Note: Descriptions are shown in the official language in which they were submitted.
CA 02219299 1997-10-24
TITLE OF THE INVENTION
P-40, A NEW MEMBER OF THE MULTIDRUG
RESISTANCE GENE FAMILY
FIELD OF THE INVENTION
The present invention relates in general to multidrug
resistance (MDR) in cells. In particular, the present invention relates to
the identification of a new member of the MDR gene family, P40, as well
as to the idenliricalion of P40 related genes (homologs) as being further
",en,be,~ of the MDR gene family. The present invention therefore relates
to nucleic acid molecules encoding P40 protein and P40 proteins
homologs, to multidrug resistant cells containing these nucleic acid
molecules; to hybridomas containing antibodies to P40 and P40
homologs; to nucleic acid probes for the detection of these nucleic acid
",olec~'Q~; to a method of detection of such nucleic acid molecules or of
the P40 protein or P40 homologs; to bioassays comprising the nucleic
acid molecules encoding P40 or P40 homologs, P40 protein or P40
protein homologs, or antibodies of the present invention to diagnose,
assess or prognose MDR in an animal; to therapeutic uses of the nucleic
acid molecules of the present invention (i.e. antisense), protein or
antibodies of the present invention; and to methods of preventing MDR
in an animal.
BACKGROUND OF THE INVENTION
The ability of malignant cells to develop resistance to
multiple anticancer drugs is a major obstacle in the treatment of cancers
[Ferguson et al., 1989, Cancer Bulletin 41(1): 7-13]. Studies using in vitro
CA 02219299 1997-10-24
model systems have led to the identification of several proteins which
confer resistance to dirrere, It cl~sses of anticancer drugs [Pastan et al.,
1987, New England Joumal of Medicine 316(22): 1388-1393;Bradley et al.,
1988, Biochimica et Biophysica Acta 948: 87-128]. The overexpression of
5 P-glycoprotein (P-gp) and the multidrug resistance associated protein
(MRP) in cells selected with hydrophobic cytotoxic drugs ( Vinca alkaloids,
anthracyclinses and epipodophyllotoxins) have been shown to confer a
multidrug resistance phenotype [Gottesman et al.,1993, Annual Review of
B[~cl1eh,;~tly 62: 385-427; Endicott et al.,1989, Annual Review of Biochemistry
58: 137-171; Cole et al., 1996, Cancer Treatment & Research. 87: 39-62].
Both P-gp and MRP belong to a large family of ATP trafficking proteins
that are evolutionary conserved and mediate the transport of numerous
ligand ranging from ions to large polypeptides [Higgins et al.,1992, Annual
Review of Cell Biology 8: 67-113]. In tumor cell lines, P-gp and MRP reduce
15 the accumulation of drugs via an energy dependent drug efflux
mechanism [Shapiro et al., 1994, Joumal of Biological Chemistry 269(5):
3745-3754; Doige et al.,1992, Biochimica et Biophysica Acta 1109: 161 -171].
P-gp and MRP ex~),ession has been detected in normal
tissues and is thought to mediate the transport of normal cell metabolites
20 and xenobiotics [Cordon-Cardo et al., 1990, Joumal of Histochemistry and
Cytochemistry 38(9): 1277-1287; Bradley et al., 1990, Journal of Cellular
Physiology 145: 398 408; Thorgeirsson et al.,1987, Science 236: 1120-1122;
Thiebaut et al.,1987, Proceedings of the National Academy of Sciences USA
84: 7735-7738]. Consistent with these speculations, inactivation of both
25 alleles of P-gp from the mouse genome has led to the accumulation of
drugs and natural products in many organs where P-gp is highly
expressed [Schinkel et al., 1997, Proceedings of the National Academy of
Sciences of the United States of America. 94(8): 4028-4033; Schinkel et al.,
CA 02219299 1997-10-24
1994, Cell 77: 491-502]. High levels of P-gp has been shown in 20 - 70%
of tumors from different cancers [Tishler et al., 1992, Joumal of
Neurosurgery 76: 507-512; Abe et al., 1994, Japanese Joumal of Cancer
Research 85(5): 536-541; Baker et al., 1989, ?: 87-97; Belloni et al., 1989,
Cancer and A~ st~-sis Review 8: 353-389; Charpin et al., 1994, Joumal of the
National Cancer Institute 86(20): 1539-1545; Fojo et al., 1987, Proceedings of
the National Academy of Science USA 84: 265-269; Henson et al., 1992,
Joumal of Neur~Oncology 14: 37-43; Hijazi et al., 1994, American Joumal of
Clinical Pathology 102(1): 61-67; Mattem et al., 1994, Anticancer Research
14(2A): 417-419], and in some tumors (e.g. haemepoitic tumors and
childhood malignancies) P-gp expression has been shown to predict
clinical drug resistance and long term survival [Nooter et al., 1994,
Leukemia Research 18(4): 233-243; Chan et al.,1995, Hematology - Oncology
Clinics of North America 9(2): 275-318; Chan et al., 1991, New England
Joumal of Medicine, 325:160~1614]. However, the lack of P-gp expression
in other multidrug resistant tumors indicates other cellular changes that
confer resistance to anticancer drugs [Lee et al., 1997, Joumal of Cellular
Biochemistry. 65(4): 513-526; Baggetto, Bulletin du Cancer. 84(4): 385-390;
Linn et al., 1994, Journal of Clinical Oncology 12(4): 812-819; Lonn et al.,
1994, Intemational Journal of Cancer 58(1): 40-45; Sognier et al., 1994,
Biochemical Pharmacology 48( 2): 391-401]. Some of the cellular changes
identified in drug resistant cells include the overexpression of MRP
[Zaman et al., 1994, Proceedings of the National Academy of Sciences USA
91(19): 8822-8826], alterations in glutathione-S-transferase activity or
GSH levels [Tew, 1994, Cancer Research 54(15): 4313-4320], reduction in
topoisomerase ll levels or activity [Frelich et al., 1995, J. Biological
Chemistry 270(15): 21429-21432], overexpression of LRP (the Lung
Resista, lce Protein, a the compo, lent of human vaults) [36] and alteration
CA 02219299 1997-10-24
in fun~tions or levels of proteins mediating apoptosis or proy,~n,med cell
death [37, 38].
There thus remains a need to identify other cellular
changes that confer drug resistance.
The overex~ression of a 40 kDa protein (P40) alone or
together with P-gp or MRP in MDR cell lines [39] has been previously
reported. However, further studies were required to demonstrate a direct
role for P40, if any, in drug metabolism and multidrug resistance (MDR).
Indeed, it was disclosed that it was unknown and unclear whether P40
could modulate a MDR phenotype directly or indirectly [39].
There thus remains a need to determine whether P40
is indeed directly implicated in MDR. In the arri",1ali~e, there also remains
a need to identify the molecular determinant of this MDR, in the form of
a nucleic acid and for protein in order to open the way for the obtention
of diagnostic, therapeutic and research tools in the field of multidrug
resistance.
The present invention seeks to meet these and other
needs.
The present description refers to a number of
documents, the content of which is herein incorporated by reference.
SUMMARY OF THE INVENTION
The invention conce" ,s the demGnsl, dlion that P40 has
a direct role in multidrug resistance.
Further, the invention relates to the identification of P40
as a member of the MDR gene family and to the identification of
CA 02219299 1997-10-24
P40/Annexin I related genes as members of this broadened MDR gene
family.
In addition, the invention relates to the identification of
Annexin ll and IV as potential MDR determinants. Thus, the invention
relates to the identifications of Annexins (I to Xl, also refe"ed thereto
herein P40 and P40 homolgs) as potential members of the MDR gene
family. Broadly therefore, the present invention also relates to the
identification of Annexin-based MDR in cells.
The present invention further relates to the isolated
nucleic acid molecules encoding P40 or fragment thereof and to the
identification of P40 as Annexin 1.
The invention in addition relates to purified P~0
polypeplides, homologs thereof, or epitope binding portions thereof and
the use thereof in multidrug resistance. The invention also provides a
specific dete~lion method for P~0 nucleic acids encoding P~0 proteins
or homologs thereof, polypeptides or fragments thereof in a sample.
