Note: Descriptions are shown in the official language in which they were submitted.
CA 0221~048 1997-09-10
Solution for Electrolytic Deposition of Zinc or Zinc Alloy Coatings
Specification:
The invention relates to an aqueous alkaline cyanide-free solution for electrolytic
deposition of brilliant and bubble-free zinc or zinc alloy coatings with a uniform coating
thickness on curved substrate surfaces.
Electrolytic zinc or zinc alloy baths are used for depositing zinc coatings or their alloys.
The coatings on the one hand fulfil a decorative purpose. Therefore the coatings must
be uniformly brilliant. This includes the fact that in a wide range of current densities,
for example from 0.1 A/dm2 to 15 A/drn2, no coarse granular metal coatings (so-called
scorching) are deposited, and that no matt zinc coatings and pores are formed in the
covenng.
Zinc and zinc alloy coatings are predomin~ntly used as an anti-corrosion measure for
baser substrate materials which are liable to corrosion, such for example as femc
metals. In particular, products forrned from wire and screws, nuts, metal coverings, for
example for motor parts, made of iron or steel are covered with zinc coatings in order to
protect them from rust.
The rust protection is in many cases improved by a subsequent treatment of the zinc and
zinc alloy coatings with chromating solutions, chromated coatings being formed on the
CA 0221~048 1997-09-10
zinc coatings. These are coloured, and depending on the composition of the chromating
solutions can be produced in various colour tones. In this way further possibilities of
decorative finish are afforded. Therefore the zinc coatings must in addition enable a
good facility of chromating. This includes the fact that the chromating coating on the
zinc coating adheres well and forms no cracks, as otherwise the resistance to corrosion
of the chromated zinc and zinc alloy layers would be very low.
An extremely important factor is also that the zinc coatings deposited should adhere
well to the base and form no bubbles.
Zinc baths for electrolytic deposition of such zinc coatings are described in various
publications.
There have been known for a long time cyanidic bath types, in which zinc salts are
dissolved as cyanide complexes. These baths are intensely :~lk~line. The coatings
deposited from these solutions are sufficiently smooth and brilliant, particularly if
organic additive compounds such for example as gelatine, peptones, sodium sulphite,
thiourea, polyvinyl alcohol, aldehydes, ketones or salts of organic acids are used.
Due to the high toxicity of these solutions and due to the entailed high outlay on work
safety when h~n~lling these solutions, and to the waste water treatment, these bath types
however are no longer practical. For this reason cyanide-free solutions were developed.
CA 0221~048 1997-09-10
The document DE 25 25 264 C2 describes an alkaline cyanide-free zinc bath Cont~ining
a zinc salt, alkali hydroxide, at least one organic brightener additive and the reaction
product of a uns~ led heterocyclic hydrocarbon compound Cont~ining at least two
nitrogen atoms with a ring, with an epihalogenhydrin, or with a glycerol halogen
hydrine.
By means of this bath, brilliant to extremely brilliant, smoothed zinc coatings can be
deposited.
In the document US-A 40 30 987 there are described cyanide-free baths for depositing
zinc which contain, in addition to the zinc compounds, an alkali hydroxide for setting
the high pH value, brighteners from the group of the aromatic aldehydes and of a
diallylammonium sulphur dioxide copolymer with the general structural formula
n
Cl- N \ IV
By means of these baths, uniforrnly bright zinc coatings can be produced which smooth
surface roughnesses of the substrate. In addition deposition rates for the zinc coatings at
a predetermined cathodic current density comparative to those of cyanide baths are
achieved.
CA 0221~048 1997-09-10
The document US-A 41 34 804 discloses cyanide-free baths, which in addition to the
zinc compounds and alkali hydroxide, contain the diallylammonium sulphur dioxide
copolymers described in US-A 40 30 987. In addition to the diallylammonium sulphur
dioxide copolymers, the baths described in this document contain, instead of aldehydes,
a quaternary pyridine compound, for example N-benzyl-3-methyl carboxylate
pyridinium chloride and nicotinic acid-N-oxide.
In the document US-A 38 56 637 there are described cyanide-free or extensively
cyanide-free zinc baths, cont~inin~ soluble zinc compounds, a brightener and an
alkaline metal silicate for avoiding matt, smeary or dirty metal coatings. Amine-
epichlorhydrin reaction products are indicated as examples of brighteners.
US-A 38 69 358 discloses an aqueous alkaline zincate bath, which contains less than 15
g of cyanide/litre of solution and in addition a soluble zinc compound as well as at least
one water-soluble compound with tertiary and/or quaternary arnine groups, produced by
polymerisation of aliphatic amine with epihalohydrin.
By means of the above named zinc baths, bright zinc coatings can in fact be deposited,
but they are not uniformly thick.
