Note: Descriptions are shown in the official language in which they were submitted.
CA 02216731 1997-09-25
WO 96/36394 PCT/US96/06098
1
1 ELECTROTRANSPORT DEVICE HAVING REUSABLE
2 CONTROLLER
3
= 4 TECHNICAL FIELD
6 The invention relates to electrotransport drug delivery systems having
7 a drug containing assembly and a reusable controller having an electrically
8 powered control circuit, the assembly and the controller being separably
9 connected by a coupler which establishes electrical connection of the
assembly to the controller.
11
12 BACKGROUND ART
13
14 The term "electrotransport" as used herein refers generally to the
delivery of an agent (e.g., a drug) through a membrane, such as skin, mucous
16 membrane, or nails. The delivery is induced or aided by application of an
17 electrical potential. For example, a beneficial therapeutic agent may be
18 introduced into the systemic circulation of a human body by
electrotransport
19 delivery through the skin. A widely used electrotransport process,
electromigration (also called iontophoresis), involves the electrically
induced
21 transport of charged ions. Another type of electrotransport,
electroosmosis,
22 involves the flow of a liquid, which liquid contains the agent to be
delivered,
23 under the influence of an electric field. Still another type of
electrotransport
24 process, electroporation, involves the formation of transiently-existing
pores in
a biological membrane by the application of an electric field. An agent can be
26 delivered through the pores either passively (i.e., without electrical
27 assistance) or actively (i.e., under the influence of an electric
potential).
28 However, in any given electrotransport process, more than one of these
29 processes may be occurring simultaneously to a certain extent. Accordingly,
the term "electrotransport", as used herein, should be given its broadest
CA 02216731 1997-09-25
WO 96/36394 PCT/US96106098
2
1 possible interpretation so that it includes the electrically induced or
enhanced
2 transport of at least one agent, which may be charged, uncharged, or a
3 mixture thereof, whatever the specific mechanism or mechanisms by which
4 the agent actually is transported.
Electrotransport devices use at least two electrodes that are in
6 electrical contact with some portion of the skin, nails, mucous membrane,
7 or other surface of the body. One electrode, commonly called the "donor"
8 or "active" electrode, is the electrode from which the therapeutic agent is
9 delivered into the body. The other electrode, typically termed the "counter"
or "return" electrode, serves to close the electrical circuit through the
body.
11 For example, if the agent to be delivered is positively charged, i.e., a
cation,
12 then the anode is the active or donor electrode, while the cathode serves
13 to complete the circuit. Alternatively, if an agent is negatively charged,
14 i.e., an anion, the cathode is the donor electrode. Additionally, both the
anode and cathode may be considered donor electrodes if both anionic and
16 cationic agent ions, or if uncharged or neutrally charged agents, are to be
17 delivered.
18 Furthermore, electrotransport delivery systems generally require at
19 least one reservoir or source of the agent to be delivered, which is
typically in
the form of a liquid solution or suspension. Examples of such donor
21 reservoirs include a pouch or cavity, a porous sponge or pad, and a
22 hydrophilic polymer or a gel matrix. Such donor reservoirs are electrically
23 connected to, and positioned between, the anode or cathode and the body
24 surface, to provide a fixed or renewable source of one or more agents or
drugs. Electrotransport devices also have an electrical power source such as
26 one or more batteries. Typically, one pole of the power source is
electrically
27 connected to the donor electrode, while the opposite pole is electrically
28 connected to the counter electrode. In addition, some electrotransport
29 devices have an electrical controller that controls the current applied
through
the electrodes, thereby regulating the rate of agent delivery. Furthermore,
CA 02216731 2006-09-13
67696-241
3
, passive flux control membranes, adhesives for maintaining device contact
2 with a body surface, insulating members, and impermeable backing members
3 are other optional components of an electrotransport device.
4 All electrotransport agent delivery devices utilize an electrical
circuit to electrically connect the power source (e.g., a battery) and the
6 electrodes. In very simple devices, such as those disclosed in Ariura et al
7 US Patent 4,474,570, the "circuit" is merely an electrically conductive wire
8 used to connect the battery to an electrode. Other devices use a variety
9 of electrical components to control the amplitude, polarity, timing,
waveform shape, etc. of the electric current supplied by the power source.
11 See, for example, McNichols et al US Patent 5,047,007.
12 To date, commercial transdermai electrotransport drug delivery devices
13 (e.g., the Phoresor soid by iomed, Inc. of Salt Lake City, UT; the Dupei
14 lontophoresis System sold by Empi, Inc. of St. Paul, MN; the Webster Sweat
Inducer, model 3600, sold by Wescor, Inc. of Logan, UT) have generally
16 utilized a desk-top electrical power supply unit and a pair of skin
contacting
17 electrodes. The donor eiectrode contains;a drug solution while the counter
18 electrode contains a solution of a bio-compatible electrolyte salt. The
19 "satellite" electrodes are connected to the electrical power supply unit by
long
(e.g., 1-2 meters) electrically conductive wires or cables. Exampies of desk-
21 top eiectrical power supply units which use "satellite" electrode
assemblies
22 are disclosed in Jacobsen et al US Patent 4,141,359 (see Figures 3 and 4);
23 LaPrade US Patent 5,006,108 (see Figure 9); and Maurer et al
24 US Patent 5,254,081 (see Figures 1 and 2). The power supply units in
such devices have electrical controls for adjusting the amount of electrical
26 current applied through the eiectrodes. Existing commercial
electrotransport
27 devices are approved for operation only by trained medical technicians.
