Note: Descriptions are shown in the official language in which they were submitted.
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/US96/09614
TE~ EUTIC MICRODEVICES
AND METHODS OF MAKING AND USING SAME
FIEID OF ~E INVENTION
The present invention relates to microstructural devices, and more particularly to
iclu~L~Ilctural devices for use in drug delivery to a body site.
BACKGROUND OF ~E INVENTION
A variety of self-assembling particles have been used or proposed for drug delivery
10 to a target site in a body. Lipid micellar particles formed of a surfactant provide certain
advantages in solubilizing and delivering hydrophobic drugs, e.g., for intravenous drug
delivery.
Liposome delivery systems have been employed for delivery of drugs to tissues and
organs, e.g., liver and spleen, rich iri llla~ilv~hages of the reticuloendothelial (RES), for
15 depot drug release in the bloodstream, and for site specific zl~cllmnl~tion and drug release in
solid tumors and sites of infecti--n
Liposomes offer a number of advantages over simpler micellar particles. The lipid
bilayer shell in a liposomes provides an internal aqueous CO~ ,dlL,IIcllL that is Pc~nti~lly
isolated from bulk phase aqueous m~ m, allowing hydl~uhilic drugs to be se.~ d at
20 high concc;llLIdlion within liposomes, and further p~ . ."i~ .g loading of ionizable drugs by
use of ion gradients across the liposomal membrane. Liposomes can also be process to
have selected sized in the 30-200 nm size range for intravenous drug delivery. Finally, the
outer lipsome surfaces may be coated with a hydl~hilic polymer, such as polyethylene
glycol, to extend blood circulation time of the particles, and/or may be d~signPd to carry
25 surface-bound ~ntilig~nf1 molecules for selective binding to target cells cont~ining cell-
specific surface ligands.
The use of synthetic polymers in drug delivery devices has focused on "smart
polymers" a term given to polymers that form gels that have the ability to expand or
contract in response to a specific stim~ c, such as light, lelll~eldlul~; or pH. Typically,
30 such polymers will pl~cil~ildL~ in solution or collapse with collcolllil~lL expulsion of gel pore
contents. Synthetic polymers may be based on a number of types of IllOIlOIII.,.iC units,
inrlu-ling vinyl ",ono",~.~, N-alkyl substituted acrylamides and the like. Copolymers have
also been utilized in an attempt to combine or m~ re the ~tim--lus responsive properties
of one or more known smart polymers. Polymer particles formed of negatively charged
35 polymers have been ~IP~ign~d for (i) rapid c~n-lPn~tion (for drug ellLld~lllenL) and
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/US96109614
~l.oc~n-lçn.c~tion (for drug release) or controlled-rate swelling and drug release, and (ii) high
drug t~ d~lllellL by an ion exchange m~ h~ ."
The collct;~L~ of smart polymer particles and lipid-bilayer vesicles have been
COlll'L h1ed in drug-delivery particles of the type having a contl~n.ced-phase polymer core
S encased in a lipid-bilayer lllcllll~lal1e, giving advantages of both types of drug-delivery
systems. In particular, the polymer core of the particle can be loaded to high drug
concell~ldlion, for rapid release by particle decon-l~nc~ti-)n, and the lipid coating on the
particles can be dçsign~d for targeting and/or for a target-specific triggering event which
can in turn leads to rapid particle decon-l~nc~tion.
It is evident that it is possible to design self-a~ssembling lipid and/or polymer
particles having various drug loading, targeting and triggering capabilities. Non~th.olçss,
self-assembling particles of this type present two ~ignific~nt limitations. First, since the
particles are typically spherical, the physical-contact area between the particles and target-
site cells, for example, for particle binding to the cell, is more limited than would be the
15 case with a particle having more planar surfaces. Secondly, and more importantly, the
functioning of self-assembling particles, in terms of Idl~t;lh~g and drug release, are limited
in terms of (i) the particle materials (and Lh~;lcrvle material properties) that can be
employed, (ii) the types of drug storage and drug release ll.~ that can be realized,
(iii) the number of Idl~C:Lillg and therapeutic functions that can be built into the particles,
20 and (iv) the ability to place dirr~lc;llL functions at discrete locations on the particles.
It is the purpose of the present invention to provide microdevices that signifi~ntly
expand the shape, materials, and functions versatility and capabilities over self-assembling
drug-delivery particles.
25 SU~ARY OF THE INVENTION
The invention includes ~.u~el~ion of microdevices for use in a~h~ g a
therapeutic agent to a selected target site in a subject. The microdevices have a selected
non-spherical shape and uniform ~lim~nci~ns and contain the thcldpcuLic agent in a form in
which the activity of the therapeutic agent is ~ essed by exposure of the microdevice to
30 the biochemical enviiul~lllclll of the target site after ~lhll;lli~ldlion to the subject. The
microdevices may have surface-bound, marker-binding molecules effective to bind to a
marker carried on the surface of cells at such target site.
Where the suspension is used for targeting selected cells or tissue via the
bloodstream, the microdevices may be coated with a hydrophilic polymer, such as
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/US96/09614
polyethylene glycol or glycocalyx polymer, effective to enhance m~ e..~l~ee of the
microdevices in suspension. The hydrophilic polymer may be conjugated to vesicle-forming
lipids, where the microdevices are coated with a lipid film containing such vesicle-forming
lipids. The microdevices may contain a marker-binding molecule bound to the free ends of
5 at least a portion of the polymer, this molecules being effective to bind to a marker carried
on the surface of such target cells or tissue.
Where the suspension is used in pa~ Aminictration to a subject, the
microdevices may have a selected m~ximnm dimension in the range between 0.1 and 3
microns. For solid-tumor ~lgt:~hlg such microdevices preferably have ~ m~imnm
10 dimension less than about 150 nm.
Where the suspension is used in delivering a theld~uLic cu~ uulld to the interctiti~l
space of a target region characterized by a target-specific marker on the b~c~
bl~1e forming the v~cc~ h~re of the target region, the microdevices may contain
surface-bound marker-binding molecule effective to bind to such marker, and an enzyme
15 effective to lyse the b~c~...~.l lllc:lll'~l~ule. One p~fell~1 enzyme is a type IV collagenase.
The enzyme may be covalently attached to a surface region of the microdevices, or may be
contained in the microdevices in rel~c~hle forrn, e.g., upon erosion of the microdevices.
In one general embodiment, the microdevices are formed of a material designed toerode in body fluid at a selected bioerosion rate. As examples, the microdevices may be
20 formed of iron, tit:lnillm~ gold, silver, platinurn, copper, and alloys and oxides thereof.
Alternatively the microdevices may be formed of a biodegradable polymer material.
In one embodiment, the microdevices are composed of a conAPnced-phase polymer
material effective to AeConAe~l~e7 at a selected rate, when exposed to plasma.
In another embodiment, the microdevices are ~ lly disc-shaped, and have a
25 l~min~ted struchure colll~illillg two or more layers, where ~Aj~rent layers are formed of
dirre,~ materials. As example, the microdevices may have a tril~min~te structurecomposed of an interior layer sandwiched between a pair of exterior coating layers, where
the coating layers have a slower rate of bioerosion than the interior layer, and the
thcla~ uLic compound is embedded in said interm~oAi~te layer, for release as the interm.oAi~tf-
30 layer is eroded.
In still another general embodiment, the microdevices have shielded wall surfacesthat are ,ub~ ulLially in~( ctDcci't)le to biological cells. These wall surface may be coated with
therapeutic agents, such as therapeutic antibodies or the like. Exemplary microdevices of
this type include ring shaped and cup-shaped devices.
CA 02222~9~ 1997-ll-27
WO 96/41236 PCT/US96/09614
For use in directing the microdevices to a target site by a m~gn--tic field, or
retrieving the devices from a site by a m~gn~tic field, the microdevices contain a m~gn~ti~
material.
