Note: Descriptions are shown in the official language in which they were submitted.
CA 02219656 2006-02-01
1
ANTIBACTERIAL CEPHALOSPORINS
The present invention relates to antibacterial compounds which are 7-acylamino-
3-
(imino)methyl cephalosporins.
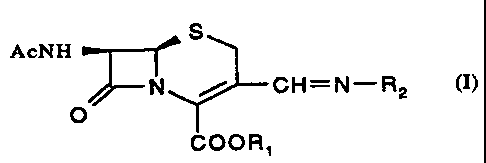
Particularly the present invention proves a compound of formula
S
AcNH
N / CH=N-RZ
O
COOR1
wherein
Rl denotes hydrogen or an ester moiety,
R2 denotes a group of formula
-O-Y -N R
' or ---NR
Rs
Ila Ilb Ilc
wherein
Y denotes hydrogen, alkyl, alkenyl, acyl, carbamoyl or aryl
R4 denotes hydrogen, alkyl, alkenyl, cycloalkyl, aryl, acyl or heterocyclyl
R5 denotes hydrogen, alkyl, alkenyl, cycloalkyl, aryl, heterocyclyl, or a
group of
formula
or -C Z
-C\ R' --C Z
NRB N R Ri,
Rip
lid Ile Ilf
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
2
wherein
R7 denotes alkyl or aryl
R$ denotes hydrogen, cycloalkyl or alkyl
Rg denotes hydrogen or alkyl
R,o denotes hydrogen, alkyl, hydroxy, amino, phenyl, alkenyl, cycloalkyl,aryl,
heterocyclyl or a group of formula
-N=CH-Phe
wherein Phe denotes aryl
R9 and RIO together with the nitrogen atom denote heterocyclyl,
Z denotes oxygen, sulphur or N-R13, wherein
R13 denotes hydrogen, alkyl or cycloalkyl
R11 denotes hydrogen, alkyl, aryl, cycloalkyl or heterocyclyl, or
R4 and R5 together with the nitrogen atom denote heterocyclyl,
R6 denotes heterocyclyl, and
Ac denotes
(i) a group
-CO-CH2 N
s NH2
(ii) a group of formula
Z2,, Z3
C B-D-Z~
or
-CO - C - Zi -COC-Zi
.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
3
wherein
B denotes N or CH
Z, denotes aryl, cycloalkyl, 1,4-cyclohexadienyl or heterocyclyl
= Z2 denotes hydrogen, alkyl or -CH2COOZ5, wherein
Z5 denotes hydrogen, alkyl or cycloalkyl
Z3 denotes hydrogen or alkyl
Z4 denotes hydrogen or an organic radical
D denotes oxygen or CH2.
A subgroup of the invention comprises any of the individual groups of
significances
mentioned therein.
R, may be hydrogen or an ester moiety. An ester moiety includes alkyl,
preferably
C1.6alkyl; arylalkyl, for example benzyl, alkoxybenzyl, such as 4-
methoxybenzyl; indanyl,
phthalidyl, alkoxymethyl, e.g. methoxymethyl; (Cl.b)alkanoyloxy(C,_6)alkyl,
(C,-6)alkoxy-
carbonyl-oxy(C,.6)alkyl, glycyloxymethyl, phenylglycyloxymethyl, (5-methyl-2-
oxo-1,3-
dioxolen-4-yl)methyl and ester moieties which form with the COO- group a
physiologically hydrolysable and acceptable ester, e.g. such known to be
hydrolysable
ester groups in the field of cephalosporins. A compound of formula I may thus
be in the
form of an physiologically-hydrolysable and -acceptable ester. By
physiologically-
hydrolysable and -acceptable esters as used herein is meant an ester in which
the COO-
group is esterified and which is hydrolysable under physiological conditions
to yield an
acid which is itself physilogically tolerable at dosages to be administered.
The term is thus
to be understood as defining regular pro-drug forms. An ester moiety may be
preferably a
group which is easily hydrolysable under physiological conditions. Such esters
may be
administered preferably orally. Parenteral administration may be indicated if
the ester per
se is an active compound or, if hydrolysis occurs in the blood.
Y may be preferably hydrogen, unsubstituted alkyl or alkyl substituted by e.g.
hydroxy or,
preferably the residue of a carboxylic acid. The residue of a carboxylic acid
includes the
residue of a carboxylic acid in free form or in salt form, of a carboxylic
acid ester and of
a carboxylic acid amide. The carboxylic acid is, for example, a C,.,
carboxylic acid,
preferably a C1 .S aliphatic carboxylic acid, an alkyl part thereof including
lower alkyl. The
alkoxy group of a carboxylic acid ester includes C,.6, preferably C,,alkoxy.
Alkyl is
preferably lower alkyl. The alkyl group is preferably unsubstituted or
substituted by
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
4
carboxylic acid residues.
R4 may be preferably hydrogen or alkyl, for example lower alkyl.
R5 may be preferably hydrogen; unsubstituted alkyl; alkyl substituted for
example by oxo,
alkyl or halogenated alkyl; amino; one or several fold substituted
heterocyclyl; or a group
of formulae Ild, IIe, IIf. Heterocyclyl includes unsaturated or saturated
heterocyclyl
having, e.g. 5 or 6 ring members and, for example, 1 to 3 hetero atoms, such
as N, 0, S,
preferably N, or condensed heterocyclyl, such as benzothiazolyl.
R4 and R5 together with the nitrogen atom may be heterocyclyl, having
preferably 5 or 6
ring members and having preferably 1 to 3 heteroatoms, for example N atoms;
which may
be unsubstituted heterocyclyl; or one or several fold substituted
heterocyclyl, for example
by oxo, amino, alkyl.
R6 may be saturated or unsaturated heterocyclyl; having preferably 5 or 6 ring
members
and having for example 1 or 2 nitrogen hetero atoms; for example unsubstituted
heterocylclyl; or one or several fold substituted heterocyclyl, for example by
amino, alkyl
or thiono.
R7 may be preferably alkyl.
R. may be preferably alkyl or cycloalkyl.
R9 may be preferably hydrogen or lower alkyl.
R13 may be preferably alkyl.
R,o may be preferably hydrogen; aryl; alkenyl; cycloalkyl; unsubstituted
alkyl; substituted
alkyl, for example by hydroxy, halogen, heterocyclyl, such as pyridyl, amino,
for example
N(alkyl)Z or N'(alkyl)3; or a group
- N=CH -Ar
wherein Ar denotes heterocyclyl; unsubstituted aryl; or substituted aryl, for
example by
hydroxy or alkoxy; preferably aryl which may be preferably phenyl.
R9 and R,o together with the nitrogen atom may be heterocyclyl having
preferably 5 or 6
ring members and 1 to 3 hetero atoms such as N, S, 0, for example N. 0;
preferably
saturated heterocyclyl. Heterocyclyl includes unsubstituted heterocyclyl, or
substituted
heterocyclyl, for example by acyl, formyl, alkyl, for example lower alkyl.
Examples
include pyrrolidine. morpholine, piperazine, preferably piperazine.
CA 02219656 1997-10-28
WO 96/35692 PCr/EP96/02023
Rl, may be preferably hydrogen; unsubstituted alkyl; substituted alkyl, for
example by
aminoalkyl, diaminoalkyl, triaminoalkyl; aryl, such as dihydroxyphenyl;
cycloalkyl; or
unsubstituted heterocyclyl; or substituted heterocyclyl, for example by alkyl,
thiono
heterocyclyl; heterocyclyl having preferably 5 or 6 ring members and 1 to 3
hetero,
preferably N atoms.
If not otherwise stated therein any carbon containing group may contain up to
20 carbon
atoms, e.g. alkyl includes C,_,A, e.g. C,-8 alkyl. Lower alkyl includes e.g.
Cl-4alkyl, preferably CI-Zalkyl. Alkenyl includes CZ-20, e.g. C2-S alkenyl.
Lower alkenyl
includes e.g. C3-5alkenyl, preferably C3alkyl. Cycloalkyl includes, for
example,
C3-6cycloalkyl, particularly C3, C5 or C6cycloalkyl. Alkyl, alkenyl and
cycloalkyl include
unsubstituted alkyl, alkenyl and cycloalkyl; and, substituted alkyl, alkenyl
and cycloalkyl,
for example, by halogen, a sulphonic acid derivative, such as SO3H, CF3,
hydroxy, alkoxy,
acyl, alkylamino, pyridyl. Cycloalkyl is preferably unsubstituted. Acyl
includes C,-lZ, e.g.
Cl.bacyl, particularly C,-4acyl. Acyl includes unsubstituted acyl and
substituted acyl, for
example, by hydroxy, alkoxy, amino. Aryl includes phenyl. Aryl may be
unsubstituted aryl
or substituted aryl, for example by alkyl, alkoxy, acyl, halogen, hydroxy,
unprotected or
protected amino. Alkoxy includes alkoxy wherein the alkyl part is as defined
above.
Heterocyclyl includes heterocyclyl having 5 or 6 ring members and 1 to 3
nitrogen,
sulphur and/or oxygen hetero atoms including, for example, condensed
heterocyclyl, such
as for example benzthiazolyl. Heterocyclyl includes further unsubstituted
hetercyclyl and
substituted heterocyclyl, for example by oxo, alkoxy, hydroxy, thiono,
mercapto, alkylthio,
imino, alkylamino, alkylimino, amino, halogen, acyl, CF31 CHO, alkyl,
cycloalkyl.
Carbamoyl includes the carbamoyl group or carbamoyl having alkyl and aryl
residues.
Z, denotes unsubstituted cycloalkyl, 1,4-cyclohexadienyl, heterocyclyl or
aryl; and one or
several fold substituted cycloalkyl, 1,4-cyclohexadiene, heterocyclyl or aryl;
for example
by carboxyl, amino, nitro, cyano, lower alkyl, lower alkoxy, hydroxy, halogen,
-CO-N(Z5Z6), -N(Z6)-COOZ,, Z6CO-, Z6OCO-, Z6COO-.
ZZ denotes hydrogen; CH2COOZ5; unsubstituted lower alkyl; one or several fold
substituted lower alkyl, for example by carboxyl, amino, nitro, cyano, lower
alkyl, lower
= alkoxy, hydroxy, halogen, -COZSZ6, -N(Z6)-COOZ,, Z6CO-, Z6OCO- or Z6COO-.
Z3 denotes hydrogen or lower alkyl.
Z. denotes hydrogen or an organic radical; preferably hydrogen; lower alkyl;
cycloalkyl;
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
6
aralkyl; acyl; carboxyalkyl; Z6CO-, -C(Z,ZB)COOZ6 or, preferably in the case
that Z, in
group
B-D-Z4 =
- CO C. - Zi
denotes a group 2-amino-thiazol-4yl or 2-amino-thia-3,5-diazol-4y1,
Z4 denotes a- group of formula
OZII Zli Z17
1
I H /-\ OZ11 or - i /Z16
Z9 Z 13
Zlo Z14 Z15
or
ZIg ~ ~ Zls
/ i
-C(CO-NH-NH-CO)n or -C-COOH
Z19 Z24 Zi2 1
7 Z19
or
O Zs7 O
Z25
C OH or - CH: Z28
N N
Z ~ I
26 Z
OH OH z9
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
7
wherein
Z9 and Z,o independently of one another denote hydrogen or protected or
unprotected
carboxyl
= Zõ denotes hydrogen or acetyl,
Z12 denotes unprotected or protected carboxyl,
Z13 denotes hydrogen or methyl,
Z14 denotes hydrogen; chloro; unprotected or protected carboxyl; methyl;
isopropyl;
hydroxy; methoxy; acetoxy,
Z15 and Z16 denote independently from one another hydrogen, hydroxy, methoxy,
ethoxy, 2-methoxyethoxymethoxy, acetoxy, chloroacetoxy, butanoyloxy,
methansulfonyloxy, p-toluenesulfonyloxy, amino, acetylamino,
benzyloxycarbonylamino or methansulfonyl; or,
Z15 and Z16 denote together ethylendioxy or carbonyldioxy,
Z,7 denotes hydrogen, hydroxy, acetoxy, methyl, methoxy, chloroacetoxy,
with the proviso, that not all of Z14, Z,s, Z16 and Z17 denote hydrogen,
Z,g and Z19 denote independently of one another hydrogen or methyl,
Zm, Z2,, Z,Z, Z23 and Z24 denote independently of one another hydrogen,
halogen or
hydroxy,
Z., and Z26 denote independently from one another hydrogen; C,-5alkyl;
unsubstituted
phenyl; or substituted phenyl,
Z27 denotes unsubstituted lower alkyl; or substituted lower alkyl,
Z28 and Z29 denote independently of one another hydrogen or hydroxy, and
n denotes 0 or 1,
Z. denotes hydrogen, alkyl, preferably lower alkyl,
Z6 and Z7 independently of one another denote hydrogen or alkyl, preferably
lower alkyl,
Z6 and Z7 together with the carbon atom denote cycloalkyl, and
Z5 and Z6 together denote cycloalkyl.
Z. may be selected from the following groups:
OH OH
-CH OH -CH: OH
I - -
COOH
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
8
O
- CHZ OH
N
OH
or
CH3 OH
I
-C-CO-NH-NH-CO ~ ~ OH
I -
CH3
For example, Ac may denote a group of formula
- CO - CH - NH - CO - N' N- CZHS or I I
,/-,\(' -CO-C O
O O II
CH3O- N
OH
Preferably Ac denotes a compound of formula
V
'I-R3
~ IC-CO-
H2N S~W
~
wherein
W denotes CH or N
V denotes CH or N-0 and
CA 02219656 1997-10-28
WO 96/35692 PGT/EP96/02023
9
R3 denotes hydrogen, acyl, carboxyl, alkyl.
The configuration of R3 in group of - C = V - R3 may be syn [(Z)] and anti
[(E)] and is
= preferably syn [(Z)].
If R3 denotes alkyl, R3 includes unsubstituted alkyl or substituted alkyl, for
example by
halogen, carboxyl. Preferably W denotes CH.
In another aspect the present invention provides a compound of formula
I I V-R3
S
----C-CO-HN ~
H2N----~~5~'W' TN CH=N-R2
O
COOR1
wherein
W denotes CH or N
V denotes CH or N-O
R, denotes hydrogen or an ester moiety,
R2 denotes a group of formula
-O-Y -N ' or NR6
R5
Ila Ilb Ilc
wherein
Y denotes hydrogen; unsubstituted lower alkyl; or substituted lower alkyl, by
the
residue of a carboxylic acid, a carboxylic acid ester or a carboxylic acid
amide,
R, denotes hydrogen, phenyl, cycloalkyl or lower alkyl
R. denotes hydrogen, lower alkyl, heterocyclyl or a group of formulae
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
-C,-ISR, -C10~ Z or -CZ
NR8 P(Rg \R
Rio
lid lie ilf
whemin
R7denotes lower alkyl
R. denotes hydrogen, cycloalkyl or lower alkyl
R9 denotes hydrogen or lower alkyl,
RIo denotes hydrogen, hydroxy; amino; phenyl; alkenyl; cycloalkyl;
heterocyclyl;
unsubstituted alkyl; substituted alkyl, by CF3, OH, alkoxy, carboxyl,
halogen, amino, monoalkylamino, dialkylamino, trialkylamino, pyridyl or a
a sulfonic acid residue; a group of formula
OR 12
-N=CH <?
OR12
wherein
R,Z denotes hydrogen or lower alkyl,
Z denotes oxygen, sulphur, or N-R13, wherein
R13 denotes hydrogen or lower alkyl, and
R11 denotes hydrogen; dihydroxyphenyl; cycloalkyl; heterocyclyl; unsubstituted
lower alkyl; substituted lower alkyl by pyridyl or monoalkylamino,
dialkylamino or trialkylamino; and,
R, and R. and/or R9 and R,o independently of one another together with the
nitrogen denote heterocyclyl,
R. denotes heterocyclyl, and
R3 denotes hydrogen; acyl; carboxyl; unsubstituted alkyl; substituted alkyl by
halogen or
carboxyl.
CA 02219656 1997-10-28
WO 96135692 PCT/EP96/02023
11
In another aspect the present invention provides a compound of formula
S
AcNH
N CH=N-R2p IP
O
COOR1 p
wherein
Rlp is the same as R, in formula I,
Ac is as defined in formula I,
R2 P denotes a group of formulae
-OY or -N--~ R'P
R5a
Ilap Ilbp
wherein
Yp is the same as Y in formula IA,
R4 p is the same as R4 in formula IA, and
R5 P denotes hydrogen, cycloalkyl, lower alkyl or a group of formula
SR~ IZp
-C or -C
NRep NR9c
RIo 0
Ildp Ilep
wherein
Rg p is the sarhe as R. in formula IA,
ZD is the same as Z in formula IA,
R9 p is the same as R9 in formula IA,
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
12
R7 P denotes methyl,
R,o p denotes hydrogen, lower alkyl or hydroxy, and
R4 p and R5 P and/or R9 p and Rlo p independently of one another together with
the nitrogen denote heterocyclyl, and
a compound of formulae IIbp, IIdp and IIep denote any tautomeric form, in free
form, or, where such a form exists, in form of an acid addition salt, inner
salt,
quaternary salt or hydrate thereof.
In another aspect the present invention provides a compound of formula
S
AcNH
Iq
O N / CH=N-R2q
COOR, q
wherein
Ac is as defined in formula I
Rl q is he same as Rl in formula IA, and
R2 q denotes a group of formula
-OY or N---~ R'Q
q
R5q
Ilaq llbq
wherein
Yq is the same as Y in formula IA,
R4 q is the same as R4 in formula IA, and
RS q denotes hydrogen, cycloalkyl, lower alkyl or a group of formulae
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
13
SR7q q
-C or -CN, R
NR8q N-11 aq
RloQ
= Ildq Iieq
wherein
R7 q is the same as R7 in formula IA,
R8 Q is the same as Rg in formula IA,
Zq is the same as Z in formula IA,
R9 q is the same as R9 in formula IA,
Rto q denotes hydrogen, lower alkyl or hydroxy, and
R4 q and R5 q and/or R9 q and Rlo q independently of one another together with
the nitrogen
denote heterocyclyl, and
a compound of formulae IIbp, IIdp and IIep denote any tautomeric form, in free
form, or,
where such a form exists, in form of an acid addition salt, inner salt,
quaternary salt or
hydrate thereof.
In a further aspect the present invention provides a compound of formula
V;-- R3.
II S
~ S ~ C-CO-HN Is
HZN ~'W' s O N CH=N-R26
COORI
wherein
R, s is the same as R, in formula IA,
V, is the same as V in formula IA,
W, is the same as W in formula IA
R3, denotes hydrogen, lower acyl; unsubstituted alkyl; substituted lower alk-,
I. by
CA 02219656 1997-10-28
WO 96135692 PCT/EP96/02023
14
carboxyl and/or fluoro; and
R2 S denotes a group of fonmula
R
-OYS -N~ 4 s or -N-R65 ,
Rss
Ilas Ilbs Ilcs
wherein
Y, denotes hydrogen; unsubstituted lower alkyl; or substituted alkyl by
carboxyl,
R4 s denotes hydrogen or lower alkyl, and
R5 S denotes hydrogen; saturated or unsaturated, unsubstituted heterocyclyl
having
or 6 ring members and I to 3 nitrogen hetero atoms; saturated or
unsaturated one or several fold substituted heterocyclyl by oxo, lower alkyl,
amino or CF3, having 5 or 6 ring members and 1 to 3 nitrogen hetero atoms;
benzothiazolyl; or a group of formula
/SR-is _ /~ ~Z
or - ~
CNR s \NRg$ C S
\ Rll s
R1o s
lids Iles Ilfs
wherein
ZS is the same as Z in formula I,
R7 s denotes lower alkyl,
Rg = denotes hydrogen, cycloalkyl or lower alkyl,
R9 , denotes hydrogen or lower alkyl,
R,o s denotes hydrogen; phenyl; allyl; cycloalkyl; unsubstituted alkyl;
substituted alkyl by CF3. dialkylamino, trialkylamino, hydroxy, pyridyl
or SOjH, and
Rõ , denotes hydrogen; pyridyl; cycloalkyl; unsubstituted lower alkyl.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
substituted lower alkyl by pyridyl or trialkylamino; saturated or
unsaturated heterocyclyl 'having 5 or 6 ring members and 1 to 3
nitrogen hetero atoms; or one or several fold substituted heterocyclyl by
= lower alkyl and/or thiono, having 5 or 6 ring members and 1 to 3
nitrogen hetero atoms;
R4 s and R5 S together with the nitrogen atom denote heterocyclyl selected
from saturated, unsubstituted heterocyclyl having 5 or 6 ring members and 1
or 2 nitrogen hetero atoms; saturated, one or several fold substituted
heterocyclyl by oxo or lower alkyl, having 5 or 6 ring members and I or 2
nitrogen hetero atoms; and/or
R9 S and R,o S together with the nitrogen atom denote saturated, unsubstituted
heterocyclyl having 5 or 6 ring members and 1 or 2 nitrogen and/or oxygen
hetero atoms; unsaturated, one or several fold substituted heterocyclyl by
CHO or lower alkyl, having 5 or 6 ring members and 1 or 2 nitrogen and/or
oxygen hetero atoms.
In another aspect the present invention provides a compound of formula
V-R3
S
N------C-CO-HN ~
H2N-I~~S"IWI N / CH=N-R2
O
COOR,
wherein
W denotes CH or N
V denotes CH or N-0
R, denotes hydrogen or an ester moiety, and
R2 denotes a group of formula
- N (R,Rs) IIb
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96l02023
16
wherein
R4 is as defined in claim 1 and
R5 denotes a group of formula
z
iI
- C~ N - R io Iie
Rg
wherein
Z denotes -N-R13, wherein
R13 is as defined in claim 1, and
R9 and Rlo together with the nitrogen atom denote heterocyclyl which is a
piperazinyl.
In another aspect the present invention provides a compound selected from
7-[[(2-amino-4-thiazolyl)-(Z)-(hydroxyimino)acetyl]amino]-3-
[[(aminoiminomethyl)-
hydrazono]methyl]-3-cephem-4-carboxylic acid (compound of Example 2),
7-[[(2-amino-4-thiazolyl)-(Z)-(hydroxyimino)acetyl]-amino]-3-
[[(piperazinoiminomethyl)-
hydrazono]methyl]-3-cephem-4-carboxylic acid (compound of Example 96)
7-[[(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-(fluormethoxyimino)acetyl]amino]-3-
[[(piperazinoiminomethyl)hydrazono]methyl]-3-cephem-4-carboxylic acid
(compound of
Example 139).
A compound of formulae I, IA, IP, Iq ,Is, IVi, IVa and VIa may exist in
equilibrium with
tautomeric forms. The present invention includes a compound of formulae I, IA,
Ip, I4, IS
IVi, IVa and VIa in any tautomeric form in which it may exists.
In another aspect the present invention provides a process for the production
of a
compound of formula I by reaction of a compound of formula
CA 02219656 1997-10-28
WO 96/35692 PGT/EP96/02023
17
s
AcNH
/ Rb II
7:N CH
N~11 Rc
COORd
wherein Ac is as defined in formula I and
a) either
a) Rb denotes hydroxy and Rc and Rd together form a bond, or
Rd denotes hydrogen, a cation, an ester forming group or a silyl group, and
Rb and Rc together denote oxo,
in free form or in form of an acid addition salt
with a group of formula
HZN - R2 IV
wherein R2 is as defined in formula I, or
b) reacting a compound of formula
S
H2N
TON CH-N=R2 VI
COORI
wherein R, and R2 are as defined in formula I, with a compound of formula
Ac-X' VII
wherein Ac is as defined in formula I and X' denotes a leaving group.
If desired reactive groups may be protected with protecting groups, which may
be, or.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
18
which are split off under the reaction conditions, or after termination of the
reaction
described above. A compound of formula I wherein R, denotes hydrogen may be
converted into a compound of formula I, wherein Rl denotes an carboxylic acid
ester
group. A compound of formula I may be isolated from the reaction mixture in
conventional manner.
Process a) may be carried out as follows:
A compound of formula III in a solvent which is inert under the reaction
conditions, such
as water, a mixture of water and a lower alcohol and/or dioxane, or a dipolar
aprotic
solvent, for example dimethylformamide or dimethylsulfoxide, optionally mixed
with an
alcohol or water is reacted with a compound of formula IV at a temperature of
about -20
to 50 C. An optimal pH may be adjusted by addition of an inorganic or organic
acid or
base. A compound of formula I thus obtained may be isolated in conventional
manner, for
example by addition of an anti-solvent or by chromatographic techniques.
Process b) may be carried out as follows:
The reaction may be carried out as conventional, e.g. a compound of formula VI
may be
reacted with a compound of formula VII in a solvent, for example dissolved or
suspended
in a mixture of acetone/water, for example at room temperature.