28 One important consideration when connecting the "satellite" electrodes to
the
29 power supply unit is to make sure that the electrodes are connected with
the
correct polarity, i.e., a satellite donor electrode which contains a cationic
CA 02216731 1997-09-25
ARC 2380 CIP1
1 therapeutic agent must be connected to the positive output of the controller
2 whereas a satellite donor electrode which contains an anionic therapeutic
3 agent must be connected to the negative output of the controller. In order
to
4 assist the medical technician to make the correct polarity connections,
s two approaches have been used. In the first approach, the outputs of the
6 controller have been color coded to the appropriate satellite electrode.
7 In the second approach (used in the CF Indicator sold by ScandiPharm, Inc.),
8 the controller is provided with electrodes in the form of metal (e.g.,
stainless
9 steel) plates which are positioned on one side of the controller housing.
The two electrode plates have different geometric shapes (e.g., one square
>> and one circular). The drug-containing donor gel and the electrolyte-
12 containing counter gel each have a different shape which corresponds to the
13 respective electrode plate shape in order to ensure that the donor and
14 counter gels are placed in contact with the correct (i.e., correct
polarity)
is electrodes.
16 More recently, small self-contained electrotransport delivery devices
17 adapted to be worn on the skin, sometimes unobtrusively under clothing,
18 for extended periods of time have been proposed. The electrical components
19 in such miniaturized electrotransport drug delivery devices are also
preferably miniaturized, and may be either integrated circuits (i.e.,
21 microchips) or small printed circuits. Electronic components, such as
22 batteries, resistors, pulse generators, capacitors, etc., are electrically
23 connected to form an electronic circuit that controls the amplitude,
polarity,
24 timing, waveform shape, etc. of the electric current supplied by the power
source. Such small self-contained electrotransport delivery devices are
26 disclosed for example in Tapper US Patent 5,224,927; Sibalis et al US
Patent
27 5,224,928 and Haynes et al US Patent 5,246,418. European Patent
28 Application 0 337 642 discloses an iontophoretic device that is light in
weight,
29 , easily manufactured and assembled, and directly and simply applied to the
patient's skin. The device includes two quick connectors.
AMENDED SHEET
CA 02216731 1997-09-25
WO 96/36394 PCI1US96/06098
1 There have recently been suggestions to utilize electrotransport
2 devices having a reusable controller which is adapted to be used with
multiple
3 drug-containing units. The drug-containing units are simply disconnected
4 from the controller when the drug becomes depleted and a fresh drug-
5 containing unit is thereafter connected to the controlier. In this way,
6 the relatively more expensive hardware components of the device
7 (e.g., batteries, LED's, circuit hardware, etc.) can be contained within the
8 reusable controller, and the relatively less expensive donor reservoir and
9 counter reservoir matrices can be contained in the disposable drug
containing
unit thereby bringing down the overall cost of electrotransport drug delivery.
11 Examples of electrotransport devices comprised of a reusable controller
12 adapted to be removably connected to a drug-containing unit are disclosed
in
13 Sage, Jr. et al, US Patent 5,320,597; Sibalis, US Patent 5,358,483;
14 Sibalis et al, US Patent 5,135,479 (Fig. 12); and Devane et al UK Patent
Application 2 239 803.
16 Electrotransport devices having reusable controllers and which are
17 adapted to be used with multiple drug-containing units are particularly
well
18 suited for drug administration to patients outside of clinic/doctor's
office
19 settings (e.g., for those patients requiring long term medication).
Unfortunately, the existing schemes for ensuring that the drug reservoir
21 of an electrotransport device is connected to the electrode of the correct
22 polarity are not foolproof. This becomes an even greater problem in
settings
23 outside of the clinic/doctor's office where the patient is expected to
24 periodically replace the drug-containing unit him/herself. The problem
becomes still greater in cases where the patient population tends to be
26 more elderly.
= E
CA 02216731 2007-08-01
67696-241
6
DESCRIPTION OF THE INVENTION
It is an aspect of the present invention to ensure
correct polarity electrical connection between the drug
reservoir of a drug-containing assembly and the reusable
controller of an electrotransport device comprised of a
reusable controller adapted to be used with a plurality of
drug-containing assemblies.
The present invention is directed to ensuring
correct polarity electrical connections in an
electrotransport device comprised of a reusable electronic
controller adapted to be used with a plurality of single use
(e.g., disposable) drug-containing units. After the drug
has been depleted from the drug-containing unit, the unit is
disconnected from the controller and discarded, and then
replaced with a fresh one. The controller includes a
bipolar power source (e.g., one or more batteries), and
optionally a circuit for controlling the timing, frequency,
magnitude, etc. of the current applied by the device. The
drug-containing unit has first and second electrodes, at
least one of which contains the therapeutic agent
(i.e., drug) to be delivered.
It will be appreciated that the "agent" or
"therapeutic agent" suitable for use in the invention means
in the broadest sense any pharmaceutically-acceptable agent,
and preferably therapeutically active substances, such as
drugs or prodrugs, which are delivered to a living organism
to produce a desired, and usually beneficial, effect.
Examples of suitable agents are described in Gyory, et al.
U.S. Patent 5,169,383, Sorenson, et al. U.S. Patent
5,207,752, Sage, Jr., et al. U.S. Patent 5,320,597,
Myers, et al. U.S. Patent 5,405,317, and Myers, et al.