Also disclosed is a microfabrication method for producing microdevices of the type
5 described. The method includes exposing a sheet of microdevice material to a photoablating
light source through a pht~tl~m~k, to form a reticular lattice pattern on the sheet
corresponding to the desired microdevice size and shape. The photoablating is colllhlucd
until the desired degree of ablation is aclli~v.,d.
These and other ob ects and features of the invention will become more fully
10 alJpdl~lll when the following detailed description is read in conjullclion with the
~comr~nying dldwillgs. '
BRIEF DESCRIPrION OF THE DRAWINGS
Figs. 1-7 illustrate various embodiments of microstructures and microdevices
15 constructed in accordance with the invention, shown in pel~pe.;li~1e views (Figs. lA-7A),
and cross-sectional views (.~ i..i.,g figures);
Fig. 8 is an enlarged section of the disk-shaped microstructure in Fig. 7C, showing
surface bound antibody and therapeutic agents on the inner wall surface thereof;Figs. 9A-9E illustrate steps in the photolithographic fabrication of Illi~lu~lluctures
of the type illustrated generally in Fig. 2A;
Figs. lOA-lOD illustrate steps in the photolithographic fabrication of microstructures
of the type illu~llaled generally in Fig. SA;
Figs. llA-llD illustrate steps in the fabrication by laser ablation of microstructures
of the type illustrated generally in Fig. 3A;
Fig. 12 is a sectional view of a coated microdevice.
Figs. 13A and 13B are section~l views of a lipid coated microdevice having a
surface coating of hydlophilic polymers, where in Fig. 13B, a portion of the polymers have
antibodies ~tt~h~d to their distal ends;
Fig. 14 is a sectional view of a microdevice having an upper surface coating of
antibodies and a hydrophilic polymer;
Fig. 15 is a sectional view of a microdevice of the type shown in Fig. 6A,
illu~lldlhlg how the device structure shields antibodies carried on the device from contact
with an immune-response cell;
CA 02222~9~ 1997~ 27
WO 96/41236 PCT/US96/0961
Figs. 16A and 16B are sectional views of a microdevice of the type illustrated in
Fig. 3A, showing more rapid erosion of a middle drug-carrying layer in the microdevice;
Figs. 17A and 17B illustrate the greater surface contact between a drug-deliveryparticle or capillary wall surface in a disc shaped particle constructed in acco~ ce with the
5 invention (17A) than in a conventional self-assembly type spherical drug-delivery particle
(17B);
Figs. 18A-18C are embodiments of the invention suitable for I~ LIllell~ of hl~ ial
tissue.
Figs. l9A and 19B illustrates ste?s in the movement of a microdevice co~ d inaccordance with one embodiment of the invention across an epithelial ~ Ialle; and
Fig. 20 is a cross section view of a microdevice cont~ining a m~gnlotic layer.
DETAILED DESCRI~rlON OF THE INVENTION
I. DeflnitiOnS
Unless in-lir~trd otherwise, the terms below have the following mr~ning.
"Microstructures" or "microparticles" or "microfabricated structures" or
"microfabricated particles" are particles formed by microfabrication methods.
"Microdevices" or "microfabricated devices" are microstructures that have been
additional prepared to include biological agents as coatings and/or therapeutic agents;
"Microfabrication mPthorl.c" refer methods employing photom~cking or pa~el.led
beam irradiation of a substrate to produce desired surface pattern features in the substrate;
The "biorh~mir~l ~"lvhulllllent of the target site" refers to one or more intrinsic
physiological conditions at the target site, such as pH, salt conditions, t~ ;;ldlule, or the
presence of target-specific cell surface markers or enzymes, effective to initiate and promote
released of a therapeutic agent from the microdevices of the invention.
The target site may be a specific cell, tissue or organ type, the hllel~liliulll of a tissue of
organ, a vascular site, or blood or plasma.
"Bioerodable" refers to a materials, such as an erodable metal, that is dissolvable in
physiological mP~inm, or a biocolllL~d~ible polymeric material that can be degraded under
physiological conditions, by physiological enzymes and/or chrrnic~l conditions, e.g., in a
reducing or reduced-pH environment.
CA 02222~9~ 1997-11-27
WO 96/41236 PCTtUS96/09614
II. Microdevices
The present invention provides microdevices that are useful therapeutically in avariety of in vitro, in vivo and ~x vivo applications, in particular, intravascular applications.
The microdevices have a selected non-spherical shape, uniform ~limrnci~ns and contain a
5 Lll~ldp~ ic agent in a form where the activity of the agent is expressed by exposure of the
microdevice to the biorhPmir~l en~ilol--ll~llL of a selected target site.
A. Representative Embodiments
The shape, size and composition of a microdev.ce of the present invention depend10 on the selected application. For example, devices designPd to be used in typical
d~,ds~ ar applications are preferably ~ ls~ lly disk-shaped, cup-shaped, or ring-
shaped. Exemplary embodiments of such disk-, cup- or ring-shaped devices are illustrated
in Figures 1-7, in top perspective views (Figs. lA-7A) and cross-sectional views (Figs.. lB-
7B and lC-7C).
Figs. lA and lB show a disk-shaped microstructure 20 composed of a thin disk
with did l.~:Lel D between about 50 nm to about 3 microns and a thil~knPcc T between about
10 nm and about 1 microns. The disk is formed of a single material 22 which may contain
the therapeutic agent (e.g., a drug in a polymer matrix). Although non-erodable materials
may be used, particularly for ex vivo applications, the plert~ d devices are formed of
20 bioerodable materials, as described below.
The device may further be coated partially or completely with a coating 24 of
hydrophilic polymer chains, such as chains 26, to form a coated microdevice 27. Typically
these chains are added after formation of the microstructures groups. Preferred hydrophilic
chains are polyethylene glycol (PEG) chains and synthetic glycocalyx, which are int~Pn~lPd to
25 either enhance the solubility of the device or, in the case of PEG, to extend blood
circulation of the device. Methods of derivatizing a variety of metal surface with polymer
chains are well known. For example, chains conldillillg thiol end groups at one chain end
can be reacted with a metal surface under conditions effective to form thioethe} linkages.
Similarly, where the disk material is a polymer, a variety of surface ~tt~rhmPnt rhPrni.ctrie
30 e.g., involving carboxyl groups in the disk material and amine or hydroxyl groups in the
polymer chains, are available for covalent linkage to the disk material. Other approaches to
~tt~rhing l-ydlol)hilic polymer chains to microstructures are described below.
Microstructures of the present invention may be formed of two or more layers of
dirre~e,lL materials. For example, Figs. 2A and 2B show a microstructure 28 formed of two
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/US96/09614
layers 30, 32. As indicated in Fig 2C, the structure may have dirrclcll~ biological coatings
on the two dirr~ L-material surfaces, forming a coated microdevice. For example, the
upper surface of the device may be coated with molecules, such as molecules 34, of a
thc.dpcu~ic agent, and the lower surface, with a molecules, such as molecules 36, of an
5 antibody, forming a microdevice 37. The antibodies illustrated are intt n~'ed for targeting
the microdevices to selected target sites, e.g., selected blood, tissue or organ cells.
Methods for ~tt~rhin~ antibody or other polymer binding agents to an inorganic or
polymeric support are d~t~ile(l, for example, in Taylor, R., ed., Protein Immobilization
F..l,d~."~ and Ap~lications (1991), at 109-110.
Figs. 3A and 3B show a disk-shaped tril~min~te microstructure 38 having outer
layers 40, 42, and an center layer 44. In the embodiment shown, the outer layers are
composed of one material, while the center layer consists of a dirrclclll material, for
example, a material that gives faster bioerosion than the outer layers. Other embodi nents .
may contain ~ ition~l layers. As indicated in Fig. 3C, the microstructure may be coating
15 with a film 48, such as a corrosion delay film, forming a microdevice 48. The corrosion
delay layer is typically made of a material that gradually dissolves in the biochP~ni
en~dlolllllcllL of the target. Examples of such coatings include tit~ninm~ gold, silver,
platinum, copper, and alloys and oxides thereof.
