Note: Descriptions are shown in the official language in which they were submitted.
CA 02211971 1997-07-30
WO 96/25043 P{;T/EP96/00398
-1-
Herbicidal composition and method of controlling weeds
The present invention relates to a novel herbicidal composition comprising a
herbicidal
active ingredient combination that is suitable for the selective control of
weeds in crops of
useful plants, for example in crops of cereals, rape, sugar beet, sugar cane,
plantation
crops, rice, cotton and especially maize and soybeans.
The invention relates also to a method of controlling weeds in crops of useful
plants and to
the use of the novel composition for that purpose.
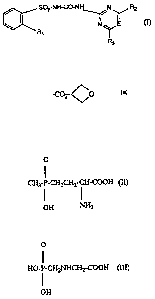
The compounds of formula I
S02-NH-CO-NH N R2
N E
R1
R3
wherein Rl is -C02-CO ' -CO2CH3 or -CH2CH2CF3,
R2 is methyl, methoxy or -OCHF2,
R3 is methyl or -OCHF2 and
E is =CH- or =N-, with E being =N- when R2 is methoxy,
and salts thereof, have herbicidal action. This is described, for example, in
EP-A-84 020,
EP-A-496 701 and EP-A-120 814.
The following compounds of formulae II and III
0
II
CH3-P-CH2CH2-CH-COOH (II)
I I
OH NH2
and
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-2-
0
II .
HO-P-CH2NHCH2-COOH (III)
I
OH
and their agrochemically acceptable salts, especially the alkali metal,
ammonium and
amine salts, are likewise known as herbicides (gluphosinates and glyphosates),
for
example in the "The Pesticide Manual", Tenth Edition 1994, Crop Protection
Publications,
and are also commercially available.
It has now surprisingly been found that a combination of variable proportions
of at least
one of the compounds of formula I with one of the above-mentioned compounds of
formula II andlor III produces a herbicidal action that is capable of
controlling the
majority of the weeds that occur especially in crops of useful plants, pre-
emergence and
especially also post-emergence, without any significant damage being done to
the useful
plant.
There is therefore proposed in accordance with the present invention a novel
composition
for the selective control of weeds which, in addition to customary formulation
adjuvants,
comprises as active ingredient at least one compound of formula I
S02-NH-CO-NH N 2
\ I ~ II (I)~
R1
R3
wherein Rl is -C02-CO '-CO2CH3 or -CH2CH2CF3, R2 is methyl, methoxy or
-OCHF2, R3 is methyl or -OCHF2 and E is =CH- or =N-, with E being =N- when R2
is
methoxy, or an agrochemically acceptable salt of at least one of the compounds
of
formula I, and a compound of formula II
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-3-
0
II
CH3-P-CH2CH2-CH-COOH (II)
I I
OH NH2
and/or of formula III
0
II
HO-P-CH2NHCH2-COOH (III),
I
OH
or an agrochemically acceptable salt of the compound of formula II and/or III,
in
admixture with one another.
Examples of agrochemically acceptable salts of the compound of formula I are
given in
the afore-mentioned EP-A-84 020, EP-A-496 701 and EP-A-120 814, in each case
on
page 4. The amines described therein and also alkali metal hydroxides and
ammonium
hydroxide are likewise examples of suitable salt-forming agents for the
compounds of
formulae II and III.
The herbicide mixture according to the invention can be used against a large
number of
agronomically important weeds, such as Veronica, Galium, Papaver, Solanum,
Cheno-
podium, Amaranthus, Xanthium, Abutilon, Ambrosia, Sagitaria, Ipomoea,
Cassiastora,
Datura stramonium, Sesbania exaltata and Sida spinosa, in crops of useful
plants, espe-
cially in crops of maize and soybeans.
The compositions according to the invention are suitable for all methods of
application
customary in agriculture, for example pre-emergence application, post-
emergence applica-
tion, which is preferred, and seed dressing.
The herbicide mixture according to the invention is suitable especially for
controlling
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-4-
weeds in crops of useful plants such as cereals, rape, sugar beet, sugar cane,
plantation
crops, rice, cotton and especially maize and soybeans.
