Note: Descriptions are shown in the official language in which they were submitted.
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
ENZYME SYSTEMS FOR GAS PROCESSING
TECHNICAL FIELD
This invention relates to a process utilizing natural, modified or
engineered enzymes as agents, alone or immobilized in conjunction with
membranes or other techniques or cells or cell fragments for the extraction of
one or more specific molecules from a mixture of molecules in a first gaseous
phase and moving at least one specific molecule to a second phase. Preferably
the mixture of molecules is in a first gas phase and a specific molecule type
is
transported to a second mobile fluid phase. The specific gases to be separated
may be present in a gas mixture, or in solution of mixed gases. In a mixed gas
solution in a liquid phase, the specific gases can be treated either in their
dissolved or solvated molecular state or after they have been converted to an
ionic or ionized state either by reaction with water, reaction with another
solvent,
or by other prior chemical reaction. The invention process may also be used to
regenerate a specific gas from the second phase. The invention further relates
to the extraction of gases, molecules or ions from a liquid solution, aqueous
or
non-aqueous, to concentrate or isolate specific materials alone or in chemical
combination and in ionic or non-ionic form.
BACKGROUND OF THE INVENTION
Traditional means of isolating selected gases from a mixed stream involve
physical, chemical reactions or a combination thereof, and inert semipermeable
membranes. Among such processes are cryogenic, gas-liquid and gas-solid
sorptive techniques (e.g. pressure swing adsorption, amine treatment, iron
sponge, etc.), and immiscible liquid-liquid extraction (for recent summaries
see
Michaels AS: New vistas for membrane technology. Chemtech. 19:160-172,1989;
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
and Babcock RE, Spillman RW, Goddin CS & Cooley TE: Natural gas cleanup:
A comparison of inembrane and amine treatment processes. Ener~v Prog.
8(3):135-142,1988.) Newer technologies focus on the use of inert semipermeable
membranes but these do not offer a separation solution that is particularly
unique over existing processes (Spillman RW : Economics of gas separation
membranes. Chem. Engr. Prog. 85:41-62,1989). Membrane systems have been said
never to achieve complete separation (Spillman, id. 1989). Prior art physical
or chemical means do not readily allow segregation among gases with similar
physical or chemical properties or those in low concentrations. In general
prior
art does not effectively deal with extracting gases or gas equivalents from a
dissolved or ionized state to regenerate a purified gas. The prior art
generally
treats gases already dissolved in water such as carbon dioxide or oxygen in
Bonventura et al., U. S. Patent 4,761,209 and 4,602,987and carbon dioxide in
Henley and Chang U. S. Patent 3,910,780. No reference has been located in
which the enzyme contacts a gas in a gas stream, separates the gas and in a
subsequent step regenerates a purified gas.
Traditional gas separation means commonly exhibit one or more of the
following problems: they are energy inefficient, commonly nonspecific, quite
slow,
require a relatively pure feedstock, depend on a significant pressure head, or
use
ecologically questionable or toxic compounds. The relatively pure feed stock
requirement may result in a geographical restriction of available feed
materials.
The geographic availability may require shipment from distant locations such
that
transportation costs may be high, and even prohibitive for some uses. The
preceding limitations present restrictions on the growth and application of
gas
extraction/purification systems. A gas separation or enrichment process that
did
not require highly concent.rat.ed feed-stocks thus eliminating or reducing
transportation requirements would be beneficial.
In contrast to the disadvantages enumerated above for traditional
physical/chemical methods, biological catalysts (enzymes) present several
advantages including enhanced efficiency, speed, and increased specificity.
2
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
Enzymes also commonly distinguish optical isomers. Further, they can be used
at moderate temperatures and pressures, enhancing safety.
Prior use of enzymes has foeused very largely on the food processing
industry, cleansing or detergent applications, or processing of sewerage.
Industrial applications in the gas field have been limited. Frior application
of
enzymes to gas extraction are found in patents to Bonaventura et al, U. S.
Patents 4,761,209and 4,602,987and Henley and Chang U. S. Patent 3,910,780.
Bonaventura uses membranes impregnated with carbonic anhydrase to facilitate
transport of CO2 across a membrane into water in an underwater rebreathing
apparatus. Henley and Chang make a similar use carbonic anhydrase. Both
processes operate on dissolved carbon dioxide. Neither taught fixation of the
enzyme with the active site exposed to the gaseous phase with sufficient
hydration to m.aintain a reactive conformation. Neither taught modification of
DNA coding for enzymes to build in specific structure for fixation or enhanced
catalysis. Indeed Bonaventura took for granted that the crude coupling
techniques disclosed would deactivate a large fraction of the active enzyme.
