Note: Descriptions are shown in the official language in which they were submitted.
= =
CA 02217990 1997-10-31
W O 96/34868 PCTAEP96101880
ESTERS OF CARBAPENEMS
This invention relates to novel antib~ct~ri~l eompounds, processes for their
preparation, pharmaceutical and veterinary compositions comprising them, and their
5 use in antibacterial therapy.
Carbapenems such as imipenem, the compound of formula (A):
Ho H H
~-\
o~ ~S(CH2)2NHCH=NH
CO2H
(A)
have a potent, broad spectrum of antibacterial aetivity (see US 3 950 357 and
US 4 194 047; Merck and Co). Such carbapenems however tend to be vulnerable to
hydrolysis by the enzyme renal dehydropeptidase-l (DHP-l) and this limits their use
in chemotherapy. In the case of imipenem, this problem may be overcome by the co-
lmini.ctration of an inhibitor of DHP-l.
Stability towards DHP-l may also be imparted by chemical modif1cation of
the carbapenem nucleus, for instance by incorporating a l~-methyl substitutent, as in
the compound mel~,pene,l" the compound of formula (B):
HO H H CH3 ~ CON(CH3k
~S --C~NH
CO2H
(B)
(see Shih D.H. et al., Heterocycles, 1984, 21, 29 and Sunagawa M. et al.,
J. Antibiotics, 1990, 43, 519). More recently, this has been extended to a 1~-
aminoalkyl substituent ~see EP 0 433 759, Bristol-Meyers Squibb).
An alternative approach to imparting improved stability to DHP-l utilises 2-
carbon~substituted carbapenems, for in.ct~nce, 2-aryl, 2-heteroaryl and 2-
heteroaromatic carbapenems (US 4 543 257, US 4 260 627, US 4 962 101,
US4978659,EP014493, EP0414489,EP0010316andEP0030032Merck&
Co) and 2-(substituted)methyl carbapenems (Schmidt et al, J.Antibiotics, 41, 1988,
780).
UK Patent 1 593 524, Merck & Co. discloses a number of 5-membered
heteroaromatic carbapenem derivatives including diazolyl and tetrazolyl compounds.
However, in the case of the pyrazolyl derivatives the heterocyclic compound is
attached to the carbapenem nucleus through the C-4 position.
CA 02217990 1997-10-31
W 096/34868 PCTAEP96/01880
Other structural modifications introduced at position-2 include a substituted
vinyl group -C(Ra)=CHRb in which, for in.~f~nce, Ra is hydrogen or methyl and Rb is
hydrogen or lower alkyl (EP 0 330 108; Fujisawa) or Ra and Rb are selected from
hydrogen, lower alkyl, aminocarbonyl, lower alkoxy, cyano, nitro and lower
alkoxycarbonyl (EP 0 430 037, Banyu Pharmaceutical Co.). In the absence of a l,B-
methyl substituent, such a mo-lific~tion does not however appear to impart DHP-1stability.
TntP.rn~tional Patent Application No. PC r/GB94/02347 describes compounds
of the general formula (C): -
d
H H R
c-~ ~ R
o~
CO2 R
(C)
in which R is:
N--N
~ 'R~;
wherein
Ra is hydrogen, optionally substituted (Cl 6)alkyl or optionally substituted aryl;
R~ is hydrogen, optionally substituted (Cl 6)aL~yl or optionally substituted aryl; or
20 Ra and R13 together form an optionally substituted 5 or 6 membered heterocyclic ring
with or without additional heteroatoms;
Rc is (Cl 6)aLlcyl which is unsubstituted or substituted by fluoro, a hydroxy group
which is optionally protected by a readily removable hydroxy protecting group, or by
an amiRQ group which is optionally protected by a readily removable amino
25 protecting group;
Rd is hydrogen or methyl; and
-CO2Re is carboxy or a carboxylate anion or the group Re is a readily removable
carboxy protecting group.
CA 02217990 1997-10-31
W 096/34868 PCTAEP96/01880
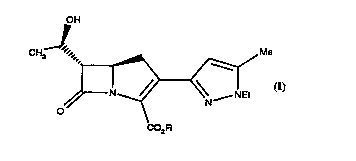
According to the present invention, there is provided the compound of
formula (I):
OH
CH3 ~'", ~Me
~~ --~ N--NEt
CO2R
(I)
wherein R is selected from the group consicting of isobutyryloxymethyl, (5-methyl-2-
oxo-1,3-dioxolen-4-yl)methyl, and benzoyloxymethyl.
