Note: Descriptions are shown in the official language in which they were submitted.
CA 02221472 1997-11-18
W O 96/39338 PCT~US96/10036
AN OXYGEN ABSORBING CONTAINER CAP LINER
FIELD OF THE INVENTION
This invention relates in general to oxygen
absorbents. It relates in particular to an oxygen
absorbent dispersed in a liner of a container cap.
BACKGROUND OF THE INVENTION
Many products are susceptible to putre~action,
denaturation, mold growth, spoilage, rancidity,
oxidation, or other deterioration when brought into
contact with oxygen. Examples o~ such products include
bee~, wine, ~uice, vinegar, sauces, seasonings, processed
~oods, bread, produce, meats, and certain pharmaceuticals
and chemicals, among a variety o~ others. Preservation
of such products is disturbed when molds, bacteria, and
CA 02221472 1997-11-18
WO 96/39338 PCTAJS96/10036
other organlsms that thrive in the presence of oxygen are
present. These organisms cause the putrefaction and
change in the taste or quality o~ the product. In
addition, some of the products themselves are liable to
be affected by oxidation that changes the taste or
quality o~ the product. To prevent such oxidation and
growth of organisms and thus increase the preservation
stability of these products, the oxygen must be removed
from the container in which the products are stored.
One technique ~or avoiding or reducing the
presence of oxygen is vacuum packing. This involves
evacuating a container before charging it with the
product. Another technique is gas displacement. Here,
an inert yas such as nitrogen is used to displace the air
and hence the oxygen in a container. The displacement
can be per~ormed before or a~ter the product is charged
to the container.
Still another technique is a foaming method.
Particularly applicable to products such as beer, a jet
~oamer can be used to inject a small amount o~
pressurized water to foam the beer after charging it to
the container. The foam acts as a mechanical
deoxygenizer, forcing the oxygen from the container.
Common disadvantages associated with all of the
above techniques are the requirement o~ large-scale
apparatus and operation and the difficulty of removing r
oxygen dissolved in the product. Also, in general, these
techniques leave between C.2~ and 5.0~ of the oxygen in
CA 02221472 1997-11-18
W O 96/39338 PCTAUS96/10036
the container. This amount of oxygen in the container is
enough to adversely a~ect most products.
A simpler, more efficient techni~ue for oxygen
removal involves placing an oxygen absorbent in the
container with the product. For this purpose, it is
known to attach an oxygen absorbent to the underside of a
container cap. For example, in U.S. Patent No.
4,287,995, issued to Moriya, an oxygen absorbent is
placed on the underside of a cap. The oxygen absorbent
is held in place by a cover layer o~ gas permeable film
that prevents contact between the absorbent and the
contents o~ the container.
U.S. Patent No. 5,143,763, issued to Yamada et
al., discloses a multi-layer composition adapted to be
attached to a liner on the underside of a container cap.
The layers of the composition include (1) an adhesive
layer that attaches the multi-layer structure to the cap
liner, (2) an oxygen absorbing layer consisting of an
oxygen absorbent dispersed in a resin, and (3) an oxygen
permeable ~ilm layer covering the absorbent layer. The
oxygen permeable film layer prevents the oxygen absorbent
~rom leaching out ~rom the resin into the contents o~ the
container. The adhesive layer is disposed between the
cap liner and the oxygen absorbing layer, comple~ely
separating the cap liner ~rom the oxygen absorbing layer.
U.S. Patent No. 5,274,024, issued to Koyama et
al., also discloses a multl-layer composition adapted to
be attached to the underside of a container cap. The
CA 02221472 1997-11-18
W O 96/39338 PCTAUS96/10036
patent discloses an adhesive layer, used to attach an
oxygen absorbent layer to the cap, and an outer layer
over the oxyyen absorbing layer. The outer layer
prevents direct contact between container contents and
the oxygen absorbent. Again, the adhesive layer is
disposed between the cap and the oxygen absorbent layer,
completely separating the cap ~rom the oxygen absorbent
layer.
In all o~ the known devices, however, separate
layers are used to accomplish the ~unctions of lining the
container cap, adhering the oxygen absorbent to the cap,
absorbing the oxygen, and covering the oxygen absorber to
prevent it ~rom contact~ing the contents within the
container.
SUMMARY OF THE I~v~NllON
The present invention provides a container cap
having a base portion, a substantially cylindrical
portion extending perpendicularly ~rom a perimeter o~ the
base portion to de~ine an inner sur~ace o~ the container
cap, and an uncovered liner disposed directly on the
inner sur~ace o~ the container cap, the liner having an
oxygen absorbent dispersed therein. The container cap is
adapted to seal an opening in a container and to absorb
oxygen within the container.
The present invention also provides a method
~or removing oxygen ~rom a container by dispersing an
oxygen absorbent in a liner, attaching the liner directly
CA 02221472 1997-11-18
W O 96/39338 PCTAUS96/10036
to an inner sur~ace o~ a container cap, and placing the
cap over an opening in the container such that the liner
in the container cap seals the opening and absorbs oxygen
within the container.
