Note: Descriptions are shown in the official language in which they were submitted.
CA 02223246 1997-12-02
W O 96/40959 PCTAUS96/09629
Cell line producing analgesic co~pounds for treating pain
Field of the Invention
The present invention relates to a cell line
useful for the treatment of pain. More particularly,
the cell line of this invention has been genetically
engineered to produce at least one analgesic compound
from each of the groups consisting of endorphins,
enkephalins, and catecholamines.
o Background of the Invention
Pain is a common symptom of disease. The
superficial dorsal horn of the spinal cord, where
primary afferent fibers carrying nociceptive
information terminate, contains enkephalinergic
interneurons and high densities of opiate receptors.
In addition, there is a dense concentration of
noradrenergic fibers in the superficial laminae of the
spinal cord.
Acute pain arises in response to acute
noxious stimuli. Chronic pain is predominantly due to
neuropathies of central or peripheral origin. This
~ ul~ SNEEr (RULE ~6)
CA 02223246 1997-12-02
WO ~f'4C35~ PCT~US96/09629
neuropathic pain is the result of aberrant
somatosensory processing that can result in increased
sensitivity to a painful stimulus (hyperalgesia) and
pain associated with a stimulus that does not usually
provoke pain (allodynia).
Intrathecal injection of morphine into the
spinal subarachnoid space produces potent analgesia.
Similarly, intrathecal administration of norepinephrine
or noradrenergic agonists also produces analgesia.
o See, e.g., Sagen et al., Proc. Natl. Acad. Sci. USA,
83, pp. 7522-26 (1986).
Co-administration of subeffective doses of
opiates, such as enkephalins, and catecholamines, such
as norepinephrine, may synergize to produce analgesia.
Ibi~. Chromaffin cells in the adrenal medulla produce
and release several neuroactive substances including
norepinephrine, epinephrine, met-enkephalin, leu-
enkephalin, neuropeptide Y, vasoactive intestinal
polypeptide, somatostatin, neurotensin, cholecystokinin
and calcitonin gene-related peptide. See, e.g., Sagen
et al., Proc. Natl. Acad. Sci. USA, 83, pp. 7522-26
(1986); Sagen et al., Joux. Neurochem., 56, pp. 623-27
(1991).
Because chromaffin cells produce both opioid
peptides and catecholamines, one approach to reduction
of nociceptive response or pain sensitivity has
investigated transplanting adrenal medullary tissue, as
well as isolated adrenal chromaffin cells, directly
into CNS pain modulatory regions, in attempts to
provide analgesia. See, e.g., Sagen et al., 1~ in
Research, 384, pp. 189-94 (1986); Vaguero et al.,
Neuroreport, 2, pp. lg9-51 (1991); Ginzberg and
SE~p~Ul~26)
CA 02223246 1997-12-02
W O 96/40959 PCT/U',G/O~C29
Seltzer, Brain Research, 523, pp. 147-50 (1990); Sagen
et al., ~in, 42. pp. 69-79 (1990).
Attempts to produce analgesic have been made
using both allogeneic and xenogeneic chromaffin tissue
s or cells transplants. Allograft tissue is in limited
supply, and is not readily available, particularly for
in human pain treatment programs. In addition,
allogeneic human tissue carries the risk of pathogenic
contamination. See e.g., Hama and Sagen, Brain
o Research, 651, pp. 183-93 (1994).
Xenogeneic donors may provide large
quantities of material that can be readily obtained.
For this reason, bovine adrenal tissue has been used.
See, e.g., Hama and Sagen, Brain Rese~rch, 651,
pp. 183-93 ~1994).
However, potentially serious host
consequences, as well as ultimate graft rejection, are
inherent problems in transplantation between disparate
species. Complete graft rejection of whole or
dissociated tissue may occur even in the CNS, normally
thought to be immunologically privileged, due to
presence of highly antigenic cells in the xenografts,
particularly endothelial cells. In addition, the donor
tissue must be carefully screened to avoid introduction
of viral contaminants, or other pathogens, to the host.
To overcome graft rejection, immunosuppression is
required typically using cyclosporine A.
Some reduction in pain sensitivity has been
reported resulting from these transplants, particularly
for the reduction of low intensity chronic pain. In
most reports, significant differences between control
and transplanted animals were noted only after nicotine
SUB~ S~t~l (RU1~26)
CA 02223246 l997-l2-02
WO 96/40959 PCT~US96/09629
administration to stimulate opioid peptide production.
However, there have been some reports that analgesia
has been observed in a rat chronic pain model from
basal level activity of chromaffin tissue allografts.
See, e.g., Vaquero et al., NeuroReport, 2, pp. 149-51
(1991) and Hama and Sagen, ~rain Rese~rch, 651, pp.
183-93 (1994).
Bovine adrenal chromaffin cells have been
encapsulated to form a bioartificial organ ("BAO") for
o implantation into rats for the treatment of acute and
chronic pain. See, e.g., Sagen et al., J. Neurosci.,
13, pp. 2415-23 (1993) and Hama et al., 7th Wo~ld
Con~ress P~in, Abstract 982, Paris France (1993).
Initial trials in human subject have been conducted
using encapsulated bovine chromaffin cells. See,
Aebischer et al., Tr~nsplantation, 58, pp. 1275-77
(1994).
There have also been attempts to induce
antinociception using other cells, e.g., AtT-20 cells.
AtT-20 cells were originally derived from a mouse
anterior pituitary tumor. These cells synthesize and
secrete ~-endorphin. See, e.g., Wu et al., J. Neur~l
Tr~nspl. & Pl~sticity, 5, pp. 15-26 (1993).
AtT-20/hENK cells are AtT-20 cells that have been
genetically engineered to carry the entire human pro-
enkephalin A gene (i.e. containing 6 met-enkephalin
sequences and one leu-enkephalin sequence) with 200
bases of 5'-flanking sequence and 2.66 kilobases of 3'-
flanking sequence. See Wu et al., supra, Comb et al.,
F~MRO J , 4, pp. 3115-22 (1985).
Wu et al., J. Neural Transpl. ~ Plasticity,
5, pp. 15-26 (1993) refers to rat hosts transplanted
SU~IIlul~:SN~ (RU1~26)
CA 02223246 1997-12-02
W O 96~40959 PCT~US96/09629
with AtT-20 or AtT-20JhENK cells. Unstimulated AtT-
20/hENK cells produced more antinociception (tail flick
test) than produced by AtT-20 implants. In contrast,
isoproterenol stimulation produced more antinociception
with AtT-20 cells than with AtT-20/hENK cells. Ibid.
In mice hosts, AtT-20 or AtT-20/hENK implants
did not affect basal response to thermal nociceptive
stimuli. Mice receiving AtT-20 implants developed
tolerance to ~-endorphin and a u-opioid agonist
lo (DAMGO). Mice receiving AtT-20/hENK implants developed
tolerance to an ~-opioid agonist (DPDPE). In ~esponse
to repeated doses of an u opiate agonist, mice
receiving AtT-20/hENK implants developed less tolerance
compared to mice receiving AtT-20 cells or controls.
The antinociceptive effect of isoproterenol
treatment appeared equal in mice receiving AtT-20 or
AtT-20/hENK cell implants. See, Wu et al., J.
Neuroscience, 14, pp. 4806-14 (1994). Wu et al.
speculated that one reason for the absence of
additional antinociception in mice implanted with
enkephalin producing AtT-20/hENK cells may be due to
lack of sensitivity of the behavioral assays. Another
possible reason was that met-enkephalin's known
antagonist effect on morphine induced antinociception
offset the potentiating effect of the single
leu-enkephalin, particularly since there are 6 met-
enkephalin sequences for each leu-enkephalin sequence
in pro-enkephalin A.
SUBSI~ tl (R~26)
CA 02223246 1997-12-02
W O ~ 59 PCT~US96/09629
Sllmm~ry of the Invention
The present invention provides a cell line
that has been genetically engineered to produce at
least one analgesic compound from each of the groups
s consisting of endorphins, enkephalins, and
catecholamines. The cell line may be used in the
treatment of pain.
There are advantages to using a cell line
over the use of primary cells. Expensive and time
o consuming testing to ensure safety and performance
criteria for cells must be performed for individual
isolations of primary cells. Less testing is required
of a cell bank. There is no need to isolate primary
cells. Output of the desired analgesics may be more
stable since the performance of primary cells may be
dependent on the age, sex, health or hormonal status of
the donor animal. It is also possible to achieve
higher output of the desired products, as well as to
engineer specifically modified peptides into the cell
line. This permits delivery of multiple analgesics
simultaneously. Expression of one or more of the
analgesics can be regulated (by using a regulatable
promoter to drive expression). In addition, for
safety, a "suicide" gene can be incorporated into the
cell line. Further, for encapsulation purposes
proliferating cells have the advantage that they divide
to replace dying or dead cells.
SUBSIIIUI~ SHEEr (RUIE 2~)
CA 02223246 1997-12-02
WO 96/40959 PCTAJS96/09629
Rrief Description of the Drawina
Figure 1 is a plasmid map of vector pBS-
hPOMC-027, pBS-IgSP-hPOMC-028 and pBS-IgSP-hPOMC-~ACTH-
029.
s Figure 2 is a plasmid map of vectors pCEP4-
hPOMC-030, pCEP4-hPOMC-031, pcDNA3-hPOMC-034 and
pcDNA3-hPOMC-035.
Figure 3 is a plasmid map of vectors pCEP4-
hPOMC-~ACTH-032, pCEP4-hPOMC-~ACTH-033, pcDNA3-hPOMC-
~ACTH-36 and pcDNA3-hPOMC-~ACTH-037.
Figure 4 is a plasmid map of vectors pcDNA3-
rTH-044, pcDNA3-rTH~-045, and pcDNA3-rTHDKS-075 (also
represented as pcDNA3-rTH~KS-075).
Figure 5 is a plasmid map of vectors pcDNA3-
rTH~-IRES-bDBH-088 and pcDNA3-rTH~KS-IRES-bDBH-076.
Figure 6 is a plasmid map of vector pZeo-
Pcmv-rTH~KS-IRES-bDBH-088.
Figure 7 is a plasmid map of vector pBS-Pcmv-
rTHAIRES-bDBH-067.
Figure 8 is a plasmid map of vector pBS-
hPOMC-~ACTH-IRES-rTH~IRES-bDBH-068.
Figure 9 is a plasmid map of vector pcDNA3-
hPOMC-~ACTH-IRES-rTH~-IRES-bDBH-069.
Figure 10 is a plasmid map of vector pcDNA3-
IRES-Zeocin-072.
Figure 11 is a plasmid map of vector pcDNA3-
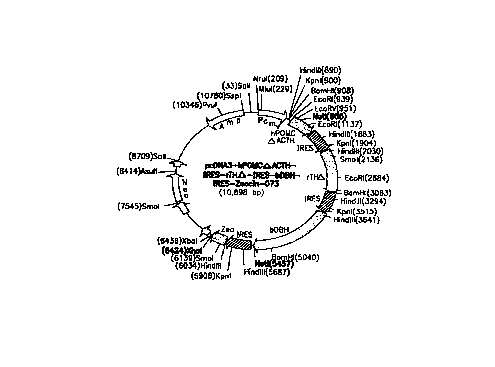
hPOMC-~ACTH-IRES-rTH~-IRES-bDBH-IRES-Zeocin-073.
Figure 12 is a plasmid map of vector pcDNA3-
hPROA+KS-091.
SUB~ ultSnttl (~UIE26)
CA 02223246 l997-l2-02
W O ~G/1~ PCTAUS96/09629
Det~ile~ DescriDtion of the Invention
In order that this invention may be more
fully understood, the following detailed description is
set forth.
Any suitable cell may be transformed with the
recombinant DNA molecules of this invention. Among the
contemplated cells are chromaffin cells, including
conditionally immortalized cnromaffin cells such as
those described in WO 96/02646, Neuro-2A, PC12, PCI2a,
o SK-N-MC, AtT-20, and RIN cells including RINa and RINb.
Preferably the cell has endogenous prohormone
convertases and/or dopa decarboxylases.
SK-N-MC cells, a neuroepithelioma cell line,
co-expresses several neuropeptides, including
15 enkephalin, cholecystokinin and gastrin-releasing
peptide. See, e.g., Verbeeck et al., J. Biol. Chem.,
265, pp. 18087-090 (1990). The pro-enkephalin A gene
has been expressed in SK-N-MC cells. See, e.g.,
Folkesson et al., Mol. Brain Res., 3, pp. 147-54
20 (1988). We prefer AtT-20 and RIN cells, most
preferably RIN cells.
RIN cells are a pancreatic endocrine cell
line derived from rat. See, e.g., Horellou et al.,
J. Physiol., 85, pp. 158-70 (1991). RIN cells are
25 known to endogenously produce GABA and B-endorphin.
Some of the characteristics of various
contemplated cells are shown in Table 1.
SU~ h~ RULE26)
CA 02223246 1997-12-02
W O~/10~5~ PCT/U',G/'~3~29
Table 1
Çells Analqesic SuL,:,lances Other Com~onents
Chl'~llldrl I NE, met-enkephalin TH, DDC, D,BH, PC
PC12, PC12a low NE 8 met-enkephalin DDC, D~H, PC
5 AtT-20 ~-endo~h- . DDC, PC
RlNa ~-endor~ub " GABA DDC, PC
RlNb ~-endorphin DDC, PC
Neuro 2A DDC, D~H, PC
TH = Tyrosine hydroxylase converts tyrosine - I-dopa
10 DDC = Dopa,l, Ie dec~ll,uAyla3e converts l~opa - dopa,ll ,e (DA)
D~H = Dopamine ~-Hy.l,uAylase converts DA- norepinephrine (NE)
PC = r~uhGrlllone Convertases process POMC to ,B-endo" l~, and Pro-
enkephalin A (ProA) to met-en':~,ch-' ,.
AtT20 = Mouse pituitary co,~ uph cell line that endogenously secretes ~-endol,ul,,
via o~ sa.on of Pro-opiomelanocortin (POMC).
RIN = Ratin~l~ ,o,lla
Neuro 2A = Mouse ne~Jrubld~lo~lla
The primary delivery products include at
least one each of an endorphin, an enkephalin and a
20 catecholamine.
Enkephalins and endorphins are endogenous
opioid peptides in humans. These opioid peptides
comprise approximately 15 compounds ranging from 5 to
31 amino acids. These compounds bind to and act at
25 least in part via the same ~ opioid receptor as
morphine, but are chemically unrelated to morphine. In
addition, these compounds stimulate other opiate
receptors. Yaksh and Malmberg, Textbook of Pain, 3rd
Ed. (Eds. P. Wall and R. Melzack), "Central
Pharmacology of Nociceptive Transmission," pp. 165-200,
1994 (New York).
The opioid peptides have common chemical
properties, but are synthesized in different pathways.
SUB~Ialu~ JIE26)
_
CA 02223246 1997-12-02
WO 96/40959 PCTAUS96/09629
-- 10 --
~ -endorphin, the most abundant endorphin, is
synthesized as part of a larger precursor molecule,
pro-opiomelanocortin t"POMC"). The POMC molecule
contains the full sequence of adrenocorticotrophic
s hormone ("ACTH"), a-melanocyte-stimulating hormone
("a-MSH"), ~-MSH, and ~-lipotropin. The POMC precursor
molecule also has the potential to generate other
endorphins, including a-endorphin and gamma-endorphin.
Processing of the POMC precursor occurs differently
within various tissues according to the localization of
cleavage enzymes, such as prohormone convertase's,
within those tissues.
In the pituitary, POMC is cleaved to produce
ACTH and ~-endorphin, and the ACTH is not further
processed. In contrast, in the hypothalamus, ACTH is
converted to ~-MSH. While different cell types may
synthesize the same primary gene product, the final
profile of hormone secretion may differ widely.
This invention contemplates use of a DNA
sequence encoding any suitable endorphin that has
analgesic activity. In addition, analogs or fragments
of these endorphins that have analgesic activity are
also contemplated. Thus the endorphin to be produced
by the cells of this invention may be characterized by
amino acid insertions, deletions, substitutions and
modifications at one or more sites in the naturally
occurring amino acid se~uence of the desired endorphin.
We prefer conservative modifications and substitutions
(i.e., those having a minimal effect on the secondary
or tertiary structure of the endorphin and on the
analgesic properties of the endorphin). Such
conservative substitutions include those described by
S~ ul~:S~ U~26)
=
CA 02223246 1997-12-02
W O ~G/4~3S9 PCTAUS96/09629
Dayhoff in Atl~s of Protein Sequence and Structure, 5,
~1978) and by Argos, ~m~o J., 3, pp. 779-85 (1989).
- Techniques for generating such variants of
naturally occurring endorphins are well known. For
example, codons in the DNA sequence encoding the wild
type endorphin may be altered by site specific
mutagenesis.
This invention contemplates using a DNA
sequence encoding the entire POMC precursor molecule.
This embodiment takes advantage of the host cell's
cleavage enzymes (i.e., Prohormone convertase 2) to
generate a suite of endorphins, some or all of which
may have analgesic properties.
This invention also contemplates use of DN~
fragments of the POMC gene that encode a particular
desired endorphin.
The DNA and amino acid sequence of POMC are
well known. Cochet et al., Nature, 297, pp. 335-9
(1982); Takahashi et al., Nucl. Acids Res., 11,
pp. 6847-58 (1983).
We prefer a DNA sequence encoding POMC in
which the ACTH coding region has been deleted. The
preferred endorphin encoded by this construct is
~-endorphin.
Some enkephalins are synthesized in the
adrenal glands as part of a large protein, pro-
enkephalin A, that contains six repeats of the Met-
enkephalin sequence and one Leu-enkephalin structure.
Met-enkephalin, as well as Met-enkephalin-Arg-Phe and
Met-enkephalin-Arg-Gly-Leu have significant
antinociceptive activity. See, e.g., Sagen et al.,
Brain Res., 502, pp. 1-10 (1989).
SU~ ultSHEr~t~llE26)
CA 02223246 1997-12-02
WO 96/40959 pcT/us96/os629
-- 12 --
Other enkephalins, i.e., dynorphins and neo-
endorphins are derived from a distinct molecule, pro-
enkephalin B. Additional "cryptic" peptides are also
encoded within the structure of these precursor
s proteins, and may be released by "pro-hormone-type"
cleavage. See, e.g., Harrison's "Principles Of
Internal Medicine", 12th Edition, pp. 1168-69 (1991).
This invention contemplates use of a DNA
sequence encoding any suitable enkephalin that has
analgesic activity. Analogs and active fragments that
have analgesic properties are also contemplated. Such
analogs or fragments may thus have amino acid
insertions, deletions, substitutions at one or more
sites in the naturally occurring amino acid sequence.
Such variants may be generated as described above.
This invention contemplates use of a DNA
sequence encoding a desired enkephalin in its "mature"
form. In addition, this invention contemplates using a
DNA sequence encoding the entire pro-enkephalin A
precursor, or the entire pro-enkephalin B precursor.
Further, we also contemplate using DNA encoding a
fusion, or fragment of these sequences, that upon
expression yields one or more enkephalin-like molecules
that have analgesic properties.
We prefer use of a DNA sequence encoding the
entire pro--enkephalin A precursor molecule. The DNA
and amino acid sequence of pro-enkephalin A are well
known. Folkesson, supra. This embodiment takes
advantage of the host cell's cleavage enzymes, such as
prohormone convertase, to generate a suite of
enkephalins, some or all of which may have analgesic
SI~ t SHEEr ~RIIL~ 26)
CA 02223246 l997-l2-02
W O 96/40959 PCT~US96/09629
- 13 -
properties. The pre~erred enkephalin encoded by thisconstruct is Met-enkephalin.
There are three naturally occurring
catecholamines which function as neurotransmitters in
the central nervous system; norepinephrine ("NE"),
epinephrine ("E"), and dopamine. NE is associated with
postganglionic sympathetic nerve endings. NE exerts
its effects locally in the immediate vicinity of its
release.
Catecholamines are synthesized from the amino
acid tyrosine, which is sequentially hydroxylated to
form dihydroxyphenylalanine (dopa), decarboxylated to
form dopamine, and then hydroxylated on the beta
position of the side chain by dopamine beta hydroxylase
to form NE. Harrison's, supra, pp. 380. NE is
N-methylated to E by phenylethanolamine-N
methyltransferase ("PNMT").
Hydroxylation of tyrosine by tyrosine
hydroxylase ("TH") is the rate limiting step in NE
synthesis. Regulation of dopa and NE synthesis in the
adrenal medulla may be accomplished by changes in the
amount and the activity of TH.
