Note: Descriptions are shown in the official language in which they were submitted.
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
IMAGE-GUIDED BIOPSY APPARATUS
WITH ENHANCED IMAGING AND METHODS
Field Of The Invention
The present invention relates to apparatus
and methods for performing biopsy of biological tissue,
and more particularly, to performing biopsy of
biological tissue guided by ultrasound imaging.
Background Of The Invention
Apparatus and methods are known to identify
tumorous masses suspected of being malignant, for
example, by radiographic and sonographic techniques.
It is typical for such tissue masses to then be
biopsied to determine status as, or degree of,
malignancy, to determine further course of treatment.
For example, a region of a mammogram suspected to
contain a lesion may be biopsied to determine whether
the lesion is benign or malignant, and if malignant,
the course of treatment appropriate for the degree of
malignancy, e.g. mastectomy, radiation treatment or
chemotherapy.
Previously known biopsy methods range from
minimally invasive techniques, such as fine needle
aspiration using a 21 gauge hypodermic needle and large
core biopsy using a 14 gauge needle mounted in an
automated biopsy gun, to open-procedures in which the
lesion is surgically excised. Minimally invasive
techniques are faster, less expensive, safer and less
traumatic for the patient than surgical excision, and
begun developing widespread acceptance.
CA 02217976 2006-05-17
74702-6
- 2 -
A concern common to previously known
minimally invasive biopsy techniques, however, is
ensuring that the biopsy needle actually obtains a
tissue sample from the suspected lesion, rather than
adjacent healthy tissue. Previously known techniques
that attempt to ensure that the biopsy needle
trajectory enters the region of the suspected lesion
are described, for example, in Fornage et al.,
"Ultrasound-Guided Needle Biopsy Of The Breast And
Other Interventional Procedures," Radiologic Clinics Of
North America, Vol. 30, No. 1(January 1992), Fornage
et al. "Breast Masses: US-Guided Fine Needle Aspiration
Biopsy," Radiology, 162:409-414 (February 1987), Parker
et al., "US-guided Automated Large-Core Breast Biopsy,"
Radiology, 187:507-511 (May 1993), and Parker and Jobe,
"Large-Core Breast Biopsy Offers Reliable Diagnosis,"
reprinted from Diagnostic Imaging (October 1990).
The foregoing articles describe a free-hand
ultrasound technique, in which insertion of a biopsy
needle into a suspected lesion is performed by holding
a linear array ultrasound transducer in one hand and
inserting the needle into the tissue with the other
hand. In particular, the ultrasound transducer is held
above the midline of the suspicious mass and the needle
(or needle of the automated biopsy gun) is then
inserted in the tissue near the base of the transducer,
so that the tip of the needle appears in the ultrasound
scan. In addition, when a biopsy gun is employed,
additional personnel may be required to steady the
biopsy gun during use or to hold the ultrasound
transducer.
As described in the Fornage et al. articles
and Parker et al. article, difficulties arise using the
free-hand technique where the suspected lesion is
located near the patient's chest wall, or in proximity
to a prosthesis. These articles also emphasize that the
CA 02217976 1997-10-09
WO 96/32066 PCT/L7S96/05123
- 3 -
practitioner's level of skill in using the free-hand
technique can dramatically influence the results
obtained. All of the foregoing articles reject the use
, of biopsy needle guides that can be attached to the
ultrasound transducer, because the guides interfere
with the flexibility and maneuverability required to
obtain satisfactory results.
The Parker and Jobe article also describes
stereotactic mammographic biopsy systems. In such
systems, two X-ray images of the breast tissue are made
at different angles, thereby permitting the coordinates
of a lesion to be calculated. The biopsy needle,
typically an automated biopsy gun (e.g., Biopty from
C.R. Bard, Inc., Bard Urological Division, Covington,
Georgia) mounted in a rigid housing attached to the
biopsy table, is moved to the calculated coordinates
and actuated. Two additional X-ray views of the breast
tissue are then taken to confirm that the needle has
actually sampled the region of the suspected lesion.
The Parker and Jobe article further describes
the drawbacks of add-on stereotactic systems -- namely,
the potential for breast movement that renders earlier
stereo calculations worthless. That article also
describes the Mammotest system sold by Fischer Imaging
Corporation, Thornton, Colorado, as overcoming some of
the problems of add-on stereotactic systems, but at a
considerable cost differential.
A drawback common to all of the stereotactic
systems, however, is the need for multiple X-rays of
the tissue, thus exposing the tissue to potentially
unhealthful ionizing radiation. These systems also
provide no real-time imaging of the needle trajectory,
so as described in the Parker and Jobe article,
intervening movement of the breast tissue may render
the calculated coordinates useless and result in a
potentially misleading biopsy sample. Indeed, the
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 4 -
clinician is not even aware that the biopsy needle
missed the intended target until after the follow-up
stereotactic views are taken.