In addition, the invention provides a recombinant nucleic
acid molecule comprisi,1g P40 (or homologs thereof) operationally linked
to a promoter, efficient in initiating transcription thereof in a host cell as
well as to such a host cell.
As well, the invention provides a non-human organism
containing the nucleic acid molecule mentioned above. Further, the
invention provides an antisense P40 or P40-related (Annexin l-related)
nucleic acid molecule.
The invention further provides an antibody having
specific binding affinity towards P~0, P40 homologs or an
e,c ilope-containing-region thereof. In one embodiment, the antibody is a
CA 02219299 1997-10-24
monoclonal. The invention also provides the hybridoma producing the
monoclonal antibody.
The invention also seeks to provide a method for the
detection of P-40 or P-40 homologs protein or portion thereof in a
5 sample. In one embodiment, such a method is quanlilali~/e.
Furthermore, the invention seeks to provide a diagnostic
kit comprising a first contained means containing the above-mentioned
antibody, and second container means co"lai"iilg a conjugate comprising
a binding partner of the monoclonal antibody and a label.
The invention seeks to provide diagnostic methods for
human ~~ise~se and particularly for cancer and the multidrug resistant of
cancer cells. Preferably, a method for evaluating the predisposition of a
cancer tumor to be and/or become multidrug resistance is provided
herein.
The invention further seeks to provide therapeutic
methods involving the P-40 nucleic acid homologs, variants or parts
thereof, antisense thereof, P40 protein, P-40 protein homologs or P-40
antibodies.
It shall be understood that in certain situations it might
20 be beneficial to render a cell MDR by providing thereinto at least one
nucleic acid encoding an Annexin (or at least one Annexin protein) so as
to give this cell a growing advantage with respect to wild-type cells in
certain growth conditions (i.e. presence of drug).
It is shown herein that P-40 is Annexin 1. Annexin I is a
25 member of a large family of Ca2+ phospholipid binding proteins [for
review see [40]], implicated in several cellular mechanisms including
intracellular membrane vascular trafficking and exocytosis process
CA 02219299 1997-10-24
[4143]. However, Annexin I has not been previously implicated in drug
resi~la"ce or suggested to be implicated l1era..)to. The inventors are then
the first to show the role of Annexin I in expression of drug resistance to
anticancer drugs. Thus, the present invention is the first to show an
5 Annexin-based mutlidrug resistance pathway in cells.
Nucleotide sequences are presented herein by single
strand, in the 5' to 3' direction, from left to right, using the one letter
nucleotide symbols as commonly used in the art and in accordal1ce with
the recommendations of the IUPAC-IUB Biochemical Nomenclature
10 Commission.
The present description refers to a number of routinely
used recombinant DNA (rDNA) technology terms. Nevertheless,
definitions of selected examples of such rDNA terms are provided for
clarity and consistency.
As used herein, "isol-~ed nucleic acid molecule", refers
to a polymer of nucleotides. Non-limiting examples thereof include DNA
and RNA molecules purified from their natural environment.
The term "recombinant DNA" as known in the art refers
to a DNA molecule resulting from the joining of DNA segments. This is
20 often referred to as genetic engineering.
The term "DNA segment", is used herein, to refer to a
DNA molecule comprising a linear stretch or sequence of nucleotides.
This sequence when read in accorclance with the genetic code, can
encode a linear stretch or sequence of amino acids which can be referred
25 to as a polypeptide, protein, protein fragment and the like.
The terminology "amplification pair" refers herein to a
pair of oligonucleotides (oligos) of the present invention, which are
CA 02219299 1997-10-24
selected to be used together in amplifying a selected nucleic acid
sequence by one of a number of types of amplification processes,
preferably a polymerase chain reaction. Other types of amplification
processes include ligase chain reaction, strand displacen,ent
5 amplification, or nucleic acid sequence-based amplification, as explained
in greater detail below. As commonly known in the art, the oligos are
designed to bind to a complementary sequence under selected
conditions.
The nucleic acid (i.e. DNA or RNA) for practising the
10 present invention may be obtained according to well known methods.
Oligonucleotide probes or primers of the present
invention may be of any suitable length, depending on the particular
assay format and the particular needs and targeted genomes employed.
In general, the oligonucleotide probes or primers are at least 12
15 nucleotides in length, prererably between 15 and 24 molecules, and they
may be adapted to be especially suited to a chosen nucleic acid
amplification system. As commonly known in the art, the oligonucleotide
probes and primers can be designed by taking into consideration the
melting point of hydrizidation thereof with its targeted sequence (see
20 below and in Sambrook et al., 1989, Molecular Cloning - A Laboratory
Manual, 2nd Edition, CSH Laboratories; Ausubel et al., 1989, in Current
Protocols in Molecular Biology, John Wiley & Sons Inc., N.Y.).
"Nucleic acid hybridization" refers generally to the
hybridization of two single-stranded nucleic acid molecules having
25 complementary base sequences, which under appropriate conditions will
form a thermodynamically favored double-stranded structure. Examples
of hybridization conditions can be found in the two laboratory manuals
CA 02219299 1997-10-24
, efe" ed above (Sarnbrook et al., 1989, and Ausubel et al., 1989) and are
commonly known in the art. In the case of a hybridization to a
nitrocellulose filter, as for example in the well known Southern blotting
pr~cedure, a nitrocellulose filter can be inc~ ~hAted overnight at 65~C with
a labeled probe in a solution containing 50% for",amide, high salt ( 5 x
SSC or 5 x SSPE), 5 x Denhardt's solution, 1% SDS, and 100 ,ug/ml
denatured carried DNA ( i.e. salmon sperm DNA). The non-specifically
binding probe can then be washed off the filter by several washes in 0.2
x SSC/0.1% SDS at a temperature which is selected in view of the
desired stringency: room temperature (low stringency), 42~C (moderate
sll ingei1cy) or 65~C (high stringency). The selected temperature is based
on the rnelling temperature (Tm) of the DNA hybrid. Of course, RNA-DNA
hybrids can also be formed and detected. In such cases, the conditions
of hybridization and washing can be adapted according to well known
methods by the person of ordinary skill. Stringent conditions will be
preferably used (Sambrook et al., 1989, supra).
Probes of the invention can be utilized with naturally
occurring sugar-phosphate backbones as well as modified backbones
including phosphorothioates, dithionates, alkyl phosphonates and
~-nucleotides and the like. Modified sugar-phosphate backbones are
generally taught by Miller, 1988, Ann. Reports Med. Chem. 23:295 and
Moran et al., 1987, Nucleic acid molecule. Acids Res., 14:5019. Probes
of the invention can be constructed of either ribonucleic acid (RNA) or
deoxyribonucleic acid (DNA), and preferably of DNA.
The types of detection methods in which probes can be
used include Southern blots (DNA detection), dot or slot blots (DNA,
RNA), and Northern blots (RNA detection). Although less prepared,
CA 022l9299 l997-l0-24
labeled proteins could also be used to detect a particular nucleic acid
sequence to which it binds. Other detection methods include kits
containing probes on a dipstick setup and the like.
Although the present invention is not specifically
dependent on the use of a label for the detection of a particular nucleic
acid sequence, such a label might be beneficial, by increasing the
sensitivity of the detection. Furthermore, it enables automation. Probes
can be labeled according to numerous well known methods (Sambrook
et al., 1989, supra). Non-limiting examples of labels include 3H, 14C, 32p,
and 35S. Non-limiting examples of detectable markers include ligands,
fluoruphores, chemiluminescent agents, enzymes, and antibodies. Other
detect~hle markers for use with probes, which can enable an increase in
sensitivity of the method of the invention, include biotin and
radionucleotides. It will become evident to the person of ordinary skill that
the choice of a particular label dictates the manner in which it is bound
to the probe.
As commonly known, radioactive nucleotides can be
incorporated into probes of the invention by several methods.
