Note: Descriptions are shown in the official language in which they were submitted.
CA 02213197 1997-08-15
WO 96/25411 PCTlUS96101906
Certain Bridged 4-Phenyl-2-aminomethylimidazoles;
New Dopamine Receptor Subtype Specific Ligands
BACK rROLTND OF THE INVENTION.
Field of the Invention
This invention relates to certain bridged .~-phenyl-2-aminomethylimidazoles
which
selectively bind to dopamine receptor subtypes. This invention also relates to
pharmaceutical
compositions comprising such compounds. It further relates to the use of such
compounds in the
treatment of affective disorders such as schizophrenia, depression as well as
certain movement
disorders such as Parkinsonism and dystonia. Furthermore compounds of this
invention are
useful in treating the extrapyramidal side effects associated with the use of
conventional
neuroleptic agents.
Description of the Rela pry Art
Schizophrenia or psychosis is a term used to describe a group of illnesses of
unknown
origin which affect approximately 2.5 million people in the United States.
These disorders of the
brain are characterized by a variety of symptoms which are classified as
positive symptoms
(disordered thought, hallucinations and delusions) and negative symptoms
(social withdrawal
and unresponsiveness). These disorders have an age of onset in adolescence or
early adulthood
and persist for many years. The disorders tend to become more severe during
the patient's
lifetime and can result in prolonged institutionalization. Within the United
States of America.
approximately 4U% of all hospitalized psychiatric patients suffer from
schizophrenia.
During the 1950's physicians demonstrated that they could successfully treat
psychotic
(schizophrenic > patients with medications called neuroleptics. This
classification of antipsychotic
medication was based largely on the activating (neuroleptic) properties of the
nervous system by
these drugs. Subsequently, neuroleptic agents were shown to increase the
concentrations of
dopamine metabolites in the brain. This finding suggested that altered
neuronal firing of the
dopamine system contributed in some way to the aberrant behavior observed in
schizophrenic
patients. Additional evidence indicated that dopamine could increase the
activity of adenylate
cyclase in the corpus striatum, an effect reversed by neuroleptic agents.
Thus, cumulative
.. . - _
CA 02213197 1997-08-15 pC~L~s96/01906
WO 96/25411
evidence from these and later experiments strongly suggested that the
neurotransmitter dopamine
was involved in schizophrenia.
One of the major actions of antipsychotic medication is the blockade of
dopamine
receptors in brain. Several dopamine systems appear to exist in the brain and
at least five classes
of dopamine receptors appear to mediate the actions of this transmitter. These
dopamine ,
receptors differ in their pharmacological specificity and were originally
classified on the basis of
their ability to bind various dopaminergic ligands.
The butyrophenones are a class of drugs containing many potent antipsychotic
drugs.
Perhaps the most prominent member of this class of compounds is the
antipsychotic drug
haloperidol (1-(3-p-fluorobenzoylpropyl)-4-p-chlorophenyl-4-
hydroxypiperidine). Haloperidol
binds relatively weakly at the major dopamine receptor subtype which activates
adenylate cyclase
(commonly classified as the DI dopamine receptor). In contrast, haloperidol
displayed binding
affinity at a dopamine receptor subtype which suppressed the activity of
adenylate cyclase
(commonly classified as D~ receptors) in the subnanomolar range.
Recently, three additional dopamine receptor subtypes have been identified
using the
often congruent sciences of receptor phamnacology and molecular biology. These
new dopamine
receptors have been labeled as D3, Da, and D5. The D3 and D4 subtypes are
pharmacologically
related to the D~ receptor via their ability to suppress the activity of
adenylate cyclase.
Conversely, the D5 receptor is classified as a "DI-like" dopamine subtype
through its ability to
stimulate cyclase activity.
Recently, a new group of drugs (such as sulpiride and clozapine) have been
developed
with a lesser incidence of extrapyramidal side effects (EPS) than classical
neuroleptics. In
addition, there is some indication that they may be more beneficial in
treating negative symptoms
in some patients. Since all D~ blockers do not possess a similar profile,
hypotheses underlying
the differences have been investigated. Major differences have been detected
in the
anticholinergic actions of these drugs. It has also been suggested that the
dopamine receptors in
motor areas may differ from those in the limbic areas which are thought to
mediate the
antipsychotic responses. The existence of the D3, D.t and D5 and other as yet
undiscovered
dopamine receptors may contribute to this profile. Some of the atypical
compounds possess
-2-
CA 02213197 1997-08-15
WO 96!25411 PC"~IUS96101906
similar activity at D~. D3 and D.t receptors. The examples of this patent fall
into this general class
of molecules.
Using molecular biological techniques it has been possible to clone cDNAs
coding for
each of the pharmacologically defined receptors. There are at least two forms
of Di which have
been identified as D 1 and D>, and two forms of D~, identified now as D~ and
D,t dopamine
receptors. In addition, there is at least one form of D3 dopamine receptor.
International Publication No. WO 94/22839 describes certain 2-
aminomethylbenzimidazoles as having affinity at dopamine receptors. The
compounds of the
present invention differ from those in WO 94/22839 in that the compounds of
this invention
possess a aromatic benzene ring fused in a [4,5-eJ fashion to the
benzimidazole substructure.
-3-
CA 02213197 1998-09-10
WO 96125411 PCTlUS96/J 1906
This invention provides novel compounds of Formula 1 which interact with
dopamine
receptor subtypes.
The invention provides pharmaceutical compositions comprising compounds of
Formula
I. The invention also provides compounds useful in treating affective
disorders such as
schizophrenia and depression as well as certain movement disorders such as
Parkinsonism.