Particular compounds in accordance with the present invention are:
isobutyryloxymethyl (SR, 6S)-2-[ 1-ethyl-5-methylpyrazol-3-yl]-6-[( 1 R)- 1-
10 hydroxyethyl]-carbapen-2-em-3-carboxylate,
(5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl (5R, 6S)-2-[1-ethyl-5-
methylpyrazol-3-yl]-6 [(lR)-l-hyd,~,~yeLhyl]-carbapen-2-em-3-carboxylate, and
benzoyloxymethyl (5R, 6S)-2-[1-ethyl-5-methylpyrazol-3-yl]-6-[(lR)-1-
hydroxyethyl] -carbapen-2-em-3-carboxylate.
Since each carbapenem compound of the present invention is intended for use
in ph~rm~ceutic~l compositions, it will be understood that it is provided in
sllbst~nti~lly pure form, for ~Y~mple at least 50% pure, more suitably at least 75%
pure and preferably at least 95% pure (% are on a wt/wt basis). Impure preparations
of the compound may be used for p~pa,ing the more pure forms used in the
20 pharmaceutical compositions. Preferably, whenever possible, each compound of the
present invention is obtained in crystalline form
When each compound of this invention is allowed to crystallise or is
recrystallised from organic solvents, solvent of cryst~lli.c~tion may be present in the
crystal~ine product. This invention includes within its scope such solvates. Similarly,
25 the compounds of this invention may be crystallised or recrystallised from solvents
cont~ining water. In such cases water of hydration may be present in the crystalline
product. This invention includes within its scope stoichiometric hydrates.
The carbapenem antibiotic compounds according to the invention may be
formulated for ~-lmini.ctration in any convenient way for use in human or veterinary
30 medicine, according to Lechniques and procedures per se known in the art withreference to other antibiotics, and the invention therefore includes within its scope a
ph~rm~ eutical composition complising an antibiotic compound according to the
present invention together with a ph~rm~ceutically acceptable carrier or excipient.
-- 3 --
CA 02217990 1997-10-31
W 096/34868 PCTAEP9GI01880
The compositions may be formulated for a~lmini.etration by any suitable route, such as
oral, pa elllel~l or topical application, although the oral route is particularly preferred.
The compositions may be in the form of tablets, capsules, powders, granules,
lozenges, creams or liquid preparations, such as oral or sterile parenteral solutions or
5 suspeneione Tablets and caps-lles for oral a~1minictration may be in unit dosepresrnt~tion form and may contain convention~l excipients such as binding agents,
for example, syrup acacia, gelatin, sorbitol, tr~g~ nth, or polyvinylpyrollidone;
fillers, for example lactose, sugar, mai~-starch, calcium phosph~te, sorbitol orglycine; tabletting lubricants, for example m~gn~eillm stearate, talc, polyethylene
10 glycol or silica; disintegrants, for example potato starch; or acceptable wetting agents
such as sodium lauryl sulphate. The tablets may be coated according to methods well
known in normal ph~rrn~eutical practice. Oral liquid preparations may be in the
form of, for example, aqueous or oily suspensions, solutions, emulsions, syrups or
elixirs, or may be presented as a dry product for reconstitution with water or other
15 suitable vehicle before use. Such liquid preparations may contain conventional
additives such as suspending agents, for example sorbitol, methyl cellulose, glucose
syrup, gelatin, hy~o~ye~llyl cellulose, carboxymethyl cellulose, ~ minium stearate
gel or hydrogenated edible fats; emulsifying agents, for example lecithin, sorbitan
monooleate, or acacia; non-aqueous vehicles (which may include edible oils), for20 example almond oil, oily esters, glycerine, propylene glycol, or ethyl alcohol;
preservatives, for example methyl or propyl p-hydlu~yl,enzoate or sorbic acid; and, if
desired conventional flavouring or colouring agents. Suppositories will contain
conventional suppository base, eg cocoa-butter or other glyceride.
For parenteral arlminietration~ fluid unit dosage forms are prepared utili.cing
25 the compound and a sterile vehicle, water being preferred. The compound, depending
on the vehicle and concentration used, can be either suspended or dissolved in the
vehicle. In preparing solutions the compound can be dissolved in water for injection
and filter sterilised before filling into a suitable vial or ampoule and se~1ing.
Advantageously, agents such as local an~sthf~.tic, preservative and buffering agents
30 can be~dissolved in the vehicle. To enhance the stability, the composition can be
frozen after filling into the vial and the water removed under vacuum. The dry
Iyophilised powder is then sealed in the vial and an accompanying vial of water for
injection may be supplied to reconstitute the liquid prior to use. Parenteral
suspensions are prepared in substantially the same manner except that the compound
35 is suspended in the vehicle instead of being dissolved and sterilisation cannot be
accomplished by filtration. The compound can be sterilised by exposure to ethylene
oxide before suspending in the sterile vehicle. Advantageously, a surfactant or
wetting agent is included in the composition to facilitate uniform distribution of the
compound.