Moreover, because the biopsy needle is
secured in a fixed housing so as to provide a fixed
trajectory for biopsy needle, stereotactic systems
provide no freedom of movement for the biopsy needle
relative to the target tissue. Consequently, several
needle insertions and withdrawals are required to
adequately characterize the tissue.
A major disadvantage of the above-described
previously known methods and apparatus arises due to
the inability of the clinician to estimate, in real-
time, the correct trajectory of the biopsy needle from
the breast surface to the region of the suspected tumor
or lesion. Even when guided by free-hand ultrasound
scanning, the clinician typically must insert and
withdraw the biopsy needle ten to fifteen times or more
to improve the confidence level that a portion of the
suspected lesion has been collected. Then, each of the
needle aspiration samples must be separately tested,
significantly increasing the overall cost of the
procedure.
Likewise, in stereotactic systems, the
inability to monitor tissue movement and to manipulate
the biopsy needle once inserted, creates the need for
multiple needle insertions to obtain adequate
characterization of the suspected lesion. And again,
each of these multiple samples must be individually
tested to properly characterize the suspected lesion.
Such repetitive insertion and withdrawal of
the biopsy needle may cause significant patient
discomfort. Moreover, in those cases where the biopsy =
indicates no need for treatment by surgical methods,
the repeated biopsy needle insertion may nevertheless
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 5 -
leave the patient with cosmetically unappealing scar
, tissue.
A further disadvantage of these previously
known methods and apparatus is the potential for
seeding the needle tracks with potentially malignant
tumor cells. For example, because the clinician in
previously known methods must make several needle
insertions to confirm that he or she has sampled cells
from the target tissue, there is the potential that
malignant cells may be dispersed along a needle track
which was not believed by the clinician to have entered
the region of the suspected tumor, but which in fact
did so.
In view of the foregoing, it would be
desirable to provide apparatus and methods by which a
biopsy needle could be positioned for insertion so as
to have a real-time, predetermined trajectory to a
targeted tissue region, thereby reducing the need for
repetitive needle insertion and withdrawal to obtain a
biopsy sample.
It would also be desirable to provide
apparatus and methods by which a biopsy needle could be
positioned for insertion in real-time with a high
degree of confidence that the needle trajectory will
enter a targeted tissue region, thus reducing the risk
of spreading potential malignant tumor cells by
dispersing them along multiple needle tracks.
It would also be desirable to provide
apparatus and methods by which a biopsy needle could be
positioned for insertion into tissue along a
predetermined trajectory, and which enables the
clinician to alter that trajectory once the needle has
been inserted, so as to reduce the number of scars
resulting from repetitive skin punctures.
A yet further drawback-of previously known
biopsy systems, including those employing ultrasonic
CA 02217976 2006-05-17
74702-6
- 6 -
imaging of the biological tissue, is the inability to assess
tissue features located near, or extending within, the chest
wall. Such features typically have been inaccessible to
previously known radiographic and sonographic imaging
techniques due to the inability, for example, to direct such
X-radiation to the X-ray film, while in sonographic systems,
complicated structures including submersing the tissue in a
water bath have been required.
It therefore would be desirable to provide a
biopsy system having enhanced imaging capability to provide
images of biological features located near or within a
patient's chest line.
Summary of the Invention
In view of the foregoing, it is an object of an
embodiment of this invention to provide an apparatus and
methods by which a biopsy needle may be initially positioned
in real-time for insertion so as to have a predetermined
trajectory to a targeted tissue region. In this manner, the
need for repetitive needle insertion and withdrawal to
obtain a biopsy sample is reduced, improving the efficiency
of the medical procedure, and reducing patient distress
during the medical procedure.
It is another object of an embodiment of this
invention to provide apparatus and methods by which a biopsy
needle may be positioned for insertion in real-time with a
high degree of confidence that the needle trajectory will
enter a targeted tissue region, thereby reducing the risk of
spreading potential malignant tumor cells by dispersing them
along multiple needle tracks.
It is yet another object of an embodiment of the
present invention to provide apparatus and methods by which
CA 02217976 2006-05-17
74702-6
- 7 -
a biopsy needle may be positioned for insertion into tissue
along a predetermined trajectory, and which enables the
clinician to alter the needle trajectory once the needle has
been inserted, thus reducing the number of scars resulting
from repetitive skin punctures as well as patient
discomfort.