U.S. Patent 5,543,098. In U.S. Patent 5,169,383, for
CA 02216731 2007-08-01
67696-241
6a
example, suitable therapeutic agents for electrotransport
are defined to include: anti-infectives such as antibiotics
and antiviral agents; analgesics such as fentanyl,
sufentanil, carfentanil, lofentanil, alfentanil,
hydromorphone, oxycodone, propoxyphene, pentazocine,
methadone, tilidine, butorphanol, buprenorphine,
levorphanol, codeine, oxymorphone, meperidine,
dihydrocodeinone, opioids, cocaine and analgesic
combinations; anesthetics; anorexics; antiarthritics;
antiasthmatic agents such as terbutaline; anticonvulsants;
antidepressants; antidiabetics agents; antidiarrheals;
antihistamines; anti-inflammatory agents; antimigraine
preparations; antimotion sickness preparations such as
scopolamine and ondansetron; antinauseants; antineoplastics;
antiparkinsonism drugs; antipruritics; antipsychotics;
antipyretics; antispasmodics including gastrointestinal and
urinary; anticholinergics; sympathomimetrics; xanthine
derivatives; cardiovascular preparations including calcium
channel blockers such as nifedipine; beta-agonists such as
dobutamine and ritodrine; beta blockers; antiarrythmics;
antihypertensives such as atenolol; ACE inhibitors such as
ranitidine; diuretics; vasodilators including general,
coronary, peripheral and cerebral; central nervous system
stimulants; cough and cold preparations; decongestants;
diagnostics; hormones such as parathyroid hormones;
hypnotics; immunosuppressives; muscle relaxants;
parasympatholytics; parasympathomimetrics; prostaglandins;
proteins; peptides; psychostimulants; sedatives and
tranquilizers.
Additional agents include fentanyl hydrochloride,
pilocarpine nitrate, lidocaine hydrochloride, hydrocortisone
derivatives, sodium salicylate, acetic acid, fluoride anion,
lithium, antibiotics such as penicillin and cephalosporin
CA 02216731 2007-08-01
67696-241
6b
and dexamethasone sodium phosphate, hydromorphone, diazepam
salts, antihypertensive agents, bronchodilator agents,
peptide hormone and regulatory agents and proteins.
Also described in U.S. Patent 5,169,383 are
suitable agents including peptides, polypeptides, proteins,
and other macromolecules, which are otherwise difficult to
deliver transdermally or transmucosally because of their
size. As indicated in U.S. Patent 5,169,383, these
macromolecular substances typically have a molecular weight
of at least about 300 Daltons, and more typically, a
molecular weight in the range of about 300 to 40,000
Daltons. However, smaller and larger peptides are also
described as being deliverable by electrotransport.
Examples of peptides and proteins given and which may be
delivered by electrotransport include, without limitation,
LHRH, LHRH analogs such as buserelin, gonadorelin, naphrelin
and leuprolide, GHRH, GHRF, insulin, insulinotropin,
heparin, calcitonin, octreotide, endorphin, TRH, NT-36
(chemical name: N-[[(S)-4-oxo-2-azetidinyl]carbonyl]-L-
histidyl-L-prolinamide), liprecin, pituitary hormones,
e.g. HGH, HMG, HCG, desmopressin acetate, follicle luteoids,
alpha-ANF, growth factor releasing factor (GFRF), beta-MSH,
somatostatin, bradykinin, somatotropin, platelet-derived
growth factor, asparaginase, bleomycin sulfate, chymopapain,
cholecystokinin, chorionic gonadotropin, corticotropin
(ACTH), erythropoietin, epoprostenol (platelet aggregation
inhibitor), glucagon, hirulog, hirudin analogs,
hyaluronidase, interferon, interleukin-2, menotropins,
e.g. urofollitropin (FSH) and LH, oxytocin, streptokinase,
tissue plasminogen activator, urokinase, vasopressin,
desmopressin, ACTH analogs, ANP, ANP clearance inhibitors,
angiotensin 11 antagonists, antidiuretic hormone agonists,
antidiuretic hormone antagonists, bradykinin antagonists,
CA 02216731 2007-08-01
67696-241
6c
CD4, ceredase, CSF's, enkephalins, FAB fragments,
IgE peptide suppressors, IGF-1, neurotrophic factors, colony
stimulating factors, parathyroid hormone and agonists,
parathyroid hormone antagonists, prostaglandin antagonists,
pentigetide, protein C, protein S, renin inhibitors,
thymosin alpha-1, thrombolytics, TNF, vaccines, vasopressin
antagonist analogs, alpha-1 antitrypsin (recombinant), and
TGF-beta, as an agent-enhancer compound.
In accordance with one embodiment of the present
invention, the reusable controller is adapted to be
electrically coupled to a more limited use (e.g., a single
use) drug-containing unit by at least two electrically
conductive snap connectors. The snap connectors have
different sizes and/or are arranged with different
male/female parts in the different respective units, so that
the controller and the drug-containing unit may be coupled
in only one way, i.e., with the correct polarity
connections.
In an alternative embodiment of the present
invention, a projecting member is provided on either the
controller or the drug-containing unit with a
correspondingly shaped hole on the other unit. The
positioning of the projecting member and the correspondingly
shaped hole are such that the controller and the drug-
containing unit may be coupled in only one way, i.e., with
the correct polarity connections.
