Note: Descriptions are shown in the official language in which they were submitted.
CA 0221362l 1997-08-22
w ~96126734 PCTAEP96/00736
ANTIVIRAL COMBINATIONS OF BCH-189 AND RITOVANIR
The present invention relates to co" ,L,inations of antiviral agents. More
specifically it is concerned with combinations of 1,3-oxathiolane nucleoside
5 analogues with prolease inhibitors effective against HIV.
Human immunodeficiency virus (HIV) causes a variety of clinical conditions
including the acquired immune deficiency syndrome (AIDS) and chronic
neurological disorders.
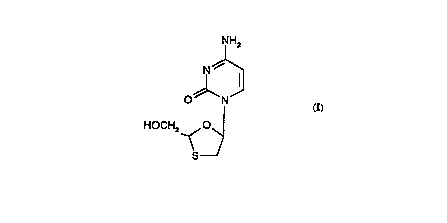
The compound of formula (I)
IH2
0 N
HOCH2_.<0
S~
15 also known as BCH-189 has been described as having antiviral activity in
particular against the human immunodeficiency viruses (Hl~s), the cA~s~tive
agents of AIDS (5th Anti-Aids CG,Irert:"ce, Monlreal, Canada 5th-9th June
1989: Abstracts T.CØ1 and M.G.P 63; European Patent App!ication
Publication No. 0382562). The compound of formula (I) is a racemic mixture of
20 the two enantiomers of formulae (1-1 ) and (I-2):-
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W 096/26734 PCT~EP96100736
NH2 NH2
~ 2) -- (1-1)
HOCH~S ~ HOCH=~S y-
Although the enantiomers of the compound of formula (I) are equipotent against
HIV one of the enantiomers (the(-)-enantiomer) has considerably lower
5 cytotoxicity than the (+) enantiomer.
The (-) enantiomer has the chemical name (-)-_4-amino-1-(2-hydroxymethyl-
1,3-oxathiolan-5-yl)-(1H)- pyrimidin-2-one. It has the absolute stereochemistry
of the compound of formula (1-1) which has the name (2R,cis)4-amino-1-(2-
hydroxymethyl-1 ,3-oxathiolan-5-yl)-(1 H)-pyrimidin-2-one. - This compound is
now known as lamivudine and 3TCTM.
The compound of formula (Il):
H3C~CH3
H3C>--<S~ NJ~NH H~HN O~ > (Il)
has the chemical name (2S, 3S, 5S)-5-(N-(N-methyl-N-((2-isopropyl4-
thiazolyl)methyl)amino)c~rL,o"~fl)valinyl)amino)-2-(N-
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W 096/26734 PCT;~3GJ~r736
((thiazolyl)methoxycarbonyl)amino)1,6-diphenyl-3-hydroxyhexane and is also
known as A-84538, ABT-538 or ritonavir. The compound of formula (Il) has
been described as having HlV-protease inhibitor activity (International Patent
Application Publication No. 94/14436).
We have now found that the compound of formula (I) and, in particular its (-)-
enantiomer exhibits unexpected advantages when used in combination with the
compound of formula (Il). In particular the compound of formula (I) shows a
synergistic antiviral effect and/or a reduction in cytotoxicity when used in
10 combination with the compound of formula (Il).
There is thus provided in a first aspect of the invention a combination
comprising the cor"~,ound of formula (I) or a pharmaceutically acceptable
derivative thereof and the compound of formula (Il) or a pharmaceutically
15 acceptable derivative thereof.
P,ererably the compound of formula (I) is in the form of its (-)-enantiomer,
lamivudine.
20 When the Compound formula (I) is in the form of the (-)-enantiomer it will
normally be provided substantially free of the corresponding (+)-enantiomer,
that is to say no more than about 5% w/w of the (+)- enantiomer, preferably no
more than about 2%, in particular less than about 1% w/w will be present.
25 By "a pharmaceutically acceptable derivative" is meant any pharmaceutically
acceptable salt, ester, or salt of such ester, of a parent co""~ound or any other
compound which, upon administration to the recipient, is capable of providing
(directly or indirectly) the parent compound or an antivirally active metabolite or
residue thereof.
It will be appre(,i~ted by those skilled in the art that the co""~ound of formula (I)
may be modified to provide pharmaceutically acceplable derivatives thereof, at
functional groups in both the base moiety and at the hydroxymethyl group of the
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W 096/26734 PCT/~l~G~ 6
oxathiolane ring. Modification at all such functional groups are included withinthe scope of the invention. However of particular interest are pharmaceutically
acceptaL.le derivatives obtained by modification of the 2-hydroxymethyl group ofthe oxathiolane ring.