In addition, regulation of synthesis of E
from NE may occur by changes in the amount and the
2s activity of phenylethanolamine-N-methyltransferase
("PNMT"). PNMT is inducible by glucocorticoids from
the adrenal cortex. Ibid.
Catecholamines are maintained in high
concentration in adrenal medullary chromaffin tissue,
mostly as E. Opioid peptides are also stored in the
adrenal gland.
SUI~ u~ tl (RU1~26)
CA 02223246 1997-12-02
WO ~C/1035~ PCT~US96/09629
- 14 -
NE and E have similar affinities at ~2
receptors and therefore both potentially contribute to
analgesia. Bylund, FA~R J., 6, PP. 832-39 (1992).
The enkephalin peptides that predo~;n~ntly include met-
s enkephalin selectively activate delta (~) opioid
receptors. Reisine and Bell, Trends Neurosci., 16,
pp. 506-10 (1993). Activation of a2 adrenergic and o
opioid receptors in the spinal cord each result in
antinociception and are potentially synergistic. Yaksh
o and Malmberg, Progress in Pain Research and Management,
Vol. 1, Ed. Fields and Lisbeskind, IASP Press, Seattle,
pp. 141-71 (1994). Activation of ~ versus (~) opioid
receptors in experimental animals results in fewer
adverse side effects including constipation and
addiction liability (Lee et al., J. Pharmacol. Exp.
Ther., 267, pp. 883-87 (1993). The combined delivery
of different opioidergic and adrenergic agents may
decrease the magnitude of tolerance that develops to a
single agent and lead to sustained pain relief. Yaksh
20 and Reddy, Anesthesiol., 54, pp. 451-67 (1981).
This invention contemplates use of a DNA
sequence encoding catecholamine biosynthetic enzymes or
analogs or fragments thereof to obtain catecholamines
that have analgesic properties. The preferred
2s catecholamines in this invention are NE and E.
In one embodiment, the host cell is
transformed with the genes necessary to accomplish
production of NE or E, as desired. The selection Oc
heterologous gene sequences required depends upon the
complement of catecholamïne synthesizing enzymes
normally occurring in the host cell. For example, RIN
cells, and AtT-20 cells lack tyrosine hydroxylase
tShtt~ (~IIL~26)
CA 02223246 l997-l2-02
W O 96/40959 PCT~US96/09629
- 15 -
~ ("TH") and dopamine beta hydroxylase ("DBH"). However,
RIN and AtT-20 cells contain endogenous dopa
decarboxylase ("DDC"). If the desired catecholamine is
E, then the gene encoding PNMT is also required. The
gene encoding PNMT is known. Baetge et al., Proc.
N~t'l Ac~. Sci., 83, pp. 5455-58 (1986).
The gene encoding TH is known. See, e.g.,
United States patent 5,300,436, incorporated herein by
reference. Modified TH variants are also known.
o United States patent 5,300,436. In addition, truncated
versions of TH that contain the necessary C-terminal
catalytic domains are also known. See, e.g., Daubner
et al., Protein Science, 2, pp. 1452-60 (1993).
AtT-20 cells have been transformed with wild
type TH, as well as various TH muteins. See, e.g., Wu
et al., J. Biol. Chem., 267, pp. 25754-758 (1992).
The sequence of the DBH gene is also well
known. See, e.g., Lamoroux et al., EMBO J., 6,
pp. 3931-37 (1987).
It will be appreciated that in addition to
the preferred DNA sequences described herein, there
will be many degenerate DNA sequences that code for the
desired analgesics.
Secondary compounds with potential analgesic
action may also be produced by the cells of this
invention. Such compounds include galanin and
somatostatin. In addition, neuropeptide Y, neurotensin
and cholecystokinin may be produced by the transformed
cells of this invention. The cells of this invention
may normally produce some or all of these compounds, or
may be genetically engineered to do so using standard
techniques.
SUBSIll~lk S~EEr (~UL~ 26)
CA 02223246 l997-l2-02
WO ~C/~095~ PCTAJS96/09629
- 16 -
Standard methods may be used to obtain or
synthesize the genes encoding the analgesic compounds
to be produced by the cells of this invention.
For example, the complete amino acid sequence
of the desired compound may be used to construct a
back-translated gene. A DNA oligomer containing a
nucleotide sequence coding for the desired analgesic
compound may be synthesized. For example, several
small oligonucleotides coding for portions of each
desired polypeptide may be synthesized and then
ligated. The individual oligonucleotides typically
contain 5' or 3' overhangs for assembly.
The DNA sequence encoding each desired
analgesic compound, may or may not also include DNA
sequences that encode a signal sequence. Such signal
sequence, if present, should be one recognized by the
cell chosen for expression of the analgesic compound.
It may be prokaryotic, eukaryotic or a combination of
the two. It may also be the signal sequence of the
native compound. It generally is preferred that a
signal sequence be encoded and most preferably that the
native signal sequence be used.
Once assembled, the DNA sequences encoding
the desired compounds will be inserted into one or more
expression vectors and operatively linked to expression
control sequences appropriate for expression in the
desired transformed cell.
Proper assembly may be confirmed by
nucleotide sequencing, restriction mapping, and
expression of a biologically active polypeptide in the
transformed cell. As is well known in the art, in
order to obtain high expression levels of a transfected
SUBSlllUlt SNER (RUIE26~
CA 02223246 l997-l2-02
W 0~6/~C~, PCTAJS96/09629
- 17 -
gene in a host, the gene must be operatively linked to
transcriptional and translational expression control
sequences that are functional in the chosen expression
cell.
The choice of expression control sequence and
expression vector will depend upon the choice of cell.
A wide variety of expression host/vector combinations
may be employed. Useful expression vectors for
eukaryotic hosts, include, for example, vectors
o comprising expression control sequences from SV40/
bovine papilloma virus, adenovirus and cytomegalovirus.
We prefer pcDNA3, pCEP4, pZeoSV (InVitrogen,
San Diego) and pNUT.
Any of a wide variety of expression control
sequences may be used in these vectors. Such useful
expression control sequences include the expression
control sequences associated with structural genes of
the foregoing expression vectors. Examples of useful
expression control sequences include, for example, the
early and late promoters of SV40 or adenovirus, the
promoter for 3-phosphoglycerate kinase or other
glycolytic enzymes, the promoters of acid phosphatase,
e.g., Pho5, the promoters of the yeast ~-mating system
and other sequences known to control the expression of
genes of eukaryotic cells or their viruses, and various
combinations thereof.
It should of course be understood that not
all vectors and expression control sequences will
function equally well to express the DNA sequences
described herein. Neither will all cells function
equally well with the same expression system. However,
one of skill in the art may make a selection among
SUB~ b~t~ (RULE26)
CA 02223246 1997-12-02
WO 96/40959 PCTAUS96/09629
- 18 -
these vectors, expression control sequences and cells
without undue experimentation. For example, in
selecting a vector, the host cell must be considered
because the vector must replicate in it. The vector's
copy number, the ability to control that copy number,
and the expression of any other proteins encoded by the
vector, such as antibiotic markers, should also be
considered.
In selecting an expression control sequence,
a variety of factors should also be considered. These
include, for example, the relative strength of the
sequence, its controllability, and its compatibility
with the actual DNA sequence encoding the desired
analgesic compounds, particularly as regards potential
secondary structures. Host cells should be selected by
consideration of their compatibility with the chosen
vector, the toxicity of the product coded for by the
DNA sequences, their secretion characteristics, their
ability to fold the polypeptides correctly, and their
culture requirements. If the host cell is to be
encapsulated, cell viability when encapsulated and
implanted in a recipient should also be considered.
Within these parameters, one of skill in the
art may select various vector/expression control
sequence/host combinations that will express the
desired DNA sequences in culture.
In one embodiment, cells (e.g., RIN cells)
are sequentially transformed with 4 separate expression
vectors containing the POMC gene, the pro-enkephalin A
gene, the TH gene and the DBH gene. In such a
transformed host cell, amplification of copy number of
the heterologous genes is more difficult to achieve.
SUBSlllul~ Snt~ L~26)
CA 02223246 1997-12-02
wo 96/409s9 PCT/Uss6/os629
-- 19 --
Thus use of fewer expression vectors is preferred. Most
preferably, a single expression vector, containing all
4 heterologous genes, is used.
In a particular embodiment RIN cells are
5 sequentially transformed with 3 expression vectors.
The first vector contains the POMC gene operably linked
to the CMV promoter. Preferably a truncated version of
the POMC gene is used, having the ACTH coding region
deleted. The second vector contains the pro-enkephalin
10 A gene operably linked to the CMV promoter. Preferably
the proA construct contains the Kozak sequence
immediately upstream of the start codon. The third
vector contains both the TH gene (preferably truncated
and having the Kozak consensus sequence immediately
upstream of the start codon) and the DBH gene. In this
embodiment, the TH gene is operably linked to the CMV
promoter. The DBH gene is operably linked to an
internal ribosome entry site promoter sequence. RIN
cells are then transformed sequentially with each
20 expression vector according to known protocols.
In another embodiment, a single expression
vector containing the pro-enkephalin A gene, the POMC
gene, the TH gene, and the DBH gene is constructed.
Preferably, the ACTH region of the POMC gene is
25 deleted. Preferably the TH gene is truncated.
Multiple gene expression from a single
transcript is preferred over expression from multiple
transcription units. One approach for achieving
expression of multiple genes from a single eukaryotic
30 transcript takes advantage of sequences in picorna
viral mRNAs known as internal ribosome entry sites
("IRES"). These sites function to facilitate protein
SUBS~ lt Shetl (RUlE26~
CA 02223246 1997-12-02
W O 96/40959 PCT/U~5~ G29
- 20 -
translation from sequences located downstream from the
first AUG of the mRNA.
Macejak and Sarnow reported that the 5'
untranslated sequence of the immunoglobulin heavy chain
binding protein (BiP, also known as CRP 78, the
glucose-regulated protein of molecular weight 78,000)
mRNA can directly confer internal ribosome binding to
an mRNA in mAmm~ lian cells, in a 5'-cap independent
manner, indicating that translation initiation by an
o internal ribosome binding mechanism is used by this
cellular mRNA. Nature 353, pp. 90-94 (1991).
W0 94/24870 refers to use of more than two
IRES for translation initiation from a single
transcript, as well as to use of multiple copies of the
same IRES in a single construct.
This invention also contemplates use of a
"suicide" gene in the transformed cells. Most
preferably, the cell carries the TK (thymidine kinase)
gene as a safety measure, permitting the host cell to
be killed in v vo by treatment with gancyclovir.
Use of a "suicide" gene is known in the art.
See, e.g., Anderson, published PCT application
W0 93/10218; Hamre, published PCT application
W0 93/02556. The recipient's own immune system
provides a first level of protection from adverse
reactions to the implanted cells. If encapsulated, the
polymer capsule itself may be immuno-isolatory. The
presence of the TK gene (or other suicide gene) in the
expression construct adds an additional level of safety
to the recipient of the implanted cells.
Preferred vectors for use in this invention
include those that allow the DNA encoding the analgesic
S~ iu~t~ (RI~L~26)
-
CA 02223246 1997-12-02
W O 96/409S9 PCTAUS96/09629
compounds to be amplified in copy number. Such
amplifiable vectors are well known in the art. They
include, for example, vectors able to be amplified by
DHFR amplification (see, e.g., Kaufman, United States
Patent 4,470,461, Kaufman and Sharp, "Construction Of A
Modular Dihydrafolate Reductase cDNA Gene: Analysis Of
Signals Utilized For Efficient Expression", Mol. Cell.
Biol., 2, pp. 1304-19 (1982)) or glutamine synthetase
("GS") amplification (see, e.g., United States patent
5,122,464 and European published application 338,841).
Such amplification can be used to increase output of
the desired analgesic compounds.
Other techniques for increasing the output of
the desired analgesic compounds are contemplated. For
example, subcloning existing polyclonal cell lines is
contemplated. Cells are cloned by limiting dilution to
a single cell in each well. Cell clones are cultures,
and the clones are tested to select the clone with the
highest output of analgesic substances.
Another technique for increasing the output
of the desired analgesic compounds involves cloning
altered forms of biosynthetic enzymes with higher
activity than the wild type form (i.e., the truncated
TH 1-155). Some truncated forms of TH have 4-6 times
increased activity over the wild type form of TH. See,
e.g., Daubner et al., "Expression and characterization
of catalytic and regulatory domains of rat tyrosine
hydroxylase" Protein Science, 2, pp. 1452-60 (1993).
In addition, use of tyrosine-free media to
select to increase tetrahydrobiopterin cofactor levels
may potentially increase tyrosine hydroxylase activity.
See, e.g., Horellou et al., "Retroviral transfer of a
SUB31llultSHEEI (RUIE26)
CA 02223246 1997-12-02
WO 96/40959 PCT/U~ 529
- 22 -
human tyrosine hydroxylase cDNA in various cell lines;
regulated release of dopamine in mouse anterior
pituitary AtT-20 cells", Proc. Natl. Acad. Sci. USA,
86, pp. 7233-37 (1989).
Preferably, the output of B-endorphin ranges
between 1 and 10,000 pg/10b cells/hr. Preferably, the
output of met-enkephalin ranges between 1 and 10,000
pg/106 cells/hr. Preferably, the output of
catecholamines ranges between 1 and 1,000 pmoles/106
cells/hr.
The cells of this invention may be implanted
into a mammal, including a human, for the treatment of
pain. If implanted unencapsulated, any suitable
implantation protocol may be used, including those
outlined by Sagen el- al., United States patent
4,753,635, incorporated herein by reference.
It may be desirable to encapsulate the
genetically modified cells of this invention before
implantation. Such encapsulated cells form a
bioartificial organ ("BAO"). BAOs may be designed for
implantation in a recipient or can be made to function
extra-corporeally. The BAOs useful in this invention
typically have at least one semipermeable outer surface
membrane or jacket surrounding a cell-containing core.
The jacket permits the diffusion of nutrients,
biologically active molecules and other selected
products through the BAO. The BAO is biocompatible.
In some cases, the membrane may serve to also
immunoisolate the cells by blocking the cellular and
molecular effectors of immunological rejection. The
use of immunoisolatory membranes allows for the
implantation of allo and xenogeneic cells into an
S~ ul~Shttl (RULE~6)
CA 02223246 1997-12-02
W O 96/40959 PCTAUS96/09629
individual without the use of immunosuppression. If
biologically active molecules are released from the
isolated cells, they pass through the surrounding
semipermeable membrane into the recipient's body. If
metabolic functions are provided by the isolated cells,
the substances to be metabolized enter the BAO from the
recipient's body through the membrane to be acted on by
the cells.
A variety of types of membranes have been
used in the construction of BAQs. Generally, the
membranes used in BAOs are either microporous or
ultrafiltration grade membranes. A variety of membrane
materials have been suggested for use in BAOs,
including PAN/PVC, polyurethanes, polysufones,
polyvinylidienes, and polystyrenes. Typical membrane
geometries include flat sheets, which may be fabricated
into "sandwich" type constructions, having a layer of
living cells positioned between two essentially planar
membranes with seals formed around the perimeter of the
device. Alternatively, hollow fiber devices may be
used, where the living cells are located in the
interior of a tubular membrane. Hollow fiber BAOs may
be formed step-wise by loading living cells in the
lumen of the hollow fiber and providing seals on the
ends of the ~iber. Hollow fiber BAOs may also be
formed by a coextrusion process, where living cells are
coextruded with a polymeric solution which forms a
membrane around the cells.
BAOs have been described, for example, in
30 United States patent Nos. 4,892,538, 5,106,627,
5,156,844, 5,158,881, and 5,182,111, and PCT
Application Nos PCT/US/94/07015, WO 92/19195, Wo
SUBSmUlE S~lEEr ~RUEE 26)
CA 02223246 l997-l2-02
W 096/40959 PCTAUS96/09629
- 24 -
93/03901, and WO 91/00119, all of which are
incorporated herein by reference.
BAOs may contain other components that
promote long term survival of the encapsulated cells.
5 For example, WO 92/19195 refers to implantable
immllnoisolatory biocompatible vehicles having a
hydrogel matrix for enhancing cell viability.
The encapsulating membrane of the BAO may be
made of a material which is the same as that of the
core, or it may be made of a different material. In
either case, a surrounding or peripheral membrane
region of the BAO which is permselective and
biocompatible will be formed. The membrane may also be
constructed to be immunoisolatory, if desired. The
core contains isolated cells, either suspended in a
liquid medium or immobilized within a hydrogel matrix.
The choice of materials used to construct the
BAO is determined by a number of factors and is
described in detail in Dionne WO 92/19195. Briefly,
various polymers and polymer blends can be used to
manufacture the capsule jacket. Polymeric membranes
forming the BAO and the growth surfaces therein may
include polyacrylates (including acrylic copolymers),
polyvinylidenes, polyvinyl chloride copolymers,
2s polyurethanes, polystyrenes, polyamides, cellulose
acetates, cellulose nitrates, polysulfones,
polyphosphazenes, polyacrylonitriles,
poly(acrylonitrile/covinyl chloride), as well as
derivatives, copolymers and mixtures thereof.
BAOs may be formed by any suitable method
known in the art. One such method involves coextrusion
of a polymeric casting solution and a coagulant which
SUBSIIlul~ SHEEr (RUI~ ~6)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
can include biological tissue fragments, organelles, or
suspensions of cells and/or other therapeutic agents,
as described in Dionne, WO 92/19195 and United States
Patents 5,158,881, 5,283,187 and 5,284,761,
s incorporated herein by reference.
The jacket may have a single skin or a double
skin. A single-skinned hollow fiber may be produced by
quenching only one of the surfaces of the polymer
solution as it is co-extruded. A double-skinned hollow
lo fiber may be produced by quenching both surfaces of the
polymer solution as it is co-extruded.
Numerous capsulè configurations, such as
cylindrical, disk-shaped or spherical are possible.
The jacket of the BA0 will have a pore size
S that determines the nominal molecular weight cut off
(nMWC0) of the permselective membrane. Molecules
larger than the nMWCO are physically impeded from
traversing the membrane. Nominal molecular weight cut
off is defined as 90~ rejection under convective
conditions. In situations where it is desirable that
the BAO is immunoisolatory, the membrane pore size is
chosen to permit the particular factors being produced
by the cells to diffuse out of the vehicle, but to
exclude the entry of host immune response factors into
the BAO. Typically the nMWC0 ranges between 50 and 200
kD, preferably between 90 and 150 kD. The most
suitable membrane composition will also minimize
reactivity between host immune effector molecules known
to be present at the selected implantation site, and
the BAO's outer membrane components.
The core of the BAO is constructed to provide
a suitable local environment for the particular cells
SUB~ u~ ILE26)
CA 02223246 l997-l2-02
WO 96/40959 PCT~US96/09629
- 26 -
isolated therein. The core can comprise a liquid
medium sufficient to maintain cell growth. Liquid
cores are particularly suitable for maintaining
transformed cell lines like PC12 cells. Alternatively,
the core can comprise a gel matrix. The gel matrix may
be composed of hydrogel (alginate, "Vitrogen~", etc.)
or extracellular matrix components. See, e.g., Dionne
WO 92/19195.
Compositions that form hydrogels fall into
0 three general classes. The first class carries a net
negative charge (e.g., alginate). The second class
carries a net positive charge (e.g., collagen and
l~in;n) . Examples of commercially available
extracellular matrix components include Matrigel~ and
Vitrogen~. The third class is net neutral in charge
(e.g., highly crosslinked polyethylene oxide, or
polyvinylalcohol).
Any suitable method of sealing the BAO may be
used, including the employment of polymer adhesives
and/or crimping, knotting and heat sealing. These
sealing techniques are known in the art. In addition,
any suitable "dry" sealing method can also be used. In
such methods, a substantially non-porous fitting is
provided through which the cell-containing solution is
introduced. Subsequent to filling, the BAO is sealed.
Such a method is described in copending United States
application Serial No. 08/082,407, herein incorporated
by reference.
One or more in vitro assays are preferably
used to establish functionality of the BAO prior to
implantation in vivo. Assays or diagnostic tests well
known in the art can be used for these purposes. See,
ult SH~Er (RUIE2¢)
CA 02223246 l997-l2-02
W O ~f'4~35~ PCTAUS96/09629
- 27 -
e.g., Metho~s In Fnzymoloay, Abelson [Ed], Academic
Press, 1993. For example, an ELISA (enzyme-linked
immllnosorbent assay), chromatographic or enzymatic
assay, or bioassay specific for the secreted product
can be used. If desired, secretory function of an
implant can be monitored over time by collecting
appropriate samples (e.g., serum) from the recipient
and assaying them. If the recipient is a primate,
microdialysis may be used.