Furthermore. compounds of this invention are useful in treating the
cxtrapyramidal side effects
associated with the use of conventional ncuroleptic agents. Since particularly
dopamine D3 and
D,~ receptor subtypes arc concentrated in the limbic system (Science,'~,5:
1034 (Taubes. 1994j>
which controls cognition and emotion, compounds which interact with these
receptors ;ire useful
in the treatment of cognitive disorders. Such disorders include cognitive
deficits which arc a
significant component of the negative symptoms (social withdrawal and
unresponsiveness) of
schizophrenia. Other disorders involving memory impairment or attemion deficit
disorders can
also be treated with the compounds of this invention that interact
specifically with dopamine D3
andlor D4 receptor subtypes.
An object of the present invention is to provide certain bridged 4-phenyl-2-
aminomethylimidazoles; new dopamine receptor subtype specific ligands.
Accordingly, a
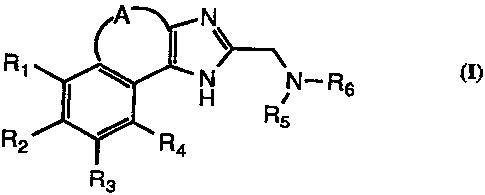
broad embodiment of the invention is directed to compounds of Formula I:
N
Rt N N-Rs
H
Rs
R2
R3
I
wherein
A represents -CH=CH-, or A represents -X-CH2-. where X is carbon or oxygen,
provided that
when X is oxygen. the oxygen is adjacent the 6-membered ring:
R,. R~. R3 and Ra are the same or different and represent hydrogen. halogen,
hydroxy, amino,
aminosulfonyl, alkylanunosuifonyl, alkylsuifonyl. arylaLkylsulfonyl. alkyl of
1 to 6
carbon atoms or alkoxy of 1 to 6 carbon atoms: and
-4.
CA 02213197 1998-09-10
RS reprexnts alkyl having 1-3 carbon atoms and R6 is benzyl, optionally
substituted with alkyl
having 1-6 carbon atoms, atkoxy, hvdroxv, or halogen: or
NRSR6 represents heterocyclic six membered ring optionally substituted with
alkyl having l-6
carbon atoms. hydroxyl, halogen. aryl, alkylaryl where the alkyl portion is
alkyl having
1-6 carbon atoms, or hetcroarvl.
The invention also pertains to the use of compounds of general Formula I in
the treatment
of neuropsychological disorders. The pharmaceutical utility of compounds of
this invention is
indicated by the assays described below for dopamine receptor subtype
affinity.
In accordance with an aspect of the present invention there is provided, a
compound
of the formula:
N
R ~ Rya
' N N
H
R»
Rz Y
R3 (CH2)s 2\ Rs
W /
Rs
R7
or the phamlaceuticallv acceptable salts thereof wherein:
A represents -CH=CH-, or A represents -X-CHI-: where X is carbon or oxygen,
provided that
when X is oxygen, the oxygen is adjacent the 6-membered ring:
Y represents nitrogen or CH:
W. Y and 2 are the same or different and reprcxnt either carbon or nitrogen.
Ri, R,. R;, Ra, RS. R~, and R~ independently represent hydrogen, halogen.
hydroxy. amino.
aminosulfonyl. arylalkvisulfonyl, alkylsulfonyl. alkylaminasulfonyl, alkyl
having 1 to 6
carbon atoms, or alkoxv of 1 to 6 carbon atoms:
Rip and R~ t are the same or different and represent alkyl groups having 1 to
6 carbon atoms:
g is an integer from 0 to 4: and
W and Z are both nitrogen: or
-S-
CA 02213197 1998-09-10
W is CRs and Z is CR9; or
W is CR8 and Z is nitrogen: or
Z is CR9 and W is nitrogen,
where R8 and R9 are the same or different and represent hydrogen. halogen,
alkyl
having from 1 to 6 carbon atoms, or alkoxy having from I to 6 carbon atoms.
In accordance with another aspect of the invention, there is provided a
compound of
the formula:
N
Rt ~ Rte
H N
R R~ ~
2 Y
R
R3 (CH2)9 Z\ 5
W
Rs
R7
or the pharmaceutically acceptable salts thereof wherein:
X is carbon or oxygen.
Y represenu nitrogen or CH:
W. Y and Z are the same or different and represent eichcr carbon or nitrogen.
Rt, R,. Rz. R.~, Rs, R~,, and R~ independently represent hydrogen. halogen.
hvdroxy. amino.
aminosulfonyl, arylalkylsulfonyl, alkylsulfonyl. alkylaminosulfonyl. alkyl
having 1 to 6
carbon stems, or alkoxy of 1 to b carbon atoms:
Rto ~d R~ ; are the same or different and represent alkyl groups having 1 to 6
carbon atoms:
g is an inteLer from 0 to 4: and
W and Z are both nitrogen: or
W is CRR and Z is CR9: or
W is CRg and Z is nitrogen: or
Z is CR9 and W is nitrogen,
where Rn and R9 arc the same or different and represent hydrogen. halogen,
alkyl
having from 1 to 6 carbon atoms, or alkoxy having from 1 to 5 carbon acorns.
-Sa-
CA 02213197 1998-09-10
In accordance with yet another aspect of the invention, there is provided a
compound
of the formula:
N
R~ ~ Rto
H N
R»
R2 Y
1
Rs ~CH2)9 Z~ R5
w
Rs
R7
or the pharmaceutically acceptable salts thereof wherein
Y represents nitrogen or CH:
W, Y and Z are the same or different and represent either carbon or nitrogen.
R~, Ra, Rz. R.~. R5. R6. and R7 independently represent hydrogsn. halogen.
hvdroxy. amino.
aminosulfonyl, arylalkylsuifanyl alkylsulfonyl, alkylaminosulfonyl, alkyl
having 1 to 6
carbon atoms. or alkoxy of 1 to 6 carbon atoms:
R~q and R~ t are the saint or different and represent alkyl groups having l to
6 c;trbon atoms:
g is an integer from U to :~: and
W and Z are both nitrogen: or
W is CRR and Z is CR9: or
W is CRx and Z is nitrogen: or
Z is CR9 and W is niQOgen.
where R~ and R9 are the same or different and represent hvdroLCn, halogen,
alkyl
having from 1 to b carbon atoms, or alkoxv having from 1 to 6 carbon atoms.
