Note: Descriptions are shown in the official language in which they were submitted.
CA 02222970 1997-11-28
Corrosion Monitoring Probe
For Reinforced Concrete Structures
The present invention relates to a corrosion
monitoring probe that may be installed in reinforced
concrete structures, and especially to a corrosion
monitoring probe that may be utilized in a variety of
measurements relating to the monitoring of corrosion of
reinforcing steel in concrete structures. In particular,
a corrosion measuring system is provided for assessing
both the onset and the rate of corrosion of reinforcing
steel in concrete structures.
Concrete structures typically have steel reinforcing
bars, frequently referred to as rebar, which may be in a
variety of shapes and sizes, including steel girders,
rods or other reinforcing parts formed of steel. Good
quality, sound concrete structures provide an ideal
environment for steel reinforcing bar i.e. rebar, because
the high pH of the cement paste pore solution in the
concrete passivates the steel, reducing the natural
corrosion rate of the steel to insignificant levels,
typically less than 1 micron (micrometre) per year. Such
protection against corrosion does not depend upon the
impermeability of the concrete, but on the high pH of the
cement pore solution which forms a thin firmly bonded
oxide layer on the surface of the steel. The oxide layer
provides sufficient protection to permit the use of steel
in many structures that are exposed to weathering.
However, under some conditions, the oxide layer on
the steel may be broken down, permitting corrosion to
occur. For instance, chloride ions from marine
atmospheres or from de-icing salts that are applied to
roads in winter can penetrate the concrete cover and
break down the passivity of the steel, thereby permitting
active corrosion to occur. In addition, reactions may
occur between the cement paste and atmospheric carbon
dioxide or other acidic gases, which causes a reduction
CA 02222970 1997-11-28
.
in the pH of the cement paste pore solution to a level at
which the steel readily corrodes. Carbonation and
chloride attack can occur independently or can act in
synergy.
Corrosion has two detrimental consequences.
Firstly, it reduces the cross-section and thereby the
load- bearing capacity of the rebar. Secondly, the
corrosion products tend to occupy a much larger volume
than the steel from which they are formed, causing
tensile stresses in the concrete that result in cracking
and spalling of the concrete cover. As used herein, the
concrete cover is the layer of concrete covering the
outer layer of rebar through which aggressive species
e.g. chlorides, must be transported in order to initiate
rebar corrosion.
The lifetime of a reinforced concrete structure
exposed to chlorides and/or carbonation can be described,
in the absence of preventative maintenance, as consisting
of two periods. The first period is one in which there
is no active corrosion but aggressive species such as
chlorides or carbon dioxide penetrate the concrete cover.
The second period starts at corrosion initiation and
continues until the damage to the concrete and steel are
sufficient to warrant remedial action.
U.S. Patent No. 5,015,355 of P. Schiessel describes
a corrosion measuring cell that has several electrodes
consisting of structural steel or reinforcing steel
arranged at different distances from the external surface
of the concrete structural part. Each of these
electrodes are connected to the electrode functioning as
a cathode e.g. a more noble material, via a measuring
element. This allows for macrocell measurements
described below.
A corrosion monitoring system that is adaptable for
use with a variety of measurement techniques has now been
found, and especially provides for measurement techniques
to assess both the onset of corrosion and the rate of
CA 02222970 1997-11-28
corrosion.
Accordingly, an aspect of the present invention
provides a corrosion measuring system for assessment of
the onset and rate of corrosion of reinforcing steel
embedded in a concrete structure having an exterior
surface, comprising:
(a) a set of at least three elements formed from
said reinforcing steel and located in a spaced apart
relationship at the same depth from said exterior
surface;
(b) each of said elements having electrical
connections extending therefrom through said exterior
surface, said electrical connections being adapted for
connection to apparatus for measurement of
electrochemical properties of said elements for
assessment of corrosion of said reinforcing steel.
In a preferred embodiment of the corrosion measuring
system of the present invention, there is additionally at
least one element of electrical conductive material less
susceptible to corrosion in said concrete, said
electrical conductive material being embedded in said
concrete and having an electrical connection extending
through said exterior surface adapted for connection to
said apparatus.
In another embodiment, there are more than one set
of said at least three elements, each of said sets of at
least three elements being located at differing distances
from said exterior surface.
In a further embodiment, said elements are located
on corrosion-resistant electrically non-conductive
supports.
In yet another embodiment, said supports are tubular
and said electrical connections pass through said tubular
support.
In still another embodiment, a reference electrode
and/or a hygrometer is embedded in the concrete.
In another embodiment, said electrically non-
CA 02222970 1997-11-28
conducting supports are further supported by a second
electrically non-conductive tubular support that is
attached to said reinforcing steel, said electrical
connections passing through said second tubular support
to said exterior surface.
