Note: Descriptions are shown in the official language in which they were submitted.
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
1
DESCRIPTION
TITLE OF THE INVENTION
METHOD AND APPARATUS FOR PRODUCING DEIONIZED WATER
TECHNICAL FIELD
The present invention relates to a method and an'
apparatus for producing deionized water, which are useful
for producing pure water or ultrapure water useful for
the production of pharmaceuticals or semiconductors or
water for boilers for power generation.
BACKGROUND ART
As a method for producing deionized water, it is
common to employ a method of obtaining deionized water by
passing water to be treated through a packed bed of ion
exchange resins so that impurity ions are removed as
adsorbed on the ion exchange resin. Here, it is common
to employ a method of regenerating the ion exchange resin
having its adsorbing ability lowered, by means of an acid
or alkali. However, such a method has a problem that a
waste liquid of the acid or alkali used for the
regeneration, is discharged. Therefore, a method for
producing deionized water which requires no such
regeneration is desired. From such a viewpoint, an
attention has been drawn in recent years to a self-
s 25 regenerating type electrodialytic deionized water
producing method wherein ion exchange resins are used in
combination with ion exchange membranes. This method is
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
2
a method wherein a mixture of an anion exchange resin and
a ration exchange resin is packed in demineralizing
compartments of an electrodialyzer having anion exchange
membranes and ration exchange membranes alternately '
arranged, and while supplying water to be treated to the
demineralizing compartments, a voltage i:a applied to
carry out electrodialysis to produce deionized water. In
this method, it is common that the ion exchange resins in
a wet condition are accommodated in the demineralizing
compartments, whereby there have been drawbacks that the
contact among the ion exchange resins to one another or
between the ion exchange resins and the ion exchange
membranes, is inadequate, and if it is attempted to
increase the thickness of the mineralizing compartments
to reduce the effective membrane area, electrical
resistance tends to increase.
As a method for overcoming these drawbacks, it has
been proposed to prevent the increase of the resistance
by adjusting the width of each demineralizing compartment
to a level of from about 0.762 to 10.16 cm (from about
0.3 to 4 inches} and the thickness to a level of from
about 0.127 to 0.635 cm {from about 0.05 to 0.25 inch) in
JP-B-4-72567 and JP-H-6-20513. However, this method has
had drawbacks that since the thickness of the
demineralizing compartment is thin, it is difficult to
pack the ion exchanger into the demineralizing
compartment, and the amount of water produced per unit
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97l00896
3
area is small.
DISCLOSURE OF INVENTION
It is an object of the present invention to provide a
novel apparatus for producing deionized water whereby, in
a self-regenerating type electrodialytic deionized water
producing apparatus wherein ion exchangers and ion
exchange membranes are used in combination, the increase
of electrical resistance is small even if the thickness
of a demineralizing compartment is made thick, and pure
water card be constantly obtained over a long period of
time without the above-mentioned drawbacks of the prior
art, and to provide a method for producing deionized
water by ,using such an apparatus.
The present invention provides an apparatus for
producing deionized water comprising an electrodialyzer
having canon exchange membranes and anion exchange
membranes alternately arranged between a cathode and an
anode to form demineralizing compartments and
concentrating compartments, and ion exchangers
accommodated in the demineralizing compartments, wherein
a gressure of from 0.1 to 20 kg/cm2 is exerted between
the ion exchangers accommodated in the demineralizing
compartments and the cation exchange membranes and anion
' exchange membranes defining the demineralizing
2~ compartments.
BRIEF DESCRIPTION OF DRAWINGS
Figure 1 is a view illustrating a method for
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
4
measuring the pressure between an ion exchanger and a
container wall.
Figure 2 is a view illustrating the construction of
an electrodialyzer used in Test Example.
Figure 3 is a schematic view illusti:ating an
embodiment of a self-regenerating type electrodialytic
apparatus.
Figure 4 is a view illustrating an apparatus used for
measuring the pressure formed by supplying water to a
dried ion exchanger in Examples.
Figure 5 is a view illustrating an embodiment of a
porous body of ion exchange resins wherein porous cation
and anion exchanger sheets are arranged in a sea-island
pattern (as used in Example 3).
Figure 6 is a view illustrating an embodiment of a
porous body of ion exchange resins wherein porous cation
and anion ion exchanger sheets are arranged in a multi-
layer pattern (as used in Example 4).
BEST MODE FOR CARRYING OUT THE INVENTION
In the present invention, the ion exchanger may have
its state preliminarily changed and then put into the
demineralizing compartments of the electrodialyzer, or
may be put into the demineralizing compartments and then
have its state changed. In this specification, the state
of the ion exchanger will hereinafter be described by -
using the following terms. Namely, the term "operation
state" means a state Where the ion exchanger is
CA 02221709 1997-11-20
WO 97!34696 PCT/JP97/00896
accommodated in tie demineralizing compartments and is
used for electrodialysis, and it is in equilibrium With
the environment for operation. The term "shrinked state"
means a state where the apparent volume of the ion
5 exchanger is shrinked by some method. The term "free
state" means a state where the ion exchanger is in
equilibrium with the environment for operation, but is
free from restriction by the demineralizing compartments:
In the present invention, the thickness of each
demineralizing compartment is usually at least 0.2 cm,
preferably at least 0.7 cm. If the thickness of the
demineralizing compartment is less than 0.2 cm, the
effect of reducing the effective membrane area tends to
be indistinct, and it tends to be difficult to pack the
ion exchanger therein. On the other hand, if its
thickness exceeds 80.0 cm, the effect of reducing the
effective membrane area is large, but the increase of
electrical resistance tends to be large, such being
undesirable. It is particularly preferred that the
thickness of the demineralizing compartment is within a
range of from 1.1 to 30.0 cm, whereby the increase of the
resistance is small, and the effect of reducing the
ef~'ective membrane area is large.