In accordance with a further aspect of the invention, there is provided a
compound
of the fonrtuia:
-Sb-
CA 02213197 1998-09-10
N
H N
Y
R3 Z\ R5
W
Rs
R7
or the pharn~aceuticallv acceptable salts thereof wherein:
Y represents nitrogen or CH:
R~. R~. Rz. and R., arc the same or different and represent hydrogen or alkvi
groups having l to
6 carbon atoms:
R~. R~,. and R7 independenty represent hydrogen, halogen, hvdroxy, amino.
alkyl having 1 to 6
carbon atoms, or alkoxv of 1 to 6 carbon atoms: and
w and Z are the same or different and represent nitrogen or CRy where Ry
represents hydrogen.
halogen, alkyl having from l to 6 carbon atoms, or alkoxy having from 1 to 6
carbon
atoms.
In accordance with a further aspect of the invention, there is provided a
compound
of the formula:
N
Rt
RAN'' Rs
s
R2
R3
or the pharmaceutically acceptable salts thereof wherein:
X represents oxygen~or mcthylene:
R~, R,. R3, and R,~ arc the same or different and represent hydrogen or alkyl
groups having I to
6 carbon atoms: and
RS represents alkyl having I-3 carbon atoms and R6 is benzvl, optionally
substituted with alkyl
having I-6 carbon atoms, alkoxy, hydroxy, or halogen.
-5 c-
CA 02213197 1998-09-10
In accordance with a further aspect of the invention, there is provided a
compound
of the formula:
N
R \
N
H
,
R2 Y
Rs Z
W ,~
R~
or the pham~aceuticallv accepwble salts thereof wherein:
Y represents nitrogen or CH:
R~. R~, Rz. :.trtd R1 are the same or different and represent hydrogen or
alkyl ~_roups having 1 to
6 carbon atoms:
R6 represents hydrogen, halogen. hydroxy, amino, alkyl having 1 to 6 carbon
atoms. or alkoxy
of 1 to 6 carbon atoms: and
W and Z ;v-e the same or different and represent nitrocen or CR9 where R9
repcesenu hydrogen.
halogen, alkyl having from 1 to 6 carbon atoms. or alkoxy having from ! to 6
carbon
atoms.
-Sd-
CA 02213197 1997-08-15
WO 96/25411 PCT/L1S96/01906
DETAILED DESCRIPTION OF THE INVENTION
In addition to compounds of general formula I described above, the present
invention
further encompasses compounds of Formula II:
N
R1 ~ Rib
H N
R R»
2 Y
R3 ~CH2)9 Z~ R5
R6
R~
11
or the pharmaceutically acceptable salts thereof wherein:
A represents -CH=CH-, or A represents -X-CH2-; where X is carbon or oxygen,
provided that
when X is oxygen, the oxygen is adjacent the 6-membered ring;
Y represents nitrogen or CH;
W, Y and Z are the same or different and represent either carbon or nitrogen,
R1, R2, R3, R,~, R5, R6, and R7 independently represent hydrogen, halogen,
hydroxy, amino,
aminosulfonyl, arylalkylsulfonyl or alkylsulfonyl where each alkyl portion has
1 to 6
carbon atoms, alkylaminosulfonyl where the alkyl portion has 1 to 6 carbon
atoms, alkyl
having 1 to 6 carbon atoms, or alkoxy of 1 to 6 carbon atoms;
Rlo and R11 are the same or different and represent alkyl groups having 1 to 6
carbon atoms:
g is an integer from 0 to 4; and
W and Z are both nitrogen; or
W is CRg and Z is CR9; or
W is CR8 and Z is nitrogen; or
Z is CRy and W is nitrogen,
where RR and R9 are the same or.different and represent hydrogen. halogen,
alkyl
having from 1 to 6 carbon atoms, or alkoxy having from 1 to 6 carbon atoms.
-6-
CA 02213197 1997-08-15
WO 96/25411 PCTIUS96/01906
The invention also encompasses compounds of formula III:
N
Ri N Rio
H N
R R1i
2 Y
(CH2)9 Z~ Rs
W /
R6
R~
III
or the pharmaceutically acceptable salts thereof wherein:
X is carbon or oxygen,
Y represents nitrogen or CH;
W, Y and Z are the same or different and represent either carbon or nitrogen,
R1, R2, R3, R4, R5, R6, and R7 independently represent hydrogen, halogen,
hydroxy, amino,
aminosulfonyl, arylalkylsulfonyl or alkylsulfonyl where the alkyl portion has
1 to 6
carbon atoms. alkylaminosulfonyl where each alkyl portion has 1 to 6 carbon
atoms,
alkyl having 1 to 6 carbon atoms. or alkoxy of 1 to 6 carbon atoms:
Rio ~d Rt W'e the same or different and represent alkyl groups having 1 to 6
carbon atoms;
g is an integer from 0 to 4; and
W and Z are both nitrogen; or
W is CR8 and Z is CRg; or
W is CRg and Z is nitrogen; or
Z is CR9 and W is nitrogen,
where R8 and R9 are the same or different and represent hydrogen, halogen.
alkyl
having from 1 to 6 carbon atoms, or alkoxy having from 1 to 6 carbon atoms.