In yet another embodiment, the distance between
adjacent sets of elements is 5-15 mm, as measured from
said exterior surface.
The present invention will be illustrated with
reference to the embodiments shown in the drawings, in
which:
Figure 1 is a schematic representation of a
corrosion monitoring system of the invention;
Figure 2 is a schematic representation of another
corrosion monitoring system of the invention;
Figure 3 is a schematic representation of
measurements using a set of elements;
Figure 4 is a graphical representation of an example
of electrochemical noise; and
Figure 5 is a graphical representation of
electrochemical noise in a concrete structure.
The corrosion monitoring probe of the present
invention has sections of reinforcing steel, generally
referred to herein as elements and preferably formed from
the specific steel used for the main reinforcement of the
structure that is to be monitored for effects of
corrosion. The sections of steel are placed in sets of
three with the sets being at increasing distances from
the concrete surface of the structure and preferably
supported by plastic tubing which must be both chemically
inert with respect to the concrete and electrically non-
conducting. Each element is independently wired with a
single, suitably insulated, wire. The wire/element
junction should be coated to prevent galvanic corrosion.
The wires pass into the tubing, which provides additional
protection during installation. In preferred
embodiments, a length of stainless steel is provided
CA 02222970 1997-11-28
within the concrete structure, which can be used as a
counter electrode in three electrode measurements, and as
a second electrode for macro cell measurements. In
addition, it is preferred that a stable reference
electrode e.g. a Mn/MnO2 electrode, and preferably a
hygrometer and a thermocouple, are also embedded in the
structure. The sets of probe elements at each level are
staggered in position to minimize any effects of
corrosion of the outermost sets on the initiation of
corrosion of elements deeper inside the cover of
concrete.
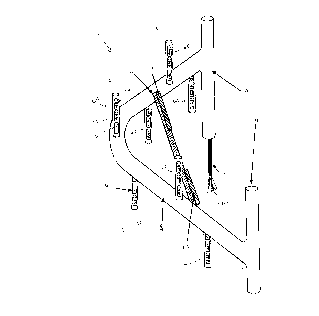
Figure 1 shows a corrosion monitoring probe,
generally indicated by 1. Probe 1 has a plastic tube 2
which, as shown, may be of relatively large diameter
compared to other plastic tubing referred to below.
Probe 2 has two foot sections, 3 and 4, that are
interconnected with intermediate section 5. Intermediate
section 5 is shown as extending upwardly from each of
foot sections 3 and 4, and is generally in the shape of
an inverted V. However, it will be appreciated that the
shape may be any convenient shape such that intermediate
section 5 is not in the same plane as foot sections 3 and
4.
Intermediate section 5 has a plurality of sets of
probe element thereon, indicated by 6A, 6B, 6C, 6D, 6E,
6F and 6G. The sets of probe elements 6A-6G are located
at different heights with respect to the plane of foot
elements 3 and 4. In particular, probe elements 6A
through 6G are at different levels between apex 14 of the
inverted V of intermediate section 5, it being understood
that apex 14 could be extending towards an exterior
surface of the concrete structure, as shown in Fig. 1, or
extending away from the exterior surface. Consequently,
the sets of probe elements 6A-6G would be located at
different distances from the exterior surface of the
concrete structure. Each of sets of probe elements 6A-6G
has three elements, referred to as 7A, 7B and 7C for
CA 02222970 1997-11-28
element 6A. Each of elements 7A, 7B and 7C are located
on the surface of tubing 8, and are at the same distance
from the exterior surface i.e. they are in a plane
parallel to the exterior surface.
Tubing 2 and tubing 8 would normally be of different
diameters, although not necessarily so, and are formed
from a material that is chemically inert with respect to
the pore solution within concrete and furthermore which
is electrically non-conductive or is electrically
isolated from the probe elements. An example of a
preferred material is a plastic material e.g. polyvinyl
chloride (PVC).
Wires, not shown in Figure 1, are attached to each
of elements 7A, 7B and 7C, and correspondingly each of
the elements in the other sets of probe elements 6B-6G.
The wires pass from each of the individual elements
through tubing 8 into tubing 5. It is to be understood
that in the preferred embodiment of Fig. 1, tubing 8 and
tubing 5 are utilized for passage of electrical wires for
protection of the wiring, but that it is not necessary to
do so. In addition, tubing 5 and tubing 8 provides a
convenient method for location and spacing of the sets of
probe elements. The electrical wires are shown as
extending from tubing 5 as an electrical conductor 9
having a plurality of wires 10, and would pass to the
exterior of the concrete structure.
Intermediate section 5 is shown as having a
stainless steel counter electrode extending across the
width of the inverted V. It is to be understood that the
counter electrode 11 could be of any suitable material
that is more corrosion resistant than the rebar,
especially substantially more resistant to corrosion
within concrete, and thus may be of materials other than
stainless steel.