A reactive group may be protected, preferably by silyl protecting group
technology.
Suitable solvents include solvents which are inert under the reaction
conditions, such as
chlorinated hydrocarbons, nitriles, such as acetonitrile, ethers, such as
tetrahydrofuran or a
mixture of such solvents. Further suitable solvents include dipolar aprotic
solvents, e.g.
N,N-dimethylformamide. Protecting groups may be split off in conventional
manner.
A starting compound of formula II may, for example, be obtained by
a) reaction of a compound of formula
S
R' T /R lllc N CH
0 Rc
C'iOORa
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
19
wherein either
(x) R, denotes a salt of -NH2 with an inorganic or organic acid, R'b denotes
hydroxy, and
R'c and R'd denote together a bond, or
= ~) R. denotes NH2, R'd denotes hydrogen and R'b and R'c together denote oxo,
with a silylation agent and,
a compound obtained in step a) of formula
S
Sil-HN
/ R b Illd
T N CH
O R .
0
COOR d
wherein Sil denotes a silyl group and either
(x) R"b denotes -OSil and R"c and R"d together denote a bond
R"d denotes Sil and R"b and R"c together denote oxo
is acylated either directly in the reaction mixture or after isolation from
the reaction
mixture.
Acylation may be carried out in conventional manner.
A compound of formula IIlc may be obtained
a) for the production of a compound of formula
S
H2N / R-b II le
N / C\
O R
0
COOR d
which is an the form of a salt of an inorganic or organic acid and wherein
R"'b denotes hydroxy and R"', and R"'a together denote a bond,
reacting a salt of an inorganic or organic acid of a compound of formula
CA 02219656 1997-10-28
WO 96/35692 PCTIEP96/02023
S
H2N
T /R1a V
N / CH= CIN,
O
R1s
COOH
wherein
RM and R15 are the same or different and each denote hydrogen or an organic
residue
in an organic solvent optionally in the presence of water with ozone
b) for the production of a compound of formula
S
H2N
CHO Ili9
T?N---
COOH
treating a compound of fonmula IIIe wherein R"'b, R"'r and R"'d ar as defined
above,
with a base.
Compounds of formulae IV are partially new and may be obtained analogously to
conventional methods, or, as described in the examples.
In another aspect the present invention provides a compound of formula
H2N - Ri; IVi
wherein
Ri, denotes a group of formula
- N (R4,RS,) IIbi
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
21
wherein
R4i is the same as R4 in formula I and denotes preferably hydrogen or alkyl
and
R5i denotes a group of formula
Zi
C IIei
N-Rioi
R9 i
wherein
Zi denotes N-R130 wherein
R13i is the same as R13 in formula I and denotes preferably hydrogen or
alkyl, and
Rq; and R,g together with the nitrogen atom denote heterocyclyl which is a
piperazinyl; or
R,,; is the same as R4 in formula I and denotes preferably hydrogen, and
R5i denotes a group of formula
C /SR
--z* NRai
Ildi
wherein
R8i denotes alkyl, preferably at least C2 alkyl; or cycloalkyl, preferably
cyclopropyl, and
R7i denotes alkyl, preferably methyl; or
R4, is the same as R, in formula I and denotes preferably hydrogen or alkyl
and
Rs, denotes a group bf formula
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
22
zi
11
- C" N- R IIei
io,
R9 i
wherein
Z; denotes N-R13i, wherein
R13i denotes hydrogen, alkyl or cycloalkyl, preferably hydrogen or alkyl
R,.,; denotes hydrogen and
R,(); denotes CH2CF3, C(CH3)3, OH or an alkyl group having at least 2 carbon
atoms
which is substituted by dialkyl amine or trialkyl ammonium, hydroxy; or
R4; is the same as R4 in formula I and denotes preferably hydrogen
R5i denotes a group of formula
Zi
11
- C aIei
N- Rioi
R9i
wherein
Zi denotes N-R11, wherein
R13i denotes alkyl or cycloalkyl, preferably alkyl, and
R9i and R,g together with the nitrogen atom denote heterocyclyl which is
morpholyl
or pyrrolidinyl; or
R4t is the same as R4 in formula I and denotes preferably hydrogen
R5, denotes a group of formula
Z,
I I
- C Ilei
N - RIoI
R91
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
23
wherein
Zi denotes N-R13i, wherein
R13i denotes hydrogen, alkyl or cycloalkyl, preferably hydrogen, and
R9i denotes hydrogen, and
R,a denotes cycloalkyl, preferably cyclopropyl; or
R4i is the same as R4 in formula I and denotes preferably hydrogen
R5i denotes a group of formula
zi
11
- C ,' IIei
N- Rioi
R9i
wherein
Zi denotes N-R13;, wherein
R13i is the same as R13 in formula I and denotes preferably hydrogen,
Rg; denotes hydrogen or alkyl, preferably hydrogen, and
R,g denotes a group
-N=CH - Phe
wherein Phe denotes phenyl, preferably a dihydroxy phenyl, or
R,; is the same as R4 in formula I and denotes preferably hydrogen
R5i denotes a group of formula
Z,
I I
- C IIfi
R>>I
wherein
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
24
Zi denotes N-R13i, wherein
R13i denotes hydrogen, alkyl or cycloalkyl, preferably hydrogen,
Rll; denotes a dihydroxyphenyl or substituted pyrrolidyl by alkyl; or
Z; denotes oxygen and
R,,; denotes the group of formula
---N
N H)IIII,N-CH3
s
In another aspect the present invention provides a compound of formula
R
Ily
H2N-N-C-RX IVa
I
RZ
wherein
R. is a group of formula
+
NH - C(CH3)3 or - NH-CH2CF3 or - NH - (CH2)2 - N (CH3)3
or
CH3
N NH Qr. . - N N - C:H3 or N N CH or 3
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
OH
H
i
N--Q or -NH-N=CH OH or
OH
N or
~
k
i
CH3 OH
Ry is NH and
RZ is hydrogen; or
R. is a group of formula
- N N- CHO or - N NH or - NH - (CH2)2 - N (CH3)3
Ry is NH and
R= is CH3; or
R. is - SCH3
Ry is a group of formula
= N or = N - C4H9
and
R. is hydrogen, or
R} is a group of formula
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
26
- '% or - \NH or - NHOH
or -NH-CHZ-CH2-OH
Ry is N- CH3 and
RZ is hydrogen; or
R,, is the group
- N
RY is N- C2HS and
RZ is hydrogen; or
R. is the group
N
/ N CH3
NH
RY is oxygen and
R. is hydrogen.
Compounds of formulae VI are partially new and may be obtained analogously to
conventional methods, or, as described in the examples.
In another aspect the present invention provides a compound of formula
CA 02219656 2006-02-01
27
s
H2N
TN CH-= N- RXx VIa
0
COORI
wherein
R1 is as defined in formula I and
RX,, denotes the group
R
y
II
-N - C - Rx
f
RZ
wherein
R, Ry and RZ as defined above.
In this specification unless otherwise indicated terms such as "compound of
formula I,
IA,1S, IP, Iq, IVi, IVa and VIa" embrace the compound in any form, for example
in salt
form and free base form. The present invention thus includes a compound in
free or base
form or, where such forms exist, in salt form, for example in form of an acid
addition salt,
inner salt, quatemary salt and/or in solvate, for example hydrate form
thereof. A salt may
be a pharmaceutically acceptable salt of a compound of formulae I, IA, IS, Ip,
Iy such as a
metal salt or an amine salt. Metal salts include for example sodium,
potassium, calcium,
barium, zinc, aluminum salts, preferably sodium or potassium salts. Amine
salts include
for example trialkylamine, procaine, dibenzylamine and benzylamine salts. A
free form
of a compound of formulae I, IA, I, Ip, Iy, IVi, IVa and VIa may be converted
into a salt
form and vice versa.
In a further aspect the present invention provides a compound of formulae I,
IA, IS6 Ip, Iq,
IVi, IVa and VIa in free form; or in salt form, for example in acid addition
salt form or in
metal salt form; and a compound of formulae I, IA, I, IP, Iq, IVi, IVa and VIa
in solvate
form.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
28
A compound of formula I may also be obtained analogously to other processes
conventional in the (3-lactam chemistry.
The compounds of formula I, hereinafter designated as "active compound(s) of
the
invention" exhibits pharmacological activity and are therefore useful as
pharmaceuticals. In
particular, the active compounds of the invention show antimicrobial, e.g.
antibacterial,
activity against gram negative and gram positive bacteria such as Pseudomonas,
e.g.
Pseudomonas aeruginosa, Pseudomonas fluorescens; Enterobacter, e.g.
Enterobacter
cloacae; Enterococcus, e.g. Enterococcus faecalis; Moraxella, e.g. Moraxella
catarrhalis;
Haemophilus, e.g. Haemophilus influenza; Klebsiella, e.g. Klebsiella
edwardsii, Klebsiella
pneumoniae; Streptococcus, e.g. Streptococcus pneumoniae, Streptococcus
durans,
Streptococcus faecium, Streptococcus pyogenes; Staphylococcus, e.g.
Staphylococcus
aureus, Staphylococcus pyogenes; Escherichia, e.g. Escherichia coli; and
Proteus, e.g.
Proteus mirabilis in vitro in the Agar Dilution Test according to National
Commitee for
Clinical Laboratory Standards (NCCLS) 1993, Document M7-A3Vo1.13, No. 25:
"Methods
for dilution Antimicrobial Susceptibility Tests for Bacteria that Grow
Aerobically - Third
Edition, Approved Standard" and in vivo in the septikaemic mouse model. The
active
compounds of the invention show activity in the mouse when administerd at
dosages from
about 0.05 to 50 mg/kg body weight (ED50 values).The active compounds show an
MHK( g/ml) in the Agar Dilution Test from about 0.005 to ca. 50. The active
compounds
of the invention show an surprising overall activity spectrum.
It has, for example, been determined that the MHK ( g/ml) ot the compound of
Example
139 against, for example Enterococcus faecalis strains ATTC 29212 or ATCC
51299, is
of ca. 0.08 to 0.25; ainst Staphylococcus aureus strains ATCC 29213 or ATCC
9144 is of
ca. 0.2 to 0.4 and against Pseudomonas aeruginosa strain 27853 is ca. 0.8.
The active compounds of the invention are, therefore, useful for the treatment
of
microbial, e.g. bacterial diseases.
In another aspect the present invention provides a compound of claim I for use
as a
pharmaceutical, preferably as an antimicrobial agent. such as an antibiotic.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
29
In a further aspect the present invention provides a compound of claim 1 for
use in the
preparation of a medicament for the treatment of microbial diseases, for
example of
diseaeses caused by bacterias selected from Pseudomonas, Enterobacter,
Enterococcus,
Moraxella, Haemophilus, Klebsiella, Streptococcus, Staphylococcus,
Escherichia, and
Proteus.
In a further aspect the present invention provides a method of treatment of
microbial
diseases which comprises administering to a subject in need of such treatment
an effective
amount of a compound of formula I.
For this indication, the appropriate dosage will, of course, vary depending
upon, for
example, the compound of formula I employed, the host, the mode of
administration and
the nature and severity of the conditions being treated. However, in general,
for
satisfactory results in larger mammals, for example humans, an indicated daily
dosage is
in the range from about 0.05 to 5 g, for example 0.1 to about 2.5 g, of an
active
compound of the invention conveniently administered, for example, in divided
doses up to
four times a day.
An active compound of the invention may be administered by any conventional
route, for
example orally, e.g. in form of tablets or capsules, or parenterally in the
form of
injectable solutions or suspensions, e.g. in analogous manner to cefotaxime.
The compound 7-[[(5-Amino-1,2,4-thiadiazol-3-yl)-(Z)-
(fluormethoxyimino)acetyl]amino]-
3-[[(piperazinoiminomethyl)hydrazono]methyl]-3-cephem-4-carboxylic acid
(compound of
Example 139) is the preferred compound of the invention for use as an
antimicrobial
agent.
It has, for example been determined that the MHK ( g/ml) of the compound of
Example
139 (tested in form of the trihydrochloride) against, for example
Streprococcus
pneumoniae, strain ATCC 49619 is ca. 0.01 whereas, for example ceftriaxone
shows an
MHK ( g/ml) of ca. 0.02. It is therefore, indicated that for the treatment of
microbial
diseases, e.g. bacterial diseases the preferred compounds of the invention may
be
administered to larger mammals, for example humans, by similar modes of
administration
at similar dosages than conventionally employed with ceftriaxone.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
The compounds of formula I may be administered in pharmaceutically acceptable
salt
form, e.g. acid addition salt form or base addition salt form or in the
corresponding free
forms, optionally in solvate form. Such salts exhibit the same order of
activity as the free
forms.
The present invention also provides pharmaceutical compositions comprising a
compound
of formula I in pharmaceutically acceptable salt form or free form in
association with at
least one pharmaceutical carrier or diluent.
Such compositions may be manufactured in conventional manner.
Unit dosage form may contain, for example 10 mg to about 1 g, for example 10
mg to
about 700 mg.
In the following Examples the temperatures indicated are in degree Celsius.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
31
Example 1
Dihydrochloride of 7-[(2-Amino-4-thiazolyl)-(Z)-(methoxyimino)acetyl]amino-
-3-[[(aminoimino-methyl)hydrazono]methyl]-3-cephem-4-carboxylic acid (Process
a)
= 1.24 g of the hydrogen carbonate of aminoguanidine are dissolved in 9.15 ml
of
2 N HCl and added under stirring to a solution of 3.2 g of the
trifluoroacetate of
N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]-
thiazin-6-yl)-2-(2-aminothiazol-4yl)-(Z)-2-methoxyimino acetic acid amide in
125 ml of
4% aqueous acetonitrile. After ca. 90 minutes the precipitated dihydrochloride
of
7-[(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino-3-[[(aminoimino-methyl)-
hydrazono]methyl]-3-cephem-4-carboxylic acid is filtered off, washed with
acetonitrile
and dried.
Example 2
Dihydrochloride of 7-[[(2-Amino-4-thiazolyl)-(Z)-(hydroxyimino)acetyl]amino]-
3-[[(amino-iminomethyl)hydrazono]methyl]-3-cephem-4-carboxylic acid (Process
a)
a) 10 g of the hydrochloride of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-
3H,7H-
azeto[2,1-b]furo[3,4-d][ 1,3]-thiazin-6-yl)-2-(2-aminothiazol-4-yl)-(Z)-2-
(acetoxyimino)-acetic acid amide are suspended in 160 ml of acetonitrile and
treated
with 53 ml of water and 11 ml of 8 N HCI. The reaction mixture is stirred for
ca.
14 hours at room temperature. A clear solution is obtained in which the
acetoxyimino group being hydrolyzed to give the hydroxyimino group.
b) 3 g of the hydrogen carbonate of aminoguanidine are dissolved in 11 ml of I
N HCI
and added dropwise to the solution obtained in step a) which is cooled to 0 .
After
ca. 30 minutes the reaction mixture is warmed to room temperature and stirred
for
ca. another 2.5 hours. The dihydrochloride of 7-[j(2-Amino-4-thiazolyl)-(Z)-
(hydroxyimino)acetyl]amino]-3-[[(amino-iminomethyl)hydrazono)methyl]-
-3-cephem-4-carboxylic acid precipitate, is filtered off, washed with a
mixture of
acetonitrile and water, acetonitrile and with ether and dried.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
32
Example 3
Sodium salt of 7-[(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino-3-[(methoxy-
imino)methyl]-3-cephem-4-carboxylic acid (Process b)
0.5 g of 7-amino-3-[(methoxyimino)methyl]-3-cephem-4-carboxylic acid and 0.75
g
of (2-amino-4-thiazolyl)(methoxyimino)acetic acid mercaptobenzthiazolyl ester
are
suspended in a mixture of 2.4 ml of water and 4.8 ml of acetone. Ca. 1.8 ml of
2N
sodium hydroxide solution are added dropwise in such a way that a pH of 8.0 is
kept.
The reaction mixture is stirred at 20 for ca. 1 hour. 2.4 ml of acetone are
added
dropwise. A clear solution is obtained within 3 hours. 120 ml of acetone are
slowly
added. A suspension is obtained which is cooled to 0 . After ca. 5 hours the
precipitate
formed is filtered off and redissolved in 4 ml of water. The clear solution is
treated with
0.2 g of active charcoal and stirred for ca. 15 minutes. Active charcoal is
filtered off
and 100 ml of acetone are added within ca. 1 hour at 0 . The sodium salt of 7-
[(2-
amino-4-thiazolyl)(methoxyimino)acetyl]amino-3-[(methoxy-imino)methyl]-3-
cephem-4-
carboxylic acid is obtained in form of colourless crystals, which are filtered
off, washed
with ca. 5 ml of acetone and dried.
The compounds of the following TABLE 1 of formula IA (V is =N-O- in all of the
Examples; and W is CH in Examples 4 to 68 and 70 to 138; and W is N in
Examples
69 and 139 of TABLE 1) may be obtained in analogous manner to that described
in
Examples I to 3. Salt forms are exemplified. The configuration of R3 in group
- C = N - R3 is syn [(Z)].
CA 02219656 1997-10-28
WO 96135692 PCT/EP96102023
33
Table 1
Ex- R3 R2 R, Salt
ample
4 CH3 OH H -
-CH2COOH ~ NH H 2HC1
-NH-C~
NH2
6 CH3 -OCH2COONa Na -
7 CH3 -NH-CO-NH2 H -
8 CH3 ,NH-CH3 H 2HCI
-NH-C~ NH
9 CH3 -NH-C6H5 H -
COCH3 N H H 2HCI
--NH-C , NH2
11 CH3 /NH-C2H5 H 2HC1
-NH-C~NH
Q
H 2HCI
12 CH3 II H
-NH-C-N
13 CH3 /NH-CH3 H 2HCI
-NH-C~N-CH
3
14 CH3 -NH ---TI-NH H 2HC1
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
34
15 CH3 I i-C2H H 2HC1
-NH-C-N
16 H -NH-C /NH-CH3 H 2HCl
NH
17 H 'iH C
H 2HCI
-NH-C-N
18 H NH-CH3 H 2HCI
-NH-C
N-CH3
19 H -NH N~ H H 2HCI
20 CH3 -NH-CS-NH2 H 2HCI
21 CH3 s _ H HCI
11
-NH-C-NH ~ ~
22 CH3 O _ H HCI
-NH-C-NH ~ ~
23 CH3 s H HCI
11
-NH-C-NH-CH3
24 CH3 ~\ H 2HC1
-N~~N-CH3
CA 02219656 1997-10-28
WO 96135692 PCT/EP96102023
25 CH3 ~NHH 2HCl
-NH-C~ NH
26 CH3 -NH ND H H 2HCI
-'<x
N
~NH~/ H 2HCI
27 CH3 -NH-C~
~NH
28 CH3 S H HCI
-N-C-NH-CH3
CH3
29 CH3 il-CH3/--\ H 2HCl
-NH-C-N%
30 CH3 ,NH-C(CH3)3 H 2HCI
-NH-C~ NH
31 CH3 CH3 H 2HCI
N-CH3
-NH-C~
N-CH3
32 CH3 /NH- CH2 CF3 H 2HCI
-HN-C'zz:~' NH
33 CH3 NH ~\ H 2HC1
-NH-C-N\--/ 0
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
36
34 CH3 0 CH3 H CI-
-NH-C-CH2 N CH3
CH3
35 CH3 NH-< H 2HCl
i
NH
36 CH3 / NH-OH H 2HCI
-NH-C ~ NH
37 CH3 CH3 H 2HCI
,N-CH3
NH
38 CH3 / ~ H 2HC1
,NH-CH2--~
-NH-C~ NH N
39 CH3 ~ H 2HCI
,NH-CHz-~
-NH-C~ NH N
40 CH3 /-~ H 2HCI
/NH-CH2 N
-NH-C~ NH
41 CH3 0 H HCI
-N NH
0
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
37
42 CH3 ~-CH3~N H 3HC1
-NH-C-N'-/ NH
43 CH3 H 2HC1
-NH
N-
44 CH3 NHZ H HC1
-NN'-f S
NH-NH
45 CH3 NH H 2HCI
-NH-C-N- j
46 CH3 H 3HCI
-NH-C-N\--/NH
47 CH3 NH-OH H 2HCI
-NH-C N-CH
3
48 CH3 CKH3 H 2HC1
-N==< Nj
N
CH3
49 CH3 O \ H HCI
-NH-C---N
CA 02219656 1997-10-28
WO 96/35692 PCTIEP96/02023
38
50 CH3 N-CH3 H 2HCI
~I /-\
-NH-C-N N-CHO
51 CH3 O H Cl-
II ~
-NH-C-CH2 {N~
52 CH3 o H HCl
-NH-C--F-- N
HN)rN-CH3
S
53 CH3 N-N H 2HCI
-NH--~ Z CH3
i NNH2 0
54 CH3 ~CH3 H 2HC1
-NH-C~NH
55 CH3 NH-(CH2)2 OH H 2HCI
N-CH
3
56 CH3 s H HCI
-NH<\ ~
N
57 CH3 / NH H 2HCI
-N-CNH
CH3 2
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
39
58 CH3 NH H 2HC1
-N-C~NH- CH3
CH3
59 CH3 NH H 2HCI
11
-NH-C
60 CH3 NH H 2HCl
-NH-C ~ ~
N
61 CH3 o H HCI
-NH-C ~- ~
N
62 CH3 NH CH3 H CI-
-NH-C-N \-/ N CH3 2HCI
63 CH3 H Ci-
II-cH' cH 3 2HCI
-NH-C- NH-(CH2)2 N CH3
CH3
64 CH3 -NH ~N H HCl
N
CF3
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
65 CH3 O H HCI
-NH ~ NH
HN-~
O
66 CH3 I NH ~\ H 3HCI
-N-C-N NH
CiH3
67 CH3 H 2HCI
11 NH
/--\
-N-C-N N-CHO
CH3
68 CH3 NH H 2HCI
-NH-C
/ N
H3C
CH3
69 CH2F ~NH H 2HCI
-NH-C~
N-6
70 H ~NH-CZHS H 2HCI
-HN-C~ NH
71 H S H HCI
It
-NH-C-NH- CH3
72 H S H HCI
11
-NH-C-NH2
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96102023
41
73 H i~-C2H H 2HC1
-NH-C-N
74 H lolNHH 2HCl
NH
75 H NH H 2HCI
-NH-<
N
76 H H 2HCI
,NH~~
-NH-C NH
77 H OCH3 H HCI
78 H ~NH-C(CH3)3 H 2HCl
-NH-CNH
79 H CH3 H 2HC1
, N-CH3
-NH-C~
~ N- CH3
80 H ~NH-CHZ CF3 H 2HCI
-HN-C NH
81 H NH ~\ H 2HCI
-NH-C-N 0
82 H I-INH ~1 H 2HCI
-NH-C~ NH~!