The number of BAOs and BAO size should be
sufficient to produce a therapeutic effect upon
implantation is determined by the amount of biological
activity required for the particular application. In
the case of secretory cells releasing therapeutic
substances, standard dosage conslderations and criteria
known to the art are used to determine the amount of
secretory substance required. Factors to be considered
are discussed in Dionne, WO 92/19195.
Implantation of the BAO is performed under
sterile conditions. Generally, the BAO is implanted at
a site in the host which will allow appropriate
delivery of the secreted product or function to the
host and of nutrients to the encapsulated cells or
tissue, and will also allow access to the BAO for
retrieval and/or replacement. The preferred host is a
primate, most preferably a human.
A number of different implantation sites are
contemplated. These implantation sites include the
- central nervous system, including the brain, spinal
cord, and aqueous and vitreous humors of the eye.
Preferred sites in the brain include the striatum, the
cerebral cortex, subthalamic nuclei and nucleus Basalis
SUI~llllJltSnttl ~RUIE26)
CA 02223246 1997-12-02
W O 96/40959 PCT/U~,G~09G29
- 28 -
of Meynert. Other preferred sites are the
cerebrospinal fluid, most preferably the subarachnoid
space and the lateral ventricles. This invention also
contemplates implantation into the kidney subcapsular
site, and intraperitoneal and subcutaneous sites, or
any other therapeutically beneficial site.
In order that this invention may be better
understood, the following examples are set forth.
These examples are for purposes of illustration only,
and are not to be construed as limiting the scope of
this invention in any manner.
F.xi lrr~les
Construction of Pol~cistronic Expression Vectors
Construction of IgSP-POMC Fusion
The SmaI-SalI fragment containing the human
POMC exon 3 was subcloned into pBS cloning vector
(Stratagene). See Takahashi, supra; Cochet, sup~a.
The resulting plasmid was named as pBS-hPOMC-027. See
Fig. 1.
A PCR fragment was generated using two
oligonucleotide primers, termed oCNTF-003 (SEQ ID
NO: 1) and oIgSP-018, (SEQ ID NO: 2) and the pNUT
plasmid containing the human CNTF gene. See Baetge
et al., Proc. Natl. Acad. Sci. USA, 83, pp. 5454-58
25 (1986). Both primers oCNTF-003 and oIgSP-018, contain
synthetic BamHI and SmaI restriction sites,
respectively, at the 5' ends.
The 196 base pair (bp) PCR fragment was
digested with restriction endonucleases BamHI and the
SmaI-isoschizomer XmaI, and electrophoresed through an
~UBSII~ul~SH~ llLE26)
CA 02223246 1997-12-02
W O 9G/4C3S~ PCTAUS96/09629
- 29 -
1% SeaPlaque agarose. The 193 bp HindIII/XmaI DNA
fragment was excised and purified using the FMC
SpinBind DNA purification kit (FMC BioProducts,
Rockland, ME).
pBS-hPOMc-027 was also digested with BamHI
and XmaI and purified from 1~ SeaPlaque agarose using
the FMC SpinBind DNA purification kit (FMC BioProducts,
Rockland, ME). The ligation mixture was transformed
into E. coli DH5~ (Gibco BRL, Gaithersburg, MD).
Positive sub-clones were initially identified
by the cracking gel procedure (Promega Protocols and
Applications Guide, 1991). Minilysate DNA was then
prepared using the FMC SpinBind DNA purification kit
(FMC BioProducts, Rockland, ME) and subject to BamHI
and SmaI restriction digestions. The positive sub-
clone was named as pBS-IgSP-hPOMC-028. See Fig. 1.
The nucleotide sequence of the fusion junction in pBS-
IgSP-hPOMC-028 was determined by the dideoxynucleotide
sequence determination using the Sequenase kit (USBC,
Cleveland). The sequence of the IgSP-hPOMC fusion is
shown in SEQ ID NO: 3.
Construction o~ IgSP-POMC Expression Vectors
The IgSP-hPOMC DNA fragment in pBS-IgSP-
hPOMC-028 was subcloned into pcDNA3 (Invitrogen Corp.,
San Diego, CA) and pCEP4 (Invitrogen Corp., San Diego,
CA) in sense and anti-sense orientations.
The NotI-SalI IgSP-hPOMC fragment from pBS-
IgSP-hPOMC-028 was ligated with the NotI-XhoI digested
pCEP4 resulting in the sense orientation clone named as
pCEP4-hPOMC-030. Fig. 2. The BamHI-SalI IgSP-hPOMC
fragment from pBS-IgSP-hPOMC-028 was ligated with the
tSk~l ~RULE26~
CA 02223246 1997-12-02
WO 9G/4~S9 PCTAJS96/09629
- 30 -
8amHI-XhoI digested pCEP4 resulting in the anti-sense
orientation clone named as pCEP4-hPOMC-031. Fig. 2.
The insert orientation in pCEP4-hPOMC-030 and -031 was
confirmed by BamHI, NotI, SalI and NotI/SalI
s restriction digestions as well as by dideoxynucleotide
sequence determination using the Sequenase kit (USBC,
Cleveland).
The BamHI-SalI IgSP-hPOMC fragment from pBS-
IgSP-hPOMC-028 was ligated with the BamHI-XhoI digested
pcDNA3 resulting in the sense orientation clone named
as pcDNA3-hPOMC-034. Fig. 2. The NotI-HindIII IgSP-
hPOMC fragment from pBS-IgSP-hPOMC-028 was ligated with
the NotI-HindIII digested pcDNA3 resulting in the anti-
sense orientation clone named as pcDNA3-hPOMC-035.
Fig. 2. Restriction digestion using SmaI, BamHI,
EcoRI, and BamHI/EcoRI was used to confirm the insert
orientation in pcDNA3-hPOMC-034, whereas HindIII, NotI
and SalI were used for pcDNA3-hPOMC-035.
Construction of ACTH Deleted IgSP-POMC
The ACTH coding region in the POMC gene in
pBS-IgSP-hPOMC-028 was deleted. pBS-IgSP-hPOMC-028 was
first digested with XmaI restriction enzyme and treated
with pfu DNA polymerase (Promega, Madison, WI). The
XmaI-pfu DNA polymerase treated pBS-IgSP-hPOMC-028 was
2s then digested with StuI restriction enzyme and purified
from 1~ SeaPlaque agarose using the FMC SpinBind DNA
purification kit (FMC BioProducts, Rockland, ME). The
self-ligation mixture was transformed into E. coli DH5
(Gibco BRL, Gaithersburg, MD). Positive sub-clones
were identified by BamHI/HindIII restriction digestion
and named as pBS-IgSP-hPOMC~ACTH-029. See Fig. 1. The
SUB~lllult SHEE~ ~ULE 26)
CA 02223246 1997-12-02
W O 96/40959 PCTAUS96/09629
- 31 -
nucleotide sequence of the ACTH deletion region in pBS-
IgSP-hPOMC-~ACTH-029 was confirmed by the
~ dideoxynucleotide sequence determination. The sequence
of the IgSP-hPOMC-~ACTH fusion is shown in SEQ ID
NO: 4.
Construction of ACTH Deleted IgSP-POMC
Expression Vectors
The IgSP-hPOMC-~ACTH DNA fragment in pBS-
IgSP-hPOMC-l~ACTH-029 was subcloned into pCDNA3
(Invitrogen Corp., San Diego, CA) and pCEP4 (Invitrogen
Corp., San Diego, CA) in sense and anti-sense
orientations. The NotI-SalI IgSP-hPOMC-~ACTH fragment
from pBS-IgSP-hPOMC-~ACTH-029 was ligated with the
NotI-XhoI digested pCEP4 resulting in the sense
orientation clone named as pCEP4-hPOMC-~ACTH-032
(Fig. 3). The BamHI-SalI IgSP-hPOMC-~ACTH fragment
from pBS-IgSP-hPOMC-~ACTH-029 was ligated with the
Ba~HI-XhoI digested pCEP4 resulting in the anti-sense
orientation clone named as pCEP4-hPOMC-~ACTH-033
(Fig. 3). The insert orientation in pCEP4-hPOMC-~ACTH-
032 and -033 was confirmed by BamHI and EcoRI
restriction digestions as well as by dideoxynucleotide
sequence determination using the Sequenase kit (USBC,
Cleveland~.
The BamHI-SalI IgSP-hPOMC-~ACTH fragment from
pBS-IgSP-hPOMC-QACTH-029 was ligated with the BamHI-
XhoI digested pcDNA3 resulting in the sense orientation
- clone named as pcDNA3-hPOM~ACTH-036 (Fig. 3). The
NotI-HindIII IgSP-hPOMC-~ACTH fragment from pBS-IgSP-
- 30 hPOMC-~ACTH-029 was ligated with the NotI-HindIII
suBslnultsEEr (RULE26)
-
CA 02223246 l997-l2-02
W O 96/40959 PCTrUS96/09629
digested pcDNA3 resulting in the anti-sense orientation
clone named as pcDNA3-hPOMC-~ACTH-037 (Fig. 3).
Restriction digestion using PvuII and EcoRI
was used to confirm the insert orientation in pcDNA3-
s hPOMC- ACTH-036, whereas SalI and EcoRI were used for
pcDNA3-hPOMC-~ACTH-037.
Cloning of Full Length and Truncated TH cDNA
Total RNA from PC12 cells was prepared using
the guanidinium thiocyanate-based TRI reagent
(Molecular Research Center, Inc., Cincinnati, OH).
Five hundred ng of PC12 total RNA was reverse
transcribed at 42~C for 30 minutes in a 20~1 reaction
volume containing 10 mM Tris.HCl (pH 8.3), 50 mM KC1,
4 mM of each dNTP, 5 mM MgC12, 1.25 ~M oligo (dT) 15-
15 mer, 1.25 l~M random hexamers, 31 units of RNase Guard
RNase Inhibitor (Pharmacia, Sweden) and 200 units of
SuperScript II reverse transcriptase (Gibco BRL,
Gaithersburg, MD). Two micro-liters of the above
reverse transcribed cDNA was added to a 25 ul PCR
20 reaction mixture containing 10 mM Tris.HCl (pH 8.3),
50 mM KCl, 800 of each nM dNTP, 2 mM MgC12, 400 nM of
primers #1 and #2, and 2.5 units of Thermus aquaticus
(Taq) DNA polymerase (Boehringer Mannheim, Germany).
To generate the full length TH cDNA,
25 oligonucleotide primers orTH-052 (SEQ ID NO: 5) and
orTH-053 (SEQ ID NO: 6) were used. For the truncated
TH, primers orTH-054 (SEQ ID NO: 7) and orTH-053 (SEQ
ID NO: 6) were used instead. These oligonucleotides
were constructed based on published TH sequence
30 information in Grima et al., ~ture, 326, pp. 707-11
(1987); US patent 5,300,436, and Paubner, supxa.
SU~lllul~ SHEE~ ~RUIE26~
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 33 -
Pri~ers orTH-052 (SEQ ID N0: 5) and orTH-054
(SEQ ID NO: 7) have synthetic HindIII restriction site
at the 5' end where orTH-053 has BamHI at the 5' end.
The PCR reaction mixtures were subject to 30
amplification cycles consisted of: denaturation, 94~C
30 seconds ~first cycle 2 minutes); annealing, 50~C 1
minute; and extension, 72~C 3.5 minutes tlast cycle 5
minutes). The 1537 bp full length and 1087 bp
truncated rat TH PCR fragments were digested with
o restriction endonucleases BamHI and HindIII and
resolved on an 1% SeaPlaque agarose gel. The 1531-bp
and 1081-bp HindIII/BamHI DNA fragments were excised
and purified using the FMC SpinBind DNA purification
kit (FMC BioProducts, Rockland, ME).
pcDNA3 expression vector was also digested
with BamHI and HindIII and purified from 1~ SeaPlaque
agarose using the FMC SpinBind DNA purification kit
(FMC BioProducts, Rockland, ME). The ligation mixture
was transformed into E.coli DH5~ (Gibco BRL,
Gaithersburg, MD).
Cracking gel procedure ~Promega Protocols and
Applications Guide, 1991) was used to screen out the
positive sub-clones. The identity of the correct
clones was further verified by BamHI/HindIII double
digestion.
The positive sub-clones for the full-length
and truncated rat TH in pcDNA3 were named as pcDNA3-
rTH-044 (Fig. 4) and pcDNA3-rTH~-04S (Fig. 4),
respectively. The nucleotide sequence of both full-
length and truncated rat TH PCR clones was determined
by the dideoxynucleotide sequence determination using
SUBSIl~ul~nttl (RULE26)
CA 02223246 1997-12-02
WO 96/40959 PCT/US96/09629
-- 34 --
the Sequenase kit (USBC, Cleveland). The sequence of
the rTH~ construct is shown in SEQ ID NO: 16.
To optimize the translation efficiency of the
truncated rat TH, oligonucleotide primer orTH-078 (SEQ
ID NO: 8) was designed so that the consensus Kozak
sequence is immediate up stream to the start codon ATG.
pcDNA3-rTH~-45 was used as the template in a 50 ~1 PCR
reaction mixture with reagent composition identical to
the one described above with the exception that the
o oligonucleotide primers were replaced with orTH-078
(SEQ ID NO: 8) and orTH-053 (SEQ ID NO: 6). The 1097
bp PCR product was cloned into pcDNA3 in the same
manner as described above. The resulting sub-clone was
named pcDNA3-rTH~KS-75 (Fig 4). The sequence of the
rTH~KS construct is shown in SEQ ID NO: 17.
Construction of rTH-IRES-bDBH Fusion Gene
Recombinant PCR methodology was used to
generate the rTH-IRES-bDBH fusion gene.
Oligonucleotides oIRES-057 (SEQ ID NO: 9) and obDBH-065
20 (SEQ ID NO: 10) are specific for IRES and bDBH gene
sequences, respectively, and contain synthetic BamHI
and NotI restriction sites at the 5' end, respectively.
Oligonucleotides oIRES-bDBH-064 (SEQ ID NO: 11) and
oIRES-bDBH-066 (SEQ ID NO: 12) are complementary to
25 each other. Furthermore, oligonucleotide primer oIRES-
bDBH-064 (SEQ ID NO: 11) has its 5' 16 nucleotides
identical to the IRES sequence and its 3' 18
nucleotides identical to the bDBH sequence; and vice
versa for oIRES-bDBH-066 (SEQ ID NO: 12).
Two first PCR reactions were carried out
using oligonucleotide pairs oIRES-057/oIRES-bDBH-066
h~l (R11~26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 35 -
and oIRES-bDBH-064/obDBH-065 on templates pCTI-001
(with an insert containing the IRES sequence shown in
SEQ ID NO: 30) and pBS-bDBH-006 (containing the bovine
DBH gene cloned from bovine adrenal chromaffin cells,
Lamoroux et al., EMBO J., 6, pp. 3931-37 (1987))
plasmids, respectively. One hundred ng of template DNA
was added to a 50 ~l PCR reaction mixture containing
10 mM Tris.HCl (pH 8.3), 50 mM KC1, 800 of each nM
dNTP, 2 mM MgCl2, 400 nM of primers #1 and #2, and 2.5
units of Therm-ls aquaticus (Taq) DNA polymerase
(Boehringer Mannheim, German).
The PCR reaction mixtures were subject to 30
amplification cycles consisted of: denaturation, 94 ~C
for 30 seconds (first cycle 2 minutes); annealing,
50 ~C 1 minute; and extension, 72 ~C 30 seconds (last
cycle 5 minutes). The PCR products were resolved on 1%
TrivieGel 500 (TrivieGen). Two agarose plugs
containing each one of the first PCR products were
transfer to a tube containing 50 ul of PCR reaction
mixtures identical to the one described above with the
exception that the oligonucleotides oIRES-057 and
obDBH-065 were used.
The second PCR reaction was subject to 30
amplification cycles consisted of: denaturation, 94 ~C
for 30 seconds (first cycle 2 minutes); annealing,
60 ~C 30 seconds (second to fourth cycles 37 ~C 2
minutes); and extension, 72 ~C 30 seconds (last cycle 2
minutes). The 2407 bp IRES-bDBH fusion PCR product and
the cloning vector pcDNA3-rTH~-45 were digested with
BamHI and NotI restriction enzymes and subsequently
purified from 1% SeaPlaque agarose gel using the FMC
SU~IIlul~SHE~ (Rlll~26)
CA 02223246 1997-12-02
WO 96/40959 PCT/US96/09629
- 36 -
SpinBind DNA purification kit (FMC BioProducts,
Rockland, ME).
The ligation of IRES-bDBH/BamHI/Notl and
pcDNA3-rTHa-045/BamHI/NotI would generate a rTHa-IRES-
bDBH expression vector named as pcDNA3-rTH~-IRES-bDBH-
066 (Fig. 5) whereas that of IRES-bDBH/BamHI/NotI and
pcDNA3-rTHaKS-075/BamHI/NotI would generate a rTHaK
IRES-bDBH expression vector, named as pcDNA3-rTHaKs-
IRES-bDBH-076 (Fig. 5), where the start codon ATG in
rTHa is preceded with a consensus Kozak sequence. The
sequence of the rTHa-IRES-bDBH construct is sho~n in
SEQ ID NO: 18. The sequence of the rTHaKs-IREs-bDBH
construct is shown in SEQ ID NO: 19. The ligation
mixture was transformed into DH5~ (Gibco BRL,
Gaithersburg, MD). The positive clones were identified
by the cracking gel procedure (Promega, Madison, WI)
and restriction digestions using HindIII, BamHI,
HindIII/BamHI, SmaI and NotI.
The 4114 bp NruI-XhoI fragment containing the
CMV promoter-rTHaKS-IRES-bDBH was excised out of
pcDNA3-rTHaKS-IRES-bDBH-076 and subcloned into pZeoSV
cloning vector (Invitrogen Corp., San Diego, CA)
digested with ScaI and XhoI in the multiple cloning
site. The resulting expression vector was named as
pZeo-Pcmv-rTHaKS-IRES-bDBH-088 (Fig. 6).
Construction of IgSP-hPOMC ACTH-
rTHD-IRES-bDBH Fusion Gene
The 4100 bp NruI-NotI fragment containing the
CMV promoter, rTHD-IRES-bDBH fusion gene, and BGH
30 polyadenylation sequence was excised out of pcDNA3-
SllBS~ t SEE~ IIE 26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
rTHQ-IRES-bDBH-066 and subcloned into the pBS
(Stratagene, La Jolla, CA) cloning vector.
~ The resulting plasmid pBS-Pcmv-rTH~-IRES-
bDBH-067 (Fig. 7) was used as the intermediary
s construct to which the recombinant PCR IgSP-hPOMCDACTH-
IRES fragment would be inserted.
Oligonucleotide oIgSP-068 (SEQ ID NO: 13),
containing a synthetic EcoRV restriction site, is
specific for the IgSP sequence.
Oligonucleotide primer orTHQ-073 (SEQ ID
NO: 14) is specific for the rTHQ sequence and contains
an endogenous SmaI restriction site.
Oligonucleotide primers ohPOMC-IRES-069 (~EQ
ID NO: 15) and ohPOMC-IRES-070 (SEQ ID NO: 20) are
complementary to each other. Furthermore,
oligonucleotide primer ohPOMC-IRES-069 has its 5', 18
nucleotides identical to the hPOMC sequence and its 3'
12 nucleotides identical to the IRES sequence; and vice
versa for ohPOMC-IRES-070.
Oligonucleotide primers oIRES-rTH~-071 (SEQ
ID NO: 21) and oRIRES-rTH~-072 (SEQ ID NO: 22) are
complementary to each other. In addition,
oligonucleotide primer oIRES-rTH~-071 has its 5' 15
nucleotides identical to the rTHQ sequence and its 3'
2s 18 nucleotide identical to the IRES sequence; and vice
versa for oRIRES-rTHQ-072.
Three sets of first PCR reactions were
carried out.
PCR reaction A: template pBS-IgSP-hPOMCDACTH-029,
oligonucleotides oTgSP-068/ohPOMC-IRES-069;
PCR reaction B: template pCTI-001,
oligonucleotides ohPOMC-IRES-070/oIRES-rTHQ-071; and
SUBSIllult~h~tl (RlJl~26)
CA 02223246 l997-l2-02
W O ~f/40~5~ PCTAUS96/09629
- 38 -
PCR reaction C: template pcDNA3-rTH~-045,
oligonucleotides orIRES-rTH~-072/orTH~-073.