The pressure formed between the ion exchangers
accommodated or packed in the demineralizing compartments
and the cation exchange membranes and anion exchange
membranes defining the demineralizing compartments, is
CA 02221709 1997-11-20
WO 97/34696 - PCT/JP97/00$96
6
adjusted within a range of from 0.1 to 20 kg/cm2. If the
pressure is less than 0.1 kg/em2, the contact of the ion
exchanger particles to one another or between the ion
exchangers and the ion exchange membranes tends to be '
inadequate, whereby electrical resistance is likely to
increase, or a short path of water to be treated is
likely to form and the purity of the resulting water
tends to be low, such being undesirable. On the other
hand, if the pressure exceeds 20 kg/cm2,, the contact of
ZO the ion exchange resin particles to one another or
between the ion exchangers and the ion exchange membranes
will be adequate, but the amount of water treated tends
to decrease, and the ion exchange membranes used, are
likely to be damaged by the pressure. ~'he above pressure
15 is preferably from 0.5 to 10 kg/cm2, more preferably from
0.8 to 2 kg/cm2.
In the present invention, the pressure may be formed
between the ion exchangers packed in the demineralizing
compartments and the ion exchange membranes preferably in
2p such a manner that (1) the ion exchangers to be,
accommodated in the demineralizing compartments axe
converted to a form having their volume reduced smaller
than the volume of their regenerated form and then packed
in the demineralizing compartments in an amount such that
2.~ the volume of the regenerated form of the ion exchanger .
in a free state would be larger than the volume of the
demineralizing compartments, followed by supplying water
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
7
and conducting an electric current to let the ion
exchanger expand to increase the volume thereby to
increase the pressure, or (2) the ion exchanger is
accommodated in the demineralizing compartments, and then
the volume of the demineralizing compartments is
mechanically reduced to increase the pressure.
In the above manner (1), it is preferred that the ion
exchanger is packed in the demineralizing compartments in
an amount such that the volume of the ion exchangers in
the regenerated form in a free state would be from 103 to
170 relative to the volume of the demineralizing
compartments. If this amount is less than an amount for
a free state volume of 103$, the contact of the ion
exchangers tend to be poor. On the other hand, if the
l5 amount exceeds an amount for a free volume of 170$, the
contact will be good, but the pressure loss in passing
water through the ion exchangers tend to be large, such
being undesirable. Among them, it is particularly
preferred that the amount of the ion exchangers are such
that the free state volume would be from 111 to 150 of
the volume of the demineralizing compartments.
As a method for reducing the volume of the ion
exchanger to a level smaller than the volume of its
' regenerated form, (i) a method of reducing the water
content by drying, (ii) a method of changing the counter
ion to an ion species for other than the regenerated form
to convert the ion exchanger to a loaded form, or (iii) a
CA 02221709 1997-11-20
WO 97/34696 - PCT/JP97/00896
8
method of immersing the ion exchanger in an organic
solvent for solvent substitution, may be mentioned.
However, a method for using the method (i} and the method
(ii} in combination, is preferred, since it can readily '
be applied irrespective of the kind or structure of the
ion exchanger, and the volume decreasing amount is large.
When the water content is to be reduced by drying, it
is preferred to reduce the water content (weight) to a
level of from 1 to 30~. If the water content is less
l0 than 1$, it takes a long time for drying, such being
undesirable. If the water content is larger than 30~,
the effect for increasing the volume by supplying water
and conducting an electric current tends to be small,
such being undesirable. It is particularly preferred
that the water content is from 5 to 15$, whereby drying
will be easy, and the effect for increasing the volume by
supplying water and conducting an electric current will
be large. As the type of the counter ion during drying,
a Na type is preferred for a cation exchanger, and a CI
type is preferred for an anion exchanger, since such type
is thermally stable. The drying temperature is
preferably from 30 to 80°C. If it is lower than 30°C, it
takes a long time for drying, and if it is higher than
80°C, the ion exchange groups tend to decompose, such
being undesirable.
In the case of a method of changing the counter ion
to an ion species for other than the regenerated form to
CA 02221709 1997-11-20
WO 97!34696 PCT/JP97/00896
s
convert the ion exchanger to a loaded form, a Na type is
preferred for a cation exchanger, and a C1 type is
preferred for an anion exchanger, as mentioned above. As
other ion species, a K type or a Li type is preferred for
a cation exchanger, and a monovalent counter ion such as
a N03 type is preferred for an anion exchanger. In this
respect, a Ca type or an A1 type, or bivalent or higher
valent counter ion such as a S04 type, is not preferred,
since the conversion to a regenerated form tends to be
not easy.
In the above-mentioned method (2) wherein the ion
exchangers are packed in the demineralizing compartments,
and then the volume of the demineralizing compartments is
mechanically reduced to increase the pressure, it is
preferred to interpose a spacer which is shrinkable by
pressure between demineralizing compartment frames and
the ion exchange membranes, and exerting pressure from
outside to compress the spacer after packing the ion
exchangers, so that the volume of the demineralizing
compartments is reduced by from 5 to 60 volt. If the
reduced volume of the demineralizing compartments is less
than 5 vol$, the contact of the accommodated ion
exchangers tend to be poor. On the other hand, if the
' reduced volume of the demineralizing compartments exceeds
60 volg, the contact will be good, but the pressure loss
when water is passed through the ion.exchanger tends to
be large, such being undesirable. As the material for
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
such shrinkable spacer, a foam sheet of e.g.
polyethylene, polypropylene or polystyrene, is preferably
employed.