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
42
83 H NH-OH H 2HCI
NH
84 H CH3 H 2HCl N-CH3
-NH-CNH
85 H ~ ~ H 2HCI
NH-CH2-(' _J
-NH-C4NH N-
86 H ~~ H 2HCl
,NH-CH-'~
-NH-C NH N
87 H ~-~ H 2HCI
-CH2 N
-NH-C~ NH NH
88 H 0 H HCI
-N N H
L---~
O
89 H S _ H HCI
-NH-C-NH ~ ~
90 H v ~ H 2HCl
-NH
N-
9l H NH2 H HCI
-NNS
NH-NH
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
43
92 CH3 -NH-C'NH-CH3 H 2HC1
-C-COOH NH
CH3
93 H NH-OH H 2HC1
-NH-C~ N-CH3
94 H -NH ,NH-(CH2)2 OH H 2HCl
-C
N-CH3
95 H N-N H 2HC1
-NH~~ CH3
N
NH2 0
96 H NH H 3HC1
-NH-C-N NH
97 H CH3 H 2HC1
N
-N=< NJ
CH3
98 H NH H 2HCI
-N
I -C,_NHZ
CH3
99 H l'- CH, H 2HCI
-NH-C.z* NH
100 H -NH-CH=NH H 2HCI
CA 02219656 1997-10-28
WO 96/35692 PCTJEP96/02023
44
101 CH3 -NH-CH=NH H 2HC1
102 CH3 -NH-C~NH2 H 2HC1
-C-COOH 'Z*NH
1
CH3
103 CH3 oeNH-OH H 2HCI
-C-COOH -NH-C~ NH
CH3
104 CH3 H 2HC1
-C-COOH ,NH-CHZ
I -NH-C--~*NH N-
CHs
105 ~H3 ~NH~ H 2HCI
-C-COOH -NH-C~
I NH
CH3
106 CH3 ~N-CH3 H 2HCI
-C-COOH -NH-C
I \
CH3 NH-CH3
H 2HCI
107 ~H, 11 N H C
- i -COOH -NH-C-N
CH3
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
108 CH3 S-CH3 H 2HC1
--NH-C
NH
109 CH2COOH /NH~I H 2HC1
-NH-C~NH~I
110 CH2COOH -NH-CN-CH3 H 2HC1
NH-CH3
111 CH2COOH ) H H 2HCl
-NH-C-NQ
112 H I NH H 2HC1
-NH-C ~ -~
N
113 H i~H H 2HCI
-NH-C
114 H I NH H 2HC1
-NH-C-N~
115 H I NH -~ H 2HC1
-NH-C-N N-CHO
116 H II N -cH3 CH H 3HCI
-NH-C-NH- (CHz)z N,
CH3
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
46
117 H Ii-CH3 CH3 H CI-
-NH-C-NH- (CHZ)2 -N CH3 2HCI
CH3
118 H IiH - H 2HC1
,
-NH-C ~ OH
OH
119 H NH H H 2HCl
-NH-C
H3C
CH3
120 H il H 2HCI
-NH-C ~-\N
121 H I NH /__~ /~~ H CI-
-NH-C-N /Nt 2HCI
'~/ '-CH3
122 H iI H-~ H 3HCI
-NH-C-N ~N-CH3
123 H -N-CNH H 2HCI
I "NH- CH3
CH3
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
47
124 H I NH H 2HC1
.CH3
-NH-C-NH- (CH2)2 -N~CF6
125 H NH H 3HC1
11 ~CH3
-N-C-NH- (CHZ)2-N~
CH, CH3
126 H ~H - H 3HCI
-NH-C-NH-N=CH ~ ~ OH
OH
127 H H H 3HCI
-NH-C-NH- NH2
128 H iIH ~~ H 3HCI
-N-C-N NH
CH3
129 CH3 i H HCI
-NH-C-NH-Q'
130 CH3 I H HCI
-NH-C-NH- (CH2)3-CH3
131 CH3 -CH, H 2HC1
-NH-C=N~
132 CH3 s-CH, H 2HCI
-NH-C=N-(CH2)3-CH3
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
48
133 CH3 S H -
-NH-C-NH-(CHZ)Z S03H
134 H S H HC1
-NH-C-NH
135 H S H HCI
-NH-C-NH- (CHZ)3-CH3
136 CH3 Ii H 2HCI
-NH-C- N \~J -CH3
137 CH3 S H HCI
-N-C-NHZ
CH3
138 CH3 -NH-COC(CH3)3 H HCI
139 CH2F I H .\ H 3HCI
-NH-C-NNH
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
49
Example 140
Dihydrochloride of 7-[2-(2-aminothiazol-4-yl)-(Z)-2-pentenoylamino]-
3-[[(aminoimino-methyl)hydrazono]methyl]-3-cephem-4-carboxylic acid
1 g of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-
d] [ 1,3 ]-thiazin-6-yl)-2-[2-(tert.-butoxycarbonylamino)thiazol-4-y 1]-(Z)-2-
pentenoic
acid amide is dissolved in a mixture of 30 ml of methanol and 30 ml of
acetonitrile
and 0.3 g of the hydrogencarbonate of aminoguanidine are added. A pH of 2.0 is
adjusted by addition of methanolic HCI. Stirring is continued at room
temperature.
Within ca. 30 minutes a light coloured precipitate forms, which is filtered
off after
ca. 3 hours, washed with acetonitrile and ether and dried. The dihydrochloride
of
7-[2-(2-aminothiazol-4-yl)-(Z)-2-pentenoylamino]-3-{ [(aminoimino-
methyl)hydrazono]-methyl }-3-cephem-4-carboxylic acid is obtained in the form
of a
light yellow powder.
Example 141
Trifluoroacetate of 7-[(2-Amino-4-thiazolyl)-(Z)-(methoxyimino)acetyl]amino-
-3-(hydrazonomethyl)-3-cephem-4-carboxylic acid
A suspension of 3 g of 7-[(2-amino-4-thiazolyl)-(Z)-(methoxyimino)acetyl]amino-
3-
[[2-(1,1-dimethylethoxy)-2-oxoethoxy]hydrazonomethyl]-3-cephem-4-carboxylic
acid
in 75 ml of inethylenchloride is treated at ca. 0 with 0,6 ml of anisol. 10
ml of
trifluoro acetic acid are added dropwise under stirring. The solution obtained
is
stirred for ca. further 3 hours at 0 . The reaction mixture is poured into 600
ml of
ether. The trifluoroacetate of 7-[(2-Amino-4-thiazolyl)-(Z)-
(methoxyimino)acetyl]-
amino-3-(hydrazono)-3-cephem-4-carboxylic acid precipitates, is filtered off
and
dried.
Example 142
Hydrobromide of 7-[(2-amino-4-thiazolyl)-(Z)-(methoxyimino)acetyl]amino-3-[(4-
methylthiazol-2-yl)hydrazonomethyl]-3-cephem-4-carboxylic acid
I g of 7-[(2-amino-4-tl'iiazolyl)-(Z)-(methoxyimino)acetyl]amino-3-
[(aminothioxome-
thyl)-hydra2onomethyl]-3-cephem-4-carboxylic acid is suspended in 30 ml of
acetonitrile and stirred after addition of 2.5 ml of N.O-
bistrimethylsilylacetamide for
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
ca. 20 minutes. The clear solution obtained is treated with 0.6 g of
bromoacetone and
stirred overnight. 1 ml of water are added. The precipitate is filtered off
and dried.
The hydrobromide of 7-[(2-amino-4-thiazolyl)-(Z)-(methoxyimino)acetyl]amino-3-
[(4-
methylthiazol-2-yl)hydrazonomethyl]-3-cephem-4-carboxylic acid is obtained as
a
yellow solid.
Example 143
Hydrobromide of 7-[(2-amino-4-thiazolyl)-(Z)-(methoxyimino)acetyl]amino-3-[(4-
methylthiazol-2-yl)methylhydrazonomethyl]-3-cephem-4-carboxylic acid
1 g of 7-[(2-amino-4-thiazolyl)-(Z)-(methoxyimino)acetyl]amino-3-
[(aminothioxome-
thyl)- methylhydrazonomethyl]-3-cephem-4-carboxylic acid is reacted in
analogous
manner as described in Example 142 with N,O-bistrimethylsilylacetamide and
with
bromoacetone. The hydrobromide of 7-[(2-amino-4-thiazolyl)-(Z)-(methoxyimino)-
acetyl]amino-3-[(4-methylthiazol-2-yl)methylhydrazonomethyl]-3-cephem-4-
carboxylic acid is obtained as a yellow solid.
Example 144
Dihydrate of 6R-trans (Z)-7-[(2-Amino-4-thiazolyl)(methoxyimino)-acetyl]ami-
no-3[[(imino(methylamino)methyl)hydrazono]methyl]-3-cephem-4-carboxylic acid
1.1 g of the dihydrochloride obtained according to Example 8 are dissolved in
25 ml
of water, treated with 0.5 g of active charcoal and stirred for ca. 5 minutes.
The
almost colourless filtrate is poured into 5 ml of water under stirring. A pH
of about 7
is kept by addition of 2.5% aqueous ammonia. The precipitate obtained is
filtered
off and dried. The dihydrate of 6R-trans (Z)-7-[(2-Amino-4-thiazolyl)-
(methoxy imino)-acetyl]amino-3 [[(imino(methylamino)methyl)hydrazono]-
methyl]-3-cephem-4-carboxylic acid is obtained as a yellowish powder.
Example 145
6R-trans (Z)-7-[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino-3-[[(imi-
no(methylamino)methyl)hydrazono]methyl]-3-cephem-4-carboxylic acid 1-
(isopropoxycarbonyloxy)ethyl ester
5.5 g of the dihydrate obtained according to Example 144 are dissolved in 55
ml of
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
51
dimethylacetamide under addition of 1.43 ml tetramethylguanidine. This
solution is
cooled to 0 , treated with a solution of 4.4 g of 1-iodoethyl-
isopropylcarbonate in 30
ml of toluene and stirred for ca. 90 minutes at 0 . The reaction mixture is
poured
into 1 liter of diethylether. The precipitate obtained is filtered off and
stirred twice
each with 500 ml of acetonitrile. The acetonitrile phases are combined,
filtered and
evaporated to a volume of ca. 10 ml. The oily residue is treated with 400 ml
of
water. A precipitate forms which is filtered off and dried. The precipitate is
stirred
with 700 ml of ethyl acetate. After evaporation of the ethyl acetate yellow
coloured
6R-trans (Z)-7-[(2-amino-4-thiazolyl)-(methoxyimino)acetyl]amino-3-[[(imi-
no(methylamino)methyl)hydrazono]methyl]--3-cephem-4-carboxylic acid 1-
(isopropoxycarbonyloxy)ethyl ester is obtained in the form of a diastereomeric
mixture in the ratio of ca. 1:1.
Example 146
6R-trans (Z)-'7-[((Acetoxyimino)-2-amino-4-thiazolyl)acetyl]amino-3-
[[(aminoimi-
nomethyl)hydrazono]methyl]-3-cephem-4-carboxylic acid 1-(isopropoxycarbo-
nyloxy) ethyl ester
0,72 g of the hydrogen carbonate of aminoguanidine are dissolved in 5.2 ml of
2 N
HC1. This solution is added to a solution of 2 g of 6R-trans (Z)-7-
[((acetoxyimino)-
2-amino-4-thiazolyl)acetyI]amino-3-formyl-3-cephem-4-carboxylic acid 1-
(isoprop-
oxycarbonyloxy)ethyl ester in 14 ml of acetonitrile containing 1.3 ml of
water. The
reaction mixture is stirred for ca. 45 minutes at room temperature and poured
into
100 ml of acetonitrile. The precipitate formed is filtered off and dissolved
in 100 ml
of water. The pH of the solution obtained is adjusted to 7 by addition of 0.5
N
aqueous sodium hydrogen carbonate. A yellow suspension is obtained which is
extracted twice with a mixture of 200 ml of ethyl acetate and 40 ml of
acetonitrile.
The organic phases are combined, dried over Na.~SO4 and evaporated. 6R-trans
(Z)-
7-j((acet-oxyimino)-2-amino-4-thiazolyl)acetyl]amino-3-[[(aminoiminome-
thyl)hydrazono]-methyl]-3-cephem-4-carboxylic acid 1-(isopropoxycarbonyloxy)
ethyl
ester is obtained in the form of a yellow diastereomeric mixture in the ratio
of ca.
1:1.
CA 02219656 1997-10-28
WO 96/35692 PGT/EP96/02023
52
Example 147
Ditosylate of 6R-trans (Z)-7-[(2-amino-4-thiazolyl)(hydroxyimino)acetyl]amino-
3-
[[(aminoiminomethyl)hydrazono]methyl]-3-cephem-4-carboxylic acid (isoprop-
oxycarbonyloxy)ethyl ester A solution of 0.6 g of a compound obtained
according to Example 146 in a mixture
of 50 ml of acetonitrile and 20 ml of isopropanol is treated with 0.66 g of
the
monohydrate of toluene-4-sulfonic acid and stirred overnight at 25 . The
reaction
mixture is poured into 150 ml of tert.butyl-methylether. The precipitate
obtained is
filtered off, washed with tert.butyl-methylether and dried. The ditosylate of
6R-trans
(Z)-7-[(2-amino-4-thiazolyl)(hydroxyimino)acetyl]amino-3 [[(aminoiminomethyl)-
hydrazono]methyl]-3-cephem-4-carboxylic acid (isopropoxycarbonyloxy)ethyl
ester is
obtained in the form of a light coloured diastereomeric mixture in the ratio
of ca.
1:1.
Example 148
Dihydrochloride of 7-[[(2-amino-4 thiazolyl)-(Z)-[(carboxymethoxy)imino]actyl]-
amino]-3-[[(aminoiminomethyl)hydrazono]methyl]-3-cephem-4-carboxylic acid
(Compound of Example 5)
al hihydrochloride of 7-Amino-3-ff(aminoiminomethyl)hvdrazonolmethyll-3-
cephem-4-carboxylic acid
To 1.0 g of 7-amino-3-formyl-3-cephem-4-carboxylic acid in a mixture of 50 ml
of
acetonitrile and 5 ml of 2N HCl are added dropwise 0.6 g of the hydrogen
carbonate
of aminoguanidine, dissolved in 2.2 ml of 2N HCI. The dihydrochloride of 7-
amino-
3-[[(aminoiminomethyl)hydrazono]methylJ-3-cephem-4-carboxylic acid
precipitates, is
filtered off, washed with acetonitrile and dried.
121 lysirochloride of 7-ff(2-amino-4-thiazolvl)-(Z)-ff2-(1 1-dimethvlethoxv)-2-
oxoetoxvliminolacetvllaminol-3-f f (aminoiminomethvl )h,ydrazonolmethyll-3-
cephem-4-carboxylic acid
4 g of the dihydrochloride of 7-amino-3-[[(aminoiminomethyl)hydrazono]methyl)-
3-
cephem-4-carboxylic acid are dissolved in 80 ml of methanol. The solution is
cooled
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
53
to 00 and treated with a solution of 7 g of (2-amino-4-thiazolyl)-(Z)-[2-(1,1-
dimethylethoxy)-2-oxoethoxy]imino]thioacetic acid S-benzothiazol ester in 50
ml of
methylene chloride. The reaction mixture is stirred for about 2.5 hours at 20
. About
a third of the solvent is evaporated off and 120 ml of ether are added to the
residue.
The hydrochloride of 7-[[(2-amino-4-thiazolyl)-(Z)-[[2-(1,1-dimethylethoxy)-2-
oxoetoxy]imino]-acetyl]amino]-3-[[(aminoiminomethyl)hydrazono]methyl]-3-cephem-
4-carboxylic acid precipitates, is filtered off, washed with ether and dried.
gl Dihydr ehloride of 7-ff(2-amino-4 thiazolvl)-(Z)-
1(carboxymethoxy)iminolactvll-
aminol-3-f((aminoiminomethy.l)hydrazonolmethvll-3-cephem-4-carboxvlic acid
3.5 g of the hydrochloride of 7-[[(2-amino-4-thiazolyl)-(Z)-[[2-(1,1-
dimethylethoxy)-
2-oxoetoxy]imino]acetyl] amino]-3-[[(aminoiminomethyl)hydrazono]methyl]-3-
cephem-4-carboxylic acid are dissolved in 20 ml of trifluoroacetic acid at 0 .
The
solution is stirred for ca. 15 minutes at 0 and for ca. 1 hour at 20 . The
reaction
mixture is treated with 40 ml of ether. A precipitate forms, is filtered off,
washed
with ether, dried, dissolved in 15 nil of 2N HCl and stirred for ca. 1 hour at
20 . A
light brownish precipitate of the dihydrochloride of 7-[[(2-amino-4 thiazolyl)-
(Z)-
[(carboxy-methoxy)imino]actyl]-amino]-3-[[(aminoiminomethyl)hydrazono] methyl]-
3-
cephem-4-carboxylic acid is obtained, filtered off and dried.
The compounds of Examples 1 to 146 may be obtained as described in Example 147
using the appropriate starting material.
Compounds used as starting material may be prepared as follows:
Example A)
Trifluoroacetate of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-
b]furo[3,4-d][ 1,3]-thiazin-6-yl)-2-(2-aminothiazol-4-yl)-(Z)-2-methoxyimi
noacetic
acid amide
a) Hydrochloride of 6-amino-] 4 5a 6-tetrahydro-3-h roxy-l 7-dioxo-3H 7H-ace-
f? t-blfurof3 4-dlf 1.31thiazin (hydroxylactone of the hydrochloride of 7-
amino-
3-formyl-3-cerhem-4-carboxvlic acid)
CA 02219656 1997-10-28
WO 96/35692 PGT/EP96/02023
54
13.8 g of the hydrochloride of 7-amino-3-[(Z/E)-prop-l-en-1 yl]-3-cephem-4-
carboxylic acid are dissolved in 200 ml of methanol. The solution is cooled to
-50 and 8 1 of 02 containing ca. 2 percent v/v ozone are introduced per
minute
under stirring at this temperature. After ca. 20 minutes the hydrochloride of
6-amino-
1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-aceto[2,1-b]furo[3,4-d] [
1,3]thiazin
being practically quantitatively formed and ozonolysis is terminated as
determined by
HPLC. 8 1 of N2 are bubbled through the reaction mixture within ca. 2 minutes.
The
slight cloudy solution is poured into 1400 ml of tert.butyl-methyl ether. The
precipitate is filtered off under N21 washed with a little of tert.butyl-
methyl ether and
acetonitrile and dried. The hydrochloride of 6-amino-1,4,5a,6-tetrahydro-3-
hydroxy-
1,7-dioxo-3H,7H-aceto[2,1-b]furo[3,4-d][1,3]thiazin is obtained in the form of
a white
powder (HPLC content of more than 95%).
121 (6R-trans)-7-Amino-3-formvl-8-oxo-5-thia-l-azabicyclof4.2.Oloct-2-en-2-
carboxylic acid (7-amino-3-formvl-3-cephem-4-carbox ic acid)
2.64 g of the hydrochloride of 6-amino-1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-
3H,7H-aceto[2,1-b]furo[3,4-d][1,3]thiazin are dissolved in 50 ml of methanol.
To this
solution a solution of 0.78 g of pyridin in 10 ml of methanol is added
dropwise
under ice cooling and stirring. The precipitate obtained is filtered off
moisture free
under nitrogen, washed with a little methanol and dried. (6R-trans)-7-Amino-3-
formyl-8-oxo-5-thia-l-azabicyclo[4.2.0]oct-2-en-2-carboxylic acid is obtained
in the
form of a light brownish powder.
IR (KBr): 1799 ctr' (B-lactam), 1672 ctri' (CHO), 1606 and 1542 cm''
(carboxylate)
UV-Spectnim: X. in H20 = 302 nm.
pl N-(1 4 5a 6-Tetrahvdro-3-hydroxv-I 7-dioxo-3H 7H-azetof2 1-blfuro(3 4-
dl(1.31thiazin-6-yl)-2-(2-tritylaminothiazol-4-yl)-(Z)-2-methoxyiminoacetic
acid
amide
g of 7-amino-3-formyl-3-cephem-4-carboxylic acid in 200 ml of acetonitrile
methylenchloride (1:1) are treated with 37.4 ml of N,O-
bis(trimetltylsilyl)acetamide at
room temperature within ca. 5 minutes. After ca. 30 minutes the reaction
mixture is
cooled to -10 and 21 g of 2-(2-tritylaminothiazol-4-yl)-(Z)-2-
methoxyiminoacetic
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
acid chloride are added in 3 portions. The temperature raises to -5 . After
ca. 45
minutes the reaction mixture is treated with 4 ml of water. The temperature
raises to
20 . The reaction mixture is stirred for ca. 10 minutes and filtered. 15 g of
active
charcoal are added to this filtrate and stirring is continued for ca. 10
minutes. After
filtration the solvent of the filtrate obtained is evaporated. The evaporation
residue is
treated with tert.butyl-methyl ether. A crystalline, almost colourless
precipitate is
obtained, filtered off and dried. Crystalline N-(1,4,5a,6-Tetrahydro-3-hydroxy-
1,7-
dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][ 1,3]thiazin-6-yl)-2-(2-tritylaminothiazol-
4-yl)-2-
methoxyiminoacetic acid amide is obtained in form of a diastereomeric mixture
in
the ratio of ca. 1:1.
i~ Trifluoroacetate of N-(1.4.5a.6-tetrah ro-3-hydroxv-l.7-dioxo-3H.7H-
azetof2.l-
blfurof3.4-dlf 1.3lthiazin-6-vl)-2-(2-aminothiazol-4-vl)-(Z)-2-
methoxyiminoacetic
acid amide
5 g of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-
d][1,3]-thiazin-6-yl)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid amide
are
introduced into 20 ml of trifluoro acetic acid at 0 . The temperature raises
to 10 .
The reaction mixture is stirred for ca. 30 minutes at 0 and added dropwise
into 200
ml of diethylether. The mixture obtained is stirred for ca. 5 minutes and
filtered. A
crystalline, diastereomeric mixture of the trifluoroacetate of N-(1,4,5a,6-
tetrahydro-3-
hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][ 1,3]thiazin-6-yl)-2-(2-
aminothiazol-
4-yl)-2-methoxyiminoacetic acid amide in the ratio of ca. 1:1 is obtained.
Example B)
Trifluoroacetate of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-
b]furoj3,4-d]j1,3]-thiazin-6 yl)-(Z)-2-(2-aminothiazol-4-yl)-2-
(hydroxyimino)acetic
acid amide
is obtained in form of a light yellow powder analogously as described in
Example A)
c) to d) but using in step c) 2-(2-tritylaminothiazol-4-yl)-(Z)-2-hydroxyimino
acetic
acid chloride instead of 2-(2-tritylaminothiazol-4-yl)-(Z)-2-methoxyimino-
acetic acid
chloride.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96l02023
56
Example C)
Hydrochloride of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-
b]furo[3,4-d] [1,3]-thiazin-6-yl)-2-(5-amino-1,2,4-thiad'aazol-3-yl)-(Z)-2-
(fluoro-
methoxyimino)acetic acid amide
A suspension of 3.73 g of 7-amino-3-formyl-3-cephem-4-carboxylic acid in a
mixture
of 80 ml of methylenchloride and 30 ml of acetonitrile is stirred at 0 with
16 ml of
N,O-bis(trimethylsilylacetamide). Within ca.15 minutes a clear solution is
obtained to
which 3.9 g of (5-amino-1,2,4-thiadiazol-3-yl)-(Z)-2-fluormethoxyiminoacetic
acid
chloride, obtainable for example as described in Example 1 of EP-0 590 681,
are
added. The reaction mixture is stirred for ca. 1 hour at 0 . 500 ml of
acetonitrile
containing 10 g of water are added and the mixture is filtered to remove
insolubles.
The filtrate is evaporated. The residue is treated with 500 ml of
acetonitrile, the
mixture is filtered and the filtrate is evaporated. The solid obtained is
treated with
tert.butyl-methyl ether and dried. The hydrochloride of N-(1,4,5a,6-Tetrahydro-
3-
hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d] [ 1,3]-thiazin-6-yl)-2-(5-
amino-1,2,4-
thiadiazol-3-yl)-(Z)-2-(fluoro-methoxyimino)acetic acid amide is obtained in
the form
of a light brownish powder.
Example D)
Hydrochloride of N-(1,4,5a,6-tetrahydro=3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-
b] furo[3,4-d][1,3]-thiazin-6-yl)-2-(2-aminothiazol-4-yl)-(Z)-2-
(acetoxyimino)acetic
acid amide
40 g of 7-amino-3-formyl-3-cephem-4-carboxylic acid are suspended in 1500 ml
of
aeeianitcile and cooled to 0'. Within ca. 20 retinuies 170 ml of N,O-
bis(trimethylsi-
lyl)acetamide are added under stirring. Within ca.15 minutes at 00 a clear
solution is
obtained, which is cooled to -10 and to which 48 g of (2-aminothiazol-4-yl)-
(Z)-
(acetoxyimino)acetic acid chloride are added in portions in such a way that
the
temperature of the reaction mixture does not exceed - 8 . Stirring is
continued for
ca. 60 minutes at -10 and 168 ml of water are added. Stirring is continued
for ca.
further 20 minutes at 00 and for ca. 2 hours at room temperature. A
crystalline
precipitate forms. is filtered off, washed with ca. 350 ml of acetonitrile and
ca. 100
ml of ether and dned. The hydrochloride of N-(1,4.5a.6-tetra-hydro-3-hydroxy-
l,7-
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
57
-dioxo-3H,7H-azeto[2,1-b]furo-[3,4-d][ 1,3]-thiazin-6-yl)-2-(2-aminothiazol-4-
yl)-(Z)-
-2-(acetoxyimino)acetic acid is obtained in form of a diastereomeric mixture
in the
ratio of ca. 1:1.
Example E)
Hydrochloride of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-
b]furo[3,4-d] [ 1,3]-thiazin-6-yl)-2-(2-aminothiazol-4-yl)-(Z)-2-
(hydroxyimino)acetic
acid amide
g of the hydrochloride of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-
azeto[2,1-b]furo[3,4-d] [ 1,3]thiazin-6-yl)-2-(2-aminothiazol-4-yl)-(Z)-2-
(acetoxyimino)-
acetic acid amide are suspended in 160 ml of acetonitrile and treated with 53
ml of
water and 11 ml of 8 N HCI. The reaction mixture is stirred for ca. 14 hours
at room
temperature. A clear solution is obtained which is diluted with water-free
acetonitrile
to obtain the 3-fold volume. The solvent is evaporated off to obtain a volume
of ca.
