Note: Descriptions are shown in the official language in which they were submitted.
CA 02217702 1997-10-07
METHOD FOR THE CHE~ICAL VAPOUR INFILTRATION OF A MATERIAL CONSISTING
OF CARBON AND SILICON AND/OR BORON.
The present invention relates to a method of
densifying a porous substrate by means of a material
obt~;ne~ by chemical vapor infiltration and comprising
carbon together with silicon and/or boron. Such a
material may be silicon carbide, boron carbide, and any
intermediate Si-B-C ternary system.
A particular application of the invention lies in
the field of making parts out of composite material by
densifying a fiber substrate or "preform" with a matrix
obtained by chemical vapor infiltration and constituted
at least in part by a material comprising carbon together
with silicon and/or boron.
Methods and an apparatus for chemical vapor
infiltration and serving in particular to manufacture
composite material parts having an SiC matrix are
described, for example, in documents FR-A-2 401 888,
FR-A-2 567 874, and W0 87/04733, while densification of
preforms by a matrix constituted at least in part by an
Si-B-C ternary system is described in document
FR-A-2 668 477.
The fiber preforms to be densified are placed in the
reaction chamber of an infiltration oven. A gas that is
a precursor of the matrix to be formed is admitted into
the chamber, e.g. into the top thereof. The co~~o~-y-
used precursor gas for SiC is methyltricholosilane (MTS)
together w;ith hydrogen (H2). The cc o~ly-used precursor
gas for boron carbide is a mixture of boron chloride
(BCl3) and a gaseous hydrocarbon or mixture of
hydrocarbons, e.g. propane (C3H8) and/or methane (CH4) or
natural gas. A matrix or a portion of matrix comprising
an Si-B-C ternary system is obt~ , for example, from a
mixture of MTS and of BC13 in appropriate proportions
together with H2. Under determined conditions of
temperature and pressure, the gas diffuses into the
accessible pores of the preforms, penetrates to the cores
CA 02217702 1997-10-07
of the preforms, and reacts to form the desired deposit
on the fibers. On entry into the chamber, the gas may be
preheated, e.g. by passing through perforated plates
raised to the temperature that obtains inside the
chamber. The residual gases are extracted by pumping
through an outlet situated at the base of the chamber.
Such methods give results that are satisfactory
providing the volume actually occupied by the preforms in
the reaction chamber is small. This applies when the
preforms require support tooling to be present in order
to conserve their shapes, and when they are thin or very
widely spaced apart from one another inside the oven.
Examples of such preforms are those designed to
constitute thermal protection elements that fit closely
over the shape of space vehicle fairings, or to
constitute flaps for aircraft jet engines. It is then
common for the effective occupancy rate of the oven, i.e.
the volume percentage of the reaction chamber actually
occupied by the preforms, to be less than 5%.
However, the performance of those known methods
falls off considerably when the occupancy rate of an oven
is significantly increased. Such an increase is possible
with preforms that are simple in shape, that do not
require supporting tooling, or that are thick. This
applies in particular to thick needled preforms designed
to constitute friction parts, in particular airplane
brake disks made of composite material having a matrix of
SiC or Si-B-C, or at least partially of SiC or Si-B-C.
Such preforms are of a shape that enables them to be
stacked and to achieve an occupancy rate of greater than
25% or even greater than 30~. The observed lowering of
performance in known infiltration methods consists, in
particular, in very marked non-uniformity of the
densification in the longitudinal direction, i.e. in the
flow direction of the gas from its inlet into the
reaction chamber to the outlet thereof. It is observed
that the preforms closest to the gas inlet are densified
CA 02217702 1997-10-07
much more and much more quickly than those further away
therefrom.
Another drawback encountered is marked non-
uniformity of the densification of thick preforms, i.e.
the existence of a steep densification gradient between
the core of a part into which less matrix is admitted,
and zones of the same part close to its outside surface,
where a larger amount of gas is admitted.
For obvious reasons of throughput in infiltration
ovens and of producing high quality parts, it is
necessary to reduce such densification non-uniformities
as much as possible.
Longitll~; n~ non-uniformity of densification is the
result essentially of the gas being depleted as it moves
through the reaction chamber. It might be envisaged that
that could be remedied by increasing the flow rate of the
gas admitted into the oven. Unfortunately, that gives
rise to even faster and greater densification of the
preforms situated close to the gas inlet, without
attenuating the densification gradient within the parts.
An object of the present invention is to provide a
method of chemically infiltrating a vapor of a material
comprising carbon together with silicon and/or boron,
which enables the effective occupancy ratio of
infiltration ovens to be increased while greatly limiting
densification non-uniformity in the reaction chambers of
ovens between their gas inlets and their residual gas
outlets.
