Note: Descriptions are shown in the official language in which they were submitted.
CA 02219280 1997-10-27
WO 96/33719 PCTIUS96105241
METHOD FOR REDUCING INTRAOCULAR PRESSURE
IN THE MAMMALIAN EYE BY ADMINISTRATION
OF POTASSIUM CHANNEL BLOCKERS
BACKGROUND OF INVENTION
Field of the Invention
i
The present invention is directed to pharmaceutical
1 o compositions, and primarily to topically applied ophthalinic
compositions comprising as the active ingredient one or more
compounds having the ability to block potassium channels in the ciliary
epithelium, e.g. to inhibit the transport of potassium ions and fluid
secretion in epithelia. The pharmaceutical compositions are useful for
reducing intraocular pressure in animals of the mammalian species. In
another aspect, the present invention is directed to administering such
formulations and compositions to animals of the mammalian species
(including humans) for reducing intraocular pressure in the eye.
2 0 Brief Description of the Art
Glaucoma is an optical neuropathy associated with elevated
intraocular pressures which are too high for normal function of the eye,
and results in irreversible loss of visual function. It is estimated in
2 5 medical science that glaucoma afflicts approximately 2 per cent of the
population over the age of forty years, and is therefore a serious health
problem. Ocular hypertension, i.e. the condition of elevated intraocular
pressure, which has not yet caused irreversible damage, is believed to
represent the earliest phase of glaucoma. Many therapeutic agents have
3 o been devised and discovered in the prior art for the treatment or
amelioration of glaucoma and of the condition of increased intraocular
pressure which precedes glaucoma.
Primary open angle glaucoma (POAG) is associated with a rise in
intraocular pressure (IOP). This increase in IOP is believed to contribute
3 5 to the loss of optic nerve function which ultimately leads to blindness.
CA 02219280 1997-10-27
WO 96/33719 PCT/US96/05241
Reduction of IOP is therefore a crucial component in the management
of POAG.
In principle, IOP can be reduced by inhibiting aqueous humor
inflow or conversely by stimulating aqueous outflow. Aqueous humor
inflow is mediated by ion transport across the ciliary epithelium. The
above secretion of aqueous humor produced by the ciliary epithelium is
then drained from the eye (aqueous outflow) via the trabecular
meshwork into Schlemm's canal.
Because ion transport mediates secretion of aqueous humor,
blocking or modulating the relevant ion channels or carriers will
consequently inhibit or reduce aqueous formation and thus lower IOP.
On the other hand, since the trabecular meshwork (TM) is a major
obstacle (resistance pathway) to aqueous outflow, reducing its resistance
to the passage of fluid should enhance outflow and lower IOP. Thus, by
reducing the volume or size of TM cells it should be possible to enhance
outflow by lowering the resistance to the passage of ocular fluid. Cell
volume/size is determined by a balance between ion uptake and efflux
mechanisms. Therefore, it follows that reducing TM cell volume can be
accomplished by either stimulating the ion efflux or inhibiting the ion
2 o uptake mechanisms in this cell type.
The drugs currently utilized in the treatment of glaucoma include
miotics (e.g., pilocarpine, carbachol, and acetylcholinesterase inhibitors),
sympathomimetrics (e.g., epinephrine and dipivalylepinephrine), beta-
blockers (e.g., betaxolol, levobunolol and timolol), alpha-2 agorusts (e.g.,
para-amino clonidine) and carbonic anhydrase inhibitors (e.g.,
acetazolamide, methazolamide and ethoxzolamide). Miotics and
sympathomimetics are believed to lower intraocular pressure by
increasing the outflow of aqueous humor, while beta-blockers, alpha-2
agonists and carbonic anhydrase inhibitors are believed to lower
3 0 intraocular pressure by decreasing the formation of aqueous humor. All
five types of drugs have potential side effects. Miofics, such as
pilocarpine, can cause blurring of vision and other visual side effects
which may either decrease patient compliance or require termination of
miotic drug therapy. Carbonic anhydrase inhibitors can also cause
3 5 serious side effects which affect patient compliance and/or necessitate
2
CA 02219280 2006-08-09
WO 96133719 PCTlUS96/05241
withdrawal of the drug therapy. At least one beta-blocker, timolol, has
increasingly become associated with serious pulmonary side effects
attributable to its effect on beta-2 receptors in pulmonary tissue.
As a result additional antiglaucoma drugs are being developed,
e.g., prostaglandin derivatives, muscarinic antagonists, etc.