Non-limiting examples thereof include kinasing the 5' ends of the probes
using gamma 32p ATP and polynucleotide kinase, using the Klenow
fragment of Pol I of E coli in the presence of radioactive dNTP (i.e.
uniformly labeled DNA probe using random oligonucleotide primers in
low-melt gels), using the SP6/T7 system to transcribe a DNA segment in
the presence of one or more radioactive NTP, and the like.
As used herein, "oligonucleotides" or"oligos" define a
molecule having two or more nucleotides (ribo or deoxyribonucleotides).
The size of the oligo will be dictated by the particular situation and
CA 02219299 1997-10-24
ultimately on the particular use thereof and adapted accordingly by the
person of ordinary skill. An oligonucle~tide can be synthetised chemically
or derived by cloning according to well known methods.
As used herein, a Uprimer~ defines an oligonucleotide
5 which is c~p~ of annealing to a target sequence, thereby creating a
double stranded region which can serve as an initiation point for DNA
synthesis under suitable conditions.
Amplification of a selected, or target, nucleic acid
sequence may be carried out by a number of suitable methods. See
generally Kwoh et al., 1990, Am. Biotechnol. Lab. 8:14-25. Numerous
amplification techniques have been described and can be readily
adapted to suit particular needs of a person of ordinary skill. Non-limiting
examples of amplification techniques include polymerase chain reaction
(PCR), ligase chain reaction (LCR), strand displacement amplification
(SDA), transcription-based amplification, the Q~ replicase system and
NASBA (Kwoh et al., 1989, Proc. Natl. Acad. Sci. USA 86, 1173-1177;
Lizardi et al., 1988, Biotechnology 6: 1197-1202; Malek et al., 1994,
Methods Mol. Biol., 28:253-260; and Sanlbrook et al., 1989, supra).
Prererably, amplification will be carried out using PCR.
Polymerase chain reaction (PCR) is carried out in
accordancewithknowntechniques. See, e.g., U.S. Pat. Nos. 4,683,195;
4,683,202; 4,800,159; and 4,965,188 (the disclosures of all three U.S.
Patent are incorporated herein by reference). In general, PCR involves,
a treatment of a nucleic acid sample (e.g., in the presence of a heat
stable DNA polymerase) under hybridizing conditions, with one
oligonucleotide primer for each strand of the specific sequence to be
detected. An extension product of each primer which is synthesized is
CA 02219299 1997-10-24
12
co",ple."enla,y to each of the two nucleic acid sl,ands, with the primers
sufficiently complementary to each strand of the specific sequence to
hybridize therewith. The extension product synthesi7ed from each primer
can also serve as a template for further synthesis of extension products
using the same primers. Following a sufficient number of rounds of
synthesis of extension products, the sample is analysed to ~ssess
whether the sequence or sequences to be detected are present.
Detection of the amplified sequence may be carried out by visualization
following EtBr staining of the DNA following gel electrophores, or using
a detect~ label in accordance with known techniques, and the like. For
a review on PCR techniques (see PCR Protocols, A Guide to Methods
and Amplifications, Michael et al. Eds, Acad. Press, 1990).
Ligase chain reaction (LCR) is carried out in accordance
with known techniques (Weiss, 1991, Science 254:1292). Adaptation of
the protocol to meet the desired needs can be carried out by a person of
ordinary skill. Strand displacement amplification (SDA) is also carried out
in accorda"ce with known techniques or adaptalions thereof to meet the
particular needs (Walker et al., 1992, Proc. Natl. Acad. Sci. USA
89:392-396; and ibid., 1992, NucleicAcids Res. 20:1691-1696.
As used herein, the term "gene" is well known in the art
and relates to a nucleic acid sequence defining a single protein or
polypeptide. A "structural gene" defines a DNA sequence which is
transcribed into RNA and translated into a protein having a specific
amino acid sequence thereby giving rise the a specific polypeptide or
protein. It will readily recognized by the person of ordinary skill, that the
nucleic acid sequence of the present invention can be incorporated into
CA 02219299 1997-10-24
13
anyone of numerous established kit formats which are well known in the
art.
The term "vector" is co",r"only known in the art and
defines a plasmid DNA, phage DNA, viral DNA and the like, which can
5 serve as a DNA vehicle into which DNA of the present invention can be
cloned. Numerous types of vectors exist and are well known in the art.
The term "expression" defines the process by which a
structural gene is transcribed into mRNA (transcription), the mRNA is
then being translated (translation) into one polypeptide (or protein) or
1 0 more.
The terminology "expression vector" defines a vector or
vehicle as described above but designed to enable the expression of an
inserted sequence following transformation into a host. The cloned gene
(inserted sequence) is usually placed under the control of control element
15 sequences such as promoter sequences. The placing of a cloned gene
under such control sequences is often referred to as being operably
linked to control elements or sequences.
Expression control sequences will vary depending on
whether the vector is designed to express the operably linked gene in a
20 prokaryotic or eukaryotic host or both (shuttle vectors) and can
additionally contain trans~ lional elements such as enhancer elements,
termination sequences, tissue-specificity elements, and/or translational
initiation and termination sites.
As used herein, the designation "functional derivative"
25 denotes, in the context of a functional derivative of a sequence whether
an nucleic acid or amino acid sequence, a molecule that retains a
biological activity (either function or structural) that is substantially
CA 02219299 1997-10-24
14
similar to that of the original sequence. This functional derivative or
equivalent may be a natural derivatives or may be prepared synthetically.
Such derivatives include amino acid sequences having substitutions,
deletions, or additions of one or more amino acids, provided that the
5 biolcgicAI activity of the protein is conserved. The same applies to
derivatives of nucleic acid sequences which can have substitutions,
deletions, or additions of one or more nucleotides, provided that the
biological activity of the sequence is generally maintained. When relating
to a protein sequence, the substituting amino acid as chemico-physical
10 properties which are similar to that of the substituted amino acid. The
similar chemico-physical properties include, similarities in charge,
bulkiness, hydrophobicity, hydrophylicity and the like. The term
"functional derivatives" is intended to include "fragments", "segmentsn,
"variants", "analogs" or"chemical derivatives" of the subject matter of the
15 present invention.
Thus, the term "variant" refers herein to a protein or
nucleic acid molecule which is substantially similar in structure and
biological activity to the protein or nucleic acid of the present invention.
The functional derivatives of the present invention can
20 be synthesized chemically or produced through recombinant DNA
technology. all these methods are well known in the art.
As used herein, "chemical derivatives" is meant to cover
addilional chemical moieties not normally part of the subject matter of the
invention. Such moieties could affect the physico-chemical characteristic
25 of the derivative (i.e. solubility, absor~lion, half life and the like, decrease
of toxicity). Such moieties are exel"pliried in Remington's Pharmaceutical
CA 02219299 1997-10-24
Sciences (1980). Methods of coupling these chemical-physical moieties
to a polypeptide are well known in the art.
The term "allele" defines an alternative form of a gene
which occupies a given locus on a chromosome.
As commonly known, a "mutation" is a detect~l~le
change in the genetic material which can be transmitted to a daughter
cell. As well known, a mutation can be, for example, a detectable change
in one or more deoxyribonucleotide. For example, nucleotides can be
added, delete;l, substituted for, inverted, or transposed to a new position.
Spontaneous mutations and ex,ueri,,,en(ally induced mutations exist. The
result of a mutations of nucleic acid molecule is a mutant nucleic acid
",ol cl~'e. A mutant polypeptide can be encoded from this mutant nucleic
acid molecule.
As used herein, the term "purified" refers to a molecule
having been separated from a cellular component. Thus, for example, a
"purified protein" has been purified to a level not found in nature. A
Usubstantially pure" molecule is a molecule that is lacking in all other
cellular components.
While the property of a host cell to become MDR is
demonstrated with human P-40/Annexin I other Annexins (Il-XI), non-
human Annexins, other biologically functional genes/cDNA-related to
Annexins can also be used in the context of the present invention. For
example, Mouse Annexin I could be used in some embodiments of the
present invention as will be recognized by a person of ordinary skill.