In one embodiment of the present invention, there
is provided an electrotransport device for delivering a
therapeutic agent through a body surface of a patient, the
device comprising: a therapeutic agent-containing unit
including first and second reservoirs electrically connected
to first and second electrodes provided for being
CA 02216731 2007-08-01
67696-241
6d
electrically connected to first and second poles of a
bipolar electrical power source, wherein at least one of the
reservoirs contains the therapeutic agent to be delivered;
and, a controller having the bipolar electrical power source
for providing electric current to the electrodes, the
controller having first and second connectors for
electrically connecting the first and second poles of the
bipolar power source to the first and second electrodes,
respectively, characterized in that the device further
includes a coupler capable of requiring electrical
connection between the first electrode and first pole and
between the second electrode and second pole, respectively,
when the controller is mated to the therapeutic agent-
containing unit.
According to another aspect of the present
invention, there is provided an electrotransport device for
delivering an analgesic through a body surface of a patient,
the device comprising: a therapeutic agent-containing unit
including first and second reservoirs electrically connected
to first and second electrodes provided for being
electrically connected to first and second poles of a
bipolar electrical power source, wherein at least one of the
reservoirs contains the therapeutic agent to be delivered;
and, a controller having the bipolar electrical power source
for providing electric current to the electrodes, the
controller having first and second connectors for
electrically connecting the first and second poles of the
bipolar power source to the first and second electrodes,
respectively, characterized in that the device further
includes a coupler capable of requiring electrical
connection between the first electrode and first pole and
between the second electrode and second pole, respectively,
when the controller is mated to the therapeutic agent-
CA 02216731 2007-08-01
67696-241
6e
containing unit. Preferably, the analgesic is fentanyl,
fentanyl hydrochloride, sufentanil, carfentanil, lofentanil,
alfentanil, hydromorphone, oxycodone, propoxyphene,
pentazocine, methadone, tilidine, butorphanol,
buprenorphine, levorphanol, codeine, oxymorphone,
meperidine, dihydrocodeinone, an opioid, cocaine, an
analgesic analogue or an analgesic combination.
According to still another aspect of the present
invention, there is provided an electrotransport device for
delivering insulin or insulinotropin through a body surface
of a patient, the device comprising: a therapeutic agent-
containing unit including first and second reservoirs
electrically connected to first and second electrodes
provided for being electrically connected to first and
second poles of a bipolar electrical power source, wherein
at least one of the reservoirs contains the therapeutic
agent to be delivered; and, a controller having the bipolar
electrical power source for providing electric current to
the electrodes, the controller having first and second
connectors for electrically connecting the first and second
poles of the bipolar power source to the first and second
electrodes, respectively, characterized in that the device
further includes a coupler capable of requiring electrical
connection between the first electrode and first pole and
between the second electrode and second pole, respectively,
when the controller is mated to the therapeutic agent-
containing unit.
According to yet another aspect of the present
invention, there is provided an electrotransport device for
delivering a peptide, polypeptide, protein, macromolecule or
combination thereof, through a body surface of a patient,
the device comprising: a therapeutic agent-containing unit
including first and second reservoirs electrically connected
CA 02216731 2007-08-01
67696-241
6f
to first and second electrodes provided for being
electrically connected to first and second poles of a
bipolar electrical power source, wherein at least one of the
reservoirs contains the therapeutic agent to be delivered;
and, a controller having the bipolar electrical power source
for providing electric current to the electrodes, the
controller having first and second connectors for
electrically connecting the first and second poles of the
bipolar power source to the first and second electrodes,
respectively, characterized in that the device further
includes a coupler capable of requiring electrical
connection between the first electrode and first pole and
between the second electrode and second pole, respectively,
when the controller is mated to the therapeutic agent-
containing unit.
According to a further aspect of the present
invention, there is provided an electrotransport device for
delivering a therapeutic agent through a body surface of a
user, the device comprising: drug unit (DU) having a first
end, a second end, and including one or more reservoirs one
of which contains the therapeutic agent for electrotransport
through the body surface, the DU including DU first
electrical coupler and second electrical coupler, the DU
having a surface for facing the skin for drug delivery; and
an electronic unit (EU) having a first end and a second end
corresponding to the first end and second end of the DU, the
EU having EU first electrical coupler and second electrical
coupler for coupling with the DU first electrical coupler
and second electrical coupler respectively for correct
polarity; at least one of the DU and EU has a projecting
member that is not one of said electrical couplers to
provide a visual fit with corresponding only one matching
portion of the other unit such that the EU and DU can be
CA 02216731 2007-08-01
67696-241
6g
aligned to be secured together face to face, the EU
including circuitry for electrically driving the therapeutic
agent for electrotransport.
According to yet a further aspect of the present
invention, there is provided an electrotransport device for
delivering an analgesic through a body surface of a user,
the device comprising: drug unit (DU) having a first end, a
second end, and including one or more reservoirs one of
which contains the therapeutic agent for electrotransport
through the body surface, the DU including DU first
electrical coupler and second electrical coupler, the DU
having a surface for facing the skin for drug delivery; and
an electronic unit (EU) having a first end and a second end
corresponding to the first end and second end of the DU, the
EU having EU first electrical coupler and second electrical
coupler for coupling with the DU first electrical coupler
and second electrical coupler respectively for correct
polarity; at least one of the DU and EU has a projecting
member that is not one of said electrical couplers to
provide a visual fit with corresponding only one matching
portion of the other unit such that the EU and DU can be
aligned to be secured together face to face, the EU
including circuitry for electrically driving the therapeutic
agent for electrotransport. Preferably, the analgesic is
fentanyl, fentanyl hydrochloride, sufentanil, carfentanil,
lofentanil, alfentanil, hydromorphone, oxycodone,
propoxyphene, pentazocine, methadone, tilidine, butorphanol,
buprenorphine, levorphanol, codeine, oxymorphone,
meperidine, dihydrocodeinone, an opioid, cocaine, an
analgesic analogue or an analgesic combination.