Particularly in the case of workpieces to be coated, which on the one hand are sharp-
edged and/or have points and corners, and on the other hand have concave surface areas
Iying opposite the anode. the most uniform possible thicknesses of coatings must be
CA 0221~048 1997-09-10
deposited, so that the quantity of metal to be deposited in all is as small as possible. In
the case of larger differences in coating thickness at the various surface areas with
varying curvature, therefore, with the known baths, under unfavourable conditions,
extremely thick coatings are generated at some points, while at other points the zinc
coating has still scarcely formed. With thin coatings, sufficient smoothing of
rollghnesses present on the substrate surface cannot be achieved. Therefore withpreviously known baths, normally large quantities of metal must be deposited, so that
operation with these baths is expensive. In addition, the workpieces must be treated for
a long time in this electroplating bath, and large quantities of bath additives are
consumed.
Moreover, long electrolytic met~llising times lead to an increased tendency to the
formation of bubbles. As at some points on the workpiece relatively thick zinc layers
must necessarily be formed, the adhesive strength of the zinc coating on the workpiece
base is reduced. Among other things, this can be caused by low internal stresses in the
deposited zinc coating.
In the German disclosure document 37 21 416 a process for zinc plating of metallic
objects is described, which makes use of a cyanide-free ~Ik~line bath for zinc plating,
which contains a product as organic additive being produced by polymerization ofdimethyl amrnonium chloride or -bromide in the presence of sulfur dioxide as
polymeri7~tion initiator at a telllp~ Lule between 85 and 1 15 ~C.
Therefore the problem underlying the present invention is to avoid the disadvantages in
prior art and to f~nd an aqueous solution for electrolytic deposition of brilliant zinc or
zinc alloy coatings being bubble-free also after aging, and in particular to produce a
uniform distribution of coating thickness of the zinc coatings, even on curved surfaces.
This problem is solved by claims l and 15. Preferred embodiments of the invention are
CA 02215048 1997-09-10
-
indicated in the sub-claims.
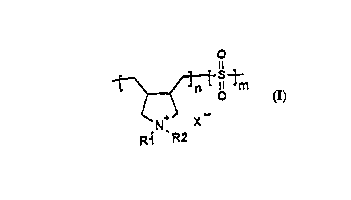
The purpose is in particular achieved by an aqueous ~lk~line cyanide-free solution for
electrolytic deposition of zinc or zinc alloy coatings, cont~ining at least
a zinc ion source, an :~lk~ ing agent and a diallylammoniurn sulphur dioxide
copolymer with the general structural formula'
~! ~m
.~ ~ X--
Ri R2
with an average molecular weight of 240 g/Mol to 10,000 g/Mol, Rl and R2 being
selected indep~n~1~ntly of one another and are hydrogen, alkyl, hydroxyalkyl, alkenyl,
alkinyl, aralkyl or phenyl and X = F, Cl, Br, and I .
The solution can also contain an amine-epichlorhydrin copolymer, obtainable by
conversion of epichlorhydrin with compounds selected from the group of amines,
diamines and triarnines.
CA 02215048 1997-09-10
Particularly suitable are amine-epichlorhydrin-copolymers with the general structural
formula
R1 OH
,N NH~ ~1 C OH N1 R2 1
R1 and R2 being selected independently of one another and are hydrogen, alkyl,
hydroxyalkyl, alkenyl, alkinyl, aralkyl or phenyl
X =F,Cl ,Br ,I and
O ~ y ~ 20
The diallylammonium sulphur dioxide copolymers and amine epichlorhydrin
copolymers according to the invention are added to the bath as basic additives.
By means of this bath liquor it is possible to coat even complexly shaped workpieces
with a substantially uniform coating thickness at all points of the workpiece surface.
Particularly at sharp-edged places on the surface, for exarnple at points and corners~
no~nally high metal coating thicknesses are generated, while the coating thicknesses at
CA 0221~048 1997-09-10
-
the concave surface areas lying opposite the anodes, when normal baths are used, can be
extraordinarily thin. With the above named bath, on the other hand, substantially
uniformly thick layers are obtained at the places indicated on the surface.
In order to determine the uniformity of the distribution of coating thickness, for
example, the thicknesses of zinc or zinc alloys can be determined at various points on
metal plates coated in a so-called Hull cell, which have been coated with different
current densities. The proportion of the thickness of a coating deposited at a high
current density (for example 3 A/dm2) to a layer deposited at a low current density (for
example 0.5 A/dm2), in previous bath types has a high value. In the case of the current
densities indicated, this ratio reaches values of at least 3.4.
With the bath according to the invention, at the points indicated of the metal plate
coated in the Hull cell test, coatings with coating thickness ratios of less than 2.4 and
frequently values smaller than 2 are obtained. This means that in relation to points on
complexly shaped workpieces, at which high current densities form due to sharp-edged
surface structures, even at points with a low current density, for exarnple at the concave
surface areas lying opposite the anodes, a suff1ciently thick zinc or zinc alloy coating
can be generated.
