Note: Descriptions are shown in the official language in which they were submitted.
CA 02213906 1997-08-2~
TITLE: PHARMACEUTICAL COMPOSITION AND METHOD FOR NEURON RESCUE IN
ISCHEMIC STROKE
FIELD OF THE INVENTION
The present invention relates generally to ",. -ospheres and pharmaceutical
compositions which contain one or more cytokines or other growth factors for potenlidli, ,9
neuronal survival. The invention can be used to treat individuals having neurological diseases
or disorders or who are susceptible to certain neurological diseases or disorders. The invention
also relates to methods for preparing the microspheres.
0 BACKGROUND OF THE INVENTION
Stroke is an example of one neurological disorder. It occurs when blood circulation fails
in an area of the brain. Stroke falls into two broad categories: those caused by blockage of
blood flow and those caused by bleeding. The most common type is stroke caused by
disruption of the blood supply leading to an ischemic (lack of oxygen) condition in which the
affected neurons in the area die (focal ischemia).
At the core of the ischemic lesion, neurons die rapidly and cannot be rescued. The core
zone of the ischemic lesion is called the infarct. The zone of ischemically threatened tissue
adjacent to the infarct is called the penumbra. Neurons may be injured sublethally in the
penumbra. These neurons may still be viable. The neurons in the penumbra closest to the
20 core of the ischemic infarct are at greatest risk and, over time, the core of the infarct may
increase in size as more and more of the neurons of the penumbra die (Sweeney et al., Can. J.
Physiol. Pharrnacol. 73:1525-1535, 1995). This invention is directed to stopping neuronal
death.
There are other neurological disorders in which neurons may be injured sublethally. For
example, individuals who have been diagnosed with disorders such as Alzheimer's disease,
Parkinson's dise~se, Todd's paralysis or Huntington's dise~se, may have neurons which are
sublethally injured and have the potential to be rescued.
The therapeutic strategies for the rescue of neurons from neurological disorders vary.
As one example, the rescue of neurons from death in ischemic-stroke is based pri",arily on
30 knowledge of changes in the neuron microenvironment when the blood supply is interrupted.
CA 02213906 1997-08-2~
The changes include a fall in cellular adenosine 5' triphosphate (ATP) (energy production)
resulting in release of potassium (K+) and the excitatory amino acids, glutamate and aspartate.
Glutamate activates N-methyl-D-aspartate (NMDA) and a-amino-3-hydroxy-5-
methylisox~a!ep~rop..,oic acid (AMPA) receptors and associated ion channels. The receptor
activation causes massive calcium (Ca ) influx into the neurons, which has a major role in
neuronal death in brain ischemia. The surrounding glial cells produce free oxygen radicals
(reactive oxygen species) which also mediate neuronal degeneration. The dying cells release a
number of proteases during the degenerative process. Thus cell death is due to three key
mechanisms: release of excitatory amino acids, release and effflux of calcium and production of
0 free radicals, all linked in a chain reaction. (The American Nimopipine Study Group Stroke
23:3, 1992; Landis, Annu. Rev. Neurosci. 17:133, 1994, Kaste et al., Stroke 25:1348, 1994).
In the case of individuals having had an ischemic-stroke, the therapeutic strategies
attempt to interfere with these links by stopping the release of glutamate by means of
hypothermia, or by inhibiting glutamate activation of NMDA receptors (in focal ischemia) and
AMPA receptors (in global ischemia) using glutamate antagonists of NMDA and AMPA/kinate
receptors and calcium channel blockers to block Ca2+ influx into the cells (Choi, D.W., Science
258:241, 1992; Kaku, D., et al., Science 260:1516, 1993; Hartley, J. Neurosci. 13, 1993). The
glutamate antagonists of the NMDA receptors, however, have been associated with
neuropsychiatric and cardiovascular side effects. In clinical trials calcium channel blockers also
20 had side effects, cardiovascular effects in particular, and generally did not demor,~l, dle patient
improvement (Sweeney et al., Can. J. Physiol. Pharmacol. 73:1525-1535, 1995). Buffers that
chelate Ca2+ and can penetrate into the cell are being developed (Tymianski et al., Neuron
11:211, 1993). However, they must be administered immediately after the occurrence of
ischemic-stroke and their effects have not yet been evaluated clinically.
Another therapy for individuals having had an ischemic-stroke is to use free radical
scavengers to reduce the effect of free oxygen radicals. Preliminary results, however, are not
encouraging (STIPAS Invesligalors Stroke 25:418, 1994).
Still another strategy for individuals having had an ischemic-stroke is to use thrombolytic
agents, such as tissue plasminogen activator, urokinase and streptokinase, to reestablish blood
30 circulation. Although results are promising, there is a risk that the agents will convert an
CA 02213906 1997-08-2~
ischemic-stroke into a hemorrhagic stroke. (Levy et at., Stroke 25: 291, 1994; Fischer, Eur.
Neurol. 35:3, 1995).
Another therapy which has been suggested for neuron injury is the use of trophic factors
which affect either trophic or neuroprotective activity of neurons. This work is still in the
experimental stages. The factors studied include NGF (Varon and Conner, J. Neurotrauma
1 1:473, 1994; Conner and Varon, Exp. Neurology 136:123, 1995) CNTF (Hag and Varon, Proc.
Natl. Acad. Sci. USA 90:6315, 1993; Dale et al., Exp. Neurol. 135:67, 1995) FGF-2 (Yamada et
al., J. Cereb. Blood Flow Metab. 11:472, 1991) TGF-b1 (Krieglstein et al., EMBO J. 14:786,
1995) IL-6 (Hama et al., Neurosci. 40:445, 1991) and IL-1 ra and LC-1 (Rathwell and Relton,
lo Cerebrovasc. Brain Metabl. Rev. 5:178, 1993). Their mode of action has not been defined.
Some of the factors have neuronal type specificity.
Another therapy for ischemic-stroke involves the use of the monoamine oxidase B
(MAO-B) inhibitor (-)deprenyl. This inhibitor has been implicated in rescuing pyramidal neurons
in forebrain ischemia (Barber et al., Sr.) Neuron. Abstr. 19:1646, 1993. There is evidence that
(-)deprenyl may exert its effect through glia cells (astroglia) by stimulating secretion of trophic
factors (Skibo et al., Am. Soc. Cell Biol. Abst. H23, 1992).
There are two major obstacles to the delivery of substances to the central nervous
system (CNS) for therapeutic purposes. The first is the blood-brain barrier. The second is the
instability of peptide drugs and the ease with which they are degraded by proteolytic enzymes.
20 Moreover, peptide drugs are short-acting, necessitating repeated injections even though this
does not always provide suffficient concentration at the site of action (Maysinger et al., Rev.
Neurosci. 6:15, 1995).
An alternative method of therapy for ischemic-stroke or brain injury includes the
insertion of a cannula into the brain and injecting the drug repeatedly or delivering it by osmotic
pump into the cannula (Maysinger et al., Brain Res. 577:300, 1992; Hagg, Methods in Neurosci.
21:201, 1994). This approach is technically diffficult and causes mechanical damage to the
brain tissue.
To overcome the diffficulty in delivering drugs to the CNS, drugs have been
encarsu~ated in microspheres. The polymers used to make ",i~ ospheres are biodegradable
30 and biocompatable and their byproducts on degradation are non-toxic and readily excreted via
normal physiological pathways. They have suitable permeability characteristics and are
CA 02213906 1997-08-2~
mechanically stable. Many polymer materials and techniques to make microspheres are known
but the proper combination of these is critical for the successful control of drug release
(Donbrow, M., Microspheres and Nanoparticles in Medicine and Pharmacy. Boca Raton: CRC
Press, 1992).
Transplantation of microspheres containing cells which release bioactive molecules has
been extensively used. There are several well-established techniques for encapsulating and
i",planling cells in vivo (Aebischer et al., Nature Medicine, 1996; Donbrow, M., Microspheres
and Nanoparticles in Medicine and Pharmacy. Boca Rata: CRC Press, 1992; Lim and Mass, J.
Pharm. Sci. 70:351, 1981; Chang, ASAIO J. 38: 128, 1992). However, implantation of
lo microspheres containing cells for delivery of drugs to the CNS has been very limited so far.
Prerequisites for encapsu~ting cells in mic,ospheres are that cells producing trophic
factors be administered to the CNS with minimal damage to the brain tissue, that the cells
survive for a relatively long time (several months or longer) and that the secreted gene product
retain its biological activity (Maysinger et al., Reviews Neuroscience 6:16, 1995).
Microspheres have been used in the rat Parkinson's disease model (Lindner et ai.J Exp.
Neurol. 132:62, 1995; Winn et al., Exp. Neurol. 133:322, 1991) and in aged animals (Emerich,
Proj. Neuropsychopharmacol. Biol. Psychiatry 18:935, 1994; Emerich et al., Exp. Neurol.
122:37, 1992) by delivery of catecholamines by cells of the cell line PC12.
Genetically engineered encapsulated baby hamster kidney cells (BHK) have been used
20 in the rat Alzheimer's disease model (Winn et al., Proc. Natl. Acad. Sci. USA 91 :2324, 1994)
and the subprimate Alzheimer's disease model (Emerich et al., J. Comp. Neurol. 349:148,
1994), as well as in the rat Parkinson's liise~se model (Lindner et al., Exp. Neurol. 132:62,
1995; Emerick et al., Exp. Neurol. 130:141, 1994). The BHK cells were engineered to produce
glia cell derived neu,ul,uphic factor (GDNF), tyrosine hydrozylase and NGF. A number of other
cells have been genetically engineered to produce NGF, enc~pslJ~ated in microspheres, and
implanted into the CNS. Such cells include schwannoma cells, and cell lines 3T3 and 208F
(Schir,~li"e et al., Cell Transpl. 4:93, 1995).
Another approach that has been used is to encarsu'-te the actual drug in a microsphere
and deliver it in encapsulated form into the CNS from where the drug or factor is gradually
30 released over a prolonged period of time. The drugs and factors used in this manner are
dopamine, norepinephrine (McRae et al., Mol. Neurobiol. 9: 191, 1994; McRae and Dahlsll un "
CA 02213906 1997-08-2~
Neurochem Int. 25:27, 1994) and delargin, a Leu-eukephalin analog. The latter drug was
targeted on the brain in nanoparticles coated with polysorbate 80, thereby making the particles
permeable through the blood-brain barrier (Schroder and Sabel, Brain Res. 710:121, 1996).
Several trophic factors have been encapsulated in microspheres including CNTF
(Maysinger et al., Exp. Neurol., in press), BDNF (Mittal et al., NeuroReport 5:2577, 1994), LIF
(Maysinger et al., NSN Annual Meeting Abstr., 1994) and NGF (Aebischer et al., 1996;
Maysinger et al., Neuroscience Letters 140:71, 1992). Of all these factors only encarsu~ated
NGF, NGF- and CNTF-producing cells were used in situ to rescue neurons; all the others were
tested only in vitro. Only limited studies were done on cytokines add,essi"g the question of
l0 neuronal survival e.g. with CNTF (Aebischer et al., 1996); TNF (Bruce et al., Nature Medicine
1996).
Despite the advances in these therapies for the treatment of neurological disorders, of
which ischemic-stroke is an example, there is no effective treatment for many neurological
diseases and disorders. Therefore, a need exists for: (1) a product which potentiates neuronal
survival, (2) a product which can be delivered to the central nervous system, the brain for
example, of an individual who has a neurological disorder without damaging the nervous
system of the individual, (3) a product which acts directly on cell-specific receptors, thus
minimizing adverse side effects, (4) a product which is long-acting and remains stable for
sufficient duration to achieve its intended effect in the treatment of neurological disorders, thus
20 avoiding the need for repeated administration to the site of action, and (5) enc~psu~2ted cells
which secrete such a product with minimal damage to the CNS.
In addition, no one before us has determined (1) whether cytokines, such as colony-
stimulating factor-1 (CSF-1), can potentiate neuronal survival, (2) whether cytokines, such as
CSF-1, and other growth factors can be delivered to the central nervous system, the brain for
example, of an individual who has a neurological disorder without damaging the nervous
system of the individual, (3) whether cytokines, such as CSF-1, and other growth factors, or
CSF-1- or SCF-mimetics (i.e. small molecules exerting similar biological effects as parent
cytokines) act directly on neurons, thus minimizing adverse side effects, (4) whether cytokines,
such as CSF-1, and other growth factors, or CSF-1 or SCF-mimetics can be delivered in a form
30 which remains stable for suffficient duration to achieve their intended effect in the treatment of
neurological disorders, thus avoiding the need for repeated administration to the site of action,
CA 02213906 1997-08-2~
and (5) whether cells enc~psulated in microspheres can secrete cytokines, such as CSF-1, and
other growth factors, or CSF-1 or SCF-mimetics without damage to the CNS.