Crops are to be understood as including those crops which have been made
tolerant to
herbicides or classes of herbicide, for example to glyphosates or
gluphosinates, by
conventional methods of breeding or gene technology.
The active ingredient combination according to the invention comprises the
compound or
compounds of formula I and the compound of formula II and/or III in any
desired mixture
ratio, generally with an excess of one component over the other. Preferred
mixture ratios
between the compound(s) of formula I and the mixing partner(s) of formula II
and/or III
are generally from 1: 20 to 1: 5.
The compositions according to the invention preferably comprise as compound of
formula I a compound of formula Ia
S02-NH-CO-NH N CH3
C02___<O N (Ia),
CH3
of formula Ib
S02-NH-CO-NH N OCH3
CH2CH2CF3 ~
CH3
and/or of formula Ic
-
CA 02211971 1997-07-30
WO 96/25043 FCT/EP96/00398
-5-
S02-NH-CO-NH N OCHF2
~ \ ( N
CO2CH3
OCHF2
Other preferred compositions comprise a compound of formula Ia, Ib or Ic and
the
compound of formula II and/or III. Of those, compositions comprising a
compound of
formula Ia, Ib or Ic and the compound of formula II or III are especially
suitable.
The rate of application may vary within wide limits and depends upon the
nature of the
soil, the type of use (pre- or post-emergence; seed dressing; application to
the seed furrow;
no tillage application, etc.), the crop plant, the weed to be controlled, the
prevailing
climatic conditions and other factors determined by the type of use, time of
use and target
crop. Generally the active ingredient mixture according to the invention can
be applied at
a rate of application of from 250 to 2500 g, especially from 500 to 1000 g,
active
ingredient mixture/ha.
In the composition according to the invention, the compound or compounds of
formula I
is/are present in a ratio by weight of from 1: 100 to 1: 0.00 1 with respect
to the
compound of formula II and/or III.
The mixtures of the compound or compounds of formula I with the compound of
formula
II and/or III may be used in unmodified form, that is to say as obtained in
the synthesis,
but they are preferably formulated in customary manner together with the
adjuvants
customarily employed in formulation technology e.g. into directly sprayable or
dilutable
solutions, dilute emulsions, wettable powders, soluble powders, dusts,
granules or micro-
capsules. As with the nature of the compositions, the methods of application,
such as
spraying, atomising, dusting, wetting, scattering or pouring, are chosen in
accordance with
the intended objectives and the prevailing circumstances.
The formulations, i.e. the compositions, preparations or mixtures comprising
the
compounds (active ingredients) of formulae I and II and/or III and, where
appropriate, one
or more solid or liquid formulation adjuvants, are prepared in a manner known
per se, e.g.
by homogeneously mixing and/or grinding the active ingredients with the
formulation
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-6-
adjuvants, e.g. solvents or solid carriers. It is also possible to use surface-
active
compounds (surfactants) in the preparation of the formulations.
Suitable solvents are: aromatic hydrocarbons, preferably the fractions
containing 8 to 12
carbon atoms, such as mixtures of alkylbenzenes, e.g. xylene mixtures or
alkylated
naphthalenes; aliphatic and cycloaliphatic hydrocarbons, such as paraffins,
cyclohexane or
tetrahydronaphthalene; alcohols, such as ethanol, propanol or butanol; glycols
and their
ethers and esters, such as propylene glycol or dipropylene glycol ether;
ketones, such as
cyclohexanone, isophorone or diacetone. alcohol; strongly polar solvents, such
as N-
methyl-2-pyrrolidone, dimethyl sulfoxide or water; vegetable oils and esters
thereof, such
as rape oil, castor oil or soybean oil; and, where appropriate, also silicone
oils.
The solid carriers used, e.g. for dusts and dispersible powders, are normally
natural
mineral fillers, such as calcite, talcum, kaolin, montmorillonite or
attapulgite. In order to
improve the physical properties it is also possible to add highly dispersed
silicic acid or
highly dispersed absorbent polymers. Suitable granulated adsorptive carriers
are porous
types, for example pumice, broken brick, sepiolite or bentonite, and suitable
nonsorbent
carriers are, for example, calcite or sand. In addition, a great number of
pregranulated
materials of inorganic or organic nature can be used, e.g. especially dolomite
or pulverised
plant residues.