The invention further encompasses compounds of formula IV:
CA 02213197 1997-08-15
WO 96/25411 PCT/LTS96/01906
N
R~ ~ Rio
H N
R R»
2 Y
R
R3 (CH2)9 Z~ s
W /
Rs
R7
N
or the pharmaceutically acceptable salts thereof wherein
Y represents nitrogen or CH;
W, Y and Z are the same or different and represent either carbon or nitrogen,
R1, R2, R3, R.~, R5, R6, and R7 independently represent hydrogen, halogen,
hydroxy, amino.
aminosulfonyl, arylalkylsulfonyl or allcylsulfonyl where each alkyl portion
has 1 to 6
carbon atoms, alkylaminosulfonyl where the alkyl portion has 1 to 6 carbon
atoms, alkyl
having 1 to 6 carbon atoms, or allcoxy of 1 to 6 carbon atoms;
Rlo and R11 are the same or different and represent alkyl groups having 1 to 6
carbon atoms;
g is an integer from 0 to 4: and
W and Z are both nitrogen; or
W is CRg and Z is CR9; or
W is CRR and Z is nitrogen; or
Z is CR9 and W is nitrogen,
where R8 and R9 are the same or different and represent hydrogen, halogen.
alkyl
having from 1 to 6 carbon atoms, or alkoxy having from 1 to 6 carbon atoms.
The invention also provides compounds of formula V:
_g_
CA 02213197 1997-08-15
WO 96125411 PCTlUS96101906
1
Rs
R~
V
wherein,
X is carbon or oxygen; and
R1, R~, R3, R4, R5, R6, R7, Rg and R9 are the same or different and represent
hydrogen,
halogen, hydroxy, amino, aminosulfonyl, arylalkylsulfonyl, alkylsulfonyl,
alkylaminosulfonyl, alkyl of I to 6 carbon atoms or alkoxy of I to 6 carbon
atoms.
The invention also provides compounds of formula VI:
R11
N
R~ N .
H
R2 /
R3 Rs
Rs
_ Rs
' lU R8 R~
vi
wherein,
-9-
CA 02213197 1997-08-15
WO 96/25411 PCT/US96/01906
R 1, R2, Rz. R,~, R5, R6, R7, Rg, R9, R Ip and RI i are the same or different
and represent
hydrogen. halogen, hydroxy, amino. aminosulfonyl, arylalkylsulfonyl,
alkylsulfonyl.
alkylaminosulfonyl. alkyl of 1 to 6 carbon atoms or alkoxy of 1 to 6 carbon
atoms.
The invention also provides compounds of formula VII:
N
R' H N
R2
R -OH
3
R5
VII
wherein
X is carbon or oxygen; and
Ri, R3, R3, R.~ and RS are the same or different and represnt hydrogen.
halogen, hydroxy,
amino, aminosulfonyl, arylalkylsulfonyl, alkylsulfonyl, alkylaminosulfonyl,
alkyl of 1 to
6 carbon atoms or alkoxy of one to six carbon atoms.
The invention also provides compounds of fomnula VIII:
-10-
CA 02213197 1997-08-15
WO 96/25411 PCTlL1S96101906
R~
VIII
OH
wherein
X is carbon or oxygen; and
R1, R2, R3, R,~, R5, R6 and R7 are the same or different and represent
hydrogen, halogen,
hydroxy, amino, aminosulfonyl, arylalkylsulfonyl, alkylsulfonyl,
alkylaminosulfonyl,
alkyl of 1 to 6 carbon atoms or allcoxy of 1 to 6 carbon atoms.
The invention also provides compounds of formula IX:
N
R' H N
R2
R3
IX
wherein
X is carbon or oxygen; and
-11-
CA 02213197 1997-08-15
WO 96/25411 PCT/US96/01906
R1, R2. R3, and R.1 are the same or different and represent hydrogen, halogen.
hydroxy, amino,
aminosulfonyl, arylalkylsulfonyl, alkylsulfonyl, alkylaminosulfonyl. alkyl of
1 to 6
carbon atoms or alkoxy of 1 to 6 carbon atoms.
The invention also provides compounds of formula X:
Rs
R3
N
N
N
H
X
wherein
R1, R~, R3, R4, R5, and R6 are the same or different and represent hydrogen,
halogen, hydroxy,
amino, aminosulfonyl, arylalkylsulfonyl, alkylsulfonyl, alkylaminosulfonyl,
alkyl of 1 to
6 carbon atoms or alkoxy of 1 to 6 carbon atoms.
The invention further provides compounds of the formula:
N
R
N N
H
R2 Y
R3 Z\ R5 ,
R6
R7
XI
-12-
CA 02213197 1997-08-15
W~ 96/25411 PCTlUS961a~906
or the pharmaceutically acceptable salts thereof wherein.
Y represents nitrogen or CH;
R1, R~. R~, and R.~ are the same or different and represent hydrogen or alkyl
~~roups having 1 to
6 carbon atoms;
~ R5, R6, and R~ independently represent hydrogen. halogen. hydroxy, amino,
alkyl having 1 to 6
carbon atoms. or allcoxv of 1 to 6 carbon atoms: and
W and Z are the same or different and represent nitrogen or CR9 where R9
represents hydrogen.
halogen, alkyl having from 1 to 6 carbon atoms, or alkoxy having from 1 to 6
carbon
atoms.
In addition, the invention provides compounds of the formula:
N
R
H R~N~ Rs
5
R2
R3
XII
or the pharmaceutically acceptable salts thereof wherein:
X represents oxygen or methylene;
R1, R2, R3, and R.~ are the same or different and represent hydrogen or alkyl
groups having 1 to
6 carbon atoms; and
RS represents alkyl having 1-3 carbon atoms and R6 is benzyl, optionally
substituted with alkyl
having 1-6 carbon atoms, alkoxy, hydroxy, or halogen.