In light of the foregoing circumstances, it is clear that a need exists
for new, more potent antiglaucoma compositions which avoid or reduce
the above-cited side effects and enhance patient compliance, since the
foregoing and other anti-glaucoma and ocular hypotensive compounds
and agents of the prior art do not provide a treatment or cure for
glaucoma and ocular hypertension which is satisfactory in all respects.
Therefore, the pharmacological and related arts and sciences continue
searching for additional and better anti-glaucoma and ocular
hypotensive agents.
Chloride channel blockers such as 5-vitro-2-(3-
phenylpropylamino)-benzoate (NPPB) have been shown to inhibit Cl-
transport and fluid secretion/absorption in rat intestine. (See for
example, Acta Physiol Scand: No. 149,1993: pp. 365-376, Fryklund et al.,
"The effects of potassium transport inhibitors on intestinal fluid and ion
2 0 transport in vivo and in vitro".)
The use of chloride-channel blockers for reducing the intraocular
pressure in the eye of a mammal is disclosed and claimed in U.S. Patent
No. 5,559,151 issued on September 24, 1996.
In addition, PCT Patent WO 89/10757 discloses the use of
potassium channel openers for treating glaucoma.
SUNLMARY OF THE INVENTION
3 0 Surprisingly it has been discovered in accordance with the present
invention that potassium channel blockers are effective as anti-
glaucoma agents and as agents for reducing intraocular pressure, when
such agents are applied to the mammalian eye in a pharmaceutical
composition, preferably in a topical ophthalmic composition.
3 5 Accordingly, the present invention relates to a method of treating
3
CA 02219280 1997-10-27
w0 96!33719 PCT/US96/05241
glaucoma, or ocular hypertension by topically administering to the
mammalian eye an ophthalmic composition which contains an
effective amount of a potassium channel blocker. In particular, to
inhibit aqueous humor production (inflow inhibition), the potassium
channel that resides at the basolateral membrane of the nonpigmented .
ciliary epithelial cell (NPE) may be blocked. It is believed that blocking
the potassium channel of the NPE cell will inhibit net solute and H20 ,
efflux and therefore aqueous secretion that will in turn will lower IOP.
Some preferred examples of potassium channel blockers are quinine,
1o tremogenic indole alkaloids such as Perutrem A and paspalicine, and
insect toxins such as charybdotoxin and iberiotoxin. In particular
tremogeruc indole alkaloids should be especially potent in blocking the
potassium channels of NPE cells since these compounds are highly
specific in blocking Ca2+ -gated Maxi potassium channels: the potassium
channel of the NPE cell appears to be a Ca2+ -gated Maxi potassium
channel. Thus, aqueous secretion is inhibited and hence intraocular
pressure (IOP) is lowered by blocking potassium channels in the NPE
cells.
The ophthalmic compositions of the invention contain the active
2 0 ingredient in a concentration range of approximately 0.0001 to 0.1 per
cent weight by volume. The composition itself includes, in addition to
the active ingredient, such excipients which are per se well known in
the art for preparing ophthalmic compositions, particularly ophthalmic
solutions. In accordance with the method of the invention the
ophthalmic compositions, preferably ophthalmic solutions are applied
topically to the mammalian eye approximately 1 or 2 times daily.
BRIEF DESCRIPTION OF THE DRAWINGS
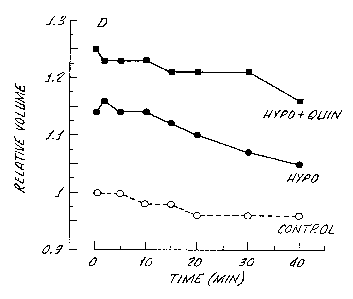
Figure 1 is a graph showing the effect of the presence of the drug
3 0 quinine on the regulatory volume decrease (RVD; a readout for net ion
and H20 efflux), on bovine nonpigmented ciliary epithelial (NPE) cells.
Inhibition of RVD by quinine is consistent with the notion that Ca2+ _
gated K channels are important in solute and water movement
(secretion) in ciliary epithelium.
4
CA 02219280 1997-10-27
WO 96/33719 PCT/US96/05241
Figure 2 is a graph showing the effect of intracameral
administration of the drug quinine on the intraocular pressure (IOP) in
the rabbit eye.
DETAILED DESCRIPTION OF THE INVENTION
The compounds which are utilized in accordance with the
method of the present invention, and in the pharmaceutical
compositions of the present invention, are potassium channel blockers.