The three sets of first PCR reactions were
carried in 50 ,ul PCR reaction mixture containing 100 ng
of template DNA, 10 mM Tris. HCl (pH 8.3), 50 mM KCl,
800 of each nM dNTP, 2 mM MgC123, 400nM of primers #1
and #2, and 2.5 units of Thermus a~u~ticus (Taq) DNA
polymerase (Boehringer Mannheim, Germany).
The PCR reaction mixtures were subject to 30
amplification cycles consisted of: denaturation, 94 ~C
for 30 seconds (first cycle 2 minutes); annealing,
50 ~C 1 minute; and extension, 72 ~C 30 seconds (last
cycle 5 minutes).
The PCR products were resolved on 1~o
TrivieGel 500 (TrivieGen). Two agarose plugs
containing each one of the PCR products from PCR
reactions B and C were transferred to a tube containing
50 ul of PCR reaction mixtures identical to the one
described above with the exception that the
oligonucleotides ohPOMC-IRES-070 and orTHA-073 were
used.
The second PCR reaction was subject to 30
amplification cycles consisted of: denaturation, 94 ~C
for 30 seconds (first cycle 2 minutes); annealing,
2s 60 ~C 30 seconds (second to fourth cycles 37 ~C 2
minutes); and extension, 72 ~C 30 seconds (last cycle 2
minutes).
The PCR products were treated as described
above. Agarose plugs containing the PCR products from
the second PCR reaction and the PCR reaction A were
combined and subjected to a third PCR amplification
using oIgSP-068/rTH~-073. The 1203 bp IgSP-hPOMC-IRES-
SUBSllll~lk SHE~ ~RUI~ 26)
CA 02223246 1997-12-02
W096/40959 PCT/US96/09629
-- 39 --
rTH~ fusion PCR product and the cloning vector pBS-
Pcmv-rTH~-IRES-bDBH-067 were digested with EcoRV and
XmaI restriction enzymes and subsequently purified from
1% SeaPlaque agarose gel using the FMC SpinBind DNA
5 purification kit (FMC BioProducts, Rockland, ME). The
ligation ~Lixture was transformed into DH5O~ (Gibco BRL,
Gaithersburg, MD).
The positive clones were identified by the
cracking gel procedure (Promega, Madison, WI) and
10 restriction digestions using EcoRI, KpnI and NotI. The
resulting clone was named as pBS-IgSP-hPOMC~ACTH-IRES-
rTH~-IRES-bDBH-068. Fig. 8. The sequence of this
construct is shown in SEQ ID NO: 23.
Construction of IgSP-hPOMCACTH-IRES-
rTH~-IRES-bDBH Expression Vectors
The 4491 bp NotI fragment containing the
IgSP-hPOMC~CTH-IRES-rTH~-IRES-bDBH gene was excised
out of the pBS-IgSP-hPOMC~ACTH-IRES-rTHL~-IRES--bDBH-068
and subcloned into the pcDNA3 (Invitrogen Corp., San
20 Diego, CA) at the NotI site in the multiple cloning
site. Restriction digestion using NotI and SmaI
confirmed that the IgSP-hPOMCa~CTH-IRES-rTH~-IRES-bDBH
gene was inserted in the sense orientation resulting in
pcDNA3-IgSP-hPOMC~ACTH-IRES-rTH~-IRES-bDBH-069. See
25 Fig. 9.
Construction of IgSP-hPOMC~ACTH-IRES-rTH~-IRES-
bDBH-IRES-Zeocine Expression Vector
Recombinant PCR methodology was used to
generate the IRES-Zeocine fusion gene.
30 Oligonucleotides oIRES-074 (SEQ ID NO: 24) and oZeocin-
SU~I~lul~ ~htt~ UlE 26)
CA 02223246 1997-12-02
WO 96/40959 PCT/U~,G/O~G29
- 40 -
077 (SEQ ID NO: 25) are specific for IRES and Zeocin
gene sequences, respectively, and contain synthetic
NotI and XhoI restriction sites at the 5' end,
respectively. Oligonucleotides oIRES-Zeocin-075 (SEQ
ID NO: 26) and oIRES-Zeocin-076 (SEQ ID NO: 27) are
complementary to each other. Furthermore,
oligonucleotide oIRES-Zeocin-075 has its 5'15
nucleotides identical to the Zeocin sequence and its 3'
18 nucleotides identical to the IRES sequence; and vice
versa for oIRES-Zeocin-076.
Two first PCR reactions were carried out
using oligonucleotide pairs oIRES-074/oIRES-Zeocin-075
and oIRES-Zeocin-076/oZeocin-075 on templates pCTI-001
and pZeoSV (Invitrogen Corp., San Diego, CA) plasmids,
respectively.
One hundred ng of template DNA was added to a
50 ul PCR reaction mixture containing 10mM Tris.HCl (pH
8.3), 50 mM KCl, 800 of each nM dNTP, 2 mM MgCl2, 400
nM of primers #1 and #2, and 2.5 units of Thermus
açIu~ticus (Taq) DNA polymerase (Boehringer Mannheim,
Germany).
The PCR reaction mixtures were subject to 30
amplification cycles consisted of: denaturation, 94 ~C
for 30 seconds (first cycle 2 minutes); annealing,
50 ~C 1 minute; and extension, 72 ~C 30 seconds (last
cycle 5 minutes).
The PCR products were resolved on l~o
TrivieGel 500 (TrivieGen). Two agarose plugs
containing each one of the first PCR products were
transfer to a tube containing 50 ul of PCR reaction
mixtures identical to the one described above with the
Sll~lllul~ ~h~ 26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
~ exception that the oligonucleotides oIRES-074 and
oZeocin-077 were used.
The second PCR reaction was subject to 30
amplification cycles consisted of: denaturation, 94 ~C
for 30 seconds (first cycle 2 minutes); annealing,
50 ~C 30 seconds tsecond to fourth cycles 37 ~C 2
minutes); and extension, 72 ~C 30 seconds (last cycle 2
minutes).
The 974 bp IRES-Zeocin fusion PCR product and
the cloning vector pcDNA3 were digested with NotI and
XhoI restriction enzymes and subsequently purified from
1% SeaPlaque agarose gel using the FMC SpinBind DNA
purification kit (FMC BioProducts, Rockland, ME).
The ligation of IRES-Zeocin/NotI/XhoI and
pcDNA3/NotI/XhoI would generate an intermediate cloning
vector named as pcDNA3-IRES-Zeocin-072. Fig. 10.
The positive clones were identified by the
cracking gel procedure (Promega, Madison, WI) and
restriction digestions using HindIII, SmaI, XhoI, NotI
and NotI~XhoI.
To generate the final IgSP-hPOMCDACTH-IRES-
rTHD-IRES-bDBH-IRES-Zeocine Expression Vector, a 4491
bp NotI fragment containing the IgSP-hPOMCaACTH-IRES-
rTH~-IRES-bDBH gene was excised out of the pBS-IgSP-
hPOMCaACTH-IRES-rTH~-IRES-bDBH-068 (Fig. 8; SEQ ID
NO: 23) and subcloned in to the pcDNA3-IRES-Zeocin-072
(Fig. 10) at the NotI site in the multiple cloning
site.
Restriction digestion using NotI and SmaI
confirmed that the Igsp-hpoMc~AcTH-IREs-rTHa-IREs-bDBH
gene was inserted in the sense orientation resulting in
pcDNA3-IgSP-hPOMCaACTH-IRES-rTH~-IRES-bDBH-IRES-Zeocin-
SUBs~ SEE~ (RU13~ 26)
CA 02223246 l997-l2-02
WO ~G/10~S~ PCTrUS96/09629
- 42 -
073. The sequence of this construct is shown in SEQ ID
NO: 28. Fig. 11.
Construction of ProA+KS Fusion
A construct containing the coding region of
s the human pro-enkephalin A gene with the consensus
Kozak sequence immediately upstream to the start codon
ATG. The sequence of this construct is shown in SEQ ID
NO: 29.
Construction o~ hProA+KS Expression Vector
o The HindIII/BamHI fragment containing the
hProA+KS fusion was ligated into BamHI and Hind III
digested pcDNA3 expression vector substantially as
described above. After screening as described above, a
positive sub-clone was named pcDNA3-hProA+KS-091.
Fig. 12. Construction of the pBS-CMV Pro A vector is
detailed in Mothis, J. and Lindberg, I., Endocrinology,
131, pp. 2287-96 (1992).
Trans~ormation of Cells
RIN and AtT-20 cells were transformed as
follows.
The RINa and AtT-20 based cell lines were
grown in DMEM tGibco) with 10~ fetal bovine serum and
pen-strep-fungizone (Gibco) base media. The cells were
plated out in PlOO petri dishes (750,000 cells/dish) in
2s 10 ml of base media. 18-24 hours later, the cells were
transfected using calcium phosphate method with a kit
made by Stratagene (San Diego, CA). A 10 ug amount of
the plasmid vector DNA was diluted in 450 ul of
deionized sterile water. Then, 50 ul of a lOx buffer
SU~ U~ IEEr(RV~E26)
CA 02223246 l997-l2-02
W O~6/10~ PCT~US96/09629
- 43 -
(solution #l) was added to the plasmid DNA. A 500 ~1
amount of solution #2 was immediately added to the DNA
containing solution and mixed gently. This was
incubated at room temperature for 20 minutes and then
the 1.0 ml solution was added to the cells in the petri
dish. The cells were incubated overnight and 18-24
hours later the cells were washed 2x with Hanks
balanced salt solution without calcium and magnesium.
Then, the cells were cultured in base media + selection
drugs. The cells were selected in either 600 ~g/ml
geneticin (Gibco) or 400 ug/ml hygromycin (Boehringer
Mannheim) or 500 ug/ml Zeocin (In Vitrogen, San Diego,
CA). Cells were sequentially transfected and selected
to obtain the final cell line.
The RINa cells were transfected with plasmid
pCEP4-hPOMC-030 containing the POMC gene. This is a
hygromycin resistant vector. The cells were also
transformed with plasmid pcDNA3-hProA+KS-091. This is
a geneticin resistant vector. Finally, the cells were
transfected with plasmid pZeo-PCMV-rTH~KS-IRES-bDBH-088
which conferred Zeocin resistance.
The AtT-20 cells were transfected with
plasmid pBS-CMV-ProA and pCEP4-POMC-~ACTH-32 which
conferred geneticin and hygromycin resistance,
respectively. Finally, the cells were transfected with
plasmid pZeo-Pcmv-rTH~KS-IRES-bDBH-088.
We have tested a number of media for cell
growth. Surprisingly we have found that in certain
serum-free medias, the above cell lines have enhanced
neurotransmitter output, compared to serum-containing
media. We prefer CHO-Ultra (Biowhitaker) for the
SU~ ltSnttl (RUL~26)
CA 02223246 1997-12-02
WO ~G/4C95~ PCT~US96/09629
- 44 -
growth of AtT-20 cells, and Ultra-Culture (Biowhitaker)
for the growth of RINa cells.
Output of various analgesics from one
transformed RINa cell line (RINa/ProA/P030/P088) is
s shown in Table 2. All values represent unstimulated
cells. Output of ~-endorphin and met-enkephalin is in
pg/106 cells/hr. ~-endorphin and met-enkephalin were
measured by radioimmunoassay using Incstar kits
(Stillwater, Minnesota). Catecholamine output is in
o pmoles/106 cells/hr. The numbers in parentheses
represent values from cells that were preincubated 18
hours with 100 ~M tetrahydrobiopterin. Catecholamines
were measured by high performance liquid chromatography
as described in Lavoie et al., "Two PC12
pheochromocytoma lines sealed in hollow fiber-based
capsules tonically release l-dopa in vitro", Cell
transplant~tion, 2, pp. 163-73 (1993). GABA output
from these RINa cells was 28 ng/10 cells/hrs.
Table2
Cell I i-le Cndoaenous [~-endorPhin Met-enk DA E
Analaesic
SI~L,aldnces
RIN a/ ProA/ 13-endor~ 22 17 3 0
POMC/ GABA (6) (2)
TH-IRES-D~H
There are encrypted enkephalin fragments which are not
fully processed from the pro-enkephalin precursor
molecule. These encrypted enkephalins have opioid
receptor binding activity. We digested these encrypted
enkephalins to measure opioid activity. The trypsin
digest protocol is as follows. A 2 ~g/ml trypsin
(Worthington #34E470) solution is added to media
ultSH~(RUlE26)
CA 02223246 1997-12-02
W O ~ 35~ PCTAUS96/09629
- 45 -
samples on ice. Samples are vortexed, then incubated
for 20 minutes in a 37~C waterbath. After the 20
~ minute digest, samples are returned to ice and 100
ng/ml carboxypeptidase B (Sigma #C-7011) is added.
Samples are mixed by vortexing, and returned to the
37~C waterbath for 15 minutes. Samples are placed on
ice once more and 10 ug/ml trypsin inhibitor is added.
At this stage, samples are either extracted for met-
enkephalin or immediately frozen for future extraction.
This results in the full enzymatic cleavage to free all
met-enkaphalin from the longer encrypted fragments. A
met-enkaphalin radioimmunoassay of the digested sample
gives total met-enkaphalin from the supermatant. The
transformed RINa cells appear to have greater than 5
fold more encrypted enkaphalins compared to fully
processed met-enkaphalin.
Fiher ca~sule formation and characteristics
Hollow fibers are spun from a 12.5-13.5~
poly(acrylonitrile vinylchloride) solution by a wet
spinning technique. Cabasso, Hollow Fiber Membranes,
vol. 12, Kirk-Othmer Encyclopedia of Chemical
Technoloqy, Wiley, New York, 3rd Ed. pp. 492-517
(1980), Unites States patent 5,158,881, incorporated
herein by reference.
The resulting membrane fibers may either be
double skinned or single skinned PAN/PVC fibers. In
order to make implantable capsules, lengths of fiber
are first cut into 5 cm long segments and the distal
extremity of each segment sealed with an acrylic glue.
Encapsulation hub assemblies are prepared by providing
lengths of the membrane described above, sealing one
S~ ul~S~kl (RUIE26)
_
CA 02223246 1997-12-02
W O ~f'4C35~ PCTAJS96/09629
- 46 -
end of the fiber with a single drop of LCM 24 (Light
curable acrylate glue, available from ICI), curing the
glue with blue light, and repeating the step with a
second drop. The opposite end is previously attached
to a frangible necked hub assembly, having a silicone
septum through which the cell solution may be
introduced. The fiber is glued to the hub assembly by
applying LCM 22 to the outer diameter of the hub
assembly, pulling the fiber up over it, and curing with
0 blue light. The hub/fiber assemblies are placed in
sterili~ation bags and are ETO sterilized.
Following sterilization with ethylene oxide
and outgassing, the fibers are deglycerinated by
ultrafiltering first 70~ EtOH, and then HEPES ~uffered
saline solution through the walls of the fiber under
vacuum.
Prepar~tion and Fncapsulation of Transformed Cells
The transformed cells are prepared and
encapsulated as follows:
A matrix solution is prepared using a
commercially availab~e alginate, collagen or other
suitable matrix material. The cell solution was
diluted in the ratio of two parts matrix solution to
one part cell solution containing the transformed cells
described above. We prefer Vitrogen (Celtix, Santa
Clara) as a matrix for AtT-20 cells.
We prefer Organogen (Organogenesis, Canton,
MA) as a matrix for RINa cells. The RINa based cells
are prepared for encapsulation by the following method.
The cells are grown in base media of DMEM + 10% fetal
bovine serum during the proliferation phase. These
SUBSTITUTE SHEET (RULE 26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 47 -
cells can be removed from the tissue culture flasks by
two washes in Hanks balanced salt solution without
~ calcium and magnesium. Then the cells are incubated in
0.25% trypsin + EDTA for 1 minute. This is removed and
the cells are rinsed free of the flask using Hanks
balanced salt solution without calcium and magnesium
solution. The cells are placed in 10 mls of base media
and centrifuged at 100 x g for 2 minutes. The cells
are resuspended in 10 mls of the preferred serum free
media (Ultra culture, Biowhitaker, Walkersville, MD).
Surprisingly, the RINa cells secrete more analgesiç
substances when cultured in this serum free media
relative to serum continuing base media.
The cells are centrifuged at 100 g twice in
the preferred serum free media before the cells are
concentrated 1:1 with the preferred Organogen matrix.
Organogen is a 1% bovine tendon collagen obtained as a
sterile solution. 8 parts of this solution are mixed
with 1 part 10X DPBS. 0.5 N sodium hydroxide is added
until physiological pH is attained (approximately
2S0 uls).
The final concentration of the cell + matrix
solution used for encapsulation can range from 20,000 -
50,000 cells/~l. The cells are counted in a standard
manner on a hemocytometer.
The cell/matrix suspension is placed in a
1 ml syringe. A Hamilton 1800 Series 50 microliter
syringe is set for a 15 microliter air bubble, is
inserted into a 1 ml syringe containing the cell
solution and 30 microliters are drawn up. The cell
solution is injected through the silicone seal of the
hub/fiber assembly into the lumen of a modacrylic
SUB~ Shttl (RULE26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 48 -
hollow fiber membrane with a molecular weight cutoff of
approximately 50,000-100,000 daltons. Ultrafiltration
should be observed along the entire length of the
fiber. After one minute, the hub is snapped off the
sub-hub, exposing a fresh surface, unwet by cell
solution. A single drop of LCM 2~ is applied and the
adhesive cured with blue light. The device is placed
first in HEPES buffered NaCl solution and then in CaC12
solution for five minutes to cross-link the alginate.
Each implant is about 5 cm long, 1 mm in diameter, and
contained approximately 2.5 million cells.
After the devices are filled and sealed, a
silicone tether (Speciality Silcone Fabrication, Paso
Robles, CA) (ID: 0.69, OD: 1.25) is then placed over
the proximal end of the fiber. A radiopaque titanium
plug is inserted in the lumen of the silicone tether to
act as a radiographic marker. The devices are then
placed in 100 mm tissue culture dishes in 1.5 ml PC-1
medium, and stored at 37~C, in a 5~ CO. incubator for
in vitro analysis and for storage until implantation.
The encapsulated cells are then implanted
into the human sub-arachnoid space as follows:
Sura~l Procedure
After establishing IV access and
administering prophylactic antibiotics (cefazolin
sodium, 1 gram IV), the patient is positioned on the
operating table, generally in either the lateral
decubitus or genu-pectoral position, with the lumbar
spine flexed anteriorly. The operative field is
sterily prepared and draped exposing the midline dorsal
lumbar region from the levels of S-1 to L-1, and
SU~IIIUIE SHEEr (RUl~26)
-
CA 02223246 1997-12-02
WO 96/40959 PCTAUS96/09629
- 49 -
allowing for intraoperative imaging of the lumbar spinewith C-arm fluoroscopy. Local infiltration with 1.0%
lidocaine is used to establish anesthesia of the skin
as well as the periosteum and other deep connective
tissue structures down to and including the ligamentum
flavum.
A 3-5 cm skin incision is made in the
parasagital plane 1-2 cm to the right or left of the
midline and is continued down to the lumbodorsal
lo fascia using electrocautery for hemostasis. Using
traditional bony landmarks including the iliac crests
and the lumbar spinous processes, as well as
fluoroscopic guidance, and 18 gauge Touhy needle is
introduced into the subarachnoid space between L-3 and
L-4 via an oblique paramedian approach. The needle is
directed so that it enters the space at a shallow,
superiorly directed angle that is no greater than 30-
35~ with respect to the spinal cord in either the
sagittal or transverse plane. Appropriate position of
the tip of the needle is confirmed by withdrawal of
several ml of cerebrospinal fluid (CSF) for
preimplantation catecholamine, enkephalin, glucose, and
protein levels and cell counts.
The Touhy needle hub is reexamined to confirm
that the opening at the tip is oriented superiorly
(opening direction is marked by the indexing notch for
the obturator on the needle hub), and the guide wire is
passed down the lumen of the needle until it extends 4-
5 cm into the subarachnoid space (determined by
premeasuring). Care is taken during passage of the
wire that there is not resistance to advancement of the
wire out of the needle and that the patient does not
SU~IIIU~E S~lEEr ~RUIE~6)
CA 02223246 1997-12-02
W O 96/40959 PCTAUS96/09629
-- 50 --
complain of significant neurogenic symptoms, either of
which observations might indicate misdirection of the
guide wire and possible impending nerve root or spinal
cord injury.
