Note: Descriptions are shown in the official language in which they were submitted.
CA 02214049 1997-08-27
WO 96/26897 PCT/FR96/00244
Plant for feedinq hYdroqen peroxide to a processinq
unit on an industrial site.
The present invention relates to a plant for
feeding hydrogen peroxide to a processing unit on an
industrial site.
It applies in particular to the feed of
hydrogen peroxide to a unit for bleaching paper pulp on
a paper production site.
Many industrial sites consume large quantities
of hydrogen peroxide, in particular paper pulp
production sites. In practice ~he hydrogen peroxide
employed is always delivered to the processing unit in
the form of a concentrated aqueous solution, for
example of 50 to 70 ~ by weight, by tanker or by
container. This method of supplying hydrogen peroxide
has numerous disadvantages, especially sa~ety problems
linked with the many operations of unloading the
concentrated solutions of hydrogen peroxide and costs
associated with conveying it and with diluting it to
the working concentration on the processing unit.
The applicant has envisaged the production of
hydrogen peroxide in less highly concentrated aqueous
solution on the processing unit on the industrial site
itself. However, most o~ the sites operate virtually
continuously throughout the year, o~ten at least 360
days per year and do not permit the interruption of the
supply of hydrogen peroxide. Such an interruption is
liable to take place, for example during the operations
of maintenance of the hydrogen peroxide production
plant.
The aim of the invention is to permit an
uninterrupted feeding of the processing unit with
hydrogen peroxide while allowing the usual maintenance
operations of the feeding plant.
To this end the subject-matter of the invention
is a plant for feeding hydrogen peroxide to a
processing unit on an industrial site, characterized in
that it includes a main plant for producing hydrogen
peroxide at a working concentration, arranged on the
CA 02214049 1997-08-27
.
WO 96/26897 2 PCT/FR96/00244
actual site o~ the processing unit, and a backup plant
intended to feed the processing unit in the event of
stoppage of the main plant, comprising a backup reserve
of hydrogen peroxide at a storage concentration higher
than the working concentration and means for diluting
the stored hydrogen peroxide which are connected
between the reserve and the processing unit and which
allow the concentration of stored hydrogen peroxide to
be reduced to the working concentration.
10According to other characteristics of the
nventlon: _
- the backup plant additionally comprises means
for in~ecting a stabilizer into the dilute hydrogen
peroxide;
15- the working concentration of the hydrogen
peroxide is between 5 and 15 ~ by weight;
- the concentration of the hydrogen peroxide in
the reserve is between 50 and 70 ~ by weight;
- the weight of the volume of the hydrogen
20peroxide in the reserve is between 100 and 200 metric
tons;
- the main production plant is of the type
using an anthraquinone process ~or the production o~
hydrogen peroxide;
25- the processing unit is a unit for bleaching
paper pulp on a paper production site.
The invention will be understood better on
reading the description which is to follow and which is
given solely by way of example, for the understanding
of which re~erence will be made to the drawings, in
which:
- Figure 1 is a synoptic diagram of a plant for
feeding hydrogen peroxide according to the invention;
- Figure 2 is a diagrammatic view of a main
plant for the production of hydrogen peroxide,
integrated into the plant of Figure 1, according to a
preferred embodiment.
CA 022l4049 l997-08-27
WO 96/26897 3 PCT/FR96/00244
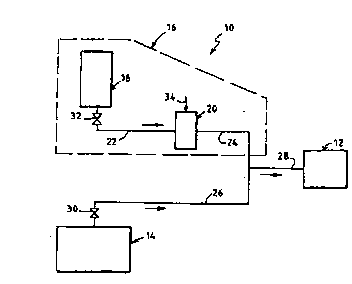
A plant 10 ~or ~eeding hydrogen peroxide to a
processing unit 12 on an industrial site has been shown
in Figure 1.
This processing unit 12 is, ~or example, a unit
~or bleaching paper pulp o~ a paper production plant or
site.