SUMMARY OF THE INVENTION.
This invention satisfies these needs. It relates to (1 ) a product which potenliales
neuronal survival, (2) a product which can be delivered to the central nervous system, the brain
for example, of an individual who has a neurological disorder without damaging the nervous
system of the individual, (3) a product which acts directly on neurons, thus minimizing adverse
side effects, (4) a product which is long-acting and remains stable for suffficient duration to
achieve its intended effect in the treatment of neurologica/ disorders, thus avoiding the need for
10 repeated admi"i~,l,dlion to the site of action, and (5) cells encapsulated in microspheres which
secrete such a product without damage to the CNS.
It also relates to cytokines, such as CSF-1, other growth factors, CSF-1 and SCF-
mimetics which (1) potentiate neuronal survival, (2) can be delivered to the central nervous
system, the brain for example, of an individual who has a neurological disorder without
damaging the nervous system of the individual, (3) act directly on neurons, thus minimizing
adverse side effects, (4) can be delivered in a form which remains stable for sufficient duration
to achieve its intended effect in the treatment of neurological disorders, thus avoiding the need
for repeated admil ,i~l, dlion to the site of action, and (5) are secreted by cells encarsul2ted in
microspheres without damage to the CNS.
In the case of ischemic-stroke, for example, the use of a cytokine, such as CSF-1, or
other growth factor normally produced in the brain by astrocytes, for neuron rescue in ischemic-
stroke, is original and provides a novel approach to ischemic-stroke therapy.
The microspheres described above are used to treat a neurological disease or disorder
of in an individual. CSF-1 is also used to treat neurological disease or disorder. The disease or
disorder that is treated includes ischemic-stroke, brain trauma, spinal cord trauma, Alzheimer's
disease, Parkinson's dise~-se, Todd's paralysis and Hu"li"~ton's disease. In a preferred
embodiment, CSF-1 is implanted outside the CNS of the individual. CSF-1 or the ", rrospheres
described above are used in a pharmaceutical composition for treatment of ischemic-stroke,
brain trauma, spinal cord trauma, Alzheimer's ~ise~se, Parkinson's disease, Todd's paralysis
and Huntington's ~isease.
CA 02213906 1997-08-2~
This invention relates to microspheres which potentiate neuronal survival. The
microspheres include (1) a cytokine or other growth factor, and (2) a biocompatible and
biodegradable matrix material membrane surrounding and enclosing the core. In forming the
microspheres, the cytokine or other growth factor is reconstituted in a first solvent including
sterile water and chitosan. The first solvent is then combined with poly-lactic-poly-glycolic acid
(PLGA) in an organic solvent. Then an emulsion is formed by ultrasonification. The emulsion is
incorporated into an aqueous solution, thereby forming a double emulsion. The membrane
forms and hardens in the double emulsion. The membrane is permeable to the cytokine or
other growth factor, biocompatible with the tissues of the central nervous system,
10 biodegradable within those tissues without producing toxic degradation by-products, and has
biodeyl addlion kinetics which may be manipulated to allow for the permeation of the factor
through the polymer at a controlled rate.
The microspheres are stable when stored at 37~C. The delivery system for implantdlion
in an individual can include a gel foam in addition to the microspheres. The cytokine or other
growth factor included in the ",icrospheres can be selected from a group consisli"g of rhCSF-1,
CSF-1, SCF, GM-CSF, PDGF, NGF, CNTF, FGF, and TGF, their mimetics, or a com~..,dlion
thereof. The microspheres are suitable for implantation in an individual having had or who is
susceptible to a neurological ~ise~se or disorder. The disease or disorder may be ischemic-
stroke, brain trauma, spinal cord trauma, Alzheimer's disease, Parkinson's c~ise~se, Todd's
paralysis and Huntington's ~isease.
This invention also includes the use of a cytokine or other growth factor or small
molecules mimicking their effects for the treatment of a neurological disease or disorder. The
cytokine or other growth factor may be encarsu'ated in a microsphere as described above or
may be part of a pharmaceutical composition. The cytokine or other growth factor can include
one selected from a group consisting of CSF-1, SCF, GM-CSF, PDGF, NGF, CNTF, FGF and
TGF, their mimetics or a combination thereof. The pharmaceutical composition would include
the cytokine or other growth factor and a pharmaceutically accepi-~le carrier, auxiliary or
excipient.
This invention also relates to a method of treating an individual who has a neurological
30 disease or disorder, or who is susceptible to a neurological ~~isease or disorder, by
administering the pharmaceutical composition or implanting the microsphere of this invention
into the nervous system of the individual. In one instance, the microsphere can be i")plar,led
CA 02213906 1997-08-2~
on top of the pia of the individual. In addition, the microsphere may be implanted into the
peritoneal cavity of the individual to reinforce the effects of the primary graft on top of the pia.
The ~lisease or disorder may be selected from a group consisting of ischemic-stroke, brain
trauma, spinal cord trauma, Alzheimer's dise~se, Parkinson's dise~se, Todd's paralysis and
Huntington's dise~se.
Furthermore, this invention includes a method of encars~ ting a Iyophilized cytokine or
other growth factor in a microsphere. The method includes the steps of (1) reconstituting the
cytokine or other growth factor in a first solvent including sterile water and chitosan, (2)
combining the first solvent with poly-lactic-poly-glycolic acid (PLGA) in an organic solvent, (3)
lo forming an emulsion by ultrasonification, and (4) incorporating the emulsion into an aqueous
solution, thereby forming a double emulsion. The microspheres form and harden in the double
emulslon.
The method also includes the further steps of (5) centrifuging the microspheres, (6)
evaporating the organic solvent under reduced pressure, and (7) Iyophilizing the microspheres.
The organic solvent may be methylene chloride. The sterile water is double distilled and
endotoxin-free. The aqueous solution can be 0.5% aqueous polyvinyl alcohol solution. The
microspheres may be stored at 4~C or room temperature. The microspheres may be packed
into gel foam for implantation into an individual. In the method, the cytokine or other growth
factor may be selected from a group consisting of rhCSF-1, CSF-1, SCF, GM-CSF, PDGF,
20 NGF, CNTF, FGF and TGF, their mimetics or a combination thereof.
Additionally, this invention includes a method of encapsulating in a microsphere cells
secreting a cytokine or other growth factor, including the steps of (1 ) tr~.si, 'i~i"g the cells, (2)
centrifuging the cells, (3) resuspending the cells in alginate solution, (4) extruding the cells
dropwise into a solution of calcium chloride, whereby the calcium ions cross-link with algineic
acid and form a semipermeable membrane which co",prise the microspheres, (5) removing the
excess calcium chloride, (6) washing the microspheres with chitosan solution, (7) incubating the
microspheres in chitosan solution, and (8) washing the microspheres in a fresh medium. The
cell density in the alginate extruded into the calcium chloride solution is 10E-6/ml. The chitosan
preferably has a high viscosity. In one case, the chitosan is chitosan #311. Furthermore,
30 preferably the reaction time between chitosan and Ca-alginate is 10 minutes. The
microspheres may be packed into gel foam for implantation into an individual.
CA 02213906 1997-08-2~
In the method, the cytokine or other growth factor may be selected from a group
consisli,lg of rhCSF-1, CSF-1, SCF, GM-CSF, PDGF, NGF, CNTF, FGF and TGF, their
mimetics or a combination thereof. Moreover, microencapsulated chelating agents ~e.g.
BAPTA), as well as stimulators of cytokine synthesis can be considered for encapsulation and
local administration in stroke.
FIGURES
The invention will now be described in relation to the figures in which:
Fig.1. Growth response of microglia to recombinant human CSF-1 (rhCSF-1) measured by
3[H]TdR incorporation. Microglia, plated in 96-well plates (5X10E-3 cells perwell), were
lo incubated in mMEM + 5% HS for 24 h and then for an additional 24 h with serial dilutions of
CSF-1 for 24 h. 3[H]TdR uptake was measured for the final 4 hr of incubation. Vertical bars
represent S.D. (Fedoroff et al., in Biology and Pathology of Astrocyte-Neuron Interactions Eds.
Fedoroff et al. Plenum Press, N.Y. 1993, pp 247-261 .
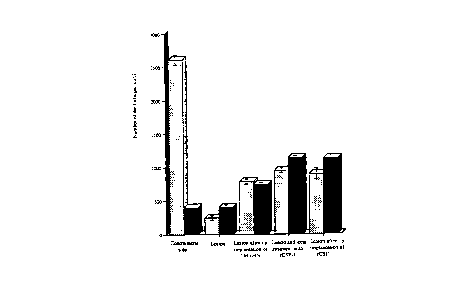
Fi~. 2. Number of glia/mm2 in cerebral cortex ischemic lesions in C3H/HeJ mice (ipsilateral
side) and in lesioned mice in which microspheres containing rhCSF-1 were implanled at the site
and time of lesioning or intraperitoneally (i.p.) 2 weeks before lesioning, compared to the
number of glia/mm2 in the corresponding area in the unoperated contralateral side. The
number of glia increased by a factor of 2.9. Data presented as mean number of glia/mm2 _
s.e.m. Data analyzed using two-way (2P) ANOVA analysis.
20 Fia. 3. Numbers of viable neurons and glial cells on the unlesioned conlldlaleral side and in the
area of cerebral cortex ischemic lesion in op/op mice with and without delivery of extraneous
CSF-1. Encapsulated LM-10 cells or rhCSF-1 were incorporated in a sterile piece of gelfoam
and applied either directly onto the site of cortical lesion at the time of lesioning or
ir,llaperiloneally two weeks before the lesion was made in CSF-1 deficient op/op mice. After 6
days, the cerebral cortex ischemic lesion in op/op mice leads to the death of approximately 90%
of neurons, glia reaction in the area of the lesion was very mild. Recombinant CSF-1 delivered
to the lesioned animals in microspheres either at the lesion site or by implantation into the
peritoneal cavity caused a highly significant (2P<0.0001) increase in neuronal survival in op/op
mice (by a factor of 3.8 and 3.6, respectively) in comparison to that in lesioned non-treated
30 op/op mice. The data were analyzed by 2-way ANOVA and chi square tests.
CA 02213906 1997-08-2~
Fia. 4. Histological comparison of cerebral cortex ischemic lesion in normal C3H/HeJ mouse
with lesion in CSF-1-deficient op/op mice with and without delivery of extraneous CSF-1. Areas
of cerebral cortex ischemic lesions, six days after disruption of pia-arachnoid blood vessels.
Cresyl violet Nissl stain. Arrows point to viable neurons with nuclei and Nissl substance;
arrowheads point to glial cells. (a,d) Areas in cerebral cortex in C3H/HeJ mouse; (a) area of
ischemic lesion and (d) contralateral side; insert: typical viable neuron with Nissl substance.
(b,e) Areas of cerebral cortex in op/op mice; (b) area of ischemic lesion and (e) contralateral
side. (c) Area of ischemic lesion in op/op mouse grafted with CSF-1-producing astroglia three
weeks before lesion was made. (fl Area of ischemic lesion in op/op mouse that had received
l0 implant of CSF-1-producing LM-10 cells into peritoneal cavity three weeks before lesion was
made. More glial cells are seen in the lesion in C3H/HeJ mouse (a) than in op/op mouse (b).
There are significantly more viable neurons in ischemic lesion in op/op mouse grafted with CSF-
1 producing astrocytes (c) and in lesion in op/op mouse that received intraperitoneal implant of
encapsulated CSF-1-producing LM-10 cells (fl than in untreated lesion in op/op mouse (b). Bar
= 50 mm. (Berezovskaya et al. Int. J. Devl. Neuroscience 13:294, 1995).
Fia. 5. Number of viable neurons/mm2 in cerebral cortex ischemic lesion in C3H/HeJ mice
(ipsilateral side) and in lesioned mice in which microspheres containing rhCSF-1 were
implanted at the site and time of lesioning or intraperitoneally (i.p.) 2 weeks before lesioning,
compared to the number of viable neurons/mm2 in unoperated contralateral side. Data
20 presented as mean number of neurons/mm2 + s.e.m. The data were analyzed using two-way
(2P) ANOVA analysis. ~=p~0.0001.
Fi~. 6. Infarct size in C3H/HeJ CSF-1-producing mice with cerebral cortex ischemic lesions, 25
days after lesioning. A Group: received no treatment; B Group: received encapsulated rhCSF-1
on top of pia at the lesion site at the time of lesioning. The infarct size in the treated mice (B
Group) is 3.8 times smaller than that in the non-treated mice (A Group) (P=0.0001).