2U
The invention also provides compounds of the formula:
-13-
CA 0 2 21319 7 19 9 7 - 0 8 -15 pCT/LTS96/01906
WO 96/25411
N
R~ N N
H
R2 Y
R3 Z
W /
Rs
XIII
or the pharmaceutically acceptable salts thereof wherein:
Y represents nitrogen or CH;
RI, R~, R3, and R,t are the same or different and represent hydrogen or alkyl
~~roups having 1 to
6 carbon atoms;
R6 represents hydrogen, halogen, hydroxy, amino, alkyl having 1 to 6 carbon
atoms, or alkoxy
of 1 to 6 carbon atoms; and
W and Z are the same or different and represent nitrogen or CR9 where R9
represents hydrogen,
halogen, alkyl having from 1 to 6 carbon atoms, or alkoxy having from 1 to 6
carbon
atoms.
-14-
CA 02213197 1997-08-15
WO 96/25411 PCT/US96I01906
Preferred "NRSR6" soups of formula I above include the followin~_
I I I I I I
N N N N N N CH
alkyl
N N N ~CH3
/ ~ / ~ ~ ~ / I N~ I
y
alkoxy O R
I I
N N N CH3 N CH3 N N
~CH3 ~ CH3
/ ~ i
N I N/ I N/ I N/ I N J
hatoge ~~ ~\OR
_ _
N N N N N N
/ / /
HO HO N
HO
N~ N / N / N~ N / / Ni 'N
~ I
haloge ~~ ~ OR
In the above preferred NRSR6 groups, OR represents hydroxy or alkoxy.
Particularly preferred NRSR6 substituents are N-benzyl-N-methyl, 4-(2-
pyrimidinyl)-
piperazinyl, and 4-phenylpiperidinyl groups.
Representative compounds of the present invention, which are encompassed by
Formula
I, include, but are not limited to the compounds in Figure I and their
pharmaceutically acceptable
salts. Non-toxic pharmaceutically acceptable salts include salts of acids such
as hydrochloric,
phosphoric. hydrobromic. sulfuric. sulfinic. formic. toluene sulfonic,
hydroiodic, acetic and the
like. Those skilled in the art will recognize a wide variety of non-toxic
pharmaceutically
acceptable addition salts.
-15-
CA 02213197 1997-08-15
WO 96/25411 PCT/US96/01906
The present invention also encompasses the acylated prodrugs of the compounds
of
Formula I. Those skilled in the art will recognize various synthetic
methodologies which can be
employed to prepare non-toxic pharmaceutically acceptable addition salts and
acylated prodrugs
of the compounds encompassed by Formula I.
When A represents -CH=CH- in the formulas set forth above. the resulting
unsaturated
system is a 1H-naphth[ 1,2-d]imidazole.
By "aryl" and "Ar" is meant an aromatic carbocyclic group having a single ring
(e.g.,
phenyl), multiple rings (e.g., biphenyl), or multiple condensed rings in which
at least one is
aromatic, (e.g., 1,2,3,4-tetrahydronaphthyl, naphthyl, anthryl, or
phenanthryl), which can
optionally be unsubstituted or substituted with e.g., halogen, lower alkyl,
lower alkoxy, lower
alkylthio, trifluoromethyl, lower acyloxy, aryl, heteroaryl, and hydroxy.
By "alkyl" and "lower alkyl" is meant straight and branched chain alkyl groups
having
from 1-6 carbon atoms.
By "lower alkoxy" and "alkoxy" is meant straight and branched chain alkoxy
groups
having from 1-6 carbon atoms.
By "heteroaryl" is meant S, 6, or 7 membered aromatic ring systems having at
least one
hetero atom selected from the group consisting of nitrogen, oxygen and sulfur.
Examples of
heteroaryl groups are pyridyl, pyrimidinyl, pytrolyl, pyrazolyl, pyrazinyl,
pyridazinyl, oxazolyl,
furanyl, quinolinyl, isoquinolinyl, thiazolyl, and thienyl, which can
optionally be unsubstituted
or substituted with e.g., halogen, lower alkyl, lower alkoxy, lower alkylthio,
trifluoromethyl,
lower acyloxy, aryl, heteroaryl, and hydroxy.
By halogen is meant fluorine, chlorine, bromine and iodine.
By alkylsulfonyl is meant a sulfonyl group substituted with a lower alkyl
group.
By arylalkylsulfonyI is meant a sulfonyl group substituted with an arylalkyl
group.
?5 By aminosulfonyl is meant a sulfonyl group substituted with an amino group.
By alkylaminosulfonyl is meant a sulfonyl group substituted with a lower
alkylamino, or
di-lower alkylamino group. ,
Representative examples of bridged 4-phenyl-2-aminomethylimidazoles according
to the
invention are shown in Table 1 below.
-16-
CA 02213197 1997-08-15
WO 96!25411 PCTlIIS96101906
Table 11
N N
(
~N N 'N
H ~~ H / N
/ N /
N~N
1
N ~ N
~I ~I
I H /N 'H N
/ ~ / ~N
OMe
4
N
N N ~ N
H H N
The number below each compound is its compound number.
The pharmaceutical utility of compounds of this invention are indicated by the
following
assays for dopamine receptor subtype affinity which demonstrate the
interaction of the
compounds with dopamine receptor subtypes. The interaction results in the
pharmacological
activities of these compounds which thus can be exploited in the treatment of
affective disorders
such as schizophrenia, depression as well as certain movement disorders such
as Parkinsonism
and dystonia. Furthermore, compounds of this invention are useful in treating
the extrapyramidal
side effects associated with the use of conventional neuroleptic agents.
-17-
CA 0 2 21319 7 19 9 7 - 0 8 - 15 pC.i~~s96/01906
WO 96/25411
Assay for D~ an~receytor binding activity
Pellets of COS cells containing recombinantly produced D' or D3 receptors from
African
Green monkey were used for the assays. The sample is homogenized in 100
volumes (w/vol) of
0.05 M Tris HCl buffer at :~° C and pH 7..~. The sample is then
centrifuged at 30,UUU x g and
resuspended and rehomogenized. The sample is then centrifuged as described and
the final tissue
sample is frozen until use. The tissue is resuspended l:'_'U (wt/vol) in U.US
M Tris HCI buffer
containing 100 mM NaCI.