Furtherrnore it is possible with the bath according to the invention to deposit brilliant
coatings without bubbles in a large range of current densities. Within a large range of
current densities, the coatings reveal no fogging, no coarsely crystalline layers are
CA 0221~048 1997-09-10
produced, and the adhesion of the coatings on the substrate surface is sufficiently high
so that bubbles and other lift-offphenomena do not form between the coating and the
substrate.
In addition, the coatings are ductile. Therefore workpieces coated in this way can be
mechanically processed without problems, for example by bending. The corrosion
resistance of the coatings is very good, and the resistance is extremely good against the
formation of so-called white rust, i.e. the coating is itself resistant to corrosion, and also
to red rust, which can occur during the coating of iron substrates, and is caused by
corrosion of the substrate.
The coatings in addition may be efficiently chromated. The chromated layer formed on
the coatings adheres well and has a high degree of corrosion resistance. Blue, yellow,
green and also black chromated layers can be produced.
A further advantage of the bath according to the invention resides in the high stability of
the additive compounds used. During down times of the bath, the effectiveness of these
compounds is not reduced. The bath liquor can be used at high temperatures, e.g. at
35~C to 40~C, or at low telnp~.d~ s, for example at ambient temperature or below. By
appropriate selection of the concentration of the additive compounds contained and
suitable further compounds, both high-brightness and semi-bright coatings can be
produced. The current yield during metal deposition is extremely high. even at zinc
contents of 8 g/l to 10 g/l solution. The working range with 4 g zinc ions/l to 40 ~/1
CA 0221~048 1997-09-10
-
solution, is without the formation of scorching, and particularly from 5 g/l to 18 g/l
solution, is extremely good with excellent distribution of coating thickness in the entire
range of current densities. It is further possible to deposit coatings of a thickness of up
to 30 llm without the formation of bubbles.
In a preferred embodiment of the invention, a coating solution is used, containing
diallylammonium sulphur dioxide copolymers with an average molecular weight of 240
- 10,000 g/Mol or a little above and preferably with a molecular weight of 500 g/Mol to
about 6,000 g/Mol. Particularly suitable are diallylammonium sulphur dioxide
copolymers with n 2 m > 0 and with R1 = R2 = methyl. When diallylammonium
sulphur dioxide copolymers are used with too high a molecular weight, the tendency to
bubble formation in the coatings increases. For example, with a bath containing
diallylammonium sulphur dioxide copolymers with a molecular weight of 100,000. no
bubble-free coatings are obtained.
Diallyldimethylammonium chloride sulphur dioxide copolymer is particularly suitable
as a diallylammonium sulphur dioxide copolymer.
The coating solution can further in addition contain a homopolymeric compound with
the general structural formula
CA 0221~048 1997-09-10
~ X-
R~ R2
with an average molecular weight of 500 to 100,000, R1 and R2 being selected
independently of one another and being hydrogen, alkyl, hydroxyalkyl, alkenyl, alkinyl,
aralkyl or phenyl, and diallyldimethylammonium halogenide and
diallyldimethylammoniurn hydrogen sulphite, origin~ting from the production of the
diallylammonium sulphur dioxide copolymers and the homopolymeric compounds,
formed during m~nufacture of the diallyldimethylammonium sulphur dioxide
copolymers from diallyldimethylammonium halogenide and introduced sulphur dioxide.
In the group of compounds selected from the diallylammonium sulphur dioxide
copolymers, the homopolymers and the monomers not converted during the reaction, for
example diallyldimethylammonium chloride, the diallylammonium sulphur dioxide
copolymers are contained in a proportion of 1% by weight to 100% by weight relative to
the mixture of the compounds in this group. The proportion of the homopolymers is
from 0% by weight to 60% by weight relative to the mixture of compounds, and the
CA 0221~048 1997-09-10
12
proportion of the unconverted monomers is 0% by weight to 70% by weight. Typical
mixtures of these compounds contain 80% by weight of diallylarnmonium sulphur
dioxide copolymers, 5% by weight of homopolymer and 15% by weight of unconverted
monomer or 70% by weight of diallylammonium sulphur dioxide copolymer, 5% by
weight homopolymer and 25% by weight of unconverted monomer.
Alternatively, for sufficient distribution of coating thickness, up to 100% of monomers
can be used, although however no brilliant coatings are achieved.
In many cases it was discovered that by means of the copolymers alone, e.g. XP- 104
according to exarnple 11, even with a reinforced addition, no substantially improved
distribution of coating thickness, i.e. better than 2.4 to about 1.6 was achievable. By a
slight addition of monomers, such for example as diallyldimethyl ammonium chloride
and the diallyldimethyl ammonium hydrogen sulphite arising in the acidic synthesis
solution for example at pH values of 1 - 2, after the end of reaction, the coating
thicknesses could be further evened out to index values below 1.6.
The reaction solution cont~inin~ copolymers can also be treated in such a way that the
polymers ple-;ipitale as a crystalline solid matter, cont~inin~ no free diallyldimethyl
ammoniurn, but only the pure copolymer.
