Note: Descriptions are shown in the official language in which they were submitted.
CA 02217194 1997-10-01
W O 9~/31474 PCT~DK96/00151
Novel heterocvclic Comoounds
Field of the Invention
The present invention relates to novel N-substituted azaheterocyclic carbox-
ylic acids and esters thereof in which a substituted alkyl chain forms part of
5 the N-substituent or salts thereof, to methods for their preparation, to
compositions containing them, and to their use for the clinical treatment of
painful, hyperalgesic and/or inflammatory conditions in which C-fibers play a
pathophysiological role by eliciting neurogenic pain or inflammation. The
invention also relates to the use of the present compounds for the treatment
10 of insulin resistance in non-insulin-dependent diabetes mellitus (NIDDM) or
aging, the present compounds knowing to interfere with neuropeptide con-
taining C-fibres and hence inhibit the secretion and circulation of insulin an-
tagonizing peptides like CGRP or amylin.
Backaround of the Invention
15 The nervous system exerts a profound effect on the inflammatory response.
Antidromic stimulation of sensory nerves results in localized vasodilation and
increased vascular permeability (Janecso et al. Br. J. Pharmacol. 1967, 31,
138-151) and a similar response is observed following injection of peptides
known to be present in sensory nerves. From this and other data it is postu-
20 lated that peptides released from sensory nerve endings mediate manyinflammatory responses in tissues like skin, joint, urinary tract, eye, menin-
ges, gastro-intestinal and respiratory tracts. Hence inhibition of sensory nervepeptide release and/or activity, may be useful in treatment of, for example
arthritis, dermatitis, rhinitis, asthma, cystitis, gingivitis, thrombo-phlelitis,
25 glaucoma, gas~ro-intestinal diseases or migraine.
CA 02217194 1997-10-01
W O96/31474 PCT~DK~6/00151
Further, the potent effects of CGRP on skeletal muscle glycogen synthase ac-
tivity and muscle glucose metabolism, together with the notion that this
peptide is released from the neuromuscular junction by nerve excitation,
suggest that CGRP may play a physiological role in skeletal muscle glucose
5 metabolism by directing the phosphorylated glucose away from glycogen
storage and into the glycolytic and oxidative pathways (Rossetti et al. Am. J.
Physiol. 264, E1-E10, 1993). This peptide may represent an important
physiological modulator of intracellular glucose trafficking in physiological
conditions, such as exercise, and may also contribute to the decreased insulin
10 action and skeletal muscle glycogen synthase in pathophysiological condi-
tions like NIDDM or aging-associated obesity (Melnyk et al. Obesity Res. 3,
337-344, 1995) where circulating plasma levels of CGRP are markedly
increased. Hence inhibition of release and/or activity of the neuropeptide
CGRP may be useful in the treatment of insulin resistance related to type 2
15 diabetes or aging.
In US Patent No. 4,383,999 and No. 4,514,414 and in EP 236342 as well
as in EP 231996 some derivatives of N-(4,4-disubstituted-3-butenyl)-
azaheterocyclic carboxylic acids are claimed as inhibitors of GABA uptake. In
EP 342635 and EP 374801, N-substituted azaheterocyciic carboxylic acids in
20 which an oxime ether group and vinyl ether group forms part of the N-
substituent respectively are claimed as inhibitors of GABA uptake. Further, in
WO 9107389 and WO 9220658, N-substituted azacyclic carboxylic acids are
claimed as GABA uptake inhibitors. EP 221572 claims that 1-aryloxyalkyl-
pyridine-3-carboxylic acids are inhibitors of GABA uptake.