It is yet a further object of an embodiment of
this invention to provide apparatus and methods by which a
clinician can image biological features within tissue that
are located near, or extend within, a patient's chest wall,
thereby enabling more thorough examination of the tissue and
more thorough biopsy, if indicated.
These and other objects of the invention are
accomplished in accordance with the principles of the
invention by providing apparatus and methods in which a
biopsy needle is guided to an initial insertion position by
correlating, in real-time, the actual needle position prior
to insertion with its probable trajectory once inserted. In
a preferred embodiment, the needle location is tracked
electronically and projected over a previously stored or
real-time image of the tissue. The clinician may then
observe which features of the imaged tissue the biopsy
needle is likely to intersect when inserted. Additionally,
ultrasound scanning of a selected trajectory may also be
provided to assess depth of penetration of the biopsy
needle, when inserted.
The ultrasound scanning provided by the apparatus
of the present invention may include the capability, by
angling either the ultrasound transducer or the upper
compression plate relative to the chest wall, to provide
imaging of biological features located near, or extending
within, the chest line.
CA 02217976 2006-05-17
74702-6
- 7a -
Another embodiment of the invention provides
apparatus for positioning a tip of a biopsy device for
insertion into a selected region of a tissue mass, the
apparatus comprising: an ultrasonic scanner that provides
an image of the tissue mass, the image of the tissue mass,
including the selected region, displayed on a display means;
a support for holding a tip of a biopsy device with a
trajectory; means, other than the ultrasonic scanner,
coupled to the support for generating a signal corresponding
to a current location of the tip of the biopsy device, prior
to insertion into the tissue mass, the signal being
displayed on the display means by a symbol representative of
the current location of the tip of the biopsy device
relative to the selected region, wherein aligning the symbol
with the selected region corresponds to moving the support
to position at which the trajectory of the tip of the biopsy
device will intersect the selected region.
A further embodiment of the invention provides
apparatus for positioning a tip of a biopsy device for
insertion into a selected region of a tissue mass, the
apparatus for use in a system including an ultrasonic
scanner that provides an image of the tissue mass, the
apparatus comprising: a support for holding a tip of a
biopsy device with a trajectory; means, other than the
ultrasonic scanners, coupled to the support for generating a
signal corresponding to a current location of the tip of the
biopsy device, prior to insertion into the tissue mass, a
display means displaying the image of the tissue mass
including the selected region and a symbol representative of
the current location of the tip of the biopsy device
relative to the selected region, so that aligning the symbol
with the selected region corresponds to moving the support
CA 02217976 2006-05-17
74702-6
- 7b -
to position at which the trajectory of the tip of the biopsy
device will intersect the selected region.
A further embodiment of the invention provides a
use of an apparatus comprising an ultasonic scanner, a
support, a generation means, and a display means wherein the
ultrasonic scanner is for generation of an ultrasonic image
of a tissue mass which includes a selected region and for
display of the ultrasonic image of the tissue mass including
the selected region; the support is for provision of a
support for a tip of a biopsy device with a trajectory; the
generation means is for the generation of a signal
corresponding to a current location of the tip of the biopsy
device, prior to insertion of the tip of the biopsy device
into the tissue mass; the display means is for display of a
symbol representative of the current location of the tip of
the biopsy device relative to the selected region,
responsive to the signal; and the support is moveable to a
location at which the symbol is aligned with the selected
region, so that the trajectory of the tip of the biopsy
device is for intersection with the selected region.
Further features of the invention, its nature and
various advantages will be more apparent from the
accompanying drawings and the following detailed description
of the preferred embodiments.
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 8 -
Brief Description Of The Drawinas
FIG. 1 is a perspective view of an illustrative embodiment of the biopsy
system of the
present invention;
FIG. 2 is an exploded perspective enlarged
view of a portion of the biopsy system of FIG. 1,
indicated in inset 2 of FIG. 1;
FIGS. 3A-3C are perspective and elevational
views, respectively, of an alternative needle support
assembly in the open and closed positions;
FIG. 4 is a perspective view from beneath of
an alternative arrangement of the biopsy system of
FIGS. 1 and 2;
FIG. 5 is a perspective view of compressed
breast tissue showing the reference axes employed with
the biopsy system of the present invention;
FIG. 6 is an illustrative Y-Z display of the
compressed breast tissue of FIG. 5, taken along line
6--6 of FIG. 5;
FIG. 7 is an illustrative X-Y display of the
compressed breast tissue of FIG. 5, taken along line
7--7 of FIG. 5, showing a biopsy needle partially
inserted into the tissue;
FIG. 8 is a cross-sectional view of an
ultrasound scanning system constructed in accordance
with the present invention that enables imaging of
biological features located near, or extending within,
the patient's chest line;
FIG. 9 is a cross-sectional view of an
alternative embodiment of the system of FIG. 8.