The thic~kn~c~ of the corrosion delay layer may be selected to, for example, provide the
20 desired lifetime of the device in the bloodstream, or to allow the device to bind to its target
before therapeutic agent is released. These layers may be applied by standard metal
deposition procedures.
It will be d~.lc-;iaLed that the surface coatings and films in the microdevices can
have any of a number of dirrclcllL function. For example they can serve as structural
25 elements of the device itself, e.g., an anticorrosive coating; as the therapeutic moiety, e.g.,
in the case of antibodies directed against a foreign antigen, as the targeting moiety (e.g.,
tumor-specific antibodies); as an element which confers other selected properties on the
microdevice, e.g., glycocalyx coating; or a thc.dl~culic agent ~l~sign-od to erode over time.
It will be recognized that various p~,.llluLaLions of l~min~te structures and coatings, such as
30 those illustrated in Figs. 1-3, are collLelll~lated.
Figs. 4A and 4B show a microstructure 50 constructed according to another general
embodiment of the invention. This structure includes a generally cup-shaped body 52
having a cavity 54. Referring to Fig. 4C, the cavity can be filled with one or more
materials, such as material 56, which forrn the "core" of the microdevice, and may contain,
CA 02222~9~ 1997-11-27
W O 96/41236 PCT/U'.,G~614
for example, the therapeutic agent, forming a microdevice 58. The device may also contain
coating(s) dirr~;lcll- from coating(s) on other parts of the device, such as the coating of
antibodies, such as antibodies 60, for targeting the device to a selected body site.
Figs. 5A and 5B show a similar type of cup-shaped microstructure 62. but here
5 co~ osed of a ring-shaped wall member 64 formed of one material and a planar bottom
member 66, forming an internal cavity 68. This construction may facilitate, for example,
the ~tt~rhmPnt of dirr~ moieties to the top and bottom portions of the device, as
intlir~ted by ~tt~-~hmrnt of target-specific antibodies, such as at 68, to form a microdevice
70 shown in Fig. 5C. The cavity in the microdevice is filled with a selected material 70, as
10 above.
A similar type of cup-shaped miclo~ u~e is shown at 74 in Figs. 6A and 6B. In
this mi~lo~ ;lulG, the bottom portion is formed of a l~min~te 76 composed of twodirr~,~.ll-material layers 78,80. For example, layer 80 may be formed of a relative low-
density erodable polymer, to impart an overall density to the particle close to that of an
aqueous ~ e--~ion m~ m, to achieve a more stable ~.u~ .ion of microparticles fortherapeutic applications. The other layer may be, for example, an easily erodable material
and/or contain an ~ ed therapeutic colll~uulld, and/or be formed of a ferr~-m~gn--tir
material.
As shown in Fig. 6C, the internal cavity of the structure, intlir~trd at 78, may be
20 constructed to contain a wall coating of biological molecules, such as antibodies 81, to form
a microdevice 82. According to an hll~olldlll feature of the invention, and as well be
~lr~rrj'~ ed below, the antibodies are effectively ~hirl~lrd sequestered from a body's immune-
le~ onse system, thus F ~ g the antibodies to carry out desired binding functions, e.g.,
removal of toxins, viral particles, cholesterol-colllah~ g particles, without the antibodies
25 themselves provoking an immune le..~ol~.e in the host OL~;dlli lll.
Figs. 7A and 7B illustrate a ring-shaped microstructure 84 having an internal core
86 defined by an annular ~LIll~;Luldl member 88. The core can contain, for example, a
coating of a thtla~ t;ulic agent or mixture of agents, as in-lir~trd at 90, forming a
microdevice 92. An exemplary coating is shown in enlarged view in Fig. 8, and includes a
30 colllbhldlion of antibodies, such as antibody molecules 94, and enzymes, such as enzyme
molecules 96. In a particular embodiment, the antibodies are directed against a lipoprotein
particle, e.g., Lp(a), and the enzyme is an esterase or protease enzyme capable of at least
partially degrading the particles, once bound within the core of the microdevice.
CA 02222~9~ 1997-11-27
wo 96/41236 PCT/US96/09614
The optimal dimensions of microdevices of the present invention similarly dependon the application. The ,.,~xi~.llll dimension of the devices (the diameter of the disk in the
case of disk-shaped devices) is typically in the range between 0.05 and 5 microns. For
example, in applications where the device is ~sign~d to extravasate through pores lining
S the walls of capillaries supplying a tumor, the m~ximllm ~lim~n~ion of the device is
preferably less than about 150 nm, to allow passive movement of the device through the
v~cnl~hlre. In applications where the devices are f~ n~d to circulate or to bind to a
target without extravasation, they may be larger. It is recognized, however, that particles
larger than 200400 nm may be rapidly cleared from the bloodsLIcdlll and in addition,
10 cannot be sterilized by filter sterilization.
The Illil~;llll~lll dimensions of the microdevices are constrained only by the
microfabrication process itself. Certain layers and coating which may be contained in a
device such as described above (e.g., a layer of targeting antibodies) can be as thin as a
single layer of molecules. The ,ni"i",..~" size again depends on the application. For
15 example, in the case of devices made from biodegradable materials, the smaller the device,
the faster it will dissolve. The stability of device of the present invention in a particular
application may be readily ~ ?cl by one of skill in the art using tagged (e.g.,
fluorescent or radiolabelled) devices in a model system.
Another important property of microstructures and microdevices is the
20 bioerodability of the material employed in making the microstructure. Some metals, such
as iron, are rapidly dissolved in serum, whereas others, such as gold, are much more
slowly eroded. Therefore, to achieve a desired rate of erosion, metals may be mixed in
alloy.
A variety of bioerodable polymers, inrl~ ing polyglycolic, polylactic, polyu~ dlle,
25 celluloses, and derivatized celluloses may be sPlecteA~ and a variety of charged polymers,
such as heparin-like polysulfated or polycarboxylated polymers are suitable in forming one
or more of the mi~au~llu~;Lule layers. The latter polymers have the advantage of being
swellable and shrinkable under selected ionic conditions, and can be loaded to high drug
conr~ntr~tion by ion-exchange effects, for drug release under physiological conditions.
30 Such polymers are described in PCT application WO/US94/01924 for "Condensed-Phase
Microparticle Composition and Method" which is hlcoll~oldled by herein by reference.
Any of the above-described microstructure materials may be selected to enhance the
detectability of the microdevices, e.g., as tli~gnt~r,tic microdevices or for purposes of
tracking the biodi~ il)uLion of therapeutic microdevices. For example, the microdevice may
CA 02222~9~ 1997-ll-27
WO 96/41236 PCTtUS96/09614
be fabricated with X-ray or MRI-resolvable material. X-ray-resolvable materials include
iron, silicon, gold and gadolinium. MRI-resolvable materials include gadolinium and iron.
Further, the microdevices can be tagged so as to allow detection or vi~ li7~tion.
For example, microdevices are rendered radioactive by implantation or surface atr~rhmt~nt
of radioactive isotopes such as I-123, I-125, I-131, In-111 and Tc-99m. Radioactive
devices cullc~ làl~d at a site of disease can be i~ ntifiPd by a radiation detectors such as
the ~y-ray cameras ~;u~ llly used in scintigraphy (bone scans), resulting in j-lPntifir~ti~n and
localization of such regions. Microdevices can also be tagged with fluol~,sc~llL molecules or
dyes, such that a conc~ alion of microdevices can be detected visually.