25 Descriction of the Invention
The present invention relates to novel N-substituted azaheterocyclic carbo-
xylic acids and esters thereof of formula I
CA 02217194 1997-10-01
W O96/31474 PCTADK96/00151
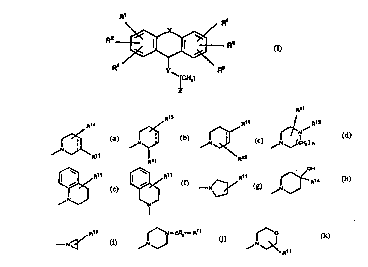
R'
R2 RGRs (I)
ICH2) r
wherein R', R2, R3, R4, Rs and R6 independently are hydrogen, halogen,
trifluoromethyl, hydroxy, C1.6-alkyl, C1 6-alkoxy, -NR7R3 or -SO2NR7R8 wherein
R7 and R3 independently are hydrogen or C1 6-alkyl; and
5 X is completion of an optional bond, -CH2-, -CH2CH2-, -CH=CH-, -O-,
-S(O)z- wherein z is 0, 1 or 2, or -N(R9)- wherein R9 is hydrogen or C1 6-alkyl;and
Y is -O-, -S(O)q- wherein q is 0, 1 or 2, or -N(R10)- wherein R'~ is hydrogen orCl ~-alkyl; and
10 ris 1, 2, 3 or4; and
Z is selected from
R14 R13
11 ~ ~ ~N~ ~N~CH2) A
R1 1 R13
[~R~
~ R11 ~9~N--C~12--Rl 1
CA 02217194 1997-10-01
W 096/31474 PCTADK96/OOlSl
wherein n is 1 or 2; and
R" is -(CH2)mOH or-(CH2)tCOR'2 wherein m is 0, 1, 2, 3, 4, 5 or 6 and t is O
or 1 and wherein R12 is -OH, -NH2, -NHOH or C, 6-alkoxy; and
R13 is hydrogen, halogen, trifluoromethyl, hydroxy, C, 6-alkyl or C, 6-alkoxy;
5 and
R14 is hydrogen, C, 6-alkyl, C, 6-alkoxy or phenyl optionally substituted with
haiogen, trifluoromethyl, hydroxy, C, 6-alkyl or C, 6-alkoxy; and
R15 is hydrogen or C, 6-alkyl; and
... is optionally a single bond or a double bond;
or a pharmaceutically acceptable salt thereof.
The compounds of formula I may exist as geometric and optical isomers and
all isomers and mixtures thereof are included herein. Isomers may be separ-
ated by means of standard methods such as chromatographic techniques or
fractional crystallization of suitable salts.
Preferably, the compounds of formula I exist as the individual geometric or
optical isomers.
The compounds according to the invention may optionally exist as pharma-
ceutically acceptable acid addition salts or - when the carboxylic acid group isnot esterified - as pharmaceutically acceptable metal salts or - optionally
alkylated - ammonium salts.
Examples of such salts include inorganic and organic acid addition salts such
as hydrochloride, hydrobromide, sulphate, phosphate, acetate, fumarate,
maleate, citrate, lactate, tartrate, oxalate or similar pharmaceutically accept-able inorganic or organic acid addition salts, and include the pharmaceutically
acceptable salts listed in Journal of Pharmaceutical Science, 66, 2 (1977)
whlch are hereby incorporated by reference.
CA 022l7l94 l997-lO-Ol
W O 96/31~74 PCTADK~6/00151
The term "Cl.6-alkyl" as used herein, alone or in combination, refers to a
straight or branched, saturated hydrocarbon chain having 1 to 6 carbon
atoms such as e.g. methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl,
isobutyl, tert-butyl, n-pentyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 4-
5 methylpentyl, neopentyl, n-hexyl, 1,2-dimethylpropyl, 2,2-dimethylpropyl and
1, 2, 2-trimethylpropyl .
The term "C1 6-alkoxy" as used herein, alone or in combination, refers to a
straight or branched monovalent substituent comprising a Cl 6-alkyl group
linked through an ether oxygen having its free valence bond from the ether
10 oxygen and having 1 to 6 carbon atoms e.g. methoxy, ethoxy, propoxy,
isopropoxy, butoxy, pentoxy.
The term "halogen" means fluorine, chlorine, bromine or iodine.
Illustrative examples of compounds encompassed by the present invention
include:
1 5 1-(2-(10,1 1-Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-3-
piperidinecarboxamide;
1-(2-(10,1 1-Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-4-
piperidinecarboxylic acid;
1-(2-(10,1 1-Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-2-
20 piperidinecarboxylic acid;
(1-(2-(10,1 1-Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)piperidin-3-
yl)methanol;
4-(4-Chlorophenyl)-1-(2-(10,1 1-Dihydro-5H-dibenzo[a,d]cyciohepten-5-yloxy)-
CA 02217194 1997-10-01
WO 96/31474 PCT/DK96/00151
ethyl)-4-piperidinol;
4-(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-4-
piperazinecarboxylic acid;
(2S,4R)-1 -(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-4-
5 hydroxy-2-pyrrolidinecarboxylic acid;
4-(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-2-morpholine-
carboxylic acid;
1 -(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-2-
aziridinecarboxylic acid;
2-(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-1,2,3,4-
tetrahydro-4-isoquinolinecarboxylic acid;
1 -(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-4-methyl- 1,4-
diazepane-6-carboxylic acid;
2-(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-1,2,3,4-
15 tetrahydro-3-isoquinolinecarboxylic acid;
1 -(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)-3-piperidine-
carboxylic acid hydroxamide;
(4-(2-(10,11 -Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)ethyl)piperazin-1 -
yl)acetic acid;
20 or a pharmaceutically acceptable salt thereof.