Incubations are carried out at 48°C and contain 0.4 ml of tissue
sample. 0.5 nM 3H-YM
lU 09151-2 and the compound of interest in a total incubation of 1.0 ml.
Nonspecific binding is
defined as that binding found in the presence of 1 ~tM spiperone; without
further additions,
nonspecific binding is less than 20% of total binding. The binding
characteristics of examples of
this invention for the D? and D3 receptor subtypes are shown in Table ? for
Rat Striatal
Homogenates.
TABLE 2
Compound Numbers j~~ l~tM l D3 K1 luM )
l, 4.200 7.800
Z. 0.350 0.210
0.220 0.440
~ Compound numbers relate to compounds shown in Table 1.
Assay for D.t receptor binding activity
Clonal cell lines expressing the human dopamine D,~ receptor subtype were
harvested in
2U PBS and the cells centrifuged and the pellets stored at -80° C until
used in the binding assay. The
pellets were resuspended and the cells lysed at 4° C in 50 mM Tris pH
7.4 buffer containing 120
mM NaCI, 1 mM EDTA and 5 mM MgCI~. The homogenate is centrifuged at 48UUU x g
for lU
minutes at .~° C. The resulting pellet is resuspended in fresh buffer
and centrifuged again. After ,
resuspension of the pellet in fresh buffer a 100 ml aliquot is removed for
protein determination.
'?5 The remaining homogenate is centrifuged as above. the supernatant removed
and the pellet stored
at 4° C until needed at which time it is resuspended to a final
concentration of 625 mg/ml (250 m~=
per sample) with 50 mM Tris buffer (pH 7.4) and 1''0 mM NaCI just prior to
use. Incubations
-18-
CA 02213197 2000-OS-OS
were carried out for 60 minutes at 25° C in the presence of 0.1 nM [3H~
YM-09151-2. The
incubation was terminated by rapid filtration through Whatman~rF~C filters and
rinsed with ? x 4
ml washes of chilled 50 mM Tris (pH 7.4) and 130 mM NaCI. Non-specific binding
was
determined with 1 ~tM spiperone and radioactivity determined by counting in an
LKB beta
counter. Binding parameters were determined by non-linear least squares
regression analysis.
from which the inhibition constant Kv could be calculated for each test
compound. The binding
characteristics of some examples of this invention are shown in Table 3 for
the dopamine D~
binding assay. In general, compounds of the accompanying Examples were tested
in the above
assay, and all were found to possess a K~ value for the displacement of (3H]YM-
09151-2 from
IU the human dopamine D.r receptor subtype of below 500 nM. Some specific data
is indicated in
Table 3.
Table 3
Com~und Numhert j~.(uM)
1 0.012
0.081
o.I45
t Compound numbers relate to compounds shown in Table I .
Compounds 1 and ~ are particularly preferred embodiments of the present
invention
because of its potency in binding to dopamine receptor subtypes.
The compounds of the invention, or a pharmaceutically acceptable srrll
thereof, i.c., the
"active ingredient", can be used alone or in combination with various
excipients, stabilizers or
agents to designed to prolong the action of the active ingredient in the
treatment of
3U neuropsychochological disorders such as, example. schizophrenia, dementia.
depression.
:utxiety. Parkinson-like motor disorders and motion disorders related to the
use of neuroleptic
agents.
The compounds of general Formula I may be administered orally, topically,
parcntcrally.
by inhalation or spray or rectally in dosage unit formulations containing
conventional non-toxic
'_'S pharmaceutically acceptable carriers, adjuvancs and vehicles. The term
parenteral as used herein
-19-
CA 02213197 1997-08-15
WO 96/25411 PCT/US96/01906
includes subcutaneous injections. intravenous. intramuscular. intrasternal
injection or infusion
techniques. In addition. there is provided a pharmaceutical formulation
comprising a compound
of general Formula I and a pharmaceutically acceptable carrier. One or more
compounds of
general Formula I may be present in association with one or more non-toxic
pharmaceutically
acceptable carriers and/or diluents and/or adjuvants and if desired other
active ingredients. The
pharmaceutical compositions containing compounds of general Formula I may be
in a form
suitable for oral use, for example. as tablets. troches, lozenges, aqueous or
oily suspensions,
dispersible powders or granules, emulsion, hard or soft capsules, or syrups or
elixirs.
Compositions intended for oral use may be prepared according to any method
known to
the art for the manufacture of pharmaceutical compositions and such
compositions may contain
one or more agents selected from the group consisting of sweetening agents,
flavoring agents,
coloring agents and preserving agents in order to provide pharmaceutically
elegant and palatable
preparations. Tablets contain the active ingredient in admixture with non-
toxic phammceuticallv
acceptable excipients which are suitable for the manufacture of tablets. These
excipients may be
for example, inert diluents, such as calcium carbonate, sodium carbonate,
lactose, calcium
phosphate or sodium phosphate; granulating and disintegrating agents, for
example, corn starch,
or alginic acid; binding agents, for example starch, gelatin or acacia, and
lubricating agents, for
example magnesium stearate, stearic acid or talc. The tablets may be uncoated
or they may be
coated by known techniques to delay disintegration and absorption in the
gastrointestinal tract and
30 thereby provide a sustained action over a longer period. For example, a
time delay material such
as glyceryl monosterate or glyceryl distearate may be employed.
Formulations for oral use may also be presented as hard gelatin capsules
wherein the
active ingredient is mixed with an inert solid diluent, for example, calcium
carbonate. calcium
phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient
is mixed with water
?5 or an oil medium, for example peanut oil, liquid paraffin or olive oil.