After the guide wire appears to be
appropriately placed in the subarachnoid space, the
Touhy needle is separately withdrawn and removed from
the wire. The position of the wire in the midline of
the spinal canal, anterior to the expected location of
o the caud equina, and without kinks or unexplainable
bends is then confirmed with fluoroscopy. After
removal of the Touhy needle the guide wire should be
able to be moved freely into and out of the space with
only very slight resistance due to the rough surface of
lS the wire running through the dense and fibrous
ligamentum flavum.
The 7 French dilator is then placed over the
guide wire and the wire is used to direct the dilator
as it is gently but firmly pushed through the fascia,
paraspinous muscle, and ligamentum flavum, following
the track of the wire toward the subarachnoid space.
Advancement of the 7 French dilator is stopped and the
dilator removed from the wire as soon as a loss of
resistance is detected after passing the ligamentum
flavum. This is done in order to avoid advancing and
manipulating this relatively rigid dilator within the
subarachnoid space to any significant degree.
After the wire track is "overdilated" by the
7 French dilator, the 6 French dilator and cannula
sheath are assembled and placed over the guide wire.
The 6 French dilator and cannula are advanced carefully
into the subarachnoid space until the opening tip of
~ ultSh~ RUL~26)
CA 02223246 1997-12-02
WO ~C/403S9 PCT~US96/09629
-- 51 --
the cannula is positioned 7 cm within the space. As
with the 7 French dilator, the assembled 6 French
t dilator and cannula are directed by the wire within the
lumen of the dilator. Position within the subarachnoid
s space is determined by premeasuring the device and is
grossly confirmed by fluoroscopy. Great care is taken
with manipulation of the dilators and cannula within
the subarachnoid space to avoid misdirection and
possible neurologic injury.
o When appropriate positioning of the cannula
is assured, the guide wire and the 6 French dilator are
gently removed from the lumen of the cannula in
sequence. Depending on the patient's position on the
operating table, CSF flow through the cannula at this
point should be noticeable and may be very brisk,
requiring capping the cannula or very prompt placement
of the capsule implant in order to prevent excessive
CSF.
The encapsulated (transformed cells) is
provided in a sterile, double envelope container,
bathed in transport medium, and fully assembled
including a tubular sillcone tether. Prior to
implantation through the cannula and into the
subarachnoid space, the capsule is transferred to the
insertion kit tray where it is positioned in a location
that allowed the capsule to be maintained in transport
medium while it is grossly examined for damage or major
defects, and while the silicone tether is trimmed,
adjusting its length to the pusher and removing the
hemaclip~ that plugs its external end.
The tether portion of the capsule is mounted
onto the stainless steel pusher by inserting the small
SUllSIllul~ SH~ RU1~26)
CA 02223246 l997-l2-02
W O 96/40959 PCT~US96/09629
- 52 -
diameter wire portion of the pusher as the membrane
portion of the device is carefully introduced into the
cannula. The capsule is advanced until the tip of the
membrane reaches a point that is 2-10 mm within the
cranial tip of the cannula in the subarachnoid space.
This placement is achieved by premeasuring the cannula
and the capsule-tether-pusher assembly, and it assures
that the membrane portion of the capsule is protected
by the cannula for the entire time that it is being
o advanced into position.
After the capsule is positioned within the
cannula, the pusher is used to hold the capsule in
position (without advancing or withdrawing) in the
subarachnoid space while the cannula is completely
withdrawn from over the capsule and pusher. The pusher
is then removed from the capsule by sliding its wire
portion out of the silicone tether. Using this method
the final placement of the capsule is such that the 5
cm long membrane portion of the device lay entirely
within the CSF containing subarachnoid space ventral to
the cauda equina. It is anchored at its caudal end by
a roughly 1-2 cm length of silicone tether that runs
within the subarachnoid space before the tether exits
through the dura and ligamentum flavum. The tether
continues externally from this level through the
paraspinous muscle and emerges from the lumbodorsal
fascia leaving generally 10-12 cm of free tether
material that is available for securing the device.
CSF leakage is min;m; zed by injecting fibrin
glue (Tissel~) into the track occupied by the tether in
the paraspinous muscle, and by firmly closing the
superficial fascial opening of the track with a purse-
SIJBSlllUlkSIlttl (I~UIE26)
CA 02223246 1997-12-02
W O ~G/~O~S~ PCT~US96/09629
- 53 -
string suture. The free end of the tether is then
anchored with non-absorbable suture and completely
covered with a 2 layer closure of the skin and
subcutaneous tissue.
The patient is then transferred to the
neurosurgical recovery area and kept at strict bed
rest, recumbent, for 24 hours postoperatively.
Antibiotic prophylaxis is also continued for 24 hours
following the implantation procedure.
Se~uences
The following is a summary of the sequences
set forth in the Sequence Listing:
SEQ ID NO:1 -- DNA sequence of oligo oCNTF-003
SEQ ID NO:2 -- DNA sequence of oligo oIgSP-018
SEQ ID NO:3 -- DNA sequence of IgSP-hPOMC fusion
SEQ ID NO:4 -- DNA sequence of IgSP-hPOMC-~ACTH fusion
SEQ ID NO:5 -- DNA sequence of oligo orTH-052
SEQ ID NO:6 -- DNA sequence of oligo orTH-053
SEQ ID NO:7 -- DNA sequence of oligo orTH-054
SEQ ID NO:8 -- DNA sequence of oligo orTH-078
SEQ ID NO:9 -- DNA sequence of oligo oIRES-057
SEQ ID NO:10 -- DNA sequence of oligo obDBH-065
SEQ ID NO:11 -- DNA sequence of oligo oIRES-bDBH-064
SEQ ID NO:12 -- DNA sequence of oligo oIRES-bDBH-066
SEQ ID NO:13 -- DNA sequence of oligo oIRE-068
SEQ ID NO:14 -- DNA sequence of oligo orTH~-073
SEQ ID NO:15 -- DNA sequence of oligo ohPOMC-IRES-069
SEQ ID NO:16 -- DNA sequence of rTH~1-155
SEQ ID NO:17 -- DNA sequence of rTH~+KS
SEQ ID NO:18 -- DNA sequence of rTH~-IRES-bDBH
SEQ ID NO:19 -- DNA sequence of rTH~KS-IRES-bDBH
SUB~I~lUlt Shttl (RU~ 26)
CA 02223246 1997-12-02
WO ~G/40~5~ PCTrUS96/09629
SEQ ID NO:20 -- DNA sequence of oligo ohPOMC-IRES-070
SEQ ID NO:21 -- DNA sequence of oligo oIRES-rTHa-071
SEQ ID NO:22 -- DNA sequence of oligo orIRES-rTHa-072
SEQ ID NO:23 -- DNA sequence of IgSP-hPOMCaACTH-IRES-
rTH~-IRES-bDBH-068 fusion
SEQ ID NO:24 -- DNA sequence oIRES-074
SEQ ID NO:25 -- DNA sequence of oligo oZeocin-077
SEQ ID NO:26 -- DNA sequence of oligo oIRES-Zeocin-075
SEQ ID NO:27 -- DNA sequence of oligo oIRES-Zeocin-076
SEQ ID NO:28 -- DNA sequence IgSP-hPOMC~ACTH-IRES-rTHa
-IRES-bDBH-IRES-Zeocin-073
SEQ ID NO:29 -- DNA sequence of proA+KS
SEQ ID NO:30 -- DNA sequence of IRES fragment
Deposits
RINa/ProA/POMC/TH-IRES-DBH ceLls, transformed
to produce a catecholamine, an enkephalin and an
endorphin, as described above in the example (and in
Table 2), named RINa/ProA/P030/P088, have been
deposited. The deposit was made in accordance with the
Budapest Treaty and was deposited at the American Type
Culture Collection, Rockville, Maryland, U.S.A. on June
7, 1995. The deposit receive~ accession number
CRL 11921.
The foregoing description has been for the
purpose of illustration and description only. This
description is not intended to limit the invention to
the precise form exemplified. It is intended that the
scope of the invention be defined by the claims
appended hereto.
SUBSlllUl~llt~l (RULE26)
CA 02223246 l997-l2-02
w o 96/40959 PCT~US96/09629
- 55 -
SE~ LIS~
.
(i) ~E~LIC~NT: Cyt~Thpr~r~ltir~r Inc. (Ebr ~lr?ncPs of ~ll
~P~i~r~t~i states excqpt US)
S~u ~ g (Ebr Fllr~s~ Of l~i ~ly)
J~1 Say~Dff (Ebr ~l~?~qP~ of US anly)
~ii) IITLE OF DN~nlCN: E~IN ~ ~ LINE
(iii) N~MEER OF 5B1E~ES: 30
(iv) ~LKK~U~N~ ALLX~
~: James F. Haley, Jr./I~or R. F.lrifi
FISH & NE~VE
(B) ~ 1251 Ave. of ~e p~ri~.c
(C) CITY: N~w York
(D) SI~IE: New York
(E) CCUNDgY: US~
(F) ZIP: 10020-1104
(v) C~MEUIER ~L~Y~ E~RM:
~ MEDIUM TYFE: EloFpy disk
(B) C~: ~M EC ~tihl P
~C) ~'1~ SY~;I~I: FC--m~;/~i--~i
(D) SOFT~KE: F~t~ntTn Release #1.0, Version #1.30
(vi) CUggENT APPLICPIION n~
~ ~EEIICPIION NUM8ER:
(B) FILIN~ r~IE:
(C) CL~
(vii) FRIOR AEP$ICPIION n~
~ ~EEIIC~IICN NUMEER: US 08/481,917
~) FILING ~IE: 07-JUNE-1995
(viii) ATIngNEY/PCENT IN~ CN:
~ N~ME: Elrifi, Ivor R
(B) K~ ~lON NUMEER: 39,529
(C) FEFa~rE~DCCKET NUM3ER: CII-29 CIP FCT
(ix) ISLB32~LIIC~IION IN~Y~D~CN:
Q~ ~E: 212 596-9000
(B) IELEE~X: 212 59~-9090
S~ SH~Er (RULE 26)
CA 02223246 l997-l2-02
W 096/40959 PCTAJS96/09629
(2) IN~ 3ICN ~UK SEQ ID N~
(i) SE2UENCE CH~R~U~
IENGDH: 33 ~ase ~;r~
~) TYEE: ~ ~l~;r acid
(C) 51~: sin~le
(D) I~GY: lir~r
(;;;) ~JIHI~.1'1~;91~: ~
(iv) ~NII-SENSE: N~
(vii) I~D~E SC~ROE:
~) CLCNE: oCNTF-003
(Xi) SE~ENCE ~ E~CN: SEQ ID N~:l:
~ uu~ C~n~ TlY~E~G~EC T~T 33
(2) IN~IP=~CN ~UK S~Q ID N~:2:
LEN~rH: 23 kase ~ S
~) TYEE: ~ ~l~ir 2Cid
(C) SI~S: sir~le
(D) T~ Y: lin32r
(ii) ~n~ : TYE~E: c~
(iv) ~Nll-SE~;E: ND
qO
(vii) l~lEDIP~E SalRCE:
(B) CLLNE: oIgSP 018
(xi) SEl~'CE L~l~llU~: SE;Q ID NC):2:
lllUJU~2~PP331;YIT OE 23
SUBSlllu~ SHEr (RlllE26)
CA 02223246 l997-l2-02
W O 9Gt4~959 PCTAJS96/09629
(2) IN~r~3ICN E~R 5EQ ID N~:3:
(i) S~EN~E CH~Rh-~L~KL~L~
~ IENErH: 849 base Fairs
(C) S~S: sir~e
(D) IW~Y: lir~r
(vii) ~ ~E:
~) CICNE: I
(iX) ~lUK~:
~ NPMEJKEY: 5'UIR
(B) Im~LlCN: 1..43
(iX) I~.UKL:
~ N~MEJKEY: exon
(B) Im~LlCN: 44 . . 89
~ ~: in~
3 0 (B) Im~LlCN: 90 . .168
(ix) ~;:
~ N~: 3 'UlR
(B) Im~lCN: 807 . . 849
(iX) ~LUK~;:
N~: misc f~h lre
(B) ~ICN: 43..186
(D) aI~ ~CN: /~ "Ig5p regicn"
(iX) I~UK~;:
(A~ N~: misc f~h lre
(B) ID~ICN: 187 . . 806
(D) aI~ ~ICN: /~rc~ '~
(xi) SE~E L~Lt'll~: ~ ID N~:3:
SllBSI~lult SHEEr (RULE 26~
CA 02223246 1997-12-02
WO 9GMO9S9 PCTAUS96/09629
- 58 -
r_rr~lil C~C3~C~hG~ G~OG~ T GhU~j1UL'11'~ G5PJ~1UW1 60
L~lUllUllU Ul~Hlu~L~G T3iL~hG~G~ ~L~lU CC2~GIC~}~ APCr~Y{~ 1Z0
mc~l~Pcr ~l~l~ffLHGr G3~YD~2CT llr~ TDC~CE~ GD~Y~ 180
~IllW l~ 2~ LJhC GY3~Y~3C IGK~I~G~ PG I~LJl~ 240
~U ~ llUL~ c~aa~E~ lr~ Y~Y~G C~3~Y~Er~
ChGG3~GY~G333~E~C ~DCPLnG~ C1~4~CI~ ~ o~GY~}nG 360
~UU~lfG~ c3a~Y~r~ GGn3l~iCrl~}~ul~lj CGh~i~PPG C~LlU L~Cr 420
~L~ Ha~ wlr~ lriiG3C~Y~rEG I33~G2~r~r-rlr~ GnGYY~T 480
}Co~hOEG C31~YE2C Gh~l~'-r~"iA~L~1'1UL~ C=~Y~T~ ~PGh3~CC 540
20 IG2CnG3~C~ G~}Y~rGG Gh3a~ G ~U~ll r ~E~ r~ Ghl3~E~PG 600
LL~ EC CGY3~-E2G C~CY~rI~C lWlWL~i~ CG Y2PY2P.G Ghl~YEECC 660
CCEYPii~T G~h3~Y~qC u~ r~ r r~ 1 ~A G3~}~Y~EC ~ r-rr~ 720
1 wr,iJ lr_ CGY~YY~CC Ch~h3ECCCC Ta3~YEiT Grn~Y~P.C WL~ 780
~L~POE~ C~h~YEEC Gh~YEEC ~G~-rr~ri~rr CC~ PC LLlU~ll~rj 840
30 Ghi~E2C 849
(2) D~ ICN E~R 5EQ ID N~:4:
(i) S~: C~i:
U~ IEN~IH: 525 kase Fairs
~) TYFE: ~ acid
(C) SI~~S: single
(D) IWC~GY: lir~r
4 0 ~ii) ~ N l r ~ ' T5~
(iv) ANTI-SENSE: N~
(vii) ~D~ SaFOE:
~III~Sh~tl (RULE26)
CA 02223246 1997-12-02
W O 96/40959 PCTAUS96/09629
- 59 -
~) CIoNE: IgSP-hE~ C~H
N~ME/KEY: 5'UIR
~) LCC~lIoN: 1.. 43
N~: ex~
~ ) LLCPIICN: 44..89
N~: inbm
~ ) IDC~IICN: 90..168
(iX) ~h~UK~:
N~Y: ~1
~ ) IDCPIICN: 169..482
~ NPME~KEY: 3'UmR
~ ) LLCPIICN: 483..525
~ NPME/KEY: ~ feahure
~) LLCPIICN: 44.... 188
(D) CIHER DN~}~ICN: /rnY~r~= IgSP LegiQn
(ix) ~;:
~ NPMEJKEY: misc f~hlne
~3) L~C~IICN: 189.. .482
(D) oIHER D~ ICN: /rr~t~ M~ regicn
(xi) SE~UENCE L~}l~llU~: SEQ ID N~:4:
GL~lUU~uil CP3nCC~;G~ Gn3G~3~T GPL~1UL11 P~PIG2D~r ~ ;1 60
1H1~11~11~ ~l~tl~L~G ~33r~Y~G~ l~ CC~Gro~C~Cr~YEG 120
40 13~ YT cna~}Y~Y~ T ll~L-lll~l Tn~y~yE~ lU~ 180
~lll~L~ ~ll~LLL l~ G~Gr~P~G~ G3~Y~PC ~a3~cP3~ ~luul~*G~ 240
G~X1~1L OEi~ li~ 1~ L W iXl ~ G1 ~ ~! ~ C CI OE ~ 300
~L_W lWl (~ fG APGYY~YG P~ U_l~ C~3~X~2G ChLlluu; l 360
SU~ LE 26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 60 -
G33~r~c A~ErEr~PCG ~Liilll~l G~3~n~rPG PPE2E3~G~ 420
~ [1 I T ~ ~11~;1' G~l~ll~; iY ~P ~ ~ ~ G} ~ 480
GbLhL-r~AG~ Gi~4r~l~ C33~YiPL~ IOE~C 525
(2) IN~}~ CN ~UK SE2 ID N~:5:
~V IENGrH: 30 kase ~ S
~) TYPE: ~ ~l~ir ~id
(C) 5}~: single
(D) IW~GY: lin~r
(ii) ~I N
(iv) ~--~SE:
~) CloNE: orl~-052
~Xi) ~E~UEN~E rE3~UE~lCN: q ID N~:5:
occ~rllG Ch L~l~LL~l~r~ncP33G 30
(2) INB I~ICN FCR SE2 ID ND:6:
(i) SE~ E CHP~LL~L1~:;:
IE~I: 30 k~se pairs
(B) TYEE: ~ ~l~ir acid
(C) SI~IE33: single
(D) TC~: 1in~r
(;ii) E~VIH~.I'I(~: N~
(iV) ~II--SE~E: NC)
(vii) l~qED~E: S~RCE:
(B) CL~NE: OrI~OS3
sal~"~k~htt~ ULE2B)
CA 02223246 l997-l2-02
W O 96/40959 PCTAUS96/09629
- 61 -
(xi) S~IEN~E LL~Kl~ C~ ID N~:6:
~1 A~LtllL~G C~KY~PC 30
(2) IN~E~f~ICN EoR SEQ ID N~:7:
~A) ~: 30 ~se ~i r~
o (B) 1~: rn~lPi~ id
(C) Si~: single
(D) l~y: lin~r
(ii) ~ N ~ TYE~: C~.
~) CL~: or~O54
(xi) SBIE~E L~_Xl~ : 5EQ ID N~:7:
C~CP~GCT~lwl~LJLl~ C~T~C~YG~ 30
(2) D~IY$~CN ECR SEQ ID N~:8:
LENGnH: 33 h~.
