Note: Descriptions are shown in the official language in which they were submitted.
CA 02214996 1997-09-09
TITLE OF THE INVENTION
PROCESS FOR PRODUCING FORMED PRODUCT OF DEPROTEINIZED NATURAL
RUBBER LATEX AND DEPROTEINIZING AGENT FOR NATURAL RUBBER LATEX
BACKGROUND OF THE INVENTION
This invention relates to a deproteinizing agent for
natural rubber latex which is used for producing a rubber product
derived from natural rubber latex and decreased in content of
allergy inducer to a level sufficient to keep it from being
harmful to the human body, such as a surgical glove, a catheter,
a condom, a foam rubber product or the like, as well as a method
for producing a formed product of deproteinized natural rubber
latex suitable for production of the above-described rubber
product derived from natural rubber latex.
Natural rubber latex is commonly used as a material for
a foam product such as a foam rubber product, a dipped product
such as a glove, a condom or a catheter, a pressure-sensitive
adhesive, an adhesive, and the like on an industrial scale. The
natural rubber latex is obtained in the form of sap of a gum
tree such as hevea brasiliensis or the like cultivated in a
plantation and contains a rubber content in an amount of about
30%, as well as a non-rubber content including protein, fatty
acids, polysaccharides, minerals and the like. The non-rubber
content is contained in an amount of several percent. The
natural rubber latex is called field latex. In order to permit
the field latex to be used for an industrial material, it is
required to be purified. For this purpose, the ffield latex is
purified while being condensed to a degree sufficient to have a
rubber content to be as high as about 60~ and ammonia is added to
the thus condensed and purified latex in an mount of 0.2 to 0.7%
based on the latex in order to prevent putref action or rotting of
the latex.
Such condensation and purification of the ffield latex
may be carried out, for example, by creaming or centrifuging. In
general, centrifuging is predominantly used to this end because
it accomplishes purification of the field latex with increased
efficiency. The centrifugal procedure permits purified natural
rubber latex which is decreased in protein content to a level as
- 1 -
CA 02214996 1997-09-09
low as about 2% to 3% by weight to be provided. About half of
protein left in the purified natural rubber latex is water-
extractable, to thereby act as a protective colloid in the latex,
resulting in contributing to stabilization of the latex. The
remaining protein is chemically bonded to rubber particles, to
thereby be rendered hydrophobic. The hydrophobic protein bonded
to the rubber particles permits the rubber particles to be
stabilized in water and is readily oxidized to prevent oxidation
and deterioration of the rubber.
In recent years, it is reported that use of medical
supplies made of natural rubber such as a surgical glove and the
like causes immediate hypersensitivity, to thereby give rise to a
problem. The immediate hypersensitivity includes Type IV allergy
represented by urticaria and Type I allergy causing dyspnea or
anaphylaxis. It is conffirmed that Type IV allergy is induced by
a vulcanization accelerator blended in unvulcanized natural
rubber and Type I allergy is induced by protein contained in the
rubber.
Such allergies are caused when a patient having an
antibody produced in the body due to contact with an allergy
inducing material (hereinafter referred to as "allergen") such as
protein or the like contained in natural rubber contacts with the
allergen again. Thus, there would be likelihood that many latent
patients are found in people commonly using a natural rubber
product containing the allergen. For example, it is reported
that an incident rate at which the medical interest commonly
using a surgical glove or an examination glove made of natural
rubber takes an allergic disease is increased to a level as high
as 10%. The Food and Drug Administration (FDA) appeals
manufacturers of such a natural rubber product to reduce a
content of protein in a natural rubber material to be used.
Thus, it will be noted that generation of allergies due to use of
a natural rubber product gives rise to a serious social problem.
Although it is thus indicated that natural rubber gives
rise to the above-described problem, natural rubber is
significantly advantageous in that it is decreased in cost,
exhibits increased toughness and permits a product made of
- 2 -
m
CA 02214996 2004-04-27
7537-8
natural rubber to exhibit satisfactory workability and
fittingness. Unfortunately, there has not been found any
substitute for natural rubber exhibiting such excellent
properties. Thus, it is highly desirable to develop natural
rubber which is decreased in allergen content to a level
sufficient to keep it form being harmful to the human body.
Conventionally, a decrease in content of protein, which
is a major allergen, in natural rubber is carried out by cleaning
natural rubber latex or a natural rubber product with hot water
or dipping it in a cleaning tank for~~a suitable length of time.
Alternatively, it is carried out by subjecting a natural rubber
product to a surface treatment using chemicals such as chlorine
or the like. Unfortunately, such a'procedure fails to remove the
allergen from natural rubber 'to a degree sufficient to
significantly reduce generation of allergies.
Also, techniques of providing deproteinized natural
rubber latex using protease and a surf actant are proposed as
disclosed in Japanese Patent Application Laid-Open Publications
Nos. 56902/199 (6-56902), 56903/199 (6-56903), 5690~+/199~ (6-
?0 56904), 56905/1994 (6-56905) and 56906/1991 (6-56906).
The techniques disclosed accomplish removal of protein
from natural rubber to a certain degree, however, it encounters
another problem due to a surf actant used for removal of protein.
The surfactant exhibits an important function for stabilization
of the latex and cleaning of the protein. However, when it is
left in an excess amount in natural rubber during a forming step,
it deteriorates film forming properties of the rubber
and decreases strength of a formed product. In particular, it is
known in the art that a deterioration in film forming properties of the
30 rubber appears in straight dip forming practiced for formation of
a thin film such as a condom i~r the like or when an anionic
surfactant is used as the surf octant. Also, when the surfactant
i s a eft in a formed product of natural rubber ; the surfactant i.tsel f
deteriorates safety of the product.
I~'urther, the techniques disclosed require both operation
f~aae d ~ 1 ut i n~, natural rubber with water and operation for
cc~ncentrai:ing it by centrifuging or the like, to thereby cause an
CA 02214996 1997-09-09
increase in the number of steps, leading to a decrease in yields
of deproteinized natural rubber latex and a deterioration in
qual ity thereof .
SUMMARY OF THE INVENTION
The present invention has been made in view of the
foregoing disadvantage of the prior art.
Accordingly, it is an object of the present invention to
provide a method for producing a formed product of deproteinized
natural rubber latex which is capable of reducing a content of
allergen in natural rubber to a level sufficient to permit the
natural rubber to be harmless to the human body while keeping
yields of the product and a quality thereof from being reduced or
deteriorated.
It is another object of the present invention to provide
a deproteinizing agent for natural rubber latex which is capable
of being suitably used for production of a formed product of
deproteinized natural rubber latex which is intended or desired
to be decreased in allergen content thereof to a degree
sufficient to keep the product from being harmful to the human
body.
In accordance with the present invention, a method for
producing a formed product of deproteinized natural rubber latex
is provided. In a first aspect of the present invention, the
method includes a protein decomposition step of adding protease,
a surfactant and water to natural -rubber latex to decompose
protein contained in the natural rubber latex, a prevulcanization
step of subjecting the natural rubber to prevulcanization, a
forming step of subjecting the natural rubber to forming, and a
cleaning removal step of removing a non-rubber content from the
natural rubber latex using extracting cleaning liquid. In a
preferred embodiment of this aspect of the present invention, a
postvulcanization step of subjecting the natural rubber to
postvulcanization may be carried out after the cleaning removal
step.