The
Bonaventura patents contain computations showing that only a small fraction
(19b) of the carbonic anhydrase need retain its activity in the bonded
membrane
to provide adequate capacity to remove carbon dioxide from the proposed
apparatus in the illustrative uses. Henley and Chang do not discuss activity
losses
nor provide any description of fixation techniques to enhance enzyme activity
when in the active site is directly exposed to a nonaqueous environment.
Despite some significant advantages, a variety of major problems have
limited the application of enzymes in industrial settings. These include short
lifetime of either free or immobilized enzyme, fouling and biofouling,
separation
of the enzyme from the immobilization surface, limited availability of enzymes
in sufficient quantity, and expense of manufacture.
These problems have resulted in relatively few efforts to use cnzymes for
manipulation of gases. Further, physical/chemical means are in place
-3-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
commercially; they are understood and represent established technology and
significant investment.
Despite these historic considerations a number of recent developments
now allow broad based enzymatic applications. First, the development of DNA
libraries and the techniques needed to generate such libraries so that large
amounts of enzyme can be made economically. Previously, and even today, many
enzymes are derived by purification from a biological source. Second,
development of techniques to generate membrane expression of enzymes and
even direct secretion such that harvesting the enzymes is easier and
economically
feasible. Third, the development of new immobilization techniques which allow
long lifetime and high efficiency.
SUNdMARX OF THE INVENTION
The invention provides a process for gas separation wherein a selected gas
in a mixed gas stream is contacted by an enzyme, and the selected gas is
~L5 removed from the gas stream. The selected gas may also be recovered as a
separated gas. In one embodiment the invention provides a process for gas
treatment wherein a selected gas in a gas phase is contacted by a solvated
enzyme wherein the enzyme active site is in direct contact with the gas phase,
the
selected gas is converted to a first product in a condensed phase by contact
with
2,Q the solvated enzyme. In a preferred embodiment the hydrated enzyme is
partially covered with a hydrocarbon film. In another preferred embodiment the
hydrocarbon film is a lipid layer, more preferably the lipid layer is a
phospholipid
and most preferably the phospholipid is a bilayer. In a preferred embodiment,
the first product is further contacted with a second enzyme in the condensed
2,1 phase and converted to a second product, which maybe the originally
selected gas
or a different material. In an alternative embodiment the invention provides
a process for gas treatment wherein a selected gas in a gas phase is contacted
by
a solvated enzyme wherein the enzyme active site is in direct contact with the
gas
phase, the selected gas is converted to a first product in a condensed phase
by
contact with the solvated enzyme, the first product is further contacted with
a
-4-
CA 02222030 1997-11-24
WO 96/40414 PCTIUS96/09610
second enzyme in the condensed phase and converted to a second product. In
an alternative embodiment a simplified bioreactor is provided which comprises
buoyant beads coated with enzyme, floating on the surface of a condensed fluid
phase, in contact with a gas phase such that the active site of a portion of
the
enzyme coating is in direct contact with the gas phase, and the beads are free
to
rotate in response to motion in either phase, and means for producing motion
in at least one phase such that portions of the beads alternately contact the
gas
phase and the fluid phase, bringing a selected gas into the condensed fluid
phase.
In a preferred embodiment the enzyme is immobilized on the bead surface. In
1Q an especially preferred embodiment the enzyme is carbonic anhydrase, and
the
condensed fluid phase is water.
The invention also provides novel apparatus for practicing the process for
gas scparations. The method couples use of selective enzymes and
immobilization by several distinct means in conjunction with a variety of
support
15 structures or membranes to achieve gas enrichment, purification, separation
and
processing of gas mixtures. The process of the invention can be used in a
selective or subtractive manner, and provides on location separation and
purification, thereby avoiding transportation problems. The invention also
encompasses advanced enzyme fixation methods resulting in higher enzyme
20 activity than the crude cross linking techniques fixation of Bonaventura et
al.
The present invention is the first to provide enzyme immobilization with
active
site exposure directly to gas phase substrates.
The invention is a process to achieve vectorial movement and separation
of one or more gas substrates admixed with several other gases, thus isolating
a
25 selected gas from a mixture. The invention may be used to detect and
measure
the gas, to subsequently purifying the selected gas, or to convert selected
gases
to new chemical entities.
In apparatus provided for practicing the invention, an appropriate enzyme
or enzymes are present at the supply side to effect a change in state or
chemistry
30 of the selected gas and thereby generate a reaction product and reduce or
-5-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
prevent exit of the enzyme reaction product back to the gaseous supply side.
Solubility and concentration factors favor movement of the enzyme reaction
product into a second phase which also contacts the enzyme. The second phase
may be a second gas stream, or a solvent or a solvent doped with ions or other
chemical materials to facilitate retention of the enzyme reaction product.
Further treatment of the second phase may recover the selected gas as a
purified
or concentrated stream, or further react the selected gas or gases to produce
a
desired chemical entity.