The plant 10 includes a main plant 14 for
producing hydrogen peroxide arranged on the actual site
o~ the processing unit 12.
The main plant 14 iS interLded to produce
hydrogen peroxide at a relatively low working
concentration which is preEerably between 5 and 15 ~ by
weight.
The plant 10 for ~eeding hydrogen peroxide also
comprises a backup plant 16 intended to ~eed the
processing unit 12 in the event o~ stoppage o~ the main
plant 14.
The backup plant 16 comprises a reserve oi~
backup hydrogen peroxide 18 and means 20 for diluting
stored hydrogen peroxide oE known type. These means oi~
dilution 20 are connected between the reserve 18 and
the processing unit 12 through the intermediacy o~ an
upstream conduit 22 and a downstream conduit 24.
The hydrogen peroxide stored in the reserve 18
iS o~ the type usually supplied on the market. The
hydrogen peroxide in the reserve has a concentration
that is higher than the working concentration,
pre~erably between 50 and 70 ~ by weight, and a volume
the weight oi~ which is pre~erably between 100 and 200
metric tons, so as to make it possible to supply, ~or
example, a conventional unit ~or bleaching paper pulp
~or approximately one week.
The main plant 14 iS connected to the
processing plant 12 through the intermediacy o~ a
conduit 26.
The conduit 24 downstream oE the reserve 18 and
the conduit 26 downstream o~ the plant 14 are connected
to the same conduit 28 Eor i~eeding the processing unit
12.
CA 02214049 1997-08-27
WO 96/26897 4 PCT/FR96/00244
Valves 30, 32 make it possible to isolate,
respectively, the main plant 14 and the backup plant 18
from the processing unit 12.
The general flow direction of the fluids is
shown by arrows in Figure 1
The operation of the plant for feeding hydrogen
peroxide according to the invention can be implemented
according to the manner described below.
Hydrogen peroxide has [sic] the desired working
concentration is usually supplied directly to the
processing unit by the main production plant 14. IP
this case the latter plant is connected to the
processing unit 12 by opening the valve 30 while the
reserve 18 is isolated from the processing unit 12 by
closing the valve 32.
When the main plant 14 is stopped, especially
~or performing maintenance operations thereon, this
plant is isolated from the processing unit 32 [sic] by
closing the valve 30, the feeding of hydrogen peroxide
to the unit 12 being done by the reserve 18 after
opening the valve 32.
The concentration of the stored hydrogen
peroxide is reduced by virtue of the means o~ dilution
20, to obtain . the working concentration normally
supplied by the main production plant 14.
The backup plant 16 preferably comprises means
34 for injecting into the dilute hydrogen peroxide a
stabilizer o~ a known type, chosen, for example, from
carboxylic, aminomethylenecarboxylic, phosphonic and
aminomethylenephosphonic compounds, in particular
ethylenediaminetetraacetic acid (EDTA) or its salts,
diethylenetriaminepentaacetic acid (DTPA) or its salts,
polyacrylic acids, diethylenetriaminepentamethylene-
phosphonic acid or its salts, 1,2-diamino-N,N,N'N'-
tetramethylenephosphonic acid or its salts and hydroxy-
ethylbismethylenephosphonic acid or its salts.
The stabilizer makes it possible to avoid the
decomposition of the hydrogen peroxide to a relatively
CA 02214049 1997-08-27
WO 96/26897 5 PCT/FR96/00244
- low concentration until the injection of this hydrogen
peroxide into the processing unit 12.
The main plant 14 for the production of
hydrogen peroxide is preferably of the type employing
an anthraquinone process. A plant of this type is shown
in Figure 2.