The diallylammonium sulphur dioxide copolymers claimed contain heterocyclic ring
structures in a 5-ring system. Only these are suitable to produce coatings with the
CA 0221~048 1997-09-10
required uniform coating thickness. Similar diallylammonium sulphur dioxide
copolymers, such for example as the polyamine sulphones described in the US Patent 41
34 804 with 6-ring systems, do not have the same advantages. In particular it is not
possible with these compounds to deposit coatings with the required regularity.
Moreover, the tendency to bubble formation is clearly reduced in comparison to the
compounds disclosed in the US Patent.
Such diallylammonium sulphur dioxide copolymers with a 5-ring system are obtainable
by suitable reaction management and substitution of the monomers during their
manufacture. These compounds are in particular obtained by polymerisation in water as
a solvent. The selection of the polymer starter coul'd also have led to the formation of
the 6-ring in the US Patents. When ammonium persulphate is used instead of other
peroxide starters, for example di-tert.- butyl peroxide or di-benzoyl peroxide, the
diallylammonium sulphur dioxide copolymers according to the invention are formed.
Formation of the 5-ring systems may be simply proved by nuclear magnetic resonance
spectroscopy (NMR). For example the NMR spectra of the 5-ring structures and of the
6-ring structures can be simply differenti~te~ from one another in a ~C-NMR-spectrum.
This was already proved by r ~n(~ter, Baccei and Panzer in Polymer Letters, Edition
14, Page 549 (1976).
Instead of the halogenides, preferably chlorides, other anions, e.g. also nitrates.
chlorates, perchlorates and sulphates can be used as counter-ions.
CA 0221~048 1997-09-10
14
The amine epichlorhydrin copolymer contained in the solution preferably has an
average molecular weight above 500 g/Mol and particularly about 1,500 g/Mol. The
amine epichlorhydrin copolymers are produced according to known methods by
polycondensation for example of 3-dimethylamino- 1 -propylamine with epichlorhydrin.
Particularly suitable are coating solutions with copolymers of amines, diamines,
triamines, hydrazines and/or heterocylic compounds containing nitrogen, with bis-
electrophilic compounds, such for example as bis-glycidyl ether, di-halogen alkanes and
epihalogen hydrines. Particularly suitable is 3-dimethylamino-1-propylamine-
epichlorhydrin copolymer as an amine epichlorhydrin copolymer. In this case Rl and
R2 are respectively methyl groups and X is chlonde in the abovementioned general
structural formula II. In order to produce a high-brilliance zinc or zinc alloy coating, the
solution can further contain l-benzylpyridinium-3-carboxylate as a brightener.
Carboxylates of the lower alkanoic acids, such for example as acetate, are considered as
a carboxylate group. Sulphonate betains, such for example as N-benzylpyridinium
sulphonate, represent compounds which are just as effective.
Zinc oxide is normally used as a zinc ion source, which dissolves as zincate in the
~lk~line coating bath. In principle however other zinc ion sources such for exarnple as
zinc salts can be used, and further anions, which pass into the bath by addition of the
zinc salts, but do not impair deposition. Zinc sulphate is for example suitable.
In order to deposit zinc alloy coatings, further metals can be deposited along with zinc.
For example it is possible to generate zinc coatings with nickel, cobalt or iron
- -
CA 0221~048 1997-09-10
-
admixtures. For this purpose the baths contain in addition to the zinc ion source also
compounds of these metals. In addition, co-ordinated complex former combinations are
necessary in the coating solution, in order to control the deposition potentials. Such
combinations are known.
As an alkalising agent, sodium hydroxide is normally added in a concentration of 50 g/l
to 200 g/l solution and in particular from 80 g/l to 150 g/l solution. However, other
alkali and earth alkali metal hydroxides and tetraalkyl ammonium hydroxides are
suitable. However, in the latter case, care should be taken that any complexing effect of
the ammonium hydroxides does not impair the deposition effect.
In addition to the named compounds, the coating solutions according to the invention
further contain other components which are added to the bath for various purposes.
For example, in order to control and stabilise the pH value, the bath can additionally
contain sodium carbonate. In order to reduce the sensitivity of the bath to extraneous
ions, particularly to calcium and magnesium in the tap water, the bath can further
contain sodium gluconate or other complex formers. Aldehydic aromatic compounds
such for example as anisaldehyde can be added to the bath as brighteners. Under certain
circumstances these compounds impair the distribution of coating thickness. These
compounds are preferably used in the form of their bisulphite adducts. in order to
increase their solubility in the bath. Thio compounds. such for e~cample as thiourea.
mercaptoben7thi~701 and mercaptotriazole, are added to the bath in order to achieve a
CA 0221~048 1997-09-10
_
16
smoothing of the metal deposition in the low current density range.
The temperature of the bath can be set within a wide range. For example, good results
are obtained with temperatures from 15~C to 40~C.
The current density usable lies between 0.01 Aldm2 and 15 A/dm2, preferably in a range
from 0.1 A/dm2 to 6 A/dm2. In this range brilliant, smoothed, uniformly thick and
bubble-free coatings are obtained.