BRIEF DESCRIPTION OF THE DRAWING
Fig 1 is a cross-sectional side view o~ an
oxygen absorbing container cap in accordance with an
exemplary embodiment o~ the present invention;
Fig. 2 is a perspective view in partial cross-
section o~ an oxygen absorbing container cap inaccordance with an exemplary embodiment o~ the present
invention;
Fig. 3 is a partially cross-sectional side view
o~ an oxygen absorbing container cap attached to a
container in accordance with an exemplary embodiment o~
the present invention;
~ Fig. 4 is a cross-sectional side view of an
oxygen absorbing cap in accordance with another exemplary
embodiment o~ the present invention; and
Fig. 5 is a top view o~ the embodiment shown in
Fig. 4.
CA 02221472 1997-11-18
W O 96/39338 PCTrUS96/10036
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides an oxygen
absorbing cap with an oxygen absorbent dispersed in a
liner of the cap, the liner being directly attached to
the inside surface of the cap. No cover layer is used
over the liner. When such a cap is secured to a
container, the oxygen absorbing liner in the cap acts
both as a sealant, providing a seal between the cap and
the container, and as an oxygen absorber, removing oxygen
from inside the container.
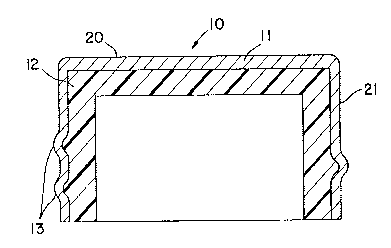
Fig. 1 shows a side view o~ an exemplary
embodiment of the present invention. Cap 11 includes a
base portion 20 and a cylindrical portion 21 that is
typically integrally ~ormed with base portion 20. Cap 11
may be a plastic material, such as polyvinylchloride,
polystyrene, or polycarbonate. Cap 11 may also be a
metallic material, such as aluminum or iron. Grooves 13
are formed on the inner surface of cap 11 to mate with
threads on a container opening (not shown).
~iner 12 is disposed on the inner surface of
cap 11. Liner 12 in the illustrated embodiment includes
a carrier resin with an oxygen absorbing material
dispersed therein.
The oxygen absorbing materials useful in the
present invention include iron, solid electrolytic salts,
and glucose oxidase. The iron may be hydrogen-reduced
iron, electrolytically reduced iron, or chemically
CA 02221472 1997-11-18
W O 96~9338 PCT~US96/10036
reduced iron. Although iron is preferred as the metallic
oxygen absorbing agent, it will be appreciated that other
metals may be used. These are, by way of example and not
limitation, aluminum, copper, zinc, titanium, magnesium,
and tin. These other materials do not, however, absorb
oxygen as fast as iron or have its oxygen absorbing
capacity. Also, other elements which can be used in
elemental or partially oxidized form are sodium,
manganese, iodine, sulfur, and phosphorous. These
elements are also not as effective as iron because they
do not have the oxygen absorbing capacity of iron, the
rate of oxygen absorption of iron, or both. The oxygen
absorbing salt may be sodium chloride or any other
suitable food compatible salt including, but not-limited
to, sodium sulfate, potassium chloride, ammonium
chloride, ~mm~n;um sulfate, calcium chloride, sodium
phosphate, calcium phosphate, and magnesium chloride.
For non-~ood products, other non-food compatible salts
may be used.
A carrier resin for the oxygen absorbing
material is preferably polyvinylchloride plastisol.
Polyvinylchloride plastisol is a known resin for lining
the inner surface of container caps. Other resins that
may be used as the carrier resin for the oxygen absorbing
material and can also serve as suitable sealants include,
without limitation, high density polypropylene, high
density polyethylene, acrvlic, vinyl acetate ethylene
copolymer, ethylene vinyi acetate, vinyl acetate
homopolymer, acetate ethylene copolymer, plasticized
~ 30 vinyl chloride, oxidized polyethylene homopolymer, and
CA 02221472 1997-11-18
W O 96/39338 PCTAUS96/10036
polyurethane. When polyvinylchloride plastisol is used
as the carrier resin, up to 75~ by weight of liner 12 may
be 200 mesh iron, a preferred oxygen absorbent.
~iner 12 is prepared by dispersing the oxygen
absorbents within the carrier resin (in a viscous liquid
state) by mixing in an electric, high-speed mixer. Liner
12 is then sprayed onto the inner surface of cap 11 in
liquid form according to methods known in the art. ~iner
12 adheres to cap 11. Cap 11 and liner 12 are then
heated (to about 400~F for 2 1/2 minutes) to solidify
liner 12. Fig. 2 is a perspective view showing liner 12
coated on the inner surface of cap 11.
Cap 11 is attached to a container by first
heating cap 11 until liner 12 softens. Cap 11 is then
threaded onto the container, forming threads in liner 12
corresponding to grooves 13 in cylindrical portion 21 of
cap 11. As shown in Fig. 3, cap 11 securely fits over
the opening of container 15. Liner 12 seals cap 11 to
container 15. Thus, liner 12 insures a tight fit between
cap 11 and container 15 and ~acilitates the sealing
~unction.