-- 4 --
CA 02217990 1997-10-31
W 096/34868 PCT~EP9G/01880
The composition may contain from 0.1% to 99.5% by weight, preferably from
10-60% by weight, of the active m~t~ri~l, depending on the method of admini~tration.
Where the compositions comprise dosage units, each unit will preferably contain
from 50-500 mg of the active ingredient. The dosage as employed for adult human
5 treatment will preferably range from 100 mg to 12 g per day for an average adult
patient (body weight 70 kg), for instance 1500 mg per day, depending on the route
and frequency of atlm;ni.~tration. Such dosages correspond to approximately 1.5 to
170 mg/kg per day. Suitably the dosage is from 1 to 6g per day.
The daily dosage is suitably given by ~lmini.~tering a compound of the
invention several times in a 24-hour period. Typically, 250 mg is ~-lmini.ctered 4
times a day although, in practice, the dosage and frequency of ~dmini.ctration which
will be most suitable for an individual patient will vary with the age, weight and
response of the p~tiP.nt.~, and there will be occasions when the physician will choose a
higher or lower dosage and a different frequency of ~minist-ration. Such dosage
regimens are within the scope of this invention.
No toxicological effects are inc1icated when any compound of the invention is
mini.~tered in the above mentioned dosage range.
The present invention also includes a method of treating bacterial infections inhumans and ~nim~l.c which method comprises ~lminicte.ring a therapeutically
effective amount of an antibiotic compound of the present invention of the formula
(I).
In a further aspect, the present invention also provides for the use of a
compound of formula (I) for the manufacture of a meAi~ment for treating bacterial
infection.
The compounds of the present invention of formula (I) are active against a
broad range of Gram-positive and Gram-negative bacteria, and may be used to treat a
wide range of b~teri~l infections including those in immunocompromised patients.Amongst many other uses, the compounds of the invention are of value in the
tre~tment of skin, soft tissue, respiratory tract and urinary tract infections in hllm~n.c
and mày also be used to treat mastitis in cattle.
A particular advantage of the antibacterially active compounds of this
invention is their stability to ,~-lact~m~ce enzymes and they are th'erefore effective
against ,13-lactamase producing org~ni.~m.c
The present invention further provides a process for the preparation of a
compound of formula (I) wherein R is isobutyryloxymethyl or benzoyloxymethyl,
which process comprises treating a corresponding compound of formula (I), wherein
R is an aL~ali metal cation, with a compound of formula XCH20COCHMe2 or
XCH2OCOC6Hs, wherein X is a leaving group such as halogen, more particularly
bromine or iodine.
- 5 -
CA 02217990 1997-10-31
W 096/34868 PCT~P96/01880
The reaction is typically carried out at between 0 and 60 C, for example at
ambient temperature, under an inert, for example argon, atmosphere, in a suitable
organic solvent, for e~r~mplP, N-methylpyrrolidin-2-one. Other suitable solvent
systems include N, N'-dimethylfommamide and N, N'-dimethyl~et~mi~e
The present invention further provides a process for the preparation of a
compound of fommula (I) wherein R is (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl,
which process comprises treating a corresponding compound of formula (I), wherein
R is an alkali metal cation, with a compound of fommula:
Me
XCH2
wherein X is a leaving group such as halogen, more particularly bromine or iodine.
The reaction is typically carried out at between 0 and 60 C, for example at
ambient temperature, in a suitable organic solvent, for e~mphP, N-methylpyrrolidin-
2-one, and in the presence of an acid-binding agent, such as 2,6-lutidine. Othersuitable acid-binding agents include pyridine and diisopropylethylamine. Other
suitable solvents include N, N'-dimethylfnrm~mide and N, N'-dimethyl~et~mi~P
Compounds of formula (I) wherein R is an aLkali metal cation" such as
sodium~ are described in TntPm~tion~l Patent Application No. PCT/GB9~/02347, andhereinbélow in Preparation 1.
The compound of formula ICH2OCOCHMe2 (iodomethyl isobutyrate) and
iodomethyl benzoate can be prepared from the corresponding chlorides via the
FinkPlstein reaction, which is well known to those skilled in the art.
Chloromethyl isobutyrate and chloromethyl benzoate can be prepared by
e~ iLying isobutyric acid or benzoic acid respectively with chloromethyl
chlorosulphate (Binderup et al., Synthetic Comme~n., 14(9), 857-864 (1984)).
The fo~owing Examples illustrate the present invention further:
General Instructions - Solutions were dried using anhydrous m~nesillm sulphate
and solvents were removed by evaporation under reduced pressure using a rotary
evaporator. Column chromatography on silica gel used Merck silica gel 60, particle
size <0.063mm.