Annexins are part of a gene family of multifunctional
calcium - and phospholipid - binding protein (for a review see Raynald et
al., 1994, Biochem., Biophys. Acta., 1197:45~2). They have been
CA 02219299 1997-10-24
16
des~ ibed in many organisms from mammals, to molds and even plants,
and their similar functional properties in Ca2+ and phospholipid are
explainable by their common primary structure (Raynald et al., 1994,
supra). Indeed, some of the Annexins are thought to have originated from
5 a co"""on ancestor (Raynald et al., 199, supra). Moreover, the family of
Annexin genes shows very significant identity between human, rat, and
mouse homologs (Raynald et al., 1994, supra). It will be clear to the
person of ordinary skill that the present invention is not to be limited to
human Annexins as homologs having the biological function of Annexin-
10 based MDR can be used within the context of the present invention.
Furthermore, since Annexins are found in diverseevolutionary distant organisms such as plants, yeasts, and parasites
(Raynald et al., 1994, supra), the present invention has very broad
implications. For example, the present invention opens the way to use
15 Annexins form diverse organisms, such as yeast (i.e. Candida albicons)
and pa~dsiles as therapeutic targets. Antifungal drugs, for example, could
be identified by using yeast Annexins as therapeutic targets.
The presence of Annexins in plants could find utility in
the development of specific crop resistance, by for example, increasing
20 the expression level of at least one Annexin.
In accordance with yet another aspect of the present
invention, there is provided a method of reducing Annexin-based MDR in
a cell or animal, comprising the step of administering a therapeutically
effective amount of a phan~aceutical composition according to the instant
25 invention.
For phar"~aceutical administration, the said polypeptide
may be inco"..oraled into preparations in either liquid or solid forms using
CA 02219299 1997-10-24
carriers and excipients conventionally employed in the pharmaceutical
art, optionally in combination with further active ingredients. The
preparalion may, for example, be applied orally, parenterally, enterally or
preferably topically. Preferred forms include, for example, solutions,
5 emulsions, gels, sprays, lotions, ointments, creams or powders.
One of ordinary skill can readily determine the amounts
of Annexin-based MDR reducing agent to be administered. It is apparent
that the dosage will be dependent on the particular treatment used. It
should also be clear that the dosage should be chosen to display the
10 biological activity without causing adverse effects. It will be understood
that age, sex, type of disease, of formulation and other variables known
to the person of ordinary skill will affect determination of the dos~ge to be
used.
The pha""aceutically acceplable carriers and excipients
15 are well known in the art. A representative textbook thereon is
Remington's Pharmaceutical Sciences, 1980, 16th Ed., Mack Eds.
Advant~geously the cor"positions may be formulated as
dosage units, each unit being adapted to supply a fixed dose of active
ingredient. The total daily dose may, of course, be varied depending on
20 the subject treated and the particular use of the composition. This can
obviously be adapted by the treating professional.
In general, techniques for preparing antibodies
(including monoclonal metabolism and hybridomas) and for detecting
antigenes using antibodies are well known in the art (Campbell, 1984, In
25 ~Monoclonal Antibody Technology: Laboratory Techniques in
Biochemistry and Molecular Biology", Elsevier Science Publisher,
CA 02219299 1997-10-24
18
Amsterdai", The Netherlands) and in Harlow et al., 1988 (in: Antibody -
A Laboratory Manual, CSH Laboratories).
The present invention opens the way to the ide"lification
of agGnisls and anlagonists of Anne,~i,)s with respect to their role in MDR.
5 An assay for Annexin-based MDR activity in cells can be used to assess
the effect of agents on Annexins function in drug resistance and therefore
identify such agonists or antagonists. Non-limiting examples of such
agents include nucleic acid molecules, peptides, antibodies,
carbohydrates, or other pharmaceutical agents.
The present invention also provides polyclonal,
monclonal antibodies, or h~""ani~ed versions thereof, chimeric antibodies
and the like which inhibit or neutralize their respective Annexin targetted
antigens in vivo and/or specific thereto.
Treatments comprise parenterval administration of
multiple or single doses of the above listed antibodies and derivatives
thereof. The dosage will be varied by the practitioning professional
depending on the usual parameters such as pharmacodynamic
characteristics the route of administration, recipient's characteristics,
symptoms and/or disease thereof and the like. A daily dosage of active
ingredient can be for example about 0.1 to 100 mg/kg of body weight,
ordinarily 0.5 - 50 and prererably 1-10 mg/kg per day, i.e. divided doses
ranging from 1~ times per day or alternatively in sustained release form.
The non-human animals of the present invention having
a transgenic interruption or alteration of the Annexin endogenous gene(s)
(knock-out animal) and/or into the genome in which transgenes directing
expression of Annexin(s) has been introduced include vertebrates such
as ,udenls, non-human pri",ales, amphibians, repliles and the like. These
CA 02219299 1997-10-24
19
animals are prepared in accordance with known methods. The same
applies to transgenic plants.
BRIEF DESCRIPTION OF THE DRAWINGS
Having thus generally desc;, ibed the invention, rererence
will now be made to the accompanying drawings, showing by way of
illustration a preferred embodiment thereof, and in which:
Figure 1A shows a restriction map of the gene encoding
P-40. The map for the 1.4 kb fragment isolated from Agt11 expression
librarywith IPM96 monoclonal antibody. The restriction enzyme sites are
indicated by the arrows. The solid bar indicates the coding sequence for
P-40 or Annexin 1. The thin lines to the left and right of the solid bar
indicate the 5' and 3' non-coding regions. Figure 1 B shows the nucleic
acid sequence of P40/Annexin 1. Figure 1C shows the amino acid
sequence of P40/Annexin 1. Figure 1 D shows the nucleic acid sequence
of both strands of P40/Annexin I and hence shows examples of
antisense nucleic acid molecules which can be used in accordance with
the present invention.
Figure 2 shows the in vitro expression of P40. The in
vitro expression of P40 gene was performed using the T7 promoter
directed t,ans~i~Jtion in TA PCRII cloning vector. Figure 2a shows in vitro
transcribed and translated mixes containing PCRII vector only
immunoprecipitated with IPM96 mAb or PCRII plus 1.4 kb insert
immunoprecipit~ted with IPM96 mAb or an irrelevant IgG2b (lanes 2 and
3, respectively). Lanes 14 of figure 2b show the same samples as in
lanes 1 and 2 but transferred to nitrocellulose membrane and probed with
CA 02219299 1997-10-24
a specific antibody to P40 (IPM96 mAb) or an irrelevant IgG2b,
respectively.
Figure 3 shows the analysis of protein and mRNA levels
in drug sensitive and resislant cells. Total cell extract from dn~g sensitive
5 (MCF7, SKOV3 and H69) and resistant human MDR cells (MCF7/AR,
SKOV3NLB'~ and H69/AR) were fractionated on SDS-PAGE and
l,an~re"ed to nitrocellulose membrane. The membrane was probed with
P40 specific monoclonal antibody, lPM96. P40 is seen in extracts from
drug resistant cells (Lanes 4-6). Low levels of P40 is detected in SKOV3
drug sensitive cells (lane 2) but not in MCF7 or H69 cells (1 and 3). For
mRNA levels in the same cell lines, total RNAs were resolved on agarose
gel and l(ansre"ed to nylon membrane and probed with 32P labeled 1.4
kb fragment encoding P40 or Annexin I and actin.
Figure 4 shows the post-translational modification of
15 P-40 or Annexin I in MCF-7/AR cells. Cells were metabolically labeled
with 35~ ll ,ionine or 32P inorganic phosphate and radiolabeled protein
were immunoprecipitated with an irrelevant IgG2b or IPM96 rnAb (lanes
1 and 3 or 2 and 4, respectively).
Figure 5 shows the expression of Annexins l, ll and IV
20 in drug sensitive and resistant cells. Total cell extract proteins from drug
sensitive (MCF-7, H69, SKOV3 and AuxB1) and resistant (MCF-7/Adr,
H69/AR, SKOV3NLB' ~, SKOV3NLB0.06 and CH C5) or revertant
(H69/PR) are resolved on SDS-PAGE and transferred to nitrocellulose
membrane. The nitrocellulose me,nbranes are then probed wit
25 anti-Annexin 1, ll or IV (figures 5a, 5b and 5c, respectively).