In accordance with a second aspect of the present
invention, the method further includes a mechanical removal step
of mechanically removing an impurity from the natural rubber,
- 4 -
CA 02214996 1997-09-09
which is incorporated between the protein decomposition step and
the prevulcanization step.
In accordance with a third aspect of the present
invention, the method further includes a mechanical removal step
of mechanically removing an impurity from the natural rubber,
which is incorporated between the prevulcanization step and the
forming step.
In each of the first to third aspects of the method of
the present invention, the extracting cleaning liquid used in the
cleaning removal step may be prepared in a specified manner.
Also, in accordance with the present invention, a
deproteinizing agent for natural rubber latex is provided, which
constitutes a fourth aspect of the present invention. The
deproteinizing agent contains a protease and a nonionic
surfactant in which LD 50 is 5000 mg/kg or more.
Now, the present invention will be described hereinafter
in connection with the first to fourth aspects described above in
order. The natural rubber latex employed in each of the first to
fourth aspects is not limited to any specif is one. Thus, any
suitable natural rubber latex such as high-ammonia natural rubber
latex, low-ammonia natural rubber latex or the like which is
commercially available may be used in the present invention.
First, the method for producing the formed product of
deproteinized natural rubber latex according to the first aspect
of the present invention will be described hereinafter.
The first step or protein decomposition step is executed
after natural rubber latex which is a material for the formed
product is charged in any suitable reaction vessel.
The first step is to add a protease and a surfactant to
natural rubber latex to decompose protein contained in the
natural rubber latex. In the first step, the protein is
decomposed into low-molecular weight substances by an action of
the protease, so that the protein which has been bound to or
adsorbed on rubber particles may be readily transferred to an
aqueous phase. The rubber particles have been stably dispersed in the
presence of the protein. The surfactant keeps rubber particles stable and
prevents the rubber particles from a coagulation after the removal of
_ 5 _ .
CA 02214996 1997-09-09
protein.
The protease used in the first step may be the same as
that used for the deproteinizing agent defined in the fourth
aspect of the present invention briefly described above and
detailedly described hereinafter.
The protease is used to ensure satisfactory
decomposition of protein contained in the natural rubber latex.
For this purpose, it may be used in an amount of 0.0005 to 5.0
parts by weight based on 100 parts by weight of the solid content
of the natural rubber latex, preferably 0.001 to 1.0 parts by
weight and more preferably 0.01 to 0.1 parts by weight.
The surfactant used in the present invention may be
selected from the group consisting of (a) an anionic surfactant,
(b) a nonionic surfactant, (c) an amphoteric surfactant and any
combination of the surfactants (a) to (c).
(a) Anionic surfactant
The anionic surfactants include a carboxylic type
surfactant, a sulfonic type surfactant, a sulfate type
surfactant, a phosphate type surfactant and the like.
The carboxylic type surfactants may include salts of
fatty acid, polyvalent carboxylates, polycarboxylates,
rosinates, salts of dimer acid, salts of polymeric acid, salts of
tall oil fatty acid, polyoxyalkylene alkylether acetates,
polyoxyalkylene alkylamide ether acetates and the like.
The sulfonic type surf actants may include alkylbenzene
sulfonates, alkylsulfonates, alkylnaphtalene sulf onates,
naphthalene sulfonates, naphthalene sulfonic aldehyde
condensates, arylsunfonic aldehyde condensates,
alkyldiphenylether disulfonates, dialkylsulfosuccinates, p(-olefin
sulfonates and the like.
The sulfate type surfactants may include alkyl sulfates,
polyoxyalkylene alkyl sulfates, polyoxyalkylene alkyl phenylether
sulfates, mono-, di- or tri-styrylphenyl Sulfonates,
polyoxyalkylene mono-, di- or tri-styrylphenyl sulfates and the
like.
The phosphate type surfactants may include alkyl
- 6 -
CA 02214996 1997-09-09
phosphates, alkylphenol phosphates, polyoxyalkylene alkylether
phosphates, polyoxyalkylene alkylphenylether phosphates,
polyoxyalkylene mono-, di- or tri-styrylphenyl ether phosphates
and the like.
Salts of the surfactants described above may include
metal salts thereof (their salts of Na, K, Ca, Mg, Zn and the
like), ammonium salts thereof, alkanol salts thereof (triethanol
amine salts thereof and the like), and the like.
(b) Nonionic surfactant
The nonionic surfactants may include a polyoxyalkylene
ether surfactant, a polyoxyalkylene ester surfactant, a
polyhydric alcohol fatty acid ester surfactant, a suger fatty acid ester
surfactant, an alkylpolyglycoside surfactant and the like.
The polyoxyalkylene ether surfactants may include
polyoxyalkylene alkylether, polyoxyalkylene alkylphenylether,
polyoxyalkylenepolyol alkylether, polyoxyalkylene mono-, di- or
tri-styryl phenylether and the like. The polyols described above
may include polyhydric alcohols having 2 to 12 carbon atoms such
as propylene glycol, glycerin, sorbitol, glucose, sucrose,
pentaerythritol, sorbitan and the like.
The polyoxyalkylene ester surfactants may include
polyoxyalkylene fatty acid ester, polyoxyalkylene alkylrosinate and
the like.
The polyhydric fatty acid ester surfactants may include fatty
acid esters of polyhydric alcohol having 2 to 12 carbon atoms, fatty acid
esters of polyoxyalkylene polyhydric alcohol and the like. More
specifically, the surfactants may include sorbitol fatty acid ester,
sorbitan fatty acid ester, glycerin fatty acid ester, polyglycerin fatty
acid ester, pentaerythritol fatty acid ester and the like, as well as
polyalkyleneoxide adducts thereof such as, for example, polyoxyalkylene
sorbitan fatty acid ester, polyoxyalkylene glycerin fatty acid ester and
the 1 i ke.
The suger fatty acid ester surfactants may include fatty acid
esters of sucrose, glucose, maltose, fructose, polysaccharide and
the like, as well as polyalkyleneoxide adducts thereof.
The alkylpolyglycoside surfactants may include
alkylglucoside, alkylpolyglucoside, polyoxyalkylene
-
CA 02214996 1997-09-09
alkylglucoside, polyoxyalkylene alkylpolyglucoside and the like
which posses glycoside in the form of glucose, maltose, fructose,
sucrose and the like, as well as fatty acid esters thereof. Also, the
surfactants may include polyalkyleneoxide adducts as well.
Further, in addition~to the above, polyoxyalkylene
alkylamine, alkylalkanol amide and the like may be used for this
purpose.