Accordingly, the several objects the invention are to provide a process
IQ wherein gas mixtures are effectively separated so that at least one species
may
be isolated and/or enriched. Another object is to extract a selected gas for
independent use or to serve as a start material or substrate for another
physical,
chemical or biological operation. Another object is to process ionic
equivalents
of a gas, present in a solution, to extract the ionic gas equivalents in the
form of
a gas or to use the material as a substrate for another physical, chemical or
biological operation. Another object of the present invention is to allow
serial,
parallel or sequential processing of gas mixtures for sequential isolation or
enrichment of gases. Another object is to allow very large volumes of gas to
be
produced, not heretofore possible. Another object is to allow enrichment or
removal from gas mixtures in which the object gas species is in low
concentration.
Another object is to allow location of the separation facility at or near to
the site
of fmal use to reduce or eliminate transportation. Another object of the
invention is to allow appropriately sized production facilities to meet the
needs
of each user. Another object is to allow placement of gas generating
facilities
anywhere in the world, or in space.
Further objects of the present invention are to utilize a low purity gas
source. Yet another object is to treat the effluent from any kind of smoke
stack,
stationary or mobile, to process gases including carbon dioxide, oxides of
nitrogen, oxides of sulfur, hydrogen sulfide, methane, ozone, or
-6-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
chlorofluorocarbons. Carbon dioxide removal from gas streams is a particularly
preferred objective for gas processing.
The invention also provides apparatus for practicing the processes of the
invention selccted embodiments of which are illustrated by the drawings. Those
skilled in the art will recognize that many changes and substitutions may be
made
without departing from the spirit of the invention as described herein. The
drawings and descriptions are provided for illustration and not for
limitation.
The invention is defined and limited by the claims set out below.
BRIEF DESCRtPTION OF THE DItAW21GS:
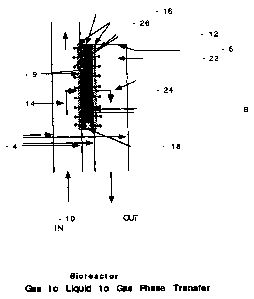
~Q Fig. 1 illustrates a simplified bioreactor for separation of a selected
gas.
Fig. 2 shows a reactor set up for recovery of carbon dioxide from a flue gas
stream.
Fig. 3 shows a multienzyme reactor for production of urea.
Fig. 4 shows a reactor for conversion of oxygen and water to hydrogen
peroxide.
15 Fig. 5 illustrates buoyant beads for positioning enzyme at a liquid surface
to
facilitate transfer of a selected gas to a liquid phase.
Fig. 6 Phase rotating immobilized enzyme carrier, plan view.
Fig. 7 Phase rotating immobilized enzyme carrier, side view.
DETAII" DESCRIP'TION OF THE INVENTION
Figure I illustrates an apparatus for practicing the invention as a process
for gas separation wherein a selected gas in a mixed gas stream is contacted
by
an enzyme, and the selected gas is removed from the gas stream. The apparatus
allows the selected gas to be recovered as a separated gas.
The bioreactor comprises a vessel having at least one first wall 2 and at
least one second wall 4, and an optional end wall 6 which closes a portion of
the
volume enclosed by first wall 2 and second wall 4. A portion 9 of second wall
4 is permeable to at least one selected gas and also comprises a support
surface
8 for enzyme immobilization; a mixed gas stream 10, from a gas source not
shown, enters a space between first wall 2 and second wall 4 wherein the mixed
-7-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
gas stream 10 contacts immobilized enzyme 12 fixed to support surface 8 which
removes selected gas 14 from mixed gas stream 10; selected gas 14 interacts
with
immobilized enzyme 12 and is caused to enter second phase 16 which is in fluid
contact with immobilization support 8 and immobilized enzyme 12 and confmed
with the volume enclosed by second wall 4 and optionally end wall 6; second
phase 16 may be a low pressure carrier gas stream or vacuum line, or a liquid
or
gas permeable gel, alternatively second phase 16 may be stirred by optional
stirrer 18 or circulated by an optional pump, not illustrated. An appropriate
enzyme or enzymes are present at permeable portion 9 to effect a change of
~Q state or chemistry of selected gas 14 and thereby reduce of prevent exit of
the
selected gas 14 back to the supply side. The desired vectorial movement of
selected gas 14 into phase 16 may be promoted by concentration effects,
solvation, chemical conversion, ionization, or other means to favor movement
of
selected gas 14 or its enzyme reaction product 15 into second phase 16.
If desired, second phase 16 may flow through the area enclosed by second walls
4 by use of a suitable port, not shown, and second phase 16 may also be
recirculated through a recycling loop not shown. The selected gas 14 or its
enzyme reaction product 15 may be used in second phase or may optionally be
further processed. Additional support surface 8, petmeable area 9 and
2Q immobilized enzyme may be provided in second wall 4 to connect second phase
16 to a recovery zone 22 enclosed by first wall 2, or second wall 4, or a
combination thereof, and optionally end wall 6 and recovered as a further
reacted product or as a concentrated gas by, for example, reverse reaction
with
the same intmobilized enzyme to generate a recovered purified selected gas 14.