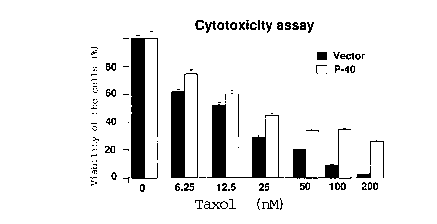
Figure 6 shows the drug sensitivity assays for P-40
transient transfectants. MCF-7 cells transfected with pCDNA3 vector
CA 02219299 1997-10-24
21
without or with P-40 (Annexin 1) gene are incubated in the absence and
presence of i"cr~asing concer,~atiol-s of Taxol (figure 6a) or doxorubicin
(figure 6b). The level of P-40 (Annexin 1) expression in MCF-7 transient
transfectant cells was determined by Westem blotting (figure 6c) and
immuno fluorescence analysis (figure 6d).
Other objects, advantages and features of the present
invention will become more apparent upon reading of the following
non-restrictive description of prefe" ed embodiments with reference to the
acco",panying drawing which is exe")plary and should not be inter~.reted
as limiting the scope of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT
MATERIALS AND METHODS
Cell culture and metabolic labelling
Drug sensitive ( MCF-7, H69, SKOV3 and HL60) and
resisla,1l (MCF-7/Adr, H69/Adr, SKOV3NLB' ~ and HL60/AR) cells were
grown in the absence of antibiotics in a-MEM or RPMI-1640 media
supplemented with 5% to 15% fetal calf serum (Hyclon. Inc.) as
previously described [4447]. Briefly, cells were grown at 37~C in humid
atmosphere of 5% C02 and 95% air. Cells were passaged when 70~0%
confluent for adherent cells and 1X106 cell/ml for cells in suspension.
Drug resistant cells were grown continuously with the appropriate
concentrations of cytotoxic drugs 24 hours following sl ~ha ~'tl lring. All cells
were examined for Mycoplasma contamination using the Mycoplasma
PCR method (Strategen Inc. San Diego, CA). For metabolic labeling of
oells, MCF-7/Adr cells at 70~0% confluency were metabolically labeled
CA 02219299 1997-10-24
22
with [35Sl methionine (100 ,uCi/ml; 1000 Ci/mmol; Amersham Life
Sciences, Inc.) or Carrier free 32P inorganic phosphate (8 mCi/ml;
Amer~hall, Life Sciences, Inc.) for 34 hours at 37~ C in methionine- or
phosphate-free a-MEM. Cells were Iysed and the cell Iysates were
5 immunoprecipitated with IgG2b, or IPM96 mAb.
Screening an expression library with IPM96 monoclonal antibody
A 5' stretch cDNA expression library of HeLa cells
constructed into Agt11 vector was obtained from Clontech (Palo Alto, CA).
10 About 1X10fi plaque forming units were plated using Eschenchia coR
Y1090 as host and screen with IPM96 monoclonal antibody. Briefly,
plates containing phage plaques were incubated at 42~C for 4 hours and
then overlayed with a dried nitrocellulose filter saturated in 10 mM X-gal.
The plates were continuously incubated at 37~C for another 3 hours and
the filters were rinsed with TBST buffer (50 mM Tris-HCL pH 7.9, 150
mM NaCI, 0.05% Tween-20) and incubated in TBST containing 5% of
skin milk for 30 minutes with gentle agitation. The nitrocellulose disks
were inu ~hAted with TBST buffer containing 2 ,ug/ml of Protein G purified
IPM96 monoclonal antibody. The reactive plaques were detected with a
20 second goat anti-mouse IgG conjugated to horseradish peroxidase and
visualized chemiluminescence using Amersham ECL kit. The
immunoreactive plaques were verified by duplicate lifts and purified by
subsequent rounds of screening using decreased plaque density.
Immunor~Aive plaques were eluted in ddH20 and utilized as template
25 for PCR directed by the 3' and 5' insert screening amplimer sequence of
Agt-11 and the fragment from PCR was cloned into PCR ll vector
(InVitrogen Inc.) following standard procedures [48].
CA 02219299 1997-10-24
N~cleotide Sequencing and Computer ~;el!~ence Analysis
The cloned insert into the T/A PCR ll vector was
sequenced by the dideoxy chain termination method using M13 universal
primer and sequence speciric prime~ via the automated DNA sequenci"g
5 servioe at the Sheldon Biotech Centre at McGill University. Both sl~ar,ds
of two different pick clones were completely sequenced. Computer
analysis of the DNA and protein sequence was done using the Blast DNA
search progra,ns.
10 Northem blot and DNA slot blot analyses
For Northern blot, total RNAs from drug sensitive and
resistant cells were extracted with the Trazol solution (GibcoBRL,
Gaithersburg, U.S.A). Approximately 10 ~Jg of RNA from each cell line
was run on a 1% formaldehyde-denatured agarose gel and transrer,ed
15 to HybondsTM-N nylon me~,brane (Amersham, Oakvill, Ont.) by
pressure blotting with 20X SSC (1X = 140 mM NaCI, 32 mM Sodium
Citrate, pH 7.4). The blot was incubated in the presence of 32P labeled
P-40 cDNA probe by nick l,a~1slation or actin gene in 50% formamid, 2.5X
Denhardtis solution, 25 mg/ml denatured Salmon sperm DNA, 1% SDS
20 and 1.25X SSPE ovemight. The blot was washed in low stringency wash
with 0.5X SSC for 30 minute at room temperature. A higher stringency
wash was applied only if it was necessary. RNA integrity and equal
loading was assessed in all cases by hybridization with actin or rRNA
probes. Quantiricalion of rAd QA~tive signals was carried out by scanning
25 the resultant autoradiography and analysis with NIH imaging software
[49].
CA 02219299 1997-10-24
24
For slot blotting, genomic DNA from MCF-7 and
MCF-7/Adr cells was prepared as described [48] and 10 ~9 DNA from
each sample was denatured by 1 M NaOH. Following 10 minute boiling
at 100~C. The denatured DNA was diluted serially loaded onto Nylon
membrane by using Manifold Slot Blot apparatus (Pharmacia Inc.). The
blot was then hybridized with a 32P-labeled probe at 42~C in 50%
fo""amide, 2.5XDenhardt's solution, 25 mg/ml denatured summon sperm
DNA, 1% SDS and 1 .25X SSPE overnight. The highest stringent wash
of the blot was 0.5X SSC with 1% SDS at 65~C for 30 minutes.
In vitro Translation and Immunoprecipitation
In vifro transcription and translation reactions were
carried out using a rabbit reticulocyte Iysate (Promaga Cor,uoration.
Madison, Wl) and 35S-methionine (DuponVNEW, Mississ~u3P Ont.)
according to the manufacturer's protocol. Briefly, cDNA clone encoding
full length P40 was cloned into Notl and Xhol sites of a pCDNA3
expression vector containing T7 and SP6 promotor sequences (In
Vit,ogene, Inc.). The pCDNA3 with and without P~0 insert were added
to a coupled reticulocyte Iysate ~ anscription and translation system in the
presence of 135S]-methionine. Following a 2 hour incubation at 30~C, in
vitro synthesized proteins were immunoprecipitated with IPM96
monoclonal antibody as previously described [50].
DNA Tra..sf~ction
MCF-7 cells were transfected with the pCDNA3 vector
or vector containing P40 coding sequence using lipofectAMlNE (Gibco,
Burlington, Ontario, Canada) accordi,1g to the manufacturer's procedure.