The ~LIucLuldl material used in forrning the micl.)sLI~l~;Lule is selected to achieve
desired erodability and drug release properties. In the case of drug release, the structural
material may be one or more biodegradable polymers. Classes of biodegradable polymers
include polyorthoesters, polyanhydrides, polyamides, polyalkylcyanoacrylates,
polyphosl)}laLclles, and polyesters. Exemplary biodegr~ ble polymers are described, for
15 example, in U.S. Patent Nos. 4,933,185, 4,888,176, and 5,010,167. Specific examples of
such biodegradable polymer materials include, for example, poly(lactic acid), polyglycolic
acid, polycaprolactone, polyhydroxybutyrate, poly(N-palmitoyl-trans~-hydroxy-L-proline
ester) and poly(DTH callJollal~).
As in~ tPd above, the polymer matrix may also contain a hydrogel, such as a
20 cross-linked polymer of hydroxyethyl methacrylate and ethylene (~im~-th~rylate or a
cellulose ether-type hydrogel (Doelker, in Hydrogels in Medicine and Pharmacy, Vol. Il,
Peppas, N., Ed., CRC Press, Boca Rotan, 1987, pp. 115-154). Hydrogels are typically
water-swellable matrices cullL~illillg dispersed or dissolved drug. Hydrogels are preferably
used in combination with water-insoluble drugs, such as steroids (Zentner, G. M., et al., J.
Pharm. Sci, 68, 970 (1979)), and high molecular weight drugs such as insulin (Davis, B.
K., Experientia 28, 348 (1972)), enzymes (Torchilin, V.P., et al., J. Biomed. Mater. Res.
11, 223 (1976)), and vaccine antigens (Bernfield, P., et al., Science 142, 678 (1963)).
The solubility of an active agent in a particular polymer can be P,~ ed by the use
of solubility parameters (Hildebrandt, J. H., et al., THE SOLUBILITIES OF
NoNELEcrRoLrrEs; Reinhold: New York (1950)), where close solubility paldlllcLel values O
between an active agent and polymer tend to favor compatibility and solubility. The active
agent will typically be di~ ed or dissolved in the polymer matrix. Alternatively, the
active agent may be covalently att~h~d to the polymer backbone through reactive pendant
chains that can be cleaved, typically by hydrolysis. In utilizing this approach, the device
CA 02222~9~ l997-ll-27
wo 96/41236 PCT/US96/09614
11
will also contain a means for providing controlled release of active agent from the polymer
backbone, such as by cleavage of the covalent ;.~ h",~"~ The diffusion or release
properties of the polymer matrix can be modified by various terhniq--Ps, such as cross-
linking, ch~mir~l structure motlific~fion, blending of two or more polymers, or by the
5 addition of pl~tiri7Prs (e.g., butylb~l~yl~ te, trioctylphosphate, dio~;lyl~,~.ll.AI~te7
glycerol, polyethylene glycol, and polypropylene glycol) or solvents. The matrix may also
contain additional colll~o~ , such as pl~,selvàliv-es or bacteriostatic agents, e.g., methyl
hydro~yl,~l~.,aLe, chlorocresol, benzalkonium chlorides, and the like.
Typically the therapeutic agent is attached to or incorporated ir~to the polymer layer
10 after microfabrication, to prevent exposure of the agent to the rh.omic~l etching procedures
.li~;..~5ed in the section below.
B. Microfabrication of the Microstructures
The structural portion or layer (i.e., microstructure) of the microdevices of the
15 present invention may be microfabricated using any suitable Illi~ rablication method, such
as the photolithography and photoablation methods detailed below.
Figs 9A-9E illustrate the steps in forming a disk-shaped microstructure 100 (Fig.
9E) by photolithographic t~rhniq~les As shown, the structure includes two layers 102, 104,
which will be formed of two i-l~ntir~lly llu~-lbel~d layers (102 and 104) forming a planar
20 expanse 106. This l~min~te expanse is formed according to conventional methods for
deposition of metal layers, e.g., rhPmir~l vapor deposition, ~ull.,lhlg or the like, and/or
mrth()tlc for producing thin polymer sheet material.
As a first step in the process, the l~min~te expanse is attached or otherwise bonded
to a sacrificial layer 108, such as pho~l,holou~ doped silicon dioxide, deposited by rhrmir~l
25 vapor deposition, and the top of the l~min~te is coated with a photoresist layer 110.
Suitable negaliv~- or positive-resist material are well known, e.g., "Introduction to
Microlilho~-a~lly", Thompson, L.F., et al., eds, ACS Symposium Series, Washington D.C
(1983). Additional details on microfabrication methods useful in the .. i.. ri.. l.. e of devices
according to the present invention are described in, e.g., co-owned PCT patent publications
30 WO 95/24261, WO 95/24472 and WO 95/24736.
The coated l~min~te is irradiated through a photomask 112 having a series of
circular openings, such as opening 116, corresponding in size to the desired size of the
microstructures, e.g., 50-200 mn tli~m~t.or. Methods for forming photomasks having
desired phol~ k patterns are well known.
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/U~.~G/~0~14
12
In the embodiment shown in Figs. 9A-9D, the photoresist is a negative resist,
m~ninE that exposure of the resist to a selected wavelength, e.g., UV, light produces a
chPmic~l change (in~ t~d by cross h~trhing) that renders that altered resist resistant to
etching by a suitable etchant. The dl~pe~u~lce of the coated l..,.i,..l~ after ph-~tt)m~c'~
5 irradiation UV and etching is shown in Fig. 9B. As seen, I~ i"~t~ 106 is now covered by
a plurality of discrete disk-shaped resist ~ m~-nt~, such as elements 118, col.c~.~olldi.lg in
size to the planar dimensions of the desired Illicl~ uctures.
The l~min~t~ is now treated with a second etchant material effective to dissolve the
l~min~t~ in the exposed areas of the l~min~te In the case of a l~",i"~l.o metal layer, ~he
10 etchant may be a suitable acid solution; in the case of a l~min~t-- biodegradable polymer
layer, the etchant could be an enzyme solution, an aqueous solution having a pH effective to
break down the polymer, or an organic solvent known to dissolve the particular polymer. It
will be al!~lccidlcd that in the case of l~."i".l~c, more than one etchant solution may be
required. The l~min~t~, after complete etching, has the appearance of Fig. 9C, which
lS shows a series of disk-like, resist-coated ~lPmPnt~ on the sacrificial layer.In the final plc~JdlaLion steps, the resist is removed by suitable rhPmi~l Llcd~ lL
(Fig. 9D), and then the sacrificial layer is removed, again by conventional ch~omi~
callllclll, leaving the individual microstructures, such as microstructure 100.
Figs. lOA-lOE illustrate further photolithographic processing effective to produce
20 cup-shaped Illi.,lo.l-uctures, such as shown at 122 in Fig. lOE. In this processing, the
etched l~min~t~/sacrificial layer structure or ~ul~.lldlc shown in Fig. 9D is further coated
with a positive resist material 124, as shown in Fig. lOA. The coated l~min~te is then
irradiated through a photomask 126 having a series of circular openings, such as opening
128, whose ~ lllrlr~:~ co~c~olld to the desired "internal" ~ m.ot~rs of the microdevices.
25 The mask is aligned with the ~L~. Lldl~, as shown, so that the mask openings are in registry
with the already formed discs in the snbstr~
Irradiation of the ~ub~ lldlc through the photomask causes photo-induced changes in
the resist (in~ ttod by cross h~t~hing) that render the irradiated regions susceptible to a
selected etchant. The d~ea~dllce of the coated l~min~te after photomask irradiation UV and
30 etching is shown in Fig. lOB. As seen, this l.caLlllclll has produced a cylill(llical opening,
such as opening 130, in the center of each Illicl~Ll~lcLule 100 in the ~.ub~ dLe.
The l~min~t~- is now treated with a second etchant material effective to dissolve the
upper l~min~t~ layer in the exposed areas of the l~min~te, producing the series of
CA 02222~9~ l997-ll-27
WO 96/41236 PCT/US96/09614
13
microstructures 122 seen in Fig. 10C. Removal of the photoresist (Fig. 10D) and sacrificial
layers produces the free microstructures 1~ shown in Fig. 10E.