25 A concentration gradient or other means will be used to favor vectorial
movement of selected gas 14 or enzyme reaction product 15 from second phase
16 to recovery zone 22. Any of the means previously listed can be also be
applied at this stage to provide movement into recovery zone 22. If desired,
one
or more different enzymes or other catalysts may be placed between second
20 phase 16 and recovery zone 22 to further convert setected gas 14 to a new
-8-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
product. Additional stages may be added to further change or process selected
gas 14 or its enzyme reaction product 15 as may be desired. Figure 2
illustrates
a reactor removing carbon dioxide from a mixed gas stream such as animal
respiratory gases by contact with carbonic anhydrase, conversion of carbon
dioxide to bicarbonate, and subsequently reconverting bicarbonate to carbon
dioxide as a concentrated gas stream. Figure 3 illustrates a multienzyme
reactor
system which converts carbon dioxide and ammonia to urea.
In a reactor of the type illustrated by Fig. 1, elements and areas maybe
interchanged to provide many alternative structures as will be apparent to
those
JLO skilled in the art. The mixed gas stream 10 may be pretreated to provide
an
optimal temperature or pressure, or to remove components that would lower the
reactor's efficiency, or contribute components that may pass through the
selection
media to contaminate purified selected gas stream 24, or recovered product 25.
Examples of pretreatment include mechanical screening by filters, chemical
j~ screening by adsorbents or absorbents, or scrubbing, and use of heat
exchangers,
waste heat recovery processing, compression, expansion and other gas
processing
steps known in the art.
The immobilization support 8 maybe of any conventional material such
as polysaccharide surfaces or gels, ion exchange resin, treated silicon
oxides,
porus metal structures, carbon rods or tubes, graphite fibers, silica beads,
cellulose membranes, gel matrices such as polyacrylamide gels, poly(acryloyl
morpholine) gels, nylon or other polymer meshes, or other suitable binding
surface. Alteratively the enzyme may be wholly or partially encapsulated in a
suitable material such as cellulose nitrate capsules, polyvinyl alcohol
capsules,
starch capsules or liposome preparations. In another alternative the enzyme
may
be fixed at a phase boundary, as illustrated in Figures 5, 6 and 7 or by use
of
nonionic surfactants as described, for example in Li et al., U.S. Patent
3,897,308.
The enzyme may be fixed to the surface by binding, covalent bonding,
physical attraction, coordination bonds, chelation, or other binding means or
mechanical trapping or other means known to those skilled in the art. The
-9-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
support may also be a membrane of selective permeability. The selectivity may
be by size, or other characteristic. The membrane may be a lipid bilayer doped
with passive porins, channels or ionophores of the co-porter or antiporter
type
which commonly rely on properties such as charge and/or hydrated radius for
i separation. Further examples of suitable materials for permeable portion 9
and support surface 8 include permeable membranes, natural and artificial,
including
semipermeable plastic membranes, black lipid membranes, alternatively doped
with ionophores to provide ion conducting channels. In yet another embodiment
the porins may be active, i.e.,dependent on an energy flux. For example with
iQ a cell wall membrane, the energy flux may be tied to an endogenous high
energy
compound such as a labile triphosphate bond or to an exogenous supply of
energy via photons, electrons or protons. In all these cases the
immobilization
support works as membrane with selective permeability to maintain separation
of the mixed gas stream 10 from the second phase 16 while passing selected gas
15 14 into contact with second phase 16.
The enzymes immobilized on support surface 8 are of two types, simple
enzymes and those requiring cofactors for activity. Simple enzymes may be
fixed
to the support surface by any of the means known to the art. Preferably the
enzymes are modified by adding an amino acid sequence that binds to the
support without substantially reducing enzyme activity. In a preferred
embodiment the enzyme is modified by altering the DNA segment coding for the
enzyme to add a sequence coding for an amino acid sequence that yields a
binding moiety to the enzyme in a manner that enhances enzyme binding. The
modification may provide a sequence that binds to a metal such as a poly-
histidine sequence, or may code for an epitope or antigen moiety that binds to
an antibody or may be a portion of an antibody that binds a known antigen. For
example, a polyhistidine sequence can be added to an enzyme such as carbonic
anhydrase by splicing a DNA fragment coding for the desired polyhistidine
sequence into the DNA coding for the enzyme at either terminus of the protein
-10-
CA 02222030 1997-11-24
WO 96/40414 PCTIUS96/09610
sequence, and expressing the DNA in a suitable organism and recovering the new
enzyme.