Detailed Description Of The Invention
The present invention is directed to a system ~
for performing biopsy of biological tissue, as
indicated by, for example, a sonogram or mammogram. In
overview, the apparatus of the present invention uses a
CA 02217976 2006-05-17
74702-6
- 9 -
previously stored or real-time ultrasound image to
determine an initial position for a biopsy needle so
that there is a high degree of confidence that the
needle trajectory will intersect a target tissue
region, fDr example, a suspected lesion.
In a first illustrative embodiment described
herein, the biopsy system includes stand-alone
sonography apparatus. In an alternative embodiment,
the biopsy system may be used in conjunction with a
sonomammography system as described in commonly
assigned U.S. Patent No. 5,479,927.
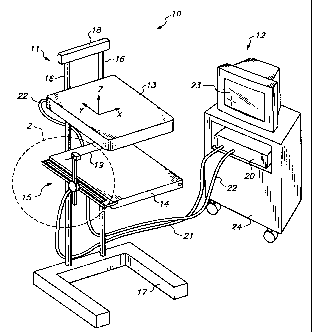
Referring now to FIG. 1, biopsy system 10
constructed in accordance with the principles of the
present invention is described. System 10 comprises
biopsy table 11 and computer-based display system 12.
Biopsy table 11 includes ultrasonic scanner 13, tissue
support table 14, and needle support system 15 movably
mounted on support members 16 between base 17 and top
block 18. Biopsy needle 19 is releasably carried by
needle support system 15, as described in detail
hereinafter. Needle support system 15 is detachably
coupled to computer 20 of computer-based display system
12 by cable 21, so that movement of biopsy needle is
displayed by monitor 23 of computer-based display
system 12.
Ultrasonic scanner 13 may be constructed as
described with respect to FIG. 7 of
U.S. Patent No. 5,479,927, so as to
include an annular or linear array ultrasonic
transducer mounted for movement in an X-Y plane as
indicated by the axes shown in FIG. 1 herein. In
particular, the ultrasound transducer may be mounted on
a carriage that is driven by a system of belts, cables,
drive screws, or similar mechanisms to provide scanning
along a series of planes sufficient to generate a
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 10 -
three-dimensional data model of the tissue to be
biopsied. =
Ultrasonic scanner 13 includes a lower
surface that functions as an upper compression plate, =
for immobilizing tissue against tissue support table
14. While the compression plate of ultrasonic scanner
13 may be constructed of any of the materials described
in the above-mentioned patent, such as polyimide, a
sodium-based ionomer resin, or a polymethyl pentene, it
will of course be understood that radiolucency of the
compression plate is not required for stand-alone
sonographic applications of the present invention.
Ultrasonic scanner 13 may also incorporate certain
improvements, described in detail hereinafter, that
enable imaging of biological features located near, or
within, the patient's chest line. Imaging data
generated by ultrasonic scanner is provided to
computer-based display system 12 via cable 22.
Tissue support table 14 of the illustrative
embodiment of FIG. 1 comprises a sturdy material, e.g.,
metal, fiberglass or plastic, such as UHMW plastic
(e.g., ultra high molecular weight polyethylene), or
combinations thereof, and serves to support the lower
surface of the biological tissue in compression. In
particular, tissue support table 14 and ultrasonic
scanner 13 may be releasably and adjustably mounted to
support members 16 to provide both adequate compression
of the tissue and to provide height adjustment to
accommodate the size of the patient.
Computer-based display system 12 is
illustratively shown comprising monitor 23,and computer
20 disposed on movable cart 24. Computer 20 may be a
general purpose personal computer, having for example,
an 80386 or greater microprocessor, or similar
processor, and a hard disk drive, or similar memory
device sufficient for storing software programs, to
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 11 -
manipulate imaging data generated by ultrasonic scanner
= 13 and positioning data generated by needle support
system 15. As will of course be understood, system 12
includes one or more additional cards for processing
data received from ultrasonic transducer 13 and needle
support system 15, the implementation details of which
employ routine application of ultrasound signal
acquisition principles, and which therefore form no
part of the present invention.
Referring now to FIG. 2, an illustrative
embodiment of needle support system 15 is described.
Needle support system 15 includes anchor bar 25,
support block assemblies 26 and 27, Y-axis track 28, Y-
axis linear encoder 29, support plate assembly 30, Z-
axis track 31, Z-axis linear encoder 32, and needle
support block assembly 33.