Another object of the invention is to provide a
chemical vapor infiltration method that enables the
densification gradient within thick parts to be reduced.
According to a method of the invention, chemical
vapor infiltration within a porous substrate is performed
at a temperature not greater than 1050~C by means of a
gas cont~; n; ng a gaseous precursor of the material
comprising carbon together with silicon and/or boron, and
also cont~in;ng hydrogen chloride (HCl). The gaseous
CA 02217702 1997-10-07
precursor is constituted by a gas or a mixture of gases.
The volume proportion of HCl relative to the gaseous
precursor for silicon and/or boron is preferably at least
10%, e.g. not less than 25%, and, for example, the
silicon precursor may be MTS, and the boron precursor may
be BCl3.
It has been found that including HCl in the gas
makes it possible to avoid premature depletion thereof.
The presence of HCl slows down matrix formation in the
parts first exposed to the gas penetrating into the
infiltration oven. This makes it possible to increase
the flow rate of the precursor gas, and consequently the
occupancy ratio of the oven, without encountering the
above-mentioned drawbacks.
Chemical vapor infiltration can be performed with a
temperature gradient, i.e. by heating the porous
substrate in such a manner that it presents a higher
temperature in portions that are remote from its exposed
surfaces than it does at its exposed surfaces.
Since matrix formation is ~nhA~ in those portions
of the substrate where the temperature is higher,
establishing a temperature gradient serves to counter the
non-uniformity of densification within the substrate.
Substrate heating can be performed by contact
between a surface of the substrate and a heated body,
such that a temperature gradient is established between
the surface of the substrate which is in contact with the
heated body and the surfaces of the substrate which are
exposed to the flow of gas. The heated body may be a
heater core electromagnetically coupled to an inductor.
When the substrate is made of an electrically-
conductive material, e.g. carbon, it can be heated by
induction by direct coupling with an inductor.
These chemical vapor infiltration t~rhniques using a
temperature gradient are described in document
FR-A-2 711 647.
CA 02217702 1997-10-07
Implementations of methods of the invention are
described below in greater detail.
Reference is made to the ~ccomranying drawings, in
which:
~ Figure 1 is a highly diagrammatic view of an
installation enabling a method of chemical infiltration
to be implemented using the vapor of a material
comprising carbon together with silicon and/or boron, and
at constant temperature; and
~ Figure 2 is a highly diagrammatic view of an
installation enabling a method of chemical infiltration
to be implemented using the vapor of a material
comprising carbon together with silicon and/or boron, and
with a temperature gradient.
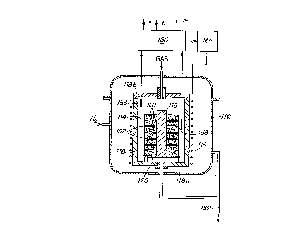
The installation shown in Figure 1 is of the same
type as that described in above-mentioned document
W0 87/04733.
A graphite heater core lO housed inside a sealed
metal enclosure 12 defines a reaction chamber 14. Inside
the enclosure 12, the core 10 is surrounded by a metal
inductor 16 with thermal insulation 18 being interposed
between them. The core 10 is in the form of a vertical
axis cylinder closed in sealed ~nne~ by a bottom wall
lOa and by a removable top cover lOb.
Inside the chamber 14, the substrates to be
densified are supported by a base turntable 20 capable of
rotating about a vertical axis which coincides with the
axis of the core 10 and of the inductor 16. Support
trays 22 and 24 supported by the turntable 20 with
interposed spacers 25 make it possible to load substrates
at several levels, at least three in the example shown.
Rotation of the turntable 20 is controlled by a motor
(not shown) coupled to a shaft 26 passing through the
bottom wall of the enclosure 12 and through the bottom
wall lOa of the core, and it is fixed to the bottom face
of the turntable 20.
CA 02217702 1997-10-07
The gas for forming the matrix material which is to
densify the substrates is admitted via a pipe 28 which
leads to the top of the chamber 14, passing through the
cover lOb. The gas comprises a mixture of a gaseous
precursor for the matrix material together with HCl. The
gaseous precursor depends on the nature of the matrix.
For an SiC matrix, the gaseous precursor is MTS together
with H2. For a boron carbide matrix, the gaseous
precursor is a mixture of BCl3 and a carbon precursor.