It will be a~lccidlcd that the mi-;lu~Lluclulcs formed as just described may be
further treated by standard photolithographic technirll-ec to produce other desired surface
S features and or layers. Further, inflf-nt~rif~ns or cavities may be filled with a material
dirr~ from the microstructure material by known methods. Thus, for example, U.S.Patent No. 5,200,051 describes photolithographic methods for depositing perm-selective and
plvlei~.~reuus layers on a planar ~ub~tlate. These same technifluf-s can be applied to the
present invention, to h.coll,olatc such layeAs in the microdevices of the invention.
In another general a~ oach, the microdevices are p,.~ llf-d from a s~lbstr~t~f- by
excimer laser photoablation t~orhniq~lf-~ Methods of laser micrù,~ ;,,;,,g or dry etching
have been described, e.g., U.S. Patent Nos. 5,368,430, 4,994,639, 5,018,164, 4,478,677,
5,236,551, and 5,313,043. This method is most suited to a polymeric ~ulJ~ldl~, because of
the ease with which a laser beam can photoablate polymer structures.
Figs. 11A-llD illustrate the method as applied to producing a disk-shaped
tril~min~tP microstructure such as shown at 134 in Fig. 1 lD. In this illustration, a
tril~minzlte expanse 136, formed on layers 138, 140, 142, is formed on a sacrificial layer
144. The expanse is then irradiated with a focused excimer laser beam, such as in-lic~trd at
146, which is p~tternf-d (by u~Llcalll photu",~ki"~ and imaging) to give a beam that is
20 effective to photoablate the l~min~tP in the areas corresponding to spaces between desired-
size circular flemrnt~ on the l~min~te, as in-lir~tf-d in Fig. 11B. This ablating is continued
until the beam has cut through the entire l~min~tto depth, as shown in Fig. 1 lC, forrning the
disc shaped mi-,lu~lluclulcs on the sacrificial layer. Removal of the sacrificial gives the
plurality of structures 134.
C. Microstructure Surface Slluclulcs
The term "molecular coating" is used herein to describe a coating which is bound to
the surface (outer or interior) of a microstructure. The molecular coating may be bound
directly to the surface of the device or bound to a lipid or a resin such as an electron
30 donating group, e.g. -NH2, OH or the like derivatized onto or associated with the surface of
a structural layer of the device. The molecular coating may cncullllJass all or a portion of
the surface area of the device. Molecular coatings that can confer various f h~ . islics or
impart selected functional properties are described below.
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/US96/09614
14
Figure 12 illustrates a general embodiment of a coated microdevice 150. A central
layer 152 in the device is coated with a corrosion delay layer 154, such as gold or nickel,
which is in turn coated with a layer of various molecules 156 that comprise the biorhf mi
interface to the blood. The lower side of the device in the figure that will bind to the cell
5 or tissue target (e.g., a capillary wall) is coated with ligand molecules 158, such as IgG
antibodies, that are effective to specifically bind to the marker molecules on the target (e.g.,
tumor capillaries).
In one general embodiment, the molecular coating is a marker-binding agent,
typically an antibody of antibody fragment, for targeting the microdevice to a specific cell
10 type, e.g., tumor cell. A number of monoclonal antibodies to tumor-specific antigens are
known to the art. See, for example, pages 301-323 of Cancer, 3d Ed., edited by V. T De
Vita, S. Hrllm~nn, and S.A. Rosemberg. Among the specific anti-tumor antibodies known
are those that bind to pallcal~;h~u-lla antigens (PCAs), an example of which is the tumor-
associated glycùplotein antigen known as TAG-72. Such antibodies thus bind to a broad
15 spectrum of cancers, and to difr~ cellular mutations in the mf~ct~tir cascade. Examples
of antibodies of this type are B-72.3 and the CC49 antibodies. Microdevices coated with
anti-PCA antibodies are particularly useful in the diagnosis and treatment of dineoplasms.
To f~rilit~tr tracking of a I~ dllt;uLic microdevice of the present invention, and/or
20 to enable the use of ~ gn~stic devices, one of the structural or coating elements of the
microdevice may be decign~d to be detectable using, for example, X-radiation, scintigraphy,
nuclear m~gn~tic. resonance, optical inspection (e.g., color, fluorescence), or ultrasound, as
noted above.
A molecular coating may also be used to prevent or inhibit ,~ r-~l of serum
25 opsonins to the devices, to extend the blood circulation lifetime of the devices. As
described below, such hydlu,ullilic polymers may be conjugated to vesicle-forming lipids,
and the microdevices can be coated with a lipid film COIIIdillillg such vesicle-forming lipids.
The microdevices may also be coated with a natural or synthetic glycocalyx to
enhance its solubility. Other hydrophilic natural polymers, such as glycogen or polyserine,
30 are also useful for this purpose, as are biocrmr~tible detergents such as non-ionic
poly~ li,ed oxyethylene ethers, e.g., "TRITON-X 100".
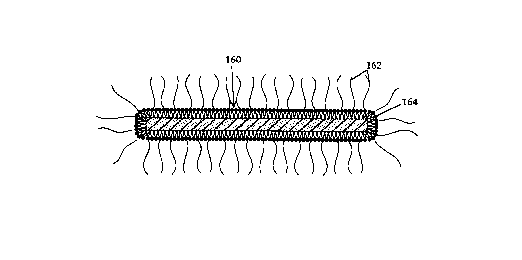
In one embodiment of a microdevice 160, shown in Figure 13A, hydrophilic
polymer chains, such as chains 162, are conjugated to vesicle-forming lipids, such as lipids
162 (see for example, U.S. Patents 5,013,556 and 5,213,804), which in turn are anchored
CA 02222~9~ 1997-ll-27
WO 96/41236 PCT/US96/09614
in a lipid film 164 formed on the exterior surface of the microstructure. Methods for
forming lipid films, such as lipid bilayer films, on particles are detailed for example, in
above cited WO/US94/01924.
Examples of hydlo~ ilic molecules suitable for conjugating to vesicle-forming
5 lipids include polyethylene glycol chains having molecular weights between about 1,000 and
10,000 daltons. Other suitable hydrophilic molecules are polyvillylpy"olidone (PVP),
poly~ Lhyloxazoline, polyethyloxazoline, polyhydroxypropyl methacrylamide,
poly",~ll,a~l.ylamide and polydimethylacrylamide, polylactic acid, polyglycolic acid, and
derivatized celluloses, such as hydroxymethylcellulose or hydroxyethy!cellulose.Referring to Figure 13B, a portion of the hydrophilic polymer chains may
additionally contain a marker-binding molecule 166 (i.e., a ligand) at their free end. Such
marker-binding molecules may be conjugated to activated free ends of the hydrophilic
molecules using known methods.
Figure 14 shows how a gradient of hl.;l~l.7illg hydrophobicity can be established by
15 de~..,ash.g the number of hydluphobic groups, such as groups 170, 172, and hl-,l.,a~ lg the
number of hydrophilic groups, such as groups 174, per unit volume as a function of
inclcasillg distance from the surface of a device, here in~lic~ted at 176. A synthetic
glycocalyx can thus be tailored to provide the functional coat desired for a particular device.
A hydrophobic ~llvilol--llcillL can be ~ in-~d near the surface of the device to harbor
20 mobile hydrophobic groups, while the entire device is kept in suspension in an aqueous
envi-u-~-llt;~-l by hydlol)hilic groups on long chains.
D. The,~,u~ulic Agents
Microdevices of the present invention consist of microfabricated structural çl~mPn
25 (microstructures) in association with a therapeutic agent and any other functionality-
e.lhdllch~g agent or coating. The therapeutic agent may be (i) incorporated into the
microdevice during microfabrication (either as a structural ~.ub~.Ll~le or as a coating), or (ii)
associated with the device after it is mi~;lu~ r;~rtl~red (e.g., by coating or conjugating it
onto the device). As is described below, therapeutic agents such as drugs can be30 incorporated into matrices (e.g., polymer matrices) which may be deposited into wells or
pits m~mlf~rtmed into the devices.
