Note: Descriptions are shown in the official language in which they were submitted.
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
1
1 "BIODEGRADABLE DEVICE"
2
3 The present invention relates to a biodegradable device
4 to aid healing.
6 Advances in surgical techniques, particularly micro-
7 surgical techniques, have enabled operations for re-
8 joining or aligning severed nerves and blood vessels to
9 be undertaken. However, to be successful such
operations still rely upon the natural healing and
11 regeneration processes of the body. Thus, even where
12 the surgeon has exerted considerable skill in aligning
13 nerve ends, there will be cases where the parts of
14 nerves fail to re-join, or where the healing process is
so slow that the effector muscle has atrophied by the
16 time that the motor nerve connection becomes effective.
17
18 Healing, for example nerve regeneration, remains an
19 essentially biological process. Even the most advanced
micro-surgical techniques for repairing damaged tissue
21 members merely optimise the environment for the natural
22 process. It is now believed that micro-surgery has
23 maximised the mechanical processes for body repair, but
24 a need still exists for enhancing the healing process
still further.
CA 02215200 2006-02-06
- 2 -
Tubes have been used to repair severed nerves, but have
enjoyed little success because the non-biodegradable tubes
remained after the regenerating nerve had been established
and impeded subsequent maturation of the nerve.
GB-A-2,099,702 describes a structural support member for
skeletal and tissue members comprised of a biodegradable
glass. However, for the healing process to be successful
it is essential that the correct chemical environment is
created to optimise the regeneration of the damaged body
part, whilst protecting that part from the body's own
defence system which can be activated against implanted
foreign bodies.
SUMMARY OF THE INVENTION
In one aspect, there is provided a biodegradable device
being of hollow construction having first and second
apertures, each aperture being adapted to receive a cut
end of a tissue member, said device having fixant means
adapted to secure the cut ends of the tissue member and a
free space between the apertures, said free space
containing a substance to facilitate healing of said
tissue member, said device formed at least in part from
biodegradable glass which biodegrades over a pre-selected
period, and said device comprising an injection port
enabling access to the interior volume of the device.
In another aspect, there is provided use of a device as
defined herein for treating a cut tissue member of a human
or non-human animal body.
CA 02215200 2006-09-22
- 2a -
DETAILED DESCRIPTION
Generally the device will be tubular. For example the
device may be an open-ended tube, the two open ends
forming the apertures for receiving the ends of the cut
tissue member.
For convenience of manufacture the device may be
essentially an open-ended cylinder of uniform internal
cross-section. Alternatively, the device may incorporate a
reservoir portion, in which reserves of the substance are
located. In this embodiment the device may be tubular, but
have an internal cross-
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
3
1 section of varying diameter, for example of increased
2 diameter in the portion between said apertures. To
3 optimise the healing together of the two cut ends
4 secured in the device, the apertures may be arranged to
face each other. However, in certain instances this
6 arrangement may not be essential, and the aperatures
7 need not be aligned.
8
9 The device of the present invention may be formed from
a biodegradable glass. Such glasses are known to those
11 skilled in the art and the composition of the glass may
12 be adjusted to produce a glass composition that
13 biodegrades over the period required, for example 1 to
14 6 months, or 1 to 3 months. Desirably the products
resulting from degradation of the glass are
16 physiologically compatible.
17
18 Additionally, the glass composition may itself be used
19 as a vehicle to deliver biologically active agents in a
controlled release manner over the period during which
21 healing occurs. Controlled Release Glasses (CRG) are
22 inorganic polymers, normally based on phosphates of
23 sodium and calcium, which have been converted into a
24 glassy form by melting the constituents at about
1000 C. CRGs dissolve in water completely leaving no
26 solid residue.
27
28 The rate of dissolution can be selected by adjustment
29 of the composition and physical form of the CRG and is
constant for as long as any of the material remains.
31 The product can be produced in many physical forms; as
32 a powder or granules, fibre or cloth, tubes, or as cast
33 blocks of various shapes.