CA 02219299 1997-10-24
Briefly, 4 X 105 cells in a 60 mm plate with 5 ml of serum-free minimal
essential medium were overlaid with 200 IJI of serum-free essential
medium containing 5 ,ug of supercoiled DNA mixed with 10 ~l of
lipofectAMlNE. After 5 hour incubation, the medium was replaced with
5 5 ml of minimal essential medium supple,nented with 10% fatal calf serum
(Hyclone Laboralo, ies) and cells were further cultured at 37~C for 24 - 48
hours. For the transient transfection, cells were collected and the
expression of P~0 was detected by western blot and immuno-
fluorescence. For stable transfectants, G418 was added to the cells at
10 1 mg/ml and continuously selected for another two weeks. The individual
transfectant clones were obtained by the limited dilution under the G418
treatment. A population exhibiting highly expressed P40, designated
BM-1, and a population of the cells transfected with pCDNA3 alone,
designated AM-1 were expanded for further analysis by Western blot or
15 immunoprecipitation.
Immunofluorescence Staining of Cells
Cells were washed with PBS and smeared onto glass
slides by brief centrifugation at 1,500 rpm. The cytospins were air-dried
20 and fixed for 10 minutes in ice-cold acetone. Cells were rinsed twice with
PBS and incubated in 1% bovine serum albumin (BSA)/PBS for 30
minutes at room temperature. Slides were incubated with the IPM96 mAb
(5 ~g/ml 1% BSAIPBS) for 30 minutes followed by three two minutes
rinses with PBS. FlTC-conjugated goat-anti-mouse IgG (1:50 dilution)
25 was added to slides and allowed to incubate for 30 minutes. After several
washes, slides were mounted in PBS containing 50% glycerol and
examined with a Nikon UFX-DX fluorescent microscope fitted with a 60X
CA 02219299 1997-10-24
26
oil immersion objective. Photographs were taken with Kodak Tri-X pan
film (400 ASA) at 800X magnification.
Drug Sensili~ Assay
Transient transfectants were harvested 48 hours later
and aliquoted at 7.5X103 cells per well of a 96-well plate. Drugs were
added to cells 24 hours later and ina Ih~ted for another 48 hours at 37~C.
The assay was dcveloped by adding 50 ,ul (5 mg/ml) of an MTT dye and
allowed to incubate for four hours at 37~C as previously described [51].
SDS-PAGE and Westem Blotting
Total cell Iysates cell cultures or from
l,ans~i,vtion/l,d,1sldlion reaction mixture were mixed with equal volumes
of sample buffer containing SDS and the denatured proteins were
resolved on 10% polyacrylamide gels according to the method of
Laemmeli [52]. For acrylamide gels containing 35S-methionine labeled
proteins, gels were fixed in 50% methanol/water and soaked in
AmplifyTM (Amersham, Oakvill, Ont.) for 30 minutes prior to drying and
exposing to Kodak film at -70~C. For Western blot analysis, proteins were
transferred to nitrocellulose membrane for 1 hour at 50 volts according
to the method of Towbin et al., [53]. The nitrocellulose membrane was
blocked with 5% skin milk/PBS and incubated overnight at 4~C with 1
~g/ml of IPM96 ",onoclonal antibody. The immunoreactive proteins were
detected with horseradish peroxidase conjugated goat anti-mouse
antibody and visualized by chemoluminence using Amersham ECL kit
(Amersham, Oakvill, Ont.).
CA 02219299 1997-10-24
27
RESULTS
Isolation and characleri~alion of P 40 cDNA clones
In a previous report [39], we had demonstrated the
over~ression of a 40 kDa protein (P40) in several MDR cell lines alone
5 or together with P-gp and MRP. In this study, we have used the
monoclonal antibody IPM96 which binds specifically to P40 to screen a
cDNA expression library made from HeLa cells. A total of 50,000 plaques
were screened and several positive plaques were identified following the
initial screening. Of the latter positive plaques t\,vo positive clones were
10 obtained after sequential plaque purification and both inserts were
isolated by PCR (see Material and Methods). Both positive clones
encoded for a 1.6 kb fragments that were subsequently cloned into a T/A
PCRII vector. Sequence analysis of both clones showed an open reading
frame of 346 amino acids which is consistent with the molecular mass of
the protein (38.7 kDa versus 40 kDa) (figure 1A). Comparison of P40
nucleotide and amino acid sequences (figures 1B and 1C) to other
sequences in the DNA data bank, using the DNA search programs
TBlast, showed it to be identical to Annexin I sequence [54].
To confirm the identify of the isolated cDNA, a 1.4 kb
20 encoding the full length of P40 (Annexin 1) was expressed in vitro using
T7 promotor directed transcription-translation reticulocyte Iysate with
35S-methionine. Figure 2a shows the immuno- precipitation of proteins
with IPM96 mAb from an in vitro expression reactions containing vector
only (lane 1) or vector plus 1.4 kb insert (lane 2). As a control for the
25 IPM96 antibody, an irrelevant IgG2b was used to immunoprecipitate
proteins from a reaction mix containing vector plus the 1.4 kb insert
(figure 2a, lane 3). The results of figure 2a show a 35S-methionine
CA 02219299 1997-10-24
28
l~heled 40 kDa protein immunoprecipitated with IPM96 mAb but not with
an irrelevant IgG2b (lanes 2 and 3, respectively). Figure 2b, also shows
Western blotting of the proteins identical to those in lanes 1 and 2 of
figure 2a, but probed with IPM96 mAb (lanes 1 and 2) or an irrelevant
5 IgG2b (lanes 3 and 4). Taken together, these results confirm the identity
of the 1.4 kb fragment as the gene encoding P40 or Annexin 1.
To determine if the amino acid sequence of P40 (or
Annexin 1) cloned from HeLa cells is similar or different from that found
in MCF-7/AR cells, Annexin I was cloned from MCF-7/AR cells by reverse
10 PCR using 5' and 3' primers encoding Annexin 1. Sequence analysis of
Annexin I clones from MCF-7/AR cells rcvoalod no differences from that
isolated from HeLa cells cDNA expression library (data not shown).
Northem blot analyses
In an earlier study [39], the levels of P40 was compared
between drug sensitive and resistant cell lines. Figure 3a shows a
Western blot analysis of total cell Iysates from drug sensitive (MCF-7,
SKOV3 and H69) and their resistant (MCF-7/AR, SKOV3NLB'~ and
H69/AR) cells probed with IPM96 mAb. The results of the latter Western
20 blot analysis shows clearly the increase in P~0 expression in resistant
cells relative to the parental drug sensitive cells. The SKOV3 cells show
lower levels of P~0 than the resistant SKOV3NLB'~. However, it is
interesting that the SKOV3 cell line was derived from a patient with
ovarian tumor that was considered to be clinically resistant Cis-platinum
25 and adriamycin [55]. To determine if the increase in P~0 or Annexin I
protein ex~ression in the above MDR cells is due to an increase in mRNA
levels, Northern blot analysis were performed with total RNA extracted
CA 02219299 1997-10-24
29
from drug sensitive (MCF-7, SKOV3 and H69) and resistant (MCF-7/AR,
SKOV3NLB' ~ and H69/AR) cells and blotted membrane probed with a
32P labeled 1.4 kb fragment. The results in figure 3b shows the 1.8 kb
mRNA band in resistant MCF-7/AR and H69/AR but not in the parental
5 drug sensitive cell lines, MCF-7 and H69/AR. The level of 1.8 kb mRNA
hybridizing band was 4-fold higher drug resistant SKOVNLB' ~ cells
relative to drug sensitive SKOV-3 cells (figure 3b). Taken together, the
Northern blot demonstrate clearly that the observed increase in P40 or
Annexin I cells is due to an increase in mRNA of P-40. Furthermore, the
10 Northem blot results are consistent with the Western blot data, especially
those relating to the protein and mRNA levels of P40 or Annexin I in
SKOV3 versus SKOV3NLB' ~ cells (figure 3).
To determine if the above increase in mRNA levels in
MDR cells relative to drug sensitive cells is at the transcriptional level or
15 is due to gene amplification, genomic DNA was isolated the above cells
lines and analyzed qua"tildli~ely using slot blot. The results (data not
shown) show no gene amplification for P~0 or Annexin I between drug
sensitive and resistant cells.