Aqueous suspensions contain the active materials in admixture with excipients
suitable for
the manufacture of aqueous suspensions. Such excipients are suspending agents,
for example -
sodium carboxymethylcellulose. methylcellulose, hydropropyl methylcellulose,
sodium alginate,
polyvinylpyrrolidone, gum tragacanth and gum acacia: dispersing or wetting
agents may be a
-20-
CA 02213197 1997-08-15
WO 96/25411 PCT1US96101906
naturally-occurring phosphatide. for example. lecithin, or condensation
products of an alkylene
oxide with fatty acids, for example polyoxvethylene stearate, or condensation
products of
ethylene oxide with long chain aliphatic alcohols, for example
heptadecaethyleneoxycetanol, or
condensation products of ethylene oxide with partial esters derived from fatty
acids and a hexitol
z ~ such as polyoxyethylene sorbitol monooleate. or condensation products of
ethylene oxide with
partial esters derived from fatty acids and hexitol anhydrides, for example
polyethylene sorbitan
monooleate. The aqueous suspensions may also contain one or more
preservatives, for example
ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one or more
Ylavoring
agents, and one or more sweetening agents. such as sucrose or saccharin.
Oily suspensions may be formulated by suspending the active ingredients in a
vegetable
oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a
mineral oil such as liquid
paraffin. The oily suspensions may contain a thickening agent, for example
beeswax, hard
paraffin or cetyl alcohol. Sweetening agents such as those set forth above,
and flavoring agents
may be added to provide palatable oral preparations. These compositions may be
preserved by
the addition of an anti-oxidant such as ascorbic acid.
Dispersible powders and granules suitable for preparation of an aqueous
suspension by
the addition of water provide the active ingredient in admixture with a
dispersing or wetting
agent, suspending agent and one or more preservatives. Suitable dispersing or
wetting agents
and suspending agents are exemplified by those already mentioned above.
Additional excipients.
2U for example sweetening, flavoring and coloring agents, may also be present.
Pharmaceutical compositions of the invention may also be in the form of oil-in-
water
emulsions. The oily phase may be a vegetable oil, for example olive oil or
arachis oil, or a
mineral oil, for example liquid paraffin or mixtures of these. Suitable
emulsifying agents may be
naturally-occurring gums, for example gum acacia or gum tragacanth, naturally-
occurring
?~ phosphatides, for example soy bean. lecithin. and esters or partial esters
derived from fatty acids
and hexitol, anhydrides. for example sorbitan monoleate, and condensation
products of the said
. partial esters with ethylene oxide, for example polyoxyethylene sorbitan
monoleate. The
emulsions may also contain sweetening and flavoring agents.
-21-
CA 02213197 1997-08-15
WO 96/25411 PCT/US96/01906
Syrups and elixirs may be formulated with sweetening agents, for example
~Tlycerol.
propylene glycol, sorbitor or sucrose. Such formulations may also contain a
demulcent, a
preservative and flavoring and coloring agents. The pharmaceutical
compositions may be in the
form of a sterile injectable aqueous or oleaginous suspension. This suspension
may be
formulated according to the known art using those suitable dispersing or
wetting agents and ..
suspending agents which have been mentioned above. The sterile injectable
preparation may also
be sterile injectable solution or suspension in a non-toxic parentally
acceptable diluent or solvent,
for example as a solution in 1,3-butanediol. Among the acceptable vehicles and
solvents that
may be employed are water, Ringer's solution and isotonic sodium chloride
solution. In
addition, sterile, fixed oils are conventionally employed as a solvent or
suspending medium. For
this purpose any bland fixed oil may be employed including synthetic mono or
diglycerides. In
addition, fatty acids such as oleic acid find use in the preparation of
injectables.
The compounds of general Formula I may also be administered in the form of
suppositories for rectal administration of the drug. These compositions can be
prepared by
mixing the drug with a suitable non-irritating excipient which is solid at
ordinary temperatures but
liquid at the rectal temperature and will therefore melt in the rectum to
release the drug. Such
materials are cocoa butter and polyethylene glycols.
Compounds of general Formula I may be administered parenterally in a sterile
medium.
The drug, depending on the vehicle and concentration used, can either be
suspended or dissolved
in the vehicle. Advantageously, adjuvants such as local anaesthetics,
preservatives and buffering
agents can be dissolved in the vehicle.
Dosage levels of the order of from about 0.1 mg to about 140 mg per kilogram
of body
weight per day are useful in the treatment of the above-indicated conditions
(about 0.5 mg to
about 7 g per patient per day). The amount of active ingredient that may be
combined with the
?5 carrier materials to produce a single dosage form will vary depending upon
the host treated and
the particular mode of administration. Dosage unit forms will generally
contain between from
about 1 mg to about 500 mg of an active ingredient. ,
It will be understood, however, that the specific dose level for any
particular patient will
depend upon a variety of factors including the activity of the specific
compound employed, the
-22-
CA 02213197 1997-08-15
WO 96/25411
PCT/US96/01906
age, body weight, general health. sex. diet, time of administration, route of
administration. and
rate of excretion, drug combination and the severity of the particular disease
undergoing therapy.
Representative procedures suitable for the preparation of compounds of the
present
invention are illustrated in Schemes 1 and 2. Those having skill in the art
will recognize that the
starting materials may be varied and additional steps employed to produce
compounds
encompassed by the present invention.
Scheme 1 depicts a representative route for the preparation of ethyl and
oxomethyl
bridged 4-phenyl-2-aminomethylimidazoles of the invention.
-23-
i
CA 02213197 1997-08-15
WO 96/25411 PCT/US96/01906
Scheme 1
Br
Br2, AcOH
O ---~ I ~ O
/ /
X = CH2, O
formamide
N N
CH20. HNRR'
NRR' AcOH, heat
/ /
Scheme 2 shows a representative route for the preparation of the ethylene
bridged 4-
phenyl-2-aminomethylimidazoles of the invention.