34
As stated above, suitable biodegradable glasses are
36 known in the art, but particular mention may be made of
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
4
1 the glasses disclosed in WO-A-90/08470 of Giltech
2 Limited. Typically, the glass compositions may
3 comprise:
4
Na20 7-33 mole%
6 K20 0-22 mole%
7 CaO 0-21 mole%
8 MgO 0-22 mole%
9 P205 46-49 mole%
11 Such glass compositions may achieve solution rates of
12 from 0.03 to 3.0 mgcm'2hr-t in de-ionised water at 37 C.
13
14 Elements other than sodium and calcium, including most
metals as their oxides and a limited number of
16 inorganic anions, can be included in the composition of
17 the glass. These elements, which may be biologically
18 active, can then be delivered at a constant rate into
19 an ambient aqueous medium (for example a physiological
fluid) as the CRG dissolves. This has found
21 application in veterinary medicine as a means of
22 delivering such diverse substances as trace elements,
23 anthelmintics and vaccines. Incorporation of a silver
24 source (for example silver orthophosphate) into the
Na20-CaO-P2O5 systems offers the possibility of producing
26 a CRG capable of releasing silver ions over a highly
27 defined time, into biological systems with safety.
28
29 In the course of developments of this type the
biocompatibility and absence of toxicity of CRG based
31 on Na20-(Ca,Mg)O-PZ05 with and without other constituents
32 have been investigated. In applications differing as
33 widely as use in orthodontics devices [see Savage, 34 Brit. J. of
Orthodontics 9: 190-193 (1982)], and in
controlled supply of Cu, Co and Zn in cattle [see Drake
36 et al, Biochem. Soc. Trans. 13 : 516-520 (1985)], no
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00754
1 ill effects were observed.' When CRG pellets were
2 implanted subcutaneously, intramuscularly and
3 intraperitoneally in rats, sheep and cattle, reaction
4 at the implant site was limited to a sterile fibrous
5 encapsulation less well developed than that expected
6 from biocompatible surgical materials [see Allen et al,
7 Vet. Soc. Commun 2 : 78-75 (1978)]. Other application
8 of CRG in the Na2O-CaO-PzO5 system have been found as
9 potential bone graft adjuncts/substitutes. No sign of
cytotoxicity was observed after soft tissue
11 implantation in sheep [see Burnie et al, Biomaterials 2
12 : 244-246 (1981)]. In further experiments with bone no
13 ill effects nor bioincompatibility could be detected
14 [see Burnie et al, "Ceramics in Surgery" Ed Vincenzini,
Elseveier Scientific, 1983, pages 169-176; Burnie et
16 al, J. Bone & Joint Surgery 65B 3: 364-365 (1983);
17 Duff et al, Strathclyde Bioengineering Seminars,
18 Biomaterials in Artificial Organs, and Paul et ai,
19 Macmillan Press, 1984, pages 312-317].
21 The glass composition may include one or more metal
22 ions which are slowly released from the composition to
23 facilitate healing. Mention may be made of K, Mg, Zn,
24 Al, Se, Si, Fe, Ag, Cu, Mn, Ce and/or Au.
26 In particular the glass composition may be manufactured
27 to provide a potassium-rich environment, which may be
28 useful in aiding healing of the tissue member,
29 especially nerves.
31 The substance located in the device will be selected to
32 facilitate healing of the cut tissue member. The
33 viscosity, osmolality and pH of the substance should
34 therefore be chosen to be physiologically compatible
with the type of tissue to be healed. The substance
36 may optionally contain one or more physiologically
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
6
1 active agents and mention may be made of growth factors
2 (especially growth factors specific for the type of
3 tissue concerned, such as nerve growths factors for
4 nerve re-generation), anti-coagulants, agents to combat
infections (for example antibiotics, silver ions etc)
6 and the like. Mention may be made of platelet
7 released and PDGF, Nerve growth factor, Keratinocyte
8 stimulation factors, Insulin-like growth factor,
9 Interleukins, peptides, enzymes and other topical
agents, oxygenators and free radical scavengers,
11 enzymes and nutritional agents such as proteins and
12 vitamins. Optionally the surfaces of the glass device
13 may be coated with silicone to reduce thrombogenesis.