P(erer,ed esters of the compound of formula (I) include the compounds in which
the hydrogen of the 2-hydroxymethyl group is replaced by an acyl function R-C-
in which the non-carbonyl moiety R of the ester is selected from hydrogen,
straight or branched chain alkyl (e.g. methyl, ethyl, n-propyl, t-butyl, n-butyl),
10 alkoxyalkyl (e.g. methoxymethyl), aralkyl (e.g. benzyl), aryloxyalkyl (e.g.
phenoxymethyl), aryl (e.g. phenyl optionally substituted by halogen, C1 4 alkyl
or C1 4 alkoxy); sulphonate esters such as alkyl- or aralkylsulphonyl (e.g.
methanesulphonyl); amino acid esters (e.g. L-valyl or L-isoleucyl) and mono-,
di- or tri-phosphate esters.
With regard to the above described esters, unless otherwise specified, any alkylmoiety present advantageously contains 1 to 16 carbon atoms, particularly 1 to
4 carbon atoms. Any aryl moiety present in such esters advantageously
comprises a phenyl group.
In particular the esters may be a C1 16alkyl ester, an unsubstituted benzyl ester
or a benzyl ester substituted by at least one halogen (bromine, chiorine, fluorine
or iodine), C1 6alkyl, C1 6alkoxy, nitro or trifluoromethyl groups.
25 Pharmaceutically acce,ulable salts of the compound of formula (I) include those
derived from pharmaceutically acceptable inorganic and organic acids and
bases. Examples of suitable acids include hydrochloric, hyd,obr.",ic, sulphuric,nitric, perchloric, fumaric, maleic, phosphoric, glycollic, lactic, salicylic, succinic,
toluene-p-sulphonic, tartaric, acetic, citric, methanesulphonic, formic, benzoic,
30 malonic",aphll,alene-2-sulphonic and benzenesulphonic acids. Other acids
such as oxalic, while not in themselves ,uhar~l~aceutically acceplable, may be
useful as i"le""ediates in obtaining the compounds of the invention and their
pl ,d""aceutically acce,l~lable acid addition salts.
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W 096/26734 PCT~EP96/00736
Salts derived from appropriate bases include alkali metal (e.g. sodium), alkaline
earth metal (e.g. magnesium), ammonium and NR4+ (where R is C14alkyl)
salts.
The advantageous effects of the cor",uounds of formula (I) and formula (Il) are
realised over a wide ratio for example 1:250 to 10:1 ,.~referably 1:50 to 1:1,
particularly about 1:20 to 1:1. Conveniently each compound will be employed in
the combination in an amount at which it exhibits antiviral activity when used
alone.
The combinations of the invention may contain other co,l,,lJo"ents in addition to
the compounds of formulae (I) and (Il). In particular, the combinations
according to the invention may contain other antiviral agents, in particular other
agQnts active ayainst H!V, suGh as other inhibitors of H!V rep!iGation. The
inhibitor may comprise any inhibitor of HIV replication no matter its method of
inhibiting HIV replication. Such inhibitors include for example those which
inhibit HIV reverse transcriptase, HIV protease, HIV integrase, TAT and the like.
Such inhibitors include for example 3'-azido-3'-deoxythymidine ( AZT,
zidovudine), 2',3'-dideoxycytidine (ddC), 2',3'-dideoxyinosine (ddl), N'-[1 (S)-benzyl-3-[4a(S), 8a(S)-3(S)-(tert-butylcar6~" ,oyl)decahydroisoquinoline-2-yl]-
2(R)-hydroxypropyl]-N"-(quinolin-2-ylca,L,G"yl)-L-asparaginamide (Ro 31-8959)
stavudine (D4T), nevirapine (Bl-RG-587), loviride (a-APA), delavuridine
(BHAP), (1 S, 4R)-cis4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-
26 cyG!opentene-1-methano! (1592U)j L~68229j MK~39j 3S-tet!ahydro-3-furyl N-
((1 S,2R)-3-(4-amino-N-isobutylbenzenesulru"a"lido)-1-benzyl-2-hydroxypropyl)
ca, 6amd~e (VX478) and (+)-S4,5,6,7-tetrahydro-5-methyl-6-(3-methyl-2-
butenyl)-imidazo(4,5, 1 jk)(1 ,4)-benzodiazepin-2(1 H)thione(R-82150) or a
pharmaceutically acceptable derivative thereof.