CA 02217194 1997-10-01
wo 96/31474 PcT/DKg6/00151
As used herein, the term "patient" includes any mammal which could benefit
from treatment of neurogenic pain or inflammation or insulin resistance in
NIDDM. The term particularly refers to a human patient, but is not intended
to be so limited.
5 It has been demonstrated that the novel compounds of formula I inhibit
neurogenic inflammation which involves the release of neuropeptides from
peripheral and central endings of sensory C-fibres. Experimentally this can be
demonstrated in animal models of formalin induced pain or paw oedema
(Wheeler and Cowan, Agents Actions 1991, 34, 264-269) in which the novel
10 compounds of formula I exhibit a potent inhibitory effect. Compounds of
formula I may be used to treat all painful, hyperalgesic and/or inflammatory
conditic~ns in which C-fibers play a pathophysiological role by eliciting
neurogenic pain or inflammation, i.e.:
Acutely painful conditions exemplified by migraine, postoperative pain, burns,
15 bruises, post-herpetic pain (Zoster) and oain as it is generally associated with
acute inflammation; chronic, painful and/or inflammatory conditions exemp-
lified by various types of neuropathy (diabetic, post-traumatic, toxic), neural-gia, rheumatoid arthritis, spondylitis, gout, inflammatory bowel disease,
prostatitis, cancer pain, chronic headache, coughing, asthma, chronic
20 pancreatitis, inflammatory sicin disease including psoriasis and autoimmune
dermatoses, osteoporotic pain.
Further, it has been demonstrated that the compounds of general formula I
improves the glucose toierance in diabetic ob/ob mice and that this may
result from the reduced release of CGRP from peripheral nervous endings.
25 Hence the compounds of general formula I may be used in the treatment of
NIDDM as well as aging-associated obesity. Experimentally this has been
demonstrated by the subcutaneous administration of glucose into ob/ob mice
with or without previous oral treatment with a compound of general formula
1.
CA 02217194 1997-10-01
W O96/31474 PCTADK~6/00151
The compounds of formula I may be prepared by the following method:
R~
> (I)
R Y R6
\ ~CH2) r
W
(Il) (111)
A compound of formula ll wherein R', R2, R3, R4, R5, R6, X, Y and r are as
defined above and W is a suitable leaving group such as halogen, p-toluene
sulphonate or mesylate may be reacted with an azaheterocyclic compound of
5 formula lll wherein Z is as defined above. This alkylation reaction may be carried
out in a solvent such as acetone, dibutylether, 2-butanone, methyl ethyl ketone,ethyl acetate, tetrahydrofuran (THF) or toluene in the presence of a base e.g.
sodium hydride and a catalyst, e.g. an alkali metal iodide at a temperature up to
reflux temperature for the solvent used for e.g. 1 to 120 h. If esters have been10 prepared in which R12 is alkoxy, compounds of formula I wherein R12 is OH maybe prepared by hydrolysis of the ester group, preferably at room temperature in
a mixture of an aqueous alkali metal hydroxide solution and an alcohol such as
methanol or ethanol, for example, for about 0.5 to 6 h.
Compounds of formula ll and lll may readily be prepared by methods familiar to
15 those skilled in the art.
Under certain circumstances it may be necessary to protect the intermediates
used in the above methods e.g. a compound of formula lll with suitable
protecting groups. The carboxylic acid group can, for example, be esterified.
Introduction and removal of such groups is described in "Protective Groups in
20 Organic Chemistry" J.F.W. McOrnie ed. (New York, 1973).
CA 022l7l94 l997-lO-Ol
W O96/31'174 PCTADK~6/00151
Pharmacoloqical Methods
Release of neurooeDtides from r herir heral and central endinqs of sensorv
C-fibers
A
Values for in vivo inhibition of formalin induced pain or oedema for the com-
5 pounds of the present invention were assessed in mice essentially by the
method of Wheeler-Aceto and Cowan (Agents Action 1991, 34, 265-269).