CA 022l7990 l997-lO-3l
W 096/34868 PCT~EP96/01880
FY~n~PIe 1
Isobutyryl~ yl-(5R, 6S~2-(1-ethyl-5-~-u:ll-yl~ ~ol-3-yl)-~t(lR)-l-
S l~y~l~ oxyethyl]carbapen-2-em-3-carboxylate
The product from Preparation 1 (200 mg, 0.61 mmol) was dissolved in N-
methylpyrTolidin-2-one (2 ml). A solution of isobutyTyloxymethyl iodide (360 mg, 1.6
mmol) in N-methylpylTolidin-2-one (0.5 ml) was added to this solution at room
10 temperature under argon and after 0.5 h the reaction mixture was diluted with ethyl
acetate (15 ml) and the solution was washed with water (3 x 15 ml), 5% sodium
thios~llf~t~ solution (15 ml) and then saturated brine (15 ml). The organic extract was
dried (Na2SO4) and concentrated to an oil which was purified by chromatography on
silica gel eluting with an ~etonP/toluene gradient ~ Lult: to yield a pale yellow solid
15 which was recrystallised from diethyl ether to afford the title compound as pale
yellow microneedles (100 mg, 40%), m.p. 107-8~C; (Found: M+, 405.1899.
C20H27N3o6 requires M 405.1900); vmax(cHcl3) 3458, 3019, 1772 and 1734 cm~
l; ~H(CDC13) 1.19 (6H, d, n.l Hz), 1.36 (3H, d, J6.1 Hz), 1.40 (3H, t, r7.2 Hz),1.76 (lH, d, J4.9 Hz), 2.29 (3H, s), 2.62 (lH, septet, J7.1 Hz), 3.18 (lH, dd, J2.7, 6.5
20 Hz), 3.30 (lH, dd, J9.0, 18.8 Hz), 3.64 (lH, dd, J9.8, 18.8 Hz), 4.08 (2H, q, J7.2 Hz),
4.16-4.30 (2H, m), 5.92 (lH, d, J5.6 Hz), 5.98 (lH, d, J5.6 Hz) and 7.02 (lH, s).
Example 2
25 (5-Methyl-2-oxo-1,3-clioxolen~-yl)methyl (5R,65)-~[(R)-l-lly~ Jx~ethyl]-2-(1- ethyl-5-methyl-pyrazol-3-yl)carbapen-2-em-3-carboxylate
Sodium (5R,65)-6-[(R)- 1 -hydroxyethyl] -2-(1 -ethyl-5-methylpyrazol-3-yl)carbapen-2-
em-3-carboxylate (300 mg) in N-methylpyrrolidinone (4 ml) was treated with 2,6-
luti-iine (ca. 30 mg) foIlowed by 4-bromomethyl-5-methyl-1,3-dioxol-2-one (290
30 mg) [P.~Sakamoto, S. Ikeda and G. Tsukamoto, Chem. Pharm Bull. 32, (6), 2241 -
2248 (1984)] in tetrahydrofuran (1 ml). The mixture was stirred for 1.25 h. Ethyl
acetate and water were added and the layers separated. The aqueous layer was re-extracted with ethyl acetate and the combined ethyl acetate layers were washed with
water, followed by saturated brine solution, and then dried (MgSO4). The filtered
35 solution was stored in a refrigerator ovemight and then evaporated and
chromatographed on silica gel (230 - 400 mesh ASTM) (2.5 x 12 cm column),
loading in CH2C12 / h~x~n~ and eluting with ethyl acetate. Practions cont~ining the
product were combined and evaporated to give a colourless solid which was
recrystallised from acetone to give crystals of (5-methyl-2-oxo-1,3-dioxolen-4-
- 7 -
CA 02217990 1997-10-31
W O 96/34868 PCTAEP96/01880
yl)methyl (SR,65)-6-~(R)-l-hydroxyethyl]-2-(l-ethyl-S-meLhyll,ylazol-3-yl)carbapen-
2-em-3-carboxylate, m. p. 148 - 151~C; ~maX(cH2cl2) 3605, 2975, 1823, 1776,
1737 (sh), 1721, 1598, 1546, 1314, 1231, and 1182 cm~l; ~max(EtOH/nm 325 (~
/dm3mol~lcm~l 14,654), 260.5 (~/dm3mol~lcm~l 3404); ~CDC13) 1.36 (3H, d, J
6.2 Hz), 1.40 (3H, t, J ca 7.3 Hz), 1.76 (lH,d, J4.9 Hz), 2.21 (3H, s), 2.30 (3H, s),
3.19 (lH, dd, J2.7 & 65 Hz), 3.31 (lH, dd, J9.0 & 18.7 Hz), 3.61 (lH, dd, J 9.9 &
18.7 Hz), 4.08 (2H, q, J7.3 Hz), 4.16 - 4.30 (2H, m), 5.00 & 5.07 (2H, ABq, J 13.9
Hz), 6.88 (lH, s) ppm; Found (EI ms) m/z 417 (M+).