In the present invention, the ion exchangers to be
5 accommodated in the demineralizing compartments, may, for
example, be an ion exchange resin, an ion exchange fiber
or a formed product thereof. Among them, an ion exchange
resin and a porous ion exchanger prepared by bonding ion
exchange resin particles with a binder polymer into a
10 sheet or a plate, are preferred from the viewpoint of the
ion exchange performance, durability, etc. Particularly
preferred is a porous ion exchanger sheet, since the
contact of the ion exchange resin particles in itself is
good, and it can readily be accommodated into the
demineralizing compartments.
The porosity of the ion exchanger as it is
accommodated in the demineralizing compartments, is
preferably such that the continuous porosity governing
passage of the liquid is at least 5 volt. If the
porosity is 3ess than 5 vol$, the flow rate of the liquid
decreases, and the pressure loss increases, such being
undesirable. It is particularly preferred that the
porosity is from 10 to 40 volt, since the water
permeation will be good, the demineralizing performance
will be excellent, and treated water with a high purity
can be obtained. This porosity is the value when the ion
exchangers are accommodated in the demineralizing
CA 02221709 1997-11-20
WO 97/34696 PCTlJP97/00896
l I.
compartments and water is supplied and an electric
current is conducted.
As the ion exchanger, a canon exchanger, an anion
- exchanger or a mixture thereof, or a porous formed
product thereof, can be employed. The ion exchanger may
have a structure in Which domains (regions) of a cation
exchanger and domains (regions) of an anion exchanger are
combined. In such a case, the patterns of the respective
domains which are in contact with the ion exchange
membrane, may be various patterns. For example, a sea-
island pattern, a layered pattern, a mosaic pattern or a
lattice pattern may be employed. Particularly preferred
is a sea=island pattern or a layered pattern, since the
ion exchanger with such a pattern can readily be
accommodated into the demineralizing compartments, and
demineralization can efficiently be carried out.
However, the overall proportions of the cation exchanger
and the anion exchanger used are preferably such that the
total ion exchange capacity ratio of the cation
exchanger/the anion exchanger is within a range of from
20/80 to 80/20.
When a porous ion exchanger is used as the ion
exchanger, the weight ratio of the binder polymer based
- on the porous ion exchanger is preferably at most 20$.
If the weight-ratio exceeds 20$, the binder polymer is
likely to cover the surface of the ion exchange resin
particles, whereby the adsorbing ability tends to be low,
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
12
and the porosity tends to be low, whereby the flow rate
of the liquid to be treated tends to decrease, and the
pressure loss tends to increase. The above weight ratio
is particularly preferably from 1 to S~. The binder
polymer is preferably a thermoplastic polymer or a
solvent-soluble polymer from the viewpo:~nt of the
preparation of the porous ion exchanger"
As such a binder polymer, the follocaing is preferably
employed. Firstly, as the thermoplastic polymer, a low
density polyethylene, a linear low density polyethylene,
an ultra high molecular weight high density polyethylene,
a polypropylene, a polyisobutylene, 1,2-~polybutadiene, a
polyvinyl acetate or an ethylene-vinylacetate copolymer
may, for example, be mentioned. As the solvent-soluble
polymer, natural rubber, butyl rubber, polyisoprene,
polychloroprene, a styrene-butadiene rubber, nitrile
rubber or a vinyl chloride-fatty acid vinyl ester
copolymer may, for example, be mentioned.
The thickness of the porous sheet having the ion
exchange resin particles bound by the binder polymer, is
preferably such that the thickness in a form having the
volume reduced for packing into the demineralizing
compartment will be from 50 to 100 of the thickness of
the demineralizing compartment. If this thickness is
less than 50~ of the thickness of the demineralizing
compartment, the porous sheet will not closely contact
with the ion exchange membranes when water is supplied
CA 02221709 1997-11-20
WO 97/34696 _ PCT/3P97/0~$96
13
and an electric current is conducted, such being
undesirable. If the thickness exceeds 100, such a sheet
can not be accommodated in the demineralizing
compartment. The thickness of the porous sheet in a form
having the volume reduced is particularly preferably from
70 to 90g of the thickness of the demineralizing
compartment.
The following method is preferred as a method for
binding the ion exchange resin particles by the binder
polymer to form a porous sheet. Namely, preferred is (1)
a method wherein ion exchange resin particles and a
binder polymer are heat-kneaded and then formed into a
sheet by thermal forming such as flat plate pressing, (2)
a method wherein a binder polymer solution is coated on
the surface of the ion exchange resin particles, and the
solvent is evaporated for curing the binder polymer, (3)
a method wherein a binder polymer, a pore-forming
material and ion exchange resin particles are heat-mixed
and sheeted and then the pore-forming material is
extracted, or (4) a method wherein a binder polymer
having a pore-forming material dispersed therein, is
coated on-the surface of ion exchange resin particles and
cured, and then the pore-forming material is extracted.
Among them, method (1) and the method (3), are preferred
from the viewpoint of the forming processability or the
specific resistance of the obtained porous ion exchanger.