In the case of enzymes requiring cofactors the cofactor maybe supplied
in second phase 16 or also fixed to the support surface 8 or supplied by
pretreatment of surface 8 with the cofactor to activate bound enzyme. If
multiple materials are to be bound to the support surface 8 they may be
exposed to a prepared surface sequentially or as a mixture.
The second phase 16 may be selected from the group consisting of gases,
aqueous solvents, protic solvents, aprotic solvents, hydrocarbon solvents,
aromatic
~Q hydrocarbon solvents and supercritical fluids. The second phase may be
stirred
or a flow introduced by a pump to move fresh material into contact with the
immobilized enzyme at the support surface. A flow may be introduced into
second phase 16 by a stirrer or pump or other conventional means. Flow or
other means is applied to maintain a concentration or other gradient to
produce
vectorial movement of the selected gas 14 into condensed phase 16. In some
embodiments immobilization support 8 and film 26 maybe combined as, for
example, in a lipid bilayer wherein a portion of the enzyme extends into the
lipid
bilayer to provide both support and an evaporation barrier.
In a preferred embodiment a film 26 is provided in which the selected gas
2Q is soluble, and which decreases the escape of second phase 16 into mixed
gas
stream 10. The film maybe a gel, hydrocarbon layer, or preferably a lipid or
phospholipid layer or bilayer.
When the recovery zone 22 is used, the condensed phase 16 is contacted
by a second surface which may carry a catalyst, enzyme or simply be
selectively
permeable such that selected gas 14 or its equivalent or derivative is
transported
to recovery zone 22. Again a concentration or other gradient may be used to
enhance vectorial movement from second phase 16 into recovery zone 22.
Gases are defined as materials which are in the gas phase at ambient
temperature and pressure (taken to be 20 degrees C. and one atmosphere).
Suitable gases include nitrogen, oxygen, oxides of carbon, nitrogen, sulfur,
-b1-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
methane, ammonia, hydrogen sulfide and the like. Any gas that interacts with
an enzyme directly from the gas phase may be a selected gas 14 in combination
with a suitable enzyme 12 which interacts with the selected gas 14. An enzyme
is a protein or peptide that selectively binds or reacts with a gas molecule.
An
enzyme is composed of amino acids with an active site which binds a specific
molecule and facilities a change in the bound molecule. An enzyme may include
nonnaturally occurring amino acids, and maybe natural or artificial. Examples
of suitable enzymes include:
ENZYME EC NUMBER
glucose oxidase 1.1.3.4
aldehyde oxidase 1.2.3.1
hydroxylamine oxidase 1.7.3.4
sulfite oxidase 1.8.3.1
sulfur-ferric ion 1.8. 99. -
oxidoreductase
catechol oxidase 1.1.3.14
(dimerizing)
laccase 1.10.3.2
L-ascorbate oxidase 1.10.3.3
2Q catalase 1.11.1.6
sulfur dioxygenase 1.13.11.18
superoxide dismutase 1.15.1.1
B galactosidase 3.2.1.23
urease 3.5.1.5
25 carbonate dehydratase 4.2.1.1
(carbonic anhydrase)
lactic acid oxygenase
inositol oxygenase
lysine oxygenase
2Q octane oxygenase
pyrocatechase
3-hydroxyanthranilate
oxygenase
tryptophan oxygenase
homogentisate oxygenase
~
-12-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
CLASS II - GAS ENZYMES REQUIRING COFACTORS OR COENZYMES
ENZYME E NUMBER COFACTOR/COENZY14iE
formate dehydrogenase 1.2.1.2 NADH
fotmate dehydrogenase 1.2.2.1 ferricytochrome bl
(cytochrome)
carbon monoxide- 1.2.3.- methylene blue
methylene blue oxidoreductase
carbon monoxide 1.2.99.2 methyl viologen
dehydrogenase
nitrate reductase (NADH) 1.6.6.1 NADH
nitrate reductase 1.6.6.2 NAD(P)H
(NAD(P)H)
nitrate reductase 1.6.6.3 NADPH
(NADPH)
1~.. nitrite reductase 1.6.6.4 NAD(P)H
(NAD(P)H)
superoxide-forming 1.6.99.- NADPH
enzyme
nitrite reductase 1.7.2.1 ferricytochrome c
2Q (cytochrome)
ferredoxin-nitrate 1.7.7.1 ferredoxin
hydroxylamine reductase 1.7.99.1 p yo c y a n i n e; m e t h y l e n e
blue;flavins
nitric-oxide reductase 1.7.99.2 pyocyanine
~.5 nitrite reductase 1.7.99.3 pyocyanine; flavins
nitrate reductase 1.7.99.4 benzyl viologen
sulfite reductase 1.8.1.2 NADP
(NADPH)
sulfite reductase 1.8.7.1 ferredoxin
IQ (ferredoxin)
sulfite reductase 1.8.99.1 methyl viologen
adenyl sulfate reductase 1.8.99.2 methyl viologen
cytochrome c oxidase 1.9.3.1 ferrocytochrome
Pseudomonas cytochrome 1.9.3.2 ferrocytochrome
c oxidase
nitrate reductase 1.9.6.1 ferrocytochrome
methane monooxygenase 1.14.13.25 NAD(P)H
nitrogenase 1.18.2.1 ferredoxin + ATP
carbamoyl-phosphate 6.3.4.16 ATP
40 synthetase
-13-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
One skilled in the are will add other enzymes as need to satisfy the intent of
this
invention. As used herein the term enzyme should be taken to mean the enzyme
per se , its cofactors and co-enzymes, i.e., all of the elements needed for a
functional enzyme system to effect biochemical transformations.