~0 Post-translational modification of P40 orAnnexin I in r~sistant cells
Annexin I is a phosphoprotein phosphorylated at serine
and tyrosine amino acids [56, 57]. Further, it has been shown that
phosphorylation of Annexin I at its N-terminal domain decreases its
affinity for negatively charged phospholipids or membrane vesicle
25 aggregation [56]. Given the above results, it was of interest to examine
the post-translational modification of P-40 or Annexin I in MDR cells. The
results in figure 4 show immuno-precipitaton of P-40 or Annexin I with an
CA 02219299 1997-10-24
irrelevant IgG2b or IPM96 mAb from MCF-7/AR cells that have been
",etabolically l~l~eled with 35-methionine (lanes 1 and 2) or 32P inorg~nic
phosphate (lanes 3 and 4). Interestingly, P40 or Annexin I was not
phosphorylated in MCF-7/AR cells. Similarly, it was not possible to
5 de",onsl,dle basal level of P40 or Annexin I phosphorylation in the other
MDR cells (data not shown).
Overe~cl,ression of Annexins ll and IV in MDR cells
To determine if other member of the annexin family are
10 similarly overexpressed in MDR cells relative to the parental drug
sensitive cells, total cell proteins from drug sensitive (MCF-7, H69 and
SKOV3) and resislanl (MCF-7/AR, H69/AR and SKOV3NLB' ~) cells were
separated by SDS-PAGE and transferred to nitrocellulose membrane.
Figure 5 shows the results of the Western blots probed with
15 anti-Annexin 1, ll and IV. The results of the Western blot probed with
anti-Annexin I show similar results to the Westem blot in figure 3a probed
with IPM96, co,1rir"~ing the antigen specificity of IPM96 mAb towards to
Annexin 1. Furthermore, the expression of Annexin ll and IV is also
increased in MDR cells relative to the parental cell lines, however to a
20 lesser extend as that of Annexin 1. For example, both Annexin ll and IV
are expressed at lower levels in drug sensitive cells compared to MDR
cells (figure 5). Of considerable interest is the levels of Annexin 1, ll and
IV in a revertant cell line (H69/PR) derived from H69/AR cells that are
less resistant to doxorubicin (figure 5). cDNA transfections (transient and
25 stable) of Annexins Il-XI, as described below will be carried out to verify
the drug resistance of Annexin ll-XI-transfected cells.
CA 02219299 1997-10-24
31
cDNA tra~;.reclion and drug sensitivity analyses
In an allar"pl to investigate the role of P40 or Annexin I overexpression
in drug resistance, the full length gene encoding P40 or Annexin I was
cloned into a mammalian expression vector, pCDNA3 and transfected
5 into MCF-7 cells. MCF-7 cells transfected transiently with pCDNA3 alone
or with P40 gene are cultured for 3 days prior to analysis. The results in
figure 6a show a Western blotting of total cell proteins from MCF-7 cells
transfected with pCDNA3 vector only or pCDNA3 vector plus P40 or
Annexin I gene (lanes 2 and 3). The results in lane 3 of figure 6a show a
40 kDa protein in MCF-7 cells lldnsrt:~Aed with pCDNA3 vector plus P40
or Annexin 1. To determine more quantilali~lely, the level of P40
transfection following a three day culturing, MCF-7 cells transfected with
pCDNA3 plus P40 or Annexin I full length cDNA were analyzed by
indirect immunofluorescence with IPM96 mAb. The results in figure 6b
15 show the relative number (~5%) of MCF-7 cells that overexpress P40 or
Annexin 1. The efficiency of the transient transfection to -5% of the cells
was conri",~ed following transfection with pCDNA3 containing a beta-gal
gene (data not shown).
Having established the expression of P40 or Annexin I
20 in MCF-7 cells, it was of interest to examine the effect of P40 or
Annexin I overexpression (albeit <5%) on the sensitivity of MCF-7 cells
to anticancer drugs. The results in figure 6c and 6d show MCF-7 cells
transfected with vector only and with vector plus P40 incubated with
increasing concenll dlions of Taxol or adriamycin, respectively.
25 Surprisingly, overexpression of P40 or Annexin I in MCF-7 cells
decreases their sensitivity to Taxol and adriamycin. Although it is
e~e~ed, whether higher levels of P-40 or Annexin I expression will lead
CA 02219299 1997-10-24
to a larger decrease in the sensitivity of transfectant cells to anticancer
drugs will have to be formerly tested using for example stable cell lines
expressing PCDNA3 alone or with P-40 gene.
The transient transfected cells were incubated with a
~1eldlor (EGTA) or a calcium channel blocker (Verapamil). P40-protein
was shown to be released by the men,brane and the EGTA or Verapamil-
treated cells were also shown to have reduced drug resistance to taxol
or adriamycin. This results suggest that small molecules find utility in the
context of the present invention.
DISCUSSION
In this study we have used the monoclonal antibody
IPM96, previously shown to detect a 40 kDa protein in MDR cells to
screen a Agt11 expression library. Two positive Agt11 clones were
identified and their cDNA insert was isolated by PCR and cloned into a
TA PCRII vector. Analysis of the nucleotide and amino acid sequence of
the 1.4 kb cDNA insert revealed an open reading frame of 346 amino
acids that are identical to Annexin I [54]. In vitro expression of IMP96
positive cDNA clone using a transcription-translation retic, Iysate followed
by immuno precipitation and Western blot analyses of the expressed 40
kDa protein confirmed the identify of the 1.4 kb fragment as P40. In
addition, Northern blot analysis using total RNA from drug sensitive and
resislanl cells confirmed the overexpression of Annexin I or P40 mRNA
in MDR cells relative to their parental drug sensitive cells.
Besides the similarities in the P40 and Annexin I
molecule masses on SDS-PAGE and the cross-reaction of IPM96 with
Annexin I expressed in vitro, the identity of the P40 as Annexin I is
CA 02219299 1997-10-24
33
consistent with our earlier observations where P40 was shown to be
found both in the me",brane and soluble fractions [39]. Fu,lher",ore
extraction of membrane associated P40 was resistant to high salt and
EDTA and sUggestC the possibility that some of P40 may be an integral
5 ",er"brane protein. Intereslingly a similar conclusion was i"dependerltly
suggested for Annexin I in an earlier study . The latter possibility is likely
given that annexins 1, V, Vl and Vll possess ion channel activity [58 59].
Also consislenl with our assignment of P40 as Annexin 1 is the fad that
the 35 kDa proteolytic product which has been previously demonstrated
10 to represent the head domain of Annexin 1 is highly sensitive to
proteolysis [56].
Annexin I is a member of a large family of calcium
dependent membrd,1e binding proteins that are sometimes referred to as
lipocortin calpectins endonexins (for review [40]). Annexins share a
15 similar core domain with four or eight conserved 70 amino acid repeats
and an amino terminal domain that varies in length and sequence
between the different members of the annexin family Although the
physiological function(s) of annexins is not clear they have been
implicated in calcium-regulated exocytosis [60 61]. Annexin I has also
20 been shown to mediate the calcium-dependent fusion of liposomes with
isolated neutrophil plasma membranes [62]. In intact cells annexins are
generally phosphorylated in response to varieties of stimuli. Annexin I is
phosphorylated by EGF receptor-kinase at tyrosine residues in found in
the N-terminal head domain [63] and by protein kinases C and A [57].
25 Interestingly phosphorylation of Annexin I at the amino terminal domain
by protein kinase C inhibits its ability to aggregate chromaffin granules
[64]. In adrenal chromaffin cells Annexin I was shown to be rapidly
CA 02219299 1997-10-24
34
phosphorylated upon stimulation of cells to secrete normal cell
metabolites [65]. Taken together, our finding that Annexin I is not
phospl ,orylated in MDR cells is consistent with its increased capacity to
cause aggregation of membrane vesicles.
In this report we show, for the first time, a direct role of
Annexin I overexpression in tumor cells resistance to anticancer drugs.
Using transient transfections of MCF-7 tumor cells, we showed that
transfection of P40 or Annexin I cDNA confers resistance to Taxol and
adlia~ycin. Although the levels of resistance towards the latter drugs are
only 1.2 to 2.0-fold more than control cells (l,ansrec~ed with vector alone),
the results are consistent and are not surprising in light of the perce"lage
(~5%) of transfected cells (figure 6a and 6b). Furthermore, similar
l,ansrection studies of P-gp or MRP cDNA have also shown much lower
levels of drug resistant in transfectant cells when compared to selected
MDR cells that overexpress similar amounts of these proteins [33, 66-68].