The ion exchange groups of the ion exchanger are
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
14
preferably a strongly acidic sulfonic acid type for
canon exchange groups and a strongly basic quaternary
ammonium salt type or pyridinium salt ty3?e for anion
exchange groups from the viewpoint of the ion exchange
performance and the chemical stability. The ion exchange
capacity of the ion exchanger is preferably from 0.5 to 7
meq/g dry resin. If the ion exchange capacity is lower
than 0.5 meq/g dry resin, adsorption of ions or
demineralization in the demineralizing compartments will
not sufficiently be carried out, whereby the purity of
treated water is likely to be low, such being
undesirable. It is particularly preferred that the ion
exchange capacity is from 1 to 5 meq/g dry resin, whereby
treated water with a high purity can be obtained, and the
stability in performance will be excellent.
In the present invention, as an apparatus for
producing deionized water, it is preferred to use an
electrodialyzer having the following construction, as
described in e.g. JP-A-3-186400, JP-A-2-2J7526, JP-A-5-
64726, US Patent 4,632,745 and US Patent 5,425,866.
The electrodialyzer comprises an anode compartment
provided with an anode and a cathode compartment- provided
with a cathode, and a plurality of cation exchange
membranes and anion exchange membranes which are
alternately arranged between the anode compartment and
the cathode compartment preferably via compartment frames
to form demineralizing compartments each defined by an
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
anion exchange membrane on the anode side and by a cation
exchange membrane on the cathode side, and concentrating
compartments each defined by a cation exchange membrane
on the anode side and by an anion exchange membrane on
5 the cathode side, alternately, preferably in a total
number of from 2 to 50 units. The thickness of a picture
frame-like compartment frame having an opening at its
center, which is present between a cation exchange
membrane and an anion exchange membrane, determines the
10 thickness of the demineralizing compartment or the
concentrating compartment. The thicknesses of the
compartment frames of the demineralizing compartment and
the concentrating compartment may not necessarily be the
same. The ion exchange membranes may be of a homogeneous
15 type or a heterogeneous type, and in order to increase
the mechanical strength, the one reinforced by a woven
fabric or a non-woven fabric, may be used. In a
concentrating compartment, it is preferred to insert a
spacer of a network-form, preferably made of a plastic,
in order to maintain the thickness of the concentrating
compartment preferably thinner than the thickness of the
demineralizing compartment and within a range of
preferably from 0.05 to 10 cm. Demineralization can be
carried out by conducting an electric current while
supplying water to be treated to the demineralizing
compartments and supplying water to the concentrating
compartments to discharge the concentrated salts. To
CA 02221709 1997-11-20
WO 97/34696 PCT/,Tf97/00896
16
each unit cell, a voltage of from 4 to 20 V is applied to
conduct an electric current preferably at a current
density of from 0.00001 to 0.05 A/cm2.
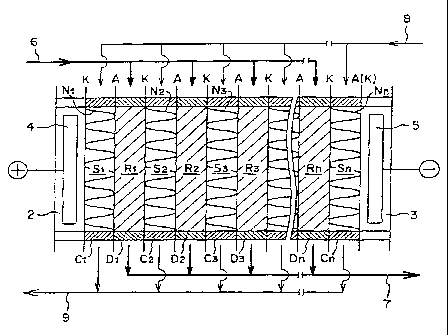
Figure 3 is a schematic view illustrating an '
embodiment of an electrodialyzer of such a type. In
Figure 3, A is an anion exchange membrane, and K is a
cation exchange membrane. As shown, the anion exchange
membranes A and the canon exchange membranes K are
arranged in the electrodialyzer 1 via demineralizing
compartment frames D1, D2, D3 ... Dn and concentrating
compartment frames Cl, C2, C3 ... Cn at predetermined
distances, to form an anode compartment 2, concentrating
compartments S1, S2 ... Sn, demineralizing compartments
R1, R2 ... Rn and a cathode compartment 3. In the
demineralizing compartments R1, R2 ... Rn, anion and
cation exchange resins are accommodated or packed. In
the concentrating compartments, spacers N1, N2, N3 ... Nn
are inserted.
In Figure 3, reference numeral 4 indicates an anode
and numeral 5 indicates a cathode, and a predetermined
voltage is applied across the two electrodes during the
operation, whereby anions in water to be treated which is
introduced into the demineralizing compartments R1, R2
... Rn from a conduit 6, will permeate and move to a
concentrating compartment on the anode side through an
anion exchange membrane A, while canons in water to be
treated will permeate and move to a concentrating
CA 02221709 1997-11-20
WO-97/34696 PCT/JP97/00896
17
compartment on the cathode side through a cation exchange
membrane K, and water to be treated itself will be
deionized and discharged via a conduit 7. Further, water
or an aqueous solution is introduced into the respective
concentrating compartments S1, S2 ... Sn from a conduit
8, and the anion and cation components permeated and
moved as described above, will be collected and
discharged as a concentrated solution from a conduit 9. -
Cations in the water to be treated, which are captured by
the cation exchangers in a demineralizing compartments,
will have a driving force given by the electric field,
will reach cation exchange membranes via cation
exchangers which are in contact with the cation
exchangers which captured the cations, and further, they
will pass through the membranes and move to a
concentrating compartments. Likewise, anions in the
water to be treated which are captured by the anion
exchangers will move to a concentrating compartments via
an anion exchangers and an anion exchange membranes.
Accordingly, it is more preferred that the cation
exchanger and the anion exchanger are, respectively,
gathered to form domains or gathered regions, whereby
contact points of exchanger particles of the same ion
type increase remarkably, so that movement of ions is
facilitated, and the deionization performance will be
improved.