Fig. 7. Disaggregated cerebral cortex neurons from 16-day-old C3H/HeJ embryos were grown
in low concentration, free of astroglia and microglia, in serum-free medium. The experimental
group received 500 U/ml of rhCSF-1 on days 2, 3 and 4. The neurons in the cultures were
counted under a phase contrast microscope. Cultures that received rhCSF-1 had significantly
30 higher numbers of surviving neurons on days 3 and 4, indicating that rhCSF-1 potentiates
neuronal survival in cultures.
CA 02213906 1997-08-2~
Fiq. 8. Double immunofluorescent staining of neurons in 7 day culture of mouse cerebral cortex.
(a): phase contrast; (b): anti-70 kD neurofilament (NF) immunoreactivity; (c): CSF-1R
immunoreactivity. NF and CSF-1 R immunreactivities are co-localized in the same neurons.
Fia. 9. Absorption of antisera for CSF-1R with CSF-1R-positive cells (strain 5/10.14) and CSF-
1 R-negative cells (strain EL4). Immunocytochemical staining of cerebral cortex neurons in
culture with anti-CSF-1 R antibody. (a): Cells treated with non-absorbed anti-CSF-1 R
antiserum. Neurons are positive for CSF-1 R. (b): Cells treated with antiserum absorbed with
CSF-1 R-positive 5/10.4 cells. Neurons are negative for CSF-1 R. (c): Cells treated with
antiserum absorbed with CSF-1 R-negative cells . Neurons are positive for CSF-1 R. See text
0 for details. ABC-HRP immunostaining.
Fiq. 10. CSF-1 antibody binding to CSF-1R in neurons. CSF-1 immunoreactivity of neurons in
culture in the presence (a) or absence (b) of CSF-1 in the culture medium. Positive
immunostaining on the surface membrane of neuron in the presence of CSF-1 and negative in
the absence of CSF-1 ind c~tes that the CSF-1 immunoreactivity is due to binding of CSF-1 to
its active receptor on the neuronal membrane. ABC-HRP immunostaining.
Fiq. 11. In situ hybri.li~dlion with CSF-1 R oligoprobe cerebral cortex neurons in culture. (a):
Neurons grown with CSF-1 are negative . (b): In situ hybridization by omitting the CSF-1 R
oligoprobe; neurons are negative. (c): In situ hybridization with CSF-1 R oligoprobe; neurons
are positive. This indicates that neurons in culture express CSF-1 R mRNA.
20 Fig. 12. Effect of human recombinant CSF-1 on apoptosis of cerebral cortex neurons in
cultures. The cultures were stained with ApoTag IN Situ Apoptosis Detection Kit and stained
neurons were counted. Note the significant decrease in the numbers (means i S.D.) of
apoptotic neurons in cultures to which hrCSF-1 was added (500 lU/ml). (* p < 0.5; ** p < 0.01).
Fiq. 13. Effect of human recombinant CSF-1 on survival of cerebral cortex neurons in culture.
Note that cultures to which hrCSF-1 was added (600 lU/ml) had significantly greater numbers
(means ~ S.D.) of neurons present than did the control cultures without addition of hrCSF-1. (*
p<o,5;**p~oo1 )
Fiq. 14. Effect of concentration of CSF-1 on survival of cerebral cortex neurons in culture.
Note that the numbers of neurons (means i S.D.) in cultures in the presence of rhCSF-1 were
30 always greater than in control cultures. The survival of neurons is dose-dependent. HrCSF-1
was added to the cultures every day beginning at culture day 0.
CA 02213906 1997-08-2~
Fiq. 15. Presence of CSF-1 R in cerebral cortex. Immunostaining for CSF-1 R of cerebral cortex
of normal adult mice and cortex 7 days after focal ischemic lesioning. (a): Cerebral cortex from
normal animal. Note the positively stained microglia and a few scattered positively stained
neurons. (b): Cerebral cortex 7 days post-lesioning. The neurons close to the lesion (upper left
corner) are intensely immunostained. Neurons more distal from the lesion are less intensely
stained. Note the positively stained microglia. (c): Higher magnification of (b). Note the heavily
labeled neurons.
Fiq. 16. Presence of CSF-1R in hippocampus. Immunostaining for CSF-1R in normal mice and
mice 7 days after cerebral cortex ischemic lesioning. (a): Hippocampus from normal animal.
10 Note positively stained microglia and some stained neurons. (b): Hippocampus 7 days post-
lesioning. The neurons are intensely immunostained. (c): Higher may"ificalion of (b).
Fiq. 17. Presence of CSF-1R in cerebellum. Immunostaining for CSF-1R in normal mice and
mice 7 days after cerebral cortex ischemic lesioning. (a): Cerebellum from normal animal.
Note positively stained Purkinje cells and some positive cells in the molecular layer and
granular layer. (b): Cerebellum from animal 7 days post-lesioning. The Purkinje cells and cells
in the molecular and granular layer are more intensely immunostained. (c): Higher
magnification of (b). Note the intensely stained Purkinje cells. Immunostained cells in the
molecular layer are probably stellate cells and cells close to the Purkinje cells are probably
basket cells. Intensely stained neurons in the granular layer are probably Golgi cells.
20 Fiq. 18. Presence of CSF-1R in facial nucleus. Immunostaining for CSF-1R in normal mice
and in mice 7 days after cerebral corex ischemic lesioning. (a): Facial nucleus from normal
animal. Note the positively stained cells in the nucleus. (b): Facial nucleus from animals 7
days post-lesioning. The motor neurons and microglia are more intensely stained. (c): Higher
may"i~icaLion of (b). Note the intensely stained neurons and microglia.
Fiq. 19. Expression of CSF-1R mRNA in cerebral cortex. In situ hybridi~dlion with 48-mer DNA
oligonucleotide probe of cerebral cortex of normal mice and mice 7 days after cerebral cortex
ischemic lesion. (a): Note the positive signals from positively labeled cells in the cortex
compared to (b). (b): Note the higher frequency of positive signals from labeled cells in the
cortex distal from the lesion. A considerably larger number of cells are labeled, indicating that
30 cells in the cerebral cortex express CSF-1 R mRNA and that it is expressed in many more cells
after lesioning.
CA 02213906 1997-08-2~
DETAILED DESCRIPTION OF THE INVENTION
One cytokine which can be used in this invention is colony stimulating factor-1 (CSF-1),
also known as MCSF. CSF-1 was originally discovered as a hematopoietic cell lineage-specific
cytokine that stimulates the proliferation, differentiation and survival of cells of the monocyte-
macrophage lineage. More recently it was found that CSF-1 also has a regulatory function in
the reproductive system, especially during pregnancy (Arceci et al., Proc. Natl. Acad. Sci.
U.S.A. 86:8818, 1989; Pollard et al., Nature 330:484, 1987) and is involved in bone metabolism
(Felix et al., J. Cell Biochem. 55:340, 1994; Stanley et al., Stem Cells 12:15, 1994). A few
years ago it was found that CSF-1 also acts as a growth factor in the nervous system (Chang et
al., J. Neuroimmunol. 52:2525, 1993; Théry et al., J. Neurosci. Res. 26:129, 1990; Frei et al., J.
Neuroimmunol. 40: 189, 1992; Hao et al., J. Neurosci. Res. 27:314, 1990; Lee et al., J.
Immunol. 150:594, 1993; Fedoroff et al., In: Fedoroff et al., eds. Biology and Pathology of
Astrocyte-Neuron Interactions. Plenum Press, N.Y., 2471, 1993). It acts in a very different
manner from trophic factors, particularly NGF, which is the only trophic factor to be
enc~ps~ ted and used in neuronal rescue. CSF-1 acts at the level of microglia to rescue
neurons by stimulating the neuroglia to produce beneficial agents. CSF-1 also acts directly on
neurons through the c-fms receptor. This is different from the mechanism of action of NGF
which acts only on cholinergic neurons in the CNS.
In the CNS the main source of CSF-1 is astroglia, which secrete the factor constitutively
(Hao et al., J. Neurosci. Res. 37, 314, 1990). Microglia express a receptor for CSF-1 (c-fms)
and are modulated by CSF-1/c-fms signaling. CSF-1 regu~ates microglial function and
response to injury (Raivich et al., Europ. J. Neurosci. 6:1615, 1994; Berezovskaya et al., Int. J.
Dev. Neurosci. 1:285, 1995; Zhang and Fedoroff, NSN Annual Meeting Abstr. 1996).
The human CSF-1 gene is approximately 21 kb in length, comprising 10 exons, (Ladner
et al., EMBO J. 6:2693, 1987). Alternative splicing yields several cytoplasmic mRNAs, derived
from a common nuclear transcript. The complete coding region of the human CSF-1 gene
specifies a membrane-spanning molecule of 522 amino acids. However, only the first amino
terminal 150 amino acids are required for biological activity (Heard et al., Oncogene. Res.
1 :423, 1987; Ladner et al., EMBO J. 6:2693, 1987). The CSF-1 crystal structure (amino acids
4-158) has recently been determined (Pandit et al., Science 258: 1358, 1992) and could be used
to develop a cluster of drugs with CSF-1 activity.
CA 02213906 1997-08-2~
CSF-1 is secreted or retained as a membrane-spanning molecule. It is secreted either
as a glycoprotein or proteoglycan (Price et al., J. Biol. Chem. 267:2190, 1992) and its half-life is
approximately 40 min. The half-life of the cell surface form of CSF-1 is approximately 11 hours
(Price et al., J. Biol. Chem. 267:2190, 1992). Human cDNAs for CSF-1 have been cloned and
CSF-1 is available from Sigma Chemical Co., St. Louis, MO, in a recombinant form. Although
large numbers of cytokines and other growth factors s are being produced by many companies
and many are being tested in clinical trials, CSF-1 has not been clinically tested and its
availability is limited.
We researched the CSF-1/CSF-1 R signaling communication pathways in the CNS by
l0 demonslldli"g by immunocytochemistry and in situ hybridization the presence of CSF-1R
mRNA and protein in CNS neurons in culture and in situ. In normal mice the ex~ression of
CSF-1 R in most neurons is very low and it is difficult to demonstrate its presence except in the
cerebellum, brain stem and in some motor neurons in the spinal cord. In response to focal
ischemic injury to the cerebral cortex, the expression of CSF-1 R mRNA and protein is
upregulated. The upregulation of CSF-1 R is not only in the vicinity of the core of the ischemic
lesion but is widespread and is seen distally from the ischemic core in the ipsilateral cortex, as
well as in the contralateral cortex and many other areas of the brain distant from the cortex.
This reflects response to direct injury to neurons in the lesion and its immediate vicinity and
secondary injury to neurons in other areas mediated by deafferentiation/de-efferentiation of
20 pathways with the cerebral cortex. A similar wide response to ischemic injury to cortex caused
by occlusion and reperfusion of middle cerebral artery was previously observed, in which the
injury to neurons was ~ssessed by staining neurons with cresyl violet, Luxol fast, silver
impregnation and immunochemistry (Nagasawa and Kogure, 1989, 1990; lizuka et al., 1989,
1990; Yamamoto et al., 1987; Kataoka et al., 1989) or by assessing neuron injury by microglial
reaction by means of lectin histochemistry or immunocytochemistry (Morioka et al., 1993;
Gehrmann et al., 1992). Upregulation of CSF-1 R may be a very sensitive indicator of sublethal
injury to neurons.
CSF-1 R is encoded by the protooncogene c-fms. The upregulation of c-fms gene
expression in microglia and neurons in response to neuron ischemic injury is in line with reports
30 of upregulation of c-fos and cjun genes after focal cerebral ischemia (An et al., 1993; Uemura
et al., 1991; Onodera et al., 1989), upregulation of CSF-1R in microglia but not in neurons in the
facial nucleus in response to axotomy of the facial nerve (Raivich et al., 1991) and upregulation
14
CA 02213906 1997-08-2~
in other nuclei and spinal cord in response to injury (Raivich et al., 1993) as well as in EAE
(Hulkoweretal., 1993).
The extent of functional involvement of this signaling pathway among neural cells is not
known; there is evidence, however, of its importance in nervous tissue development and in
pathology. The op/op mice which express CSF-1 R normally but do not have the biologically
active ligand, CSF-1, because of point mutation, have a number of abnormalities. These
animals have abnormal development of auditory and visual pathways and cortex (Michaelson et
al., 1996) and their cerebral cortical neurons are considerably more vulnerable to ischemia than
those of normal animals. The delivery of biologically active CSF-1 to op/op mice potentiates the
lo resistence of neurons to ischemia (Berezovskaya et al., 1995).