10 ml, which is treated with ca. 200 ml of acetonitrile. A precipitate forms
which is
treated with ether, filtered off and dried. The hydrochloride of N-(1,4,5a,6-
tetrahydro-
3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d] [ 1,3]-thiazin-6-yl)-2-(2-
aminothiazol-4-yl)-(Z)-2-(hydroxyimino)acetic acid amide is obtained in
yellowish
coloured form.
Example F)
Trifluoracetate of N-(1,4,5a,6-tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-
b]furo[3,4-d][1,3-]-thiazin-6-yl)-2-(2-aminothiazol-4-yl)-(Z)-2-[(1-carboxy-l-
methylethoxy)imino]acetic acid amide
is obtained in form of a light brownish powder analogously as described in
Example
A) a) to c) but using 2-(2-tritylaminothiazol-4-yl)-(Z)-2-[(1-carboxy-l-
methylethoxy)-
iminoacetic acid chloride instead of 2-(2-tritylaminothiazol-4-yl)-(Z)-2-
methoxyimino-
acetic acid chloride.
Example G)
N-(1,4,5a,6-Tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]-
thiazin-6-yl)-2-[2-(tert.-butoxycarbonylamino)thiazol-4-yl]-(Z)-2-pentenoic
acid
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
58
amide
is obtained in form of a light brownish powder analogously as described in
Example
A) c) to d) but using 2-(2-(tert.butoxycarbonylamino)thiazol-4-yl)-(Z)-2-
pentenoic
acid chloride instead of 2-(2-tritylaminothiazol-4-yl)-(Z)-2-methoxyimino-
acetic acid chloride.
Example H)
Dihydrochloride of 1-(hydrazinoiminomethyl)piperazine
al H roiodide of 4-formvl-l-fimino(methvlthio)methyllnineazine
25.5 g of 4-formyl-l-piperazinecarbothioamide are suspended in 80 ml of
methanol,
treated with 22 g of methyliodide and refluxed. Within ca.10 minutes a clear
solution
is obtained. The mixture is cooled to room temperature. The solvent is
evaporated.
Crystalline hydroiodide of 4-formyl-l-[imino(methylthio)methyl]piperazine is
obtained.
b) Hydrochloride of 4-fonmyl-l-(hvdrazinoiminomethvl)pinerzine
48.1 g of the hydroiodide of 4-formyl-l-[imino(methylthio)methyl]piperazine
are
dissolved in 100 ml of water, run through a column filled with 800 ml of a
strong
basic ion exchanger in chloride form and eluated with 850 ml of water. The
solvent
is evaporated to obtain a volume of ca. 100 ml which is treated with 7.35 g of
hydrazinehydrate. The reaction mixture is stirred for ca. 1 hour at room
temperature
and the solvent is evaporated off. The oily hydrochloride of 4-formyl-l-
(hydrazinoiminomethyl)piperazine crystallizes on drying.
IZI Dihydrochloride of l -(hvdrazinoiminomethvl)piperazine
11 g of the hydrochloride of 4-formyl-l-(hydrazinoiminomethyl)piperazine are
dissolved in 400 ml of methanol and treated with 50 of HCI.... The reaction
mixture is stirred for ca. 14 hours at room temperature. A white precipitate
forms, is
filtered off, washed with methanol and ether, dried and recrystallized with
water/ethanol. The dihydrochloride of 1-(hydrazinoiminomethyl)-piperazine is
obtained in crystalline, colourless form.
CA 02219656 1997-10-28
WO 96/35692 PCTIEP96/02023
59
Analogous in the manner as described in Example H) compounds of formula IV of
TABLE 2 may be obtained:
TABLE 2
Ex. R2 Salt Process
j) ~ N-CZHS HCI H) a) to c)
CN-C
\
NH-NHz
J) ~--~ N-CH3 HCl H) a) to c)
O ~N-C
NH-NHZ
K) NH HCI H) a) to c)
(CH3)3C -NH~**-I
NH-NHZ
L) NH 2HC1 H) a) to c)
CH' - N \-/N-C
NH-NHZ
M) + N-CH3 2HCL H) a) to c)
(CH3)3 - N - (CH2)2- NH - C N,
NH-NHZ
N) N-CH3 HCI H) a) to b)
OHC-N\--/ N-C
NH-NHI
0) N-CH3 3HCl H) c)
HN N-C
~-~ ~ NH-NH,
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
Example P)
1-Amino-3-(2-hydroxyethyl)-4-methylgua nidine
12.7 g of 2-methylamino-2-oxazoline ar dissolved in 50 ml of water, treated
with 3
g of hydrazinehydrate and stirred for ca. 17 hours at room temperature. The
solvent
is evaporated and 1-amino-3-(2-hydroxyethyl)-4-methylguanidine is obtained as
oily
residue crystallizing upon cooling.
Example Q)
Hydrochloride of 1,1-dimethyl-4-(hydrazinoiminomethyl)piperaziniumchloride
gl Hydroiodide of 1 1-dimethyl-4-fimino(methvlthio)methvllnineraziniumiodide
3.2 g of 4-methyl-l-piperazinecarbothioamide are suspended in 100 ml of
methanol. 6.2 g of methyliodide are added and the mixture is refluxed for ca.
2
hours and cooled to 20 ab. The hydroiodide of 1,1-dimethyl-4-[imino-
(methylthio)methyl]piperaziniumiodide precipitates, is filtered off and dried.
b) Hydrochloride of I 1-dimeth l~fhydrazinoiminomethyI)nineraziniumchloride
6.57 g of the hydroiodide of 1,1-dimethyl-4-[imino(methylthio)methyl]pipe-
raziniumiodide are dissolved in 70 ml of water, run through a column filled
with
150 ml of a strong basic ion exchanger in chloride form and eluated with 250
ml
of water. Water is evaporated off the eluate to obtain to a volume of ca. 50
ml,
which is treated with 0.9 ml of hydrazinehydrate and stirred overnight. The
solvent is evaporated off and the residue obtained is treated with n-hexane.
The
hydrochloride of 1,1-dimethyl-4-(hydrazinoiminomethyl)piperaziniumchloride is
obtained.
Example R)
Trihydrochloride of 1-[hydrazino(methylimino)methyl]piperazine
al Hydrochloride of S-methyl-2-methvlisothiosemicarbazide A solution of 239.8
g of the hydroiodide of S-Methyl-2-methylisothiosemi-
carbazide in 100 ml of water is run through a column filled with 1500 ml of a
CA 02219656 1997-10-28
WO 96/35692 PCTIEP96/02023
61
strong basic ion exchanger in chloride form and eluated with water. The eluate
is
lyophilized and the lyophilization residue is treated with ether. The
precipitate is
filtered off and dried. The hydrochloride of S-methyl-2-methylisothiosemi-
carbazide is obtained as a white solid.
Melting point: 116
HXdrochloride of 4-form 1-v 1-fhvdrazino(methvlimino)methvllpinerazine
A mixture of 20 g of freshly distilled formylpiperazine and 27.3 g of the
hydrochloride of S-methyl-2-methylisothiosemicarbazide in 250 ml of ethanol is
refluxed overnight and the solvent is evaporated. The oily residue is
dissolved in
70 ml of hot isopropanol and the solution is slowly cooled to 20 . A
precipitate
forms and the mixture is allowed to stand for ca. 2 hours at 4 . The
hydrochloride
of 4-formyl-l-[hydrazino(methylimino)methyl]piperazine is filtered off and
recrystallized from isopropanol.
Cl T~ri lvdrochloride of 1-fhydrazino(methylimino)methvllpiperazine
g of the hydrochloride of 1-fonmyl-4-[hydrazino(methylimino)methyl]piperazine
are dissolved in 250 ml of methanol. 50 ml of HCIcc are added, the mixture
obtained is stirred overnight and the solvent is evaporated. A solid residue
is
obtained which is dried over solid KOH. The trihydrochloride of 1-jhydrazino-
(methylimino)methyl]piperazine is obtained in form of a white product.
In analogous manner as described in Example R) but reacting the hydrochloride
of
S-methyl-isothiosemicarbazide or the hydrochloride of S-methyl-2-
methylisothiosemi-
carbazide or the hydrochloride of S-methyl-4-methylisothiosemicarbazide with a
corresponding amine compounds of formula IV of TABLE 3 may be obtained.
TABLE 3
Ex. R, Salt
CA 02219656 1997-10-28
WO 96135692 PCT/EP96/02023
62
S) NH HCI
CF3CHz NH-C~
NH-NH2
T) NH HCI
>-NH-C '
NH-NH2
U) HONH-C/ N-CH3 HCI
NH-NHZ
V) CH3 NH 2HCI
jN-(CH2)2-NH-C
CH3 N-NH2
CH3
Example W)
Hydrochloride of 1-Amino-3-(3,4-dihydroxybenzylidenamino)guanidine
1 g of the hydrochloride of diaminoguanidine are dissolved in 10 ml of 4 N HCI
and
diluted with 20 ml of methanol. This solution is treated quickly with a
solution of
I g of 3,4-dihydroxybenzaldehyde in 40 ml of methanol. The reaction mixture is
stirred for some minutes at room temperature and the solvent is evaporated
off. The
residue is suspended in 50 ml of acetonitrile. The precipitate formed is
filtered off
and dried. The hydrochloride of 1-amino-3-(3,4-dihydroxybenzylidenamino)-
guanidine is obtained.
Example X)
Hydroiodide of S-Methyl-4-cyclopropylthiosemicarbazide
295 mg of 4-cyclopropyjthiosemicarbazide are dissolved in 5 ml of dry methanol
and
treated with 154 ml of methyliodide. The mixture is stirred at 40 under
nitrogen for
ca. 5 hours, cooled and treated with diethylether. A colourless precipitate of
the
CA 02219656 1997-10-28
WO 96/35692 PGT/EP96/02023
63
hydroiodide of S-Methyl-4-cyclopropylthiosemicarbazide is formed, filtered
off,
washed with diethylether and dried.
Example Y)
Hydroiodide of S-methyl-4-n-butylthiosemicarbazide
147 mg of 4-n-butylthiosemicarbazide in 2,5 ml of dry methanol are treated
with 149
mg of methyliodide. The mixture is stirred under nitrogen for ca. 5 hours,
cooled and
treated with diethylether. A colourless precipitate of the hydroiodide of S-
methyl-4-n-
butylthiosemicarbazide is formed, filtered off, washed with diethylether and
dried.
Example Z)
1-Methyl-5-mercapto-1,2,4-triazol-3-carboxylic acid hydrazide
0.48 g of 1-methyl-5-mercapto-1,2,4-triazol-3-carboxylic acid methyl ester are
dissolved in 10 ml of methanol, treated with 450 l of hydrazinehydrate and
stirred
for ca. 2 hours at 20 . A precipitate of 1-methyl-5-mercapto-1,2,4-triazol-3-
carboxylic acid hydrazide is formed, filtered off, washed with methanol and
dried.
IR (KBr): 1669 cm', 1608 cm', 1517 ciri'
13C-NMR (300 MHz, DMSO-d6): 35.4 (NCH3); 143.3, 154.3 and 166.7
Example AA)
Hydroiodide of 1,5-dimethyl-2-(hydrazinoiminomethyl)pyrrol
al I.5-Dimethylovrrol-2-carbothioamide
g of 2-cyano-1,5-dimethylpyrrol are dissolved in 40 ml of ethanol and treated
with 10 ml of triethylamine. 50 ml of an ethanolic HZS solution (3.8 g/100 ml)
are
added and the mixture is heated for ca. 15 hours in an autoclave at 70 . The
reaction mixture is cooled and the solvent is evaporated off to obtain ca. a
quarter
of its volume. 1,5-dimethylpyrrol-2-carbothioamide crystallizes upon cooling
at 00
in the form of a light yellow precipitate.
Hvdroiodide of 1.5-dimethvl-2-fimino(methvlthio)methv]]Qyrrol
1 g of 1.5-dimethylpyrrol-2-carbothioamide are dissolved in 20 ml of methanol
CA 02219656 1997-10-28
WO 96/35692 PGT/EP96/02023
64
and treated with 1.7 g of methyliodide. The reaction mixture is stirred for
about 5
hours at room temperature. The solvent is evaporated until crystallization
starts.
The residue is cooled to ca. 0 . The crystalline hydroiodide of 1,5-dimethyl-2-
[imino(methylthio)methyl]pyrrol is filtered off, washed with methanol and
dried.
c) Hydroiodide of 1.5-dimethvl-2-(hvdrazinoiminomethvl)pvtrol
1.3 g of the hydroiodide of 1,5-dimethyl-2-[imino(methylthio)methyl]pyrrol are
dissolved in 20 ml of methanol. 0.28 g of hydrazinehydrate are added. The
reaction
mixture is stirred for ca. 3 hours, the solvent is evaporated off and the
residue is
recrystallized from acetonitrile/ether. The hydroiodide of 1,5-dimethyl-2-
(hydrazinoiminomethyl)pyrrol is obtained.
Example AB)
Hydroiodide of 3,4-dihydroxy-2-(hydrazinoiminomethyl)benzene
is obtained in analogous manner as described in Example AA), but using 3,4-
dihydroxy-thiobenzamide instead of 1,5-dimethylpyrrol-2-carbothioamide.
Example AC)
7-Amino-3-[[(carboxymethoxy)imino]methyl]-3-cephem-4-carboxylic acid
A solution of 1.86 g of the hydrochloride of aminooxyacetic acid in 20 ml of
water
is treated under stitring at 0 with 3.16 g of the hydrochloride of 6-amino-
1,4,5a,6-
tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin. The
mixture is stitred for ca. 8 hours at 0 . 7-Amino-3-
[[(carboxymethoxy)imino]methyl]-
3-cephem-4-carboxylic acid precipitates in form of colourless crystals, which
are
filtered off, washed with 5 ml of cold water and 5 ml of acetone and dried.
Example AD)
7-Amino-3-[(methoxyimino)methyl]-3-cephem-4-carboxylic acid
A solution of 0.5 g of the hydrochloride of 0-methylhydroxylamine in 10 ml of
water is treated under stirring at 0 with 1.38 g of 7-amino-3-formyl-3-cephem-
4-
carboxylic acid and stirred for ca. 8 hours at 0 . 7-Amino-3-
[(methoxyimino)methyl]-
3-cephem-4-carboxylic acid precipitates in form of almost white crystals,
which are
CA 02219656 1997-10-28
WO 96/35692 PC.T/EP96/02023
filtered off, washed with 5 ml of cold water and 5 nil of acetone and dried.
Example AE)
7-Amino-3-[(hydroxyimino)methyl]-3-cephem-4-carboxylic acid
a) A solution of 1.26 g of the hydrochloride of hydroxylamine in 7.5 ml of
water is
treated under stirring at 0 with 4.74 g of the hydrochloride of 6-amino-
1,4,5a,6-
tetrahydro-3-hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin and
stirred for ca. 8 hours at 0 under nitrogen. The pH of the reaction mixture
is
adjusted to 3.5 using solid sodium hydrogen carbonate. 7-Amino-3-[(hydroxy-
imino)methyl]-3-cephem-4-carboxylic acid precipitates in form of colourless
crystals, which are filtered off, washed with ca. 5 ml of cold water and 5 ml
of
acetone and dried.
b) A suspension of 0.79 g of the hydrochloride of 6-amino-1,4,5a,6-tetrahydro-
3-
hydroxy-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin in 10 nil of
dichloromethane is treated under stirring at 4 with 2.67 g of N,O-bis-
(trimethylsilyl)acetamide. A clear solution is obtained within 10 minutes.
0.21 g
of the hydrochloride of hydroxylamine are added. The reaction mixture is
stirred
for ca. 2 hours under nitrogen at 4 and the solvent is evaporated off. The
residue
is treated with 10 ml of isopropylalkohol, precooled to 1 . 7-Amino-3-
[(hydroxy-
imino)methyl]-3-cephem-4-carboxylic acid precipitates in form of almost
colourless crystals which are filtered off, washed with 5 ml of acetone and
dried.
Analoguously as described in Examples AC) to AE) the compounds of Table 4 of
formula VI may be obtained.
Table 4
Bsp: R, R2 Salz
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
66
AF) H NH 2HCI
NHz
AG) H -NH-CO-NH2 2HCI
AI-1) H -NH-CS-NH2 2HCI
Al) H NH HCI
-NH-C
\S-CH3
AJ) H NH 2HCI
-NH-CNl NH-CH3
AK) H -NH-CaHs H2N-NH-C6H5
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
67
1H-NMR-Snectra of the compounds obtained according to the Examples (Ex.)
Ex. Spectrum
1 (300 MHz, CD3OD): 8.43 (s, 1H, CH=N); 6.96 (s, 1H, CH); 5.99 (d,
J=4.9 Hz, 1H, CH); 5.22 (d, J=4.9 Hz, 1 H, CH); 4.04 (s, 3H, OCH3);
3.99 and 3.56 (AB quartet, J=17.8 Hz, 2H, SCH2).
2 (90 MHz, DMSO-db + D20): 3.6 and 4.3 (AB quartet, J=18 Hz, 2H,
SCH2); 5.3 (d, J=5.1 Hz, 1H, B-lactam-H); 5.95 (d, J=5.1 Hz, 1H,
B-lactam-H); 6,95 (s, 1H, thiazolyl-H); 8.35 (s, 1H, CH=N).
3 (300 MHz, CD3OD): 7.97 (s, 1 H, CH=N); 6.84 (s, 1 H, CH); 5.69 (d,
J=4.9 Hz, 1H, CH); 5.13 (d, J=4.9 Hz, 1H, CH); 4.13 and 3.93 (AB
quartet, J=16.8 Hz, 2H, SCH2), 3.81 (s, 3H, OCH3); 3.67 (s, 3H, OCH3).
4 (300 MHz, CD3OD): 8.36 (s, 1H, CH=N); 6.87 (s, 1H, CH); 5.88 (d,
J=4.9 Hz, 1H, CH); 5.29 (d, J=4.9 Hz, 1 H, CH); 4.00 (s, 3H, OCH3);
3.95 and 3.60 (AB quartet, J=17.8 Hz, 2H, SCHZ).
(300 MHz, DMSO-d6): 3.57 and 4.43 (AB quartet, J=18.2 Hz, 2H,
S-CH2); 4.71 (s, 2H, O-CH2); 5.30 (d, J=5.0 Hz, 1H, 0-lactam-H); 5.91
(dd, J=5.0 and 7.9 Hz, 1H, P-lactam-H); 7.02 (s, 1H, CH thiazol); 7.9
(broad 4H, NH); 8.29 (s, 1H, CH=N); 9.88 (d, J=7.9 Hz, 1H, NH); 12.25
(s, 1 H, OH).
6 (300 MHz, CD3OD): 8.10 (s, IH, CH=N); 7.01 (s, 1 H, CH); 5.84 (d,
J=4.9 Hz, 1 H, CH); 5.29 (d, J=4.9 Hz, I H, CH); 3.98 (s, 3H, OCH3);
3.96 and 3.59 (AB quartet, J=16.8 Hz, 2H, SCH2).
7 (300 MHz, CD3OD): 8.26 (s, 1 H, CH=N); 7.04 (s, 1 H, CH); 5.90 (d.
J=5.1 Hz. IH, CH); 5.24 (d, J=5.1 Hz. I H, CH); 4.05 (s, 3H, OCH,);
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
68
4.32 and 3.65 (AB quartet, J=17.8 Hz, 2H, SCH2).
8 (300 MHz, CD3OD): 8.46 (s, 1H, CH=N); 6.99 (s, 1H, CH); 5.95 (d,
J=5.2 Hz, 1H, CH); 5.27 (d, J=5.2 Hz, 1 H, CH); 4.01 (s, 3H, OCH3);
4.37 and 3.63 (AB quartet, J=18.1 Hz, 2H, SCH2); 2.95 (s, 3H, N-CH3).
9 (90 MHz, DMSO-d6): 9.78 (d, J=8.0 Hz, 1H, CONH); 8.26 (s, 1H,
CH=N); 6.91 (s, 1H, CH); 7.32 (dd, J=7.3 Hz, 2H, H.); 7.05 (d, J=7.3
Hz, 2H, Ho); 6.78 (t, J=7.3 Hz, 1H, Hp); 5.76 (dd, J,=4.8 Hz, J2=8.0 Hz,
1H, CH); 5.25 (d, J=4.8 Hz, 1 H, CH); 3.91 (s, 3H, OCH3); 4.16 and 3.76
(AB quartet, J=17.4 Hz, 2H, SCH2).
(90 MHz, DMSO-d6): 2.25 (s, 3H, CH3CO); 3.65 and 4.55 (AB quartet,
J=18 Hz, 2H, SCH2); 5.4 (d, J=5 Hz, 1 H, B-lactam-H); 5.95 (dd, J=5Hz
and 8 Hz, 1 H, B-lactam-H); 7,32 (s, 1 H, thiazolyl-H); 8.4 (s, 1 H, CH=N);
10,2 (d, J=8.0 Hz, 1 H, NH).
11 (90 MHz, DMSO-d6): 1.12 (t, J=7.1 Hz, 3H, CH3); 3.29 (q, 2H, CH2);
3.56 and 4.50 (AB quartet, J=18.1 Hz, 2H, SCH2); 3.93 (s, 3H, N-O-
CH3); 5.30 (d, J=5Hz, 1H, CH); 5.9 (q, J=6Hz, and 8Hz, IH, CH); 6.90
(s, IH, thiazolyl-H); 8.32 (s, 1H, CH=N); 9.86 (d, J=8.OHz, NH).
12 (90 MHz, DMSO-d6): 1.85-2.15 (m, 4H); 3.25-3.7 (m, 5H, -CH2-N-CH2-
and 1H of SCH2); 4.0 (s, 3H, N-O-CH3); 4.5 (part of the AB quartet,
J=18Hz, 1H of SCH2); 5.3 (d, J=5Hz, 1H, CH); 5.9 (q, J=5Hz and 8Hz,
IH, CH); 7.0 (s, 1 H, thiazolyl-H); 8.8 (s, IH, CH=N); 10.1 (d, J=7.9Hz,
NH).
13 (90 MHz, DMSO-d6): 2.9 (broad s, 6H, N-CH3), 3); 3.6 and 4.5 (AB
quartet, J=18Hz, 2H, SCH2); 3.9 (s, 3H, Iv'-O-CH3); 5.3 (d, J=5Hz, IH, CH));
(q, J=5Hz and 8Hz, 1H, CH); 6.95 (s, 1H, thiazolyl-H); 8.75 (s.
I H, CH=N); 9.95 (d. J=8Hz, NH).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
69
14 (90 MHz, DMSO-d6): 3.65 (broad s, 4H, N-CH2-CH2-N); 3.5 and 4.4 (AB
quartet, J=18Hz, 2H, SCHZ); 3.9 (s, 3H, N-O-CH3); 5.3 (d, J=5.OHz, 1H,
CH); 5.85 (q, J=5Hz and 8Hz, 1H, CH); 6.9 (s, 1H, thiazolyl-H); 8.35 (s,
1H, CH=N); 9.9 (d, J=8Hz, NH).
15 (90 MHz, DMSO-d6): 1.16 (t, J=7.lHz, 3H, CH3); 1.8-2 (m, 4H); 3.32 (q,
2H, CH2); 3.45-3.65 (m, 5H, -CHZ-N-CH2- and 1H of SCH2); 3.91 (s, 3H,
N-O-CH3); 4.1 (part of the AB quartet, J=18Hz, 1H of SCHZ); 5.27 (d,
J=5Hz, 1 H, CH); 5.9 (q, J=5Hz and 8Hz, 1 H, CH); 6.86 (s, 1 H, thiazolyl-
H); 8.56 (s, 1H, CH=N); 9.82 (d, J=8Hz, NH).
16 (90 MHz, DMSO-d6): 2.86 (broad s, 3H, N-CH3); 3.5 and 4.5 (AB
quartet, J=18Hz, 2H, SCHZ); 5.3 (d, J=6Hz, 1H, CH); 5.9 (q, J=5Hz and
8Hz, IH, CH); 6.85 (s, 1 H, thiazolyl-H); 8.4 (s, 1 H, CH=N); 9.8 (d,
J=8Hz, NH).
17 (90 MHz, DMSO-d6): 1.85-2.15 (m, 4H); 3.25-3.8 (m, 5H, -CH2-N-CH2-
and 1H of SCH2); 4.5 (part of the AB quartet, J=18Hz, 1H of SCH2); 5.3
(d, J=5Hz, 1 H, CH); 5.85 (q, J=5Hz and 8Hz, 1 H, CH); 6.85 (s, IH,
thiazolyl- H); 8.7 (s, 1H, CH=N); 9.8 (d, J=7.9Hz, NH).