The alkyl groups contained in the nonionic surfactants
described above may include, for example, straight-chain or
branched saturated or unsaturated alkyl groups having 4 to 30
carbon atoms. Also, the polyalkylene groups described above may
include those having alkylene groups having 2 to 4 carbon atoms
such as, for example, ethylene oxide of which the number of moles
added is about l to 50. Also, the fatty acids described above
may include, for example, straight-chain or branched saturated or
unsaturated fatty acids having 4 to 30 carbon atoms.
(c) Amphoteric surfactant
The amphoteric surf actants may include an amino acid
type surfactant, a betaine type surfactant, an imidazoline type
surfactant, an amineoxide type surfactants and the like.
The amino acid type surfactants may include salts of
acylamino acid, salts of acylsarcosine, acyloylmethyl
aminopropionates, alkylamino propionates, acylamidoethyl
aminohydroxyethylmethyl carboxylates and the like.
The betaine type surfactants may include alkyldimethyl
betaine, alkylhydroxyethyl betaine, acylamidopropyl hydroxypropyl
ammoniosulfobetaine, amidopropyl dimethylcarboxymethyl
ammoniobetaine ricinoleates and the like.
The imidazoline type surfactants may include
alkylcarboxymethyl hydroxyethyl imidazolinium betaine,
alkylethoxy carboxymethyl carboxymethyl imidazolinium betaine and
the like.
The amineoxide type surfactants may include
alkyldimethyl amineoxide and the like.
In order to ensure that the surfactant exhibits the
above-described function and maintains the strength of a product,
it is preferably used in an amount of 0.1 to 10 parts
_ g _
CA 02214996 1997-09-09
by weights based on 100 parts by weights of the solid content of
the natural rubber latex and more preferably 0.5 to 5 parts by
weight.
In the first step, an excessive increase or decrease in
concentration of the solid content of the natural rubber latex
fails to ensure smooth progress of a decomposition reaction of
the protein. In order to avoid the disadvantage, the natural
rubber latex is preferably selectively diluted or concentrated
with water so as to keep the solid content of the natural rubber
latex at a concentration within a range of about 10 to 60% by
weight.
Conditions under which the procedure of the first step
takes place are not limited to any specific ones so long as the
conditions promote satisfactory progress of the enzyme
reaction. For example, a smooth decomposition reaction of the
protein may take place at a temperature at about 5 to 90'C and
preferably about 20 to 60'C for about 2 minutes to about 24 hours
while being left to stand or stirred. Although addition of the
surfactant may be carried out either during the decomposition
procedure or thereafter, it is preferably carried out during the
decomposition procedure. Also, prior to the reaction, the enzyme
is preferably adjusted to an optimum pH value by means of a
suitable pH adjustor. In this instance, a dispersing agent may
be used in combination therewith.
In the first step, such a deproteinizing agent as in the
fourth aspect of the present invention detailedly described
hereinafter may be used.
The second step or prevulcanization step is to improve
workability in the forming step subsequent thereto.
In the second step, prevulcanization may takes place
using any suitable techniques known in the art such as, for
example, a sulfur vulcanization system, a non-sulfur
vulcanization system, a peroxide vulcanization system or a
radiation vulcanization system.
Vulcanizing agents may include sulfur, sulfur chloride,
precipitated sulfur, insoluble sulfur, selenium and tellurium, as
well as sulfur-containing organic compounds such as tetramethyl
_ g _
CA 02214996 1997-09-09
thiuram sulfide, tetraethyl thiuram sulfide and the like, organic
peroxides such as benzoyl peroxide, dicumyl peroxide and the
like, and metal oxides such as zinc oxide, magnesium oxide, zinc
peroxide and the like: Also, vulcanization accelerators may
include those known in the art such as aldehyde ammonias,
aldehyde amines, guanidines, thioureas, thiazoles, thiurams,
sulfenamides, dithiocarbamates, xanthates and the like. Also,
any suitable vulcanization supplement accelerator, plasticizer,
curing agent, filler, antioxidant and the like which are known in
the art may be added as required.
Conditions for the prevulcanization are suitably
determined depending on the amount of natural rubber latex and
the like. Normally, the prevulcanization is preferably carried
out at a temperature of about 20 to 60'C for about 0.1 to 24
hours. Radiation vulcanization may take place in a manner known
in the art using a sensitizer such as acrylic ester. In this
instance, intensity of the radiation may be suitably determined
depending on a composition of the natural rubber latex, a forming
manner and the like. Normally, it is preferably about 1.0 to 5
Mrad.
The third step or forming step is to make an
intermediate formed product of the natural rubber latex. Prior
to the third step, aqueous ammonia may be added to the natural
rubber latex in order to stabilize it as required. The forming
step is not limited to any specif is manner and may employ any
suitable techniques known in the art such as, for example,
straight dip forming, casting, extrusion or the like depending on
a form of a product to be formed, applications thereof and the
like.
The fourth step or cleaning removal step is to clean the
formed product with the cleaning liquid to remove the non-rubber
content therefrom.
The cleaning removal step is carried out in order to
remove the non-rubber content from the formed product by
extraction and clean a surf ace of the product. The term "non-
rubber content" used in connection with the cleaning removal step
herein indicates, of a non-rubber ingredient added during
- 10 -
CA 02214996 1997-09-09
production of the product and originally contained in the natural
rubber latex, a part harmful to the human body or unnecessary to
keep satisfactory quality of the product. For example, the non-
rubber contents include the protease, surfactant, vulcanization
accelerator and Proteolysis products.
The cleaning liquid may be at least one selected from
the group consisting of (i) aqueous alkali solution, ammonia,
(ii) water containing free chlorine in an amount of 0.005 to
0.02% by weight and (iii) alcohol-water mixed liquid containing 5
to 80% by weight of alcohol. In the fourth step, it is desirable
that the amount of cleaning liquid used and conditions for the
cleaning are suitably varied depending on a type of the cleaning
liquid. Now, details of the cleaning liquid and conditions for
the cleaning will be described hereinafter.
(i) Aqueous alkali solution, ammonia
An aqueous solution of alkali such as sodium hydroxide,
potassium hydroxide or ammonia which is used for the cleaning
procedure may be 0.1 to 1.0% aqueous NaOH solution, 0.1 to 1.0%
aqueous KOH solution or 0.001 to 1.0% aqueous ammonia solution.
The cleaning liquid may have a silicone emulsion or a surfactant
added thereto in an amount of 0.01 to 1.0% so as to function as a
detackifier. Also, in order to further decrease tackiness of the
product, a fine powder of talk, cornstarch, silica or the like
may be applied in a dry state or in the form of slurry to a
surface of the intermediate formed product. Alternatively, the
surface of the formed product may be subject to a chlorine gas
treatment for this purpose.
The cleaning treatment or procedure is not limited to
any specif is manner so long as it permits the formed product to
be fully contacted with the cleaning liquid. For example, the
cleaning procedure may be carried out by placing the formed
product and cleaning liquid at a weight ratio of about 1:10 to
1000 in a suitable container and leaving them to stand therein
while being stirred as required.
The cleaning treatment using the above-described aqueous
alkali solution or ammonia solution preferably takes place at a
temperature of 20 to 100'C for several minutes to 24 hours.