About 20 9 NMRI female mice were injected 20 ~l 1 % formalin into the left hind
paw. The animals were then placed on a heated (31~C) table, and the pain
response was scored. After 1 h they were killed and bled. Left and right hind
10 paws were removed and the weight difference between the paws was used as
indication of the oedema response of the formalin injected paw.
Reduced release of CGRP from r herir heral nervous endings
ob/ob female mice, 16 weeks of age, where injected glucose (2g/kg) subcutane-
ously. At times hereafter blood glucose was determined in tail venous blood by
15 the gluGose oxidase method. At the end of the study the animals were decapita-
ted and trunck blood collected. Immunoreactive CGRP was determined in plasma
by radio-immuno-assay. Two groups of animals were used. The one group was
vehicle treated, whereas the other group received a compound of formula I via
drinkin~ water (100 mg/l) for five days before the test.
20 For the above indications the dosage will vary depending on the compound of
formula I employed, on the mode of administration and on the therapy desired.
However, in general, satisfactory results are obtained with a dosage of from
about 0.5 mg to about 1000 mg, preferably from about 1 mg to about 500 mg
of compounds of formula 1, conveniently given from 1 to 5 times daily,
25 optionally in sustained release form. Usually, dosage forms suitable for oraladministration comprise from about 0.5 mg to about 1000 mg, preferably from
CA 022l7l94 l997-lO-Ol
W O 96/31471 PCTADK~6/00151
about 1 mg to about 500 mg of the compounds of formula I admixed with a
pharmaceutical carrier or diluent.
The compounds of formula I may be administered in a pharmaceutically accept-
able acid addition salt form or where possible as a metal or a lower alkylammo-
5 nium salt. Such salt forms exhibit approximately the same order of activity asthe free base forms.
This invention also relates to pharmaceutical compositions comprising a com-
pound of formula I or a pharmaceutically acceptable salt thereof and, usually,
such compositions also contain a pharmaceutical carrier or diluent. The composi-
10 tions containing the compounds of this invention may be prepared by conven-
tional techniques and appear in conventional forms, for example capsules,
tablets, solutions or suspensions.
The pharmaceutical carrier employed may be a conventional solid or liquid
carrier. Examples of solid carriers are lactose, terra alba, sucrose, talc, gelatin,
15 agar, pectin, acacia, magnesium stearate and stearic acid. Examples of liquid carriers are syrup, peanut oil, olive oil and water.
Similarly, the carrier or diluent may include any time delay material known to the
art, such as glyceryl monostearate or glyceryl distearate, alone or mixed with awax.
20 If a solid carrier for oral administration is used, the preparation can be tabletted,
placed in a hard gelatin capsule in powder or pellet form or it can be in the form
of a troche or lozenge. The amount of solid carrier will vary widely but will
usually be from about 25 mg to about 1 9. If a liquid carrier is used, the prepara-
tion may be in the form of a syrup, emulsion, soft gelatin capsule or sterile
25 injectable liquid such as an aqueous or non-aqueous liquid suspension or
solution .
CA 02217194 1997-10-01
W O9G/31474 PCT~D~6100151
1 1
Generally, the compounds of this invention are dispensed in unit dosage form
comprising 50-200 mg of active ingredient in or together with a pharmaceuti-
cally acceptable carrier per unit dosage.
The dosage of the compounds according to this invention is 1-500 mg/day, e.g.
about 100 mg per dose, when administered to patients, e.g. humans, as a drug.
A typical tablet which may be prepared by conventional tabletting techniques
contains
Core:
Active compound (as free compound 100 mg
or salt thereof)
Colloidal silicon dioxide (Areosil$) 1.5 mg
Cellulose, microcryst. (Avicel0) 70 mg
Modified cellulose gum (Ac-Di-Sol~) 7.5 mg
Magnesium stearate
1 5 Coating:
HPMC approx. 9 mg
Mywacett~ 9-40 T approx. 0.9 mg
Acylated monoglyceride used as plasticizer for film coating.
The route of administration may be any route which effectively transports the
20 active compound to the appropriate or desired site of action, such as oral orparenteral e.g. rectal, transdermal, subcutaneous, intranasal, intramuscular,
topical, intravenous, intraurethral, ophthalmic solution or an ointment, the oral
route being preferred.