Thclaptulic agents associated with the microdevice after it is microfabricated
include coatings of therapeutic biomolecules, such as Ihe-~ulic antibodies (e.g., antibodies
directed against LDL or a viral antigen).
CA 02222~9~ 1997-ll-27
WO 96/41236 PCT/US96/09614
16
The activity of the therapeutic agent is ~ cssed by exposure of the microdevice to
the biorhpm~ enviiulllllGIlL of the target site. The target site can be a particular location
in the body, or the site of ~ .ation. For example, in the case where the target is a set
of molecules circulating in the bloodstream, the bio~hPmir~l ell~ilulllllell~ of the target site
5 is the blood. In cases where the target is a non-ch.;uldlil,g tissue, such as a tumor, the
bioçhP~nir~l envilulllll.,.lL of the target site is the envhulllllt;llL in proximity to the tumor.
The lh~ldl)tuLic agent cont~inPd in the th~.dp~ulic devices of the present invention
may be a releasable agent or an immobilized agent. A releasable agent is a therapeutic
col~lpuulld, such as a drug, that is designPd to be released at a selected target in order to
10 exert its therapeutic efficacy. An immobilized therapeutic agent is one that pelroll~ls its
therapeutic function while immobilized on the microdevice. For example, microdevices
cont~ining therapeutic antibodies as described above are an example of an immobilized
the.d~)~ulic agent.
Microdevices of the present invention may be fashioned to deliver a selected
15 therapeutic agent or ligand to a selected target in a form where the Llleld~ulic agent is
shielded from recognition by the subject's immune system. In one such embodiment, the
therapeutic agent (e.g., an antibody directed against LDL or against a viral antigen) is
attached to the bottom portion of a cup-shaped microdevice, as exemplified in Figure 15.
The microdevice shown, inflir~tPd at 180, has a tri-l~min~te structure consi-,~illg of two
20 bottom layers 182, 184, and an upper layer 186, which defines an internal cavity 188. The
exposed surface of layer 184, which forms the inside bottom of the cup-shaped
microdevice, has a surface layer of covalently bound antibodies, such as antibodies 190.
The material forming the device is selected for surface specific ~tt~hmPnt of antibodies to
the upper surface of layer 184. The exposed surfaces of layer 186 can be optionally coated
25 with hydlu,ullilic molecules, as inflic~te(~
The d;~ . D of such a device is small enough (e.g., less than about 150 nm) and
the thirknpcs of layer 102 large enough (e.g., greater than about 30 nm) so that the cell
m~,lll,~dlles of an immune-response cell, such as in~ ted at 192, that may contact the
device while it is in circulation cannot contact the layer of antibodies on the exposed surface
30 of layer 184. By preventing such contact, the host immune response directed against
therapeutic antibodies 128, while they are associated with the microdevice, will be decrease
or eli...i. ~ed
.
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/US96/09614
17
E. Microdevice Suspension
The invention includes a suspension of microdevices of the tyye described above for
use in ~lmini~rering a therapeutic agent to a selected target site in a subject. To form the
sllcllPn~ion7 microdevices as described above are sllcrenrlPd in an aqueous medium at a
5 selected c<J,lcc~ alion. The optimal col-r~ rion will depend on the ~;hdldc~ lics (e.g.,
solubilization properties) of the microdevice, type of therapeutic application and mode of
a.l",i"i~/,alion. For example, c~ posilions for oral ~h~ dlion can be relativelyviscous, and may Illelcrole contain a high Collcclllldlion (e.g., >50%~ of the microdevices.
Solutions for bolus injections ~crc.~bly contain a relatively c.l~-r~ od suspension of
10 microdevices (e.g., 10-50%), but not so collcel.l~dled that it has an ~yp~l,ciably higher
viscosity than saline (to ",i"i",i~.~ need for large-bore needles). Solution used for
continuous intravenous infusion typically contain a relatively low cunrr'.l.dlion (e.g., 2-10%
su~ycl~ion) of microdevices, due to the relatively large volumes of fluid that are
~llllilli~l~ lcd.
~ 15 The microdevices can be su~pPn~lPd in any suitable aqueous carrier vehicle. A
suitable ph~"~rc~.lir~l carrier is one that is non-toxic to the recipient at the dosages and
Collc~illllalions employed and is compatible with other ingredients in the formulation.
Examples of suitable carrier vehicles include but are not limited to water, saline, Ringer's
solution, dextrose solution, and 5% human serum albumin. Suspensions for use in
injectable formulations are preferably isotonic with the subject's blood. Generally, the
carrier can contain minor amounts of additives such as suhst~nrPc that enhance isol~llicily
and rh~mic~l stability, e.g., buffers and preservatives, as well as low molecular weight (less
than about 10 residues) polypeptides, proteins, amino acids, carbohy-lldles including glucose
or dPl~tr~n~, chPI~ting agents such as EDTA, or other excipients.
Prior to ~ dlion to a subject, the suspension of microdevices is sterilized by asuitable sterilization method. Heat-stable microdevices can be heat-sterilized, e.g., using an
autoclave. Alternatively, microdevices that are not heat-stable may be sterilized by passage
through a cull,lllcl-;ially-available sterilization filter, e.g., a 0.2 ~m filter. Of course,
filtration may be used only in cases where the microdevice is smaller than the pores of the
sterilizing filter.
III. Applications
Microdevices of the present invention can be ~lmini~Pred to a subject in need oftherapeutic intervention via any suitable ~lmini~tration method. The particular method
CA 02222~9~ l997-ll-27
WO 96/41236 PCT/US96/09614
18
employed for a specific application is cl~ ".i.,rd by the att~n-ling physician. Typically, the
microdevices will be ~.I.,.i"i.~l~ .ed by one of the following routes: topical, palellL~;lal,
inhalation, oral, vaginal and anal.
As ~ cu~ed above, microdevices of the present invention are particularly useful in
S Lll,a~ ,.ll of m~lign~nt tumors. In these applications, the type of tumor inflllPn~es the mode
of ~ dlion. For example, skin cancer may be treated by topical application of a
preferably viscous suspension; lung cancer may be treated by inh~l~ti~m of an aerosolized
aqueous microdevice ~"~ ion; cervical cancer may be treated by vaginal ..-l",i,-i~l,d~ion
of a microdevice ~u~l~ension; and colon cancer may be treated by rectal a.l"~ l,.lion of
10 such a suspension.
The majority of thc.dl~eulic applications involve some type of pal~llLt;ldl
.dLion, which includes intravenous (i.v.), hlll,..l"lc~ r (i.m.) and sub-c~t~nf ouc
(s.c.) injection. Microdevices suitable for parenteral a.l"~ l,dlion preferably have a
selected m~ximllm dimension in the range between 0.1 and 3 microns. Specific examples
15 of devices useful for pal~ le..dl ~ dlion~ particularly intravascular ~.l",i";:~il,alion, are
described below.
Further, the ~-l",i"i,.l~alion can be systemic or local. The non-pdl~,.ll.,.dl examples
of ~.llllilli.~l,dlion recited above, as well as i.m. and s.c. injections, are examples of local
~.l",i"i~ lion. Intravascular ~imini~tration can be either local or systemic. Local
20 illllavds~;ular delivery can be used to bring a therapeutic ~ e to the vicinity of a
known lesion by use of guided catheter system, such as a CAT-scan guided catheter.
General injection, such as a bolus i.v. injection or contimlon~/trickle-feed i.v. infusion are
typically systemic.
In a pl~rtlled embodiment, the microdevices of the present invention are injected
25 into the blood stream and allowed to circulate and localize to their target. Exemplary
targets include ~ ,.ll types of freely-circulating molecules, cells and/or tissues accessible
via the circulatory system. The effectiveness of the microdevices in these applications is
potentially affected by several factors, in~ln-ling the subject's immune response directed
against the microdevices, and the solubility of the devices in circulation.