Depending upon the nature of the compound of formula I to be formulated,
suitable
surface-active compounds are non-ionic, cationic and/or anionic surfactants
having good
emulsifying, dispersing and wetting properties. The term "surfactants" will
also be under-
stood as comprising mixtures of surfactants.
Both water-soluble soaps and water-soluble synthetic surface-active compounds
are
suitable anionic surfactants.
Suitable soaps are the alkali metal salts, alkaline earth metal salts or
unsubstituted or
substituted ammonium salts of higher fatty acids (Clo-C22), e.g. the sodium or
potassium
salts of oleic or stearic acid, or of natural fatty acid mixtures which can be
obtained e.g.
from coconut oil or tallow oil. Mention may also be made of fatty acid
methyltaurin salts.
More frequently, however, synthetic surfactants are used, especially fatty
alcohol
sulfonates, fatty alcohol sulfates, sulfonated benzimidazole derivatives or
alkylaryl-
CA 02211971 1997-07-30
WO 96/25043 FCT/EP96/00398
-7-
sulfonates.
The fatty alcohol sulfonates or sulfates are usually in the form of alkali
metal salts,
alkaline earth metal salts or unsubstituted or substituted ammonium salts and
contain a
C8-C22alkyl radical, which also includes the alkyl moiety of acyl radicals,
e.g. the sodium
or calcium salt of lignosulfonic acid, of dodecyl sulfate or of a mixture of
fatty alcohol
sulfates obtained from natural fatty acids. These compounds also comprise the
salts of
sulfated and sulfonated fatty alcohol/ethylene oxide adducts. The sulfonated
benz-
imidazole derivatives preferably contain.two sulfonic acid groups and one
fatty acid
radical containing 8 to 22 carbon atoms. Examples of alkylarylsulfonates are
the sodium,
calcium or triethanolamine salts of dodecylbenzenesulfonic acid,
dibutylnaphthalene-
sulfonic acid, or of a condensate of naphthalenesulfonic acid and
formaldehyde.
Also suitable are corresponding phosphates, e.g. salts of the phosphoric acid
ester of an
adduct of p-nonylphenol with 4 to 14 mol of ethylene oxide, or phospholipids.
Non-ionic surfactants are preferably polyglycol ether derivatives of aliphatic
or cyclo-
aliphatic alcohols, saturated or unsaturated fatty acids and alkylphenols,
said derivatives
containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the
(aliphatic) hydro-
carbon moiety and 6 to 18 carbon atoms in the alkyl moiety of the
alkylphenols.
Further suitable non-ionic surfactants are the water-soluble adducts of
polyethylene oxide
with polypropylene glycol, ethylenediaminopolypropylene glycol and
alkylpolypropylene
glycol containing 1 to 10 carbon atoms in the alkyl chain, which adducts
contain 20 to 250
ethylene glycol ether groups and 10 to 100 propylene glycol ether groups.
These
compounds usually contain 1 to 5 ethylene glycol units per propylene glycol
unit.
Examples of non-ionic surfactants are nonylphenol polyethoxyethanols, castor
oil poly-
glycol ethers, polypropylene/polyethylene oxide adducts,
tributylphenoxypolyethoxy-
ethanol, polyethylene glycol and octylphenoxypolyethoxyethanol.
Fatty acid esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan
trioleate, are
also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which contain,
as
N-substituent, at least one C8-C22alkyl radical and, as further substituents,
unsubstituted or
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-8-
halogenated lower alkyl, benzyl or hydroxy-lower alkyl radicals. The salts are
preferably
in the form of halides, methyl sulfates or ethyl sulfates, e.g.
stearyltrimethylammonium
chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in formulation technology, which may also
be used
in the compositions according to the invention, are described inter alia in
"Mc Cutcheon's
Detergents and Emulsifiers Annual", MC Publishing Corp., Ridgewood, New
Jersey,
1981; Stache, H., "Tensid-Taschenbuch" (Surfactant Handbook), Carl Hanser
Verlag,
Munich/Vienna 1981; and M. and J. Ash, "Encyclopedia of Surfactants", Vol. I-
III,
Chemical Publishing Co., New York, 1980-198 1.