F.Y~mp~ 3
Benzoyloxyl-.elllyl (5R,6S)-2~ ethyl-5-~,~lhyl~ ~ol-3-yl)-6-[(lR)-l-
hr~lroxy~lllyllcal b~e~l-2-em-3-carbox-ylate
The product from Preparation 1 (200 mg, 0.61 mmol) was dissolved in N-
methylpyrrolidin-2-one (2 ml). A solution of benzoyloxymethyl iodide (336 mg, 1.28
mmol) in N-methylpyrrolidin-2-one (0.5 ml) was added to this solution at room
tempelature under argon and after 0.5 h the reaction mixture was diluted with ethyl
acetate (15 ml) and the solution was washed with water (3 x 15 ml), 5% sodium
thioslllf~tP solution (15 ml) and then saturated brine (15 ml). The organic extract was
~ 20 dried (Na2SO4) and concentrated to an oil which was purified by chromatography on
silica gel eluting with an ~etonP/toluene ~ liPnt ~ Ul~ to yield a pale yellow solid
which was recrystallised from acetone/diethyl e~er to afford the title compound as a
white solid (143 mg, 53%), m.p. 131-4~C; (Found: M+, 439.1743. C22H2sN3O6
requires M439.1743); ~max(CHCl3) 3021, 1740, 1602, 1452 and 1440 cm~l; ~
H(CDC13) 1.34 (6H, m), 1.70 (lH, d, J4.9 Hz), 2.25 (3H,s), 3.19 (lH, dd, J2.4, 6.3
Hz), 3.30 (lH, dd, J8;8, 18.8 Hz), 3.64 (lH, dd, J10.1, 18.8 Hz), 4.07 (2H, q, J7.2
Hz), 4.18-4.31 (2H, m), 6.20 (2H, s), 7.42-7.48 (2H, m), 7.56-7.61 (lH, m), and 8.10
(2H, d, J7.1 Hz) ppm.
Prep~flon 1
Sodium (SR,65)-6-[(R)-l-hydroxyelhyl]-2-(1-ethyl-5-methyl~y~ol-~
yl)carbapen-2-em-3-carboxylate
(a) Ethyl l-ethyl-5-melllyl~yl~;ole-3-carboxylate
N-Ethylhydrazine oxalate (12 g) in glacial acetic acid (100 ml) was cooled in an ice-
bath and treated with ethyl 2,4-dioxovalerate (11.24 ml). After addition was complete
-- 8 --
CA 02217990 1997-10-31
W O 96/34868 PCTAEP96/01880
the mixture was stirred at room temperature; after ca. 45 min the mixture was
warmed to dissolve insoluble ethylhydrazine oY~l~tP The mixture was stirred for a
further 2 h and then poured into water( ca. 300 ml) / ethyl acetate (ca. 700 ml) and
solid K2CO3 was carefully added, with stirring, untiLthe pH was neutral. After
5 ~paration the aqueous layer was re-extracted with ethyl ~cet~t~o The combined ethyl
acetate extracts were dried (MgSO4), and the solvents removed to leave an oil.
Chromatography on silica gel, loading in CH2C12/hexane and eluting with a gradient
elution of ethyl acetate/hexane mixtures (from 2:8 to 1:1) gave ethyl 1-ethyl-5-methylpyrazole-3-carboxylate as an oil (13.2 g); 1)max (CH2C12) 1717, 1446, 1389,
and 1219 cm~l; ~(CDC13) 1.38 (3H, t, J7.2 Hz), 1.42 (3H, t, J7.3 Hz), 2.30 (3H, s),
4.17 (2H, q, J7.3 Hz), 4.38 (2H, q, J7.1 Hz), 6.55 (lH, s); (Found m/z 182.1055.CgH14N202 requires m/z 182.1055).
(b) l-Ethyl-5-metl-yl~yl azole-3-carboxylic acid
Ethyl l-ethyl-S-methylpyrazole-3-carboxylate (10.93 g) in ethanol (70 ml) was
treated with KOH (3.69 g), followed by water (30 ml), and the mixture was stirred
and heated under reflux for 6 h. The ethanol was removed using a rotary evaporator
and ethyl acetate/water were added. The pH of the mixture was adjusted to 3.0 and
the layers were ~p~r~tl~-i The aqueous layer was re-extracted with ethyl ~cet~te20 The combin~.d ethyl acetate layers were extracted with excess aqueous NaHCO3. The
NaHCO3 extract was poured into excess acid, and the pH was then adjusted to 3, and
NaCl was added to the solution. The mixture was then repeatedly extracted with
ethyl ~et~t~., and the combined extracts were dried (MgSO4) and evaporated. The
residue was triturated~~ith diethyl ether to give the acid as a solid (5.65 g); l~max
(CH2C12) 2754, 2598, 1698, 1498, 1464, 1387, and 1233 cm~l; ~(CDC13) 1.40
(3H, t, J7.3 Hz), 2.32 (3H,s), 4.19 (2H, q, J7.3 Hz), 6.61 (lH,s) ppm; (Found n2/z