- 11 -
CA 02214996 1997-09-09
The cleaning step may be repeated twice or more
depending on the amount of the non-rubber content to be removed
and the like. The second-time and subsequent executions of the
cleaning step are preferably at a temperature equal to or higher
than that of the first-time execution. Also, when the cleaning
step is repeated twice or more, a stripping step is carried out
between each two of the executions of the cleaning step. The
stripping step is practiced manually or using any suitable means
such as a rotary brush, pressurized water, compressed air or the
like.
(ii) Water containing 0.005 to 0.02% by weight of free
chlorine:
The amount of free chlorine contained in the cleaning
liquid is 0.005 to 0.02 by weight and preferably 0.005 to 0.01
by weight for such reasons as indicated below.
Such water containing free chlorine may be prepared by
blowing chlorine gas into water or charging hypochlorite in
water. A content of free chlorine in water may be measured by
placing the cleaning liquid in an aqueous solution containing an
excessive amount of potassium iodide to isolate iodine and
subjecting the thus isolated iodine to back titration.
A treatment of natural rubber with chlorine water has
been conventionally carried out in order to enhance lubricating
properties of a natural rubber glove and improve fittingness
thereof. Also, it is known in the art that the treatment
contributes to a decrease in protein constituting an allergen.
However, chlorine water conventionally used for this purpose has
a chlorine content as high as 0.06% by weight or more. Cleaning
of a formed rubber product and extraction of proteolysis products which
are carried out using chlorine water of such an increased
chlorine content render a surface of the formed rubber product
coarse due to chlorination, to thereby cause an increase in
surface area of the product, resulting in the proteolysis products being
extracted at an increased speed during an initial stage of the
cleaning treatment. However, as chlorination advances, the
surf ace of formed rubber product is changed in properties, to
thereby impede molecular motion on the surface or amino acids
- 12 -
CA 02214996 1997-09-09
which are residues of decomposition of the protein are
polymerized by oxidation to form a film on the surface of the
product. This causes insufficient extraction of the
proteolysis products, resulting in a failure in satisfactory cleaning of
the product. On the contrary, the present invention effectively
eliminates such a deterioration in cleaning effect with progress
of chlorination as encountered with the prior art, because the
chlorine content is kept at a level as low as 0.02% by weight or
less.
The cleaning liquid is used in an amount of 10 to 1000g
per gram of the intermediate formed product of natural rubber
latex. The formed latex product is kept dipped in the cleaning
liquid for 1 to 24 hours while being left to stand or stirred,
resulting in being cleaned. The cleaning liquid is kept at a
temperature below a boiling point thereof and normally at 25 to
50°C.
(iii) Alcohol-water mixed liquid containing 5 to 80% by
weight of alcohol:
Alcohol contained in alcohol-water mixed liquid may be
selected from aliphatic alcohol having 1 to 5 carbon atoms and
aliphatic alcohol having 1 to 5 carbon atoms which is replaced
with an alkoxy group having 1 to 2 carbon atoms. More
specifically, the alcohols may include methanol, ethanol, n-
propanol, isopropyl alcohol (IPA), 2-methyl-1-propanol, 2-methyl-
2-propanol, n-butanol, n-pentanol, the above-described alcohols
replaced with a methoxy or ethoxy group, and the like. In
particular, methanol, ethanol, isopropyl alcohol (IPA) and 3-
methyl-3-methoxybytanol (MMBA) are preferably used.
Such alcohol-water mixed liquid has an alcohol content
of 5 to 80 ~ by weight so as to ensure swelling of the formed
product and exhibit a satisfactory cleaning effect. An alcohol
content in the cleaning liquid is preferably within a range
between 10~ by weight and 50% by weight.
The alcohol-water mixed liquid is used at a ratio of 10
to 1000g per gram of the latex formed product. The formed latex
product is kept dipped in the cleaning liquid for 1 to 24 while
being left to stand or stirred, resulting in being cleaned. The
- 13 -
CA 02214996 1997-09-09
cleaning liquid is kept at a temperature below an azeotropic
point thereof and normally at 25 to 50~C.
Such cleaning of the formed natural rubber latex product
with the alcohol-water mixed liquid permits the non-rubber
content to be removed with increased efficiency. Now, the reason
will be considered hereinafter.
In general, it is considered that in cleaning of natural
rubber latex, cleaning liquid (water) leads to swelling of a
formed latex product to a degree Buff icient to facilitate
extraction of a non-rubber content from the product. However,
although cleaning of the product with only water permits the
product to initially swell, it gradually fails to swell with
removal of hydrophilic substances such as hydrophilic protein and
the like, leading to a deterioration in cleaning effect of the
water., On the contrary, use of the alcohol-water mixed liquid as
the cleaning liquid in the present invention permits alcohol
contained in the liquid to exhibit affinity for the formed
product; so that even when such hydrophilic substances as
described above are removed, the affinity permits the formed
latex product to be kept swollen, resulting in the cleaning
liquid continuously exhibiting a stable cleaning function.
The alcohol-water mixed liquid may contain free
chlorine. The chlorine content is preferably 0.005 to 0.02% by
weight and more preferably 0.005 to 0.01 by weight. The
alcohol-water mixed liquid containing free chlorine may be used
for the cleaning treatment in substantially the same manner as
the alcohol-water mixed liquid. When a temperature for the
cleaning is kept at a high level, the chlorine content is
increased; whereas when it is low, the chlorine content is
decreased. This results in the cleaning conditions being
rendered appropriate.
The fifth step or postvulcanization step is to subject
the intermediate formed product from which the non-rubber content
including t he proteolysis products has been removed by the cleaning
treatment described above to a postvulcanization treatment, to
thereby provide a final formed product. When the formed product
which has been subject to the cleaning treatment may take the
- 14 -
CA 02214996 1997-09-09
form of a final formed product desired, the postvulcanization
step may be eliminated.
Conditions employed in postvulcanization step are not
limited to any specific ones. Normally, the postvulcanization
treatment is preferably carried out at a temperature of about 70
to 120~C for about 0.1 to 24 hours.
The first aspect of the present invention eliminates
dilution and concentration steps which are required in the prior
art, to thereby simplify production of the formed natural rubber
latex product, resulting in a period of time required for the
production being significantly reduced and yields of the product
being substantially improved. Also, the first aspect of the
present invention permits an allergen content of the product to
be decreased to a level sufficient to render the product harmless
to the human body.
Now, the method for producing a formed product of
deproteinized natural rubber latex according to the second aspect
of the present invention will be described hereinafter. The
second aspect, as noted from the above, is featured in that the
mechanical removal step for mechanically removing an impurity
from the latex is interposedly incorporated between the protein
decomposition step and the prevulcanization step each described
above in connection with the first aspect of the present
invention. The term "impurity" used in connection with the
second aspect of the present invention has substantially the same
meanings as the term "non-rubber content" indicated above, except
that it is free of the vulcanization accelerator and the like
because the mechanical removal step takes place prior to the
prevulcanization step.
A mechanical removal treatment in the step may be
executed by centrifuging or ultrafiltration. The centrifuging
procedure is carried out in a manner to subject the latex to
centrifuging to obtain a serum (heavy liquid component) and then
concentrate a rubber content contained in the serum to purify it.