A. Targetin~ Circulatin~ Molecules and Viruses
In one general application of the present invention, the microdevices described
herein are dc;livaLi~ed to contain an antibody or ligand e~fective to bind to a circulating
blood molecule, and are used Lll~ ;r~lly to reduce the co~ lion of the circulating
CA 02222~9~ 1997-11-27
WO 96/41236 PCT/US96/09614
19
blood molecule. The microdevice used for such an application p~ere:lably contains the
antibody or ligand in a manner that protects the antibody or ligand from attack by the
subject's immune system. An example of such a configuration is shown in Figure 15.
By way of example, such microdevices may be derivatized to contain antibodies
5 directed against very low density li~u~Loleills (VLDLs) and/or low density lip~lo~l;hls
(LDLs), elevated levels of which are associated with increased risk of coronaly heart
disease in hurnans. The therapeutic agents in such microdevices are the anti-LDL and anti-
VLDL antibodies. The activity of the thc~a~,c.lLic agents (i.e., antibodies) is expressed by
e~û~ul~ of the microdevice to the bioch~mic~l environment of the target site, i.e., upon
10 injection into the bloodstream. The expression of thelap~uLic activity is lllallir~ d in this
example by the antibodies' binding of VLDLs and LDLs in the bloodstream. Such
antibody-containing microdevices are pl~r~lably microfabricated using structuralcompositions that biodegrade after a selected period of time, e.g., several hours to several
days. Once the structural portions of the microdevices have degraded, the LDL-antibody
15 and/or VLDL-antibody complexes are released and degraded by lllaclvl,hages.
B. Tar~etin~ Cells and/or Tissues
Microdevices of the present invention may be used to target and deliver thela~t;uLic
compound(s) to selected cellular or tissue targets. The cells may be circulating cells, such
20 as monoml~ t~d blood cells (MBCs), bacterial cells in a systemic infection, etc., or non-
circulating cells, such as cells Cv~ g a fixed tumor mass, or the epithelial cells forming
the lining of vessel or capillaries. Any cell-specific molecular marker may be detected by
the m.othn-ls of the invention so long as the device is fabricated with a marker-specific
ligand or antibody. ln a pl~,r~llc;d embodiment, the molecular markers to be i-lPnti~led are
25 tumor-specific antigens. In selecting specific antibodies for in vivo applications, an efficient
strategy is to use human antibodies specific for the COIlllllOll generic molecules that are
relatively more plentiful in target diseased tissue cell lllelllblalles than in healthy cell
S.
An exemplary microdevice 194 useful for targeting a tissue in need of exposure to a
30 therapeutic agent is shown in Figs. 16A and 16B. The device has a tril~min~t~ structure
cvl1si~ g of a Lhela~ulic-agent-~;-lltaillillg layer 196 sandwiched between two
targeting/support layers 198, 200. The exposed surfaces of the support layers can be coated
with, e.g., (i) hydrophilic molecules, such as molecules 202, to improve the su~pPn~ion or
CA 02222~9~ 1997-ll-27
WO 96/41236 PCT/U'.~C~ 14
circulation characteristics of the device, and (ii) binding molecules, such as antibody
molecules 204, directed against a marker on the target cell or tissue.
In one embodiment, the therapeutic-c~ aillillg layer erodes at a faster rate than the
outer, targeting/support layers. This feature, illustrated by the partially-eroded middle layer
S in Figure 16B, allows the microdevice to remain securely ~ttArhrd to the target even as the
therapeutic layer dissolves away. A related embodiment has a bilaminar structure coll7i,lillg
of one therapeutic layer and one targeting/support layer as described above, where the
therapeutic layer dissolves at a faster rate than the targeting/support layer.
When targeting n; ncirculating cells inside capillaries (e.g., the f-mloth.oliAl lining),
10 the binding between the device and the molecular marker should be snfficiently strong to
overcome the drag force exerted by the flowing blood. This objective can be ~Aticfi~d by
having a relatively large, relatively planar surface area for specific binding and a relatively
low profile in the capillary's blood-flow space.
Referring to Figure 17A, a disk-shaped microdevice 202 iS attArh~-d to capillary15 wall 204 via interactions between marker molecules 206 on the capillary wall and ligand
molecules 208 on the device's surface. Epithelial cell markers which are specific for
certain pathologies, e.g., tumors, have been itl.ontifird
The total shear strength, IG~les~,l.led by arrow 210 in the figure, is due to the total
binding interaction at the interface between the device and the capillary wall. As seen, this
20 shear force is greater than the sum of the blood flow forces, represented by arrow 212,
acting to move the device in a dowlL~7ll~,dlll direction (toward the right in the figure). This
favorable state is due to the high ratio of total area of device contact with the capillary wall
to the device's profile in the region of blood flow.
By C~JIl.~d.;soll, Figure 17B shows a cross section of a spherical microparticle 214
25 bound by ligand-specific interactions on a capillary wall 216. Here the ratio of area of
device contact with the capillary wall to the device's profile in the region of blood flow is
quite low, as can be d~le-,ialGd. Accoldill~;ly the shear force holding the particle to the
capillary wall, in-1ir~t~d by arrow 218, iS smaller than the flow force on the particle,
in~licAtPd by arrow 220, giving an unstable binding condition.
The same factors, particularly the high surface area of contact, produces ~.~hAnred
binding between microdevices of the invention having ~ ,1A~ 11Y planar surfaces, and cell
surfaces.
CA 02222~9~ 1997-ll-27
WO 96/41236 PCT/US96/09614
21
C. Tr~n~lumin:~l Tar~etin~
The invention further provides a therapeutic method in which a microdevice crosses
the vascular ~ lllI,ldne to a preselected site for delivery of a Ihel~p~llLic agent. In one
embodiment, the microdevice is coated with a ligand to the endothelial or b~
5 I~ llblane of a neoplastic cell. Examples of ligands l~ ,.hlg the b~çmrnt Ill~l..I,.d,-e are
an anti-collagen type I, IV, V, anti-rlbloneclill, anti-proteoglycans and anti-laminin
antibodies. In a pl~r~ll~ e.llbodi~ , the ligand is anti-collagen IV antibody. The device
is designed so that, upon binding to the target cell, it migr~teC into the underlying tissue and
delivers the Ih~ld~c;uLic agent.
For the illustration of these uses, l~fe.ellce is made to a microdevice 224 in Figure
18A, composed of a microstructure 226 and a core 228. A cross-sectional view of the
device is given in Figure 18B. The device may be protected with an anticorrosion layer,
such as layer 230, which may be selected to have dirr~.e~l- thi~ l.Rcsçs in its .l.i.;.~L-~Icture
and core surface areas. Also as shown, the outer surface may be coated with hydrophilic
15 polymers, such as described above, and antibody molecules, such as molecules 232, for
capillary-wall targeting.
Another embodiment of the microdevice, in~ir~ted generally at 234, is shown in
Figure 18C, with an anti-corrosion coating 236 present only on the inferior surface,
hydrophilic polymer chains only on the lateral surface, and targeting antibodies, such as at
20 238, only on upper portions of the device, as shown.
Figs. l9A and l9B illustrate the method by which the microdevices are able to cross
a vascular-wall Ill~.llbldllC or wall, such as in-lir~trd at 245. The illn~tr~tion is with respect
to microdevice 234 shown in Fig. 18A, and co~t~h h~g anti-collagen IV antibodies on its
upper surface in that figure. After i.v. a~ll.,illi~l,~Lion, and migration to the target capillary
25 wall surface, the anti-collagen IV antibodies adhere to the subendothelial b~r~
-b~ c (Fig l9A). The en~loth~ l cells tend to retract when contacted from their
luminal surface, and s~~bseq~~~ntly to migrate over bodies co..~;li..g the contin---~us
b~rmt?nt lllt;lllblane~ so as to separate such bodies from the circulation. These processes
underlie well-known biological actions such as the repair of cuts in the vessels walls, and
30 the extravasation of tumor cells from both venules and arterioles to initiate ,.,~ ic
c~r~-lrc. The time required for the reaching of a configuration such as shown in Figure
l9A is about 24 hours. The microdevice may be coated with an anti-corrosion layer
~lesignrd to allow for such a time interval, with an a~p.op.iate safety factor. The
CA 02222~9~ l997-ll-27
WO 96/41236 PCT/US96/09614
microdevice may be complexed on its passive (open) side by fibrin 240, platelets 242, and
immune cells 244, as shown.