154.0740. C7HloN202 requires n2/z 154.0742).
(c)N-Metho~y-N-methyl- l-ethyl-5-metl.yl~yl a~ole-3-carboY~m: lle
1-Ethyl-S-methylpyrazole-3-carboxylic acid (5.25 g) in dry dichloromethane (100 ml)
cont~ining N,N-dimethylform~mi~ (0.26 ml) was cooled in an ice-bath and treated
with a solution of oxalyl chloride (3.27 ml) in dichloromethane (25 ml), added
dropwise. The mixture was stirred in the cold for 25 min, and then allowed to warm
to room temperature, when evolution of a gas was observed. After 10 min the solvent
was removed by evaporation in vacuo and toluene was added and removed (x 2) to
g
CA 02217990 1997-10-31
W O 96/34868 PCTAEP96/01880
- ensure any residual HCl and oxalyl chloride had been removed. The reslllt~nt acid
chlnritle was redissolved in dry dichlornmeth~n~- and then treated with N,O-
dimethylhydroxylamine hydrochloride (3.61 g). The mixture was cooled in an ice-
bath and treated with pyridine (6.0 ml). the mixture was then allowed to stir at room
5 temperature for 1.5 h and then diluted with ether (100 ml) and washed with brine.
The organic layer was then dried (MgSO4) and evaporated to leave an oil. This was
the chromatographed on silica gel, loading in dichloromethane, and eluting with ethyl
acetate / hexane mixtures to give, after evaporation of requisite fractions, the ~=
hydroY~m~te (5.2 g) as a solid;
10 ~maX(cH2cl2) 2982, 2937, 1641, 1489, 1445, 1379,and 975 cm~l; ~(CDC13) 1.43
(3H, t, J7.3 Hz), 2.29 (3H, s), 3.42 (3H, s), 3.76 (3H, s, ), 4.13 (2H, q, J7.3 Hz),
6.49 (lH, s); (Found m/z 197.1164. CgHlsN3O2 requires m/z 197.1164).
(d) 3-Acetyl-l-ethyl-5-methylpyrazole
15 N-Methoxy-N-methyl-l-ethyl-5-methylpyrazole-3-carboxamide (3.12 g) in dry
tetrahydrofuran (60 ml) was cooled in an ice-bath and treated with a 3.0M solution of
methylm~gn~ci-lm bromide in ether (11.08 ml). After stirring for 1.5 h the mixture
was poured into a mixture of methanol (100 ml) and SM aqueous HCl (10 ml) in an
ice-bath. The mixture was then evaporated to lower volume and treated with a
20 mixture of dichloromethane, water and saturated bAne. After separation the aqueous
layer was re-extracted with dichlornmeth~n,q. The combined dichloromethane
extracts were dried (MgS04) and evaporated to leave an oil (2.26 g), which solidified
on standing; l)max (CH2C12) 16~0, 1446, 1425, 1380, 1324, 1208, and 945 cm~l;
(CDC13) 1.44 (3H, t, J7.3 Hz), 2.30 (3H, s), 2.53 (3H, s), 4.13 (2H, q, J7.3 Hz,),
25 6.51 (lH,s); (Found: nz/z 152.0949. CgH12N2O requires m/z 152.090).
(e) (3S,4R)-4-t(l-ethyl-5-me~l-yl~yr~ol-3-yl)carbonylmethyl]-3-[(R)-l-tert-
butyl~lim~thylsilyloxyethyl]azeffdin-2-one
3-Acetyl-l-ethyl-5-methylpyrazole (3.51 g) in dry tetrahydrofuran (THF) (150
ml) under an argon atmosphere was cooled in an acetone / solid carbon dioxide bath
and then treated with a lM solution of lithium bis(trimethylsilyl)amide (50 ml). The
mixture was stirred for 45 minutes and then (3R,4R)-4-acetoxy-3-[(lR)- l-tert-
butyldimethylsilyloxyethyl]azetidinone (6.6 g) was added as a solid under a blanket
- 10-
CA 02217990 1997-10-31
W 096/34868 PCTAEP96/01880
OI argon. The mixture was stirred in the cold for 3.~n. ~hlr~tecl aqueous ammonium
chloride was then added, followed by ethyl acetate, and the mixture was allowed to
warm to room temperature. A little water was added and the layers were separatedand the aqueous layer was re-extracted with ethyl acetate. The combined ethyl
5 acetate extracts were washed with ~tnr~ted brine, dried and evaporated.