CSF-1 binds with CSF-1 R in neurons and that this binding potentiated neuron survival in
a dose-dependent manner in cultures. This supports the observation by Michaelson et al.,
(1996) that treatment of hippocampal neurons with CSF-1 in cultures promotes their survival
and process outgrowth in a dose-dependent mannen
CSF-1 R which originally was believed to be restricted to hemopoietic macrophages, is
expressed in the CNS in microglia, astroglia, oligodendroglia and neurons, with the highest
levels of expression in microglia. The CSF-1 is produced mainly by astroglia, but some may
also be produced by microglia and probably by some neurons. In response to injury there is a
rapid and extensive upregulation of CSF-1R indicating that CSF-1/CSF-1R signaling must be a
20 very important means of neuron-glia communication.
The examples which follow describe in detail the microsphere encarsu~ation of a
cytokine, such as CSF-1, its mimetics and cells secreting this cytokine or its mimetics. The
procedures used in these examples may also be used for microsphere encapsulation of other
cytokines, growth factors, their mimetics, chelating agents, stimulators of cytokine synthesis
and cells secreting other cytokines and growth factors.
Pharmaceutical compositions of this invention used to treat patients having neurological
dise~ses or disorders could include a cytokine or other growth factor and an acceptable carrier,
auxiliary or excipient. The compositions can be for oral, topical, rectal, parenteral, local,
inhalant or intracerebral use. They may be in solid or semisolid form, for example pills, tablets,
30 creams, gelatin capsules, capsules, suppositories, soft gelatin capsules, gels, or membranes.
The compositions of the invention may also be conjugated to transport molecules to facilitate
transport of the molecules. A pharmaceutical composition comprising a cytokine or other
CA 02213906 1997-08-2~
growth factor encars~ ted in a microsphere may be incorporated into gel foam and grafted
over the lesion site or intraperitoneally implanted.
The pharmaceutical composition can be administered to humans or animals. Dosagesto be administered depend on individual needs, on the desired effect and on the chosen route
of administration.
The pharmaceutical compositions can be prepared by known methods for the
preparalion of pharmaceutically acceptable compositions which can be administered to
patients, and such that an effective quantity of the active substance is combined in a mixture
with a pharmaceutically acceptable vehicle. Suitable vehicles are described, for example in
l0 Remington's Pharmaceutical Sciences (Remington's Pharmaceutical Sciences, Mack
Publishing Company, Easton, Pa., USA 1985).
On this basis, the pharmaceutical compositions include the cytokine or other growth
factor in association with one or more pharmaceutically acceptable vehicles or diluents, and
contained in buffered solutions with a suitable pH and iso-osmotic with the physiological fluids.
The methods of binding the compound to the vehicles or combining them with diluents is well
known to those skilled in the art. The composition could include a targeting agent for the
transport of the active compound to specified sites within cells, tissues or organs.
The invention also relates to the use of the cells (fibroblasts) secreting CSF-1 to
introduce cytokines or other growth factors into the ~liseased or traumatized central nervous
20 system. The cells may be encapsulated in biodegradable or nonbiodegradable but
biocompatible polymers. The microspheres may be incorporated into gel foam and applied
over a lesion or intraperitoneally implanted. The cells act as a vector to transport a recombinant
molecule, for example, or to transport a sense or antisense sequence of a nucleic acid
molecule. In the case of a recombinant molecule, the molecule would contain suitable
transcriptional or translational regulatory elements.
Suitable regulatory elements may be derived from a variety of sources, and they may be
readily selected by one or ordinary skill in the art. If one were to upregulate the ex~,lession of
the gene, one would insert the sense sequence and the appropriate promoter into the vehicle.
If one were to downregulate the expression of the gene, one would insert the antisense
30 sequence and the appropriate promoter into the vehicle. These techniques are known to those
skilled in the art.
16
CA 02213906 1997-08-2~
Examples of regulatory elements include: a transcriptional promoter and enhancer or
RNA polymerase binding sequence, a ribosomal binding sequence, including a translation
i"ilidlion signal. Additionally, depending on the vector employed, other genetic elements, such
as selectable markers, may be incorporated into the recombinant molecule. The recombinant
molecule may be introduced into cells of a patient using in vitro delivery vehicles such as
retroviral vectors, adenoviral vectors, DNA virus vectors and liposomes. They may also be
introduced into such cells in vivo using physical techniques such as microinjection and
electroporation or chemical methods such as coprecipitation and incorporation of DNA into
liposomes. The compositions may also be delivered in the form of an aerosol or by lavage.
10 Example 1 - Encar~s~ tion of CSF-1
By this invention, we have determined that a cytokine, such as CSF-1, or other
cytokines and growth factors, can bind to neurons and rescue them from death in the animal
ischemic-stroke model. We have devised a method for delivery of extraneous cytokine, such as
CSF-1, or other cytokines and growth factor, to lesions in the CNS, including the brain.
To prepare microspheres containing recombinant human CSF-1 (rhCSF-1 ) we used
phase separation by emulsification and subsequent organic solvent evaporation. Briefly, the
rhCSF-1 (Sigma) was reconstituted in 20 mL of double distilled, sterile, endotoxin-free water
and chitosan (#311). This was combined with poly-lactic-poly-glycolic acid (PLGA) in
methylene chloride and the emulsion (w/o) was formed by ultrasonircdlion. The emulsion was
20 then incorporated into a 0.5% aqueous polyvinyl alcohol solution, thereby forming a (wlo)lw
double emulsion. In the emulsion the microspheres formed and hardened. The microspheres
were centrifuged, the organic solvent (methylene chloride) was evaporated under reduced
pressure, and the microspheres were Iyophilized. In the Iyophilized form the microspheres
containing rhCSF-1 can be stored between 4~C and room temperature.
For implantation into the CNS, dry microspheres containing rhCSF-1 were packed into
gel foam and the gel foam was placed on top of the pia over the ischemic lesion or implanted
into the peritoneum. Scanning electron microscopy showed microspheres with a smooth
surface and relatively uniform shape and size. Image analysis showed the mean diameter of
the microspheres to be 2.0117 + 4.912E-001 mm.
We enc~ps~ ted commercially available recombinant human CSF-1 in chitosan and
poly-lactic-poly-glycolic acid and placed the microspheres on top of the pia, above the lesion
17
CA 02213906 1997-08-2~
site. CSF-1 was released from the microspheres, penetrated the lesion, became bound to the
neurons and exerted its trophic effect.
We also implanted encapsulated CSF-1 intraperitoneally. The CSF-1 diffused out of the
carsu'es and was carried in the blood to the lesion site in the brain. This was possible because
in ischemic-stroke the blood-brain barrier is compromised.
The grafting of microspheres on top of the pia over the lesion site is an effective way to
deliver a cytokine, such as CSF-1, or other growth factor quickly. Intraperitoneal implantation is
slower but can be used to reinforce administration of a cytokine, such as CSF-1, and other
growth factor, such as CSF-1, over the lesion for a longer period of time. CSF-1 in
10 microspheres is stable and can be stored at 4~C until needed.
This method of encapsulation could be used with any other cytokine or growth factor,
such as SCF, GM-CSF, PDGF, NGF, CNTF, FGF and TGF, or a combination thereof.
Example 2 - Encapsulation of CSF-1 Producin~ Cells
Another way to deliver extraneous CSF-1 is to use encapsulated cells of a high CSF-1-
producing cell line. By this method, cells in microspheres survived for over a month and
throughout this period they rele~sed CSF-1~
In this approach, we used immortalized or genetically selected cells, which synthesize
and secrete the cytokines and were enc~psu'~ted in biodegradable polymeric polymers. The
encapsulation protected the cells from proteolysis and immunological rejection but at the same
20 time the cells continuously secreted the factor. Moreover, implantation of the encapsulated
cells onto the pia of the CNS was not diffficult.
The LM mouse fibroblast-like cells were obtained from American Type Culture
Collection, Rockville, Maryland (ATCC (CCL 1.2)). In our laboratory the cells were cloned and
a high CSF-1-producing clone (LM-10) was selected. Selection of a high CSF-1 producing cell
and cloning of the cell is well known to those skilled in tissue culturing techniques. The clone
was stable and has been maintained in our laboratory for several years. The cells are grown in
a serum-free medium, Medium 199 with 0.5% Bactopeptone.
To prepare the cells for encarslJlation in microspheres they were trypsinized, then
centrifuged and resuspended in 50 ~ul of fresh medium. Sterile 3% alginate solution was used
30 to resuspend the cells in 50 microlitres and then they were extruded dropwise into a solution of
CA 02213906 1997-08-2~
calcium chloride. The calcium ions cross-linked with algineic acid and formed a sen~ipermeable
membrane. The calcium chloride was then removed and the microspheres were washed briefly
with diluted chitosan (#311) solution followed by incubation in chitosan (#311). Finally, the
microspheres were washed in fresh medium and used for implanlalion.
Cell viability assays were done to determine the survival of cells in the microspheres.
The microspheres were disintegrated with citrate, thus liberating the cells. Trypan blue assay
and colorimetric assay with tetrazolyl was used. There was a striking difference in the survival
of LM-10 cells depending on the cell density in the alginate extruded into the calcium chloride
solution. The optimal cell density was 10E-6/ml, with 85-90% survival and a sustained high
0 viability (80%) for a period of one month. In addition to cell density, selection of chitosan was
particularly critical when encarsu'ating living cells. After testing chitosans with various
densities, we concluded that the best chance for cell survival was obtained when the cells were
incorporated in mi~ ospheres with high viscosity chitosan #311. In addition, the reaction time
between chitosan and Ca-alginate played an important role in forming an optimally stable and
sufficiently strong semipermeable membrane. The time of 10 minutes has been found to be
optimal. The microspheres were very stable and the integrity of the semipermeable membrane
was retained for as long as four weeks.
The amount of CSF-1 released into the medium is determined regularly using a
tic~ssay. The cells retain their ability to secrete CSF-1 for 38 days after encapsulation.
This method of encapsulation could be used with any other cytokine or growth factor,
such as SCF, GM-CSF, PDGF, NGF, CNTF, FGF and TGF, or factor producing-cells or a
combination thereof.
Example 3 - ExPerimental Ischemic-stroke Model in Mice
We adapted as our test model the mouse ischemic-stroke model originally developed by
Sofroniew et al. (Brain Res. 289:370, (1983), which was modified by Maysinger et al. (Brain
Res. 577:300, 1992) and adapted for our experimental conditions by Berezovskaya et al., (Int.
J. Devel. Neurosci. 13:285, 1995). Small, focal, cerebral cortex ischemic lesions of
reproducible size were produced, in which reaction to injury at the cellular and molecular levels
was analyzed and quantified.
The middle cerebral artery ramifies over the lateral surface of the cerebral hemisphere
and anastomoses with branches from the anterior and posterior cerebral arteries to form an
19
CA 02213906 1997-08-2~
arteriolar network (plexus) in the pia mater. From this network fine endarteries penetrate the
underlying cerebral tissue at right angles. Such penetrating arteries supply the rich capillary
network of the cortex. It is possible to disrupt the penetrating arterioles, thus producing
ischemic lesions of reproducible size and limited to specific areas of cortex. Because the
mouse brain has been mapped and the areas responsible for various sensory or motor
functions are known (Li and Waters, J. Neurol. Sci. 18:28, 1991), it is possible to affect known
sensory and motor functions.
To make a lesion, we bore a hole in the skull, 1-2 mm in diameter, depending on the
lesion size desired, in a stereotaxically defined area, then cut through the dura and expose the
lo pial vascular plexus. We disrupted the penetrating vessels that entered the cerebral cortex in
the exposed area, thereby causing a cerebral infarct, approximately 1-1.2 mm in diameter and
approximately 0.25 to 0.85 mm in depth. The lesions were of reproducible size; variation in
lesion size in the operated mice was not significant (P~0.1162) and two independent workers
could produce lesions which did not vary significantly in size.
We have used this ischemic-stroke model in mice extensively to study the effects of
CSF-1 and other factors on neuron survival.
The effects are evaluated by comparing the infarct size, which is determined from serial
cryostat sections through the lesion. By knowing the thickness of the sections and by
determining by computer image analysis the damaged area in each section, it was possible to
20 ~ te the size (volume) of the infarct.
Another parameter was determined by counting the number of neurons in sections cut
through the middle of the lesion, after staining the sections with cresyl violet. The reaction of
microglia was determined after immunostaining the microglia with Mac-1 antibody to CR3 which
is the receptor for the C3 component of complement. The reaction of astroglia was ~ssessed
after immunostaining the astroglia with antibody to GFAP, the protein of the intermediate
filaments of astrogia.