Anchor block 25 is dimensioned to fit with
close tolerances into grooves 34 provided in the
lateral sides of tissue support table 14. Positioning
holes 35 are provided in the grooved portion of tissue
support table 14 so that locking pegs (not shown) can
be extended through holes 35 and into holes 36 of
anchor block 25, thus positively locking anchor block
(and thus needle support system 15) into known
25 relation with tissue support table 14.
The combination of groove 34 and anchor block
25 provides a high degree of rigidity to the overall
needle support system, while provision of grooves 34 on
each side of tissue support table 14 enables the
clinician to obtain access to the tissue from either
the left or right side. In addition, ultrasonic
scanner 13 and tissue support table 14 may be
adjustably mounted, for example, to a block that is in
turn pivotally connected to support members 16, so as
to enable the entire biopsy system to be rotated
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 12 -
relative to the biological tissue, thus offering
additional areas of access to the tissue. =
= Support block assemblies 26 and 27 rigidly
fasten Y-axis track 28 to anchor block 25, and thus
tissue support table 14. Y-axis linear encoder 29
comprises, for example, an incremental binary counter,
and is slidably movable along the length of Y-axis
track 28. In a preferred embodiment, the Y-axis track
has disposed within it a printed circuit board
arrangement of parallel, spaced-apart copper strips,
while Y-axis linear encoder 29 includes a head that
senses the static capacitance of the copper strips as
the encoder is manually slid along Y-axis track 28, and
circuitry for interpolating between adjacent copper
strips. As Y-axis linear encoder 29 is moved along
track 28, it outputs a signal corresponding to its
displacement from a preset reference point, preferably,
a hard stop at a distal-most position from the
patient's chest wall. The signal output by linear
encoder 29 is provided to computer 20 via connecting
cable 21, which connects to encoder 29 through jack
21a.
Support plate assembly 30 rigidly connects Z-
axis linear encoder 32 to Y-axis linear encoder 29. Z-
axis track 31 is slidably engaged in linear encoder 32,
so that linear encoder 32 generates a signal
corresponding to the displacement of Z-axis track
relative to linear encoder 32 when track 32 is raised
and lowered. The signal output by linear encoder 32 is
provided to computer 20 via connecting cable 21, which
connects to encoder 32 through jack 21b. Linear
encoder 32 may use, for example, either the upper
surface of tissue support table 14, or the lower surface of ultrasonic scanner
13, as its reference
point. Linear encoders 29 and 32 preferably have a
displacement accuracy of about plus/minus 0.05 mm, and
CA 02217976 1997-10-09
WO 96/32066 PCTIUS96/05123
- 13 -
can be reset via switches on encoders 29 and 32, or via
= software control.
Linear.encoder 29 includes means, not shown,
for locking the encoder in position along Y-axis track
28, while linear encoder 32 includes means (not shown)
for locking Z-axis track 31 in position in encoder 32.
Linear encoders 29 and 32, and mating tracks 28 and 31,
are available from Sylvac S.A., Crissier, Switzerland,
and distributed in the United States by Fowler Company,
Inc., Chicago, Illinois, as Part Nos. 54-050-035 (for
Y-axis encoder 29) and 54-050-000 (for Z-axis encoder
32).
In addition, as will of course be understood
by persons of skill in the art, linear encoders 29 and
32 may also comprise suitably designed rotary encoders,
for example, as are used in computer mice and videogame
joysticks, or other suitable displacement sensing
components, such as linear variable displacement
transducers or linear potentiometers.
Needle support assembly 33 comprises a biopsy
needle holder that holds the biopsy needle securely
during initial positioning and insertion, but
detachably releases biopsy needle to allow free-hand
movement of the biopsy needle once it has been inserted
into a patient's tissue.
In the illustrative embodiment of FIG. 2,
needle support assembly includes upper block 33a and
lower block 33b. Needle support member is detachably
coupled to the upper end of Z-axis track 31, for
example, by a slot (not shown) in the lower surface of
lower block 33b. Upper block 33a includes semi-
circular channel 33c in its lower surface while lower
block 33b includes semi-circular channel 33c in its
upper surface. The channels in upper block 33a and
lower block 33b mate when the two pieces are positioned
CA 02217976 1997-10-09
WO 96/32066 PCT/I7S96/05123
- 14 -
together, thus forming a bore through which biopsy
needle 19 may be slidably disposed. =
Upper block 33a and lower block 33b
preferably also have mating projections and
concavities, for example, in the illustrative
embodiment of FIG. 2, block-like projection 33e and
corresponding indentation 33f. Bore 33g aligns across
upper block 33a and lower block 33b when the two blocks
are mated together, to permit pin 33d to be slidably
disposed in bore 33g. In this manner, upper block 33a
and lower block 33b may be rigidly fastened together by
pin 33d to carry biopsy needle 19 during initial
positioning and insertion of biopsy needle 19. When
inserted in the bore formed by channels 33c, the biopsy
needle has a trajectory aligned with the bore.