14
Over a number of years a great deal of evidence has
16 emerged from in vitro experiments to suggest that the
17 group of substance known as 'nerve growth factors' or
18 'nerve cell rescue factors' may enhance the
19 regeneration process which takes place after a nerve is
injured and repaired. There are now many such
21 substances awaiting evaluation. Some are thought to
22 act preferentially on either motor or sensory nerves
23 and the potential for their use in chemically
24 manipulating and improving the results of surgical
nerve repair is enormous. Despite at least 20 years of
26 study in the laboratory little or no success has been
27 achieved in the method of delivery to this site of
28 injury and also because the tests which are used to
29 quantify nerve repair are insufficiently sensitive to
resolve the small (but most useful) benefits which
31 growth factors may bring. For a substance to have
32 maximal effect is must be delivered at the site of
33 regeneration, at an appropriate and maintained
34 concentration and at the time at which its effect on
the growing nerve axons will be most effective. To
36 achieve this, delivery must be constant at the site of
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
7
1 injury over the growing period and diffusion away from
2 this site must be insufficient for the local
3 concentration to fall below effective values. Lundborg
4 [see G. Lundborg, Nerve Injury and Repair, 1988,
Edinburgh Churchill-Livingston] has to a small extent
6 achieved this by wrapping the site in silicon tubes
7 containing growth factors. However there is still an
8 inadequate concentration over time and the permanent
9 tube constricts the growing nerve in its maturation
phase. The end result is worse rather than better and
11 no surgeon in human practice would contemplate a second
12 operation to remove a silicon tube.
13
14 The biodegradable device of the present invention
offers two features which address these issues. First
16 the device can be made to dissolve over a timecourse
17 which would include the period of growth in length when
18 growth factors could be delivered to an isolated
19 environment but dissolution would occur before the non-
growth-factor-dependant phase of maturation (growth in
21 diameter). Secondly, growth factors could be delivered
22 into the device through a side hole by means of an
23 osmotic pump. If the outlet silicon rubber tube is
24 glued into the device a watertight system is effected.
Using proprietary osmotic pumps, growth factors can be
26 delivered in appropriate constant concentration for
27 four weeks after repair. This encompasses the time for
28 growth factor-dependant regeneration. At the end of
29 this time the device will biodegrade and the pump and
its tubing can be removed from its remote subcutaneous
31 site under local anaesthetic in a very small and simple
32 operation. The nerve is thus left unimpeded to mature.
33
34 The substance may be any means to facilitate healing,
including cellular matrices which encourage and
36 mechanically guide regeneration e.g. of nerve or
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
8
1 muscle, and/or humeral substances such as chemical
2 growth factors. By increasing the concentration of the
3 supplied substance at the site of injury and
4 regeneration the latter may be enhanced and its
specificity improved.
6
7 The fixant may be any means of securing the cut end of
8 the tissue member into an aperture of the device.
9 Desirably the fixant substantially seals the tissue
member end into the aperture. Mention may be made of
11 sutures, clips and other mechanical means, but
12 desirably the fixant should be biodegradable. Thus
13 physiologically compatible "glues" may be preferred.
14 One particular example is a fibrin-based tissue glue.
16 The device itself may comprise means to secure a tissue
17 member end in an aperture of the device. For example,
18 the internal diameter of the device may decrease in the
19 proximity of the aperture. In one preferred embodiment
the device includes internal barbs which grip the
21 tissue member once inserted. Desirably however a
22 physiologically acceptable "glue" is used to seal the
23 aperture after insertion of the tissue member. Thus
24 the glue can be used to protect the damaged ends of the
tissue member from the body's defence mechanisms.
26
27 The device of the present invention is particularly
28 useful for enhancing the healing of severed nerves,
29 including individual nerve fibres as well as nerve
bundles. The device may also be of utility for aiding
31 the healing of tissue members such as tendons, blood
32 vessels (especially capillary blood vessels), muscle
33 fibres and ducts.