Example 4 - CSF-1 RecePtor
CSF-1 receptor (CSF-1 R) is present on microglia in the brain and on neurons in the
cerebral cortex. It is encoded by the c-fms proto-oncogene product (Sherr et al., Cell 41 :665,
30 1985; Coussens et al., Nature 32:277, 1986). The CSF-1 R gene is 58kb in length and
comprises 21 introns and 22 exons (Hampe et al., Oncogene Res. 4:9, 1981; Sherr, Blood
CA 02213906 1997-08-2~
75:1, 1990). The receptor is an integral transmembrane glycoprotein and has a highly
glycosylated extracellular ligand-binding domain (493 amino acids), a hydrophobic
transmembrane domain (25 amino acids), an intracellular tyrosine kinase domain (436 amino
acids) and an interkinase domain (73 amino acids) which interrupts the tyrosine kinase domain
(Cousins et al., Nature 32:277, 1986; Rathwell and Rohrschneider, Oncogene Res. 1 :311,
1987; Borycki et al., Growth Factors 6:209, 1992).
According to the classification by Ulrich and Schlessinger (Cell61:203, 1990), CSF-1R
belongs to the group of protein tyrosine kinase receptors, subclass lll, which also includes
receptors for PDGF AIB (Yarden et al., EMBO J. 6:3341, 1987; Claeson-Welsh et al, Mol. Cell
Biol. 8:3476, 1988; Claesson-Welsh et al., Proc. Natl. Acad. Sci. USA 86:4917, 1989; Matsui et
al., Science 243:800, 1989), protooncogene c-kit product, stem cell factor receptor (SCF-R),
also known as Steel factor or mast cell growth factor receptor (Yarden et al., EMBO J. 6:3341,
1987; Chabot et al., Nature 335:88, 1988; Geissler et al., Cell 55: 185, 1988; Qui et al., EMBO J.
7: 1003, 1988), flk-2 (Matthews et al., Cell 65: 1143, 1991) and flt-3 (Rosnet et al., Oncogene
6:1641, 1991) receptors. These receptors are characterized by extracellular regions having five
immunoglobulin-like domains and an intracellular region containing a tyrosine kinase domain
that is interrupted by a kinase insert region composed of hydrophilic amino acids. This group of
receptors, because of their extracellular structure, is also related to the immunoglobulin
superfamily of receptors. All the protein tyrosine kinase, subclass lll, receptors are expressed
20 in the brain.
In the brain, the CSF-1 receptor is expressed in microglia (Hao et al., J. Neurosci. Res.
27:314, 1990; Sawadaetal., BrainRes. 509:119, 1990; Leeet al., J. Immunol. 150:594, 1993;
Akiyama et al., Brain Res. 639: 171, 1994). Astroglia are the main source of its ligahd, CSF-1.
(Hao et al., J. Neurosci. Res. 27:314, 1990; Lee et al., J. Immunol. 150:594, 1993; Frei et al., J.
Neuroimmunol. 40:189, 1992). Therefore a great deal of attention is paid to the regulation of
microglia by astroglia by means of paracine CSF-1 signaling.
By this invention, we have determined that CSF-1 also reg~ tes the response of
microglia to CNS injury and that CSF-1 R is also present on cerebral cortex neurons (see
Example 7).
CA 02213906 1997-08-2~
Example 5 - Effects of CSF-1 on Microglia in Cultures and in situ
By this invention, we have determined that CSF-1 also regu~ates the respon$e of
microglia to CNS injury. The microglia that we developed from their progenitor cells in cultures
of neopallial cells responded to CSF-1 in low dosages without proliferation, in higher dosages
(100-1000 U/ml) with logarithmic pr~';r~:rdlion, and in even higher dosages of CSF-1, the
microglia entered into a stationary phase (see Fig. 1).
We have shown that CSF-1 is required for the development of microglia from theirprogenitor cells in culture (Richardson et al., Neuropathol. App. Neurobiol. 20:183, 1994;
Richardson et al., Glia 7:25, 1993). CSF-1 mRNA in the brain is expressed early in
lo dcvelopl"ent, at E13, and lack of CSF-1 because of mutation impairs brain development
(Chang et al., J. Neuroimmunol. 52:9, 1994; Michaelson et al., Abstr. Soc. Neurosci. 20: 1690,
1994; Berezovskaya et al., Int. J. Dev. Neurosci. 13:285, 1995).
We have shown that in natural knock-out mice which lack CSF-1 systemically (op/op
mice), the microglia developed and their number and morphology appeared to be normal
(Blevins and Fedoroff, J. Neurosci. Res. 40:535, 1995) but the microglia were unable to
respond to injury (Berezovskaya et al., Int. J. Dev. Neurosci. 13:285, 1995). However, by
implanting syngeneic CSF-1-producing astrocytes or microspheres with CSF-1-producing LM-
10 cells into mice that have devascularizing ischemic brain lesions (ischemic-stroke) the
function of microglia was restored in mutant op/op mice (Berezovskaya et al., 13:285, 1995)
20 (see Table 1), incorporated herein by reference.
Thus our observations indicated that CSF-1 regulates microglia in situ as it does in
culture. When the devascularizing ischemic lesions were produced in normal CSF-1-producing
mice (e.g., C3H/HeJ) and the mice were implanted with microspheres containing C~;F-1-
producing LM-10 cells, the number of microglia and the degree of morphological transformation
in response to the ischemic lesion did not change. This indicated that microglia were regulated
by CSF-1 in situ in the same way as in culture. Microglia responded to the injury by
proliferation if CSF-1 was available to the microglia but if the CSF-1 was increased above the
levels produced by the normal animal, the microglia did not continue to proliferate but rather
entered a steady state, as they did in cultures. We also found that replacing LM-10 cells with
30 recombinant human CSF-1 resulted in the same response as with CSF-1-producing cells. This
indicated that the factor that regulated microglia in situ was indeed CSF-1 (see Fig. 2).
CA 02213906 1997-08-2~
Example 6 - Effect of CSF-1 on Neurons.
In the course of our studies of the regulation of microglia by CSF-1 in natural CSF-1
knock-out mice (op/op) that had devascularizing cerebral cortex ischemic-stroke lesions, we
observed that CSF-1, in addition to its effect on microglia, had an effect on neuron survival.
The number of viable neurons in the lesion in op/op mice (CSF-1 deficient) was decreased by a
factor of 10.5 when compared to the number in a corresponding area on the non-operated
contralateral side (Berezovskaya et al., Int. J. Dev. Neurosci 1 :285-299, 1995).
When we delivered CSF-1 by grafting syngeneic astrocytes onto the cerebrum before
making the ischemic-stroke lesion, or when we implanted alginate-chitosan microenr.~rsu~ted
lo LM-10 cells (high CSF-1-producing) in mutant op/op mice with cerebral cortex ischemic-stroke
lesions at the time of lesioning, the number of neurons that survived ischemic damage
increased by a factor of 3.7 (P~0.0001) and the size of the ischemic infarct decreased by a
factor of 2 (P<0.0001) (Table 1).
op/op mice C3H/HeJ mice
Neurons Glial cells Neurons Glialcells
cont,~laleral side 2612.39 i 75.07 402.55 i 3.64 2416.07 i 57.22 437.55 i 15.67
lesion 247.51 i 2.97 405.57 i 38.86 578.25 + 46.23 1275.11 i 89.99
(9.5%) (100.75%) (23.93%) (291.42%)
lesion in animal 913.20 i 22.76 748.8 i 25.39
with grafted (34 95%) (186%)
astroglia
lesion in animal 544.0 i 32.43 567.2 + 39.84
with implanted (20.82%) (140 9%)
microspheres
Table 1. Number of viable cells per mm2 in op/op and C3H/HeJ mice on conl,~lal~ral side
and in the area of cerebral cortical ischemic lesion. The numbers are expressed as
means ~ s.e.m. The numbers in the brackets indicate the percentage of viable cells
23
CA 02213906 1997-08-2~
in the lesion vs that on the contralateral side. (Berezovskaya et al. Int. J. Devl.
Neuroscience 13:285-299, 1995).
Similar results were obtained when we delivered CSF-1 in the form of rhCSF-1 in
microspheres (Fig. 3 and 4). These observations strongly indicate that the availability of CSF-1
in the ischemic-stroke lesions has a significant effect on minimizing the ischemic darnage in the
cerebral cortex.
The above experiments clearly indicate that the availability of CSF-1 in mammals that
are systemically deficient in CSF-1 is very important for microglial response to injury and for
potentiation of neuron survival in ischemic-stroke lesion.
o The question is what effect delivery of extraneous CSF-1 has on neuronal survival in
ischemic-stroke lesion in CSF-1-producing mice. In normal mice implanted with chilk.san
microspheres containing rhCSF-1 at the time and site of the lesion or intraperitoneally two
weeks before lesioning, neuron survival in the lesion increased 2-fold (P<0.0001) (F g. 5).
Microglial response, as mentioned above, did not increase significantly in comparisan to that in
lesioned normal mice that did not receive any extraneous CSF-1 (P>0.9) (Fig. 2).
The size of the infarct was determined in CSF-1-producing normal mice which received
extraneous encarsu~ted rhCSF-1 and the size was compared to that of infarcts in controls 7
days and 25 days after lesioning. After 7 days the infarct size as compared to controls, which
did not receive extraneous CSF-1, was approximately twice as small. After 25 days the infarct
size in animals that received CSF-1 remained 3.8 times smaller than in the controls ~see Fig. 6).
This indicates that the rescue of neurons by CSF-1 in ischemic-stroke lesions is not a
temporary effect but lasts for at least one month. It is most unlikely that the infarct size would
suddenly increase after one month.
Example 7 - Presence of CSF-1 Receptor (CSF-1 R) in Neurons
If rhCSF-1 rescues neurons from injury, then neurons should have CSF-1 R. We
immunoreacted cryostat sections of cerebral cortex and cerebral cortex neurons in culture with
four different antibodies for CSF-1 R which were produced against different mouse CSF-1 R
epitopes. All four antibodies immunoreacted positively with cerebral cortex neurons and the
reaction was more intense on the lesioned ipsilateral side of the cortex than on the
cor,esponding contralateral side. To verify the specificity of the immunoreactivity, we adsorbed
the antisera with cells (EL4) which did not have CSF-1R and cells (5/10.14) which did express
24
CA 02213906 1997-08-2~
CSF-1 R. The immunoreactivity after adsorption of antisera with cells which did not have CSF-
1 R was only slightly weaker than with non-adsorbed antisera. The immunoreactivity of the
antisera adsorbed with cells which did express CSF-1 R was completely negative, indicating the
specificity of immunoreactivity to CSF-1 R and the presence of CSF-1 R in neurons. Neurons in
the cerebral cortex express CSF-1 R (Fig. 9).
The neurons in culture immunoreacted positively to CSF-1 after CSF-1 was added to
the cultures. Neurons that were not exposed to CSF-1 had no immunoreactivity to CSF-1,
indicating that CSF-1 can bind to CSF-1 R in neurons.
To confirm that the CSF-1 R was indeed localized in the neurons, we double-
o immunostained neurons with antibody to extrac~ r domain CSF-1 R and antibody to 70 kD
neurofilaments, specific to neurons. Both were colocalized in the same neurons, indicating that
cerebral cortex neurons in culture express CSF-1 R (Fig. 8).
We also probed neurons in cryostat sections of cerebral cortex and in cultures with in
situ hyL, idi~alion using 48-mer DNA oligonucleotide probe complementary to, and selective for,
bases 904-951 of mouse CSF-1 R mRNA. The neurons in the cerebral cortex cryostat sections
and in cultures were labeled positively. In lesioned cerebral cortex the labeling was
considerably more intense and larger numbers of neurons were labeled.
To see whether CSF-1/CSF-1 R signaling has any biological activity, we investigated the
effect of CSF-1 on neuron survival and neurite outgrowth in cultures. The addition of CSF-1 to
20 pure cultures of cerebral cortex neurons significantly potenlialed neuron survival (see Fig. 7)
and neurite outgrowth, in every case that we tested.
We thus determined that cerebral cortex neurons express CSF-1 R mRNA and produceactive protein receptor which binds CSF-1 and CSF-1/CSF-1 R signaling potentiates neuron
survival and neurite outgrowth in cultures and potentiates neuron survival in ischemic-stroke
lesion in mice. In ischemic-stroke lesions neurons and microglia upregulate the ex,u,ession of
CSF-1 R.
Example 8 - Expression of CSF-1 R mRNA in cerebral cortex neurons in cultures
We used oligonucleotide probe labeled at the 3'end with Digoxigenin-ddUTP to
demonsl,ate the presence of CSF-1R mRNA in cerebral cortex neurons in cultures. Three-day-
30 old neuron cultures were hybridized in situ using DIG Oligonucleotide 3'End Labeling Kit
CA 02213906 1997-08-2~
(Boehringer Mannheim). All neurons were labeled for CSF-1 R mRNA (Fig. 11), indicating that
cerebral cortex neurons in culture express CSF-1 R mRNA as well as receptor proteln.