18 (90 MHz, DMSO-d6): 2.86 (broad s, 6H, N-CH3), 3); 3.55 and 4.47 (AB
quartet, J=18.9Hz, 2H, SCH2); 5.31 (d, J=5.1 Hz, 1 H, CH); 5.91 (q ,
J=5.lHz and 7.9Hz, 1 H, CH); 6.8 (s, IH, thiazolyl-H); 8.58 (s, IH,
CH=N); 9.72 (d, J=7.9Hz, NH).
19 (90 MHz, DMSO-d6): 3.7 (broad s, 4H, N-CH2-CH2-N); 3.55 and 4.35
(AB quartet, J=18.lHz, 2H, SCHZ); 5.31 (d, J=5.OHz, 1 H, CH); 5.9 (q,
J=5.1 Hz and 8Hz, 1 H, CH); 6.8 (s, 1 H, thiazolyl-H); 8.38 (s. 1 H, CH=N);
9.73 (d, J=8.OHz, NH).
20 (300 MHz, CD3OD): 8.34 (s, 1H, CH=N); 7.06 (s, 1H. CH); 5.93 (d.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
J=4.9 Hz, 1H, CH); 5.32 (d, J=4.9 Hz, 1 H, CH); 4.09 (s, 3H, OCH3);
4.33 and 3.64 (AB quartet, J=18.2 Hz, 2H, SCH2).
21 (90 MHz, DMSO-d6): 3.65 and 4.7 (AB quartet, J 18 Hz, 2H, SCH2);
3.95 (s, 3 H, O-CH3); 4.2 (part of the AB quartet, J 18 Hz, 1H of
SCH2); 5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz,
B-lactam-H); 7.0 (s, 1 H, thiazolyl-H); 7 to 7.7 (m, 5 H, aromatic H); 8.45
(s, 1 H, CH=N);.9 (d, J = 8 Hz, NH).
22 (90 MHz, DMSO-d6):3.55 and 4.6 (AB quartet, J = 18 Hz, 2H, SCH2);
3.93 (s, 3 H, O-CH3); 4.2 (part of the AB quartet, J = 18 Hz, 1 H of
SCH2); 5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz,
B-lactam-H); 7.0 (s, 1 H, thiazolyl-H); 7 to 7.7 (m, 5 H, aromatic-H); 8.3
(s, 1 H, CH=N); 9.9 (d, J = 8 Hz, NH).
23 (90 MHz, DMSO-d6): 3.05 (d, J = 4 Hz, 3 H, NHCIJ3); 3.55 and 4.5
(AB quartet, J 18 Hz, 2H, SCH2); 3.93 (s, 3 H, O-CH3); 4.2 (part of the
AB quartet, J 18 Hz, 1H of SCHZ); 5.25 (d, J = 5 Hz, I H, B-lactam-
H); 5.8 (dd, J 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, 1 H, thiazolyl-H);
8.25 (s, 1 H, CH=N);8.4 (d, J = 4 Hz, NHCH3);9.85 (d, J = 8 Hz, NH).
24 (90 MHz, DMSO-d6): 2.85 (s, 3H, NCH3); 3.1 to 3.7 (m, 9 H, 8 piperazi-
nyl-H's and 1H of SCHZ); 3.95(s, 3 H, OCH3); 4.1 (part of the AB
quartet, J = 18 Hz, 1H of SCHZ); 3.95 (s, 3 H,O-CH3); 5.3 (d, J = 5 Hz, 1
H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H);6.95 (s, I H,
thiazolyl-H); 7.95 (s, 1 H, CH=N); 9.9 (d, J = 8 Hz, NH).
25 (90 MHz, DMSO-d6): 3.6 and 4.55 (AB quartet, J = 18 Hz, 2H, SCH2);
3.9 to 4.1 (m, 5 H, -OCH3 and -N-CI:j2-CH=CH2); 5.1 to 5.5 (m, 3 H. B-
lactam-H and -N-CH,-CH=CjJ2); 5.7 to 6.1 (m,2H, B-lactam-H and N-
CH:-CH=CH2);6.95 (s, I H, thiazolyl-H);8.3 (s, I H, CH=N); 9.95 (d. J
8 Hz, NH).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
71
26 (90 MHz, DMSO-d6): 1.7 to 2 (m, 2 H, -CH2-C~,-IZ-CH3); 3.1 to 3.5 (m,
4 H); 3.55 and 4.5(AB quartet, J= 18 Hz, 2H, SCH2); 3.95 (s, 3 H, 0-
CH3); 5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J= 5 Hz and 8 Hz, B-
lactam-H); 6.95 (s, 1 H, thiazolyl-H); 8.3 (s, 1 H, CH=N); 9.9 (d, J = 8
Hz, NH).
27 (90 MHz, DMSO-d6): 0.8 to 1.1 and 1.1 to 1.7 (m, 7 H, -CH2-CH2-CH3);
3.15 to 3.45 (m, 2 H, -NHCIJ2-); 3.6 and 4.55 (AB quartet, J = 18 Hz,
2H, SCH2); 3.95 (s, 3 H, O-CH3); 5.3 (d, J= 5 Hz, 1 H, B-lactam-H);
5.85 (dd, J= 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, 1 H, thiazolyl-H); 8.4
(s, 1 H, CH=N); 9.95 (d, J = 8 Hz, NH).
28 (90 MHz, DMSO-d6): 3.05 (d, J = 4 Hz, 3 H, NHCH3); 3.65 (s, 3 H,
NCH3); 3.55 and 4.6 (AB quartet, J= 18 Hz, 2H, SCH2); 3.93 (s, 3 H,
O-CH3); 4.2 (part of the AB quartet, J = 18 Hz, 1H of SCH2); 5.3 (d, J=
Hz, 1 H, B-lactam-H); 5.85 (dd, J= 5 Hz and 8 Hz, B-lactam-H); 7.0 (s,
1 H, thiazolyl-H); 8.6 (s, 1 H, CH=N); 9.4 (d, J= 8 Hz, NH).
29 300 MHz, DMSO-d6): 2.93 (d, J= 4.6 Hz, 3 H, NCH3); 3.4 to 3.6 (m,
5H); 3.6 to 3.8 (m, 4H); 3.93 (s, 3 H, O-CH3); 4.2 (part of the AB
quartet, J= 18 Hz, 1H of SCHZ); 5.3 (d, J= 5 Hz, I H, B-lactam-H); 5.85
(dd, J= 5 Hz and 8 Hz, B-lactam-H); 6.93 (s, I H, thiazolyl-H); 8.6 (s, I
H, CH=N); 9.92 (d, J= 8 Hz, NH).
30 (90 MHz, DMSO-d6):1.3 (s, 9 H, -C(CH3)3); 3.55 and 4.55 (AB quartet, J
= 18 Hz, 2H, SCH2); 5.3 (d, J= 5 Hz, I H. B-lactam-H); 5.85 (dd, J = 5
Hz and 8 Hz, B-lactam-H); 6.95 (s, 1 H, thiazolyl-H); 8.25 (s, I H,
CH=N); 9.95 (d, J = 8 Hz, NH).
31 (90 MHz, DMSO-d6): 2.9 (s, 3 H. NCH3); 3.0 (s, 6 H, N(CH3)2); 3.6 and
4.2 (AB quartet, J = 18 Hz, 2H. SCH2); 3.95 (s. 3 H. O-CH3); 5.3 (d. J =
5 Hz, I H. B-lactam-H); 5.85 (dd. J = 5 Hz and 8 Hz, B-lactam-H). 7.0 (s.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
72
1 H, thiazolyl-H); 8.55 (s, 1 H, CH=N); 9.95 (d, J = 8 Hz, NH).
32 (90 MHz, DMSO-d6) : 2.85 (s, 2 H); 3.55 and 4.6 (AB quartet, J = 18
Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH3); 5.3 (d, J = 5 Hz, 1 H, B-lactam-H);
5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, I H, thiazolyl-H); 8.65
(s, 1 H, CH=N); 9.9 (d, J = 8 Hz, NH). '
33 (90 MHz, DMSO-d6): 3.4 to 3.8 (m, 9 H, morpholino H's and 1H of
SCH2); 3.95 (s, 3 H, O-CH3); 4.6 (part of the AB quartet, J = 18 Hz, 1H
of SCH2); 5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8
Hz, B-lactam-H); 6.95 (s, 1 H, thiazolyl-H); 8.7 (s, 1 H, CH=N); 9.9 (d, J
= 8 Hz, NH).
34 (300 MHz, DMSO-d6): 3.32 (s, 9H,-N+(CH3)3); 0.4 to 1(m, 4 H, -CH2-
CH2-); 2.5 to 2.8 (m, 1 H); 3.65 and 4.17 (AB quartet, J = 18.1 Hz, 2H,
SCH2); 3.94 (s, 3 H, O-CH3); 4.8 (q, J = 17 Hz, 2H); 5.3 (d, J = 5 Hz, I
H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.94 (s, I H,
thiazolyl-H); 8.26 (s, I H, CH=N); 9.93 (d, J = 8 Hz, NH).
35 (90 MHz, DMSO-d6): 0.4 to 1(m, 4 H, -CHZ-CHZ-); 2.5 to 2.8 (m, I H),
3.55 and 4.6 (AB quartet, J = 18 Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH3);
5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, Li-
lactam-H); 6.9 (s, I H, thiazolyl-H); 8.35 (s, 1 H, CH=N); 9.85 (d, J = 8
Hz, NH).
36 (90 MHz, DMSO-d6): 3.6 and 4.55 (AB quartet, J = 18 Hz, 2H, SCHZ);
3.95 s, 3 H, O-CH3); 5.3 (d, J = 5 Hz, I H, B-lactam-H); 5.85 (dd, J = 5
Hz and 8 Hz, B-lactam-H); 6.9 (s, I H, thiazolyl-H); 8.5 (s, 1 H. CH=N);
9.85 (d, J = 8 Hz, NH); 10.4 (broad singulet, I H, -NH-OH).
37 (90 MHz, DMSO-db): 3.1 (s. 3 H. N-CH3); 3.55 and 4.6 (AB quartet. J
18 Hz, 2H. SCH:); 3.9 (s. 3 H. O-CH3); 5.3 (d. 3 = 5 Hz, I H, B-lactam-
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
73
H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.9 (s, 1 H, thiazolyl-H);
8.7 (s, 1 H, CH=N); 9.85 (d, J = 8 Hz, NH).
38 (300 MHz, DMSO-d6): 3.56 and 4.54 (AB quartet, J = 18.1 Hz, 2H,
SCH2); 3.91 (s, 3 H, O-CH3); 4.87 (d, J = 6.5 Hz, 2 H); 5.3 (d, J = 5 Hz,
1 H, B-lactam-H); 5.89 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.88 (s, 1 H,
thiazolyl-H); 7.6 (m, 2 H, pyridinyl-H); 8.15 (m, 1 H, pyridinyl-H); 8.39
(s, 1 H, CH=N); 8.86 (m, 1 H, pyridinyl-H); 9.83 (d, J = 8 Hz, NH).
39 _(300 MHz, DMSO-d6): 3.57 and 4.52 (AB quartet, J = 18.1 Hz, 2H,
SCH2); 3.91 (s, 3 H, O-CH3); 4.87 (d, J = 6 Hz, 2 H); 5.3 (d, J = 5 Hz, 1
H, B-lactam-H); 5.89 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.88 (s, 1 H,
thiazolyl-H); 8.15 (m, 1 H, pyridinyl-H); 8.38 (s, 1 H, CH=N); 8.45 (m, 1
H, pyridinyl-H); 8.8 (m, 1 H, pyridinyl-H); 8.85 (s, 1 H, pyridinyl-H);
9.91 (d, J = 8 Hz, NH).
40 (300 MHz, DMSO-d6): 3.58 and 4.57 (AB quartet, J = 18.3 Hz, 2H,
SCH2); 3.9 (s, 3 H, O-CH3); 5.06 (broad singulet, 2 H); 5.3 (d, J = 5 Hz,
1 H, B-lactam-H); 5.88 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.94 (s, 1 H,
thiazolyl-H); 8.02 (d, J = 6.6 Hz, 2 H, pyridinyl-H); 8.4 (s, I H, CH=N);
8.92 (d, J = 6.6 Hz, 2 H, pyridinyl-H); 9.91 (d, J = 8 Hz, NH).
41 (90 MHz, DMSO-d6): 3.65 and 4.35 (AB quartet, J = 18 Hz, 2H, SCH2);
3.9 (s, 3 H, O-CH3); 4.2 (d, J = 7 Hz, 2H); 5.2 (d, J = 5 Hz, I H, B-
lactam-H); 5.75 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, I H,
thiazolyl-H); 7.85 (s, I H, CH=N); 9.8 (d, J = 8 Hz, NH).
42 (90 MHz, DMSO-d6): 2.95 (broad duplet, 3 H, N-CH3); 3.0 to 3.3 (m, 4
H, -CH2-N-CH2); 3.4 to 3.8 (m, 5 H, -CHZ-NH'-CH2- and 1 H of SCH2);
3.85 (s, 3 H: O-CH3); 4.1 (part of the AB quartet, J= 18 Hz, l H of
SCH2); 5.25 (d, J= 5 Hz, I H. B-lactam-H); 5.85 (dd, J= 5 Hz and 8 Hz.
B-lactam-H); 6.8 (s, 1 H. thiazolyl-H). 8.65 (s, I H, CH=N); 9.75 (d. J
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
74
8 Hz, NH).
43 (90 MHz, DMSO-d6): 3.7 and 4.85 (AB quartet, J = 18 Hz, 2H, SCH2);
3.95 (s, 3 H, O-CH3); 5.35 (d, J = 5 Hz, 1 H, B-lactam-H); 5.95 (dd, J = 5 Hz
and 8 Hz, B-lactam-H); 7.0 (s, 1 H, thiazolyl-H); 7.2 (t, J = 6 Hz, I
H, pyridinyl-H); 7.4 (d, J = 8 Hz, 1 H, pyridinyl-H); 8.15 (t, J = 6 Hz, 2
H, pyridinyl-H); 8.55 (s, 1 H, CH=N); 9.95 (d, J = 8 Hz, NH).
44 (90 MHz, DMSO-d6): 3.6 and 4.05 (AB quartet, J = 18 Hz, 2H, SCH2);
3.95 (s, 3 H, O-CH3); 5.25 (d, J = 5 Hz, 1 H, B-lactam-H); 5.75 (dd, J
Hz and 8 Hz, B-lactam-H); 6.9 (s, 1 H, thiazolyl-H); 8.5 (s, 1 H,
CH=N); 9.85 (d, J = 8 Hz, NH).
45 (300 MHz, DMSO-d6): 1.4 to 1.7 (m, 6 H); 3.4 to 3.7 (m, 5 H, -CH2-N-
CH2- and 1H of SCH2); 3.92 (s, 3 H, O-CH3); 4.55 (part of the AB
quartet, J = 18 Hz, 1H of SCH2); 5.29 (d, J = 5 Hz, 1 H, B-lactam-H);
5.89 (dd, J = 5 Hz and 7.8 Hz, B-lactam-H); 6.89 (s, 1 H, thiazolyl-H);
8.6 (s, I H, CH=N); 9.84 (d, J = 7.8 Hz, NH).
46 (90 MHz, DMSO-d6): 3.1 to 3.4 (m, 4 H, -CH2-NH'-CH2-); 3.65 and
4.65 (AB quartet, J = 18 Hz, 2H, SCH2); 3.85 (s, 3 H, O-CH3); 4 to 4.3
(m, 4 H, -CH2-N-CH2-); 4.65 (part of the AB quartet, J = 18 Hz, 1 H of
SCH2); 5.2 (d, J = 5 Hz, 1 H, B-lactam-H); 5.8 (dd, J = 5 Hz and 8 Hz,
B-lactam-H); 6.75 (s, 1 H, thiazolyl-H); 8.5 (s, I H, CH=N); 9.7 (d, J = 8
Hz, NH).
47 (300 MHz, DMSO-d6): 2.85 (broad singulet, 3 H, N-CH3); 3.54 and 4.52
(AB quartet, J = 18.1 Hz, 2H, SCH2); 3.93 (s, 3 H, O-CH3); 5.3 (d, J = 5
Hz, I H, B-lactam-H); 5.89 (dd, J = 5 Hz and 7.9 Hz, B-lactam-H); 6.91
(s, I H. thiazolyl-H); 8.62 (s, 1 H, CH=N); 9.88 (d, J = 7.9 Hz, NH);
12.0 (s, 1 H, OH).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
48 (90 MHz, DMSO-d6): 3.2 (s, 6 H, NCH3); 3.7 (s, 4 H, -N-(CH2)2-N-);
3.65 and 4.0 (AB quartet, J = 17.8 Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH3);
5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-
lactam-H); 6.95 (s, 1 H, thiazolyl-H); 8.8 (s, 1 H, CH=N); 9.9 (d, J = 8
Hz, NH).
49 (300 MHz, DMSO-d6): 3.7 and 4.13 (AB quartet, J = 17.8 Hz, 2H,
SCH2); 3.9 (s, 3 H, O-CH3); 5.31 (d, J = 5 Hz, 1 H, B-lactam-H); 5.89
(dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.92 (s, 1 H, thiazolyl-H); 8.13 (d, J
= 6 Hz, 2 H, pyridinyl-H); 8.7 (s, 1 H, CH=N); 8.93 (m, 3 H, pyridinyl-
H); 9.88 (d, J = 8 Hz, NH).
50 (300 MHz, DMSO-d6): 2.94 (d, J = 4.7 Hz, 3 H, N-CH3); 3.29 (broad s, 6
H, N+(CH3)2); 3.3 to 3.7 (m, 9H, piperazinyl-H's and 1H of SCH2); 3.93
(s, 3 H, O-CH3); 4.2 (part of the AB quartet, J = 18 Hz, 1H of SCH2);
5.28 (d, J = 5 Hz, 1 H, B-lactam-H); 5.89 (dd, J = 5 Hz and 7.6 Hz, B-
lactam-H); 6.9 (s, 1 H, thiazolyl-H); 8.1 (s, 1 H, formyl-H); 8.6 (s, I H,
CH=N); 9.9 (d, J = 8 Hz, NH).
51 (300 MHz, DMSO-d6): 3.7 and 4.2 (AB quartet, J = 18 Hz, 2H, SCH2);
3.93 (s, 3 H, O-CH3); 5.35 (d, J = 5 Hz, I H, B-lactam-H); 5.85 (dd, J =
5 Hz and 8 Hz, B-lactam-H); 6.0 (AB quartet, J 9 Hz, 2H); 6.93 (s, 1
H, thiazolyl-H); 8.2 (t, J = 7 Hz, 2 H), 8.7 (t, J 7 Hz, 1 H) and 9.1 (d, J
= 6 Hz, 2H), pyridinium-H; 8.32 (s, I H, CH=N); 9.95 (d, J = 8 Hz, NH).
52 (90 MHz, DMSO-d6): 3.7 (s, 3 H, N-CH3); 3.65 and 4.1 (AB quartet, J
18 Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH3); 5.35 (d, J = 5 Hz, I H, B-
lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 7.0 (s, I H,
thiazolyl-H); 8.75 (s, I H, CH=N); 9.9 (d, J = 8 Hz, NH).
53 (90 MHz, DMSO-db): 2.25 (s. 3 H. triazinyl-CH3); 3.5 and 4.65 (AB
quartet, J = 18 Hz. 2H, SCH2); 4.0 (s, 3 H. O-CH,); 5.35 (d. J = 5 Hz. I
CA 02219656 1997-10-28
WO 96135692 PCT/EP96/02023
76
H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, 1 H,
thiazolyl-H); 8.85 (s, 1 H, CH=N); 9.95 (d, J= 8 Hz, NH).
54 (90 MHz, DMSO-d6): 2.3 (s, 3 H, CH3); 1.8 to 2.1 (m, 1 H); 3.6 and 4.55
(AB quartet, J= 18 Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH3); 5.35 (d, J= 5
Hz, 1 H, B-lactam-H); 5.9 (dd, J= 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, 1
H, thiazolyl-H); 8.65 (s, 1 H, CH=N); 9.9 (d, J= 8 Hz, NH).
55 (90 MHz, DMSO-d6): 2.8 (broad duplet, 3H, N-CH3); 3.2 to 3.7 (m, 5 H,
-N-CHZ-CH2-O and 1H of SCH2); 3.95 (s, 3 H, O-CH3); 4.5 (part of the
AB quartet, J= 18 Hz, 1H of SCH2); 5.3 (d, J= 5 Hz, 1 H, B-lactam-H);
5.85 (dd, J= 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, 1 H, thiazolyl-H); 8.65
(s, 1 H, CH=N); 9.9 (d, J = 8 Hz, NH).
56 (90 MHz, DMSO-db): 3.7 and 4.15 (AB quartet, J = 18 Hz, 2H, SCH2);
4.0 (s, 3 H, O-CH3); 5.35 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J= 5
Hz and 8 Hz, B-lactam-H); 7.0 (s, 1 H. thiazolyl-H); 7 to 7.8 (m, 4 H.
aromatic-H); 8.45 (s, 1 H, CH=N); 9.95 (d, J= 8 Hz, NH).
57 (90 MHz, DMSO-d6): 3.35 broad singulet, 3 H, NCH3); 3.55 and 4.55
(AB quartet, J= 18 Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH3); 5.3 (d, J= 5
Hz, I H, B-lactam-H); 5.85 (dd, J= 5 Hz and 8 Hz, B-lactam-H); 6.95 (s,
1 H, thiazolyl-H); 8.15 (s, 1 H, CH=N); 9.85 (d, J= 8 Hz, NH).
58 (90 MHz, DMSO-d6): 2.95 (broad duplet, 3 H, NCH3); 3.35 (broad
singulet, 3 H, NCH3); 3.65 and 4.65 (AB quartet, J = 18 Hz, 2H, SCH2);
3.95 (s, 3 H, O-CH3); 5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5
Hz and 8 Hz, B-lactam-H); 6.95 (s, I H, thiazolyl-H); 8.1 (s, 1 H,
CH=N); 9.85 (d, J = 8 Hz, NH).
59 (90 MHz, DMSO-d6): I to 1.5 (m, 4 H, -CH:-CH2-); 1.8 to 2.1 (m, I H);
3.55 and 4.55 (AB quartet, J= 18 Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH,);
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
77
5.3 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-
lactam-H); 6.9 (s, 1 H, thiazolyl-H); 8.65 (s, 1 H, CH=N); 9.9 (d, J = 8
Hz, NH).
60 (90 MHz, DMSO-d6): 3.7 and 4.8 (AB quartet, J = 18 Hz, 2H, SCH2); 4.0
(s, 3 H, O-CH3); 5.35 (d, J = 5 Hz, 1 H, B-lactam-H); 5.95 (dd, J = 5 Hz
and 8 Hz, B-lactam-H); 7.0 (s, 1 H, thiazolyl-H); 7.85 (dd, J = 4 Hz and
6 Hz, pyridinyl-H); 8.2 (dt, J = 2 and 8 Hz, pyridinyl-H); 8.5 (d, J = 6
Hz, pyridinyl-H); 8.9 (d, J = 4 Hz, pyridinyl-H); 8.95 (s, 1 H, CH=N);
9.95 (d, J = 8 Hz, NH).
61 (90 MHz, DMSO-d6): 3.6 and 4.15 (AB quartet, J = 18 Hz, 2H, SCH2);
3.85 (s, 3 H, O-CH3); 5.25 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J
Hz and 8 Hz, B-lactam-H); 6.75 (s, 1 H, thiazolyl-H); 7.5 (dd, J = 5
Hz and 8 Hz, pyridinyl-H); 8.25 (broad duplet, J = 8 Hz, pyridinyl-H);
8.65 (broad triplet, J = 6 Hz, pyridinyl-H); 9.05 (s, I H, CH=N); 9.7 (d, J
= 8 Hz, NH).
62 (300 MHz, DMSO-d6): 3.13 (broad duplet, 3 H, N-CH3); 3.29 (broad s, 6
H, N+(CH3)2); 3.4 to 3.75 (m, 5 H, -CHZ-N+-CH2- and IH of SCH2); 3.85
(s, 3 H, O-CH3); 4 to 4.3 (m, 4 H, -CH2-N-CH2-); 4.65 (part of the AB
quartet, J = 18 Hz, 1H of SCH2); 5.27 (d, J = 5 Hz, 1 H, B-lactam-H);
5.85 (dd, J = 5 Hz and 7.6 Hz, B-lactam-H); 6.78 (s, 1 H, thiazolyl-H);
8.75 (s, I H, CH=N); 9.75 (d, J = 7.6 Hz, NH).