34
The ends of the tissue member may be inserted into the
36 aperture of the device by any suitable means. For
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
9
1 example, the aperture may be large enough for the
2 tissue member end to be simply placed therein; the end
3 then being secured by any suitable means, preferably a
4 4 physiologically acceptable glue. However in certain
circumstances it may be desirable for the aperture to
4 6 be of similar internal diameter to the external
7 diameter of the tissue member. In this instance a
8 suture, threaded through the device is drawn through
9 the tissue member end which can then be pulled through
the aperture as required.
11
12 In one embodiment the device has a semi-porous or
13 porous region, preferably located between said
14 aperatures. Prior to implantation the device is
exposed to physiologically useful agents which may be
16 taken up into the porous or semi-porous region of the
17 device for release after implantation. The agents may
18 facilitate the healing of the tissue member. Thus, the
19 same device could be used to facilitate healing for
different types of tissue members, but will be adapted
21 specifically for each depending on the physiologically
22 useful agents taken up into the porous or semi-porous
23 region. Following implantation, said physiologically
24 useful agent(s) can be injected adjacent to the
implant, pass through the porous region and onto the
26 tissue member under repair.
27
28 In a further embodiment, the device may include an
29 opening to enable introduction of a substance into the
device before implantation and/or after implantation.
31 The opening may optionally also be used for exit of the
32 suture pulling the end of the tissue member through the
33 aperature. In one particular embodiment the device of
34 the present invention may be replenished with the
substance after implantation. Thus, for example, the
36 device could be connected to a reservoir external to
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
1 the patient and/or a time-operated pump to
2 automatically replenish the substance in said device.
3
4 In a further aspect, the present invention provides a
5 method to facilitate healing of a cut tissue member,
6 said method comprising inserting each end of said
7 tissue member into a separate aperture therefor in the
8 device of the present invention and securing the tissue
9 member ends into said apertures by means of a fixant.
11 The technique of inserting the tissue member ends, for
12 example nerve ends, into a tube and securing them there
13 with fibrin-based tissue glue is very simple. This
14 technique dispenses with the need for an operating
microscope, expensive microsurgical sutures and
16 instruments and the need for a trained microsurgeon.
17 It may thus have considerable implications for current
18 surgical practice and could further extend the repair
19 of nerves to underdeveloped countries where at present
nerve injuries may be untreatable.
21
22 In a further embodiment the device of the present
23 invention may be used totest theeffect of different
24 factors on tissue healing. For example the device may
be considered as a model system in which growth factors
26 may be tested to find out whether and to what extent
27 such factors may be helpful in promoting and directing
28 the natural process of regeneration.
29
In a yet further embodiment the present invention
31 provides a kit to aid healing of a cut tissue member,
32 said kit comprising a device of hollow construction
33 having two apertures adapted to receive the cut ends of
34 a tissue member; said kit further comprising a
physiologically acceptable fixant and a substance to
36 aid healing of said tissue member.
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
11
1 The device of the present invention may also be used in
2 vitro to promote growth of a tissue member; the
3 regenerated tissue member may subsequently be used for
4 transplantation, for example to replace a damaged
tissue member.
6
7 In a further aspect, the present invention provides a
8 method of treating a human or non-human animal body
9 having a cut tissue member, said method comprising
inserting the cut ends of said tissue member into
11 separate aperatures of the device according to the
12 invention. Optionally the device may be used in
13 conjunction with an external reservoir of the substance
14 and/or with a time operated pump to deliver the
substance to the device.
16
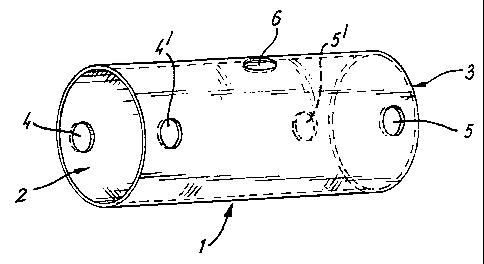
17 Fig. 1 illustrates a biodegradable glass tube suitable
18 for nerve repair.
19
Fig. 2 illustrates the biodegradable glass tube of Fig.
21 1 having a rubber tubing attached thereto.