The carbon precursor may be an alkane, an alkyl, or an
alkene, on its own or in a mixture, e.g. a mixture of C3H8
and of CH4 (or of natural gas). For a matrix constituted
by an Si-B-C ternary system, the gaseous precursor is a
mixture of MTS and BCl3 together with H2. The MTS, BCl3,
C3H8 + CH4, Hz, and HCl gases come from sources 30, 31, 32,
33, and 34 via feed ducts 36, 37, 38, 39, and 40 provided
with injection valves 42, 43, 44, 45, and 46 l~;ng to
the pipe 28.
Residual gas is extracted from the chamber 14 by
opening a valve 48 which puts the chamber 14 into
communication with pumping apparatus 50 via at least one
exhaust pipe 52. By way of example, the pumping
apparatus 50 can be a water ring pump. The pipe 52
~o~lln;cates with the bottom portion of the chamber 14
via an annular passage 54 formed around the shaft 26.
Signals delivered by a pressure sensor 56 and a
temperature sensor 58 represent the pressure and the
temperature in the chamber 14 and are transmitted to a
controller 60. The controller controls the pump 50 and
the valve 48 to establish the desired pressure in the
chamber 14 prior to admitting the gas, and it controls a
generator 62 feeding the inductor 16 so as to maintain
the temperature inside the enclosure at the desired
value. The controller 60 also controls the valves 42,
43, 44, 45, and 46 to control the respective flow rates
of MTS, BCl3, CH4 + C3H8, H2, and HCl as a function of the
predetermined composition of the gas.
CA 02217702 1997-10-07
The gas penetrating into the reaction chamber 14
comes initially into contact with a preheater 64, e.g. in
the form of superposed perforated plates. Since the
preheater plates are inside the reaction chamber, they
are continuously at the temperature which obtains
therein. This makes it possible to raise the gas to the
desired temperature before it comes into contact with the
substrates that are to be densified.
The volume situated around the core 10 inside the
enclosure can be swept continuously by an inert gas such
as nitrogen (Nz). This comes from a gas source 65 via a
duct 66 provided with a valve 68 under the control of the
controller 60. The nitrogen thus forms a blanket of
inert gas around the reaction chamber. It is extracted
via a duct 69 leading to the exhaust pipe 52 outside the
enclosure 12.
Tests conc~ning chemical vapor infiltration of an
SiC matrix have been performed with the above-described
installation. During each test, on each of the three
loading levels in the oven, there was placed the same set
of porous substrates constituted by:
~ a cylindrical sample A comprising a needled carbon
fiber texture having a diameter of 90 mm and a thickness
of 35 mm;
~ three cylindrical samples B of the same texture,
having a diameter of 35 mm and a height of 35 mm;
~ a cylindrical sample C of the same texture having
a diameter of 15 mm and a height of 35 mm;
~ a cylindrical sample D of the same texture, having
a diameter of 15 mm and a height of 8 mm; and
~ a sample E in the form of a cube having a side of
about 2 cm3, and comprising a substrate of carbon fibers
partially densified by vacuum suction of a powder.
The texture of samples A, B, C, and D was made by
stacking and needling two~directional sheets of carbon
fibers progressively, as described in document
FR-A-2 584 106. The texture is identical to that
CA 02217702 1997-10-07
constituting the preforms for the carbon-carbon composite
material brake disks fitted to airplanes of the "Airbus"
type.
The substrate of sample E was constituted by a
carbon fiber felt partially densified by vacuum suction
of carbon powder, as described in document
FR-A-2 671 797.
Each of the tests was performed at a pressure (P) in
the reaction chamber of 10 kPa, with a ratio between the
flow rates of H2 and of MTS (Q(H2) and Q(MTS)) equal to 6
for a total duration (d) of 20 hours (h).
A first series of three tests I, II, and III was
performed at a temperature T in the reaction chamber of
1010~C, with Q(MTS) e~ual to 150 st~n~d cm3/min (sccm),
and with Q(H2) equal to 900 sccm, while giving the HCl
flow rates (Q(HCl)) of 0, 37.5 sccm, and 75 sccm
respectively, i.e. proportions successively equal to 0,
25%, and 50~ of HCl flow rate relative to that of MTS.
A fourth test IV was performed by doubling Q(MTS)
and Q(H2) compared with test III, leaving the other
parameters unchanged.
A fifth test V was performed under the same
conditions as test IV, except that the temperature T was
lowered from 1010~C to 950~C.
A sixth test VI was performed under the same
conditions as test V, with the exception of the HCl flow
rate Q(HCl) being doubled from 75 sccm to 150 sccm.