In the ultrafiltration procedure, only proteolytics are filtered
out by means of an ultrafilter.
Thus; it will be noted that the second aspect of the
- 15 -
CA 02214996 1997-09-09
present invention permits the natural rubber product to be
further decreased in allergen content.
Now, the method for producing a formed product of
deproteinized natural rubber latex according to the third aspect
of the present invention will be described hereinafter. The
third aspect, as described above, is featured in that the
mechanical removal step for mechanically removing an impurity
from the latex is interposedly incorporated between the
prevulcanization step and the forming step described above in
connection with the first aspect of the present invention. The
term "impurity" used in connection with the third aspect of the
present invention has substantially the same meanings as the term
"non-rubber content" indicated above.
The mechanical removal treatment in the third aspect may
be practiced in substantially the same manner as that in the
second aspect.
Thus, it will be noted that the third aspect of the
present invention likewise permits the formed natural rubber
latex product to be further decreased in allergen content.
Now, the deproteinizing agent for natural rubber latex
(hereinafter referred to as "deproteinizing agent") according to
the fourth aspect of the present invention will be described
hereinafter.
The protease used in the fourth aspect of the present
invention may comprise any suitable protease known in the art.
In particular, an alkali protease is preferably used in the
fourth aspect of the present invention. A source or derivation
of the protease is not limited to any specific one. Thus, the
proteases may include a bacteria-derived protease, a mold-derived
protease, a yeast-derived protease and the like. Of such
proteases, the bacteria-derived is preferably used for this
purpose. In the fourth aspect of the present invention, the
protease may be used in combination with any suitable other'
enzyme such as, for example, cellulase, amylase, lipase, esterase
or the like as required.
The nonionic surfactants having LD 50 of 5000 mg/kg or
more which is suitable for use for the deproteinizing agent may
- 16 -
CA 02214996 1997-09-09
include an activator of the polyhydric alcohol ester type of
which LD 50 is 5000 mg/kg or more, an activator of the
polyoxyalkylene type having LD 50 of 5000 mg/kg, an activator of
the polyhydric alcohol ether type having LD 50 of 5000 mg/kg and
the like.
The activators of the polyhydric alcohol ester type may
include polyoxyalkylene sorbitan fatty acid ester, polyoxyalkylene
glycerin fatty acid ester, polyglycerin fatty acid ester, sorbitan fatty
acid ester, glycerin fatty acid ester, sucrose fatty acid ester and the
like. The activators of the polyoxyalkylene type may include
polyoxyethylene fatty acid ester, polyoxyethylene-oxypropylene block -
copolymer, polyoxyalkylene alkylether and the like. The
activators of the polyhydric alcohol ether type may include alkyl
(poly)glycoside, polyoxyethylene alkyl (poly)glycoside and the
like. The surfactants may be used solely or in combination with
each other.
In the deproteinizing agent, the protease and nonionic
surf actant are preferably combined together at a weight ratio
within a range between 1:1 and 1:5000.
The deproteinizing agent may have an excipient and/or a
filler incorporated therein, resulting in taking any desired form
such as a powder-like form, a liquid form containing water, a
solid-like form or,the like, as required. The deproteinizing
agent may be charged in the natural rubber latex material during
the deproteinizing treatment.
The amount of deproteinizing agent used may be suitably
adjusted depending on a content of protein in the natural rubber
latex material and a composition of the protease and surfactant
in the deproteinizing agent. For example, in order to ensure a
suitable protein decomposition reaction, and stability and
cleaning properties of the product, as well as satisfactory
formability of the product, the components of the deproteinizing
agent are preferably used in amounts within ranges indicated
below.
More specifically, the protease may be used in an amount
of 0.0005 to 5.0 parts by weight based on 100 parts by weight of
the solid content of the natural rubber latex, preferably 0.001
- 17 -
i a,
CA 02214996 2004-04-27
27537-8
to 1.0 par t s by weight, and more preferably 0.01 to 0.1 parts by
weight. Th a surfactant may be used in an amount of 0.1 to 10
parts by w a fight based on 100 parts by weight of the solid content
cf the nat a r al rubber latex and preferably 0.5 to 5 parts by
weight.
Application of the deproteinizing agent to the protein
decomposit i on step in production of the deproteini2ed natural
rubber late x contributes to an improvement in protein cleaning
and stabil ity of the latex , as well as an improvement in film
1Q forming prop erty in the subsequent forming step, in particular, the
forming ste p in which straight dipping is employed. Also, the
surf actant contained in the deproteinizing agent has LD 50 as
high as 5000 mg/kg, resulting in exhibiting increased safety:
Thus, even when the surfactant remali,~s in the product, it is kept
from being harmful to the human body.
BRIEF DESCRIPTION OF THE DRAWINGS
These and other objects and many of the attendant
advantages of the present invention will be readily appreciated
as the same becomes better understood by reference to the
20 following detailed description when considered in connection with
the accompanying drawings; wherein: .
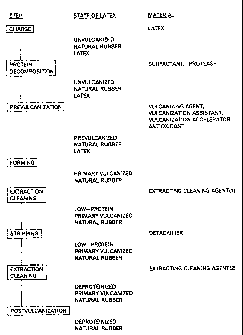
Fig. 1 is a flow chart showing a method for producing a
formed natural rubber latex product practiced in each of Examples
1 to 5;
Fig. 2 is a flow chart showing a method practiced in
Comparative Example 1;
Fig. 3 is a flow chart showing a method practiced in
each of Comparative Examples 2 and 3; and
Fig. ~4 is a flow chart showing a method practiced in
30 Comparative Example 4.
DETAILED DESCRIPTION OF THE INVENTION
The invention will be understood more readily with
reference to the following example; however, these examples are
intended to illustrate the invention and are not to be construed
to limit the scope of the invention.
Example 1 A
(2) Protein decomposition step
-18-
CA 02214996 1997-09-09
10g of aqueous potassium laurate solution (solid content
concentration: 20~) acting as a surf actant and 0.5g of protease
were added to 167g of high-ammonia natural rubber latex (from
Malaysia, solid content concentration: 60%, total nitrogen
content: 0.200%) to prepare a reaction system, which was then
uniformly dispersed and kept at 50~C for 5 hours.
(2) Prevulcanization step
Then, the reaction system was left to stand, resulting
in being cooled and thereafter 4g of sulfur dispersion (solid
content concentration: 50%), 2g of zinc oxide dispersion (solid
content concentration: 50%), 1g of zinc di-n-butyl dithiocarbamate
(solid content concentration: 50~, total nitrogen content: 0.06%)
acting as a vulcanization accelerator and lg of phenolic type
antioxidant dispersion (solid content concentration: 50%, total
nitrogen content: 0~) acting as an antioxidant were added to the
reaction system. Subsequently, the reaction system was heated at
50~C for l5 hours while being stirred, resulting in latex which
contains a non-rubber content (protease, surfactant,
vulcanization accelerator, proteolysis products and the like) being
obtained.