According to still a further aspect of the present
invention, there is provided an electrotransport device for
delivering insulin or insulinotropin through a body surface
CA 02216731 2007-08-01
67696-241
6h
of a user, the device comprising: drug unit (DU) having a
first end, a second end, and including one or more
reservoirs one of which contains the therapeutic agent for
electrotransport through the body surface, the DU including
DU first electrical coupler and second electrical coupler,
the DU having a surface for facing the skin for drug
delivery; and an electronic unit (EU) having a first end and
a second end corresponding to the first end and second end
of the DU, the EU having EU first electrical coupler and
second electrical coupler for coupling with the DU first
electrical coupler and second electrical coupler
respectively for correct polarity; at least one of the DU
and EU has a projecting member that is not one of said
electrical couplers to provide a visual fit with
corresponding only one matching portion of the other unit
such that the EU and DU can be aligned to be secured
together face to face, the EU including circuitry for
electrically driving the therapeutic agent for
electrotransport.
According to another aspect of the present
invention, there is provided an electrotransport device for
delivering a peptide, polypeptide, protein, macromolecule or
combination thereof, through a body surface of a user, the
device comprising: drug unit (DU) having a first end, a
second end, and including one or more reservoirs one of
which contains the therapeutic agent for electrotransport
through the body surface, the DU including DU first
electrical coupler and second electrical coupler, the DU
having a surface for facing the skin for drug delivery; and
an electronic unit (EU) having a first end and a second end
corresponding to the first end and second end of the DU, the
EU having EU first electrical coupler and second electrical
coupler for coupling with the DU first electrical coupler
CA 02216731 2007-08-01
67696-241
6i
and second electrical coupler respectively for correct
polarity; at least one of the DU and EU has a projecting
member that is not one of said electrical couplers to
provide a visual fit with corresponding only one matching
portion of the other unit such that the EU and DU can be
aligned to be secured together face to face, the EU
including circuitry for electrically driving the therapeutic
agent for electrotransport.
According to yet another aspect of the present
invention, there is provided a method of making an
electrotransport device for delivering a therapeutic agent
through a body surface of a user, comprising matching a
projecting member from one of an electronic unit (EU) and a
drug unit (DU) to corresponding only one matching portion of
the other unit, pressing together the EU and the DU; the DU
having a first end, a second end, and including one or more
reservoirs one of which contains the therapeutic agent for
electrotransport through the body surface, the DU including
DU first electrical coupler and second electrical coupler,
the DU having a surface for facing the skin for drug
delivery, the EU having a first end and a second end
corresponding to the first end and second end of the DU, the
EU having EU first electrical coupler and second electrical
coupler for coupling with the DU first electrical coupler
and second electrical coupler respectively for correct
polarity; the projecting member providing a visual fit with
the corresponding only one matching portion of the other
unit such that the EU and DU can be aligned to be secured
together face to face, the projecting member being not one
of said electrical couplers and the EU including circuitry
for electrically driving the therapeutic agent for
electrotransport.
CA 02216731 2007-08-01
67696-241
6j
In another embodiment of the present invention
there is provided a use of the electrotransport device
described herein for the delivery of an analgesic to the
body surface of the patient for the treatment of pain.
In another embodiment of the invention there is
provided a use of the electrotransport device described
herein for the delivery of insulin or insulinotropin for the
treatment of diabetes mellitus.
CA 02216731 1997-09-25
ARC 2380 CIP1
i
~ BRIEF DESCRIPTION OF THE DRAWINGS
2
3 In the figures, wherein like parts are given like reference numerals and
4 wherein;
Fig. 1 is a perspective view of an electrotransport device comprised of
6 a reusable controller and a separate drug-containing unit, in an uncoupled
7 configuration, which controller and unit are couplable in accordance with
prior
s art iontophoretic devices;
s Fig. 2 is a cross sectional view of the device shown in Fig. 1, showing
the controller and the drug-containing unit in a coupled configuration;
11 Fig. 3 is a perspective view of an electrotransport device comprised of
12 a reusable controller and a separate drug-containing unit, in an uncoupled
13 configuration, which controller and drug unit are couplable in accordance
with
14 prior art iontophoretic devices;
Fig. 4 is a perspective view of an electrotransport device comprised of
16 a reusable controller and a separate drug-containing unit, in an uncoupled
17 configuration, which controller and drug unit are couplable in accordance
with
18 one embodiment of the invention;
19 Fig. 5 is a perspective view of an electrotransport device with the
controller and the drug-containing unit in an uncoupled configuration,
21 the drug-containing unit adapted to slidably engage the controller in
22 accordance with another embodiment of the invention;
23 Fig. 6 is a bottom view of the device shown in Fig. 5 with the controller
24 and the drug-containing unit in a coupled configuration;
Fig. 7 is a sectional view of the device shown in Figs. 5 and 6, taken
26 along line 7-7 shown in Fig. 6;
27 Fig. 8 is a perspective view of an electrotransport device including a
28 reusable controller and a separate drug-containing unit in an uncoupled
29 configuration, wrerein the controller and drug-containing unit are
couplable in
accordance with another embodiment of the invention;
A%1Pt4g'D SHEET
CA 02216731 1997-09-25
WO 96/36394 PCT/US96/06098
8
1 Fig. 9 is a top view of a drug-containing unit in accordance with
2 another embodiment of the present invention;
3 Fig. 10 is a side view of the drug-containing unit shown in Fig. 9;
4 Fig. 11 is a perspective view showing the coupling of a reusable
controller to the drug-containing unit illustrated in Figs. 9 and 10;
6 Fig. 12 is a top view of the coupled system shown in Fig. 11;
7 Fig. 13 is a top view of a coupled electrotransport system in
8 accordance with another embodiment of the present invention;
9 Fig. 14 is a top view of the drug-containing unit shown in Fig. 13;
Fig. 15 is a side view of the drug-containing unit shown in Fig. 14,
11 with parts shown in section;
12 Fig. 16 is a top view of another electrotransport system in accordance
13 with the present invention;
14 Fig. 17 is a top view of the drug-containing unit of the system shown in
Fig. 16; and
16 Fig. 18 is a perspective view showing the coupling of the reusable
17 controller to the drug-containing unit of the system shown in Figs. 16 and
17.