Once the needle is inserted in the patient,
pin 33d may be removed from bore 33g, permitting
removal of upper block 33a and movement of lower block
33b out of the clinician's way. This arrangement
permits the clinician to thus remove the biopsy needle
from needle support assembly 33 and manipulate it
manually, while observing movement of the needle tip
via display 23. Accordingly, needle support assembly
33 permits the biopsy needle to be initially positioned
with the accuracy of a stereotactic biopsy system,
while providing the flexibility and maneuverability of
free-hand ultrasound techniques.
An alternative illustrative embodiment of
needle support assembly 33' is described with respect
to FIGS. 3A-3B. As shown in FIG. 3A, needle support
assembly 33' comprises a block 37 having a V-shaped
channel formed in elements 37a and 37b, and integrally
formed locking arm 38. Locking arm 38 includes ridge
38a disposed to engage biopsy needle 19 when in a
closed position. Locking arm also includes latch
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 15 -
portion 38 including serrations 38c that interengage
serrations 37c on block 37.
Locking arm 38 is dimensioned so that it fits
with close tolerances between elements 37a and 37b,
thereby enabling ridge 38a to engage biopsy needles
having a wide range of diameters. As illustrated in
FIGS. 3B and 3C, needle support block 33' may include
slot 37d disposed therein for coupling the support to
Z-axis track 31. Locking arm 38 securely engages
biopsy needle 19 within the V-shaped channel formed in
elements 37a and 37b for image-guided positioning of
biopsy needle 19 relative to the patient's tissue, but
permits the biopsy needle to be readily released from
needle support assembly 33' by lifting locking arm 38
up and away from block 37.
Needle support assemblies 33 and 33' are
preferably comprised of sturdy, lightweight materials
that are capable of being sterilized. For example,
needle support assemblies 33 and 33' may comprise
machined aluminum that can be repeatedly sterilized, or
injection-molded plastic elements that are disposed of
after a single use. Needle support assembly 33' is
preferably integrally molded from a suitable plastic,
such as, polyethylene.
Referring now to FIG. 4, an alternative
embodiment of the biopsy system of FIGS. 1-3 is
described, in which elements similar to those of the
system of FIGS. 1-3 are indicated by reference numerals
increased by 100, e.g., biopsy needle support system
115.
The biopsy system of FIG. 4 differs from that
of FIGS. 1-3 in that tissue support table 114 comprises
a grid-like structure having a multiplicity of
apertures 114' and needle support system 115 is
disposed beneath tissue support table 114. As will be
apparent from FIG. 4, the arrangement of the system of
CA 02217976 1997-10-09
WO 96/32066 PCTIUS96/05123
- 16 -
FIG. 4 permits biopsy of the tissue to be performed
through the lower surface of the breast, thus reducing the prominence of
scarring associated with needle
punctures. It is also to be understood that the
multiplicity of apertures 114' extends over the entire
area of tissue support table 114 used to support the
patient's tissue (indicated in FIG. 4 by dots).
In preferred embodiment of the system of FIG.
4, tissue support table 114 has a thickness of about
0.5 inch (12.7 mm) and is formed of a suitable rigid
plastic, metal alloy, or combination thereof. Tissue
support table 114 includes a multiplicity of square
apertures about 1.5 inches (38 mm) on a side, at a
spacing sufficient to enable access to most of the
underside of the patient's tissue with a minimum of
repositioning.
With reference to the directional axes shown
in FIGS. 1 and 2, needle support system 115 of FIG. 4
lies in the X-Y plane, and comprises Y-axis track 128,
Y-axis linear encoder 129, X-axis track 131, X-axis
linear encoder 132, and needle support assembly 133.
X-axis track 131 is mounted to tissue support table 114
by support block assemblies 126, with Y-axis linear
encoder 132 slidably engaged thereon. Y-axis track 128
is slidably engaged in Y-axis linear encoder 129, which
is in turn coupled to X-axis linear encoder as
described above with respect to FIG. 2. Y-axis track
128 carries needle support assembly 133 that engages
biopsy needle 119.
Needle support system 115 is connected to
computer-based display system 12 via cable 121. Except
for the different physical arrangement of the
components of the system of FIG. 4 as just described,
the description provided hereinabove with respect to
the system of FIGS. 1-3 otherwise applies to the system
of FIG. 4.