The cathodic current yield of the bath lies between 80% and 95%, relative to the
quantity of metal deposited in the case of pure zinc coatings.
The solution according to the invention can also be used for coating workpieces in the
so-called frame technique, in which the workpieces are secured to a frame and
immersed in the bath. Small parts however, due to the complexity of assembly, are not
metallised by the frame technique, but by a drum process in which the parts to be
metallised are filled into a drum located in the bath liquor and are metallised in the
drum. This procedure is also possible without problems in the solution according to the
invention.
Both soluble zinc anodes and also insoluble anodes, for example of iron, iron alloyed
with nickel or titanium can be used as anodes.
CA 0221~048 1997-09-10
In order to even out the distribution of coating thickness on the workpiece to be coated,
and in the case of the frame technique, also on the individual workpieces to be coated
simultaneously, air can be blown into the coating solution and in this way the solution
can be kept in vigorous motion.
Some examples are described in the following in order to explain the invention.
Example 1:
A cyanide-free alkaline bath with the composition:
zinc as zinc oxide 10 g,
sodium hydroxide 130 g,
sodium carbonate 20 g
per litre aqueous solution
as basic composition, and
1-be,~ylpyridinium-3-carboxylate 40 mg,
mercaptoben7thi~701 0.1 g
per litre aqueous solution
CA 0221~048 1997-09-10
as additives
was used for zinc deposition.
The bath was tested, zinc being deposited on plate iron cathodes in a Hull cell at 22~C
with an overall current of 1 A for 15 minutes. The distribution of coating thickness at
various current densities was determined by zinc dissolution at a point on the iron plate
coated, on which zinc had been deposited with a current density of 3 A/dm', and at
another point on which zinc had been deposited with a current density of 0.5 A/dm~. As
a measurement figure, an index was calculated, which indicates the ratio of the coating
thickness deposited at 3 A/dm2, to the coating thickness deposited at 0.5 A/dm'.
Added to the basic bath were S ml/l of an aqueous solution at 25% by weight of diallyl
dimethylammonium chloride sulphur dioxide copolymer in water and 0.5 ml/l of an
aqueous solution at 38% by weight of 3-dimethylamino-1-propylamine-epichlorhydrin
copolymer. Uniformly coated, semi-brilliant zinc coatings were obtained. The coating
thicknesses at the measurement points were 5.7 ~lm (3 A/dm2) and 3.3 ~lm (0.5 A/dm').
The index was 1.7.
Example 2 (Co~l~p;~tive example):
The test of Example I was repeated, but instead of the compounds diallyl dimethyl
amrnoniumchloride-sulphur dioxide-copolymer and 3-dimethylamino-1-propylamine-
CA 0221~048 1997-09-10
-
19
epichlorhydrin copolymer, other bath additives were used, which are also described in
the publication DE 25 25 264 C2 (5 ml of an aqueous solution at 35% by weight of
imidazole-epichlorhydrin-copolymer (IMEP), 1 -benzylpyridinium-3-carboxylate,
thiourea, anisaldehyde-bisulphite adduct per litre of bath liquor).
The iron plates were covered with a brilliant zinc coating. The coating thicknesses were
5.9 ~lm (3 A/dm2) and 2.0 ,um (0.5 A/dm2), and the index was 3Ø
Example 3:
The test of Example 1 was repeated. In addition, 1-benzylpyridinium-3-carboxylate was
added in a concentration of 80 mg/l to the bath solution.
A zinc coating was obtained which was brilliant in the entire current density range, with
a uniforrn distribution of coating thickness. After treating the coated plate for 1 hour in
a tempering furnace at 120~C, the plates were unchanged and revealed no bubbles.
Example 4:
The test of Example 1 was repeated. The temperature during deposition was increased
to 30~C.
Brilliant to semi-brilliant zinc coatings without scorching were obtained. The
CA 0221~048 1997-09-10
-
distribution of coating thickness was just as regular.
Example 5:
The test of Example 1 was repeated in a 5 litre beaker with pure zinc anodes. The iron
plate cathodes were bent at right angles longitudinally several times, and were coated
with zinc at a mean current density of 2 A/dm2 for 1 hour. During this time air was
passed through the coating solution.
The coating thicknesses varied from 25 llm to 32 ~m. The coating was semi-brilliant to
brilliant.
Subsequent blue chromating with the chromating bath ICP 33L of the Firm Atotech
Espana, S.A., Spain, which contains hexavalent chrome compounds and inorganic salts,
and generates a bluish finish, could be carried out without difficulty.
After treating the coated plate for 1 hour in the tempering furnace at 120~C, the plates
were unaltered and revealed no bubbles.
Example 6:
The test of Example 3 was repeated. However, an additional 50 mg/l of anisaldehyde-
bisulphite adduct was added to the coating solution. Coating was carried out at an
CA 0221~048 1997-09-10
21
overall current of 1 A in the Hull cell for a coating time of l S minutes.