(3) Forming step
Then, the latex thus prepared was cast on a glass plate
and left to stand at a room temperature for 24 hours, so that an
intermediate product formed into a film-like shape was provided
in an amount of 106.7g.
(4) Cleaning step
Subsequently, 106.7 g of film-like intermediate formed
product and 10 kg of 0.1~ aqueous sodium hydroxide solution were
added to an extraction cleaning tank (volume: 20 liters) and kept
at 40~C for 2 minutes while being stirred.
(5) Postvulcanization
Then, the intermediate formed product was removed the
extraction cleaning tank and then subject to postvulcanization at
90'C for 30 minutes, resulting in a final formed product in the
form of a film being obtained in an amount of 104.2g. Results of
measurement of yields of the film and a total nitrogen content
thereof were as shown in Table 1.
- 19 -
i
CA 02214996 2004-04-27
27537-8
(Protein Content)
The protein content was measured according to a Kj eldahl
method ( Y . Tanaka et al , J . Nat . Rubb . Res . 7 ( 2 ) , pp 152-155
(1992)). , .
( y fields )
The yields were calculated according to the following
expression:
Yields (%) _ (weight of product after postvulcanization)/
(solid content concentration in latex material) x 100
Examples 1B to lE
In each of Examples lB tolE, a formed natural rubber
latex product was produced according to a process shown in Fig .
1. A natural rubber latex material for the formed product was
the same as that in Example lA and subject to the same treating
procedure and conditions as those in Example 1A , A cleaning step
was executed twice under conditions shown in Table 1 and a
stripping step was executed between the two executions of the
cleaning step. A treatment in the stripping step was carried out
using a detackifier containing 0.1~ of silicone emulsion and 1~
of cornstarch. The yields and total protein content were as
shown in Table 1.
Example 1 F
A formed natural rubber product was obtained according
to a process shown in Fig. 2. A natural rubber latex material
for the formed product was the same as that in Example 1A and
subject to the same treating procedure and conditions as those in
Example lA. Centrifuging was carried out using a De. Laval type
centrifugal separator (acceleration during centrifuging: about
10000G) , resulting in a solid content thereof being concentrated to
about 65~. The yields and total protein content were as shown in
Table 1.
Examples 1G and 1H
A formed natural rubber product was obtained according
to a ~ process shown in Fig . 3. A natural rubber latex material
therefor was the same as that in Example lA and subject to the
same treating procedure and conditions as those in ExamplalA.
Centrifuging was carried out under the same conditions as in
-20-
CA 02214996 2004-04-27
2'753-8
_a ,- r a __ . '~'":e _,:_..~c~s and total protein conte:-:t were
aS ~riown i:a '='able i .
Comparative Example 1I .
A f ormed naturalrubber product was tainedaccording
ob
to a proces . 4. A natural rubberhatex material
s shown in
Fig
therefor was that in Ex amplelA subject to the
the same and
as
same treat nd conditions as in amplelA. The
i ng procedure those Ex
a
yields and total protein content were as in
shown Table
1.
Table 1
Example Conditions for cleaning treatment Total N
Yields
1st time 2nd time (%) content ( )
1A p.l~ NaOH, --- 98 0.045
X10C, 2 min ~ ',
1B O , l~ NaOH 0~..1~ NaOH ~ 98 0.039
40C, 2 min 40C, 24 hr
1C 0.1~ NH3 0.1~ NH3 98 0.042
40C, 2 min 40C, 24 hr
O - 1~ NaOH 0 . 1~ -NaOH 98 0.01
1D
40C, 2 min 100C, 0.5 hr
2G 0.1% NH 0.1% NH 98 0.026
i='' 3 3 .
40C, 2 min 100C, 0.5 hr
1F 0.1% NaOH, --- 85 0.037
40C, 2 min
1G 0.1~ NaOH, --- 85 0.022
40C, 2 min
1;~ 0.1~ NH3 0.1% NH3 85 0.020
40C, 2 min 40C, 24 hr
Comparative Example
1I 0.1~ NaOH --- 98 0.280
30 40°C, 2 min
As will be noted from Table 1, the formed natural rubber
product obtained in ExamplelA was 98~ in yields. Also, it was
decreased in total nitrogen content to a level as low as 0.045x.
The formed product obtained in each of Examples 1B to 1E in which
the cleaning treatment took place twice was further decreased in
total nitrogen content while having yields kept at a level as
-21-
27537-8
CA 02214996 2004-04-27
high as 98~-
pm the contrary; although the formed product obtained in
Example lF was decreased in total nitrogen content, it
was deteriorated in yields because of being increased in the
number of steps for production and it disadvantageously required
a long period of time for production. Thus , Example
1F was disadvantageous from an industrial pint of view.
Also, Examples 1G and 1H each substantially '
reduced a total nitrogen content of the formed product, however,
those led t o both an increase in capital investment and a
decrease in yields because of requiring the centrifuging step.
Comparative ExamplelI increased the yields
because of being decreased in numbe~'of steps, however, it caused
a total nitrogen content remaining in~the formed natural rubber
product to be excessive, to thereby fail to provide the formed
product with satisfactory safety:
Examples 2A to 2I, Comparative Examples 2J to 2M
(i) Protein decomposition step
Commercially available high-ammonia natural rubber latex
(rubber solid content: 60~, ammonia content: 0.7~) was employed.
and a nonionic-anionic composite surfactant and protease were
added to the latex in amounts of 1~ by weight and 0.02 by weight
based on the rubber solid content of the latex, respectively,
resulting in a reaction system being prepared. Then, the
reaction system was subject to an enzyme reaction at 40'C for
2tl hours .
Emai*E-70C and alkali protease each manufactured by Kao
Corporation were used as the nonionic-anionic composite
surfactant and protease, respectively.
(2) Prevulcanization step
1 part by weight of sulfur, 1 part by weight of zinc
oxide and 0.6 part by weight. of zinc di-n-butyl dithiocarbamate were
added to the latex thus subjected to the enzyme reaction,
which was then subject to prevulcanization at 30'C for 24 hours,
resulting in prevulcanized latex being obtained.
(3) Forming step
The prevulcanized latef> thus obtained was then subject
* Trade-Mark -22-
11
CA 02214996 2004-04-27
27537-8
to straight dip forming, to thereby provide a glove made of a
rubber film having an average thickness of 0.25mm.
( 4 ) Cleaning step
The rubber glove thus formed was cleaned in each of . ,
cleaning liquids (see Table 2) while stirring the liquid under
conditions shown in Table 2. The cleaning liquids each were used
in an amount of 300g per gram of the rubber glove. Comparative
Example 5 did not carry out the cleaning treatment. The cleaning
liquid used in Comparative Example 6 was pure water .
( 5 ) Postvulcanization
Then, the rubber glove was removed from the cleaning
liquid and then subject to postvulcanization at 90'C for 30
minutes, to thereby obtain a final formed product.