~) TYF~: ~ ~lPir acid
(C) 51~:35: sir~le
(D) IWCa~Y:
(iv) ANll--S~iE: N~
~vii) I~EDIP~ SaRCE:
(B) CL~NE: orl~O78
lllUlts~ E26)
CA 02223246 1997-12-02
WO 9~/4J359 PCT~US96/09629
- 62 -
(xi) SE~EN~E L~YUKl~l'lU~: SEQ ID ND:8:
~ ~!m~ 'l[_~_T I ' I 1 i; I ~33
(2) IN~P~CN ~UK ~r~ ID ~:9:
~ IENGDH: 30 ~ase
(C) 5~;5: siT~le
(D) ICPo~DGY: linear
(Vii) IMMEDI~IE SCURCE:
~) CILNE: oI~ES-057
(xi) SE~ ~P~CN: 5~ ID ~:9:
~2~Hl~L~ UUJ_l~lUL~'lUJ~TT~T~: 30
(2) DN~ CN FCR CF~ ID N~:10:
(i) g~lEN~E CH~RhLL~KI~
~A) IE~IH: 30 ~ase pairs
(B) TY~E: r~ ~id
(C) SI~SS: single
(D) IW~DGY: linear
(ii) ~1 N ~ Jl ~ E: c~
(i; i ) H~J~HI~'I'I~ ~L,: NC)
4 o (iv) ~l~r-SE~3E: ND
(vii) D~5EDIP~E SaRCE:
(B) CI~E: ~065
~s
(xi) 9~1EN~E LLYUKl~l'lU~: SEQ ID ND:10:
SlllblllUI~ SHEE~ (RUIE26)
CA 02223246 1997-12-02
W O 96/40959 pcT~us96/os62s
~rl~rriCC~CErDs~ ~LLlll ~ 30
(2) DN3~3IoN ~R 5EQ ID N~
~ LENGrH: 30 base palrs
(B) TYE~: rnlrl~ir acid
(C) SIR~ E~S: single
lo (D) ICECa~GY: linear
(iv) ~-SE2~E: ~
~) CLCNE: oIRES-baeH-064
(xi) SE~UENCE ~E3~E~ICN: SEQ ID N~:ll:
25 ~ll~LL~L~Lpl~LHo3G CkIIr~ 30
(2) DN~ CN E~R SEQ ID N~:12:
~ LENGTH: 30 kase
(B) I~E: r~ ;r a~id
(c) sr~ sNEss: sm~le
(D) T~ GY: linear
(ii) M~ ~lrF TYE~: C~
(i;; ) H~IH~ lr
(iv) P~--SE~E: N~
(vii) ~MED~E: SalRCE:
~) CL~NE: oIRES~ 066
(xi) SE~ L~ N: S~Q ID N~):12:
SUB~ ult~hs~l (RlJlE26)
CA 02223246 l997-l2-02
WO 96/40959 PCT/U~~.'09C29
- 64 -
1~ T~ 7 I~L~lWll~ I3l~yy~TT 30
~2) IN~ CN E~R 5EQ ID N~:13:
(i) SB~EN~E CH~R~LL~KL~
o~ LENGrH: 30 kase p~;r~
(B) T~: rn~l~ir a~
(C) S~RYN~INESS: single
(D) ~ LCY: linear
(iv) ~NII-SEN5E: N~
(vii) 1~ ~E:
(B) CC~E~ oIgSP 068
(xi) S~IEN~E L~Y~Kl~ : 5E2 ID N~:13:
~2~ffltlu~ r~lil~ ~o~X~2G 30
(2) IN~P~loN ECR SEQ ID N~:14:
(i) 5B~ a~;:
~ LEN~rH: 25 base p~;r~
OE3) TYFE: ~ ~l~ ~d
(C) S~g~NEIhE~S: single
(D) T~[DGY: lin~r
TYE~E:
(iV) ~ 5E~IE: NC)
(vii) l~!EDIP$E Sa~E:
(B) C~NE: orl~}O73
(xi) 5E~~ L~l'lC~: 5~Q ID N~:14:
A~ ~: C~3;3G 25
SUB~ ultSHEEr(RI~LE26~
=
CA 02223246 l997-l2-02
W O ~ 359 PCTAUS96/09629
- 65 -
(2) DN~IMÇ~lCN EoR ~ ID NO:15:
S ~ IENGDH: 30 kase p~;r~
~B) 1~: r~ ~ir. ~r;d
(C) SDKYNI~NE~S: single
(D) IO~GY: linear
(B) CIoNE: chPCM~-IRES-069
~J~i~7CE~ PG~ l~l~LLLl 30
25 (2) IN~ ICN E~K SE~ ID N~:16:
OE~ LENEIH: 1030 kase ~ s
~B) TYE~:: m~ . acid
(C) S~Y~i~NE~S: sin~le
(D) IO~LCY: linear
/l N ~ TYE~ g~mir)
(iV) .~--g~E: N3
40 ~vii) I~D~E Sa~OE::
~B) C~: ~D
~iX) ~L~;:
OE~ N~Y: 5'UI~R
4 5 ~B) LO~ICN: 1. . 6
SlleSI~lul~ S~kl (RUIE26)
CA 02223246 1997-12-02
WO 96/40959 PCT~US96/09629
- 66 -
o3) ICCPIICN: 7..1017
(iX) ~1~;:
~ N~ME/REY: 3'~IR
~3) ICC~IIoN: 1018..1030
(~i) SEEUEN~E L~iKl~ : ~FQ ID N~:16:
~LlL~ 1 CCCPPGD~ r D3EP~GIG ICPCCP~CDG 60
GT~Y:CPPGr IIG~C~CnG~ IC~GP3~IG cPcrP333aG ~ CCPYG~GqP~ 120
15 CEi CP3oGIC CEPPGi nG2r IECPGPGPrT ~LL'l'lUL~G~ }CPP~CP33G I G~hCCPPrT 180
~LLL~ }Y~PCPCP3C GEPPG~GPrT ~CIPi CI3G~ }L~ rC~ ICTCPLECIG 240
~ }3~3Y~3~ r ~ G~GCP3CTG~ P~Wl'l'lLL~ ~ 300
CEGr2C53~G ~CIP33GY3~ GEPLPYC~IC CCPCP3CI3G ~YEP~EI3DC ~L~L'l'l~'l'l~ 360
}YYi7Y313G~ L~ Gi T3CEP30C (;ll hL'WlULLL CCEDGP~TTT 420
25 ~'lWl~~ LL'l'l~ Wlt:~l'l'l~A TG~GT Pil~U~ 'l~L'lLL'l~A 480
LL'L~3 1~1'L CP~[OEGCC ~ Ll~ CP~T TGG~Y[GT ~L~:il'l~ 540
GCD~ ~ 11~ ~,l lWU~ GF~III~C 11~ lL 1 ( i ~ ~1~ 600
GP~A TT~YW3PLT Cl~TG IPL1W1~1~ CD3D334~TT U~lL'LAl~'l' 660
P~G GG~ ~LllP~r GCDQ~GC 'l~ilW'lLL'Lf~ CGGD~C 720
35 C3~C CCC TG~ ~It~C C~:lllti }~C P~l~IG 780
C Y~CC }~PC ClPaY~T GT~I'ITG TGI~G Cl~C 840
GCCP}Y3~ ~ C~ -LL'lUl' CE3~1~C W~llLl~ TG~[T 900
GP~ G~L'1WJ~L' TLPCl;lPCrG GP~IC P~ (i 1~ ~ILI 1~ 960
G~l~l: P~T GCDL~GIG G03~PC 1~1~ TP~PIG 1020
45 CP~ ICC 1030
(2) 3NE~110N E~ SE~ ID ~):17:
SUBSlllult SHEEI (RUIE26)
CA 02223246 l997-l2-02
W O ~6/409J9 PCTAUS96/09629
- 67 -
IEN3rH: lOY base ~ s
pir a~id
(C) SIR;N~IIE55: single
(D) TOEC~LDGY: linear
(ii) ~/1 N ~ TYE~ ; r.)
(Vii) ~ED~ S~lE~:
~) c[~~: r~
(iX) ~;PiLU~;:
OE~ NPME~KEY: 5'UmR
20. ~) LfCPIICN: 1.. 13
(ix) ~U~;:
o~ N~ME~KEY: excn
~ ) IDCPIICN: 14..1024
2s
(ix) ~;:
OE~ N~ME~KEY: 3'UmR
~) I~CPIICN: 1025..10Y
(Xi) SE~UENoE DE3~ ioN: SEQ ID N~:17:
C~'l~il~; Pm~DGlTl~ PL~'1~3;1~,'1' ~C a~ U }~{ 'I' '1~ ,'1~ 120
WWlf~L~; OEC{ ~ {~ ~l~l lW Pl~l~ Tlm~G~ }~ 180
aY13~ C~ LI, l~ j C~ L C_l~ ~!GI~ C~ 1U~ 300
~l~Y~ ~'1~;1~{{ '1' }~ a)L~3;1~ ~~ ilC~ 360
~ ,l'l~G (~ ~ 'l'lU~'l' C l~~ ~'L~ l l rj 420
SUBSIIlul~ Shttl (RUI E 26)
CA 02223246 1997-12-02
W O 96/40959 PCTAJS96/09629
- 68 -
~LH11~C CD~PGnC3~ 1'G~Ll~l'l~ GYC~ L~ 540
'G~31i~ ll~LLLhGTT C~ EaC}~IG~Z~rIG ~ 600
~U~ G~r G~Y3YY31~}2P~LD~IC C~ PC~lWl'l~ffCTG D3G~F~EG 660
~LLH~ C~G~Y~E~G}G~IGP~GE~~lL~lWl~LA(~ ;I~crD3~YEG 720
10 ~G~lcI-1~ C~ ;1 CbGY3~Y~ IGP3GI~D~L_lll~OC C~3~Pi~ 780
.
~i1~;1l~G ooc~Y~P~G PICPPP3C~Cch~L~l~'l~ I~L'll'l~l~l oDG~Y~lT 840
C~Y~LECC APiGK~G~ Icy3Gp~r~ wLLl~lwL}~cp33Ecc ~l'lW~l~l' 900
G~Y~ PC C33~Y~CPC~l~LLHl'l~ CG~3~EP~ PEirCIC~ o~YnIlYiG 960
20 c~YY~rP~ ~EPIo~ io37
(2) D~l ro~IcN ~ 5E2 ID N~:18:
(i) SBIE~E CH~R~LL~
ov IEN~DH: 3425 h~CP FalrS
(B) TY~: Tll~l ~i l- i lri d
(C) SE~: single
~D) l~f: lin~r
(ii) ~r~F~F~TyEE nN~ (~Pnnmir)
(iv) ~-S~iE:
(vii) ~ED~
(iX) ~A~U~L:
NPME/KEY: 5'UIR
~) LDC~IloN: 1..6
(iX) ~;~;1~;:
~ N~~EY: exr,n
~) ICC~IICN: 7..1017
SUB~ ul~ Sh~t~ llLE 26)
CA 02223246 l997-l2-02
WO 96/409s9 PCTrUS96/09629
- 69 -
~ N~: in~
n3) ILCPIICN: 1018..1617
(iX) ~ UKL:
p3) ILC~IICN: 1618..3411
(ix) ~;:
0 ~ N~ EY: 3'1nER
~3) ICCPIICN: 3412..3425
o~ N~ EY: nL~3c feature
(lB) ICCPIICN: 1025. .1617
(D) CIHER I~E lRVDIICN: /pnp~Y~ k~ sP~ P"
(Xi) SEr~E3~E L~Y~K~ : SEQ ID N~:18:
U,I_I '1- lil l Cm~A ~ilWU~ ~DGI~ ~m~I~ 60
25 ~~L~ l~ CE~YY~nG4r ~P~;Y~YrT ~U ll~L~GT PL~YY~Y~3~ YYI~4YrT 180
~JLLH~ PY~Y~Y~Y3~ CE~YY~YG~rr C~I~YI~13~ }~Yi ~C~ YI3Cn~ 240
P~ JLl~l ~ Y~ L l~LUi~ cPY~YI~na~ Pf~ L~ f~ 300
CrG31Y3I3D~ CCI~YII}Y;~ CE~YlY3:Yr~ C~Y~Y3~3~ }LE~YI~nGD~ ~L~ ll~ 360
35 ~l~LL~ r~ Lll~L~ Wl~lll~ L~ aT ~ L~ 480
G~ CP~iY3~DGr ~EE~Y~Gr PLLL~l~ll~ 540
~ Ll~tLLL~i~ ~Hl l l~L~ L _~G C~Y l~r I3G Y~ l l~L~ I ~ 600
C~YI~YY~Y~ TTC~YYYYY~T CTCCP3~3DG I~LlWll~ C~G~GGP~TT LUU~L'LHl~l' 660
~DYDG C~ WL'l'l:t~lWl' G~iL'lW 'l~ilL'l'lLL'l:A CGGP~rc 720
45 C~ Y~DC ~C ~ Y~Y3G~ GCCDGP 3G~C CG~~UYl l l~ PL~I ~Yi YC h~ PL3~Y3~ ~G D~ 780
CPLrl~ ~ ~CC ~YY~Yn~ Y~C CI PCC~ 3CCT G~GI PCIqTG IG~ YE~G CI IC~G~C 840
SllBSIllutt SHEEr (RUL~ 26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 70 -
OE~P~A C 'L~C LL'l~ 'l' Wlf~lU~ C~ T 900
OE~ oeL'l~:L~L' 1~1~ (~1~ ~a~ C~'l~l'll~ 960
GP~ a~T C~GYrrIG Gnl}Yr~PC T~Jl~LLtl ~3C~D~ 1020
cYmY~U~C~iJ~ ,lUL(I~ oc~YYr~ U~ f~ 1080
10 I~ C;l~ ;IC_~l~ llll~L~oG~IP~1~LL~1~ 1111~ 1140
~r~ cIPYC22CrDC ~ar~ LcClll~L~3a~ ~cn3aY~rc ol~YI~X~C1~20
G~C~3~ }~ ~}~~LirPCEr OE ~Y~ CKI~ A 1380
20 0~C~3C ~L~ Tl3E~'r~GTT GD3~Yi~.G D~Y~E~ CrO~EC 1440
G~Pl~U3CA P~ ~}~ 1 'I~A ~.w~; ~CC ~ ~'L~ 500
3~Kr~CE~ GE~LilU~'ll' 'l'lUL'l'll~ P~AL~CEPrG ~K~ C CK~YYrPIG 1620
~YI~LCG(r~ }~;I ~llULl~hl~ A~L1U~1~ CrG~K~CA ~LlU~;Ll 1680
~ LI CrD~K~C ~LL~ 0~ OI~Y~i~P.C O~n~Y~G 1740
TC~A~CA ICP~L'L~1~L G~Y3~i~CC ~DC~YI~CC PLLlU~lWL G~33~Y~C 1800
~'I~;I~i 'l~'l~l'll~.li (~:L~ilU~ 0~33PLC ~7~C ~ L'llWl~i 1860
C;l~ L'lUl~ CrGK~;A ( ~1:1 1 'I'YC 'l-llW~ill:~ O~EA O~G 1920
CA~!CC Da~CA GOK~IP~C ~L'l'lUl~: G~G GA~AA 1980
40(~ L'lUl'l~ GPl 1~1;1111 G~G Pa~A C~PLL'lU';lU 2040
GA~ O~ WWLA~l~ 'l'lUL'l~ pr } r T } ~,l~; (; I ~ r } ~ I ~ } ~, 2100
D~CA ~W~LL'll' GCK ~.0333G Cl~~G D3~ GA~YC 2160
~EC ( r~ } r, 03~ACACG 03~GG P{~:luu~ om~c '~0
SUBSIIlul~Sh~tl (R~E~6)
CA 02223246 l997-l2-02
W O96/40959 PCT~US96/09629
lULLU~ GI~Y~ C C~IG~Y3~ ~3~EYI~G~ Ca~K3~ G~L~ 2280
( 1 l l r~-r ~ k~LW~ ;lWl~i P~ (-r-r ~ ; 2390
5 t~ P~ 1 r r 1~ L;~ C ~ 2400
~r;l~I{~r ~cm3~ C~ ~1~ (-r~I~rr}~r1: 2460
1~ rl-r-r I ~ r j ~ cm~c C~; Cp~ lr} ~ Wl~ l r-r-r~-ri 2520
r{ r ~r ~ C~llUl~ ~1~ Gl ~ ~ (~1 ~ 2580
r ~ I ~_1 l I j PL~lU,lU~i C~ l I -r r ~ r l -r ' 1~ ~i ~'l'lU~ 2640
15 ~Jl~l~A ~3~4~E~ ULl C P~ I LL~WL C3~311~G 2700
p ~ _llU'3 ~I EG CI~CG OE~ ~1~ ~ r 1: 2760
1 ~ 1~;P ~ ~llW_ Cl ~ C CPalm ~ 1 ~ I_Ii~li~ ~ L:~l~l~; 2820
~Y2~ j CC~Cr}P.CEG C~E~ Y~ GP~H1W1~A ~P{3~Y~Y~ CCPC~PCPEC 2880
~1~ P~G ~-,ll~i ~hl~lWl~l Cll~I ~ C G3~1G 2940
Cl ~ Cll ~ CZ~P ~ A GD~OEC ~ ~ r~i~ l lU 3000
W~tlU_l~Li }i3~ i~3IG~G CG3~Y~r GI3CY~rDCT PCrrrCPE~C G~PCC~CGPG 3060
Wl1 ~ ( ~ Wl~ A(ll Ir~-r 'l'lU CI~G~ PL'1'1U~'1' ~EG 3120
TIW~G ~GI~I~ aLLl~ lL CP ~ lW~i ~ 11 ~ (illlW lUL 3180
~lWLLl~f~lWll~ CrrCrPLC~G CICPY33~CC ~ YX33CTr CE~PYIr~D~ 3240
35 1U~L~ GCP~[C ( W;~1U U~11U~; G3~ CEECP.GCCC 3300
(~r~ ~ lWl,lu;~i Gr1~G CCCDLm~C P~I~~C CPL~POEr 3360
CP~.GCCCCG ( I ri~ UJ11jL1~ P~GI~G G~CDG ~ 1;~ 3420
G ~ 3425
(2) 11~ E~ g~ ID ~ 9:
~i) 5B;~E C~ERIS~C~j:
IE~I: 3432 ~CQ ~;~
(B) IYFE: ~ ~l~i~ ~id
Sll~lllult S~tetl ~RULE 26)
CA 02223246 1997-12-02
WO 9C/~333 PCT/U~,G/09G29
(C) S~i: single
(D) lWCa~ i 7'3ear
(il i ) H~_1lHI~I'IC ~L~: N~ )
(iv) ~--SE~;E: N~
(vii) ~ ~~:
(B) a~~: ~_~j-~H
~A) N~: 5'UIR
(B) ID~ICN: 1.~13
(iX) ~L~;:
~ N~Y: ~n
(B) Im~lCN: 14.. 1024
(ix) EEP~RE:
~ NPM~: intr~n
(B) L~LICN: lOQ5..1624
(ix) ~E~:
N~: ex~n
~B) Im~ICN: 1625..3418
~ix) ~L~;:
~ N~: 3'UIR
(B) LC~ICN: 3419..3432
~Al .~Y: nisc f~tL~re
~B) Im~ICN: 1032..1624
~xi) SE~ ~Lt'llC~: Sl~ ID 7,~ ):19:
P~lWl~ Cl'lWl'lW_; 1~22?~ ~ }~1~ 60
ultSHEr ~RIIIE26)
CA 02223246 l997-l2-02
WO 96/40959 PCT/US96/09629
~IOE~; ~I~;lr~ I Am~ CPL~1WA (~ P~ 1;1~.1~11. 360
~ r 'l~ CWY~ P~l~ ~ 'lWl'l~IG ~ 660
iLAZ~A CPL~ l-r~ p~P~ Tl~ ~'l~l~il' Cl~ 720
~Pill~l~L ~ I~ l~'l~'lWL ~ C~l'l~ ltiL 900
(~1~ C~ ~1~ (I3~CI~ }~1~ a~l~ 960
~ lU ll~i (1~1~ ~ CZ~ I r ~ I oea~CI~ ~l~L'L~ 1020
~ lil'l'l~L'LA l~T~ ~ 'lW~il~,l'l'L 1140
ll~l:l~l~ I~A ~ Gl~ GI~G C~L~11U-1~ 1 1260