Example 9 - CSF-1 bindin~ to CSF-1 R in cerebral cortex neurons in culture
We observed that in 2-day cultures of cere~ral cortex neurons, in which some astroglia
were present, there was a weak immunoreactivity of neurons with antibody to CSF-1. In 7-day
neuron cultures, in which the number of astroglia was greatly increased, the immunoreactivity of
neurons with antibody to CSF-1 was considerably more pronounced than in 2-day-old cultures
with astroglia. Astroglia secrete CSF-1 and therefore it is possible that it did bind to the CSF-
1 R in neurons (Hao et al., 1990; Lee et al., 1993).
To show that CSF-1 R in cerebral cortex neurons is actually biologically active and binds
CSF-1, neuron cultures free of astroglia were set up according to the procedure described by
Fedoroff and Richardson (1996). To eliminate astrocytes from the cultures, the cer~bral cortex
neuron cultures were treated with arabinoside C (10 mg/ml) which su,upr~sses astroglial
pr~!if~,rdlion. We added 1000 lU/ml of recombinant human CSF-1 (rhCSF-1) to the arabinoside
C-treated 7-day neuron cultures and incubated them for 3-5 min at 37~C. After thorough
washing with ice-cold DPBS the cultures were immunoreacted with the monoclonal antibody to
CSF-1. Approximately half of the neurons showed strongly positive immunoreactivity. The
immunoreactivity was seen on the surface of the neuron cell body and along the surface of the
neurites. The neurons in sister cultures, to which no rhCSF-1 was added, did not immunoreact
20 with the antibody to CSF-1 (Fig. 10).
The positive immunoreactivity of neurons with the monoclonal antibody to C' F-1 in the
presence of astroglia and in astroglia-free cultures to which rh-CSF-1 was added showed that in
cultures CSF-1 R in cerebral cortex neurons can actually bind CSF-1. The loc~ tion of the
immunoreactivity to the surface of the neuronal cell bodies and neurites indicates the reaction
to the cell-bound CSF-1.
Interestingly, although all cerebral neurons in culture immunoreacted positively to
antibodies to CSF-1 R, only about half of the neurons were seen to bind CSF-1 to their surfaces.
This could be attributed to the sensitivity of the method or to the possibility that neurons
express different numbers of receptors in their cell membranes. Some neurons may' have too
30 few receptors to detect CSF-1 binding. It is also possible that CSF-1 R is not active ih some
neurons and therefore does not bind rhCSF-1. This phenomenon requires further invesligdlion.
CA 02213906 1997-08-2~
Example 10 - Effect of CSF-1 on aPoPtosis and survival of cerebral cortex neurons in culture
To show that CSF-1 binding to CSF-1 R activates the intracellular pathway of signaling,
we inve~ligated the effect of CSF-1 on neuron apoptosis and survival in culture in serum-free
medium. We used the ApoTag In Situ Apoptosis Detection Kit (Onco, Gaithersburg, MD) to
demonstrate apoptotic neurons in culture. The stained apoptotic cells were counted. Already
at the first and second days of culturing we observed a significant decrease in the number of
apoptotic neurons in the presence of 500 lU/ml of CSF-1 (Fig. 12).
In cerebral cortex neuron cultures in serum-free medium, generally the number of viable
neurons decreased over the 5-7 days of culturing. When we added 600 lU/ml of CSF-1,
0 although the number of neurons still declined, a significantly larger number of neurons survived
in the 5-day cultures (Fig. 13). The effect of CSF-1 on neuronal survival was dose-dependent.
In the presence of 300 lU/ml of CSF-1, approximately 1.3 times more neurons survived after 3
days of culturing and in the presence of 900 lU/ml of CSF-1, more than twice as many neurons
survived (Fig. 14). These observations showed that the presence of CSF-1 R has biological
significance for neurons and that CSF-1 can activate receptor-mediated intracellular signal
pathways.
Example 11 - Expression of CSF-1 R in neurons in situ
Brain and spinal cord from normal C3H/HeJ mice and mice that had received
devascularizing focal ischemic cerebral injury were fixed in situ, then quickly frozen. Cryostat
20 sections were cut and immunostained for CSF-1 R. We found that microglia throughout the
brain expressed CSF-1 R but only individual scattered neurons were immunopositive for CSF-
1 R, except in the cerebellum and brain stem where larger numbers of neurons expressed CSF-
1 R. In the cerebellum, Purkinje cells were strongly immunopositive for CSF-1 R. In addition,
closely associated cells, probably some basket cells and some Golgi cells, were also positive.
Neurons in cerebellar nuclei were also CSF-1 R immunopositive (Fig. 17). However, only a few,
if any, granule cells were CSF-1 R-negative.
In the brain stem, neurons in motor nuclei: hypoglossal, occulomotor, trigeminal, facial
and ambiguous; and in sensory nuclei: trigeminal, cochlear, vestibular, parabigeminal, raphe,
lateral lemniscus; and neurons in the reticular formation, were positive for CSF-1 R. In the
30 spinal cord, some neurons in the ventral and dorsal horns were weakly CSF-1 R-positive (Table
2).
CA 02213906 1997-08-2~
In sections from animals 7 days after focal ischemic cerebral injury, the microglia around
the lesion were very strongly positive for CSF-1 R. The intensity of CSF-1 R immunopositivity in
neurons varied in various areas of the brain. The neurons in the zone near the ischemic core
were strongly CSF-1R immunoreactive (Fig. 14). Neurons in the ipsilateral cortex remote from
the ischemic territory upregulated CSF-1 R to a lesser extent and the levels of upregulation were
comparable to those in neurons in the contralateral hemisphere. It is of interest that neurons in
many areas remote from the cerebral cortex also upregulated the expression of CSF-1 R (Table
2; Figs. 1 6,1 7,1 1 ).
When we used very diluted anti-CSF-1 R antibody we observed that in animals thatl0 received focal ischemic cerebral injury, the CSF-1 R immunostained more intensely in section of
cerebellum and brain stem, where neurons constitutively express CSF-1 R. This showed that
such neurons also upregulate the expression of the receptor in response to injury .
ExamPle 12 - Ekl,rt:ssion of CSF-1R mRNA in the cerebral cortex neurons in vitro and in situ
We probed cerebral cortex cryostat sections with 48-mer DNA oligonucleotide probe.
The probe labeled a few neurons and all microglia, indicating that some cerebral cortex neurons
in normal animals do express CSF-1 R mRNA (Fig. 19). In the zone immediately surrounding
the ischemic core, the number of labeled neurons increased, as did the intensity of labeling.
Example 13 - Active Site of CSF-1
The CSF-1 crystal structure (amino acids 4-158) has recently been determined (Pandit
20 et al., Science 258:1358, 1992) and this is used to develop a cluster of drugs with CSF-1
activity. In this study, we determine the mechanism by which CSF-1 rescues neurons from
lethal injury. It acts as a cytokine or is involved in ameliorating Ca2 influx damage after
ischemic-stroke injury. Efforts are directed to production of small synthetic peptides to be used
as drugs with the activity of the CSF-1 terminal fragments and to test how easily such drugs
penetrate the blood-brain barrier and then bind and activate the CSF-1 receptor on neurons and
microglia. Our work determines whether CSF-1 and its closely related cytokines GM-CSF, SCF
and PDGF bind to human neurons and whether they have a biological effect on human
neurons, such as potentiation of neuron survival and branching.
CA 02213906 1997-08-2~
Example 14 - Degree of Improvement that CSF-1 Produces on Grafting to Ischemic- Stroke
Animals
We evaluate the degree of improvement in behavioral terms that CSF-1 produces ongrafting to ischemic-stroke lesioned animals. This study indicates the extent to which neurons
are rescued and continue to function normally after an ischemic-stroke episode.
Example 15 - Testing of Other CYtokines and Other Growth factors
By this experiment, we test other cytokines belonging to the same cytokine group as
CSF-1, such as GM-CSF, SCF and PDGF for their effect on rescue of neurons in ischemic-
stroke injury, individually and in various combinations.
10 Example 16 - Dosin~
This work determines the dose of cytokines required for neuronal rescue and the length
of time the microspheres remain functional in situ.
MATERIALS AND METHODS
Neuronal cultures.
Enriched cultures of cerebral cortex neurons were prepared from neopallium of E15
C3H/HeJ mouse embryos. A pregnant mouse was killed by CO2 inhalation and embryos were
removed aseptically. The cerebral hemispheres were separated from the brain and the
neopallia were dissected out by carefully removing the olfactory bulbs, basal ganglia,
20 hippocampus and meninges. The neopallial tissue was placed into Ca2+- and Mg2~-free
balanced salt solution and cut into 1 mm3 cubes, then incubated with 0.25% trypsin for 3-5 min,
washed in Hanks' BSS (HBSS) and disaggregated by careful pipetting up and down in
Dulbecco's Minimum Essential Medium (DMEM). The cells were plated on poly-L-lysine coated
coverslips placed in 60 mm culture dishes at a density of 5 x 105 cells per dish, in serum-free
DMEM or Honegger's medium. After 10 min the medium was removed and cells attached to
the substratum were washed with HBSS and fresh serum-free DMEM or Honegger's medium or
fresh MEM containing 5% horse serum was added (3 ml/dish). The cultures were grown at
37~C in a humidified atmosphere of 5% CO2 in air. After 7 days in vitro the cells were fixed,
washed, and used for immunocytochemistry.
CA 02213906 1997-08-2~
Assay for neuron survival in vitro
Non-culture Petri dishes containing six cerebral cortical neuron coverslip cultures per
dish were divided into two groups of 5 dishes each. To the first group hrCSF-1 (M9667, Sigma)
at a concentration of 600 lU/ml was added immediately after the cells attached to the substrate
and at 2 and 4 days in culture. To the control group, instead of CSF-1, BSA (B2518 Sigma) at
a concentration of 1 mg/ml was added.
After 1, 2, 3, 4 and 5 days of culturing, the number of viable neurons bearing processes
longer than their cell body diameter were counted in 10 microscopic fields chosen at random
from each neuron coverslip culture, using a 20x phase contrast objective lens on an Olympus
lo inversion microscope. The number of neurons surviving at each culture day was expressed as
mean i S.D.
To determine the dose response 300, 600 or 900 lU/ml of hrCSF-1 were added to
neuron coverslip cultures immediately after cell attachment and after 2 days of culturing. After
1, 2 and 3 days of culturing the number of neurons was determined as described above.
Assay for ~rGIJtot;c neurons
Non-tissue culture Petri dishes, each containing six cerebral cortex neuron c~verslip
cultures, were divided into two groups of four dishes each. The experimental group received
500 lU/ml of hrCSF-1/dish each day. The control group, received 1 mg/ml of BSA daily, instead
20 of hrCSF-1. After 1 and 2 days of culturing, the cultures in 2 dishes from each group were
gently washed twice in PBS and fixed in formalin-80 % alcohol-acetic acid fixative (1:45:1) for
30 min. The cultures were washed in PBS twice after fixation and then treated with 0.2%
trypsin in Puck's BSS at room temperature for 30 min. The cultures were post-fixed in ethanol-
acetic acid (2:1 ) for 5 min at -20~C and then rinsed twice in PBS.
The cultures were stained using the ApoTag In Situ Apoptosis Detection Kit ~S71 00-kit,
Oncor, Gaithersburg, MD). Some coverslip cultures were counterstained with hemotoxylin or
nuclear fast red.
To determine the number of apoptotic neurons present after 1 and 2 days of culturing,
the total cell number and the number of stained apoptotic cells were counted from 5
30 microscopic fields chosen randomly for each coverslip culture, using a 20X objective lens on a
Leitz microscope. The data were expressed as mean i S.D.
CA 02213906 1997-08-2~
Antibodies
Four different rabbit polyclonal antibodies to CSF-1 R (c-fms) were used for
immunocytochemical staining: i.e., antibody to whole CSF-1 R (Cambridge ResearchBiochemicals, England); antibody to extracellular domain of CSF-1 R; and two different
antibodies to cytoplasmic domain of CSF-1 R, one human and one mouse-specific (Upstate
Biotechnology Inc., N.Y.). Monoclonal antibody to rat CSF-1 was obtained from Oncogene
Science Inc. (N.Y.). Monoclonal antibody 8A1 to 70 kD neurofilaments was a gift from Dr. D.J.
Barnstable, Yale University, Buffalo, N.Y.