Whether Annexin I confers lower levels of drug resistance as compared
to P-gp or MRP drug efflux mechanisms remains to be determined. In
any event, unlike in vitro tumor selected cell lines, low levels of drug
resistance conferred by P40 or Annexin I are likely to be clinically
relevant. Work is in progress to isolate and characterize the drug
resistant profile of stable transfectants of Annexin 1.
Although, our data thus far shows a direct correlation
between P40 or Annexin I overexpression and resistance to anticancer
drugs, it is no clear how is P40 mediated this phenolype. However, given
the role of annexins in promoting aggregation of membrane vesicles
through calcium phospl~olipid binding, it may be spea ~ ed that Annexin I
confers a drug resistance phenotype by promoting the aggregation of
CA 02219299 1997-10-24
drug filed ,ne"lbrane vesicle or exocytosis of such drug filed vesicles [69j.
Increased me"~bra~e vacuolization has been observed in many MDR
selected cell lines [70 71]. Furthermore P-gp and MRP have been
observed in endosomal membranes in in vitro selected MDR cell lines
5 [72-74] In the latter possibility Annexin I or other members of the annexin
family (which are also overexpressed in MDR cells; figure 5) could
function together with P-gp or MRP to cause the aggregation and
possibly exocytosis of drug filled vesicles.
Of importance the analysis of Annexin I RNA levels in
10 mouse and hamster was shown to also positively correlated with MDR.
Although the present invention has been described
hereinabove by way of preferred embodiments thereof it can be
modified without departing from the spirit and nature of the subject
invention as defind in the appended claims.
CA 02219299 1997-10-24
36
REFERENCES
1. Ferguson et al.,1989, Cancer Bulletin 41(1): 7-13
2. Pastan et al., 1987, New England Joumal of Medicine 316(22): 1388-
1393
3. Bradley et al.,1988, Biochimica et Biophysica Acta 948: 87-128
4. Gottesman et al.,1993, Annual Review of Biochemistry 62: 385-427
5. Endicott et al.,1989, Annual Review of Biochemistry 58: 137-171
6. Cole et al.,1996, Cancer Treatment & Research. 87: 39-62
7. Higgins et al.,1992, Annual Review of Cell Biology 8: 67-113
8. Shapiro et al.,1994, Journal of Biological Chemistry 269(5): 3745-3754
9. Doige et al.,1992, Biochimica et Biophysica Acta 1109: 161 -171
10. Cordon-Cardo et al.,1990, Joumal of Histochemistry and Cytochemistry
38(9): 1277-1287
11. Bradley et al.,1990, Joumal of Cellular Physiology 145: 398-408
12. Thorgeirsson et al.,1987, Science 236: 1120-1122
13. Thiebaut et al.,1987, Proceedings of the National Academy of Sciances
USA 84: 7735-7738
14. Schinkel et al.,1997, Proceedings of the National Academy of Sciences
of the United States of America. 94(8): 4028-4033
15. Schinkel et al.,1994, Cell 77: 491 -502
16. Tishler et al.,1992, Joumal of Neurosurgery 76: 507-512
17. Abe et al., 1994, Japanese Journal of Cancer Research 85(5): 536-541
18. Baker et al.,1989, ?: 87-97
19. Belloni et al.,1989, Cancer and Metastasis Review 8: 353-389
20. Charpin et al., 1994, Journal of the National Cancer Institute 86(20):
1539-1545
21. Fojo et al.,1987, Proceedings of the National Academy of Science USA
84: 265-269
CA 02219299 1997-10-24
22. Henson et al., 1992, Joumal of Neuro-Oncology 14: 37-43
23. Hij~i et al., 1994, American Joumal of Clinical Pathology 102(1): 61-67
24. Mattem et al., 1994, Anticancer Research 14(2A): 417-419
25. Nooter et al., 1994, Leukemia Research 18(4): 233-243
26. Chan et al., 1995, Hematology - Oncology Clinics of North America 9(2):
275-31 8
27. Chan et al., 1991, New England Joumal of Medicine, 325:1608-1614
28. Lee et al., 1997, Journal of Cellular Biochemistry. 65(4): 513-526
29. Baggetto, Bulletin du Cancer. 84(4): 385-390
30. Linn et al., 1994, Joumal of Clinical Oncology 12(4): 812-819
31. Lonn et al., 1994, Intemational Journal of Cancer 58(1): 40-45
32. Sognier et al., 1994, Biochemical Pharmacology 48( 2): 391-401
33. Zaman et al., 1994, Proceedings of the National Academy of Sciences
USA 91(19): 8822-8826
34. Tew, 1994, Cancer Research 54(15): 4313-4320
35. Frelich et al., 1995, J. Biological Chemistry 270(15): 21429-21432
36. Schefferetal., 1995, Nature Medicine 1(16): 578-578
37. Lowe et al., 1993, Cell 74(6): 957-967
38. Lowe et al., 1994, Science 266(5186): 807-810
39. Wang et al., 1997, Biochemical & Biophysical Research
Communications. 236(2): 483-488
40. Raynal et al., 1994, Biochimica et Biophysica Acta. 1197: 63-93
41. Creuk, 1992, Science. 258: 924-931
42. Lin et al., 1992, Cell 70: 283-291
43. Creuk et al., 1992, Journal of Cell Science. 103: 1 177-1192
44. Mirski et al., 1987, Cancer Research 47: 2594-2598
45. McGrath et al., 1987, Biochemical & Biophysical Research
Communications. 145(3): 1171-1 176
46. Bradley et al., 1989, Cancer Research 49: 2790-2796
CA 02219299 1997-10-24
38
47. Batist et al., 1986, Joumal of Biological Chemistry. 261(33): 15544-
15549
48. Sambrook et al., 1989, Molecular cloning: A laboratory Manual. Cold
Spring Harber Laboratory Press, Cold Spring Harbor, N.Y.
49. Wayne R,1992, NIH Imaging Software
50. Georges et al.,1991, Journal of Cellular Physiology 148: 479-484
51. Pouliot et al.,1997, Biochemical Pharmacology 53(1): 17-25
52. Laemmli et al.,1970, Nature 227(259): 680-685
53. Towbin et al., 1979, Proceedings of the National Academy of Sciences
of the United States of America 76(9): 4350-4354
54. Wallner et al.,1986, Nature 320: 77-81
55. Fogh et al.,1975, Plenum Press, New York: 155-159
56. Wang et al.,1994, BIOCHEMISTRY 33: 276-282
57. Varticovski et al.,1988, BIOCHEMISTRY 27: 3682-3690
58. Pollard et al., 1988, Proc. Natl. Acad. Sci. USA 85: 2974-2978
59. Rojas et al.,1990, Joumal of Biological Chemistry 265(24239-24245)
60. Drust et al. ,1988, Nature 331: 88-91
61. Creut_ et al.,1987, Joumal of Biological Chemistry 262: 1860-1865
62. Meersetal.,1986, Nature321(81-84.)
64. Wang et al.,1986, Joumal of Biological Chemistry 261 (6548-6553.)
66. Cole et al., 1994, Cancer Research 54(22): 5902-5910
67. Ueda et al., 1987, Proceedings of the National Academy of Sciences
USA 84: 3004-3008
68. Gros et al.,1986, Nature 323: 728-731
69. Chauffert et al., Cancer Research 46: 825-830
70. Beck,1987, Biochemical Pharmacology 36(18): 2879-2887
71. Sehested et al.,1987, British Joumal of Cancer 56: 747-751
72. Abb~-c7~degan et al.,1997, Cancer Research 56(23): 5435-5442
CA 02219299 1997-10-24
39
73. Slapak et al. 1992 Joumal of Biological Cl,ei"isl"~. 267(15): 10638-
10644
74. Klohs et al. 1988 Molecular Pharmaco'og~ 34: 180-185