A hygrometer/thermocouple 12 is shown as attached to
stainless steel counter electrode 11 on one end thereof,
and a reference electrode is shown as attached to the
CA 02222970 1997-11-28
other end thereof. Hygrometer/thermocouple 12 and
reference electrode 13 may be located at any convenient
location. A typical reference electrode is a Mn/MnO2
electrode.
Figure 2 shows a cross-section of a concrete
structure showing an alternate arrangement of the sets of
rebar elements within the concrete structure. Concrete
structure 20 has an exterior surface 21 and a supporting
rebar 22. Located between surface 21 and rebar 22 is
support 23, which would be chemically inert and
electrically non-conductive or is electrically isolated
from the probe elements formed of e.g. plastic (PvC)
tubing. Support 23 is shown as having three sets of
rebar 24, 25 and 26. The set 24 of rebar has three
elements 24A, 24B and 24C that are in a plane that is
parallel to surface 21 of the concrete. Similarly, set
25 has three elements 25A, 25B and 25C that are also in
the plane of surface 21. Set 26 is similarly
constructed. A plurality of spacers are located between
each of the individual elements of the sets of rebar e.g.
between elements 24A, 24B and 24C and support 23, are
similarly located between the individual elements of sets
25 and 26 and rebar 22.
In a typical example, the sets of the elements would
be located at intervals of 5-15 mm between the exterior
surface and the rebar.
Figure 3 shows a concrete structure 30 having an
exterior surface that has been exposed to a solution 31
containing chloride ions. Section 32 of concrete
structure 30 is shown as having three elements of rebar,
indicated by 33A, 33B and 33C, which are located in a
plane parallel to that of the plane of the exterior
surface. Two of the elements 33A and 33B, are shown as
being connected through an ammeter 34 using connections
35. Similarly, rebar elements 33B and 33C are shown as
interconnected with a voltmeter 36 and connections 37.
Such a system could be used for the measurement of
CA 02222970 1997-11-28
electrochemical noise.
A sample of a trace of current noise in
microammeters over a period of two hours is shown in
Fig. 4 for both stainless steel and carbon steel which
have been placed in a synthetic pore solution containing
4% chloride in the form of sodium chloride. Similar
behavior is observed for steel embedded in concrete.
Electrochemical current and potential noise traces
for probes in cracked and non-cracked regions of a
concrete reinforced structure are shown in Figure 5.
When steel reinforcement in concrete corrodes, the
iron atoms become ionised releasing electrons which are
consumed by the reduction of dissolved oxygen (or, in
deaerated or anaerobic concrete, by the evolution of
hydrogen).
The flow of electrons between the areas where the
anodic and cathodic reactions take place constitutes a
current known as the corrosion current. These locations
can be adjacent on the atomic level, in which case the
cell is known as a micro-cell. At the other extreme,
they can be widely separated constituting a macro-cell,
for example, the corroding anodic area may be on the top
rebar mat of a structure while the bottom of the rebar
mat acts as the cathode. The principle is the same in
both cases. According to Faraday's law, the corrosion
rate, measured in depth of material removed from the
surface per unit of time, is related to the corrosion
current as follows:
d = i .t. A
n .F. p
where "d" is the depth of corrosion in ~m/year; "i"
is the corrosion current density in ~A/cm2; "t" is the
time in seconds; "A", "n" and "p" are the atomic mass,
the valency and the density of iron, respectively and "F"
is Faraday's constant (96,500 coulombs/equivalent). As
an example, a corrosion current is 1 ~A/cm2 would be
CA 02222970 1997-11-28
equivalent to a corrosion rate of 11.6 ~m/year dissolving
or oxidizing from the steel surface.
Using these principles, and the probe elements
installed in the concrete as described herein, a number
of electrochemical techniques can be used to monitor the
corrosion, including half cell potential, macro-cell
current, linear polarization resistance, and
electrochemical noise.
One or more of these techniques may be employed to
continuously monitor the probe elements, which would
allow variations in corrosion behaviour with ambient
temperature and relative humidity to be determined.
Since the data will be received as electrical signals, it
can be transmitted to a remote location for analysis.
One technique for measurement of rebar corrosion
damage is the measurement of half cell potential. Such a
measurement procedure is described in ASTM C876-80,
which uses a reference electrical and an electrical
connector to the rebar. The basis of the test is that
the corrosion potential of the rebar will shift in the
negative direction if the surface changes from the
passive or non-corroding state to the active or corroding
state.
In the measurement of half cell potential mapping
using the present invention, either the reference
electrode (indicated by 13 in Fig. 1) or the stainless
steel bar incorporated in the probes (indicated by 11 in
Fig. 1) can act as a reference electrode and the
potential difference between each probe element, and
either the reference electrode or the stainless steel bar
can be used as half-cell potential. Such a technique is
simple, but the results obtained are qualitative, and no
actual rebar corrosion rate is established. Furthermore,
results obtained can be misleading e.g. data suggestive
of corrosion may be recorded in submerged concrete
structures where the corrosion rate is negligible due to
the absence of oxygen.