Now, the present invention will be described in
CA 02221709 2005-03-23
7416-137
18
further detail with reference to Examples. However, it
should be understood that the present invention is by no
means restricted to such specific Examples.
TEST EXAMPLE
Preparation of ion exchangers
A spherical canon exchange resin (Diaion SKlH,
trademarks Mitsubishi Chemical Corporation) having an
average diameter of 50D ,um and a spherical anion exchange
resin (Diaion SAlOA, trademark, manufactured by
Mitsubishi Chemical Corporation) having an average
diameter of 500 ~m were mixed in a volume ratio of 50/50
and dried at 50°C. By the drying, the weight of the
mixture decreased to 55 wt% of the original weight. As a
binder, a linear low density polyethylene used in Example
2 mentioned below of a pellet form having a diameter of
from 2 to 6 mm and a length of from 4 to 9 mm, was added
in an amount shown in Table 1 as the amount of the binder
to the total amount of the binder and the ion exchange
resins, and the mixture was kneaded by a kneader at 140°C
for 40 minutes. This kneaded product was put into a
metal mold of a rectangular parallelopiped with an
opening 'side surface of 250 mm x 150 mm and pressed under
a condition of 120°C x 25 kgw/cm2 to obtain a porous
molded sheet of a rectangular parallelepiped.
Hy changing the amount of the mixture to be filled in
the mold, the molded sheets having thicknesses of 6.7 mm,
7 mm and 7.5 mm were, respectively, obtained from kneaded
CA 02221709 1997-11-20
WO 97/34696 - PCT/JP97/00896
19
products with the respective blend ratios as identified
in Table 1. Depending upon the thickness, each molded
sheet was cut so that the width and length of the molded
sheet would in a ratio of length: width:thickness of
140:100:8. Thus, ion exchangers 1 to 9 were obtained.
When immersed in pure~water at room temperature for 8
hours, these ion exchangers swelled in substantially the
same proportion in the length, width and thickness
directions and reached an equilibrium state. The
ZO increase in the length over the original length is shown
as the swelling rate
Table 1
Ton fount of Swelling
exchanger binder Size (mm) rate
(wt$) ($)
1 5 117 x 84 x 6.7 23
2 5 123 x gg x 7 22
3 5 131 x 94 x ?.5 23
4 2 117 x 84 x 6.7 26
5 2 123 x 88 x 7 27
6 2 131 x 94 x 7.5 25
7 1 117 x 84 x 6.7 27
8 1 123 x gg x 7 2g
9 1 131 x 94 x 7.5 25
Measurement of the swelling pressure
As shown in Figure 1, an ion exchanger 13 in a dry
state is put into a metal container 11 of a rectangular
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
2fl
parallelopiped (bottom width: 7.00 mm, bottom length: 140
mm), and a metal plate 12 is placed thereon, whereupon
the position of aload cell 14 is adjusted so that the
forward end of the load cell 14 will contact the metal -
plate 12 when the ion exchanger 13 swells to a thickness
of 8 mm. Namely, the position of the load cell is set so
that when the ion exchanger 13 is in a dry state, the sum
of the space a between the forward end of the load cell
14 and the metal plate 12 and the thickness b of the ion
exchanger 13 will be 8 mm. Then, water is supplied from
a water supply inlet 15, and from a load exerted to the
load cell 14 when absorption of water reaches
equilibrium, the pressure between the ion exchanger 13
and the metal plate 12 was obtained. Then, the volume
ratio of the ion exchanger in an operation state to that
in a free state i.e. volume in operation-like
state/volume in free state x 100, was obtained. These
results are shown in Table 2.
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
21
Table 2
Ion Pressure Volume ratio
exchanger (kgw/cm2) ($)
I 0.22 91
2 1.10 , 7g
3 4.51 65
4 0.43 g5
S 1.95 74
6 6.52 61
7 0.47 83
8 2.06 72
9 6.68 59
Evaluation by an electrodialvzer
One of ion exchangers 1 to 9 was put in a
demineralizing compartment 27 of an electrodialyzer
having a construction as shown in Figure 2 and clamped to
a prescribed size. The shape of the demineralizing
compartment 27 was a rectangular parallelopiped, whereby
the length in the water flow direction was 140 mm, the
width Was 100 mm, and the space between an anion exchange
membrane 28 and a canon exchange membrane 25 was 8 mm.
In each of two concentrating compartments 26, a spacer
net made of polypropylene was inserted, so that even when
the ion exchanger in the demineralizing compartment 27
expanded, the space between the anion and cation exchange
membranes would not substantially change. Accordingly,
also in this demineralizing compartment, the ian
exchanger exhibits the same pressure as shown in Table 2.
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
22
Further, for the purpose of comparison, as ion exchanger
20, a molded product of 111 mm x '79.4 mm x 6.3 mm
prepared in the same manner as ion exchangers 1 to 9 and
having a binder amount of 2 wt~, was permitted to absorb -
water adequately and adjusted to have the same size as
the demineralizing compartment 27. and such a molded
sheet was accommodated in the demineralizing compartment
27.
Then, an electric current of 1.0A was conducted under
the same condition, after supplying 0.18 ~/hr of water
having an electrical conductivity of about 10 ,ccS/cm to
the demineralizing compartment, 20 ~/hr of water having
an electrical conductivity of about 1 mS/cm to the
concentrating compartments 26 and 1 ~/hr of water having
an electrical conductivity of about 200 ~rS/cm to the
anode compartment 24 and the cathode compartment 21 for
one hour. The dialyzer was continuously operated for 40
hours, and when the operation was stabilized, the flow
rate in the demineralizing compartment wa.s adjusted to
28.8 P/hr, whereupon the pressure loss at the upper and
the lower end portions of the demineralizing compartment
of the electrodialyzer, the electrical conductivity of
deionized water discharged from the demineralizing
compartment and the resistivity of the demineralizing
compartment, were measured. The results are shown in
Table 3. In Figure 2, reference numeral 21 indicates a
cathode, and numeral 22 indicates an anode.