63 (90 MHz, DMSO-d6 +TFA): 3.0 (broad duplet, 3 H, N-CH3); 3.2 (s, 9 H,
N'(CH3)3); 3.5 to 3.8 (m, 5 H, N-CH,-CH2-N' and I H of SCH2); 3.90 (s,
3 H. O-CH3); 4.65 (part of the AB quartet, J = 18 Hz, 1 H of SCHZ); 5.3
(d, J = 5 Hz, I H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-
H); 6.8 (s, 4 H. thiazolyl-H); 8.75 (s, I H, CH=N); 9.75 (d, J = 8 Hz,
NH).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
78
64 (90 MHz, DMSO-d6): 3.65 and 4.15 (AB quartet, J = 18 Hz, 2H, SCH2);
4.0 (s, 3 H, O-CH3); 5.25 (d, J = 5 Hz, 1 H, B-lactam-H); 5.8 (dd, J = 5
Hz and 8 Hz, B-lactam-H); 7.0 (s, 1 H, thiazolyl-H); 7.2 (d, J = 5 Hz, 1
H, pyrimidinyl-H); 8.45 (s, 1 H, CH=N); 8.8 (d, J = 5 Hz, 1 H,
pyrimidinyl-H); 9.9 (d, J = 8 Hz, NH).
65 (90 MHz, DMSO-d6 +TFA): 4.0 (s, 3 H, O-CH3); 3.6 and 4.65 (AB
quartet, J = 18 Hz, 2H, SCH2); 5.25 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85
(dd, J = 5 Hz and 8 Hz, B-lactam-H); 7.0 (s, 1 H, thiazolyl-H); 8.3 (s, 1
H, CH=N); 9.85 (d, J = 8 Hz, NH).
66 (90 MHz, DMSO-d6): 3.2 (broad singulet, 3 H, N-CH3); 3.0 to 3.4 (m, 4
H, -CH2-N-CH2); 3.4 to 3.8 (m, 5 H, -CH2-NH+-CH2- and 1H of SCH2);
3.95 (s, 3 H, O-CH3); 4.3 (part of the AB quartet, J= 18 Hz, 1 H of
SCH2); 5.35 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J= 5 Hz and 8 Hz,
B-lactam-H); 7.0 (s, 1 H, thiazolyl-H); 8.15 (s, 1 H, CH=N); 9.9 (d, J= 8
Hz, NH)-
67 (90 MHz, DMSO-d6): 3.3 (s, 3 H, N-CH3); 3.3 (broad s, 6 H, N+(CH3)2);
3.3 to 3.7 (m, 9H, piperazinyl-H's and 1H of SCHZ); 3.85 (s, 3 H, 0-
CH3); 4.25 (part of the AB quartet, J= 18 Hz, IH of SCH2); 5.25 (d, J=
Hz, 1 H, B-lactam-H); 5.8 (dd, J= 5 Hz and 8 Hz, B-lactam-H); 6.8 (s,
I H, thiazolyl-H); 8.1 (s, I H, forrnyl-H); 8.15 (s, 1 H, CH=N); 9.75 (d, J
= 8 Hz, NH).
68 (90 MHz, DMSO-d6): 2.25 (s, 3 H); 3.65 (s, 3 H, N-CH3); 3.7 and 4.6
(AB quartet, J= 18 Hz, 2H, SCH2); 3.9 (s, 3 H, O-CH3); 5.3 (d, J = 5
Hz, I H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.05 (d,
J = 4 Hz, pyrrol-H); 6.85 (d, J= 4 Hz, pyrrol-H); 6.9 (s, 1 H, thiazolyl-
H); 8.75 (s, I H, CH=N); 9.9 (d, J = 8 Hz, NH).
69 (90 MHz. DMSO-d6): 3.5 and 4.45 (AB quanet, 3 = 20 Hz, 2H, SCH2);
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
79
5.25 (d, J= 5 Hz, 1 H, B-lactam-H); 5.75 (d, J = 55 Hz, 2H, -CH2F); 5.85
(dd, J = 5 Hz and 8 Hz, B-lactam-H); 8.25 (s, I H, CH=N);9.85 (d, J= 8
Hz, NH).
70 (300 MHz, DMSO-d6): 1.13 (t, J=7.1 Hz, 3H, CH3); 3.31 (qd, J=7.1 and
ca. 6 Hz, 2H, CH2); 3.55 and 4.47 (AB quartet, J=18.1 Hz, 2H, S-CH2);
5.29 (d, J=5.0 Hz, 1H, P-lactam-H); 5.89 (dd, J=5.0 and 7.9 Hz, 1H,
P-lactam-H); 6.78 (s, 1H, CH thiazol); 8.01 (broad, 2H, NH); 8.19 (broad
t 1H, NH) ; 8.32 (s, 1H, CH=N); 9.70 (d, J=7.9 Hz, 1H, NH); 12.03 (s,
1 H, OH).
71 (300 MHz, DMSO-d6): 2.98 (d, J=4.6 Hz, 3H, N-CH3); 3.56 and 4.46
(AB quartet, J=18.1 Hz, 2H, S-CH2); 5.28 (d, J=4.9 Hz, 1H, (3-lactam-H);
5.87 (dd, J=4.9 and 7.9 Hz, 1H, (3-lactam-H); 6.83 (s, 1H, CH thiazol);
8.22 (s, 1 H, CH=N); 8.48 (q broad J=4.6 Hz, 1 H, NH); 9.75 (d, J=7.9 Hz,
1 H, NH); 11.63 (s, 1H, OH); 12.28 (s, 1H, OH).
72 (300 MHz, DMSO-d6): 3.53 and 4.47 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.26 (d, J=5.0 Hz, 1 H, (3-lactam-H); 5.88 (dd, J=4.9 and 7.9 Hz,
1H, P-lactam-H); 6.84 (s, IH, CH thiazol); 8.00 (s, IH, NH); 8.23 (s, IH,
CH=N); 8.28 (s, 1 H, NH); 9.76 (d, J=7.9 Hz, 1 H, NH); 11.56 (s, 1 H,
OH); 12.31 (s, 1 H, OH).
73 (300 MHz, DMSO-d6): 1.16 (t, J=7.1 Hz, 3H, CH3); 1.90 (m broad, 4H,
CH2); 3.39 (qd, J=7.1 and ca. 6 Hz, 2H, CH2); 3.56 (m broad, 4H, CH2);
3.63 and 4.07 (AB quartet, J=18.0 Hz, 2H, S-CH2); 5.28 (d, J=5.0 Hz,
1 H, P-lactam-H); 5.88 (dd, J=5.0 and 7.8 Hz, IH, (3-lactam-H); 6.81 (s,
1 H, CH thiazol); 7.97 (t broad, J= ca. 6 Hz 1 H, NH); 8.60 (s, 1 H,
CH=N); 9.76 (d, J=7.8 Hz, 1 H, NH); 11.70 (s, 1 H, OH); 12.26 (s, 1 H,
OH).
74 (300 MHz, DMSO-d6): 3.57 and 4.48 (AB quartet, J=18.1 Hz, 2H,
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
S-CH2); 3.97 (broad, 2H, N-CH2-C=C); 5.1-5.3 (m, 2H, C=CH2); 5.30 (d,
J=5.1 Hz, 1H, P-lactam-H); 5.8-5.9 (m, 1H, C-CH=C); 5.89 (dd, J=4.9
and 8.2 Hz, 1H, P-lactam-H); 6.83 (s, 1H, CH thiazol); 8.10 (s, 2H, NH);
8.34 (s, 1H, CH=N); 8.41 (s, 1H, NH); 9.77 (d, J=8.0 Hz, 1 H, NH); 12.26
(s, 1H, OH); 12.38 (s, 1H, OH).
75 (300 MHz, DMSO-d6): 1.89 (m broad 2H, CH2); 3.33 (s broad, 4H,
N-CH2); 3.54 and 4.42 (AB quartet, J=18.1 Hz, 2H, S-CH2); 5.29 (d,
J=5.0 Hz, 1H, P-lactam-H); 5.89 (dd, J=5.0 and 8.0 Hz, 1H, P-lactam-H);
6.76 (s, 1H, CH thiazol); 8.29 (s, 1H, CH=N); 8.38 (s, 2H, NH); 9.66 (d,
J=8.0 Hz, 1H, NH); 11.90 (s, 1 H, OH); 12.03 (s, 1 H, OH).
76 (300 MHz, DMSO-d6): 0.89 (t, 3H, C-CH3); 1.2-1.4 (m, 2H, C-CH2-C);
1.4-1.6 (m, 2H, C-CH2-C); 3.2-3.4 (m, 2H, N-CH2-C); 3.56 and 4.47 (AB
quartet, J=18.1 Hz, 2H, S-CH2); 5.30 (d, J=5.0 Hz, 1H, j3-lactam-H); 5.89
(dd, J=5.0 and 7.9 Hz, 1H, (3-lactam-H); 6.83 (s, 1H, CH thiazol); 8.04 (s,
2H, NH); 8.24 (s, 1 H, CH=N); 8.32 (s, 1 H, NH); 9.76 (d, J=7.9 Hz, 1 H,
NH); 12.13 (s, 1H, OH); 12.36 (s, IH, OH).
77 (300 MHz, DMSO-d6): 3.66 and 3.92 (AB quartet, J=17.9 Hz, 2H,
S-CH2); 3.86 (s, 3H, O-CH3); 5.27 (d, J=5.0 Hz, 1H, (3-lactam-H); 5.88
(dd, J=5.0 and 7.8 Hz, 1H, P-lactam-H); 6.84 (s, 1H, CH thiazol); 8.22 (s,
1 H, CH=N); 9.78 (d, J=7.8 Hz, IH, NH); 12.34 (s, 1H, OH).
78 (300 MHz, DMSO-d6): 1.39 (s, 9H, C-CH3); 3.56 and 4.47 (AB quartet,
J=18.0 Hz, 2H, S-CH2); 5.29 (d, J=5.0 Hz, 1H, P-lactam-H); 5.89 (dd,
J=5.0 and 7.9 Hz, IH, P-lactam-H); 6.81 (s, 1H, CH thiazol); 7.90 (s
broad 2H, NH); 7.99 (s broad 1 H, NH); 8.25 (s, 1 H, CH=N); 9.68 (d,
J=7.9 Hz, IH, NH); 12.03 (s, 1 H, OH); 12.16 (s, 1 H, OH).
79 (300 MHz, DMSO-d6): 2.92 (d. J=4.8 Hz. 3H. N-CH3); 3.03 (s, 6H,
N-CH3); 3.61 and 4.17 (AB quartet. J=18.0 Hz. 2H, S-CH:); 5.29 (d.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
81
J=5.0 Hz, 1H, P-lactam-H); 5.88 (dd, J=5.0 and 7.8 Hz, 1H, P-lactam-H);
6.81 (s, 1 H, CH thiazol); 8.20 (q broad J=4.8 Hz, 1 H, NH); 8.55 (s, 1 H,
CH=N); 9.76 (d, J=7.5 Hz, 1H, NH); 11.83 (s, 1 H, OH); 12.28 (s, 1 H,
OH).
80 (300 MHz, DMSO-d6): 2.75 (s, 2H, N-CH2); 3.55 and 4.54 (AB quartet,
J=18.1 Hz, 2H, S-CH2); 5.32 (d, J=5.1 Hz, 1H, P-lactam-H); 5.93 (dd,
J=5.1 and 7.9 Hz, 1H, (3-lactam-H); 6.79 (s, 1 H, CH thiazol); 8.59 (s, 1 H,
CH=N); 9.73 (d, J=8.0 Hz, 1 H, NH); 12.13 (s, 1 H, OH).
81 (300 MHz, DMSO-d6): 3.55 and 4.54 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 3.5-3.6 (m, 4H, CH2); 3.6-3.7 (m, 4H, CH2); 5.30 (d, J=5.1 Hz,
1H, 0-lactam-H); 5.89 (dd, J=5.0 and 7.9 Hz, 1H, (3-lactam-H); 6.84 (s,
1 H, CH thiazol); 8.35 (broad, 2H, NH); 8.65 (s, 1 H, CH=N); 9.80 (d,
3=7.9 Hz, 1 H, NH); 12.27 (s, 1 H, OH); 12.51 (s, 1 H, OH).
82 (300 MHz, DMSO-d6): 0.64 (m, 2H, cyclopr. CH2); 0.83 (m, 2H, cyclopr.
CH2); 2.62 (m, 1H, cyclopr. CH); 3.53 and 4.49 (AB quartet, J=18.1 Hz,
2H, S-CH2); 5.29 (d, J=5.0 Hz, 1 H, P-lactam-H); 5.89 (dd, J=5.0 and
8.0 Hz, 1H, P-lactam-H); 6.79 (s, 1H, CH thiazol); 8.09 (s, 2H, NH); 8.35
(s, 1 H, CH=N); 8.59 (s, 1 H, NH); 9.70 (d, J=8.0 Hz, IH, NH); 12.08 (s,
1 H, OH); 12.13 (s, 1 H, OH).
83 (300 MHz, DMSO-d6): 3.54 and 4.48 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.29 (d, J=5.0 Hz, 1 H, P-lactam-H); 5.89 (dd, J=4.9 and 7.8 Hz,
IH, R-lactam-H); 6.83 (s, 1H, CH thiazol); 8.15 (s, 2H, NH); 8.39 (s, 1H,
CH=N); 9.79 (d, J=7.9 Hz, 1 H, NH); 11.21 (s, 1 H, OH); 12.15 (s, I H,
OH); 12.44 (s. 1 H, OH).
84 (300 MHz, DMSO-d6): 3.09 (s, 6H. N-CH,); 3.55 and 4.55 (AB quartet,
J=18.1 Hz, 2H, S-CH2); 5.30 (d. J=5.0 Hz. IH, P-lactam-H); 5.89 (dd,
J=5.0 and 7.8 Hz, 1H, P-lactam-H); 6.84 (s, IH. CH thiazol). 8.07 (s. 2H.
CA 02219656 1997-10-28
WO 96/35692 PCr/EP96/02023
82
NH); 8.65 (s, 1 H, CH=N); 9.81 (d, J=7.9 Hz, 1 H, NH); 11.86 (s, 1 H,
OH); 12.53 (s, 1H, OH).
85 (300 MHz, DMSO-d6): 3.58 and 4.50 (AB quartet, J=18.0 Hz, 2H,
S-CH2); 4.90 (d, J=6.4 Hz, 2H, N-CH2); 5.31 (d, J=5.1 Hz, 1H,
P-lactam-H); 5.90 (dd, J=5.2 and 7.8 Hz, 1H, P-lactam-H); 6.84 (s, 1H,
CH thiazol); 7.6-7.8 (m, 2H, CH aromatic); 8.1-8.3 (m, 1H, CH
aromatic); 8.35 (s broad, 1H, NH); 8.39 (s, 1H, CH=N); 8.7-8.8 (m, 2H,
CH aromatic); 9.3 (broad, 1 H, NH); 9.78 (d, J=7.8 Hz, 1 H, NH); 12.42
(s, 1H, OH); 12.49 (s, 1H, OH).
86 (300 MHz, DMSO-d6): 3.57 and 4.52 (AB quartet, J=18.0 Hz, 2H,
S-CH2); 4.85 (d, J=6.6 Hz, 2H, N-CH2); 5.30 (d, J=5.0 Hz, 1H,
P-lactam-H); 5.90 (dd, J=5.0 and 7.8 Hz, 1 H, P-lactam-H); 6.82 (s, 1 H,
CH thiazol); 7.9-8.1 (m, 1H, CH aromatic); 8.38 (s, 1H, CH=N); 8.4-8.6
(m, 1 H, CH aromatic); 8.8-8.9 (m, 1 H, CH aromatic); 8.9-9.0 (m, 1 H, CH
aromatic); 8.7-8.8 (m, 2H, CH aromatic); 9.77 (d, J=7.9 Hz, 1 H, NH);
12.32 (s, 1H, OH); 12.45 (s, 1 H, OH).
87 (300 MHz, DMSO-d6): 3.57 and 4.52 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 4.98 (d, J=6.2 Hz, 2H, N-CH2); 5.30 (d, J=5.0 Hz, 1 H,
(3-lactam-H); 5.90 (dd, J=5.0 and 7.9 Hz, 1 H, P-lactam-H); 6.81 (s, 1 H,
CH thiazol); 7.9-8.0 (m, 2H, CH aromatic); 8.40 (s, 1 H, CH=N); 8.8-9.0
(m, 1 H, CH aromatic); 9.00 (s broad 1 H, NH); 9.76 (d, J=7.9 Hz, 1 H,
NH); 12.30 (s, 1 H, OH); 12.56 (s, IH, OH).
88 (300 MHz, DMSO-d6): 3.68 and 4.05 (AB quartet, J=17.9 Hz, 2H,
S-CH2); 4.19 and 4.38 (AB quartet, J=16.4 Hz, 2H, N-CH2-C=O); 5.30 (d,
J=5.0 Hz, IH, P-lactam-H); 5.87 (dd, J=5.0 and 7.7 Hz, 1H, P-lactam-H);
6.87 (s, IH, CH thiazol); 7.86 (s, IH, CH=N); 9.82 (d, J=7.7 Hz, 1H,
NH); 11.35 (s, IH, OH); 12.45 (s, 1 H. OH).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
83
89 (300 MHz, DMSO-d6): 3.58 and 4.64 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.29 (d, J=4.9 Hz, 1H, P-lactam-H); 5.89 (dd, J=4.9 and 7.9 Hz,
1 H, P-lactam-H); 6.84 (s, 1 H, CH thiazol); 7.1-7.6 (m, CH aromatic);
8.33 (s, 1H, CH=N); 9.78 (d, J=7.9 Hz, 1H, NH); 10.03 (s, 1H, NH);
11.86 (s, 1H, OH); 12.35 (s, 1H, OH).
90 (300 MHz, DMSO-d6): 3.66 and 4.70 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.34 (d, J=5.0 Hz, 1 H, P-lactam-H); 5.92 (dd, J=5.0 and 7.9 Hz,
1H, P-lactam-H); 6.84 (s, 1H, CH thiazol); 7.0-7.2 (m, 1H, CH aromatic);
7.2-7.3 (m, 1 H, CH aromatic); 8.0-8.2 (m, 2H, CH aromatic); 8.49 (s, 1H,
CH=N); 9.79 (d, J=8.0 Hz, 1 H, NH); 12.32 (s, 1 H, OH); 13.41 (s, 1 H,
OH).
91 (300 MHz, DMSO-d6): 3.65 and 4.03 (AB quartet, J=17.8 Hz, 2H,
S-CH2); 5.27 (d, J=4.9 Hz, 1H, P-lactam-H); 5.83 (dd, J=4.9 and 7.7 Hz,
1H, P-lactam-H); 6.88 (s, 1H, CH thiazol); 8.52 (s, 1H, CH=N); 9.77 (d,
J=7.7 Hz, 1H, NH); 11.08 (s, 1 H, OH); 12.35 (s, 1H, OH).
92 (300 MHz, DMSO-d6): 1.51 (s, 3H, C-CH3); 1.54 (s, 3H, C-CH3); 2.86 (d,
J=4.9 Hz, 3H, N-CH3); 3.55 and 4.50 (AB quartet, J=18.2 Hz, 2H,
S-CH2); 5.32 (d, J=5.1 Hz, 1H, P-lactam-H); 5.96 (dd, J=5.0 and 8.2 Hz,
IH, P-lactam-H); 6.95 (s, 1 H, CH thiazol); 8.03 (s broad 2H, NH); 8.18
(s broad 1 H, NH); 8.32 (s, 1 H, CH=N); 9.74 (d, J=7.9 Hz, 1 H, NH);
12.19 (s, 1 H, OH).
93 (300 MHz, DMSO-db): 2.9 (broad, 3H, N-CH3); 3.54 and 4.50 (AB
quartet, J=18.1 Hz, 2H, S-CH2); 5.30 (d, J=5.1 Hz, IH, (3-lactam-H); 5.89
(dd, J=5.0 and 7.8 Hz, 1 H, j3-lactam-H); 6.83 (s, IH, CH thiazol); 8.62 (s,
1 H, CH=N); 9.79 (d, J=7.9 Hz, I H, NH); 11.98 (s, 1 H, OH); 12.42 (s,
1 H, OH).
94 (300 MHz, DMSO-db): 3.39 (m broad 2H. CHz); 3.54 (m broad 2H. CH2);
CA 02219656 1997-10-28
WO 96135692 PCTlEP96/02023
84
2.89 (d, J=4.6 Hz, 3H, N-CH3); 3.55 and 4.49 (AB quartet, J=18.0 Hz,
2H, S-CH2); 5.30 (d, J=5.0 Hz, 1H, P-lactam-H); 5.90 (dd, J=5.0 and
7.9 Hz, 1H, P-lactam-H); 6.79 (s, 1H, CH thiazol); 8.61 (s, 1H, CH=N);
9.71 (d, J=7.9 Hz, 1 H, NH); 11.73 (s, 1H, OH); 12.10 (s, 1H, OH).
95 (300 MHz, DMSO-d6): 2.26 (s, 3H, CH3); 3.57 and 4.70 (AB quartet,
J=18.0 Hz, 2H, S-CH2); 5.33 (d, J=5.0 Hz, 1H, P-lactam-H); 5.93 (dd,
J=5.0 and 7.9 Hz, 1H, 04actam-H); 6.83 (s, 1H, CH thiazol); 8.77 (s, IH,
CH=N); 9.80 (d, J=7.9 Hz, 1H, NH); 12.40 (s, 1H, OH).
96 (300 MHz, DMSO-d6): 3.22 (m broad 4H, N-CH2); 3.55 and 4.52 (AB
quartet, J=18.1 Hz, 2H, S-CH2); 3.85 (m broad 4H, N-CH2); 5.30 (d,
J=5.0 Hz, 1H, P-lactam-H); 5.90 (dd, J=5.0 and 7.9 Hz, 1H, P-lactam-H);
6.82 (s, 1H, CH thiazol); 8.5 (broad, 2H, NH); 8.65 (s, 1H, CH=N); 9.76
(d, J=7.9 Hz, 1H, NH); 9.82 (s, 2H, NH); 12.31 (s, 1H, OH); 12.47 (s,
1 H, OH).
97 (300 MHz, DMSO-d6): 3.14 (s, 6H, N-CH3); 3.64 and 3.94 (AB quartet,
J=17.9 Hz, 2H, S-CH2); 3.68 (s 4H, N-CH2); 5.28 (d, J=5.0 Hz, 1H,
P-lactam-H); 5.89 (dd, J=5.0 and 7.7 Hz, IH, P-lactam-H); 6.81 (s, 1H,
CH thiazol); 8.64 (s, 1H, CH=N); 9.77 (d, J=7.7 Hz, 1H, NH); 12.29 (s,
1 H, OH); 12.36 (s, IH, OH).
98 (300 MHz, DMSO-db): 3.33 (s, 3H, N-CH3); 3.54 and 4.55 (AB quartet,
J=18.3 Hz, 2H, S-CH2); 5.29 (d, J=5.0 Hz, 1H, P-lactam-H); 5.91 (dd,
J=5.1 and 7.9 Hz, IH, P-lactam-H); 6.76 (s, 1 H, CH thiazol); 8.10 (s, 1 H,
CH=N); 8.2 (s, NH); 9.67 (d, J=7.8 Hz, 1H, NH); 11.92 (s, IH, OH).
99 (300 MHz, DMSO-d6): 2.80 (s. 3H, CH3); 3.57 and 4.48 (AB quartet,
3=18.1 Hz, 2H, S-CHZ); 5.34 (d, J=5.1 Hz. IH, P-lactam-H); 5.94 (dd,
J=5.1 and 7.9 Hz, IH. P-lactam-H ); 6.82 (s, I H, CH thiazol); 8.55 (s, IH.
CH=N); 9.28 (s, IH. NH); 9.80 (d. J=7.9 Hz, 1H. NH); 9.90 (s, 1H. NH).
CA 02219656 1997-10-28
WO 96/35692 PGT/EP96/02023
12.39 (s, 1H, OH); 13.52 (s, 1H, OH).
100 (300 MHz, DMSO-d6): 3.58 and 4.46 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.34 (d, J=5.1 Hz, 1H, (3-lactain-H); 5.94 (dd, J=5.1 and 7.9 Hz,
1H, 04actam-H); 6.82 (s, 1H, CH thiazol); 8.28 (dd, J=6.7 and 14.8 Hz,
1H, N-CH=N); 8.58 (s, 1H, CH=N); 9.58 (d, J=14.8 Hz 1H, NH); 9.77
(d, J=8.0 Hz, 1H, NH); 9.9 (d, J=6.7 Hz 1H, NH); 12.29 (s, 1H, OH).
101 (300 MHz, DMSO-d6): 3.57 and 4.48 (AB quartet, J=18.0 Hz, 2H,
S-CH2); 3.9 (s, 3H, O-CH3); 5.33 (d, J=5.1 Hz, 1H, P-lactam-H); 5.92
(dd, J=5.1 and 8.0 Hz, 1H, P-lactam-H); 6.87 (s, 1H, CH thiazol); 8.27
(dd, J=6. 9 and 14.6 Hz, 1H, N-CH=N); 8.60 (s, 1H, CH=N); 9.55 (d,
J=14.4 Hz 1H, NH); 9.79 (d, J=8.0 Hz, 1H, NH); 9.91 (d, J=6.5 Hz 1H,
NH).