A brilliant, uniform zinc coating was obtained. The coating thicknesses were 5.8 llm (3
A/dm2) and 3.3 ~Lm (O.S A/dm7), and the index was 1.7. No bubbles could be seen even
after heating in the furnace.
Example 7:
There were added to the basic composition of Example 1, per litre of aqueous solution,
6 ml of diallyldiethylammonium chloride sulphur dioxide copolymer, 1 ml 3-
dimethylamino-1-propylamine-epichlorhydrin copolymer, S0 mg 1-benzylpyridinium-3-
carboxylate and 100 mg thiourea.
At a temperature of 30~C and an overall current of 1 A, after l S minutes' coating time in
a Hull cell a uniformly thick zinc coating with good smoothing and without scorching
was obtained. The plate was totally covered with zinc and had coating thicknesses of
6.6 llm (3 A/dm2) and 5.4 ~m (O.S A/dm~). The index was 1.3.
The zinc coating was coated without difficulty by means of the yellow chromating bath
ZP 1 C of the Firm Atotech Espana, S.A., which contains hexavalent chrome compounds
and inorganic salts, and gives an iridescent finish, and after tempering treatment.
showed no bubbles.
CA 0221~048 1997-09-10
-
Example 8:
Example 7 was repeated. The concentration of l-benzylpyridinium-3-carboxylate in the
bath was increased to 100 mg/l. The coating conditions corresponded to those of
Example 7.
The zinc coating was highly brilliant without bubble formation, even after tempering
treatment. The coating thickness index obtained was 1.6 to 1.7.
Example 9:
Following Exarnple 8, 4 ml/l of a solution of imidazole-epichloryhydrin copolymer
(IMEP) was added to the bath at 35% by weight.
The plate coated with zinc was highly brilliant without bubble forrnation after tempering
tre~tment The index of distribution of coating thickness was 1.6 to 1.7.
Example 10:
A zinc coating was deposited with a bath according to Example 1. Yet, instead of 3-
dimethylamino- 1-propylamine-epichlorhydrin copolymer, one of the compounds
a) 1,3-tetramethyldiaminopropane-epichlorhydrin copolymer in water or
CA 0221~048 1997-09-10
'_
b) diethylenetriamino-epichlorhydrin copolymer in water
was used. The coatings had a similarly good distribution of coating thickness to the
coatings produced according to Exarnple 1.
Example 11:
(Distribution of coating thickness using various diallyldimethylamrnonium chloride
sulphur dioxide copolymers):
Bath composition:
zinc as zinc oxide 10 g,
sodium hydroxide 130 g,
sodium carbonate 20 g,
1-benzylpyridinium-3-carboxylate 10 mg,
sodium gluconate 1.5 g,
3-mercapto-1.2.4-triazol 0.1 g,
additive: 3-dimethylamino-l-
propylamine-epichlorhydrin copolymer 0.5 ml
per litre of aqueous solution.
The diallyldimethylammonium chloride sulphur dioxide copolymers (XP I xx) were
produced with varying sulphur dioxide and initiator quantities (see synthesis example)
CA 0221~048 1997-09-10
24
and used as solutions at 25% by weight in water. The compounds PAS A5 and PAS 92
are products of the Company Nitto Boseki Co., Ltd., Tokyo, Japan, and are, according
to the information of the manufacturer, likewise diallyldimethylammonium chloride-
sulphur dioxide copolymers, however with a 6-ring system (according to structural
formula IV) not according to the invention.
The coating results obtained by means of a Hull cell are given in the following. The
coating conditions correspond to those of Example 1. The values in Table 1 indicate the
coating thickness distribution indices.
Table 1
Concentration 4.0 ml/l 6.0 ml/l 8.0 ml/l lO.Oml/l
PAS A5 3.04 3.0 2.94 2.8
PAS 92 3.85 3.82 3.67 3.62
XP 101 2.1 2.0 2.0 1.8
XP 103 2.3 2.0 2.0 2.1
XP 104 2.0 1.9 1.9 1.6
XP 105 2.1 2.0 1.8 2.1
XP 106 2.4 2.0 1.9 2.0
XP 114 2.3 2.5 2.2 1.9
Example 12:
The same bath composition as in Example 11 was used. However, there were used as
an additive various compounds and combinations of compounds according to the
following Table (BP3C:I-benzylpyridinium-3-carboxylate~ IMEP: imidazole-
CA 0221~048 1997-09-10
-
epichlorhydrin copolymer, compound II: 3-dimethylamino-1-propylamine-
epichlorhydrin copolymer). The coating conditions correspond to those of the
preceding Example.