(Evaluation of cleaning effect)
Evaluation of a cleaning effect in each of Examples 2A to
2I and Comparative Examples2J to 2M was made in a manner described
below using a sample made by cutting the rubber glove subjected
to the cleaning step into a size of 2cm x 2cm. A sample obtained
each of the gloves prior to the cleaning step and the sample
cleaned in each of the examples and comparative examples were
dried and then subject to protein extraction at 40'C for 1 hour
using pure water in an amount of 5ml per u00mg of each sample . A
protein-analogous material extracted was subject to 750nm
absorbance measurement according to a direct determination
preventing precipitation of protein by means of a protein
determining kit (Procedure No. 5656) of SIGMA using an improved
Lowry reagent. Then, a protein content of the extracted liquid
was calculated in terms of albumin based on a calibration curve
prepared using albumin as a standard material. Then, the protein
content thus calculated was turned into a value per the formed
product sample, which was employed as a residual protein content.
-23-
,.
CA 02214996 2004-04-27
27537-8
Table 2
Example Alcohol '~1 ~'2 ~3 ~4 ~5
' Cwt%) (wt%) ('C) (hr) (ug/g)
2A ethanol 50 0 40 24 70
2B ethanol 50 0.005 25 24 55
2C ethanol 50 0.010 25 24 50
2D ethanol 50 0.020 25 24 60
2E ethanol 25 0.010 25 24 45
ethanol 75 0.010 25 24 52
2C methanol 50 0.010" 25 24 75
2H IPA 50 0 40 24 55
2I MMBA 10 0 40 24 60
Comparative w
Example
2J ___ __ ___ __ __ 7864
2K ___ p p 40 24 700
2L~ --- 0 0.044 25 24 1360
2M ethanol g0 0.010 25. 24 1200
~1 Alcohol content in cleaning- liquid
~2 Concentration of free chlorine in cleaning liquid
~3 Cleaning temperature
~4 Cleaning period
~5 Residual protein content
Examples 3A to 3C, Comparative Example 3D to 3F
The examples and comparative examples were practiced in
substantially the same manner as Examples2A tc 2I and Comparative
Examples2J to2M described above, to thereby produce a rubber
glove made of a rubber film having an average thickness of
0.25mm. A composition of a cleaning liquid, a cleaning
temperature and a cleaning period which were employed in each of
the examples and comparative examples are shown in Table 3.
Comparative Example3D did not carry out cleaning and Comparativ a
'Example 3E used pure water as the cleaning liquid.
(Evaluation of cleaning effect)
Evaluation of a cleaning ef f e,t in each of Examples 3A
to 3C and Comparative Examples 31~ t.. 3H ras made in substantially
the same manner as Examples 2A to 2I using a sample made by
-24-
CA 02214996 2004-04-27
cutting the rubber glove after the cleaning step into a size of
2cm x 2cm. The results were as shown in Table 3.
Table 3
Example Concentration Cleaning Cleaning Residual .
of free temperature period protein
chlorine in (°C) (hr) content
treating liquid (~g/g)
(wt~)
3A 0.020 25 24 253
3B 0.010 25 '~ 24 50
3c 0.005 40 24 30
Comparative
Example
3D ___ . __ ~~ __ 796p
3E 0 25 24 1485
3F 0.044 25. 24 1360
Example 4A
(1) Protein decomposition step
A field latex (rubbe,r solid content: 30~) was used as a
natural rubber latex material. Then, a nonionic-anionic
composite surfactant and protease were added to the latex in .
amounts of 1~ by weight and 0.02 by weight based on the rubber
solid content of the latex, respectively, resulting in a reaction
system being provided. Then, the reaction system was subject to
enzyme reaction at 40°C for 24 hours.
Latex manufactured by FEL~DA in Malaysia was used as the
field latex material. Also, Emai* E-70C (sodium polyoxyethylene laaryl
ether sulfate) and alkali protease each manufactured by Kao
Corporation were used as the nonionic-anionic composite
surfactant and protease, respectively.
(2) Centrifuging step
After the enzyme reaction, the rubber latex material
was diluted with water, resulting in the rubber solid content
being 10~ and then subject to concentration and purification by
means of a De. Laval type centrifugal separator (acceleration
during centrifuging: about 10000G), resulting in the rubber solid
content being 65~. A cream obtained by the concentration and
* Trade-Mark -25-
,,
CA 02214996 2004-04-27
27537-8
purification step was diluted with water so as to reduce the
rubber content to a level as low as TO% and then centrifuged
again. This resulted in deproteinized natural rubber latex. of
which the rubber solid content is 65% and a nitrogen (N) content
in raw rubber is 0.007% being obtained.
(3) Prevulcanizat ion step
1 part by weight of sulfur, 1 part by weight of zinc
oxide and 0.6 part by weight of zinc di-n-butyl dithiocarbamate were
added to the latex thus obtained, which was then subject to
prevulcanization at 30'C for 24 hours, resulting in prevulcan ized
latex being obtained.
(4) Forming step
The prevulcanized latex thus obtained was then subject
to straight dip forming, to thereby provide a glove made of a
rubber film having an average thickness of 0.25mm.
(5) Cleaning step
The rubber glove thus formed was cleaned in each of
cleaning liquids while stirring the liquid. The cleaning liquids
each were used in an amount of 300g per gram of the rubber glove.
Conditions and the like for the cleaning step were as shown in
Table 4. .
(6) Postvulcaniz ation
Then, the rubber glove was removed from the cleaning
liquid and then subject to postvulcanization at 90'C for 30
minutes, to thereby obtain a final formed product.
(Evaluation of cleaning effect)
Evaluation of a cleaning effect in .example uA was made
in substantially the same manner as Examples 2A to 2I using a
sample made by cutting the rubber glove after the cleaning step
30 into a size of 2cm x 2cm. The results were as shown in Table u.
Examples 4B to 9I and Comparative Examples 9J to 4 N
The examples and comparative ex ampies were practiced in
substantially the same mariner as Example 4A described above , to
thereby produce a rubber glove of deproteinized natural rubber
latex. Conditions theref or were as shown in Tabl a ~.
Comparative Example 4J did not carry out cleaning and Comparative
Example 9K used pure water as the cleaning liquid. Then, samples
-2 6-
CA 02214996 2004-04-27
27537-~
were prepared in substantially the same manner as .Example 4A
described above and evaluation of a cleaning effect in each of
the examples and comparative examples was made. The results . were
as shown in Table a .