SUBSTITUlE SHEET (RULE 26
=
CA 02213621 1997-08-22
W 096/26734 PCTAEP96/00736
Preferably the further inhibitor of HIV replication is zidovudine: it has been found
that there are particular advantages associated with a triple combination of
lamivudine, ritonavir and zidovudine.
5 In a preferred embodiment the present invention accordingly provides a
combination comprising lamivudine, ritonavir and zidovudine.
In a particularly preferred embodiment, the present invention provides a
combination of lamivudine, ritonavir and zidovudine in a ratio of about 1:4:2.
It is expected that the present combinations will be generally useful against viral
infections or virus-associated tumours in humans, and the method of their use toinhibit viral infectivity or tumour growth in vitro or in vivo is also within the scope
of the present invention.
Thus there is provided in a further aspect a method for the treatment of a viralinfection in a mammal, including man, comprising co-administration of an
antiviral compound of formula (I) or a pharmaceutically acce~able derivative
thereof and an inhibitor of HIV ~urolease of formula (Il) or a pharmaceutically
20 acceptable dèrivative thereof and optionally zidovudine or a pharmaceutically acceptable derivative thereof.
It will be appreciAted that the compounds of formulae (I) and (Il) may be
acl" ,inislered either simultaneously, sequentially or in co" ,bination. If
25 administration is sequential, the delay in administering the second of the active
ingredients should not be such as to lose the benefit of the synergistic effect of
the combination. P, eferably administration will be simultaneous.
For combinations comprising the compound of formula (I), the compound of
30 formula (Il) and zidovudine, it will be appreciated that each agent may be
administered simultaneously, sequentially or in alternative paired combinations.Preferably all three agents will be administered simultaneously. In a ,.rer~r,ed
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W O 96126734 PCT/~l3~ 7~6
embodiment, lamivudine and zidovudine may be administered as a fixed
combination, for example as a tablet.
It will be appreciated by those skilled in the art that rerere"ce herein to
5 treal,ne"l extends to prophylaxis as well as the treatment of established
infections or sy" "~to" ,s.
It will be further appreciated that the amount of a combination of the inventionrequired for use in treatment will vary, with the route of administration, the
10 nature of the condition being treated and the age and condition of the patient
and will be ultimately at the discretion of the attendant physician or veterinarian.
In general however a suitable dose will be in the range of from about 1 to about100mg/kg e.g. from about 1 to about 50-mg/kg of bodyweight per day,
~rererably in the range of 2 to 30mglkg/day of each of the active ingredients of15 the combination.
The desired dose may conveniently be presented in a single dose or as divided
doses administered at appro~,riate intervals, for example as two, three, four ormore sub-doses per day.
The combination is conveniently administered in unit dosage form; for example
containing 10 to 1000mg, conveniently 20 to 500mg, most conveniently 100 to
300mg of each active ingredient per unit dosage form.
25 A prerer,ed dosage regimen comprises administration to a human of a unit doseof lamivudine (150mg) twice per day, a unit dose of zidovudine (300mg) twice
per day and two doses of ritonavir (100mg) six times per day.
While it is possible that, for use in therapy, the active ingredients of the
30 cor"bil ,dlion may be administered as the raw chemical it is preferable to present
combinations as a ,~,1,ar",aceutical formulation.
SUBSTITUTE SHEET (RULE 26
CA 02213621 1997-08-22
W 096/26734 PCTAEP96/00736
The invention thus further provides a pharmaceutical formulation comprising a
compound of formula (I) or a pharmaceutically acceptable derivative thereof and
inhibitor of HIV replication together with one or more pharmaceutically
5 acceptable carriers therefor and, optionally, other therapeutic and/or
prophylactic ingredients. The carrier(s) must be 'acceptable' in the sense of
being compatible with the other ingredients of the formulation and not
deleterious to the recipient thereof.
10 Pharmaceutical formulations include those suitable for oral, rectal, nasal, topical
(including buccal and sub-lingual), vaginal or parenteral (including
intramuscular, sub-cutaneous and intravenous) administration or in a form
suitable for administration by inhalation or insufflation. The formulations may,where appropriate, be conveniently presented in discrete dosage units and may
15 be prepared by any of the methods well known in the art of pharmacy. All
methods include the step of bringing into association the active compound with
liquid carriers or finely divided solid carriers or both and then, if necess~ry,shaping the product into the desired formulation.