10 Immunohistoche,.,istry
Mice without treatment and mice seven days after cerebral cortex ischemic lesioning
were deeply anesthetized with metofane and perfused transcardially with PBS followed by 4%
paraformaldehyde. The brains were removed, post-fixed for 2-3 h in the same fixative,
cryoprotected in 20% sucrose overnight, embedded in OCT compound and rapidly frozen in
isopentane pre-cooled by liquid nitrogen. Cryostat coronal sections were cut at 12 mm
thickness at -10~C. Immunostaining was performed using anti-CSF-1R (c-fms) antibodies.
Before immunostaining, sections were treated for 20 min in PBS containing 2% non-fat milk
powder and 0.1% Triton X-100 (for CSF-1 R extracellular domain, Triton X-100 was omitted).
Endogenous peroxidase activity was eliminated by exposing the sections to 0.6% H2~2 in
20 methanol for 15 min. The sections were incubated overnight at 4~C with primary antibody (anti-
CSF-1 R, dilution 1:400), then for 1 h with biotinylated secondary antibody (dilution 1:500 ) and
then for 1 h with avidin-biotin complex (ABC), followed by a horseradish peroxidase substrate
solution containing 0.05% 3,3'-diaminobenzidine (DAB), 1.5% NiSO4 and 0.06% H2O2. Some
sections were counterstained with nuclear fast red for 3 min, dehydrated and mounted. In the
cor,l,ols the primary antibody was repl?ced with non-immune IgG (Sigma), the same isoform as
the primary antibody.
The cultures were rinsed with cold Dulbecco's physiological balanced solution (DPBS),
fixed in -20~C cold methanol for 10 min, then rinsed with DPBS followed by incubation with
DPBS containing 2% non-fat milk powder (Carnation Milk, Nestlé, Canada) for 20 min to
30 suppress unspecific immunoreactivity. The four types of primary antibodies against CSF-1 R
described above were used. All four antibodies were used in 1:200 dilution.
CA 02213906 1997-08-2~
To identify neurons, 8A1 monoclonal antibody to 70 kD neurofilament (gift from D. J.
Barnstable) was used in dilution 1:100. ForABC-HRP staining, the coverslips with the cells
were incubated with primary antibody overnight at 4~C and then for 30-45 min with biotinylated
secondary antibody, dilution 1 :500 (Vector Laboratories, Inc. Burlington, CA, USA). The
coverslips with cells were then incubated for 1 h with avidin-biotin complex (Vectastain Elite
ABC-kit, Vector Laboratories, Inc., CA), followed by a horseradish peroxidase (HRP) substrate
solution containing 0.05% 3,3'-diaminobenzidine (DAB), 1.5% NiSO4 and 0.06% H2O2. The
cultures were dehydrated and mounted.
For fluorescence immunoreactivity coverslips with cells grown for 2 or 7 days in culture
l0 were fixed as described above, then incubated with primary antibody for 1 h at room
temperature, followed by incubation with fluorescein isothyocyanate (FITC) or rhodamine
conjugated secondary antibodies (Jackson Research laboratory) diluted 1 :100, for 1 h at room
temperature. As a control the primary antibody was omitted or non-immune IgG (Sigma) of the
same species as the primary antibody was used instead of primary antibody.
Preparation of probe
A 48-mer DNA oligonucleotide probe complimentary to, and selective for, bases 904-
951 of murine CSF-1 receptor (c-fms) mRNA (Rothwell and Rohrschneider, 1987) wassynthesized (UCDNA Services, Calgary, AB, Canada). The probe was labeled at the 3'-end
20 with [35S]dATP (NEN, Boston, Mass, USA) using terminal deoxynucleotide transferase
(Amersham, Canada) in a buffer containing 10 mM CoC12, 1 mM dithiothreitol (DDT), 300 mM
Tris base and 1.4 M-potassium cacodylate (pH 7.2), purified through NENSORB-20 columns
(New England Nuclear, USA) and DDT added to a final concentration of 10 nM. The specific
activities obtained ranged from 2 to 5 x 106 dpm/ng oligonucleotide.
In situ hyl,ri.li~alion
Deeply anesthetized animals were perfused via aorta with 50 cc 0.1 M phosphate-
buffered saline solution (pH 7.4) to clear the blood, followed by rapid dissection and freezing of
the brains embedded in OCT Compound (Tissue Tek, Miles Laboratories, Elkhart, IN, USA) in
30 isopentane pre-cooled by liquid nitrogen in a Cryomold (Tissue Tek, Miles Laboratories, Elkhart,
IN, USA). Sections were cut at 10 mm on a Micron cryostat (Zeiss, Canada), thaw-mounted
CA 02213906 1997-08-2~
onto Probe-ON slides (Fisher Scientific, Pittsburgh, PA, USA) and stored with desicc~n~ at -
20~C until hybridization.
Hybridization was carried out according to published procedures (Dagerlind et al., 1992;
Verge et al., 1995). The sections were brought to room temperature, air dried, and without any
additional treatment, covered with a hybridization buffer containing 50% formamide, 4 x SSC (1
x SSC-0.15 M NaCI, 0.015 M sodium citrate), 1 x Denhart's solution (002% bovine serum
albumin and 0.02% Ficoll), 1% sarcosyl (N-laurylasarcosine), 0.02M phosphate buffer (PH 7.0),
10% dextran sulphate, 500 mg/ml heat-denatured salmon sperm DNA, 200 mM DDT and 107
dpm/ml of probe. The slides were placed in a box humidified with 1 x SSC and incubated at
0 42~C for 18 h. After hybridization, the slides were washed four times for 15 min in 1 x SSC at
55~C and then brought to room temperature over 30 min while in the final rinse, dipped twice in
distilled water, dehydrated in 60% and 95% ethanol and dried with an airstream.
To generate radioautograms, the incubated slides were dipped in NTB2 nuclear track
emulsion (Kodak, Rochester, N.Y., USA) diluted 1 :1 with distilled water and stored in the dark
with desiccant at 4~C. The sections were exposed for 2-4 weeks, then developed in Kodak
D19 for 3 min, fixed and mounted with glycerol and coverslip for analysis on a Zeiss
photomicroscope equipped with darkfield capabilities, or stained with Toluidine blue and
mounted with Permount (Fisher Scientific, Canada) and a coverslip for viewing under
brightfield.
Cells were considered labeled if they had more than five times background levels of
silver grains, as determined by averaging grain counts over defined areas of the neuropil devoid
of positively labeled cell bodies.
The specificity of hybridization signal for the probe used in the study was ascertained by
hybridi~dlion of adjacent 10 mm sections of experimental mouse brain. Series of sections were
hybridized with labeled probe, labeled probe with a 800-fold excess of cold probe, or labeled
probe with a 800-fold excess of another, dissimilar cold probe of the same length and similar G-
C content.
In situ hyL ri~ dlion in culture with Digoxigenin-l~hele~l probe
The probe described above that was used for in situ hyLridi~alion in situ was labeled at
the 3'end with Digoxigenin-ddUTP using the DIG Oligonucleotide 3'End Labeling Kit (1362 372,
Boehringer Mannheim, Laval, PQ, Canada)
CA 02213906 1997-08-2~
Cerebral cortex neuron coverslip cultures after 3 days of culturing were rinsed with
0.03M PBS prepared with DEPC-treated distilled water and were fixed in a solution of 5% acetic
acid with 4% paraformaldehyde. The coverslips were washed in 0.03M PBS at 4~ C for 30 min
followed by dehydration with ethanol. They were stored with desiccant at -20~ C until use.
Before hybridization the coverslips were rehydrated with ethanol, rinsed in 4X SSC and
then covered with the hybridization buffer, as described above in the isotope method, and
digoxigenin labeled probe. They were kept in a humidified box at 42~ C for 18 hours.
After hybridization the coverslips were washed four times for 15 min in 1X SSC at 42~ C
and rinsed in DPBS followed by incubation in a blocking solution (1210 220, Digoxigenin
lo Detection Kit, Boehringer Mannheim, Laval, PQ, Canada ) for 30 min. The coverslips were
then incubated with anti-digoxigenin-AP (dilution 1:500) at room temperature for 1 h, followed
by an AP substrate solution containing 1 ml Tris buffer (pH 9.5), 5 nl NBT, and 3.75 nl X-
phosphate (Digoxigenin Detection Kit, Boehringer Mannheim).
Procedure for cerebral cortex ischemic lesion
Four- to six-week-old male C3H/HeJ mice bred in our animal facilities were used. All
surgical procedures were performed aseptically under somnatol anesthetic injected i.p. at a
concentration of 0.015 ml per 209 body weight. Following anesthesia mice were immobilized in
a stereotaxic frame and a midline incision of the skin over the skull was made. A hole (1 mm2)
20 was bored into the skull with a dental drill, about 2.5-3 mm caudal to the bregma and 1 mm to
the right of the midline. The dura was exposed, cut, and a few pial-arteriolar plexus arteries
were clipped with a Dumont forceps. Blood around the wound was removed using surgical
spears (Merocel Corp., Mystic, CT). A small piece of sterile gelfoam was placed over the
wound and the skin was sutured. Postsurgically, all experimental animals were kept warm for
1 h under a lamp and injected with 0.03 ml of the analgesic Temgesic (Reckitt & Colman, Hull,
England).
The present invention has been described in detail and with particular reference to the
preferred embodiments; however, it will be understood by one having ordinary skill in the art
that changes can be made thereto without departing from the spirit and scope thereof.
All publications, patents and patent applications are herein incorporated by reference in
their entirety to the same extent as if each individual publication, patent or patent application
34
CA 02213906 1997-08-2~
was specifically and individually indicated to be incorporated by reference in its entirety.
CA 02213906 1997-08-2~
REFERENCES
Akiyama H, Nishimura T, Kondo H, Ikeda K, Hayashi Y, McGeer PL (1994): Ex,urt:ssion of the
receptor for macrophage colony stimulating factor by brain microglia and its upregulation in
brains of patients with Altzheimer's dise~se and amyotrophic lateral sclerosis. Brain Res
639: 171 -174.
Alliot F, Lecain E, Grima B, Pessac B (1991): Microglial progenitors with a high proliferative
potenlial in the embryonic and adult mouse brain. Proc Natl Acad Sci USA 88:1541-1545.
o Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G,
Peschle C (1992): Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating
factors) by normal human astrocytes in response to IL-1 b and tumor necrosis factor-a. J
Immunol 149:2358-2366.
An G, Lin TN, Liu JS, Xui JJ, He JJ, Hsu CY (1993): Expression of c-fos and cjun family genes
after focal cerebral ischemia. Ann Neurol 33:457464.
Arceci RJ, Shanahan F, Stanley ER, Pollard JW (1989): Temporal expression and location of
colony stimulating factor-1 (CSF-1) and its receptor in the female reproductive tract are
20 consistent with CSF-1 regulated placental development. Proc Natl Acad Sci USA 86:8818-8822.
Bartocci A, Pollard JW, Stanley ER (1986): Regulation of colony stimulating factor-1 during
pregnancy. J Exp Med 163:956-61.
Berezovskaya O, Maysinger D, Fedoroff S (1995): The hematopoietic cytokine, colony
stimulating factor-1, is also a growth factor in the CNS: congenital absence of CSF-1 in mice
results in abnormal microglial response and increased vulnerability to injury. Int J Dev Neurosci
13:285-299.
30 Cecchini MG, Dominguez MG, Mocci S., Wetterwald A, Felix R., Fleisch H, Chisholm O.,
Hofstetter W, Pollard JW, Stanley ER (1994): Role of colony stimulating factor-1 in the
est~l.shment and regulation of tissue macrophages during postnatal development of the
mouse. Development 120: 1357-72.
Chambers SK, Wang Y, Gertz RE, Kacinski BM (1995): Macrophage colony-stimulating factor
mediates invasion of ovarian cancer cells through urokinase. Cancer Res 55:1578-1585.
CA 02213906 1997-08-2~
Chambers SK, Wang Y, Gilmore-Hebert M, Kacinski BM (1994): Post-transcriptional regulation
of c-fms proto-oncogene ex,ur~ssion by dexamethasone and of CSF-1 in human breast
carcinomas in vitro. Steroids 59:514-22.
Chang Y, Albright S, Lee F (1994): Cytokines in the central nervous system: expression of
macrophage colony stimulating factor and its receptor during development. J Neuroimmun
52:9-17.
Chen HE, Chang S, Trub T, Neel BG (1996): Regulation of colony stimulating factor 1 receptor
lo signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol 16:3685-
3697.
Cheung AN, Srivastava G, Pittaluga S, Man TK, Ngan H, Collins RJ (1993): Expression of c-
myc and c-fms oncogenes in trophoblastic cells in hydatidiform mole and normal human
placenta. J Clin Pathol 46:204-207.