In order to characterize the performance obt~; n~
concerning SiC densification of the substrates, the
following characteristics were evaluated:
~the relative mass increase ~m/m of each substrate,
~m being the difference between the mass m' of the
substrate at the end of the test (after densification)
and the initial mass _ of the substrate;
~ longit~-~; n~ non-uniformity of densification
(between the gas inlet and outlet), i.e. variation of
densification as a function of the location of the
CA 02217702 1997-10-07
substrates in the reaction chamber, evaluated for each
type of substrate by measuring the ratio between the mass
increase ~m/m at the "top" level (closest to the gas
inlet) and the mass increase ~m/m at the "bottom" level
(furthest from the gas inlet);
~ non-uniformity of infiltration, i.e. the gradient
of densification between the core and the surface of each
substrate evaluated by measuring the ratio between the
mass uptake ~m/m of substrate A having the greatest
volume and the mass uptake ~m/m of the substrate C having
the smallest volume of substrates A, B, and C having the
same nature and height, with this being done at the
"top", "middle", and "bottom" levels of the reaction
chamber;
~ the thickness of the SiC deposit on the fibers,
evaluated by laser diffraction, to accuracy of the order
of 0.1 ~; and
~ densification efficiency evaluated by calculating
the ratio of the mass uptake actually achieved by the
substrates to the total mass uptake that would
theoretically be possible as a function of the quantity
of MTS consumed.
The results obt~;n~ are brought together in Tables
I to IV below.
CA 02217702 1997-10-07
TABLE I
Mass increase
P(kPa) 10 10 10 10 10 10
d(h) 20 20 20 20 20 20
Operating T(~C) 1010 1010 1010 1010 950 950
conditions Q(MTS)sccm150 150 150 300 300 300
Q(H2)sccm900 900 900 1800 1800 1800
Q(HCl)sccm 0 37.5 75 75 75 150
T33.131.1 22.8 57.3 36.2 32.3
A M 17.5 10.1 3.8 33.8 20.6 23.6
B 1.8 0.6 0.7 15.7 10.9 12.5
T 72.4 76 68.9 137 89 56.8
B M 27.1 25.8 16.6 54.7 34.1 33.4
B 4.4 0.9 1.3 32.2 20.2 21.1
T 113 110 111 208 134 68.6
Substrate C M 29.6 18.3 9.4 52.5 25 26.2
~m/m(~) B 2.7 0.9 1.2 32.8 15.9 18.8
T 231 106.5 106 313 196 91.8
D M 29.6 18.3 4.1 123107.386.6
B 2.7 0.8 1.9 24.5 67.7 61
T 10.9 10.1 7.8 11.2 10 9.9
E M 1.4 0.9 1.7 6 10 8.5
B 0.2 1 1.4 0.5 6.8 7.5
Where the letters T, M, and B identify the "top",
"middle", and "bottom" levels in the reaction chamber.
Clearly, the cycles performed by doublîng the MTS
and H2 flow rates gave rise to greater mass uptakes. The
most uniform densifications were those performed at the
lower temperature (950~C) in particular in the presence
of HCl.
CA 02217702 1997-10-07
TABLE II
Longitudinal non-uniformity
P(kPa) 10 10 10 10 10 10
d(h) 20 20 20 20 20 20
Operating T(~C) 10101010 . 1010 .1010 ~ 950 950
conditions Q(MTS)sccm150 150 150 300 300 300
Q(H2)sccm900 900 900 18001800 1800
Q(HCl)sccm0 37.5 75 75 75 150
A18.451.832.6 3.6 3.3 2.6
B16.484.4 53 4.3 4.4 2.7
Substrates C 41.9 122.292.5 6.3 8.4 3.6
D 85.6133.1 55.812.8 2.9 1.5
E 54.5 10.1 5.622.4 1.5 1.3
The results ~om; n~ closest to the ideal optimum
value (1) were for the cycle performed at doubled flow
rates of MTS and H2, at the lower temperature and in the
presence of HCl.
TABLE III
Densification non-uniformity
_
P(kPa) 10 10 10 10 10 10
d(h) 20 20 20 20 20 20
Operating T(~C) ,1010 10101010 .1010 950 . 950
conditions Q(MTS)sccm150 150 150 300 300 300
Q(Hz)sccm900 900 900 1800 1800 5 1800
Q(HCl)sccmO 37.5 75 75 75 150
T0.290.280.210.280.27 0.47
Level M 0.59 0.56 0.410.640.82 0.9
B 0.66 0.67 0.580.480.68 0.66
As before the best results were obt~i n~ with the
cycle having doubled flow rates of MTS and H2, at low
temperature, and in the presence of HCl.