18
19 MODES FOR CARRYING OUT THE INVENTION
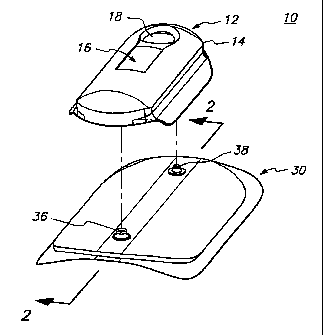
21 Fig. 1 is a perspective view of electrotransport device 10 having a
22 reusable electronic controller 12 which is adapted to be coupled to and
23 uncoupled from, drug-containing unit 30. The controller 12 is reusable,
24 i.e., it is adapted to be used with a plurality of drug units 30, e.g., a
series of
identical and/or similar'drug units 30. On the other hand, drug unit 30
26 typically has a more limited life and is adapted to be discarded after use,
27 i.e., when the drug contained therein has been delivered or has been
28 depleted.
CA 02216731 1997-09-25
WO 96/36394 PCTIUS96/06098
9
1 The controller 12 is comprised of a housing 14, typically formed of a
2 molded plastic material. With reference to Fig. 2, there is shown a
sectional
3 view of the device 10 with the drug unit 30 coupled to the controller 12.
4 The controller 12 includes a battery 20, e.g. a button cell battery, for
powering the circuit board 22. The circuit board 22 is formed in a
6 conventional manner, having conductive traces patterned for interconnecting
7 component(s) 24 thereon. Electrical component(s) 24 control the
8 magnitude, timing, frequency, waveform shape, etc., of the electric current
9 applied by device 10. Although not critical to the invention, controller 12
includes a push button switch 18 which can be used to start operation of
11 device 10 and a liquid crystal display (LCD) 16 which can display system
12 information such as current level, dosing level, number of doses delivered,
13 elapsed time of current application, battery strength, etc.
14 The drug unit 30 is configured to be removably coupled to the
controller 12, with the top of drug unit 30 adjacent to and facing the bottom
of
16 the controller 12. The top of drug unit 30 is provided with the male parts
of
17 two snap type connectors, the male parts being posts 36 and 38 which
18 extend upwardly from drug unit 30. The bottom of housing 14 is provided
with
19 receptacles 26 and 28 (shown in Fig. 2) which are electrically connected
to the outputs of the circuit on circuit board 22 by through-board connectors
21 23 and 25, respectively. Receptacle 26 is positioned and sized to receive
22 donor post 36 and receptacle 28 is positioned and sized to receive counter
23 post 38. Receptacles 26, 28 and posts 36, 38 are made from an electrically
24 conductive material (e.g., a metal such as silver, brass, stainless steel,
platinum, gold, nickel, beryllium, copper, etc. or a metal coated polymer,
26 e.g., ABS with a silver coating). The donor post 36 is electrically
connected to
27 the donor electrode 31, which in turn is electrically connected to the
donor
28 reservoir 32 which typically contains a solution of the therapeutic agent
29 (e.g., a drug salt) to be delivered. The counter post 38 is electrically
connected to the counter electrode 33, which in turn is electrically connected
CA 02216731 1997-09-25
WO 96136394 PCT/US96/06098
1 to the counter reservoir 34 which typically contains a solution of a
2 biocompatible electrolyte (e.g., buffered saline). The electrodes 31 and 33
3 are typically comprised of electrically conductive materials, most
preferably a
4 silver (e.g., silver foil or silver powder loaded polymer) anodic electrode
and a
5 silver chloride cathodic electrode. The reservoirs 32 and 34 typically
include
6 hydrogel matrices which hold the drug or electrolyte solutions and are
7 adapted to be placed in contact with the body surface (e.g., skin) of a
patient
8 (not shown) when in use. The electrodes 31,33 and the reservoirs 32,34 are
9 isolated from each other by foam member 35. The bottom (i.e., patient
10 contacting) surface of foam member 35 is preferably coated with a skin
11 contact adhesive. A release liner 39 covers the body contacting surfaces of
12 the two reservoirs 32 and 34 and the adhesive coated surface of foam
13 member 35 before the unit 30 is put in use. The release liner 39 is
preferably
14 a silicone coated polyester sheet. The release liner 39 is removed when the
device 10 is applied to the skin of a patient (not shown).