Table 4
Example Alcohol ~1 ~2 ~3 ~~ ~5
(wt%) (wt%) ( 'C) (min) (~ag/g)
4A ethanol 50 0 40 30 CIO
4B ethanol 50 0.005 25 15 32
4C ethanol 50 0.010 ' 25 15 30
1 ~
9D ethanol 50 0.020 15 38
25
a~ ethanol 25 0.010 25 15 25
~F ethanol 75 0.010 ~ 25 15 32
methanol 50 ~ o ~ ~ ' 25 15 35
~
IPA 50 40 30 25
0
MMBA 10 0 40
30 30
C ompar at iv a
Example
___ __ _ . __ ._ 180
9K ___ 0 0 X10
30 l00
9L ~. -- 0 0.066 25 15 120
uN ethanol 1 0.010 25 15 90
4N methanol 90 0.010 25 15 100
~1 Alcohol content in cleaning
liquid
'~2 Concentration of free chlorine in eaning1 iquid
cl
~3 Cleaning temperature
~~! Cleaning period
~5 Residual protein content
Examples 5A
(1) Protein decomp osition step
.,
3 parts by weight of polyoxyethylene sorbitan
(20)
monooleate (nonionic surfact ant, LD 50 >
15000 mg/kg)
and 0 . 05
part by weight of protease a cting as a deproteinizing
agent were
added to 100 parts by weight of high-ammonia
natural rubber
latex
(from Malaysia, solid conten t concentration: total nitrogen
60%,
content: 0.2000 to prepare a reaction system,
which was then
uniformly dispersed and kept at 50'C f or hours.
5
-27-
CA 02214996 1997-09-09
(2) Prevulcanization step
Then, the reaction system was left to stand, resulting
in being cooled and thereafter 2 parts by weight of sulfur, 1
part by weight of zinc oxide, 0.5 part by weight of zinc di-n-
butyl dithiocarbamate (total nitrogen content: 0.06%) acting as a
vulcanization accelerator and 0.5 part by weight of phenolic type
antioxidant (total nitrogen content: 0%) acting as an antioxidant
were added to the reaction system. Subsequently, the reaction
system was heated at 50'C for 15 hours while being stirred,
resulting in latex which contains a solid content of about 60%
being obtained.
(3) Centrifuging step
Then, the latex thus obtained was left to stand,
resulting in being cooled and then pure water was added to the
latex so that a solid content thereof is 20%. Thereafter, the
latex was centrifuged by means of a De. Laval type centrifugal
separator (acceleration during centrifuging: about 10000G),
resulting in the rubber solid content being 65%. Subsequently,
the latex was diluted with pure water so that the solid content
is 20%, followed by centrifuging again under the same conditions.
(4) Forming step
Then, 1% ammonia was added to the latex thus centrifuged
to dilute the latex so that the solid content is 60%. Then, a
glass mold formed into a shape like a test tube was directly
dipped in the latex and then drawn up therefrom, followed by
heating at 90'C for 5 minutes in an oven for drying of the latex,
resulting in an intermediate formed product being obtained.
(5) Cleaning step
Subsequently, the intermediate formed product (as
adhered to the glass mold) and 0.1% aqueous sodium hydroxide
solution were added at a weight ratio of 1:100 (based on weight
of only the formed product other than the glass mold) to an
extraction cleaning tank and kept at 40'C for 2 minutes while
being stirred.
(6) Stripping step
A stripping step was executed using a detackifier
containing 0.1% of silicone emulsion and 1% of cornstarch, to
- 28 -
CA 02214996 2004-04-27
27537-8
thereby st rip the formed product from the glass mold.
( 7 ) Cleaning step
A cleaning procedure was carried out under substantially
the same conditions as the foregoing cleaning step (5) except ,
that the intermediate formed product was kept at 100'C for 0.5
hour.
( 8 ) Postvulcanization
Then, the intermediate formed product was removed from '
- the extrac t ion cleaning tank and then subject to
postvulcanization at 90'C for 30 minutes, resulting in a final
formed product. A total nitrogen content of the final formed
product was determined according to a Kjeldal method, resulting
in being indicated to be less than 0,.05%.
Stability of the latex after each of the
prevulcanization step and centrifuging step and filmization of
the latex in the forming step .using straight dip forming were
visually ob served according, to criteria indic aced below. The
results were as shown in Table 5.,'
( Stability? v
In Table 5, "0" indicates that the latex was uniformly
dispersed without coagulating and causing an increase in
viscosity and "X" indicates that the latex coagulated and was
increased in viscosity.
(Film forming property)
In table 5, 0 indicates that the latex exhibited improved
film forming property and was uniform in thickness and X indicates that
the latex exhibited excessive sagging, was decreased in speed of film
forming property and was non-uniform in thickness.
Examples 5B and 5C
A formed rubber product was produced .ace girding to
substantially the same procedure in Example 5A described above,
except that a content of polyoxyethylene (20) sorbitan monooleate
therein was L parts by weight in Example 5B and 5 parts by weight
in Example 5C . A total nitrogen content of the formed product
obtained in each of Examples 5B and 5C was determined according
to a Kjeldal method, resulting in being indicated to be less than
0.05. Also, the formed products of the examples were subject to
-29-
,;
CA 02214996 2004-04-27
27537-8
SubStc:l~ially ~;~e same tests as in Example 5A described above.
The results were as shown in Table 5.
Examples 5D to 5F
In each of these examples, a formed rubber
product was produced according to substantially the same
procedure in Example 5A described above, except that
sodium polyoxyethylene (3) lauryl ether sulfate which nonionic
is a
surf octant was used yn an amount of parts b~T weight in
Example 5D, in an amount of 9 parts by weight in
I
I -
E}.._ample 5E and in Gn amount of 5 pa~t-ts by ~ weignt
ir:
Example 5F. Also, the formed products of these examples
were subject to the same' tests as 5A
The results were as shown in Table 5.
Table 5
Example Surfactant ~'1 Stability
~2
~3
5A polyoxyethylene (20) 3 0 0 0
sorbitan monoo3eate .
5B polyoxyethylene (20) 4 0 0 0
sorbitan monooleate
5C polyoxyethylene (20) . 5 0 0 0
sorbitan monooleate
5 D sodium polyoxyethylene (3) 3 0 0 X
lauryl ether sulfate
' SE sodium polyoxyethylene (3) 4 0 0 X
lauryl ether sulfate
5 F sodium polyoxyethylene (3) 5 0 0 X
lauryl ether sulfate
~1: Amount of surf octant used
~2: Film forming property during dip forming step
straight
~3: Prevulcanization step
~4: Centrifuging step
As will be apparent from Table 5, Examples 5A to 5C
wherein the nonionic surfactant was usedas a component of the
deproteinizing latex to exhibit
agent each permitted
the
-30-
27 5 3 ~' ~ CA 02214996 2004-04-27
increased stabiist>~ ~n both prevulcanization step and
centrifuging step, to thereby ensure smooth workability. Also,
the examples each improved film forming property in the straight dip
forming step to a degree to permit a formed product of a uniform
thickness to be rapidly provided.
On the contrary, although~each of Examples SD to 5F
contributed to an improvement in stability of the latex,
it caused excessive sagging of the latex during the straight dip
forming step; requ_red an increased per iod of time for film ,
lp forming property Gnd rendered a thickness, of the product non-uniform,
resulting in the product being defective.
As can be seen from the foregoing, the method of the
present invention can be applied to'tlae conventional natural
rubber product producing apparatus without modifying it and used
for production of a foam product such as a foam rubber product or
the like and a dspped product such as a glove, a condom, a
catheter or the like.
Obviously many modificat~.o~ns and variations of the
present invention ire possible in~~light of the above teachings.
It is therefore to be understood that within the scope of the
appended claims the invention may be practiced otherwise~than as
specifically described. ..
-31-