CA 02217702 1997-10-07
TABLE IV
Efficiency
P(kPa) 10 10 10 10 10 10
d(h) 20 20 20 20 20 20
Operating T(~C) 1010. 1010.1010. 1010 950 950
conditions Q(MTS)sccm 150 150 150 300 300 300
Q(H2)sccm 900 900 900 1800 1800 1800
Q(HCl)sccm 0 37.5 75 75 75 150
T 28.1 26.5 23.3 22.9 17 13
10 Level M 13 8.7 4.7 11.5 8 9
B 1.6 0.5 0.1 5.8 4.4 5.1
Total42.7 35.7 28.1 40.2 29.4 27.1
For cycles at doubled flow rates of MTS and H2, and
at low temperature (950~C), the presence of HCl reduces
efficiency, but it is better distributed throughout the
load in the oven, and another point which is also
important industrially, there were practically no
unwanted deposits on the preheating plates.
The results brought together in the above tables
~ show conclusively the advantage in terms of reducing non-
uniformity of SiC densification both throughout the
volume of the oven and throughout the volume of a single
part, and of performing chemical vapor infiltration at a
relatively low temperature, preferably less than 100~C,
in the presence of HCl, and with an increased flow rate
of MTS.
The process of chemically infiltrating SiC vapor can
be implemented with a temperature gradient, e.g. by means
of an installation such as that shown diagrammatically in
Figure 2.
This installation is more particularly designed for
densifying annular preforms 100 such as the brake disk
preforms made of a material that conducts heat, such as
carbon in fiber form. The preforms are stacked around a
central cylindrical graphite core 110 constituting a
heater core, with the assembly being supported by a
CA 02217702 1997-10-07
stationary insulating tray 120. The preforms 100 may be
slightly spaced apart from one another by means of
spacers 102 so as to facilitate access by the gas to the
main faces of the preforms. The reaction chamber 114 in
which the core and the preforms to be densified are
received is defined by an insulating wall 118 that does
not conduct electricity, having a bottom wall 118a and a
cover 118b. An inductor 116 surrounds the wall 118,
while still being inside an enclosure 112.
The means for f~;~g the chamber 114 with MTS + H2
+ HCl gas and for extracting residual gas are similar to
those of the installation of Figure 1 and they are not
shown. Nevertheless, it may be observed that the exhaust
pipe 152 leads directly to the bottom portion of the
chamber 114 through the bottom wall 118a. In addition,
the chamber 114 is not provided with means for preheating
the incoming gas.
The core 110 is heated by electromagnetic coupling
with the inductor 116. The annular preforms are heated
by having their inside cylindrical surfaces in contact
with the core 110. A temperature gradient is then
established between these inside surfaces and the exposed
outside surfaces thereof, which surfaces are cooled by
radiation and by convection in contact with the gas that
has penetrated into the chamber. This gradient depends
in particular on the ~;me~ional and heat conductivity
characteristics of the preforms. The generator 162
fee~; ng the inductor 116 is controlled so that, at least
at the beg; nn; ng of the infiltration process, the
temperature of preform portions adjacent to the core is
significantly higher than the minimum temperature for SiC
deposition, i.e. about 700~C. SiC densification then
takes place preferentially in those portions of the
preforms. This prevents portions of the preforms close
to their outside surfaces densifying too quickly, since
that could lead to premature clogging of the pores,
preventing densification in the cores of the preforms and
r CA 02217702 1997-10-07
14
generating a steep densification gradient within the
resulting parts.
The concept of performing chemical vapor
infiltration with a temperature gradient is known. Here
it constitutes a particularly advantageous application
because of the presence of HCl in the gas, which presence
has a particularly beneficial effect on the uniformity of
the matrix.
In the installation of Figure 2, the substrates 100
are heated by means of the heater core 110 with which
they are in contact. When the conductive nature of the
substrates makes it possible (e.g. substrates made of
carbon or graphite having a relatively high fiber
content), it is possible to envisage heating the
substrates at least in part by direct coupling with the
inductor, in which case the core may optionally be
omitted. Although the induced currents occur mostly in
the vicinity of the surfaces of the substrates, the
desired temperature gradient is established because the
exposed surfaces of the substrates are cooled by
radiation and convection.
The method of the invention is suitable for
chemically infiltrating the vapor of a material made of
carbon together with silicon and/or boron into any type
of substrate capable of withstanding the operating
conditions and chemically compatible with the gas. The
method can be used to perform densification with a matrix
constituted solely or partially of this material. In
which case, the matrix may also include one or more
matrix phases constituted by other materials deposited
before and/or after the material made of carbon together
with silicon and/or boron.