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
23
Table 3
Electrical Resistivity
Ion Pressure conductivity of
loss of deionized demineralizing
exchanger (~gw/cm2} water
( !-~s/cm ) compartment
( ~cm )
1 0.08 0.481 520
2 0.12 0.368 330
3 0.18 0.213 210
4 0.10 0.439 450
5 0.14 0.327 300
6~ 0.20 0.200 120
7 0.10 0.436 360
8 0.14 0.320 280
9' 0.21 0.197 110
10 0.02 2.12 1350
With ion exchangers 1 to 9, deionized water of a high
purity was obtained constantly, and the resistivity was
low. Further, such a tendency was observed that with an
ion exchanger having a higher pressure shown in Table 2,
the properties were better. Whereas, with ion exchanger
10, the purity of deionized water was not high. From the
measurement of the pressure loss, it was found that
spaces were formed between the ion exchangers and the
compartment frames or between the ion exchangers~and the
ion exchange membranes, towards the outlet from the inlet
of each demineralizing compartment.
EXAMPLE 1
CA 02221709 2005-03-23
7416-137
24
A sulfonic acid type (B-type) canon exchange resin
having a particle size of from 400 to 600 ~m and an ion
exchange capacity of 4.5 meq/g dry resin (Diaion SK-1B,
trademark, manufaetured by Mitsubishi Chemical
Corporation) and a quaternary ammonium salt type (OH-
type) anion exchange resin having a particle size of from
400 to 600 ~m and an ion exchange capacity of 3.5 meq/g
dry resin (Diaion SA-10A, trademark, manufactured by
Mitsubishi Chemical Corporation) were subjected to hot
air drying at a temperature of 50°C to bring the water
content to 8 wt% and then mixed in a ratio of cation
exchange resin/anion exchange resin ~ 44/6 (weight ratio
in a dried state) to obtain a mixture having an ion
exchange capacity ratio of 50/50. This dried ion
exchange resin mixture was packed into each
demineralizing compartment of an electrodialyzer wherein
the thickness of the demineralizing compartment Was 1.2
cm and the thickness of a concentrating compartment
having a spacer net made of polypropylene (thickness: 0:2
cm) was 0.2 cm, to a volume packing ratio of 60%. After
supplying water for 60 minutes and electric current
conducting pretreatment for 24 hours, the resistivity in
water with 10 ~S/cm was measured and found to be 1051
n~cm at a current density of 0.0025 A/cm2. Using such an
electrodialyzer shown in Figure 3, pa~oduction of
deionized water was carried out as follows. The
electrodialyzer was the one consisting of a filter press
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
type dialyzer having cation exchange membranes (strongly
acidic heterogeneous membranes, thickness: 500 Wit, ion
exchange capacity: 2.7 meq/g dry resin) and anion
. exchange membranes (strongly basic heterogeneous
membranes, thickness: 500 ,ccm, exchange capacity: 2.1
meq/g dry resin) alternately arranged and clamped via
demineralizing compartment frames (made of polypropylene
having a thickness of 1.2 cm) and concentrating
compartment frames (made of polypropylene having a
10 thickness of 0.2 cm) and having an effective surface area
of 507 cm2 (width: 13 cm, length: 39 cm) x 5 pairs of the
demineralizing compartment and the concentrating
compartment.
Using water having an electrical conductivity of 5
15 /-~S/cm as feed water, demineralization was carried out at
a current density of 0.004 A/cm2 (voltage = 5 V per unit
cell), whereby treated water having an electrical
conductivity of 0.062 ~.eS/cm was obtained constantly at a
production rate of 0.4 m3/hr. In this case, the
20 effective surface area of the membrane per 1 m3/hr of
produced water was 1.27 m2. After the measurement, the
ion exchange resin was taken out from the demineralizing
compartments, and the volume of the ion exchange resin
mixture in a free state was measured and found to be 122
25 of the volume of the demineralizing compartments.
Further, using the measuring apparatus as shown in Figure
4, the same dried ion exchanger as in this Example was
CA 02221709 2005-03-23
71416-137
26
put in the metal container l0' in the same volume packing
ratio, and water was supplied, whereupon the pressure
formed was measured and found to be 2.1 kg/cma. In
Figure 4, reference numeral 11' is a metal plate, numeral
32' a water supply inlet, numeral 13' a water discharge
outlet, numeral 14' a load cell and numeral l5' a dried
ion exchanger.
EXAMPLE 2
A sodium sulfonate type (Na-type) canon exchange
resin having a particle size of from 400 to 600 ~m and an
ion exchange capacity of 4.5 m~q/g dry resin (Diaion Sit-
1H, trademark, manufactured by Mitsubishi Chemical
Corporation) and a quaternary ammonium salt type (Cl-
type) anion exchange resin having a particle size of from
400 to 600 ,um and an ion exchange capacity of 3.5 meq/g
dry resin (Diaion SA-10A, trademark, manufactured by
Mitsubishi Chemical Corporation) were subjected to hot
air drying at a temperature of 50°C to bring the water
content to 8 wt~ and then mixed in a ratio of citron
exchange resin/anion exchange resin = 44/56 (weight ratio
in a dried state) to obtain a mixture having an ion
exchange capacity ratio of 50/50.