102 (300 MHz, DMSO-d6): 1.49 (s, 3H, C-CH3); 1.50 (s, 3H, C-CH3); 3.54
and 4.48 (AB quartet, J=18.1 Hz, 2H, S-CH2); 5.31 (d, J=4.9 Hz, 1H,
(3-lactam-H); 5.97 (dd, J=4.9 and 8 Hz, 1H, P-lactam-H); 6.84 (s, 1H, CH
thiazol); 8.29 (s, 1H, CH=N); 9.65 (d, J=8 Hz, 1H, NH); 12.06 (s, 1H,
OH).
103 (300 MHz, DMSO-d6): 1.51 (s, 3H, C-CH3); 1.53 (s, 3H, C-CH3); 3.52
and 4.52 (AB quartet, J=18.3 Hz, 2H, S-CH2); 5.30 (d, J=5.0 Hz, 1H,
P-lactam-H); 5.95 (dd, J=5.0 and 8.1 Hz, 1H, P-lactam-H); 6.94 (s, 1H,
CH thiazol); 7.61 (s broad 2H, NH); 8.15 (s broad 2H, NH); 8.38 (s, 1 H,
CH=N); 9.74 (d, J=8.1 Hz, 1 H, NH); 11.20 (s, 1 H, OH); 12.16 (s, I H,
OH).
104 (300 MHz, DMSO-db): 1.49 (s, 3H, C-CH3); 1.51 (s, 3H, C-CH3); 3.56
and 4.52 (AB quartet, J=18.3 Hz, 2H, S-CH2); 4.90 (d, J=6.3 Hz, 2H,
CH2); 5.32 (d, J=5.0 Hz, 1H, P-lactam-H); 5.97 (dd. J=5.0 and 8.1 Hz.
1H, a-lactam-H); 6.91 (s, 1H, CH thiazol). 7.6-7.8 (m 2H. CH aromatic);
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
86
8.2-8.3 (m 1H, CH aromatic); 8.38 (s, 1H, CH=N); 8.6-8.8 (m 1H, CH
aromatic); 9.71 (d, J=8.2 Hz, 1 H, NH); 12.48 (s, 1 H, OH).
105 (300 MHz, DMSO-d6): 0.64 (m broad 2H, CHZ); 0.84 (m broad 2H, CH2);
1.50 (s, 3H, C-CH3); 1.52 (s, 3H, C-CH3); 2.61 (m broad 1H, N-CH);
3.53 and 4.53 (AB quartet, J=18.2 Hz, 2H, S-CH2); 5.31 (d, J=5.0 Hz, 1H, P-
lactam-H); 5.96 (dd, J=5.0 and 8.2 Hz, 1H, P-lactam-H); 6.90 (s,
1H, CH thiazol); 8.10 (s broad 2H, NH); 8.34 (s, 1H, CH=N); 8.60 (s
broad 1H, NH); 9.70 (d, J=8.2 Hz, 1H, NH); 12.08 (s, 1H, OH).
106 (300 MHz, DMSO-d6): 1.50 (s, 3H, C-CH3); 1.52 (s, 3H, C-CH3); 2.87
(broad 6H, N-CH3); 3.54 and 4.51 (AB quartet, J=18.1 Hz, 2H, S-CH2);
5.33 (d, J=5.0 Hz, 1H, P-lactam-H); 5.96 (dd, J=5.0 and 8.1 Hz, 1H,
P-lactam-H); 6.91 (s, 1 H, CH thiazol); 8.06 (s broad 1H, NH); 8.30 (s
broad 1 H, NH); 8.62 (s, IH, CH=N); 9.71 (d, J=8.4 Hz, 1 H, NH); 11.76
(s, 1 H, OH).
107 (300 MHz, DMSO-d6): 1.51 (s, 3H, C-CH3); 1.53 (s, 3H, C-CH3); 1.8-2.0
(m, 4H, C-CH2); 1.8-2.0 (m, 4H, N-CH2); 3.54 and 4.55 (AB quartet,
J=18.1 Hz, 2H, S-CH2); 5.31 (d, J=5.0 Hz, 1H, P-lactam-H); 5.95 (dd,
J=5.0 and 8.3 Hz, 1H, P-lactam-H); 6.90 (s, IH, CH thiazol); 7.70 (s
broad NH); 7.93 (s broad NH); 8.63 (s, 1 H, CH=N); 9.62 (d, J=8.2 Hz,
1 H, NH); 9.75 (s, 1 H, NH); 11.71 (s, 1 H, OH).
108 (300 MHz, CD3OD): 8.59 (s, 1 H, CH=N); 6.94 (s, 1 H, CH); 5.95 (d,
J=5.0 Hz, 1H, CH); 5.29 (d, J=5.0 Hz, I H, CH); 4.02 (s, 3H, OCH3);
4.34 and 3.61 (AB quartet, J=18.0 Hz, 2H, SCH2); 2.73 (s, 3H, SCH3).
109 (300 MHz, DMSO-db): 0.64 (m broad 2H, CHZ cyclopr); 0.84 (m broad
2H, CH2 cyclopr); 2.62 (m broad I H. N-CH cyclopr); 3.54 and 4.51 (AB
quartet, J=18.1 Hz, 2H, S-CH2); 4.66 (s, 2H, O-CH2); 5.30 (d, J=5.0 Hz,
1H, P-lactam-H); 5.93 (dd, J=5.0 and 8.1 Hz, IH, R-lactarn-H); 6.93 (s.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
87
1H, CH thiazol); 8.09 (broad 2H, NH); 8.35 (s, 1H, CH=N); 8.58 (broad,
1 H, NH); 9.77 (d, J=8.0 Hz, 1 H, NH); 12.04 (s, 1 H, OH).
110 (300 MHz, DMSO-d6): 2.87 (s, 6H, N-CH3); 3.56 and 4.50 (AB quartet,
J=18.0 Hz, 2H, S-CH2); 4.67 (s, 2H, O-CH2); 5.32 (d, J=5.0 Hz, 1H,
P-lactam-H); 5.92 (dd, J=5.0 and 8.1 Hz, 1H, (3-lactam-H); 6.94 (s, 1H,
CH thiazol); 8.1 (broad 1H, NH); 8.35 (broad 1H, NH); 8.63 (s, 1H,
CH=N); 9.80 (d, J=8.1 Hz, 1H, NH); 11.77 (s, 1H, OH).
111 (300 MHz, DMSO-d6): 1.93 (broad, 4H, C-CH2); 3.47 (broad, 4H,
N-CH2); 3.55 and 4.54 (AB quartet, J=17.9 Hz, 2H, S-CH2); 4.67 (s, 2H,
O-CH2); 5.30 (d, J=5.0 Hz, 1H, (3-lactam-H); 5.92 (dd, J=5.0 and 7.9 Hz,
1H, P-lactam-H); 6.94 (s, 1H, CH thiazol); 7.96 (broad 2H, NH); 8.62 (s,
1 H, CH=N); 9.79 (d, J=7.8 Hz, 1 H, NH); 11.72 (s, IH, OH).
112 (300 MHz, DMSO-d6): 3.62 and 4.67 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.37 (d, J=5.1 Hz, 1H, P-lactam-H); 5.96 (dd, J=5.1 and 7.9 Hz,
1H, P-lactam-H); 6.83 (s, 1H, CH thiazol); 7.7-7.9 (m, 1H, CH aromatic);
8.1-8.3 (m, 1 H, CH aromatic); 8.4-8.6 (m, 1 H, CH aromatic); 8.8-8.9 (m,
1 H, CH aromatic); 8.97 (s, 1 H, CH=N); 9.79 (d, J=7.9 Hz, 1 H, NH); 9.85
(s, 1 H, NH); 10.37 (s, 1 H, NH); 12.31 (s, 1 H, OH).
113 (300 MHz, DMSO-d6): 1.1-1.3 (m, 2H, CH2 cyclopr); 1.2-1.4 (m, 2H,
CH2 cyclopr); 1.9-2.0 (m, 1H, CHZ cyclopr); 3.54 and 4.49 (AB quartet,
J=18.1 Hz, 2H, S-CH2); 5.32 (d, J=5.1 Hz, 1H, P-lactam-H); 5.93 (dd,
J=5.1 and 8.0 Hz, 1H, P-lactam-H); 6.76 (s, 1H, CH thiazol); 8.59 (s, 1H,
CH=N); 9.07 (s, 1 H, NH); 9.23 (s, I H, NH); 9.67 (d, J=8.0 Hz, I H, NH);
11.92 (s, 1H, OH); 13.27 (s, IH, OH).
114 (300 MHz, bMSO-d6): 1.59 (broad, 6H, C-CH2); 3.53 (broad 4H. N-CH2);
3.6 and 4.52 (AB quartet, J=18.4 Hz, 2H, S-CH2); 5.29 (d, J=5.0 Hz. 1 H.
(3-lactam-H); 5.89 (dd, J=5.0 and 7.6 Hz. 1 H. P-lactam-H); 6.82 (s, I H.
CA 02219656 1997-10-28
WO 96135692 PCT/EP96/02023
88
CH thiazol); 8.16 (s 2H, NH); 8.60 (s, 1H, CH=N); 9.75 (d, J=7.6 Hz,
1 H, NH); 11.94 (s, 1 H, OH); 12.30 (s, 1 H, OH).
115 (300 MHz, DMSO-d6): 3.5 and 4.53 (AB quartet, J=17.9 Hz, 2H, S-CH2);
3.4-3.7 (m, 8H, N-CH2); 5.31 (d, J=5.0 Hz, 1H, (3-lactam-H); 5.90 (dd,
J=5.0 and 7.9 Hz, 1H, (3-lactam-H); 6.82 (s, 1H, CH thiazol); 8.08 (s, 1H,
CH=O); 8.38 (broad 2H, NH); 8.62 (s, 1H, CH=N); 9.75 (d, J=7.9 Hz,
1 H, NH); 12.18 (s, 1H, OH); 12.28 (s, 1H, OH).
116 (300 MHz, DMSO-d6/D2O): 2.81 (s, 6H, N-CH3); 2.92 (s, 3H, C=N-CH3);
3.54 and 4.58 (AB quartet, J=18.1 Hz, 2H, S-CH2); 3.6 (broad, 2H,
N-CH2); 3.97 (broad, 2H, N-CH2); 5.30 (d, J=4.8 Hz, 1H, (3-lactam-H);
5.90 (d, J=4.8 Hz, 1 H, P-lactam-H); 6.81 (s, 1 H, CH thiazol); 8.55 (s, 1H,
CH=N).
117 (300 MHz, DMSO-d./DZO): 2.91 (s, 3H, C=N-CH3); 3.19 (s, 9H, N-CH3);
3.29 (broad, 2H, N-CH2); 3.56 and 4.48 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 3.82 (broad, 2H, N-CH2); 5.31 (d, J=5.0 Hz, 1 H, (3-lactam-H);
5.90 (d, J=5.0 Hz, IH, P-lactam-H); 6.84 (s, 1H, CH thiazol); 8.56 (s, 1H,
CH=N).
118 (300 MHz, DMSO-d6): 3.61 and 4.59 (AB quartet, J=18.0 Hz, 2H,
S-CH2); 5.35 (d, J=5.1 Hz, 1H, (3-lactam-H); 5.59 (dd, J=5.1 and 7.9 Hz,
1H, P-lactam-H); 6.82 (s, 1H, CH thiazol); 6.9-7.1 (m, 1H, CH aromatic);
7.2-7.4 (m, 2H, CH aromatic); 8.74 (s, 1H, CH=N); 9.31 (s 1H, NH/OH);
9.76 (s 1 H, NH/OH); 9.78 (d, J=7.9 Hz, 1 H, NH); 12.25 (s, 1 H, OH);
13.03 (s, 1 H, OH).
119 (300 MHz, DMSO-d6): 2.30 (s, 3H, C-CH3); 3.59 and 4.57 (AB quartet.
J=18.1 Hz, 2H, S-CH2); 3.67 (s, 3H, N-CH3); 5.34 (d. J=5.1 Hz, 1 H,
P-lactam-H); 5.94 (dd, 3=5.1 and 8.0 Hz. IH. R-lactam-H); 6.13 (d.
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
89
J=3.9 Hz 1 H, CH Pyrrol); 6.77 (s, 1 H, CH thiazol); 6.86 (d, J=3.9 Hz 1 H,
CH Pyrrol); 8.66 (s, 1 H, CH=N); 9.25 (s 1 H, NH); 9.46 (s 1 H, NH); 9.70
(d, J=8.0 Hz, 1H, NH); 11.96 (s, 1H, OH); 12.90 (s, 1H, OH).
120 (300 MHz, DMSO-d6): 3.71 and 4.12 (AB quartet, J=17.9 Hz, 2H,
S-CH2); 5.32 (d, J=5.1 Hz, 1H, (3-lactam-H); 5.91 (dd, J=5.0 and 7.9 Hz,
1H, (3-lactam-H); 6.84 (s, 1H, CH thiazol); 7.9-8.0 (m, 2H, CH aromatic);
8.66 (s, 1 H, CH=N); 8.8-8.9 (m, 2H, CH aromatic); 8.8 (broad 1H, NH);
9.76 (d, J=8.0 Hz, 1H, NH); 12.17 (s, 1H, OH); 12.37 (s, 1H, OH).
121 (300 MHz, DMSO-d6): 3.22 (s, 6H, N-CH3); 3.54 and 4.55 (AB quartet,
J=18.5 Hz, 2H, S-CH2); 3.6 (broad, 4H, N-CH2); 4.0 (broad, 4H, N-CH2);
5.30 (d, J=5.0 Hz, 1H, P-lactam-H); 5.91 (dd, J=5.0 and 7.9 Hz, 1 H,
P-lactam-H); 6.82 (s, 1H, CH thiazol); 8.73 (s, 1H, CH=N); 9.75 (d,
J=7.6 Hz, 1 H, NH); 12.30 (s, 1 H, OH); 12.76 (s, 1 H, OH).
122 (300 MHz, DMSO-d6): 2.76 (s, 3H, N-CH3); 3.1-3.3 (broad, 2H, N-CH2);
3.4-3.6 (broad, 2H, N-CH2); 3.5-3.7 (broad, 2H, N-CH2); 3.55 and 4.53
(AB quartet, J=18.1 Hz, 2H, S-CH2); 4.2-4.4 (broad, 2H, N-CH2); 5.31 (d,
J=5.0 Hz, 1 H, P-lactam-H); 5.90 (dd, J=5.0 and 7.8 Hz, IH, P-lactam-H); =
6.82 (s, 1 H, CH thiazol); 8.66 (s, 1H, CH=N); 9.77 (d, J=7.8 Hz, IH,
NH); 11.74 (s, 1 H, NH); 12.36 (s, 1 H, OH); 12.56 (s, IH, OH).
123 (300 MHz, DMSO-db): 2.90 (d, J=4.7 Hz 3H, N-CH3); 3.34 (s, 3H,
N-CH3); 3.55 and 4.59 (AB quartet, J=18.1 Hz, 2H, S-CH2); 5.30 (d,
J=5.0 Hz, IH, (3-lactam-H); 5.91 (dd, J=5.0 and 7.9 Hz, 1 H, P-lactam-H);
6.81 (s, 1H, CH thiazol); 8.09 (s, IH, CH=N); 8.25 (s, 2H, NH); 8.37 (s,
1 H, NH); 9.72 (d, J=7.9 Hz, IH, NH); 12.14 (s, IH, OH).
124 (300 MHz, DMSO-d6): 2.81 (d, J=4.3 Hz 6H, N-CH3); 3.2-3.4 (m broad
2H, N-CH2); 3.56 and 4.55 (AB quartet. J=18.0 Hz, 2H. S-CH2); 3.7-3.9
(m broad 2H. N-CH2); 5.30 (d. 3=4.9 Hz. 1H, P-lactam-H); 5.9 (dd. J=4.9
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
and 7.9 Hz, 1H, (3-lactam-H); 6.82 (s, 1H, CH thiazol); 8.3 (broad, NH);
8.38 (s, 1 H, CH=N); 8.47 (broad, NH); 9.76 (d, J=7.9 Hz, 1 H, NH);
10.84 (s, 1 H, NH); 12.31 (s, 2H, OH).
125 (300 MHz, DMSO-d6): 2.82 (d, J=4.5 Hz 6H, N-CH3); 3.2-3.3 (m broad
2H, N-CH2); 3.40 (s, 3H, N-CH3); 3.56 and 4.73 (AB quartet, J=18.3 Hz,
2H, S-CH2); 3.8-3.9 (m broad 2H, N-CH2); 5.29 (d, J=5.0 Hz, 1H,
P-lactam-H); 5.91 (dd, J=5.0 and 7.8 Hz, 1H, P-lactam-H); 6.82 (s, 1H,
CH thiazol); 8.11 (s, 1H, CH=N); 8.68 (s, 2H, NH); 8.74 (m broad 1H,
NH); 9.77 (d, J=7.9 Hz, 1H, NH); 10.91 (s, 1H, OH); 12.32 (s, 1H, OH).
126 (300 MHz, DMSO-d6): 3.60 and 4.56 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.32 (d, J=5.0 Hz, 1H, P-lactam-H); 5.92 (dd, J=5.0 and 7.9 Hz,
1 H, P-lactam-H); 6.83 (s, 1 H, CH thiazol); 6.8-6.9 (m, 1 H, CH aromatic);
7.1-7.2 (m, 1 H, CH aromatic); 7.3-7.4 (m, 1 H, CH aromatic); 8.23 (s, 1 H,
CH=N); 8.37 ( 2H, NH/OH); 8.51 (s, 1H, CH=N); 9.78 (d, J=7.9 Hz, 1H,
NH); 12.27 (s, 1H, OH).
127 (300 MHz, DMSO-d6): 3.53 and 4.49 (AB quartet, J=18.1 Hz, 2H,
S-CH2); 5.29 (d, J=5.0 Hz, 1H, P-lactam-H); 5.89 (dd, J=5.0 and 7.9 Hz,
1H, P-lactam-H); 6.79 (s, 1H, CH thiazol); 7.93 (broad, 2H, NH); 8.37
(broad, 1 H, CH=N); 9.73 (d, J=7.8 Hz, 1 H, NH); 12.15 (s, 1 H, OH).
128 (300 MHz, DMSO-d6): 3.25 (broad, 4H, N-CH2); 3.31 (s, 3H, N-CH2);
3.62 and 4.27 (AB quartet, J=18.0 Hz, 2H, S-CH2); 3.74 (broad, 4H,
N-CH2); 5.30 (d, J=5.0 Hz, 1H, P-lactam-H); 5.89 (dd, J=4.9 and 7.9 Hz,
IH, P-lactam-H); 6.79 (s, IH, CH thiazol); 8.11 (s, IH, CH=N); 9.03
(broad, 1H, NH); 9.31 (broad, 1H, NH); 9.67 (d, J=7.9 Hz, 1H, NH); 9.87
(s, 2H, NH); 12.07 (s, 1 H, OH).
129 (300 MHz, DMSO-d6): 0,70 (m; 4H, -CH,-CH2-); 3,05 (m, I H); 3,51 and
4,49 (AB quartet, J=18 Hz, 2H. SCH=); 4.38 (s. 3H. O-CH3); 5,24 (d,
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
91
J=4,9 Hz,1H, B-lactam-H); 5,84 (dd, J=7,9 Hz and 4,9 Hz, 1H, B-lactam-
H); 6,86 (s, 1 H, thiazolyl-H); 8,19 (d, J=3,9 Hz, 1 H); 8,21(s, 1 H, CH=N);
9,72 (d, J=8,0 Hz, 1H, NH);11,58 (s, 1H).
130 (300 MHz, CD3CN + D20): 1,26 (t, J=7 Hz, 3H); 1,68 (sextet, J=7 Hz,
2H); 1,93 (quintet, J=7 Hz, 2H); 3,93 (t, J=7,1 Hz, 2H); 3,95 (s, 3H, 0-
CH3); 3,98 and 4,57 (AB quartet, J=18 Hz, 2H, SCH2); 5,59 (d, J=4,9 Hz,
1 H, B-lactam-H); 6,18 (d, J=4,9 Hz, 1 H, B-lactam-H); 7,40 (s, IH,
thiazolyl-H); 8,63 (s, 1 H, CH=N).
131 (300 MHz, D20): 0,74 (m, 2H); 0,88 (m, 2H); 2,58 and 2,38 (two singu-
lets, 3H, SCH3); 2,68 (m, 1H); 3,45 and 3,94 (AB-system, broad, 2H,
SCH2); 3,95 (s, 3H, O-CH3); 5,23 (d, J=4,7 Hz, 1H, B-lactam-H); 5,75 (d,
J=4,7 Hz, 1H, B-lactam-H); 7,03 (s, 1H, thiazolyl-H); 8,36 (s, broad, IH,
CH=N).
132 (300 MHz, D20): 0,82 (t, J=7,3 Hz, 3H); 1,29 (sextet, J=7 Hz, 2H); 1,56
(quintet, J=7 Hz, 2H); 2,61 and 2,46 (two singulets, 3H, SCH3); 3,46 (t,
J=7,1 Hz, 2H); 3,55 and 4,01 (AB quartet, J=18 Hz, 2H, SCH2); 3,98 (s,
3H, O-CH3); 5,25 (d, J=4,9 Hz, 1H, B-lactam-H); 5,78 (d, J=4,9 Hz, 1H,
B-lactam-H); 7,05 (s, 1H, thiazolyl-H); 8,39 (s, IH, CH=N).
133 (300 MHz, DMSO-d6): 2,68 (m, 2H); 3,73 (m, 2H); 3,57 and 4,23 (AB
quartet, J=18 Hz, 2H, SCH2); 3,96 (s, 3H, O-CH3); 5,29 (d, J=4,9 Hz, 1H,
B-lactam-H); 8,48 (dd, J=8 Hz and J=4,9 Hz, 1H, B-lactam-H); 6,91 (s,
IH, thiazolyl-H); 8,24 (s, IH, CH=N); 9,20 (s, 1 H); 9,90 (d, J=8,0 Hz,
1 H NH).
134 (300 MHz, DMSO-d6+ D20): 0,68 (m, 2H); 0,84 (m, 2H); 2,91 (m, I H);
3.62 and 4.22 (AB quartet, J=18 Hz, 2H. SCHZ); 5,28 (d, J=4,9 Hz, 1 H,
B-lactam-H); 5.85 (d, J=4,8 Hz. IH, B-lactam-H); 7.06 (s, I H, thiazolyl-
H); 8.23 (s. 1H, CH=N).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
92
135 (300 MHz, DMSO-d6): 0,89 (t, J=7 Hz, 3H); 1,29 (sextet, J=7 Hz, 2H);
1,54 (quintet, J=7 Hz, 2H); 3,51 and 4,47 (AB quartet, J=18 Hz, 2H,
SCH2); 3,52 (m, 2H); 5,24 (d, J=4,8 Hz, 1H, B-lactam-H); 5,85 (dd,
J=7,9 Hz and 4,8 Hz, 1H, B-lactam-H); 6,69 (s, 1H, thiazolyl-H); 8,21 (s,
1H, CH=N); 8,47 (m, 1H); 9,55 (d, J=7,9 Hz, 1H, NH); 11,44 (s, 1H);
11,54 (s, 1H).
136 (300 MHz, DMSO-d6): 2,77 (s,3H, NCH3); 3,0-3,2 (m, 4H); 3,35-3,6 (m,
4H); 3,63 and 4,03 (AB quartet, J=18 Hz, 2H, SCH2); 3,95 (s, 3H,
OCH3); 5,26 (d, J=4,9 Hz, 1H, B-lactam-H); 5,84 (dd, J=7,9 Hz and J=4,9
Hz, 1H, B-lactam-H); 6,85 (s, 1H, thiazolyl-H); 8,40 (s, 1H, CH=N); 9,69
(d, J=8,0 Hz, 1H, NH); 11,67 (s, 1H).
137 (300 MHz, DMSO-d6): 3.59 and 4.54 (AB quartet, J = 18.2 Hz, 2H,
SCH2); 3.66 (d, J = 4 Hz, 3 H, NHCH3); 3.95 (s, 3 H, O-CH3); 5.26 (d, J
= 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-lactam-H);
6.95 (s, 1 H, thiazolyl-H); 7.96 (s, I H, CH=N); 8.4 (d, J = 4 Hz,
NHCH3); 9.84 (d, J = 8 Hz, NH).