The production plant 14 in Figure 2 includes
three main units of equipment in the form of columns: a
catalytic hydrogenator 101, a countercurrent oxidizer
102 and a water extractor 103. It also comprises many
item~s of equipment associated with these three units of
equipment, only some of which have been shown: a
supercharger-condenser unit 104 for recirculating the
gaseous mixture containing hydrogen, associated with
the hydrogenator; a filter 106, a pump 107, a heat
exchanger 108, a water cooler 109, a head condenser 110
and an air compressor 111 which are associated with the
oxidizer; a conduit 112, as short as possible,
connecting the base of the oxidizer 102 to that of the
extractor 103; and a coalescer I14 and a pump 115 for
recycling the working solution.
Also shown in Figure 2 iS a conduit 116 for
feeding the hydrogenator with make-up hydrogen, a
conduit 117 for feeding air to the compressor 111, a
conduit 118 for feeding the extractor 103 with
demineralized water, a hydrogen peroxide output conduit
119, which starts from the base of the extractor 103
and ends at the processing unit 12 which consumes
hydrogen peroxide at the same concentration, and a
conduit 121 for recycling the working solution. The
conduit 119 is connected to the conduit 26.
The plant for producing hydrogen peroxide
includes many items of equipment which are well known
in the art and are not shown, such as means for
regenerating decomposed products of the working
solution, for using the catalyst, for recovering
solvent, and the like.
In operation, the working solution, consisting
of at least one anthraquinone derivative and at least
=
CA 02214049 1997-08-27
Wo 96/26897 6 PCT/FR96/00244
one organic solvent, is introduced at the base of the
hydrogenator 101 via the recycling conduit 121
connected to the delivery of the pump 115, and a stream
of gas containing hydrogen is also introduced at the
base of the hydrogenator. This gas stream consists, on
the one hand, of the gas stream drawn off at the top of
the hydrogenator, recirculated by the supercharger-
condenser unit 104 and, on the other hand, of make-up
hydrogen delivered via the conduit 116.
The working solution is thus partially reduced.
The reduced solution--drawn ~rom-~ ~She base of the
hydrogenator by the pump 107 via the filter 106
therefore contains hydroquinone derivatives (for
example 80 ~ o~ tetrahydroanthrahydroquinone and 20
of anthrahydroquinone).
The anthraquinone derivative constituent of the
working solution is preferably chosen from 2-alkyl-
9,10-anthraquinones in which the alkyl substituent
contains from 1 to 5 carbon atoms, such as the methyl,
ethyl, sec-butyl, tert-butyl and tert-amyl radicals, as
well as the corresponding 5,6,7,8-tetrahydro deriva-
tives, or from 9,10-dialkylanthraquinones in which the
alkyl substituents, which are identical or dif~erent,
contain from 1 to 5 carbon atoms, such as the methyl,
ethyl, tert-butyl radicals, for example 1,3-dimethyl,
1,4-dimethyl, 2,3-dimethyl, 2,7-dimethyl, 1,3-diethyl,
2,7-di-tert-butyl, 2-ethyl-6-tert-butyl and the
corresponding 5,6,7,8-tetrahydro derivatives. The
organic solvent constituent of the working solution is
preferably a mixture o~ a nonpolar compound and of a
polar compound. The nonpolar compound is preferably
chosen from petroleum cuts with a boiling point higher
than 140~C containing predominantly aromatic
hydrocarbons containing at least 9 carbon atoms, such
as trimethylbenzene isomers, tetramethylbenzene
isomers, tert-butylbenzene, methylnaphthalene isomers
and dimethylnaphthalene isomers. The polar compound is
preferably chosen from saturated alcohols preferably
containing ~rom 7 to 11 carbon atoms, such as
CA 02214049 1997-08-27
.
WO 96/26897 7 PCT/FR96/00244
- diisobutylcarbinol, 3,5,5-trimethylhexanol, isohep-
tanol, carboxylic acid esters such as methylcyclohexyl
acetate marketed under the name o~ "Sextate", heptyl
acetate, butyl benzoate, ethyl heptanoate, phosphoric
acid esters such as tributyl phosphate, tri(2-
ethylbutyl) phosphate, tri(2-ethylhexyl) phosphate,
tri(n-octyl) phosphate, and tetrasubstituted ureas such
as tetra-n-butylurea.