To this mixture, 3 wt% of linear low density
polyethylene (Affinity SM-1300, trademark, manufactured
by Dow Chemical) was mixed based on the product obtained
and kneaded at a temperature of from 120 to 130°C. The
obtained kneaded product was thermally formed by a flat
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
27
plate press at 130°C to obtain a porous ion exchanger
sheet having a thickness of 0.6 cm. The porosity of
continuous pores in this porous sheet was 23 vol$. This
porous ion exchanger sheet was accommodated into the
demineralizing compartments of an electrodialyzer having
the same construction as in Example 1 except that the
thickness of each demineralizing compartment was changed
to 0.8 cm, at a volume packing ratio of 54g. After
supplying water for 60 minutes and electric current
conductii~g pretreatment for 24 hours, the resistivity in
water with 10 ~S/cm was measured and found to be 116452~cm
at a current density of 0.0025 A/cm2.
After the measurement of the resistivity, production
of deionized water was carried out. As the
electrodialyzer, the same electrodialyzer as used in
Example 1 except for the thickness of the demineralizing
compartments, was used. Using water having an electrical
conductivity of 5 ,uS/cm as feed water, demineralization
was carried out at a current density of 0.004 A/cm2
(voltage = 5 V per unit cell), whereby treated water
having an electrical conductivity of 0.060 ~eS/cm was
obtained constantly with a production rate of 0.45 m3/hr.
In this case, the effective surface area of the membrane
per 1 m3/hr of produced water was 1.13 m2. After the
operation, the ion exchanger sheet was taken out from the
demineralizing compartment, and the volume in a free
state was measured and found to be 111 of the volume of
CA 02221709 2005-03-23
71416-137
a8
the demineralizing compartment. Further, the same dried
ion exchanger sheet as in this Example was put into the
metal container 10' of the measuring apparatus shown in
Figure 4 at the same volume packing ratio, and water was
supplied, whereupon the pressure formed was measured and
found to be 1.2 kg/cm2.,
EXAMPLE 3
A sodium sulfonate type (Na-type) can on exchange
resin having a particle size of from 40fl to 600 hem and an
ion exchange capacity of 4.5 meq/g dry resin (Diaion SK-
1B, trademark, manufactured by Mitsubishi Chemical
Corporation) and a quaternary ammonium salt type (C1-
type) anion exchange resin having a particle size of from
400 to 600 ~cm and an ion exchange capacity of 3.5 meq/g
dry resin (Diaion SA-10A, trademark. manufactured by
Mitsubishi Chemical Corporation) were subjected to hot
air drying at a temperature of 50°C to bring the wager
content to 8 wt%~ To the respective ion exchange resins,
3 wt% of linear low density polyethylene (Affinity S~M-
1300, trademark, manufactured by Dow Chemical) was mixed
based on the product obtained and kneaded at a
temperature of from 120 to 130°C. The obtained
respective kneaded products were thermally formed by a
flat plate press at a temperature of 130°C to obtain a
porous canon exchanger sheet and a porous anion
exchanger sheet each having a thickness of 0.6 cm. The
porosity of continuous pores of the obtained porous
CA 02221709 1997-11-20
WO 97/34696 - PCT/JP97/00896
29
cation exchanger sheet was 24 vol$, and the porosity of
the porous anion exchanger sheet was 23 volt.
Using these two porous ion exchanger sheets, a
- combination of domains (regions) of the cation exchanger
and domains (regions) of the anion exchanger in the
pattern as shown in Figure 5(a) and 5(b), was prepared
and then accommodated into the demineralizing
compartments with a thickness of 0.8 cm of the same
electrodialyzer as used in Example 2 at a volume packing
30 ratio of 66g. Figure 5(a) shows a plan view, and Figure
5(b) is a cross sectional view taken along line Y-Y in
Figure 5(a). In Figure 5, reference numeral 16 indicates
an anion exchanger domain, and numeral 1~ ;"~;~~~o~
___.~_.~... ~.~~ Q-
canon exchanger domain. After supplying water for 60
minutes and electric current conducting pretreatment for
24 hours, the resistivity in water with ZO ~cS/cm was
measured and found to be 911 S2-cm at a current density of
0.0025 A/cm2.
After the measurement of the resistivity, production
of deionized water was carried out. As the
electrodialyzer, the same electrodialyzer as used in
Example 2 was used. Using water having an electrical
conductivity of 5 ~S/cm as feed water, demineralization
' was carried out at a current density of 0.004 A/cm2
- 25 (voltage = 5 V per unit cell), whereby deionized water
having an electrical conductivity of 0.057 ~eS/cm was
obtained constantly at a production rate of 0.47 m3/hr.
CA 02221709 2005-03-23
71416-137
In this case, the effective surface area of the membrane
per 1 m3/hr of produced water was 1.08 mZ. After the
measurement, the ion exchange resin was taken out from
the demineralizing compartment and the volume was
5 measured and found to be 134% of the volume of the
demineralizing compaztment. Further, the same dried ion
exchanger as in this Example was put into the metal
container 10° of the measuring apparatus as shown in
Figure 4, and water was supplied, whereupon the pressure
10 formed was measured and found to be 4.2 kg/cm2.