The herbicidal compositions usually comprise 0.1 to 99 %, preferably 0.1 to 95
%, of an
active ingredient mixture of the compound(s) of formula I with the compound of
formula II and/or III, 1 to 99 % of a solid or liquid adjuvant, and 0 to 25 %,
preferably
0.1 to 25 %, of a surfactant.
Whereas commercial products will preferably be formulated as concentrates, the
end user
will normally employ dilute formulations.
The compositions may also comprise further auxiliaries, such as stabilisers,
e.g. vegetable
oils or epoxidised vegetable oils (epoxidised coconut oil, rape oil or soybean
oil), anti-
foams, e.g. silicone oil, preservatives, viscosity regulators, binders and
tackifiers, as well
as fertilisers or other active ingredients.
Preferred formulations have especially the following composition (throughout,
percentages are by weight):
Emulsifiable concentrates:
active ingredient mixture: 1 to 90 %, preferably 5 to 20 %
surface-active agent: 1 to 30 %, preferably 10 to 20 %
liquid carrier: 5 to 94 %, preferably 70 to 85 %
Dusts:
active ingredient mixture: 0.1 to 10 %, preferably 0.1 to 5 %
solid carrier: 99.9 to 90 %, preferably 99.9 to 99 %
CA 02211971 1997-07-30
WO 96/25043 FCT/EP96/00398
-9-
Suspension concentrates:
active ingredient mixture: 5 to 75 %, preferably 10 to 50 %
water: 94 to 24 %, preferably 88 to 30 %
surface-active agent: 1 to 40 %, preferably 2 to 30 %
Wettable powders:
active ingredient mixture: 0.5 to 90 %, preferably 1 to 80 %
surface-active agent: 0.5 to 20 %, preferably 1 to 15 %
solid carrier: 5 to 95 %, preferably 15 to 90 %
Granules:
active ingredient mixture: 0.1 to 30 %, preferably 0.1 to 15 %
solid carrier: 99.5 to 70 %, preferably 97 to 85 %
The following Examples further illustrate, but do not limit, the invention.
Formulation Examples:
Mixtures of compounds of formulae I and II and/or III (throughout, percentages
are by
wei ht :
F 1. Emulsifiable concentrates a) b) c) d)
mixture of compound(s) of
formula I with one of the 5 % 10 % 2 5% 5 0%
compounds of formula II and/or III
calcium dodecyl benzenesulfonate 6 % 8 % 6 % 8 %
castor oil polyglycol ether 4 % - 4 % 4 %
(36 mol of ethylene oxide)
= octylphenol polyglycol ether - 4 % - 2 %
(7-8 mol of ethylene oxide)
cyclohexanone - - 10 % 2 0 %
aromatic hydrocarbon mixture 8 5% 7 8% 5 5% 16 %
C9-Ci2
Emulsions of any desired concentration can be obtained from such concentrates
by
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-10-
dilution with water.
F2. Solutions a) b) c) d)
mixture of compound(s) of
formula I with one of the 5 % 10 % 5 0% 90 %
compounds of formula II and/or III
1-methoxy-3-(3-methoxy-propoxy)-
propane - 20 % 20 % -
polyethylene glycol MW 400 20 % 10 % - -
N-methyl-2-pyrrolidone - - 30 % 10 %
aromatic hydrocarbon mixture 75 % 60 % - -
C9-Ci2
These solutions are suitable for application in the form of microdrops.
F3. Wettable powders a) b) c) d)
mixture of compound(s) of
formula I with one of the 5 % 25 % 5 0% 80 %
compounds of formula II and/or III
sodium lignosulfonate 4 % - 3 % -
sodium lauryl sulfate 2 % 3 % - 4 %
sodium diisobutylnaphthalene-
sulfonate - 6 % 5 % 6 %
octylphenol polyglycol ether - 1% 2 % -
(7-8 mol of ethylene oxide)
highly dispersed silicic acid 1 % 3 % 5 % 10 %
kaolin 88 % 62 % 35 % -
The active ingredient is thoroughly mixed with the adjuvants and the mixture
is
thoroughly ground in a suitable mill, affording wettable powders which can be
diluted
with water to give suspensions of any desired concentration.