Chromatography on silica gel, eluting with ethyl acetate/hexane mixtures gave the
title compound (3.65 g), ~max (CH2C12) 3411, 1761, 1678, 1376, 1151, and 838 cm~l; o~CDC13) 0.064 (6H, s), 0.86 (9H, s), 1.20 (3H,d, J 6.3 Hz), 1.44 (3H, t, J 7.3
Hz), 2.31 (3H, s), 2.89 (lH, dd, J 1.8 & 4.9 Hz), 3.15 (lH, dd, J 10.0 & 17.1 Hz),
3.50 (lH, dd, J3.5 & 17.0 Hz), 4.06 - 4.25 (4H, m), 6.11 (lH, s), 6.53 (lH, s).
(Found m/z 379.2296. ClgH33N303Si requires m/z 379.2291).
(f~ Allyl (2R and 25)--2-{(35,4R)-4-[(1-ethyl-5-metl.yll,yla~ol-3-
yl)carbonylmethyl]-3-[(R)-l-tert-butyl~im~thylsilylox,~ll.yl]-2-oxo~7eti 1inyl}-2-
hy~ll oxy ~et~te
(3S,4R)-4-[( 1-Ethyl-5-methylpyrazol-3-yl)carbonylmethyl] -3-[(R)- l-tert-
butyltiimethylsilyloxyethyl]azetidin-2-one (3.6 g) and allyl glyoxylate hydrate (1.66
g) in toluene (100 ml) were heated under reflux in a Dean and Stark apparatus under
an atmosphere of argon for 3.5h. T.l.c. of the reaction mixture showed the reaction
had almost prceeded to completion, so more allyl glyoxylate hydrate (190 mg) wasadded and the mixtur~ was heated under reflux for a further 45 min. The mixture was
cooled, the toluene was removed to give crude allyl (2R and 25)-2-{(3S,4R)-4-[(1-
ethyl-5-methylpyrazol-3-yl)carbonylmethyl]-3-[(R)- l-tert-
butyldimethylsilyloxyethyl]-2-oxoazetidinyl }-2-hydroxy~cet~t~, which was used in
the next stage; 1)max (CH2C12) 3681, 3518, 1758, 1676, 1448, 1376, 1326, 1209,
1148, 1092, 954, and 836 cm~l; ~(CDC13) inter alia 0.035 (s), 0.061 (s) (together
6H,), 0.858 (s), 0.865 (s) (together 9H), 1.21 (d, J 6.2 Hz), 1.24 (d, J 6.2 Hz),
(togethe~ 3H), 1.44 (3H, t, J7.2 Hz), 2.31 (3H, s), 2.95 - 3.00 (lH, m), 3.25 - 3.64
(2H, m), 6.53 (s), 6.56 (s) ppm.
(g) Allyl 2-{(35,4R)4-[(1-ethyl-5-methyl~yr~ol-3-yl)carbonylmethyl]-3-t(R)-l-
tert-butyldimethylsilyloxyethyl]-2-oxo~eti~linyl}-2-(tri-n-
butylphosphoranylidene)~ret~t~
Allyl (2R and 25)-2-{(35,4R)-4-[(1-ethyl-5-methylpyrazol-3-
yl)carbonylmethyl]-3-[(R)- l-tert-butyldimethylsilyloxyethyl]-2-oxoazetidinyl }-2-
- 11 -
CA 02217990 1997-10-31
W 096/34868 PCTAEP96/01880hydroxyacetate (crude from the above preparation) ~n dry THF (12~ ml) under argon
was cooled to -20~C and treated with 2,6-lutidine (l.g8 ml), followed by thionylchloAde (1.24 ml). The mixture was stirred at -20~C for 30 minl-t~s, and then
allowed to warm to room temperature and filtered, washing the residue with THF (20
ml). The filtrate was evaporated in vacuo, toluene (70ml) was added and removed in
vacuo and the residual oil was dried in vacuo. The oil was then taken up in 1,4-dioxan (40 ml) under an argon atmosphere, and treated with tri-n-butylphosphine
(3.11 ml). The mixture was stirred for 1 h. 2,6-Lutidine (1.59 ml) was then added
and the mixture was stirred for a further 30 minutes. The mixture was diluted with
ethyl acetate, washed with water, then with bAne, and dried (MgS04). After
removal of the ethyl acetate the crude product was chromatographed on silica gel,
eluting with ethyl acetate/hexane mixtures to give the phosphorane, which was used
in the next stage.