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 17 -
Operation of the system of FIGS. 1-4 is now
described with reference to FIGS. 5-7. Referring now
to FIG. 5, a mass of biological tissue 100,
specifically a human breast, is shown as it would
appear when compressed between the upper surface of
tissue support table 14 and the lower surface of
ultrasonic scanner 13 (for clarity, ultrasonic scanner
13 and tissue support table 14 are not depicted in FIG.
5). It will of course be understood that tissue 100
remains connected to the patient. Tissue 100 contains
within it region 101 corresponding to a suspected
lesion.
FIG. 5 illustrates the reference axes
referred in the following description. In particular,
the Y-axis is the direction extending perpendicularly
from the patient's chest wall (plane 110), the z-axis
direction is elevational, and the x-axis direction
extends in a parallel manner along the patient's chest
wall. Projected needle trajectory 40 contacts the skin
of tissue 100 at location 41 and intersects region 101
of the suspected lesion.
In operation, a patient's tissue mass is
compressed between a lower compression surface of
ultrasonic scanner 13 and the upper surface of tissue
support table 14, thereby taking on the shape of tissue
mass 100 of FIG. 5. A gel pad may be used to
distribute the compressive loading over the tissue
mass, to ease the patient's discomfort, and to improve
coupling of the ultrasonic scanner to the tissue mass.
The clinician may then conduct a thorough
ultrasound examination of the tissue by operating
ultrasonic scanner 13 to generate a series of two-
dimensional slices in the Y-Z or X-Z planes. These
slices may then be digitally manipulated to provide a
holographic image on display 23 of the interior
CA 02217976 1997-10-09
WO 96/32066 PCTIUS96/05123
- 18 -
features of the tissue mass, or to provide a view of
any desired plane through the tissue. =
When a biopsy is indicated, the clinician
couples a fresh needle support assembly 33 or 33' to Z-
axis track 31 and engages the biopsy needle with the
needle support assembly. The clinician then selects a
viewplane for viewing on display 23, such as an
elevation view (i.e., in the Y-Z plane) through tissue
100, as shown in FIG. 6. As the biopsy needle is
manually moved by the clinician in the Y-Z plane
adjacent to tissue 100, encoders 29 and 32 of needle
support system 15 output a signal that is processed by
computer 20 and projected on the ultrasound image of
the selected viewplane as, for example, cross-hair 45.
It will of course be understood that the dimensions of
needle support assembly are well controlled so that
parallax between the location of the biopsy needle and
the location of encoders 29 and 32 is properly taken
into account.
By manually moving biopsy needle 19 adjacent
to tissue 100, needle support system 15 provides
computer 20 with corresponding coordinates that enable
the clinician to align cross-hair 45 with region 101 of
the suspected lesion (shown in FIG. 6 by dotted arrow
moving cross hair 45 to dotted cross-hair 45').
Once cross-hair 45 is aligned with region
101, the clinician may select additional views through
tissue 100 to assess the trajectory of the biopsy
needle. For example, the clinician may choose a plan
view (i.e., in the X-Z plane) through tissue 100, as
shown in FIG. 7, to indicate the trajectory of biopsy
needle 19. In a plan view such as that of FIG. 7, the
biopsy needle is preferably projected onto the
ultrasound image as line 46, rather than a cross-hair.
Biopsy system 10 further provides for
continually updating the ultrasound image of the entire
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 19 -
tissue mass 100, or of a selected portion thereof, by
operating ultrasonic scanner 13 to continually generate
images of the tissue interior. Thus, for example, when
the clinician has aligned the biopsy needle with region
101, he or she may lock needle support system 15
against further movement in the Y-Z plane, and issue
appropriate commands to the ultrasonic scanner to scan
only that portion of tissue 100 in the vicinity of the
biopsy needle trajectory, for example, within dotted
lines 47 shown in FIG. 7.
The clinician then extends biopsy needle 19
into tissue 100 by sliding the needle in the X-
direction through the needle support assembly to enter
the patient's tissue. If the plan view of the
ultrasound image is displayed, as shown in FIG. 7, the
clinician may then monitor the progress of the biopsy
needle as it penetrates the tissue mass.
After the clinician has obtained a sample of
region 101 along line 46 of the trajectory of biopsy
needle 19, he or she may then remove pin 33d to release
biopsy needle 19 from needle support assembly 33 or
locking arm 38 of needle support assembly 33'. The
clinician may then manipulate the biopsy needle to
collect additional samples of region 101 under
ultrasound image guidance, without having to create
additional puncture wounds in the skin of tissue 100.