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-11-
F4. Coated granules a) b) c)
mixture of compound(s) of
formula I with one of the 0.1 % 5 % 15 %
compounds of formula II and/or III
highly dispersed silicic acid 0.9 % 2 % 2 %
inorganic carrier 99.0 % 93 % 83 %
(diameter 0.1 - 1 mm)
for example CaCO3 or Si02
The active ingredient is dissolved in methylene chloride and the solution is
sprayed onto
the carrier, and the solvent is subsequently evaporated off in vacuo.
F5. Coated granules a) b) c)
mixture of compound(s) of
formula I with one of the 0.1. % 5 % 15 %
compounds of formula II and/or III
polyethylene glycol MW 200 1 . 0% 2 % 3 %
highly dispersed silicic acid 0.9 % 1 % 2 %
inorganic carrier 98.0 % 92 % 80 %
(diameter 0.1 - 1 mm)
for example CaCO3 or Si02
The finely ground active ingredient is uniformly applied, in a mixer, to the
carrier
moistened with polyethylene glycol. Non-dusty coated granules are obtained in
this
manner.
F6. Extruder granules a) b) c) d)
mixture of compound(s) of
formula I with one of the 0.1 % 3 % 5 % 15 %
compounds of formula II and/or III
sodium lignosulfonate 1. 5% 2 % 3 % 4 %
carboxymethylcellulose 1.4 % 2 % 2 % 2 %
kaolin 97.0 % 93 % 90 % 79 %
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96/00398
-12-
The active ingredient is mixed and ground with the adjuvants, and the mixture
is
moistened with water. The mixture is extruded and then dried in a stream of
air.
~
F7. Dusts a) b) c)
mixture of compound(s) of
formula I with one of the 0.1 % 1% 5 %
compounds of formula II and/or III
talcum 39.9 % 4 9,% 35 %
kaolin 60.0 % 50 % 60 %
Ready-for-use dusts are obtained by mixing the active ingredient with the
carriers, and
grinding the mixture in a suitable mill.
F8. Suspension concentrates a) b) c) d)
mixture of compound(s) of
formula I with one of the 3 % 10 % 25 % 50 %
compounds of formula II and/or III
ethylene glycol 5 % 5 % 5 % 5 %
nonylphenol polyglycol ether - 1 % 2 % -
(15 mol of ethylene oxide)
sodium lignosulfonate 3 % 3 % 4 % 5 %
carboxymethylcellulose 1 % 1 % 1% 1 %
37% aqueous formaldehyde 0.2 % 0.2 % 0.2 % 0.2 %
solution
silicone oil emulsion 0.8 % 0.8 % 0.8 % 0.8 %
water 87 % 79 % 62 % 38 %
.
The finely ground active ingredient is intimately mixed with the adjuvants,
giving a
suspension concentrate from which suspensions of any desired concentration can
be
obtained by dilution with water.
It is often more practical for the compound(s) of formula I and the mixing
partner of
formula II and/or III to be formulated separately and to be brought together
only shortly
CA 02211971 1997-07-30
WO 96/25043 PCT/EP96100398
-13-
before application in the applicator in the desired mixture ratio in the form
of a "tank
mixture" in water.
Biological Examples:
Example B 1: Post-emergence test:
In a greerihouse, monocotyledonous and dicotyledonous test plants are grown in
plastic
pots containing standard soil and in the 4- to 6-leaf stage are sprayed with
an aqueous
suspension of the test compounds of formula I, prepared from a 25 % wettable
powder
formulation (Example F3, b)) corresponding to a rate of application of 2000 g
a.i./ha
(5001 water/ha). The test plants are then grown on in the greenhouse under
optimum
conditions. After about 18 days the test is evaluated in accordance with a
scale of nine
ratings (1 = total damage, 9 = no action). Ratings of from 1 to 4 (especially
from 1 to 3)
indicate a good to very good herbicidal action. In this test the compounds of
formula I
exhibit a strong herbicidal action. The same results are obtained when the
compounds of
formula I are formulated in accordance with Examples Fl and F2 and F4 to F8.