(h) Allyl 2-{(3S,4R)-4-t(l-ethyl-5-J.Ietl~yl~yl ~ol-3-yl)carbonylmethyl]-3-t(R)- 1-
I-,y~ x~ethyl]-2-n~o~ inyl~-2-(tr~-n-butylphosphoranylidene)?ret~te
The phosphorane prepared above was taken up in 1,4-dioxan (60 ml) and
treated with 5M HCl (20 ml). After 1 h the mixture was carefully treated with ca. 40
ml saturated aqueous NaHCO3, followed by solid NaHCO3 until the pH was slightly
~lk~lin.o Saturated brine was added and the mixture was extracted twice with ethyl
acetate. The combined extracts were dried (MgSO4) and evaporated. The residue
was chromatographed on silica gel, eluting with ethyl acetate/hexane mixtures to give
the hydroxy compound, (2.60 g). l)max (CH2C12) 3454, 1741, 1667, 1606, 1448,
1403, 1379, 1155, 1087, 953, and 811 cm~l.
(i) Allyl (SR,6S)-6-[(R)-l-l-ylll o~y~ ,yl]-2-(1-ethyl-5-melllyi~yl azol-3-
yl)carbapen-2-em-3-carboxylate
- 12-
CA 02217990 1997-10-31
W 096/34868 PCTnEP96/01880
Allyl 2- { (3S,4R)~[(l-ethyl-5-meth~l~yl~zol-3-yl)carbonylmethyl]-3-[(R)- 1-
hyd~ yethyl~-2-oxoazetidinyl}-2-(tri-n-butylphosphoranylidene)acetate (2.6 g), in
toluene (120 ml) co~t~ining hydroquinone (20 mg) was heated under reflux in an
argon atmosphere for 4 h, allowed to stand for 64 h, and then heated under reflux for
a further 2 h. The mixture was cooled and then loaded onto a column (4.5 x 12 cm)
of silica gel (particle si~ 0.040 -0.063 mm), eluting with ethyl acetate/hexane
mixtures; 1:1; 6:4; 7:3; 8:2; 9:1 (250 ml of each), followed by ethyl ~et~te This gave
~, the carbapenem (436 mg); ~maX(CH2C12) 3604, 2976, 1774, 1716, 1600, 1546,
1311, 1189 cm~l; ~max(EtOH)/nm 321.5 (~/dm3mol~lcm~l 14,856), ~(CDC13)
1.36 (d, J 6.3 Hz), 1.39 (t, J7.3 Hz) (together 5H), 1.80 (lH, d, J5.0 Hz), 2.28(3H,s), 3.19 (lH, dd J 2.7 & 6.7 Hz), 3.28 (lH, dd, J9.0 & 18.6 Hz), 3.60 (lH, dd, J
9.9 & 18.5 Hz), 4.08 (2H, q, J 7.3 Hz), 4.16 - 4.30 (2H, m), 4.68 - 4.90 (2H, m), 5.27
(lH, m, approx d, J ca 12 Hz), 5.46 (m, approx d, J ca 17 Hz), 5.93 - 6.08 (lH, m),
7.00 (lH, s) ppm; [Found m/z 345.1693. ClgH23N304 requires m/7 345.1689].
(j) Sodium (5R,65)-6-[(R)-1-1~yl1l oxy~ yl]-2-(1-ethyl-5-methyl-pyrazol-3-
yl)carbapen-2-em-3-carbox-ylate
Allyl (5R,6S)-6-[(R)-l-hydlo~yethyl]-2-(1-ethyl-5-methylpyrazol-3-
yl)c~l,apen-2-em-3-carboxylate (267 mg) in dichlorom~pth~np (3ml) and ethyl acetate
(3 ml) under argon was treated with sodium 2-ethylhexanoate (183 mg), followed by
triphenylphosphine (24 mg), followed by tetrakis(triphenylphosphine)p~ m(O)
(35 mg) and the mixture was stirred for 45 min. Diethyl ether (lOOml) was then
added, and after stirring for 90 minutes, the mixture was centrifuged. The residual
solid was dried under a stream of argon, and then in a desiccator. The solid was then
taken up in water cont~ining sodium chloride and chromatographed on DIAION
HP20SS resin, eluting with water, followed by water/THF mixtures; 1 %, 2%, and
3%,THF. Fractions were monitored by HPLC, and those containing the product were
combined, reduced in volume and freeze-dried to give sodium (SR,6S)-6-t(R)-l-
hy~ yethyl]-2-(1-ethyl-5-methylpyrazol-3-yl)carbapen-2-em-3-carboxylate as a
solid (168 mg); ~max(KBr) 1761, 1608, 1577, 1381, 1225 cm~l; ~max(H20)/nm 298
(~/dm3mol~lcm~l 8,531); ~(D20) 1.26 (d, Jca. 6 Hz), 1.27 (d, Jca. 7 Hz) (together
SH), 2.23 (3H, s), 3.17 (2H, approx d, J ca. 9 Hz), 3.44 (lH, dd, J 2.9 & 6.0 Hz),
- 4.04 (2H, q, J7.3 Hz)~ 4.15 - 4.25 (2H, m), 6.41 (lH, s) ppm.
- 13-