~P~ CPPLl~l~-l~l P~l~ ~ (~PmII~ 1320
k~ ,l (~ CmY~I~ l~C Cl~DPOE 1380
(~W~ ~G~ T~ (~ (~G~ ~:1~1~_1~: 1440
C~ 1~ C;~l~ ~Li Gl~LL~:~'l ~iL~l~l~ 1500
'l~l~_l~i CL1~_W1~ ~ LlL~G I~ IW~ 1560
i~ Pm~ WlWl'l'l'l~; ClT~A (}~ P1~'1'1~L:P~ 1620
ul~SHEEl (~IIIE26)
CA 02223246 l997-l2-02
WO 96/409S9 PCTrUS96/09629
- 74 -
PP~C~D3IK~ GB~Ir~r~ l~T~ l~ll U~lWl W ~ ~'lW1~L1 7 C~C~3~{E~ 1680
~lU~ LL Gnl~Y~C~C U_llU~L_ll o5~ULLL cn3~3~n~ ~33~ T 1740
7~ GE~Y:YDC~ ~LL~l~L~L~ GGKGY~ ~cr~P~ lULlWl~L~ 1800
~H~Ll ~ jr-t~ Hl GD13~}E~ G33~Y~ P~PP13C~G~ 1860
Ull~Til~ Ul~wLH-IG}c~iE~cEG CEn:~i~TT ~1~W_L GG43~CC~ 1920
G~hi~ G G~l~YI~ qCOChaC~ GG~r~YrPG ~ll~l~U~j C~C~KiPC 1980
WLLLllU~ ~LLl~l~a~ CC~CEK:r~ 2040
~LlU~lU~G G~O~CnG ~}4I~ET ~L~LY1l~ CD3~Y3~YC ULLlt'l~;lU 2100
GC~}Y~CC ~D~W~Y~ ~L~Lll~L~ G~ ~j C~GY3~ t ~. 2160
GII~GG~TC Cn~Y~rEG ULLlULLLLL G~4~YIiCC ~IP~3GYG~ 0
CEhJLlU_l~ P~LI ~ L-LI:pGcY3sIpc GT~C~GC T~3G~}~CG P~LlLLL~i~ 2280
U~L-llUlL 033~rr2C~ 'lWl~tl~'LA O~Y3I~TC G~YIIPEG G~YYI~GEC 2340
~ ~C C~G~I~PGG 'lT-l-lu~GlG 1_1~ L1}l I~G TD~GhGhOC~ TO~3IPrlT 2400
G~-LI~}{~I~ T;OG~CQO~GY~rCC G~Y3IECTC ~PL~ ~LC GD~hI~CT 2460
30 (~ }ir l~ , O~Y~CTT TE~P~IC~ G43~YY~PG U~LlU~U~ll' 2520
I } ~ I L ~LlULlU_~ ~Y~-l lUlU~7 CCD33Wr~T C~CErIPC~rIIP~laGT 2580
G~yypfrC ~ IL~Tf~-T ULlU~l~' I 11~l ~ jI;I~C TPCPCEE~TG C~L1l~ T~ T- 2640
CrTO~PCECG wL~ L7pr~ G31G~rPCG UUUil~Y1U~ U~U~l~L 2700
G ~ OECG ~:l lWlU_ T~ CI13~DC P~GI~C P~ Ll~Ll' 2760
40 C~ .11U;~L1~ T;~C PL~Q ~IL~ ~A 2820
~;I~};I~YC~71~ ~ Ga3Y~CCG G~Y~YP~G PUIC~GPhG~ G~GP~Dh0C~ 2880
CDY~h311C~ CYCr~IPGG P~H1U~i~ GrDG~Y~PG ~1W1~1~1~ To~43II~G 2940
PÇPYL71~L1L ~CPCCTCTT G~Y~PLW~ CP~3~Y-PC ~{EY3i~EG CCAn~rr. 3000
Sh~tl (RU1~26)
CA 02223246 l997-l2-02
W O96/40959 PCTAJs96/09629
(}I~I-lr~ HG~ ~l~'l~LJl CPPLL~1~1~ C~C~Y~C~ C~G~}YEC~ 3060
GCn~}YiTC ~3~K~CiG c~an3~{~ 1~ 1'1~1~ CKGYK~CT l~L~LL'lWL 3120
5 G9Y3Y~IC}~iPGCEP3GP~C3CDG~PC(~ ~G ~L~l~l~l~L Cn}K~P~T 3180
'l~L~l~L~l~ cn~ T C~T~LO~ CGP~'l~Ll~ A~ ;1'2~L'1'1~ 3240
~LLtl~l~L A3C~bCT3C~C~ U~L TDI~K~E~GPGr3GYD~G 3300
G~ C~D3Y~G ~3n~Y{~T GG4K}K{~PYCCCq~Pir GD~GY~I~G 3360
CCK133~PG ~}~ C~3~ C~ G~n}~YP~C ~1 ~ P233~ C 3420
15 (ilrl-r}l}~; GC 3432
(2) D~ CN ECR SEQ ID N~:20:
o~ LENGDH: 30 base Fairs
(C) S~33: s~yle
(D) I~GY: lin~r
(ii) ~r~ F TyEE C
(iv) ~ SE~:
(vii) 'i~D'i~ Sa~E:
~) CLLNE: ohEoMC-~gES-070
(xi) SB;~ E I~lPlICN: SE~ 'iD N~:20:
A~AGC ~{ } ~ l~ 30
40 (2) D~E~CN E~R ~F~ 'iD ~D:21:
(i) SE~E C~RPL~-ll~:
W IE~H: 30 ~se F~is
(C) S~gYN}INES3: single
(D) ~g~I~Y: linear
SU~ u~ tl (RULE26)
CA 02223246 l997-l2-02
WO 9C/40~5~
- -- PCT~US96/09629
- 76 -
r~F~F~TyEE: d3N~
(iV) }~
(vii) ~EDIP~ ~:
(B) CL~NE: oIgE~i-rl~071
(Xi) SBIE~E rE3~E~oN: SEQ ID N~:21:
~PP3CP3E3G ~LL~lWl'l~ I33}YY~IT 30
(2) I~ CN ECR SEQ ID N~:22:
(i) SEI~ C~I~CS:
a~) I~I~I: 30 ~se ~s
2 0 (B) TYE~: m ~l ~; ~ acid
(C) Sl~S: single
(D) IQ~DGY: linear
(ii) ~/1 N ~r ~
IHI-, I I C ~,
(iv) P~-SE~iE:
(V~ i ) ~ Sa~OE:
~) CICNE: oIgES-rTHD-072
(Xl) SE4~ENCE LkYUKl~llCN: SEQ ID N~:22:
C ll~PA C ~LWlW_: C lt~l'lW~; 30
(2) ll~llCN E~ SB2 I~ N~):23:
(i) SE~E OEF~CS:
~ LENEDH: 4499 ~ase p~jrS
(B) T~: r~ ir a~id
(C) SnR~N~IIESS: single
(D) TCEoa~GY: linear
r F TYE~: ~. (g~c)
SUB~ Ult~EE~ (RIJI~2~)
CA 02223246 l997-l2-02
W O 9~/40959 PCT~US96/09629
(vii) ~ED~ ~~:
(B) CICNE: Fcmc-thrdbh fusicn
1 o (ix)
OE~ N~MEtKEy: 5'UmR
(B) L3~1CN: 1..43
(ix) EE~:
~ N~ME~KEY: exnn
(B) LLCPIICN: 44..89
(iX) ~L:
OE~ N~ME/KEY: intr~n
(B) ~X~ICN: 90.. 168
(ix) ~;:
(B) ~X~ICN: 169..482
2s
(ix) ~u~;:
OE~ NPME/KEY: intron
(B) L3~1CN: 483..1080
~iX) 1-~;:
~ NP~: ~n
(B) LX~ICN: 1081..2091
(ix) ~
3s OE~ N~ME/KEY: intran
~) L~CPIICN: 2092.. 2691
(ix) EE~:
OE~ N~ME/KEY: exan
(B) LCCPIICN: 2692. 4485
(ix) ~U~;:
OE~ N~ME~KEY: 3'U~R
(B) L~CPIICN: 4486..4499
.
(xi) S~IE~E DE3~E~ICN: SEQ ID N~:23:
SUBSIllultSllEE~ (~UIE26)
CA 02223246 l997-l2-02
W O ~6/4~359 PCT/U~,GI'~3G29
- 78 -
~ ChC3:~;G~ ~'l~Ll~l'G~ U'11 }CPPIGPD~r GC~ ;1' 60
~L~lCll~llC Cl~t~LHG ~33rE~G~ ~l~ CCPhGDC~}~h~r~YEG 120
~ CT CTa~YJG~C~HCT'll~LLlll~l TD~K~{i~ Gn~4~1~E~ 180
Cl'l~ C~~ GY3~G~ GB3Y3~PC IC3~CPG~3~c1~ f~ 240
10 G2~H1C;JLUL oG~ G Gn3~Y~{E~ o~4~3rI2c CD3~Y~ 300
~l'll~,l~il'[~ ~G A~YP2~ G ~i~III'1'~ Chi~ HG ChL'll~U~'l' 360
15 }PC~E~IPOG ~L~J'~ GhLL'luL~H~ PPLPCD~G~ 42
~rl~LI~I[l;l' GP~ PP330G~ Ic~n~ ~G~A C31C~ ~PG P~G 331~Y~ 480
GP~G ~ I}t~ U'lULL'lW WL G~!ACG 1~ l~ i 540
20 GP~POEC ~}il~ill} lil-l 'lCil~l;L~lf~Lti 'll~A~L'l'l'lU;~ Uf~L~:LlWL(ilU'l'l'l'lWL 600
C,l~l~; Ul~XPP~C ~I~}i L I 'llilC, Ti~LEA ~ L'lC_L'l~G W~ilUl'llUL 660
~!L1C_1U~ ~tlA PL~1UW11~ ~;LC~1U~1~ P~hT 'lUL'lt'l W A 720
GL'1'1C'1'1 ~ GP~AC G~I~ECG PLLL'111~1;~ G3G~A CG~ T 780
G~T 1~ I 11 Il} 11i CGD~PPGCCA CG~.G P IPOEICC ADP~A 840
30 CP~GT GG ~I~,T G~Lj11~l~ ~all~l~ G~I~G ~lUlULlU~ 900
llC~Pa~T G~L~ LL CPI ~ C co~r W~LU1W 960
1 ll}}~ U IIL GaIt3}YP~G CTI~T Gllq~ I~GA G~AA CG~IPOEC 1020
CG~A CGGGGpmCG ~il'l'l'lU'l'l'L GADA~CG ~P13Cr T~CC 1080
A:1W1 ~ L'l' Wll~G ~CG GPA~;DCA PGI~I~CCA CL'lWl~DG 1140
40 ~l~C ul~lc 1~ C~I~D.c ~ll~l CL~GGT GTP~G 1200
C~lu~ l~l~ ~lc~LllL CP~~PGC ~3I~aCC PA~lllU~ 1260
G~CA CP~EA ~l'l~L'LPOG T~3G ~l~l~l~ GCI~OEC 1320
C_l~LL~ W~Ll~J CG~!C CI~IT T~l'ICT G~C 1380
SU~lllult SHEEI (~UIE 26)
~ = ~
CA 02223246 1997-12-02
W O96/40959 . PCT~US96/09629
- 79 -
L~ ~G GK3~}~PG C~D~X~{~G CD3GY~CG lWULL~l'l CTDG~Y~PG 1440
A~ LL~ hYn3~D~ C~l~Y~ L~LLl~ CT~YI~G 1560
c~r~7Y~ CooEE2C~G ~l~LL~ G ~ C AD3~YI~ Ll~C 1620
~ LL~Jll~l~ C~3~Y~T GE~Lll~Ltl ~lt~ :c~y}D~p~ 1680
~AP~ P~L1~1~LHC GGD3~Y~G TD~ EG P~1U~ D3~YYYPG 1740
~ Hi~ ~2P3ECr~ ~G3~YE3 ~ 1'1 CCIICE~K}~ l~L~C 1800
'l~L~ G PÇEPCC~T;~ WlU~Ha~ TTn~YX~PG ~CKCK~YC ~3n3~Y~XC 1860
IKI~YY~ P2PCCIPX~ t~ ;1 ~C 111~ L~ PL23~Tn~ GKI~r2~G 1:920
GD~ G~GC ~1~1U~U CK~ 1U1~1~1~ Gr~G 1980
T2DK~G u~Ll~r ~ 2EC C~ T;~ C~G ZO40
G~G }~2C LL1W~LHC G~G C~ PA~ÇG 2100
2 5 ~ Lr~ LL1~: U11~ 1 IP~ G~C GH~-L11 G3~C 2160
G~C ~ ;l (llUl'l ~ ~ ;lll'llU W~l~lU;~; 2280
P~ P~1~1t,11 G~1W1~j }~CPG 1'1W1U1~ P~-L11U11~ 2340
~A ~l; l ~ I ~; l ~ G2~LL111~ ~ PLmm~.CC Ta~2EG 2400
35 ~ G~ CC P~A GYPa~G C~PP~LEC P~2G 2460
TG~CPCE T~G TG~l lU~L PLl WlU~ }Y PL ICPA~T ~L-LlUl LlU P~LL~L~ll~ 2520
~ PYPhCLEEC T~PPi E2DGC COY}YL~I~ UUl~l~L~ WU~YlUl~'lUl~ }l 2580
U~il~ G~l~'IG TGITl~GICG P~A P~C C~P.CC 2640
PLLLJLRJhL WllllLLl'L TGYWY~CPC G~}Y~.GC TD31C4GW~C C2~G~D33C 2700
45 ~ ~i l~ LJl~llLLl Wl ~lU_l~ t;l~ Lt~ ~C TaCP333CTC ~L-LlULL W~ 2760
GK~Y~rCCT l~LLLllULA L~llr~ G~XX~r~CG G3~ ~G~ ~-Ll~l~LWtj 2820
SII~SIIlu~t SHEEr ~RULE26)
CA 02223246 l997-l2-02
WO 96/40959 PCTAUS96/09629
- 80 -
Ppcp~23~rk~uLuLHGG~ G~OGK~C Tn~GY~ r~ G~nG~y}~T 2880
Wl~l~Ll~l ll~tl~l~ GG411~EG ~P3CD3E9G~}3C~ LIT WlWl~l~ 2940
I3G4C~KC~ G3~YI~E~ CIhLlll~c,~ CD~PnCYG~G333~ 3000
Chl~ CT CnC~GCY3~T~l~i3TTCl~Uil~C AG~3~Y~ AG~4i311~ 3060
0 IPl r~r ~ ~ ~ P~c~r ~ C 31~0
~G~KIr~llULl~ Gh3~3IEC~lUL~JlU~Ll GG~Jl~Ltl~ 3180
P2~PCaDGI~ ~Lll~LffC~C(~ ~G ~ L~G~ D3Cn~ C C~ UUL~ 3240
Ah~ rJ~ C~1313~L~ Cj~lULl~l~ 3300
C I l l li l f~ p~ C 'lliil~ aC GD~OE ~ C 'l'lC T r T T r~. ' 3360
20 CKI~Y~G DG~3PLE~ C~Ul~Wl~: P~rCP33EG~ }Cr~GEC3~r G~n3~4IPC 3420
T~CGC CG~C G~rP3rC CG~l~rPG (~ ; 3480
G~C~ G~ T~PDLI~G~L~L'l~A~C'l'l~ lTI;l~ ff'J~'l~Ll~ }II: 3540
~ lIr~ LL~ LA C~13}Y~G G~Y ~ rC W~LLllW~ UI~}: 3600
'W_~lU_HG~r~ G~2 p 3rD32c TAC~ ~}~CC c2cr; GDG~T A~c~33rEG 3660
30 CG~T u~u~ ccn3Pc~2c ~ l~ ~ :11-1 I{ '11~ 1 3720
~LU~ ~ G~rc GTa~ l~ ~ G~-rc 3780
~ r~ ~ 013~2CrG C~lr~Pc22G TaC~IrPGC'l~LLL'lUUL U~LL'l~HGEG 3840
P3~ C~CP D~T 'lU~LL~lUl~ Gcnl~2{G C~3C~ ~LrG G~CEEPPGGT G~YC2GrG 3900
UlU~LLHG3G ~r~-r~ G~G4~4~C Gn~Y~LEG ~LPP{ChC~ C2G~I~C2C 3960
4 o TD~PG~hG~ lU~l L G~4~4Y ETC ~wlulwu ~{C CE 3G~G~ Wl~Ll ~lU 4020
ALL1U11ULA CK~{~C2C G~ Y~G ~i~ Wl ~ L'l-lU~U 9080
CD3~4~G~ l~LJ~l~A ~'L~l~l~L~C TPC~YlIrC PLP{Ei2{CT GEhj~l~'l~L 4140
~PL~Gn33rG Ta~4113EG Ul'lULlULWC P~G~PLTICC ~-LL'lWl~A Ch33rD~DPC 4200
SUBSII~ult SHEE~ (RIJIE26)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 81 -
IYi~GPYiaYY~ ~3~3~YlCr~ C~ 33~G l~l~l~L_l~ lY~lY~rTT3~ ~1[~4260
CPY:DG:PPYi~ W l ~ lU~ LI~_ll~ CPY~i3I~YGr CE~2~I33~ i I 'I' 4380
C~i,H~ l c~Pi3~n3~ PS~Y3IIC~CC CI3~Y~n33~ CPY~rPY3I~ CE~tlY~3~4440
~ I}~ L cr~Y~ l C~YY~D~hGr CEIi33~YY~ C~ YY~aD~ : 4499
(2) IWE~1l5YrICN E~R CFQ ID ~ 24:
~V IE~I: 30 base ~ir~;
~3) I~Z: ~ Y-l~ir a~id
(C) S~;s: single
(D) IC$X~UX~Y: 1ilY#~r
(iv) ANll-SE~;E: 2
~) CLCNE: olK~-074
(xi) SEÇUENCE L~Kl~llr~: SEQ ID N3:24:
A~rl~-rl~ CUULl~l~LL l~ rllll: 30
(2) ~N~r~CN F~R ~T~ ID N~:25:
(i) SE~CEN~E CHPRh
IENGrH: 30 kase p~ir~
~) TYEE: ~ ~l~ir acid
(C) SD~3N~INE55: single
4 o (D) T~DGY: }r
(iv) ~-g~;E:
SUB~I~Iult S~t~ U~E 26)
CA 02223246 1997-12-02
W O 9C/~J~3, PCT~US96/09629
- 82 -
(vii) ~D~E~
~) CICNE: ~7Fn~in,077
(xi) SE~UEN~E L~Y~Kl~l'l~: SE~ ID N~:25:
PY~CD~Gr Ch~l~Ll~l ~L'l~LL~ 30
(2) I~ Ç~CN E~R x~ ID ND:26:
LENGrH: 30 ~ase r~;r~
(C) 5~5: single
(D) IC~Y: linear
(ii) ~ N ~-1 1 Jl ~' TYE~ cCN;~.
(i i i ) H~Y~I1~-1'1~ ~L,: ~
(iv) ~-~E ~
(Vli) I~EDIP~E: SaKE:
~) CICNE: OI~ES-Z~xin~075
(xi) g~lEN~E rE3$UE~CN: ~n rD N~:26:
GaDG~YI~ ~LL~lWl'l~ ~33CYY~T 30
(2) IN~1~$3ICN ~R ~r1ID N~:27:
(i) gBlEN~E C~R~L~
IENE~H: 30 kase Fairs
~) TYEE: ~ ~l~ir acld
(C) SE~S: single
(D) IWC~Y: lin~r
(iv) l~Nll--SE~iE: ND
SUBSulul~Sh~tl (RIIIE26)
CA 02223246 1997-12-02
W O 96/40959 PCTAUS96/09629
- 83 -
(B) CICNE: olK~-Z~xinrO76
(Xl) SB;aE~ ~ll(~: S!~ ID ~:27:
~'l'lW~ ~l;~L~ Grl~m~ 30
(2) D~a~M~ICN ~UK SEQ ID N~:28:
~ LENGIH: 5540 base
(B) TYEE: ~ ~l ~; r ~i d
(C) SD~IINE~S: single
(D) ~ Y: linear
(iv) ~--SE~;E: ~
(vii) l~ED~ Sa~OE:
(B) CLCNE: PQ13~Y~H-IgES-THD-IgES-~8H-IRES-Zeocin
(ix) ~u~;:
NPME~KEY: 5'UmR
(B) I~CPIICN: 1..118
(ix) ~;: .