Exnmple 1
To immobilize the enzyme on a membrane or mesh surface, such as, for
example, nylon, one proceeds as follows: The material is activated by exposure
to a 0.2 M nitric acid wash. The activated surface is exposed to 2 M sodium
carbonate solution containing 20 g of disodium iminodiacetie acid (IDA) for 24
~Q h at 60-65 C. The material is then rinsed with 0.1 M sodium carbonate, 0.01
M
sodium acetate and water. It is then charged using zinc chloride at 1 mg/ml.
Additional immobilized metal affinity chromatography (IMAC) procedures are
found in Smith MC, Furman TC, Ingolia TD, and Pidgeon C (1988) Chelating
Peptide-immobilized Metal Ion Affinity Chromatography: a new concept in
affinity chromatography for recombinant proteins. J. Biol. Chem. 263:7211-
7215.,
and Sulkowski E (1987) Immobilized metal ion affinity chromatography of
proteins. In: Burgess R (ed.) Protein Purification. Micro to Macro. Alan R.
Liss,
Inc. New York pp. 149-162. The basis of protein retention is binding of amino
acids on the protein surface (notably histidine) to the vacant coordination
sites
IQ of metals (e.g., copper, zinc) complexed to chelating groups such as
N-(carboxymethyl) glycine. The IDA ligand is favored because it is resistant
to
Ph between 2 and 13, and temperatures to at least 120 C.
EXANITLE2
In a reactor as illustrated by Figure 2, a carbonic anhydrasc (CA) (E
4.2.1.1)is immobilized on both sides of a reservoir of aqueous buffer. As
noted
above CA is the one of the fastest enzymes known with a turnover number of
600,000 katals. Thus it has the ability to catalyze the hydration of 600,000
molecules of carbon dioxide per second per molecule of CA. Carbonic
anhydrase is a diffusion limited enzyme. Thus, any CO2 molecule which comes
into contact with the enzyme will be converted virtually immediately into HCO3-
.
-14-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
Any carbon dioxide containing mixed gas supply might be used. The scale
of the equipment to be used must be adjusted accordingly. In this example, the
mixed gas supply is taken from the exhaust stack of a gas fired electric power
plant following appropriate heat exchangers and after passage of the exhaust
through an air house. The make up of a typical gas is N2 - 64.92%, 02 - 2.04%,
CO2 - 7.06 %, and H20 - 25.98 %.
Any heavy metals which could foul the enzyme are first extracted from the
mixed gas supply 10. Such metals could be present in the exhaust of a coal
fired
furnace. CA contains a zinc atom and other divalent cations can, under correct
circumstances, displace the zinc and alter the reactivity of the enzyme.
Cobalt is
a particularly potent divalent cation of this class.
The enzyme is covered with a thin oil film 26 to prevent water loss from
the second phase 16. 1 this example the second phase is an aqueous 20 mM
sodium phosphate buffer. CA catalyzes the hydration of CO2 to HCO3-. The
~S bicarbonate is delivered to the flowing buffer streani of the second phase
16 and
carried to contact the permeable wall area adjacent recovery one 22 where it
contacts a second layer of CA fixed to support surface 8 at permeable wall
area
9. The CA layer reverses the reaction and converts HCO3- to CO2. The second
phase 16 buffer stream is constantly flowing to deliver lean buffered medium
to
ZQ the bicarbonate rich zone adjacent to the mixed gas supply 10 and then
carry the
bicarbonate rich fluid to the surface adjacent the recovery zone 22. The flow
removes bicarbonate from the first surface where it can act as a competitive
inhibitor of CA and slow the reaction. The overall effect is to create a
continuous removal of product adjacent the enzyme surfaces and maintain a
gradient that favors vectorial movement of CO2 into solution as bicarbonate
adjacent mixed gas stream 10 and from bicarbonate to CO2 adjacent zone 22.