In this regard the term potassium channel blocker is defined as those
1o compounds or agents which inhibit net potassium flux (current)
through a potassium specific pathway (channel, integral membrane
protein) within biological membranes. Specific and preferred examples
of potassium channel blockers which are utilized in accordance with the
present invention are provided.
Pharmaceutically acceptable salts of the potassium channel
blockers can also be used in accordance with the present invention. A
pharmaceutically acceptable salts may be any salt which retains the
activity of the parent compound and does not impart any deleterious or
untoward effect on the subject to which it is administered and in the
2 0 context in which it is administered.
Such a salt may be derived from any organic or inorganic acid or
base. The salt may be a mono or polyvalent ion. Of particular interest
where the acid function is concerned are the inorganic ions, such as
alkali ions, e.g. sodium, potassium, etc. Organic amine salts may be
2 5 made with amines, particularly ammonium salts such as mono-, di- and
trialkyl amines, e.g. alkyl amines wherein each alkyl group may
comprise up to six carbon atoms, or ethanol amines. Salts may also be
formed with caffeine, tromethamine and similar molecules. It is only
important that the cation of any salt of a potassium channel blocker
3 o utilized in the compositions or methods of this invention be able to
block potassium channels in the ciliary epithelium.
For reducing intraocular pressure in a mammalian eye, and
particularly for treatment of glaucoma in humans suffering from that
condition, the active compounds (or mixtures or salts thereof) are
3 5 administered in accordance with the present invention to the eye
5
CA 02219280 1997-10-27
WO 96/33719 PCT/US96/OS241
admixed with an ophthalmically acceptable carrier. Any suitable, e.g.,
conventional, ophthalmically acceptable carrier may be employed. A
carrier is ophthalinically acceptable if it has substantially no long term or
permanent detrimental effect on the eye to which it is administered.
Examples of ophthalmically acceptable carriers include water (distilled or
deionized water), saline and other aqueous media. In accordance with
the invention, the active compounds are preferably soluble in the carrier
which is employed for their administration, so that the active
compounds are administered to the eye in the form of a solution.
Alternatively, a suspension of the active compound or compounds (or
salts thereof) in a suitable carrier may also be employed.
In accordance with the invention the active compounds (or
mixtures or salts thereof) are administered in an ophthalmically
acceptable carrier in sufficient concentration so as to deliver an effective
amount of the active compound or compounds to the eye. Preferably,
the ophthalmic, therapeutic solutions contain one or more of the active
compounds in a concentration range of approximately 0.0001% to
approximately 1% (weight by volume) and more preferably
approximately 0.0005% to approximately 0.1% (weight by volume).
2 0 Any method of administering drugs directly to a mammalian eye
may be employed to administer, in accordance with the present
invention, the active compound or compounds to the eye to be treated.
By the term "administering directly" is meant to exclude those general
systemic drug administration modes, e.g., injection directly into the
patient's blood vessels, oral administration and the like, which result in
the compound or compounds being systemically available. The primary
effect on the mammal resulting from the direct administering of the
active compound or compounds to the mammal's eye is preferably a
reduction in intraocular pressure. More preferably, the active useful
3 o compound or compounds are applied topically to the eye or are injected
directly into the eye. Particularly useful results are obtained when the
compound or compounds are applied topically to the eye in an
ophthalmic solution, i.e. as ocular drops.
Topical ophthalmic preparations, for example ocular drops, gels
3 5 or creams, are preferred because of ease of application, ease of dose
6
CA 02219280 1997-10-27
WO 96/33719 PCT/L1S96/05241
delivery and fewer systemic side effects, such as cardiovascular
hypotention. An exemplary topical ophthalmic formulation is shown
below in Table I. The abbreviation q.s. means a quantity sufficient to
effect the result or to make volume.
TABLE I
Ingredient Amount(% W /V~
Active Compound in accordance about 0.0001 to
1 o with the invention, about 1
Preservative 0-0.10
Vehicle 0-40
Tonicity Adjustor 1-10
Buffer 0.01-10
pH Adjustor q.s. pH 4.5-7.5
antioxidant as needed
Purified Water as needed to
make 100%
V arious preservatives may be used in the ophthalmic preparation
described in Table I above. Preferred preservatives include, but are not
limited to, benzalkonium potassium, chlorobutanol, thimerosal,
phenylmercuric acetate, and phenylmercuric nitrate. Likewise, various
preferred vehicles may be used in such ophthalmic preparation. These
vehicles include, but are not limited to, polyvinyl alcohol, povidone,
hydroxypropyl methyl cellulose, poloxamers, carboxymethyl cellulose
and hydroxyethyl cellulose.