22
23 Fig. 1 shows a biodegradable glass tube 1 suitable for
24 use in the present invention, especially for nerve
repair. Tube 1 consists of a hollow, essentially
26 cylindrical, glass body having aperatures 2, 3 at the
27 ends thereof. Two diametrically opposed suture holes
28 4,4' are located in tube 1, close to aperature 2. Two
29 similar diametrically opposed suture holes 5,5' are
also located in tube 1, close to aperature 3.
31 Approximately mid-way down the length of tube 1 is an
32 injection port 6, which enables access to the interior
33 volume of tube 1, even when tube 1 is in place within a
34 patient.
36 Fig. 2 illustrates a similar tube 1 to that shown in
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
12
1 Fig. 1, having flexible tubing 7 (for example silicone
2 tubing) passed through injection port 6 into the
3 interior volume of tube 1. Tubing 7 may be connected
4 to a pump or reservoir (not shown) containing a {
substance or active agent capable of promoting healing
6 of the body part in question. Once sufficient healing
7 has taken place tubing 7 may be simply removed, without
8 disturbing tube 1.
9
In use, one of the ends of the damaged body part will
11 be inserted into aperature 2 of tube 1, optionally
12 after trimming the end of the body part. A suture will
13 then be passed through a first suture hole 4, through
14 the end of the body part inserted through aperature 2
and out through suture hole 4'. The ends of the suture
16 will then be securely fastened. Optionally a tissue
17 glue may then be used to seal the body part into the
18 aperature 2 of tube i.
19
The process described above will then be repeated with
21 the other end of the damaged body part, aperature 3 and
22 suture holes 5,5' of tube 1.
23
24 Optionally tubing 7 may be passed through injection
port 6 into the interior volume of tube 1 and an
26 appropriate substance fed into the free space within
27 tube 1 to provide an environment suitable for healing
28 the body part. The two ends of the body part will
29 gradually grow down the interior of tube 1 and, on
meeting will knit together. Alternatively the
31 substance may be simply injected into the free volume
32 within tube 1 by any suitable means (e.g. syringe).
33
34 For very small body parts (e.g. the sciatic nerve of
rats, the common peroneal nerve of rabbits or similarly
36 sized body parts of other animals), the length of the
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
13
1 glass tube may be 20-26mm (e.g. 22mm) with an outer
2 diameter of 4-5mm. The tube itself may have a
3 thickness of 1-2mm (e.g. 1.2mm) and the suture holes
4 and injection ports may each typically have a diameter
of 0.5-lmm (e.g. 0.7mm).
6
7 For slightly larger body parts, a larger dimensioned
8 tube will be required, and the dimensions recited above
9 may be adapted as required. For example in sheep, a
tube length of 30mm having an outer diameter of 8-9mm
11 and inter diameter of 7mm, with suture hole and port
12 diameter of 1.2-1.3mm may be sufficient.
13
14 The invention will be further described with reference
to the following, non-limiting, examples.
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
14
1 Example 1
2
3 All procedures were performed on rats and under sterile
4 conditions.
6 1. The biceps femoris muscle was retracted. Care was
7 taken not to involve the medial femoral circumflex
8 artery which supplies these muscles.
9
2. The sciatic nerve was cut about 2cm from the
11 sciatic notch. (Midway down the nerve).
12
13 3. A biodegradable glass tube (as illustrated in
14 Figure 1) was cut to size enabling 2mm of nerve to
extend into the centre of the tube.
16
17 The glass of the tube was composed as follows:
18
19 Mole %
Na20 32.0
21 CaO 21.0
22 P205 47.0
23
24 The glass had a solution rate when annealed of
0.4mgcm'2hr'1 in de-ionised water at 37 C. The tube
26 had a physiological life expectancy of
27 approximately 40-50 days.
28
29 4. The tube was secured by either suture, clip or
glue.
31
32 5. The animal was kept for over 60 days before
33 undergoing electrophysiological studies and
34 microscopic analysis under anaesthesia.
36 6. EMG was taken to measure conduction velocity. The
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
1 sciatic nerve was exposed as in step 1 and
2 dissected out 2cm above the graft and 2cm below.