EXAMPLES
CA 02217194 1997-10-01
W O96/31474 PCT~DK~6/00151
12
The process for preparing compounds of formula I and preparations containing
them is further illustrated in the following examples, which, however, are not
to be construed as limiting.
Hereinafter, TLC is thin layer chromatography and THF is tetrahydrofuran, CDCI3
5 is deuterio chloroform and DMSO-d5 is hexadeuterio dimethylsulfoxide. The
structures of the compounds are confirmed by either elemental analysis or NMR,
where peaks assigned to characteristic protons in the title compounds are
presented where appropriate. 'H-NMR shifts (~) are given in parts per million
(ppm). M.p. is melting point and is given in ~C and is not corrected. Column
10 chromatography was carried out using the technique described by W.C. Still etal, J. Org. Chem. (1978), 43, 2923-2925 on Merck silica gel 60 (Art. 9385).
Compounds used as starting materials are either known compounds or com-
pounds which can readily be prepared by methods known ~er se.
EXAMPLE 1
1-(2-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)-1-ethyl)-4-piperidine-
carboxylic acid hydrochloride
~ ,HC
A mixture of 10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ol (10.0 9, 0.047
mol), 2-bromoethanol (8.6 9, 0.069 mol) and concentrated suiphuric acid (1.4
ml) in benzene (120 ml) was stirred for 0.5 h at room temperature. After cooling
CA 02217194 1997-10-01
W O 96/31474 PCTADK~6/00151
13
(water/ice), crude 5-(2-bromoethoxy-10,11 -dihydro-5H-dibenzo[a,d]cyclohepten
was filtered off, washed with petroleum ether and dried. This afforded 10.7 9
(72 %), which was used without purification for further reaction.
M.p. 62 - 70 ~C.
A mixture of 5-(2-bromoethoxy)-10,11-dihydro-5H-dibenzo[a,d]cycloheptene
(9.5 g, 0.03 mol), ethyl 4-piperidinecarboxylate (4.7 9, 0.03 mol), potassium
carbonate (8.1 9, 0.06 mol) and dimethylsulfoxide (120 ml) was stirred on a
water bath at 40 - 50~C for 4 h. The mixture was cooled, filtered and the solid
was washed with dimethylsulfoxide (10 ml) . The combined filtrates were diluted
with water (720 ml) and extracted with diethyl ether (2 x 200 ml). The ether
extract was washed with water, dried (MgS04), and the solvent was evaporated
under vacuum. The residue was dissolved in 2-propanol, and a hot solution of
oxalio acid (4.0 g) in 2-propanol was added. After cooling, crystals of ethyl
1 -(10,11 dihydro-5H-dibenzo[a,d]cyclohepten-5-yloxy)-1-ethyl-4-piperidinecar-
boxylate hydrogen oxalate precipitated. This afforded after filtration and drying
8.3 g (57 ~/0), which was used for further reaction without purification.
The above oxalate suspension (7.3 9, 0.015 mol) in water was made alkaline
with a 25 % ammonia solution and extracted with diethyl ether (2 x 100 ml).
The organic layer was washed with water (2 x 50 ml), dried (MgS04 ) and
evaporated in vacuum. This afforded 5.7 9 (96 %) of free ethyl ester as an oil.
A mixture of the above ester 2.01 9 (0.005 mol), 2.4 ml 20 % aqueous sodium
hydroxide and ethanol (15 ml) was stirred at room temperature for 4 h and
overnight. Dichloromethane (250 ml) was added, and under magnetic stirring
and cooling (water/ice bath), ~ Z.5 N HCI was carefully added dropwise to pH
25 1. The water layer was separated, and the organic layer was dried (MgS04) and evaporated under vacuum. The solid residue was re-evaporated twice with
acetone. This afforded 1.68 9 (83 %) tne title com~ound after crystallisation
-
CA 02217194 1997-10-01
W O 96/31474 PCTADK96/00151
14
from a mixture of ethanol and ether. Dichloromethane, which was present in
the crystalline sample was removed under vacuum at elevated temperature.
M.p.192 -1 94~C.
'H NMR (DMSO-d6): 7.42 (bd, 2 H); 7.19 (m, 6 H); 5.62 (s, 1 H); 3.83 (t, 2 H);
3.28 (t, 1 H); 4.96 (d, 1 H); 2.53 (m, 1 H); 3.37 (m, 6 H); 3.00 (m, 4 H); 1.99
(m, 4H).