Tonicity adjustors may be added as needed or convenient. They
3 0 include, but are not limited to, salts, particularly sodium potassium,
potassium potassium etc., mannitol and glycerin, or any other suitable
ophthalmically acceptable torucity adjustor.
Various buffers and means for adjusting pH may be used so long
as the resulting preparation is ophthalmically acceptable. Accordingly,
3 5 buffers include but are not limited to, acetate buffers, citrate buffers,
7
CA 02219280 1997-10-27
WO 9E/33719 PCTILTS96/05241
phosphate buffers, and borate buffers. Acids or bases may be used to
adjust the pH of these formulations as needed.
In a similar vein, ophthalmically acceptable antioxidants include,
but are not limited to, sodium metabisulfite, sodium thiosulfate,
acetylcysteine, butylated hydroxyanisole, and butylated hydroxytoluene.
The ophthalmic solution (ocular drops) may be administered to
the mammalian eye as often as necessary to maintain an acceptable level .
of intraocular pressure in the eye. In other words, the ophthalmic
solution (or other formulation) which contains the potassium channel
blocker as the active ingredient, is administered to the mammalian eye
as often as necessary to maintain the beneficial hypotensive effect of the
active ingredient in the eye. Those skilled in the art will recognize that
the frequency of administration depends on the precise nature of the
active ingredient and its concentration in the ophthalmic formulation.
Within these guidelines it is contemplated that the ophthalmic
formulation of the present invention will be administered to the
mammalian eye approximately once or twice daily.
Specific examples of potassium channel blockers which are used
as the active effective ingredients in the ophthalmic compositions of the
2 o present invention are described and cited above.
A potassium channel blocker, in accordance with the present
invention, may be identified by the method disclosed in Single-Channel
Recording, Sakmann et al, published by Plenum Press. (See Chapter 21,
by Camardo et al entitled Single-Channel Analysis in Aplysia Neurons
A Specific K+ Channel Is Modulated by Serotonin and Cyclic AMP.)
Potassium Channel blockers may also be identified in accordance
with the method disclosed below in the Example.
EXAMPLES
The present invention is demonstrated by in vitro and in vivo
3 0 data. In Figure 1, 100. M of quinine were found to depress the
regulatory volume decrease (RVD) that occurs following hyposmotic
swelling of bovine non-pigmented ciliary epithelial (NPE) cells. In this
example, NPE cells were suspended in an isosmotic (295 mOsm)
solution containing 100~.M quinine for 30 minutes prior to suspension
3 5 in a hyposmotoic (198 mOsm) solution. Control cells were subjected to
8
CA 02219280 2006-08-09
Wfl 96133719 PC'T/US96/OSZ41
the same hyposmotic solution but without quinine in the medium.
Changes in cell volume were measured using a Coulter Counter
interfaced to a Coulter Channelyzer. It is noted that, following osmotic
swelling, control cells regulate towards their original isosmotic volume
while quinine-treated cells remain swollen. The above findings indicate
that quinine, via blocking of the potassium channel, inhibits solute and
osmotically obliged H20 efflux. Because the potassium-dependent ion
flux pathways, activated following osmotic cell swelling of NPE cells, are
involved in aqueous secretion, quinine will inhibit aqueous humor
1 o formation and, thus, lowex IOP.
In the in vivo studies normotensive rabbits were injected
intracamerally with 1 m M quinine. Figure 2 shows that lm M quinine
lowered IOP by 7 mm of Hg and IOP remained depressed for 24 houzs.
Taken together, the above zn vitro and in vivo experiments
demonstrate that blocking the potassium channel in the ciliary
epithelium will reduce IOP.
One advantage potassium channel inhibition has over other IOP
lowering therapies is that the effector, i.e. the ion channel or carrier, is
targeted rather than the receptor. Since effector blockage is direct, it
2 0 should be the most potent and effective way of inhibiting aqueous
secretion and hence lowering IOP. On the other hand, targeting a
receptor to block an effector is indirect and relies on modulation of a
series of cellular events (intracellular messengers/signals) prior to
effector inhibition.
2 5 In view of the above, it is clear that the scope of the present
invention should be interpreted solely on the basis of the following
claims, as such claims are read in light of the disclosure.
* Trade-mark