Carbonic anhydrase has a dehydrating rate constant which is slightly
slower than the hydrating rate constant. For equal molar volumes of carbon
dioxide and bicarbonate this favors the hydration of carbon dioxide. However,
-15-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
adjusting the relative surface area of the two enzyme surfaces will compensate
for this difference. The newly generated CO2 is drawn into recovery zone 22 at
low pressure for extraction. This guarantees a vectorial flow for the entire
system.
The kinetics and the specificity of the enzyme greatly favor the removal
of CO2 from the mixed gas supply above all other gases. Carbonic anhydrase
does not recognize or interact with any of the other gases in the flue gas
mixture.
However, under steady state conditions the concentration and solubility of the
several gases in the mixed gas stream will determine the degree to which they
may pollute the purified gas stream. The solubility of these gases in oil and
in
><,Q water at 37U0s given in Table I. The permeability of COz in water is 210
* 10-9
* cm3 (STP) * cm/sec * cm2 * cm-Hg. The diffusivity of CO2 in water is 1.96 *
10-5 * cm2/sec. The solubility of CO2 in water is 3.39 * 10'5 gmoles * cm *
10'3/amz.
TABLE I
j~ GAS OIL RATIO to COZ WATER RATIO to COZ
ml gas/ml oil mi gas/ ml water
Nitrogen 0.067 0.037:1 0.014 0.023:1
Oxygen 0.13 0.099:1 0.027 0.043:1
Carbon dioxide 1.34 1:1 0.63 1:1
~ Carbon monoxide 0.097 0.073:1 0.012 0.034:1
Methane 0.31 0.231:1 0.029 0.046:1
Given the solubility ratio, vis-a-vis CO2 dissolving into the system and the
respective concentrations the expected contamination of purified gas stream by
each gas in the exhaust stream of the electric power plant in a single pass
is: N2
ZE - 0.9%, 2 - 0.006%, H20 - 0 k, CO - 0%, CH4 - 0% The buffer and other
salts
do not appreciably alter the gas solubility and the rate of dissolution of
carbon
-16-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
dioxide in water is 10~ times slower in the absence of carbonic anhydrase. In
the absence of enzymatic conversion over 99% of the CO2 remains in the
dissolved gas form and less than 1% is spontaneously converted to bicarbonate,
and very little gas would be recovered in recovery zone 22.
EXAMPI.E 3
In Figure 3 a two stage reactor of the same general design and operation
as that illustrated in Example 2 is shown. The inlet mixed gas 10 is
preferably
a carbon dioxide rich stream. On the mixed gas stream side carbonic anhydrase
is the inimobilized enzyme 12 which is covered by a lipid film 26 as in
Example
2. Carbon dioxide is converted to bicarbonate adjacent the mixed gas side also
as described in Example 2. A separate supply line 28 injects ammonia into the
aqueous phase 16, which is converted to ammonium carbonate by conventional
chemical reaction with the bicarbonate. The ammonium carbonate rich aqueous
phase is circulated to contact urease 12 immobilized on a semipermeable
membrane 9 adjacent urea recovery zone 22. The ammonium carbonate from
the aqueous buffer is converted to urea by urease and transported across the
membrane to the recovery zone via absorption. An organic solvent in the
recovery zone dissolves the urea which is conveyed away from permeable zone
9, for recovery. The urea stream is flash evaporated to concentrate the urea
while the solvent is retumed to zone 22 and recycled.
EXAMPLE 4
The operation of a gas phase to gas phase bioreactor is illustrated in Fig
4. The immobilized enzyme 12 is catalase and the selected gas from mixed gas
stream 10 is oxygen. Catalase normally catalyzes the reaction H202 = = > 1/2
02 + HZO. Catalase is a diffusion limited or "perfect enzyme". Its reaction
rate is about 5.6*106 mol/mol/min. eG for the reaction of hydrogen peroxide to
produce oxygen and water in the uncatalyzed state is 18 kcal/mol. When
catalyzed by a typical physical catalyst, platinum, this value drops to 13
kcal/mol,
an improvement of only 38%. However, when catalyzed by catalase it declines
-17-
CA 02222030 1997-11-24
WO 96/40414 PCTIUS96/09610
dramatically to 7 kcal/mol, an improvement over platinum of 86% and a total
improvement of 257%. This reaction is exothalmic in the amount of 45.68
kcal/gmol in aqueous phase and 25.97 kcal/gmol in gas phase.
The reaction can be forced to proceed in the opposite direction, using 02
and H20 vapor to produce H202. If the raw materials 02 and H20 vapor are
supplied at elevated temperature, or other means, sufficient to overcome the
energy barrier, then H202 can be generated. It is important to maintain this
reaction in the gas phase first to minimize the energy differences and second
to
prevent the deleterious effect of even moderate concentrations of peroxide on
JQ the integrity of the enzyme. It is also important that the second side is
at lower
pressure than the first side to provide vectorial flow to the system and
remove
the peroxide from the enzyme before an appreciable concentration accumulates.