EXAMPLE 4
A sodium sulfonate type (Na-type) cation exchange
resin having a particle size of from 400 to 600 ~cm and an
ion exchange capaeity of 4.5 meq/g dry resin (Diaion SIt-
15 1B, trademark, manufactured by Mitsubishi Chemical
Corporation] and a quaternary ammonium salt type (C1-
type) anion exchange resin having a particla size of from
900 to 600 arm and an i:on exchange capacity of 3.5 meq/g
dry resin (Diaion SA-1OA, trademark, manufactured by
20 Mitsubishi Chemical Corporation) were subjected to hot
air drying at a temperature of 50°C to bring the water
content to 8 wt%. To the respective ion exchange resins,
3 wt% of 1,2-polybutadiene (RB-820, manufactured by Japan
Synthetic Rubber Co., Ltd.) was mixed based on the
25 product obtained and kneaded at a temperature of from 120
to 130°C. The obtained respective kneaded products were
thermally formed by a flat plate press at a ~temiaerature
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97100896
31
of 130°C to obtain a porous cation exchanger sheet and a
porous anion exchanger sheet each having a thickness of
0.6 cm. The porosity of continuous pores of the obtained
porous cation exchanger sheet was 24 volg, and the
porosity of the porous anion exchanger sheet was 23 volt.
Using these two porous ion exchanger sheets, a
combination of domains (regions) of the cation exchanger
and domains (regions) of the anion exchanger in the
pattern as shown in Figure 6(a) and 6(b), was prepared
and pack'red into the demineralizing compartments having a
thickness of 0.8 cm at a volume packing ratio of 55~.
Figure 6(a) indicates a plan view, and Figure 6(b) is a
cross sectional view taken along line Z-Z in Figure 6(a).
In Figure 6, reference numeral 16 indicates an anion
exchanger domain, and numeral 17 indicates a cation
exchanger domain. After supplying water for 60 minutes
and electric current conducting.pretreatment for 24
hours, the resistivity in water with 10 ~eS/cm was
measured and found to be 1206 S2~cm at a current density
of 0.0025 A/cm2, which was lower than 1362 S2~cm in a case
where non-dried regenerated type ion exchange resins
having the same blend ratio were put into the cell and
measured.
After the measurement of the resistivity, production
of deionized water was carried out. As the
electrodialyzer, the same as used in Example 2 was
employed. Using water having an electrical conductivity
CA 02221709 2005-03-23
7.416-137
32
of 5 ~S/cm as feed water, demineralization was carried
out at a current density of 0.004 A/cm2 (voltage = 5 V
per unit cell), whereby treated water having an
electrical conductivity of 0.057 ~S/cm was obtained
' constantly at a production rate of 0.46 m3/hr. In this
case, the effective surface area of the membrane per 1
m3/hr of produced water was 1.10 m2. After the
measurement, the ion exchange resin was taken out from
the demineralizing compartment, and the volume was
measured and found to be 113% of the volume of the
demineralizing compartanent. Further, the same dried ion
exchanger as in this Example was put into the metal
container 10' of the measuring apparatus as shown in
Figure 4, and water was supplied, whereupon the pressure
formed was measured and found to be 1.3 kg/cm2.
COMPARATIVE EXAMPLE
A sulfonic acid type (H-type) ration exchange. resin
having a particle size of from 400 to 600 ~cm and an ion
exchange capacity of 4.5 meq/g dry resin (Diaion SK-1$,
trademark. manufactured by Mitsubishi Chemical
Corporation) and a quaternary ammonium salt type (OH-
type) anion exchange resin having a particle size of from
400 to 600 ~m and an ion exchange capacity of 3.5 meq/g
dry resin (Diaion SA-10A, trademark, manufa~ctur~ed by
Mitsubishi Chemical Corporation) were regenerated with
hydrochloric acid and an aqueous sodium hydrflxide
solution, and then mixed in a ratio of ration exchange
CA 02221709 1997-11-20
WO 97/34696 PCTlJP97/00896
33
resin/anion exchanger resin = 40/60 (volume ratio in a
wet state) to obtain a mixture having an ion exchange
capacity ratio of 50/50.
This regenerated ion exchange resin mixture in a wet
state, was packed into the demineralizing compartments of
an electrodialyzer having the same construction as in
Example 1 except that the width of each demineralizing
compartment was 0.8 cm, at a volume packing ratio of
100. After supplying water for 60 minutes and electric
current conducting pretreatment for 24 hours, the
resistivity in water with 10 ~cS/cm was measured and found
to be 1362 S2~cm at a current density of 0.0025 A/cmz.
After the measurement of the resistivity, production
of deionized water was carried out. As the
electrodialyzer, the same as used in Example 1 except
that the thickness of the demineralizing compartments was
different, was used. Using water having an electrical
conductivity of 5 ~S/cm as feed water, demineralization
was carried out at a current density of 0.005 A/cm2
(voltage = 5 V per unit cell), whereby treated water
having an electrical conductivity of 0.07 ,uS/cm was
obtained only at such a small production rate as 0.04
m3/hr. The amount of water treated rn"~~ "~+. ~,~
increased, since if the amount of water treated was
increased, the electrical conductivity increased. In
this case, the effective surface area of the membrane per
1 m3/hr of produced water was as large as 12.68 m2.
CA 02221709 1997-11-20
WO 97/34696 PCT/JP97/00896
34
According to the apparatus for producing deionized
water of the present invention, the contact of the ion
exchanger particles with one another and with the ion
exchange membranes, is increased as accommodated in the
demineralizing compartments of an electrodialyzer,
whereby the resistivity can be reduced, and the thickness
of the demineralizing compartments can be made large.
Accordingly, it is possible to obtain an apparatus having
a large production rate of deionized water with a
relatively small effective surface area of the membrane.