Operation of the system of FIG. 4 is similar
to that described above, except that needle support
system 115 is manipulated in an X-Y plane located
beneath the patient's breast. Once the clinician has
obtained a complete ultrasound image of the interior
features of the tissue 100, a view in the X-Y plane is
selected, which view would appear similar to that of
FIG. 6. As needle support system 115 is manipulated,
corresponding cross-hair 45 moves about on the
displayed ultrasound view plane, as depicted in FIG. 6.
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 20 -
Biopsy needle may then be inserted through
the nearest aperture 114' in tissue support table 114
to perform the biopsy. Similar to the system of FIGS.
1-3, progression of the biopsy needle as it is inserted
in tissue 100 can be obtained by subsequent imaging
using ultrasonic scanner 13. In particular, images
generated in the Y-Z plane using the system of FIG. 4
will have an appearance similar to that of shown in
FIG. 7.
As described above, biopsy system 10 of the
present invention provides significant benefits over'
previously known systems and techniques. Unlike free-
hand ultrasound techniques, the present invention
provides precise initial positioning of the biopsy
needle, so that the clinician has a high degree of
confidence that the proposed trajectory of the biopsy
needle will intersect the region of interest, thus
reducing the number of needle insertions/withdrawals
and the risk of seeding malignant cells along multiple
needle tracks.
Moreover, the present invention provides
continuous monitoring of the biopsy needle over the
entire extent of the needle track, as opposed free-
hand techniques that display only the portion of the
needle that comes within the ultrasound scan. For
example, free-hand ultrasound techniques provide no
information about the biopsy needle trajectory until
the needle has already punctured the skin; by contrast,
the present invention enables prediction of the needle
trajectory before it is even inserted.
Biopsy system 10 of the present invention
likewise provides significant advantages over
stereotactic X-ray systems. For example, the biopsy
system of the present invention eliminates the use of
hazardous ionizing radiation attendant with use of X-
rays, eliminates the need for calculating coordinates
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 21 -
for needle placement, provides real-time monitoring of
the actual needle trajectory without the need for
follow-up imaging, and enables the clinician to sample
multiple areas within a target region through a single
puncture wound. Moreover, the system of the present
invention is less complex than stereotactic X-ray
systems, and proportionately less expensive to build,
use and maintain.
It will further be understood that the biopsy
system of the present invention may be used with other
ultrasonic scanning apparatus, for example, in
conjunction with the sonomammography apparatus
described in the above-mentioned U.S. patent 5,479,927.
For example, to use the biopsy system 15 of the present
invention in an X-ray system including an ultrasonic
scanner as described in that patent, needle support
system--15 riay-be anchored-to-a-dummy X=ray film
cassette. The dummy X-ray film cassette is installed
in the film holder/diffraction grid assembly of the X-
ray system (often called a "Bucky"), to permit combined
use of the ultrasonic scanner and biopsy needle support
system as described hereinabove.
Referring now to FIGS. 8 and 9, further
features are described which are suitable for use in
the biopsy system of the present invention. Referring
to FIG. 8, a cross-section of ultrasonic scanner 50 is
shown which is essentially similar in design to the
ultrasonic scanner 13 described above and in the above-
mentioned U.S. Patent 5,479,927. Ultrasonic scanner 50
differs from the above-described embodiments-in that
transducer 51 is not coupled to compression plate 52
directly, but is instead canted at an angle e away from
the compression plate. In addition, both compression
plate 52 and front panel 53 are constructed of a rigid
sonolucent material.
CA 02217976 1997-10-09
WO 96/32066 PCT/US96/05123
- 22 -
Proper acoustic coupling between transducer
51 and compression plate 52 is obtained in ultrasonic
scanner 50 by filling it with water 54 or another
suitable acoustically transmissive medium. This
arrangement is expected to enable ultrasonic scanner 50
to provide imaging not only of tissue disposed directly
below the scanner, but also to provide imaging of
tissue located near, or within, the patient's chest
wall.
FIG. 9 provides an alternative embodiment of
an ultrasonic scanner designed to provide enhanced
imaging. In particular, ultrasonic scanner 60 of FIG.
9 is similar in design to ultrasonic scanner 13
described hereinabove, except that ultrasonic
transducer 61 and compression plate 62 are canted at an
angle 6' to the horizontal. Since transducer 61 and
compression plate 62 are both angled, transducer 61 may
be acoustically coupled to compression plate 62 using
coupling means described in the above-mentioned U.S.
Patent 5,479,927. The inclined angle of compression
plate 62 is also expected to enable ultrasonic imaging
of internal features located near, or extending within,
the patient's chest wall.
While preferred illustrative embodiments of
the present invention are described above, it will be
obvious to one skilled in the art that various changes
and modifications may be made therein without departing
from the invention and it is intended that the appended
claims cover all such changes and modifications which
fall within the true spirit and scope of the invention.