Different functions may be assigned to the device's core, in~lir~tlqd at 246. The first
of these is Iysis or dissolution of the b~clom~nt membrane. For this purpose, the core may
5 consist exclusively or partially of ~h~ lre5 or biological entities that degrade such
Ill~,llI)ldnes. Examples are matrix-degrading enzymes among serine prulcinases, neutral
metallu~ ehlascs, and cysteine protch~ases. In a plerellcd embodiment, microdevice core
contains type IV collagenase, a metall~)~loleillase that is specific to collagen type IV, and
thus particularly efficient for the Iysis of the b~c~mPrt ~llclllblane
After binding of the microdevice to the walls of the target v~cc~ tllre, the
membrane-degrading agent within the core is released, and gradually dissolves the bacr",~
.,lbldlle. The device thus pcncLldles the cell stroma, while mcl~ ldlle lccon~ ction
takes place by natural processes. A configuration such as shown in Figure l9B is reached,
with the microdevice stably lodged within the intcl~Liliulll. The extravasation process
15 requires about 2-12 hours.
At any time following binding, Illcllll~ldne Iysis, and/or tr~nchlmin~l migration,
other functions may be performed, in~hl~ling the delivery from the microdevice of
thl,lapculic agents d~plu~lidle to the pathology being treated. The diffusion of such
therapeutic agents to the targeted tissue in indicated by arrows 248 in Figure l9B. For the
20 treatment of neopla,cms, preferred thc~dp~.llic agents include anti-cancer drugs, including
~"li",~ l~holites, alkylating agents, plant alkaloids, and ~ntitllm~r antibiotics; biological
agents, inrlll~ling monoclonal antibodies, hll~,~rclons, and interleukins; çh~""~s~
i.e., rh~-mic~l.c that decrease the ~ re of cells to drugs, such as misonidazol and its
analogs; and compounds which enhance the scl~ilivily of tumor cells to radiation, e.g.,
25 halogenated pyrimi~linrc~ and compounds that radios~ hypoxic cells, e.g.,
nilloillladizole compounds.
D. Tumor Tar~eting
In one embodiment, microdevices are clesignPd for the in vivo lledllllcll~ or detection
30 of tumors by extravasation. This targeting occurs by circulation of the microparticles
through the bloodstream, over an extended time period, and passive extravasation of the
devices through colllpl~lllised regions of the vaccnl~tl-re, which tend to correspond to
vascular regions servicing a solid tumor The microdevices therefore have a preferred
maximum dimension of less than about 150-200 nm, to allow for passive transit through the
CA 02222~9~ 1997-11-27
W O 96/41236 PCT/U5~CI'0~614
cu~ ised vacc.ll~ture, and a hydrophilic surface coating to ensure prolonged blood
circulation lifetime.
E. Tar~etin,e bY External Ma~netic Field
In another embodiment of the invention, the microdevices are fabricated to include a
strong m~gnPti~ subdomain. An example is illustrated in Fig. 20 showing a microdevice
250 having a core which in this case contains two dirr~,~e..~ core layers, such as layers 252,
254, and a m~gnPtic layer 256 by which the particles can be guided in the body by an
external rnagnetic field.
Magnetic removal of particles from biological fluids for in vitro applications is
taught in U.S. Patent Nos. 4,018,886 and 3,970,518.
When a~~ .iale, the devices may be limited to circulate within localized diseased
areas by application of localized m~gnPtic fields. For example, a device containing a
m~gnPti~ core as well as d~.iale therapeutic agent(s) is a.l."i,.i~l~red locally or
15 ~y~Lt:ically, and a local magnetic field applied to the ~iicç~ced region. This is a useful
procedure for the Llt.d~ of some lesions for cases in which surgical excision is not
desirable, or possible. Such cases include melanoma with multiple superficial mPt~ct~Cçs
and penile cancer. The concentration of radioactive tags with magnetic particles in vitro is
taught in U.S. Patent No.3,993,997.
For rhpnnothprapeutic therapies, localized m~gnPtic fields can be used as described
above for collcc;llLIdlillg radiotherapeutic microdevices in known tlicç~.ced sites. This is a
useful procedure for the Ll~aLIll~.ll of superficial lesions for cases in which surgical excision
is not desirable, or possible.
.
F. Tar~eetin~ IntraoperativelY
The device of the invention may be used for intra-operative diagnosis and intra-operative Ll~dL---enL of mPt~ct~ti- d--m~inc. For this application, the device carries
antibodies to target antigens expressed on the surface of neoplastic tissue. Examples of
such antibodies include anti-collagen IV antibodies, for the cases of breast and colon
cancers, as well as Iymph node mPt~ct~cPc of the former. The device core can contain any
desired combination of therapeutic agents, such as ~nfimpt~bolites~ alkylating agents, plant
alkaloids, antihlmor antibiotics, monoclonal antibodies, h~le~r~lolls, interleukins, chemo-
s~ , and the like. In the course of surgery directed to the removal of an ~ Pntifipd
large lesion, a suspension of microdevices in physiological fluid is applied to a region
CA 02222~9~ 1997-11-27
W O 96/41236 PCT~US96/09614
24
adjacent or related to the main lesion, if such area is ~u.~e~;~cd of ,.,P~ ;c infiltration.
The microdevices that do not bind to the tumor antigens in the area of exposure are
removed, e.g., by washing, suction, or m~gn~tir~lly, as described above. The bound
devices identify the exi~tenre of Illr~ lic domains, which the surgeon may elect to
5 remove. However, even if these are not removed, therapeutic action against them is
provided by the LhcldpcuLiC agents loaded within the microdevices. The limits or rlimin~tes
the necessity for follow-up or adjuvant chemotherapeutic or radio~llcldl.cu~ic ~lcd~ enl of
mrt~ct~tir colonies.
It will be dlJplecidled from the foregoing that the present invention provides anumber of advantages in therapeutic delivery- composition over prior art particle
compositlons.
The microdevice particles employed can be design~od with selected shapes,
preferably having at least one planar surface, for enh~nred ligand-specific binding to target
cells. Further the particle sizes and shapes are effectively uniform, and can be made as
small as existing types of "nanoparticles".
The devices can be ~ signPd with a number of discrete functio~ls, include surface
binding, ~u~ nsion and RES-evasion functions, and one or more dirr~c~l therapeutic
agents contained in separate Cu~ alllllcllL for dirr~.c.lL release rates.
The particles may be constructed of a variety of dirr~cllt materials, to achieveoptimal and dirrclelllial drug release at a target site. One of these materials may be a
radiopaque material, such as a metal for vi~ li7ing the biodistribution of the particles, or a
ferrom~gn-otic material to allow m~gn-otir field particle guidance.
In one embodiment, the microdevice has a core in which foreign lhcld~cuLic agents,
such as antibodies and/or cl~yllles can be shielded from the host's immune system, allowing
a variety of novel applications for clearing undesired serum components.
In still another embodiment, the microparticles are designed for either passive
e~lld~asdlion into solid tumors, or active tr~n~lllmin~l movement through a culllbi-ldlion of
capillary-specific binding and partial Illclllb~dlle degradation.
Although the invention has been described with respect to specific embodiments and
applications, it will be appreciated that a variety of changes and modifications may be made
without departing from the invention as rl~imr(l