16 Thus, the donor post 36 and the receptacle 26 comprise a snap type
17 connector which electrically connects an output of the circuit on circuit
board
18 22 to the drug-containing donor electrode 32. Similarly, the counter post
38
19 and the receptacle 28 comprise a snap type connector which electrically
connects an output of the circuit on circuit board 22 to the electrolyte
21 containing counter electrode 34. In addition to providing the above
described
22 electrically connections, the two snap connectors also provide a separable
23 (i.e., not permanent) mechanical connection of the drug unit 30 to the
24 controller 12.
The two outputs of the circuit on circuit board 22 have different
26 polarities, i.e., one output is positive and is adapted to be connected to
the
27 anodic electrode in drug unit 30 whereas the other circuit output is
negative
28 and is adapted to be connected to the cathodic electrode in drug unit 30.
29 It is important to ensure that the connections of the two electrodes in
drug
CA 02216731 1997-09-25
WO 96/36394 PCT/US96/06098
11
1 unit 30 are connected to the controller outputs of the correct polarity,
since if
2 the connections are reversed (i.e., if the positive circuit output is
connected to
3 the cathodic electrode and the negative circuit output is connected to the
4 anodic electrode), little if any drug would be delivered by
electrotransport.
The present invention ensures correct polarity connections by making it
6 substantially impossible to make incorrect (i.e., reversed) polarity
connections
7 between the controller 12 and the drug unit 30. As is clearly shown in
8 Figs. 1 and 2, the diameter of post 36 is larger than the diameter of post
38.
9 Similarly, the inside diameter of receptacle 26 is larger than the inside
diameter or receptacle 28. Preferably, the inside diameter of receptacle 28 is
11 smaller than the diameter of post 36 so that it is not possible to insert
post 36
12 into receptacle 28.
13 In accordance with this embodiment of the present invention, each of
14 posts 36 and 38 has a different size. Those skilled in the art will
appreciate
that in addition to the size (i.e., diameter) of posts 36, 38 being made
16 different, the shape (e.g., cross-sectional or other shape) of posts 36, 38
17 could be made sufficiently different to ensure only correct polarity
connections
18 between controller 12 and drug unit 30.
19 An alternate means for ensuring correct polarity connections between
a drug unit having donor and counter electrodes and a controller is
illustrated
21 in Fig. 3. Reusable controller 12' is adapted to be separably connected to
22 one or more drug units 40. Drug unit 40 has a donor post 42 which performs
23 a similar function as donor post 36 illustrated in Figs. 1 and 2. However,
24 unlike drug unit 30, drug unit 40 has a receptacle 44 which is electrically
connected to the counter electrode (not shown) in the drug unit 40.
26 Receptacle 44 is adapted to engage a post (not shown) extending from the
27 underside of controller 12'. Thus, drug unit 40 contains both a male part
28 (i.e., post 42) of a first snap connector and a female part (i.e.,
receptacle 44)
29 of a second snap connector. The two snap connectors provide both electrical
3o and mechanical coupling of the drug unit 40 to controller 12'. By having a
CA 02216731 1997-09-25
WO 96/36394 PCT/US96/06098
12
1 male connector and a female connector in each of the drug unit 40 and the
2 controller 12', the coupling of the controller 12' to the drug unit 40 can
only be 3 accomplished in one way, i.e., with the correct polarity
connections.
4 Referring now to Fig. 4, there is shown an electrotransport device
comprised of a reusable electronic controller 12" and a drug unit 50.
6 Unlike device 10 illustrated in Figs. and 2, the reusable controller 12" has
a
7 third snap type receptacle adapted to receive a third post 56 on drug unit
50.
8 Thus, posts 52 and 54 perform substantially the same function as posts 36,
9 38 in device 10. The positioning of the third post 56, as well as the
positioning of the receptacle (not shown) for post 56 in the bottom of
11 controller 12", should not be equidistant from posts 52 and 54 assuming
that
12 the posts and receptacle are all the same size and shape. By positioning
13 post 56 closer to post 54 than to post 52, there is only one way to connect
the
14 drug unit 50 to the controller 12", i.e., with correct polarity
connections.
1s An alternative way to ensure correct polarity connections between a
16 controller 62 and a drug unit 80 is illustrated in Figs 5 to 7.
Electrotransport
17 device 60 is comprised of a reusable controller which is adapted to be
18 coupled to a plurality of same or similar drug units 80 in succession.
19 The body of the controller 62, shown in section in Fig. 7, is shown as a
solid
cross section to simplify the drawing. Those skilled in the art will
appreciate
21 that controller 62 contains an electrical power source and a current
control
22 circuit similar to that illustrated in Fig.2. Controller 62 has two circuit
outputs
23 68 and 70 which need to make electrical connection to electrode contacts
24 82 and 84, respectively in order to ensure correct polarity electrical
connection of electrodes 88 and 90 to controller 62. Controller 62 includes a
26 clasp 64. The drug unit 80 is adapted to be slid into the space between
clasp
27 64 and the body of controller 62. A post 66 engages notch 86 in drug unit
80
28 when the drug unit 80 is slid into place and helps position drug unit 80
relative
29 to controller 62 so that circuit output 68 touches electrode contact 82 and
circuit output 70 contacts electrode contact 84. In addition to the siding
CA 02216731 1997-09-25
WO 96/36394 PCT/US96/06098
13
1 engagement of drug unit 80 with controller 62, there is also provided a snap
2 type connector which provides secure, but separable, mechanical connection
3 of drug unit 80 to controller 62. The snap connector is comprised of a
4 receptacle 72 in the body of controller 62 and a post 92 on drug unit 80.