20 Pharmaceutical formulations suitable for oral administration may convenientlybe presented as discrete units such as capsules, cachets or tablets each
containing a predetermined amount of the active ingredient; as a powder or
granules; as a solution, a suspension or as an emulsion. The active ingredient
may also be presented as a bolus, electuary or paste. Tablets and capsules for
25 oral administration may contain conventional excipients such as binding agents,
fillers, lubricants, disintegrants, or wetting agents. The tablets may be coatedaccording to methods well known in the art. Oral liquid preparations may be in
the form of, for example, aqueous or oily suspensions, solutions, emulsions,
syrups or elixirs, or may be presented as a dry product for conslilution with
30 water or other suitable vehicle before use. Such liquid preparalions may
contain conventional additives such as suspending agents, emulsifying agents,
non-aqueous vehicles (which may include edible oils), or preservatives.
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W 096JZ6734 PCT/~,G/00736
The co"1lJounds according to the invention may also be formulated for
~, parenteral administration (e.g. by injection, for example bolus injection or
continuous infusion) and may be presented in unit dose form in ampoules, pre-
filled syringes, small volume infusion or in multi-dose containers with an added5 preservative. The compositions may take such forms as suspensions, solutions,
or emulsions in oily or aqueous vehicles, and may contain formulatory agents
such as suspending, stabilising and/or dispersing agents. Alternatively, the
active ingredient may be in powder form, obtained by aseptic isolation of sterile
solid or by Iyophilisation from solution, for constitution with a suitable vehicle,
10 e.g. sterile, pyrogen-free water, before use.
For topical administration to the epidermis the compounds according to the
invention may be formulated as ointments, creams or lotions, or as a
transdermal patch. Ointments and cre~n,s may, for example, be formulated with
15 an aqueous or oily base with the addition of suitable thickening and/or gelling
agents. Lotions may be formulated with an aqueous or oily base and will in
general also contain one or more emulsifying agents, stabilising agents,
dispersing agents, suspending agents, thickening agents, or colouring agents.
20 Formulations suitable for topical administration in the mouth include lozenges
comprising active ingredient in a flavoured base, usually sucrose and acacia or
tragacanth; pastilles comprising the active ingredient in an inert base such as
gelatin and glycerin or sucrose and ac~ci~ and mouthwashes comprising the
active ingredient in a suitable liquid carrier.
Pharmaceutical formulations suitable for rectal administration wherein the
carrier is a solid are most preferably presented as unit dose suppositories.
Suitable carriers include cocoa butter and other materials commonly used in the
art, and the suppositories may be conveniently formed by admixture of the
30 active compound with the softened or melted carrier(s) followed by chilling and
shaping in moulds.
SUBSTITUTE SHEET (RULE 26
-
CA 022l362l l997-08-22
W 096/26734 PCT/~l3Gloo736
Formulations suitable for vaginal administration may be presented as pessaries,
tar"pons, creams, gels, pastes, foams or sprays containing in addition to the
active ingredient such carriers as are known in the art to be ap~,rop, iate.
5 ~or intra-nasal administration the compounds of the invention may be used as a liquid spray or dispersibie powder or in the form of drops.
Drops may be formulated with an aqueous or non-aqueous base also
comprising one more dispersing agents, solubilising agents or suspending
10 agents. Liquid sprays are conveniently delivered from pressurised packs.
For administration by inhalation the compounds according to the invention are
conveniently delivered from an insufflator, nebuliser or a pressurised pack or
other convenient means of delivering an aerosol spray. Pressurised packs may
15 comprise a suitable propellant such as dichlorodifluoromethane,
trichlorofluoromethane, dichlorotel~ drluoroethane, carbon dioxide or other
suitable gas. In the case of a pressurised aerosol the dosage unit may be
determined by providing a valve to deliver a metered amount.
20 Alternatively, for administration by inhalation or insufflation, the compounds
according to the invention may take the form of a dry powder composition, for
example a powder mix of the compound and a suitable powder base such as
lactose or starch. The powder composition may be presented in unit dosage
form in, for example, capsules or cartridges or e.g. gelatin or blister packs from
25 which the powder may be administered with the aid of an inhalator or insufflator.
When desired the above described formulations adapted to give sustained
release of the active ingredient may be employed.
30 The pharmaceutical compositions according to the invention may also contain
other active ingredients such as antimicrobial agents, or preservatives.
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W 096126734 PcT/~~ oo736
The compound of formula (I) may be obtained as desc~ ed in European Patent
Application Publication No. 0382562.
Methods for the ~r~,,aralion of lamivudine are described in International PatentApplication Publication No. WO91/17159.