Table 2 gives the indices of coating thickness, combination number 5 being a
co~llpa~dlive exarnple not according to the invention:
Table 2
Comb. XP 104 BP3C IMEP Cpd.II layerthickness
index
[ml/l] [mg/l] [ml/l] [ml/l]
- 2.0
2 5 40 - - 1.6
3 5 80 - - 1.6
4 5 80 - 0.5 1.65
- 40 - 0.5 2.86
6 5 - 0.3 - 1.85
7 5 - 0.6 - 1.95
8 5 - 0.6 0.5 2.20
9 5 - 1.0 0.5 2.05
- 2.0 0.5 2.0
Example 13:
Synthesis example for diallyldimethylammonium chloride-sulphur dioxide copolymer
ofthe type XP 104:
An aqueous solution at 60% by weight of 1260 g of di~llyldimethylammonium chloride
(Company Aldrich, Germany) was diluted with 540 ml water. 200 g sulphur dio.Yide at
-
CA 02215048 1997-09-10
-
26
up to 30~C were slowly introduced to this solution. At 28~C, 8.7 g ammonium
peroxodisulphate in 43 ml water was added as an initiator. After 2.5 hours a further 13
g of arnmonium peroxodisulphate, dissolved in 65 ml water, was added. Then heating
was carried out for 5 hours at 70~C.
A product is obtained with an average molecular weight of about 1400 g/Mol in the
polymeric proportion. The molecular weight was determined by gel permeation
chromatography (GPC) with Ultrahydrogel 120/250; refractometric determination,
pullulane and maltooligosaccharide standard.
In addition to the further compounds (XP 1 xx) quoted in Table 1, the synthesis data are
to be seen in the following Table 3; production is effected, if not stated otherwise,
according to the above synthesis example for ~P 104.
Table 3:
Type diallyldimethyl- SO2 ammonium reaction
ammonium chloride persulphate conditions
XP 101 87g of a 60% 13.3 g 0.6 g in 3 ml 24 hrs @ 50~C
solution with water
37 ml water
XP 103 85 g of a 60% 9.1 g 0.4 g in 2 ml 16 hrs ~67~C
solution with water, then 0.6
25 ml water in 3 ml water
XP105 87~,ofa60% 13.9g 0.6~,in3ml 16hrs~68''C
solution water
XP 106 69.9g of a 60% 10.9 g 0.5 g in 2.4 ml 18 hrs ~ 1 08~C
solution with water
15 ml water
XP 114 58g of a 60% 9.4 g 0.6 g in 3 ml ~6 hrs @. 77~C
solution with water
50 ml water
CA 0221~048 1997-09-10
-
27
Similarly to the measuring method described in Lancaster, Baccei and Panzer in
Polymer Letters, Edition 14, Page 549 (1976), the chemical structure ofthe compound
was obtained in D20 as a solvent and trimethylsilyl propanosulphonate (TSP) as a
standard. In the '3C-NMR spectrum the compound gave signals which must be
associated with a 5-ring:
In the following a typical example is presented:
C-NMR (D20,TSP): 34.9 (ring-CH-).
54.5 (-CH2-S02-)
55.5. 57.1 and 57.7 (CH3-N+-).
71.1 und 71.4 (ring-CH2-)-
These signals agree well with the NMR data for 5-ring polymers indicated in the literary
source cited above.
The association to a 5-ring system discovered receives further support as to the number
and position of the signals by agreement of the spectra measured with calculated
spectra.
In addition, in the '3C-NMR spectra, the signals for the monomer
diallyldimethylammonium chloride and for a poly-diallyldimethylammonium chloride
sequence or a diallyldimethylammonium chloride homopolymer were recognisable.
CA 02215048 1997-09-10
28
Poly-diallyldimethyl ammonium chloride signals:
'3C-NMR (D20,TSP): 29,2 (skeleton-CH2-)
41.0 and 41.4 (ring-CH-),
55.2 and 56.9 (CH3-N+-),
73.2 (ring-CH2-)
Example 14
Synthesis example of 3-dimethylamino- 1 -propylamine-epichlorhydrin-copolymer:
1.088 kg 3-Dimethylamino-l-propylamine
0.709 kg epichlorhydrin
2.7 litres water
3-dimethylamino-1-propylamine was provided. With stirring, water was added in 500
rnl portions. Epichlorhydrin was also added in portions to the reaction mixture while
being stirred. The reaction vessel was then heated to an internal temperature of 1 00~C,
and the reaction mixture stirred for 5 hours. The mixture was then brought to pH 4 with
sulphuric acid.
The overall weight of the reaction mixture was then 7.25 kg, the polymer content 1.8 kg,
the overall volume 6.4 litres and the dry weight of the product 38% by weight of the
reaction mixture.
CA 02215048 1997-09-10
-
29
Example 15
Bath Composition per litre solution
zinc 10 g/l
sodium hydroxide 130 g/l
sodium carbonate 25 g/l
sodium gluconate 1.2 g/1
3-mercaptotriazole 0,1 g/1
3 -dimethylamino- 1 -propylamine-
epichlolllydlin copolymer,38~/O/wt. 0,5 ml/l
XP- 104, 25%/wt. 4ml/1
1-benzylpyridinium-3-carboxylate 40 mg/1
residue water
On a Hull cell plate coated at 1 ampere, after 15 minutes the coating thicknesses were
6.35 and 3.8 ,um respectively at high and low current density. The ratio of coating
thickness is 1.65.