"Hydrogen peroxide equivalent/' will hereina~ter
be understood to mean the quantity o~ hydrogen
peroxide, expressed in grams, which one liter o~
~ working solution is capable o~ supplying at the end o~
the oxidation stage i~ the yield o~ this stage in the
oxidizer 102 is 100 ~. This potential mass
concentration o~ peroxide corresponds to a molar
concentration which is equal to the molar concentration
o~ all the reoxidizable anthrahydroquinone ~orms in the
working solution. It depends, on the one hand, on the
concentration o~ anthraquinone ~orms o~ the working
solution at the start and, on the other hand, on the
hydrogenation conditions in 101.
In the present case the hydrogenation is
per~ormed at a temperature o~ between 50 and 70~C, with
a pressure in the gas headspace o~ the hydrogenator
(pressure which controls the ~low rate o~ hydrogen)
approximately ~rom 0.8 to 1.5 bar and the hydrogen
peroxide equivalent is adjusted to a value o~
approximately between 7 and 9 g/l, by adjusting the
residence time in the hydrogenator ~or a given
concentration o~ anthraquinone ~orms.
The reduced working solution drawn ~rom the
hydrogenator is ~iltered at 106 to remove any trace o~
catalyst and then cooled in 108 and then in 109 to a
temperature o~ the order o~ 35 to 40~C. The pressure in
the gas headspace o~ the oxidizer is maintained at a
value o~ between 2 and 4 bars. The reduced working
solution is thus oxidized in 102, the head ~luid of the
oxidizer being partially condensed in 110.
CA 02214049 1997-08-27
wo 96/26897 8 PCT/FR96/00244
- The hydrogen peroxide ~ormed by the oxidation
reaction is drawn ~rom the base o~ the oxidizer in a
quantity equal to the product o~ the abovementioned
hydrogen peroxide equivalent times the yield o~ the
oxidizer, as a mixture with the working solution which
is oxidized again. This liquid is conveyed directly via
the conduit 112, by virtue o~ the propelling pressure
dif~erence to the base o~ the extractor 103, which
operates slightly above the atmospheric pressure. A
liquid-liquid extraction is per~ormed in the extractor
by means o~ the demineralized water introduced at the
top of the extractor.
A water-hydrogen peroxide solution is drawn
~rom the base o~ the latter, and its hydrogen peroxide
concentration is adjusted to the value necessary ~or
its direct use in the processing unit 12.
The working solution separated ~rom the
hydrogen peroxide is drawn ~rom the top o~ the
extractor 103, ~reed ~rom the droplets o~ aqueous phase
which it has entrained in the coalescer 114, and then
conveyed by the pump 115 to the heat exchanger 108, in
which it is warmed up and, ~rom there, recycled to the
base o~ the hydrogenator 101.
The oxidizer 102 comprises an outer enclosure
containing an organized packing or simple per~orated
trays or trays o~ the distillation tray type, that is
to say each with a liquid seal, ori~ices ~or bubbling
gas rising through this seal and liquid downcomers ~rom
one tray to the next, or else a combination o~ an
organized packing and o~ such trays.
The invention comprises numerous advantages.
The plant according to the invention makes it
possible to ~eed a processing unit with hydrogen
peroxide without interruption, while permitting plant
maintenance operations. Thus, when the main plant ~or
the production of hydrogen peroxide is stopped, the
backup plant ~eeds the processing unit instead o~ the
main plant.
CA 02214049 1997-08-27
WO 96/26897 9 P~T/FR96/00244
The dimensions of the reserve 18 are relatively
small, given the high concentration of the hydrogen
peroxide stored therein.
The plant according to the invention makes it
possible to circumvent the disadvantages due to the
supply of hydrogen peroxide in the form of concentrated
aqueous solution, in particular the risks associated
with the unloading operations.