138 (300 MHz, DMSO-d6): 1,43 (s, 9H, -OC(CH3)3); 3.62 and 4.02 (AB
quartet, J = 17.8 Hz, 2H, SCH2); 5.25 (d, J = 4.9 Hz, 1 H, j3-lactam-H);
5.83 (dd, J= 4.9 and 8,0 Hz, 1H, P-lactam-H); 6,93 (s, 1H, thiazolyl-H);
8,20 (s, 1 H, CH=N); 9,69 (d, J= 8,0 Hz, 1 H, NH).
139 (300 MHz, DMSO-d6): 3.21 (broad singulet, 4 H); 3.89 (broad singulet, 4
H); 3.50 and 4.53 (AB quartet, J= 18.1 Hz, 2H, SCHZ); 5.27 (d, J= 5
Hz, I H, B-lactam-H); 5.77 (d, J= 58 Hz, 2H, -CH2F); 5.90 (dd, J = 5 Hz
and 8.2 Hz, B-lactam-H); 8.66 (s, 1 H, CH=N); 9.85 (d, J= 8.2 Hz, NH).
140 (300 MHZ, DMSO-d6): 1.02 (t, J=7.4 Hz. 3H, C-CH3); 2.32 (qd, J=7.4
and 7.5 Hz, 2H, C=C-CH:-C); 3.52 and 4.15 (AB, J=17.7 Hz, 2H, S-
CH2): 5.17 (d. 3=5.2 Hz, 1H. P-lactam-H); 5.73 (dd, J=5.2 and 8.8 Hz,
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
93
1H, P-lactam-H); 6.48 (s, 1H, CH thiazol); 6.61 (t, J=7.5 Hz, 1H,
C=CH-C); 8.93 (s, 1H, CH=N); 9.14 (d, J=8.8 Hz, 1H, NH).
141 (90 MHz, DMSO-d6): 2.3 (s, 3 H, CH3); 1.8 to 2.1 (m, 1 H); 3.95 (s, 2H,
SCH2); 3.9 (s, 3 H, O-CH3); 5.35 (d, J = 5 Hz, 1 H, B-lactam-H); 5.9 (dd,
J = 5 Hz and 8 Hz, B-lactam-H); 6.95 (s, 1 H, thiazolyl-H); 8.65 (s, 1 H,
CH=N); 9.9 (d, J = 8 Hz, NH).
142 (90 MHz, DMSO-d6): 2.3 (s, 3 H, thiazolyl-CH3); 4.0 (s, 3 H, O-CH3);
3.75 and 4.3 (AB quartet, J = 18 Hz, 2H, SCH2); 5.4 (d, J = 5 Hz, 1 H,
B-lactam-H); 5.95 (dd, J = 5 Hz and 8 Hz, B-lactam-H); 6.7 (s, 1 H,
thiazolyl-H); 7.05 (s, 1 H, thiazolyl-H); 8.55 (s, 1 H, CH=N); 9.95 (d, J=
8 Hz, NH).
143 (90 MHz, DMSO-d6): 2.25 (s, 3 H, thiazolyl-CH3); 3.60 (s, 3 H, N-CH3);
3.7 and 4.15 (AB quartet, J = 18 Hz, 2H, SCH2); 3.95 (s, 3 H, O-CH3);
5.35 (d, J = 5 Hz, 1 H, B-lactam-H); 5.85 (dd, J = 5 Hz and 8 Hz, B-
lactam-H); 6.7 (s, 1 H, thiazolyl-H); 7.02 (s, 1 H, thiazolyl-H); 8.15 (s, 1
H, CH=N); 9.95 (d, J= 8 Hz, NH).
144 (300 MHz, DMSO-d6): 2.83 (d, 3H, NCH3); 3.55 and 4.23 (AB quartet, J
= 19.8 Hz, 2H, SCH2); 3.84 (s, 3H, =N-OCH3); 5.21 (d, J= 5.5 Hz, 1 H,
B-lactam-H); 5.70 (dd, J= 5.5 Hz and 9 Hz, B-lactam-H); 6.77 (s, I H,
thiazolyl-H); 9.28 (s, 1 H, CH=N); 9.63 (d, J= 9 Hz, 1 H, NH).
145 (300 MHz, DMSO-d6):
Diastereomer A: 1.25 (d, J = 6 Hz, 3H); 1.24 (d, J= 6 Hz, 3H); 1.53 (d,
J= 5.4 Hz, 3H, -O(Cli3)CH-O-); 2.9 (d, J = 4,9 Hz, 3H, NCH3); 3.62
and 4.61 (AB quartet, J = 18.3 Hz, 2H, SCHZ); 3.94 (s, 3H, =N-OCH3);
4.75 to 4.84 (m, I H, -O-CH(CH3)2); 5.34 (d, J = 5 Hz, IH, B-lactam-H);
5.94 (dd, J = 5 Hz and 7.8 Hz, B-lactam-H); 6.9 (q. J = 5.3 Hz, I H. -
O(CH3)Cli-O-); 6.92 (s, I H. thiazolyl-H); 8.3 (s. I H. CH=N); 9.96 (d. J
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
94
=7.8Hz, 1H, NH).
Diastereomer B: 1.24 (d, J = 6 Hz, 3H); 1.22 (d, J = 6 Hz, 3H); 1.51 (d, J
= 5.5 Hz, 3H, -O(CH3)CH-O-); 2.9 (d, J = 4,9 Hz, 3H, NCH3); 3.60 and
4.65 (AB quartet, J = 18.3 Hz, 2H, SCH2); 3.93 (s, 3H, =N-OCH3); 4.75
to 4.84 (m, 1 H, -O-CH(CH3)Z); 5.30 (d, J = 5 Hz, 1H, B-lactam-H); 6.04
(dd, J = 5 Hz and 7.6 Hz, B-lactam-H); 6.8 (q, J = 5.3, 1 H; O(CH3)CH,-
O-); 6.92 (s, 1 H, thiazolyl-H); 8.14 (s, 1 H, CH=N); 9.95 (d, J = 7.6 Hz,
1 H, NH).
146 (300 MHz, DMSO-d6):
Diastereomer A: 1.25 (d, J = 6 Hz, 6H); 1.50 (d, J = 5.4 Hz, 3H, -
O(CH3)CH-O-); 2.18 (s, 3H, CH3CO); 3.76 and 4.48 (AB quartet, J
17.9 Hz, 2H, SCHZ); 4.7 to 4.9 (m, 1 H, -O-CH(CH3)Z); 5.31(d, J = 4.8
Hz, 1H, B-lactam-H); 5.88 (dd, J = 4.8 Hz and 7.6 Hz, B-lactam-H);
6.87 (q, J = 5.3 Hz, 1 H, -O(CH3)CH-O-); 7.1 (s, 1 H, thiazolyl-H); 8.28
(s, 1 H, CH=N); 9.93 (d, J = 7.6 Hz, 1H, NH).
Diastereomer B: 1.23 (d, J = 6 Hz, 6H); 1.49 (d, J = 5.4 Hz, 3H, -
O(CH3)CH-O-); 2.17 (s, 3H, CH3CO); 3.70 and 4.38 (AB quartet, J = 18
Hz, 2H, SCH2); 4.7 to 4.9 (m, 1 H, -O-CH(CH3)2); 5.28 (d, J = 4.8 Hz,
1H, B-lactam-H); 5.83 (dd, J = 4.8 Hz and 7.6 Hz, B-lactam-H);6.80 (q,
J= 5.2, 1 H,-O(CH3)CH-O-); 7.1 (s, 1 H, thiazolyl-H); 8.18 (s, 1 H,
CH=O); 9.91 (d, J = 7.6 Hz, 1 H, NH).
147 (300 MHz, DMSO-d6 )
Diastereomer A: 1.26 (d, J = 6.2 Hz, 6H); 1.53 (d, J = 5 Hz, 3H, -
O(CH3)CH-O-); 2.29 (s, 6H, 2 aryl-CH3); 3.60 and 4.54 (AB quartet, J
18.5 Hz, 2H, SCH2); 4.75 to 4.84 (m, I H, -O-CH(CH,)Z); 5.34 (d, J = 5
Hz, 1 H, B-lactam-H); 5.97 (dd, J = 5 Hz and 7.7 Hz, B-lactam-H); 6.91
(q, J = 5.3 Hz, I H, -O(CH3)CH-O-); 6.92 (s, I H, thiazolyl-H); 7.12 and
7.49 (AB quartet, J = 8 Hz, 2 x 4 aromatic-H); 8.34 (s, I H, CH=N); 9.69
(d. J = 7.7 Hz, 1 H. NH).
Diastereomer B: 1.24 (d, J = 6.2 Hz. 6H); 1.52 (d, J = 5.5 Hz. 3H.
-
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
O(CH3)CH-O-); 2.29 (3. 6 H, 2 Aryl-CH3); 3.59 and 4.51 (AB quartet, J
= 18.4 Hz, 2H, SCH2); 4.75 to 4.84 (m, 1 H, -O-CH(CH3)2); 5.31 (d, J=
5 Hz, 1H, B-lactam-H); 5.93 (dd, J = 5 Hz and 7.7 Hz, B-lactam-H); 6.83
(q, J= 5.3, 1 H; O(CH3)CH-O-); 6.84 (s, 1 H, thiazolyl-H); 7.12 and 7.49
(AB quartet, J= 8 Hz, 2 x 4 aromatic-H); 8.24 (s, 1 H, CH=N); 9.69 (d, J
= 7.7 Hz, 1H, NH).
A) a) (D20 + DCI): 3.62 (AB quartet, J=16Hz, 2H, S-CH2); 5.10 (2d, J=5Hz,
2H, B-lactam-H); 6.20 (s, broad, 1H, O-CH-O).
A) c) (DMSO-d6): 3.55 and 3.73 (AB quartet, J=18Hz) resp. 3.70 (s), (2H,
S-CH2); 3.87 (s, 3H, N-O-CH3), 5.11 (d, J=5Hz, B-lactam-H); 5.87 (m,
1H, B-lactam-H); 6.20 resp. 6.26 (s, 1H, O-CH-O); 6.77 resp. 6.78 (s, 1H,
thiazolyl-H);7.27-7.35 (m, 15H, Ar-H); 9.6 (s, broad, 1H, NH-thiazolyl);
9.72 resp. 9.74 (d, J=8Hz, 1 H, NH).
A) d) (DMSO-d6): 3.58 and 3.76 (AB quartet, J=18Hz) resp. 3.72 (s), (2H,
S-CH2); 3.88 (s, 3H, N-0-CH3), 5.15 (d, J=5Hz, B-lactam-H); 5.94 (dd,
J=8Hz and 5Hz, 1 H, B-lactam-H); 6.21 resp. 6.28 (s, 1 H, O-CH-O); 6.81
resp. 6.82 (s, 1H, thiazolyl-H); 9.77 resp. 9.78 (d, J=8Hz, 1H, NH).
B) c) (CDC13): 3.2- 3,5 (m, 2H, S-CH2); 5.05 (d, J=5Hz, B-lactam-H); 6,0 (dd,
J= 5 and 8Hz, 1H, B-lactam-H); 6.4 (s, 1H, O-CH-O); 7-7.4 (m, 30H,
Ar-H).
B) d) (DMSO-d6): 3.72 (m, 2H, S-CH2); 5.15 (d, J=5Hz, B-lactam-H); 5.95
(dd,J=8Hz and 5Hz, 1H, B-lactam-H); 6.3 (broad s, 1H, O-CH-O); 6.8 (s,
IH, thiazolyl-H); 9.75 (d,J=8Hz, IH, NH).
C) (300 MHz; DMSO-d6): 3.55 and 3.77 (AB quartet, J=18Hz) resp. 3.71 (s),
(2H, S-CH2); 5.14 (d, J=SHz, B-lactam-H); 5.97 (m, 1H, B-lactam-H); 5.79
(d, J=55 Hz, 2H, -CHZF); 6.20 resp. 6.27 (s, IH, O-CH-O); 9.81 resp. 9.84
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
96
(d, J=8Hz, 1H, NH).
D) (300 MHz, DMSO-d6): 2.20 resp. 2.21 (s, 3H, O=C-CH3); 3.63 and 3.80 (AB
quartet, J=18 Hz) resp. 3.76 (s) (2H, S-CH2); 5.20 (d, J=5Hz, 1H, B-lactam-
H); 6,00 (dd, J=8Hz and 5Hz, 1H, B-lactam-H); 6,23 resp. 6,29 (s, 1H, O-
CH-O); 7.16 resp. 7.17 (s, 1H, CH thiazol); 10,04 resp. 10,05 (d, J=8Hz, 1H,
NH).
E) (300 MHz, DMSO-d(,): 3.58 and 3.79 (AB quartet, J=18.2Hz) resp. 3.75 (s)
(2H, S-CH2); 5.17 (d, J=5Hz, 1H, B-lactarn-H); 5.94 (dd, J=8Hz and 5Hz,
1H, B-lactam-H); 6,21 resp. 6,28 (s, 1H, O-CH-O); 6.85 resp. 6.86 (s, 1H,
CH thiazol); 9.74 (d, J=8Hz, 1 H, NH); 12.38 (s, 1 H, OH).
F) c) (300 MHz, DMSO-d6): 1.4 (2s, 6H, C-(CH3)2); 1.5 (s, 9H, C-(CH3)3);3.6
and
3.7 (AB quartet, J=18 Hz) resp. 3.7 (s) (2H, S-CH2); 5.2 (d, J=5Hz, 1H, B-
lactam-H); 5.9 (dd, J=8Hz and 5Hz, 1H, B-lactam-H); 6,2 resp. 6,3 (s, 1H,
O-CH-O); 6.8 (s, 1H, CH thiazol); 7.2-7.5 (m, 15H, CH aromatic); 9.6 (d,
J=8Hz, 1H, NH).
F) d) (300 MHz, DMSO-d6): 1.48 resp. 1.50 (s, 6H, C-(CH3)2,); 3.60 and 3.77
(AB
quartet, J=18 Hz) resp. 3.74 (s) (2H, S-CH2); 5.19 (d, J=5.2Hz, 1H, B-lac-
tam-H); 6,01 (dd, J=8.5Hz and 5.2Hz, 1H, B-lactam-H); 6,23 resp. 6,29 (s,
1H, O-CH-O); 6.87 resp. 6.88 (s, 1H, CH thiazol); 9.67 (d, J=8.5Hz, IH,
NH).
G) (300 MHz, DMSO-db): 1.00 (t, J=7.5 Hz, 3H, C-CH3); 1.47 (s, 9H,O-C(-
CH3)3); 2.27 (qd, J=7.5 Hz, 2H, C=C-CHZ-C); 3.57 and 3.74 (AB
quartet, J=18,3 Hz) resp. 3.73 (s) (2H, S-CH2); 5.11 (d, J=5.1 Hz, 1H, ~-
lactam-H); 5.88 (dd, J=8.5 Hz and 5.1 Hz, 1 H, P-lactam-H); 6,22 resp.
6,26 (s, I H, O-CH-O); 6.56 U. J=7.5 Hz, 1 H, C=CH-C); 7.05 (s, 1 H, CH
thiazol); 8.80 resp. 8.81 (d, J=8.4 Hz. 1H. NH).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
97
H) a) (300 MHz, DMSO-d6): 2.7 (s, 3H, S-CH3); 3.5-3.6 (m, 4H, N-CH2); 3.7-
3.8 (m, 2H, N-CH2); 3.8-3.9 (m, 2H, N-CH2); 8.1 (s, 1H, CH=O); 9.6
(broad, 2H, NH).
H) b) (300 MHz, DMSO-d6): 3.42 (s, 4H, N-CH2); 3.4-3.6 (m, 4H, N-CH2); 4.8
(broad, 2H, NH); 7.9 (broad, 2H, NH); 8.1 (s, 1 H, CH=O); 9.5 (broad,
1H, NH).
H) c) (300 MHz, DMSO-d6): 3.1-3.2 (s, 4H, N-CH2); 3.7-3.8 (m, 4H, N-CH2);
4.8 (broad, 2H, NH); 8.0 (broad, 2H, NH); 9.6 (broad, 1H, NH); 10.0
.(broad, 2H, NH).
I) (90 MHz, D20): 1,2 ppm (t, 3H); 1,9 - 2,1 ppm (m, 4H); 3,3 - 3,7 ppm
(m, 6H).
J) (90 MHz, DMSO-d6): 2,9 ppm (d, J = 5 Hz, 3H, NCH3), 3,4 - 3,8 ppm
(m, 8H), 7,55 ppm (broad quartet, 1 H, NH).
K) (90 MHz, D20): 1,3 ppm (s, 9H).
L) (90 MHz, DMSO-d6 + D20): 2,8 ppm (s, 3H, NCH3); 3,4 - 3,65 ppm
(m, 4H); 4,0 - 4,4 ppm (m, 4H).
M) (300 MHz, DMSO-d6): 2.74 (s, 3H, C=N-CH3); 3.15 (s, 9H, N(CH3)3);
3.49 (m broad, 2H, N-CH2); 3.64 (m broad, 2H, N-CH2); 4.8 (broad, 2H,
NH); 7.8 (broad, 3H, NH).
N) (90 MHz, DMSO-d6): 2.85 ppm (s, 3H, NCH3); 3,2 - 3,65 ppm (m. 8H);
8,1 ppm (s, 1 H, CH=O).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
98
0) (90 MHz, DMSO-d6 + D20): 2,85 ppm (s, 3H, NCH3); 3,2 - 3,5 ppm
(m, 4H); 3,5 -3,9 ppm (m, 4H).
P) (300 MHz, D20): 2.84 (s, 3H, N-CH3); 3.3-3.4 (m, 2H, N-CH2); 3.7-3.8
(m, 2H, N-CH2). ,
Q) a) (90 MHz, DMSO-d6): 2,65 ppm (s, 3H, S-CH3); 3,35 ppm (s, 6H,
NCH3)2); 3,65 - 4,0 ppm (m, 4H); 4,0 - 4,3 ppm (m, 4H), 9,45 ppm
(broad singulet, 1 H, NH).
Q) b) (90 MHz, DMSO-d6): 3,3 ppm (s, 6H, NCH3)2); 3,5 - 3,8 ppm (m, 4H);
3,8 - 4,2 ppm (m, 4H).
R) a) (90 MHz, DMSO-d6): 2,55 ppm (s, 3H, SCH3); 3,45 ppm (s, 3H,
NCH3).
R) b) (90 MHz, DMSO-d6): 3,15 ppm (s, 3H, NCH3); 3,2 - 3,28 ppm (m, 2H);
3,28 - 3,35 ppm (m, 2H); 3,4 - 3,55 ppm (m, 4H); 5,18 ppm (broad
singulet, 2H); 8,05 (s, 1H, -CH=O); 8,1 - 8,3 (broad singulet, 2H).
R) c) (300 MHz, DMSO-d6): 3,16 ppm (m, 3 + 4 H); 3,63 ppm (m, 4H); 6,7
ppm (broad singulet, 5H); 8,5 (broad singulet, 1 H); 10,0 ppm (broad
singulet, 2H).
S) (300 MHz, DMSO d6): 2.55 (s, 2H, N-CH2); 5.92 (s, 2H, NH).
T) (300 MHz, DMSO d6): 0.5 (m, 2H, CH2); 0.7-0.8 (m, 2H, CH2); 2.4-2.5
(m, IH, N-CH); 4.7 (broad, 2H. NH); 7.5 (broad, 2H, NH); 8.2 (broad,
1 H. NH); 8.9 (broad, 1 H, NH).
U) (300 MHz, DMSO d6): 2.7 (s. 3H. N-CH3); 4.7 (broad. 2H. NH); 7.7
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96/02023
99
(broad, 1H, NH); 9.2 (broad, 1 H, NH/OH); 9.8 (broad, 1 H, NH/OH).
V) (300 MHz, DMSO d6): 2.79 (d, J=4.8Hz 6H, N(CH3)2); 3.20 (s, 3H, N-
CH3); 3.2 (m, 2H, N-CH2); 3.6 (m, 2H, N-CH2); 4.7 (very broad, 2H,
NH); 7.7 (broad, 2H, NH); 10.4 (broad, 1H, NH).
W) (300 MHz, D20): 6.75-6.85 (m, 1 H, CH aromatic); 6.9-7.0 (m, 1 H, CH
aromatic); 7.1-7.15 (m, 1 H, CH aromatic); 7.7 (s, 1 H, CH=N).
X) (300 MHz, D20): 2,0 (m, 1H); 2,47, 2,35 (s, s, together 3H, -SCH3); 0,84
(m, 2H); 0,69 (m, 2H).
Y) (300 MHz, D20): 3,36 (t, J=7 Hz, 2H); 2,51, 2,43 (s, s, together 3H, -
SCH3); 1,55 (quintet, J=7 Hz, 2H); 1,29 (sextet, J=7 Hz, 2H); 0,85 (t, J=7
Hz, 3H).
Z) (90 MHz, DMSO-d6): 3,65 ppm (s, 3H, NCH3).
AA) a) (300 MHz, DMSO d6): 2.19 (s, 3H, C-CH3); 3.84 (s, 3H, N-CH3); 5.84 (d,
J=3.8Hz 1H, C=CH); 6.58 (d, J=3.8Hz 1Hz, C=CH); 8.76 (s, 1H, NH);
8.93 (s, 1 H, NH).
AA) b) (300 MHz, DMSO d6): 2.3 (s, 3H, C-CH3); 2.75 (s, 3H, S-CH3); 3.65 (s,
3H, N-CH3); 6.2 (d, J=4Hz 1 H, C=CH); 7.1 (d, J=4Hz 1 Hz, C=CH); 10.6
(s, broad 5H, NH).
AA) c) (300 MHz, DMSO d6): 2.2 (s, 3H, C-CH3); 3.1 (s, 3H, N-CH3); 5.95 (d,
J=4Hz 1H, C=CH); 6.5 (d, J=4Hz 1Hz, C=CH); 7.2 (very broad 5H, NH).
~ - -
AB) (300 MHz, DMSO d(/DZO): 6.75-6.85 (m, 1H, CH aromatic); 7.00-7.05
(m, I H. CH aromatic); 7.05-7.10 (m. 1 H. CH aromatic).
CA 02219656 1997-10-28
WO 96/35692 PCT/EP96102023
100
AC) (300 MHz, DMSO d6/D20): 8.24 (s, 1H, CH=N); 5.20 (d, J=5.2 Hz, 1H,
B-lactam-H); 4.07 (d, J=5.2 Hz, 1H, B-lactam-H); 3.89 and 3.61 (ABq,
J=17.8 Hz, 2H, SCH2). AD) (300 MHz, DMSO d6/CD3CO2D+CF3COOD): 8.67 (s, 1H,
CH=N); 5.38-
5.40 (2d, 2H, 2B-lactam-H); 4,01 (s, 3H, CH3-O); 3,98-4.00 (ABq, 2H,
SCHZ).
AE) (300 MHz, DMSO d6): 8.14 (s, 1H, CH=N); 5.33 (d, J=5.6 Hz, 1H, CH);
4.80 (d, J=5.6 Hz, 1H, CH); 3.88 and 3.58 (ABq, J=17.8 Hz, 2H, SCH2).
AF) (300 MHz, DMSO d6): 7.96 (s, 1H, CH=N); 5.17 (d, J=5.2 Hz, 1H, CH);
5.02 (d, J=5.2 Hz, 1H, CH); 3.96 and 3.47 (ABq, J=17.7 Hz, 2H, SCHZ).
AG) (300 MHz, DMSO d6): 8.35 (s, 1H, CH=N); 5.31 (d, J=5.1 Hz, 1H, CH);
5.14 (d, J=5.1 Hz, 1H, CH); 4.28 and 3.84 (ABq, J=17.9 Hz, SCH2).
AH) (300 MHz, DMSO d6): 8.41 (s, 1H, CH=N); 5.34 (d, J=5.1 Hz, 1H, CH);
5.18 (d, J=5.1 Hz, 1H, CH); 4.37 and 3.80 (ABq, J=17.9 Hz, SCHZ).
AI) (300 MHz, DMSO d6): 8.61 (s, 1H, CH=N); 5.36 (d, J=5.1 Hz, IH, CH);
5.18 (d, J=5.1 Hz, 1H, CH); 4.42 und 3.71 (ABq, J=18.0 Hz, SCH2); 2.74
(s, 3H, SCH3).
AJ) (300 MHz, DMSO d6): 8.52 (s, 1H, CH=N); 5.36 (d, J=5.1 Hz, IH, CH);
5.21 (d, J=5.1 Hz, 1H, CH); 4.46 and 3.79 (ABq, J=17.7 Hz, 2H, SCH2);
2.95 (s, 3H, N-CH3).
AK) (300 MHz, DMSO d,): 8.51 (s, I H, CH=N); 7.56-6.84 (m, l OH. 2Ph);
5.28 (d, J=4.8 Hz, 1H, CH); 5.00 (d, J=5.1 Hz, 1H, CH), 4.13 and 3.93
(ABq. J=16.8 Hz. 2H, SCH2).