CA 02222970 1997-11-28
A second technique is the macro-cell current
technique, also known as the zero resistance ammetry,
which measures the current flowing between individual
embedded probe elements and the stainless steel bar, and
serves as a more quantitative indication of corrosion
than half cell potential. The stainless steel will act
as the cathode of a galvanic cell with the probe element
acting as the anode. When chlorides penetrate to the
level of the probe element and initiate active corrosion,
the current flowing between the rebar and the stainless
steel will increase significantly, typically by more than
two order of magnitude. The principle of macro-cell
current technique is used in ASTM G102-92 corrosion test
procedure, where the current flows between rebar embedded
near a surface exposed to chlorides and rebar at greater
depths of cover.
The concept of measuring a macro-cell current as
indicator of corrosion severity may also be applied using
two of the three probe elements of each set described
herein. However, the magnitude of the current will tend
to be lower than that obtained using one rebar element
and the stainless steel, particularly if both rebar
elements are corroding.
Under natural, equilibrium corrosion conditions, the
number of electrons released by the anodic reaction is
exactly equal to that consumed by the cathodic reactions.
Therefore, there is no net current which can be measured.
In order to determine the corrosion current, the system
must be biased away from equilibrium and the resulting
net current is then measured. In corrosion, the current
and electrochemical potential are proportional for small
(<20 mV) deviations from equilibrium. Therefore, in this
range of potential, the system can be biased by applying
a potential, ~E and measuring the resultant current, ~i,
(potentiostatic control), or by applying a current and
measuring the resultant potential gradient (galvanostatic
control).
CA 02222970 l997-ll-28
The ratio (~ E) is termed the Polarization
Resistance, Rp, as described in ASTM 659, and is related
to the actual corrosion current, iCorr by the relationship:
B
icorr = Rp
where B is a constant of the order of 3OmV.
While this technique does give a significant
indication of corrosion rate, it is not a passive
technique: a potential (or current) must be applied
between the probe elements and the resultant current (or
potential) measured.
Unlike the other electrochemical techniques,
electrochemical noise measurements do not rely on any
"artificial" signal imposed on a rebar probe element or
establishment of an artificial galvanic cell. In
contrast, natural fluctuations in the corrosion potential
and current are monitored to characterize the severity
and type of corrosive attack, which requires a three
electrode system for simultaneous measurement of current
potential and current noise. The three nominally
identical rebar probe elements of each set are used for
such measurement.
For electrochemical noise measurements on rebar
probes embedded in concrete, sensitive instrumentation is
required, for example with minimum current and potential
resolution levels around 0.1 ~A and 0.1 mV respectively.
The current and potential noise levels tend to increase
with increasing electrochemical activity on the rebar
surface. Furthermore, the noise data can be particularly
useful for identifying the initiation and propagation of
corrosion pits, a distinct advantage over all of the
other techniques. The noise transients characteristics
of rebar pit initiation and repassivation on a
microscopic scale are illustrated in Fig. 4 and 5. These
"signatures" of the initiation of corrosion pits are
evident long before the attack is observable by visual
means, or detectable by other electrochemical techniques,
CA 02222970 1997-11-28
indicating the "early warningn capabilities of this
sensitive technique.
The corrosion monitoring probe of the present
invention utilizes sets or groups of three rebar elements
at the same depth within the concrete, with a plurality
of sets at different depths. It is to be understood that
more than three such elements could be utilized in each
set for additional measurements although it is not
necessary to do so. However, the sets of rebar elements
must have at least three rebar elements for the
measurements described herein.
The corrosion surveillance system of the present
invention permits prediction of (i) when, in the absence
of any remedial treatment, corrosion will start, and (ii)
the rate of corrosion after initiation. This will allow
maintenance and rehabilitation processes to be scheduled
in advance and suitable corrosion protection systems to
be installed at an appropriate time. Such a system may
be designed to allow the progress of chloride penetration
through the concrete cover to be monitored and, thereby,
to predict when corrosion of the main rebar will be
initiated. By constructing the probe elements from the
same steel as the rebar, the actual corrosion rates after
initiation can be determined and applied to protect the
life of the structure.
The present invention provides a versatile concrete
monitoring probe system that is capable of being utilized
for a wide variety of measurements. It is understood
that such elements would be embedded in the concrete at
the time of erection of the structure, immediately prior
to pouring of the concrete, but once installed could not
be relocated, inserted, repaired or otherwise disturbed,
as any such disturbance would negate any subsequent
measurements of corrosion or potential corrosion.