Due to its specificity catalase is unreactive to other gases commonly found in
air,
for example. Water vapor is supplied to the enzyme by increasing the humidity
of the mixed gas stream 10, preferably to near 100%. Water vapor at near 100%
humidity provides sufficient water to hydrate the active site of the enzyme.
The
consequence of the exemplified operation is the production of enriched
peroxide
in the second phase 16.
EXA1VH'~~ 5
N A simplified bioreactor is illustrated in Fig. 5 wherein an enzyme 12 such
as carbonic anhydrase is coated on the surface of a buoyant bead 30 which
floats
at the surface of a fluid. A gas stream contacts the exposed enzyme on the
exposed surface of the floating bead, and the enzyme binds its gas substrate.
Turbulence of the fluid at the surface rotates the bead to immerse enzyme with
25 bound substrate and wash away the product of the enzymatic reaction into
the
fluid phase. In the case of carbonic anhydrase, carbon dioxide in a mixed gas
such as the atmosphere is bound and converted to bicarbonate. Rotation of the
bead immerses enzyme carrying bound carbon dioxide or bicarbonate and
exposes fresh enzyme to the atmosphere. When the beads float on a water
surface, the net effect is to facilitate the conversion of carbon dioxide in
the
-18-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
mixed gas to bicarbonate in aqueous solution. Such a bead reactor may be
incorporated for example into a conventional scrubber to reduce the amount of
carbon dioxide released into the atmosphere by a fossil fuel fired power
generation plant. Beads floating on the surface of a pond may be useful to
remove carbon dioxide from the atmosphere and increase the available
bicarbonate concentration in the water. Also shown in Figure 5 are oblong
beads
32 which may be partially coated with enzyme and weighted to maintain the
enzyme exposed to the gas phase. An alternate embodiment uses fully coated
beads 30 of a selected density to float at the fluid surface with a portion of
the
nQ enzyme directly exposed to the gas phase. While any fluid may be employed
provided that the enzyme remains active and product is washed off into the
fluid
phase, water or aqueous buffers are the preferred fluids.
An alternative embodiment uses a structure similar to that shown in Fig.
6. Beads having enzyme immobilized on the surface are fixed to a lattice 34 in
15 such a manner that the beads can rotate around a lattice element. The
lattice
34 is positioned such that the beads are fixed at the surface of a fluid phase
in
contact with a gas stream. The gas stream impinges on the beads such that the
force of the gas stream 10 is sufficient to rotate the beads exposing fresh
enzyme
surface to the gas and carrying bound gas into contact with the fluid phase
16.
The turbulence is such that the bead 30 carrying the immobilized enzyme
rotates
to be successively exposed to the gas phase and the fluid phase 16.
EXAMPLE 6
The bioreactor illustrated in Figs. 6 and 7 utilizes countercurrent flow of
the two phases on opposite sides of the immobilized enzyme affixed to axially
tethered bead-like immobilization surfaces. Under these circumstances the
beads
will rotate moving the surface from contacting the first fluid to contacting
the
second fluid and vice versa. Gases or products will be delivered from the
first
fluid to the second fluid where they will be deposited. The effect of the
immobilized enzyme at the bead surface is to facilitate transfer of a gas or
~ ~~ product from the first fluid to the second fluid. Preferably the
countercurrent
-19-
CA 02222030 1997-11-24
WO 96/40414 PCT/US96/09610
fiuids are inuniscible or are separated by a semipermeable layer which may
partially overlay the bead support lattice 34 to separate the countercurrent
fluids.
The beads are sized to extend beyond the separating layer and contact each
fluid.
In another embodiment the beads of Figures 6 or 7 may include vanes to
facilitate rotation by the fluid flow. Disks or other shaped supports may be
substituted for spherical beads in this embodiment.
In the bioreactors of Examples 5 and 6, material choices for beads,
immobilization methods, solvents, buffers and enzymes are the same as those
discussed above for the other systems. In the floating bead reactor of
Examples
IQ 5 and 6 much of the structure is eliminated while the essential features of
the
invention are retained.
In each of the preceding examples, the choice of fluid, buffer and salt
content is deterrnined by both the solubility and ionization potential of
ionic
equivalents of the gas of interest in the fluid, on both an absolute scale and
in
~ relation to the solubility and formation of ionic equivalents of other gases
in the
mixed gas stream. The following factors guide the choice. One is to increase
the
fractional concentration of the gas of interest. Another is to decrease the
fractional concentration of contaminants. Yet another is to increase the
ability
of chemical reactions to occur in the medium and to increase the ability of
the
enzyme to generate ionic equivalents. Another is to optimize the miscibility
of
the gas with the phase surrounding the enzyme.
Many variations of structure and design may be made to combine the
elements of the disclosed invention in many configuration without departing
from
the scope and spirit of the present invention as defmed by the appended
claims.
-20-