The post 92 snaps into receptacle 72 as best shown in Fig. 7.
6 An alternative way to ensure correct polarity connections between a
7 controller 112 and a drug unit 130 is illustrated in Fig. 8.
Electrotransport
8 device 110 is comprised of a reusable controller 112 which is adapted to be
s coupled to a plurality of same or similar drug units 130 in succession.
Controller 112 contains an electrical power source and a current control
11 circuit similar to controller 12 illustrated in Figs. I and 2. Controller
112 has
12 two receptacles (not shown in Fig. 8) adapted to engage posts 136 and 138
13 in drug unit 130. Unlike the device illustrated in Figs. 1 and 2, posts 136
and
14 138 have the same size. In order to ensure that the posts 136 and 138 are
snapped into the correct receptacles on the underside of controller 112,
16 a projecting member 134 is provided on the surface of drug unit 130 which
17 abuts against the underside of controller 112. As shown in Fig. 8,
projecting
18 member 134 has a square shape which engages a square shaped hole
19 (not shown in Fig. 8) on the underside of controller 112. Those skilled in
the
art will appreciate that projecting member 134 may have any number of
21 different shapes such as triangular, rectangular, circular, half-moon, etc.
and
22 should preferably project out a sufficient distance from the surface of
drug unit
23 130 to ensure that post 138 cannot engage the incorrect receptacle in
24 controller 112 in the event the patient attempts to couple the drug unit
130 to
the controller with incorrect polarity connections. Preferably, the projecting
26 member 134 is provided on a spine member 132 having increased rigidity.
27 It is important that projecting member 134 be positioned on spine 132 at a
28 location other than the midpoint between the two posts 136, and 138 in
order
29 to ensure that only one (i.e., the correct) polarity connection between the
drug
unit 130 and the controller 112 can be made.
CA 02216731 1997-09-25
WO 96/36394 PCT/US96106098
14
1 Referring now to Figs. 9 through 12, there is shown an alternate
2 embodiment of an electrotransport device 210 comprised of a controller 212
3 which is adapted to be coupled to a plurality of same or similar drug units
230
4 in succession. As best shown in Figs. 9 and 10, drug unit 230 has a pair of
posts 236, 238 adapted to engage receptacles (not shown) in the underside
6 of controller 212. The posts 236, 238 are preferably provided on a rigid
spine
7 member 232. Also provided on spine member 232 is a wedge-shaped
8 projecting member 234. As best shown in Figs. 11 and 12, the controller 212
9 has a wedge-shaped opening 235 with a size and shape which is adapted to
mate with the wedge-shaped projecting member 234. The projecting member
11 234 and the opening 235 provide a visual lock and key mechanism which
12 visually guides the user to couple the controller 212 to the drug unit 230
with
13 the correct polarity connections therebetween. If further certainty is
required,
14 the controller 212 may be made in a manner wherein the projecting member
234 engages and closes a switch contained in controller 212 thereby closing
16 a circuit pathway which enables the device to deliver electrotransport
drive
17 current to the patient. When the projecting member 234 is disengaged from
18 the opening 235, the switch is opened and electrotransport drug delivery is
19 not possible.
Referring now to Figs. 13 through 15, there is shown an
21 electrotransport device 310 comprised of a reusable controller 312 adapted
to
22 be coupled to a series of same or similar drug units 330. The drug unit 330
23 has a receptacle 334 which is adapted to accept and engage an end of
24 controller 312. The snap connections are provided in a position which
insures
that the controller 312 can be electrically coupled to drug unit 330 only when
26 one of the two ends of controller 312 is inserted into receptacle 334.
27 Alternatively, the receptacle 334 can be sized and/or shaped to accept only
28 one of the two ends of controller 312. The selective engagement of
controller
29 312 can be accomplished through any number of known means including
appropriately varying the size and/or shape of the respective ends of
CA 02216731 1997-09-25
WO 96/36394 PCTlUS96/06098
1 controller 312 and/or providing some type of appropriate keying mechanism
2 (not shown). In this way, only one end of the controller 312 may be engaged
3 within receptacle 334, thereby ensuring correct polarity connections between
4 the controller 312 and the drug unit 330 by means of the two snap connectors
5 of the kind described hereinbefore.
6 Referring now to Figs. 16 through 18, there is shown another
7 embodiment of the present invention. Like the system shown in Figures 13
8 through 15, electrotransport device 410 is comprised of a controller 412
which
9 is adapted to fit in a single orientation within receptacle 434 on drug unit
430
10 due to the dissimilarly shaped ends (one end is flat and the other end is
11 rounded) of controller 412 and receptacle 434. By shaping the receptacle
12 434 to "match" the shape of only one of the two ends of controller 412,
13 only one (i.e., the correct) polarity connection between controller 412 and
the
14 drug unit 430 can be made.
15 While the foregoing detailed description has described several
16 embodiments for ensuring correct polarity coupling of an electrotransport
17 controller to a drug unit having donor and counter electrodes, it is to be
18 understood that the above description is illustrative only and not limiting
of the
19 disclosed invention. It will be appreciated that it is possible for one
skilled in
the art to modify the materials, dimensions, type and shape of the couplers
21 disclosed herein, or to include or exclude various elements, and yet remain
22 within the scope and spirit of this invention. Thus the invention is to be
limited
23 only by the following claims.