3 EMG was then taken at each point to determine the
4 speed of conduction:
5
6 (EMG time proximal - EMG time distal)
7. Distance between points
8
9 The Extensor digitorum longus muscle was chosen
10 for the EMG because the nerve supply is the Deep
11 Peroneal Nerve which is a direct tributary of the
12 Sciatic-Common Peroneal Division.
Results
Type of Graft Length (mm) Conduction Healing
(if removed) Velocity time
(M/s) (days)
Tube and Clip 13 4.33 46
Tube and Clip 24 25.26 67
Tube and Clip 25 31.25 114
Tube and Suture 12.5 8.06 47
Tube and Suture 38 19.46 68
Tube and Suture 27 31.76 68
Tube and Suture 15 21.43 90
Tube and Suture 18 21.18 90
Tube and Suture 23 17.04 96
Normal 18 36 -
13 Example 2
14
15 A further study was conducted to establish:
16
17 a) that a biodegradable glass tube (BGT) was
18 compatible with effective nerve repair; and
19
1 b) that the BGT was not toxic to the regenerating
2 nerve or to the surrounding tissue and that the BGT
3 did not provoke a fibrotic tissue reaction or
4 immune response likely to affect nerve regeneration
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
16
1 adversely.
2
3 The experiments were performed in rats. The sciatic
4 nerve was divided and a BGT (as used in Example 1)
placed over it. With the BGT pushed to one side the
6 nerve stumps were repaired by epineurial suture. The
7 BGT was then placed at the repair site and fixed in
8 place with epineurial sutures and fibrin glue.
9 Electrophysiological and morphometric assessment was
carried out at 100 days. It was found that normal nerve
11 regeneration had taken place and that the BGT had
12 completely dissolved. There was no sign of any adverse
13 reaction.
14
Example 3
16
17 This experiment was conducted on New Zealand large white
18 rabbits. In eac+ rabbit the common peroneal nerve was
19 divided and repaired in the upper thigh. The tibial
nerve was left intac-_. ?GTs were all as described in
21 Example 1 and all of 1.5cm in length. Each of the
22 methods of repair represented by the contents of the
23 tube are accepted clinical techniques for nerve repair
24 with the exception of the gap which was a control and
which would not be expected to be compatible with
26 recovery of nerve function.
27
28 1) BGT + lcm gap in nerve (control)
29 2) BGT + lcm freeze-thawed muscle autograft (FTMG)
3) BGT + lcm nerve autograft
31 4) BGT + nerve and FTMG short lengths in series to
32 length of lcm
33 5) FTMG without tube (control).
34
There were 5 rabbits in each group.
36
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
17
1 Each animal was reviewed 6 months after nerve repair.
2 Under anaesthesia the repair site was re-exposed and the
3 nerve was subjected to a number of electrophysiological
4 tests. Some of these tests have become well established
as a means of assessing recovery after nerve repair.
6 Others are new tests which are currently being evaluated
7 in an attempt to find tests which will resolve the small
8 but important improvements in nerve regeneration which
9 may be expected where nerve growth factors are used. In
all cases the opposite limb was used as a control.
11
12 After electrophysiological assessment, the segments of
13 repaired and control nerve were excised and processed
14 for microscopic examination. Computerized morphometric
assessment was used to measure indices of nerve
16 regeneration such as axon and fibre diameter and G-
17 ratio.
18
19 In group 1 above it was surprising to find that
regeneration had taken place albeit to a limited extent.
21 It seems likely that isolating the regenerating nerve
22 within the tube may have improved its chances of
23 crossing the gap. This result speaks well for the fact
24 that the tube does not impede nerve regeneration.
26 In groups 2, 3 and 4 all of the indices of recovery
27 showed comparability with the best results obtained by
28 conventional means. This means that as a supporting
29 medium for either direct repair or repair using short
neural and FTMG grafts the BGT system performs as well
31 as anything else currently available.
32
33 Group 2 demonstrated the best results, with all groups
34 1, 2 and 3 giving successful regeneration of the
peripheral nerve. There were no signs of neuroma in any
36 of the groups and the BGT was completely dissolved after
CA 02215200 1997-09-11
WO 96/31160 PCT/GB96/00784
18
1 the 6 month test period.