation of this embodiment
is the same as the one discussed above for the third
version in Figure 7, except in this embodiment there are
two additions, namely (a) effluent detector 28 and (b)
sample collector port (at #6). The drain line 15 is now
located at port #7.
The effluent detector comprises of a light source
28a facing a light detector 28b. Variations in the
light intensities are detected by the light detector and
the signals transmitted to microprocessor for the
appropriate actions.
The patient line 12 passes between the light source
28a and the light detector 28b of the effluent detector
28. Hence during DR~IN if the patient's effluent is
cloudy (due to an onset of peritonitis; infection-
production of white blood cells), the light beam to thelight detector is diffused. The detector transmits the
message accordingly to the microprocessor. Both audio
and visual alarms are initiated. When this happens the
machine automatically, at the point of emptying the
CA 02211848 1998-07-27
- 20 -
metering pump P1, opens port #6 and sends a sample of the
cloudy effluent solution into the sample collector
container 30 (which couid be a bag or a syringe). Then
port #6 will be closed and the normal drain procedure
will be continued by operating port #7.
If the apparatus had been set up to initiate
peritonitis treatment, rapid peritoneum flush or flushes
(fills immediately followed by drains), will be carried
out. Then followed by treatment fill volumes containing
medication or medications, automatically metered from the
medication containers such as M1, by the metering pump
P1.
The effluent detector, similarly, will detect
excessive amount of blood in the effluent (usually with
new catheter operations or with new catheter break-ins),
and could be programmed to automatically reduce the
amount of heparin additive and or reduce the dialysate
infusion volumes.
While particular embodiments of this invention have
been described in relation to the accompanying drawings,
it will be evident to those skilled in the art that
changes and modifications may be made therein, without
departure from the spirit of the invention as defined in
the appended claims.