Methods for the preparation of ritonavir are desc;,iL,ed in International PatentApplication Publication No. WO94/14436.
Zidovudine can be prepared for example, as described in US Patent 4 724 232.
Zidovudine can also be obtained from Aldrich Chemical Co. Milwaukee
Wl53233 USA.
The following examples illustrate the invention but are not intended as a
limitation thereof.
Antiviral Activities Alone or in Combination
Example 1
Compounds are first serially-diluted in 2-fold decrements in 96-well microtitre
plates. Chequerboard titrations are prepared by mixing 25~11 aliquots from each
co",pound dilution both alone or in combination (to a final volume of 50~1 in new
96-well microtitre plates). Aliquots of MT4 cells (106 cells/~l) in RPMI 1640
growth medium are inrecled with HIV-1 strain RF at a moi of 2 x 10-3 infectious
doses/cell. Virus is adsorbed at room temperature for 90 minutes after which
the cells are washed in RPMI 1640 growth medium to remove unadsorbed virus
and resuspended at 106 cells/~l in RPMI 1640 growth medium. 50u1 of infected
cell suspension are inoullAted into wells containing cG"Ipound or growth
medium only. 50~1 of mock-i"ractad cell suspension are inoc~ ted into wells
not containing compound. The plates are then incl~h~ted for 7 days at 37~C in
5% C02/air.
SUBSTITUTE SHEET (RULE 26)
CA 022l362l l997-08-22
W 096/26734 PCT/~l~G~oo736
After incubation, 10~11 of 3-[4,5-dimethyl thiazol-2-yl]-2,5-diphen~llel, ~oliumbromide (MTT) at 7.5mg/~l are added to all wells and the plates incubated for a
further 90 minutes at 37~ C. 150~1 of 10% (v/v) Triton X-100 in isopropanol are
then added and the cells resuspended. After 15 minutes at room temperature
the plates are analysed in a Multiskan MC (Flow Laboratories, Irvine, UK)
reader at 405nm. Conversion of yellow MTT to its formazan derivative is
maximum in the uninfected untreated cells, and absent in untreated infected
cells.
Dose response curves are plotted for each compound alone (IC50% values)
and for reciprocal titrations of each compound at a fixed conce"l,alion of the
second compound and isobolograms plotted.
Example 2
The human T-cell Iymphotropic virus type 1-transformed cell line MT4 is grown
and infected with HIV-1 strain 3B or strain MN (Advanced Biotechnoiogies Inc.,
Columbia, Maryland) at 10 times the amount necess~ry to cause a 50%
reduction of MT4 cell growth (10 x TCID50, 2 x 104 plaque forming units/cell).
Mock-infected cells are also prepared. Following I hour incubation, the cells are
pipetted onto 96-well dishes at 1 x 104 cells/well. The wells contain various
conceul,alions of zidovudine, and peak or trough plasma levels of lamivudine
and ritonavir. The infected T-lymphoblastoid cells are inu~h~ted for 5 days to
allow for HIV-1 me~ ted growth inhibition. Plates are then treated with 28 ~LI of
5% Nonident P-40 (Sigma) in phosphate-buffered saline (PBS) and 60 ,~LI
samples are l,dnsrerred to filter-bottomed, 96-well plates (Idexx Corp.). Platesare placed in an automated assay instrument (Idexx Screen Machine) which
adds propidium iodide to each well, performs a series of washes, and
determines the resulting fluoroscence (E). Fluorescence has been shown to
correlate directly with cell number, allowing for the quanlildlion of HIV-1
mediated cytopathic effect (CPE). Uninfected cells are determined to have 0%
CPE and infected u"l,ealed cells are determined to have 100% CPE. Percent
SUBSTITUTE SHEET (RULE 26)
CA 02213621 1997-08-22
W ~96/26734 PCTAEP96/00736
inhibition of HIV-1 induced CPE and ICg~;s (95% inhibitory concentration) are
determined.
The antiviral activities of combinations comprising lamivudine and ritonavir and5 lamivudine, ritonavir and zidovudine are tested and compared with the active
agents used alone. Both combinations are found to exhibit synergy.
Cvtotoxicities of ComPounds Alone or in Combination
10 ExamPle 3
Cytotoxicities of lamivudine and ritonavir and lamivudine, ritonavir and
zidovudine alone and in combination are compared in uninfected peripheral
blood Iymphocytes and an established T-lymphocyte cell line: cytotoxicity is
15 measured using a [3H]-thymidine uptake assay.
SUBSTITUTE SHEET (RULE 26