In addition, liner 12 absorbs oxygen within
container 15 without the need for additional layers, such
as cover layers or adhesive layers. The invention
provides an economical ard practical method for absorbing
oxygen withln a container by combining the adhesive
function (affixing liner ~~2 to cap 11), the oxygen
CA 02221472 1997-11-18
W O 96/39338 PCT~US96110036
g
absorbing ~unction, and the container sealing ~unction in
a single element: liner 12.
Fig. 4 is a side view o~ an alternative
embodiment o~ the present invention. In the illustrated
embodiment, cap 11 has a peripheral liner 30 disposed
around the intersection o~ base portion 20 and
cylindrical portion 21. Peripheral liner 30 extends over
grooves 13 such that grooves will be ~ormed in peripheral
liner 30 as cap 11 is attached to a container. This
ensures sealing cap 11 to the container Peripheral
liner 30 does not include an oxygen absorbent.
The embodiment illustrated in Fig 4 also
includes a central liner 31. Central liner 31 is
disposed on the inner sur~ace o~ base portion 20.
Central liner 31 is centrally disposed and circular in
the illustrated embodiment, but any shape or thickness
may be used. Central liner 31 contains an oxygen
absorbent that absorbs oxygen within the container to
which cap ll is attached The oxygen absorbent is
dispersed in central liner 31 as discussed above.
Peripheral liner 30 and central liner 31 are both sprayed
into cap 11 according to methods known in the art, and
then heated to solidi~y.
Fig. 5 is a top view o~ the embodiment
illustrated in Fig. 4 This embodiment conserves both
the carrier resin material used ~or the liners and the
oxygen absorbents.
v
CA 02221472 1997-11-18
W O 96/39338 PCT~US96/100~6
- 10
The ~ollowing examples are presented to
illustrate the present invention; they are not intended
to limit it.
Example 1:
Polyvinylchlorlde plastisol in an amount o~
10.35 grams was blended with 12.51 grams of 200 mesh iron
containing 2~ sodium chloride. The blending was done in
an electric, high-speed mixer. A sample o~ the resulting
liner material was coated on the inner surface o~ a
container cap. The container cap was placed ln a 500cc
mason jar containing lOOcc o~ oxygen. A one-eighth-inch
hole was drilled in the lid o~ the mason jar with a
septum placed over the hole to prevent oxygen ~rom
leaking out o~ the container. The container was le~t at
room temperature at 100~ relative humidity within the
jar, and the amount o~ oxygen absorbed over time by the
cap liner was measured This procedure was repeated two
times using di~erent weights o~ liner material inside
the cap. The results are tabulated below.
Oxygen Absorbed Over Time (cc)
Sample Sample Sample Average
#1 #2 #3
Time (1.47 gms*~(1 71 qms*)(1.51 qms*)(1.56 Gms*)
22 hrs 10 10 10 10
46 hrs 15 14 15 15
96 hrs 24 22 24 23
184 hrs 37 32 34 34
234 hrs 37 32 37 35
330 hrs 51 41 48 47
* Weight o~ liner coated on inner sur~ace o~ cap.
CA 02221472 1997-11-18
W O 96/39338 P~CT~US96/10036
Example 2:
Polyvinylchloride plastisol in an amount of
8.40 grams was blended with 5.17 grams of 200 mesh iron
cont~;n;ng 2~ sodium chloride in an electric, high-speed
mixer. A sample of the resulting liner composition was
coated on the inner surface of a container cap, which was
placed in a 500cc mason jar containing 100cc of oxygen.
A one-eighth-inch hole was drilled in the lid o~ the
mason jar with a septum placed over the hole to prevent
oxygen from leaking out o~ the container. The container
was le~t at room temperature at 100~ relative humidity
within the jar, and the amount of oxygen absorbed by the
liner over time was measured. The procedure was repeated
two times using different weight samples of liner within
the cap. The results are tabulated below.
Oxygen Absorbed Over Time (cc)
Sample Sample Sample Average
#1 #2 #3
Time (1.47 qms*)(1.71 qms*)(1.51 qms*)(1.56-qms*)
22 hrs 8 8 8 8
46 hrs 12 12 12 12
96 hrs 26 19 21 22
184 hrs 46 30 30 35
234 hrs 52 33 30 38
330 hrs 61 43 41 48
* Weight of liner coated on inner surface of cap.
The results of both Examples 1 and 2 show good
oxygen absorption using the present invention. Using the
invention, the results are achieved with reduced material
; and fabrication time.
CA 02221472 1997-11-18
W O 96/39338 PCTAUS96/10036
Although illustrated and described herein with
re~erence to certain specific embodiments, the present
invention is nevertheless not intended to be limited to
the details shown. Rather, various modifications may be
made in the details within the scope and range of
equivalents o~ the claims and without departing from the
spirit of the invention.