Example 16:
1 ml/l of XP-104 was added to the bath according to Example 15. The measured
coating thicknesses, with similar treatment, were 6.41 and 3.86 ~Lm, and the resultant
ratio of coating thickness is 1.66.
CA 02215048 1997-09-10
-
Example 17:
Example 16 was repeated, but diallyldimethylammonium chloride (DADMAC) was
added as a solution at 60% by weight at 0.2 ml/l. The measured coating thicknesses
after 15 minutes were 6.21 and 4.38 ,um; this corresponds to a coating thickness ratio of
1.4.
Example 18:
To the bath composition according to Example 16, but without
dimethylaminopropylamine-epichlorhydrin copolymer, XP-104 and benzylpyridinium
carboxylate, 2 - 20 ml/l (DADMAC) were added. The results can be seen from the
following Table 4.
Table 4
DADMAC layer thickness llm ~ current density distrib./relative
ml/l high low
2 1.27 1.05 1.21 1.21
4 1.29 3.97 0.32 3.13
8 1.14 3.96 0.28 3.57
12 2.37 3.82 0.62 1.61
8.12 2.18 3.72 3.72
This example shows that monomers alone can intensely influence a distribution of
coating thickness, so that the exemplary additions of 2 and 12 ml/l still come under the
solution according to the invention. However the version of E,xample 17 is the preferred
CA 0221~048 1997-09-10
embodiment, as according to Example 17 brilliant and uniform coatings were achieved,
while in Example 18 the result on a Hull cell plate was worse in that the coating was
matt and dark and at high additions of monomer, scorching was in part to be observed.
In addition, a deposition of pulverulent zinc was noted on the plate.
Example 19:
This Example shows the effect of the pure polymer.
A reaction solution of XP- 104 was produced according to Example 12, was part-
lyophilised to a moist oil, and mixed with a multiple volume of methanol. A crystalline
solid matter was precipitated. This was separated and then re-crystallised from
methanol-water. According to H-NMR, the colourless crystals were free of
diallyldimethylammonium monomer and contained only the diallyldimethylammonium
chloride-sulphur dioxide copolymer. The average molecular weight was 5,300 g/Mol,
determined according to the method already described.
This material, XP-104 re-cr,vstallised, was, as described above, subjected to a Hull cell
test at 1 A for 15 mimlt~c and at 24~C.
Bath Composition per litre solution
zinc 10 g/l
sodium hydroxide 130 g/l
CA 02215048 1997-09-10
sodium carbonate 25 g/l
Trilon D (complexing triacetic acid by
BASF Co.), instead of sodiurn gluconate 1.25 ml/l
3-mercaptotriazole 0.1 g/l
XP-104-recrystallised in divergent quantities with divergent results according to Table 5
residue water
Table 5
XP- 104 layer thickness llm ~ Index Layer feature
-recryst. current density layer length 100 mm
g/l high low
0.36 8.34 3.5 2.38 grey-matt to 90-95 mm
0.50 8.25 3.65 2.26 grey-matt to 70-75 mm
0.75 7.06 3.43 2.05 semi-bright to bnght
1.0 7.09 4.09 1.93 uniform
The last coating is regarded as technically satisfactory in the sense of the invention, and
contains as an organic bath matrix only one component, 3-mercaptotriazol, in addition
to the claimed polymer XP-104.
The complexing compounds gluconate or Trilon D are used by the person skilled in the
art for water softening, and a~e not an inherent ingredient of the bath matrix.
Examples 20 to 22:
To the bath according to E~cample I there were added 50 ppm iron as iron sulphate
CA 0221~048 1997-09-10
'_
relative to zinc.
The bath was tested in the same way as Example 1. The coating thicknesses at the
measuring points were 5.7 to 3.4 llm. The index was 1.7; the Fe content of the coating
was 0.55%. A semi-brilliant and uniform covering was produced, which is easily
chromated with Ecopas Black 8000, a bath of the Company Atotech Espana, S.A.,
cont~ining a chrome salt and inorganic acids. A uniformly black decorative finish is
produced.
Example 20 was repeated as Examples 21 and 22, yet once with an addition of 50 ppm
iron as iron saccharate, and once with iron gluconate. The results corresponded to those
of Example 20.
Example 23:
Added to the bath according to Example 3 are 50 ppm iron as iron sulphate relative to
zmc.
A uniform and semi-brilliant coating was produced. The coating thickness came to 6.8
to 5.5 llm; the index was 1.2. The iron content of the covering was 0.65%. The
covering is easy to chromate within 30 seconds with Tridur Yellow, a bath containing
chrome salts of the Firm Atotech Espana, S.A., in order to produce a yellow iridescent
colouring.
CA 02215048 1997-09-10
34
After tre~trnent for one hour of the coated plates according to Examples 20 to 22 in the
tempering furnace at 1 80~C, the plates were unaltered and had no bubbles.