Dagerlind A, Friberg K, Bean AJ, Hokfelt T (1992): Sensitive mRNA detection using unhxed
tissue: Combined radioactive and non-radioactive in situ hybridization histochemistry.
Histochemistry 98:39-49.
Daiter E, Pampfer S, Yeung YG, Barad D, Stanley ER, Pollard JW (1992): Expression of colony
stimulating factor-1 in the human uterus and placenta. J Clin Endocrinol Metab 74 :850-858.
Fedoroff S, Ahmad 1, Young VW, Guilbert LJ, Antel J (1991): A subpopulation of human
astroglia expresses the receptor for colony-stimulating factor-1 which is encoded by c-fms. J
Neuroimmunol 1: 100.
FedoroffS, Hao C, Guilbert LJ (1993): Paracrine and autocrine signaling in regulation of
microglial survival. In Fedoroff S, Juurlink BHJ, Doucette R (eds): Biology and Pathology of
Astrocyte-neuron Interactions. New York: Plenum Press, pp. 247-61.
Fedoroff S and Richardson A. (1997): Generation of mouse astroglia and microglia cultures
from mouse neopallium. In Fedoroff S, Richardson A (eds): Protocols for Neural Cell Culture
2nd Ed. Totowa NJ: Humana Press, pp 131-141.
Felix R, Hofstetter W, Watterwald A, Cecchini MG, Fleisch H (1994): Role of colony stimulating
factor-1 in bone metabolism. J Cell Biochem 55:340-349.
Frei K, Nohava K, Malipiero UV, Schwerdel C, Fontana A (1992): Production of macrophage
colony-stimulating factor by astrocytes and brain macrophages. J Neuroimmunol 40:189-196.
CA 02213906 1997-08-2~
Gehrmann J, Bonnekoh P, Miyazawa T, Hossmann K-A, Kreutzberg GW (1992):
Immunochemical study of an early microglial activation in ischemia. J Cerebral Blood Flow
Metab 12:257-269.
Giulian D, Ingeman JE (1988): Colony-stimulating factors as promoters of ameboid microglia. J
Neurosci 8:4707-4717.
Giulian D, Young DG, Woodward J, Brown DC, Lachman LB (1988): Interleuki-1 is an astroglial
lo growth factor in the developing brain. J Neurosci 8:709-714.
Hamilton JA (1997): CSF-1 signal transduction: what is of functional significance? Immunol
Today 18:313-317.
Hao C, Guilbert LJ, Fedoroff S (1990): Production of the colony stimulating factor-1 (CSF-1) by
mouse astroglia in vitro. J Neurosci Res 27:314-23.
Hofstetter W, Wetterwald A, Cecchini MC, Felix R, Fleisch H, Mueller C (1992): Detection of the
transcripts for the receptor for macrophage colony-stimulating factor, c-fms, in murine
20 osteoclasts. Proc Natl Acad Sci USA 89:9637-9641.
Hopkins SJ and Rothwell NJ (1995). Cytokines and the nervous system l: expression and
recognition. TINS 18:83-88.
Hulkower K, Brosnan C.F, Aquino D.A, Cammer W, Kalshrestha S, Guide M.P, Rapoport DA,
Berman JW (1993): Expression of CSF-1, c-fms and MCP-1 in the central nervous system of
rats with experimental allergic encephalomyelitis. J Immunol 150:2525-2533.
Iizuka H, Kaoru MD, Sakatani K, Young W (1989): Corticofugal axonal degeneration in rats
30 after middle cerebral artery occlusion. Stroke 20:1396-1402.
Iizuka H, Sakatani K, Young W (1990): Neural damage in the rat thalamus after cortical infarcts.
Stroke 21 :790-794.
Jokhi PP, Chumbley G, King A, Gardner L, Loke YW (1993): Expression of the colony
stimulating factor-1 receptor (c-fms product) by cells at the human uteroplacental interface. Lab
Invest 68:308-320.
38
CA 02213906 1997-08-2~
Kacinski BM (1997): CSF-1 and its receptor in breast carcinomas and neoplasn,s of the female
reproductive tract. Mol Reprod Devel 46:71-74.
Kacinski BM, Scata KA, Carter D, Yee LD, Sapi E, King BL, Chambers SK, Jones MA, Pirro
MH, Stanley ER, Rohrschneider LR (1991): Fms (CSF-1 receptor) and CSF-1 transcripts and
proteins are expressed by human breast carcinomas in vivo and in vitro. Oncogene 6:941-952.
Kataoka K, Hayakawa T, Yamada K, Mushiroi T, Kuroda R, Mogami H (1989): Neuronalnetwork disturbance after focal ischemia in rats. Stroke 20: 1226-1235.
Lee SC, Liu W, Roth P, Dickson D, Berman JW, Brosnan CF (1993): Macrophage colony
stimulating factor in human fetal astrocytes and microglia . Differential regulation by cytokines
and lipopolysaccharide, and modulation of class ll MHC on microglia. J Immunol 150:594-604.
Leiserowik GS, Harris SA, Subramaniam M, Keeney GL, Podratz KC, Spelsberg TC (1993):
The proto-oncogene c-fms is overexpressed in endometrial cancer. Gynecol Oncol 4 9: 190-
196.
Liu W, Brosnan CF, Dickson DW, Lee SC (1994): Macrophage colony stimulating factors
20 mediates astrocyte-induced microglial ramification in human fetal central nervous system
cultures. Am J Pathol 145:48-53.
Mallat M, Chamak B, Thery C, Leroy D (1989): Microglial cell functions during brain
dcvelopi"ent. Hormones Cell Regen 198:71-75.
Mehler MF and Kessler JA (1995): Cytokines in neural differentiation. Critical Rev Neurobiol
9:419-446.
Michaelson MD, Bieri PL, Mehler MF, Xu H, Arezo JC, Pollard JW, Kessler JA (1996): CSF-1
30 deficiency in mice results in abnormal brain development. Development 122:2661-2672.
Morioka T, Kaleuhua AN, Streit WJ. (1993): Characterization of microglial reaction after middle
cerebral artery occlusion in rat brain. J Comp Neurol 327: 123-132.
Mundy GR (1993): Cytokines and growth factors in the regulation of bone remodeling. J Bone
Mineral Res 8. Suppl 2:9505-9510.
Nagasawa H and Kogure K (1989): Correlation between cerebral blood flow and histological
changes in a new rat model of middle cerebral artery occlusion. Stroke 20:1037-1043.
39
CA 02213906 1997-08-2~
Nagasawa H and Kogure K (1990): Exo-focal postischemic neuronal death in the rat brain.
Stroke 21 :790-794.
Nohava K, Malipiero U, Frei K, Fontana A (1992): Neurons and neu,oblastoma as a source of
macrophage colony-stimulating factor. Eur J Immunol 22:2539-2545.
Onodera H, Kogure K, Ono Y, lgarashi K, Kiyota Y, Nagaoka A (1989): Proto-oncogene c-fos is
transiently induced in the rat cerebral cortex after forebrain ischemia. Neurosci Lett 98:101-104.
Pollard JW (1997): Role of colony-stimulating factor-1 in reproduction and development. Mol
Reprod Devel 46:54-61.
Pollard JW, Bartocci A, Arceci R, Orlofsky A, Lander MB, Stanley ER (1987): Apparent role of
the macrophage growth factor, CSF-1, in placental development. Nature 330:484-486.
Raivich G, Gehrmann J, Kreutzberg GW (1991): Increase of macrophage colony-stimulating
factor and granulocyte-macrophage colony-stimulating factor receptors in the regenerating rat
facial nucleus. J Neurosci Res 30:682-686.
Roberts M, Shapiro LH, Ashmun RA, Look T (1992): Transcription of the human colony-
stimulating factor -1 receptor gene is regulated by separate tissue-specific promoters. Blood 79:
586-593.
Roth P and Stanley ER (1992): The biology of CSF-1 and its receptor. Cur Top Microbiol
Immunol 18:141-167.
Rothwell NJ and Hopkins SJ (1995): Cytokines and the nervous system ll: actions and
mechanisms of action. TINS 18:130-136.
Rothwell VM, and Rohrschneider LR (1987): Murine c-fms cDNA: cloning, sequence analysis
and retroviral expression. Oncogene Res 1 :311-324.
Roussel MF (1994 ): Signal transduction by the macrophage colony stimulating factar receptor
(CSF-1 R). J. Cell Sci Suppl 18: 105-108.
Sawada M, Itoh Y, Suzumura A, Marunouchi T (1993): Expression of cytokine receptors in
cultured neuronal and glial cells. Neurosci Lett 160:131-134.
CA 02213906 1997-08-2~
Sawada M, Suzumura A, Marunouchi T (1995): Cytokine network in the central nervous system
and its roles in growth and differentiation of glial and neuronal cells. Int J Devl Neurosci 13:253-
264.
Sawada M, Suzumura A, Yamamoto H, Marunouchi T (1990) Activation and proliferation of the
isolated microglia by colony stimulating factor-1 and possible involvement of protein kinase C.
Brain Res 509: 119-124.
o Scholl SM, Bascou CH, Mosseri V, Olivares R, Magdelenat HG, Dorval T, Palangie T, Validire
P, Pouillart P, Stanley ER (1994): Circulating levels of colony stimulating factor-1 as a
prognostic indicator in 82 patients with epithelial ovarian cancer. Br J Cancer 69:342-346.
Shafit-Zagardo B, Sharma N, Berman JW, Bornstein MB, Brosman CF (1993): CSF-1
ex~r~ssion is upregulated in astrocyte cultures by IL-1 and TNF and affects microglial
proliferation and morphology in organotypic cultures. Internatl J Devl Neurosci 11:189-198.
Stanley E.R (1994): Colony stimulating factor-1 (Macrophage colony-stimulating factor). In
Thompson A (ed): The Cytokine Handbook, 2nd ed. San Diego: Academic Press, pp 382-418.
Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmer SH (1983): CSF-1, a mononuclear phagocyte
lineage-specific hematopoietic growth factor. J Cell Biochem 21:151-159.
Suzumura A, Sawada M, Yamamoto H, Marunouchi T (1990): Effects of colony stimulating
factors on isolated microglia in vitro. J Neuroimmunol 30:111-120.
Tada M, Diserens AC, Desbaillets 1, deTribolet N (1994): Analysis of cytokine receptor
messenger RNA expression in human glioblastoma cells and normal astrocytes by reverse-
transcription polymerase chain reaction. J Neurosurg 80:1063-1073.
Tamura A, Graham Dl, McCulloch J, Teasd-'E GM (1981): Focal cerebral ischemia in the rat:
Description of technique and early neuropathological consequences following middle cerebral
artery occlusion. J Cereb Blood Flow Metab 1 :53-60.
Thery C, Hetier E, Evrard C, Mallat M (1990): Expression of macrophage colony stimulating
gene in the mouse brain during development J Neurosci Res 26:129-133.
Thery C, Stanley ER, Mallat M (1992): Interleukin 1 and tumor necrosis factor-a stimulate the
production of colony-stimulating factor 1 by murine astrocytes. J Neurochem 59:1183-1186.
CA 02213906 1997-08-2~
Tkachuk M and Gisler RH (1997): The promotor of macrophage colony stimulating factor
receptor is active in astrocytes. Neurosci Lett 225:121-125.
Troutt AB, Lee F (1989): Tissue distribution of murine hemopoietic growth factor mRNA
production. JCell Physiol 138:38-44.
Uemura Y, Kowall NW, Moskowitz MA (1991): Focal ischemia in rats causes time-dependent
expression of c-fos protein immunoreactivity in widespread regions of ipsilateral cortex. Brain
lo Res 552:99-105.
Verge VMK, Richardson PM, Wiesenfeld-Hallin Z, Hokfelt T (1995): Differential influence of
nerve growth factor on neuropeptide expression in vivo - a novel role in peptide suppression. J
Neurosci 15:2081-2096.
Wesselingh SL, Gough NM, Finlay-Jones JJ, McDonald PJ (1990): Detection of cytokine mRNA
in astrocyte cultures using the polymerase chain reaction. Lymphokine Res 9:177-183.
Yamamoto K, Akai F. Yoshimine T, Yanagihara T (1987): Immunochemical investigation of
20 cerebral ischemia after middle cerebral artery occlusion in gerbils. J Neurosurg 67:414-420.
Yue X, Favot P, Dunn TL, Cassady Al, Hume DA (1993): Expression of mRNA encoding in
macrophage colony-stimulating factor receptor (c-fms) is controlled by a constitutive promoter
and tissue-specific transcription elongation. Mol Cell Biol 13:3191-3201.
42