OE~ N~EY: excn
(B) ILC~IICN: 119164
3s (ix) ~lUP~:
OE~ N~ EY: intr~n
(B) ~o~ICN: 165243
(ix) ~W:
o~ N~ EY: excn
(B) L~C~IICN: 244..557
OE~ N~ME/KEY: in~-
45 (B) LCC~IICN: 558.. 1155
(iX) ~hP~UK~;:
SUB~ ul~Shttl (RIJIE26)
CA 02223246 1997-12-02
WO 96/4W59 PCT~US96/09629
- 84 -
(B) IDC~IICN: 1156..2166
~ N~MEJKEY: intron
(B) LDC~IICN: 2167.. 2766
~ N~: 0~m
(B) LCC~IICN: 2767.. 4560
(iX) ~I~;P;ll~!;:
W ~: inbm
(B) IDC~IICN: 4561..5159
(B) LLC~IICN: 5160..5534
(lX) ~ UK~:
~V N~MEtKEY: 3'UIR
(B) LDC~IICN: 5535..5540
(Xi) 5~ LLj~Kl~l'lU~: 5EQ ID N~:28:
llWLA CnGY~3~EG P~r~cDPG~ rncI~G 60
~r I~: C~l~ C3~ G~I~EG ~ 120
35 CP~A ~ L~; ~ll~L C~GI~ C~G C~ 300
P~rG G}~T (-r-r ~.LI 1~ (-r ~ r r-r 1 1 ~; 360
C~ CI~Y313G ( l~~ c~ G~ CC~ 420
~rG C~r 1~ ;1 11~ 480
C~G C~l m ~,lWl~3~ ~[qaY~ W~L~: P~ 540
45 P~G CGD~LEG C~ W_lW~l U I I I I I ~ I r ~1~ 600
c~ ~ pr i r I 1 ~ ~l l lWW ~PiL~,l ~ L 1~ 660
S~IIIUIE SHEr ~tUlE 26)
CA 02223246 1997-12-02
WO 96/40959 PCT/U:,r~'09G29
-- 85 --
'l'lW~l~_lL 111~ G~ r-r l~ ,ll ~ 720
~illU l~ I~l~ T~P~II~I~ ~ ~G 840
~ r I r-r r ~ I~D 2:~ 900
10 C~ C~4~ ~ ~0
~3;1~1~1 GIt~ P~l~ ~i ~:lWl~A (~ 1020
P~I~ p( r -r I I U ~ I i ~ P~l~l l l l C~ll~ P~ 1140
~11~ ~LWl ~ r;i-l~ C~P~ ~:[ ~ 1200
20 ~ ~IT l~ltxL CI~I~ IYI~; ~ ,l~C 1260
CP~1~LP~1~; (~; ~L~l~xll ~1~ C~l~ a~ 1320
(~1~ ~l~l~il~ P3~ (~1~ ~ C~i~l' 1380
G~P~;l- ~ ~ ~1 i~ I r-r{i p~. (~lllU~ 1440
( ll~ l~C ~1~ ClPm~ ~ ~1~ (~Wl~l~L 1500
30 ~11~,11~ P~ 1~11~ C~ 1~1~1 PLl~il~ 1560
WI~Ll~ Cl~ ~i;lll~ 111 lW:~i }Y~ 1~1~ lW~ 1740
~ r-r r~ r l~ ~ 1~1~ ~ P~lWll~C l13I~IC 1800
40 (~~ il~AZ~ ~ ~lL~Wlti a~:l~l ~l~llU_l;~C 1860
~: ~CI~ GI~G ~1~ GD~ll-ll~ ~ 1920
~-r I~ l~lT~ Glm~ 1980
11~ ~POE~ ~1~ I~LW 1~ 1~ GK~ ~1~_1 .1 2040
SUBSIllult~n~l (RUIE26)
CA 02223246 l997-l2-02
W O 96/40959 PCTAUS96/09629
- 86 -
GD~YK~13~ P30I~iPC ~Ll~l G~3G~Y3~ PCPY33CD~ C~ L~G 2100
cr-tlt~ [}i ~i-t}~ ~ Ga~Y~ C~YIr~GG Co~ PLT G~~wLL~ll 2160
~L~ti~ P~ t~Ji UUULltlt~JL lt~~ ; C~13~PC ~3333~G~ 0
A~i}~;1~'l~L~lll~il~ L~l ~ TT~3~YIP~ ~ll~LU~l~l 2280
'lllUUL~Hlt~ TG~t~triG~Y~ E~ ULWl~llt_l ~Y3}y~p~ IO~ ET 2340
~lll~LLLlt_ ~3311~G~ PPn3~YYET cn3rE~P~ T~5}PYG~A~4Gr~rT 2400
G~33~YnC Tr~3~YE~ G33G~YrrC 2460
15 oC~ }}~ ~c~ r~ l~A PP~~n~Yr~G I~ PC ALrIECPPPG 2520
Gn3 i~Y~rC CrC~GT3X~ W hGT I3~ 3~Y}GT CPP~ 2S80
T2 ~GCG TATI~PA~-r~ ~ ~iL~AAG3~ ll~l~lW~ 2640
l~l~ltlUi ~-LLlUWl~i C ~ WLlll PL~11;1~111- AGIOE~T A~C 2700
T~ ~ ~ I 111~ G~GG G~lWl l l luLlll~pA A~ ~ iLll ~; 2760
25 ACP~T A033~GC Wl~ ~ lt_ llLLlWl~A lU lWlW~ T~Cl~rPG 2820
~lU L'Ll~ 03~Y~P.G ~Lllwl; Tr~C 03~C CG~YC 2880
CTa~T C~P~T C~iL~ltTT~CP OE ~ T~ ~_lU I~ j 2940
033~p(~ Ll~ U;~ GD{~T G3Y~YECT 3000
G~llWlUi l~iLl~l ~ T~C ti~ CT Tr~YEc CTa~-PC 3060
35 C~EGC AG3r~T G~C~i CP~YC P~-Lllt l~i G3~ G 3120
ACl~PG G3~T ULlW 1~3P~G P~l l l ltj G~l~ cnl~PLr-pc 3180
TPLL1 ~ Ui AG3D~C 031m~rG ~,l~l~t~l To~ ~luu~j 3240
lU~LlU~Gr n~Y~Chc ~ I lt; CD~C Ta ~ T ~{~ , 3300
APf~A T~Yrc ~ t l;lt~t_ G3~C G~ ~ilUl~; 3360
45 C03~mlnC lt~L~.I r r r~ O~CC A031P~I~;T G~IY-PC O~L~LlUUl~ 3420
G~L~1'1U_ UUll/r{C~ ~lU~ 2~ TP~Y~rr~ WEDC~OC~ G33~YYXPG 3480
Sll~lllul~ SHEEr (RUIE 2C)
CA 02223246 1997-12-02
W O 96/40959 PCT~US96/09629
- 87 -
~I}:I~}~ Y~C~IG3~ Wl~l'l~L~7-1~}lilT~ CE~GDC ~ LLLLHC 3540
TD~K~r~ ULl~L~HCr~ CWYGY~P2G C13~Y~rE~ cn~Yr~ 3600
C~ U~l~ }~ o3o~YrEn~ TTT~Y~hC~ C~GY~PG~ A~LL1~L~ 3660
~1'1-17~}~}~ 1~1~ CP~H11'1~1~(1}I,l~f~7 Tl~KCrY~C~ C~hIl~YI~ 3720
GD~ G~}1l~}1~ Ul~ 71'PCI~P33EC'1~}1}_1~}1~. 3780
U~'1'1U t~7 U~l~' ~(~ 'I~}~} ~ I7~ 1' ~ 3840
C~E~h3aYG~ U~L-l'lWl o~ncKIIEc ~Ycn3~Yr~ ACPPG~E~DC o~ }~l~ 3900
Ull~.~ I T~ I i ~1~ (~lLll~L IC~ PoeCE~ . 3960
A~ ~lWl~ ~ G~i l - } l ~ } ~ C~3~ ~Y EE~ C33~Y~Y~G ~ Wl~ c~a 3aY~ ~c 4020
2 0 CPCI~ECC CF~ c~ P~ AwlwlWL: D3I~3IG 4080
~C D33 W~IC Tn3~c PP~G P~GaY-EcT G~IG 4140
C- ~ 'l'lU:~C ~ l Ll ~ C ~ 1~71~; G~C~G D~ CImY~G 4200
C~EC D~G ~7 l l;l~}f'C W l~'l'lW Da3Y~ C,ll ~ Ll~ 4260
~,T D~ (~GlaEC P~ } T U l: AW~,lw~,l C3~G 4320
l'Ll~}TI~II~ 'l~-Ul'l-}f~ C,lU'l'l ~ C C31~3I~C D~T C ~ 'l'lU 4380
C ~ L~.l U ~1 ~ IG, CD~TCC 1~ T}} I T;lW ~11'1 ~ 7 C~T 4440
Ca3~LCCCC Dall~T Wl~,l~; Tn3~EC C }~Ia~ CI13~ECC 4500
P~[C A~ r}} llr ~ GD~II~ l~l~i CD~I~ 4560
klil~}}}l7~jcll}l~ l Ul'lW ~ L GD 3~D OE IPLl~ AW~ll~, 4620
40 P~G ~ }r;lllC71UI;~1~1~71 L~l'll~C (~L~1'1 ~ 7'1~'1'1'1'11~ A 4680
P~EC C333~AFccT ~}} r~ 1 D~l~OEPG C~l'lLLl ~7 Wl~l'l'lW~; 4740
~ l~,lW~ A~Lw~A ~71~,1~1'1~ Alil~l ~ ~ G~T UL'1~1~i~ 4800
C,llLll~; ~KG D31~ Ulll~l~ OE~PC Cm~I~; 4860
SUBSIllul~ SHEEr (RUIE 26)
CA 02223246 1997-12-02
WO 96/40959 PCT/US96/09629
- 88 -
c~r~ 33~ ~Ll~l~-T T~i CP~v~Y31C~ C~ 4 i}~ ~P{P~r~3~ AY~LL~Jl.q~ 4920
}~ 43D~ CX~ G T~ Y~ P{I~4Y~n3~ 4980
5 ~UiLH~ C~Y~}~ ~H~ ~ }Y~YY3G~Y3~ ~L~l~l~L~ 1~ 5040
~ C~ Y~Y~3~ Y~YPGD~ Y~I3Y~ C~l~YYYY~Y~ C~ Y33CCC 5100
cr~l~YY~CY~ crr;~YI3~3~ 'l'l'l'l~J 'l'l'l~i }YYYYY}~ X~Y~YY3~rT C~P{~YY~C~ 5160
I~;;I~5hGrT c~YI~Y~llGo~ ~llUJ1~l~L IC~ ~ } ~ Ii CE~UJl-~L~ G~ ilU~ 5220
~ C cr~ L~ CE;~Y~Fn~ r CE~C~Y~ i 5280
15l~ }~C~PYI~I;Y~ C~PYr~Y3GD~ f~ 5340
I r,l~ {~ }~ YIiY3Cr C~PYI;I~Y~ lWl~ 3~ 5400
'll lilTilT 1 ;~ G~L'1'1UU ~ L'lU Ii (-1-11 I-li 1;'3:1' OEm~ ~EC 5460
( lil~}} ~}} Ij ~Lil'lU LL C '~ I~Dc I lii I Ti~ ~'A L 'l~'lt~ 'l~lu;~ I Ij 5520
P~ CI~G 5540
(2) lNE~LCN E~ SE~ 29:
(i) ~ C~L~L~L1~:
~ IE~I: 829 ~se pairs
(C) S~SS: s~gle
(D) IW[~iY: linear
35 (iii) H~l~t~ l l( -~
(iv) PiNll-SE~E: ~
40 (vii) ~DIP~E: SalK~:
~) c~~: E~;
(ix) ~;:
~ N~: 5'UIR
(B) IDoe~I~: 1.. 16
(ix) ~URE:
SUB~ SHT~ (RUIE 26)
CA 02223246 l997-l2-02
W O 96/40959 PCTrUS96/09629
- 89 -
P3) I~C~IICN: 17..820
o~ N~E/KEY: 3'UIR
~3) ICC~lIoN: 821..829
(Xi) SE~UEN~E LLYUKl~llCN: SEQ ID N~:29:
CCCPP3CTDC GCC~C~P~a~ ~LiJll~Ll G~CP~TTD3C AL11~ L l~ll~Ll~J 60
~ L~HO0~ }~PGC~hG ~ L~L~ CGnGCP3C~ 120
CCG~CrP3DG ~r~ t~}c~cppcTT ~lW ~ G~2PI3EPYr C~G2~G3I~ 180
~Ll~LLll~l CDGPPAPrTT CGEPAYOCDG CPYEED3CDC CDGiP3CD3r C{PPPCCPG~ 240
~ L~3C~ G{PC3CDC~}~PPA~P3C }22CCEGA2G ~ LLh~ll 300
GC~GCC~A~ PLLLH1U~ Lll~ Ll l~Pl~ PG2222I3G~ 360
IG2~L'l'l'LHl' ~LL~ WGC C2L;YY~YYG~ GG3C~2~33~}~AGPICC TCGCCP2ECG 420
~1~ Trcp~A2G~ ~GE2~3C~G~ GGPLEACC-2C l~ '~ P~ LlL~G~ 480
CC~GCrK2A~ GP~L'1'1U1~ A2YY~Y33iG~ CPPCCE2GAG CGTA CCP~CC P{C~EGATGG 540
CPGT~2I~T GA E22G22G TGAiiPPE2G ~ WW~L TrCP~12{~G GC3~P2PCAG 600
}YL~ W~ CDGE2PGDrG }YY~I~YYY3~ G3DGCPG2AG ~ lU~ ~L~l~lL~ G 660
}P{2{~PGGT CG~CCPG2GT WlULtl~ CIhC~GAA~ PlU~G ~'l'l'l~L'l~ 720
3 5 W.~L'l'l'l~iLL G~L~ L'lU'l ~L L'l~ }~ }Y~I1~ API~ICC 780
TGP~YYI~3Y~}PPU{2~CG GP~1'1'LP1' C2G2rTTr~ lUL~ 829
(2) INE~FYPrICN EaR SEQ ID N~:30:
(i) SE~ENCE CH~R~YL~Kl~
IEN~DH: 598 base ~qirc
p3) TYE~E: rn~l~ir aci.d
(C) S~RPNCEINE33: single
(D) TC~oLCGY: lin3ar
(ii) ~ F~lr F, TYE~E: ~ (~li r)
SUBSIIlul~ SHEr (RUIE26)
CA 02223246 l997-l2-02
WO 9G/~C359 PCT~US96/09629
-- 90 --
(iv) .P~-g~E: ~
(vii)
1 o ~ix) ~L~;:
N~: in~
~) ICC~IIoN: 1..598
(Xl) S~IE~E ~ l'lCN: SEQ rD N~:30:
L~LL U~'l~'l~LL'l~ UL~ l'APo~ G G~ nCG CTn~mP~ 60
~ i ~il'll~lU'LA I~n;~TT D~ 'lW WlUlll'~G3G~}~G 120
k-~ r r r lt ~l~; 180
G~C~b3G~ ~3~r~T GT~3Y~ Gn~Y~P~G C~~lluLl~l GG~Y~ T 240
25 ~G~9GYCYY~ C~hJJl~l~L ~G3GYl~TT ~Gi~3~GC ~G4YI~rrC ~ ~C 300
P~ ~1 G{3G~CY~A~ CCC~C3DG~ i 2C CD3~Y~G~ CEC~}Y~rC 360
C~ 3r2CG TDGD32GTDG G~L~ il~ GP~ A~:lWLlW~; CD ~ 420
TD~F~!GG GG~ T~PG GTP~l ~1 (il~LWL~l~ lti"iLLl~i 480
L~ Lll.L~C P;l~l~llli~G T~ P~l~ (~ l l l l~ 540
35 }~ C~ilWllll: cm~ CPL~1~LA P~jL11~C P}~ 598
S~llrulESE~ (RUlE26)
CA 02223246 1997-12-02
W O 96/40959 PCTAUS96/09629
90/1
~ 5Or~genlslllCcTI~2s CIP PCT
rence numbcr
INDICA I IONS RElJ~'rlNG TO A DEPOSITE D MlcRooRGANlsM
(PCr Rul~ 13L is)
A. rhe m--Jc t-c---w ~ c ~ m~ em:d lo .n Ibe ~'
on p~c 54 N~n~ 5 14 ~2 3
1~. ID~r~--~.T~NOFD~:r-)!ilT Funbe~ rc~d n~Gedon~n~
N~m~ o~ orv ~nsu~u~un
American Type Culture Ccllection
Addr~ss o~ d~pos~rv In~ u~on ~ n
12301 Parklawn Drive
Roc~ville, Maryland 20852
United States of America Cell Line, RINa/ProAJ
Identification Reference by DeDositor:P03o/po88
e ~ CDo~ ~on Numb~r
07 June 1995 (07.06.95) CRL 11921
C. AD~ O~ INl)lC,~'rlON~ ~-f~-~ic~ ~Auouedon~n~l_ ~
In recpect of the designation of the EPO, samples of the de-
posited microorganisms will be made available until the pub-
lication of the mention of the grant of the European patent ~r
until the date on ~-~hich the application is re~used or~ithdrawn
or is deemed to be uithdrawn, as provided in Rule ~~(3) o~ the
Implementing Regulations under the EPC only b~ the _ssue of
samDle to an eXDert nominated bv requester (Rule 2~ 4) ~c~
D. DESIGNA'rED ST~.TES FOR WHICH INDICATIONS ARE MADE ~;fll~ ~f.,~
EPO
E. SEP~RATE r ~ OF INDICATIONS ~ ;f ~ .~
~ ~, . "~ - ~O~C e ~ ~. 5 - rL~ ~-__
__ fD_~
For ~nl~ O~c- ~ onl~ Fo~ ~ dr
~Th.~ ~be~ w~ ved ~ b ~ ' ., ' O Thi~ ~he~ w~ reel~ed br Ihe ' E~ on
~J
SUBSTITUTE SHEET (RULE 26)
CA 02223246 1997-12-02
WO 95/433S~ PCTAJS96/09629
90/2
¦ A!~pltc~n~sor~s~nlslllc .. ~ pp~lc~loni~o.
reterencenumi~er CT~/29 CIP PCT ~ l
INDI~ I IONS RElATlNG TO A DEPOSITED MICROORGANISM
(PCrRulc 13bis)
A. Ibc - - -~ m~uc DCI~W rcl~le lo u~ re~er~eu lo ~n Ibe
n ~g~ ~i 4 ~ line 5 i4--23
~ ~CATION ~F Ul l'(~IT Furtber deparrs ~re identified on ~n ~ddi~ul sbc~ ~1
N~me ol depos~urv ms~Uul on
American Type Culture C_.lection
l~ddress ~1 dcoosl~rv Ins~l~u~lon ~ ~n~ o~ ~oa~n~ rAL V)
12301 Parkla~n Drive
Rockville, Maryl~nd 20852
United States of America Cell Line, RINa/ProA/
Identification Reference by De70sitor: P030/P088
i~le I Icpos~ ~clon i~lumbcr
G7 June 1995 (07.06.95) _ CRL 11921
C ADDlTll~NALlNolcArloNs~l~a~eo~AiifAo~pdir~e~ Ibi~i iscontinaedonsnsddi~l~
In respec~ of the de.c~.gnation of Finland, until the
application has been laid open to public inspection by the
Finnish Patent Office, or has been finally decided upon by
the Finnish Patent Office ~Jithout having been laid open to
public inspection, samples of the deposited microorganisms
ill be n~ade available only to an expert in the art.
D DEslGNATED STATES FOR WHICR INDICATIONS ARE MADE ~if~ a~ , r. .
Finland
E SEPARATE ~ ui~h~n~G OF INDICAT ONS /l~o~if~dir~4
Tbe i l~ted helow w~ll be su~n~lled to lhe i - Bure~u i / 5 ~ f ~ r-an
N_ofOq~ ~
- For recc vml! OiTlce use only For BureJu us- ooly
~/is shee~ w~ recelved wl~h lhe ' O Thi~ sieel w~s received by ~he Buresu OiL
A _ officer (~) ~ officer
Su~ snttl (RULE26)
CA 02223246 1997-12-02
W 096/40959 PCTAUS96/09629
90/3
¦Aetpe!re~cntsorr grnlSlilecTI/29 CIP PCT j No.
INDl~-A l IONS REIATING TO A OEPOSITED MICROORGANISM
(PCl Rulc 13bis)
A. Ibc ~ - rrl~uc r~clt~w rcl~e ro ~ne ~ . re~enera ro m Ihe
~np ge 54 line 5 14-23
B.l .~r ~CATION O F Dl~rOSlT l:unher depo-~rs re idemif ed OA sn sddi~ i ~beet a
N~ ne ot depos~u~v ~n~nlul~oo
American Type Culture Collection
Address or dcposn~rv Ins:llunon ~ A~pas~ r~
12301 Parkla~ln Drive
Rockville, Maryland 20~52
United States of America Cell Line, RINa/ProA/
Identification Reference bv De~osi~r~ P030/P088
D~e ~ Jcpos-~ ~slon Number
07 June 1995 (07.06.95) CRL 11921
C. ADDrrlONALlNlllCArl<)N!;~ Ivem~ r~ bis ~scon~inuedonsn~i~
Applicant(s) hereby give notice of my/our intention that
samples of the above-identified culture shall be available
only to experts in accordance with paragraph 3 of the
Fourth Schedul-_ to the Patents Rules 1995.
D- DESIGNATED STATES FOR WHIC~ INDICATIONS ARE M~DE fifr~,r~fO~ . . r. .
Singapore
E. SEPARATEFl~n~ OFINDICATIONS ~t~il.~if~ppiiccbte~
Tlre . Ii~red below wlll be sub m~ted ro rhe l~ur~u l~cr / . ~r O - r
N_ ef Oq~
For recelvmg Office use only For ~ - ~ Bure u us d~
~/This sheet w~s rece~ved w~lb Ihe - ~ r ~ This sheet w~5 rer eived by ~he I Hu ou or~
off cer f~ ~ur~nz o offic~
SUBSTITUTE SHEET (RULE 26)