Scheme 2
/ NH2 / N
Chloroacetic acid
NH . . ~ ~CI
SN hydrochlonc acid
N
/ / H
HNRR'
/ ~ K2C03
~NRR'
N
/ H
lU In each of Schemes 1 and 2 above, NRR' represents the group NRSR6 defined
above or a
protected precursor thereof.
This invention is further illustrated by the following examples which are not
to be
consuued as limiting the invention in scope or spirit to the specific
procedures and compounds
described therein.
-24-
CA 02213197 1997-08-15
WO 96J25411 PCTIUS96J01906
Example 1
N
N N
~H
~N
~- N
N \,
1. A solution of bromine (5.5~~) in 25 ml of acetic acid was added dropwise to
a
solution of 1-tetralone in ?5 ml of acetic acid at 60°C. After the
addition was complete, the
reaction was cooled and most of the acetic acid was removed in vacuo. The
residue was
partitioned between water and ethyl acetate. The organic layer was washed with
a dilute solution
of sodium bisulfite. dried with sodium sulfate and concentrated. A portion of
the resulting 2-
bromo-1-tetralone (3g) was mixed with 30 ml of formamide and the resulting
solution was heated
at 160°C for 12 hours. Upon cooling. 100 ml of water was added followed
by 10 ml of 3N
hydrochloric acid solution. The mixture was washed with diethyl ether and the
ether layer was
discarded. The remaining solution was made basic with saturated sodium
carbonate solution and
extracted with methylene chloride. The organic extracts were dried with sodium
sulfate and
concentrated to provide 1.2 g of 4,5-dihydronaphth[1.2-d]imidazole.
2. A solution of 100 mg of 4.5-dihydronaphth( 1?-d]imidazole, 51 mg of 37% w/w
IS aqueous formaldehyde, 103 mg of 1-(2-pyrimidinyl)piperazine in 5 ml of
acetic acid was
refluxed for 1 hour, cooled and the solvent removed under reduced pressure.
Ethanol (3 ml) and
ammonium hydroxide (0.1 ml) were added and the solution was reconcentrated.
Purification of
the product on silica gel using 10% v/v methanol/chloroform as the eluent
provided 1H-2-(1-(4-
(2-pyrimidyl)piperazinyl))methyl-(5,6-dihvdrol-naphth[ 1.2-d]-imidazole as an
oil. The addition
of a saturated solution of hydrogen chloride in isopropanol to the product in
isopropanol yielded
the amine salt which was isolated by filtration to provide 151 mg of: IH-2-( 1-
(4-(2-
pyrimidyl)piperazinyl))methyl-(5,6-dihydrol-naphth[1,2-d]imidazole
dihydrochloride
~ (Compound 1, m.p. 212-214 °C).
-25-
CA 02213197 1997-08-15
WO 96/25411 PC'T/US96/01906
Example 2
The following compounds are prepared essentially according to the procedure
described
in Example 1.
(a) 1H-{ 2-(N-benzyl-N-methyl)aminomethyl j-(5.6-dihydro)-naphth[ 1.2-
djimidazole
dihydrochloride (Compounds 2. m.p. 225-227°C).
(b) 1H-{2-(N-benzyl-N-methyljaminomethyl]chromano[3.4-d]imidazole
dihydrochloride (Compound 3, m.p. 198-201°C dec.;).
Example 3
lU Monochloroacetic acid (15g, 0.16 mol) and diaminonaphthlene (22.18, 0.14
mol) were
refluxed in 60 ml of 5 N HCl for 6 hours. The reaction was cooled in ice and
neutralized with
aqueous ammonium hydroxide. The precipitated product was collected by
filtration and
recrystallized from benzene/hexane (18.78, 62%).
To a solution of the isolated 2-chloromethyl-1H-naphth[1,2-d]imidazole (1.4g,
6.5
15 mmol) in dimethylformamide (20m1) was added potassium carbonate (2g) and
the resultant
mixture was refluxed for 2 hours. After cooling to 20°C, the reaction
mixture was poured onto
ice water and extracted with chloroform. The organic extracts were dried and
concentrated to
give an oil. The oil was dissolved in lOml of isopropyl alcohol and 48% HBr
was added
dropwise until the pH of the solution was less than 3.0 as indicated by pH
paper. A precipitate
20 developed upon standing which was isolated by filtration providing 1 H-2-(
1 (4-(2-
methoxyphenyl j-piperazinyl)]methylnaphth[ 1,2-d]imidazole dihydrobromide
(Compound 4,
2.7g, 77%).
Example 4
The following compounds are prepared essentially according to the procedure
described
in Example 3.
(a) 2-[1-(4-phenyl)piperidinyl]methyl-1H-naphth[1,2-d]imidazole
dihydrochloride
(Compound 5).
-26-
CA 02213197 1997-08-15
WO 96/25411 PCTJUS961D1906
(b) ?-[1-{4-phenyl-1.?.3.6-tetrahydro}pyridyl]methyl-IH-naphth(1.'_'-
d]imidazole
dihydrochloride (Compound 6).
(c) ?-[1-{.~-hydroxy-4-(4-chlorophenylj}piperidinyl]methyl-1H-naphth[1,2-
d]imidazole dihydrochloride (Compound 7).
The invention and the manner and process of making and using it are now
described in
such full, clear. concise and exact terms as to enable any person skilled in
the art to which it
pertains, to make and use the same. It is to be understood that the foregoing
describes preferred
embodiments of the present invention and that modifications may be made
therein without
departing from the spirit or scope of the present invention as set forth in
the claims. To
particularly point out and distinctly claim the subject matter regarded as
invention. the following
claims conclude the specification.
-27-